Note: Descriptions are shown in the official language in which they were submitted.
CA 02263500 2002-05-15
MALONATE DRIVATIVBS AND THEIR PRODUCTION
This is a divisional application of Canadian Patent
Application Ser. No. 2,157,032 filed December 26, 1994.
Technical Field
The subject matter of the parent application has
been restricted to novel isoxazolidlnedione derivatives which
have hypoglycemic action and hypolipidemic action, and are
useful as therapeutic agents for diabets and the complications
thereof, and as therapeutic agents for the related diseases
such as hyperlipidemia.
The subject matter of this divisional application is
restricted to malonate derivatives of formula (XIV) described
hereinunder and their production processes.
However, it should be understood that the expression
"the present invention" or the like includes the subject
matter of bath the parent and divisional applications.
Background Art
In general, the treatment of non-insulin-dependent
diabetes mellitus (NIDDM) involves a combination of
alimentotherapy, kinesitherapy, and administration of insulin
or oral hypoglycemic agents. As the oral hypoglycemic agents,
there are currently known sulfonylureas such as tolbutamide,
chlorpropamide, acetohexamide, glibenclamide and tolazamide
and biguanides such as phenformin, buformin and metformin.
While the sulfonylureas have strong hypoglycemic
action, they sometimes induce severe and prolonged hypo-
- 1 -
27103-138D
CA 02263500 2002-05-15
glycemia, and chronic use thereof may impair their effective-
ness. In addition, the biguanides frequently induce severe
lactic acidosis. For these reasons, the use of these
medications has required considerable attention.
Japanese Patent Unexamined Publication No.
85372/1986 teaches that thiazolidinedione derivatives such as
~5_ C4_ L2_ ~5_
- la -
27103-138D
CA 02263500 1999-02-22
methyl-2-phenyl-4-oxazolyl)ethoxy]benzyl]-2,4-thiazolidinedione]
have hypoglycemic action, and Japanese Patent Unexamined
Publication No. 51189/1985 teaches that thiazolidinedione
derivatives such as [(~ )-5-[4-(6-hydroxy-2,5,7,8-
tetramethylchroman-2-yl-methoxy)benzyl]-2,4-thiazolidinedione]
have hypoglycemic action. It has been also taught that
oxazolidinedione derivatives such as [5-[4-[2-(2-phenyl-5-
methyloxazol-4-yl)ethoxy]benzyl]-2,4-oxazolidinedione]
described in Japanese Patent Unexamined Publication No.
170478/1991 and [5-[4-[2-[N-(2-benzoxazolyl)-N-methyl]amino-
ethoxy]benzyl]-2,4-oxazolidinedione] described in W092/02520
possess hypoglycemic action and cholesterol lowering action.
However, these compounds are not necessarily satisfactory
in terms of activity, and the use thereof rather causes anxiety
when their side effects (e. g. toxicity) are taken into
consideration. These publications do not include a description
suggesting an isoxazolidinedione derivative such as the
compounds of the present Invention.
Disclosure of the Invention
The present inventors have conducted intensive studies in
an effort to provide a compound effective as a therapeutic drug
for diabetes, the complications thereof and hyperlipidemia, and
found novel low toxic isoxazolidinedione derivatives having
superior hypoglycemic action and hypolipidemic action, which
resulted in the completion of the invention.
Accordingly, the present invention relates to novel
2
CA 02263500 1999-02-22
isoxazolidinedione derivatives of the formula (I)
n
R --
R4
wherein
R is an optionally substituted aromatic hydrocarbon, an
optionally substituted alicyclic hydrocarbon, an
optionally substituted heterocyclic group, an optionally
substituted condensed heterocyclic group or a group of
the formula
R3
R1-X
wherein R1 is an optionally substituted aromatic hydro-
carbon, an optionally substituted alicyclic hydrocarbon,
an optionally substituted heterocyclic group or an
optionally substituted condensed heterocyclic group, R2
and R3 are the same or different and each is a hydrogen
atom or a lower alkyl, and X is an oxygen atom, a sulfur
atom or a secondary amino;
R4 is a hydrogen atom, a lower alkyl or a hydroxy;
R5 is a lower alkyl optionally substituted by hydroxy; and
- 3 -
27103-138D
CA 02263500 1999-02-22
P and Q are each a hydrogen atom or P and Q together form a
bond, and pharmaceutically acceptable salts thereof.
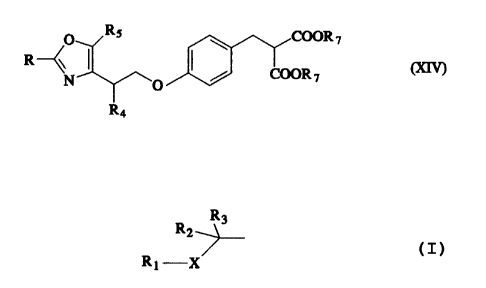
The present invention also provides malonate
derivatives of the formula (XIV): a malonate derivative of
the formula (XIV):
O RS COOR ~
R ~ ~ ~ ~ c~
O / COORS
R4
(wherein
R is optionally substituted aromatic hydrocarbon, an
optionally substituted alicyclic hydrocarbon, an
optionally substituted heterocyclic group, an optionally
substituted condensed heterocyclic group or a group of
the formula
R3
R2-~-
R 1-X
wherein R1 is an optionally substituted aromatic
hydrocarbon, an optionally substituted alicyclic
hydrocarbon, an optionally substituted heterocyclic group
or an optionally substituted condensed heterocyclic
group, R2 and R3 are the same or different and each is a
hydrogen atom or a lower alkyl, and X is an oxygen atom,
- 4 -
27103-138D
CA 02263500 2002-05-15
' ' 27103-138D
a sulfur atom or a secondary amino;
R4 is a hydrogen atom or a lower alkyl;
R5 is a lower alkyl; and
R~ is a lower alkyl),
The present invention further provides processes for
producing the malonite derivatives of the formula (XIV), which
comprise: [A] reacting compounds of the formula (XIII):
O Rs \
I I Y cx~
N O /
R4
(wherein R, R4 and R5 are as defined above with respect
to the formula (XIV) and Y is a halogen atom)
with malonic di-lower alkyl esters, in organic solvents in the
presence of bases under cooling to.under heating, or
[B] treating compounds of the formula (XXZ):
O Rs \ \ COORS
R I I / COORS
N O
R4
(wherein R, R4, R5 and R~ are as defined above)
with hydrogenation catalysts in organic solvents under
hydrogen atmosphere at normal temperature to under heating.
CA 02263500 1999-02-22
As used here in the present specification, "aromatic
hydrocarbon" means phenyl, biphenyl, naphthyl and the like.
It may be aralkyl such as benzyl, with preference given to
phenyl.
"Alicyclic hydrocarbon" means that having 3 to 7
carbon atoms, and is exemplified by cyclopropyl, cyclobutyl
- 6 -
27103-138D
CA 02263500 1999-02-22
cyclopentyl, cyclohexyl, cycloheptyl, cyclopropenyl,
cyclobutenyl, cyclobutadienyl, cyclopentenyl, cyclopentadienyl,
cyclohexenyl, cyclohexadienyl, cycloheptenyl and
cycloheptadienyl. Preferred is alicyclic hydrocarbon having 5
to 7 carbon atoms, such as cyclopentyl, cyclohexyl, cycloheptyl,
cyclopentenyl, cyclopentadienyl, cyclohexenyl, cyclohexadienyl,
cycloheptenyl and cycloheptadienyl, with preference given to
cyclopentyl and cyclohexyl.
"Heterocyclic group" is a 5- or 6-membered heterocyclic
group, preferably aromatic heterocyclic group having, as an
atom constituting the ring besides carbon atom, 1 to 3,
preferably 1 or 2, hetero atoms selected from nitrogen atom,
oxygen atom and sulfur atom. Specific examples include thienyl,
furyl, pyrrolyl, imidazolyl, pyrazolyl, thiazolyl,
isothiazolyl, oxazolyl, isoxazolyl, oxadiazolyl, thiadiazolyl,
triazolyl, pyridyl, pyrazinyl, pyrimldinyl, pyridazinyl,
triazinyl, dithiazolyl, dioxolanyl, dithiolyl, pyrrolidinyl,
dithiadiazinyl, thiadiazinyl, morpholinyl, oxazinyl, thiazinyl,
piperazinyl, pyperidinyl, pyranyl and thiopyranyl, with
preference given to thienyl, furyl, pyrrolyl, imidazolyl,
pyridyl and pyrimidinyl, and particular preference given to
pyridyl, pyrimidinyl and imidazolyl.
