Note: Descriptions are shown in the official language in which they were submitted.
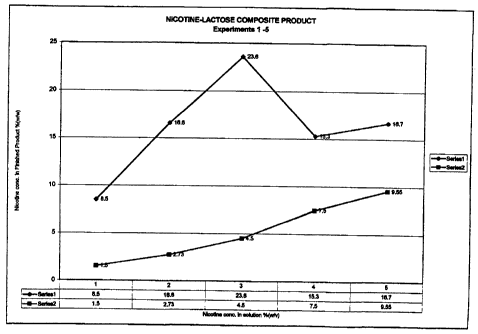
CA 02265198 1999-03-10Title: METHOD OF PRODUCING A NICOTINE MEDICAMENTAND A MEDICAMENT MADE BY THE METHODFIELD OF THE INVENTIONThis invention relates to nicotine medicaments. Inparticular, the invention relates to a method of producing a nicotinemedicament which is suitable for inhalation.BACKGROUND OF THE INVENTIONSmoking has been determined to be a contributory orcausative factor in a number of diseases including respiratory diseasessuch as emphysema, chronic bronchitis, lung infections and lungcancer. Most regular smokers become addicted to, or dependent upon,the pharmacological effects of nicotine in tobacco smoke. Nicotine israpidly absorbed across the blood/brain barrier and exerts a directaction on nicotine receptors in the spinal cord, autonomic ganglia andadrenal medulla.Various nicotine replacement therapies have beendeveloped. Some of these utilize a nicotine substitute. Nicotinesubstitutes generally contain nicotine in a solid form, in a vapour or insolution. For example, nicotine replacement therapy has included theuse of nicotine gum. One disadvantage with nicotine gum is thatlower steady state nicotine levels are achieved from chewing nicotinegum compared to smoking cigarettes and the rate of rise of bloodnicotine levels is substantially lower as compared to smokingcigarettes. Further, the gum has been associated with gastrointestinalside effects, hiccups, mouth ulcers and sore throat. The amount ofnicotine absorbed is also highly variable and is dependent upon thechewing and swallowing actions of the user over a prolonged period oftime.Nicotine patches have also been developed. Onedisadvantage of nicotine patches is that they have been associated withskin irritation at the site of application. Further, they result in a slowCA 02265198 1999-03-10-2-absorbtion of nicotine which may not be effective in satisfying aperson's craving for cigarettes.Selfâpropelled aerosols (also known as pressurizedaerosols) which contain nicotine in solution have been proposed ascigarette substitutes. An example is the selfâpropelled formulation ofJacobs (United States Patent No. 4,635,651). As shown in Jacobs, thesedelivery systems contain a water based aerosol formulation and apropellent such as freon which are stored in a pressurized container.When actuated, Jacobs delivers nicotine and a solid carrier to themouth of the user. Thus the aerosol created by Jacobs contains, incombination, a mixture of nicotine and the solid carrier. The nicotineis not formed as a composite part of the solid carrier. Further, theparticle size of the aerosol created by Jacobs was variable. Therefore, thedose which is administered by using such pressurized aerosols maynot be accurately controlled.It has also been proposed to produce a dry powder inhalerfor delivering a nicotine containing medicament via inhalation (seePCT application PCT/CA95/00562). While nicotine formulations inthe form of salts and complexes have been developed, there is still aneed for a nicotine formulation which is adapted for inhalation intothe alveoli and smaller airways of the lungs.SUMMARY OF THE INVENTIONCigarette smoke is an aerosol comprising discrete particlesof tar with which the nicotine is associated. The tar particles are of asize which makes them capable of travelling to the alveoli and lowerairways of a person. Upon study, it has been determined that thenicotine is effectively conveyed to the alveoli and lower airways of aperson by the tar particles. Current tobacco replacement therapies havenot been effective in satisfying a person's craving for cigarettes.According to the instant invention, a nicotine formulation whichmore closely simulates cigarette smoke is provided which may be used........ -.._.-._...............u_......_..._........".......H.