Note: Descriptions are shown in the official language in which they were submitted.
CA 02264429 1999-03-03
t
- 1 -
Preparation of 9-Hydrocarbyl-9-Phosphabicyclononanes
Background of the Invention
United States Patent No. 3,501,515 (Van Winkle et al)
discloses the use of 9-alkyl-9-phosphabicylononanes as superior
ligands when combined with cobalt for the hydroformylation of
olefins. The 9-alkyl-9-phosphabicyclononane is currently
prepared in a two step reaction process. In a first step,
phosphine is added in a free radical reaction to 1, 5-
cyclooctadiene (COD), to form a 9-phosphabicyclononane.In a
subsequent step an olefin is added to the 9-phosphabicyclo-
nonane in a free radical reaction, to form 9-alkyl-9-
phosphabicyclononane.
The product of the first step is a mixture of 9-
phosphabicyclononane isomers, 9-phosphabicyclo [3.3.1] nonane
(the symmetrical isomer) and 9-phosphabicyclo [4.2.1] nonane
(the unsymmetrical isomer). These are obtained in a mixture
that is approximately 60 parts symmetrical isomer and 40 parts
unsymmetrical isomer. The desired isomer is the symmetrical
one, as it is more hindered at the phosphorus atom and
therefore more sensitive when used as a ligand in a catalyst.
Otherbyproducts of this reaction are several 1:2 adducts of
phosphine and cyclooctadiene and some 2:2and 2:3 adducts of
phosphine and cyclooctadiene.. Also formed is a small amount
(usually about 1.5 to 1.8~, based on the two main isomers) of
trans 5-phosphinocyclooctene.
In the subsequent step the mixture of the symmetrical
and the unsymmetrical 9-phosphanonanes and other byproducts
obtained from the first step is allowed to react with an olefin
under free radical conditions. Unwanted higher oligomers of
phosphine and cyclooctadiene are then removed by vacuum
stripping. The mixture of the symmetrical and unsymmetrical 9-
alkyl-9-phosphabicyclononanes is then combined with Co2+ to
form a catalyst system for the hydroformylation of long chain
olefins.
As stated the symmetrical isomer is believed to be
the active component of the mixture. Efforts to increase the
content of the symmetrical isomer at the expense of the
CA 02264429 1999-03-03
- 2 -
unsymmetrical isomer have been unsuccessful, however. In
practice the symmetrical isomer has constituted between about
58~ and about 61~ of the isomer mixture, and obtaining the
value of about 61~ has necessitated some loss of yield when
calculated on the amount of cyclooctadiene used.
United States Patent No. 3,400,163 (Mason et al)
discloses some bicyclic heterocyclic phosphines and their
production. In Example IV eicosylphosphine is reacted with 1,
5-cyclooctadiene at a temperature of 135°C-145°C in the
presence of di-(tert.-butyl) peroxide. The product is said to
be a mixture of 9-eicosyl-9-phosphabicyclo[4.2.1]nonane and 9-
eicosyl-9-phosphabicyclo[3.3.1]nonane. The relative proportion
of these two isomers is not stated.
Summary of the Invention
The present invention provides a process for
preparing a 9-hydrocarbyl-9-phosphabicyclo[3.3.1]-nonane which
comprises the addition of a primary hydrocarbyl phosphine to
1,5-cyclooctadiene in the presence of a free radical initiator
at a temperature not greater than 100°C.
The reaction of the hydrocarbyl phosphine with the
1,5-cyclooctadiene still results in a mixture of the desired
symmetrical[3.3.1] isomer and the undesired unsymmetrical
[4.2.1] isomer. The ratio of the isomers is shifted markedly
in favour of the desired isomer, however. In some instances
the mixture has had a symmetrical content of 74~. This is a
25~ improvement in yield over the approximately 60~ that was
the best that could previously be achieved. Furthermore, the
amount of other undesired byproducts is reduced.
Description of the Preferred Embodiments
The process is preferably carried out in a reactor
under autogenous pressure in the presence of a free radical
initiator that is an azo compound. The reaction is carried out
at a relatively low temperature, preferably below say 80°C,
more preferably below about 60°C and most preferably below
about 40°C. It has been found that as the reaction temperature
is reduced the quantity of undesired byproducts, particularly
the traps 1:1 phosphine:COD adduct, is much reduced and the
CA 02264429 1999-03-03
- 3 -
ratio of the desired symmetrical isomer to the undesired
unsymmetrical isomer is much enhanced. At lower temperature,
however, the reaction does take longer.
