Note: Descriptions are shown in the official language in which they were submitted.
CA 0226~981 1999-03-16
WO 98/11944 PCT/US97/16120
METHOD AND APPARATUS FOR CONTROLLED
CONTRACTION OF SO~Y TISSUE
CROSS-REFERENCE TO RELATl~D APPLICATIONS
BACKGROUND OF THE INVENTION
Related Inventions
This application is a continuation in part of Serial No. 08/637,095, filed
April 24, 1996, entitled METHOD AND APPARATUS FO~ CONTROLLED
CONTRACTION OF SOFT TISSUE, which is a continuation of Serial No.
08/389,924, filed February 16, 1995, entitled METHOD AND APPARATUS
FOR CONTROLLED CONTRACTION OF SOFT TISSUE, which is a
continuation of Serial No. 08/238,862, filed May 6, 1994, entitled METHOD
AND APPARATUS FOR CONTROLLED CONTRACTION OF SOFT
TISSUE.
Field ofthe Invention
This invention relates generally to a method and apparatus for delivering
thermal energy to a selected collagen cont~ining tissue and effecting a
contraction of at least a portion of the collagen cont~ining tissue, and more
particularly to a method and apparatus for contracting a collagen co~ ;"g
tissue that is at least partially adj~c~nt to a fluid medium.
Description of the Related Art
Instability of peripheral joints has long been recognized as a ~ignifir.~nt
cause of disability and functional limitation in patients who are active in their
daily activities, work or sports. Diarthrodial joints of the musculoskeletal
system have varying degrees of intrinsic stability based on joint geometry and
lig~m~nt and soft tissue investment Diarthrodial joints are comprised of the
. .
CA 0226~981 1999-03-16
WO 98/11944 PCT/US97/16120
articulation of the ends of bones and their covering of hyaline cartilage
surrounded by a soft tissue joint capsule that In~ the constant contact of
the cartilage surfaces. This joint capsule also Ill~ c within the joint the
synovial fluid that provides nutrition and lubrication of the joint surfaces.
~ .ig~ e~ s are soft tissue con-len~tions in or around the joint capsule that
reinforce and hold the joint together while also controlling and restricting
various movements of the joints. The lig~m~nts~ joint capsule, and connective
tissue are largely comprised of collagen.
When a joint becomes unstable, its soft tissue or bony structures allow
for excessive motion of the joint surfaces relative to each other and in directions
not normally permitted by the lig~m~nt~ or capsule. The two main forms of
joint instability are called subluxations and dislocations. A subluxation occurswhen one surface of a joint slides out of position relative to the other surfacewhile ret~ining some contact belween the surfaces. A dislocation occurs when
one surface of the joint completely di.~eng~ges and loses contact with the
opposing surface. Generally, joints with a larger range of motion have more
inherently loose soft tissue investments surrounding the joint and as a result are
more prone to instability than others. For example, the shoulder (glen~humeral)
joint has the greatest range of motion of all peripheral joints and has long been
recognized as having the highest subln~tiQn and dislocation rate.
Instability ofthe shoulder can not only occur congen;l~lly and
development~lly but also traumatically. Furthermore, this inq~bility often
becomes recurrent and requires surgical repair. In fact, subluxations and
dislocations are a common occurrence and cause for a large number of
orthopedic procedures each year. Joints which require repair are characterized
by symptoms which include pain, instability, weakness and limit~tion of
function. If the in~t~hility is severe and recurrent, functional ine~raÇity and
arthritis may result. Surgical all~"~)ls are directed toward ti~htt?ning soft tissue
~ es~ which have become loose. These procedures are typically perforrned
CA 0226~981 1999-03-16
WO 98/11944 PCT/US97/16120
through open surgical approaches that often require hosrit~li7~tion and
prolonged rehabilitation programs.
More recently, endoscopic (arthroscopic) techniques for achieving these
same goals have been explored with variable success. FndoscQpic techniques
have the advantage of being performed through smaller incisions and are
usually less painful, performed on an outp~ti~.nt basis, are associated with less
blood loss and lower risk of infection and have a more co~mPtic~lly ~cceptAble
scar. Recovery is often faster postoperatively than using open technirlues.
However, it is often more technically d~m~n-~in~ to advance and tighten capsule
or 1ig~ ou~ tissue arthroscopically because ofthe difficult access to
pathologically loose tissue and because it is very hard to determine how much
tightçning or advancement of the lax tissue is clinically necessary. In addition,
fixation of advanced or tight~ned soft tissue is more difficult arthroscopicallythan through open surgical methods.
Collagen co~ g tissue is ubiquitous in the human body and provides
the cohesiveness of the musculoskeletal system, the structural integrity of the
viscera as well as the elasticity of inte~lment Collagen also demonstrates
unique characteristics not found in other tissues. A previously recogrlized
property of collagen is shrinkage of collagen fibers when elevated in
temperature. Collagen fibrils are at their greatest length in the native state of a
triple helix. Thermal energy to the collagen molecules disrupts the bonds which
st~bi1i7~ the triple helix. The loss of the triple helix structure causes the fibrils
to decrease in length or contract, giVillg the collagen co~ ing tissue the
appearance of contracting. The degree of contraction is a function of both the
height of temperature elevation as well as the length of te.lll)el alLlre elevation.
Thus, the same degree of contraction may be achieved by a high temperature
elevation of short duration or by a lower temperature elevation for an extended
duration.
Investigators have taken advantage of the unique collag~n features to
effect positive ch~nges in non-vascularized collagen conlai~ .g structures. For
........ ..
CA 0226~981 1999-03-16
WO 98/11944 PCT/US97/16120
in.ct~nc~ the use of infrared laser energy to shrink collagen in the cornea of the
eye relates to laser keratoplasty and has been described by Sand in U.S. Patent
No. 4,976,709. Further, radio frequency (RF) electrical current has been used toreshape the cornea. Such shaping has been reported by Doss in U.S. Patents No.
4,326,529 and 4,381,007.
