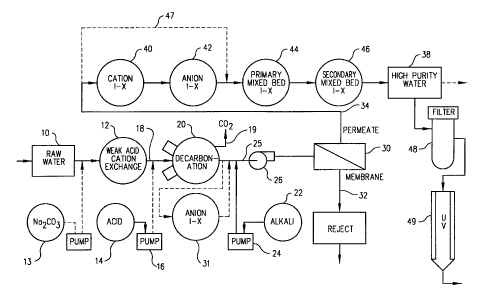
Note: Descriptions are shown in the official language in which they were submitted.
10152025CA 02264619 1999-02-09WO 98/06483METHOD AND APPARATUSFOR HIGH EFFICIENCY REVERSE OSMOSIS OPERATIONTECHNICAL FIELDMy invention relates to a method for the treatmentof water in membrane based water treatment,purification, and concentration systems, and toapparatus for carrying out the method. In oneembodiment, my invention relates to methods forfeedwater pretreatment and for operation of reverseosmosis ("RO") equipment, which achieve increased soluterejection, thereby producing very high purity (lowsolute containing) product water, while significantlyincreasing on the onâstream availability of the watertreatment equipment.BACKGROUNDA continuing demand exists for a simple, efficientand inexpensive process which can reliably provide waterof aa desired purity, in equipment which requires aminimum of maintenance. In particular, it would bedesirable to improve efficiency of feed water usage, andlower both operating costs and capital costs for highpurity water systems, as is required in variousindustries, such as semiconductors, pharmaceuticals,biotechnology, steamâelectric power plants, and nuclearpower plant operations.In most water treatment systems for theaforementioned industries, the plant design and-1-PCT/US97/ 1423910152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239operational parameters generally are tied to finalconcentrations (usually expressed as total dissolvedsolids, or "TDS") which are tolerable in selectedequipment with respect to the solubility limits of thesparingly soluble species present. In particular,silica, calcium sulfate, and barium sulfate often limitfinal concentrations achievable. In many cases,including many nuclear power plants and many ultrapurewater plant operations, boron or other compounds ofsimilarly acting ampholytes have a relatively lowrejection across membranes in conventionally operated'ROsystems, and may dictate design or operatinglimitations. More commonly, the presence of suchcompounds result in sufficiently poor reverse osmosisproduct water, known as permeate, that additional postRO treatment is required to produce an acceptably purewater. In any event, to avoid scale formation andresulting decreases in membrane thruput, as well aspotential deleterious effects on membrane life, thedesign and operation of a membrane based water treatmentplant must recognize the possibility of silica and othertypes of scale formation, and must limit water recoveryrates and operational practices accordingly. In fact,typical RO plant experience has been that declines inpermeate flow rates, or deterioration of permeatequality, or increasing pressure drop across themembrane, require chemical cleaning of the membrane atregular intervals. Such cleaning has been historicallyrequired because of membrane scaling, particulate-2-10152025WO 98/06483CA 02264619 1999-02-09PCT/U897/14239fouling, or biofouling, or some combination thereof.Because of the cost, inconvenience, and productionlosses resulting from such membrane cleaning cycles, âitwould be advantageous to lengthen the time betweenrequired chemical cleaning events as long as possible,while nevertheless efficiently rejecting undesirableionic species and reliably achieving production of highpurity permeate.Since the introduction and near universal adoptionof thin film composite membranes in the mid to late1980s, the improvements in R0 technology have beenevolutionary in nature. Operating pressure needed toachieve desired rejection and flux (permeate productionrate per unit of membrane surface area, commonlyexpressed as gallons per square foot of membrane perday, or liters per square meter per day) has been slowlyreduced, while average rejection of thin film compositemembrane has improved incrementally.Historically, brackish water RO systems have beenlimited in their allowable recovery and flux rates bythe scaling and fouling tendencies of the feedwater. Itwould be desirable to reduce the scaling and foulingtendencies of brackish feedwater to the point whererecovery limits would be dictated by osmotic pressure,and where flux rates can be increased substantially,compared to limits of conventional brackish water ROsystems.From a typical end user's point of view, severalareas of improvement in R0 technology â chlorine-3-l0152025W0 98l06483CA 02264619 1999-02-09tolerance being one of them â are still sought. Thinfilm composite membranes, at least partly due to theirsurface charge and characteristics, are relatively proneto biological and particulate fouling. With certainfeedwaters, particularly from surface water sources,membrane fouling and the frequent cleaning required tocombat fouling can present some arduous, costly, andtimeâconsuming operational challenges.It is known that rejection of weakly ionizedspecies, such as total organic carbon ("TOC"), silica,boron, and the like, is significantly lower thanrejections for strongly ionized species as sodium,chloride, etc. Since the efficiency of post~RO ionexchange is largely determined by the level of the weakanions present in the R0 permeate, it would beadvantageous to remove (reject) as many weak anions aspossible in the RO unit operation. In other words, byremoving (rejecting) more silica (and boron) in the R0step, ea higher throughput is achievable in the ion-exchange unit operation that follows the RO unit.With the exception of an R0 process disclosed inU.S. Patent No. 4,574,049, issued Mar. 4, 1986 Pittnerfor 2a REVERSE OSMOSIS SYSTEM, which reveals a doublepass (product staged) RO system design, carbon dioxidetypically represents the largest fraction of the anionload jJ1 R0 permeate. However, a nmltiple pass R0configuration provides very little benefit underconventional RO system operating conditions, since thecarbon dioxide content of permeate stays at the same-4-PCT/US97/ 14239l0152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239(absolute) level and represents an even bigger fractionof the anion load. High rejection of weak anions in asingle pass RO system is, therefore, considered to beanother area where significant improvement is stillsought.In addition to increasing the rejection of theweakly ionized species, the increased rejection ofstrongly ionized species is also desired.Recovery rate, or volumetric efficiency, is anotherparameterâ where improvements iJ1 RO systenx performancewould be advantageous. A typical R0 system operates atabout 75 percent recovery, where only 75 percent of theincoming feed to RO is used beneficially, and the rest(25 percent) is discharged. With water becoming bothmore scarce and more costly throughout the world,increasing the maximum achievable recovery rate in an R0system is an important goal.Increasing the operating flux is always importantto end users, as increased flux reduces capital costs.Simplification and cost reduction for postâRO unitoperations is also sought by end users. This is becauseallowable levels of impurities in ultrapure water hascontinually decreased with the ever tightening designrules in semiconductor device geometry. Thus, lowercontaminant levels in the ultrapure water system arerequired. As a result, the cost and complexity of thepost-RO system components have dramatically grown inrecent years.High purity water processing procedures and the-5-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239hardware required for carrying them out are complex andexpensive. In fact, the regenerable ndxed bed ionexchange system represents, by far, the most expensive(and complicated) single unit operation/process in theentire ultrapure water treatment system. Thus,significant improvement in the characteristics of the ROtreated water would appreciably reduce the overallultrapure water system cost and complexity.I am aware of various attempts, some in high puritywater treatment applications and some in wastewatertreatment applications, in which an effort has been madeto improve the efficiency of the rejection of certainions which are sparingly soluble in aqueous solution atneutral or near neutral pH. Such attempts are largelycharacterized by conventional hardness removal and thenraising the pH with chemical addition. One such methodis shown in U.S. Patent No. 5,250,185, issued October 5,1993 ix) Tao, et al., for REDUCING AQUEOUS BORONCONCENTRATIONS WITH REVERSE OSMOSIS MEMBRANES OPERATINGAT HIGH PH. In a preferred embodiment, his inventionprovides use of a conventional zeolite softener followedby a weak acid cation ion-exchanger operated in sodiumfornl to remove divalent cations. Due both. equipmentlimitations and process design considerations, hispretreatment steps are followed by the somewhat costlyand otherwise undesirable step of dosing the feedwaterwith aa scale inhibitor to further prevent hardnessscales from forming. Also, although his method doesprovide a simultaneous hardness and alkalinity removal-6-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239step, which is of benefit in many types of applicationswhich are of interest to me, his method does not providefor a high efficiency in that removal step, as isevidenced In! the fact that two additional downstreamsoftening steps are required in his process. Moreover,his application pertains to, and is described andclaimed with respect to oil field produced waterscontaining hydrocarbon compounds (containing carbon andhydrogen only, and generally not ionizable), whereas inapplications which are of interest to me, such compoundsare almost totally lacking. In applications of primaryinterest to me, a variety of naturally occurring organicacid such as humic and fulvic acids are present,particularly in surface waters presented for treatment.Also, method used in high purity water applicationsis disclosed in Japanese KOKAI No. Sho 58âll2890,Published June 29, 2984 by Yokoyama, et al., for METHODOF DESALINATION WITH A REVERSE OSMOSIS MEMBRANE UNIT.His examples show reverse osmosis units utilizing apretreatment process of strong acid cation exchangeresin ("SAC") for softening in one example, and withoutsoftening in the other example. While his process willwork for certain feedwaters, it does not teach howoperation at higher pH levels may be employed whilestill avoiding scaling of RO membranes.In order to better understand my process; it isuseful to understand some basic water chemistryprinciples. With respect to calcium carbonate (CaCOg),for example, the likelihood of occurrence of..7_10152025WO 98/06483CA 02264619 1999-02-09PCT/U S97! 14239precipitation on an RO membrane in the final reject zonemay be predicted by use of the Langelier Index,sometimes known as the Langelier Saturation Index (LSI).See the Nalco Water Handbook, copyright 1979, by McGraw-Hill. This index is generally formulated as follows:LSI = PHreject âPHSwhere pHs = the pH at saturation of CaCO3 (reject)and pHs = pCa + pAlk + Cand wherein:pCa = âlog of Ca++ ion concentration (moles/liter)pAlk = âlog of HCO3â ion conc. (moles/liter)C = a constant based on total ionic strengthand temperature of the RO rejectha a given R0 reject water, in order to avoidcarbonate scaling, it most preferable to keep the LSInegative, i.e. in a condition so that CaCO3 willdissolve. However, in the field, it has been found thatunder some conditions, with use of certain types ofantiâscalant additives, an LSI of up to about +1.5 canbe tolerated, without CaCO3 scale formation resulting.In any event, at the pH of any given R0 reject, pHs mustbe minimized in order to avoid undesirable scaleformation. To put this into perspective, consider thatin any R0 pretreatment operation, it can be anticipatedthat there will always be at least some leakage ofcalcium from the softening step. Thus, depending uponthe raw feedwater hardness and the pretreatment process-8-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239scheme practiced, a lower limit on the achievable valueof the pCa term, due to the concentration of the Ca++ion present in the treated RO feedwater, can beanticipated. Furthermore, in all events, the value of Cis fixed by the total ionic strength and by thetemperature. Thus, to keep the LSI in an acceptablerange â in order to provide scale free R0 operation -the leakage of calcium (as well as other hardness suchas magnesium) becomes a critical factor. The Tao et al.patent, identified above, approaches this problem byproviding various types of softeners in series.Specifically, he simply accepts the inevitably highcapital and operating costs associated therewith.Yokoyama, on the other hand, evidently decided to limitRO operation to a pH which is consistent with the degreeof calcium removal. When he operates with R0 reject ata pH of 9, assuming 0.1 ppm of Ca++ leakage from the ionexchange train disclosed, and a concentration factor of5 ("5X") in the RO, his RO operation may be expected toprovide an R0 reject with an LSI of about -0.5. ThatLSI is acceptable for non-scaling operation, with orwithout scale inhibitors. However, if the pH inYokoyamaâs example were increased to :11, for example,given the same pretreatment method, an LSI of about +2.4might be expected. In such a case, the LangelierSaturation Index of the reject water would be well abovethe level where current antiâscalants have the abilityto provide scale free RO operation.Thus, for the most part, the prior art methods-9-l0152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239known to me have one or more of the followingshortcomings: (a) they do not reliably achieve theextremely low hardness and nonâhydroxide alkalinitylevels