The free radical initiator can be, for example a
peroxide or an azo radical initiator, or it can be radiation,
for example W radiation or gamma radiation. The peroxide and
azo initiators are temperature sensitive. Furthermore
peroxides, for example di-(tert.-butyl)peroxide, tend to
require a higher reaction temperature, and also to cause
formation of phosphine oxide, so their use is not preferred.
Radiation is not temperature sensitive, but phosphine
is not a good UV or gamma radiation absorber, so, on their own,
the use of these radical sources is not preferred. The
preferred initiators are azo compounds, for instance 2,2'-
azobis-(2-methylbutyronitrile) (also known as azobis
isovaleronitrile) and 2,2'-azobis-(2,4-dimethylvaleronitrile),
available from Du Pont under the trademarks Vazo 67 and Vazo
52, respectively. These normally decompose thermally to yield
free radicals that initiate the desired reaction. Different
compounds, of course, decompose at different temperatures and
different rates, and the number following the trademark
indicates the temperature at which the compound has a half life
of 10 hours. Thus Vazo 67 has a half life of 10 hours at 67°C
and Vazo 52 has the same half life at 52°C. Other suitable azo
free radical initiators are commercially available under the
trade-marks Vazo 88 and Vazo 64 and have 10 hour half lives of
88°C and 64°C, respectively. The initiator should be selected
with the intended reaction temperature in mind, so that for
reactions in the vicinity of 70 to 100°C Vazo 67 is preferred
and for reactions in the range of 40 to 70°C Vazo 52 is
preferred.
It is possible to use a combination of an initiator
and radiation. Because the azo initiators are good W
absorbers, the radiation causes decomposition of the azo
initiator to yield free radicals to initiate the desired
reaction. In this embodiment the rate of decomposition of the
azo initiator and hence the rate of reaction, are not
CA 02264429 1999-03-03
- 4 -
temperature dependent. This advantage must of course be
balanced against the cost of providing both initiator and a
suitable W reactor.
The reaction is carried out in an inert, e.g.,
nitrogen, atmosphere.
The substituent at the 9-position of the product is
determined by the primary phosphine reactant. The primary
phosphine reactant can be represented by the formula
RPH2
so that the reaction yielding the desired symmetrical product
can be represented by the equation -
RPH2 +
R can be alkyl, straight chained or branched, suitably
containing up to about 36 carbon atoms, or cycloalkyl or
arylalkyl. A preferred alkylphosphine is eicosylphosphine.
The group R can be substituted provided that the substituents
do not interfere with the reaction. As possible substituents
there are mentioned hydroxyl, amino, monoalkyl, dialkylamino,
alkanoyloxy, alkoxycarbonyl, cycloalkyl, phenyl and pyridyl
groups. The group R can be cycloalkyl containing from 3 to 8,
preferably 5 or 6, carbon atoms.
The reaction is normally carried out in the liquid
phase. Depending upon the value of R, this may require the use
of pressure or the use of a solvent. If R is a lower alkyl
group, for instance, a methyl, ethyl or propyl group, then
increased pressure, up to about 100 psig or possibly higher,
may be used. COD is itself a liquid but as-the desired product
is formed the melting point of the reaction mixture may
increase and the reaction mixture may freeze or crystallize.
This is undesirable, so a solvent, or mixture of solvents, may
be used to lower the freezing point of the reaction mixture.
Examples of suitable solvents include aliphatic hydrocarbons
CA 02264429 1999-03-03
z
- 5 -
such as octane or kerosene, alkylaromatic hydrocarbons such as
toluene, xylene, ethylbenzene, tert.-butyl-toluene and the
corresponding halogenated aromatic hydrocarbons in which the
halogen, e.g., chlorine, atom is attached to a carbon atom of
the aromatic ring, alcohols such as isopropanol and ethers such
as tetrahydrofuran (THF). The hydrocarbyl primary phosphine or
COD may be used in excess and this excess may serve as solvent.