"Condensed heterocyclic group" means a ring wherein 5- or
6-membered heterocyclic groups, preferably aromatic
heterocyclic groups having, as an atom constituting the ring
besides carbon atom, 1 to 3, preferably 1 or 2, hetero atoms
7
CA 02263500 1999-02-22
selected from nitrogen atom, oxygen atom and sulfur atom have
been condensed, or a ring wherein such heterocyclic group,
preferably aromatic heterocyclic group and a 4- to 6-membered
aromatic hydrocarbon ring, preferably phenyl, have been
condensed. Specific examples include furoisoxazolyl,
imidazothiazolyl, thienoisothiazolyl, thienothiazolyl,
thienothiazolyl, imidazopyrazolyl, cyclopentapyrazolyl,
pyrrolopyrrolyl, cyclopentathienyl,.thienothienyl,
oxadiazolopyrazinyl, benzofurazanyl, thiadiazolopyridinyl,
triazolothiazinyl, triazolopyrimidinyl, triazolopyridinyl,
benzotriazolyl, oxazolopyrimidinyl, oxazolopyridinyl,
benzoxazolyl, thiazolopyridazinyl, thiazolopyrimidinyl,
benzoisothiazolyl, benzothiazolyl, pyrazolotriazinyl,
pyrazolothiazinyl, imidazopyrazinyl, purinyl, pyrazolo-
pyridazinyl, pyrazolopyriminidyl, imidazopyridinyl, pyrano-
pyrazolyl, benzimidazolyl, indazolyl, benzoxathiolyl, benzo-
dioxalyl, dithioropyrimidinyl, benzodithiolyl, indolidinyl,
indolyl, isoindolyl, furopyrimidinyl, furopyridinyl, benzo-
furanyl, isobenzofuranyl, thienopyrazinyl, thlenopyrlmidinyl,
thienodioxynyl, thienopyridinyl, benzothienyl, isobenzothienyl,
cyclopentaoxazinyl, cyclopentafuranyl, benzothiadiazinyl,
benzotriazinyl, pyridoxazinyl, benzoxazinyl, pyrimidothiazinyl,
benzothiazinyl, pyrimidopyridazinyl, pyrimidopyrimidinyl,
pyridopyridazinyl, pyridopyrimidinyl, cinnolinyl, quinazolinyl,
quinoxalinyl, benzoxathiinyl, benzodioxynyl, benzodithiinyl,
naphthylidinyl, isoquinolinyl, quinolinyl, benzopyranyl, benzo-
s
CA 02263500 1999-02-22
thiopyranyl, chromanyl, isochromanyl and indolinyl, with
preference given to benzoxazolyl, benzoisothiazolyl, benzo-
thiazolyl, benzimidazolyl, indazolyl, benzoxathiolyl, benzo-
dioxalyl, benzodithiolyl, indolyl, isoindolyl, benzofuranyl,
isobenzofuranyl, benzothienyl, isobenzothienyl, benzothia-
diazinyl, benzotriazinyl, benzoxazinyl, benzothiazinyl,
cinnolinyl, quinazolinyl, quinoxalinyl, benzoxathiinyl, benzo-
dioxynyl, benzodithiinyl, isoquinolinyl, quinolinyl, benzo-
pyranyl, benzothiopyranyl, chromanyl, isochromanyl and indolinyl,
and particular preference given to indolyl, isoindolyl, benzo-
furanyl, isobenzofuranyl, benzothienyl, isobenzothienyl,
isoquinolinyl and quinolinyl.
"Lower" means that the number of the carbon atoms
constituting the group is 1 to 6, preferably 1 to 4.
"Lower alkyl" means alkyl having 1 to 6 carbon atoms, such
as methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl,
tert-butyl, pentyl, isopentyl, neopentyl, tert-pentyl, hexyl,
isohexyl and neohexyl. Preferred is alkyl having 1 to 4 carbon
atoms such as methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec-butyl and tert-butyl, with particular preference
given to methyl.
"Optionally substituted" means that the group may be
substituted by 1 to 3 same or different substituents. Examples
of the substituent include lower alkyl such as methyl, ethyl,
propyl, butyl and tert-butyl; lower alkoxy such as methoxy,
ethoxy, propoxy, butoxy and tert-butoxy; halogen atom; nitro;
s
CA 02263500 1999-02-22
cyano~ hydroxyls acyl including lower alkanoyl (such as
formyl, acetyl, propionyl, butyryl, and isobutyryl), benzoyl
and naphthoyli acyloxy including lower alkanoyloxy (such as
formyloxy, acetyloxy, propionyloxy, butyryloxy and
isobutyryloxy) and benzoyloxyr aralkyloxy such as benzyloxy,
phenethyloxy and phenylpropyloxyl mercapto~ lower alkylthio
such as methylthio, ethylthio, propylthio, butylthio,
isobutylthio and tart-butylthio~ amino lower alkylamino such
as methylamino, ethylamino, propylamino, isopropylamino and
butylaminot di-lower alkylamino such as dimethylamino,
diethylamino, dipropylamino, diisopropylamino and
dibutylamino~ carboxyl lower alkoxycarbonyl such as
methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
isopropoxycarbonyl, butoxycarbonyl and tart-butoxycarbonyl~
carbamoyll trifluoromethyl~ phosphoryl~ sulfo~ sulfamoylt
lower alkylphosphonamido such as methylphosphonamido,
ethylphosphonamido, propylphosphonamido and
isopropylphosphonamido~ methylenedioxy~ lower alkoxyphosphoryl
such as methoxyphosphoryl, ethoxyphosphoryl, propoxyphosphoryl
and isopropoxyphosphoryl~ lower alkylsulfonyl such as
methylsulfonyl, ethylsulfonyl, propylsulfonyl, butylsulfonyl
and tart-butylsulfonyl~ and lower alkylsulfonylamino such as
methylsulfonylamino, ethylsulfonylamino, propylsulfonylamino,
butylsulfonylamino and tart-butylsulfonylamino. Preferred are
hydroxyl, lower alkyl, lower alkoxy, aralkyloxy, mercapto,
lower alkylthio, vitro, halogen atom, trifluoromethyl, amino,
di-lower alkylamino, lower alkylamino, acyl, cyano, carbamoyl,
27103-138
CA 02263500 1999-02-22
acyloxy, sulfo, carboxyl and lower alkoxycarbonyl, with
particular preference given to hydroxyl, lower alkyl and lower
alkoxy.
10a
27103-138
CA 02263500 1999-02-22
"Pharmaceutically acceptable salt" may be any as long as it
forms a non-toxic salt with a novel isoxazolidinedione deriva-
tive of the above-mentioned formula (I). Examples thereof
include alkali metal salts such as sodium salt and potassium
salt; alkaline earth metal salts such as magnesium salt and
calcium salt; ammonium salt; organic base salts such as tri-
methylamine salt, triethylamine salt, pyridine salt, pycoline
salt, dicyclohexylamine salt and N,N'-dibenzylethylenediamine
salt; and amino acid salts such as lysine salt and arginine salt.
The compound of the present invention has superior
hypoglycemic action and hypolipidemic action, and is not only
useful as an agent for the prevention and treatment of diabetes
and hyperlipidemia, but also expected to be useful as an agent
for preventing arteriosclerosis. When the compound of the
formula (I), which is the compound of the present invention, or
a pharmaceutically acceptable salt thereof is used as a
pharmaceutical preparation, it is generally admixed with a
pharmacologically acceptable carrier, excipient, diluent,
extender, disintegrant, stabilizer, preservative, buffer,
emulsifier, aromatic, coloring, sweetener, thickener, flavor,
solubilizer, and other additives known her se, such as water,
vegetable oil, alcohol such as ethanol and benzyl alcohol,
carbohydrate such as polyethylene glycol, glycerol triacetate,
gelatin, lactose and starch, magnesium stearate, talc, lanolin,
petrolatum and the like, and formulated into tablet, pill,
powder, granule, suppository, injection, eye drop, liquid,
1 1
CA 02263500 1999-02-22
capsule, troche, aerosol, elixir, suspension, emulsion, syrup
and the like which may be administered orally or parenterally.
While the dose varies depending on the kind and severity of the
disease, the compound to be administered, administration route,
age, sex and body weight of patient and the like, the compound
(I) is preferably administered orally at a dose of, in general,
0.01-1,000 mg, particularly 0.05-100 mg per day to an adult.
The compound of the formula (I) has one or more asymmetric
carbons. When it has one asymmetric carbon, a pure optically
active compound, a mixture thereof at an optional proportion or
a racemate exists; and when it has two or more asymmetric
carbons, optically pure diastereomers, racemates thereof, a
combination of these or a mixture thereof at an optional
proportion exist(s). These are all encompassed in the present
invention. Depending on the case, they may be hydrates. As is
evident from the structure, the above-mentioned compound (I) can
exist as a keto-enol type tautomer, which is also within the
scope of the present invention.
The compound of the present invention can be synthesized,
for example, by the following methods. It is needless to say
that the production method of the compound of the present
invention is not limited to them.