necessary for essentially scale free operation atvery high pH levels; (b) they rely on redundant andexpensive capital equipment, with attendant operatingcosts, to minimize hardness leakage, (c) they" dependprimarily on hardness reduction to reduce the LS1 of theRO reject (and do not include provisions for highefficiency dealkalization), and (d) they rely on anti-scaling additives to prevent scale formation. Thus, theadvantages of my simple treatment process which exploits(a) hardness removal to very low residual.levels, and(b) efficient dealkalization, to allow extended troublefree R0 operation at high pH levels, are important andselfâevident.Moreover, because «cf upper concentration factorlimits due to the tendency of scale to form, RO systemsare often unable to use about twenty five (25%) or moreof the raw feedwater. Also, at recoveries levelsgreater than approximately seventy five percent (75%) orsomewhat lower, depending upon raw water chemistry, thecontrol of chemical scaling and biological fouling inconventional RO systems becomes almost unmanageablydifficult when trying to achieve long run times.Therefore, widespread commercial use of RO systems withwater recovery in excess of about seventy five percent(75%) has not been accomplished.As water is becoming increasingly expensive, or in_10_10152025W0 98l06483CA 02264619 1999-02-09PCT/U S97/ 14239short supply, or both, it would be desirable to increasethe ratio of treated product water to raw water feed inR0 systems. Therefore, it can be appreciated that itwouhi be desirable to achieve reduced costs of watertreatment by enabling water treatment at higher overallrecovery rates rates than is commonly achieved today.Finally, it would be clearly desirable to meet suchincreasingly difficult water treatment objectives withbetter system availability and longer run times than iscommonly achieved today.In so far as It anu aware, no one heretofore hasthought it feasible to operate a reverse osmosis basedwater treatment system at higher than about pH 9, incontinuous, sustainable, long term operations to producea highly purified treated water product. Theconventional engineering approach has been to designaround or battle scale formation, by use of moderate pH,by limiting final concentration and resulting waterrecovery, by use of chemical additives. Historically,cellulose acetate membranes were limited in operation toa pH range of roughly 4 to 7. Newer polyamide and thin-film-composite type membranes have traditionally beenoperated in the pH range of roughly from about 4 toabout 8. Although higher pH operation has occasionallybeen attempted for a few special purposes, it hasusually been in non-silica related applications. And,although higher pH operation has been utilized in secondpass R0 applications where silica was of concern, in sofar as I am aware, it has only been accomplished after a_ll_1O152O25WO 98/06483CA 02264619 1999-02-09PCT/US97/14239first pass RO operation with a neutral or near neutralpH of operation. In those cases where organics are ofspecific concern, then the pH may often range to below5, and preferably, below 4.In contrast to prior art methods forâ watertreatment, the method taught herein uses the essentialdesign philosophy of virtually eliminating any possibleoccurrence of scaling phenomenon during first passoperation at the maximum feasible pH using the availablemembranes, while maintaining the desired concentrationfactor, and taking the benefit of water recovery thatresults.SUMMARYI have now invented a novel water treatment methodbased. on. aggressive hardness and alkalinity" removal,followed by membrane separation at high pH, to produce ahigh quality permeate with extremely low silicaconcentration.Sui a unique feedwater treatment process, rawfeedwaters of suitable chemical composition are treatedwith a weak acid cation ion exchange resin, operated inthe hydrogen form, to simultaneously remove hardness andalkalinity. The weak acid cation ion exchange resinscan be operated at incoming raw feedwater hardness andalkalinity levels well above those that would causeconventional ion exchange systems to fail due tohardness breakthrough.The preferred treatment train design used in my-12-1O152025WO 98/06483CA 02264619 1999-02-09PCT/US97l14239wastewater treatment plant overcomes a number ofimportant and serious problems. First, the lowhardness, combined with virtual elimination of non-hydroxide alkalinity, substantially eliminates theprecipitation of scale forming compounds associated withsulfate, carbonate, or silicate anions. Thus, cleaningrequirements are minimized. This is importantcommercially because it enables a water treatment plantto avoid lost water production which would otherwiseundesirably require increased treatment plant size toaccommodate for the lost production during cleaningcycles. Second, the preferred high pH operationalconditions enable a high degree of ionization to beachieved in various species which are sparingly ionizedat neutral or near neutral pH in aqueous solution, toenable such species to be preferentially rejected by themembrane system. Finally, operation at high pH providesprotection against biological contamination, thuspreventing undesirable contamination of product water.At the preferred high operational pH, bacteria andendotoxins are effectively destroyed. In essence, watertreatment systems operated according to the teachingsherein normally operate at conditions which mightordinarily be considered cleaning conditions forconventional RO systems.I have now developed a novel process design for usein treatment of water. In one embodiment, the processinvolves treatment of a feedwater stream which ischaracterized by the presence of (i) hardness, (ii)_13_10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239alkalinity, and (iii) molecular species which aresparingly ionized when in neutral or near neutral pHaqueous solutions, to produce a low solute containingproduct stream and a high solute containing rejectstream. The process involves effectively eliminatingthe tendency of the raw feedwater to form scale when theraw feedwater is concentrated to desired concentrationfactor at a selected pH, by effecting, in any order, oneor more of the following (i) removing hardness from theraw feedwater stream, (ii) removing alkalinity from theraw feedwater stream, or (iii) removing dissolved gasescreated during the hardness removal step. Then, the pHof the feedwater is raised to a selected pH of at leastabout 8.5, or up to 9.0, or Lg) to about 10, orpreferably (with currently available thin film compositetype Inembranes) to a :range between 10 and 11, orotherwise in excess of 11, and more preferably to about12 or somewhat more, until the benefits gained by highrejection rates of silica and other species isoutweighed by the additional cost. With currentlyavailable thin film composite membranes, controlling thepH at up to about 10.5 provides most of the benefits ofthis method without compromise of long-term membranelife. The pH increase is accomplished by adding aselected base to the softened and dealkalatedfeedstream, preferably by direct injection oralternately by the use of anion ionâexchange. The pHincrease urges the molecular species which are sparinglyionized when in neutral or near neutral pH toward-14-10152025WO 98/06483CA 02264619 1999-02-09PCT/U S97/ 14239increased ionization. An alternate concept is that theprotonatable, i.e., proton accepting substances, orbases, are increased. The pH adjusted feedwater is thensent through membrane separation equipment, typically ofthe reverse osmosis type, but alternately ofnanofiltration or other suitable type or configurationwhich is otherwise available, or which may in the futurebecome available, and in which the current method may bepracticed, to produce a reject stream and a productstream. The membrane separation equipment is ideally ofthe type which has a semi~permeable membrane which whichsubstantially resists passage of ionized speciestherethrough. It is important that in my process, themembrane separation equipment produces a product streamwhich is substantially free of the normally undesirablespecies which are sparingly ionized when in neutral ornear neutral pH in aqueous solutions.OBJECTS, ADVANTAGES, AND FEATURESFrom the foregoing, it will be apparent that oneimportant and primary object of the present inventionresides in the provision of a novel method for treatmentof water to reliably and continuously produce over longoperational cycles a water product stream of a pre-selected extremely high purity quality standard.More specifically, an important object of myinvention is to provide a membrane based water treatmentmethod which is capable of avoiding common scaling andfouling problems, so as to reliably provide a method of-15-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239high purity water generation when operating at highefficiency.Other important but more specific objects of theinvention reside in the provision of a method for watertreatment as described in the preceding paragraph which:allows the of hardness andremovalalkalinity from a selected feedwater to bedone in a simple, direct manner;has a minimum of unit process requirements;minimize or avoid complex chemical feedsystems;requires less physical space than existingtechnology water treatment plants;is easyâ to construct, to start, and toservice;has high efficiency rates, that is, theyprovide high product water outputs relativeto the quantity of feedwater input to thewater treatment plant;in conjunction with the preceding object,provide lower unit costs to the watertreatment plant operator and thus to thewater user, than is presently the case;in conjunction with the just mentionedobject, results in less chemical usage thanin most water treatment facilities, byvirtually eliminating use of some types ofheretofore commonly used chemical additives,particularly scale inhibitors.-16..l0152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239A feature (Hf one embodiment of the presentinvention is the use of a unique combination of weakacid cation ion-exchange with substantially completehardness and alkalinity removal, and subsequent high-pHRO operation, thereby enabling the water treatmentplant to minimize the percentage of reject water. Thisresults in high overall cycle efficiencies.Another feature of the present invention is the useof a high pH operation to highly ionize weaklyionizable species such as silica, boron, or TOC, thusenabling operation with silica, boron, or TOC rejectionlevels considerably exceeding the limits ofconventional RO treatment systems when treatingfeedwaters of comparable chemistry.Yet another feature of the present invention is thecapability to retrofit existing R0 plants to operateaccording to the present process design, to increasecapacity without increasing the RO membranerequirements.Another feature of the present invention is theability to provide higher purity product water whileoperating at kugher flux levels than has heretoforebeen feasible with conventional RO system designs.Other important objects, features, and additionaladvantages of my invention will become apparent tothose skilled in the art from the foregoing, and fromthe detailed description which follows, and from theappended claims, in conjunction with the accompanyingdrawing.-17-10152025WO 98/06483CA 02264619 1999-02-09PCT/U S97/ 14239BRIEF DESCRIPTION OF THE DRAWINGIn the drawing, identical features shown in theseveral figures will be referred to by identicalreference numerals without further mention.FIG. 1 illustrates the percentage ionization ofsilica ions in aqueous solution as a function of pH.FIG. 2 illustrates a first embodiment of my methodfor high efficiency reverse osmosis operation, showinguse of a weak acid cation exchange unit for simultaneoushardness and nonâhydroxide alkalinity removal.FIG. 3 shows a second embodiment of my method forhigh efficiency reverse osmosis operation, whereinhardness is reduced by sodium zeolite softening andoptional lime or lime/soda softening.FIG. 4 shows a third embodiment of my method forhigh efficiency reverse osmosis operation, showing theequipment configuration where alkalinity in rawfeedwater can be efficiently and adequately reduced byacid addition, and where hardness may optionally bereduced by lime or lime/soda softening.FIG. 5 illustrates the differential pressure, inpounds per square inch versus time (PSID v. Months) fora reverse osmosis membrane employed in pilot reverseosmosis test equipment utilizing my novel process.FIG. 6 illustrates the normalized permeate flow, inliters per nunute versus time, for a reverse osmosismembrane employed in pilot reverse osmosis testequipment utilizing my novel process.FIG. 7 illustrates the silica concentration in the-18-101520WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239reverse osmosis reject stream in pilot reverse osmosistest equipment utilizing my novel process.FIG. 8 illustrates the rejection. percentage ofsilica versus time, for a reverse osmosis membraneemployed in pilot testing of my novel process.FIG. 9 describes the use of my method of R0 systemoperation when using a nmltipass RO process tosequentially process a portion of initial feedwater toproduce a permeate which has been passed through morethan one RO membrane.FIG. 10 illustrates the use of my Inethod. of ROsystem operation for boiler feed makeup water, or forcooling tower makeup water, or for scrubber makeupwater.FIG. 11 illustrates the use of my method of ROoperation in combination with continuous electro-deionization equipment for high purity water production.FIG. 12 illustrates a process flow diagram for oneconfiguration of my high efficiency RO process.FIG. 13 illustrates a system schematic for aconventional RO system process design.FIG. 14 illustrates an exemplary process flowdiagram for my high efficiency RO process, utilizing thedesign and operational concepts taught herein.