-. .. ..CA 02265198 1999-03-10-3-with existing inhaler technology so as to improve the effectiveness oftobacco replacement or withdrawal therapies.In accordance with the method of the instant invention,there is provided a composite material comprising discrete particleswhich are a mixture of nicotine and a carrier. As with cigarette smoke,the composite material is a physical combination of both the nicotineand the carrier. The carrier effectively provides a particle having a sizeand density such that it will be conveyed on inhalation to the alveoliand lower airways of a person. The nicotine is combined with thecarrier such that it will be conveyed to the alveoli and lower airways ofa person with the carrier. In contrast, in prior art formulations, thenicotine and the carrier were merely associated or aggregated with eachother such that they separated from each other in the air stream. Thusthe carrier did not act in general to transport a dose of the nicotine tothe alveoli and lower airways of a person. In accordance with theinstant invention, the nicotine and carrier form a composite materialwhich are physically combined in such a way that they will notseparate during inhalation.In accordance with the method of the instant invention,there is provided a method of producing a nicotine medicament foruse in an inhaler comprising:(a) combining a nicotine formulation, a pharmaceuticalgrade sugar and a liquid carrier to produce a flowable mixture; and,(b) drying the flowable mixture to produce a compositematerial at conditions to produce particles of the nicotine medicamentsuitable for delivery to the alveoli and lower airways of a person.In one embodiment, the liquid carrier may comprisewater. In another embodiment, the liquid carrier additionallycomprises alcohol, particularly where the nicotine is a nicotine saltsuch as a nicotine sulphate or a nicotine tartrate. In this case, alcoholis added as a cosolvent, to expedite the solubility of the nicotine in thesolution. In such a case, the liquid carrier preferably comprises a minorCA 02265198 1999-03-10-4-proportion of the alcohol and a major proportion of water. The ratio ofalcohol to water in the liquid carrier may be from about 1:1 to 1:10,preferably from about 1:2 to 1:8 and more preferably from about 1:5 to1:7 parts by weight.The ï¬owable mixture is preferably dried by spray drying.In one embodiment of the invention, the flowable mixture isatomized prior to being dried.The ï¬owable mixture is preferably dried at conditions toform substantially spherical particles. More preferably, the flowablemixture is dried at conditions to form spherical particles which have adimpled surface. In one embodiment, the flowable mixture is dried ata temperature sufficiently high so that the liquid carrier is rapidlyremoved from the atomized particles of the flowable mixture in thespray drier.An advantage of the instant invention is that themedicament particles produced by the method disclosed herein arewell adapted for absorption into the bloodstream of a person via thealveoli and small airways of the lungs. The particles are a compositestructure. Accordingly, the nicotine will not separate from the sugar(the carrier) during inhalation. Thus the sugar will convey thenicotine to the lungs in a manner to mimic cigarette smoke. Bycontrolling the conditions at which the flowable mixture is spraydried, particles having a size from about 0.1 to about 5 mm, morepreferably from about 0.5 to about 3 um may be produced.Nicotine, if it impacts upon the throat or upper airways ofthe person, may cause irritation. Thus, the method of the instantinvention may be used to produce a powdered medicamentformulation which, by inhalation, may reach the alveoli and smallerairways of a person's lungs without causing undue, and preferably, noirritation.The method may also be used to produce particles which,not only are spherical, but have a uneven or a "dimpled" surface. TheCA 02265198 1999-03-10-5-spherical shape of the dried particles reduces aggregation of theparticles while in the inhaler, thus rendering it easier to aerosolize theparticles upon inhalation by the user. Further, by having a dimpledsurface, the aerodynamics of the medicament particles are improvedwhereby the particles may by more easily entrained in the air inhaledby the user.DESCRIPTION OF THE DRAWINGSThese and other advantages of the instant invention willbe more fully and completely understood in accordance with thefollowing description of a preferred embodiment of the invention,taken together with the drawings in which:Figure 1 is a graph of nicotine concentration in a finishedproduct made in accordance with the present invention