Note: Descriptions are shown in the official language in which they were submitted.
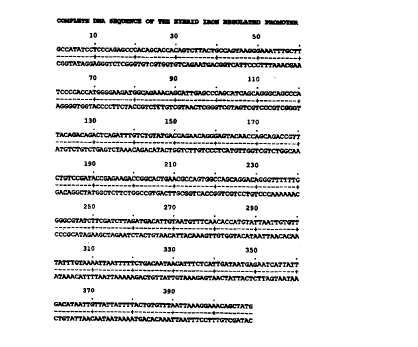
WO 98110064101520253035CA 02265883 1999-03-05PCTIAU97I00503Iron Regulated Promoter and Uses ThereofThis invention relates to promoters and their use for the expressionof polypeptides and in particular their use in live vaccines. The presentinvention also provides a method of integrating foreign genes into thechromosome of bacteria and to bacterial vaccines expressing foreign genes.It is known to construct recombinant bacterial vaccines in whichartificially introduced foreign polypeptide genes are carried by naturallyoccurring bacterial strains and expressed in the host under the control of apromoter. Live attenuated Gram-negative bacterial strains currentlyproposed for the expression of foreign antigen genes from pathogens,however, suffer from several major limitations, one of which is that in vivagene expression is significantly less than what might have been expectedfrom empirical considerations. Primarily this is believed to be due toenvironmental shock experienced by the bacterium when it encounters thedifferent and nutritionally complex environment of intestinal lumen andhost tissues. The process of adaptation to the new environment slows downbacterial gene expression and metabolic activity, with a resulting reductionin the foreign antigen dose delivered to the host immune system andcorresponding sub-optimal immune response.The Escliericlzia coli bacterium displays a characteristic response toiron starvation which is shared by many other aerobic and facultativeanaerobic microorganisms, such as Salmonella typhimurium. Underconditions where the iron concentration is less than about 5 M thetranscription of a collection of genes or groups of genes scattered through thechromosome is coordinately activated in a manner resembling an iron-controlled regulon. Iron control is in all instances mediated by negativeregulation via the product of the fur gene, the absence of which triggers theexpression of the genes to constitutive levels. The Fur protein also regulatesexpression of the aerobactin-mediated iron transport regulon.In order to attempt to overcome the reduction in gene expressionwhich occurs when a bacterium encounters a harsh environment, such as inthe intestine, the present inventors have developed a system involving theuse of a novel hybrid iron-regulated promoter which can be induced tohyper-express polypeptide encoding genes. When incorporated in a bacterial101520253035W0 98Il0064CA 02265883 1999-03-05PCTlAU97I00503vaccine, the promoter can provide an optimal polypeptide dose to the hostimmune system. In this specification, by a promoter the inventors mean aDNA molecule having a nucleic acid sequence which (as a portion of a largerDNA molecule) is effective in inducing the expression of a polypeptide-encoding gene or genes localised downstream of the promoter DNAsequence. The polypeptide may comprise one or more antigenicdeterminants of a pathogenic organism, and may be derived from a virus,bacterium, fungus, yeast or parasite.Live attenuated Salmonella strains are currently being developedworld-wide as carriers of foreign antigens from viral, bacterial and parasiteorigin. These recombinant salmonellae are being used to immunise theanimal or human host to elicit a protective immune response against therespective infection. As mentioned above these recombinant Salmonella-based vaccines suffer from a number of drawbacks. One major problem isthat they typically carry the foreign antigen gene on self-replicatingplasmids. These plasmids are often unstable and are lost from the bacterialcell in Vivo resulting in a loss of the foreign antigen gene and consequentlythe foreign protein. This plasmid loss results in a reduced antigen dosebeing presented to the host immune system with a consequent sub-optimalprotective immune response.Two specific chromosomal integration systems have been developedby other groups, one of which relies on homologous recombination into theS. typhimurium histidine operon and