-19-l0l5202530WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239DETAILED DES CRIPTI ONI have developed a new nethod for process designand operation of RO systems. This new method forprocess design and operation of RO systems has beenthoroughly tested. The process has shown that it iscapable of achieving important improvements in R0operational objectives.Attributes which characterize my HERO (tm) brand ROprocess design and operation include:(1) Very high rejection of all contaminants, especiallyweak acid anions such as TOC, silica, boron, etc.(2) Very high achievable recovery - ninety percent(90%) or higher recovery can be achieved.(3) Biological fouling is essentially eliminated.(4) Particulate fouling is substantially reduced.(5) Cleaning frequency is substantially reduced.(6) Removal of chlorine from the feedwater may not beneeded, due to the resulting chemical speciespresent at the high operating pH, or in some cases,by eliminating the need to add chlorine in thefirst place.(7) Addition of scale inhibitors is virtuallyeliminated.(8) Substantially higher flux is achieved.(9) Reduced overall capital cost, compared toconventional RO systems.(10) Reduced overall operating cost, compared toconventional R0 systems.(11) The complexityâ of an 'ultrapure water system issignificantly reduced._20_10152025WO 98/06483CA 02264619 1999-02-09PCT/U S97! 14239The HERO brand RO system is highly siteâspecific.Individual process steps are customized to fit thespecific feedwater at a specific site. Regardless ofthe difference in pretreatment process for differentsites, one process parameter is common for allapplications, namely that the RO system is operated atthe highest feasible reject pH. Consistent with thehighest allowable pH limit for currently available R0membranes (for example, pH 11.0 for FILMTEC(R) brand ROelements), a typical HERO brand RO system is designed tooperate at pH of up to approximately 11, as measured inthe R0 reject stream.Because of the very high concentration factors(i.e. percent recovery) allowed by my HERO brand ROprocess, the RO feed pH is correspondingly lower. Forexample, in a system operating at ninety percent (90%)recovery, a feed pH of 10.0 will produce a reject streamat an approximate pH of 11, provided that the RO feed isonly slightly buffered by the presence of carbonate,phosphate, etc. Unlike conventional RO systems,typically operated at about seventy five percent (75%)recovery, a HERO brand RO system can be routinelyoperated at ninety percent (90%) or greater recovery,limited only by osmotic pressure of the RO reject. ThepH increase from RO feed to reject is magnified at veryhigh recoveries. Thus, the maximum allowable pH isspecifically applicable for the R0 reject conditions.â-21..l0152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239In order to operate an R0 system with reject up tonear pH 11, or at about pH 11, or above, several processconditions must be met in order to effectively eliminatethe potential for scale formation on the RO membrane.Some of those process conditions are also necessary foroperating an R0 system at very high recovery rate. Suchprocess conditions are as follows:(1) Calcium, magnesium, strontium, and bariumconcentration in the RO feed must be substantiallyeliminated, preferably to near zero, and mostpreferably, to essentially zero.(2) Aluminum, iron, and manganese content includingorganically bound species, as well as the presenceof colloidal particles containing such materials,should be substantially eliminated, and preferablyto near zero.(3) Buffering anions (specifically bicarbonate, orcarbonate, and/or phosphate species) should bereduced to as low of a level as can be practicallyachieved.The selection of specific operations and controlpoints to fulfill the above process conditionrequirements is influenced by the characteristics ofeach specific feedwater. The percent recovery needed(or desired for a specific application) also affects theoperations and control point criteria as well. FIG. 2represents a highly cost effective R0 unit processsequence._22_10152025WO 98/06483CA 02264619 1999-02-09PCT/U S97/ 14239The first step is to adjust the hardnessâtoâalkalinity ratio of the feedwater, if needed.Optimizing this ratio, which is typically done by alkaliaddition, makes complete hardness removal feasible inthe next process step.The second step in the RO process train involvesthe utilization of a weak acid cation (WAC) resin (e.g.DOWEX(R) MACâ3, or Lewatit CNPâ80, Amberlite(R) IRCâ86).Operated in hydrogen form, the WAC resin removeshardness quantitatively, given the proper hardnessâtoâalkalinity' ratio of the influent. The hydrogen ionsliberated in the cation exchange process react with thealkalinity and produce carbonic acid (H2CO3)2, which isdissolved in the WAC effluent.The third step involves adding acid to the WACeffluent to destroy" the remaining alkalinity; if anysuch alkalinity is present. Total alkalinity removal atthis step is important in order to achieve very highrecovery across the RO system.In a fourth step, the acidified effluent,containing "virtually" zero hardness and alkalinity, isthen treated for carbon dioxide removal. This removalcan be accomplished iJ1 a forced/induced draftdecarbonator or in an existing vacuum degasifier ofeither packed bed or gas permeable membrane barrierdesign. The decarbonated, essentially" zero hardness,essentially zero alkalinity water, is then injected witha soluble alkali, preferably for adjusting pH to 10.0 orhigher, and most preferably to the 3&1 as needed to_23_10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239achieve pH up to at or near 11.0 in the RO reject.The next step consists of operating the R0 systemin such a manner that the pH of the reject isapproximately, but preferably not appreciably higherthan, 11Ø Note that this pH 11 limitation isapplicable simply with respect to currently available ROmembranes. An exemplary membrane, with the highest pHtolerance capability, is a FILMTEC type FT30 membrane.If RO membranes with a higher pH tolerance capabilitybecome available in the future, then the maximumallowable RO reject pH can be raised accordingly, withconcomitant benefits from the higher pH, in excess ofll.O.Feedwaters utilized for production of high puritywatery as âwell as those encountered iJ1 wastewatertreatment, include the presence of silicon dioxide (alsoknown as silica. or SiO2) in. one forn1 or another,depending upon pH and the other species present in thewater. For membrane separation systems, and inparticular for R0 type membrane separation systems,scaling of the membrane due to silica is to bereligiously avoided. This is because (a) silica formsrelatively hard scale that reduces productivity of themembrane, (b) is usually rather difficult to remove, (c)the scale removal process produces undesirablequantities of spent cleaning chemicals, and (d) cleaningcycles result in undesirable and unproductive offâlineperiods for the equipment. Therefore, regardless of thelevel of silica in the incoming raw feedwater, operation..24__l0l52025WO 98/06483CA 02264619 1999-02-09PCT/U S97/ 14239of conventional membrane separation processes generallyinvolves concentration of SiO2 in the high totaldissolved solids ("TDS") stream to a level notappreciatï¬gr in excess of 150 ppnl of SiO2 (as SiO2).Typically, RO systems are operated at lowered recoveryrates, where necessary, to prevent silica concentrationin the reject stream from exceeding roughly 150 ppm.Scaling due to various scale forming compounds,such as calcium sulfate, calcium carbonate, and thelike, can be predicted by those of ordinary skill in theart and to whom this specification is directed, by useof the Langlier Saturation Index, as discussed above, orother available solubility data. Operating parameters,including temperature, pH, permeate and reject flowrates, must be properly accounted for, as well as thevarious species of ions in the raw feedwater, and thosespecies added during pretreatment.I have found that by reliable hardness and non-hydroxide alkalinity removal, to levels whicheffectively avoid formation of scale at a selected pHfor R0 operation, the concentration of SiO2 in the ROreject streanl can. be safely increased to 450 ppu1 ormore. This is accomplished by increasing the pH of thefeedwater to the R0 system, and without use of scale-inhibition chemicals. Moreover, even with this increaseof silica in the R0 reject, the level of silicacontamination in the RO permeate is preferentially andsubstantially decreased, when compared to the silicawhich might be anticipated under conventional RO process-25-10152025WO 98/06483CA 02264619 1999-02-09PCT/U S97] 14239conditions.It is commonly understood that the solubility ofsilica increases with increasing pH, and that silica isquite soluble in high pH aqueous solution. Along withsolubility, the degree of ionization of silica alsoincreases with. increasing jpH. While the increase insilica solubility is not directly proportional to thedegree of ionization, the rate of increase in silicasolubilityâ is basically proportional âto the ârate ofchange in ionization. This discrepancy betweensolubility and ionization is explained by the fact thateven undissociated silica exhibits some solubility inaqueous solutions, typically up to about one hundredtwenty (120) ppm to one hundred sixty (160) ppm,depending upon temperature and other factors. Incomparison, silica solubility at pH 11 is in excess ofone thousand five hundred (1,500) ppm at ambienttemperature; silica is increasingly soluble astemperature and/or pH increases.Silica is very weakly ionized when in neutral ornear neutral aqueous solutions and. is generallyconsidered. to exist as undissociated (meta/orthoâ)silicic acid (H4SiO4) in most naturally occurring waterswith a pH of up to about 8. The dissociation constant(pKa) value forâ the first stage of dissociation ofsilica has been reported at appnmdmmtely 9.7, whichindicates that silica is approximately fifty percent(50%) ionized at a pH of 9.7; the other fifty percent(50%) remains as undissociated (ortho) silicic acid at_25-l0l52025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239that pH. A graphical representation of the relationshipbetween pH and the percent silica ionization is shown inFIG. 1. Clearly, it would be advantageous, where silicaionization is desired, to operate at a pH in excess of10, and more preferably, in excess of 11, and yet morepreferably, in excess of 12.The understanding of silica ionization phenomenonis important since the rejection of most species acrossthe membranes of membrane separation equipment isenhanced by increased ionization. Consequently, silicarejection by an RO membrane can be expected to improveas the degree of ionization increases; with respect tosilica, ionization increases at higher pH. Therefore,increasing the pH of the RO operation thus providesmajor benefits. First, silica solubility can beradically increased, even while remaining within thecurrent pH limitations of existing commercial thin filmcomposite type RO membranes. Second, silica rejectionis increased significantly at high pH levels,corresponding to the increased degree of ionization ofthe silica.To gain maximum benefit from silica ionization athigh pH, the RO system should be operated at a pH ashigh as possible, given the limitations imposed.bymembrane chemistry and by the nembrane manufacturer'swarranty. Preferably, the RO system is operated at a pHof about 10 or above, and more preferably, at 10.5 orabove, and most preferably, at a pH of 11 or higher.This contrasts with typical RO operation practice,.where_27_CA 02264619 1999-02-09WO 98/06483 PCT/US97/ 14239operating pH has been limited to about 8.5, in order toavoid scale formation, particularly silica and carbonatescales.Referring" again to FIG. 2, one embodiment of myprocess for membrane separation equipment operation isshown. In this method, raw water 10 is first treated ina weak acid cation ion exchange unit 12, where hardnessand bicarbonate alkalinity are simultaneously removed.For those cases where raw water 10 hardness is greaterthan alkalinity, operation of the weak acid cation ionexchange unit 12 must be facilitated by addition of asource of alkalinity 13, such as by addition of anaqueous solution of sodium carbonate (Na2CO3).Preferably, the WAC unit 12 is operated in the hydrogenform for ease of operation and regeneration. However,it would also work in the sodium form, followed by acidaddition. In any case, in the just mentioned case andotherwise optionally where appropriate, acid 14 is addedby pump 16 to the effluent 18 from the WAC unit(s) 12 toenhance bicarbonate destruction. Then, the carbondioxide 19 which has been created in the WAC (and/or byacid addition) is removed, preferably in an atmosphericpressure or vacuum degassifier 20. Finally, an alkali22 (base) is added, preferably by pumped 24 injection ofliquid solution, to increase the pH of the feedwater 25to a selected level. Any of a variety of convenientlyavailable and cost effective base products may be used,provided that no appreciable scaling tendency isintroduced.