Note: Descriptions are shown in the official language in which they were submitted.
CA 02261444 1999-02-11
METHOD FOR MANYNG IODII~E~lZS LOADED SUE$TRAT$9
FO~t U$E xN RADrdAGTIV$ SOURCES
9ACKGROQND 01~' '~8 INV~TTON
The preoent invention relates to a method nor a~aklng iodine-
1~5 loe~d~ad substrates for use inn radioacti.ve avurcee~. ~ Kore
particularly, tt~a present invention relateo to a dty,pracsms
~aethod sar producing iodine-Za5 loadod zeelite particles for use
in sealed braebytherapy souxces.
For decades, the usual method of commercially prodacl.ng
lodinewla5 ha~ involved the irradiation of xenoh gas with thermal
neutron~ it $ nualaat reactox. The nuclear rvactian e~loyed is
written as xenon~124(n,ga~nma)xenon-izs-~i.odine-1z5. the xenon
gas taurget is a~ually enriched in the stable isotope xenon-1~4
end ig ihradi,ated for about I duty in a container placed near or
0 ~rithfn a reactor core. The g8s is than xemaved from the
irradiation eortaiper, usually by cryogenic pumping, to a rem,ota
vessel Vhere it is held to decay nor a period v! 1 to 9 dnya_
During this perl.vd, radioactive xenon-Iz5 (ba~,p~life J,~ hours)
lvt~ted during the irradiation decey$ to form iodi,ne~125 (half-
liPe 60 days). The iodine-1~5 migrates to the decay vespel
eaZls, to w~t~ioh it adhQrea gaits ~l.rnly wlthough through poorly
undere~tood ~neahahisma. At the end of the decay period, the xenon
Qae ie pumped e.ray to be re~irradiated. The cycle mey ~
repeate6 many ti~nee to build up t?re required amount o= iodine-laS
CA 02261444 1999-02-11
i.ty the Qecay weasel. ~'he ivdine~l2s is then removed lrob the
decry vessel either by high temperature volatilisation ana
entrapment or the raGloiodi.ne vapor in an dlkallne solution, or
Ay directly applying do alkaline avlution to the interior o! the
decay veegnl~ washing the walls of the vassal, end than
xaeowrlrig the solution. tn either case) the prima=y prodttat ie
an alkaline solution usually containing iodine-128 ~rith a
conoehtration 3n the ratsge 0.5 to 1 auriea per millilf.tar.
In co~amercinl iodine-1Z5 radioactive svWrce praduativn,
eubotratss loaded with iodine-!Z5 are hermvtioal.ly aoaled in
aapeules. !lost a! this activ~.ty is i.n cvtlhactian with
brachytherapy swats~e. Th~ase are encapmu~lntsd e~ourcos with
substrates holding iodine-18S ssgled inside welded cylindrical
titanium aapeules. The teartnologies underiyiag iodine-1Z5
brechytherapy douroes era a~ariaAd in U.B. Patent tros.
3,751,0a8; a,323,055; 4,891,i65; and 4,99d,013) which patents are
ir~corpvra~ted horein bY referenen.
Currently, the substrate preparation procasaeA involve the
handling, dispensing end evaporation o= aguevus voluticns
'.e oontaining iodine-125 in high Concentrativh, such as the
aolutiona prepares bY the general method far iodine-i25
r,
production dpperibed above. It ie generally hot practical to
carry out tsese solution operations in permanently sealed Systems
at~d they always present a pubstantial radiological har~trd
because of rvl~aaas to the working environment of volatile
radioactive iodine species such as elemental iodine (1~5Z~) and
_2_
CA 02261444 1999-02-11
hydrogen l0dida (H1~6I~. Thees volatile radioiodinee are
initially generated in solution by reactivna between the iodine-
125 in the iodi.dr~ ion Zor~f~ and chemically reactive speciev such
t~s t~rdrogen and o~cygen radicals, free electrons, and hy4tog~en
peroxide produced in the solution by the rediolytia daeompositi~
of water. Much e!lott and cepital inveatmQnt are spent ih
guarding against and coping with such rele4oea.
suHMARY of Trcs r~vESrT=oN
zn aceordenc. with a broad aapact of the present invention,
there is providedl a dry~prvcesa method far proeucin~t iodine-125
108dod aVbBtrate particles for use in radioactive svurcoa. Tha
metbvd comprises prvvid~,ng a plurality of gubstrate..p4rtielas)
end aontectihg the substreto particlem with x8nvn-izs In gaseous
form during the radioaotlve decay or the xenan~las. The
subotrate partiolta retain 1,o41ne~1,2s generated by the
radivacCive Qecar of the xenon-125.