The capsule of the shoulder joint consists of a synovial lining and three
well defined layers of collagen. The fibers of the inner and outer layers extendin a coronal access from the glenoid to the humerus. The middle layer of the
collagen extends in a sagittal direction, crossing the fibers of the other two
layers. The relative thickness and degree of intermin~ling of collagen fibers ofthe three layers vary with di~lc;n~ portions ofthe capsule. The li~3~mçntouc
components of the capsule are lep[esenled by abrupt thir~toning~ of the inner
layer with a significant increase in well olgani~ed coarse collagen bundles in the
coronal plane. The capsule functions as a hammock-like sling to support the
humeral head. In pathologic states of recurrent traumatic or developmental
instability this capsule or pouch becomes attçnu~ted and the capsule capacity
increases secondary to capsule redlln~nre. In cases of conge~ AI or
developmental multi-directional laxity, the ratio of type III to type I collagenfibers is often larger than usual. An appal~ s capable of shrinking the collagencontaining tissue in the shoulder may çlimin~te many of these instabilities.
Further, if this apparatus could be used endoscopically, many of the problems
with current endoscopic techniques would be elimin~ted since fixation,
tightçning and adv~nrçmrnt would no longer be lequ;led.
The use of endoscopic devices which simply heat the colla~lon
c~ ing tissue are not s~ti~f~ctory because ofthe delivery of uncol,llulled
energy. High telllpel~lures can cause cell necrosis and may damage the tissue.
There is a need for a method and apparatus which causes coll~gçn
co..~ g tissues to contract while minimi7ing cell necrosis and damage to the
tissue as well as other organs or bodies which may be present, more particularly,
for joints and shoulder capsules. There is a need for a method and apparatus
CA 0226~981 1999-03-16
WO 98/11944 PCTrUSg7/16120
capable of causing a collagen cont~ining tissue site at least partially a~ c~nt to
a fluid media to contract a selected amount without d~ gh-g the tissue of the
- site or any of the surrounding tissues or bodies whether they contain collagen or
not.
SUMMARY OF T~E INVENTION
It is an object ofthe present invention to provide a method and appa,~lus
configured to contract at least a portion of a selected site of a collagen
cont~ining tissue.
Another object of the present invention is to provide a method and
appa~L~Is configured to deliver sufficient energy to a s~lected site of a collagen
co~ n;..g tissue to produce a contraction of at least a portion of the selected
site.
Still another object of the present invention is to provide a method and
appa,~lus configured to deliver sufficient energy to a selected site of a collagen
co..~ -g tissue to effect an increase in the thermal energy content ofthe
selected site.
Yet another object of the present invention is to provide a method and
appa~ s configured to deliver sufficient energy to a s~.lected site of a collagen
co..l~ g tissue to effect an increase in the telllpe~ re ofthe selected site to a
pre-determined level.
A further object of the present invention is to provide a method and
apphl~L~Is confi~l.red to deliver s~ffici~nt energy to a s~lected site of a collagen
co.. ~ g tissue such that the te.lll)e~ re of the selected site increases to a pre-
determined level and remains at or near that level for a selected period of time.
Yet a further object of the present invention is to provide a method and
app~L~Is configured to deliver s-lfficient energy to a selected site of a cQll~g~n
co..l~;l,ing tissue to create a contraction of collagen fibers.
CA 0226~981 1999-03-16
WO 98tll944 PCT/US97/16120
Still another object of the present invention is to provide a method and
appa al-~S with a feedbac~ control device configured to deliver sufficient and
controllable energy to a selected site of a collagen conl~ g tissue.
Another object of the present invention is to provide a method and
apparatus with a feedback control device configured to deliver sufficient energyto a selected site of a collagen cont~ining tissue positioned at least partiallyadjac~nf to a fluid medium to contract at least a portion of the selected site and
produce a therrnal feedbac~ signal representative of a composite of the therrnalenergy contents of at least a portion of the selected site and at least a portion of
the adjacçnt fluid me~ m
Another object of the present invention is to provide a method and
apparatus with a feedbac~ control device configured to deliver suff1cient
thermal energy to a selected site of a collagen co,~ ;ng tissue of an unstable
joint at least partially positioned ~ c~nt to a fluid me~ m and at least partially
repair the instability of the joint.
These and other objects of the invention are obtained with an apparatus
for effecting change in at least a portion of a selected site of a collagen
ch~ nil~g tissue that is a~ least partially adj~cent to a fluid merli--m The
appa~ s inr.lud~c an energy delivery device confi~-red to deliver a level of
energy to the selected site of the collagen containing tissue. The energy
delivery device in~ludes a distal portion where a sensor is positioned. The
sensor provides a signal indicative of the thermal energy content of at least the
selected site ofthe collagen col.l~inil-~ tissue and the adjacent fluid m~i.-m to a
feedback control unit. The signal is received by the feedb~cl~ control system
which adjusts the level of energy supplied to the energy delivery device and
delivered to the selected site based on the signal received from the sensor.
In another embodiment, the app~alus in~l~lrles an energy delivery device
configured to produce a selected thermal distribution in the s~lected site of the
collagen cont~ining tissue to effect a controllable contraction of at least a
portion of the collagen fibers. The energy delivery device inrh1des a sensor
CA 0226~981 1999-03-16
WO 98/11944 PCT/US97/16120
po~itioned at a distal portion of the energy delivery device. A ~edbncl~ controldevice is coupled to the sensor. A position of the sensor, a gen. ..el, y of thedistal portion of the energy delivery device and the feedbaclr control system
provide a controllable energy delivery to the selected site of the collagen
col-lAi~ -g tissue.
The energy delivery device is configured to deliver energy from the
distal portion to the selected site ofthe collagen co..~ ;.,g tissue. The selected
site absorbs at least a portion of the delivered energy and the thermal energy
content and temperature of the sçlected site are increased. As the thermal
energy content ofthe selected site is increased, thermal energy is con~ cted to
the collagen fibers of the s~ected site. Collagen fibers exposed to s..ffil~.içnt
thermal energy at least partially lose their triple helix shape and contract. Thus,
the delivery of energy to the selected site causes the tel,lpe~ re and the thermal
energy content of the sçlected site to hl.;l~ase and create a contraction of at least
a portion ofthe collagen co.,~ g tissue site.
In one embodiment, the sensor is located within the distal portion.
