Note: Descriptions are shown in the official language in which they were submitted.
CA 02270967 1999-OS-06
WO 98/23811 PCT/FI97/00730
I
Bleaching of cellulosic pulp with ozon and peracid
The present invention relates to a method for the production of bleached pulp
with good
strength characteristics according to the preamble of claim 1.
According to such a method, the pulp is treated in at least one bleaching
stage in which
it comes into contact with ozone.
The invention also relates to the bleaching sequences according to the
preamble of claim
28 as well as to the pulp according to the preamble of claim 32.
Compared with ECF pulp, ozone-bleached softwood sulphate pulp is known to have
a
lower viscosity and about 10 % inferior tear strength. Viscosity has been
found to
correlate closely with tear strength for a given pulp. If the pulp viscosity
could be
I S improved, this would be reflected as an improvement in pulp strength.
The fall in viscosity is thought to be due not only to depolymerization
reactions but also
to the alkali-labile carbonyl groups formed during ozonation ( 1 ). As a
result of this alkali
lability, glycosidic bonds in the cellulose chain are broken in the subsequent
alkali stage
(2).
It has been suggested that the viscosity of ozonated (Z) pulp can be improved
by
treatment with borohydride (R), for example using the sequence OZ-R-P, where O
=
oxygen and P = hydrogen peroxide (3). Treatment with borohydride is based on
that the
alkali-labile carbonyl groups formed in the pulp during ozonation are reduced
by
borohydride to alkali-stable alcohol groups (4). The alcohol groups formed in
the
reduction reaction are not stable, however, and are gradually oxidized back to
carbonyl
groups, for example on exposure to air. Other drawbacks include the high cost
of
borohydride and possible problems in treating the effluents.
Other methods put forward include reducing the carbonyl groups to alcohols
with sodium
CA 02270967 1999-OS-06
WO 98I23811 PC~/FI97/00730
2
dithionite (5) and oxidizing them to carboxyl groups with chlorite (6) or-
hydroxylamine
hydrochloride (7).
Generally speaking, there are very few bleaching chemicals that can be
employed together
in the same bleaching stage. One of the chemicals will normally be more
reactive than the
other, and its bleaching reactions will therefore dominate. To take an
example: used
together, hydrogen peroxide and chlorine dioxide will react with each other,
which means
they are less effective together than separately.
Treatment with peroxomonosulphuric acid (Caa) has been proposed either prior
to, or
immediately after, ozone bleaching, the sequence being Caa/Z-E or Z/Caa-E.
Research
shows that both sequences lead to roughly the same bleaching results (8, 9).
Ozone and peracetic acid (Paa) have been used as consecutive stages in an
OZPaa
sequence and the results compared with those from OZEZ and OZP sequences (
10). The
pulps used included pine pulp with a kappa number of 20 after the oxygen
stage. The
highest brightness achieved was 78 - 81 %, even though the ozone charge was
around 20
kg/ADt and the peracetic acid charge 20 kg/ADt. In the study cited, prior to
ozonation
the sulphate pulps were steeped in acetic acid to which dimethylsulphoxide had
been
added. The problems associated with these solvents relate to fire hazards and
the di~culty
of their recovery from the bleaching filtrates.
In a more established procedure, peracetic acid, ozone and oxygen have been
employed
under acidic conditions in a single stage (FI Patent Application No. 940412,
reference 11 ).
In this well-known procedure, the pulp is first treated with peracetic acid,
which reacts
with lignin and opens up the pulp' s fibre structure. Peracetic acid treatment
allows the
use of a smaller ozone charge, which in turn reduces fibre damage. It is
believed that
treating the pulp with only small amounts of ozone will produce a stronger
pulp than that
obtained with the three-stage treatment O-Z-Paa. In accordance with FI Patent
Application No. 940412, ozonation is followed by an alkaline extraction stage
using either
chlorine dioxide or peroxide.
CA 02270967 1999-OS-06
WO 98/23811 PCT/FI97/00730
3
In other known bleaching experiments, pulp has been treated with ozone,
peroxide and
peroxy acids such as peracetic acid. In their report, Kinell et al. compared
pulps bleached
with peroxide combined with ozonation, ozone and peracetic acid treatment, and
peroxy
acid treatment alone, with pulps bleached using chlorine dioxide (y~). The
authors
concluded that ozone bleaching is not suitable for the production of good-
strength pulps.
The use of peroxy acids and hydrogen peroxide in conjunction with ozone
bleaching has
also been studied by Nin and Ooi (1~,) using the sequences ZE(O)PaaQP and
ZE(O)QPPaa. Based on the results, the authors proposed that hydrogen peroxide
be
employed before peroxy acid in the early stages of the bleaching sequence,
where there
are still large amounts of residual lignin.
WO Published Application No. 92!2l814 describes a bleaching sequence in which
an
ozone stage and a washing stage are followed by a Paa stage employing 2 %
peracetic
acid and 3 % NaOH. The slurry is then maintained at a temperature of 70
° C for 240 min.
When the instructions in the publication are followed, the pH is 10.6 at the
start of the Paa
stage and l0.2 at the end. The alkali !ability resulting from the treatment of
ozonated pulp
under these alkali conditions causes depolymerization and a decrease in pulp
stren~h.
For the sake of completeness, it can be added that the use of a solvent such
as an alcohol,
aldehyde or carboxylic acid has been proposed as a way of improving the
selectivity of
ozonation. The main purpose of the solvent is to capture the harmful radicals
formed
during ozonation. Other proposed solvents include urea-methanol, methanol, DMF
and
DMSO. However, these are expensive and also present problems in relation to
effluent
treatment and cycle closure.
The purpose of the present invention is to overcome the drawbacks of the prior
art and
to provide an entirely novel type of a process for the ozone bleaching of
pulp.
It is also the purpose of the invention to provide new bleaching sequences and
to produce
a pulp that is new in terms of its characteristics.