1 2
CA 02263500 1999-02-22
Ra COOR61
(II)
H2N COOH
(Step 1)
ORa COORgi
~ R5 (III)
R51' ' N
H I
O
(Step 2)
O Ra COORe
O
(Step 17 ) R_ 'Y H2N R5 (IV) y y
(V) O R2 R3 (XV)
oRa cooRg (Step 3) (Step 12) oRa cooRg
Ri-XH II
R N R5 ,~ (XIX) ~ y~N R5 (XVI)
H ~ R2 Ra H O
o (Step 16)
(vi)
(Step 13)
R2 R3 O R5 (XVII)
I COORg
(Step 4) y N
Ra
(Step 14)
R Ra O R5
R--<~ ~ 5 (VII) RZ~~ I (XVIII)
N COORg
Ri-XH Y N OH
Ra (XIX) Ra
(Step 5). o R5 (Step 15)
R----y I
N ~ OOH
(VIII) Ra
(Step 6 )
1 3
CA 02263500 1999-02-22
O R5
R \ I (Ix)
N Z
R4
CHO
(Step 7)
(x>
O Rs ~ CHO
OOR~ R I
N / (XI)
COORS O
(XX) Rs
(Step 18) (Step 8)
O Rs ~ ~ COORS R5
I O ~ OH
N O / COORS R~ I I / (XII)
N ~~O
(XXI) R4
(Step 9)
O Rs ~ Y
R I I / (XIII )
N ~ w0
R4
(Step 10)
O Rs ~ COORS
R (XIV)
N I Q I / ' COORS
(Step 20)
R4
O Rs ~ COY
(Step 11) R~N I I / COY
-O
R4
(XXII)
o (Step 21)
O Rs ~
R I ~ ,NH (I)
N O / O O
Rs
1 4
CA 02263500 1999-02-22
Step 1
The compound (III) wherein Rs~ is a carboxy-protecting
group such as benzyl, R5, is a lower alkyl, an optionally
substituted aromatic hydrocarbon, an optionally substituted
alicyclic hydrocarbon, an optionally substituted heterocyclic
group, an optionally substituted condensed heterocyclic group or
a group of the formula
Rs
R
R~ X
wherein R~, RZ and R3 are as defined above, and R4 and Rs are as
defined above, can be synthesized by reacting compound (II)
wherein R4 and Rsl are as deffined above, which is an aspartate
derivative, in the presence of pyridine or a base such as
triethylamine, in acid anhydride such as acetic anhydride and
propionic anhydride, at room temperature to heating, and
treating the resulting compound with water. In this reaction,
addition of 4-dimethylaminopyridine sometimes affords better
results.
Step 2
The compound (IY) wherein Rs is an alkyl, and R, and R5 are
as defined above, is obtained by removing N-acyl such as N-
acetyl of the formula R51-CO- by heating compound (III) wherein
R4, Rs, Rsl and Rs, are as defined above, in an acidic solvent
such as hydrochloric acid. Since Rs is eliminated at the same
time, the resulting compound is esterified by reacting same in
an alcohol solvent such as methanol, ethanol and propanol, in
1 5
CA 02263500 1999-02-22
the presence of an acid such as hydrogen chloride, whereby
compound (IV) is obtained.
Step 3
The compound (YI) wherein R, R~, Rs and Rs are as defined
above, can be synthesized by reacting compound (IY) wherein R4,
Rs and Rs are as defined above, and compound (Y) wherein Y is a
halogen atom and R is as defined above, in an organic solvent
such as benzene, toluene, dichloromethane, chloroform, ether,
dioxane, tetrahydrofuran, acetonitrile, dimethoxyethane,
pyridine and acetone or a mixed solvent thereof, in the presence
of a base such as triethylamine, pyridine and N-
methylmorpholine under cooling to room temperature.
Step 4
The compound (VII) wherein R, R4, R5 and Rs are as defined
above, can be synthesized by reacting compound (YI) wherein R,
R4, Rs and Rs are as defined above, in an organic solvent such
as benzene, toluene, acetonitrile, chloroform and
tetrahydrofuran, or without solvent, in the presence of an acid
catalyst such as sulfuric acid and p-toluenesulfonic acid and a
dehydrating agent such as acetic anhydride, at room temperature
to under heating, preferably under heating.
Step 5
The compound (VIII) wherein R, R4 and R5 are as defined
above, can be synthesized by reducing compound (YII) wherein R,
R,, Rs and Rs are as defined above, by a conventional method,
using a 'reducing agent such as diisobutyl aluminum hydride, in
1 s
CA 02263500 1999-02-22
an organic solvent such as benzene, toluene, ether, dioxane and
tetrahydrofuran.
Step 6
The compound (IX) wherein Z is p-toluenesulfonyloxy,
benzenesulfonyloxy, methanesulfonyloxy or a leaving group such
as halogen atom and the like, and R, R4 and R5 are as defined
above, can be synthesized by reacting compound (VIII) wherein
R, R4 and Rs are as defined above, and sulfonyl chloride such
as p-toluenesulfonyl chloride, benzenesulfonyl chloride and
methanesulfonyl chloride, or a halogenating agent such as
phosphorus tribromide and thionyl chloride, in an organic
solvent such as benzene, toluene, dichloromethane, chloroform,
ether, dioxane, tetrahydrofuran, acetonitrile, dimethyl-
formamide, dimethylsulfoxide, acetone and ethyl acetate, or a
mixed solvent thereof, or without solvent, in the presence of a
base such as triethylamine, 4-dimethylaminopyridine and
pyridine, under cooling to under heating.
Step 7
The compound (XI) wherein R, R4 and R5 are as defined
above, can be synthesized by reacting compound (IX) wherein R,
R~, Rs and Z are as defined above, and 4-hydroxybenzaldehyde (X)
in an organic solvent such as benzene, toluene,
dichloromethane, chloroform, ether, dioxane, tetrahydrofuran,
acetonitrile, dimethylformamide, dimethylsulfoxide, sulforan
and dimethoxyethane, in the presence of a base such as sodium
hydride, potassium hydride, sodium amide, sodium alkoxide,
1 7
CA 02263500 1999-02-22
potassium alkoxide, triethylamine and sodium hydroxide, under
cooling to under heating.
Step 8
The compound (XII) wherein R, R~ and R5 are as defined
above can be synthesized by reducing compound (XI) wherein R, R4
and R5 are as defined above, using a catalyst such as sodium
borohydride, lithium aluminum hydride, lithium borohydride and
dibutyl aluminum hydride, in a solvent such as ethanol and
isopropanol.
Step 9
The compound (XIII) wherein R, R4, R5 and Y are as defined
above, can be synthesized by reacting compound (XII) wherein R,
R4 and R5 are as defined above, in a solvent such as pyridine
and dioxane in the presence or absence of a catalyst such as
zinc chloride, adding a halogenating agent such as hydrogen
bromide, phosphorus trichloride, phosphorus tribromide and
thionyl chloride, at room temperature to under heating. It can
be also synthesized by reacting compound (XII) wherein R, R4 and
Rs are as defined above, in a solvent such as anhydrous carbon
tetrachloride, adding triphenylphosphine, at room temperature to
under heating.
Step 10
The compound (XIV) wherein R, is a lower alkyl, and R, R~
and Rs are as defined above, can be synthesized by reacting
compound (XIII) wherein R, R4, R5 and Y are as defined above,
with malonic diester, in an organic solvent such as benzene,
1
CA 02263500 1999-02-22
toluene, dichloromethane, chloroform, ether, dioxane,
tetrahydrofuran, acetonitrile, dimethylformamide,
dimethylsulfoxide, sulforan and dimethoxyethane, in the presence
of a base such as sodium hydride, potassium hydride, sodium
amide, sodium alkoxide, potassium alkoxide, triethylamine and
sodium hydroxide, under cooling to under heating.
Step 11
The compound (I) wherein R, R, and R5 are as defined above,
can be synthesized by reacting compound (XIV) wherein R, R4, Rs
and R, are as defined above, with hydroxyamine in an anhydrous
alcohol solution, under cooling to under heating.
When the compound has hydroxy as the substituent for R, a
compound having methoxy and the like as the substituent is
synthesized and hydrolyzed under acidic conditions.
The compound (VIII) can be synthesized by introducing
substituent R~ after ring closure, as mentioned below.
Step 12
The compound (XYI) wherein R2, R3, R,, R5, Rs and Y are as
defined above, can be synthesized by reacting compound (IY)
wherein R4, Rs and Rs are as defined above, with compound (XV)
wherein Ra, R3 and Y are as defined above, in an organic
solvent such as benzene, toluene, dichloromethane, chloroform,
ether, dioxane, tetrahydrofuran, acetonitrile, dimethoxyethane,
pyridine and acetone, or a mixed solvent thereof, in the
presence of a base such as triethylamine, pyridine and N-
methylmorpholine, under cooling to room temperature.