During surgery, the distal portion is preferably placed in contact with a portion
of the selected site and the fluid medillm adjacçnt to the selected site. Because
of this contact, the thermal energy from the selected site and the adjacçnt fluid
medium will conduct through the thermally conductive sections of the distal
portion to the sensor. The m~gnitu(le of the resulting signal lepresellLs a
composite of the thermal energy contents of the selected site and the adjacent
fluid medi~lm
The sensor provides a signal which is le~ se.l~ e ofthe thermal
energy contents of a portion of the fluid metiil~m a~ljacçnt to the selected site as
well as at least a portion of the selected site. As a surgeon moves the distal
portion about a selected area, it is possible for a surgeon to bring the distal
portion into contact with a selected site which has previously been elevated to
- the desired temperature for the desired period of time. This second application
of energy may quickly elevate the temperature enough to cause cell necrosis or
CA 0226~981 1999-03-16
WO 98111944 PCT/US97116120
cause the temperature at the sPIected site to remain elevated for longer than the
desired period required for the desired level of collagen contraction.
Since the mAgnih1de of the signal provided by the sensor partially
l epl esellls the thermal energy of the fluid me~ m the apparatus is responsive
to changes in the thermal energy content of the fluid medi~Im Due to the nature
of delivering energy to a selected site thermal energy is more disperse in the
fluid medium. Because the apparatus responds to thermal energy in the fluid
metlillm the appal~l~s reduces cell necrosis resulting from sl~ccescive
applications of energy to the selected site. Stray contractions are contraction
which occur away from the selected site due to the fluid metlillm becoming
elevated in temperature for an PYtçnded period of time. Further response of the
apparallls to therrnal energy in the fluid mP~ m can also reduce stray
contractions.
DESCRIPTION OF T~IE DRAWINGS
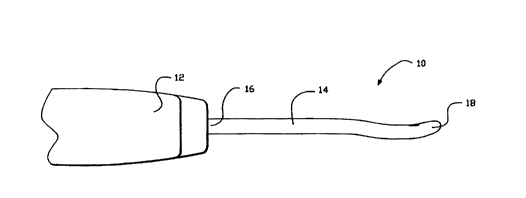
Figure 1 is a perspective plan view of an embodiment of the present
invention illustrating an appa~aLus for contracting collagen con~ g tissue.
Figure 2 is a pel ~pe~ re plan view of an embodiment of the present
invention illustrating an appa~ ~LIls coupled to an energy source for contracting
collagen co.~ ;.,g tissue.
Figure 3 is a perspective plan view of an embodiment of the present
invention illustrating an appa~lus for contracting collagen coI~Ail.;..g tissue a
desired amount in contact with a collagen co~ e tissue.
Figure 4 is a perspective plan view of an embodiment of the present
invention illusll~ling an appal~ s for contracting coIIAgPn co~ ni~ tissue a
desired amount delivering heat to a selected site within a setected area.
CA 0226~981 1999-03-16
W O 98111944 PCTrUS97/16120
Figure 5 illustrates the po~itioning of a distal end of an energy delivery
device while delivering energy to a selected tissue site and a portion of an
~-ljacent fluid medium and the measurement of a composite telllyel alul e.
Figure 6 is a cross-sectional view of a distal portion of the energy
delivery device with a sensor positioned in interior of the distal portion.
Figure 7 is a pe- ~ye~ e plan view of an embodiment of the present
invention illustrating an apparatus for contracting collagen CO"~ g tissue a
desired amount where the energy delivery surface is a composite construction.
Figure 8 is a sc.h~m~tic of an embodiment of the present invention
illustrating a feedbarlr control system.
Figures 9(a)-(d) are perspective plan views of di~relenl embodiments of
the present invention illustrating several apparatus, each configured to provide a
signal from a sensor such that the signal I eplese..ls thermal energies of di~(~-"
surfaces or me~ -m~.
Figure 10 is a perspective plan view of an embodiment of the present
invention illustrating an apparatus with a handpiece, energy delivery device andan operating cannula according to the present invention.
Figure 11 is a perspective plan view of an embodiment of the present
illustrating an invention apparatus inrlu~ing an in~ ting layer for preventing
damage to surrounding tissues, organs or bodies.
Figure 12 is a perspective plan view of an embodiment of the present
invention illustrating an apparatus inrlu-ling a handpiece, an energy delivery
CA 0226~981 1999-03-16
W O98111944 PCTrUSg7/16120
device and a sleeve that slides across the surface of the energy delivery deviceto vary the amount of energy delivery device conductive surface.
Figure 13 is a perspective plan view of an embodiment of the present
invention illustrating an apparatus inrhl~in~ a thermal in.~l~]~ting layer whichcan be positioned to specify the surface of the distal end section from which the
sensor is able to detect thermal energy.
Figure 14 is a sectional view of an embodiment of the present invention
illustrating a deflected energy delivery device with a resistive heating elementpositioned in an interior lumen of the energy delivery device.
Figure 15 is a perspective plan view of an embodiment of the present
invention illustrating an energy delivery device with a steering wire positionedon the exterior of the energy delivery device.
Figure 16 is a sectional view of an embodiment of the present invention
illustrating an energy delivery device with a lumen and a plug that is ~tt~ched to
the energy delivery device distal end.
Figure 17 is a sectional view of an embodiment of the present invention
illustrating an energy delivery device with an oval cross section and a heating
zone in the tissue.
Figure 18 is a sectional view of an embodiment of the present invention
illustrating a handle, energy delivery device, ope~ g cannula and a viewing
scope, with the viewing scope and energy delivery device po.cition~d in the
opel~Lingc~nm~l~
CA 0226~981 1999-03-16
WO 98/11944 PCT/US97/16120
Figure 19 is a cross sectional view of an embodiment of the present
invention illustrating a device of Figure 18, taken along the lines 19- 19.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Referring now to Figure 1, an apparatus for contracting collagen
co.,~ g tissue to a desired level is generally denoted as 10. Apparatus 10
incllldes a handpiece 12 that is preferably made of a thermal in.c~ tin~ material,
or an electrode that is electrically inc~ ted Types of such inc~ tin~ materials
are well known to those skilled in the art. An energy delivery device 14 is
coupled to handle 12 at a proximal end 16 of energy delivery device 14, and
may be att~ched thereto. A distal end 18 of energy delivery device 14 in~ des
a distal portion 20 which may have a geometry that delivers a controlled amount
of energy to tissues in order to achieve a desired level of contraction of collagen
fibers in a coilagen cont~ining tissue. Located at distal portion 20 is one or
more sensors 22 which provide a signal whose m~nit~lde is leplesel.lalh~e of
the amount of thermal energy sensed.
As shown in Figure 2, energy is supplied from an energy source 24
through a cable 26 to energy delivery device 14. Since several types of energy
can cause an elevation in the temperature of a collagen cnnt~ining tissues 28.