RECTIFIED SHEET (RULE 91)
CA 02270967 1999-OS-06
WO 98/238I1 PCT/FI97/00730
4
The present invention is based on the surprising fact that the treatment of an
ozonated
pulp with an oxidizing agent under acidic conditions can reduce or completely
eliminate
pulp alkali lability. The acid stage can be carried out either after washing
or without any
prior washing stage. In the next stage, residual lignin is extracted from the
pulp fibres
S under alkaline conditions without breaking the cellulose chains. The alkali
extraction can
be performed with or without prior washing using peroxide and/or peroxide
reinforced
with oxygen.
According to a preferred embodiment, fully bleached pulp (ISO brightness >
87.5 %) with
a kappa number of less than 2, a viscosity greater than 600 ml/g, a tear index
(T70) of
more than 13 mN~mz/g, and a zero-span tensile index (T70) of over 100 N~m/g
measured
for a wet sheet can be produced by means of the treatment.
The invention can be applied in practice using bleaching sequences containing
Z, Paa and
1 S P and/or E stages in this order, one essential requirement being that the
Z stage is not
followed by an alkali treatment stage before the ozonated pulp has been
treated with an
oxidizing agent, in particular a peroxy alkanoic acid.
More specifically, the method according to the invention is characterized by
what is stated
in the characterizing part of claim 1.
The bleaching sequences according to the invention are characterized by what
is stated in
the characterizing part of claim 28; the pulp according to the invention is
characterized
by what is stated in the characterizing part of claim 32.
As stated above, the invention can be used to reduce the alkali lability of
ozonated pulp
by bringing the pulp into contact with an oxidizing bleaching agent under
conditions that
are acid (including neutral or at most slightly acid conditions). Although we
do not fully
understand the mechanism underlying the bleaching process, it seems clear that
this kind
of treatment reduces the number of carbonyl groups in the pulp, which in turn
means less
hydrolysis of glycosidic bonds in the alkali stage following the acid stages.
This finding
CA 02270967 1999-OS-06
WO 98/23811 PCT/FI97/00730
has to be regarded as surprising, since it is previously known that the
carbonyl group
content of pulp increases as a result of a treatment with performic and
peracetic acids. As
the experimental results presented below will show, the carbonyl group
contents of pulps
to be bleached in accordance with the invention are doubled during ozonation,
whereafter
5 these contents clearly fall by 10 - 15 % (in same cases 20 %) as a result of
treatment with
peracetic acid subsequent to ozonation. By washing the pulp between the ozone
and
peracetic acid stages the carbonyl content can be further reduced (by just
under 30 %).
Unlike the solution according to the WO Published Application (see above), the
method
according to the invention treats Z pulp with peracetic acid under acidic
conditions prior
to the alkaline stage, thereby oxidizing some of the carbonyl groups to
carboxyl groups.
As a result, the pulp undergoes far less depolymerization during the
subsequent alkaline
stage. The pH at the start of this treatment is acid, neutral or slightly acid
(e.g., approx.
4.5 - 8), while the final pH is acid (usually approx. 4.5 - 5).
In addition to reducing the carbonyl group content, the method according to
the invention
reduces the amount of carbohydrates dissolving under alkaline conditions.
Carbohydrate
dissolution is at least 30 % lower than in cases where the pulp is treated
under alkaline
conditions after ozonation. Preventing the dissolution of carbohydrates helps
to improve
tear strength, the strengths of individual fibres (zero-span strength) and
yield, and also to
reduce the solute content of the filtrate.
The invention also offers other significant benefits. For example, the method
does not
employ organic solvents or other compounds that would capture radicals because
the
carbonyl groups formed by unwanted reactions are removed by the after-
treatment
according to the invention.
The method according to the invention produces high-brightness pulps by
reducing the
residual lignin content before ozone treatment and carrying out an alkali
treatment at the
end of the bleaching stage, yielding pulp with 85 - 92 % brightness (sequence
OOZPaaO
or OOZPaaE, for example). Performing the alkaline treatment immediately after
the Paa
treatment also allows unreacted peroxide to be utilized at the high pH, thus
leading to
CA 02270967 1999-OS-06
WO 98/23811 PCT/FI97/00730
6
higher brightness.
The method disclosed in the present invention allows the use of higher ozone
charges
than in the process described in reference 11, provided that the pulp is
subsequently
subjected to oxidation, for example with peracetic acid, under acidic
conditions. Since the
use of ozone as a bleaching agent is fairly economical in the long term, the
present
invention also offers economic benefits.
Another benefit of particular significance is the fact that the bleaching
process described
lends itself to process cycle closure. The filtrate from the alkaline
extraction stage
immediately after peroxy acid treatment can be recycled, in some cases without
treatment,
for use in oxygen delignification, or else it can be processed without
problems in a
recovery boiler.
In the following, the present invention will be examined more closely with the
help of a
detailed description and working examples. The diagrams appended provide
graphic
representations illustrating the results from the working examples, as
follows:
Figure 1 shows pulp delignification for different bleaching sequences,
Figure 2 shows the development of brightness,
Figure 3 shows hydrogen peroxide consumption in the final P stage of the
bleaching
sequence,
Figure 4 shows the tear indexes of the bleached pulps,
Figure S shows the zero-span indexes (wet) of the bleached pulps,
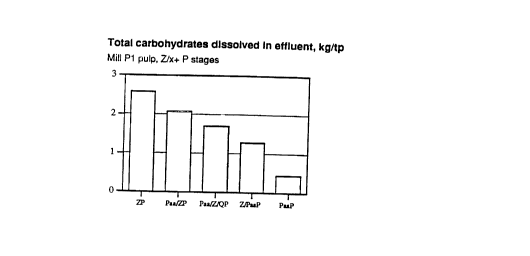
Figure 6 shows the carbohydrate contents of the bleaching filtrates
separately,
Figure 7 shows the total carbohydrate contents of the bleaching filtrates per
tonne of
bleached pulp,
Figure 8 shows the tear indexes of Z/Paa/EP pulp for different ozone and Paa
charges,
and
Figure 9 shows the zero-span tensile index (wet) of Z/Paa/EP pulp.
