Note: Descriptions are shown in the official language in which they were submitted.
CA 02261916 1999-01-27
WO 981'~5C15 PCTAUS97/13090
4-SUBSTITUTED QUINOLINE DERIVATIVES
HAVING FUNGICIDAL ACTIVITY
Back~round of the Invention
This invention provides novel compounds which are
4-substituted quinoline derivatives having plant
fungicidal activity. This invention also provides
compositions and combination products containing one or
10 more compounds of this invention as the active
ingredient. Some of the combination products exhibit
synergistic activity against plant pathogens. This
invention also provides fungicldal methods.
SummarY of the Invention
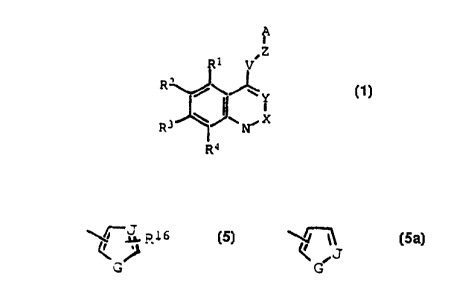
This invention provides novel compounds of formula
Z
~R V
3 J~,~ N~, X ( 1 )
wherein
X is CR5, where R5 is H, Cl or CH3;
2s Y is CR5 where R5 is H, Cl, or Br;
Z is O, S,,SO, SO2, NR6, where R6 is H, C1-C4
alkyl, C1-C4 acyl, CR7R8, where R7 and R8 are
independently H, C1-C4 alkyl, C1-C4 alkenyl, C2-C4
alkynyl, Cl-C4 acyl, CN, or OH, or R7 and R8 together
combine to form a carbocyclic ring containing four to
six carbon atoms;
CA 0226l9l6 l999-0l-27
W 098,'~ PCTAUS97/13090
R1-R4 are independently H, OH, NO2, halo, I, C1-C4
alkyl, C3-C4 branched alkyl, C1-C4 alkoxy, halo C1-C4
alkyl, halo C1-C4 alkoxy, or halo C1-C4 alkylthio, or
Rl and R2 or R2 and R3 together combine to form a
carbocyclic ring containing four to six carbon atoms;
V is CR7R8 where R7 and R8 are independently H,
C1-C4 alkyl, C1-C4 alkenyl, C2-C4 alkynyl, C1-C4 acyl,
CN, optionally substituted phenoxy, halo C,-C4 alkyl, or
10 OH, or R7 and R8 together combine to form a carbocyclic
ring containing ~our to six carbon atoms;
A is
(a) a C1-C1g saturated or unsaturated straight or
branched hydrocarbon chain, optionally including a
hetero atom selected from O, S, SO, or SO2, and
optionally substituted with halo, halo Cl-C4 alkoxy,
OH, or C1-C4 acyl;
(b) C3-Cg cycloalkyl or cycloalkenyl
(c) a phenyl group of formula (2)
R--~Rl o
ll
R13 R12 (2 )
wherein
R9-R13 are independently H, CN, NO2, OH, halo, C1-
C4 alkyl, C3-C4 branched alkyl, C2-C4 alkanoyl, halo C1-
C7 alkyl, hydroxy Cl-C7 alkyl, Cl-C7 alkoxy, halo C1-C7
CA 02261916 1999-01-27
W O 9~ '~1~ PCTrUS97/13090
alkoxy, C1-C7 aLkylthio, halo C1-C7 alkylthio, phenyl,
substituted phenyl, phenoxy, substituted phenoxy,
phenylthio, substituted phenylthio, phenyl C1-C4 alkyl,
substituted phenyl C1-C4 alkyl, benzoyl, SiR20R2lR22 or
oSiR20R21R22~ where R20 ,R21, and R22 are H, a C1-C6
straight chain or branched alkyl group, phenyl, or
substituted phenyl, provided that at least one of R20,
R21, and R22 is other than H, or R11 and R12 or R12 and
R13 combine to form a carbocyclic ring, provided that
10 unless all of R9-R13 are H or F, then at least two of
R9-R13 are ~;
~d~ a furyl group of formula (3)
~R14
0
wherein
R14 is H, halo, halomethyl, CN, N02, C1-C4 alkyl,
C3-C4 branched alkyl, phenyl, or C1-C4 alkoxy
(e) a thienyl group of formula (4)
R15
~ S~ (4)
wherein
R15 is H, halo, halomethyl, CN, N02, C1-C4 alkyl,
C3-C4 branched alkyl, phenyl, or C1-C4 alkoxy;
(f) a group of formula (5) or (5a)
~ J Rl 6
G (5) G (~a)
wherein
CA 02261916 1999-01-27
W 0 98/05645 PCTrUS97/13090
R16 is H, halo, halomethyl, CN, N02, C1-C4 alkyl, C3-C4
branched alkyl, phenyl, substituted phenyl, or C1-C4
alkoxy, and J is N or CH and G is 0, NR19 or CH,
provided that either J is N or G is NR19, where R19 is
H, C1-C4 alkyl, C1-C4 acyl, phenylsulfonyl, or
substituted phenylsulfonyl;
(g) a group selected from pyridyl or substituted
pyridyl;
(h) a group selected from pyrimidinyl or
substituted pyrimidinyl; or
(i) a group selected from 1-naphthyl, subs~ituted
1- naphthyl, 4-pyrazolyl, 3-methyl-4-pyrazolyl, 1,3-
benzodioxolyl, tricyclo~3.3.1.1(3,7)]dec-2-yl, ~-(3-
chlorophenyl~-lH-tetrazol-5-yl, pyridyl, substituted
pyridyl, or an acid addition salt of a compound of
formula (1), or an N-oxide of a compound of formula (1)
where Y is CH.