â Besides use of common sodium hydroxide,-28-l0152025WO 98/06483CA 02264619 1999-02-09PCT/U S97/ 14239other chemicals such as sodium carbonate, potassiumhydroxide, or potassium carbonate might be selected. Infact, in certain cases, an organic base, such as apyridine type compound, may be used effectively to carryout this process. In any event, pressurization offeedwater 25 for the membrane process is accomplished byhigh pressure pump 26 before transfer to the RO typemembrane separation unit 30 as shown. Alternately,alkali (base) addition to the feedwater may beaccomplished by passing the feedwater through an anionionâexchange unit 31 to increase the pH to 21 desiredlevel. The pH of the feedwater is raised to a selectedpH of at least about 8.5 or 9.0, or up to about 10, orpreferably (with currently available thin film compositetype membranes) to a range between 10 and 11, orotherwise in excess of 11, and more preferably to 12 ormore, and most preferably, to 13 or nmre. Withcurrently available thin film composite type ROmembranes, such as those sold by DOW CHEMICAL ofMidland, Michigan under their FILMTECH brand by theirFILMTEC, INC. subsidiary, controlling the pH to about10.5 provides most of the benefits of this methodwithout compromise of long-term membrane life. However,to increase silica solubility, and silica rejection,membranes allowing the pH to be raised to at least about11, or more preferably to at least about 12, or mostpreferably, to at least about 13, would be desirable.Thus, it can be appreciated that my method may be usedto even further advantage when membranes with long life-29-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239expectancy" at such. elevated. pHâs become commerciallyavailable.Reject 32 from membrane separation unit 30 may besewered or sent to further treatment, as appropriate inparticularâ site circumstances. Permeate 34 frommembrane separation unit 30 may utilized "as is" or maybe furtherâ purified. to remove residual contamination,for example, for high purity water users such assemiconductor manufacturing, where 18.2 meg ohm puritywater is desired. A conventional post-RO treatmenttrain for production. of high âpurityâ water 38 in thesemiconductor industry includes a cation exchanger 40,followed by an anion exchanger 42, with primary 44 andsecondary 46 mixed bed polisher ion exchange units.Somewhat different post R0 treatment trains may beutilized to west the particularized needs of 21 givensite, raw water chemistry, and end use, withoutdeparting from the advantages and benefits which may begained by the RO process method disclosed herein. Forexample, it may be desirable in some circumstances toomit the cation. 40 and. anion. 42 ion-exchangers, andbypass the RO permeate via line 47 to directly reach theprimary mixed bed 44 and polish mixed bed 46 ionâexchange âunits. Finally, :h1 many ultrapure waterplants, the product from the polishing mixed bed ion-exchange units 46 is currently further treated in finalfiltration units 48 and ultraviolet irradiation units 49to eliminate particulates and biofouling, respectively.Additional treatment operations may added as appropriate-30-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239to meet the needs of a particular end user.Another distinct and unique advantage of my methodof RO system operation is that it may be possible, undervarious raw feedwater chemistry and operatingconditions, to operate the entire postâRO ion exchangetrain (i.e., ion-exchange, units 40, 42, 44, and 46)without regeneration. Depending upon chemistry, it maybe possible to simply replace the cation 40 and anion 42exchangers. In the more usual case, the secondary orpolishing waxed bed unit 46 may be replaced with newresin, and the old polishing resin moved to the primarybed 44 âposition. This is possible, particularly inultrapure and boiler feed type water treatment systems,because the polishing mixed bed unit 46 is controlled byending operation when the silica, boron, or other ionleakage reaches a predetermined value. When thepredetermined ion leakage value is reached, the thenpolishing mixed bed unit 46 is substituted for, andplaced into the position of, the primary mixed bed ion-exchange unit 44. When the change over of mixed bedion-exchange units is made, the "old" primary mixed bedunit 44 resin is taken out, and either discarded or soldto other less demanding resin users. New resin is thenput into the "old" primary mixed bed ion-exchange unit44, whereupon it becomes the "new" polishing mixed bedion exchange unit 46. .In other embodiments, and as suited to meet theparticularized needs of a selected raw feedwaterchemistry, various forms of hardness removal may be-31-l0l52025W0 98/06483CA 02264619 1999-02-09PCT/US97/14239utilized, including sodium form strong acid cationexchange 50, followed by acidification (see FIG. 3) oreven the use of a lime 52 (or similar lime/soda)softener as an additional pretreatment step to eithersodium form strong acid cation exchange 50 or weak acidcation exchange 12 (see FIGS. 2 and 3).For particularly soft waters, the lime or lime/sodasoftenerâ 52 may Ix: totally inappropriate, and thismethod may proceed with no softening of the raw water,and only a simple acid 14 feed before decarbonization,as can be seen in FIG. 4. On the other hand, wheresoftening is appropriate, some raw feedwaters can beappropriately treated for reductions in hardness andalkalinity to a desired extend by softener 52.Regardless of the equipment configuration selected fortreatment of a particular raw water chemistry, the keyprocess parameters are (a) to remove those cationswhich, in combination with other species present at highpH, would tend to precipitate sparingly soluble salts onthe membrane surfaces, and (b) eliminate non-hydroxidealkalinity to the maximum extent feasible, to furtherprotect against precipitation of scales on the membranesurfaces.The weak acid cation ("WAC") ionâexchange resinsused in the first step of the preferred embodiment of mymethod, as illustrated in FIG. 2, are quite efficient inthe removal of hardness associated with alkalinity.Such a reaction proceeds as follows:-32-CA 02264619 1999-02-09WO 98/06483 PCT/U S97/ 14239Ca++ + 2RCOOH âââ> (RCOO)2Ca + 2H'''Then, the hydrogen combines with the bicarbonate toform carbonic acid, which when depressurized, formswater and carbon dioxide, as follows:H+ + HCO3- ----> HZCO3 ----> H20 + CO2Regeneration of the resin is accomplished by use-ofconveniently available and cost effective acid. It iswell known by those in the art that regeneration of WACionâexchange resins may proceed quite efficiently, atnear stoichiometric levels (generally, not more thanabout one hundred. and twenty" percent (120%) of ideallevels). Preferably, hydrochloric acid may be used,since in such cases highly soluble calcium chloridewould be produced, and the regeneration process wouldnot pose the potential danger of formation of insolublesulfate precipitates, such as calcium sulfate, even withhigh strength acids. However, by use of a stagedregeneration procedures, i.e., by using a lowconcentration acid followed tar a higher concentrationacid, it. is possible to reliably utilize other acids,including sulfuric acid (H2804), while still avoidingundesirable precipitates on the resin. In this manner,hardness ions are solubilized to form soluble salts,which are eluted from the resin bed and are typicallysewered. âUse of sulfuric acid, is particularlyadvantageous in semiconductor manufacturing operations,-33..l0152025WO 98/06483CA 02264619 1999-02-09PCT/U S97/ 14239since such plants typically use large quantities of suchacid, and. waste or spent acid xnay'.be advantageouslyutilized for regeneration of a weak acid cation exchangebed.Other polyvalent cations, most commonly iron(Fe++/Fe+++), magnesium (Mg++), barium (Ba++), strontium(Sr++), aluminum (Al+++), and manganese (Mn++/Mn++++),are also removed by the WAC resin. Since the presenceof even âvery small quantities of hardness or" otherspecies of decreasing solubility at increasing pH willresult in precipitation of sparingly soluble salts underthe process conditions present in my process, care mustbe taken to prevent precipitation on the membrane of thesubstances such as of calcium carbonate, calciumhydroxide, magnesium hydroxide, and magnesium silicate.One precaution that should be observed is that bothhardness and nonâhydroxide forms of alkalinity should beaggressively reduced in the feedwater, prior to upwardpH adjustment to selected RO operating conditions. Oncehardness and nonâhydroxide forms of alkalinity have beenremoved, then the desired pH increase may beaccomplished with any convenient alkali source, such assodium or potassium alkali, or by anion exchange. Oncethis pretreatment has been thoroughly accomplished, thenan R0 system can be safely operated at very high pHlevels, in order to take advantage of the aforementionedsilica solubility.In cases where raw water composition is such thatsodium zeolite softening is advantageous, as is depicted-34..l0152025WO 98/06483CA 02264619 1999-02-09PCT/U S97/ 14239in FIG. 3, elimination of calcium hardness proceeds asfollows:Ca+2 + Na2X âââ> CaX + 2Na+Thenq bicarbonate alkalinityâ is converted tx> carbondioxide, with a selected acid, in a manner similar tothe following:NaHCO3 + HCl âââ> NaCl + H20 + CO2For those waters where lime softening may be anacceptable or preferred method for initial hardness andalkalinity reduction, the addition of lime to the waterreduces calcium and magnesium hardness, and associatedbicarbonate alkalinity, as follows:Ca(HCO3)2 + Ca(OH)2 âââ> 2 CaCO3 * + 2 H20Mg(HCO3)2 + 2Ca(OH)2 âââ> Mg(OH)2 V + 2 CaCO3 + 2 H20This process configuration is depicted as an alternateembodiment of my method, as illustrated in FIGS. 3 and4. In the cases where lime or lime/soda softening isused, however, extreme care must be used in evaluatingthe performance of the remainder of the preâtreatmentsystem, since the solubility of hardness ions remainsappreciable in the softener 52 effluent stream 54.For most feedwaters, particularly where lime or-35-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239lime/soda softening is not employed, the use of a carbondioxide removal step significantly enhances cost-effectiveness of the process when carried out prior tothe pH increase. This also helps to maintain a lowertotal alkalinity level in the feed to the RO, thusproviding a greater margin of safety against scaling dueto hardness leakage from the cation removal step.Dealkalization by carbon dioxide removal also helps toenhance silica rejection, due to the lack of competingspecies. This is because the rejection of one weaklyionized anion is affected by the presence andconcentration of other weakly ionized anions in thefeedwater; this applies to weakly ionized anions such asboron, organic acids (TOC), cyanide, fluoride, andcertain arsenic and selenium compounds.Since the high pH operation also increasesionization of other weakly ionized anions, includingborate, organic acids (TOC), cyanide, fluoride, andcertain arsenic and selenium compounds, their rejectionrates are enhanced in an RO membrane system.Consequently, in general, my method may beadvantageously applied to reject across the membranemost weak acids with 51 pKa1 of about 7.5 or higher.Silica rejection can be increased to about 99.95%, ormore, from a conventional baseline of about 99%rejection; this amounts to at least one order ofmagnitude decrease in the amount of silica escaping intothe permeate, thus providing a ten plus (10+) foldincrease in running life for the silica scavenging ion--35-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239exchange resin bed, namely anion exchanger 42 and themixed bed units.In the case of cyanide, rejections in a first passR0 of in excess of ninety percent (90%) can be attained,in contrast with a nwre typical range of about fiftypercent (50%) or so with conventional RO processes.Similar to the case for silica, boron rejection canbe increased from a conventional baseline from a rangeof about 60-70% to 99% and higher, by operation at asuitably high pH. The beneficial effects on rejectionpercentage due to higher pH operation start at aslightly lower pH in the case of boron, since the pKafor boron is 9.14, roughly one-half pH unit higher thanthat for orthosilic acid, namely 9.7. The beneficialeffects of high pH operation are much more pronounced inthe case of boron, however, because orthosilic acid(H2SiO4) in aqueous solution typically includes sixmolecules of water of hydration, whereas boric acid(H3BO3) typically has no attached hydrating watermolecules. Thus, the orthosilic acid molecule is verylarge with respect to membrane pore size as compared.toboric acid, no matter what the pH, and as a result,silica has much higher normal rejection rates.Consequently, the increased ionization of boric acidwhen operating at aa pH in excess of about 9.1 isextremely beneficial, and increasingly so as pH rises tobetween 10 and 11, or the currently preferred controlpoint of approximately 10.5. The boron rejection effectwould be even further enhanced when operating an R0-37..1O152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239system at a pH of 12 or even 13, when commercialmembranes become available for such practice.EXAMPLE â PILOT TESTA pilot water treatment system was set up to testthe efficacy' of the method. disclosed. disclosed. Thepilot water treatment system was designed for treatingan incoming raw city water supply to provide high purityproduct water for potential future use in aa semi-conductor manufacturing plant. The objectives were (a)to increase recovery, so as to minimize water usage, (b)to increase the purity of treated water, and (c) toincrease the average time between nembrane cleanings.The pilot system performed a series of tests. In eachof the tests, the system was started up with 450 ppm orhigher silica level in the RO reject. The pilot plantsystem was operated continuously until either (a) a tenpercent (10%) decline in normalized RO permeate waterflow was experienced, or (b) a fifteen percent (15%)increase in axial differential pressure across the ROmembrane was reached. The pilot test was performed witha membrane separation unit including ea Dow/Filmtec R0Membrane Model FT30/BW4040, which was operated_atpressures from about 130 psig to about 185 psig, withfeedwater temperatures ranging from about 200 C to about25°C, and at feedwater rates of up to about 8 US gallonsper minute (30 litres per minute) maximum. As seen inFIG. 