According to the invention, the bleaching sequence for pulp includes the
following stages:
- CA 02270967 1999-OS-06
WO 98/23811 PCT/FI97/00730
7
-Z/Paa/P,
-Z -Paa/P,
-Z/Paa -P,
-Z/Paa/E,
-Z/Paa -E,
-Z/Paa/EP,
-Z/Paa/ER,
where Z = ozone stage, Paa = peroxy acid stage, P = peroxide stage, E =
alkaline
extraction stage, R = an alkaline stage involving borohydride, O = oxygen
delignification
stage, and the hyphen (-) indicates a washing stage. The Z stage should
preferably be
preceded by a peroxide (P) or pressurized peroxide (PO) stage.
According to the invention, the pulp pH is not allowed to rise to well on the
alkaline side
between ozonation and treatment with oxidizing bleaching agent in order to
avoid the
dissolution of carbohydrates that would result at alkaline pH from the
formation of alkali-
labile groups through the reactions of ozone. The pH should preferably be kept
between
2 and 8, the preferred pH being around 4.5 - 8 at the start and about 2.5 - 6
at the end.
Ozone treatment and treatment with an oxidizing bleaching agent are performed
either in
the same bleaching stage or in different stages. Ozone treatment and treatment
with an
oxidizing bleaching agent can be carried out more or less simultaneously, or
else the pulp
can first be treated with ozone and then with an oxidizing bleaching agent.
The
simultaneous use of ozone and peroxy acid has not been found to cause any
problems.
According to one preferred embodiment of the invention, the total oxidation
equivalent
to be used in bleaching is divided more or less equally between ozonation and
peroxy acid
treatment. Accordingly, ozone normally accounts for 30 - 90 % (preferably
about 40 - 70
%) of the oxidation equivalent used in bleaching, white the peroxy acid
accounts for 70 -
10 % (preferably about 60 - 30 %) of the oxidation equivalent used in
bleaching.
CA 02270967 1999-OS-06
WO 98/23811 PCT/FI97100730
8
The oxidizing bleaching agent used according to the present invention is a
peroxy acid
such as a lower peroxy alkanoic acid, more particularly performic acid,
peracetic acid or
perpropionic acid, or peroxomonosulphuric acid (Caro' s acid) or mixtures
thereof.
Peracetic acid, which is a particularly suitable peroxy alkanoic acid, is
prepared by reacting
acetic acid with hydrogen peroxide in the molar ratio 1:1 - 1:2 with a small
amount of
sulphuric acid as catalyst. Peracetic acid is used either as an equilibrium
product or in
distilled form. Typical conditions for a peracetic acid stage are: peracetic
acid charge 2 -
40 kg/BDt, pH 3 - 8, temperature 50 - 90 ° C and reaction time 30 min -
6 hours. If
necessary, the peracetic acid stage can include additives, e.g. magnesium
sulphate and/or
a chelating agent such as EDTA or DTPA, at the rate of 0.5 - 3 kg/BDt.
Particularly
suitable conditions for a peracetic acid treatment are: pH 4.5 - 7, reaction
time 30 - I 80
min, and temperature 50 - 80 ° C.
For the ozone stage, typical conditions include a pulp consistency of 1 - 40 %
(i.e. low,
medium or high consistency). The ozone gas used for bleaching is a mixture of
ozone and
oxygen, usually containing 5 - 15 % ozone. The optimum pH for ozonation is
between 2
and S, preferably about 3. The pH is usually adjusted with sulphuric acid,
although organic
acids can also be used. With very low ozone charges (under 2 %), a higher pH
(5 - 10)
can be employed. However, in this case some of the ozone decomposes and the
charge
therefore has to be increased to make good the loss. The temperature in the
ozone stage
can be 0 - 80 ° C. In industy) ozonation is performed at a temperature
of around 50
70 ° C. A high temperature has the same kind of impact as high pH. The
ozone charge is
0.5 - 15 kgBDt, usually 2 - 6 kg/BDt, the charge depending on the kappa number
of the
incoming pulp. A high kappa number requires a greater ozone charge than a low
kappa
number.
According to one preferred embodiment, the pulp is washed with water between
the
ozone and peroxy acid stages until it appears neutral or only slightly acidic.
This washing
further reduces the content of dissolved carbohydrates, improves both tear
index and
_ brightness, and appears to lower the content of carbonyl groups. Washing can
be carried
out in the usual way in one or more stages, with the water displaced from the
pulp
CA 02270967 1999-OS-06
WO 98I23811 PCT/FI97/00730
9
between washing stages by filtration.
The pulp going forward to the alkali stage has a carbonyl group content of at
least 10 %,
usually at least 1 S %, less than the pulp coming to the ozone stage. This
means that
certain typical sulphate pulps contain less than 14 milk-equivalents of
carbonyl groups per
kg of pulp when the pulp is bleached using 62S kg of oxidation equivalents
(OXE)/tonne.
In this invention, the alkaline treatment stage following the peroxy acid
stage comprises
any known treatment stage in which the pH of the pulp is made alkaline, i. e.
raised to a
pH of at least 8, preferably of around 10 - 12.
Suitable alkaline stages include alkaline extraction (usually abbreviated E),
possibly
incorporating treatment with another chemical such as borohydride (R) or
peroxide (P);
an oxygen stage (O), a hydrogen peroxide stage (P, PO), and combinations
thereof. The
conditions for the alkaline-stage correspond to those in known bleaching
sequences. The
borohydride charge is usually about 0.1 - 10 kgBDt. The amount of peroxide
used is
generally S - 60 kgBDt (0.5 - 6 %), which may be divided between the peroxide
stage
preceding ozonation and the peroxide stage following peroxy acid treatment.