Energy source 24 can include but is not limited to RF, microwave, ultrasonic,
coherent and incoherent light, thermal transfer, and reCict~nce heating.
As illustrated in Figure 3, distal portion 20 is configured to be positioned
~dj~c~nt to a collagen co~ i..;.lg tissue 28 which is at least partially ~djacçnt to
a fiuid mP~ m 30. Appropriate collagen co.,l~;nil~g tissues 28 can include but
are not limited to vascularized densely collagenous structures such as ten~lonc~lig~mentc, joints capsules and the like. Distal portion 20 is pler~l~ly in contact
with collagen cont~ining tissue 28. Fluid medium (gas, liquid, or a
combination) 30 may be flowing as would result from irrigating collagen
co~ sg tissue 28 or it may be substantially less dynamic or non-moving.
CA 0226~981 1999-03-16
WO 98/11944 PCT/US97116120
Further, fluid medillm 30 need only be partially fluid and contain bone, portions
of organs or other bodies and the like.
Referring now to Figure 4, energy delivery device 14 is configured to
deliver energy from distal portion 20 to a selected site 32 ofthe cQll~gen
co.. ~ g tissue 28. Selected site 32 receives at least a portion of the delivered
energy. Once the energy is delivered it becomes thermal energy causing the
thermal energy content and the te~l,?elalLIre of selected site 32 to increase. As
the thermal energy content of selected site 32 is increased, thermal energy is
con~ucted to the collagen fibers in and around selected site 32. Collagen fibersexposed to sufficient thermal energy loose their triple helix shape. Since the
triple helix shape of collagen fibers is the longest shape for collagen fibers,
fibers which loose their triple helix shape will contract. Thus, the delivery ofenergy to selected site 32 causes the te,l.pe~ re and the thermal energy contentof selected site 32 to increase and effects collagen fibre contractions. The
collagen fiber contraction results in a contraction of collagen co~ il-il-g tissue
28.
Energy delivery device 14 is configured to deliver a level of energy to
selected site 32. Sensor 22 provides a signal indicative of a composite
te"")e~ re of at least selected site 32 and at least a portion of at least a portion
of a(lj~cent fluid me~ m 30 to a feedback controi unit. The signal is received
by a feedbac~ control system which adjusts the level of energy supplied to
energy delivery device 14 and delivered to selected site 32 based on the signal
received from sensor 22.
Throughout the l~eal,.,e,ll, it is often be desirable to effect contractions
in a selected area 34 which is larger than a selected site 32. Further, may be
desirable to elevate the te-..?e~ re of the selected site 32 or selected area 34 to
a desired average temperature for a specified period of time. There are several
methods available for achieving these results. For in.ct~nce, one embodiment is
to "paint" distal portion 20 across selected area 34 by continually moving distal
portion 20 over the surface of the selected area 34 so that the entire selected area
CA 0226~981 1999-03-16
W O 98/11944 PCTrUS97tl6120
34 is covered. Selected area 34 can then be brought to the desired temperature
and l~ah~ed at that tenlpe.~L~lre by continually moving distal portion 20 over
s~lected area 34. In another embodiment, distal portion 20 is left at selected site
32 until the desired temperature is obtained for the desired time. Distal portion
20 is then moved to another selected site 32 for a desired time. This pattern isrepeated until the entire selected area 34 is covered. A colllbina~ion of these
techniques may also be used.
Referring now to Figure 5, the composite tell.pe.~ e is a co.l,~.in~;on
of at least two diLrerenl temperatures in some ratio. One tempe.~ re 25 is from
at least a portion of ~Ajac~nt fluid me~ m 30 and another te.. -l)el~lure 27 of at
least a portion of selected tissue site 32. This ratio is a function of different
parameters incluAing but not limited to the size, shape, dimensions and
geometry of a thermal energy delivery surface of energy delivery device 14, the
portion of the thermal energy delivery surface that is in contact with ~Aj~cent
fluid medium 30 and selected tissue site 32, the location of sensor 22 in
relationship to the thermal energy delivery surface. Current flow 29 which
creates molecular friction, and conducted thermal energy are greater in selectedtissue site 32 than in adjacent fluid me~ m 30 due to the higher recist~nce of
the tissue.
One embodiment of distal portion 20 is illustrated in Figure 6. Distal
portion 20 of energy delivery 14 in~llldes sensor 22 positioned in an interior of
distal portion 20. A thermally conductive material 31 at least partially
surrounds sensor 22 and a potting compound 33 is in~lndeA Distal end 18 is
made of stainless steel, and a nylon coating is po~itioned at an exterior surface
of distal portion 20.
At the thermal energy delivery device fluid me~illm interface there is
less resistance and a hydro dynarnic force which contribute to a lower reflectedtemperature. At the tissue interface there is a static conductive sit~tion with a
higher re~ ance producing higher reflective temperature at the interface.
CA 0226~981 1999-03-16
W O 98/11944 PCTnUS97116120
Energy delivery device 14 can be made of a number of di~erelll
materials including but not limited to stainless steel, pl~tim~m, other noble
metals and the like. Energy delivery device 14 can be made of a memory metal,
such as nickel tit~nillm, cornmercially available from Raychem Corporation,
Menlo Park, California. Energy delivery device 14 can also be a composite
construction whereby di~lent sections are constructed from di~lellL materials.
Further, it may be desirable for delivery device 14 to be a composite of a firstmaterial 36 which is not conductive to the type of energy being delivered and a
second material 38 which is conductive to the type of energy being delivered as
shown in Figure 7. Such a construction permits treatment in locations where
there are tissues, organs or other bodies present which the surgeon does not wish
to expose to the delivered energy. For example, when energy delivery device
14 is introduced into a joint where it is desirable to treat a specific section of the
joint and avoid delivery of energy outside of that section, an energy delivery
device 14 partially constructed of non-con-~usting material 36 can permit
tre~tm~nt
One embodiment of an open or closed loop fee~ba~l~ control system 40
is shown in Figure 8. The physician can, if desired, override the closed or openloop feedback control system 40. The fee(lb~c~ control system 40in~hldes an
energy source 24, (in~hl(ling but not limited to a RF source), a temperature
measuring device 44, a voltage and current measuring device 46, a user display
unit 48, a timekeeping device 50, a microprocessor 52 and a user input device
54.