versusnicotine concentration in the solution prior to being spray dried; andFigure 2 is a graph of nicotine concentration in thefinished product versus the ratio of nicotine to lactose in the solutionprior to being spray dried.DESCRIPTION OF THE PREFERRED EMBODIMENTAccording to the method of the instant invention, acomposite material comprising nicotine and lactose is produced in aform suitable for inhalation by a user. In particular, the medicamentcomprises solid discrete flowable particles which may be entrained inthe air inhaled by a person so as to travel to the alveoli and smallerairways of the lungs.According to the method of the instant invention, apharmaceutical grade sugar and nicotine are mixed with a liquidcarrier so as to form a flowable mixture which may then be dried. Theliquid carrier is an agent which mixes with the sugar and the nicotineto a degree sufficient to form a flowable mixture which may be rapidlyCA 02265198 1999-03-10-5-dried such as in a spray drier. The nicotine, sugar and liquid carriermay be combined in any order.The sugar is preferably selected from lactose, dextrose,glucose, maltose or combinations thereof, and is most preferablylactose. The sugar may be a natural or a synthetic sugar and mayinclude analogs or derivatives of sugars. It will be appreciated thatreferences herein are made to lactose, although one or more of theother sugars mentioned could similarly be employed. The lactose actsas a carrier and, therefore, any form of lactose approved as an excipientmay be used. The lactose is preferably of a pharmaceutical grade suchas CP, USP, NF, BP or BPC. The lactose which is used as a startingmaterial is therefore in the form of a dry powder which is readilysoluble in water.The nicotine may be any form of nicotine which issoluble in or miscible with the liquid carrier. For example, the nicotinemay be a nicotine base which, at room temperature, is a liquid that ismiscible in water. Alternately, or in addition, the nicotine may be a saltwhich, at room temperature, is a solid. The nicotine base is typically anoil formulation. Preferably, the nicotine comprises nicotine base. Thenicotine may be pharmacologically active analogs or derivatives ofnicotine or substances which mimic the effect of nicotine, either aloneor in combination with other active substances.The liquid carrier may be any liquid or liquids with whichthe nicotine may be mixed and the lactose may be dissolved to form aflowable mixture which is preferably of a generally uniformcomposition. Nicotine bases are generally miscible in water andnicotine salt formulations are generally soluble in water. Further,lactose is soluble in water. Accordingly, whether the nicotine is a baseand/or a salt formulation, the liquid carrier may comprise water.When a salt is used, the liquid carrier solubilizes the nicotine and thelactose. When a nicotine base is used, the liquid carrier solubilizes thelactose and mixes with the liquid base to create a generally uniformCA 02265198 1999-03-10-7-solution (eg. it is miscible with the liquid base). While water is thepreferred liquid carrier, other liquids in combination with or in placeof water may be used. For example, alternate liquids may be used,either by themselves or in combination to water, to solubilize the solidmaterial or to disperse the nicotine base in the liquid carrier.In a further preferred embodiment, the liquid carrier maycomprise a mixture of alcohol and water. The water and the alcoholform an azeotropic mixture. Nicotine base formulations are readilysoluble in an alcohol. However, the lactose is not soluble in thealcohol. Pursuant to this embodiment, the flowable mixture maycomprise less water thus assisting in the rate of drying of the ï¬owablemixture and/ or the amount of water in the dried product.Preferably, the alcohol is a primary alcohol. Further, thealcohol is preferably a lower alkyl alcohol (i.e. C1 to C5). A particularlypreferred alcohol which may used as a solvent for the nicotine basesolution is ethanol. The ethanol may be CP grade, and preferably, is,USP grade. However, it will be appreciated that it is preferable, wherepossible, to avoid the use of alcohol in the base solution.This liquid carrier preferably contains an excess amountof water compared to alcohol where