In accordance with a more specific aspect of thv prosent
invention, thrre is provided a d;y-process method of producing
oub~trate partic148 loaded with tightly bound i,od.ine-z25 !or use
aG ae the active components vt sealed radioactive sources.
rn accordance with another specific aopeat of the invention,
t~
there ie provided a method for making a plurality of iodine-Z25
loaded aubstrateB comprising the steps of providing a gourca of
xenvn~l25 in s cold trap; adding the pluraaity of pubstre~toe td a
decay vesoai~ mnd cvollng the decay vessel to reduce the
tempere~ture of the subetrete particles For improving xenon gee
-3-
CA 02261444 1999-02-11
absorption efriciency of the substrates. The cold trap and the
decay vessel are interconneoted, atl4 the oold trap ie waxmed to
cause the xenon-lZ5 tv expand in a gaseous state into the decay
vessel. For n predetermindd period vt time, rgdioactive decay of
the xenon-1z5 to iodine-lz5 i~ permfttad vhiio ire the gaseous
otatm ar'd in contact with the eub~trate particl~aa, with the
iodine-1~5 being retained by the substrata particles. Tbie
pr~sdetermined p~riod of tie ig in keeping With th,o hall-file of
xandn~i25, i.er., typically 10 to loo hours. The cold trap and
thm 4ecay vesool are diaoonnected; and then tho iodine-125 loaded
substrate p~trticlas ert reatoved. f=ore the decay veasei.
In contrast to cur7rent technology, the invention has the
following advantages. It avoids the ~xaking and handling of
radioiodine solutions at the roactor site, the dispensing and
evaporation o! redioivdine solutions nt the radioactive source
manulacturidg Dtte) and tho aeaoaiat~ad ra4iologicel hadatrds and
costs at both aites.~ The invention also pravidea source
substrates ~ohich are very uniform is their radioactivity content
relative to sub~tratea prepared by other ~nethoda.
A broad object of the invontion is to provide.subetrxtes
bearing iodine-1~5 for use in sealed radioactive sources by a
method that le radiologicaily safer and less costly than current
methods.
Another broad object of the invention ie to proviso a m~thoa
of making iodine-1Z5 b~aring sobstratas for use in sealed
radioactive sources ehnt are more consistent ~oi,thin a batch in
_4 _
CA 02261444 1999-02-11
their radioactivity content than those made by vther.aec.hode.
another broad object of the invention is to provi9e
subetrotva bearing iodi»4~i25 for ua~e in raQiodctive eouroae by
meano of a 4ry process in a seraled ay~tem uhieh Qoe6 not involve
rediaawtiva iodine so7.utiong.
A ep~oiric object o1 the invention is to provldo a t~st?~od of
inking $eoliea substrates bearing iodine-iz5 Poor use in sealed
brarhytherapy sources and other types oP sealed radioactive
6all~,'C~~ .
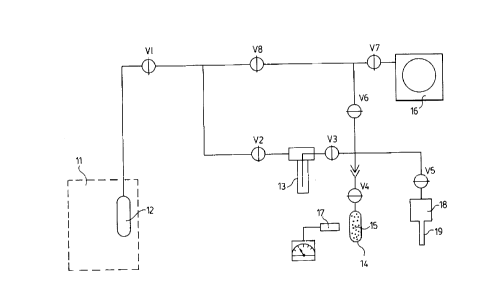
IQ BRIEF DESCArP'i'IGM OF THE DRJI~PIHO
The Figure ~ia a schematic draorirrg o! a xeno» gas hattaling
Byetem connected to a~ xenon irradiation ahanfber in the core o! a
nvcloar reactor and tv m decay ve8pel cor~ta~ining substrate
pnrtiolar.