6, long term normalized permeate flows of slightly-33-1015202530WO 98/06483CA 02264619 1999-02-09PCT/US97/14239more than 5 US gallons per minute (about 20 liters perminute) were tested. The pilot test apparatus includeda pair of weak acid cation ion exchange beds operated inparallel, utilizing Rohm and Haas Company (Philadelphia,Pennsylvania) weak acid cation resin product number IRCâ86, followed by a forced air decarbonator, sodiumhydroxide injection, separation of the treated feedwaterby the RO membrane into a reject stream and a permeatestream.Table 1 presents the chemical analyses of from thepilot plant operation for raw water, RO reject, and R0permeate. The Table 1 also shows the rejection ratesachieved in the pilot RO operation, and compares thoserates with those achieved with a conventional RO systemoperating on the same feedwater. In particular, notethe level of silica in the raw feedwater (67 ppm) and inthe RO reject (480 ppm). The silica concentration inthe IUD reject is roughly three times that normallyachievable in reject water from conventional R0 processconfigurations. Moreover, even at the highconcentration of silica in the R0 reject, improvedrejection of silica is seen, in that silica rejection of99.87% was achieved, compared with rejections rangingfrom about 95% up to about 99% with a conventional ROsystem on the same feedwater.In fact, improved rejection rates were experiencedwith all important chemical species over the rejectionrates experienced with conventional R0, as is clear fromthe data presented in Table 1. Specifically, the high-39-CA 022646l9 1999-02-09W098/06483TABLE 1PILOT TEST ANALYTICAL RESULTSPCT/U S97/ 14239V RnwFecd Pilot R0 Pilot R0 Pilot R0 Convention-a1RQ(ppm) Reject Ptrmcï¬lr Rejcctuon Rejection ("/o)(Ppm) (ppm) _ (°/«)5 sodium 299 460 0 955 99.73 95598Potassium 6,4 33.7 <0.003 99,93+ 90*95Calcium 34 <01 <0003 -~Magnesium 5.3 <0.1 <0 000âChloride 12.1 78.1 <o.o04 9999+ 97-98Nitrate 0.74 9.42 0.003 99.96 90-95Sulfate 46.1 278.4 <0_00l 99 99+ 99.9lBoron 0.083 0.62 0.007 98.51 60-70(Dissolved) Silica 67 430 0.46 99.87 .95-991 0 TOC 0.64 1.1 <o,003 99.66+ 90-95pH 3.0 10.3 10.3 -- --Concentrations in ppm as ion, unless otherwise noted.TABLE 21 5 âSodium Ion Exchange Effects' Sodium, ppbConventional New- ' R0 ProcessR0 Pcrmcatc 193 955Post Cation IX 0.431 <0.0072 0TABLE 3POST MIXED BED ION EXCHANGE RESULTSConstituent Conventional R0 New ProcessBoron Nomdctcctable NomdetoctableSilica 0.43 ppb 0.35 ppbTOC 5.9 ppb < 3.0 ppb-40-10152025WO 98106483CA 02264619 1999-02-09PCT/US97/14239rejection rates of boron and TOC also providesignificant additional benefit in reducing loading ofdownstream anion 42 and mixed bed ion exchange units 44and 46. In this regard, note that a rejection of 98.51%was achieved for boron, compared with about 60% to 70%which is achievable in conventional RO systems on thesame feedwater. Typically, termination of an anion ormixed bed exchange run is determined by silica, or incertain cases, boron leakage. In spite of higherrecovery in the pilot R0 system, silica content in theconventional R0 system permeate was three times higherthan in the pilot R0 system. Specifically, silicaconcentrations of 0.45 ppm SiO2 were achieved inpermeate from the pilot test unit of this method,compared to 1.5 ppm SiO2 in conventional RO permeate.Clearly, levels of less than 1.0 ppm SiO2 are achievablein R0 permeate when utilizing the present method, and infact, levels of less than 0.5 ppm SiO2 have been shownachievable. Also, the boron content in permeate from mynovel process was 0.007 ppm E, versus 0.06 ppm B forpermeate from a conventional R0 system. Clearly, boronlevels of less than 0.05 ppm were demonstrated, as wellas levels of less than 0.01 ppm of boron. The testresults from Table 1 also shown this result, in thatrejection of boron in a conventional RO system rangesfrom about sixty percent to seventy percent (60%-70%),whereas rejection of boron in my water treatment processwas shown to be about ninety eight and one-half percent(98.5%).In other words, in a conventional RO process-41-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239roughly thirty to forty (30 to 40) borate ions passthrough the membrane for each oneâhundred (100) presentin the feedwater, whereas in my process less than two,and specifically, only about one and oneâhalf (1:5)borate ions pass through the membrane out of every one~hundred (100) present. In other words, 30 per 100 or 40per 100 borate ions in the feedwater reach the permeatein conventional R0, versus 1.5 per 100 in this process.In certain feedwaters this number would decrease evenfurther, to as low as 1/100, or 1/1000, for boronrejection rates of ninety nine percent (99%) or ninetynine point nine percent, (99.9%), respectively. Thus,this indicates that the run times on anion exchanger 42,while not necessarilyâ proportionate to the influentsilica and boron levels, are nevertheless going to besignificantly longer when treating permeate 34 from mynew process, as compared to run times when treatingpermeate fronl a conventional RO system. Since anionexhaustion is indicated by a pmedetermined level ofleakage of silica (SiO2), and, in some cases boron, andsince the resin bed outlet concentration is related tothe mean species concentration in the resin bed, byachieving significant reduction in the concentration ofsuch anions in the influent to the anion ionâexchangeresin bed, the consequence is that longer run times areattained before the maximum allowable leakage of SiO2_orboron is reached.Importantly, the levels of boron, and particularlysilica and TOC were found to be extremely low after-42-l0l52025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239treatment of the permeate 34 in the mixed bed ionexchangers 44 and 46 in the pilot plant. A comparisonwith post mixed bed permeate from a conventional ROprocess is provided with the data in TABLE 3.Significantly, in my new process, in post mixed bed ion-exchange treated water, the TOC level was found to beless than 3.0 ppb, i.e., below detection limit.And, not to be overlooked, are the significantlyimproved rejection of sodium and potassium, whichimproved to 99.73% and 99.98%, respectively, fromconventional RO system rejection rates ranging fromninety five to ninety eight percent (95% â-98%) in thecase of sodium, and from about ninety to ninety fivepercent (90%â95%), in the case of potassium.The significantly higher rejection of stronglyionized species such as sodium, potassium, chloride, andsulfate, compared to conventional RO operations asevidenced by the data in Table 1, was a particularlyimportant and an unexpected experimental result of pilottesting. Further, even though the RO permeate in thepilot plant testing contained a higher level of sodiumthan does the permeate of a conventional RO process, asnoted in TABLE 2, the impact of the higher sodiumcontent on post RO cation exchange is relativelyinconsequential. Since the RO permeate from my novelprocess is highly alkaline (a typical pH of 10.3 duringpilot testing is shown in Table 1) and containssignificant levels of free hydroxide ions, the sodiumremoval extent, and capacity of the resin in cation-43..10152025WO 98/06483CA 02264619 1999-02-09PCT/U S97/ 14239exchange unit 40, is increased by a substantial margin.The effect of the increased hydroxide alkalinity in thepermeate to enhance removal of sodium from such permeateis shown in TABLE 2. In conventional R0 treatment ofthe same feedwater, where the RO system permeate hasonly 193 ppb of sodium, yet the cation ionâexchangeresin is only able to effect sodium removal to about0.431 ppb. In contrast, my novel process, even though955 ppb of sodium was encountered in the RO permeateafter cation ionâexchange treatment, the sodium ionconcentration was reduced to less than 0.007 ppb.The improved rejection «of total organic carbon("TOC") in my process also provides a significantbenefit to RO plant operators. It is normal for watersof natural origin to contain detectable quantities ofhigh molecular weight organic acids and theirderivatives, particularly humic, fulvic, and tannicacids. These compounds result from decay of vegetativematerials, and are usually related to condensationproducts of phenol-like compounds. Broadly, humic acidsinclude the fraction of humic substances which aresoluble in water at alkaline pH, but which precipitateat acidic pH. Fulvic acids include the fraction ofhumic substances which are water soluble at alkaline andacidic pH. These acids, and their decompositionproducts, can be carried around in the feedwater streamand form undesirable deposits on selected substrates,particularly anion selective substances. Also, theytend. to contribute to fouling ï¬x: conventional R0_44_l0152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239systems. Therefore, it is desirable to minimize theeffect of such molecules on or through the reverseosmosis membrane, so that adverse consequences of theirpresence can be avoided, particularly at the anion ion-exchange unit. As can be seen by reference to Table 1,the TOC content of the permeate 34 is substantiallylower in comparison to TOC from a conventional R0process with identical TOC in the raw feedwater.Specifically, there is rejection of ninety nine pointsixty six percent (99.66%) of TOC in the pilot plant R0system, compared to only ninety to ninety five percent(90 to 95%) recovery in conventional RO systems. As inthe cases of silica and boron, increased ionization ofTOC at the elevated pH of my new process attributes tothis important result. Thus, taking advantage of theionization range of ionizable organic carbon speciesenables effective TOC reductions when operating ROsystems according to the method set forth herein.Operational results of the pilot test unit may alsobe better appreciated by reference to FIGS. 5, 6, 7, and8. FIG. 5 illustrates the relationship between theaxial differential pressure (AP) versus time, in poundsper square inch, for the reverse osmosis membraneemployed in the pilot reverse osmosis test equipment.The differential pressure shown has not been correctedfor changes in feedwater flowrate. In comparison'toconventional R0, the pilot test results show that astable normalized permeate flow rate, a stable silicarejection rate, and a stable differential pressure have-45-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239been achieved. This indicates that fouling/scaling havebeen essentially eliminated in my new process. FIG. 6shows the normalized permeate flow, in liters perminute, versus time over a six month period, for thereverse osmosis membrane employed in the pilot reverseosmosis test equipment.FIG. 7 illustrates the silica concentration in thereverse osmosis reject stream over a six month period inpilot reverse osmosis test equipment. FIG. 8 illustratesthe rejection percentage of silica, versus time over asix month period, for the reverse osmosis membraneemployed in pilot reverse osmosis test equipment. Thissilica rejection is based on an arithmetical mean silicaconcentration in the pilot RO unit.After each shutdown of pilot plant operation due toa ten percent (10%) or more decline in normalizedpermeate flow, the membranes were inspected and cleaned.An important finding was that cleaning can be simply andeffectively accomplished by commodity membrane cleaningchemicals, such as hydrochloric acid solutions,tetrasodium EDTA, and sodium hydroxide. Expensiveproprietary chemical cleaning agents were not required.An RO membrane operated with feedwater pretreatment inthe manner set forth herein was proven to be completelyrestored to a flux of essentially one hundred percent(100%) of startup performance values. Substantially allof the cleaning was accomplished with the acidic firststep of the cleaning process, thus indicating thatcalcium carbonate, magnesium hydroxide, magnesium-45-10152025WO 98/06483CA 02264619 1999-02-09PCT/U S97/ 14239silicate, and the like, were the predominant scalingspecies. Importantly, this revealed that neither silicascaling or biofouling were major concerns under thespecified process conditions. The enhanced runnability,or increased system availability, with minimal scalingand virtually non-existent bioâfouling, is clearlyanother important benefit of my novel RO operationalmethod.Biological fouling of thin film composite membraneshas heretofore tended to be a common problem, and, withcertain specific feedwater sources, has been virtuallyinsurmountable. Although ii: was anticipated thatcontrol of biological fouling would be improved due tooperation at relatively high pH levels, the degree ofbiological fouling control actually achieved farexceeded expectations, with bacteria levels beingvirtually nonâdetectable during autopsy of RO membraneelements. This