alcohol is necessary as a cosolvent.In such an embodiment, the mixture preferably comprises a minorproportion of alcohol and a major proportion of water. Where alcoholis required, the ratio of alcohol to water in the liquid carrier may befrom about 1:1 to 1:10, preferably from about 1:2 to 1:8 and morepreferably from about 1:5 to 127 parts by weight.The liquid carrier (eg. water and/or alcohol) may bemixed with the nicotine to produce a liquid mixture to which thesugar may then be added. Accordingly, the lactose and a nicotine saltmay be dissolved in water (and optionally a water/ alcohol mixture) toform the flowable mixture. Alternately, the lactose may be dissolved inwater (and optionally a water/ alcohol mixture) and the nicotine basemay be mixed with the water (and optionally a water / alcohol mixture)CA 02265198 1999-03-10-3-to form the ï¬owable mixture. It will be appreciated that the nicotine,liquid carrier and sugar may be combined together in any desired orderto produce the dry ï¬owable mixture.According to the preferred embodiment of thisinvention, the nicotine compound is added to the alcohol and mixeduntil a relatively consistent solution is achieved. Lactose is dissolvedin water. Subsequently, the mixture of the nicotine in alcohol andadded to the aqueous lactose solution and mixed until the flowableproduct is produced. The mixing may be conducted by any meansknown in the art.The amount of liquid mixture which is utilized issufficient to produce a flowable mixture. Pursuant to the preferredembodiment, the mixture is finely divided (such as passing theflowable mixture through an orifice) on entry to a spray dryer.Accordingly, the flowable mixture is preferably in the form of a liquid,such as a syrup or the like, which may readily be finely divided such asby passing the liquid through an atomizer (preferably a rotaryatomizer).The ratio of nicotine to lactose which is dissolved in theflowable mixture will vary upon the concentration of nicotine in thespray dried product. Due to product handling limitations, it is typicalin the field that the carrier comprises a substantial portion of theweight of a powder medicament as compared to the active ingredient.The amount of lactose which is utilized, compared to the amount ofnicotine, must be sufficient such that the spray dried product can beused in association with dry powder inhalers which are known in theart. Accordingly, the ratio of lactose to nicotine in the flowablemixture may vary from about 1:10 to about 10:1, more preferably fromabout 3:7 to about 3:2 and, most preferably, about 426 parts by weight.Further, the concentration of nicotine in the flowable mixture mayvary from about 1 to about 10, more preferably from about 2 to about 5and, most preferably, about 3 % (w/v, i.e. g/ 100 ml).CA 02265198 1999-03-10-9-The flowable mixture is dried so as to produce particleswhich are sized so as to be able to travel to the alveoli and smallerairways of the lungs. Preferably, the particles have a particle size fromabout 0.1 to about 5 mn, more preferably from about 0.5 to about 5 umand, most preferably from about 0.5 to about 5 um based on the massmedian aerodynamic diameter (MMAD) of the particles. The flowablemixture is preferably rapidly dried such as by using a spray drier.However, other drying techniques capable of producing appropriatelysized particles (eg. the use of fluidized bed drying) may be used.The flowable liquid is preferably rapidly dried so as toproduce spherical or substantially spherical particles. Such particlesmay be achieved by using a rotary atomizer to feed the flowable liquidinto a spray dryer.The operating conditions of the spray dryer are adjustedso to produce particles which are sized so as to be able to travel to thealveoli and smaller airways of the lungs. The rotary atomizer may beoperated at a liquid feed rate from about 2 to about 20, more preferablyfrom 2 to about 10, and most preferably from about 2 to about 5ml / min. The rotary atomizer may be operated from about 10,000 toabout 30,000, more preferably from about 15,000 to about 25,000, andmost preferably from about 20,000 to about 25,000 rpm.The spray dryer is operated at temperatures sufficientlyhigh to cause the liquid carrier to rapidly evolve without raising thetemperature of the