Note: Descriptions are shown in the official language in which they were submitted.
CA 02272849 2007-03-21
WO 98/08991 PCT/US97/15109
TITLE OF THE INVENTION
METHOD FOR PHYTOMINING OF NICKEL, COBALT
AND OTHER METALS FROM SOIL
BACKGROUND OF THE INVENTION:
Field of the Invention
This invention pertains to a method of extracting nickel, cobalt and other
metals, including the platinum and palladium metal families, from soil by
cultivation of the soil with hyperaccumulating plants that concentrate these
metals in
above-ground portions of the plants, which can be harvested, dried and smelted
to
recover the metal (metal phytomining).
BACKGROUND OF THE PRIOR ART
It has long been known that certain types of soil and geological materials,
including serpentine, lateritic serpentine, ultramafic and meteor-impacted
soils may
be rich in nickel or cobalt, and are sites for mining of these metals. The
cost of
conventional mining for these metals remains high, and the level of metals
required
in geological materials to which current technology may be usefully applied
are
-1-
CA 02272849 2007-03-21
WO 98108991 PCT/US97/15109
much higher than most serpentine, lateritic serpentine, ultramatic and meteor-
derived soils.
This application is related to U.S. Patent Application Serial No. 08/470,440,
allowed
as U.S. Patent No. 5,711,784, and its corresponding PCT application (WO
1998/008991). In
this earlier application, recovery of Ni, Co and related metals from soil is
described through
culturing Allysum plants on Ni-enriched soil. The specific soil conditions
described in that
application include reducing calcium as far as possible, in accordance with
conventional
teachings regarding the inverse relationship between calcium concentration and
nickel
hyperaccumulation. Additionally, the application limits calcium concentrations
to a value
such that the exchangeable Ca/Mg ratio is below 0.20.
U.S. Patent 5,364,451, Raskin et al., is directed to a method of removing
metals from metal-rich soil by growing genetically altered plants of the
family
Brassicaceae in these soils, so as to remediate polluted soils at a reduced
cost.
Suitable parents for the mutants that are the subject of the Raski patent
include B.
juncea. While the patent generally describes a large number of metals that may
be
recovered, specific artificial examples are directed to recovery of chromium
and
lead.
A review of the examples of this reference, and application of the
technology proposed, illustrates continuing problems posed in rededication of
metal-
rich soil, and recovery of the metals therefrom. In particular, the examples
set
forth reflect artificial culture in sand media with intermittent feeding with
phosphate
to permit plants to grow without severe yield reduction and without severe
lead
toxicity. The patent also relies on genetic mutations that are produced by
random
"mutagenesis", that is, the creation of a library of mutants or potential
mutants from
a starting parent by indiscriminate application of a mutagen, coupled with
screening
the offspring to define acceptable hyperaccumulators. While promising, the
Raskin
patent offers little basis for an opportunity to proceed directly with soil
rededication
-2-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
through plant growth or culturing. Additionally, the patent offers little
realistic
opportunity for recovery of the metal itself, indicating only that under
circumstances (not identified) the metal can actually be reclaimed.
One of the most widely found, and technologically important metals is
nickel. Nickel is a natural constituent in all soils, being particularly high
in
concentration in serpentine, lateritic serpentine, utramafic and meteor-
derived soils.
Cobalt, which has chemical and geological characteristics very similar to
nickel, can
similarly be found in these soils, and is another valuable metal. Other metals
that
are also subjects for phytomining within the scope of the invention, including
those
of the platinum and palladium families, including palladium, rhodium,
ruthenium,
platinum, iridium, osmium and rhenium which commonly co-occur with Ni and Co.
Cultivation of plants which are hyperaccumulators of these metals, in metal-
rich
soils, or "phytomining", is a desirable alternative as a means for recovering
metals
from soil. Ordinary cultivation methods, however, without adequate preparation
and maintenance of soil conditions, does not lead to adequate
hyperaccumulation of
metals in the plants economically interesting. Additionally, specific methods
for
recovery of the metals remain to be explored.
Among the soil conditions and cultivation methods most frequently
investigated, the relationship between calcium levels and nickel uptake, as
well as
nickel tolerance, have been frequently reported. While the reports are not
uniform,
in general, the prior art has reported a negative correlation between calcium
concentration and nickel upgrowth. Gabbrielli et al., Atti. Soc. Tosc. Sci.
Nat.
B38:143-153 (1981) observed that serpentine soils typically have low levels of
calcium. An increase in calcium level was reported to reduce nickel uptake.
Similarly, increasing Mg and Ca has been reported to lower nickel tissue
concentration in nickel accumulator species endemic to serpentine soils.
Gabgriell
i
et al., Physiol. Plant. 62:540-544 (1984). See also, Vergnano et a1., The
Vegetation of Ultramafic Soil, page 319-322, (1992). Thus, in general, the art
teaches that raising calcium levels from the extremely low values normally
-3-
CA 02272849 2007-03-21
WO 98/08991 PCTIUS97/15109
encountered in serpentine soil to higher levels can be expected to yield a
reduction
in nickel uptake.
Similarly, a ratio recognized as important in maintaining the health of
various plants endemic to serpentine soils is the exchangeable Ca/Mg ratio.
Prior
art reports set a ratio of about 0.67 recommended as a fertility index.
Alexander et
aL, Soil Sci. 149:138-143 (1990). Typically, exchangeable Ca/Mg ratios in
serpentine soils are at much lower values of about 0.2. Thus, the general
teaching
of the art is that to preserve fertility, a substantial increase in available
calcium is
required, which can be expected to decrease nickel uptake.
In U. S. Patent Application Serial No. 08/470,440, a 11 owed as U. S.
Patent No. 5,711,784 a method of phytomining is disclosed which calls
for reduction of calcium levels, among other soil treatments. This is
consistent with
teachings of the prior art. Accordingly, it remains an object of those of
skill in the
art to develop a reliable system for phytomining of soils rich in nickel,
cobalt and
the other identified metals, naturally occurring or otherwise, that will lead
to a
recovery of these metals at economically acceptable levels.
SUMMARY OF THE INVENTION
By screening a wide variety of plants from the Brassicaceae family, the
inventors have identified plants in the Alyssum genus which may be
hyperaccumulators of nickel and which accumulate valuable amounts of cobalt.
By
definition, hyperaccumulator plants accumulate over 1000 mg Ni or Co/kg dry
weight growing in the soils where they evolved. Because cobalt occurs at about
3-
10% of the level of Ni in the listed soils, Ni is the dominant toxic metal
which
induced evolutionary selection of the Ni hyperaccumulator plants and Co is
accumulated to economically useful levels but Ni hyperaccumulation is the
dominant
economic benefit of the phytomining technology. Evidence suggests members of
the section Odontarrhena of the genus Alyssum are likely candidates as nickel
-4-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
hyperaccumulators. The plant may also concentrate, in the above-ground plant
tissues, metal from the platinum and palladium families, including Pd, Rh, Ru,
Pt,
Ir, Os and Re, in significant amounts. Accumulation of nickel in plant tissues
in
excess of 2.5 percent is practicable.