To summarize what has been said above, one preferred embodiment of the
invention
would be as follows: ozone stage using approx. 1 - 8 kg ozoneBDt (0.1 - 0.8
%), peroxy
acid stage using approx. 1 - 30 kg peroxy acidBDt (0.1 - 3 %), peroxide stage
preceding
ozonation using approx. 5 - 30 kg hydrogen peroxideBDt (O.S - 3 %), and
peroxide stage
following peroxy acid treatment using approx. 1 - 20 kg hydrogen peroxideBDt
(0.1 - 2
%).
The pulp to be subjected to ozone bleaching may be chemical, chemi-mechanical
or
mechanical pulp. One very common type of pulp in this context would be oxygen-
delignified sulphate pulp, more particularly oxygen-delignified softwood pulp.
In addition
to oxygen delignification, the pulp may be subjected to other treatments with
agents such
as enzymes, complexing agents and peroxide, depending on the treatment stages
following
CA 02270967 1999-OS-06
WO 98I23811 PCT/FI97/00730
ozonation. Examples of bleaching stages that may be used prior to ozonation
include the
following sequences:
-O/O-X/Q-(PO),
S -O/O-X/Q-P,
-O-O-Q-P, and
- O/O-X-ZlPaa/Q-P,
where X refers to pulp treatment with an enzyme and Q refers to a stage
involving a
10 complexing agent. The abbreviations O, P, PO, Paa and Z have the same
meanings as
given earlier.
The pulp entering the ozone stage generally has a kappa number of about 2 -
16,
preferably about 2 - 10.
1S
The treatment specified in the present invention yields a pulp with good
strength
characteristics and, depending on the bleaching sequence used, an ISO
brightness of at
least 8S units, usually over 87 units. The pulp has a kappa number of less
than 2, in some
cases under 1.8, a viscosity greater than 620 ml/g (closer to 700 ml/g,
depending on the
temperature of the peroxy acid stage), a tear index (T70) of over 14 mN~m2/g
and a zero-
span tensile index (T70), measured from wet sheets, of over 10S N~m/g. The
zero-span
tensile index (wet sheet) can be raised to almost 11 S N~m/g by washing the
pulp between
the peroxy acid stage and the alkali stage. The tear index is preferably over
14. S mN~m2/g.
2S As stated above, the bleaching sequences specified in the invention can be
made part of
cycle closure. This especially applies to sequences that do not contain a
separate
complexing agent stage (Q). The filtrate from the alkaline extraction stage is
recovered
and returned, at least in part, to pulp cooking, oxygen delignification or
bleaching.
Performing an alkali treatment immediately after the acid stages facilitates
both filtrate
handling and cycle closure. From the point of view of cycle closure, the
following
CA 02270967 1999-OS-06
WO 98/23811 PCT/FI97/00730
11
sequences according to the invention are particularly favourable:
OOPaa/Z/Paa/E,
OOQPZ/PaaO and OOQPZ/Paa/E. As the use of chelating agents in peroxide
bleaching
is essential for control of harmful metal ions and retention of viscosity, a
sequence ending
in an E or O stage, as specified in the invention, reduces the charge of
chelating agent
used.
One preferred embodiment is an alternative in which the alkali stage is
performed after
peroxy acid treatment without intermediate washing, whereby the filtrate from
the alkaline
extraction stage contains at least some peroxy acids, which form hydrogen
peroxide. This
filtrate can be recycled direct to oxygen delignification and to suitable
peroxide stages.
To avoid the use of chelating agent completely, a sequence according to the
invention can
be used, such as OOXPaa/Z/Paa/O, where X represents an enzyme treatment. The
principal enzymes used are hemicellulase or ligninase types such as xylanases
and/or
mannanases and laccase.
The following non-limiting examples illustrate the invention.
Example 1
These experiments were conducted using mill softwood sulphate pulp. An
equilibrium
solution of peracetic acid was prepared from 50 % hydrogen peroxide and 100 %
acetic
acid. The CH3COOH : HZOZ molar ratio was 1.67. The softwood sulphate pulp from
the
digester had a kappa number of 24. l, a brightness of 32.8 % and a viscosity
of l050 ml/g.
The pulp was delignified to the kappa number 10 using the sequence O/O-Q, and
bleaching was continued with a PO stage.
The PO pulp had a kappa number of about 6, a brightness of about 75 % and a
viscosity
of 760 - 790 ml/g. The pulp was then bleached using sequences according to the
invention
and also with reference sequences, as follows: Z-P, Z/Paa-P, Z-Paa-P, Z/D-P,
Z/R-P and
D-EOP-D. Calculated in terms of oxidation equivalents, the same amounts of
chemicals
CA 02270967 1999-OS-06
WO 98I23811 PCT/FI97/00730
12
were charged to the Z, Z/Paa, Z-Paa and Z/D stages, i.e. 625 kg OXE/BDt. Based
on the
total OXE charge, the amount of ozone used was 50:50 in relation to the
peracetic acid
or chlorine dioxide ratio. Based on optimization experiments, the borohydride
charge to
the Z/R stage was 10 kgBDt and the ozone charge 5 kg/BDt.
The bleaching conditions are shown in Table 1.
Table 1. Conditions used in the O/O, Q, PO, Z, Z/Paa, Z-Paa, Z/D, Z/R, P and D-
EOP-D bleaching stages.
