Note: Descriptions are shown in the official language in which they were submitted.
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
ENCAPSULATED SALT PARTICLES FOR USE
IN BAKING YEAST-RAISED BAKERY PRODUCTS
CROSS REFERENCE TO RELATED APPLICATION
This application is a continuation-in-part of copending U.S. patent
application
08J378,166, filed January 25, 1995, which is a continuation-in-part of U.S.
patent
applications S.N. 08/148,712, filed November 8, 1993, now abandoned, and S.N.
08/206,378, filed March 7, 1994, and now abandoned.
FIELD OF INVENTION
The invention is directed to a salt composition for use in baking yeast-raised
bakery products. In particular, the invention is directed to an encapsulated
salt
composition for use in baking bromate-free bakery products, such as bread.
BACKGROUND OF THE INVENTION
Most bread is made commercially in the United States by either of three basic
procedures: ( 1 ) the straight-dough method; (2) the sponge-and-dough method;
or
(3) the liquid-sponge method. In the straight-dough method, all of the
essential
ingredients of the bread (flour, yeast, salt and water) are mixed together in
a single
step to from a dough which is fermented, placed into individual pans, proofed,
and
baked. In the sponge-and-dough method, the yeast, water and 50-70% by weight
of the flour are formed into an initial dough, which is referred to as the
"sponge."
The sponge is fermented for 2-4 hours after which the remaining potion of the
flour, salt, and secondary additives are added to form a final dough. The
final
dough is then placed into individual baking pans, proofed, and baked. The
liquid-
sponge method differs from the sponge-and-dough method mainly in that the
sponge is of liquid consistency, and contains I O-60% by weight of the total
flour.
[The term "proofing" or "proofed" refers to the practice of subjecting dough
to
storage for about one hour at a temperature of 90-130F (27-54C), and high
CA 02268932 1999-02-19
WO 98/07324 PCT/LTS97/14509
humidity (60-90% rh) in order to restore the extensibility and aeration of the
dough prior to baking.] These are batch processes.
In addition to the foregoing dough-making methods, which are batches in
nature, a
S considerable volume of breadmaking is carried out using continuous dough
mixing systems. These methods are characterized by the preparation of a
pumpable liquid preliminary admixture (preferment) in which the yeast is
activated to its maximum degree of fermentation in the presence of part of the
flour and/or sources of assimilatable nitrogen with careful adjustment of pH.
The
fermented admixture, which may contain as much as 90% weight of the total
flour
content of the bread, is mixed on a continuous basis with the remaining flour
and
other dough ingredients to form an homogeneous dough. The homogeneous
dough is then intensively kneaded under pressure and anaerobic conditions to
form a degassed dough. The kneaded dough is deposited directly into baking
pans
1 S on a continuous basis. The so-called "continuous-brew method" is an
example of
such continuous systems in which the preferment contains no flour, and the
total
flour content is incorporated during dough formation.
In addition to the essential four dough components, it is customary to add one
or
more secondary additives, which are optional. The use of these materials is in
large a part a function of the particular bread being made. Such secondary
additives include yeast food, sweeteners, shortening, dairy blend, protease
enzyme., emulsifiers, dough strengtheners, preservatives, gluten, etc. For
example, a typical bread may contain as secondary additives all of the
following:
high fructose corn syrup, wheat gluten, soybean oil, calcium propionate,
potassium bromate, vinegar, ammonium sulfate, calcium sulfate, ascorbic acid,
and sodium stearoyl lactylate.
Among the most commonly used and preferred secondary additives are oxidizing
agents such as potassium bromate (KBr03), which when added to the dough at
levels up to 75 ppm by weight, reacts with the gluten, or protein, fraction of
the
2
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
wheat to improve the strength and resiliency of the dough. A substantial
portion
of this strengthening action occurs in the first several minutes the bread is
in the
baking oven as increased temperature accelerates the action of potassium
bromate.
Also, during this first portion of the baking process, the dough expands
considerably in volume due to accelerated gas production by the yeast, and
expansion of the contained gas with increasing temperature.
The strengthening action of potassium bromate works in conjunction with this
volume expansion to "set" the structure of the dough into a loaf of desired
volume
and consistency. This synergistic action is especially valued in modern
automated
production lines where mechanical shock can cause a reduction in dough volume
prior to entering the baking oven. This is especially true for bakery
products, such
a hamburger buns, which have a relatively short time in the baking oven, e.g.
7-10
minutes, as compared with 25-28 minutes for pan bread. Therefore, breads which
do not contain potassium bromate, or an equivalent oxidizing agent, tend to
have
poor volume, weak crust, poor symmetry and uneven grain and texture.
However, recent studies in Japan and the United Kingdom indicate that
potassium
bromate may not be completely converted to harmless potassium bromide during
the baking process. Moreover, it is believed that residual amounts of bromate
may
be carcinogenic. Therefore, the use of potassium bromate as a component of
bread is being curtailed or even discontinued.
For the above reasons, there is a need for a convenient, safe and effective
means of
replacing potassium bromate in yeast-raised baked goods. In this regard,
ascorbic
acid (Vitamin C) has been mentioned. Though the functions of ascorbic acid in
baking are the same as potassium bromate, it has the significant disadvantage
that
it is substantially decomposed by the moisture, oxygen, trace metals, and pH
conditions present during mixing and proofing, leaving little or none
remaining to
work with the volume expansion that occurs in the oven. This makes it
unsuitable
as a total replacement for potassium bromate.
3
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
In the technology of baking bread, salt has the primary purposes of flavor
enhancement and strengthening the gluten structure that serves to give bread
its
shape. However, it is well known that salt has the disadvantages of
interfering
with gas separation by yeast, and, through its dough-strengthening effect,
limiting
the extent to which the dough may rise. This is demonstrated in the common
practice within the baking trade of waiting until the final portion of the
dough
mixing step to add salt, as it substantially increases the energy required to
achieve
a uniform dough. The yeast inhibitory effect occurs at salt concentrations
above
approximately 1.5%, basis flour. Most commonly, salt is added to a 2%
concentration.
For these reasons, there has been a substantial need for a potassium bromate
replacement product which will (1) increase the volume of the proofed loaf by
reducing the effect of salt upon the yeast, (2) add ascorbic acid and salt in
such a
manner so that they can be released slowly during proofing, and rapidly in the
oven allow the retention of the increased dough volume, and (3) release the
bulk
of its contained salt and ascorbic acid in the early stages of baking to
support the
desirable volume expansion, and repair the effects of mechanical shock.