DESCRIPTION OF THE PREF$RRED E~ODIM~?iT8
zn principle, the ob7eate~ of the invention are~~aehlavpd
through assns of absorbing and holding xanvn gas at low
t~mperaturo within partiel~a of a Suitable substrate n~ateriii.
Ths xe»on gas must contain a suitable concentration of
~o tsdioadtiye xenon--1~~ ~rhich decays tv a Buitnble amount of
iodine-1z5. The decay of the xenon~iZ5 xuet~,tdke place at
temperatures above the xenon boiling point eo that the xenon~x~5
remains in the gag phase a»d consequently the generated lodi»e-
1~5 is urii~ormly distributed throughout the absorbing aubetra.te
masm. Tha substrate material at room temperature must later
tis~ly retain the iodine-lzs produced from the decay o~ the
xenon~125 but not iretairi the xenon gaa.
-s-
CA 02261444 1999-02-11
Zaolitee~art the prolezred substrate meterietl. Zeolftes are
a olaae of crystalline molecular s3evea that occur naturally or
oan be eynthesisad irt powder lorn~. 2~hny are otable inorganic
cvmpounde~ that have open a lumano ~ s i 1 ice to Fra~tewarke that a l.l.oWg
thaw to hoot ether che~nieal epeele~'within their gtrueturae.
Zaalita~~e arc uped in large quancitiee in sang industrial
appl3eationa. comprehenelve inlorraation oat $eolit~e is given in
the textbook 'Zeolite Molecular SSeveB' by bonald p. Hreclc, John
Wiley dad Sons Irc., New York (i974), vhiah is incorporated
herein by rei~rence.
Zlolitas have sxaollent properties for use as substrate
materials in radi0llctive aourcee. The relsvaht prvpsxtias of
zeolitee in this regard include good heat ana radiation
resistance, eativn sxehange capaoities comparable with organic
xoaine, good capacity tvr th~ edaorptivn and retsntien of
radiosctive iodine, low density and average atvmie number of
elemental certstituenta weansr~q loo attenuation or lvv-energy
photons, and with a binder ran be formed into durable pellots~ or
spherical beads iri appropriate sizes.
ao In a preferred embodit~eat, the substrate partiolQS~ are driod
gevlit~ molecular sieve particles, prelerabZ~ spheres, that beve
been silver-doped by catl0~i ~xchdnga are employed ea the
gub9~trato material. 1~ e~aalJ~ metal vo~esl lilted with the zeolite
spheres is atts~Ched tv a eetiled gas handling syst~ and ovacuata~d
through a valve on th~ ve~sel.. The vessel and zeolite spheres
aro then Cooled to abc~Vt -75aC by imaersion v! the vessel in
mol~.d carbon dioxlda powder (dry iced. The e.ctual dry foe
-6-
CA 02261444 1999-02-11
sublimation tearperature at standard (atmospheric) pressure ie -
78.5°c. The cold taolite sphere mass is pertused pith a volume
of xenon ges3 highly enriohed in the stable isotope xenon~124.
The xenon gar ha~ bean just prsvicuely irradiated with neutrons
in b nuclear reactor and contains a suitable anvunt of
radioactive xenon-lZ5 farmed 9uring the irradl.atioh via th0 ,
nuclear reaotivh xenon-lZd (n.gs~mr5) xenon~la5. Most of the xersah-
la5 (half-li!~ l7 hours) is allov4d to decay to ~.odirte-12S (hsilt-
lifo 60 days) whilst in contact with the xeolits spheres. The
iodine~1~5 so permed is entrapped within the eovllte Btrvaturg or
becomms chetsically bauOd with the silver dapant in the spherms.
