Note: Descriptions are shown in the official language in which they were submitted.
DEC.-23'98~WED) 10:33 HILL STEADMAN P.Oll
F~ I L E~ P
r
METE101~) FOR M~ ACT~l~IN G MP.lV~T-1R ~NE ~LECT~O~)E
~ITS (ME) [~Ol~ POLYl!~ ELEC rROLYTE ~JI~.l~RRA~E (PEM)
FUEL ~,T~.T.T.~
Theinve ~ o~isd~ected ~c) a co~ ItiO,l~OUS ;~d ~llto1n~t~hle mettlod for
man11~2~t1~ne M E (m~hraneelectrodl) UrUts forPE M fuel ce~ tha~ ~ cos~-
b~n~iri1l ~nds~lit~hlPfor m ~sproducti~
I~ previously kno~n~ m~thn~c f 3 r m~mlf~1n~ nn~ elect~rod~s for fuel
cells ~C), the desi~ed electrode m~teri~l is produced in a first methnfl step, this
elect~ode nl~r~ bein~ applied ~nto th, ! electrolyte ar onco a carrier such as,
for ~mr~e~ ca~hon paper ~n a fLl~her n ethod step. The applie~rion ca~ ensue
by m Prh~nif ~ tnti~ or by appl~rir ~ a s~lsF~neinn (ink or paste) onto the
membr~e a~d c~ se~llent evaporatio~ I If the solvent.
In ~itilnll to this n~hr~rl, whe~ ~in the electrode rn~t.A-ri~l must be
m~mlf~rhlred ~d insulated in a first sta ,e before it ca~ be applied o~to the
elect~olyte (polyIner me~l~ne) i~ a sec ~nd mPthn~ stage, there aIe also
mPth~ w~erein the elec~rode m~pri~l ~s p~oduced directly on the electroly~e.
Tn~ ~ t~erein i., a Inethod wb~ rein t~e electrode m~tPn~l, for
e ~ A pl~tin~l~, is rl~I ncitecl o~ the e ectrolyce from the ~apor phase. What
is di~adv~nt~f.us ab~ut this mP~h~rl is ~ar it is also poorly sui~ed for a
2~ c~ ti~ f-~frre - due to ~e ~e luired e~ tinn p~ocess -- he~ ~se a
mem~ne ~veb cannot be drawn ~hrou~ b a vacuu~ without l~ving the
VlCUUm c~ pc~ Moreover, porous cat llyst layers arelliffi-~llt~o realize gi~en
this n.~th~)d.
Another metllod is l~own whe~ ein th~ electrode ma~erial is prof~1~cerl
2 5 Ul situ on t_e me~brane. H. T;~k~n~k~ ~nd E. Torikai et al. cli~lr.~e this in
Irlt. J. Hydrogen Erle~gy 7, 397 (1982). The ~embrane i~ thereby ~tretched
be~een tWI) half cells, sntha~itsepar~ ;es the two ele~trolyte spaces of the
half-cells f~om otle another. A salt ~ tio~ c)f ~he desired electrode m~tpri~
CA 02266868 1998-12-24
DEC. -23' 98~WED) 10:34 HILL STEADMAN P. 012
(fo~ e~cample, thus, an ~I2PtCl~ snI~ nn) is placed at the one side of ~he
~er~l~rane; d sllit~hl~ rç~ rtinn agellt is 1 Ilaced at the other side. The redtlc~ion
age~t diffuses to t~e side of tlle Pt-coPtai ling solu~ion. The encouIlter of ~erell Irt-on agen~ with the PtCI6' ions the~ eby l~ads tO d precipitation of a
pl~tirllIm layer in the susface region of tl~ e ~n~h~ne. T~is metho~l is fixed in
time by the g~ven flif~Icinn rates of ~e rl ~t~nt~ Over ~d above ~his, this
mP~hnfl must be ~pplied twice in order t~ prod~ce a rnmrlPt~ ~IE) since only
o~e side of the Inembrane carl be coated i n o~e met~ad step.