means that instead of accumulating livingand dead bacteria against the membrane surface, as iscommon in conventional R0 systems, in my unique method,incoming bacteria are killed and dissolved away from themembrane surface. Thus, this method of R0 pretreatmentand operation may become useful for treating problematicwater sources. This is effective because high pHsolutions cause disinfection by cell lysing or ruptureof the cell wall. This is a quite potent and quickacting method of antiâbacterial activity, when compared,for example, with chlorination which acts by the muchslower method of diffusion through the cell wall to_47_10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239cause death by inactivation of the ndcroorganismâsenzymes. Also in contrast to chlorine sanitiredsystems, at the high pH operation preferred in thepresent method, viruses and endotoxins(lipopolysaccharide fragments derived from cell walls ofGramânegative bacteria) are effectively destroyed bylysis, thus enabling the present method to be employablefor the production of pyrogen free or sterile water. Inessence, the present method, when operated at a pH inexcess of about 10, provides sanitization (3 logreduction in bacteria and destruction of vegetativematter), and may also prove to essentially provide truesterilization (12 log reduction in bacteria and theelimination of biofilm and spores) of the processequipment, as test results showed a zero (0) bacteriacount in the permeate. Also, it should be noted thatthe increased pH of permeate in this method of operationenables similar, helpful results in the post ROtreatment equipment. Such a method of operation shouldbe of particular benefit in the production of highpurity water for pharmaceutical applications, where therequirements for United States Pharmacopeia 23 ("USP23") standards, as supplemented, must ultimately be metby" the final product water. In this regard, theavoidance of use of raw water polymers, antiscalants,and other proprietary chemicals in R0 pretreatment, asdescribed herein with respect to a preferred embodiment,can. eliminate âundesirable additives txa pharmaceuticalgrade water, and reduce costs by reducing the necessary-48-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239tests on RO product water. More concisely, theselection of a pH for R0 operating conditions which doesnot support bacteria growth, and carrying out ofhardness and alkalinity removal to a level which avoiduse of additives, is a superior method for production ofhigh purity water.A further benefit of high pH operation is withrespect increased protection of membranes, particularthe thin film composite types, which have limitedtolerance for oxidizing agents at neutral, near neutral,and moderate alkaline pHâs (up to roughly pH 9). Whenchlorine is added to EU) feedwatery gaseous chlorine(C12) or sodium hypochlorite (NaOCl) are typicallyutilized. Because of nembrane sensitivity to freechlorine, in conventional RO systems, it is normallyremoved by sulfite (SO3") injection. However, above.pH9, and particularly above pH 10, the effect of chlorineand other similar oxidants on thin film compositemembranes is significantly reduced. This is because theconcentration of the nonâionized species (such as HOCl,known as hypochlorous acid) is decreased dramatically,since such acids are relatively weak. Consequently, inmy HERO(tm) high pH reverse osmosis process, typicallyoperating at a pH of 10 or higher, chlorine removal isnot generally necessary, thus reducing system complexityand costs. This may be especially beneficial for thosesystems which utilize a nmnicipal water source as thefeedwater to the water treatment plant.Enhanced membrane life is also another benefit of-49-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239my novel membrane operation process. In membraneoperations, and in particular with respect to ROoperations, longer membrane element life may beexpected, primarily because scaling and biofouling areavoided, and thus, exposure to harsh cleaning chemicals(for instance, acid chemicals and surfactants) isreduced dramatically.RO membranes are taken out of service when therejection of critical species, for example silica,boron, or TOC, falls below an acceptable limit. Forsilica, this usually occurs when rejection falls tobetween ninety five and ninety six percent (95%â96%),from an original value of ninety nine percent (99%) orhigher. As discussed above, the initial rejectionvalues for silica in my process are significantly higherthan are achieved in conventional RO systems. Therefore,if conventional RO limitations for silica rejection wereaccepted, for example, a specific membrane element wouldlast longer before the acceptable limits were reached.Stated another way, even after a considerable term ofservice, the membrane elements utilized in the presentmethod will give silica rejections which are in excessof those provided by even new membranes operating inconventional R0 process configurations.High flux, or permeate production, is alsoachievable due to the unique operating conditions of mymethod for operating an R0 system. Several factorscontribute to this result. Flux, expressed as gallonsof water passed through one square foot of membrane in-50-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239one day, generally termed "GFD", is anticipated at about15 GPD for conventional RO systems. In pilot testing,the noted thin film composite type FILMTEC BW membranewas operated at 24 GPD, and potential for up to 30 GPDwas favorably evaluated. While the latter flux rate isbelieved to be the approximate current hydraulic limitof conventional R0 Inodule design, based. on. spacerconfigurations, it is anticipated. that even increasedflux can be achieved in this method of operation (up to50 GPD or so) when. membrane modules become availablethat can support such increased flux. This is a mostadvantageous result for R0 system operators, since, forexample, if the normal flux is doubled by use of thismethod, then the total square feet of membrane surfacerequired is reduced by a factor of two. Correspondingdecreases in capital cost (specifically, for membranesand pressure vessels) and floor space requirements aretherefore achieved. Operating cost, alreadysignificantly lowered by other benefits of the instantmethod, are further decreased by lowered membranereplacement costs. The one hundred fifty percent (150%)plus flux increase demonstrated in testing over thedesign basis for conventional RO systems provides animmediate benefit.When utilizing the present method, osmotic pressureof the RO reject represents the ultimate limitation forR0 technology. Once appropriate raw feedwater treatmenthas effectively removed sparingly soluble species, suchas calcium carbonate, calcium sulfate, barium sulfate,_51_l0152025CA 02264619 1999-02-09WO 98/06483 PCT/US97/14239silica, etc., then concentration of reject can proceeduntil the osmotic pressure limitation is reached. Atthis time, the design pressures for commercially provenRO systems are typically limited to approximately 1,200psig. If a design allowance is wade for a 200 psigdriving force with respect to the reject stream, thenthe maximum allowable osmotic pressure would beapproximately 1000 psig. For purposes of example, basedon a simplified rule of thumb that approximately one (1)psig of osmotic pressure is exerted by one hundred (100)ppm of TDS, the maximum allowable TDS of the rejectstream would be approximately 100,000 ppm. Thus, thisnew IN) operating technology, regardless of feedwaterchemistry, is potentially capable of concentrating anyfeedwater to approximately 100,000 ppm without concernwith respect to the various sparingly soluble species,amd in particular, with respect to calcium sulfate,barium sulfate, and silica.Yet another advantage of my new RO operatingtechnology is that existing RO systems, when retrofittedwith tï¬ua herein discussed pretreatment equipment forhardness and alkalinity removal, can take advantage ofthe operating benefits of this process method.Additional applications for this unique R0operating method exist in both high purity applicationssuch as semiconductor manufacturing and pharmaceuticalapplications, as well as the more traditional industrialuses for boiler feedwater, cooling tower makeup water,and scrubber makeup water. Application of my method of-52-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239reverse osmosis system operation to high purity waterproduction systems is shown in FIG. 9. In this figure,a multipass reverse osmosis process technique isutilized to sequentially process a portion of initialraw feedwater 10 feedwater to produce a final permeate34(1+N) which has been passed sequentially through anumber N of reverse osmosis membrane units, where N is apositive integer, typically two (2) or sometimes three(3), although a higher number could be utilized. Asdescribed above the raw feed water 10, if deficient inalkalinity, may have alkalinity added by any convenienttechnique, such as by sodium carbonate 13, and then thattreated stream RC is sent to the weak acid cation ion-exchange system 12. After cation exchange, acid 14 suchas hydrochloric or sulfuric may be added to produce anintermediate treated stream WA. Then, carbon dioxide isstripped in decarbonation unit 20 to produce anintermediate treated stream D. Then, the pH isincreased by a convenient and cost effective method suchas addition of alkali solution 22 or by anion ion-exchange unit 31, to produce a further intermediatetreatment stream DQH. Reject 32(N) from reverse osmosisunit N (and any intermediate RO units between the firstRO unit 30(1) and the final RO unit 3O(N) are thenrecycled into the feedwater before the RO unit 30N, toproduce a feedwater 25(1+N) containing undesirable buttolerable solute species and solvent water. Pump 26pressurizes the feedwater 25(1+N) to produce apressurized feed to the first RO unit 30(1); after-53-1O152025WO 98/06483CA 02264619 1999-02-09PCTIUS97/14239processing, permeate 34(1) results, which is then feedto the next reverse osmosis unit the the series from 1to N units. The reject from the entire R0 train is shownas reject 32(l+N). High purity treated permeate fromthe entire train is shown as permeate or product water34(1+N), and it is feed to the usual ionâexchangeequipment for final cleanup before use. Cation ion-exchange unit 40 produces a further intermediate puritystream C, which is followed by anion ionâexchange unitunit 42 to produce a further intermediate purity streamA. Before use, a primary mixed bed ionâexchange unit 44produces a yet higher purity stream P, and an optionalsecondary or polishing mixed bed ionâexchange unit 46produces a still higher purity, possible final purityproduct S, or, using the same nomenclature as above,pure product water stream 38(l+N). In semiconductormanufacturing, final filtration in subâmicron filters48, using nominally sized 0.02 micron filters, butperhaps selected from sizes ranging from about 0.02micron tn) about 0.1. micron jJ1 size, is generallypracticed, to produce a still higher product stream M.Also, biological control by passing high purity waterthrough a UV sterilizer unit 49 is customary, normallyoperating at 254 nm wavelength to kill any bacteriawhich may remain in the high purity stream M to producea final ultrapure water U. In many systems, thepositions of the final subâmicron filters 48 and the UVsterilizer unit 49 may be reversed, or a further post UVfilter may be utilized.-54-l0152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239FIG. 10 illustrates the use of my method of reverseosmosis system operation for boiler feed makeup water,or for cooling tower makeup water, or for scrubbermakeup water. The reverse osmosis unit 30 and variouspretreatment equipment is operated according to themethods set forth hereinabove, to produce a high puritypermeate 34. The product water permeate 34 is thentreated in an ionâexchange system as necessary based onspecific boiler requirements, and fed as nakeup water100 to a boiler 102. Blowdown 104 from boiler 102 issent to an accumulation tank 106 for pumping 108 throughreturn line 109 to the RO pretreatment train. Althoughthe cooling tower 110 and scrubber 112 could be fed withR0 permeate 34, more typically, the cooling tower 110and scrubber 112, for example in a steamâelectric powerplant, would be supplied by usual raw water 10 supplies,such as nmnicipal or well water. Therefore, coolingtower blowdown 114 and scrubber blowdown 116 aretypically high in both hardness and alkalinity.Likewise, this system may be used to treat water havingintimate Contact with ash, such as ash pond water or ashsluicing water from coal fired steamâelectric powerplants. In my reverse osmosis process, a significantamount of reusable water can usually be obtained by mymethod of RO pretreatment and operation, unlike the casewith conventional RO systems.Another advantageous use of my wmthod forpretreatment and operation of an R0 system isillustrated in FIG. 11, where a pmeferred embodiment-55-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239similar to that explained above is shown in use with amultipass R0 system (here, two pass with R0 units 3O(1yand 30(N), Where N=2), in pretreatment for a continuouselectrodeionization system 150. R0 permeate 34(l+N),when treated .by continuous electrodeionization, willproduce a very high quality deionized water E which,after ultraviolet treatment 46 and final filtration 48,will be of acceptable for use in the ndcroelectronicsindustryâ as ultrapure water UP. Optionally, thesecondary or polish