Ins either casA thA iodine-l25 remains rirmiy e~ttaabe~d to the
eeolite spheres vhsn) after a suits~ble deoay period, the vessel)
and ~eolite ~phsreg are returned to room temperature and the
xenon gas ~.e pumped away to ba re-irradiated in the reacto;. The
cycle may be repeated a number of tiasss4 until the~ arvunt of
iodine-Z~S ors t~ho saolite spheres reaches the desired levAl o!
rad~,oaativitY. The olos~ed vassal vorstaining the s;eolite spheres
may then be subjected to a heat treatment to t~rther s~tabiliae
o the adherence Cr the iodine-1~5 to the s3pherwr. Thnn~the
evacuated vessel, with its valve closed and containing the
reol~te l~phsres, is re~eoved free the gas handling sygt~a. The
apherae are removed from the v~sasel and may be ured withcvt
i'urther treatstent as radioactive substrates it radioactive
sources. .
Opti4nally at this stage, the zeolite apher4m xith bdeorbed
iodinG~laS may be heated to the tanperature et which the sreolite
CA 02261444 1999-02-11
etruotura collapses and vitrification oacurg. Tha vitri.=ication
tampargture for pellitizeC zeoZitve is typically ih tho range vi
trOtO about Aoo°C to aba~ut l~OV°C, vitrl,tication results in
hat'd
glassy spheres Chat are smaller than ths~ originals $r~d that have
arihanced propsstigs Eor certain appliCat~-, ~n particular,
mechanical axrahgth is i~aproved and tha potantiex tvr abrasion of
radioactlvs material from the ~aur=nc~ With attendant radiological
haaard is r~duced. The potential fen tha leaching of redioactiva
material from the sphorea is also reduced. Thi~ ie important in
io applications involving contact With liquids, and is of particulaz
importance in connection With implanted braehythera,py s~ouraee
olhere a bleak in the eneapeulativn ef A source arould oxpvev ~e
spheres to body lluids.
Xenon gas ran be strongly absoxbed by certain types of
soolitss at low te~iperature~. Romssrkably, 1 gram at either 4A nr
13X seolite at about -~ovc, rrhicri ie well above the xenon boiling
point, will bbevrlo ovor 5o milliistars of xenon gas, 2'Aia is a
vo~equerce of the special properties of seolit~ae; t?~e xenon gas
btoms are clvse~packad into the zeolite cryetel lattice almost in
ao s l.lc~uid phaaA. Zeolitee, when doprd with metallic eiesasnts such
as sllvor, are v~rY erfective in aeqvestsring nearly vhaniaal
i~
species of iodine from offlnsnt gas atreasv. Inrosmativn to this
ettedt is given in the thosts "Zaolites as scavengers for
Radioiodine 8peciea from the gas Phaee" by c.h. sa~apson and the
references therein. This thesis is incorparatad herein by
relerence.
filth ='~tere=tce tv the l~S.gure, ~ar~e is shov~n an operit~ng
_8 _
CA 02261444 1999-02-11
nuclear reactor core il vhcrein is located a xenon gas target
ohs~nber 1a connected via valves and tubing to an evacuateG xenon
gee hanQlir~q system l0. All of the valves on the eyet4m gxA
closed. The target chamber cont~eine high-pu..tity xenon ga~o
erf.riched in the sta~ole isotope xenon-~.2~. Tn~r target qaa is
ixra4lated yith neutxons so that some vt the xenon-1z4 nuclei
capture a neutron anc~ becotoe radioactive xenvn~t2s nuclei . l~tte~-
a euitaDle i,xrediation period that e~xlowe p~yfficient build-up of
xenon-»5 radioe~ctivity, a~ually about 1 day, valves ~ vi end V~
1,0 ere opeud~ and the xenon target gas end generated xenon-lZ5 are
cryo-pumped to a sold trap i3. This is acavmplieh4d by cooling
the cold trap ~rith liqu3.d nitrogon and theroby reduc~.ng the vapor
pre~sure of xenon Co essentially eero by freeaing it to the solid
state. During'the cryo-pwnping opCtation, the IImal1 dacxy vessel
14, previously f~lll~d vith suitable absorbing ~ubetrate ~rartidles
15, and puaped out using the vacuum pump ls, i$ immersed in a
guite~ble oold medium so as to reduce the temperature of the
suDatrate particles to betW~~n about -70°C and about -80°c to
thereby attain good xenon gas absorption vilicienay. valve vz is
:a thQn cloned and valve V3 eepaxating tho cold trap troe the decay
ve,sael and valve V4 on the devay vsesel era opened. The cold
trap 13 is v~atmed to room temperature allowing the frosen solid
xenon to return to the gab phase ahd to expand into the deCBy
vessel l4 ana~re it is Wrifvrmiy absorbed by the oold substrate
particles. Ve,lve Vd is theil closed. The cold medium ~,g bristly
removed tro~n around tl~e dece~y v~ssel 1~4 in ordef to faci7.itate an
accurate meeauresent o! the amount of xenon-~z5 radioactivitx in
.,g_
CA 02261444 1999-02-11
eonte~ct With Lhe substrata particleB by se8ns or 4 rbdiativn
detaotor 1'~ .