The metals listed accumulate in biomass by growing nickel
hyperaccumulating Alyssum species in the target soils. Some 48 taxa within the
section Odontarrhena of the genus Alyssum are known to be hyperaccumulators of
nickel. These include the following species already evaluated: A. murale, and
A.
pintodasilvae (A. serpyllifolium ssp.), A. malacitanum, A. lesbiacum, and A.
fallacinum. Other Ni-hyperaccumulating species which may be employed include:
A. argenteum, A. bertolonii, A. tenium, A. heldreichii. About 250 other plant
taxa
have been shown to hyperaccumulate nickel, but many of these do not exceed
10,000 mg Ni/kg d.w., and the majority are of tropical origin.
The identified metal species are accumulated by growing the Alyssum in
nickel-rich soil, under specific soil conditions. The conditions include: (1)
lowering
the soil pH, which increases the phytoavailability of nickel; (2) maintaining
moderate levels of Ca in the soil by appropriate treatments and by use of Ca,
Mg-
rich soil amendments adjusted to maintain Ca levels at levels corresponding to
solution values between 0.128 mM and 5.0 mM; (3) using ammonium constraining
or ammonium-generating nitrogen fertilizers to improve plant growth and to
increase Ni hyperaccumulation due to rhizosphere acidification; and (4)
applying
chelating agents to the soil to improve nickel uptake by the roots of the
hyperaccumulating Alyssum species. Examples of suitable chelating agents
include
nitrilotriacetic acid (NTA). Other chelating agents commonly used in
connection
with increasing soil metal mobility for plant uptake include
ethylenediaminetetraacetic acid, and ethylene glycol-bis-(P-aminoethylethehr)-
N, N-
tetraacetic acid. Maintenance of these soil-conditioning factors will improve
nickel
hyperaccumulation in Alyssum, in excess of a 2.5 percent concentration in
above-
ground portions of the plant, particularly leaves and stems or shoots, which
make
-5-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
for easy cultivation and metal recovery. This is preferable to concentration
in the
roots, discussed in Raskin et al., which may be an aid in soil rededication if
non-
leachable therefrom, but does not offer convenience for phytomining. It is
particularly surprising that intermediate values of Ca increase Ni uptake
while
values of 0.128 mM and below and 5 mM and above decrease Ni uptake. This,
combined with exchangeable Ca/Mg ratios of 0.16-0.40, much lower than that
recommended in the prior art, further increases Ni tissue concentrations.
BRIEF DESCRIPTION OF THE DRAWINGS
Figures 1 -10 are graph illustrations of experimental data obtained and
discussed below.
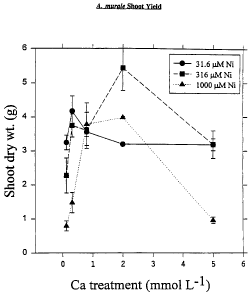
Figures 1-3 reflect shoot yield for given levels of Ni as a function of Ca
concentration for Cabbage, A.murale and A. pintodasilvae, respectively.
Figures 4-6 reflect Ni levels in shoots for given levels of Ni as a function
of
Ca concentration for Cabbage, A. murale and A. pintodasilvae, respectively.
Figures 7-8 reflect the ratio of Ni in shoots/roots for A.murale and A.
pintodasilvae, respectively.
Figures 9-10 reflect shoot Ni content at five given Ni concentration values as
a function of Ca concentration for A.murale and A. pintodasilvae,
respectively.
DETAILED DESCRIPTION OF THE INVENTION
Applicants have screened a large wild-type collection of germplasm to
identify hyperaccumulating plants. In particular, plants of the Brassicaceae
family,
particularly naturally occurring plants as opposed to those with induced
mutations,
such as those employed in the Raskin patent, are known to be Ni + Co
accumulators. Within the family, and even with the various genera, however,
wide
variations in metal accumulation, to the extent it occurs, do appear. Alyssum
-6-
CA 02272849 2007-03-21
WO 98/08991 PCTIUS97/15109
species that are preferred candidates for use in the claimed invention
concentrate
and hyperaccumulate nickel, shown enhanced uptake of cobalt and may be useful
in
accumulating other metals. Preferred species have a preference for, and a high
toxicity resistance to these metallic elements. This appears to be due to
evolutionary driving forces, which permit the plant to benefit from the
ecological
niche presented. This should be contrasted with the response of a different
Brassicaceae member, Thlaspi caerulescens, which accumulates very high levels
of
zinc and cadmium. While Alyssum exhibits a higher uptake rate at low nickel
and
cobalt concentrations than other species, Thlaspi actually grow well on soils
with
much higher Zn and Cd concentrations. Thus, while Alyssum concentrates nickel
and cobalt over a range of concentrations, Thlaspi hyperaccumulates very high
levels of Zn and Cd, some strains accumulating Ni and Co. Rather than relying
on
the unpredictable process of mutagenesis, the applicants in screening a large
library
of wild-type germplasm, have identified several Alyssum species including A.
murale, A. pintodasilvae (A. serpyllifolium ssp.), A. malacitanum, A.
lesbiacum,
A. tenium and A. fallacinum as a suitable hyperaccumulators of nickel land
useful
in the enhanced uptake of cobalt. The same plants may also accumuatte Pd, Rh,
Ru, Pt, Ir, Os and Re. While these platinum and palladium metals are
accumulated
in lower concentrations, their greater value per unit weight, makes
phytomining of
these metals economically attractive as well.
Soil Management
To improve nickel and cobalt sequestration in the above-ground tissues of
Alyssum plants, the soil in which they are grown is preferentially conditioned
taking
advantage of different factors.
These include soil pH, moderate calcium concentratiom,low to moderate
exchangeable Ca/Mg ratios, and optionally, use of ammonium containing or
generating fertilizer rather than other N-fertilizers and application of
chelating
agents. Each of these is considered in turn below.
-7-
CA 02272849 1999-02-26
WO 98/08991 PCTIUS97/15109
1olpH
The maintenance of preferred pH ranges in soil is well known in agriculture
for a variety of reasons. Typically, pH of soil is altered or modified so as
to
maintain it within a near neutral range of about 6.0-7.5. Thus, soil near a
limestone
foundation or other building may be treated with acidifying soil amendments so
as
to reduce an alkaline pH. Soil with a naturally low pH may instead be treated
with
limestone or similar amendment, so as to increase the soil pH. A reduced pH
increases the phytoavailability of nickel and cobalt. A reduced pH increases
solubility and optimizes the release of these metals for absorption by the
roots, and
translocation to the above-ground tissues of the plant. Soil pH can be
maintained in
any of a variety of established methods, and the methods themselves do not
constitute an aspect of this invention. Preferably, soil pH is managed at a
low value
by addition of sulfur and use of ammonium - N fertilizers. The Alyssum
species,
and indeed, any plant species, grows best at its evolved optimum pH
conditions.