type mixed bed ion exchange unit 46may be omitted, and the continuous electrodeionizationproduct water B may be sent directly to the UVsterilizing unit 49. This is true since the limitationsof continuous electrodeionization to reject boron,silica, TOC and the like thus limit its ability toproduce, as a direct effluent, 18.2 megohn1 water forelectronics manufacturing. Yet, the permeate 34(1;N)from the two pass RO system, when operated according tothe method disclosed herein, contains very low levels ofsuch species which are troublesome to continuouselectrodeionization, such as boron, silica, TOC and thelike. Thus, use of such permeate as feed for acontinuous electrodeionization treatment unit isbelieved to enable such electrodeionization units toproduce 18.2 megohm water without the benefit ofdownstream ionâexchange polishers 46. The advantage ofusing continuous electrodeionization over conventionalion exchange, of course, is that the continuous process(rather than the batch process of ion exchange resins)-56-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239is regenerated electrically, rather than chemically, andtherefore avoids the use of conventional regenerationchemicals.And, even in wastewaters, the instant method mayoften be used to advantage. Since an R0 system whenoperated as taught herein will substantially rejectionizable species at high. pH, high. rejection. of suchconstituents will be achievable to produce an R0permeate low jJ1 such constituents, for recycle andreuse. Wastewaters from refineries, pulping andpapermaking operations, and nmnicipal sewage treatmentplants, all are fairly high in candidate components(aliphatic and or aromatic organic acids and theirderivatives), and are most difficult for conventional ROmembranes to handle due to organic fouling and relatedbiological growth. Typical industrial uses where waterof sufficient quality may be attained when treatingwastewaters include cooling towers, boiler makeup,scrubber makeup, and the like.Benefits of HERO Brand RO Process Design and OperationMany exemplary and desirable process benefitsprovided by the HERO brand RO system process design andoperation were listed above at pages 22-23. Detailedexplanation of such benefits include:(A) High Rejection of ContaminantsAs shown in Table 4, which summarizes data from aHERO brand RO process pilot plant, rejection of allspecies is significantly higher than what can be-57-l0152025.9â: 0WO 98/06483CA 02264619 1999-02-09PCT/U S97/ 14239achieved in conventional RO operation. Particularlynoticeable is the improvement in the rejection of weakanions such as TOC, silica, and boron. Given thathumic/fulvic acid derivatives (TOC), silicic acid, andboric acid are all relatively weak acids, at highoperating pH these acids will dissociate to a muchgreater extent (compared to nearâneutral pH operation)and, therefore, will be much better rejected by the ROmembrane.The improvement in the rejection of stronglyionized (at nearâneutral pH) species was also observed.Several factors are believed to contribute to theimprovement in rejection of strongly ionized species. Achange of membrane morphology, is believed to occur. Asignificant reduction in the thickness of theconcentration polarization layer adjacent to themembrane surface (due to reduced surface tension at highfree causticity conditions) is believed to be a majorcontributor to this improvement. Also, swelling ofelastomers such as oârings, and the resultant bettersealing characteristics in the modules are also afactor.The impact of much higher rejection of silica,etc., on the behavior/operation of a postâRO ionexchange system is extremely significant. Since thevast majority of postâRO ion exchange is regenerated onthe basis of either silica or boron breakthrough, afactor of ten reduction in the influent silica/boroncontent will provide much longer run times between_53_1015202530CA02264619 1999-02-09W0 93/05433 PCT/US97/14239TABLE 4. COMPARISON op HEROTM RO vs. CONVENTIONAL RORejection Rejection Passage Passage Passage Passage(%) (%) ' (%) (%) Factor ReductionConstituent Conventional HERO Conventional HERO Conv/PERO (%)Sodium 98 99.73 2 0.27 7.4 87Potassium 90 99.98 10 0.02 500.0 99âChloride 93 99.99 2 0.01 200.0 99Silica 99 99.87 1 0.13 7.7 87Boron 70 98.51 30 1.49 20.1 95TOC .95 99.66 5 0.34 14.7 93TABLE 5. WATER ANALYSISRavJVVaU125713850130.828.2507.1R0 Reject1.3500050S01030828250010.8RO Product<100<1<1<1<1<1<110.2 Notes: 1.reject and R0 Product.Analysis of RO feed is not shown in the table, nor is the hydroxide content of R02. The chemistry is based on 90 percent R0 recovery, while maximum recoveryfeasible is approximately 96 percent. .3. Except for pH, all constituents are reported as mg/I as CaCO,.TABLE 6 . COST ESTIMATE OF A RETROFITWater/Waste Water Savings 244,000 (US $/Y1)Antiscalam Elimination 30,000 (US S/Y r)Power Savings 17,000 (US S/Yr)Additional Chemical Costs (40,000) (US S/Y r)Additional Miscellaneous Costs (20,000) (US $/Y r)Net Annual Savings 231,000 (US $fYr)Conversion (Capital) Cost 200,000 (One Time)Simple Pay-Back Period 10.4 (Months)TABLE 7 . COST COMPARISONConventional System HERO SystemEquipment Capital Cost (US SMM) 12 7.8Operating Cost (US S/1,000 US Gallon) 5_75 <4.00Note: See Section 5.0 for basis.-59-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239regenerations. Absence of carbon dioxide, as well asbicarbonate in the RO permeate (due to a high pH,typically at least 10), will also increase onâline timebefore silica/boron leakage exceeds normal thresholdvalues. Reduction of strongly ionized speciesconcentration in the RO permeate is of relatively lesssignificance, since most postâRO ion exchange isultimately silica or boron limited.Compared to 60 to 70 percent boron rejection inconventional thin film composite RO operation, the newprocess provides approximately 99 percent boronrejection. In a double pass configuration, the newprocess is capable of producing a permeate with lowerthan detectable limits of boron content.Another very significant advantage in operating ionexchange with permeate from a HERO brand RO system isthat sodium leakage from cation resin is reduced byseveral orders of magnitude, due to the high ambient pHof the influent. As a result, longer run times betweenregeneration for existing ion exchange systems meanslower chemical and manpower needs, lower regenerationwaste volume, etc. For new systems, or for existingsystems undergoing expansion, the new HERO brand ROprocess design and operation can have a strong positiveimpact on the ion exchange system capital cost as well.(B) High Recovery RatesSince hardnessâcausing ions such as calcium,magnesium, barium, strontium, aluminum, iron, manganese,etc., have been removed prior to the RO, undesirable-60-CA 02264619 1999-02-09wo 93/05433 PCT/US97/14239precipitation of species such as calcium carbonate,calcium fluoride, calcium sulfate, barium sulfate,magnesium hydroxide, aluminum/magnesium silicate, etc.,does not occur in the HERO brand R0 process, and thusthat type of precipitation no longer limits the recoveryachievable by an R0 system. Importantly, silicasolubility, is increased dramatically at the normal HERObrand RO operating pH (preferably of approximately 11) .Sustainable longâterm operation with silica levels inthe 450 to 500 ppm range (in the RO reject) has beenproven, and theoretical models indicate that levels'of1,000 ppm or higher may be achievable in this new R0operational method.Based on 25 ppm silica in the RO feed, 95 percentrecovery RO operation (approximately 500 ppm in thereject) has been proven by testing. Still, 97.5 percentrecovery (approximately 1,000 ppm silica in the ROreject) is theoretically feasible, whether or notpractical from an operational point of view. Sincesilica usually represents the ultimate limitingcriterion, in terms of maximum allowable recovery in anR0 system, increased silica solubility along withessentially total absence of species such as calcium,barium, etc., in R0 feed, should allow RO operation atvery high recovery rates (90 to 98 percent) with thevast majority of feedwaters.With feedwater relatively high in barium content,RO system recovery can be limited by barium sulfateprecipitation potential at the reject end. The HERO-51-1O152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239system eliminates this concern altogether, since bariumis quantitatively removed prior to the R0. The sameoutcome jg; also applicable for EH) systems limited (inrecovery) by strontium sulfate, calcium sulfate, calciumfluoride, and other sparingly soluble calcium,magnesium, iron, and aluminum salts.Of course, the final limit in R0 recovery,represented by osmotic pressure of the RO reject, willstill control the maximum feasible recovery achievablewith a specific feedwater, but this limit is not usuallyreached. at recoveries less than 99 percent witfx mostfeedwaters.(C) Bioloqical Foulinq is Essentially EliminatedMost commonly occurring microbial species arecompletely lysed (physically destroyed) at the highoperating pH. In fact, even virus, spores, andendotoxins are either destroyed or rendered incapable ofreproduction/proliferation at very high pH levels.Saponification of lipids (fat) is expected to play arole in the process as well since fatty acids and theircorresponding glycerides will form soluble "soaps" atthe high operating pH.In one location where longâterm tests were carriedout, biofouling was conspicuous by its absence duringthe test of the HERO technology. This pilot RO systemexhibited very stable operating performance in terms ofnormalized permeate flow and system pressure dropthroughout the test period. Further confirmation of theabsence of biofouling was obtained during autopsy of R0-62-l0152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239elements at regular intervals. A stage wise program totest and autopsy the FILMTEC FT3O based elements wasconducted over a 15 umnth period. The data showedhigher salt rejection than the initial Quality Assurancevalues under standard test conditions. Also, themembrane surface was clean and free of any evidence ofbiofouling. 'This characteristic of the new process can be ofsignificant benefit for sites with known biofoulingproblems or for the treatment of bioâcontaminated/bio-active wastewater. It can also be very effective forsystems with higherâthanâambient temperature ROoperation.(D) Particulate Fouling is Substantially ReducedIt has been known (and practiced) for almost 30years that softening of R0 feedwater destabilizescolloidal solids present in the feedwater andsignificantly reduces the associated fouling problems.Mandatory softening requirement as pretreatment forhollow fine fiber RO elements in the late 1960s andearly 1970s attests to this strategy. In addition, zetapotential is generally reduced between ea surface andfoulant particles at high pH, thus reducing thelikelihood of adhesion. This property is accentuated bythe fact that most naturally occurring particles(including bacteria) exhibit negative surface charges.While sideâbyâside zeta potential determination is yetto be carried out, the new process is expected tosignificantly reduce, if not eliminate, particulate-63-l0152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239fouling problems. The reduction of zeta potentialfurther reduces the possibility of particle adhesion tothe slightly negatively charged membrane surface. Theinâsitu formation of surfactants from bacterial lipids,if present, will further help in reducing particleadhesion to the membrane surface.This unique characteristic of the new process canbe of significant value in the design of an R0 system,particularly in the potential to reduce capital cost andoperating complexity of treating UPW. In addition tothe ability to accept a certain level of particulatefoulants, the new process may also minimize the need formultimedia filtration, coagulant/floucculant addition,Diatomaceous Earth filtration, etc., as pretreatment tothe RO system.(E) Significantly Reduction in Chemical UsageDechlorination, either by chemical addition or byactivated carbon, may very well be unnecessary as wellsince the level of free (undissociated) hypochlorousacid (HOCL) is extremely low at the very high operatingpH.(F) Elimination of Scale Inhibitor UseUse of antiscalants or scale inhibitors, while notharmful or incompatible with the new process, can becompletely eliminated, as proven by an 18-month test ata semiconductor manufacturing facility.(G) High Flux RatesGiven. the reduced. thickness of the concentrationpolarization layer, as well as the elimination of-64-l0152025WO 98/06483CA 02264619 1999-02-09PCT/US97/14239biofouling and the reduction of particulate adhesion tothe membrane surface, it is not surprising that an R0system utilizing the new process can operate at higherflux compared to conventional operation. Compared to anormal design flux of 15 gfd (gallons per square footper day), the HERO brand RO system is designed in excessof 15 gfd, and is preferably designed at about 20 gfd,and more preferably up to about 25 gfd, and, wherefeasible, in excess of 25 gfd.(H) Hiqher Product PurityIn addition to reduced capital cost for the ROsystem, the quality of the R0 permeate is improvedsignificantly due tn) the higher design flux. Forexample, at 25 gfd, the RO permeate will contain 40percent lower dissolved solids compared to a 15 gfddesign basis. The higher pH operation, in combinationwith the high product flux, provides the result that thesalt flux (which is concentration dependent, rather thanpressure dependent) is significantly reduced. The ROsystem can be expected to be about 20 percent lessexpensive due to this factor alone (or more than 20percent less expensive), all other parameters beingequal.