Detailed Descri~tion of the Invention
Throughout this document, all temperatures are
given in degrees Celsius and all percentages are weight
percentages, unless otherwise stated.
The term halo, used alone or in combination with
other terms, refers to F, Cl, or Br.
The term "alkyl" refers to a straight chain alkyl
radical.
The term "branched alkyl" refers to all alkyl
isomers containing the designated number of carbon
atoms, except the straight chain isomers.
CA 02261916 1999-01-27
W O ~ '615 PCTrUS97/13090
The term "alkoxy~ refers to a straight or branched
chain alkoxy group.
The term "halo alkyl~ refers to a straight or
branched alkyl group, substituted with one or more halo
atoms.
The term "halo alkoxy" refers to an alkoxy group,
substituted with one or more halo atoms.
The term "halo alkylthio'l refers to a straight or
branched alkylthio group, substituted with one or more
halo atoms.
The term "acyl~' refers to straight or branched
chain alkanoyl.
The term "substituted phenyl" refers to phenyl
substituted with up to three groups selected from halo,
20 C1-C1o alkyl, branched C3-C6 alkyl, halo C1-C7 alkyl,
hydroxy Cl-C7 alkyl, Cl-C7 alkoxy, halo Cl-C7 alkoxy,
phenoxy, phenyl, NO2, OH, CN, Cl-C4 alkanoyloxy, or
benzyloxy.
The term "substituted phenoxy" refers to a phenoxy
group substituted with up to three groups selected from
halo, Cl-C1o alkyl, branched C3-C6 alkyl, halo C1-C7
alkyl, hydroxy C1-C7 alkyl, C1-C7 alkoxy, halo C1-C7
alkoxy, phenoxy, phenyl, NO2, OH, CN, C1-C4
30 alkanoyloxy, or benzyloxy.
The term ~substituted phenylthio" refers to a
phenylthio group substituted with up to three groups
selected from halo, C1-Clo alkyl, branched C3-C6 alkyl,
halo Cl-C7 alkyl, hydroxy Cl-C7 alkyl, Cl-C7 alkoxy,
halo C1-C7 alkoxy, phenoxy, phenyl, NO2, OH, CN, C1-C4
alkanoyloxy, or benzyloxy.
CA 02261916 1999-01-27
W 098/05645 PCTrUS97/13090
The term "substituted phenylsulfonyl" refers to a
phenylsulfonyl group substituted with up to three
groups selected from halo, I, Cl-clo alkyl, C3-C6
branched alkyl, halo C1-C7 alkyl, hydroxy C1-C7 alkyl,
C1-C7 alkoxy, halo-C1-C7 alkoxy, phenoxy, phenyl, NO2,
OH, CN, Cl-C4 alkanoyloxy, or benzyloxy.
The term "unsaturated hydrocarbon chain" refers to
a hydrocarbon chain containing one to three multiple
10 bond sites.
The term "carbocyclic ring~ refers to a saturated
or unsaturated ring of four to seven carbon atoms.
While all the compounds of this invention have
fungicidal activity, certain classes of compounds may
be preferred for reasons such as greater efficacy or
ease of synthesis. These preferred classes include
those compounds of formula ~1), above, wherein
X is CR wherein R is H;
Y is CR wherein R is H;
Z is O
Rl-R4 are independently H, halo, or Cl -C4 alkyl, or
more preferably halo;
V is CH or C,-C4 alkyl, and
A is a phenyl group of formula (2), above, wherein
R9R13 are independently halo, C,-C~ alkyl, or halo C1-C7
alkyl, or more preferably a phenyl group of formula (2)
above, wherein R Rl' is independently halo; a pyridyl or
substituted pyridyl group; or a pyrimidinyl or
substituted pyrimidinyl group.
_: , I .,
CA 02261916 1999-01-27
W 0981'~5~5 PCTAUS97/13090
The compounds of formula (1) have been found to
control fungi, particularly plant pathogens. When
employed in the treatment or prevention of plant fungal
diseases, the compounds are applied to seeds or plants
in a disease-inhihiting and phytologically-acceptable
amount. The term "disease-inhibiting and
phytologically-acceptable amount", as used herein,
refers to an amount of a compound of the invention
which kills or inhibits the plant disease for which
10 control is desired, but is not significantly toxic to
the plant. This amount will generally be from about 1
to 1000 ppm, with 10 to 500 ppm being preferred. The
exact concentration of compound required varies with
the fungal disease to be controlled, the type of
formulation employed, the method of application, the
particu~ar plant species, climate conditions, and the
like. The compounds of this invention may also be used
to protect stored grain and other non-plant loci from
fungal infestation.
The following tests were performed to de~ermine
the fungicidal efficacy of the compounds of this
lnventlon.
Funqicide Utilitv
The compounds of the present invention have been
found to control fungi, particularly plant pathogens.
When employed in the treatment of plant fungal
diseases, the compounds are applied to the plants in a
disease inhibiting and phytologically acceptable
amount. As used herein, the term ~disease inhibiting
and phytologically acceptable amount", refers to an
amount of a compound of the present invention which
kills or inhibits the plant disease for which control
is desired, but is not significantly toxic to the
plant. This amount will generally be from about 1 to
1000 ppm, with 10 to 500 ppm being preferred. The
CA 02261916 1999-01-27
W 098/05645 PCT~US97/13090
exact concentration of compound re~uired varies with
the fungal disease to be controlled, the type
formulation employed, the method of application, the
particular plant species, climate conditions and the
like. A suitable application rate is typically in the
range from about 0.10 to about 4 lb/A. The compounds
of the invention may also be used to protect stored
grain and other non-plant loci from fungal infestation.
The following experiments were performed in the
laboratory to determine the fungicidal efficacy of the
compounds of the invention.
The test compounds were formulated for application
by foliar spray. The following plant pathogens and
their corresponding plants were employed.