IP view of the fu~ure si~lifir~-lr~ O f all fuel cells and, in parucular, of
che PEM fuel ce~ls as energy converters o the fu~ure, there is tllus a need to
offer an improved method for the autom Ited and cost-ben~fici~ rtl~e
of the core of every PEM fuel cell, nanlel r the ME. It is t~e~efo~e an object of
the present invention cn make a method .vailable ~Vit}l which ME for PEM
$uel cells can be . .~ -.L~red in a contiP llaus and ~tom~t~hlr p~ncess.
The general ~erception of t~e inv~ nti~n i that a~ ~.~t~ly ~ick salt
c~ust or a salt film (rI~penclçnt on the amo unt of rern~ ing solvent) adhere tOthe nlembra~e web as ~ result of simply ~ i~pi~ t~e electrolyte m~ml~ranP web
into a salt solutioP of the electrade mateI ial, and that, after ~nrtis
~nC~I~d, the electrode m~tPn~I ~dheres to rbe polymer i~ The desired
o penetratioll depth a~d rnass.
Tlle çubject matter of t~e l ~ese~,lt i~vemion is a ~ethod fo~ the
c~ntinl~n~ nllfar1 ~nes [sic~ of meml ~rane electrDde units for polymer-
electroly~e memb~e (E)E~I) fuel cells, ~ here~ a poly~e~-electrolyte
me~brane passing t~ough web-sh~ped
2 5 -- is drawn such ~hroug~ a solueion Df a sal~ of ~he elec~rode ~aterial in afi~st me~od ste~ *lat the ~alt ad~ e~es to both sides of the m~mhra~e in
an amo~n~ adeq~ate for forIning :lle electrodes, and, ~e~,
- t~e 9~ the ~embran~ is redu ed tO the electrc~d~ material iIl
soIIltinn or ~ the gas stream irl a second ~ethod ste~.
CA 02266868 1998-12-24
DEC. -23' 98(WED) 10:34 HILL STEADMAN P. 013
In a~ adva~ltageo~ls development )f the invetltion, a drying s~ep for
rP~oYing the solvent tllat adlleres to the membr~ne web is also iIlterp~sed
bet~veen the ~wo mPtho~l steps.
~n prefer~ed emhoclime~t the secnnd mPthhd step, i.e. the r~t l~ir n of
t~e ~ PrinE salts, is im~l~mpnted ~ the gas stre~ and, pote~ially, under
telnperature elev~tion.
However, l~e emhnrli~Pnt is adv Lnrageous w~sein the membrane .weh
is simply ~ nr~t~ rer ~lpflerrinn rolle ~ t~rough two baths, first tb.rough
- one chat cr)nt~inc the sal~ d sllbse~Pn1 ly thr~ugh one ~at cnn~inc tbe
1~ rerll~rf;on a~ePt.
W~at are lcLr~d to as "~Pmhr~ne webs" or "electrolyte webs" ~r
~'polymer mem~ane webs" are all proces ;able webs of polyl~lers rhat con~tllrt
~rotons (or fnnr~ t hydroxide io~s ~s w ~11) rhat can be employ~ as
electrolytes nf PEM f~el cells. Let sulfor ated poly~ty~l webs o~ Nafio~
(registe~ed t~ade~ark), ~vhich is a perfluc lidated polymer, be cited here by ~ray
of e~rnrl~.