the other uses transposons to transposethe antigen gene cassette randomly into the bacterial chromosome. Bothsystems suffer from the drawback of being highly cumbersome, labourintensive and are very inefficient. The latter system integrates randomlyinto the chromosome and hence suffers from the additional drawback ofcreating additional mutations in the bacterial chromosome which may bedeleterious to the vaccine strain. Additionally, the use of transposons(mobile genetic elements) in vaccine strains is not desirable since they tendto âhopâ around in the vaccine strain making the genotype of the strainill-defined. Genetically attenuated vaccine strains need to be clearly defined(genotype and phenotype) before they can be accepted for commercial use.In an attempt to solve the problem of using recombinant plasmids inbacterial vaccines, the present inventors have developed methods tointegrate heterologous polypeptide-expressing gene cassettes into a specificWO 981100641015202530CA 02265883 1999-03-05PCT/AU97/00503non-essential site in the chromosome of bacteria and especially inattenuated S. typhimuzium strains. This process eliminates the need forrecombinant plasmids and the genes of interest are expressed stably from thechromosome of the bacteria.This invention has broad spectrum applications since this site-specific recombination system can, with the appropriate modifications, beapplied to integrating foreign genes into the chromosomes of a wide range ofbacteria and eucaryotic cells.In a first aspect the present invention consists in an iron-regulatedpromoter comprising the DNA sequence shown in Fig. 1, or a fragmentthereof which includes the sequence from residues 284 to 409, or afunctionally equivalent nucleic acid sequence.It is preferred that the iron-regulated promoter includes the nucleicacid sequence from residues 284 to 409 as shown in Fig. 1.In a second aspect the present invention consists in a recombinantDNA molecule comprising a promoter having a nucleic acid sequenceincluding the DNA sequence shown in Fig. 1, or a fragment thereof whichincludes the sequence from residues 284 to 409, or a functionally equivalentnucleic acid sequence, expressively linked to a further DNA sequenceencoding a polypeptide.It is preferred that the promoter includes the nucleic acid sequencefrom residues 284 to 409 as shown in Fig. 1.In a preferred embodiment the polypeptide includes at least one atleast one epitope. It is preferred that the polypeptide includes B-cell and/orT-cell epitopes. In another embodiment the polypeptide includes at leastone CTL epitope. A preferred polypeptide is the 37 kDextracellular/secretory protein of Tr1'chostrongy1us colubriformis.In a third aspect the present invention consists in a recombinantvector, the vector comprising an iron-regulated promoter and a site forinsertion of a sequence encoding at least one polypeptide such that theinserted sequence is in frame with the iron-regulated promoter, wherein theiron-regulated promoter comprises the DNA sequence shown in Fig. 1, or afragment thereof which includes the sequence from residues 284 to 409, or afunctionally equivalent nucleic acid sequence.101520253035W0 98/10064CA 02265883 1999-03-05PC'IâIAU97I00503In a preferred embodiment the vector further includes an attPsequence and preferably the vector further includes a sequence encodingintegrase protein.It is also preferred that the vector further includes a sequenceencoding at least one polypeptide inserted at the insertion site. It ispreferred that the encoded polypeptide includes at least one epitope.Typically the encoded peptide will B and/or T-cell epitopes.In another preferred form the encoded polypeptide includes at leastone CTL epitope. As will be understood by those skilled in this area thesequence encoding the polypeptide may encode a plurality of CTL epitopes.Such an arrangement is disclosed in PCT/AU95/00461 the disclosure ofwhich is incorporated herein by reference.In yet another preferred embodiment the encoded polypeptide is the37 kD extracellular/secretory protein of Trichostrongylus