1 9
CA 02263500 1999-02-22
Step 13
The compound (XYII) wherein RZ, Ra, R4, R5, Rs and Y are as
defined above, can be synthesized by reacting compound (XYI)
wherein R2, R3, R4, Rs, Rs and Y are as defined above, in an
organic solvent such as benzene, toluene, acetonitrile,
chloroform and tetrahydrofuran or without solvent, in the
presence of an acid catalyst such as sulfuric acid and p-
toluenesulfonic acid and a dehydrating agent such as acetic
anhydride, at room temperature to under heating, preferably
under heating.
Step 14
The compound (XYIII) wherein Rz, R3, Rs, R5 and Y are as
defined above, can be synthesized by reducing compound (XVII)
wherein Ra, R3, R4, Rs and Y are as defined above, in an organic
solvent such as benzene, toluene, ether, dioxane and
tetrahydrofuran, using a reducing agent such as diisobutyl
aluminum hydride, by a conventional method.
Step 15
The compound (YIII) wherein R, R4 and R5 are as defined
above, can be synthesized by reacting compound (XYIII) wherein
R2, R3, R4, R5 and Y are as defined above, and compound (XIX)
wherein R, and X are as defined above, in an organic solvent
such as benzene, toluene, dichloromethane, chloroform, ether,
dioxane, tetrahydrofuran, acetonitrile, dimethylformamide and
dimethylsulfoxide, water or a mixed solvent thereof, in the
presence of a base such as sodium hydride, potassium hydride,
2 0
CA 02263500 1999-02-22
sodium amide, sodium alkoxide, potassium alkoxide,
triethylamine and sodium hydroxide, under cooling to under
heating.
The compound (VI) can be also synthesized by the following
steps.
Step 16
The compound (VI) wherein R, R4, R5 and Rs are as defined
above, can be synthesized by reacting compound (XYI) wherein
Rz, R3, R4, Rs, Rs and Y are as defined above, and compound
(XIX) wherein R1 and X are as defined above, in an organic
solvent such as benzene, toluene, dichloromethane, chloroform,
ether, dioxane, tetrahydrofuran, acetonitrile, dimethylformamide
and dimethylsulfoxide, water or a mixed solvent thereof, in the
presence of a base such as sodium hydride, potassium hydride,
sodium amide, sodium alkoxide, potassium alkoxide,
triethylamine and sodium hydroxide, under cooling to under
heating.
Step 17
The compound (YII) wherein R, R4, Rs and Rs are as defined
above, can be synthesized by reacting compound (III) wherein R4,
R5, R51 and Rs~ are as defined above, in an organic solvent
such as benzene, toliene, acetonitrile, chloroform and
tetrahydrofuran or without solvent, in the presence of an acid
catalyst such as sulfuric acid and p-toluenesulfonic acid and a
dehydrating agent such as acetic anhydride, at room temperature
to under heating, preferably under heating.
2 1
CA 02263500 1999-02-22
Step 18
The compound (XXI) wherein R, R4, Rs and R~ are as defined
above, can be synthesized by refluxing under heating compound
(XI) wherein R, R, and R5 are as defined above, and compound
(XX) wherein R, is as defined above, in an organic solvent such
as toluene and benzene, using a catalyst such as piperidinium
acetate, ethylene diammonium acetate and ammonium acetate,
which has been formed from acetic acid and piperidine in the
system, while removing water out from the system.
Step 19
The compound (XIY) wherein R, R~, R5 and R~ are as defined
above can be synthesized by reacting compound (XXI) wherein R,
R~, R5 and R~ are as defined above, in an organic solvent such
as methanol, ethanol, propanol, isopropanol, tetrahydrofuran,
dioxane, dichloromethane and acetic acid or a mixed solvent
thereof, using a catalyst such as palladium carbon and palladium
black under a hydrogen atmosphere at normal temperature to
under heating.
Step 20
The compound (XXII) wherein R, R4, Rs and Y are as defined
above, can be synthesized by hydrolyzing compound (XIV) wherein
R, R,, Rs and R, are as defined above, to give a dicarboxylic
acid and treating same with a halogenating reagent such as
thionyl chloride and oxalyl chloride.
Step 21
The compound (I') wherein R, R4 and Rs are as defined above
2 2
CA 02263500 1999-02-22
can be synthesized by reacting compound (XXII) wherein R, R,,
Rs and Y are as defined above, with hydroxyl amine, in the
presence of a base such as pyridine and triethylamine.
The above-mentioned methods are particularly advantageous
when P and Q are hydrogen atoms.
The compound (I) thus obtained can be isolated and purified
by known separation-purification means such as concentration,
concentration under reduced pressure, solvent extraction,
precipitation, recrystallization and chromatography.
Of the compounds (I), a compound wherein P and Q are
combined, which is represented by the formula (I')
0
0 Rs
R I ( ~ NH (I'
~N 0 ~ 0 0/
R4
wherein R, R4 and R5 are as defined above, can be synthesized
by, for example, the following method.
COOR,
I , ,1'
0 Rs HO ~ COORS
R ~ ~ (XXIII)
N Z
(IX) R, (Step 22)
0 RS ~ ~ COORS
R ~ I
N 0 ~ COOR, (Step 23)
R,
(XXIY)
2 3
CA 02263500 1999-02-22
0
0 Rs
R ~ I ~ \ \ ~H (I')
N 0 0 0
R~
Step 22
The compound (~) wherein R, R,, Rs and R, are as defined
above, can be synthesized by reacting compound (IX) wherein R,
R,, Rs and Z are as defined above, with compound (XXIT3) wherein
R7 is as defined above, in an organic solvent such as benzene,
toluene, dichloromethane, chloroform, ether, dioxane,
tetrahydrofuran, acetonitrile, dimethylformamide,
dimethylsulfoxide, sulforan and dimethoxyethane, in the
presence of a base such as sodium hydride, potassium hydride,
sodium amide, sodium alkoxide, potassium alkoxide,
triethylamine and sodium hydroxide, under cooling to under
heating.
Step 23
The compound (I') wherein R, R4 and Rs are as defined
above, can be synthesized by reacting compound~~r) wherein R,
R4, Rs and R~ are as defined above, with hydroxyamine using
sodium methoxide in an anhydrous methanol solution, under
cooling to under heating.
The present invention is explained in more detail by way of
Examples in the following, to which the present invention is
not limited.
2 4
27103-138D
CA 02263500 1999-02-22
Example 1
Step 1 : Synthesis of benzyl 3-acetamido-4-oxopentanoate
B-Benzyl L-aspartate (400 g, 1.79 mol) was suspended in
triethylamine (748 ml, 5.37 mol) and acetic anhydride (676 ml,
7.16 mol) was dropwise added at 0°C with stirring. After
stirring for 30 minutes, 4-dimethylaminopyridine (20.0 g, 0.16
mol) was portionwise added under ice-cooling. The mixture was
stirred overnight at room temperature, and ice was added under
ice-cooling. At the end of the exothermic process, water (700
ml) was added. A 7.5N aqueous solution of potassium hydroxide
was portionwise added to make its pH 9. The mixture was
extracted three times with ethyl acetate (1,000 ml), and the
organic layer was washed twice with 1N hydrochloric acid (1,000
ml), twice with a saturated aqueous solution of sodium
hydrogencarbonate (500 ml) and with saturated brine (500 ml) in
order. The layer was dried over magnesium sulfate and
concentrated to dryness to give 390 g of the title compound.
Step 2 : Synthesis of methyl 3-amino-4-oxopentanoate
hydrochloride
6N Hydrochloric acid (700 ml) was added to the compound
(390 g, 1.50 mol) synthesized in the above Step 1, and the
mixture was stirred under reflux for 2 hours. The mixture was
cooled to room temperature and the reaction mixture was washed
twice with dichloromethane (500 ml). The aqueous layer was
concentrated to dryness. A solution of hydrogen chloride in
methanol (1,500 ml) was added under ice-cooling and the mixture
2 5
CA 02263500 1999-02-22
was stirred. The mixture was gradually warmed and the mixture
was stirred overnight at room temperature. Concentration to
dryness gave 247 g of a crude product. The crude product (60
g) was recrystallized from isopropanol to give 30 g of the title
compound as a white solid.
Step 3 : Synthesis of methyl 3-(benzene-2-carboxamide)-4-
oxopentanoate
The compound (9.40 g, 51.3 mmol) synthesized in the above
Step 2 was suspended in dichloromethane (200 ml) at 0°C.
Benzoyl chloride was added thereto, and N-methylmorpholine
(20.8 g, 0.2 mol) was dropwise added gradually with stirring.
The mixture was stirred for 3.5 hours, and water (100 ml) was
added to separate an organic layer. Further, an organic layer
was extracted from the aqueous layer with dichloromethane (100
ml). The extracted organic layers were combined, washed with
1N aqueous hydrochloric acid (100 ml) and water (100 ml) in
order, and dried over magnesium sulfate. Concentration to
dryness gave 12.75 g of the title compound (yield 1000 .
