Note: Descriptions are shown in the official language in which they were submitted.
CA 02272624 1999-OS-20
WO 98122777 PCT/US97121106
-1-
HEMOGLOBIN MEASUREMENT DEVICE
Field of the Invention
The invention relates to a diagnostic medical device, and in
particular to a blood cell analyzer having a hemoglobin measurement device.
Background of the Invention
The hemoglobin level in blood is an important aid to a medical
practitioner in the diagnosis of many abnormal conditions in the body such as
anemia. The hemoglobin content in blood is known to be between 12 and 16
grams (g) per 100 milliliters (ml) of blood in females and between 14 and 18
~ g per 100 (ml) in males. Hemoglobin levels above or below these levels may
indicate an abnormal condition and should be brought to the attention of a
medical practitioner.
Several methods and devices have been developed to measure
hemoglobin. It is known that hemoglobin can be detected and measured by
chemical analysis or the analysis of color absorbance of an unknown sample
with a known sample. However, the methods and devices using color
absorbance techniques are known to require expensive, bulky, and cumbersome
equipment that require continuous inspection and maintenance. In addition,
. none of the methods or devices known in the prior art are capable of
sampling
and measuring hemoglobin with small low power Iight sources as the
hemoglobin is passing through or paused in the measuring device.
The present invention addresses and solves the problems of
conventional hemoglobin measurement equipment by providing an inexpensive,
CA 02272624 1999-OS-20
WO 98/Z2777 PCT/US97/2110b
-2-
compact, and efficient disposable device that requires little or no inspection
and
maintenance.
Summary of the Invention
The present invention relates to a diagnostic medical device, and
S in particular to a hemoglobin measurement device for analyzing the
hemoglobin
content of blood samples. A hemoglobin sample is prepared from a blood
sample and provided to the hemoglobin measurement device, for example, by
a conventional blood analyzer. The hemoglobin sample is prepared by mixing
a portion of the blood sample with diluent and lyse in a portion necessary to
release hemoglobin from red blood cells in the blood sample. The hemoglobin
sample is pumped following an appropriate interval of time through the
hemoglobin measurement device where a hemoglobin measurement is made.
The device has a chamber with a light source and light detector
located at opposite ends of the chamber and operable at an optical wavelength
that is absorbed and/or reflected by the hemoglobin. The hemoglobin level is
determined as a function of the light intensity emitted at a preselected
wavelength and transmitted through the sample and the light intensity
detected.
This measurement can be made when the hemoglobin sample is flowing
through or stationary in the chamber.
In one aspect of the invention, a hemoglobin measurement device
includes a body having a measurement chamber, a hemoglobin sample input
port, and a hemoglobin sample output port. Both ports extend through the body
into the measurement chamber to provide a flow of hemoglobin sample through
the chamber. The invention includes a light source that is directed into the
chamber which emits light at a preselected wavelength through a flowing or
stationary hemoglobin sample.
The invention further includes a light detector that is directed at
the light source and positioned such that the light passing through the
hemoglobin sample is directed onto and detected by the detector. The
CA 02272624 1999-OS-20
WO 98122777 PCTIUS97121106
-3-
hemoglobin level in the blood sample is determined from the amount of light on
the detector as a loss of light intensity from absorption by the hemoglobin.
Brief Description of the Drawings
For a better understanding of the present invention, reference is
S made to the accompanying drawings. The drawings show embodiments of the
invention as presently preferred. However, it should be understood that the
invention is not limited to the precise arrangements and instrumentality shown
in the drawings.
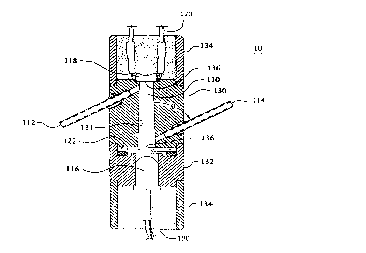
Figure 1 is a cross-section view of a hemoglobin measurement
device according to a preferred embodiment of the present invention.
Figure 2 is a cross-sectional view of a hemoglobin measurement
device having an insert and light source according to another preferred
embodiment of the present invention.
Figure 3 is a sectional view of the insert and light source of the
hemoglobin measurement device shown in Figure 2.
Detailed Description of the Invention
Referring to the drawings, where like elements are identified by
like numerals, there is shown in Figure 1 a hemoglobin measurement device 10
according to a preferred embodiment of the present invention.