colubriformis.In a fourth aspect the present invention consists in a recombinanthost cell, the host cell including a recombinant DNA molecule comprising apromoter having a nucleic acid sequence including the DNA sequenceshown in Fig. 1, or a fragment thereof which includes the sequence fromresidues 284 to 409, or a functionally equivalent nucleic acid sequence,expressively linked to a further DNA sequence encoding at least onepolypeptide.It is also preferred that the recombinant DNA molecule is insertedinto the host cell chromosome.It is preferred that the host cell is a bacterium, preferably Gramnegative, and more preferably the bacterium is Escherichia coli orSalmonella species, and preferably the Salmonella species is Salmonellatyphimurium.In a preferred embodiment the encoded polypeptide includes at leastone at least one epitope. It is preferred that the polypeptide includes B-celland/or T-cell epitopes. In another embodiment the polypeptide includes atleast one CTL epitope. A preferred polypeptide is the 37 lcDextracellular/secretory protein of Trtichostrongylus colubriformis.In a fifth aspect the present invention consists in a composition foruse in inducing an immune response in an animal, the compositioncomprising a recombinant host cell of the present invention and anacceptable carrier.WO 981100641015202530CA 02265883 1999-03-05PCTIAUMI00503As used herein the term "functionally equivalent nucleic acidsequence" is intended to cover minor variations in the promoter sequencewhich do not result in a promoter having substantially lower activity fromthe promoter defined from residues 284 to 409 in Fig. 1.As will be appreciated in a preferred form the vector of the presentinvention includes the bacteriophage P22 int gene and an attP region. Whenthis vector is introduced into a suitable bacterium, the bacteriophage P22 intgene is expressed to produce the int protein. A short DNA sequence calledattP (attachment) in the vector is homologous to the attB sequence found inthe bacterial chromosome. The bacterial chromosome expresses a proteincalled II-IF (integration host factor) and that protein interacts with theexpressed int protein from the vector which causes the homologousrecombination of the vector DNA and the chromosomal DNA between attPand attB sequences. As a result of this integration, attB and attP recombineand stably integrate the vector into the bacterial chromosome. When thevector includes a DNA molecule encoding a foreign gene, upon integration,the bacterium is capable of expressing that gene from its chromosome toproduce the foreign protein.In a preferred embodiment of the present invention, the bacterium isan attenuated vaccine bacterium and the heterologous protein is derivedfrom a virus, bacterium, fungus, yeast or parasite. More preferably, thebacterium is a S. typhimuzium strain and the polypeptide is the 37 kD extracellular/secretory protein from the nematode parasite of sheepTiichostrongylus co1ubr1'foItm1's.The present inventors have developed a site-specific chromosomalintegration system to integrate foreign antigen genes into the chromosome ofattenuated S. typhimuï¬um strains. The advantages of this system include:1. It is based on site-specific recombination into the bacterialchromosome (non-essential region) therefore the site of integration is clearlydefined.2. The system does not use mobile genetic elements (e.g. transposons)and therefore once the vaccine strain is constructed the genotype andphenotype will not be altered by genes hopping around to other sites in thebacterial chromosome.WO 98110064101520253035CA 02265883 1999-03-05PCTIAU9â7I005033. The efficiency of chromosomal integration approaches 100% i.e. allbacterial cells that are transformed by this DNA are found to have theheterologous polypeptide genes integrated in the bacterial chromosome.4. The system is "user-friendly" and requires a single step cloning eventto clone the gene of interest into the "chromosomal integration vector"followed by transformation of the attenuated S. typhimuzium strain ofchoice.5. This system is highly versatile