Thus, pH cannot be reduced so low as to substantially retard or inhibit plant
growth. An optimum pH range for phytomining using Alyssum is a pH of 4.5 to
6.2, preferably 5.2-6.2. After extraction of economically phytominable Ni and
Co
from the soil, limestone application can raise soil to pH levels required by
more
traditional farm crops.
Calcium Concentrations
Alyssum species which hyperaccumulate Ni and Co evolved in Ni-rich
ultramafic and serpentine soils which simultaneously have low soil calcium.
The
presence of extremely low and extremely high calcium concentrations in soil
inhibits nickel/cobalt hyperaccumulation by Alyssum. Acceptable calcium
concentrations in soil ranges from 0.128 mM to 5.0 mM, as set forth in the
examples below. Calcium concentrations may be maintained by any of a variety
of
known methods. One method involves acidification of the soil with sulfur,
sulfuric
acid, or other amendments and leaching, followed by use of Ca soil amendments.
-8-
CA 02272849 2007-03-21
WO 98/08991 PCTIUS97/15109
Whatever method is selected to adjust calcium concentration in soil, it should
be
selected so as to be consistent with the objective of soil phytomining.
Additional of Ammonium Fertilizer
Generally, high metal concentrations are toxic to plants, and inhibitory of
plant growth. While Alyssum has developed the ability to hyperaccumulate
nickel/cobalt in its above-ground plant tissues, nonetheless, fertilizer
support for the
growth, particularly in polluted soil, is an essential element for substantial
hyperaccumulation. Use of high-ammonium N-fertilizers is of value.
Nonetheless,
the use of ammonium fertilizers per se is well known, and acceptable
fertilizers and
protocols will be arrived at by those of ordinary skill in the art on an
empirical
basis.
Addition of ChelatingAgents
Metal chelates are commonly used in agriculture, and occur naturally in
living cells. The addition of chelating agents, such as KfA,, or any of a
variety of
amino-acetic acids known to those of ordinary skill in the art as chelating
agents, to
the soil to be phytomined for Ni/Co and Pt, Pd metals improves the movement of
soil metals to root surfaces for uptake and translocation of these materials
into the
above-ground plant tissues. Any of a variety of known chelating agents of
commerce may be used. A preferred chelating agent is NTA or EDTA. Typically,
chelating agents will be added at 5-100 kg/ha after the plants are
established. As
with the use of fertilizers, optimum additions of chelating agents can be
determined
on an empirical basis. Chelating compounds which chelate Ni in the presence of
high soil levels of Fe, Mg, and Ca selectively increase Ni uptake by the
hyperaccumulator plants.
-9-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
Metal Recovery
As noted, a principal object of this invention is the recovery of the metal
sequestered by the hyperaccumulating plant. In U.S. Patent 5,364,451, plants
are
identified which accumulate the metals in the roots. Recovery of metals from
roots
poses substantial mechanical problems, including the recovery of the root
itself, as
well as recovery of the metal from the root tissue. By cultivating selected
Alyssum
genotypes, as contemplated in the claimed invention, a very high degree of the
nickel/cobalt absorbed by the roots is translocated to above-ground tissues,
such as
stems, leaves, flowers and other leaf and stem tissues. This feature
facilitates
recovery of the metal extracted from the soil. The Alyssum can be harvested in
conventional fashion, that is, cutting of the plant at soil level. The
harvested
materials are left to dry, in much the same fashion that alfalfa is dried, so
as to
remove most of the water present in the plant tissues. After drying, the plant
material is collected from the field by normal agricultural practices of hay-
making,
incinerated and reduced to an ash with or without energy recovery. This
organic
material may alternatively be further treated by roasting, sintering, or
smelting
methods which allow the metals in an ash or ore to be recovered according to
conventional metal refining methods such as acid dissolution and
electrowinning.
With metal concentrations as high as 2.5 to 5.0% in the above-ground plant
tissues,
particularly leaves or shoots, metal recovery becomes economical, thus
satisfying
the primary objective of the invention. Conventional
smelting/roasting/sintering
temperatures of 500-1500 F are sufficient to combust the organic material in
the
dried plant biomass, leaving a residue of the accumulated metal, with few
contaminants which are known to interfere with metal refining. Indeed, it is
suspected that the other components of the ash will be lower than with
conventional
mined ore concentrates.
-10-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
EXAMPLES
Materials and Methods
Plant Material
A nutrient solution study was conducted to define the effects of Ca and Mg
on Ni uptake by two know Ni hyperaccumulator species, Alyssum murale and
Alyssum pintodasilvae, compared to the normal non-tolerant crop species,
cabbage
(Brassia oleracea var. capitata) cultivar Danish Roundhead. A varying solution
concentrations of Ni (3 levels) and Ca (5 levels) were used in a factorial
experimental design for Alyssum, while 2 levels of Ni and 5 levels of Ca were
used
in a factorial experimental design for cabbage. All solutions contained a high
concentration of Mg to simulate serpentine soils where phytomining plants
might be
grown. Seeds for Alyssum murale and Alyssum pintodasilvae were collected from
plants growing in Panorama, Thessaloniki, N. Greece and Braganca, NE Portugal.
Plant Growth
The study was conducted in an environmental growth chamber; temperature
in the chamber was maintained at 25 C day and 19 C night, and relative
humidity
was set at 70%. The day period was maintained for 16 hours periods with >400
p,Ern-2sec 1 photosynthetically active radiation at plant height from a
combination of
cool-white fluorescent and incandescent lamps.
Alyssum seeds were treated with ethanolic Arasan for 45 seconds and
germinated by placing seeds in company germination bags with a macronutrient
solution (1 mM Mg as MgSO4; 2.5 mM CaNO3 and KNO3; 0.1 mM K2HPO4). The
bags were kept moist all the time. After 2 weeks in the germination bags in
the
growth chamber, Alyssum seedlings were transferred into 8 L buckets containing
a
0.5 strength Hoagland solution (1 mM Mg as MgSO4; 2.5 mM CaNO3 and KNO3;
0.1 mM K2HPO4; 20 M Fe as FeHBED; 75 M KCI; 25 M HCI; 10 M gBO3;
2 M Mn as MnCl2; 05 M Cu as CuSO4; and 0.2 M Mo as Na2MoO4; 1.0 mM
-11-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
Zn as ZnSO4). Seedlings were maintained in these buckets for an additional 2
weeks to grow to larger or reasonable handling before transplanting to
treatment
solutions.
Cabbage seeds germination was begun 10 days before transplanting to
treatment solutions. Cabbage seeds were placed in standard seed germination
papers with the same germination macronutrient solution and showed good
germination within six days.