Hence, in its broad aspect R can be hydrocarbyl. The
term "hydrocarbyl" is used in its accepted meaning as
representing a radical formed from a hydrocarbon by removal of
a hydrogen atom. The hydrocarbyl groups represented by R in
the formula above may be any non-acetylenic organic radical
composed solely of carbon and hydrogen. The widest variation
is possible in that the (non-acetylenic) hydrocarbyl group may
be alkyl, alkenyl, cycloalkyl, cycloalkenyl, aryl, aralkyl,
alkaryl, single ring, mufti-ring, straight chain, branched
chain, large or small. Representative hydrocarbyl groups
include methyl, ethyl, methallyl, n-butyl, hexyl, hexenyl,
isooctyl, dodecyl, oleyl, octadecyl, eicosyl, hexacosyl,
octacosyl, triacontyl, hexatriacontyl, tetracontyl, cyclohexyl,
cyclooctyl, cyclooctenyl, phenyl, naphthyl, benzyl, styryl,
phenethyl, and the like. Thus, a particularly useful class of
bicyclic heterocyclic tert-phosphines is that containing only
carbon, hydrogen, and phosphorus atoms.
Substituted hydrocarbyl groups are also operable and
may contain a functional group such as the carboxyl, nitro,
amino and hydroxy (e. g. hydroxyethyl) groups. A particularly
useful group of ligands consists of those in which R is
hydrocarbyl of from 1 to 36 carbon atoms; especially preferred
are those in which R is hydrocarbyl of from 3 to 30 carbons.
The invention is further illustrated in the following
Examples and in the accompanying figures, which are a graphical
representation of results obtained in Examples 8, 9 & 10.
Example 1
Addition of Cyclohexylphosphine to 1,5-cyclooctadiene
at 95°C. A stirred jacketed glass reactor was inerted with
nitrogen and was then charged with 499 g of cyclohexyl-
CA 02264429 1999-03-03
- 6 -
phosphine.- After heating the reactor contents to 95°C, 208.3 g
of a mixture containing 3.65 g of azobisisovaleronitrile in
204.7 g of 1,5-cyclooctadiene was added over a three hour
period. This was followed by the addition of 266 g of a
solution containing 4.6 g of azobisisovaleronitrile in toluene
over three hours. Gas chromatographic analysis of the product
mixture indicated the presence of 37.9% toluene, 11.8%
cyclohexylphosphine, 2.4% 1,5-cyclooctadiene, 6.7% of the 1:1
RPH2/COD trans adduct, 10.9% unsymmetric 9-cyclohexyl-9-
phosphabicyclo[4.2.1]nonane, 25.6% symmetric 9-cyclohexyl-9-
phosphabicyclo[3.3.1]nonane and a total of 2.4% of byproduct
1:2 RPH2/COD isomers. Based on symmetric and unsymmetric
isomer content, the product mixture contains 70.1% symmetric.
The weight ratios of byproduct 1:1 RPH2/COD trans adduct and
1:2 RPH2/COD adduct isomers to the desired bicyclo nonanes are
0.184 and 0.065 respectively.
The phosphorus NMR spectrum of the product mixture
contained three major signals with the following chemical
shifts: 13.21, -25.71 and -110.92 ppm. The relative areas
were 16.13, 36.02, and 25.41 respectively. The signals at
13.21 and -25.71 ppm are due to the unsymmetric and symmetric
products. The signal at -110.92 is from unconverted
cyclohexylphosphine. A minor signal at -33.80 ppm is due to
the 1:1 RPH2/COD trans adduct. In a corresponding proton
coupled phosphorus NMR spectrum, the signals at 13.21 and
-25.71 ppm remained as ringlets while thecorresponding signals
at -33.81 and -110.92 became a doublet and a triplet
respectivelythus confirming that the assignments corresponded
to the expected tertiary, secondary and primary phosphines.
The symmetric content based on the peak areas is 69.1% which is
consistent with the G.C. data.
Example 2
Addition of cyclohexylphosphine to 1,5-cyclooctadiene
~t 60°C. Similar to Example 1, a reactor was charged with
484.5 g of cyclohexylphosphine, 202.2 g of 1,5-cyclooctadiene
and 3.6 g of azobisisovaleronitrile. After five hours at 60°C,
a further 3.5 g of radical initiator was added. Sixteen hours
CA 02264429 1999-03-03
_ 7
later the reaction mixture was cooled and analyzed by G.C. The
mixture contained 23.1% unconverted cyclohexylphosphine, <0.1%
COD, 5.19% byproduct 1:1 RPH2/COD traps adduct, 19.1%
unsymmetric isomer, 51.6% symmetric isomer and <0.3% byproduct
1:2 RPH2/COD adducts. The symmetric isomer thus made up 73.0%
of the desired bicyclic nonanes. The weight ratios of
byproduct 1:1 RPH2/COD traps adduct and 1:2 RPH2/COD isomers to
desired products were 0.074 and <0.004 respectively.