and can be adapted for integration ofgenes into the chromosomes of a wide range of Gram-negative bacteria aswell as eucaryotic cells.6. The applications of this technology range widely and can be used as(a) basic-research tool to study chromosomally integrated genes in Gram-negative bacteria and Eucaryotic cells, (b) preparation of live attenuatedvaccines, (c) tissue specific expression in gene therapy, and (d] developmentof transgenic plants and animals.In order that the nature of the present invention may be more clearlyunderstood, preferred forms will now be described with reference to thefollowing drawings and examples.Brief Description of the DrawingsFigure 1 shows the complete DNA sequence encoding a hybrid iron-regulated promoter according to the present invention;Figure 2 shows the construction of plasmids including promotersaccording to the present invention;Figure 3 shows details of the plasmid used for chromosomalintegration in Salmonella;Figure 4 shows anti-37KD serum titers;Figure 5 shows anti B-galactosidase serum antibody titers;Figure 6 shows egg counts; andFigure 7 shows worm count in sheep intestinal lumen.Constr1_1cï¬on of ï¬e PromThe promoter was initially cloned as an EcoRI/BamI-II fragment isplasmid pSU207 (Fig. 2). Plasmid pHB158 (Fig. 2) is a pBR3ZZ basedplasmid carrying a polylinker. PCR primers I-[B38 and I-[B39 were designedto amplify part of the aerobactin gene promoter between positions 10 and394 on the aerobactin promoter sequence. This region carriers minor and1015202530WO 98110064CA 02265883 1999-03-05PCTlAU97l00503major promoters P2 and P2 respectively, along with the primary andsecondary Fur binding sites. The Shine Dalgarno and downstream DNAsequence of the aerobactin promoter were not amplified. The PCR primerscarried the NotI and Pacl restriction enzyme cleavage sites and the shortenedaerobactin promoter was subcloned into the NotI/Pacl sites of pHB158 togive plasmid designated pl-IB164 (Fig. 2].PCR PRIIVIER SEQUENCESI-IB38 CTCGAATTCGCGGCCGCCATATCCTCCCAGAHB39 CTCGGGCCCTTAATTAAACACAGTAAAATAATAACThe 37 kD extracellular/secretory protein encoding gene fromnematode parasite of sheep Trichostrongylus colubriformis was cloned as aIacZ gene fusion in plasmid pHb167(Fig. 2). Upstream of the 5' region of the37 kD gene sequence was engineered a DNA sequence carrying the PacIrestriction enzyme site followed by Shine Dalgarno sequence (AGGA), a 7 bpspacer sequence (AACAGCT) and a translation start codon (ATG).The Pacl/Xbal fragment form plasmid pHB167 (Fig. 2) carrying theupstream region, along with the 37 kD/lacZ genes was subcloned into thePacI/Xbal sites of plasmid pI-IB164 to give plasmid designated pHB170(Fig.2). This fusion of the aerobactin promoter (paer) with the upstream regionprovided a novel iron-regulated promoter according to the present invention.This promoter is unique in carrying the aerobactin promoter gene sequencesfor P1 and P2, as well as designed primary and secondary Fur binding sites,and designed Shine Dalgarno, spacer and ATG sequences.The important sequence of the iron-regulated promotor (Fig. 1) liesfrom DNA sequence residues 284 to 409 inclusive. This region includes the-35 (residues 284 to 289) and -10 (residues 307 to 312) regions of minorpromotor P2, the -35 (residues 340 to 345] -10 (residues 363 to 368) regionsof major promotor P1, the primary (residues 333 to 363) and secondary(residues 364 to 382) Fur protein binding sites, the Par: I restriction enzymesite (residues 388 to 395), the Shine-Dalgarno sequence (residues 396 to 399)and the translation initiation site (residues 407 to 409).10152025WO 98110064CA 02265883 1999-03-059 PCTlAU97I00503Demonstration of Induction of the PromoterPlasmid pl-IB170 was transformed into E. coli strain HB101 andS. typh1'mur1'um aroA-strain. The strains were grown in vitro under ironârichconditions in Luria Broth with 200 p.M FeCl3. Cultures for iron starvationwere grown under identical conditions but with the inclusion of 100 pM 2,2â-dipyridyl (iron chelator). The FeCl3 and 2,2.