Botll the s~oo~h, proton-conduc ~g electrolyte ~embra~e as well as a
membrane partially provided with car~i~ r m ten~l or cl~rrent çnll~ctors such
as, f~r P~mrlP, carbon paper or fabric c ~ be eInployed in r~e inve~rive
2n m~thocl. GiveP~ the latter, the imme~sio I time of ~he me~brane ~h~nf~ctr~ n~lol~cly because the carrier m--~te~il 1 is ve~ pidly s~ ate~l with the salt
sol~lri~
What are refe~ed to aS "elec~rodl m~ten~1" or "electro-catalyst'~ a~e the
elec~odes sta~d~rd for PEMFC, parti~ arly precious me~ls such as, for
2 5 ~Ya~nl~lP~ pl~rin~l~n o~ ruthenium. Allo~ ~lectrodes ca~ be n~m~f~ red from
co~ .y~ iingly mixed s~lt solutio~
~7hat are rcfe~red to as 'lsnlltticln of a ~ of electrode m~te~alll are salt
~ollltlnn.c of thc a~oremPntinned sub~tances and compour~.ds, i.e., for ~Y
the ~nlll~on of a ~ t~ s;llt witll Pt~ io~s such as a 2-molar sol~-tinn of
CA 02266868 1998-12-24
DEC. -23' 98~WED) 10:34 HILL STEADMAN P. 014
hP~hIoropl~tin~te in ~vater. Of caurse, Pr~ r or arbitrary mixtures of metal
salrs such as, for e~rnrI~, a ~ ure of Pt !~ salts wi~ll Ru3~ salcs for prnAIlrir~e
P~JRu catalysts for ~e direc~-me~anol f~ el cell c~n be present. Neit~er the
salt cited hero l~y ~y of ~ rIP nor s~uc metal or solvent are ~nterlded ta
limit ~e scope c~f the inventiorl.
T~e salt cnnff..l,d~ioP prevailin~ he solution is, of course,
~lPpen~nt OII the desired thickn~cc or per etr.ltinn depth of the salt crust a~
well as on the ilrea that the elec~ode shou Ld occupy on the electrolyte web.
- The of~rnrqrinn of t~e ~embrane ~vith sal t can be corltrolled by the
con~ t~"ion of the salt 5nllltinn
In general, the web is pulled throu ~ the salt solution with a speed ~ O;
ho~v~., it i~ also possible rhat r~le web d wells in the salt soI~Itinn, i.e. rhat the
~nnrinllQU~ d aurom~t~ process rcpeatl dly prn~rides ho~ ne times. The
pene~ration depth ~f the electrode materii 1 in the membri~e (depth
distribution of l:he electr~-caralyst in the pnlymer elec~rolyte caIl be controlled
via the dwell time of rhe membr~e in rh~ salt bath
The prope~ies of the m~nlIfnftllre ~ ME can ~e varied i~ a ~road r~ge
by ehe s~table 5t~ nn of ~he reaction c~Inrlitinnc (salt, re~ nn agent,
~olvent, thixotropy, viscosiey, pH value, n~cpntr~ nc~ bath aIld a~bient
2 o temperature) and ~y ~ litinr~ of addi~ves (fl~r ~Yam~1P. bi~de~s). T~is
variatio~ is also rnccihI~ by ~nIhsequrn~ d~ ter-t~P~tn~nt of the n~p:~1
elec~rolyte polymer~e~ such a~ addi~g c lrre~t ~ oIl~cthr.~ (for ~mrI~, carbo
fabnc), pressillg, imp~PgJl~tinn, tempen~, ar t~e like.
All st~ qr-1 rech~ti nn agents thal leave the membr~e n~ ~teri~ d the
n~ cll~red ME .~n~ n~g~-l (specifieal] y i~ view of p~ nnin~), t~at can ~e
removed 11~e free ~d ~hereb~r quami~ ativel~ reduce the adheri~g salt under
op~ima~ly Inild ~nr~ t~ c are s~litahIP fc r the re~ tiQn of the s~t ~ nn~ to
cml~ra~l:. F~r~mrl~s :~o~ for insr:~n ~, hydrogen or clj~
CA 02266868 1998-12-24
DEC. -2~' 981WED) 10:35 HILL STEADMAN P. 015
The inventian is described i~ grea er detail below on the basis of the
t~vo Figures. The aforerrlentinn~f~ defir~t ~ons are valid rlot only far rhe
~p~rif~r~ion and cla~ms but also for the tl ImS employed in the PYF~I~ni~ri¢lr~ o~
the Figures.
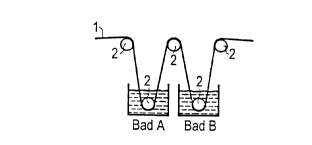
5 Fi~u~e 1 shows t~e pl e~el ~d embod iment of the method wherein t~e
occlIp~tinn of the m~m~r~I e web with salt e~sues in a bath A
and t~e ref~--ction of the ad lerirlg salt e~sues ~n a further ~ath B.