Energy source 24 supplies energy to energy delivery device 14 for
delivery to selected site 32. The voltage and current supplied to the energy
delivery device 14 are measured on voltage and current measuring device 46
and can display these to the user on user display unit 48. Tell,pelaLu~e
measuring device 44 measures the te..~pe~ re at sensor 22, in~lutling the
telllpel ~lure of adjacent fluid medium 30 and selected tissue site 32. The
14
CA 0226~981 1999-03-16
WO 98/11944 PCT/US97/16120
tel.lpel~L-Ire measured by temperature measuring device 44 can be displayed to
the user on the user display unit 48.
In one embodiment a signal produced by the sensor 22iS received by a
feedb~ control system 40. Feedbaçk control system 40 monitors the signal
produced by sensor 22 and adjusts the amount of energy or current supplied to
energy delivery device 14 according to the m~gnihlde ofthe signal. Energy is
supplied to apparatus 10 at a particular rate. The rate of energy delivery can be
expressed as power. Power supplied to energy delivery device 14 is adjusted so
the temperature at sensor 22iS elevated to a temperature which is desired by theuser and is input to the fee~iba~L control system 40. Once the desired
telllpel ~ure is reached, power is adjusted so that the temperature at sensor 22has minor fluctuations but averages to the desired telllpcl ~L~Ire over time. Thus,
the feedbac~ control system 40 m~int~in~ the desired tel.lpel~ re at the sensor
22 and correspondingly at the selected site 32.
In another embodiment, feedb~ck control system 40 also monitors time.
In this embodiment both time and temperature are inputs. Thus, once the
temperature at sensor 22iS elevated to the desired tel~lpel~ure, fee(lbacl~ control
system 40 tracks the length of time sensor 22 averages the desired temperature.
Once the temperature at sensor 22 averages the desired temperature for the
desired time, fee~bac~ control system 40 may either stop the delivery of energy
or it may inform the user on a user display screen (not shown). Thus, feedback
control system 40 can be used to m~int~in the desired temperature at selected
site 32 for the desired time.
In one embodiment, microprocessor 52 monitors voltage, current and
temperature. Microprocessor 52 car, calculate the power supplied to energy
delivery device 14 from the current and voltage and can display the power on
the user display unit 48. Microprocessor 52 can also monitor and control a
tim~keeping device 50. The microprocessor 52 can signal time~eeping device
50 to begin or stop tracking time. While timekeeping device 50 is tracking time
and microprocessor 52 can monitor the passage of time. Microprocessor 52 also
CA 0226~981 1999-03-16
WO 98/11944 PCT/US97/16120
receives input from a user input device 54. User input device 54 allows a user
to program rnicroprocessor 52 or input information such as the desired
temperature or the desired time.
Energy source 24 includes circuitry for mod~ tinp: the power supplied
to the energy delivery device 14 according to a signal received from
microprocessor 52, thus, microprocessor 52 can control the power supplied to
the energy delivery device 14. Microprocessor 52 is programrned to ad~ust the
power supplied to energy delivery device 14 so that the tenlpel~ re at sensor 22is ."~ ;"ed at the desired telllpelal~lre for the desired time. The program takes
into account at least the desired time, temperature and desired tell~ re in
making these adjustments.
Feedback control system 40 is used to obtain the desired degree of
contraction by ~ t~ i"g selected site 32, at a desired temperature for a
desired time. It has been shown that temperatures of 45 to 90 degrees C can
cause collagen fibers contractions. It has also been shown that the degree of
collagen fiber contraction is controlled by how long the temperature is elevatedas well as how high it is elevated. Thus, the same degree of contraction can be
obtained by exposing selected site 32 to a high temperature for a short period of
time or by exposing selected site 32 to a lower t~lllpe-~ re for a longer periodof time. A ~l erel ~ ed range for desired te~npe~ res is about 45 to 75 degrees C,
still a more pl~rel~ed range is 45 to 65 degrees C. Before tre~tm~nt the surgeonevaluates the characteristics of the selected site 32 to determine what degree of
contraction is necessary and also whether it is app.opl;ate to treat the selected
site 32 with a high telllpe~ re for a low period oftime or lower te...pe.~ re for
a long period of time. The surgeon then enters into the user input device 54 thedesired te ..pe- ~lure and the desire time. Feedbar.~ control system 40 uses this
information to control the delivery of energy to selected site 32 which results in
a controlled contraction of collagen colll~ining tissue fibers. The controlled
collagen fiber contraction allows for a desired degree of collagen co..~ g
tissue contraction.
16
CA 0226~981 1999-03-16
WO 98/11944 PCT/US97/16120
Additionally, fee~bar.~ control system 40 can be used to effect how
deeply within the collagen cont~ining tissue 28 the collagen fiber contractions
occur. For instance, elevating the tel.lpe~ re of selected site 32 to the low end
of the range effects contractions near the surface of collagen cont~ining tissue28. Elevating the temperature to the high end of the range effects contractions
deeper within collagen co.~ g tissue 28. Thus, if the surgeon is dealing with
a very thin collagen cont~ining tissue 28 which is adjilcent to tissue which maybe damaged by elevated temperatures, the surgeon may choose to elevate the
temperature of selected area 34 to a low temperature for longer periods of time.However, if collagen cont~ining tissue 28 is thicker, the surgeon may choose
higher temperatures to effect contractions deeper in collagen cont~ining tissue
28. Thus, the choice of the desired temperature can control the thermal energy
distribution and thus the depth of contractions.
Feedback control system 40 further allows apparatus 10 to minimi7e and
even prevent cell necrosis (ablation) resulting from exposure to high
temperatures. High temperatures can cause excessive destruction and
~icintegration of the collagen fibrillar patterns and cell necrosis. Since feedback
control system 40 can m~iht~in the temperature of sensor 22 at a desired
temperature, the temperature at selected site 32 does not exceed ablation
temperature.
Further, feedback control system 40 can prevent overshoots which may
cause cell necrosis. Overshoots occur while raising the te...pe~ re of selected
site 32 or selected area 34 to the desired level and temporarily surpassing thatlevel. Some overshoot of the desired te...~)e.~L~Ire will be inherent in most
embodiments of feedback control systems, however, it is possible to cause cell
necrosis or dissociation if the overshoot is high enough or of long enough
duration. Feedb~cl~ control system 40 reduces overshoots by reducing the rate
of energy delivery once the selected site 32 te--.l)el~ re is near the desired level.