PathogenDesignation Host
in following
Table
Erysiphe g~aminis tritici PMW wheat
(powdery mildew)
Screeninq Method for PMW:
Wheat c.v. Monon was grown in the greenhouse from
seed in a soil-less peat-based potting mixture
("Metromix"~. The seedlings were used for testing at
the 1.5 leaf stage. Compound formulation was
accomplished by dissolving technical materials in
acetone, with serial dilutions then made in acetone to
obtain desired rates. Final treatment volumes were
obtained by adding nine volumes 0.011% a~ueous Triton
X-100, resulting in test solutions with 10% acetone and
0.01~ Triton X-100. Test rates were 400, 100, 25, and
6.25ppm.
In a high volume foliar application, plants were
sprayed to runoff (using two opposing Spraying Systems
35 1/4JAUPM air atomization nozzles operated at
approximately 138 kPa. Test inoculum for wheat powdery
,
,
CA 02261916 1999-01-27
W O 98/05645 PCTrUS97/13090
mildew (E. graminis f. s~. tritici~ was produced in
vivo on stock plants in the greenhouse. The test
plants were inoculated by dusting spores from stock
plants on test plants 24 hours after spray application
After inoculation the test plants were kept in the
greenhouse for seven days, until disease on the
untreated control plants was fully developed. Seven
days after inoculation, the disease incidence on the
10 leaves was assessed visually.
The following table presents the activity of
typical compounds of the present invention when
evaluated in these experiments. The effectiveness of
1~ test compounds in controlling disease was rated using
the following scale.
NT = not tested against specific organism
0 = 0% control
~ 49% control
+ = 50-100% control
CA 02261916 1999-01-27
W O 98/05645 PCTrUS97/13090
COMPOUND RATE PMW
NU~BER (-n~)
.~no +
no +
2 +
6.~5 +
4~ +
1,
6..~
4~1 +
+
2 +
J 6. , +
4C~r~ +
~' 10~ +
2 +
6.... , +
1~
6. ,
4~,
6..-,
~, 4C~' +
7 10 +
6.~,
4~U
6.
4 +
+
6.;,
: 4C~' +
+
n 2 +
6.,,
__ 4
6.,,
4 ~ +
_ 1 +
+
_ 6.~5 +
4~ +
+
_ 6.,,
_~ 4~ rl
_L 10
~ 2
- 6.,~ _
. 4 ~' +
+
_ 6._5
_ 4C~ +
-10-
CA 02261916 1999-01-27
W O 98/05645 PCTrUS97/13090
_ 2 +
_ 6.-~ -
: 4~u +
7 1 111 +
.7 2 +
_7 6. +
_ 4 +
+
2~ +
_ 6.. ~ +
_ 4C~' +
+
2 +
- 6. ,
4~' +
+
. 2 +
_ 6. ~ +
+
;. 6. +
4l +
+
_ 6.
+
__ 6. ~ -
~ 4 ~ +
+
. . _ +
- - 6. ,
4C~ _
4 ~ +
+
6..,
.- 400 +
~ 10 +
2 +
6.25
4C~' +
- 2
6. ~ -
4 ~ -
6.
400
- 10
6..,
-- 4~ _
_
-' 6. s
'~ 4~0
.
CA 02261916 1999-01-27
W O 9~ 15 PCT~US97/13090
- lC~ _
6.~5
+
+
6.. ~ -
- 4~ +
+
+
6.. ~ +
+
+
6. ~ +
4l +
1l +
6. ~ _
- ' +
6. ~ -
+
6 -
' 6.. ~ _
6.~ _
~ 6.. 5
4_ 4~ -
~ 1 --
~_ 6. ~
4 40 +
~_ 6.~5
'' 4~ _
'- 10
'~ 2
6. ~ _
- 4~
~ 1 _
6.~ _
4~ _
1 l _
4 6.. ~ -
~ 40~ _
~7 2
~7 6._5
8 4C~ -
-12-
CA 02261916 1999-01-27
W O ~8/~'C15 PCTrUS97/13090
lC~ -
~' 6i 7 5
' 6. 5
4 ~U
~ 2
6. ~ _
lr
6.,~
6..5
4 +
2 +
6.~5 +
6..,
+
6. , +
+
2 +
6..,
4~ +
7 2 +
6.,, +
4~u +
+
6.25 +
The compounds of this invention are made using
well known chemical procedures. The required starting
materials are commercially available, or readily
synthesized utilizing standard procedures, several of
which are disclosed in U.S. Patent 5,145,843. The
compounds of formula (1) are then prepared by treatment
of the corresponding 4-V substituted lepidine
derivative with the appropriate -Z-A containing
10 derivative.
CA 02261916 1999-01-27
W O ~8i'1~ PCT~US97/13090
The following nonlimiting examples further
illustrate this invention.
ExamPle 1
4-((4-Fluoro~henYloxY)methyl~-8-Chloroauinoline
NaH/THF ~ ~F
F~--OH N
C~ Cl
4-Bromomethyl-8-chloroquinoline (0.8 g, 3.11 mmol)
was dissolved with stirring in dry THF (10 mls) and
sodium hydride (0.15 g, 60% dispersion in mineral oil,
6.23 mmol) added. The mixture was stirred at room
temperature for 15 minutes and 4-fluorophenol (0.52 g,
5 3 .11 mmol) added. The mixture was stirred at room
temperature overnight and worked up to provide the
product (0.46 g, 51.2~) as a white solid, mp 144-5~C.
Found: C,66.41; H, 3.67; N, 4.88%; calculated: C,
66.87; H, 3 . 67; N, 4.88%
Exam~le 2
4-(2-PhenYlethenvl)-7-chloroauinoline
Br ~J
~JI~ R3P/Pd(OAc)2 ~ ~.