Example 3
Addition of cyclohexylphosphine to 1,5-cyclooctadiene
at 40°C. Similar to Example 1, a reactor was charged with
495.3 g of cyclohexylphosphine, 241.3 g of 1,5-cyclooctadiene
and 4.2 g of azobisisovaleronitrile. After 15 hours at 40°C, a
further 4.0 g of radical initiator was added. The mixture was
allowed to digest for a further 48 hours. At that time it was
analyzed by G.C. The product mixture contained 33.5%
cyclohexylphosphine, 1.3% COD, 1.8% 1:1 RPH2/COD traps adduct,
15.2% unsymmetric isomer, 44.4% symmetric isomer and 1.2% 1:2
RPH2/COD adducts. The symmetric isomer thus made up 74.5% of
the desired products. The weight ratios of byproduct 1:1
RPH2/COD traps adduct and 1:2 RPH2:COD isomers to desired
products were 0.030 and 0.020 respectively. Examples 1-3
clearly demonstrate the effect of low reaction temperature on
reducing the byproduct 1:1 RPH2/COD traps adduct. An added
benefit from lower reaction temperatures is an increase in
symmetric isomer content.
The above product was subjected to vacuum
distillation to recover the bicyclic products. A 236 g
fraction distilled over with a vapour temperature of 142°C at
0.4 mmHg. The fraction solidified on cooling to room
temperature. (m. pt. approximately 50°C). G.C. analysis
indicated the fraction contained 2.8% 1:1 RPH2/COD traps
adduct, 24.3% unsymmetric isomer and 72.0% symmetric isomer.
The symmetric isomer made up 74.8% of the desired product.
Example 4 (Comparative)
Addition of phosphine to 1,5-cyclooctadiene at 95°C.
A 3.7 litre autoclave was charged with 1378 g of 1,5-
CA 02264429 1999-03-03
_ g _
cyclooctadiene and 400 g of toluene. The mixture was heated to
95°C under 550 psig of phosphine pressure. A mixture
containing 19.5 g of azobisisovaleronitrile in 250 g of toluene
was added over seven hours while maintaining the autoclave
temperature and pressure. G.C. analysis of the product mixture
indicated the presence of 0.25% COD, 26.8% toluene, 1.15% 1:1
PH3/COD trans adduct, 38.2% symmetric isomer, 26.0% unsymmetric
isomer and 4.65% 1:2 PH3/COD isomers. The symmetric isomer
makes up only 59.5% of the desired bicyclic nonanes. The
weight ratios of byproduct 1:1 PH3/COD trans adduct and 1:2
PH3/COD isomers to desired product are 0.018 and 0.072. The
above 9-phosphabicyclic nonanes can be converted to 9-alkyl-9-
phosphabicyclic nonanes by the free radical addition of an
olefin such an octene-1, cyclohexene or isobutylene. The
product mixture will only contain at best 59.5% symmetric
isomer. Examples 1 and 4 demonstrate that the vast increase in
symmetric isomer content which can be obtained by adding a
primary phosphine to 1,5-cyclooctadiene vs the two step process
of phosphine addition to COD followed by further reaction with
an olefin. In addition, while the yield losses of COD to 1:1
P/COD trans adducts are comparable, the COD yield losses to 1:2
P/COD adducts are much less.
Example 5
Addition of isobutylphosphine to 1,5-cyclooctadiene
at 60°C. As per Example 2, a reactor was charged with 462 g of
85% isobutylphosphine (remainder is isopropanol), 232 g of COD
and 3.7 g of azobisisovaleronitrile. After six hours at 60°C,
a further 3.9 g of radical initiator was added. After a
further sixteen hours at 60°C, the mixture was cooled and
analyzed by G.C. The mixture contained 2.2% IPA, 17.6%
isobutylphosphine, 3.1% 1:1 RPH2/COD trans adduct, 18.4%
unsymmetric isomer, 54.7% symmetric isomer and <0.3% 1:2
RPH2/COD isomers. The symmetric isomer formed 74.8% of the
desired product. The weight ratios of 1:1 RPH2/COD trans
adduct and 1:2 RPH2/COD isomers to the desired products are
0.042 and < 0.004 respectively.
Components of the reaction mixture were confirmed by
CA 02264429 1999-03-03
_ g _
G.C./M.S. analysis. The mass spectra of the symmetric and
unsymmetric 9-isobutyl-9-phosphabicyclononane isomers are
virtually identical and had the same molecular ion (198) as the
spectrum of the 1:1 RPH2/COD trans adduct. However the
bicyclic products are distinguished from the 1:1 RPH2/COD trans-
adduct by the rather large abundance (base peak) of the stable
142 ion. The corresponding 142 ion for the trans adduct is
only 35% of the base peak.