Step 4 : Synthesis of methyl [2-(2-phenyl)-5-methyl-4-
oxazolyl]acetate
Anhydrous acetate (70 ml) was added to the compound (12.75
g, 51.2 mmol) synthesized in the above Step 3 and the compound
was dissolved. Con. sulfuric acid (1.0 ml) was dropwise added
with stirring. The mixture was stirred at 90°C for 3 hours and
cooled to room temperature. Water (100 ml) was added to the
reaction mixture, and the mixture was neutralized with a
2 6
CA 02263500 1999-02-22
saturated aqueous solution of sodium hydrogencarbonate, and
extracted with dichloromethane (100 ml). The extract was dried
over magnesium sulfate, and concentrated to dryness to give
8.75 g of the title compound (yield 74~).
Step 5 : Synthesis of 2-[2-(2-phenyl)-5-methyl-4-oxazolyl]-
ethanol
A solution of the compound (8.75 g, 37.88 mmol) synthesized
in the above Step 4 in toluene (200 ml) was dropwise added to a
solution (133 ml, 133.20 mmol) of diisobutyl aluminum hydride
in toluene at 0°C under a nitrogen stream. Two hours later,
methanol (100 ml) was dropwise added. Then; 2N hydrochloric
acid (700 ml) was added to this gel reaction mixture to
dissolve same, and the mixture was extracted 4 times with ethyl
acetate (500 ml). The extracted organic layers were combined,
washed with saturated brine (200 ml) and dried over magnesium
sulfate. Concentration to dryness gave 7.69 g of the title
compound (yield 1000 .
Step 6 : Synthesis of 2-[2-(2-phenyl)-5-methyl-4-oxazolyl]-1-
ethyltosylate
Pyridine (30 ml) was added to a solution (15 ml) of the
compound (7.69 g, 37.88 mmol) synthesized in the above Step 5
in dichloromethane, and p-toluenesulfonyl chloride (7.58 g,
39.77 mmol) was gradually added at 0°C. After stirring for 6
hours, dichloromethane (100 ml) was added to dilute same, and
dilute hydrochloric acid (100 ml) was added. The mixture was
partitioned, and the organic layer was sequentially washed with
2 7
CA 02263500 1999-02-22
water (100 ml), a saturated aqueous solution of sodium
hydrogencarbonate (100 ml) and saturated brine (100 ml).
Drying over magnesium sulfate and concentration to dryness gave
11.63 g of the title compound (yield 86~).
Step 7 : Synthesis of 4-[2-[2-(2-phenyl)-5-methyl-4-oxazolyl]-
ethoxy]benzaldehyde
A 60~ oil of sodium hydride (3.14 g, 78.4 mmol) was washed
twice with n-hexane (20 ml) under a nitrogen stream and added
with dimethylformamide (20 ml), and the mixture was cooled to
0°C. A solution (20 ml) of 4-hydroxyaldehyde (9.57 g, 78.4
mmol) in dimethylformamide was added with stirring. After
stirring for 10 minutes, a solution (30 ml) of the compound (28
g, 78.4 mmol) synthesized in the above Step 6 in
dimethylformamide was dropwise added. The mixture was warmed
to room temperature and stirred for 60 hours. The mixture was
neutralized with 1N hydrochloric acid and extracted twice with
ethyl acetate (100 ml). The extracted organic layer was washed
twice with water (100 ml) and dried over magnesium sulfate.
The solvent was distilled away to give 24.1 g of the title
compound as a colorless solid (yield 1000 .
Step 8 . Synthesis of 4-[2-[2-(2-phenyl)-5-methyl-4-oxazolyl]-
ethoxy]benzyl alcohol
Sodium borohydride (2.46 g, 65.1 mmol) was gradually added
to a solution (300 ml) of the compound (20 g, 65.1 mmol)
synthesized in the above Step 7 in ethanol, and the mixture was
stirred for 1 hour. Ethanol was distilled away and water (200
2 8
CA 02263500 1999-02-22
ml) was added. Filtration of the resulting precipitates gave
19.5 g of the title compound as a yellow solid (yield 97%).
Step 9 . Synthesis of 4-[2-[2-(2-phenyl)-5-methyl-4-oxazolyl]-
ethoxy]benzyl chloride
Thionyl chloride (8.9 ml, 124.2 mmol) was gradually added
to a solution (300 ml) of the compound (19.18 g, 62.1 mmol)
synthesized in the above Step 8 and zinc chloride (1.74 g,
12.78 mmol) in dioxane at room temperature, and the mixture was
stirred for 1 hour. After stirring, dioxane and thionyl
chloride were distilled away under reduced pressure and water
(200 ml) was added. The mixture was extracted twice with
dichloromethane (100 ml) and dried over magnesium sulfate.
After drying, the solvent was distilled away to give 19.69 g of
the title compound as a yellow solid (yield 94%).
Step 10 . Synthesis of diethyl 4-[2-[2-(2-phenyl)-5-methyl-4-
oxazolyl]ethoxy]benzyl malonate
A 60% oil of sodium hydride (488 mg, 12.2 mmol) was washed
twice with n-hexane (5 ml) under a nitrogen stream and added
with tetrahydrofuran (20 ml), and the mixture was cooled to
0°C. Diethyl malonate (1.95 g, 12.2 mmol) was added with
stirring. After stirring for 30 minutes, the compound (4 g,
12.2 mmol) synthesized in the above Step 9 was added and the
mixture was heated at 70°C for 2 hours. The mixture was
warmed to room temperature and the mixture was neutralized with
1N hydrochloric acid. The mixture was extracted twice with
dichloromethane (100 ml) and dried over magnesium sulfate. The
2 9
CA 02263500 1999-02-22
solution was purified by fast flow liquid chromatography
(developing solvent; hexane:ethyl acetate=2:1) to give 3.13 g of
the title compound as a colorless solid (yield 57~).
Step 11 . Synthesis of 4-[4-[2-(2-phenyl-5-methyl-4-oxazolyl)-
ethoxy]benzyl]-3,5-isoxazolidinedione
A solution (4 ml) of hydroxyamine ~ hydrochloride (348 mg,
5.00 mmol) in anhydrous methanol was added to a solution (4 ml)
of sodium methoxide (540 mg, 9.99 mmol) in anhydrous methanol at
0°C. The resulting sodium chloride was filtered off and a
solution (4 ml) of the compound (1.5 g, 3.33 mol) synthesized in
the above Step 10 in anhydrous methanol was added. The mixture
was stirred overnight at room temperature: After stirring, the
solvent was distilled away, and the residue was dissolved in an
aqueous solution of sodium hydroxide and washed twice with
ether (20 ml). 1N Hydrochloric acid was added to the aqueous
layer to make same acidic. The mixture was extracted twice with
ether (50 ml), dried over magnesium sulfate. The obtained
solid was dissolved in ether and an insoluble material was
removed. Evaporation of ether under reduced pressure gave 412
mg of the title compound as a colorless solid (yield 32~).
Example 1' : Synthesis of dimethyl 4-[2-(2-phenyl-5-methyl-4-
oxazolyl)ethoxy]benzilidene malonate (Step 18)
Dimethyl malonate (1.39 g, 0.01 mol), acetic acid (0.3 ml)
and piperidine (0.3 ml) were added to a solution of the compound
(2.94 g, 0.01 mol) synthesized in Example 1, Step 7 in toluene
(30 ml), and the mixture was refluxed under heating using a Dean
3 0
CA 02263500 1999-02-22
Stark trap while removing water to outside the system. Four
hours later, toluene was distilled away and the obtained residue
was recrystallized from methanol to give a colorless solid (2.5
g, yield 6090.
Synthesis of dlmethyl 4-(2-(2-phenyl-5-3nethyl-4-oxazolyl)-
ethoxy]benzyl malonate (Step 19)
The above-mentioned compound (2.5 g, 0.06 mol) was
dissolved in a mixed solvent of methanol-dioxane (1:5, 20 ml),
and 59~ Pd-C (150 mg) was added. The mixture was vigorously
stirred under an HZ atmosphere at normal temperature and under
atmospheric pressure. Two hours later, the catalyst was
filtered off and the solvent was distilled away to give a
solid. Recrystallization from methanol gave a colorless solid
(2.15 g, yield 8590.
Synthesis of 4-[4-[2-(2-phenyl-5-methyl-4-oxazolyl)ethoxy]-
benzyl]-3,5-isoxazolidinedione (Step 11)
A solution (4 ml) of sodium methoxide (574 mg, 10.6 mmol)
in anhydrous methanol was gradually added to hydroxyamine~
hydrochloride (360 mg, 5.3 mmol) in anhydrous methanol solvent
(4 ml). The precipitated sodium chloride was filtered off, and
a solution (4 ml) of the above-mentioned compound (1.5 g, 3.5
mmol) in anhydrous methanol was added. The mixture was stirred
at 60°C for 3 hours.