In particular, it has been found that in some yeast-raised breads, which
undergo
rough handling before baking, greater loaf height is needed. An example of
such
breads is white dough bread for making buns.
SUMMARY OF THE INVENTION
In a primary aspect, the invention is therefore directed to a particulate
composition
for use in baking bromate-free yeast-raised bakery products comprising a
particulate core of crystalline sodium chloride, having a maximum dimension of
100-500 micrometers, encapsulated with an inert, water-resistant thermoplastic
shell, having a thickness of 10-300 micrometers, and a release temperature of
100-
300F (38-149C), the shell having randomly dispersed therein 1-10% by weight,
4
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
basis total particulate composition, of finely divided particles of ascorbic
acid,
having bimodal particle size distribution, in which 50-80% by weight of the
ascorbic acid particles are 1-I00 micrometers in size, and 50-20% by weight of
the
ascorbic acid particles are 200-400 micrometers in size. Preferably, the
composition also contains 1-8% by weight of finely divided particles of a
leavening agent selected from bicarbonates of Li, Na, K, NH4, and mixtures
thereof.
In a secondary aspect, the invention is directed to a dough composition for
use in
baking bromate-free, yeast-raised bread, comprising an admixture of flour,
salt,
yeast, water, and the above-described encapsulated salt composition, in which
the
weight ratio of unencapsulated salt in the dough to encapsulated salt in the
particulate composition is 1:1 to 9:1, and the encapsulated ascorbic acid
constitutes 2-220 ppm by weight of the flour component of the dough.
In a further aspect, the invention is directed to a method for baking a
bromate-free,
yeast-raised bread by the straight-dough method, comprising ( 1 ) formation of
a
dough, comprising an admixture of flour, water, free salt, and yeast, (2)
fermenting the dough, (3) dividing and placing the fermented dough into
individual pans, (4) proofing the fermented dough, and (5) baking the proofed
dough, characterized in that the above-described encapsulated salt composition
is
added to the dough, fermenting the dough in such proportions that the weight
ratio
of unencapsulated salt in the particles is I :1 to 9:1, and the encapsulated
ascorbic
acid constitutes 2-200 ppm by weight of the flour content of the dough
In a still further aspect, the invention is directed to a method for baking a
bromate-
free, yeast-raised bread by the sponge-and-dough method, comprising { 1 ) the
formation of a sponge, comprising an admixture of flour, water, and yeast, the
sponge containing I O-70% by weight of the total flour content of the bread,
(2)
fermentation of the sponge, (3) formation of a dough by admixing salt,
secondary
additives and the remainder of the flour with the fermented sponge, (4)
proofing
CA 02268932 1999-02-19
WO 98/07324 PCT/US97I14509
the dough and (5) baking the proofed dough, characterized in that the above-
described encapsulated salt composition is added to the fermented sponge or
dough in such proportions that the weight ratio of unencapsulated salt in the
dough
to encapsulated salt in the particles is 1:1 to 9:1, and the encapsulated
ascorbic
acid constitutes 2-220 PPM by weight of the flour content of the dough.
BRIEF DESCRIPTION OF THE DRAWING
The Drawing consists of two figures, which are schematic representations of
the
encapsulated salt composition of the invention. Figure 1 depicts the
composition
I O of the invention, in which ascorbic acid alone is contained in the
encapsulating
shell. Figure 2 depicts the composition of the invention, in which both
ascorbic
acid and a leavening agent are contained in the shell.
DETAILED DESCRIPTION OF THE INVENTION
A. In General: Applicants have discovered that superior results are obtained
with
the product of the invention because a minor amount of the ascorbic acid
contained in the encapsulating shell is released during the latter part of the
proofing step, while the rest of the ascorbic acid is released quite early in
the
baking oven. The minor amount of ascorbic acid released in the proofing box
allows for additional rise to the fermenting dough, but does not perceptibly
interfere with the action of the yeast, so long as the release takes place
later in the
proofing process and is limited in quantity. However, it is essential that the
remainder of the ascorbic acid be released rapidly early in the baking oven,
preferably within the first four minutes. In particular, the ascorbic acid
must be
completely released before crushing of the bread takes place. On the other
hand,
most of the salt release takes place after the ascorbic acid is substantially
completely released into the heated dough.
The attainment of both limited release of ascorbic acid in the proofing box
and
rapid release of the ascorbic acid in the baking oven is realized by
incorporating
the ascorbic acid in two particle size modes. Basically, the ascorbic acid
should
6
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
be comprised of 50-80% by weight, smaller particles having a particle size of
1-
100 micrometers, and 50-20% by weight, larger particles having a particle size
of
200-400 micrometers. Some of the ascorbic acid particles will, in many
instances,
be outside these ranges of size. However, so long as those within these ranges
are
present in suitable amounts, the admixture of such diverse particles will be
suitable for use in the invention. It is preferred that the smaller sized
particles
constitute 60-70% by weight of the admixture, and the larger sized particles
constitute 40-30% by weight of the admixture.
Even though the shell material is at least water-resistant, and preferably
water
insoluble, a small amount of the ascorbic acid is nevertheless released in the
proofing box as a result of diffusion of moisture, fats and oils from the
dough.
This is caused by defects at the interface of the large ascorbic acid
particles, and
the shell material, as well as incomplete encapsulation of some of the
particles. In
addition, some softening of the shell material may take place at the proofing
temperature (ca. 125F, 52C).
Though some softening of the shell material may take place upon contact with
the
fats and oils in the dough, there is nevertheless no major release of ascorbic
acid
during the proofing step. Because the action of the yeast is completed by the
end
of the proofing step, and the ascorbic acid release is minor, neither ascorbic
acid
nor the salt interferes with the action of the yeast.
As the shell softens upon exposure to the higher temperature in the baking
oven,
the larger particles, more of which lie at or near the surface of the shell,
are
released. As the temperature of the shell rises, the large particles are
completely
released, and are followed by the slower release of the smaller particles.
The temperature of most commercial baking ovens is on the order of 375-450F
(278-234C). Therefore, to assure that the shell material does not melt before
the
oven, it should have a melting point well above the temperatures encountered
in
7
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
the proofing step. Therefore, a melting point of at least 1 SOF (66C), and
preferably at least 200F (93C) is required. Thus, the shell materials for use
in the
invention will ordinarily have a melting point of 100-300F (43-149C), and
preferably 150-250F (66-127C). It should be noted here that the temperature
within the bread does not reach the oven temperature because of the
evaporation
of water from the bread within the oven.
It is preferred to introduce the encapsulated particles of the invention into
the
dough mixture just before going to the proofing box; they can nevertheless be
added to the sponge and dough before proofing, since no ascorbic acid is
released
during mixing of the sponge and dough.
B. Bread Components and Additives: Except for the encapsulated salt
composition of the invention, the components of the bakery products in which
the
invention can be used are conventional, and thus well known in the art. For
example, the basic constituents of breads are flour, yeast, salt and water.
However, as discussed herein above, most breads contain one or more secondary
additives such as yeast food, calcium propionate, sodium stearyl lactolate,
vitamin
C (ascorbic acid), sugar, honey, syrups, baker shortenings, dairy products,
egg
products, etc. The presence or absence of such secondary bread additives other
than those claimed therein, is not critical, with respect to the operability
of the
invention. That is, the invention is effective in a wide variety of yeast-
raised
bakery products, whether or not they contain any or all of such materials. In
addition to bread, the invention can be used in other yeast-raised bakery
products
such as rolls, doughnuts, frozen doughs, and Danish pastries.
C. Encapsulant Shell Material: A wide variety of organic thermoplastic shell
materials can be used in the invention, so long as they are suitable for
direct
addition to foods. Thus, the composition of the shell component of the
invention
must be a solid at ambient temperatures, be chemically inert in the presence
of all
the bread components, be suitable as a food component, and have suitable
melting
8
CA 02268932 1999-02-19
WO 98/07324 PCTlUS97/14509
properties, so that it is released at the appropriate temperature, and be
water
resistant at proofing temperatures. Water solubility is still further
preferred.
Such materials include vegetable fats such as mono,di- and tri-glycerides;
vegetable oils and wax blends therewith; animal fats such as lard, and beef
tallow;
blends of animal and vegetable fats, and hydrogenated derivatives of such fats
and oils. Also included are waxes, such as beeswax, candelilla wax, paraffin
wax,
and microcrystalline wax. Other suitable materials are polysaccharides, such
as
gums, gelatins, alginates, and modifications thereof. These include natural
polymers, such as carboxymethylcellulose, cellulose acetate phyalate,
ethlycellulose, gelatin, gum arabic, starch, succinylated gelatin, proteins,
alginates.
Other synthetic polymers which can be used as shell materials include
polyvinyl
alcohol) and polyvinyl acetate). Such materials are selected on the basis of
their
melting points and release characteristics in particular applications.
Mixtures of
such shell materials can also be used to obtain particular combinations of
physical
properties.
The amount of ascorbic acid or precursor thereof dispersed in the shell
relative to
the volume of the shell material (shell loading) is not critical with respect
to the
functionality of the invention in ordinary baking applications. However, it
has
been observed that the release of ascorbic acid at equivalent temperature
conditions tends to be faster when the volume of ascorbic acid is higher, than
when the volume of ascorbic acid is used. Thus, the loading level of ascorbic
acid
in the shell is likely to have an effect on release time.
D. Bicarbonate Leavening Agent: Especially in situations when the dough is
subject to severe shock as it is conveyed to the oven, it is preferred that
the
composition of the invention contains I-10% by weight of a leavening agent.
Preferred leavening agents are bicarbonates of Na, Li, K, NH4, and mixtures
thereof. Of these, sodium bicarbonate is preferred. Unlike the ascorbic acid,
the
particle size of the bicarbonate is not so critical. However, it is preferred
that the
9
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
bicarbonate be released entirely and quickly in the front part of the baking
oven.
Therefore, it will usually be preferred to use finely divided particles of
bicarbonate
within range of 1-500 micrometers, and preferably 1-200 micrometers.
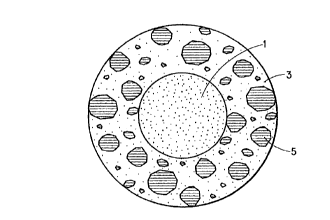
E. Formulation and Microencapsulation: The structure of the encapsulated salt
particles of the invention is illustrated by the single figure of the Drawing,
which
is a schematic representation of the particles. In particular, a crystalline
particle of
salt (1) is encapsulated within a thermoplastic shell (3), in which are
dispersed
finely divided particles of ascorbic acid (5) and sodium bicarbonate (7). It
is
preferred that the salt particles which are used in the invention have a
maximum
dimension of no more than 220 micrometers, so that they can be easily blended
and dispersed in the fermented dough. On the other hand, it is preferred that
the
salt particles have a minimum dimension no smaller than 100 micrometers,
because such small particles are more difficult to encapsulate satisfactorily.
It is
further preferred that the maximum dimension of the salt particles be in the
range
of 125-300 micrometers.
The invention has been developed primarily for use with sodium chloride,
because
of its overwhelmingly greater use. ~ Nevertheless, the invention is also
applicable
to the use of other flavoring salts, such as potassium chloride and calcium
chloride, as well as mixtures thereof with sodium chloride.
It is preferred that the thickness of the organic shell, in which the salt
particles are
encapsulated, be at least 10 micrometers, and still more preferably at least
20
micrometers, to be assured that the coating is substantially continuous, and
that it
contains few holes. However, the shell thickness should not exceed 300
micrometers, and preferably 200 micrometers, lest the encapsulated particles
become less granular in character, and thus are not free flowing. It is, of
course,
preferred that the particles be free flowing in bulk, so that they can be
dispersed
more easily in the dough.
CA 02268932 1999-02-19
WO 9$/07324 PCT/US97/14509
The ascorbic acid and bicarbonate are preferred to be of particle size such
that they
do not exceed about half the thickness of the shell, and thus can be randomly
dispersed throughout the shell. Though randomly dispersed ascorbic acid and
bicarbonate particles can be at the outer surface of the shell, it is
preferred that the
ascorbic acid particles not protrude, because too many protruding particles
would
result in too rapid release during the dough fermentation. On the other hand,
it is
preferred that the bicarbonate particles be of sufficient size and quantity,
so that
they protrude in order to facilitate early release. It is also preferred that
the
particles in the shell not be smaller than 0.5 micrometer, because they are
difficult
to handle. Therefore, the particles dispersed within the organic shell well be
0.5-
400 micrometers in size. As set out above, to obtain an optimum effect by use
of
the invention, it is preferred that the ascorbic acid particles be present in
a bimodal
particle size distribution. In particular, it is preferred that 50-80% by
weight of the
particles have a size of 1-100 micrometers, and 50-20% by weight of the
particles
have a size of 200-400 micrometers. It is still further preferred that the
finer
particles constitute 60-70% by weight, and the smaller size particles be 40-
30% by
weight of the ascorbic acid particles in the shell of the encapsulated salt
composition.
It will be appreciated that ascorbic acid derivatives, which are similar to
ascorbic
acid, can be used in the invention, as well as ascorbic acid itself.
Therefore,
compounds such as sodium ascorbate, calcium ascorbate, ascorbyl palmitate,
erythorbic acid and sodium erythorbate may also be useful in the practice of
the
invention. The term "ascorbic acid" as used in the claims is therefore
intended to
include such similar ascorbic acid compounds.
The required release temperature of the organic shell material is a function
of the
proofing and baking temperature. Since the shell materials for use in the
invention
are heat-reieased, the melting point of the shell material must be higher than
the
proofing temperature. In particular, it is preferred that the shell release
temperature be at least 25°F (14°C) higher than the proofing
temperature. Thus, if
11
CA 02268932 1999-02-19
WO 98107324 PCT/US97/14509
proofing is carried out at 1 OOF (31 C), the release shell temperature should
be at
least 125F (38C), and preferably still 150F (64C). (As used herein, the terms
"release temperature," and "melting point" are used interchangeably.) For most
applications, the shell release temperature should be 125-300F (52-I49C), and
preferably 150-250F (66-121C).
The amount of ascorbic acid in the shell of the invention particles should be
1-
I 0% by weight, basis total particle weight. If substantially less than 1 % is
used,
the oxidative effect is insufficient and the dough will lack strength and have
low
loaf volume. On the other hand, if more than 10% is used, the oxidative effect
is
excessive, and loaf volume may be diminished.
The amount of metal bicarbonate in the shell should be at least 1 % by weight,
basis total particle weight, to obtain a technical effect, and preferably at
least 2%.
No more than 10% bicarbonate should be used in order to avoid adversely
affecting the taste of the bread. Preferably, no more than 6% bicarbonate
should
be used. In white bread, 4-5% bicarbonate appears to be optimum.
The amount of bicarbonate in the shell on a molar basis should be about the
same
as the amount of ascorbic acid. The reason for this is that the acid moiety of
the
ascorbic acid serves as a reagent for decomposition of the bicarbonate with
the
concomitant release of C02. The release of C02 is believed to be an essential
feature of the bicarbonate functionality in the invention.
Though not essential for the practice of the invention, it will be recognized
that the
shell can have additional secondary additives dispersed therein, for example,
other
oxidizing agents, sodium diacetate, calcium propionate and the like. However,
it
should be noted that use of the invention in bromate-free doughs also
eliminates
the need for such secondary additives as azodicarbonamide and enzymes.
12
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/145U9
Microencapsulation of the salt can be carried out by any of several
conventional
microencapsulation methods. A preferred method for carrying out the
encapsulation involves the steps of ( 1 ) admixing the salt particles into the
molten
shell materials, (2) adding the ascorbic acid and bicarbonate to the admixture
of
salt and shell material, and (3) cooling the final admixture to create coated
granules which are free flowing. Another technique is use of a fluidized bed.
More particularly, the ascorbic acid and bicarbonate are suspended in the
molten
shell material, (2) the salt particles are fluidized, and (3) the molten shell
material
containing ascorbic acid and bicarbonate is sprayed into the fluidized salt
particles. A still further technique is centrifugal extrusion, as developed by
the
Southwest Research Institute, San Antonio, TX. In the Examples which follow,
the encapsulated salt particles were prepared in the following manner:
{ 1 ) Hydrogenated cottonseed oil was melted in a jacketed mixing tank;
(2) Fine flake salt was added to the molten cottonseed oil with stirring to
obtain a
uniform dispersion of the salt in the oil;
{3) While maintaining stirring, ascorbic acid having an average particle size
of 3
micrometers, and U.S.P. powdered NaHC03 were added to the oil/salt dispersion;
and
(4) The admixture of oil, salt, ascorbic acid and NaHC03 was slowly cooled
until
the product granulated. The granulated material was then removed from the
vessel, and screened through a 20 mesh (U.S. Standard) screen.
Ordinarily, it is preferred that the individual particles in bulk be free
flowing.
However, in some instances, it will be desirable to utilize the particles in
the form
of agglomerated particles or tablets. In those instances, a plurality of
particles is
agglomerated or tabletted by means of a lower melting binding agent.
13
CA 02268932 1999-02-19
WO 98/07324 PCT/LTS97/14509
EXAMPLES
Example 1
A quantity of encapsulated salt particles in accordance with the invention and
containing by weight 75% fine flake salt, 23% cottonseed oil flake, and 2%
ascorbic acid was prepared by the following procedure:
1. A jacketed vessel was loaded with the cottonseed oil flake and the vessel
was
heated to 90-95C to melt the oil flake; 2. the fine flake salt was added to
the
molten cottonseed oil, and the mixture heated to 100-1 lOC for 5 minutes;
3. The heated admixture of oil and salt was mixed at 85C for 15-30 minutes,
after
which the temperature was lowered to 60C;
4. Finely divided particles of ascorbic acid were added to the oil and salt
dispersion, and the admixture cooled to 30-32C with continuous agitation; and
5. The cooled admixture was screened through a 20 (U.S. Standard) mesh screen.
Figure 1 illustrates encapsulated salt particles made by the method of Example
1,
in which a particle of salt (1) is encapsulated within a shell of hydrogenated
cottonseed oil flake {3), and a bimodal mixture of ascorbic acid particles (5)
is
distributed in the cottonseed oil shell (3).
Example 2
In a commercial baking line for making whole wheat bread by the sponge-and-
dough method, 845 pounds of sponge were prepared containing bromate-free
whole wheat flour, wheat gluten, water, yeast food, sodium stearyl lactate,
creamed yeast, and ascorbic acid tablets. After fermentation, the remainder of
the
dough components and encapsulated particles made by the method of Example 1
was formed into a second dough, which was mixed into the sponge. The
additional dough components were bromate-free whole wheat flour, water
14
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
soybean oil, sugar, unencapsulated salt, particles of the composition of the
invention containing salt and ascorbic acid, honey, vinegar, calcium
propionate,
and wheat gluten. The encapsulated salt was equivalent to 0.5% by weight, and
the encapsulated ascorbic acid was equivalent to 200 ppm, basis dry flour
weight.
The weight of the final dough was 1461 pounds. After panning and proofing at
90F and 85 rh, the dough was baked at 450F (375C). The resultant bread
prepared
in accordance with the invention was found to be fully equivalent in every
property with the bread, prepared by the control method for baking this bread.
The control method differed from the experimental run, in that the dough
contained potassium bromate, and free salt replaced the encapsulated salt and
ascorbic acid.
Example 3
In a commercial baking line for making white bread by the sponge-and -dough
1 S method, I ,184 pounds of sponge were prepared, containing bromate-free
white
wheat flour, water, yeast, shortening, softener, yeast food, and ascorbic acid
tablets (44 ppm by weight, basis flour). After fermentation, the remainder of
the
dough components and encapsulated particles made by the method of Example 1
were formed into a dough, and mixed into the sponge. The additional dough
components were white wheat flour, water, whey, unencapsulated salt, particles
of
the composition of the invention containing salt and ascorbic acid, dough
conditioner, syrup, inhibitor, yeast, and sodium stearyl lactate. The
encapsulated
salt was equivalent to 0.5% by weight, and the encapsulated ascorbic acid was
equivalent to 140 ppm, basis dry flour weight. The weight of the final dough
was
1.934 pounds. After panning and proofing at 90F (32C) and 85 rh, the dough was
baked at 400-450 {204-232C). The resultant bread was found to be fully
equivalent in every property with the bread prepared by the control method for
baking bread. The control method differed from the experimental run in that
the
dough contained potassium bromate, and free salt replaced the encapsulated
salt
and ascorbic acid.
CA 02268932 1999-02-19
WO 98107324 PCT/US97/14509
Example 4
In a commercial baking line for making white bread by the sponge-and-dough
method, 1,191 pounds of sponge were prepared containing bromate-free white
wheat flour, water, yeast, shortening, softener, yeast food, and ascorbic acid
S tablets (44 ppm by weight, basis flour). After fermentation, the remainder
of the
dough components and encapsulated particles made by the method of Example 1
were formed into a dough and mixed into the sponge. The additional dough
components were white wheat flour, water, whey, encapsulated salt, dough
conditioner, syrup, inhibitor, yeast, sodium stearyl lactate, and ascorbic
acid
I 0 tablets. The encapsulated salt was equivalent to 0.5% by weight, and the
encapsulated ascorbic acid was equivalent to 99 ppm, basis dry flour weight.
The
weight of the final dough was 1,946 pounds. After panning and proofing at 90F
(38C) and 85 rh, the dough was baked at 400-450F (204-232C). The resultant
bread was found to be fully equivalent in every property with the bread
prepared
15 by a control method for baking the same bread. The control method differed
from
the experimental run in that the dough contained potassium bromate, and free
salt
replaced the encapsulated salt and ascorbic acid.
In most commercial baking operations, the oven temperature of the baking step
is
20 400-450F (204-232C); however, the baking temperature for some baked goods
may be as low as 350F (177C), depending on the baking time, and the physical
characteristics of the baked products in question.
The ratio of unencapsulated salt to encapsulated salt may vary according to
the
25 particular baking operation in which the invention is used. In some
instances, the
weight ratio of the unencapsulated salt to encapsulated salt may be as low as
1:1,
but is usually preferred to be at least 1.5: I . Nevertheless, the weight
ratio of
unencapsulated salt to encapsulated salt should not exceed 4:1, and preferably
no
higher than 3.5:1. A particularly preferred ratio for most bread applications
is
30 3.5:1.
16
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
Example 5
Four batches of encapsulated salt particles in accordance with the invention
were
made by the following procedure.
1. A jacketed vessel was loaded with the hydrogenated cottonseed oil flake,
and
the vessel was heated to 85-90C to melt the oil flake;
2. The fine flake salt was added to the molten cottonseed oil, and the heated
admixture of oil and salt was mixed at 85-90C for 15-30 minutes, after which
the
temperature was lowered to 60C;
3. Finely divided particles of an admixture of ascorbic acid and sodium
bicarbonate were added to the oil and salt dispersion, and the admixture
cooled to
30-32C with continuous agitation; and
4. The cooled admixture was screened through a 20 (U.S. Standard) mesh screen.
The composition of the particles in the four batches was as follows:
Table 1
Example No. SA ~ 5B ~ 5C I SD
Component % wt. % wt. % wt. % wt.
Fine flake 71 71 71 71
salt
Hydrogenated24 26 25 23
cottonseed
oil
Ascorbic 1 l 2 2
acid
Sodium 4 2 2 4
bicarbonate
Figure 2 illustrates encapsulated salt particles made by the method of Example
5,
in which a particle of salt (1) is encapsulated within a shell of hydrogenated
17
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
cottonseed oil flake (3}, and a mixture of bimodal ascorbic acid particles
(5), and
sodium bicarbonate particles (7) is distributed in the cottonseed oil shell
(3).
Example 6
In a commercial baking line for making white bread hamburger rolls by the
sponge-and-dough method, 882 pounds of sponge were prepared containing
bromate-free white wheat flour, water, yeast, emulsifier, azodicarbonamide and
enzymes. After fermentation, the remaining dough components were formed into
a dough and mixed into the sponge. The additional dough components were white
wheat flour, water, sugar, shortening, calcium propionate, sodium propionate,
calcium sulfate, dough conditioner (sodium stearyl lactylate),
azodicarbonamide,
emulsifier, and 6 pounds of encapsulated salt particles per Example SB (75 ppm
by weight ascorbic acid, basis flour). The encapsulated salt was equivalent to
0.5% by weight, basis dry flour weight. The weight of the final dough was
1,424
pounds. After panning and proofing at 90-115F (32-46C) and 80-110 rh, the
dough was baked at 440-460F (227-238C). The resultant bread was found to have
good height and volume, even texture, well-distributed crumb, and evenly
spaced
holes.
Examples 7-10
A series of laboratory scale tests was conducted in which bread for hamburger
buns was made by the sponge-and-dough method for the purpose of observing the
effect of varying concentrations of the encapsulated salt particles of the
invention.
Each of the tests was conducted with sponge weights of about 850 grams, and
total dough weights of about 1,3000 grams. The compositions of the sponge and
dough for this series of tests are given in Table 2 below:
18
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
Table 2
Sponge and Dough Test Formulae
INGREDIENT % WT. (BASIS FLOUR)WEIGHT, G.
Sponge
White wheat flour 70 490
Compressed yeast 3 21
Water 46 322
Dough conditioner 0.5 3.5
Nonbromated yeast 0.3 2.1
food
I Dough
White wheat flour 30 210
High fructose corn18 126
syrup
Shortening 6 42
Unencapsulated 2 14
salt
Encapsl'd salt Variable Variable
per Ex. 1 A
Water Variable Variable
Calcium propionate0.12 0.84
S The bread compositions, including a control composition, were prepared by
the
sponge-and-dough method. The test compositions in the series contained 6, 8,
10,
and 12 ounces of the encapsulated salt particles per hundred weight of flour.
The
control dough composition was the same as the Example doughs, except that it
contained unencapsulated salt particles, and no ascorbic acid or sodium
bicarbonate. The following procedure was used for the preparation of the
breads:
All sponge ingredients were mixed for 2 minutes at 79F (26C), and allowed to
ferment in a sealed container for 3.5 hours at 87F (31 C). The dough
ingredients
were mixed for 30 seconds at low speed, and the sponge was added to the
admixture, and mixed for 7.0 minutes to allow gluten development. The fully
19
CA 02268932 1999-02-19
WO 98/07324 PCT/LTS97/14509
mixed dough was allowed to rest for I 0 minutes at 87F (31 C) in a covered
container, after which the dough was removed from the container, and divided
into
56 g dough pieces, which were rounded and then panned. The panned dough was
proofed at 11 OF (43 C) and 90% rh to 3.6 cm total height, and baked for 11
S minutes at 435F (223C). The weight (g) and volume (cc by rapeseed
displacement) were measured 30 minutes after completion of baking. Four dough
batches were prepared for each encapsulated salt level, and for the control
dough
as well. To observe the effect of shock on the various dough, two of the
doughs
were subjected to shock by dropping the pan on a hard surface from a height of
3
inches (7.6 cm).
All of the doughs were evaluated with respect to their external properties --
symmetry, crust density and color. They were also evaluated with respect to
their
internal properties -- grain, texture, crumb body and color, taste/aroma and
mouthfeel, in accordance with the American Institute of Baking (AIB) Sensory
Evaluation Test. All data are based on the average of duplicate dough batches.
All of the test breads prepared using the encapsulated salt particles of the
invention, which had undergone shock, exhibited equal or better external
properties than the control bread, and better internal properties than the
control
bread.
In addition, as shown by the following loaf volume data, all of the breads
utilizing
the invention had at least equal loaf volume, and the bread containing 6
ounces/cwt. of encapsulated salt appeared to have even higher volume than the
control bread composition.
CA 02268932 1999-02-19
WO 98/07324 PCT/i1S97/14509
Table 3
Loaf Volume of Breads Exposed to Shock
EXAMPLE NO. WT. OF ENCAPSL'D LOAF VOLUME
SALT
(OZ/CWT.) (CC)
Control 292
Example 7 6 313
Example 8 8 296
Example 9 10 296
Example 10 12 289
Nevertheless, the weight ratio of unencapsulated salt to encapsulated salt
should
not exceed 4:1, and preferably no higher than 3.5:1
Examples 11-14
A still further series of bread dough compositions for use in baking hamburger
rolls was tested in which encapsulated salt particles containing different
amounts
of bicarbonate and ascorbic acid were used. The composition of these particles
is
given hereinabove (Examples SA-D). These dough compositions were then
compared with a control dough of the same composition, except the encapsulated
salt particles contained ascorbic acid, but no sodium bicarbonate in the
hydrogenated cottonseed oil shell. The breads tested in this manner were made
and evaluated in accordance with the procedure of Examples 7-10, except for
the
i 5 variations in the compositions of the encapsulated salt particles therein.
The AIB evaluation of these breads showed that the particles from Examples SA-
SD yielded rolls having the same or higher internal evaluations than the
control,
and yielded uniformly higher external evaluations than the control . The
breads
containing the Example SA-C particles all exhibited significantly higher loaf
21
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
volumes. The volume of the bread containing the Example SD particles was
slightly lower than the control bread (269 v. 276 cc.)
The foregoing examples show clearly the quite beneficial effect of making
bread
from doughs containing the encapsulated salt particles of the invention, as
compared with conventional breads made from unencapsulated salt, or from
encapsulated salt particles which contained no sodium bicarbonate. In
particular,
the addition of sodium bicarbonate to the encapsulated salt and ascorbic acid
clearly improves the resistance of the dough to shock forces incurred during
processing and handling of the dough.
In most commercial baking operations, the oven temperature of the baking step
is
400-450F (204-232C); however, the baking temperature for some baked goods
may be as low as 350F (177C), depending on the baking time, and the physical
characteristics of the baked products in question.
The ratio of unencapsulated salt to encapsulated salt may vary according to
the
particular baking operation in which the invention is used. In some instances,
the
weight ratio of unencapsulated salt to encapsulated salt may be as low as 1:1,
but
is usually preferred to be at least.l .5:1. Nevertheless, the weight ratio of
unencapsulated salt to encapsulated salt should not exceed 9:1, and preferably
no
higher than 5:1. A particularly preferred ratio for most bread applications is
5:1.
In the course of several such tests, it has been observed that bun crumb
quality
and uniformity, as well as texture, have been improved.
In a laboratory trial, white pan bread baked by the sponge-and-dough method
was
used as a model system to test the efficacy of various bimodal or unimodal
ascorbic acid compositions encapsulated in accordance with the invention. The
compositions were comprised of 60% salt, 2% sodium bicarbonate, and 6%
ascorbic acid. Twenty-seven tests were carried out, in which the particle size
modality of the ascorbic acid in the shell of the encapsulated compositions
was as
follows:
22
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
Table 4
Particle Size Modality of Oxidized Particles
WEIGHT RATIO, FINE/COARSE* SAMPLE DESIGNATION
6/0 p
4/2 g
2/4 C
0/6 p
* fine particles 1-100 pm
coarse particles 200-400 ~m
The formula and operating variables for the sponge-and-dough method are given
in Table 5, below.
Table 5
Sponge-And-Dough White Pan Bread
COMPONENT GRAMS
SPONGE
Flour 490.0
Yeast Food 3.5
Emulsifier 3.5
Yeast 15.5
Water 284.0
DOUGH
Flour 210.0
Dextrose 21, p
Sugar 38.0
Shortening 1'7,5
23
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
Table 5 Continued
Salt 15.5
Yeast 6.3
Oxidizer (ppm on variable
flour)
Percent Abs. 63.0
Water 157.0
OPERATING
VARIABLES
SPONGE
Water: 284 g
Water Temp: 78 F
Mix: 1 Min. speed 1: 1
Min.
speed 2
Sponge Temp: 78 _ 79 F
Fermentation Temp:82 - 86 F
Relative Humidity:g0~
Time: 2 Hours, 15 min.
Room Temp: 74 F
DOUGH
Temp: 81 - 82 f
Floor Time: 15 Minutes at 82
- 86 F
Panning Weight 520 g
Overhead: 6 minutes
Proof Temp: 100 F
Proof Time: To 3/4" Template
Sheeter Setting: Top, 3.4, Bottom,
1.4; Pressure
Board, 3.25 (Acme
Sheeter)
Bake: 400 F for 20 Minutes
24
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
In this series of laboratory baking trials, all the ascorbic acid compositions
were
added with the dough ingredients. Each encapsulated ascorbic acid composition
was added to the doughs over a wide range of concentrations, measured as parts
per million (ppm,) based on flour weight, to determine the optimum usage level
for each. The various additions levels were baked in a series of five test
bakes,
starting with a broad range of additions in the first test bake. In succeeding
test
bakes, a narrower range of ascorbic acid composition concentrations was added
to
confirm the optimum usage level for each unimodal or bimodal composition.
Each test bake yielded two loaves per formulation. All breads were scored
after
each test bake by a modified American Institute of Baking Universal Scoring
System. Twenty-five points were awarded for grain and texture, and volume was
scored according to the scale listed at the end of Table 7. Scores for grain
and
texture (structure) and for volume were averaged across the two loaves, and
totaled to yield a final score. The addition level scoring highest for each
composition after the first test bake was used as the mid-range addition level
for
the second test bake. The design for the second, and each succeeding, test
bake
included a narrower range of higher and lower additions than the first test.
This
scoring and design pattern continued until the optimum addition level for each
composition had been confirmed by the observation of declining volume and
structure scores for addition levels which were higher or lower than the
optimum.
In this scoring system, score differences of 2 or more are considered by
commercial bakers to be significant. Results showed that optimum usage levels
in
ppm varied across the formulations (Table 6):
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
Table 6
Oxidizer Performance in White Pan Bread-Average Total Score Based on All
Tested Addition Levels
OXIDIZERS
Range of Range of Average ScoreRange of
Scores
CompositionAscorbic
Acid
Addition Addition
~PPm)* ~PPm)*
Ascorbic
Acid
Compositions
6% Fine 307. I - 18.43 - 21.85 19 - 25
1842.8 110.57
2% Fine 4% 457.1 - 27.43 - 21.0 18 - 24
1842.8 110.57
Coarse
2% Coarse 307.1 - 18.43 - 25.28 20 - 28
3714.2 222.85
4% Fine
6% Coarse 457.1 - 27.43 - 21.83 19 - 2fi
3714.2 222.85
Performance scores for the 6% ascorbic acid composition showed a bell-shaped
distribution with lower total scores for addition levels above and below the
optimum range. The bimodal encapsulated ascorbic acid composition containing
2% coarse and 4% fine particles scored higher at the optimum usage level, and
over the entire range of concentrations tested, than did any of the other
bimodal or
unimodal 6% ascorbic acid compositions.
26
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
Table 7
Comparison of Various Oxidizer Addition Levels
on White Pan Bread Quality
OxidizerLevel Bake AverageGrain Text Struc Total
# Vol. Score* Score**
16 307.1 3 2888 7 11 18 20
17 307.1 3 2863 6 11 17 19
18 457.1 4 2850 7 12 19 25
19 457.I 4 2875 7 12 19 21
20 457.1 5 2850 6 11 17 19
21 457.1 5 2900 7 10 17 20
22 614.2 1 3063 8 12 20 26
23 614.2 4 2888 8 11 19 25
24 614.2 1 2963 7 14 21 25
25 614.2 4 2963 6 11 17 21
26 614.2 2 3113 6 11 17 24
27 614.2 5 2875 6 12 18 20
28 614.2 2 2925 8 10 18 21
29 614.2 5 2038 5 10 15 18
30 771.4 4 2925 6 II 17 24
31 771.4 4 2875 7 12 19 21
32 771.4 4 2862 6 11 17 19
33 1228.5 1 3100 7 13 20 27
34 1228.5 1 2963 6 12 18 22
35 1228.5 2 2950 7 12 19 23
36 1228.5 2 3100 6 I1 17 24
37 1842.8 1 3200 6 13 19 28
27
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
Table 7 continued
38 1842.8 1 3013 6 13 19 24
39 1842.8 2 3075 7 13 20 26
40 1842.8 2 3050 7 11 18 24
41 3714.2 5 2925 7 12 19 22
42 3714.2 5 2850 7 10 17 19
Volume Scale
3250cc = 10 3000cc = 5
* Grain and Texture 3200cc = 9 2950cc = 4
** Volume and Grain and Texture Score 3150cc = 8 2900cc = 3
3100cc = 7 2850cc = 2
3050cc = 6 2800cc = 1
The ascorbic acid compositions of this invention were able to achieve a
standard
bread score of 24 under the conditions of these tests at between 36.9 ppm and
110.57 ppm of active ascorbic acid (Table 7). Both Samples containing either
100% fine-ascorbic acid, or a high percentage (66 2/3%) of fine-ground
material
achieved total scores of 24, using less ascorbic acid (46.28 and 36.85 ppm,
respectively) than either samples, which contained between 66 2/3% and 100%
80-mesh ascorbic acid. However, the sample containing 4% fine-ground and 2%
80-mesh ascorbic acid was able to product breads with a superior score of 27
when added to the doughs at a concentration which provided 73.71 ppm of active
ascorbic acid. This indicated that compositions of the invention containing
more
than 50% fine-ground ascorbic acid showed greater efficiency as dough
conditioners than compositions containing only unimodal or bimodal particles
with a larger percentage of large mesh material (Table 7).
28
CA 02268932 1999-02-19
WO 98/07324 PCT/LTS97/14509
Table 8
Particle CompositionAscorbic Highest Total
Size ppm Acid jppm) Score
Modality
wt
%wt. m wt. m wt.
6 / 0 1614.2 56.9 (?) 25
4 / 2 1842.8 110.6 28
2 / 4 1228.5 73.7 24
0 / 6 1842.8 110.6 26
All weights based on flour weight, except particle size modality, which is
based
on encapsulated particle weight.
Results in Table 8 show that breads baked with a bimodal particle-size
distribution
of ascorbic acid in which 66 2/3% was fine-ground, and 33 1/3% was large mesh,
consistently scored higher over the entire range of addition levels tested.
For
example, the ascorbic acid compositions of the invention having a large
portion of
fine-ground particles (66 2/3%), and a lesser portion of large-mesh particles
(33
1/3%) were more efficient in producing breads of high quality (i.e., scores of
27 or
higher) than were unimodal compositions (Table 8). For comparison,
compositions containing bimodal particle-size distributions than included
higher
percentages of larger-mesh material than fine-ground material generated the
lowest total scores over the entire range of additions levels tested (Table
6).
An additional benefit of bimodal compositions containing a higher portion of
fme-
ground material is that samples receiving these compositions demonstrated a
wider tolerance or range of utility in comparison to any of the other
compositions
tested. However, the bimodal sample, which contained a large proportion of
coarse ascorbic acid, and the unimodal sample, which contained only fine-
ground
particles, produced lower total scores and showed more erratic performance
than
29
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
did the sample which contained 2% coarse and 4% fine particles. Table 8 shows
the optimum usage level, and the highest score achieved by each composition.
Breads baked with optimum usage levels for each composition were tested for
softness at days one and four after baking (Table 9).
Table 9
Effects of Various Oxidizers on the Softness of Aged Bread
TEXTURE A1VALYSIS
(kilograms/second)
PARTICLE SIZE Dal Day 4
MODALITY % wt.
SAMPLE-A 2% 80 0.1723 0.3061
Mesh Ascorbic 4%
Fine
Ground Ascorbic
SAMPLE-B 6% 0.2169 0.3373
Fine Ground Ascorbic
SAMPLE-C 6% 0.2020 0.33536
80 Mesh Ascorbic
SAMPLE-E 2% 0.2115 0.3253
Fine Ground Ascorbic
4% 80
' Mesh Ascorbic
Softness was greater when the least amount of force in kilograms per second
was
needed to depress the crumb structure. Each score represents an average of ten
( 10) crumb depressions with an Instron Texture Analyzer. Results showed that
softness was significantly better on day one in breads containing the bimodal
6%
ascorbic acid composition with 2% coarse and 4% fine-ground materials, than in
breads baked with either the unimodal compositions, or the bimodal composition
containing a majority of large particles. However, on day four, breads baked
with
bimodal samples were softer than breads baked with unimodal 6% ascorbic acid
compositions, although Sample A, containing 66 2/3% fine-ground particles, was
preferred over Sample E, which contained 66 2/3% large mesh particles. Final
CA 02268932 1999-02-19
WO 98/07324 PCT/LTS97/14509
results showed that bimodal ascorbic acid compositions comprised of 33 1/3% 80-
mesh, and 66 2/3% fine-ground particles were the preferred embodiment of the
invention. These compositions consistently produced breads which scored higher
for quality over a wider range of conditions, and which remained softer longer
than did any of the other bimodal or unimodal 6% ascorbic acid compositions
tested (see Table 9).
Table 10
Effect of Ascorbic Acid Particle Size on Total Bread Scores
6/0 19 21 23 21 22 24 -
Fine
only
4/2 20 25 26 24 27 28 22
Fine/
Coarse
2/4 - 20 20 19 24 - -
Fine/
Coarse
0/6 - 19 22 - 23 26 19
Coarse
Only
Ascorbic307 457 614 771 1229 183 3714
Acid
Fine particles -- 1 - 100 p,m Coarse particles -- 200- 400 ~tm
Example 15
A series of commercial light white breads doughs was baked on a laboratory
scale
to assess the difference between various oxidizing systems in which potassium
bromate had been omitted. The encapsulated ascorbic acid composition of this
invention was tested alone or in combination with an enzyme-based bromate
replacer, and azodicarbonamide at various salt levels. These test formulations
were compared to a control oxidation system comprising unencapsulated ascorbic
31
CA 02268932 1999-02-19
WO 98/07324 PCT/US97/14509
acid, azodicarbonamide, and an enzyme-based bromate replacer. All breads were
made by a liquid ferment system, and were scored for dough handling and baked
volume. Fiber and minor ingredients were prehydrated prior to mixing.
In comparison to a control, which contained 100% salt concentration, ascorbic
acid, azodicarbonamide, and an enzyme-based oxidizing system, breads
containing only the composition of the invention as a bromate replacer,
maintained or increased average baked volume, except in the case where salt
had
been reduced by 25%. The highest volume was achieved when salt was reduced
50%, and the composition of the invention was the sole source of oxidation.
This
combination also produced the most even dough performance among divided
doughs, and was significantly better than when either 100% or 75% also had
been
used. The doughs, however, were slightly more sticky than the control, but not
as
sticky as when a combination of the enzyme-based bromate replacer, the
composition of the invention, and azodicarbonamide was used at a 50% salt
level.
In addition, the invention was able to be substituted for powdered ascorbic
acid
without loss of volume or dough-handling characteristics. These results
indicate
that the invention, in combination with a 50% salt reduction, is capable of
producing light, white bread with greater volume than would the combination of
azodicarbonamide, ascorbic acid and an enzyme-based dough conditioned in the
presence of 100% salt concentration.
32