To initiate treatments, one plant of each species was transferred to separate
1
L polyethylene beakers containing a modified 0.5 strength Hoagland solution (2
mM MG as MgSO4; 2.5 mM KNO3; 0.1 mM K2HPO4; 20 M Fe as FeHBED; 75
M KCI; 25 M HCI; 15 M H3B03; 2 M Mn as MnC12; 0.5 M Cu as CuSO4;
0.2 M MO as NaMoO4; and 1.0 mM Zn as ZnSO4) with 2 mM MES to maintain
solution pH at 6.2, high Mg level (2 mM) and Ca and Ni treatments. FeHBED was
used because even high levels of Ni or micronutrients do not displace Fe from
this
chelate, and dicots easily obtain the Fe by reduction.
A randomized complete block design with three replications was used. The
plants were placed into polyurethane foam plant supports and inserted into a
slot
and hole in a black plexiglass cover. The beakers were covered with black
polyethylene to minimize light exposure. Each beaker was continuously aerated.
Plants were harvested six weeks after treatment initiation. At harvest, plants
were separated into roots and shoots. Shoots were rinsed with deionized water.
Roots were rinsed with 2.5 mM Ca(NO3)2 to remove extracellular metals prior to
rinsing with deionized water. All samples were dried at 65 C in a forced draft
oven.
Treatments
Ni was supplied as NiSO46H2O. Three high concentrations Ni treatments
were established for the Alyssum spp. (31.6 AM, 316 AM, and 1000 MM), and two
Ni treatments were established for cabbage (1.0 M and 10.0 AM) based on
-12-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
preliminary studies of Ni tolerance by these species. Ca was supplied as
Ca(N03)24H20 with NH4NO3 to adjust nitrogen concentration to 10 mM for all
treatments. Five Ca treatments were established for all tested species 0.128
mM,
0.32 mM, 0.8 mM, 2.0 mM, 5.0 mM Ca(NO3)2 with balancing 4.87 mM, 4.68
m<, 4.2 mM, 3.0 mM, 0.0 mM NH4NO3. Solution pH was maintained above 6.0
by the addition of 2 mM MES buffer. pH was adjusted as necessary by the
addition
of KOH. Fourteen days after initiation of treatment, all solutions were
completely
replaced, and again at 21, 28, 28 and 35 days of treatment.
Sample Anal,
Dry plant samples were ground in a stainless steel Wiley mill if necessary,
2.00 g samples were weighed into low silicate beakers and ashed in a 480 C
muffle
oven for 16 hours. Ash was digested with 2 mL concentrated HNO3 and heated to
incipient dryness; 10 mL 3N HCl was added, the beakers heated at reflux with
stirring for 2 hours. Digests were filtered, a 1.00 mL aliquot of Fisher
Scientific
Cobalt Reference Solution (1000 mg L' Co) was added to each sample as an
internal reference (40 mg L' cobalt) for subsequent analysis using Inductively
Coupled Plasma Emission Spectrophotometry (ICP-ES). Samples were brought to
25 mL in 1N HC1. Necessary dilutions were made in 1N HC1 to maintain constant
viscosity. Blanks were prepared for every 10 samples and NBS#1575 pine needles
standard reference materials were digested for every 20 samples for quality
assurance. Plant analysis was performed in duplicate when there was sufficient
sample. Ni concentration of plants were determined using a flame atomic
absorption spectrometer (AA). Zn, P, Cu, Mn, Fe, Mg, Ca, and K concentrations
were analyzed by using an ICP-ES (emission spectrometer), and all results were
corrected by use of the internal standard.
-13-
CA 02272849 2007-03-21
WO 98108991 PCT/US97/15109
Statistical Analysis
Data was analyzed using SAS-PC version 6.0 (SAS institute, 1989). Data
required lot transformation to attain homogeneity. The GLM procedure was
utilized for analysis of variance of plant yield and tissue metal
concentration for
differences of treatments. Treatment means were compared using the Duncan K-
ratio t-test after it was determined that there was a significant (P < 0.05)
treatment
effect using the GLM procedure.
Growth and Symptoms
The experiment tested for interactions between Ca and Ni in growth and
element accumulation in cabbage and two Ni hyperaccumulator Alyssum species.
Alyssum murale, A. pintodasilvae, and cabbage plants all appeared healthy at
the
start of Ni and Ca treatments.
In the first week of growth, Alyssum spp. and cabbage plants in all treatments
were green. In the second week of the trial, those Alyssum spp. plants in
highest
solution Ni level (100 M) with lowest solution Ca (0.128 mM) and highest
solution Ca (5 mM) started to show chlorotic symptom on young leaves, but the
size
was not significantly different; and those cabbage in higher solution Ni level
(10
AM) almost all showed chlorotic or blown spots symptoms with curling edge in
young leaves. At the fourth week of the trial, those Alyssum spp. plants in
highest
solution Ni level (1000 &M) with lowest solution Ca (0.128 mM) and highest
solution Ca (5 mM) were visibly smaller than others; the chlorotic and
necrotic
symptoms in those cabbage in higher solution Ni level became more severe.
In the sixth week of the trial and just before harvesting, little chlorotic
leaves
symptoms were observed on those Alyssum murale plants in lowest solution Ca
level (0.128 mM) across all three solution Ni levels. Smaller size and severe
chlorotic symptom were significantly showed on those Alyssum plants in highest
solution Ni level with lowest solution Ca level. For cabbage plants, not just
those
plants grown in higher solution Ni level showed chlorotic and necrotic with
curling
-14-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
edge symptoms, but also showed on the lower solution Ca levels (0.128, 0.32
mm)
in lower solution Ni. Root systems were less extensive in all plants shown
severe
chlorotic and/or necrotic symptoms in leaves.
For all species, low Ca (0.128-32 mM) caused reduced yield compared to
normal (0.8-2.0 mM) or high solution Ca (5.0 mM), for all Ni levels. Cabbage
was
more sensitive to Ni phytotoxicity, than the Alyssum species and low Ca caused
greater toxicity than in Alyssum. For cabbage (Fig. 1), at 1.0 M Ni, full
yield was
restored by increasing solution Ca; but at 10 M Ni, full yield was not
restored at
higher Ca levels. But for Alyssum species (Fig. 2 and 3), yield also declined
at 5
mM Ca.
In tables IA - 1D, the analysis of variance for the main factors (solution Ca,
solution Ni, plant species, block) and interactions (solution Ca-x-solution Ni
within
species, and solution Ca-x-solution Ni-x-species) are reported. All the main
factors
and interactions, except block, had significant effects (P < 0.001) on shoot
dry
yield and shoot Ni concentration.
Dry Matter Yields
Cabbage
For the lower solution Ni level (1 AM), increasing solution Ca had
hyperbolic effect with decreasing slope in increasing shoot dry yield (Fig.
1). For
the higher solution Ni (10 M), increasing Ca caused a 5 times shoot yield
increase
at 2 mM Ca, but declined at 5 mM Ca when compared to 2 mM Ca levels. The
similar pattern was observed on root dry yield.