With valves V3, V4 and V7 closod, valves V5, V6.and V8 are
opened to a~.lov n fresh cb~xrge of xenon target gas to ilv~r from
the storage ve~oeel is into th4 qae target chnmbe: La for
irradiation. Ve~lve V1 iB then closed and valves v2 and V3 ate
opened. ~,'ho lines are Gryo-pumped free vt xerian by temporarily
imtaereing 4 cold rings= 19 of the storage vvseal 18 itt liquid
~1i'ttogen. A11 peen valves are then CIOged. After a eui~table
1o daoay period, usually 1 to 3 de~yo, during which rise the decay
vessel 14 ie cvntiruouslY kept Geld at a temperelture batweer~
about -~o°e and about -eo°c in vrc~er to minimize the internal
gag
pressure) the sold finger ~9 0! the atoxage vessel 18 is again
te~tporari7,y avcled with liquid nitrogen to a temperature between
about -190dC and about --t00°C. V~tlvos V4 and V5 are opelloc3, and
the sold medium ie removed l=am around the decay ve8sel. ThH
xenon t?fan fldwu from the decay vesoel ~4 to the atorege vegsai
1A loavlng the iod,ine~laS behind oe the substrate p~rt:lClms.
Then all open vAlvas Ore clocsd. The cycle taay be repeated to
gild up the enovrtt of iodine-125 vn the substrate particles.
then the substrate particles 15 mre eutEicle~ltly loaded With
iodine--185 and any xenon has been pumped array, xhe eiosed inn
evacuated decay vassal z4 and substrate particles l5 may be
hAatod to a tempart~ture betveen about loo°c and about aOOnC to
chetaically gtabiliae the iodino~125, nrd then cooled to a
tempexa,ture batt~ean about 10°c and about 30°c and pumped upon
for
a short period to re~o~e a~~y iodlina-1a5 l~tt ire the vapor pheso.
~10-
CA 02261444 1999-02-11
The decay-veeael 14 ig valued closed, disconnected at ~o
frara the gee handling eysteea 10 and ret~cved tv s ventilated
le~aiiity fox =urther handling. The nearly radloaictiva ~ubstra~te
particles 15 are thon romovad lros the decay vasbei ld and
eaa~pled for quality assurance taste prior to being available
for further proaessi.ng such as vitrification or ivr vsa in
radioactive evurooa.
It vih be reeocpnized that the system depicted 1n the Figgie
is a ai.rplo one. In practice, multiple decay veaoel8 to hold
io substrate particles) and even multiplo reactor gas~target
chdmbero xay ba installed to add flexibility and capacity tv the
sxstem.
Ih a preferred embcdi,mcnt of the a~athod described adova) the
substrate particles are zaolite spherer sop~a oritn silver by
nation exchange and meant primarily !or use in brachythorapy
oourcae) the xenon target gag is enxiched to over 50 p~r cent in
the xenon~124 stable ievtopo, and the cold medium is aolid carbon
dioxide (dry i~co).