StageConsistencyTemperatureTime InitialChemical charge
C min H k Dt
O/O 10 100 45/60 NaOH 25/I0, MgS04 5,
OZ
pressure 8/6 bar
Q 8 80 60 5 DTPA 3
PO 12.5 90 120 Hz02/NaOH 20/20, DTPA-
/MgS04 2/2.5, 02 pressure
6
bar
Z 12.5 50 10 3 O~ 5
Paa 12.5 70 l20 5 Paa 23.8, DTPA/MgS04
2/2.5
Z/Paa12.5/10 50/50 10/1803/S O;/Paa 2.5/12.7, DTPA/Mg-
SO 2/2.5
Z-Paa12.5/10 S0/70 10/1203/5 03/Paa 2.5/12.8,
DTPA/M SO 2/2. S
Z/D 12.5/l0 50/50 10/60 3/3 O /C10 2.S/4.2
Z/R 12.5 50/50 10/60 3/I0 O /NaBH 5/10
P 12.5 90 180 10.3 HZOZ/NaOH 15/20,
DTPA/M SO 2/2.5
D 8.0 69 60 -/2.6CIO 19.2/
EOP l0.0 70 60 - NaOH, H202 3
/10.1
D l0.0 75 180 -/4 C10, 20
The characteristics of the pulps are shown in Table 2.
- CA 02270967 1999-OS-06
WO 98I23811 PCT/FI97/00730
13
Table 2. Characteristics of the Z, ZlPaa, Z-Paa, Z/D, Z/R and D-EOP-D pulps.
O/O-Q-PO Z-P Z/Paa-PZ-Paa-PZ/D-P Z/R-P D-EOP-D
Ka a no. 6 1.4 2.5 1.1 1.4 1.7 0.8
Bri htness, 75 88.981.7 88.9 91.1 89.5 88.1
%
Stand. vise, 780 610 650 680 680 680 730
ml/
Borohydride 770 670 650 680 680 680 730
vise,
ml/
D vise, ml/ 10 60 0 0 0 0 0
*)
Yield, % 99.3 89.999.1 99. 99.0 99.4 not def
l
P consumption, 10.2 5.9 2.8 4.9 5.8 4.4
k 1 Dt
*) Difference between borohydride and standard viscosities.
The results indicate that ozone, peracetic acid, chlorine dioxide (oxidizing
agents) and
borohydride (reducing agent) fianction well together. Washing between the Z
and Paa
stages improved delignification to some extent. The H202 consumption in the
final P stage
of the bleachings Z/Paa-P and Z-Paa-P was approx. 5 kgBDt. In Z-P bleaching
the
consumption was much higher. The contents of carbonyl and carboxyl groups of
the pulps
and the differences between standard and borohydride viscosities are shown in
Table 3.
Table 3. Carbonyl and carboxyl group contents, and differences between
standard
and borohydride viscosities for Z, Z/Paa, Z-Paa, Z/D and Z/R pulps.
2s
Se uence Z Z/Paa Z-Paa Z/D Z/R
Pul PO Z Z Paa Z Paa Z D Z R
Carbonyl content,8.917.2 15.212.0 l4.310.9l5.2 15.517.2 2.4
mmoUkg
Stand. vise, 780650 680 710 720 650 710
ml/
Boroh. vise, 790710 700 710 740
ml/
0 vise. (boroh.-10 60 20 0 20 60
stand. , mU
CA 02270967 1999-OS-06
WO 98/Z3811 PCT/FI97/00730
14
As can be seen from the Table; ozonation causes a doubling of the pulp' s
carbonyl
content. In the sequence Z/Paa, the carbonyl content falls from 15.8 to 12.0
mmol/kg
during the Paa treatment subsequent to the Z stage. Washing the pulp between
the ozone
and Paa stages (Z-Pa,a) lowered the carbonyl content still fi~rther (14.3 to
10.9 mmol/kg)
after the Paa treatment. The alkali lability of ozone-bleached pulp falls
significantly during
Paa treatment, which is also reflected in the fact that the difference between
borohydride
and standard viscosities is only 0 - 20 ml/g.
Table 4 shows the bleaching results and papermaking characteristics of the
pulps at a
I0 tensile index of 70 N~m/g (T70).
Table 4. Bleaching results and papermaking characteristics obtained with 2-P,
Z/Paa-P, Z-Paa-P, Z/DP, Z/R-P, Paa-P and D-EOP-D sequences.
SequenceKappa BrightnessPFI Tear indexZero-spanZero-span
number revs. (T70) dry (T70)wet (T70)
(T70 mN~m2/ N~m/ N~m/
PO pulp 6.0- 75.0
Z-P 1.4 88.9 1963 13.1 I49 91
ZlPaa-P 1.5 87.8 1577 14.1 l44 10S
Z-Paa-P 1.1 88.9 1 S33 14.5 139 114
Z/DP 1.4 91.l 1630 14.8 149 l13
Z/RP 1.7 89.5 1916 15.2 l50 l08
Paa-P 1.7 87.7 1935 14.8 156 114
D-EOP-D 0.8 88.1 1785 14.3 143 124
The results from the above Table are presented graphically in Figs. 1, 2, 4
and 5.
Figure 3 presents hydrogen peroxide consumption in the final stage of the
sequences
Z-P, Z/x-P, ZxP, x/Z-P and Paa-P.
_ The Table shows that the strength of the TCFZ pulp can be improved by 13 -
16
by including in the ozone stage an oxidative treatment at acid conditions with
CA 02270967 1999-OS-06
WO 98I23811 PCT/FI97/00730
IS
peracetic acid (Paa) or chlorine dioxide (D), or reduction with borahydride
(R)
without intermediate washing. An even better kappa reduction, brightness and
tear
index than for the Z/Paa-P pulp were obtained with the sequence Z-Paa-P, i.e.
with
intermediate washing. Zero-span tensile indexes measured from wet sheets were
14
- 23 units ( 15 - 25 %) greater for the Z-Paa-P than the Z-P pulp.
Example 2
A mill pulp subjected to the treatment O-Q-O-P 1 was bleached using the
sequences
P-Z-P, P-Paa/Z-P, P-Paa/Z/Q-P, P-Z/Paa-P, P-Z/R-P and P-Paa-P. The conditions
used in the Z/x-P sequences (x = Q, Paa, Caa and NaBH4) are shown in Table 5.
Table 5. Bleaching conditions used in the Z, Paa, Z/x, x/Z and P2 stages.