Alyssum murale
For the lower solution Ni level (31.6 MM), increasing solution Ca increased
shoot dry yield (Fig. 2) up to 0.32 mM Ca and caused a progressive decline
after
that. For the higher solution Ni levels (316 and 1000 M), increasing solution
Ca
caused shoot yield to increase 2.5-4 fold up to 2 mM Ca, but declined at 5 mM
Ca.
-15-
CA 02272849 1999-02-26
WO 98/08991 PCTIUS97/15109
The similar pattern was observed on root dry yield in lower Ca levels, but the
higher Ca levels only caused a small decline in root yield and the difference
was not
significant.
Alyssum pintodasilvae
For the lower solution Ni levels (31.6 and 316 M), increasing solution Ca
increased shoot dry yield (Fig. 3) up to 2 times up to 0.8 mM Ca and decreased
yield at 5 mM Ca. A similar pattern was observed for the highest solution Ni
level
(1000,uM), but the highest shoot yield was obtained at 2 mM. For the lower
solution Ni level (31.6 MM), increasing solution Ca had hyperbolic effect with
decreasing slope on root yield (Fig. 3A1). For the middle solution Ni level
(316
M), increasing solution Ca increased shoot yield up to 0.8 mM Ca, with a
progressive decline at higher Ca. For the high solution Ni level (1000 M.M), a
trough effect with positive slope in lower Ca levels was observed.
Across all Ni and Ca treatments (Table 3), dry matter yield of shoot, root,
and whole plant were found significantly different (P < 0.0001) for three
species
tested, except the root yield of Alyssum murale which was only significantly
difference in P < 0.05 level. the maximum shoot and root yield were attained
at
31.6 M Ni with 2 mM Ca for Alyssum murale, at 31.6 M Ni with 0.8 Ca for
Alyssum murale, and at 1.0 M Ni with 2 mM for cabbage.
Ni Concentration and Distribution in Dry Matter
Cabbage
For the lower solution Ni level (1.0 M), increasing solution Ca had no
effect on shoot Ni concentration (Fig. 4). For the higher solution Ni (10 M),
increasing Ca caused a progressive decline in shoot Ni up to 2 mM Ca but did
not
decrease further at 5 mM Ca. The similar pattern was observed on root Ni
concentration for the lower solution Ni level. A trough effect with positive
slope at
higher Ca levels was observed at higher solution Ni level (10 M).
-16-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
Alyssum murale and A. pintodasilvae
For the lower solution Ni level, increasing solution Ca decreased shoot Ni
(Figs. 5, 6) somewhat, with flat response after 0.8 mM Ca. But for the higher
solution Ni levels, increasing solution Ca decreased shoot Ni at low Ca, but
increased shoot Ni at high solution Ca. For Alyssum pintodasilvae, Ni was so
toxic
at the lowest Ca with 1000 M Ni that the reduction in shoot Ni with
increasing Ca
at low Ca levels (0.128 to 0.32 mM) was not observed, in contrast with the
pattern
for 31.6 and 316 MNi.
For the lower solution Ni level, increasing solution Ca had a flat response
on root Ni concentration of Alyssum murale, but decreased root Ni somewhat
with
flat response after 0.32 mM Ca for A. pintodasilvae. For the higher solution
Ni
levels (316 and 1000 M), increasing Ca decreased root Ni in low Ca levels and
increased root Ni after 2 mM Ca, but increasing solution Ca had no effect on
root
Ni at low Ca with 316 M solution Ni for A. murale.
Alyssum species translocated a greater percentage of Ni to shoot tissue.
Shoot contained was 84 % to 98 % of total plant Ni acrose all Ni and Ca
treatments.
Shoot Ni/root Ni concentration ratio values ranged from 1 to 10 (Figs. 7,8),
far
higher than found in cabbage or in tomato (Chaney et al. 1997).
Across Ni and Ca treatments, dry matter yield, Ni concentration, and Ca
concentration differences of shoot, root, and whole plant were found for the
three
species tested (P < 0.001), except root yield of Alyssum murale was only
significantly difference in P < 0.05 and root Ca concentration of A. murale
had no
significantly difference (Table 3).
Nutrient Composition in Shoot Dry Matter
Zn concentration
Shoot Zn concentration in Alyssum spp. (Table 5A and 5B) were
significantly higher in the highest solution Ca levels in 1000 M Ni
treatment, and
remained similar across all Ca treatments for lower solution Ni levels. The
highest
-17-
CA 02272849 2007-03-21
WO 98/08991 PCT/US97115109
shoot Zn concentration in Alyssum spp. was observed in highest solution Ca
with
highest solution Ni level. However, shoot Zn concentration in cabbage (Table
5C)
was significantly lower in the higher solution Ca levels for both solution Ni
levels,
and the highest shoot Zn concentration in cabbage was found at the highest
solution
Ni level with lower solution Ca levels. In general, these interaction did not
cause
plant Zn to be raised to toxic levels or reduced to deficient levels. In crop
plants,
Ni is commonly found to reduce shoot Zn concentration and had additive effect
to
each other when concentration is above their toxic threshold (Wallace and
Berry,
1989).
Cu and Mn concentration
Shoot Cu and Mn concentration in Alyssum spp. (Table 5A and 5B) were
highest in the highest solution Ca treatment for higher solution Ni levels
(316 and
1000 uM) and remained similar across all Ca treatments for lower solution Ni
level
(31.6 M). The highest shoot Cu concentration in Alyssum spp. was observed in
highest solution Ca with highest solution Ni level. Shoot Cu and Mn
concentration
in cabbage (Table 5C) decreased with increasing solution Ca for all solution
Ni,
except that there was no significant difference for Cu uptake in lower
solution Ni
level.
Fe concentration
For Alyssum murale (Table 5A), shoot Fe concentration was lowest in the
highest solution Ca treatment in lower solution Ni (31.6 /LM), and remained
similar
across all Ca treatments in highest solution Ni (1000 M). For all solution Ni
levels in Alyssum pintodasilvae (Table 5B) and middle solution Ni level (316
&M)
in A. murale, shoot Fe concentration was highest in the normal solution Ca
levels
(0.8-2 mM) and lower in both lower and higher Ca treatments. The highest shoot
Fe concentration in cabbage (Table 5C) was happened in lowest solution Ca with
lower solution Ni level.
-18-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
P concentration
Shoot P concentration in Alyssum spp. (Table 5A and 5B) in higher solution
Ni levels was lower in the normal solution Ca levels and higher in both higher
and
lower solution Ca levels, and remained not significantly different in lower
solution
Ni level. Increasing solution Ca level decreased shoot P concentration in
cabbage
(Table 5C) across both solution Ni levels. Shoot P was in the normal range for
healthy plant growth in all treatments, the adequate shoot P concentration is
2 g/kg
for most plants (Taiz and Zeiger, 1991).
Mg and Ca concentration
Shoot Mg concentration decreased with increasing solution Ca level across
all Ni treatments and species (Table 5A, 5B, and 5C), and shoot Ca
concentration
increased regularly with increasing solution Ca.
Correlation between Ni and All Other Elements Concentration in Shoot Dry
Matter
There was no significant correlation between Ni concentration and Mg and
Ca concentration in shoot for all species (Table 4A, 4B, and 4C), except
Alyssum
spp. had a positive correlation between Ni concentration and Ca concentration
(P <
0.05).
A positive correlation (P < 0.001) between Ni concentration and Zn, Cu,
and P concentration in shoot for all species was observed. The correlation
between
shoot yield and shoot Ni concentration was negative (P < 0.001 for Alyssum
murale and cabbage, but only P < 0.01 for Alyssum pintodasilvae).
Only Alyssum pintodasilvae has negative correlation (P < 0.001) between
Ni concentration and M n and Fe concentration in shoot. Cabbage had positive
correlation (P < 0.001) between Ni concentration and Mn concentration in
shoot.
-19-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
Ni Content
Shoot Ni content showed a similar pattern to shoot Ni concentration in
Alyssum murale (Fig. 9), but the shoot yield was reduced remarkably due to
toxicity
of 1000 M Ni combined with high Ca level (5 mM) and caused the reduction of
shoot Ni content. Shoot Ni content of Alyssum pintodasilvae (Fig. 10)
reflected the
pattern of shoot yield, except the lowest solution Ni level was no difference
due to
low Ni concentration in shoot.
The best treatment to get maximum Ni content in shoots was 316 M Ni
with 5 mM Ca for Alyssum murale (50 mg/plant) and 1000 M Ni with 2 mM Ca
for Alyssum pintodasilvae (40 mg/plant) in 6 weeks growth period. Cabbage
shoots
contained only less than 1.5 mg Ni/plant in all conditions.
-20-
T
CA 02272849 1999-02-26
WO 98/08991 PCTIUS97/15109
00
O
U H
cc
U. O~ O~ O tr1 N 00 rt n
00 n cri et ~t O
O ~D r1 =-,
O .r
.II
N
U
E
ca
u E
61 M N
co) ..r N 00 O N
N N N N O O
Z ~O et O oo O O O O O ~,
u U
O y _
U Z
-. y U x
U ~ cn
cc o
79 d O CS,
c .o rn
4
E 1..
+
+
?
A O
r~ N N d 00 t*1 N N 00 N 00
w GL
r1 . R7
U O
... C 1
V
r i U
co a
13
u z
Q Q x x- C s
N N
m 0 cc cu
-21-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
o +*- N 0\ v O 00
'~ . C ri et e~i o
C N
o h
h
ae
e,
O
0
a4
l..i
en en fn
~O N 0000 O I I N O O .cf
C C N C C O O C O
00 cl.
.C
z
O
41: S
1c; O v c N 00 en .N., 00 N 00 - 3
"~ . t
y O ..+
~ = Z 8
04
U C sy
V) 0
lLy O y y a~+ CO
64
cts
z
z
x U ~' a
y U Ln H v1 cn * ^ rr O
SJ .. Cy V V IF U
Qf)
-22-
CA 02272849 1999-02-26
WO 98/08991 PCTIUS97/15109
Table 1C. Mean squares (MS) for the combined analyses by species, Ni
treatments, Ca
treatments, and blocks on shoot yields of 2 Alyssum spp. and 1 cabbage
reference species.
Source DF MS F values
Species: Alyssum murale
Ni trt 2 2.318 47.55***
Ca trt 4 1.084 22.24***
Ni trt x Ca trt 8 0.481 9.86***
Error 30 0.9157
Source DF MS F values
Species: Alyssum pintodasilvae
Ni in 2 3.904 44.43***
Ca trt 4 2.399 27.30***
Ni in x Ca in 8 0.759 8.64***
Error 30 0.0879
Source DF MS F values
Species: Cabbage
Ni in 1 13.86 330.67
Ca trt 4 1.733 41.35
Ni trt x Ca in 4 0.8740 20.86
Error 20 0.0419
*, ***, Significant at the probability 0.05 and 0.001 levels, respectively.
Type III MS for Species x Ni trt x Ca trt was used as the error term to test
for hypotheses.
t shoot yield is log g.
-23-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
Table 1D. Mean squares (MS) for the combined analyses by species, Ni
treatments, Ca
treatments, and blocks on shoot Ni concentrationt of 2 Alyssum spp. and 1
cabbage
reference species.
Source values
Species: Alyssum murale
Ca trt 2 8.957 161.18***
Ni trt 4 0.9013 16.22***
Ni trt x Ca trt 8 0.2965 5.34***
Error 30 0.0556
Source DF MS F values
Species: Alyssum pintodasilvae
Ca trt 2 6.650 104.87***
Ni trt 4 0.2614 4.12**
Ni trt x Ca trt 8 0.2013 3.17**
Error 30 0.06341
Source DF MS F values
Species: Cabbage
Ni trt 1 133.5 1160.34***
Ca trt 4 0.1482 1.29
Ni trt x Ca trt 4 0.4358 3.79*
Error 20 0.1151
*, ***, Significant at the probability 0.05 and 0.001 levels, respectively.
Type III MS for Species x Ni trt x Ca trt was used as the error term to test
for hypotheses.
t Ni concentration is log mg L''.
-24-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
Table 2. Nickel concentration for Ni treatments additions to 0.5 strength
Hoagland solution
with 2.0 mM MgSO., respectively.
Treatment Concentration Treatment Concentration
--AM-- pNi * mol/L --- M- pCa * mol/L
-Ni- -Ca-
Cabbage
1.00 6 128 3.89
10.00 5 320 3.49
800 3.10
2000 2.70
5000 2.30
Alyssum snn.t
31.60 5.50 128 3.89
316.00 3.50 320 3.49
1000.00 3.00 800 3.10
2000 2.70
5000 2.30
t NiSO, was used as nickel treatments. Due to the death of cabbage before
nickel treatment
reaching 31.6 mM in pre-experiment, cabbage was only applied 2 lower nickel
levels.
$ Alyssum spp. are Alyssum morale and Alyssum pintodasilvae
-25-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
Table 3. Mean squares from analysis of variance of shoot, root, and whole
plant dry matter
yield, Ni concentration, and Ca concentration for A. murale, A. pintodasilvae,
and cabbage
across Ca and Ni treatments, respectively.
Dry matter yield
Source df Root Shoot Whole Plant
log g
A. murale 14 2.78* 0.92*** 0.95***
A. pintodasilvae 14 2.03*** 1.68*** 1.67***
Cabbage 9 7.30*** 2.70*** 3.00***
Ni Concentration
log mg kg-'
A. murale 14 4.03*** 1.71*** 1.82***
A. pintodasilvae 14 3.21*** 1.14*** 1.21 ***
Cabbage 9 13.73*** 15.10*** 12.42***
Ca Concentration
log mg kg''
A. morale 14 0.480+ 3.48*** 2.95***
A. pintodasilvae 14 0.329*** 2.55*** 2.31***
Cabbage 9 3.06*** 2.73*** 2.74***
+, *, *** Significant at the 0.1, 0.05, and 0.001 of probabilities,
respectively.
-26-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
0
00
* *
cs
o 00 CD IT%D-otn
L o 0 0 0 0 0 c
+ + +
.Q kn 00 oN
G b 0 N .., . r N N
= 0 0 0 0 0 0
cc
* * * *
~~pp
"It
s pr 00000 .U
oA
z~
=
(a CJ ON ~o 0% en u
Ooc4O c
coon >
O r
a
ooo a.v
E
* * 0 0
EE * * =o
00
O r ca
U 0 O 000
79
cc * 0 00
lu *e! O ~
"c N c
cc
0.3 =: ~ 8
'fl G CO
M
c:
~ 0 3 * i
-a *
~ ~ se
y ZCo NU~ci c.~um +
-27-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
0
00
Oi
+ +
=r-000NN '.0
ON -rN -- '.0
0. V C O O O C O 0 0
E
o~go~
0 0u orq en
~ ~ 0000000
00 C4
km r- 00%nCD turf
L1. 000000 U
O
Z * * H _~
h. cc
* * * * .U
tNenet
-~ ~, 00000 ~`"
U * * ~M
~ 41 C O V7 ~ .~
000 a.v
S M
M
V * * O
w C O 00 as
O to O O
U o
v co
o$ N 00 0 9
-v` c c0
ohs ., o
$ I t o
co
s c ~:
CL)
c Z 3 * ,?
v ~ * ^ V
cl O _ O C= O U po .C
-..~ Zv~NUU +V
-28-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
off!
0
V *
Oar-+M[`- {- D\
U ooooocio
0
*
C3 * * * *
0 =-=erco %ooCD
In 'IT ") rl: In IRT
0 c 0 0 0 0
2
* * * *
* * * *
A a 0000 == by
0. 2
U
cd "'~~gM ~
y (y 000
:r N
Q
y * * * M
V 000 G.M
. 0 O
rl~ O9 X000
O 0
y O
o N c ca
=
V 0
0m V
aCC .~ !.igqa~7
co 2~ 3 V
3 Z *V
~'c b
tC.Q O * U
NU r-W +U
-29-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
v v .0 m v u ,n A u u u ~, en 00 ~o
y N'00 O0~ riO
ea N V) tl- !f 0~ M ~+ W) O N M h ~' r -
U N M .. ~' M
era
Z eO A R - u cc CO Co m co co cOS M
m 00 V'1 f~ ~0 M to en O tT \D N V'f en
w r- oo co V) co v C~ t~ W ~D r- m
00 h 'Ct et tf M et V'1 M N V1 w; M M
L
.C
3
cv A m s m cu A ea .n ca ca cu .a ~ ea
C O yyn~ r~--~~NM NW O
O et O~ M M 00 Io rl~ O~ M D\ O~ l0 rl r
M N M M M M N M- M ~o et r4 N N
0
6~ I lV ep t0 A .0 .0 .a eb A u cC A e0 e0 c7
y r`1ON~oQ\ co 00 r`ItT
(L V1 D\ 00 t~ ~0 O~ 00 O V1 M ~C t~ N e!=
0\ D\ 00 00 %n VII h 00 r+ 'w 00 h %0 00
C
C
3
o -0 = - .0 " .c .0 .0 .0 c0
_ c0 R e0 m m 0~ r- %0 V1 tN 00 et r4 q*'
v '~ 3 0\ N 00 "r N O M 4 r-: el; 4 O 00 N \6
O N M %n %n et h M ~o w IV N N 00
y E ca ea ea ea ea u .0 u to Ø0Ø0 cc
- N'1 7 I.0%000^+... NN00 Vy O~0r` hoo
U ~ ~ --~ N O ~ M --~ O M 00 f` O\ M O\
W C N NNM V1~r` l'^ 0~r-%0IC10
O
c
~" e0 ci CE l0 ci cc .0 c% .0 A .0-0 .0 .0
C t. C r- N --~ et r- N ~0 M 00 00 00 N
c~etM16 owi 6 ri%6---ri
~o ~a h ~0 00 v 00 in 00 ~o in r- o
8 y
H u
e0 .0 ci ci cc u u .0 u .0 .0 .0
C r M 00 %0 - M h r- N M 00 00
p .~+ Z oo "O "0 I0 N r 0~ rl M 00 In N h "-+ 00
M --~ N Cl N V) to 00 w %0 ^ as 00 00 N
_lw i E 00
C 0
U .0 ca cOO Øa .0 Ø0 cV .0 O .0 cO R
'O rt %0 \0 01 as N M G 0\ 00 00 00 \0
C N r --+ -I N rl ' ... r et rl 0\ 0%
M' r en M M N M M h M M M
U v
u a0i c II >0N0\000 II >0 c0 CD II >0~0\CD 0
u~: C E 000r,-en So et-=r-en (U 00t ter-M
U C Z U M M M N N z U M M M N N U M M M N N
-30-
CA 02272849 1999-02-26
WO 98/08991 PCTIUS97/15109
fn ON 00 n t` n C %0 co
d' a N O '
-+ N N M V1 -- N M IV M e)' N M W) C
II
. . ca co cc ca - ca m t0 ft to to
Q~ O~ h 0~ O h et er O %M 00 h h co m
o ^ ~n vi N N v~ n to et h o~ vn 00 II
... Cy .....M ty.......~. N 1l1et^ ^N
4)
ca N ft R m cu ~~u tea m .G ty ft M N A y4y
OMCD th OOO\DO +01 hOe4 0 ~ ~~O
M N M M ~! cn M M N M O h M N
L:.
Rt t0 R A R7 to ~ ~ ~ O A N tD N N =_
00 h M ee0~ p~ O O ~O O O% v1 rte-
M tA
N Go co en en %n W N M "tr h N
N C4 e4 .~ .a .a .~ A ea to t0 N ta
ea ea to . ea v1 O b - h h ~+ h
M t ^ ^ N N 00 co e!' 00 e! 4
V'100NN"ef
ca t ea A N M om .0 .O N N N A tD N C
hMM~ON MMMpo^O ONM00
co m r% \0 c\ 00 0 rn
C4 Go %n 4n 00
A e c ea ca .a .O Ø0 E
In cc ft
" \0 \O In MOO %,A, c; N en
N ^ N N M N en C4
V
cq ea N ed
^^ m .0 .0 ea A.G.GO 1.