Thus, when energy is first delivered to a selected site 32, there can be a high rate
of energy delivery, however, once the temperature of the selected site is nearing
CA 02265981 1999-03-16
W O 98/11944 PCTrUS97/16120
the desired range, the rzte of energy delivery is reduced ir order to prevent anovershoot. Pro~ g this rampiag down effect,lito a feedbac~ control
system 40 is well known to those in the art of feedbac!c control. Note: Some
overshoots are ok, but the average ternp rnust fall within a non-dilative range.Sensor 22 can consist of, but is not lir.~ted to, a therrnocouple, a
thermistor or phosphor coated optical fibers. The se1lror 22 can be in an interior
of the distal portion 20 or on the surface of the distal portion 20 and can further
be a single sensor 22 or several sens~rs. It can als~ be a band or patch insteadof a sensor 22 which senses or,ly discrete points.
Sensor 22 provides 2 sigr.al whose m~g~litude is representative ofthe
thermal energy content of the surfaces &nd mediums in physical contact Wit}l thesurface of the sensor 22. Thus, if several surfaces or mediumc are in physical
contact with sensor 22, the m~gnitllde of the signal provided by sensor 22 will
be representative of a composite of the thermal energy contents of those
surfaces and/or mediums. Further, the effective suriace of sensor 22 can be
increased by wholly enclo~ing sensor 22 ir. a medillm which easily conducts
therrnal energy. In this embodiment, thermal energy will be conducted firom the
surface of the thermally conductive mediuln to th ser,sor 22. The magnitude
of the signal will represent a com?csite OI fl~e ther~r~l energy contents of anysurfaces and mediums in physical co~act with the surface of the thermally
conductive medium. For inct~nce~ Figure 9~a) il'ustrates an embodiment where
sensor 22 is located within distal portion 20. Fu.rther, distal portion 20 is inphysical contact with a portion Gf the selec.e~ site 32 and fluid medillm 30
atljacPnt to the selected site 32. r'ec~ r~e cf this cor.tact, the thermal energy
from selected site 32 and adjacent fluid rr.ediurn.30 cond.lcts through the
thermally conductive sections cf dirtal ~" crt.~n 23 t~ sensor 22. The m~,~nitude
of the resulting signal provide& by senrcr 22 represen~s the com~osite thermal
energy content of selected site 32 and at leas~ a ~~ortic;l of ~ ctont fluid
meditlm 30.
CA 0226~981 1999-03-16
WO 98/11944 PCT/US97/16120
By strategically positioning and configuring sensor 22, it is possible to
design the distal portion 20 such that signal represents the thermal energy
content of specific surfaces or medillm.c. For in~t~nce, Figure 9(b) illustrates an
embodiment where the sensor 22 is positioned such that the thermal energy
S con~llcted to the sensor is from substantially only sPIected site 32. Thus,
sensor 22 provides signal which is l ep~ ese"l~Live of the therrnal energy content
of substantially only selected site 32. Further, Figure 9(c) shows an
embodiment where sensor 22 is configured as a band and positioned such that
the thermal energy conducted to sensor is from substantially only fluid medil]m
30 ~1jaeçnt to selected site 32. Thus, sensor 22 provides a signal which
ep, ese"ls substantially only the therrnal energy content of the ~dj~cçnt fluid
me-lium Figure 9(d) illustrates another embodiment where fluid medillm
adjacent to selected site 32 contains other tissue, organs or bodies 56. In thisembodiment, the signal provided by sensor 22 le~ s~nls a composite ofthe
thermal energy contents of selected site 32 and ~dj~ nt fluid medillm 30 as
well as the other tissues, organs or bodies 56.
Sensor 22 provides the composite signal of thermal energy and
temperature whether fluid merlillm 30 is flowing or non-flowing. When the
surgeon chooses to deliver energy to selected area 34 by moving distal
portion 20 from selected site 32 to another, it is possible to bring distal portion
20 into physical contact with a selected site 32 which has previously been
elevated to the desired temperature for the desired period of time. This second
application of energy may quickly elevate the tenli)e,~u~e enough to cause cell
necrosis or may cause the temperature at selected site 32 to remain elevated forlonger than the desired period causing the collagen fibers to contract more thandesired.
Positioning sensor 22 to provide a signal which represel,L~ a composite
of the therrnal energy contents of selected site 32 as well as ~dj~cPnt fluid
medillm 30 reduces cell necrosis or over contraction caused by a second
application of energy. As energy delivery device 14 delivers energy to selected
19
.
CA 0226~981 1999-03-16
WO 98111944 PCT/US97/16120
site 32 it also delivers energy to fluid meriillm 30 which is in physical contact
with energy delivery device 14 adjaG~nt to selected site 32. This delivery of
energy to fluid medium 30 causes the thermal energy content of fluid medium
30 to increase. The thermal energy content of fluid me~illm 30 ~dj~cent to
S selected site 32 also rises due to conduction of thermal energy from se}ected site
32 to fluid tne~ m 30. Furthermore, due to convection resulting from the
movement of distal portion 20, thermal energy disperses through fluid m~ium
30 at a quicker rate than through collagen co..~ g tissue 28.
As a result ofthe energy ~lanar~la described above, the colleal)ol1dillg
elevations in temperature will be more dispersive in fluid me~ m 30 than in
selected site 32. Thus, the signal produced by sensor 22 is di~rent when dis~:alportion 20 is placed a(ljacçnt to a previously heated selected site 32 than whendistal portion 20 is placed in a selected site 32 away from any previously heated
selected sites 32. Although the selected sites 32 in the former and latter caseswill have similar thermal energies, in the former case, the dispersive energy influid medium 30 causes the fluid medium 30 to have a higher thermal energy
content than in the latter case. Since sensor 22 provides a signal whose
m~gnit~lde ~eplesen~s a composite ofthermal energies of fluid medi~lm 30
~djacent to selected site 32, in the former case sensor 22 provides a signal to
feedback control system 40 indicating an elevated thermal energy content and
reduces the amount of energy delivered to selected site 32. This reduced energy
delivery decreases cell necrosis or o~lco"l,action near selected site 32. The
same is true in those in~t~nres when distal portion 20 is again passed over a
previously heated selected site 32.