StageChemical DTPA- Final TemperatureReactionPulp
charge /MgS04 pH time consistency
k Dt k Dt C min
Z 5 3 50 I2.5
Z/Q 5/2 3/S 50/S0 /60 l2.5/12.5
Paa/Zvaried 2/2.5/ 3.8/3.S50/S0 60-180/12.5/l2.5
Z/Paa2.5/1l.9 /2/2.5 3/5 50/50 /l80 12.5/l0
Z/R 5/10 3/l0 50/S0 /60 12.5/12.5
Paa 23.8 2/2.5 5 180-90 120 12.5
P HzO,/NaOH 2/2.5 90 180 12.5
20% 15
30
The carbonyl contents and viscosities of the pulps are presented in Table 6.
CA 02270967 1999-OS-06
WO 98/23811 PCTIFI97/00730
16
Table 6. Pulp carbonyl contents and viscosities.
Sta a Z Paa/Z Z/R Z/Paa Paa
Chemical charge, kg/BDt5.0 11.9/2.55/5 2.5/11.924
Carbonyl content, mmol/kg15.8 l2.9 9.8 11.3 9.9
Stand. viscosity, ml/g660 690 700 720 750
Borohydride viscosity,720 730 700 740 7S0
ml/g
0 visk. boroh.- stand.60 40 0 20 0
, ml/
The high carbonyl content of the Z pulp is clearly reflected in the difference
between
borohydride and standard viscosities, which was 60 ml/g. The corresponding
difference in the case of the Z/Paa and Z/R pulps was much smaller (0 - 20
mI/g).
The kappa numbers, brightnesses and papermaking characteristics of the pulps
are
shown in Table 7.
Table 7. Bleaching results and papermaking characteristics (T70 = PFI
beating to a tensile index of 70 N~m/g) of the pulps.
Sequence Kappa BrightnessPFI Tear indexZero-spanZero-span
number revs. (T70) dry (T70)wet (T70)
(T70) mN~mz/~; N~m// N~m/
P I pulp 6.8 75.8 939 l 3 4 147 12l
ZP 1.8 88.3 1642 12.1 144 102
Paa/ZP 1.8 88.0 1840 l2.3 138 101
Paa/Z/QP 2.0 87.9 1517 12.3 142 100
Z/PaaP 1.8 88.0 1467 13.2 150 109
Z/RP 2.0 88.9 1417 13.0 1 SO 102
PaaP 1.9 88.0 l659 l2.9 138 115
As Table 7 shows, the tear index of Z/PaaP pulp bleached using the sequence
according to this invention is 1.2 units higher than that of pulp bleached
with the
reference sequence ZP.
CA 02270967 1999-OS-06
WO 98/Z3811 PCT/FI97/00730
17
Example 3
The bleaching experiments according to the invention were performed on
softwood
sulphate pulp with a kappa number of 16 and a viscosity of 810 ml/g that had
been
oxygen-delignified (O) at the mill. The pulp was subjected to chelation with
EDTA
(Q) at pH 5 and then pre-bleached with oxygen in a pressurized peroxide stage
(PO). The hydrogen peroxide charge in the peroxide stage was 20 kgBDt
(consumption 13 kgBDt), the alkali charge 20 kgBDt, pH I0.5, temperature
80 ° C, reaction time 180 min and pressure about 6 bar. The additives
were DTPA
(5.5 k~BDt) and MgS04 (2. S kgBDt). The kappa number was reduced to 5.6 by
this treatment.
Ozone bleaching (Z) was performed at medium consistency with an ozone charge
of 0.5 kgBDt (consumption 3.6 - 4.0 kgBDt) at 50 ° C and pH 3. The
peracetic
I S acid stage (Paa) was carried out, without washing, using a distilled
peracetic acid
product with charges of 5, 15 or 20 kgBDt (consumption 5, I 0 and 12 kgBDt) at
70 ° C and pH 5 using a reaction time of 180 min. In the subsequent
alkaline
extraction stage (E), which was also performed without washing, the NaOH
charge
was 20 kgBDt, which raised the pH to 10.5. The reaction time was 60 min and
the
temperature 70 ° C.
After the Z/Paa/E stages, the pulps were washed and the pH adjusted to 4.5
with
sulphur dioxide.
In one experiment, the E stage was boosted with a small (3 kgBDt) peroxide
charge (Ep stage). In another experiment, borohydride (0.5 kgBDt) was used in
the
E stage. In the experiment with the lowest peracetic acid charge (5 kgBDt),
the E
stage was replaced by a peroxide stage (P) without intermediate washing, the
hydrogen peroxide charge being I O kgBDt (consumption S kgBDt).
For comparison, the same ~zonated pulp was subjected to an alkaline peroxide
CA 02270967 1999-OS-06
WO 98/23811 PCT/FI97/00730
18
stage (P) without any oxidative treatment under acid conditions. The sequence
was
thus O/O-Q-PO-Z-P. The effects of these treatments on pulp viscosity are shown
in the Table below. A duplicate series of experiments was performed to confirm
the
results (Table 8).
Table 8. Effects of the bleaching sequences according to the invention on the
characteristics of bleached sulphate pulp. T70 = tensile index 70 N~m/g.
Kappa BrightnessStand. Kappa BrightnessStand.
no. visc. no. visc.
ml/ % ml/
Series Series
1 2
Z-P (com1.7 88.9 580 Z-I' 1.6 88.0 590
.) (com
.)
Z/Paa/E 1.2 88.7 650 Z/Paa/E 1.2 87.8 630
1.2 89.1 650
8.7 7n R 1.3 88.I 650
Z/Paa/P 1.6 88.7 620 Z/Paa/P 1.4 88.l 6I0
Tear index (T70) Z/P: I2.4 mN~m2/g
Z/Paa/E l3.4 "
Zero-span tensile index (T70) measured from wet sheet:
Z/P: 104 N~m/g
Z/Paa/E I06 "
As can be seen from the Table, after the treatment according to the invention
the
pulp viscosities were 40 - 90 viscosity units higher than those of the
reference (Z-P)
pulps.
