Note: Descriptions are shown in the official language in which they were submitted.
CA 02272667 1999-OS-25
PERMANENT EXPANDABLE INTRALUMINAL TUBULAR STENT
FIELD OF THE INVENTION
The present invention relates to expandable intraluminal
tubular stents which are applied within the peripheral or coronary
arteries of a living animal or human being to maintain patency
after a balloon angioplasty, but also relates more generally to
stents which may be applied to the pathology of other anatomical
canals, such as the venous, biliary and urinary canals.
DESCRIPTION OF THE PRIOR ART
Stents are generally tubular-shaped devices which function to
hold open a segment of a vessel in the human body. The term
"vessel" is intended to include any of the arteries and body
passageways found in the human body.
Stents are of two types: the first comprising a non-elastic,
metallic material which is radially expandable from the inside
towards the outside under the effect of an inflatable balloon; or
the second comprising an elastic metallic material made of metal
meshes that are introduced stretched, i.e under tension, into the
lumen of the vessel wherein the diameter increases when the
longitudinal extension, i.e. tension, is relaxed.
Further details of prior art stent structures may be found in
U.S. Pat. No. 5,514,154 (Lau et al): U.S. Pat. No. 5,041,126
-1-
CA 02272667 1999-OS-25
(Gainturo)~ U.S. Pat. No. 4,655,771 (Wallsten); U.S. Pat.
No. 5,496,365 (Srgo); U.S. Pat. No. 5,133,732 (Wiktor); U.S. Pat.
No. 5,382,261 (Palmaz); U.S. Pat. No. 5,102,417 (Palmaz); and U.S.
Pat. No. 5,195,984 (Schatz). These patents are incorporated
herein by reference in their entirety.
One of the difficulties encountered using known stems
involves maintaining the radial rigidity needed to hold open a
body lumen while at the same time maintaining the axial length of
the stmt. Accordingly, prior to the development of this
invention, there has been no intravascular stent capable of
increasing axial length of the stmt as it radially expands.
SUMMARY OF THE INVENTION
The present invention is directed to an expandable permanent
tubular stmt which increases in axial length as it radially
expands and is relatively flexible along its longitudinal axis to
facilitate its delivery to the body lumen.
The permanent expandable intraluminal tubular stmt includes
an arrangement of connecting members interconnected by a plurality
of radially expandable, elongatable members all disposed generally
coaxially about the stents longitudinal axis. The tubular stmt
has a substantial uniform thin-walled thickness disposed between
proximal and distal ends, a plurality of slots or axial spaces
formed between opposite longitudinal ends of the connecting
-2-
CA 02272667 1999-OS-25
members. The tubular stent has an initial constricted diameter
which permits intraluminal delivery into a body passageway. The
stmt may then be deformed by the inflation of a balloon forming
part of a catheter delivery system. The balloon expands the
diameter by applying a radially, outwardly directed force, wherein
the expanded diameter is variable and dependent upon the amount of
force applied to the tubular stmt.
A further feature of the present invention is the elongation
of the initial axial length of the tubular stmt as it radially
expands. The elongated length of the st mt is variable and also
dependent upon the amount of outward radial force applied to the
tubular stent. The stents are preferably fabricated from low
memory more plastic than elastic, bio-compatible materials, for
example: stainless steel 316L, gold, tantalum and similar
materials, which enable the stems to be plastically deformed from
their constricted diameter to their expanded diameter.
BRIEF DESCRIPTION OF THE DRAWINGS
In the drawings:
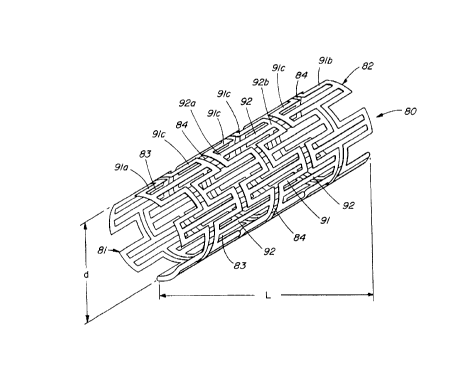
FIG. 1 is a perspective view of a permanent expandable
intraluminal tubular stmt, constructed and operable according to
the teachings of the present invention, in its relaxed and
flexible state before plastic deformation.
-3-
CA 02272667 1999-OS-25
FIG. 2 is a side elevational view of the tubular stmt of
FIG. 1 in its expanded configuration after plastic deformation.
FIGS. 3A and 3B are cross-sectional views showing the stmt
of FIG. 1 in situ before and after expansion by a balloon forming
part of its catheter delivery system.
FIG. 4 is a perspective view of an alternative embodiment of
the tubular stent, constructed and operable according to the
teachings of the present invention, in its relaxed and flexible
state before plastic deformation.
FIG. 5 is a side elevational view of the tubular stent of
FIG. 4 in its expanded configuration after plastic deformation.
FIG. 6 is a perspective view of another alternative
embodiment of the tubular stmt, constructed and operable
according to the teachings of the present invention, in its
relaxed and flexible state before plastic deformation.
FIG. 7 is a side elevational view of the tubular stmt of
FIG. 6 in its expanded configuration after plastic deformation.