Example 6
Addition of isobutylphosphine to 1,5-cyclooctadiene
at 40°C. A reactor was charged with 490 g of 85%
isobutylphosphine (remainder is isopropanol), 309 g of COD and
4.0 g of azobisisovaleronitrile. After 6 hours at 40°C, an
additional 3.6 g of radical initiator was added. Two
additional charges (3.7 and 2.0 g) of initiator were made after
24 and 30 hours respectively. Finally after 48 hours the
mixture was analyzed by G.C. The mixture contained 5.5%
isobutylphosphine, 3.3% COD, 1.97% 1:1 RPH2/COD trans adduct,
19.47% unsymmetric isomer, 63.0% symmetric isomer and 0.9% 1:2
RPH2/COD isomers. The desired products have 76.4% symmetric
content. The weight ratios of 1:1 RPH2/COD trans adduct and
1:2 RPH2/COD isomers to the desired product are 0.024 and 0.011
respectively.
The above product mixture was sujected to vacuum
distillation to recover the bicyclononane isomers. A 290 g
fraction distilled over with a vapour temperature of 126°C at 9
mmHg. The fraction (a viscous liquid) contained 2.45% 1:1
RPH2/COD trans adduct, 21.2% unsymmetric isomer and 72.9%
symmetric isomer. The desired bicyclic components contain
77.4% of the symmetric isomer. A phosphorus NMR of this
fraction indicated two major signals at -2.66 and -38.99 ppm
which had relative areas of 21.07 and 72.15. The area of the
symmetric component is 77.4% of the total. This is consistent
with the G.C. data.
Example 7
Addition of cyclopentylphosphine to 1,5
cyclooctadiene at 35°C. A reactor was charged with 615 g of
CA 02264429 1999-03-03
- 10 -
cyclopentylphosphine, 271 g of COD and 4.0 g of azobis-
isovaleronitrile. Additional 4.0 g charges of radical
initiator were made after 24 and 48 hours at 35°C. Finally
after 72 hours the mixture was analysed by G.C. The mixture
contained 7.9% cyclopentylphosphine, 1.6% COD, 1.9% 1:1
RPH2/COD trans adduct, 18.9% unsymmetric isomer, 54.4%
symmetric isomer. The symmetric content of the desired
products was 74.2%. The weight ratio of the 1:1 RPH2/COD trans
adduct to desired product was 0.026.
Examples 5, 6 and 7 further demonstrate that 74-76%
symmetric 9-alkyl-9-phosphabicyclicnonanes can be obtained by
the addition of primary phosphines to 1,5-cyclooctadiene
whether they be hindered or non hindered.
Examples 8, 9 and 10
Three reactions were carried out using
eicosylphosphine as the hydrocarbylphosphine. The reactions
were carried out at 37°, 70° and 92°C, using Vazo 52 at
the two
lower temperatures and Vazo 67 at the higher temperature. A
10-15% molar excess of COD was charged to a stirred jacketed
and inerted reactor containing eicosylphosphine. After heating
the reaction mixture to the desired reaction temperature the
initiator was added as a solution in toluene over a 4-5 hour
time period. Due to the half life of Vazo 52 at 37°C Example 8
required an additional 23 hours to convert the
eicosylphosphine, whereas the other two reactions were over
within 1-3 hours of adding the initiator.
The reactions were followed by gas chromatographic
(GC) analysis of the product mixtures with time. The actual
charges and reaction conditions are given in Table 1, and the
results are given in Tables 2A and 2B, below, the figures given
in Table 2A being based on data from GC analysis and the data
given in Table 2B being based on data from 31P NMR analysis.
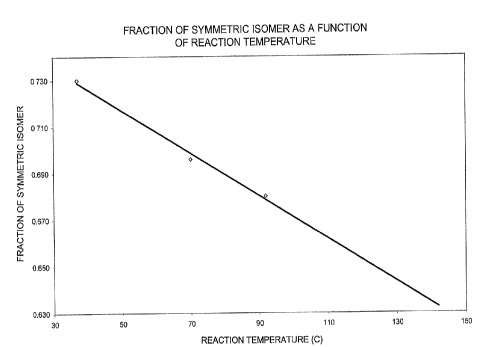
Data from Table 2A are plotted in Figures 1, 2 and 3.