2s Cl ~ N Et3N,130~C Cl ~ N
-14-
CA 02261916 1999-01-27
W O 98J'O5~15 PCTrUS97/13090
A mixture of 4-bromo-7-chloroquinoline (24.3 g,
0.1 mol), styrene (12.0 g, 0.125 mol), tri(o-tolyl)
phosphine (0.4 g, 1.3 mmol), palladium acetate (0.2 g,
0.89 mol) and triethylamine (120 mls) was charged into
a 300 ml stirred pressure vessel and heated at 120~C
for 17 hours. The mixture was cooled, filtered to
remove triethylamine hydrobromide, and the solids
washed with ethyl acetate (250 ml). Solvents were
evaporated under reduced pressure and the residue
10 dissolved in ethyl acetate (500 ml). The solution was
washed with water and brine, and dried over anhydrous
sodium sulphate. Evaporation of the solvent under
reduced pressure and recrystallisation of the residue
from ethyl acetate: hexane gave the product (13.5g,
15 51%) as an orange solid, mp 120-122~C.
Found: C, 76.50; H, 4.62; N, 5.38%; calculated: C,
76.84; H, 4.55; N, 5.27%
Exam~le 3
4-((3-Trifluoromethvl-2-~Yridinvloxv)methyl)-7-
chloro~uinoline
l~q
F3C~
~OH ~O
Cl ~N NaH/THF ,~
2~
4-Hydroxymethyl-7-chloroquinoline (O.6 g, 3.12
mmol) was dissolved with stirring in dry THF (20 mls)
and sodium hydride (0.15 g, 60% dispersion in mineral
- oil, 3.75 mmol) added. The mixture was stirred at room
30 temperature for one hour and 2-chloro-3-
trifluoromethylpyridine (0.62 g, 3.42 mmol) added. The
-15-
.
CA 02261916 1999-01-27
W 098/05645 PCT~US97/13090
mixture was stirred overnight and worked up to provide
the product (0.9 g, % as a tan solid, mp 125-7~C.
Found: C, 56.68; H, 2.90i N, 8.17%; calculated: C,
56.74; H, 2.98; N, 8.27%
Exam~le 4
4~ (4-Fluoro~hen~l)oxvlethvll-5,7-dichloroauinoline
Cl OH Cl Br
Cl J~N Cl J~)N
Bromine (15 ml, 0.30 mol) was dissolved in
acetonitrile (200 ml) and added dropwise to a
suspension of 4-hydroxy-5,7-dichloroquinoline (60 g,
0.28 mol) (Swiss Pat. CH 93-3640 931207) and
tripheny~phosphite (78 ml, 0.30 mol) in acetonitrile (l
L) over three hours. The reaction was left to stir for
24 hours at which point it was filtered to collect the
precipitate. The solid was suspended between water ~1
L) and dichloromethane (500 ml) and neutralized with
sodium bicarbonate. Extractions were performed
periodically as the aqueous layer neared neutral and
finally at pH 10 to give a total of 5X500 ml aliquots.
The organics were combined, dried (magnesium sulfate),
filtered through a plug of silica gel, and concentrated
under vacuum to a total volume of 1 L, then heated
until solid dissolved and left to crystallize 12 hours.
Filtration gave analytically pure product (46 g, 65%)
while concentration of the mother liquor gave
spectroscopically clean product ~14 g, 20%, mp 131~C).
- -16-
CA 02261916 1999-01-27
W O 98/05645 PCTrUS97/13090
Cl ~ ~ Cl ~
A solution of 4-bromo-5,7-dichloroquinoline (5.7
g, 21 mmol), Palladium(II)acetate (0.93 g, 4.2 mmol),
tri-o-tolylphosphine t2.5 g, 8.2 mmol), styrene (5 ml,
26.2 mmol), copper iodide ~0.80 g, 4.2 mmol), and
triethylamine (4 ml, 28 mmol) in acetonitrile (41 ml)
was heated at reflux for three hours. After cooling to
ambient temperature, the reaction was diluted with
l O ethyl acetate and filtered through a plug of silica
gel. This material was used as is minus an analytical
sample (178 mg, mp 242~C) used for characterization.
The resulting solid was taken up in methanol,
dichloromethane solution (1:1, 600 ml) and cooled to -
15 78~C. Ozone was passed through the system until thereaction was complete as determined by GCMS. Thiourea
was added (5 g, 600 mmol) and left to warm to ambient
temperature, filtered through a plug of silica and
rinsed with through with dichloromethane. The solvent
20 was removed under vacuum with slight heat until a white
solid precipitated. The solid was collected as several
crops to give the desired aldehyde (2.1 g, 45~ over 2
steps, mp 154~C ). Analytical samples were obtained by
recrystalization from ethylacetate.
2!~
Cl ~ Cl
Cl ~ ~ Cl ~
The aldehyde (100 mg, 0.44 mmol) was dissolved in
toluene (6 ml) and cooled to 0~C. Methylmagnesium
bromide (1.4 M in toluene/tetrahydrofuran) was dripped
in until the starting material was exhausted as judged
by TLC. The reaction was diluted with ethylacetate (50
-17-
CA 02261916 1999-01-27
W O ~8/05C15 PCTrUS97/13090
ml) and O.S N HCl (50 ml) and allowed to warm to
ambient temperature. The organic layer was
additionally extracted with 0.5 N HCl (3X50 ml). The
aqueous layers were combined and neutralized with
s sodium bicarbonate and extracted with ethylacetate
(4XS0 ml)the organics were dried (Magnesium sulfate),
filtered and concentrated to give clean product (101
mg, 94%, mp 112~C).