The solvent was distilled away, and 1N aqueous HC1 (50 ml)
was added to the residue to make same assume acidity. The
residue was extracted twice with ether (50 ml) and dried over
3 1
27103-138D
CA 02263500 1999-02-22
magnesium sulfate. The solvent was distilled away and the
obtained solid was recrystallized twice from methanol to give
650 mg of a colorless solid (yield 47~).
mp . 154.6-155.4°C
The signals at 400 MHz NMR: 2.35 (s,3H), 2.92 (t,J=6.5Hz,2H),
3.23-3.27 (m,2H), 3.50 (t,J=4.9Hz,lH), 4.11 (t,J=6.7Hz,2H),
6.77-7.95 (m,9H)
Reference Example 1 . Synthesis of diethyl 4-hydroxybenzilidene
malonate
4-Hydroxybenzaldehyde (24.4 g, 0.20 mol), diethyl malonate
(30.4 ml, 0.2 mol), benzoic acid (3.0 g) and piperidine (3.0 ml)
were dissolved in toluene (200 ml), and the mixture was
refluxed for 6 hours with dehydration using a Dean Stark trap.
After cooling the mixture to room temperature, the resulting
solid was filtrated and washed with toluene, a 0.5N aqueous
solution of citric acid, a saturated aqueous solution of sodium
hydrogencarbonate and ether in order. The obtained solid was
dried under reduced pressure to quantitatively give the title
compound as a white solid.
Example 2
Step 22 : Synthesis of diethyl 4-[2-(2-phenyl-5-methyl-4
oxazolyl)ethoxy]benzilidene malonate
A 60~ oil of sodium hydride (616 mg, 15.4 mmol) was washed
twice with n-hexane (2 ml) under a nitrogen stream and added
with dimethylformamide (20 ml). The mixture was cooled to
0°C. Diethyl 4-hydroxybenzilidene malonate (4.07 g, 15.4 mmol)
3 2
CA 02263500 1999-02-22
synthesized in the above Reference Example 1 was added to this
solution. After stirring for 10 minutes, the compound (5.00 g,
14.0 mmol) synthesized in Example 1, Step 6 was added and the
mixture was stirred overnight. The reaction mixture was
extracted with ethyl acetate, and the extract was washed with
water and saturated brine. After the washing, the organic
layer was dried over magnesium sulfate and the solvent was
distilled away under reduced pressure. The obtained residue
was purified by silica gel column chromatography (developing
solvent; chloroform: methanol=98:2) to give 5.44 g of the title
compound as a white solid.
Step 23 : Synthesis of 4-[4-[2-(2-phenyl-5-methyl-4-oxazolyl)-
ethoxy]benzilidene]-3,5-isoxazolidinedione
A solution (10 ml) of hydroxyamine ~ hydrochloride (977 mg,
14.1 mmol) in anhydrous methanol was added to a solution (10
ml) of sodium methoxide (956 mg, 14.1 mmol) in anhydrous
methanol at 0°C. The resulting sodium chloride was filtered
off and a solution (10 ml) of the compound (4.21 g, 9.37 mol)
synthesized in the above Step 17 in anhydrous methanol was
added. An equivalent of sodium methoxide was added, and the
mixture was stirred for 3 hours. After stirring, the solvent
was distilled away, and the residue was extracted with ethyl
acetate, washed with dilute hydrochloric acid and saturated
brine, and dried over magnesium sulfate. The solvent was
distilled away. The obtained solid was dissolved in ether and
an insoluble material was removed. Ether was distilled away
3 3
CA 02263500 1999-02-22
under reduced pressure, and the residue was purified by silica
gel column chromatography (developing solvent; chloroform:
methanol=95:5) to give 1.45 g of the title compound as a white
solid (yield 32~).
Examples 3 and 4
In the same manner as in Example 1, the compounds of Table
1 were obtained.
3 4
CA 02263500 1999-02-22
II II "O vi p ~ ~p' N
N "': ~_ ~ ~ ~ r ~~ N
o x
.-. v c'? ~ ~n N
..r ~ W 00 ~ ~ N pj ~
> et M x x dr x r. N x r. b
x x N N ~~ N N N
M N Ov ,~N, ~ M x
E ~ ~ ~ ~ ~ ~ ~O ~b ~D
M
t~ N ~ ~ n 11
1" w ~ In ~ V r1
V r_i M ~ ; '. x M b .~.r o t~,
M~ o~ ~_,~ ~ o .. x
O N
U M M ~C d ,~p ~ej f~ N n N E
x xx x~ xx ~ x x
x~ xx x~ xx V ~ a x
~1 t~ 00 N ~' ~ N h b
~O ~ ~p ~p ~p op I)
~~~hii~_~~~ir:rOV~..
x a
x N ~n N w.. " ~: 'y-' ~ ~ M et
Oy~ Ow-. O C = ~ ~ ~'~ N M ~ r
N vp fV ~' M rx~ N ~fj N . x x
r: ~: ~ ~ '~ N x .'T,' ~ t~ N .~~N'
cx~1 N M x cx~7 N M N N
N H x ~ ~ N
h x Vr7 x n r. I~ x ~-:
M t~ M Ov M ~ x M ~ x
N ~O CV ~ fV .-~ cV v0 N
."~ , v, v ~n o
.-~ ~'1"'., E ! a ! ~ ! ~ ! '° ! o
O O ~ 'o N o - o .~
O ~ +-~ °' ~ ~ v o
.D '~
cfl
z
z O
O z Z O z O 'O 'O
'O O 'O 'O
-O O
\O ~ \O
O
'd O O
O O O
O
t~.
H
O
U
._ - p ~z p ~z
o ~z O ~z O ~z
/ ~cn cn
i z
1
z
.-, N M
3 5
CA 02263500 1999-02-22
As mentioned above, the present invention is not limited to
the above-mentioned Examples. For example, the compounds shown
in the following Tables 2 to 17 are also encompassed in the
present invention.
3 6
CA 02263500 1999-02-22
Table 2
O
0
NH
O O
R R4 Rs R R4 Rs R R4 Rs
HO ' I H Me ~ H Me ~ I H Me
N
Ms ~ I o it Me ~ r, ,, HOOC ' ~. r, r,
N
i i
Et0 ~ r, ~~ Et0 ~ 'r o NC ' / o Et
Et0 N
HS ' I '~ o HS ~ o r, Et ' ( r,
N
y
I '~ Et HO ~ '~ '~ HZN ' I ~r Me
w
N
Met
OzN ~ ~ O 4 OpN-~ Et it Me '~ o O
N
HOOC ' I '~ '~ H3COOC ~ '~ Et Et0 ' ( Me
N
NC ' ( '' '' MeS ~ H Me Br ' I H o
N
CI ' I '~ '~ H2N ~ % ~, HO ' I . % o
N
HzN ~ I~ Me 'r Br ~ r, ,, H2N ' ~ ~. o
N
3 7
CA 02263500 1999-02-22
Tabl a 3
O
NH
o ~ 0 0
R R4 RS R R4 RS R R4 RS
N
H Me Et '~ H M >
C
e N H Me
N S
Me
~N ~ ~ Et0 rN
au ' J ,, ,, Eto ~ ,, ,, ' '~ ,, ,,
N S
N
Me0 ~j ~~ Et EtS-',', ~~ ,, HO ' '> ,, Et
~ ,
'
N S H
N
HS JN o Me HO-',', o ,, HS-',L~, , ,
~ '~ , ,
'
N S H
N
HO ~~ it 4 O2N '~ o ~, H2N ' 'J ~i Me
N S N
H
N
02N ~J ~~ r~ H3COC '~ Et ,, O2N ' '~ o
N S N
H
N
HOOC ~J O ~, NC-',', it Et HOOC ' '> Me
~
'
N S N
H
N
NC ~J ~. o H2N-',', H Me NC ' '~ H
~
'
N S H
N
fir' N '/ ,, Ct Br ' '>
'
' /' ,,
~NJ LS N
H
~,,N Et0 ~N
H2N ' Me '~ Et0 '~ % ,, HS '
J >
N N
S Et
3 8
CA 02263500 1999-02-22
Table 4
O
R H
N
R R4 RS R R4 RS R R4 Rs
H Me ' I S H Me ~ Me
\ H
0
HO 'N r, 4 HO ' I r, ,, HO ' ( \ ,, ,,
N \
S
Et 'N p Et HS ~ ( it it Et0-/ I ~~ Ef
H \ \
~ S
EtS '\ rr Me Et0 / I r, ,, HO / \
\ ~i
HO
\ \
HOOC 'N r, O MeOC ( ~r ~~ HOOC \ I r Me
\
~ S
H O
H2N LH ~~ O HOOC ' Et it OZN ~ I ~i o
I S \
O
02N L \ ~~ o Me0 '~ % Et NC \ I \ Me
H I S
Me0
CI 'N ii O H2N '~ H Me H2N \ I H b
I S \
H CI
Et2N 'N ,, ,, CI I \ rr p CI \ I \ ~~ it
H ~ S ,
O
.\ / \
BuOC 'H Me ~~ t-Bu ' r, r, Me \ ( O r, r,
( S
3 9
CA 02263500 1999-02-22
Table 5
H
R R4 RS R R4 RS R R4 RS
H Me 1 I j H Me ~ I H Me
N N O
/ ~N /
HS ' I ~ ~~ ,, HS ~ I ~ ., ,, HO \ I o O
N NJ O
/ ~ '
suo ~ I o Et suo ~ I , ~. ~, Meo ' I ~. Et
~ . N O
N
H2N ~ I ~i Me H2N ~ I J i. ., HZN \ I . .
, N O
N
/ ~ / ~N /
OZN ' I '. ~, OZN ' I ~ .' O2N ' I ~. Me
~ N O
N
HOOC-/ I
., ., HOOC ~ I Et ~i Br ~ I n O
N J O
N
~N
CI ' I N O ~~ CI ' I N~ % Et Me l~_ I Me ~i
O
CI
CI ~/ I o o CI '/ I J H Me MeS ~ I H ,,
~ CI~ N MeS~ O
CI~ N
w N M f\.~
Me0 '/ I ., ,, Me0 ~/ I ., ,~ MeS '/''r ~, o
N N J i1 0J
Me0 Me0 NC J~
/ / ~N HO
CI '/ I Me ., CI '~, I ., ., ~ ~ O ., .
N N J i T I
HzN H2N
4 0
CA 02263500 1999-02-22
Table 6
O
R2 ~..
~N H
R1- ~ O O
RI X. R2 R3 R4 Rs RI X R2 R3 Ra Rs
S H Me H Me HzN ~ I S H Me H Me
HO ~ I MeOC ~
.. .. .. .. I .. .. .. ..
W
Me \ I . ~ . . . Et2HN-' ., . H . Et
(
i
Et0 ~ I . . H Me . t_gu ~/, ., ,, . Et .
I
t-Bu
i i
HS I . ~ . . Et Me0 ~ I ., ., ., ., Me
Me0
,, ., ,.
w H Me ~I ~ I . , . , ,
O ~
CI~
i
02N w I .~ .. .~ .. ~. H2N l%: .. .. .. H ..
I
CI
t-Bu
HOOC I . . Me . . HO I . . Me .
%~
t-Bu
NC ' I . , , . H2N ~~ ., ., ., ., .,
I
HO
i
CI ~ . . . . , Me ~ . . . . .
CI~
4 1
CA 02263500 1999-02-22
Table 7
Ra O Rs
R2w ~ /
R~.-X N, ~ wOi ~ O
R1 X R2 R3 R4 RS R, X. R2 R3 R4 RS
H2N-~ S H Me H Me H2N:l J S H Me H Me
N
HO ~ , , , , . HO ~ J ., ., ., ,, ,,
N
i
Me ~ , , , . . Me ' J ,, ,, H , Et
N
i N
Et0 ~ , , H Me ~ Et0 ~J , , . Et
N
HS ~ ~ ~, , , Et HS ~j ~, ., ,, , Me
N
I
, , . H Me MeOC ~J . . . , .,
~N ~
N
i
0 . , , , N ~ .
N 0
2 , , . . .. 2 , ,. ,. H .,
~ J
N
HOOC ~ , , Me , , HOOC ~~ , . Me ,,
N
NC ~ , , . . , NC ~ j , ,, ., ., .,
N
c1 ~ ., , , , , c1 ~J ~. ~, , ,, ,,
N
4 2
CA 02263500 1999-02-22
Table 8
O
R2
NH
R1- O ~ O O
R1 X R2 R3 R4 RS R, X 'R2 R3 R4 RS
N
H2N ~ S H Me H Me CN~ S H Me H Me
I
~N
HO ~ . . ., ., ,, HO ' ~~ ,,
N
H
~N
Me ~ . . . . ~. H2N ' ~~ . . H
, , . Et
N
H
N
Et0 ~ , ~. H Me . Et ~ ~~ , . . ,
L . , , t
N ,
H
~N -
HS ~ . , , . Et Et0 ' ~~ ,, ,, ,, ., Me
N
H
r.N
HZNOC ~ . ~. ~. H Me HS LN . . ~. . .
H
rN
OZN ~ ,, ,, ,, ,, ,, NC ' ~~ ., ., ., H
N
H
r.N
HOOC ~ . . Me ~. 02N ' ~ . M . .
N
. , . . e , ,
H
N
NC ~ ., ., ., ,, ,, HOOC-~',~ ,, , . .
>
'N , , . o
H
~N
CI . . . . . CI ' ~~ , , ,
, , , , , , ,, ,,
N
H
4 3
CA 02263500 1999-02-22
Table 9
O
R Rs O Rs \
NH
R1-X N O ~ O O
Ra
R i X- R2 R3 R4 Rs R 1 X R2 R3 R4 Rs
~
Ho ~ I S H Me H Me I ~ S H Me H Me
. S
N
Me0 ' 1 ~. . . . . HO '~ . . . . .
,
N S
1 ,
~ . . . . H2N '~ ., ., H . Et
N S
~, H Me . Et '~ ,, ,, . Et .
S
C~ ~, I ., ., ., . Et Et0 ; ~ ., ,, ., .,
, Me
'
~ N
C1 S
/
Me \ I ~. . . H Me HS '~ , ., ,, ., .,
~
O S
/
e~ ' I . , . . . NC '~ , , ., H .,
~
S
H2N ~ I , Me , . OZN '~ ~ Me ~ ,
~
o S
NC \ I ., ., ,, ., ., EtOC '~ ., ., ., ., ,,
o
S
HO ~ I ~ , ~ , ~ CI '~ , . , ,
~ ~
O
S
4 4
CA 02263500 1999-02-22
Table 10
O
R2 ' NH
R~- / O O
RI X R2 R3 Ra Rs RI X R2 R3 Ra Rs
I O H Me H Me H2N ' I O H Me H Me
HO \ I . . . . o MeOC \ ., ., ., .,
I
i
Me ' I . . . . . Et2HN ' ., . H . Et
I
i i
Et0 ~ . . H Me ~ t-Bu ( . . , Et ,
~y.
t-Bu
MeO ~ ( Me
HS I ~ ~ ~ Et ,, ,, ,, .,
MeO~
O ' I . . . H Me CI ~~, ., .. . . .,
I
CI
02N ~ I ~ . . . ., HZN ~ I ., ., ., H ,
CI/
t-Bu ,
HOOC ' . , Me . . HO ~'~ ~, . Me
I t-Bu
i
NC ' I ~ o . , ., H2N ~~ ., ., ., ., .,
HO
i
CI- I . . . . . Me ~ o .
CI
4 5
CA 02263500 1999-02-22
Table 11
O
R R3 ~ R5
NH
R~-X N O ~ O O
Ra
RI X R2 R3 R4 RS R, X R2 R3 R4 RS
H2N ~ O H Me H Me H2N ' J O H Me H Me
N
HO ~ ~ ~ ~ HO
~ . . . . . N
Me ~ , , , . ~, Me ' J ,, ., H ,, Et
N
~N
Et0 H
,~N , , Me Et0 ' J . , . Et ,
N
HS ~ ~ ~ ~ ~ Et HS ~J , .. .. ,, Me
1
N
"
~
LO , . . H Me MeOC ~J , , , .
~N N
02N ~N ., ., ., ,, . 02N ~J ~ , , H .
N.
HOOC ~ . . Me . , HOOC ~J , , Me
N
i
~ Nc
~~
Nc , ,, , , ,, ' ,, , , , ,
N
c~ ~ , ,, ., ., ., ci ~J . , , ,,
N
4 6
__~..N.:_ . _. _._ __.. _._:_._..__.~..a
CA 02263500 1999-02-22
Table 12
O
NH
/. 0 O
R1 X R2 R3 R4 RS R, X R2 R3 R4 RS
N __
H2N- O H Me H Me ~N~ O H Me H Me
I
,\ . ,
O . , . . , rN , , ,
HO L
~
N , .
H
rN
Me ~ . , . . . H2N L ., ., H Et
N .
H
N
Et0 ~ , , H Me , Et L ~~ , , , Et ,
, , .
N ,
H
rN
HS , . , ., Et Et0 ' ~~ ,, ,, ,, ., Me
N
H
~N
H2NOC . . , H Me HS L ~~ . , , . .
N
H
N
02N ~ , , NC , , , H
N
L .
H
rN
HOOC ~ . . Me , , 02N L N~ , , Me .
.
H
N
NC ~ HOOC ~ ~
J
,
, , , , , 'N , o , , ,
H
rN
CI ~ . , , , . CI ' ~) . , ., ~. ,.