It will also be applec;aled that when sensor 22 is positioned where it
provides a signal representing a composite in~lu~ing adjacent fluid me~illm 30
when the surgeon chooses to paint s~lected area 34 rather than moving from one
selected site 32 to another. The surgeon will want to keep the te~ ei~ure of an
entire selected area 34 within a specific range during the pau,~h~g process. As
distal portion 20 is painted across s~lected area 34 it leaves a path which has
CA 0226~981 1999-03-16
W O 98/11944 PCT~US97116120
been heated and may intersect that path several times during the process of
keeping the temperature within the desired range. As selected area 34 is
covered and distal portion 20 intersects the heated path it is desirable to deliver
more energy to areas which are not in the path and consequently have not been
previously heated. It is also desirable to deliver less energy to areas which are
part of the path and have previously been heated. By delivering energy this
way, the thermal energy content of the selected area 34 will approach a uniform
thermal energy across the selected area.
As described above, thermal energy can be more dispersive in fluid
medillm 30. As a result, when distal portion 20 is moved toward a previously
heated path sensor 22 provides a di~erenl signal than it would if it were not
traveling toward a previously heated path. Fluid mçdillm 30 will have a higher
thermal energy content in the forrner case than in the latter case. Since sensor22 provides a signal whose m~nitllde is related to a composite of the thermal
energies of fluid m~dillm 30 adjacent to selected site 32 and the selected tissue
site 32 in the forrner case sensor 22 provides a signal to feedbar.~ control system
40 indicating an elevated thermal energy content and reduces the amount of
energy delivered to selected site 32. As a result ofthe elevated therrnal energycontent, feedback control system 40 reduces the amount of energy delivered in
the former case. The result allows the te,.l~e,al~lre across the selected area 34 to
approach uniro~ ily. U~iiro",li~y of temperature is desirable as it reduces cellnecrosis or overcontractions near path intersections.
Positioning sensor 22 such that it provides a signal which I epl t;senls a
composite ofthermal energies inr~ ing adjac~nt fluid mç~lium 30 can also
reduce stray contractions. Stray contractions are undesired contractions of
collagen fibers outside selected area 34. As described above, while energy is
delivered to selected area 34, the thermal energy content of fluid mç(lium 30
also increases. During an extended 1l ~llllenl it is possible for the thermal
energy content of fluid medium 30 to rise considerably. If the thermal energy
content of fluid mç~lillm 30 remains elevated for an extended period oftime it is
CA 0226~981 1999-03-16
WO 98/11944 PCT/US97/16120
possible for the conduction of thermal energy from fluid me~ m 30 to collagen
co.~ .g tissue 28 to elevate the te~ )e.~ re of collagen coi~Ai.~ing tissue 28
sufficiently to cause undesired contractions of collagen fibers and may occur
outside sele~cted area 34. These stray contractions are even more of a problem
when the fluid mer~il-m 30 is flowing since the flow will carry the heated fluidmedium 30 away from the selected area.
These stray contractions are reduced by positioning sensor 22 to provide
a composite signal which inr.l~ldes at least a portion of fluid medil-m adj~cPnt to
the selected site 32. For inct~nçe, when the thermal energy content of fluid
me~ m 30 is raised, the signal will be di~renl than when it is not and the
energy delivery is adjusted accordingly. Since sensor 22 provides a signal
whose m~gnit~lde is related to a composite which inr.l~ldes the fluid medium 30
adjac~nt to the sPIected site 32, in the forrner case sensor provides a signal to
feedb~cl~ control system 40 indicating an elevated thermal energy content and
reduces the amount of energy delivered to s~lected site 32. The reduced energy
delivery reduces the amount of energy delivered to fluid medium 30 and
concçquçntly reduce stray contractions.
Apparatus 10, comprising handpiece 12 and energy delivery device 14,
is adapted to be introduced through an operating cannula 58 for percutaneous
applications. It will be appreciated that apparatus 10 may be used in non-
percutaneous applications and that an operating cannula 58 is not n~cecc~ly in
the broad application of the invention.
As illustrated in Figure 10, appar~ s 10 can also include, as an integral
member, an operating cannula 58 which can be in the form of a hypodermic
trocar with tlim~ncions of about 3 to 6 mm outside ~ m~o,ter, with tubular
geometries such as those of standard col-.lllel.;ially available ope.~ling ç~nm~l~c
Operating cannula 58 can be made of a variety of biocompatible materials
innl~lding but not limited to stainless steel, and the like.
Ope-~Ling cannula 58 has a cannula p.o~umal end that att~hes to
handpiece 12 and a cannula distal end 60 which can have a sharp or piercing end
CA 0226~981 1999-03-16
W O 98/11944 PCT~US97/16120
for penetrating body structures in order to introduce energy delivery device 14
to a selected site 32. Energy delivery device 14 is positioned within an interior
lumen of operating cannula 58 and is extendable beyond cannula distal end 60
in order to reach selected site 32. Energy delivery device 14 can be advanced
and retracted in and out of operating cannula 58 by activating a deployment
button 62 which is located on the exterior of handle 12. Deployment button 62
is p~ere~al)ly activated by the operator merely by sliding it, which causes energy
delivery device 14 to advance in a direction away from cannula distal end 60.
Deployment button 62 can be pulled back, causing a retraction of energy
delivery device 14 towards cannula distal end 60. In many in~t~ncçc, energy
delivery device 14 is retracted to be positioned entirely within operating cannula
14. Energy delivery device 14 can also be deployed with fluid hydraulics,
pne~-m~tics, servo motors, linear actuators, and the like.
In Figure 11, distal portion 20 of energy delivery device 14 incl~ldes an
ing~ ting layer 64 which is substantially impenetrable to the energy delivered
to collagen co~ h~ g tissue 28. Specifically, in the case of an RF energy
source 24, electrical insulation can be used. Insulation 64 can be formed on
energy delivery device 14 such that a miniml~m of energy is delivered to tissue,organs or other bodies which the surgeon does not wish to treat. For example,
when energy delivery device 14 is introduced into a tight area, and only one
surface of the tight area is to be treated, then it is desirable to avoid delivering
energy outside of that surface. The inclusion of in.c~ ting layer 64
accomrli.~hes this result. Suitable insulation materials include but are not
limited to polyamide, epoxy varnish, PVC and the like.
The area of energy delivery device 14 that serves as a conductive surface
66 can be ~ju~ted by the inclusion of an in~ ting sleeve 68 (Figure 12) that is
positioned around energy delivery device 14. Sleeve 68 may be advanced and
retracted along the surface of energy delivery device 14 in order to increase ordecrease the surface area of conductive surface 44 that is directed to collagen
cont~ining tissue 28. Sleeve 68 can be made of a variety of materials inr.ll~(ling
_ _ . . . . . .