CA 02270967 1999-OS-06
WO 98/23811 PCT/FI97/00730
19
Example 4
The effects of borohydride (R stage) and peracetic acid (Paa stage) have been
compared using the same ozone-treated pulp (ozone charge S kgBDt). The
borohydride and peracetic acid charges used were 0, 2.5, 5, 7. S and 10 kgBDt.
Peroxide (P stage) was used for final bleaching. The pulp viscosities
resulting from
said reducing (R) and (Paa) stages according to the invention are shown in
Table
9.
Table 9. Effects on Z pulp characteristics of a reducing agent (borohydride,
R stage) and of an oxidizing agent (peracetic acid, Paa stage) according to
the
invention. Starting pulp: O/OQ{PO) pulp, kappa no. 7.3, brightness 72.8 %
and viscosity 790 mUg.
NaBH4 Kappa BrightnessViscosityKappa BrightnessViscosity
or Paa number ml/g number % ml/g
char~eBDt
Z/RP Z/RP Z/RP Z/PaaP Z/PaaP Z/Paa/P
0.0 1.9 88.1 610 2.1 87.3 630
1.0 2.3 87.4 620 2.1 87.4 670
2.5 1.9 88.8 640 2.1 87.6 670
5.0 1.9 89.l 660 88.2 660
10.0 1.9 89.4 660 1.7 88.2 670
As Table 9 shows, the borohydride (R stage) and peracetic acid (Paa stage) are
equally effective in delignifying ozone-treated pulp and removing its alkali
lability.
CA 02270967 1999-OS-06
WO 98I23811 PCT/FI97/00730
Example 5
The method according to the invention can be used in environment-friendly
processes in order to assist water cycle closure in a bleaching plant without
the need
5 for chelation and/or treatment of the acid filtrates. The use of ozone and
peracetic
acid together in the same stage was tested using the sequences O-O-Paa/Z/Paa
and
O-O-Z/Paa/E. As neither bleaching sequence involves a peroxide stage (P or
PO),
there is no need for a separate chelation stage or the special treatments that
such a
stage necessitates. Another advantage of this type of sequence is that it does
not
10 increase the sulphur load to the recovery system because pH adjustment is
performed with peracetic acid instead of sulphuric acid. The bleaching results
obtained are presented in Table 10.
Table 10. Results from environment-friendly bleaching sequences O-O-
15 Paa/Z/Paa and O-O-Z/Paa/E. Starting pulp: O-O pulp, kappa no. 8.2,
brightness 51.7 % and viscosity 780 ml/g.
Sequence Chemicals charges/consumptionsKappa Bright- Visc.
kg/BDt numberness mUg
%
Paa/Z/Paa/EO, 6I5.2, Paa l5/14.8, 20/19.9,1.5 81.2 S00
NaOH 35
20 Z/Paa/E O; 6/4.6, Paa: 20/19.9, NaOH 1.8 79.3 530
22
As can be seen from Table 10, both sequences result in a low kappa number and
thus good
brightness stability.
One of the environment-related targets at pulp mills is to reduce the loadings
from
bleaching. From this point of view, minimum carbohydrate dissolution during
the bleaching
stages would be an advantage. Figure 6 shows the carbohydrate contents of the
bleaching
filtrates individually and in total per tonne of bleached pulp.
-
CA 02270967 1999-OS-06
WO 98/23811 PCT/FI97/00730
21
As Figure 6 shows, use of the sequences according to the invention roughly
halves the
amount of carbohydrates found in the filtrates. Replacing some of the ozone
charge with
peracetic acid reduces the amount of oxalic acid formed through ozone
reactions, thus
easing the problem of oxalate precipitation. No oxalate precipitation occurs
in Paa
S bleaching. The present invention is thus particularly useful in helping to
achieve water cycle
closure at pulp mills.
Example 6
Table 11 below presents the optimum pH range for a combined ozone and
peracetic acid
stage (Z/Paa), though without restricting the invention to this. Experiments
were performed
using a softwood sulphate pulp pre-bleached using the sequence O-O-Q-(PO) to
kappa
number 5.5 and 74 % brightness. Final bleaching was performed with the
sequence Z/Paa/E.
The ozone charge was 5 kgBDt and the temperature 50 ° C. The Paa stage
was carried out
1 S with a peracetic acid charge of 15 kg/BDt at 70 ° C for 180 min.
Also shown in the Table
is the effect on bleachability of a pressurized Paa stage. In this case the
ozone charge was
only 2.5 kgBDt, the peracetic acid charge only 10 kg/BDt, the temperature 50
° C and the
reaction time 60 min.
Table 11. Effect of pH in Z/Paa stages on the bleachability of softwood
sulphate pulp
using the sequence O-O-Q(PO)-Z/Paa/E according to the invention.
03 charge/conc.Paa charge pH in Kappa Brightness,Viscosity,
/ stage
kg/BDt conc. Z Paa no., final, final,
final % mUg
k~/BDt
U_nnress.
Paa:
5.1/3.6 15/l0.2 3 3 1.1 88 660
4.9/3.7 15/11.6 3 5 1.1 87 640
5.1/3.9 15I11.9 5 5 1.3 85 640
Pressurized
Paa:
2.5/1.9 l0/5.3 3 5 1.8 85 670
CA 02270967 1999-OS-06
WO 98I23811 PCT/FI97/00730
22
As the Table shows, the highest brightness is achieved by performing the Z
stage at pH 3.
The results also reveal that the economic benefit achieved using the invention
can be
improved not just by omitting washing stages but also by pressurizing the
peracetic acid
stage. This reduces the amounts of chemicals needed and/or the reaction
temperature in the
Paa stage and/or the reaction time.
Example 7
Oxygen-delignified (O/O) mill sulphate pulp was bleached using different ozone
and
peracetic acid charges in Q-(OP)-ZlPaa/EP sequences. The bleaching conditions
are shown
in Table 12.