Referring to Figure 1, the hemoglobin measurement device 10
includes a body 130 with a chamber 110. The body 130 has a cap portion 132
for retaining a light source 116, a light detector I I 8, and a pair of collar
portions
I 34 for protecting an retaining the electrical connections 120, 190 to the
detector
118 and source 116, respectively. The body 130 and chamber 110 may have
open or closed ends 136. The chamber can be made into any shape including,
but not limited to, cylindrical, spherical, or frustoconical. During operation
of
the device 10, the chamber 110 has a flow of blood sample supplied to the
chamber by an external blood sample source such as, but not limited to,
CA 02272624 1999-OS-20
WO 98122777 PCT/US97/21106
-4-
conventional blood analyzer equipment equipped with fluid pumping and
withdrawing means.
In a preferred embodiment, a flow of hemoglobin sample is
produced in the chamber by a supply of hemoglobin sample coupled to an input
port 112 that extends through the body 130. The hemoglobin sample enters the
chamber 130 through the input port I 12, flows through the chamber where it is
removed by exiting a sample output port 114 that is coupled to a blood sample
removing means (not shown). In summary, blood samples enter the
measurement chamber 110 through the input port 1 I 4, pass through the chamber
110 and across a light path formed by the light source 116 and detector 118
vJhich measure the hemoglobin level in the blood sample, and the samples exit
out of the chamber I 10 through the output port 114. It is to be understood
that
the direction of flow of the hemoglobin sample through the chamber 110 can be
in either direction and through either port 112, 114. The direction of flow
and
I S nomenclature of the ports 112, 114 were merely selected here for
convenience.
In one aspect of the present invention, the input port 112 and
output port l I4 are tubes that exit the body 130 at a preferred angle 0 that
is
about 60 degrees with respect to the chamber's surface I31. It is under stood
that the angle 0 of the input port 112 and output port 114 need not be the
same
and can be an angle in the range of 10 to 90 degrees.
In another aspect of the invention, particularly when the angle of
the ports 112, I 14 is 60 degrees with respect to the interior surface 131 of
the
chamber 110, the blood sample creates a vortex as it enters the chamber 130.
The vortex produces a desirable effect by reducing the accumulation of air
bubbles and debris on the surface of the light detector 118 and light source
116
which improves hemoglobin measurement accuracy and reliability.
In another aspect of the invention, the interior surface 131 of the
chamber 110 is made to produce a hydrophillic condition. One way this can be
achieved is by texturing the chamber surface I31 by making it rough or
irregular. For example, the body 130 can be made from a material such as metal
or plastic that is molded, milled or drilled to produce the chamber 110 having
CA 02272624 1999-OS-20
WO 98/22777 PCT/US97/21106
-5-
a surface 131. The chamber surface 131 is typically treated to produce a
hydrophillic condition. However, it is recognized that an extremely high
hydrophillic surface can be produced without the need of additional surface
treatment.
When a hydrophillic surface treatment is required, it can be
accomplished by mechanical or chemical abrasions such as sand blasting or a
chemical enchant to produce an irregular surface. The irregular surface
improves
the wetting of the chamber surface 131 by reducing bubble formation on the
surface 131 as the sample fills the chamber 130. Thus, bubble content in the
IO chamber 130 is reduced which greatly improves hemoglobin measurement
accuracy.
At one end of the body 130 is a light source 116 that is directed
into the chamber 110. The light source emits light into the chamber 110 at a
preselected wavelength typically in the range of 520 to 580 nm. Unlike prior
art
15 systems, the present invention employs an unfiltered light source that
directly
produces emitted light at the proper wavelength. In another aspect of the
invention, the light source has a peak wavelength of 557 nm and a dominant
wavelength at about 560 nm. However, it is understood that blood hemoglobin
will absorb light in a bandwidth of wavelengths about 549 nm which is also
20 contemplated by the present invention.
Opposite the light source 116 is a light detector 118. The light
detector 118 is directed at the light source 116 and positioned such that the
light
passes through the flowing blood sample onto the detector 118 where it is
detected. A hemoglobin level is then determined as a loss of light intensity
due
25 to absorption by the hemoglobin. Therefore, the difference in intensity of
light
emitted by the light source 116 and the light received by the detector 118 is
related to the amount of hemoglobin present in the blood sample. This
phenomenon is also referred to here as the loss or attenuation of light by the
hemoglobin.
30 In a preferred embodiment of the present invention, pre-mixed
fluid, also referred to as hemoglobin sample, contains blood, diluent, and a
CA 02272624 1999-OS-20
WO 98122777 PCT/US97121106
-6-
Lysing agent, that is pumped or drawn by a pump (not shown) into the chamber
110. As described above, the hemoglobin sample may enter the body 130
through an input port i 12 and exits through an output port 114. The source of
the hemoglobin sample can be obtained from a blood sample supply or any
conventional sampling device having a pump or drawing capability.