â-dipyridyl concentrations wereoptimised to obtain maximal repression and induction respectively withoutaffecting growth rate or viability of the organisms.Whole cell extracts of the recombinant E. coli and S. typhimuriumaroA- [uninduced and induced) were electrophoresed on a 6% SDS-polyacrylamide gel and analysed by Western blotting. PurifiedB-galactosidase protein and purified 37 kD protein were also electrophoresedas controls. One of the blots was developed with anti B-galactosidasemonoclonal antibody and the other was developed using 37 kD specificpolyclonal sheep antiserum. The results demonstrated that in both E. coliand S. typhimurium aroA-, the hybrid promoter was strongly induced underiron starvation conditions.B-galactosidase assay was carried out on the uninduced and inducedcultures and the results (Table 1) show that there is about a ten-fold increasein expression of the fusion protein following induction under iron starvationconditions.TABLE 1. Quantitative analysis of fusion protein expression under thehybrid iron regulated promoterPlasmid Growth [3-GalactosidaseStrain Conditions (Units)E. Cali HB101 pl-IB170 uninduced 840E. coli HB101 pI-IB17O induced 8560S. typh1'mur1'um pl-IB170 uninduced 255aroA-S. typhimurium pl-IB170 induced 2250aroA-1015202530WO 98110064CA 02265883 1999-03-05PCTIAU97l00503Construction of chromosomal integration vector.Bacteriophage P22 integrates its genome into the chromosome ofS. typhimuzium by the following mechanism:1. P22 attaches to the bacterial cell surface and injects its DNAinto the bacterial cell.2. The injected DNA circularises and expresses the INT(integration) protein encoded by the int gene.3. The P22 genome has a short DNA sequence called the attP(attachment) which is homologous to the attB sequence found in theSalmonella chromosome.4. The bacterial chromosome expresses a protein called the IHF(integration host factor).5. The INT and II-IF proteins interact to homologously recombinethe attP and attB sequences thereby integrating the P22 genome into thebacterial chromosome (lysogeny).Based on this system an integrating plasmid was constructed.Brieï¬y, plasmid pNEB193 (purchased from New England Biolabs) was usedas the plasmid into which the different gene insertions were made.plac/Iac gene fragment which encodes the lac repressor protein, wasPCR amplified from plasmid pLOF/Ars and cloned into the Pacl/Xbalrestriction sites of plasmid pNEB193 to result in plasmid pHB178.The ptac promoter gene fragment from plasmid pKK223â3 was PCRamplified and cloned into the Sacl/Kpnl sites of plasmid pHB178 to result inplasmid pHB179.The paer/ArsA/ArsB gene fragment which encodes Arseniteresistance was PCR amplified from plasmid pLOF/Ars and cloned into theHindi II site of plasmid pl-IB179 to result in plasmid pI-IB180.The bacteriophage P22 int/attP region including its ribosome bindingsite-spacer-translation start [ATG) was PCR amplified from the P22 phagegenome. This gene fragment was cloned into the Kpnl/Pacl site of plasmidpI-IB18O to result in plasmid pl-IB181.W0 98/1013641015202530351 1 I I I ICA 02265883 1999-03-05PCTIAU97I0050310Transformation of pla§y_1_i_d nHl_3_;81 into Salmonella tvpliimgrjum straingig conï¬rmation of chromosomal integration.Plasmid pHB181 was transformed into S. typhimurium strain H4004and transformants were selected on Brain Heart Infusion agar containingAmpicillin. The transformation efficiency was 106 colony forming units(c.f.u.) per microgram of DNA and was the same as control plasmid DNA.Plasmid DNA from individual transformants were analysed by agarose gelelectrophoresis and the results showed that the plasmid had integrated intothe chromosome. DNA from control E. 0011' cells harbouring the sameplasmid revealed the presence of the plasmid. Southern hybridisation withthe plasmid as a probe was used to confirm that the plasmid had integratedinto the chromosome.Sheep Vaccination ancgrotection Trial with the Recomï¬ant Sagonellae.6 month old sheep were vaccinated with various recombinant andcontrol salmonellae. 