Cl ~ N
Neat diethyl azodicarboxylate (0.30 ml, 1.9 mmol)
was added to a solution of secondary alcohol (300 mg,
1.25 mmol), triphenylphosphine (600 mg, 2.3 mmol), and
4-fluorophenol (200 mg, 1.7 mmol) in chloroform (6
ml)over 15 minutes. The reaction was allowed to stir
for two hours, concentrated under vacuum and purified
by medium pressure chromatography, (10:1, heptane/ethyl
20 acetate). the resulting solid was recrystalized from
pentane to give the phenoxy lepidine (325 mg, 78%, mp
1 0 1 ~C )
Exam~le 5
4-~(4-fluoro~henYlamino)methvl)l-5,7-dichloroquinoline
C1 /~
Cl
-18-
CA 02261916 1999-01-27
W O 98/05645 PCTrUS97/13090
5,7-Dichloro~uinoline-4-carboxaldehyde (500 mg,
2.2 mmol) and 4-fluoroaniline (246 mg, 2.2 mmol) were
combined in 20 ml benzene with magnetic stirring. The
flask was fitted with a Dean-Stark trap and heated to
reflux overnight. Reaction was monitored by TLC and
shown to be complete after 16 hours. The benzene was
removed in vacuo and the resulting solid recrystallized
from ethyl acetate/heptane. Yield: 0.5 g (71%), mp
10 ~53~C.
C1 ~NH
C1 ~
The product above(600 mg, 1.9 mmol) was dissolved in 10
15 ml ethanol. Sodium borohydride (90 mg, 2.4 mmol) was
added and the reaction magnetically stirred overnight
at room temperature under nitrogen atmosphere.
Reaction was monitored by TLC and shown to be complete.
The reaction was diluted with water and extracted with
ethyl acetate. The organic layer was dried over
magnesium sulfate, filtered and evaporated in vacuo.
The resulting solid was recrystallized from ethyl
acetate/heptane. Yield: 180 mg (30%), mp lb6.5~C.
Exam~le 6
4-HvdroxYmethYl-5,7-dichlororoquinoline
--19--
;
CA 02261916 1999-01-27
W O~8~!~SÇ15 PCTrUS97/13090
OH
Cl Br Cl
CIJ~ Cl~
A solution of 4-bromo-5,7-dichloroquinoline (5.7
g, 21 mmol), palladium(II)acetate (0.93g, 4.2 mmol),
tri-o-tolylphosphine (2.5 g, 8.2 mmol), styrene (5ml,
26.2 mmol), copper iodide (0.80g, 4.2 mmol), and
triethylamine (16 ml, 115 mmol) in acetonitrile (41 ml~
was heated at reflux for three hours. After cooling to
ambient temperature, the reaction was diluted with
10 ethyl acetate and washed with dilute hydrochloric acid
and brine. The organic layer was dried (MgSO4) and
concentrated to a white solid. The solid was taken up
in a methanol/dichloromethane solution (1:1, 400 ml)
and cooled to -78~C. Ozone was passed through the
system until the reaction was complete as determined by
GCMS. Thiourea was added (5.0 g, 600 mmol) and left to
warm to ambient temperature. The reaction was diluted
with dichloromethane and filtered through a plug of
silica gel eluting with methanol/dichloromethane (1:1).
The solution was cooled to 0~C and sodium borohydride
was added as a solid over one hour until the reaction
was deemed complete by GCMS. The reaction was quenched
and washed with lN hydrochloric acid. The aqueous
layers were combined and filtered through a plug of
2s cotton and then neutralized with sodium bicarbonate.
The solid was collected by filtration and air dried to
give the desired product (2.8 g, in >80% purity. An
analytical sample was prepared via recrystalization
from ethyl acetate/methanol (mp 196~C).
Exam~le 7
4-HydroxYmethYl-7-chloroauinoline
-20-
;
....
CA 02261916 1999-01-27
W O 98~'~5615 PCTrUS97113090
OH
Br
CIJ~ ~ CIJ~
A 200 ml stainless steel autoclave was loaded with
4-bromo-7-chloroquinoline (1.2 g, 5.0 mmol) (Can. Pat.
CA 94-2133620 941004), bis(triphenylphosphine)palladium
chloride (0.1 g), triethylamine (3 ml) and ethanol (40
ml) and pressurized to 200 psi with carbon monoxide.
The autoclave was heated at 120~C for 12 hours, cooled,
and vented. Solids were removed by filtration through
10 celite and the mother liquor concentrated in vacuo.
The residue was taken up in chloroform (50 ml), washed
with water (3x50 ml), saturated brine (50 ml), and
dried (Na2SO4). Filtration and removal of solvent left
1.3 g of a brown li~uid. Flash chromatography on
silica using 1 vol % CH3CN in CH2Cl2 as eluent afforded
product as a colorless syrup which solidified to a waxy
solid. This solid was dissolved in methanol (50 ml)
and sodium borohydride was added over two hours until
judged complete by TLC. The reaction was diluted with
water and acidified with lN hydrochloric acid and then
neutralized with sodium bicarbonate. The resulting
solid was recovered via filtration, dissolved in ethyl
acetate and dried (MgSOg). Filtration and
concentration afforded the desired compound (mp: 160~C,
0.66 g, 68% over two steps).
Exam~le 8
8-Chlorole~idine
-21-
CA 02261916 1999-01-27
W 0~8~'~5615 PCTrUS97/13090
--NHz O~
Cl Cl
Commercially available 2-chloroaniline (200 g,
1.56 mol) was dissolved in 600 ml ethanol, and dry HCl
gas was bubbled through for 10 minutes to give 2-
chloroaniline hydrochloride. A new flask was charged
with 2-chloroaniline hydrochloride (130 g, 0.793 mol),
ferric chloride hexahydrate (25.7 g, 0.095 mol),
10 anhydrous zinc chloride (10.9 g, 0.0799 mol) and 500 ml
2B ethanol. The mixture was heated to 60~C for 10
minutes and 1,3,3-trimethoxy butane was added dropwise
over a period of one hour. The mixture was then
refluxed for two hours and allowed to stand overnight
at room temperature. Most of the alcohol was removed
by distillation and the residue was made alkaline with
25% sodium hydroxide solution. The reaction was
cooled, filtered, and the filter pad washed with
toluene. The toluene was concentrated to dryness. The
product was recrystallized from dichloromethane/pentane
to obtained a spectroscopically pure sample (mp: 110~C,
37.0 g, 25 % overall yield).
