Note: Descriptions are shown in the official language in which they were submitted.
CA 02262788 1999-02-09
W O 98/07701 PCTrEP96/03610
Non-ulcerogenic anal~esic/antiinflammatory clorliYi n
derivative
This invention refers to a novel product which is useful
in human and animal therapeutics, particularly in the
treatment of pain and/or inflammation with non-steroidal
analgesic/antiinflammatory agents and with low
ulcerogenic effect.
BACKGROUND ART
The treatment of pain and/or inflammation in mammals with
concomitant absence of adverse side-effect has been a
goal long sought. In general steroids having cortisone-
like activity suffer from the drawback of the sideeffects induced by the corticoid, such as electrolyte
imbalance, water retention, etc. Thus, the treatment with
non-steroidal analgesic/antiinflammatory agents has
emerged as a whole field in modern therapeutics.
Relatively active analgesic/antiinflammatory products are
already known in the art, and many of them are on the
market. However, virtually all of them present ulceration
of the gastrointestinal track as an adverse side-effect.
Thus, the provision of new analgesic-antiinflammatory
products that have a better balance between high
analgesic/antiinflammatory activities and low ulcerogenic
adverse effects, is an important problem of modern
therapeutics. This problem has not been solved
satisfactorily yet, despite the existence of abundant
research in the field.
From the chemical point of view, most commercial non-
steroidal analgesic/antiinflammatory active principles
are carboxylic acids, often used in the form of salts.
CA 02262788 1999-02-09
W O 98/07701 PCTAEP96/03610
One group of these products includes derivatives of
salicylic acid, such as diflunisal. Another group
includes 2-aryl-substituted propionic acids, such as
ibuprofen, ketoprofen, naproxen, suprofen, tiaprofenic
acid, flurbiprofen, and -in a broad sense- indobufen and
ketorolac. Another group includes aryl-substituted acetic
acids, such as diclofenac, etodolac, fentiazac, sulindac
and indomethacin. Another group includes substituted 2-
anilinobenzoic acids, such as ~efenamic and meclofenamic
acids. Finally, another group includes substituted 2-
anilinonicotinic acids such as clonixin or clonixic acid
(IV), the carboxylic acid which is structurally closest
to the product of the present invention.
O
~'N ~ N~
~ CH3
(IV)
Most of the attempts to ameliorate the balance between
high analgesic/antiinflammatory activities and low
ulcerogenic adverse effects of the above-mentioned
carboxylic acids have involved the preparation of esters,
such as those illustrated below.
US 5.508.301 discloses the 2-(1-pyrrolidinyl)ethyl ester
of ketorolac. US 4.548.952 discloses the carboxymethyl
ester of diclofenac, which is being used under the name
aceclofenac. Nitroxy-(C1-C10)-alkyl esters of several
CA 02262788 l999-02-09
W O 98/07701 PCT~EP96/03610
analgesic/antiinflammatory carboxylic acids, such as
ketorolac, flurbiprofen, ketoprofen, naproxen,
indomethacin and diclofenac, are claimed in WO 95/09831,
WO 94/12463 or WO 94/04484.
In respect of clonixic acid (IV), the carboxylic acid
which is structurally closest to the product of the
present invention, attempts have been made to ameliorate
the balance between high analgesic/antiinflammatory
activities and low ulcerogenic adverse effects that have
led to the preparation of several esters. Thus, US
4.273.777 discloses pivaloyloxymethyl clonixate (III) and
ph.nalidyl clonixate, and provides some pharmacological
comparative data. US 3.689.653 discloses some lower-alkyl
clonixates (methyl, ethyl, heptyl), but does not provide
pharmacological data.
~ OCH2OCOC(CHJ)3
N NH
CH3
~CI
(III)
The method of estimating the analgesic and
antiinflammatory activities of these carboxylic acids and
their esters, differ from one document to another;
therefore, significant comparisons between published
activities are often difficult to make. In any case, it
is generally observed that analgesic and antiinflammatory
activities of esters are not substantially higher than
those of the corresponding acids. Thus, for instance, US
CA 02262788 1999-02-09
W O 98/07701 PCT~EP96/0361
4.273.777 shows that pivaloyloxymethyl clonixate (III)
and phthalidyl clonixate have analgesic and
antiinflammatory activities similar to those of clonixic
acid.