Table 12. Chemical charges and reaction conditions used in the bleaching
experiments.
Conditions (O/O Q sta OP) sta Z sta Paa stage EP sta
ul a a a a
Consistency % 8 12.5 l2.5 10 10
NaOH, kgBDt 20 2-7 8-17
Ozone, kgBDt 0-10
Peracetic acid, 0-20
kgBDt
Peroxide, kgBDt 20
MgS04, kgBDt 2.5 2.5 2.5
DTPa, kgBDt 3 2 2
02 pressure, bar 6 2
For pH adjustmentHzS04 NaOH HZS04 NaOH/HZS04 NaOH
Initial pH 4.5 3.0 5.0 10.5
Temperature, C 80 90 50 70 70
Reaction time, 60 120 3-13 120-180 60
min
The results are presented in Table 13, while Figure 8 shows brightness
development and
Figure 9 the tear strengths of the resulting pulps.
CA 02270967 1999-OS-06
WO 98/23811 PCT/FI97/00730
23
Table 13. Characteristics of bleached sulphate pulps. 5/0 = reference, i.e.
O/O-Q-
(OP)-Z/EP without peracetic acid.-
03/Paa chargeKappa Bright.Visc. Tear index Zero-span wet
k Dt no. % mi/ (T70) (T70), N'm/
mN'mz/
0/0 4.6 7S.0 730 n.d. n.d.
0/l0 2.7 81.7 730 n.d. n.d.
2.S/5 2.2 83.2 6S0 n.d. n.d.
2.5/1S 1.2 86.7 660 n.d. n.d.
5/0 1.0 81.6 570 12.2 104
5/10 0.8 88.3 6l0 l3.6 114
S/1S 0.7 88.0 6S0 l3.7 108
5/20 0.7 89.7 610 12.4 n.d.
7.S/S 0.6 89.3 590 12.0 n.d.
1S 7.S/15 0.2 90.3 580 12.3 1I0
10/10 0.5 90.6 S30 11.6 l01
The results show that, in this case, the best strength characteristics and
high brightnesses
were obtained using the sequence O/O-Q-(OP)-Z/Paa/Ep according to the
invention with
an ozone charge of S kgBDt and a peracetic acid charge of 10 - 1 S kg/BDt.
Figure 8 presents the tear index of Z/Paa/EP pulp at a tensile index of 70
Nm/g for the
different ozone and peracetic acid charges. It can be seen that the sequence
2S O/OQ(PO)Z/Paa/EP led to tear index values about 2 units higher than with
the
O/OQ(PO)ZP sequence. The tear indexes compare with those of ECF pulps.
Figure 9 shows the zero-span tensile indexes (wet sheet) of Z/PaalEP pulp for
different
ozone and peracetic acid charges. The index reflects the strengths of
individual fibres. The
bleaching process according to the invention also improves the strengths of
individual
fibres.
CA 02270967 1999-OS-06
WO 98I23811 PCT/FI97/00730
24
References
1. Jacobson, B., Lindblad, P-O., Nilvebrant, N O. Lignin reactions affect the
attack of
ozone on carbohydrates. Int. Pulping and Bleaching Conf., Stockholm June I 1-
I 4, 1991,
Vol. 2, 45-58.
2. Malinen, R, Fuhrmann, A. Recent trends in bleaching of chemical pulp.
Paperi ja Puu -
Paper and Timber 77 (l995):3, 78-83.
3. Chirat) C., Lachenal, D. Minimizing pulp degradation during totally
chlorine free
bleaching sequences including an ozone stage. 1994 Int. Pulp Bleaching Conf.,
Vancouver,
British Columbia, June 13-16, 1994, Papers, 109-114.
4. Cellulose chemistry and its application. Editors: Newell, T.P., Zeronian,
S.H., ElIis
Harwood Ltd. Chichester;-England, l985, 248-262.
5. Guibault, L.J., Hache, M., Munroe, D. C., Wang, D.L.K, Teodore.rcu, G.
Kemiallisen
massan valkaisu. FI pat.hak. 945202, 4.11.1994.
6. Schleicher) H., Lang) H. Carbonyl- and Carboxylgruppen in Zellstofl'en and
Cellu-
loseprodukten. Das Papier 48 {l994): 12, 765-768.
7. Kishitomoto, T.) Nakatsubo, F., Murakami, K. Non-chlorine bleaching of
kraft pulps I.
Evaluation of ozone-polysaccharide reactions in ozone bleaching. J. Jpn. Wood
Res. Soc.
39 (I993): 9, 1049-1055.
8. Hamman, M. Menetelma kemiallisten paperimassojen valkaisemiseksi ja ASAM-
25. FI
Pat.hak. 93S422, 3.12, 1993.
9. Francis) RC., Zhange) X Z., Troughton, N.A. Parannnettu otsonilperhappo-
menetelma
lignoselluloosapitoisen materiaalin deiignifioimiseksi. FI pat.hak. 9S4463,
21.9.199S.
- CA 02270967 1999-OS-06
WO 98I23811 PCTfFI97/00730
I0. Rotherrberg, S., Roborrson, D.H , Johosonbaugh, D.K. : Bleaching of oxygen
pulps with
ozone. Tappt J. 58 (1975): 8, l82-185.
11. Parthasarathy, V R.) Rudie, G.F. Selluloosamassojen
happi/otsoni/peretikkahappo-
5 delignifiointi ja -valkaisu. FI pat.hak. 940412, 28.1.1993.
12. Kinell, P., Strom, K E., Swan, N. : Satsning p$ uthallig utveckling room
Storas
massafabriker. Svensk Papperstidning/Nordisk Cellulosa 98 (1996): 2, 37-39
10 13 . Ni, Y., Ooi, T : Laboratory study on bleaching softwood kraft pulp by
a totally
chlorinefree process including the novel ozone bleaching. Tappi Journal 79 (
1996): 10, 167-
I72
20
30