CA 0226~981 1999-03-16
W O 98/11944 PCTrUS97/16120
but not limited to nylon, polyamides, other thermoplastics and the like. The
amount of available conductive surface 44 available to deliver thermal energy
can be achieved with devices other than sleeve 68, inclu~ing but not limited to
printed circuitry with multiple circuits that can be individually activated, and the
like.
As illustrated in Figure 13, distal portion 20 of energy delivery device
14 incll-des a thermally ine~ ting layer 70 which is subst~nti~lly impenetrable
to thermal energy. Thus, thermal inc~ ting layer 70 can be used to limit the
arnount of selected site 32 that contributes to the te~llpe.~lule detected by sensor
~2. For example, by in~ ting only distal end 18 of distal portion 20
substantially only thermal energy from fluid medi~m 30 ~dj~c~nt to selected
site 32 is con~llcted to sensor 22. Thus, the m~gnitl-de ofthe signal produced
by the sensor 22 represents substantially only the thermal energy content of
fluid medium 30 adj~c.çnt to selected site 32. Thermal energy in~ ting layer
70 can also be used in conjunction with a delivered energy jn~ ting layer 64 to
cover identical areas or different areas. Thermal in~ul~ting layer 70 can be
constructed of the same material as the delivered energy in.~ tinE layer 64.
For many applications, it is necec~ry to have distal portion 20 become
deflected. In Figure 14, a resistive heating element 72 can be positioned in an
interior lumen of energy delivery device 14 which is at least partially made of
memory metal. Resistive heating element 72 can be made of a suitable metal
that transfers heat to energy delivery device 14, causing distal portion 20 to
become deflected when the temperature of energy delivery device 14 reaches a
level that the memory metal is caused to deflect, as is well known in the art.
Not all of energy delivery device 14 need be made of the memory metal. It is
possible that only distal portion 20 be made of the memory metal in order to
effect the desired deflection. When deflection is caused by heating memory
metal, it is desirable to insulate sensor 22 from the effects of the resistive
heating element 22. One method of doing this is demonstrated in Figure 12
24
CA 0226~981 1999-03-16
W O 98/11944 PCT~US97/16120
where thermal inc~ tin~ layer 70 is located between the distal portion 20 and
sensor 22 where sensor 22is a band.
Deflection can also be accomplished meçh~ni~ lly A steering wire, or
other mech~nic~l structure, is att~çhed to either the exterior or interior of energy
delivery device 14. A deflection button 74, located on handle 12 (Figure 10), isactivated by the physician, causing a steering wire 76 (Figure 15) to tighten, and
impart an retraction of energy delivery device 14, resulting in a deflection of
distal portion 20. It will be app-eciated that other mech~nical mec.l~ ,.,g can
be used in place of steering wire 76. The deflection may be desirable for
selected sites 32 that have difficult access, and it is nec~cs~,y to move about a
non-planar collagen conl~;..it-g tissue 28. By deflecting distal portion 20, theopportunity to provide more even thermal energy to se~ected site 32 is achieved,and the possibility of ablating or dissociation of collagen material is greatly
reduced.
As shown in Figure 15, steering wire 76 att~hes to a flat formed on the
exterior of energy delivery device 14. Wire EDM technology can be used to
form the flat on energy delivery device 14. A "T" bar configuration is
illustrated in Figure 15. Chemical etching may be used to create the T bar.
Steering wire 76 need not be an actual wire. It can also be a high tensile
strength cord such as Kevlar. Steering wire 76 can be made of stainless steel
flat wire, sheet material, and the like.
As shown in Figure 16 energy delivery device 14 can be tubular in
nature with a central lumen. Distal portion 20 can include a conductive plug 78
that is sealed to distal portion 20 by welding, e-beam, laser and the like.
2~ Energy delivery device 14 can have a variety of dif~ geol,lt;llicconfigurations which can vary based on the type and shape of collagen
co.,~ in~ tissue 28 to be heated. In Figure 17, energy delivery device 14 has
an oval cross section. The oval cross section provides a greater conductive
surface 66 area that is in contact with collagen col ~ g tissue 28. A larger
zone of heating to collagen co.,l~ g tissue 28 is provided. The thermal
CA 0226~981 1999-03-16
W 0 98/11944 PCTrUS97/16120
gradient within collagen CG~ g tissue 28iS more even and the possible
dissociation or breakdown of the collagen fibers is reduced.
As illustrated in Figures 18 and 19, operating cannula 58 may include a
viewing scope 80 which may be positioned adjacP-nt to energy delivery device
14. Viewing scope 80 provides a field of view 82,pe~ ing the surgeon to
view while delivering energy to selected site 32 and contracting collagen
con~ g tissue 28. Viewing scope 80 can include a bundle of light
e fibers and optical viewing elenn~.nte Alternatively, the surgeon can
view the procedure under al~h,oscopic viell~li7.~tion.
The present invention also provides a method of contracting collagen
co~ i-lg tissue 28. The collagen co"l~ g tissue 28iS contracted to a
desired shrinkage level while ll.;~l;..,;~.;i-g cell necrosis as well as damage to
surrounding organs and other bodies. It can be used in the joints such as the
shoulder, spine, cosmetic applications, and the like. It will be appreciated to
those skilled in the art that the present invention has a variety of di~l enL
applications, not merely those specifically mentioned in this specification.
Some specific applications include joint capsules, specifically the gleno-
humoral joint capsule ofthe shoulder, herniated discs, the m~niecl~.e ofthe knee,
in the bowel, for hiatal hernias, abdominal hernias, bladder suspensions, tissuewelding, DRS, and the like.
The surgeon determines which collagen cont~ining tissues 28 require
contraction and how much shrinkage should occur. The surgeon then selects an
area of the collagen cor.~ -g tissue 28 for shrinkage. The surgeon can find
the selected area 34 by using arthroscopic viewing or using the apparatus 10
include a viewing scope 80. Once the surgeon places the energy delivery device
14 next to the selected site 32, the surgeon soon begins delivery of energy.
While embodiments and applications of this invention have been shown
and described, it will be apparent to those skilled in the art that many more
modifications than mentioned above are possible without departing from the
26
CA 02265981 1999-03-16
WO 98/llg44 PCT/tJS97/16120
invention concepts herein. The invention, therefore, is not to be restricted
except in the spirit of the appended claims.
What is claimed is: