Note: Descriptions are shown in the official language in which they were submitted.
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
FABRIC SOFTENING COMPOSITIONS
Field of the invention
The present invention relates to fabric softening compositions, and more
particularly to diluted fabric softening compositions.
Background of the invention
Fabric softening compositions are known in the art for imparting benefits
such as softness and/or antistatic properties to the treated fabric.
However, nowadays, consumer acceptance of fabric softening
compositions is determined not only by the performance achieved with
these products but also by the aesthetics associated therewith. Viscosity
of the product is therefore an important aspect of the successful
formulation of such commercial products, stable medium to medium-high
viscosities being highly preferred by consumers. 8y medium-high
viscosities, it is meant viscosities of 50cps to 150cps when the fabric
softening composition is in a diluted form, that is containing less than 10%
by weight of fabric softening agents, and viscosities of 30cps to 90cps
when the fabric softening composition is in a concentrated form, that is
containing from 10% to 80% by weight of fabric softening agents.
CA 02270383 2002-04-02
2
To fulfill such need, fatty acids have been incorporated into fabric
softening compositions. Such a disclosure can be found in
European Patent No. EP~~'63592A1.
However, although a satisfactory viscosity stability was obtained with the
compositions in a concentrated form, the diluted compositions were still
found deficient in certain conditions. Indeed, under shear conditions such
as process, handling and shiprnent, a loss in the viscosity of the diluted
softening compositions was observed.
Accordingly, it is an obpect of the invention to provide a diluted softening
composition comprising fatty acids which exhibits suitable viscosity
stability under shear conditions.
The Applicant has now found that the provision of specific nonionic
compounds overcomes the problem.
It is therefore an advantage of the invention to provide diluted
compositions with tow sensitivity to shear.
By "sensitivity to shear", it is meant that the viscosity of the freshly made
product does not stay to its initial value after shear.
By "low sensitivity to shear", it is meant that the viscosity of the sheared
product is substantially the same as the viscosity of the freshly made
product.
Summary of the invention
The present invention is a diluted fabric softening composition comprising:
a!-a cationic fabric softener in an amount of less than 10°~ by weight,
b!-a fatty acid, and
c!-a nonionic compound selected from the group consisting of:
t!- block copolymers of terephthalate and ethylene oxide or propylene
oxide;
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
3
ii)- block copolymers of propylene oxide and ethylene oxide in which
the central block is polypropylene oxide; and
iii)- mixtures thereof.
In another aspect of invention, there is provided a method for treating
fabrics which comprises the step of contacting said fabrics in the rinse
cycle with an aqueous medium containing said composition.
D~taiied description of the invention
Cationic fabric softener
A cationic fabric softener is an essential ingredient of the invention.
Typical
levels of said fabric softener components within the diluted softening
compositions are from less than 10% by weight, preferably from 1 % to
9% by weight, and more preferably from 2% to 7% by weight of the
composition.
The preferred, typical cationic fabric softening components include the
water-insoluble quaternary-ammonium fabric softeners, the most commonly
used having been di-long alkyl chain ammonium chloride or methyl sulfate.
Preferred cationic softeners among these include the following:
i 1 ditallow dimethylammonium chloride (DTDMAC);
2) dihydrogenated tallow dimethylammonium chloride;
3) dihydrogenated tallow dimethylammonium methyisulfate;
4) distearyl dimethylammonium chloride;
5) dioleyl dimethylammonium chloride;
6) dipalmityl hydroxyethyl methylammonium chloride;
7) stearyl benzyl dimethylammonium chloride;
8) tallow trimethylammonium chloride;
9) hydrogenated tallow trimethylammonium chloride;
10) C12-14 alkyl hydroxyethyl dimethylammonium chloride;
11 ) C12_18 alkyl dihydroxyethyl methylammonium chloride;
12) di(stearoyloxyethyl) dimethylammonium chloride
(DSOEDMAC);
13) di(tallowoyloxyethyl) dimethylammonium chloride;
14) ditallow imidazolinium methylsulfate;
CA 02270383 1999-04-29
WO 98/18890 PCTILTS97/19794
4
15) 1-(2-tallowylamidoethyl)-2-tallowyl imidazoiinium
methylsulfate.
However, in recent years, the need has arisen for more environmentally-
friendly materials, and rapidly biodegradable quaternary ammonium
compounds have been presented as alternatives to the traditionally used di-
long alkyl chain ammonium chlorides and methyl sulfates. Such quaternary
ammonium compounds contain long chain alk(en)yl groups interrupted by
functional groups such as carboxy groups. Said materials and fabric
softening compositions containing them are disclosed in numerous
publications such as EP-A-0,040,562, and EP-A-0,239,910.
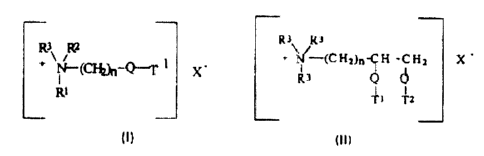
The quaternary ammonium compounds and amine precursors herein have
the formula (I) or (II), below
R2 1 + N/ (CHZ)n-CH -CH? X
~--(~WQ-T x-
R3 Q
R1 T~ T2
or
wherein Q is selected from -O-C(O)-, -C(OI-O-, -O-C(O)-O-, -NR4-C(O)-, -
C(O)-NR4-;
R1 is (CH2)n-Q-T2 or T3;
R2 is (CH2)m-Q-T4 or T5 or R3;
R3 is C1-C4 alkyl or C1-C4 hydroxyaikyl or H;
R4 is H or C1-C4 alkyl or C1-C4 hydroxyalkyl;
T1, T2, T3, T4, T5 are independently Cg-C22 alkyl or alkenyl;
n and m are integers from 1 to 4; and
X- is a softener-compatible anion.
Non-limiting examples of softener-compatible anions include chloride or
methyl sulfate.
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
The alkyl, or aikenyl, chain T1, T2, T3, T4, T5 must contain at least 6
carbon atoms, preferably at least 11 carbon atoms, more preferably at
least 16 carbon atoms. The chain may be straight or branched.
Tallow is a convenient and inexpensive source of long chain alkyl and
alkenyl material. The compounds wherein T1, T2, T3, T4, T5 represents
the mixture of long chain materials typical for tallow are particularly
preferred.
Specific examples of quaternary ammonium compounds suitable for use in
the aqueous fabric softening compositions herein include
1 ) N,N-diltallowoyl-oxy-ethyl)-N,N-dimethyl ammonium chloride;
2) N,N-di(tallowoyl-oxy-ethyl)-N-methyl, N-(2-hydroxyethyl) ammonium
methyl sulfate;
3) N,N-di(2-tallowoyl-oxy-2-oxo-ethyl)-N,N-dimethyl ammonium chloride; .
4) N,N-di(2-tallowoyl-oxy-ethylcarbonyl-oxy-ethyl)-N,N-dimethyl
ammonium chloride;
5) N-(2-tallowoyl-oxy-2-ethyl)-N-(2-tallowyl-oxy-2-oxo-ethyl)-N,N-dimethyl
ammonium chloride;
6) N,N,N-tri(tallowoyl-oxy-ethyl)-N-methyl ammonium chloride;
7) N-(2-tallowoyl-oxy-2-oxo-ethyl)-N-(tallowyi-N,N-dimethyl-ammonium
chloride; and
8) 1,2-ditallowoyl-oxy-3-trimethylammoniopropane chloride;
and mixtures of any of the above materials.
Of these, compounds 1-7 are examples of compounds of Formula (I);
compound 8 is a compound of Formula (II).
Particularly preferred is N,N-di(tallowoyl-oxy-ethyl)-N,N-dimethyl
ammonium chloride, where the tallow chains are at least partially
unsaturated.
The level of unsaturation of the tallow chain can be measured by the Iodine
Value (IV) of the corresponding fatty acid, which in the present case
should preferably be in the range of from 5 to 100 with two categories of
compounds being distinguished, having a IV below or above 25.
Indeed, for compounds of Formula (I) made from tallow fatty acids having
a IV of from 5 to 25, preferably 15 to 20, it has been found that a cis/trans
isomer weight ratio greater than 30/70, preferably greater than 50/50 and
more preferably greater than 70/30 provides optimal concentrability.
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
6
For compounds of Formula (I) made from tallow fatty acids having a IV of
above 25, the ratio of cis to trans isomers has been found to be less
critical unless very high concentrations are needed.
Other examples of suitable quaternary ammoniums of Formula (I) and III)
are obtained by, e.g. .
- replacing "tallow" in the above compounds with, for example, coco,
palm, lauryl, oleyl, ricinoleyl, stearyl, palmityl, or the like, said fatty
acyl
chains being either fully saturated, or preferably at least partly
unsaturated;
- replacing "methyl" in the above compounds with ethyl, ethoxy, propyl,
propoxy, isopropyl, butyl, isobutyl or t-butyl;
- replacing "chloride" in the above compounds with bromide,
methylsulfate, formate, sulfate, nitrate, and the like.
In fact, the anion is merely present as a counterion of the positively
charged quaternary ammonium compounds. The nature of the counterion is
not critical at all to the practice of the present invention. The scope of
this
invention is not considered limited to any particular anion.
By "amine precursors thereof" is meant the secondary or tertiary amines
corresponding to the above quaternary ammonium compounds, said
amines being substantially protonated in the present compositions due to
the pH values.
For the preceding fabric softening agents, especially with biodegradable
fabric softening agents, the pH of the liquid compositions herein is a
preferred parameter of the present invention. Indeed, it influences the
stability of the quaternary ammonium or amine precursors compounds,
especially in prolonged storage conditions. The pH, as defined in the
present context, is measured in the neat compositions at 20°C. For
optimum hydrolytic stability of these compositions, the neat pH, measured
in the above-mentioned conditions, must be in the range of from 2.0 to
4.5. Preferably, where the liquid fabric softening compositions of the
invention are in a concentrated form, the pH of the neat composition is in
the range of 2.0 to 3.5, while if it is in a diluted form, the pH of the neat
composition is in the range of 2.0 to 3Ø The pH of these compositions
herein can be regulated by the addition of a Bronsted acid.
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
7
Examples of suitable acids include the inorganic mineral acids, carboxylic
acids, in particular the low molecular weight IC1-C5) carboxylic acids, and
alkylsulfonic acids. Suitable inorganic acids include HCI, H2S04, HN03 and
H3POq,. Suitable organic acids include formic, acetic, citric, methylsulfonic
and ethylsulfonic acid. Preferred acids are citric, hydrochloric, phosphoric,
formic, methylsulfonic acid, and benzoic acids.
Fattv acid
Another essential component of the invention is a fatty acid compound.
Suitable fatty acids include those containing from 10 to 25, preferably from
12 to 25 total carbon atoms, with the fatty moiety containing from 10 to
22, preferably from 16 to 22, carbon atoms. The shorter moiety contains
from 1 to 4, preferably from 1 to 2 carbon atoms.
The level of unsaturation of the tallow chain can be measured by the Iodine
Value (IV) of the corresponding fatty acid, which in the present case
should preferably be in the range of from 5 to 100, more preferably in the
range of from 0 to 25.
Specific examples of tatty acid compounds suitable for use in the aqueous
fabric softening compositions herein include compounds selected from
iauric acid, myristic acid, palmitic acid, stearic acid, arachidic acid,
behenic
acid, oleic acid, coconut fatty acid, tallow fatty acid, partially
hydrogenated tallow fatty acid and mixtures thereof.
A most preferred fatty acid compound is tallow fatty acid with an Iodine
Value (IV) of 18.
The fatty acids will preferably be present in a weight ratio of said
biodegradable fabric softening agents to said fatty acid compounds of from
25:1 to 6.5:1, more preferably from 20:1 to 10:1 and most preferably from
20:1 to 15:1. Indeed, it is within these ratios ranges that the diluted fabric
softening compositions of the invention exhibit best storage stability as
well as viscosity.
Nonionic compound
CA 02270383 2002-04-02
8
Another essential ingredient for the purpose of the invention is a nonionic
compound selected from the group consisting of block copolymers of
terephthalate and ethylene oxide or propylene oxide; block copolymers
of propylene oxide and ethylene oxide in which the central block is
polypropylene oxide; and mixtures thereof.
i1-Blnck conolvmers of ~renhthalate and ethylene oxide or oroovlene oxide
Block copolymers of terephthalate and polyethylene oxide or polypropylene
oxide for use herein are those conventionally known as soil release
polymers and are typically present in an amount of from 0.005% to 1 °~6
by
weight, preferably from 0.01 % to 0.5% by weight of the composition.
A preferred block copolymer for use herein is a copolymer having blocks of
terephthalate and polyethylene oxide. More specifically, these polymers are
comprised of repeating units of ethylene terephthalate and polyethylene
oxide terephthalate at a molar ratio of ethylene terephthalate units to
polyethylene oxide terephthalate units of from 25:75 to 35:65, said
polyethylene oxide terephthalate containing polyethylene oxide blocks
having molecular weights of from 200 to 2000. The molecular weight of
this polymeric soil release agent is in the range of from 600 to 55,000.
Another preferred polymeric soil release agent is a crystailizable polyester
with repeat units of ethylene terephthalate units containing from 10% to
1596 by weight of ethylene terephthalate units together with from 10% to
5096 by weight of polyoxyethyiene terephthalate units, derived from a
polyoxyethylene glycol of average molecular weight of from 300 to 6,000,
and the molar ratio of ethylene terephthalate units to potyoxyethylene
terephthalate units in the crystallizable polymeric compound is between 2:1
and 6:1. Examples of this polymer include the commercially available
materials Zelcon 4780 TM (from Dupont) and Milease TT"" (from ICI).
Highly preferred soil release agents are polymers of the generic formula:
0
O
X=(TCh12CH2) ~-C-.'R~14 CI -.TRtS)u(T~-R14 _ C_T)iCH2CH2T-~-X
P
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
9
in which each T is an 0, or NH linkage, preferably O linkage, each X can
be a suitable capping group, with each X typically being selected from the
group consisting of H, and alkyl or acyl groups containing from 1 to 4
carbon atoms. p is selected for water solubility and generally is from 6 to
113, preferably from 8 to 50. a is critical to formulation in a liquid
composition having a relatively high ionic strength. There should be very
little material in which a is greater than 10. Furthermore, there should be
at least 20%, preferably at least 40%, of material in which a ranges from 1
to 6.
The R14 moieties are essentially 1,4-phenylene moieties. As used herein,
the term "the R14 moieties are essentially 1,4-phenylene moieties" refers
to compounds where the R14 moieties consist entirely of 1,4-phenylene
moieties, or are partially substituted with other arylene or alkarylene
moieties, alkylene moieties, alkenylene moieties, or mixtures thereof.
Arylene and alkarylene moieties which can be partially substituted for 1,4-
phenylene include 1,3-phenylene, 1,2-phenylene, 1,8-naphthylene, 1,4-
naphthylene, 2,2-biphenylene, 4,4-biphenylene, and mixtures thereof.
Alkylene and alkenylene moieties which can be partially substituted include
1, 2-propylene, 1,4-butylene, 1, 5-pentylene, 1, 6-hexamethylene, 1, 7-
heptamethylene, 1,8-octamethylene, 1,4-cyclohexylene, and mixtures
thereof.
For the R14 moieties, the degree of partial substitution with moieties other
than 1,4-phenylene which can generally be tolerated will depend upon the
backbone length of the compound, i.e., longer backbones can have greater
partial substitution for 1,4-phenylene moieties. Usually, compounds where
the R14 comprise from 50% to 100% 1,4-phenylene moieties (from 0% to
50% moieties other than 1,4-phenylene) are adequate. For example,
polyesters with a 40:60 mole ratio of isophthalic (1,3-phenylene) to
terephthalic ( 1,4-phenylene) acid can be used. However, because most
polyesters used in fiber making comprise ethylene terephthalate units, it is
usually desirable to minimize the degree of partial substitution with
moieties other than 1,4-phenyiene for best soil release activity. Preferably,
the R14 moieties consist entirely of (i.e., comprise 100%) 1,4-phenyiene
moieties, i.e., each R14 moiety is 1,4-phenylene.
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
For the R15 moieties, suitable moieties are ethylene or substituted ethylene
moieties which include ethylene, 1,2-propylene, 1,2-butylene, 1,2-
hexylene, 3-methoxy-1,2-propylene, and mixtures thereof. Preferably, the
R 15 moieties are essentially ethylene moieties, 1, 2-propylene moieties, or
mixtures thereof. Surprisingly, inclusion. of a greater percentage of 1,2-
propylene moieties tends to improve the water solubility of compounds.
Therefore, the use of 1,2-propylene moieties or a similar branched
equivalent is desirable for incorporation of any substantial part of the
component in the liquid fabric softener compositions. Preferably, from 75%
to 100%, are 1,2-propylene moieties.
The value for each p is at least 6, and preferably is at least 8. The value
for each n usually ranges from 6 to 113. Typically, the value fpr each p is
in the range of from 6 to 113.
A more complete disclosure of soil release agents is contained in U.S.
4,661,267; 4,711,730; 4,749,596; 4,818,569; 4,877,896;
4,956,447; and 4,976,879.
Commercially available block copolymers of terephthalate and polyethylene
oxide or polypropylene oxide, suitable for use herein, are the copolymers
sold under the tradename HOE S3639, HOE S3702 type 566 from
Hoechst, and Zelcon 7201 from Dupont.
ii)-Block cooolvmers of ~roovlene oxide and ethylene oxide in which the
central block is ~yhroovlene oxide
Block copolymers of propylene oxide and ethylene oxide in which the
central block is polypropylene oxide) for use herein, are typically present
in an amount of from 0.005% to 1 % by weight, more preferably of from
0.01 % to 0.5% by weight of the composition.
The copolymers for use herein preferably have an average molecular
weight of at least 4000 and contains 5% to 30%, preferably 5°~ to 20%,
and more preferably 10% polyethylene oxide) by weight of the copolymer.
Suitable examples of such copolymers include PLURADYNE (trademark) FL-
11, BASF-Wyandotte Corporation, a product reported to have an average
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
11
molecular weight of 3800, a density of 1.02 at 25°C and a Brookfield
viscosity of 800 cP at 25°C; PLURONIC (trademark) PE 10100, BASF
United Kingdom, Ltd., a product reported to have an average molecular
weight of 3600, a density of 1.02 at 20°C and a viscosity of 300
mm2/sec at 40°C; and SYNPERONIC (trade mark) PE L-121, Imperial
Chemical Industries, P.L.C., a product reported to have an average
molecular weight of 4400, a density of 1.02 at 25°C and a viscosity of
1600 cP at 25°C. Each such copolymer was reported to contain
10°~
polyethylene oxide).
Further commercially available block copolymers of propylene oxide and
ethylene oxide in which the central block is polypropylene oxide), suitable
for use herein, are the copolymers sold under the tradename Pluronic PE
4300, and Pluronic PE 10400 from BASF.
Mixtures of any of the above described nonionic compounds can also be
used herein.
By the use of the above mentioned specific nonionic compounds, the
resulting diluted fabric softening composition exhibits a low sensitivity to
shear.
Determination of the shear sensitivity is made as follows:
A 150m1 sample of a composition, to be tested for shear sensitivity, is
placed in a glass vial and sheared with a magnetic stirrer at maximum
speed (approximately 1000rpm). The measurement of the viscosity is then
made at 0 and after 5, 15, 30 and 60 minutes of shearing using a
Brookfield viscosity meter set to a speed of 60 rpm ( 1 Hertz).
Products which have a low sensitivity to shear are those having a viscosity
which is substantially the same as the viscosity measured with the
unsheared product, that is at 0 minutes. By "substantially", it is meant that
the viscosity of the sheared product does not vary by more than 20% after
each of the above mentioned time measurements when compared to the
viscosity of the unsheared product, that is at 0 minutes.
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
12
The diluted softening composition of the invention will also comprise a
liquid carrier. Suitable liquid carriers are selected from water, organic
solvents and mixtures thereof. The liquid carrier employed in the instant
compositions is preferably at least primarily water due to its low cost
relative availability, safety, and environmental compatibility. The level of
water in the liquid carrier is preferably at least 60%, most preferably at
least 80%, by weight of the carrier. Mixtures of water and low molecular
weight, e.g., < 200, organic solvent, e.g., lower alcohol such as ethanol,
propanol, isopropanol or butanol are useful as the carrier liquid. Low
molecular weight alcohols include monohydric, dihydric (glycol, etc.)
trihydric (glycerol, etc.), and higher polyhydric (polyols) alcohols.
Optional
The composition may also contain optional components which may be
suitable for further improving the aesthetic appearance of the fabrics
treated therewith. Suitable optional components include a polyethylene
glycol, additional fabric softening components, enzymes,
cyclodextrin/perfume complexes and free perfume delivery systems, and
mixtures thereof.
Polyethylene glycol
A polymeric material which can optionally be included is polyethylene
glycol (PEG). When used, PEG will provide an increase in the viscosity
stability upon storage of the composition of the invention. Typical
molecular weight ranges for these purposes range from 500 to 100,000,
preferably from 1,000 to 50,000, more preferably from 1,500 to 10,000.
A most preferred molecular weight is 4000. When present, typical levels of
polyethylene glycols are from 0.01 to 1 % by weight, preferably from
0:05% to 0.5% by weight of the composition.
Additional components
Additional fabric softening materials may be used in addition or
alternatively to the cationic fabric softener. These may be selected from
nonionic, amphoteric or anionic fabric softening material. Disclosure of
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
13
such materials may be found in US 4,327,133; US 4,421,792; US
4,426,299; US 4,460,485; US 3,644,203; US 4,661,269; U.S 4,439,335;
U.S 3,861,870; US 4,308,151; US 3,886,075; US 4,233,164; US
4,401,578; US 3,974,076; US 4,237,016 and EP 472,178.
Typically, such nonionic fabric softener materials have a HLB of from 2 to
9, more typically from 3 to 7. Such nonionic fabric softener materials tend
to be readily dispersed either by themselves, or when combined with other
materials such as single-long-chain alkyl cationic surfactant described in
detail hereinafter. Dispersibility can be improved by using more single-long-
chain alkyl cationic surfactant, mixture with other materials as set forth
hereinafter, use of hotter water, and/or more agitation. In general, the
materials selected should be relatively crystalline, higher melting, (e.g.
>40°C) and relatively water-insoluble.
Preferred nonionic softeners are fatty acid partial esters of polyhydric
alcohols, or anhydrides thereof, wherein the alcohol, or anhydride, contains
from 2 to 18, preferably from 2 to 8, carbon atoms, and each fatty acid
moiety contains from 12 to 30, preferably from 16 to 20, carbon atoms.
Typically, such softeners contain from one to 3, preferably 2 fatty acid
groups per molecule.
The polyhydric alcohol portion of the ester can be ethylene glycol, glycerol,
poly (e.g., di-, tri-, tetra, yenta-, and/or hexa-) glycerol, xylitol,
sucrose,
erythritol, pentaerythritol, sorbitol or sorbitan. Sorbitan esters and
polyglycerol monostearate are particularly preferred.
The fatty acid portion of the ester is normally derived from fatty acids
having from 12 to 30, preferably from 16 to 20, carbon atoms, typical
examples of said fatty acids being lauric acid, myristic acid, palmitic acid,
stearic acid and behenic acid.
Highly preferred optional nonionic softening agents for use in the present
invention are the sorbitan esters, which are esterified dehydration products
of sorbitol, and the glycerol esters.
Commercial sorbitan monostearate is a suitable material. Mixtures of
sorbitan stearate and sorbitan palmitate having stearate/palmitate weight
ratios varying between 10:1 and 1:10, and 1,5-sorbitan esters are also
useful.
Glycerol and polyglycerol esters, especially glycerol, diglycerol,
triglycerol,
and polyglycerol mono- and/or di-esters, preferably mono-, are preferred
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
14
herein (e.g. polyglycerol monostearate with a trade name of Radiasurf
72481.
Useful glycerol and polyglycerol esters include mono-esters with stearic,
oleic, palmitic, lauric, isostearic, myristic, and/or behenic acids and the
diesters of stearic, oleic, palmitic, lauric, isostearic, behenic, and/or
myristic acids. It is understood that the typical mono-ester contains some
di- and tri-ester, etc.
The "glycerol esters" also include the polyglycerol, e.g., diglycerol through
octaglycerol esters. The polyglycerol polyols are formed by condensing
glycerin or epichlorohydrin together to link the glycerol moieties via ether
linkages. The mono- and/or diesters of the polyglycerol polyols are
preferred, the fatty acyl groups typically being those described
hereinbefore for the sorbitan and glycerol esters.
Further fabric softening components suitable for use herein are the
softening clays, such as the low ion-exchange-capacity ones described in
EP-A-0,150, 531.
Enzymes
Enzymes may also optionally be included in the composition of the
invention. Enzymes suitable for use herein are selected from proteases,
amylases, lipases, cellulases, peroxidases, and mixtures thereof. A
preferred enzyme for use herein is a cellulase.
Cellulases which may be usable herein include both bacterial and fungal
types. U.S 4,435,307 discloses suitable fungal cellulases from Humicola
insolens or Humicola strain DSM1800 or a cellulase 212-producing fungus
belonging to the genus Aeromonas, and cellulase extracted from the
hepatopancreas of a marine mollusk, Dolabella Auricula Solander. Suitable
cellulases are also disclosed in GB-A-2.075.028; GB-A-2.095.275 and DE-
OS-2.247..832. CAREZYME~ and CELLUZYME° (Novo1 are especially
useful. See also WO 9117243 to Novo.
Typical amounts of enzymes are up to 5 mg by weight, more typically 0.01
mg to 3 mg, of active enzyme per gram of the composition. Stated
otherwise, the compositions herein will typically comprise from 0.001 % to
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
5%, preferably 0.01 % to 1 % by weight of a commercial enzyme
preparation.
_Ot~tional Cvclodextrin/Perfume Complexes and Free Perfume Delivery
terns
The products herein can also contain from 0.5% to 60%, preferably from
1 % to 50%, cyclodextrin/perfume inclusion complexes and/or free
perfume, as disclosed in U.S 5,139,fi87; and 5,234,610. Perfumes are
highly desirable, can usually benefit from protection, and can be complexed
with cyclodextrin. Fabric softening products typically contain perfume to
provide an olfactory aesthetic benefit and/or to serve as a signal that the
product is effective.
Suitable perfume component for use in the present invention comprise
compounds having an ester of a perfume alcohol. The ester includes at
least one free carboxylate group and has the formula
O
HOC R C:OR'
m ~ ~n
wherein R is selected from the group consisting of substituted or
unsubstituted C1-C30 straight, branched or cyclic alkyl, alkenyl, alkynyl,
alkylaryl or aryl group; R' is a perfume alcohol with a boiling point at 760
mm Hg of less than 300 oC; and n and m are individually an integer of 1 or
greater.
The perfume component may comprise from 0.01 % to 10% by weight of
the composition. Preferably, R is selected from the group consisting of
substituted or unsubstituted C1 - C20 straight, branched or cyclic alkyl,
alkenyl, alkynyl, alkylaryl, aryl group or ring containing a heteroatom. R' is
preferably a perfume alcohol selected from the group consisting of
geraniol, nerol, phenoxanol, floralol, p-citronellol, nonadol, cyclohexyl
ethanol, phenyl ethanol, phenoxyethanol, isoborneol, fenchol,
isocyciogeraniol, 2-phenyl-1-propanol, 3,7-dimethyl-1-octanol, and
combinations thereof and the ester is preferably selected from maleate,
succinate adipate, phthalate, citrate or pyromellitate esters of the perfume
alcohol. The most preferred esters having at least one free carboxylate
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
16
group are then selected from the group consisting of geranyl succinate,
neryl succinate, (b-citronellyl) maleate, nonadol maleate, phenoxanyl
maleate, (3,7-dimethyl-1-octanyl) succinate, (cyclohexylethyl) maleate,
floralyl succinate, (b-citronellyl) phthalate and (phenylethyl), adipate.
The optional perfume ingredients and compositions of this invention are the
conventional ones known in the art. Selection of any perfume component,
or amount of perfume, is based solely on aesthetic considerations. Suitable
perfume compounds and compositions can be found in the art including
U.S 4,145,184; 4,209,417; 4,515,705; and 4,152,272. Many of the art
recognized perfume compositions are relatively substantive to maximize
their odor effect on substrates. However, it is a special advantage of
perfume delivery via the perfume/cyclodextrin complexes that
nonsubstantive perfumes are also effective.
If a product contains both free and complexed perfume, the escaped
perfume from the complex contributes to the overall perfume odor
intensity, giving rise to a longer lasting perfume odor impression.
As disclosed in U.S 5,234,610, by adjusting the levels of free perfume and
perfume/CD complex it is possible to provide a wide range of unique
perfume profiles in terms of timing (release) and/or perfume identity
(character).
Optionally, longer lasting perfume odor impression can also be provided by
the use of nonionic or anionic esters of an ailylic alcohol perfume described
in WO 96/02625 and having the formula:
O
I I
R-[C-O-CR'2-CR"=CR"2]n
wherein R is selected from the group consisting of nonionic or anionic
substituted or unsubstituted C1 - C30 straight, branched or cyclic alkyl,
alkenyl, alkynyl, alkylaryl, or aryl group; each of R', R", and R"' is
independently selected from the group consisting of hydrogen, or a
nonionic or anionic substituted or unsubstituted C1 - C25 straight,
branched or cyclic alkyl, alkenyl, alkynyl, alkylaryl, or aryl group; and n is
an integer of 1 or .greater. The most preferred nonionic or anionic ester of
an allylic alcohol perfume are then selected from the group consisting of
digeranyl succinate, dineryl succinate, geranyl neryl succinate, geranyl
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
17
phenylacetate, neryl phenylacetate, geranyl laurate, neryl laurate, and
mixtures thereof. When used, the perfume component may be comprised
in an amount of from 0.01 % to 10% by weight of the composition.
Additional components
The composition may also optionally contain additional components such
as surfactant concentration aids, electrolyte concentration aids, stabilisers,
such as well known antioxidants and reductive agents, emulsifiers,
bacteriocides, colorants, perfumes, preservatives, optical brighteners, anti
ionisation agents, and antifoam agents. These ingredients, especially the
minor ingredients can be usefully added with, and preferably protected by,
"carrier materials" such as zeolites, starch, cyclodextrin or wax.
Stabilisers
Stabilisers may also optionally be used. When used, said stabiliser will help
achieving the desired finished product viscosity as well as stabilising the
finished product upon storage. Stabilisers are typically selected from single
long chain alkyl cationic surfactants, nonionic alkoxylated surfactants,
amine oxides, fatty acids, and mixtures thereof, typically used at a level of
from 0 to 15% by weight of the composition.
1 )Single long chain alkyl cationic surfactants
Such mono-long-chain-alkyl cationic surfactants useful in the present
invention are, preferably, quaternary ammonium salts of the general
formula
fR2N + R33 X_
wherein the R2 group is C10-C22 hydrocarbon group, preferably C12-C18
alkyl group of the corresponding ester linkage interrupted group with a
short alkylene (C1-C4) group between the ester linkage and the N, and
having a similar hydrocarbon group, e.g., a fatty acid ester of choline,
preferably C12-C14 (coco) choline ester and/or Clg-Clg tallow choline
ester at from 0.1 % to 20% by weight of the softener active. Each R is a
C1-C4 alkyl or substituted le.g., hydroxy) alkyl, or hydrogen, preferably
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
18
methyl, and the counterion X- is a softener compatible anion, for example,
chloride, bromide, methyl sulfate, etc.
Other cationic materials with ring structures such as alkyl imidazoline,
imidazolinium, pyridine, and pyridinium salts having a single C~ 2-Cgp alkyl
chain can also be used. Very low pH is required to stabilize, e.g.,
imidazoline ring structures.
Some alkyl imidazolinium salts and their imidazoline precursors useful in the
present invention have the general formula
N ' N+ CZH4-Y2 R7 X_
Eli \R6
R8
wherein Y2 is -C(O)-O-, -O-(O)C-, -C(O)-N(R5)-, or -N(R5)-C(O)- in which R5
is hydrogen or a C~-C4 alkyl radical; R6 is a C~-C4 alkyl radical or H (for
imidazoiine precursors); R~ and R8 are each independently selected from R
and R2 as defined hereinbefore for the single-long-chain cationic surfactant
with only one being R2.
Some alkyl pyridinium salts useful in the present invention have the general
formula
R? N+~ X
wherein R2 and X- are as defined above. A typical material of this type is
cetyl pyridinium chloride.
21-Nonionic Alkoxylated Surfactant
C ~ 2 ~ H2
Suitable nonionic alkoxylated surfactants for use herein include addition
products of ethylene oxide and, optionally, propylene oxide, with fatty
alcohols, fatty acids and fatty amines.
CA 02270383 1999-04-29
WO 98/18890 PCT/US97/19794
19
Suitable compounds are substantially water-soluble surfactants of the
general formula
R2 - Y - (C2H40)z - C2H40H
wherein R2 is selected from primary, secondary and branched chain alkyl
and/or acyl hydrocarbyl groups; primary, secondary and branched chain
alkenyl hydrocarbyl groups; and primary, secondary and branched chain
alkyl- and alkenyl-substituted phenolic hydrocarbyl groups; said
hydrocarbyl groups having a hydrocarbyl chain length of up to 20,
preferably from 10 to 18 carbon atoms.
Y is typically -O-, -C(O)O-, -C(0)N(R)-, or -C(0)N(R)R-, in which R2 and
R, when present, have the meanings given hereinbefore, and/or R can be
hydrogen, and z is of from 5 to 50, preferably of from 1- to 30.
The nonionic surfactants herein are characterized by an HLB (hydrophilic-
lipophilic balance) of from 7 to 20, preferably from 8 to 15.
Examples of particularly suitable nonionic surfactants include
-Straight-Chain, Primary Alcohol Alkoxylates such as tallow alcohol-EO(11 ),
tallow alcohol-EO(18), and tallow alcohol-EO(25);
-Straight-Chain, Secondary Alcohol Alkoxylates such as 2-C16E0(11); 2-
C20E0(11 ); and 2-C16E0(14);
-Alkyt Phenol Alkoxylates, such as p-tridecylphenol EO(11 ) and p-
pentadecylphenol EO(18), as well as
-Olefinic Alkoxylates, and Branched Chain Alkoxylates such as branched
chain primary and secondary alcohols which are available from the well-
known "0X0" process.
~)-Amine Oxides
Suitable amine oxides include those with one alkyl or hydroxyalkyl moiety
of 8 to 28 carbon atoms, preferably from 8 to 16 carbon atoms, and two
alkyl moieties selected from alkyl groups and hydroxyalkyl groups with 1 to
3 carbon atoms.
Examples ,include dimethyloctylamine oxide, diethyldecylamine oxide, bis-
(2-hydroxyethyl)dodecylamine oxide, dimethyldodecyi-amine oxide,
dipropyltetradecylamine oxide, methylethylhexadecylamine oxide, dimethyl-
2-hydroxyoctadecylamine oxide, and coconut fatty alkyl dimethylamine
oxide.
CA 02270383 1999-04-29
WO 98/18890 PCT/US97119794
A preferred stabiliser for use herein is a nonionic alkoxylated surfactant.
When used, such nonionic alkoxylated surfactant will be present in an
amount of 0.01 % to 10% by weight, preferably from 0.05% to 2% by
weight of the composition.
Electrolyte stabiliser
Inorganic viscosity control agents which can also act like or augment the
effect of the stabilisers, include water-soluble, ionizable salts which can
also optionally be incorporated into the compositions of the present
invention. Incorporation of these components to the composition must be
processed at a very slow rate.
A wide variety of ionizable salts can be used. Examples of suitable salts are
the halides of the Group IA and IIA metals of the Periodic Table of the .
Elements, e.g., calcium chloride, magnesium chloride, sodium chloride,
potassium bromide, and lithium chloride. The ionizable salts are
particularly useful during the process of mixing the ingredients to make the
compositions herein, and later to obtain the desired viscosity. The amount
of ionizable salts used depends on the amount of active ingredients used in
the compositions and can be adjusted according to the desires of the
formulator. Typical levels of salts used to control the composition viscosity
are from 20 to 2000 parts per million (ppml, preferably from 20 to 1100
ppm, by weight of the composition.
Alkylene polyammonium salts can be incorporated into the composition to
give viscosity control in addition to or in place of the water-soluble,
ionizable salts above. In addition, these agents can act as scavengers,
forming ion pairs with anionic detergent carried over from the main wash,
in the rinse, and on the fabrics, and may improve softness performance.
These agents may stabilise the viscosity over a broader range of
temperature, especially at low temperatures, compared to the inorganic
electrolytes.
Specific examples of alkylene polyammonium salts include I-lysine
monohydrochloride and 1,5-diammonium 2-methyl pentane dihydrochioride.
The present invention also encompasses a method for treating fabrics
which comprises the step of contacting said fabrics in the rinse cycle with
an aqueous medium containing a composition as defined hereinbefore.
CA 02270383 1999-04-29
WO 98/18890 PCTlUS97/19794
21
Preferably, the aqueous medium is at a temperature between 2 to 40°C,
preferably between 5 to 25°C.
The invention is illustrated in the following non limiting examples, in which
T all percentages are on an active weight % basis unless otherwise stated.
In the examples, the abbreviated component identifications have the
following meanings:
DEQA . Di-(tallowyl-oxy-ethyl) dimethyl ammonium chloride
Fatty acid . Tallow tatty acid of I V =18
PEG . Polyethylene Glycol 4000
Nonionic . block copolymers of terephthalate and ethylene
1 oxide or
propylene oxide commercially available from Hoechst
under the tradename HOE S3639
Nonionic . block copolymers of propylene oxide and ethylene
2 oxide
in which the central block is polypropylene oxide
commercially available from BASF under the tradename
Pluronic PE 4300
Nonionic . oxyalkylated amines commercially available
3 from BASF
under the tradename Pluronic PE 10400
Example
The followin
com ositions
are in accordance
with the
resent invention:
Com onent A B C D E F G
DEQA 5.0 5.0 5.0 4.0 4.0 4.0 5.0
Fatt acid 0.5 0.5 0.5 0.4 0.4 0.4 0.5
Nonionic 1 0.1 - - 0.05 - - 0.05
Nonionic 2 - 0.2 - - 0.1 - 0.10
Nonionic 3 - - 0.2 - - 0.1 -
PEG 0.2 0.2 0.2 0.2 0.2 0.2 0.2
Perfume 0.5 0.5 0.5 0.5 0.5 0.5 0.5
Water and
minors to
balance to
100