5 animals were used per group and the sheep werevaccinated by the oral or intramuscular route.The groups include:1. S. typhimurium aroA- [strain 4335] - oral (1011 organisms).2. S. typhimurium aroA- [strain 4335] carrying plasmid pI-IB170 (ironregulated promoter-37kD-1acZ) - oral (1011 organisms).3. Same as (2) but cells were induced to maximal protein production(3 7kD/B-gal fusion) in-vitro, ethanol fixed - oral [1011 organisms).4. Same as (3) - intramuscular (109 organisms).5. S. typhimurium aroA- [strain 4335] carrying "iron regulatedpromoter-37kD-lacZ" cassette integrated into the chromosome - oral(1011 organisms). The plasmid used for this integration is shownschematically in Fig 3.6. Same as (5) - intramuscular (109 organisms).The sheep received three vaccinations at two week intervals. Thesheep were challenged 2.5 weeks after the final vaccination withT. colubriformis L3 larvae over a 4 week period, the animals receiving 2000L3 two times per week.WO 98110064101520253035CA 02265883 1999-03-05PCTIAU97/0050311Serum was collected at various time points following vaccinationincluding pre-vaccination (negative control sera). The serum was analysedin ELISA assay to detect antibodies to the T. colubriformis 37kD polypeptideand to the [3-galactosidase reporter polypeptide. The serum antibody titersare shown in Figs. 4 and 5. .Following challenge of the sheep, T. calubriformis eggs were countedin fecal samples at various time points. Results of egg counts are shown inFig. 4. The sheep were euthanased about 2 months after commencement ofchallenge and the intestinal linings were scraped to collect T. colubriformisworms. The worm count data is shown in Fig. 5.The results showed the following:1. S. typhimurium aroA- carrying the plasmid pl-IB170 (iron regulatedpromoter-37kD-lacZ] showed no serum antibody response to either 37kD orthe [3-galactosidase polypeptides.2. The same salmonella (as in 1), when induced for maximal recombinantprotein expression in-vitro and ethanol fixed prior to vaccination, gave astrong serum antibody response to both recombinant proteins particularly inthe intramuscular vaccinated sheep (Figs. 2 & 3). Presumably thesesalmonellae function like a non-living carrier e.g. a liposome packed up withthe recombinant protein. The salmonellae in (1) were also induced in-vitroin the same way but were live, and our previous results had shown rapidplasmid was loss in in-viva (segregation) and presumably the vaccine lost theinitial load of recombinant proteins in-Vivo.3. Recombinant S. typI11'mur1'um aroA- carrying the same gene cassette(chromosomally integrated]as in (1) & (2), however, gave enhanced serumantibody titers to the recombinant polypeptides (Figs. 2 & 3). The orallyimmunised group produced lower titers but intramuscular immunisationgave titers that matched the fixed salmonella group. This result clearlydemonstrates that chromosomally integrated gene cassettes possibly underthe control of in-Vivo inducible promoters are effective in inducingrecombinant protein synthesis in vivo and eliciting at least antigen-specificserum antibody responses.1015WO 981100641| - â I I llCA 02265883 1999-03-05PCTIAU97I00503124. Similar results are obtained for egg counts (Fig. 4). The data for all groupsexcept for chromosomally integrated salmonellae (oral and i.m. immunised)are similar to the background level seen in S. typhimurium aroA- immunisedsheep. There is however, a 30% reduction in egg counts (compared tobackground level) in orally immunised sheep (chromosomally integratedSalmonella) and a more dramatic an significant [p< 0.05) 61% reduction inegg counts in the i.m. group (chromosomally integrated).5. The worm count data (Fig. 5) parallels that of egg counts and the i.m.vaccinated sheep (chromosomally integrated) had a significant (p<0.05)reduction of 61% in worm burden.It will be appreciated by persons skilled in the art that numerousvariations and/or modifications may be made to the invention as shown inthe specific embodiments without departing from the spirit or scope of theinvention as broadly described. The present embodiments are, therefore, to ibe considered in all respects as illustrative and not restrictive.