Methods of estimating ulcerogenicity also differ from one
document to another. In any case, it is generally
observed that ulcerogenic adver5e effects of esters,
although smaller than those of the corresponding acids,
still have a substantial value. Thus, for instance, in WO
95/09831 gastrointestinal damages of the 4-nitroxybutyl
esters of ketorolac and indomethacin are estimated as 10-
15 % of those of the acids. In WO 94/12463 the relative
ulcerogenicity of 4-nitroxybutyl esters of ketoprofen,
flurbiprofen, suprofen, indobufen and etodolac are
estimated as 20-35 % of those of the acids. WO 94/04484
mention the low ulcerogenicity of the 4-nitroxybutyl
ester of diclofenac, but it does not include data. In US
4.273.777 the ulcerogenic index of pivaloyloxymethyl and
phthalidyl clonixate were evaluated as 3.0 and 3.6,
values that are smaller than the one of clonixic acid
(4.0), but that still are substantial.
Therefore, it is clear that, despite the numerous
attempts to prepare esters of non-steroidal
analgesic/antiinflammatory carboxylic acids with a better
balance between high analgesic/antiinflammatory
activities and low ulcerogenic adverse effects, a
completely satisfactory solution has not been reached
yet. In particular, this is true for the case of esters
of clonixic acid.
nF.S~pTIoN OF THE lwv~w lON
The present invention solves the above-mentioned problem
CA 02262788 1999-02-09
W O 98/07701 PCT~EP96/03610
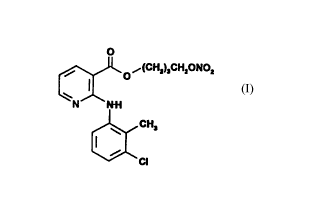
by providing 4-nitroxybutyl clonixate, a novel product of
formula (I), and its pharmaceutically acceptable solvates
(e.g. hydrates) and addition salts (e.g. hydrochloride).
,~ ~(~ H2)JCH20NO2 ~o~(CH2)3CH2X
~ 3 ~ CH
(I) (V)
The subject matter of the present invention also includes
the 4-halobutyl clonixates of formula (V), where X - Cl,
Br, or I, which are novel intermediates for the
preparation of (I). Specially preferred is 4-chlorobutyl
clonixate, i.e. intermediate (V) where X = Cl.
The subject matter of the present invention includes
pharmaceutical compositions comprising a therapeutically
effective amount of 4-nitroxybutyl clonixate (I) or a
pharmaceutically acceptable solvate or addition salt
thereof, and appropriate amounts of pharmaceutically
acceptable excipients or carriers, preferably for oral
administration. In a particular embodiment, the
pharmaceutical compositions of the present invention are
used for the treatment of pain and/or inflammation in
mammals.
Another aspect of the present invention is the use of 4-
nitroxybutyl clonixate (I) or a pharmaceutically
acceptable solvate or addition salt thereof, for the
preparation of a medicine for the treatment of pain
CA 02262788 1999-02-09
W O 98/07701 PCTIEP96/03610
and/or inflammation in mammals. In other words, the
present invention refers to a method of treatment of a
mammal suffering from pain and/or inflammation,
comprising the administration of a therapeutically
effective amount of 4-nitroxybutyl clonixate ~I) or a
pharmaceutically acceptable solvate or addition salt
thereof, together with appropriate amounts of
pharmaceutically acceptable excipients or carriers. Oral
administration is the preferred way.