N
H
4 7
CA 02263500 1999-02-22
Table 13
O
R2
NH
R1- O ~ O O
R1 X R2 R3 R4 RS R, X R2 R3 R4 RS
Ho ' I O H Me H Me I ~ O H Me H Me
, S
N
Me0 ~ I ~ , % ~. ,. HO ; ~ ,. ,, ,. o
, ~
N "S
w
CI ' I ~. ~. ,. ~. ,, H2N , ~ ,, ,, H ~. Et
N L'S
i
Ho ~ I ,, ,, H Me ,. Et-', ~ ,, ,, ,, Et ,.
, '
~
HO S
N
c1 j I ., ., ., ~. Et Et0 '~ ., ,, ,, .,
, Me
N
CI S
Me ' I ~ . ,, H Me HS '~ ~. ,. ,,
~
S
Br ~ I ~, ,. ,. ,. ,, NC '~ ~, ,, ,. H .
~
o S
H2N \ I % Me ~. , OZN '~ , , Me , ,,
~
o S
NC ' ( ,, ,, ,, ., ., EtOC '~ ., ,, ., ., ,.
~
O S
HO ~ I , o ,, ,~ , CI '~ , , , , ,,
~
O
S
4 8
CA 02263500 1999-02-22
Table 14
O
R R3 O RS \
NH
Ry-X N O ~ O O
Ra
R1 X R2 R3 R4 RS R, X R2 R3 R4 RS
I NH H Me H Me H2N ~ ( NH H Me H Me
i
HO I . . . . .,, MeOC ~ . . . . .
Me ~ I . . o ~ ~ EtyHN ' ., ., H . Et
I
Et0 ~ I ' H Me ' t-Bu ~/~ . . Et ,
I
t-Bu
i i
HS- ( '. . . Et Me0 ~ I ., ., ., ,, Me
Me0
(~ i
. . . H Me CI ~~ ' ' ' ' '
,
CI
i
02N ~ I .. .~ .~ .~ .~ H2N ~ .. .. .~ H
~
CI
t-Bu
I f~~
HOOC . . e . . HO-~ I . . e . .
'-
u
t-B
i
NC ~ I . . . '. H2N l~ ' ' '' ' '
HO
CI ~ I . . . . . Me ~ I . . ~ .
CI~
4 9
CA 02263500 1999-02-22
Table 15
O
R2 \
NH
R~- ~ p O
R1 X R2 R3 R4 RS R, X R2 R3 R4 RS
HZN ~ NH H Me H Me H2N ' j NH H Me H Me
N
HO ~ . . . , .. HO ' J . ~, . . .
N
i
Me ~ ~. ~. . . ~. Me ' j ., ,, H ~. Et
N
~i N
Et0 ~ . , H Me . Et0 ' J . , , Et ,
N
HS ~ ~ . . . Et HS ~J ., ., ., ., Me
N
. . , H Me MeOC ~J . . . . ,
N
N . , . . . N ~ .
0 0
2 , . , . , 2 , .. .. H ..
~ J
N
HOOC ~ . ~. Me , . HOOC ~J . ~. Me
N
NC ~ . . , ~. . NC ~J . . . . ,,
N
ci ~ , , ., ., , ci ~j , , , ,
N
0
CA 02263500 1999-02-22
Table 16
0
NH
O O
R1 X R2 R3 R4 RS R1 X R2 R3 R4 RS
N
H2N- NH H Me H Me CN NH H Me H Me
I
~N
HO , ~, o ~, ~, HO 'N , .
H
N
Me ~ , '~ ' '~ '~ H2N LN , ~, H ~, Et
~
H
N
Et0 ~ '~ '~ H Me '~ Et L N , ~, ~, Et ,
H
N
Et0 r ~
S ~ t L ~ , , . e
N
H
~N
_ H HS-',', .. ,, ,, .. ,,
H , , , e ~~
NOC L
2 N
H
N
0 ,~ ,~ ,~ ,~ ,~ NC ; N ,, ., ,, H .,
N ~
2
H
~N
HOOC ~ , Me ~, . OzN L ~~ . , Me ,
, N
H
N
HOOC ~ ~
C , , , , . LN . , .
H
~N
CI ~ . . ~. ., ,, CI ' ~~ ., ,, ,, .~ .,
N
H
1
CA 02263500 1999-02-22
Table 17 Q
R Ra. O Rs \
NH
R1-X . N O / O O
Ra
R1 X R2 R3 R4 Rs RI X R2 R3 R4 Rs
Ho ' I NH H Me H Me I ~ NH H Me H Me
, S
N
i
Me0 ~ I o ~. . ~. . HO , ~ . . ~. ~. .
'
N S
ct ~ I . ~. ~. ~. . HZN-',', ,, . H ~, Et
, ~
'
N S
Ho ~ I ., ,, H Me ~. Et
~ ~. , . Et
S
HO N
i
ct ~ I . . ~. . Et Et0-',', ,, ., ,, .
, ~ e
~ N ~'
CI S
nne \ I ~ . . H Me HS '~ . , , .
~
o S
Br \ I ~, ~. ~, . ~, NC '~ ~ ~, . H
~
O
S
H2N \ I . ~. Me ~, ~. OxN '~ ~ ~ Me .
~
o S
NC ' I ., ., ., ., ., EtOC '~ ,, ., ., ., ,,
o
S
HO \ I , ~, ~. , ~. CI '~ , , ,
~ S
O
2
CA 02263500 1999-02-22
Experimental Example 1
Genetically obese, hyperglycemic and hyperlipidemic
diabetic mice (C57BL/Ksj-db/db, male, Jackson Laboratories/Clea
Japan, Inc., 13 weeks of age and KK-Ay, male, Clea Japan, Inc.,
13 weeks of age) were used for the pharmacological tests. As a
reference compound, a hypoglycemic agent CS-045 [(~ )-5-[4-(6-
hydroxy-2,5,7,8-tetramethylchroman-2-yl-methoxy)benzyl]-2,4-
thiazolidinedione] [see Diabetes, vol. 37, p. 1549.(1988)] was
used.
The mice were weighed and blood samples were taken
immediately before the initiation of administration on day 1.
Serum glucose and serum triglyceride were measured, based on
which the mice were grouped (6-8 per group) in such a manner
that there existed no difference in terms of average body
weight, average serum glucose and average serum triglyceride.
The test drugs were all suspended in a solution of 0.5~
sodium carboxymethylcellulose and administered orally twice a
day (the second administration was 6 hours after the first
administration) on day 1, day 2, day 3 and day 4. To a vehicle
control group, a solution of 0.5~ sodium carboxymethylcellulose
was orally administered.
At day 5, blood samples were taken again and measured for
serum glucose and serum triglyceride. The blood sample was
taken from orbital cavity plexus by 400 u1 under
anesthetization with ether and kept at ice temperature. After
separation into serum (12000 rpm, 5 min.), serum glucose was
3
CA 02263500 1999-02-22
measured by hexokinase method (glucose-HK-test "BMY";
Bohelinger Mannheim Yamanouchi) and serum triglyceride was
measured by enzyme method (triglycolor III "BMY"; Bohelinger
Mannheim Yamanouchi) using an automatic analyzer COBAS FARA
(manufactured by Roche).
Change in percent of serum glucose and serum triglyceride
in each group was calculated using serum glucose and serum
triglyceride, respectively, of vehicle control group at day 5 as
follows:
Change in percent of serum glucose (3~) -
serum glucose of each serum glucose of vehicle
group at day 5 ~ - ~ control group at day 5
serum glucose of vehicle control group at day 5
Change in percent of serum triglyceride value (9~) -
serum triglyceride of serum triglyceride of vehicle
~~ each group at day 5 ~ -~ control group at day 5
serum triglyceride of vehicle control group at day 5
The results are shown in Table 18.
Table 18
x 100
x 100
dose serum glucose serum triglyceride
(m (9~) (9~)
/
g KK-A' mouse db/db mouse KK-Ar mouse db/db mouse
kg)
Ex. 10 -38.3 -19.8 -50.3 -29.1
1
CS-045 100 -29.4 -21.5 -22.9 -55.5
4
CA 02263500 1999-02-22
As shown in Table 18, the compound of the present invention
lowered serum glucose and serum triglyceride of both kinds of
diabetic mice more significantly than did the control compound.
From the foregoing, it is evident that the compound of the
present invention has superior hypoglycemic and hypolipidemic
actions and is useful for the treatment of diabetes and
hyperlipidemia. In addition, the compound of the invention is
expected to be efficacious for the prevention and treatment of
the complications of diabetes.
Industrial Applicability
The~oxazolidine derivative compound (I) and a salt thereof
of the present invention are novel compounds having extremely
potent and low toxic hypoglycemic action as compared with known
oxazolidine derivatives and other therapeutic agents of
diabetes, and are very useful as therapeutic agents for diabetes
and hyperlipidemia. In addition, the compounds of the present
invention are expected to be useful for the prevention of the
complications of diabetes, especially for the prevention of
arteriosclerosis.
5