(I) Reject Usable as Scrubber MakeupThe reject from the HERO brand R0 system, with highpH, low carbonate alkalinity, and virtually no hardness,can be used as makeup to acidic gas scrubbers. Due toconcerns about potential silica precipitation if the pHis lowered significantly in the scrubber, the RO reject-55-l0152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239should be used on a onceâthrough basis, and thus not beevaporation rate limited.Process ChemistrvAs discussed earlier, very high reject pH is onefactors which characterize operation of the HERO brandRO system. Extremely high rejection of the weaklyionized anions such as TOC, silica, boron, etc., can becorrelated to such characteristics. The followingexample, based on silica, can be used to explore thisrelationship.In naturally occurring waters and at nearâneutralpH range (6-8), silica is primarily present asorthosilicic acid (H4SiO4). Orthosilicic acid, commonlyreferred to as silicic acid, is one of the weakest acidspecies present in water. silicic acidâs firstdissociation constant (i.e. the dissociation cf thefirst proton from the total of four hydrogens) isapproximately 2 x 1O'1O, corresponding to a pKa value ofapproximately 9.7 at ambient temperature and very lowbackground ionic strength of the solution.A convenient way of visualizing the relativestrength of silicic acid with pKal of 9.7 is to statethat at pH 9.7, it is fifty percent (50%) percentionized, i.e. 50 percent of it is present asundissociated orthosilicic acid, while the other 50percent is dissociated and is present as nwnovalentsilicate ion, the conjugate base of orthosilicic acid.At pH 10.7, when the log of conjugate base to-66-10152025WO 98/06483CA 02264619 1999-02-09PCT/U S97/ 14239undissociated acid is unity; approximately" 91 percentexists as silicate ion, the otherâ 9 percent asundissociated acid. At pH 11.7, the distribution is 99and 1 percent respectively. Conversely, at pH of 8.7(when log of ratio is 0.1), approximately 91 percent ofthe species is present as undissociated acid and 9percent as the ionized silicate. At a gï¬i of 7.7,approximately 99 percent is present as undissociatedsilicic acid and 1. percent as the ionized nmnovalentsilicate ion.Since the majority of naturally occurringfeedwaters are at gï¬i 8 or lower, essentially all thesilica exists as undissociated silicic acid under theseconditions. Other very weak acids, such as boric acid(H3BO3, with pKa, of approximately 9.2) and hydrocyanicacid (HCN, with pKa of approximately 9.3) exhibit verysimilar properties, but of course they are both somewhatstronger acids compared to silica.Rejection characteristics of individual speciesacross the RO membrane is influenced by the size, shape,and charge density of the solute. It is generallyrecognized that an ionized solute will be rejected mustbetter compared to a solute that exists in anundissociated state, provided that their size and shapeare comparable. Rejection of fluoride, for example, isessentially zero at pH less than 3, 30 percent at pH3.5, 50 percent at pH 4, 75 percent at pH 5, and 98percent (or more) at pH 7. Hydrofluoric acid (a weakacid with pKa of 3.2) is the counterpart of the ionized-57-l0l52025WO 98106483CA 02264619 1999-02-09fluoride species and is the primary component at low pHvalues.Rejection of silica/silicic acid, however, is asurprisingly high 98 percent at pH 7, where the primaryconstituent is the undissociated silicic acid and notthe ionized silicate species. This discrepancy is atleast partially explained by the fact that the actualsize of (ortho) silicic acid l£§ much bigger thanexpected since the molecule incorporates up to sixmolecules of water of hydration. Thus, the highrejection is due to the size/shape factor, since at pH 7there is very little ionization (less than 0.2 percent)of silicic acid.Based on the factors involved, it would appear thatsilica, when substantially ionized, should haverejection comparable to that of sulfate (S04) ion. Theexpectation is based on the fact that the sulfate ionalso incorporates six waters of hydration and, ofcourse, it is completely ionized at. nearâneutral pHvalues. As a Hatter of record, sulfate rejection of99.5 to 99.9 is routinely" observed. in. normal ROoperation and the silica rejection in the HERO systemoperating at pH 10.5 to 11.0 range has actually beenbetterâ than 99.9 percent. In. otherâ words, sulfaterejection at pH 7 and silica rejection at pH above 10are quite comparable. In view of the relative strengthsof the corresponding acids and the relative size of themolecules, this effect can be rationalized as well asutilized.-68-PCT/US97/1423910152025WO 98/06483CA 02264619 1999-02-09PCT/US97I14239Another aspect of the new process that meritsfurther discussion. is the requirement for essentiallycomplete removal of alkalinity prior to pH adjustment(increase) of the RO feed. From an entirely practicalpoint of view, nearâzero alkalinity is a necessity sinceany residual alkalinity will provide a strong bufferingeffect and substantially increase the quantity of alkalineeded to raise the pH to the normal operating range.Overâ and. above the direct cost of increased. alkalirequirement, the sodium content of the RO permeate willbe much higherâ also, resulting in âunnecessarilyâ highpost-R0 ion exchange load and cost.From a conceptual point of view, however, therequirement for alkalinity removal is far more urgentbut straightforward. The following example, based oncalcium carbonate solubility, will be used to quantifythe relationship.Solubility product (Ksp) of calcium carbonate isapproximately 8.7 x loâ9 square molar at ambienttemperature and very low ionic strength. Assuming 90percent recovery across the RO is the goal, allowablemaximum CaCO3 ion product of the RO feed isapproximately 8.7 x 1Oâ11 square molar. Furtherassuming 0.1 mg/l of calcium in the softened feedwater,the allowable maximum carbonate content of the RO feedis approximately 2.1 mg/l, all expressed as ions.At pH 11.0 reject condition, approximately" 85percent of the carbonate(s) species is present ascarbonate, the rest exists as bicarbonate. Assuming 5-59-10152025WO 98106483CA 02264619 1999-02-09PCT/U S97/ 14239mg/l of total residual carbon dioxide equivalent priorto pH increase, approximately 5.8 mg/l of carbonate (asion) will be present in the RO feed. Compared to themaximum allowable 2.1 mg/l of carbonate, the achievable5.8 mg/l is three times as high.To ensure scaleâfree operation at 90 percentrecovery, one or more of the following must be achievedâ residual calcium content must be less than 0.1 mg/l,or the RO operating conditions must be changed. Whilecalcium carbonate scale inhibitors are known togenerally allow a high Ksp, I am not aware of any suchformulation which would efficiently and cost effectivelyallow continuous high pH operation of RO. Important, itshould be noted that during the longâterm testing of theHERO system, no scale inhibitors were used whatsoever.Magnesiunx hydroxide, witr1 a Ksp of approximately1.2 >< 10â11 cubic molar, is in some ways even moredemanding in terms of allowable residuals, sincemagnesium tends to leak earlier from the weak acidcation exchanger and, therefore, more care is needed toprevent magnesium hydroxide scale.Typical ExampleThe following is an. example for a. typicalapplication for the HERO system. The feedwater in theKumamoto area in Southern Japan, high in silica content,was selected for the example. Costs shown arebudgetary (+ or - 30 percent accuracy). A costprojection is based on the following assumptions:-70-10152025WO 98106483CA 02264619 1999-02-09PCT/U S97/ 14239(1) 1,500,000 US GPD system nominal capacity;(2) 75 percent normal recovery ratevs. 90 percent HERO system recovery rate;(3) UPW quality (chemical) criteria are:(a) silica <1 PPB,(b) TOC <1 PPB, and(c) Oxygen <5 PPB;(4) Consumable costs:(a) Sulfuric acid (93 percent) at US $100/ton;(b) sodium hydroxide (100 percent) at US $450/ton;(c) antiscalant at US $1.50/pound;(d) electricity at US $0.075/kwh;(e) water purchase and wastewater discharge costs(combined) at US $3/1,000 US gallons.Conversion of Existing RO SystemTable 6 below assumes that an existing 1.5 millionGPD (US) system operating at 75 percent recovery withfeedwater shown in Table 5 is converted into a 90percent recovery HERO brand RO process system and thatno changes are made beyond the R0 system.In some cases, it may also be feasible to use aHERO brand RO process design to increase overall ROrecovery rates, by processing reject from a conventionalRO system, by (a) simultaneously reducing hardness andalkalinity in a WAC system, (b) decarbonation, and (c)raising the pH, before feeding the stream into a secondRO system.Conversion of existing systems may also provide-71-10152025WO 98/06483CA 02264619 1999-02-09PCT/US97/ 14239unique opportunities to increase the capacity of an R0system. This is possible because the flux of about 15gfd in a conventional RO system can be increased up toabout 20 gfd, or perhaps up to as much as 25 gfd, ormore, when the operation is changed to a HERO brand ROprocess design and operation configuration.New RO System Design and OperationThe projection in Table 7 below is made on thebasis that two brand new UPW systems will be built, inone case utilizing the conventional approach (see FIG.13), and in the other case utilizing the HERO brand ROsystem (see FIG. 14) that includes a simplifiedpolishing loop design. Both systems will use double-pass RO, hollow fine fiber ultra filter and no dual-bedion exchangers. Approximatelyâ 40 percent of the UPWusage will be at high temperature, and the cost estimateincludes DIW heaters. The distribution piping systembeyond the ultra filtration system is not included inthese cost estimates, nor is system installation or anyPVDF lined storage tanks, since sizing of thesecomponents are very site specific.SummaryThe new HERO brand RO technology has been shown toexhibit very high rejection of all contaminants,especially weak acid anions. In addition, R0 recoveryof ninety percent (90%) or higher can be achieved withthe vast majority of feedwater. Biological fouling is-72-10152025WO 98106483CA 02264619 1999-02-09PCT/U S97/ 14239essentially eliminated while particulate fouling issubstantially reduced. A flux considerably higher thanis normally practical using conventional RO systemdesign can be achieved with the new HERO technology.Although the benefits of this new process might justifyhigher UPW system cost, just the opposite is true. lheoverall cost as well as the complexity of the UPW systemare both reduced dramatically.The method and apparatus for processing water viamembrane separation equipment, and jJ1 particular, viathe HERO brand reverse osmosis ("R0") process design asdescribed herein, provides a revolutionary, paradoxicalresult, namely, simultaneous increase in levels ofsilica in the RO reject, but with lower levels of silicain the purified RO permeate. This method of operatingmembrane separation systems, and in particular, foroperating reverse osmosis systems, represents asignificant option for reducing water use whilesimultaneously reducing capital and operating costs ofthe water treatment system. Water recovery, that is,the ratio of the quantity of the permeate product streamproduced to the quantity of the feedwater streamprovided is clearly in excess of about 50%, and easilywill be up to about 85% or more, and often, will be upto about 95%, and, at times, will reach levels of about99%. Further, given the efficiencies, dramatically lessusage of chemical reagents, either for ion. exchangeregenerant or for R0 cleaning, will be consumed per-73-CA 02264619 1999-02-09WO 98/06483 PCT/US97/ 14239gallon of pure water produced.It will thus be seen that the objects set forthabove, including those made apparent from the proceedingdescription, are efficiently attained, and, sincecertain changes may be made in carrying out the abovemethod and iJ1 construction of aa suitable apparatus inwhich to practice the method and in which to produce thedesired product as set forth herein, it is to beunderstood that the invention may be embodied in otherspecific forms without departing from the spirit oressential characteristics thereof. For example, while Ihave set forth an exemplary design for simultaneoushardness and alkalinity removal, other embodiments arealso feasible to attain the result of the principles ofthe method disclosed herein. Therefore, it will beunderstood that the foregoing description ofrepresentative embodiments of the invention have beenpresented only for purposes of illustration and forproviding an understanding of the invention, and it isnot intended to be exhaustive or restrictive, or tolimit the invention to the precise forms disclosed. Onthe contrary, the intention is âto cover allmodifications, equivalents, and alternatives fallingwithin the spirit and scope of the invention asexpressed in the appended claims. As such, the claimsare intended to cover the methods and structuresdescribed therein, and not only the equivalents orstructural equivalents thereof, but also equivalentstructures or methods. Thus, the scope of the-74..CA 02264619 1999-02-09WO 98/06483 PCT/US97/ 14239invention, as indicated by the appended claims, isintended to include variations fronl the embodimentsprovided which are nevertheless described by the broadmeaning and range properly afforded to the language ofthe claims, or to the equivalents thereof.-75-