ExamDle 9
2~
4-Bromometh~l-8-chloro~uinoline
CH3 CH28r
Cl Cl
-22-
CA 02261916 1999-01-27
W O 98/05645 PCTAUS97/13090
The 8-chlorolepidine (20.0g, 0.113 mol) and
N-bromosuccinimide, (20.0 g, 0.113 mol) were dissolved
in 200 ml dry carbon tetrachloride and the mixture
5 stirred under nitrogen for three hours, while being
exposed to a 250 watt high intensity sun lamp. The
solution was cooled to room temperature, filtered, and
evaporated to dryness. The residue was placed over a
silica gel column using ethyl acetate!pentane, to give
lO the bromomethyl lepidine (10.8 g, 37.4 % yield, mp:
92~C).
Exam~le 10
7-Chloro- a- ( tri fluoromethvl)-4-auinolinemethanol
~0 F3('~0H
Cl ~
4-Carboxaldehyde-7-chloro quinoline (1.00 g, 5.22
mmol) was dissolved in 12.5 ml of a 0.5 M solution of
trimethyl(trifluoromethyl)silane in tetrahydrofuran in
an oven dried, nitrogen swept 100 ml round bottomed
flask, and the resulting solution was cooled to 0~C
25 under nitrogen. With stirring, tetra-n-butyl ammonium
fluoride trihydrate (0.014 g, 0.052 mmol) was added as
a solid and the mixture allowed to warm slowly to room
temperature over two hours by which time thin layer
chromatography Ihexanes:ethyl acetate / 2:1) indicated
complete consumption of the starting material. The
reaction mixture was cooled to 0~C and 10% aqueous
hydrochloric acid (3 ml) was added and the mixture
stirred at room temperature until cleavage of the
trimethyl silyl ether was complete as indicated by thin
layer chromatography (1 h). The reaction mixture was
partitioned between saturated sodium hydrogen carbonate
and ethyl acetate, the layers separated and the aqueous
- -23-
CA 02261916 1999-01-27
W O 98/05645 PCTAUS97/13090
layer extracted with an additional portion of ethyl
acetate. The combined organic layers were dried
(MgSO4) filtered and concentrated to dryness.
Purification by flash silica gel chromatography
(hexanes:ethyl acetate / 2:1) provided 7-chloro-a-
(trifluoromethyl)-4-quinolinemethanol (1.32 g, 96%) as
a yellow solid. An analytical sample was prepared by
re crystallization from ethyl acetate:hexanes. (mp
161~C)
Exam~le 11
5-Fluoro-2-(methYlsulfonyl)~vrimidine
SO2Me
N ~ N
F~HC ~ H
F NMe~l~
F
N-~2,3,3-Trifluoro-1-propenyl)trimethylammonium
iodide (10.0 g, 35.6 mmol) (Tetrahedron Lett. 1995,
36(9), 1527) was dissolved in 70 ml of acetonitrile in
a oven dried nitrogen swept 250 ml round bottomed
flask. With stirring, diethylamine (15.6 g, 213 mmol)
was added via syringe, the flask was fitted with a
reflux condenser and stirred under nitrogen at 75-80~C
for one hour. The mixture was allowed to cool to room
temperature and 2-methyl-2-thiopseudourea sulfate (19.8
g, 71.2 mmol) and sodium methoxide (3.80 g, 71.2 mmol,
15 ml of a 25% solution in methyl alcohol) were added
with stirring and the mixture heated to reflux for six
hours. The cooled reaction mixture was poured into
water, the layers separated, the aqueous phase
extracted with methylene chloride (3 x 50 ml) and the
combined organics were dried ~sodium sulfate), filtered
and concentrated to low volume. Purification by flash
silica gel chromatography (hexanes:ethyl acetate /
10:1) provided the volatile 5-fluoro-2-(thiomethyl)
pyrimidine, which was immediately dissolved in 100 ml
of methylene chloride in a 500 ml round bottomed flask,
-24-
CA 02261916 1999-01-27
W O 98tOS645 PCTrUS97/13090
and treated at 0~C with 3-chloroperoxybenzoic acid
~21.6 g, 125 mmol) with good stirring. After stirring
for 15 hours at room temperature, the oxidation was
judged complete by thin layer chromatography
(hexanes:ethyl acetate / 1:2). The reaction mixture
was poured into saturated sodium hydrogen carbonate,
the layers separated and the aqueous phase extracted
with methylene chloride and the combined organics
washed with saturated sodium hydrogen carbonate and
o dried (NaSO4), filtered and concentrated. Purification
by flash silica gel chromatography (hexanes:ethyl
acetate / 1:2) provided 5-fluoro-2-(methylsulfonyl)
pyrimidine (4.3 g, 68% (from N-(2,3,3-trifluoro-1-
propenyl) trimethylammonium iodide) as colorless oil:
Analysis calcd for CsHsFlN2O2S: C, 34.09i H, 2.86; N,
15.9; S, 18.2. Found: C, 34.09; H, 2.79i N, 15.81; S,
18.3.