DETAILED DESCRIPTION OF THE INVENTION
FIGS. 1 and 2 illustrate a permanent expandable intraluminal
tubular stmt 80 which generally comprises a generally cylindrical
arrangement of a plurality of connecting members 92 disposed
longitudinally and coaxially parallel to each other. The
connecting members are oriented such that their longitudinal ends
-4-
CA 02272667 1999-OS-25
92a are proximal a first or proximal end 81 of the st mt 80 and
their opposite longitudinal ends 92a are proximal the distal or
second end 81 of the stmt 80. The connecting members 92 are
disposed in equal-numbered sets of circumferentially-spaced
connecting members, with each set being axially displaced with
respect to the adjacent set and with the connecting members 92 of
each successive set being circumferentially-interspaced with
respect to the connecting members 92 of the preceding set. In
general, the ends 92a and 92b of adjacent connecting members 92
are joined together by elongatable members 91. The
circumferentially-adjacent proximal ends 92a of the connecting
members 92 at the proximal end 81 of the stmt are attached by
means of elongatable members 91a while the circumferentially-
adjacent distal ends 92b of the connecting members 92 at the
distal end 81 of the stmt are attached by means of elongatable
members 91b. The remaining distal ends 92b of each set of the
connecting members 92 are attached to the proximal ends 92a of
each successive set of connecting members 92 by elongating members
91c. Longitudinal ends 92a and 92b of longitudinally adjacent
connecting members 92, i.e every second set of connecting members,
are axially-spaced or separated by narrow space slots 84. The
tubular stent 80 has substantial uniform thin-walled thickness
disposed between proximal 81 and distal 82 ends.
-5-
CA 02272667 1999-OS-25
The tubular stent 80 has an initial constricted diameter d
which permits intraluminal delivery of the tubular stmt 80 into
the lumen 101 of the body passageway 100 (see FIG. 3A) and is
controllably "deformed" to expanded diameter d' shown in FIG. 2
upon application of a radially, outwardly extending force from the
interior of tubular stent 80. The term "deformed" is used to
indicate that the material from which tubular st mt 80 is
manufactured and in particular, the elongatable members 91, is
subjected to a force which is greater than the elastic limit of
this material. The expanded diameter d' is variable in size, which
is dependent upon the amount of force applied to deform the
tubular stent 80. Accordingly, the elongatable members 91a, 91c
will permanently deform radially and the elongatable members 91c
will permanently deform generally diagonally, moving the
connecting members 92 coaxially away from each other in a radial
direction. At the same time connecting members 92 will move away
from each other in a longitudinal or axial direction. The
connecting members 92 retain their integrity without any deformity
which results in a widening of the space slots 84 between the ends
92b and 92a of longitudinally adjacent connecting members 92.
This widening effect creates an elongation of the initial axial
length L of the tubular stent 80 to a second axial length L' as
shown in FIGS. 1 and 2.
-6-
CA 02272667 1999-OS-25
The tubular stmt 80 is preferably fabricated from bio-
compatible, low memory, more plastic than elastic material to
permit the tubular stmt 80 to be expanded and deformed from the
configuration shown in FIG. 1 to the configuration shown in FIG.
2 and further to permit the tubular stmt 80 to retain its
expanded and deformed configuration with enlarged diameter D' and
axial length L' shown in FIG. 2 and also to resist radial collapse.
Suitable materials for the fabrication of the tubular stmt 80
would include silver, tantalum, stainless steel, gold, titanium,
NiTi alloy or any suitable plastic material, such as thermoplastic
polymers, having the requisite characteristics previously
described. Preferably, the stmt of the present invention is
fabricated from a tubular length of the selected material, which
is laser-cut and removed of unwanted material to provide the
desired structure as shown in FIGS. 1, 4 or 6.
Tubular stmt 80 is shown in FIGS. 3A and 3B overlying a
balloon 130 forming part of its catheter delivery system 131.
Tubular stmt 80 is mounted on its catheter delivery system 131 in
its constricted diameter state shown in FIG. 3A for plastic
deformation upon inflation of a balloon 130 to its expanded
diameter shown in FIG. 3B for supporting the walls 100 of a body
conduit 101. The properties of the tubular stent 80 may be varied
by alteration of the characteristics of elongatable members 91 and
connecting members 92.
CA 02272667 1999-OS-25
FIGS. 4 and 5 illustrate an alternative tubular stmt 120
which generally comprises a plurality of thin-walled radially
expandable elongate members 91 and connecting members 92 disposed
generally the same as those of FIG. 1, wherein the elongatable
members 91 of tubular stent 120 are more elongatable than those of
tubular stent 80 of FIGS. 1 and 2. The elongatable members 91 of
the stmt 120 of FIG. 4 comprise a plurality of intermediate
sections arranged in a serpentine pattern whereas the elongating
members of the stmt 80 of FIG. 1 comprise three intermediate
sections arranged at right angles. This will achieve more
flexibility for tubular stmt 120.
FIGS . 6 and 7 illustrate another alternative tubular stmt
140 which is similar to the previous embodiments but wherein the
connecting members 92 are relatively shorter and square shaped and
wherein the connecting members of each successive set are axially-
spaced. Preferably, the elongated length of the elongatable
members of stmt 140 is approximately ten times the longitudinal
length of the connecting members of stent 140, whereas the
elongated length of the elongatable members of stems 80 and 120
are approximately half the longitudinal length of the connecting
members of the respective stems 80, 120. This will achieve more
flexibility for tubular stmt 140 which is particularly important
for delivery through blood vessels having multiple curved
portions.
_g_
CA 02272667 1999-OS-25
It is to be understood that the invention has been described
with respect to a limited number of embodiments. It will be
appreciated that many variations, modifications and other
applications of the invention may be made. Accordingly, the
invention is therefore to be limited only by the scope of the
appended claims.
-8a-