A further embodiment of the present invention is a
preparation process of 4-nitroxybutyl clonixate (I) or a
pharmaceutically acceptable solvate or addition salt
thereof, that comprises the reaction, in an appropriate
solvent, of a 4-halobutyl clonixate of formula (V) where
X is Cl, Br or I, with a nitrate selected between silver
nitrate and mercurous nitrate, optionally adding the
necessary reagent to obtain the desired solvate or
addition salt. Thus, for example, when the addition salt
is the hydrochloride, the necessary reagent is HCl (g) or
HCl (aq). ~n a preferred embodiment of the process, X is
Cl, and the nitrate is silver nitrate.
In a particular embodiment of the process the 4-halobutyl
clonixates of formula (V) are prepared by a reaction
between an alkaline salt of clonixic acid, and the
compound Y-(CH2) 4-X, where X is as defined above, and Y
is a leaving group better than X. Specially preferred is
the process where the alkaline salt is the potassium one,
X is Cl and Y is Br.
The reaction steps of the processes of the present
invention can be carried out in any appropriate solvent
known in the art for reactions of the same type. In a
particular embodiment the solvent is acetonitrile.
CA 02262788 1999-02-09
W O 98/07701 PCT~EP96/03610
Compared with the non-steroidal analgesic-
antiinflammatory products known in the art, the product
of the present invention, 4-nitroxybutyl clonixate (I),
unexpectedly presents an excellent balance between high
analgesic/antiinflammatory activities and low ulcerogenic
adverse effects. This is so as a consequence of a
surprisingly high antiinflammatory activity, simultaneous
to an acceptable good analgesic activity and very low
ulcerogenic adverse effects. Actually, the ulcerogenicity
is so low that it appears as negligible (index = 0) in
the comparative pharmacological tests of the accompanying
examples.
In order to make significant comparison of analgesic
activity, antiinflammatory activity and ulcerogenicity,
products (I)-(IV) have been tested under comparable
conditions in the accompanying Examples 3-5. The identity
and origin of tested products (I)-(IV) in Table 1 is as
follows:
(I): 4-Nitroxybutyl clonixate, subject matter of the
present invention, prepared here for the first time (cf.
Example 1).
(II): Butyl clonixate, prepared here for the first time,
for comparison (cf. Example 2).
(III): Pivaloyloxymethyl clonixate, proposed as
analgesic-antiinflammatory product with low ulcerogenic
adverse effects; disclosed in US 4.273.777.
(IV): Clonixic acid, commercially available analgesic-
antiinflammatory agent, also known as clonixln.
CA 02262788 1999-02-09
W O 98/07701 PCTAEP96/03610
_________________________ __ _ ____ __ _ ____________
Table 1: Comparative tests of analgesic activities,
antiinflammatory activities, and ulcerogenic adverse
effects of clonixic acid and some of their esters.
_________
Compound ANL,% ANT,% ULC
_________________________________ ____ _______________
tI) 4-nitroxybutyl clonixate - 27 58 0
(II) butyl clonixate 26 21 8
1~ (III) pivaloyloxymethyl clonixate 27 27 8
(IV) clonixic acid 25 28 333
___________________________________ ___________________
ANL = analgesic activity as percentage of pain reduction
in a writhing test (0 % = no activity)i ANT
antiinflammatory activity as percentage of edema
inhibition (0 % = no activity); ULC = ulcerogenic index
(0 = no adverse effects = none of the animals has any
haemorrhage at all). ULC is the result of the sum of the
ulceration scorer of each animal (with scorers above
zero) multiplied by the percentage frequency of animals,
divided by the total number of animals.
____________________________ _________________________
Table 1 summarizes the results of comparative tests of
analgesic activities, antiinflammatory activities and
ulcerogenic adverse effects of clonixic acid (IV) and
clonixates (I)-(III). Parameters ANL, ANT and ULC are
defined in the footnote of the table, and they are fully
explained in Examples 3, 4 and 5, respectively. For the
desired purposes, the higher ANL value, the higher ANT
value, and the lower ULC value, the better.
Concerning the analgesic activities (ANL) in the table,
tested clonixates (I)-(III) are roughly as active as
CA 02262788 1999-02-09
W O 98/07701 PCT~EP96/03610
clonixic acid (IV).