The hemoglobin sample is pumped or drawn through the chamber
110 or made stationary in the chamber and illuminated by a light source 116.
In another aspect of the invention, the light source or the detector I 18 or
both
can be separated from the hemoglobin sample by an optically permeable barrier
122 such as a transparent window composed of glass or plastic. The light
source
116 is of a frequency that is either reflected or absorbed, or both, by
hemoglobin. The light from the light source 116 propagates through the
hemoglobin sample where it is attenuated by absorption or reflection, or both,
according to the amount of hemoglobin present in the fluid. In another aspect
of the invention the preferred light source is a green light LED such as, but
not
limited to, an LPK382 green LED manufactured by Siemens, Inc. In another
aspect of the invention the light detector 118 is a silicon photodiode.
Light from the source 1 I6 that is not reflected or absorbed by the
fluid is received by the light detector 118 and converted into an electric
signal.
The signal is carried on a cable 120 and represents the hemoglobin level of
the
fluid passing through the chamber 110. The measurement of the hemoglobin
in the fluid is made either as the fluid moves through or is paused in the
chamber 110.
In another aspect of the invention, the fluid is drawn through the
chamber 110 by a negative pressure applied to the output port 114. The output
port 114 is located proximate to the light detector 118 thereby preventing the
accumulation of air bubbles and debris on the surface of light detector 118.
As
the hemoglobin sample is drawn into the chamber 110 a vortex effect is created
by the hemoglobin sample flow and the angle of the input port 112 and output
port 114. The ports are openings into the chamber 110 which can be, but are
not limited to, tubes that are attached to the body 130 and chamber 110.
_... ~.,~n.. . ~ . _....
CA 02272624 1999-OS-20
WO 98/22777 PCT/US97/21106
Referring to Figure 2, in another aspect of the invention the
hemoglobin measurement device includes a body 130 having at least one thermal
equilibrium vent 172 . The thermal equilibrium vent 130 is passageway made
through the body 130 allowing heat produced by the light source 116 to
dissipate
outside the body 130. Typically, air surrounding the source 116 will carry the
heat produced by the source 116 by convection through the vent 130.
Referring to Figures 2 and 3, in another aspect of the invention
the light source 116 and the light detector 118 are each mounted to an insert
140
that is attached to the body 130 by an insert locking device 160. The insert
140
can be made to accept either a light source 116 or a light detector 1 I8. In
one
aspect of the invention, the light detector 116 is mounted to its insert 140
by a
bead of epoxy 170 after it is adjusted and retained in place by a retaining
device
150.
Referring to Figure 3, the light source 116 can be mounted
directly or by means of a molded or cast assembly 180. In addition, the
detector
118 can also mounted directly or by means of a molded or cast assembly (not
shown). The light source I 16 is adjusted by changing its location within the
insert I40. The adjustment is made to produce a desired amount of columnated
light when the light source I 16 emits light. To improve columnation of light
a columnating device 141 is provided which can be located in the insert 140.
The columnating device 141 has a spherical reflecting surface 142 that is
located
about the light source 116. The light source 116 is directed through the
columnating device 141 and the emitted light is reflected by the reflecting
surface 142 which forms the emitted light into a column.
In yet another aspect of the invention, the hemoglobin
' measurement device 10 has a modular design for the body 130, modular light
source 20, and modular light detector 30. The modular design allows the light
source 20 and detector 30 to have the same form and fit in the body 130. The
body 130, as explained above, provides uniform sample flow through the
chamber 110 when it is suppled with hemoglobin samples through either port
112, 114. The modular light source 20 includes a light source 160 mounted in
CA 02272624 1999-OS-20
WO 98/22777 PCT/US97121106
_g_
an insert 140. The modular light detector 30 includes a light detector I18
mounted in an insert 140. The insert 140 can be mounted to the body 130 by
commonly sized openings 200 and retained by a common locking device 160.
Accordingly, the insert with the light source and the insert with the light
detector can be interchangeably attached to the body 130 and retained by an
insert locking device. Therefore, the source 20 and detector 30 can be mounted
in the body 130 without regard to the direction of flow. This feature reduces
the
number and difference in parts and greatly improves reliability and
serviceability.
The present invention may be embodied in other variant forms
where the variation does not substantially differentiate from the essential
novelty
and uniqueness revealed in the foregoing disclosure. Reference should
therefore
be made to the appendant claims rather than the foregoing specification, as
indicating the scope of the invention. It should be understood that many
modifications, variations and changes may be made without departing from the
spirit and scope of the invention as defined in the claims.