Example 12
7-Chloro-4-~2,2,2-trifluoro-1-~(5-fluoro-2-
~vrimidinYl)oxYlethYllauinoline
rl~ ~; ' ~ F
7-Chloro-a-(trifluoromethyl)-4-quinolinemethanol
(0.25 g, 0.96 mmol) was dissolved in 5 ml of
tetrahydrofuran in a oven dried, nitrogen swept 25 ml
round bottomed flask and the resulting solution was
cooled to 0~C under nitrogen. With stirring, NaH
(0.042 g, 1.1 mmol, 60% dispersion in mineral oil) was
added all at once. After 15 minutes, 5-fluoro-2-
(methylsulfonyl) pyrimidine (0.17 g, 0.96 mmol) was
added dropwise via syringe as a solution in
tetrahydrofuran (2.5 ml) over S minutes. An additional
2 ml of tetrahydrofuran was used to rinse the flask
containing the sulfone and the syringe. The milky
CA 02261916 1999-01-27
W09~ 5 PCT~S97/13090
solution was allowed to warm to room temperature and
stir for 15 hours by which time thin layer
chromatography ~hexanes:ethyl acetate / 1:1) indicated
complete consumption of the starting materials. The
reaction mixture was partitioned between water and
ethyl acetate, the layers separated and the aqueous
layer extracted with an additional portion of ethyl
acetate. The combined organic layers were dried
(MgS04) filtered and concentrated to dryness.
10 Purification by flash silica gel chromatography
(hexanes:ethyl acetate / 2:1) provided 7-chloro-4-
[2,2,2-trifluoro-1-[(5-fluoro-2-pyrimidinyl)
oxy]ethyl]quinoline (0.32 g, 94%) as a white solid. An
analytical sample was prepared by re crystallization
from hexane. (mp 92~C).
The following table identifies compounds of
formula (1) prepared analogous to the various processes
illustrated in the preceding examples.
Cmpd R1-R4 X Y V Z A m.p
No (~C)
1 7-Cl CH CH CHCH3 O 4-~luo v~h~yl 104-106
2 7-Cl CH CH CHC6H13 o 4-fluo v~h~yl 38-40
3 7-Cl CH CH CHCN O 4-fluoLo~h~.lyl 118-123
4 7-Cl CH CH CHcH=cH2 O 4-fluo.v,vhe.. yl 186-190
7-Cl CH CH CH2 o 2,5-trifluoro- 83-85
methyl-6-pyridinyl
6 7-Cl CH CH CH2 o 4,6-methoxy-2- 134-136
pyrimidinyl
7 5,7- CH CH CH2 o 2-trifl~oro- 108.1-
diCl methyl-6-pyridinyl 110 1
8 5,7- CH CH CH2 O 4-metho~y-6 166-167
diCl methyl-2-
pyrimidinyl
9 7-Cl CH CH CH2 O 4-methoxy-6- 114-116
methyl-2-
pyrimidinyl
-26-
SIJBSTITUTE SHEET (RULE 26)
CA 0226l9l6 l999-0l-27
WO 98.'lC~4~ PCTrUS97/13090
.
5,7- CH CH CH2 NCH3 4-fluo~h_.. yl 118-122
diCl
11 5,7- CH CH CH2 o 2-trifluo,~ -Lhyl~ 176.1-
diCl 6-pyridinyl 178.1
12 5,7- CH CH CH2 o 4-methoxy-2- 211.1-
diCl pyrimidinyl 213.1
13 5,7- CH CH CHcH2cH3 0 4-fluoro~henyl 102-103
diCl
14 5,7- CH CH CH2 o 4,6-~i -thyl~1~3~ 169.4-
diCl pyrimidinyl 170.4
15 7-Cl CH CH CH2 o 4-trifluor. -thyl~ 99-101
2-pyridinyl
16 7-Cl CH CH CH2 o 4,6-dimethyl-1,3- 145-147
pyrimidinyl
17 7-Cl CH CH CH2 o 4-methoxy-2- 125-127
pyri m; ~i nyl
18 7-Cl CN CH CH2 0 ~ 61-63
N CF3
19 7-Cl CH CH CH2 o ~CF3 110-112
20 5,7- CH CH CH2 ~ CF3 134.5-
diCl ~ 136.5
21 7-Cl CH CH CH2 o 2-chloro-4-fluoro- 116.3-
phenyl 117.3
22 7-Cl CH CH CH2 o 2-trifluoromethyl- 112-115
phenyl
23 7-Cl CH CH CH2 o F3C~ 125-127
24 7-Cl CH CH CH2 o N~ 152-153
F
8-F CH CH CH2 o 2-chlorophe.. yl 122-123
26 8-F CH CH CH2 o 4-fluo ~h~yl 124-125
27 7-Cl CH CH CH2 o 2,4-fl~olvphol.yl 83.5-
85.5
28 7-Cl CH CH CH2 o ~CCH3 104.5-
106
29 7-Cl CH CH CH2 o 2-chlor~henyl 143-144
SUBSTITUTE S~1EET (RULE 26)
CA 0226l9l6 l999-0l-27
WO 98,~ ~61~ PCT/US97113090
H CH CH CH2 o ~C(CH3)3 93~5
94 5
31 H CH CH CH2 o 2-trifluoL. -~.yl~ 107.5-
phenyl 108.5
32 H CH CH CH2 o 2-chlo oph~.,yl 92.5-
94.5
33 8-F CH CH CH2 o ~C(CH3)3 78-81
34 8-Cl CH CH CH2 o 2-chlor~}.~-.yl 122-123
5,7- CH CH CHCH3 o 4-fluoro~henyl 104.5-
diCl 105 5
36 7-Cl CH CH CH2 O 4-fluol~}.er.yl 133-135
37 5,7- CH CH CH2 o 2-chloro-4-fluoro- 142-143
diCl phenyl
38 8-F CH CH CH2 o phenyl 101-103
39 8-F CH CH CH2 o 2-trifluoromethyl- 128-131
phenyl
8-F CH CH CH2 O 2~4-fluvl~h~--yl 98-100
41 8-F CH CH CH2 O ~ O ~ 96-98
42 8-F CH CH CH2 o 2-chloro-4-fluoro- 117 5-
phenyl 121.5
43 5,7- CH CH CH2 o 4 ph.-lo~y~h~l-yl 153-155
diCl
44 5,7- CH CH CH2 o 4-t-butylphenyl 136-137
diCl
8-Cl CH CH CH2 O ~C(CH3)3 107-109
46 8-Cl CH CH CH2 o 2,4-~ifl~r~he.. yl 156-158
47 8-Cl CH CH CH2 o phenyl 100-102
48 8-Cl CH CH CH2 o 2-trifluoL~I.. hyl 146-148
49 8-Cl CH CH CH2 0 4-fluoloph~.. yl 144-145
H CH CH CH2 o 2-chloro-4-fluoro- 125.5-
phenyl 126 5
51 8-Cl CH CH CH2 o ~ O ~ 120-122
52 8-Cl CH CH CH2 O 2-chloro-4-fluoro- 132-135
phenyl
53 5,7- CH CH CH-o~F O 4-fluor~h~--yl 110-112
- -28-
SUBSTITUTE SHEET (RULE 26)
CA 02261916 l999-0l-27
W O 98~561~ PCT~US97/13090
diCl
54 ~ C~ CH CH2 o 4-fluor~,~ yl 111-113
S,7- CH CH CH2 N 4-fluoro~ yl 167
diCl
56 5,7- CH CH CH2 o 2-trifluo~. ~.yl~ 145-146
diCl phenyl
S7 5,7- CH CH CH2 o 2,4-diflu~ ~}.~.. yl 154-156
diCl