Concerning the antiinflammatory activities (ANT) in the
table, butyl clonixate (II) and pivaloyloxymethyl
clonixate (III) are roughly as active as clonixic acid
(IV), as it was expected. However, unexpectedly, 9-
nitroxybutyl clonixate (IJ, the product of the present
invention, has an antiinflammatory activity that is
surprisingly high. Actually, this activity is two times
the one of clonixic acid (IV).
The table also shows remarkable differences in
ulcerogenicity. It is not surprising that all tested
esters are less ulcerogenic than the corresponding acids.
However, nothing in the art allowed to predict that 4-
nitroxybutyl clonixate (I) has such a low ulcerogenic
adverse effect. Actually, it is so low that it appears as
negligible ~value = 0) in the test, what means that none
of the treated animals showed any haemorrhage at all.
The low ulcerogenicity, together with the acceptable good
analgesic activity and the surprisingly high
antiinflammatory activity, gives to product (I) an
extraordinary good balance between high
analgesic/antiinflammatory activities and low ulcerogenic
adverse effects. This unexpected technical feature of the
product of the invention cannot be attributed to a mere
esterification of clonixic acid. It can neither be
attributed to the presence of the four carbon atoms of
the butyl group. It can neither be attributed to the
presence of the nitroxy substituent at the end of the
butyl chain.
Thus, 4-nitroxybutyl clonixate (I) is a very useful
analgesic-antiinflammatory active principle which
CA 02262788 1999-02-09
W O 98/07701 PCT~EP96/03610
represents a technical advantage in the treatment of pain
and/or inflammation, over the non-steroidal analgesic-
antiinflammatory products known in the art, and
particularly over the known esters of clonixic acid.
EXAMPLES
Exam~le 1. Pre~aration of 4-nitroxybutyl clonixate (I).
(la) 4-Chlorobutvl clonixate. Clonixic acid (10 g, 83
mmol) was suspended in 300 mL acetonitrile. Potassium
carbonate (10.5 g , 76 mmol) was added, and the mixture
was stirred for 10 min under nitrogen. 1-Bromo-4-
chlorobutane (8.8 mL , 76 mmol) was added dropwise and
slowly, and the mixture was stirred at 80 ~C for 2 h.
Solids were filtered off. Solvent evaporation from
filtrate, at 45 ~C under vacuum, yielded an oil residue.
Washing with ethanol:water 70:30, followed by solvent
evaporation under vacuum, washing with hexane, and
filtration, yielded 12.5 g of the title compound as a
solid melting at 69-70 ~C, which was used in the
following step without further purification.
(lb) 4-Nitroxybutvl clonixate (I~. A solution of silver
nitrate (5.8 g, 34 mmol) in 10 mL acetonitrile was added
dropwise onto a suspension of 4-chlorobutyl clonixate (6
g , 17 mmol) in 300 mL acetonitrile. The reaction mixture
was refluxed for 90 h; then it was filtered. Solvent
evaporation under vacuum from the filtrate yielded a
solid residue that was dissolved with dichloromethane;
the obtained solution was washed with abundant water. The
organic phase was separated from the aqueous one, it was
dried with anhydrous magnesium sulfate, and the solvent
was evaporated under vacuum. Column chromatography of the
solid residue, in silicagel with isopropyl ether:hexane
CA 02262788 1999-02-09
W O 98/07701 PCTAEP96/03610
1:1, allowed the collection of a fraction that, after
solvent evaporation under vacuum, yielded the title
compound (5.28 g , 82 %) as a solid melting at 55-7 ~C,
wlth the following spectroscopic properties: IR (v, cm~1):
1700 (C=O); 1620 (asymmetrical NO2); 1280 (symmetrical
NO~); 880 (NO stretching). NMR (~, ppm): 1.95 (m, 4H);
2.40 (s, 3H); 4.4 (m, 2H); 4.54 (m, 2H); 6.7-8.5
(aromatic); 9.9 (s, lH). Mass spec~rum (m/z, 20 eV): 379
(M+).
Exam~le 2. Pre~aration of butYl clonixate (II).