PRGPI~ir't'IC EXAMPLE
o A 5o milliliter volume or x~non gas onriahed to over 99 per
cant in xenon-1~4 ie irradiatmB in a nuciea~r~,roactor for Z4 hours
at a tDermal neutron flux of 5 x 10i3 nautrone pot square
dar~tiatetar pex second. fhe amount of xenon-12s (half-life 17
hoys'a) produced et the end of the ~.rradiation i.s approximately coo
ourieo .
At the end of the irradiation, the xenon gas i.s tranglerred
to a small decay veaael containing io,oov silver doped type ~3X
~11-
CA 02261444 1999-02-11
~eolita mpherical beode cooled tv approximately -~3°c with dry
ice. The decay ves8al is then valves closed, and kept cool With
dry ice except fox a period of about 1 minute at the beginning
xhen the dry ice ie~ xeaiovesd ao Chat an acottxate radia~tiori reading
of the deoay vea~sel c4n be taken. The total internal volume et
the 9Qcay vessel ie 1 to 2 billilitors. The dl,ameter of aach
zevl3te bead is o.65 m,illf.motars and the i.o,0o0 beads essentially
fill the decay vessel. Nearly all o! the rerun is abmorbed end
the gee p=eoeute inside the decay va9ael is les8 than 1
atao~phare.
lifter a ?~ hour decay periell, the xonvn is punpea away to a
8torsg~ vessel.. 11t this stagy there ie a total. of appraximat~ely
Z.s curies oP iodibe-ia5 on the zeolite bead substrates, or aDOut
0.15 millicuriea per bead, xt is intendod to produce
brachythorapy sources containing two beads each end having
redioactsv~,ty cvnt~ts of 7..0 miilicuries each. Tt~e
irxadiatiors/deeay cycle is therefore repeated ~ sore times over
the next nine day8, whereupon, allawfng for ~aome decay during trie
proce~ea) each bead has slightly in exces8 0! 0.5 fii111aur~,es of
2o iodine~la5 which ie oufficietlt to make too-ba~,d ~ouroQe
co~ta~iniag z miliicu~cie per source. (Note t~rat the 24 hour xenon
irradiation period associated with a partioular eycxe vvmme»eeg
48 Z'IOIlTg into the 71 hour decay period of the previova cycle).
The beadg are transferred from the decay vesse~. into a
quartt vial that is then flame healed. The rtv;artz vial is placed
in a lurnace at zoom temperature. The furnace is turned on and
ov~r a period of one hauz~ the Ylal ana beads ere heated to
~lZ-
CA 02261444 1999-02-11
l000°C. In this psocosa, there ~.s no loss of iodine-iZS) but the
beads are vitrified and ~ceduoed in diameter by about z5 I,~er cent.
The bea4e aQhexs to each other very slightly. but upon cooling
they are easily separable by shaking and are ready for uas fn
radioaCtivs sources. -
other pvrou8 materi$,lo, such as charaoai particles) possess
otrong xenon absorption and iodine retention properties and may
be substituted ror the prslezrad ieel3ts aubet~rats particles.
certain non-porous end noh-absorbing substrata Aateri.als with
surfaces having affinity ~o~ iodin~, such as ai7.ver wires) may be
substituted for the pseterrod $avlita substtato particles.
although in this case, because there ie no xenon gee pressure
reduction by absorption, decay vessels capable of sustaining
internal gas preosures of s~verai tone a! atmospheres would be
required. Also xenon-iss may ba produced by ~oaang of a charged
particle accelerator, ratter than a reactor, by irradl.ating a.
xenon gas target oontairiing et0ble xenon-~ia~ with protdne. All
of the~a modifieationa are co~tnmplated and enaaapasoed by the
invention. 111eo, the vitrification process ~aay be used for
~4 aeolites bearing other radioisotopes such as palladium-lo3 and
ytterbium-l69.
Although the invention has been described in conjunction
with epaeitic embvaiments, it is evid~ant that many alternative$
and variations dill be apparent to those sxillaa in the art in
light at the foregoing deeeri.ptier. Accordingly, the invet~tlor
ie i,ntsnded to embracs a11 of the alternatives arid variations
that fall within the spirit end scope of the eppnnded claims.
The above references are hereby incorporated by refer~nae.
Vl~