58 5,7- CH CH CH2 o 4-fluGr~ yl 133-134
diCl
The compounds of this invention are applied in the
form of compositions, which are important embodiments
of the invention, and which comprise one or more
s compounds of formula (1) with a phytologically-
acceptable inert carrier. The composition may
optionally include fungicidal combinations which
comprise at least 1% of one or more compounds of
formula (1) with another fungicide.
The compositions are either concentrated
formulations which are dispersed in water for
application, or are dust or granular formulations which
are applied without further treatment. The
15 compositions are prepared according to procedures which
are conventiona~ in the agricultural chemical art, but
which are novel and important because of the presence
therein of the compounds of this invention. Some
description of the formulation of the compositions
will, however, be given to assure that agricultural
chemists can readily prepare any desired composition.
The dispersions in which the compounds are applied
are most often aqueous suspensions or emulsions
2s prepared from concentrated formulations of the
compounds. Such water-soluble, water suspendable, or
emulsifiable formulations are either solids usually
known as wettable powders, or liquids usually known as
-29-
- . SUBSTITUTE SHEET (RULE 26)
CA 02261916 1999-01-27
W 0~8,~'Ç15 PCTrUS97/13090
emulsifiable concentrates or a~ueous suspensions.
Wettable powders, which may be compacted to form water
dispersible granules, comprise an intimate mixture of
the active compound, an inert carrier and surfactants.
The concentration of the active compound is usually
from about 10% to 90%. The inert carrier is usually
chosen from among the attapulgite clays, the
montmorillonite clays, the diatomaceous earths, or the
purified silicates. Effective surfactants, comprising
10 from about 0.5% to about 10~ of the wettable powder,
are found among the sulfonated lignins, the
naphthalenesulfonates, alkylbenzenesulfonates, the
alkyl sulfates, and non-ionic surfactants, such as, for
example,- ethylene oxide adducts of alkyl phenols.
Emulsifiable concentrates of the compounds
comprise a convenient concentration of a compound, such
as from about 10% to about 50% of liquid, dissolved in
an inert carrier, which is either a water miscible
solvent or a mixture of water-immiscible organic
solvents, and emulsifiers. Useful organic solvents
include aromatics, especially the high-boiling
naphthalenic and olefinic portions of petroleum such as
heavy aromatic naphtha. Other organic solvents may
also be used, such as, for example, terpenic solvents,
including rosin derivatives, aliphatic ketones, such as
cyclohexanone, and complex alcohols, such as 2-
ethoxyethanol. Suitable emulsifiers for emulsifiable
concentrates are chosen from conventional nonionic
surfactants, such as those mentioned above.
Aqueous suspensions comprise suspensions of water-
insoluble compounds of this invention, dispersed in an
aqueous vehicle at a concentration in the range from
about 5% to about 50%. Suspensions are prepared by
finely grinding the compound, and vigorously mixing it
into a vehicle comprised of water and surfactants
chosen from the same types discussed above. Inert
-30-
,
CA 02261916 1999-01-27
W O ~'CC~15 PCTrUS97/13090
ingredients, such as inorganic salts and synthetic or
natural gums, may also be added, to increase the
density and viscosity of the aqueous vehicle. It is
often most effective to grind and mix the compound at
the same time by preparing the aqueous mixture, and
homogenizing it in an implement such as a sand mill,
ball mill, or piston-type homogenizer.
The compounds may also be applied as granular
10 compositions, which are particularly useful for
applications to the soil. Granular compositions
usually contain from about 0.5% to about 10% of the
compound, dispersed in an inert carrier which consists
entirely of in large part of clay or a similar
15 lnexpensive substance. Such compositions are usually
prepared by dissolving the compound in a suitable
solvent, and applying it to a granular carrier which
a]has been pre-formed to the appropriate particle size,
in the range of from about 0.5 to 3 mm. Such
compositions may also be formulated by making a dough
or past of the carrier and compound, and crushing and
drying to obtain the desired granular particle.
Dusts containing the compounds are prepared simply
by intimately mixing the compound in powdered form with
a suitable dusty agricultural carrier, such as, for
example, kaolin clay, ground volcanic rock, and the
like. Dusts can suitably contain from about 1% to
about 10% of the compound.