Clonixic acid (3.0 g , 11.4 mmol) was suspended in 80 mL
acetonitrile. Potassium carbonate (3.15 g , 22.8 mmol)
was added, and the mixture was stirred for 10 min under
nitrogen. A solution of 1-bromobutane (2.5 mL , 22.8
mmol) in acetonitrile (5 mL) was added dropwise, and the
mixture was stirred at 80 ~C for 4 h. Solids were
filtered off. From filtrate, solvent evaporation at 45 ~C
under vacuum yielded a solid residue. Washing of the
residue with ethanol:water 70:30, followed by solvent
evaporation under vacuum, washing with hexane, solvent
evaporation and recrystallization from acetonitrile,
yielded 3.2 g (88 %) of the title compound as a solid
melting at 65-8 ~C, with the following mass spectrum
(m/z, 70 eV) : 318/20 (M+, 35 %); 303/5 (100 %); 261/63
(30 %); 89 (17 %).
Example 3: Com~arison of analgesic activities.
Analgesic activities were assessed in mice using a
writhing test according to R. Koster et al. (J. Fed.
Proc. 1959, vol. 18, p. 412). Writhes were induced by
acetic acid in sets of albino mice, of both sexes, from
the CWF strain, each weighing 25-8 g. Mice had no food
CA 02262788 1999-02-09
W O 98/07701 : PCTAEP96/03610
(but water ad libitum) for 12 h before treatment. Every
mouse, 30 mln before receiving 0.25 mL of 3 % aqueous
acetic acid i.v., was treated p.o. with the following
doses of the tested compounds: 8.5 mg/kg of clonixic acid
(IV) or clonixates (I)-(III). Immediately after receiving
the acetic acid, the number of writhes was counted for 20
min. The average values were compared with the control
(29.5 writhes), and the results -shown in Table 1 were
obtained, expressed as percentages of reduction in the
number of writhes. The higher the number in the ANL
column in Table 1, the better analgesic activity (0 % =
no activity).
Example 4. ComParison of antiinflammatory activities.
Antiinflammatory activities were assessed in Sprague
Dawley rats, using a carrageenin induced paw edema test
according to C.A. Winter et al. (Proc. Soc. Exp. Biol.
Med. 1962, vol. 111, pp. 544-7). Rats of both sexes,
approximately weighing 150 g each, received 0.1 mL of 1
% carrageenin saline suspension by injection in the
subplantar region of one paw. 30 min before the injection
of carrageenin, the following doses of tested compound
were administered p.o.: 22.5 mg/kg of clonixic acid (IV)
or clonixates (I)-(III). Paw volumes were measured 3 h
after the injection. The results shown in Table 1 were
obtained, expressed as percentages of edema inhibition
(volume reduction) of treated animals, compared with the
untreated (control) set. The higher the number ir. the ANT
column of Table 1, the better antiinflammatory activity
(0 % = no activity).
Example 5. ComParison of ulceroqenic adverse effects.
Ulcerogenic effects were evaluated by a macroscopic
CA 02262788 1999-02-09
W O 98/07701 PCTAEP96/03610
examination of the ulceration of gastric epithelium,
according to M. Chaumontet et al. (Arzneim. Forsch. Dru~
Res. 1978, vol. 28 (II), pp. 2119-21). Sets of Sprague
Dawley rats, approximately weighing 150 g each, received
p.o. the following doses of the tested compounds: 125
mg/kg of clonixic acid (IV) or clonixates (I)-(III).
After 4 h of the administration, animals were sacrificed,
the stomach was removed, open and examined for
macroscopic damage. For every compound an ulcerogenic
index was calculated according to the following
evaluation: 0 = no haemorrhage; 1 = small haemorrhage; 2
= large haemorrhage; 3 = small ulcers (less than 2 mm
diameter); 4 = large ulcers (more than 2 mm diameter); 5
= perforated ulcer. The obtained ulcerogenic indexes are
shown in the column ULC of Table 1. A value ULC = 0 means
no adverse effects, i.e., that none of the animals had
any haemorrhage at all.