Figure 2 shows ~nc-th~r preferred en Lbodiment of the ill~entiorl wherein
the membrane ~reb I.s first ~ pi~cl with salt in a bat~ A and the
adhe~ng salt is then reduce ~ in t~e ~gas stream.
First, the polymer-elPctr~.Iyte mesnb~e ~reb 1 passillg rhroug~ web-
like can be see~ ~ Fi~ure l. Via a fi~st de ~lecticn ~ol~er 2, the ~embra~e web
proce~eds i~o the bath A in whieh the sal~ ~r the salt ~ixture is fou~d in
illert solve~t, prefer~bly water or some 01 ~er solvellt wi~h a hig~ pctrir
co~stant. Upon passage throllgh the bat~. A, the m~mhr~P is oco~piP~ with
the salt fraIn Ihe snhltif~n on both sides, ~ nd a salt film or a salt cmst is on
both sides af the web 1 w~erl the web 1 e ~erges from the l~alh A. The web 1
covered with salt is rn~ ct~l over the n, xt ~lefl~ tif~n roller 2 i~to the bat~ B
i~ ~vhich the rPrIuctinn age~ is C~ t~ ke~vise in a~ i~ert solvent. The salt
2 o adher~ng to the web 1 is thereby reduced whe~ passing t~rau~h the bath B.
~ia the la~t deflectian rolle~ 2 at t~e extn ~e right end of ~ re l, the web 1
proceeds ~s finis~ed ME in~o furt~er pro :essi~g such as, far e~ p~, illtO a
~uttirlg m Irhin~ Later, ~he eleclrode s~r Icn~re ca~ be opti~ni7.~ with a press,
potentially with follo~in~ thermal t~edtl ~ent.
2 5 E;igure ~ likewise shows a ~eb of poly~ner-electroly~e membra~e 1
passin~ ~hrough ~N~b-likc that is stec~cd ~ efl~ctlnn rnlle~ 2 inta, firs~. rhe
bath A thal, as in Figure 1, contains the ,alt of the elec~rode material in
CA 02266868 1998-12-24
DEC.-23'98~WED) 10:35 HILL STEADMAN P.016
soluuan. Upon passage t~rough the L~atll A, a s~lt crust forms on the
membrane web, and, after leaving ~he bath A, the encn~sted me~ane web is
pulled thr~ugh a vessel 3 with reclucing at nosphere th~t potentially has a
h~t.~g device av_ilable to it. Gia~eous rec uction agent ca~ fla~r mw the vessel:~ with re~ ine ;~tTnr~sphf~rp via the ad~is lon npenin~ 4. When it emer~ es
from the vessel ~, both sides of the memb a~le web are again coated ~vith
electrode r~t~
In the em~ndimptlt ~vhere~n the re l~lction of the salt cmst on the
memb~ane web ensues in the gas stre~ is advantageous th;at tlle mi~ing of
0 ~he contenes of both ba~hs i.5 avoided in bi th B. In che embnrlimpnt acco.. ~lg
to Fi~ure l, ho~vever, this cdn also be achi ~ved in that a drying n~prhqnicln
such as, forPY~mrl~, a ventilato~ or farl t~ ~r [. .] at the level ~f the ~ pctinn
roller 2 that dries the ~eb from both side! ~nd rids rhe salt cmst of ~olvent ixi~terposed ~etween rhe eme~gerlce from t le bath A and the en~ry of the
ITIPmbr7lnp web i~to the h~th B.
The in~entive methad is clistin~ hed in that both sides of the
memb~e c~ be s;~nult~np~ usly coverec ~it~ an electroch~ y active
eleet~ode layer in a ~ ntin~ c prc~cess an d, thus, ~n ME a~ses in a sin~le
pro~sstep. ~li~ m~n~1f~nl~n~ speeds ~iven lo~v costs can thu~ be achiev~d
by the elimin~ti~n of ~ic~onn~l~ous wor~:steps ~ we~ as t~ne~ ni~g~
diffusiDn-limited steps.
, . , .. . . , . , ~
CA 02266868 1998-12-24