lactose and nicotine to a point at which thesecompounds commence to degrade. Accordingly, the spray dryer maybe operated with an inlet temperature from about 120 to about 170°Cand an outlet temperature from about 70 to about 100°C.The medicament particles are spherical or of anotheraerodynamic shape. Such particles will tend not to aggregate whenstored in a bulk form. Further, by evolving the liquid carriersufficiently rapidly during the spray drying process, the medicamentparticles may be produced with an uneven or a "dimpled" surface.CA 02265198 1999-03-10-10-The uneven surface produces turbulence as the particles travelthrough the air, thus providing the particles with aerodynamic lift.This assists the particles to be entrained, and to remain entrained, inthe air inhaled by a user thus improving the ability of the medicamentparticles to travel to the alveoli and smaller airways.The following examples are intended to be illustrativeonly, and do not limit the scope of the invention.Examples3g of nicotine and 27 g of lactose were added to 200 g ofwater. The mixture was stirred until the solution was clear(approximately 10 minutes). The mixture was spray dried in a BuchiMini Spray Dryer 190, with an air flow rate of 500 ml / minute, an inlettemperature of 165°C and an outlet temperature of 87°C. The nicotineand lactose solution was fed into the atomizer at a rate of 7 ml/ min.The results are set out in Table 1.This experimental procedure was repeated under each ofthe sets of conditions set out in Table 1. Determination of the nicotinecontent in the nicotine lactose composite product was determined byusing UV spectrophotometry at a wavelength of 262 nm. Particle sizewas determined using laser diffraction methods known in the art.A concentration of 3% (w/v) of nicotine in solution, witha 4:6 ratio of nicotine to lactose (w/w) produced the highestconcentration in the finished product. An air flow higher than 750ml/min. resulted in a wet powder being produced which wasdetrimental to the flow characteristics.Figure 1 is a graph setting out the concentration ofnicotine in the finished product as a function of the nicotineconcentration in solution for experiments 1-5. âSeries 1" is theconcentration of nicotine in the finished product after spray drying.âSeries 2" is the concentration of nicotine in solution prior to spraydrying. It will be seen that a higher concentration of nicotine inCA 02265198 1999-03-10-11-solution did not always result in a higher concentration of nicotine inthe finished product.Figure 2 is a graph setting out the concentration ofnicotine in the finished product as a function of the ratio of nicotine tolactose in the solution, for experiments 6-8. It will be seen that thehigher nicotine to lactose ratio in solution did not necessarily producea higher concentration of nicotine in the finished product. Thehighest ratio of nicotine to lactose in solution was determined to beapproximately 3:7. âSeries 1" in Figure 2 shows the concentration ofnicotine in the finished product after spray drying, while âSeries 2"shows the ratio of nicotine to lactose in solution prior to spray drying.The results show that the highest concentration ofnicotine in the finished product were achieved with a nicotineconcentration of approximately 3% (w/V) in solution, and anicotine:lactose ratio of approximately 4:6 in solution.CA 02265198 1999-03-10-12-32.m .3 2 2-m33 3% .2 cm 82 3 8,3 525 22 m 2522 26:22 m 3 82. 222 32 o2mum .3 2 2-m93 $8 .2 No 32 co 2: 2&5 ba m E22 E25 m 3 822 22 32 o2&3 .3 2 2.2233 No.8 .2 3 82 we 80 abs be m E22 2322 m mm oom m2 2 wmum .3 2 2-m$3 8% .2 2.3 82 3 So 852 be m 252 225 m 3 O3 222 32 52 2.22,3 25302 Ma 82 3 So 2.222 >2 m E22 225 m mm 8m 23 2. o$2$2 282.62 m.\. 32 3 Sm $20 52 mE_2>:â¬:m moo 3 83 m 23 22m2$2 9202302 E 82 3 com abs 5 m 252 3822 mm mm 83 m2 m2 283 .3 m233 222.90. .2 mm 32 3 8m abs 3. m E22 E25 3 Em 83 23 3 m232 952302 mm $2 3 com 322 22: m E222 222322 2.3 93 033 23 0 3m23 8o2:oz mm 82 5 8m 552 5 m 2522 E25 m2 92 83 3 m 2ï¬oio 33 as$2252 .3 £28262 5:283?, G02 CL 232 E 25: 252 @ 3 myCS \ >>v2xv 232:: axe A 9.25.21 925:2. wmmm Gw.G\7G -mb22mucou wmouumd mvmvnw Uwï¬ï¬m Uovï¬m.n_.m Cm0C««OU_Z wN2m 2032mm $250 «$5 coziom 305 .224 main. 29229 zï¬mm 923032 2m2.5ou2Z 235$ mmoï¬Ã©d m:zou_Z .02H 222222?