Figure 1 is a graph of the fraction of the symmetrical isomer,
based on the symmetrical plus unsymmetrical isomer, versus the
reaction temperature, Figure 2 is a graph showing the amount of
the undesired trans 1:1 adduct versus the reaction temperature
CA 02264429 1999-03-03
- 11 -
reaction temperature and Figure 3 is a graph of the product
purity i.e., the amount of the symmetrical plus unsymmetrical
isomer, based on the amount of alkylphosphine reactant RPH2,
versus the reaction temperature. Figure 1 clearly demonstrates
that as the reaction temperature is lowered the amount of the
desired symmetrical isomer obtained is increased at the expense
of the undesired unsymmetrical isomer. Figure 2 clearly shows
that as the reaction temperature is lowered the amount of the
undesired trans 1:1 adduct is reduced. Figure 3 clearly shows
that as the reaction temperature is lowered the amount of
symmetrical plus unsymmetrical isomers obtained is increased.
CA 02264429 1999-03-03
- 12 -
(t3 O di N ~ I~Jl
~
~
'7--1 Cp
-,1 \ u1 W
~
,~
O
~ N ~
H
O N O
lD N
N
O
N N
FC c7 ~ d~ ~ ~ c~
H
y
O
U
N
. N d~ l0 L~.a' . ~4 lflO
~,' Ln d' W ~ -~- I~ l0 l0
-1
H ~ ~ . O O O
~
l
L mn oo ~ O
\ ~ d' d''di O U1
rl rl rl C~ M rl
xN _ O H H -I N N
H W
o\o
rl ~ ~ L(1t17
~'-I +~ J> U~
O
..~ U
~1 rIi
II~ ~ M M O iQ,' O O
~
O ~-I61 M ~ H l0 N '-I
N
-rl ~ CO l0 C~ ~ O q CO 01 L(1
o\o
Wit'LflLf1
N N N
H ''-~ ~C
~
O J-
U
H
O N ~ ~ 1~ H
-ra ,.."~C~ m t U tI1
J-~ Pa o1 lflL~ o ~ 61
N l l l ~ o\ '
~
U r r r ~ M C
~ ~
U] ~ r~ r-i
~ FC
-r~- W ~,1
H
O
(~ N N L~ ''-~ N Lf700 di
"I N N l0
'
l!1117l0 ~ o\o "
,
N~ ~ O O O O O rl
O
H ~ ~ ~
W Ul
H
L~ 01 ~-1
O O rl
L~ O N N
W
o~ M L~ 01
~U L~ O N
~ 0
M L 1
H
o r-t
~z ~,
w
w
CA 02264429 1999-03-03
- 13 -
n '~ ~ o ~
c ~
o ~ ~ mo
U +
u~
0 0 0
z
U
'~3
M ''tj
N ,-I aoto ~O
~-1 N
.!-~ Lc7v-IN
N cd
~i
H
N
H o~
N
O
-rl
ri .!-~
,.Q
U
cti
rd
H N
P
4-a
O
0
O
C~O N
.~..) M C~ Ol
N
w E-~
O
J-~
U
O
p., O
z
U O
[) r~ 0001
N
Pa ?C
tx W
CA 02264429 1999-03-03
- 14 -
In the example reactants and products were
characterized by GC/MS data and 31P NMR. While the unsymmetric
and symmetric isomers have almost identical mass spectra, the
spectra are both somewhat different from that of the undesired
traps 1.1 RPH2/COD adduct. However with additional data from
31p ~R spectra, the symmetric and unsymmetric isomers as well
as the traps 1:1 RPH2/COD adduct can be identified. The proton
coupled 31P NMR of a -secondary phosphine (the traps l:l
RPH2/COD adduct) is a well defined "doublet" with a chemical
shift in the -50 to -60 ppm range while the proton coupled 31p
NMR of the symmetric and unsymmetric isomers, because they are
tertiary phosphines, shows ringlets for each isomer with
chemical shifts in the +10 to -40 ppm range. The 31P NMR
spectra of the three product mixtures of Examples 8, 9 and 10
each. have a pair of ringlets and two doublets with peak areas
corresponding roughly to the GC peak areas of the symmetric and
unsymmetric isomers and the traps 1:1 RPH2/COD adduct. Table
2B contains the traps 1:1 adduct concentrations and the
symmetric isomer fraction calculated from the 31P NMR spectra.
The data are comparable to the GC results.