Q~ po O~
M !r ~ et ~ O N O N M \0
O~ ~ N 00 Y'1
0000^ NNNNh coot m0 .4:
ea cc ea eta m m ea ea ca ea 4)
00 e C0 4'1 ~D 0000 r- N N N
v1 00 kn c C14 00000"Ca OMMel
0 0-- O 0 0 0-- 0 0 0 0 0
ll >o~o,ooo II >o~o~ooCD II >o~o~ooo
z U M M M N N Z U M M M N N cu en M M N N
-31-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
{
{ d '~ U ee 'o v ea .c cc
u M ON N N co 00 IRr ON M M
{ v'~O~O~O~ M~DNO~cn ~D V'1N OHO
!t N N V7 ^. to
Z 3
cc cc .a .0 U ea ed cOa m .a eo cC ea ft CC
~_ ~~pp ee~~ pp
tm 00 en C4 m C) co
,~ O e 0 0 m CD O M rrn
%n n V=t 00
3 oo v i Y1 ~ ~l e+i ~ cn ... . et v . .
o
{ CC lC C A A cC .0 .0 .a cc m e0O .a A
o Mr- en N%nco tn O%%DO~O
.+ n. { M M M co N 00 t, r- 10, - W)
V'1 M M d d M N M M d ~O V1 M [~
C.)
Lr Ø0 .0 CCC 1~ e0 O
C c ee ( U O cC A U
OEM 00~00~N rl~a -
NmON w mas%0 Nt0 CD 00 et VI) 00
3 w 00 0 a o r- W) r- r` qn tr N
o
as
o~ U
coo ewe u U ed ca e0 cC eC eC
-~ { e0 e0 e0 A A M d 00 =--- Ct *~D
OO Q\ ~p --~ ~p r- 00 f- M N V9 M M 'Tr M
frj ON co^ooen Oar- V=o%O V1V-) tnen cu m .0 cc to w cc .0 .0 go u
R. p
r-htn00 M W)N%n N
e ~., U E V'i v7 ~O N o0 N en M O ~D O~ ...
,~ CL { M N ^~ N en 110 kn 00 ~ -+ O r- en
43
e.3 =
S~ !
M M M M M ee M M M =G a ca
O C. M M O~ t00 d ~O
N ooItt o ~0 0 00 a N 0 00 0 co 0 0 00 eli rD~ W) 00
U 00 CD
o
Z y .0 -mm .0 -coo
0 etenNrne+1 OA co~o~o0 t~e+iM0110
-- E
c.r
U
C .~+ O O cc (C A teOC O - A
UU ~DOr-~C--- C14 NNnOr-4 00
CN
4] w
CC)
cis
m U II >cka-, o II >O~o%ooo II >a,m c)
C cod t- en _ ?? 00 - r- N OO d' r~ en
U bq O Z cC M M cn . N N Z ee M M M N N Z eC M M M N N
cc o r U U
-32-
CA 02272849 1999-02-26
WO 98/08991 PCTIUS97/15109
U e.0a ca e0 V U U O ca cc to ea ea ea
Y9 00 N 0 M en 01 wl ONO
00 -
M M `V N ~-+ N M e! d' N eV N d II
Lr
ca - . .0 .a ca ea ca ea ea ca .G .C p
to 0~ V) ~o O e! ~o 0 oo %o co 00
N W) C14 f W) M N -. 00 NN O
II
f3,
C
to ea to to ppsa~~ e0 ea to to to ea m M.0 A
oo en "toO'oc en n 00f- n
' V'1 01 01 00 ~
NMNM Men en en M
ed A CO ca CO ~`.
O ~ O~ -~ 00 ca ca eo e0 ea ca to ea eOOa N
00No~00 NNco etN
00 00 ^
N M 'et N N
- - .o CO A ea ea CO CO CO - .a .t]
a ' 00N M ^...N V7h y
Cl O fw C4 r,4 !t M 00 N lr 1 rn N N
M e+1 N <h
cc
cc
ca ca N ea A ea to ca A ea ~ .G ~ .C .O '~^,
e7~OO Vi
NN ~NO~~O - NNO .=~..
Oenenl lq NN Rt N~-+ en en
N --- ..r M N N N M M NN N N
N "C
ca
ea ca A to ea ca eta .o cc m .0 ea cc .a CV
,6 06 06
W) 0% 1100~ V~t1Mr-0n0~ Nv~co 4
'0NNNN C Nom-^N NN~QQ~-'0
ea ~.G.C O
M M ea ed ea ea OON e00 U e~0 CO
Od efi~h ~IRrtN+'f~lT 0\N0000hN
- 0000 N N'0 NN ~
CU~ V
Ca 10 ea ea m .G cc ea comet cu U .C c~0 to .C~ h
--+ M _N M M N N of %n f- e} co m tn
00 CD kn N V 00 h In O W N N
N M e}' v
O O O O O O O O O O CO O O O O
0
u u a,
II ^o0OSt ~NM ii .!!oooove0N II 0 r- en 22
Z CO M N1 M N N z w M M M N N U M M M N N
-33-
CA 02272849 1999-02-26
WO 98/08991 PCTIUS97/15109
> 'L7 '~ 0.0 c a 'O ~ .0 O ea - Ø0.G co
4:.
ed can %0 co 1 C4 W)000%rr M~000~0
U S. 00% Mtn =0' ^-+MN
G)
E
Cc .0 ,p U 'fl to .0 t) 'S7 t7 a Øc) O eo
b 1
0 -+Ot~~~ N~O~O VMt den Wl r14
' r tn%n000000
Z L= %0 0% O ~D vi In N 0% %O ',O M M cn M 4
'~~' eu .c .o eO cO ca .O m m e0 m ea
O% M O'0 O~ N N v V1 00 'D I", en r-
C a -~ ON %00 00 O0%C N N 0%0%N M In
p ta0 N0000 ~Dvft~OM ~--
.y
h
CO ft M.0 e0 td A A A A cQ CV eE e0 e0
LLI et .rQ\NN 00O 00 v7 en *0CD 00
C
ft .0 .0 .0 .a eV vs end .0 co CO CO CO ee CO
t` C%O ~O d ~C
M 00 vy O M N 4 M N 00 N -= O O'
a) -a C viNN%0 MMO'.OC~ d MN NM
V e0 CO A e0 A A e0 A CO e0 to
y.., b0
p C .SC co N 0Q%~ 00 M M d v1 %n O M M
U vC%OON OMOt~h 00N0 V1
G~ E N - NNN '0 '0NM ~N000%0'.
II
C
cO b
ea O O Ø0 CO C CO .0 cc CO e0 e7 CO CO
$ C c
"gr en -00 MN 00 f+'.N tn0
y N ~166 4C40 O; P:C\CM vi00ot~0
-r h N N N N N N N - 00 N M -+ - N
H ti
u 3 CO CO !O eO Rf
H V7N n -+N .0 0 ed eq eO A cV
t0 c0 10 C.) V
O _Q N - CM _ NNpM d'MN'.O'0 --en en In
"N 00e
L
Co co
Z a0 SSSOS en
~~ --~ OOOOO
E 0 0 0 0 C 0 0 0 O 0 0 0 0 0 0
U b0
O 0 .O ,p .0 p 0 t0 0 .a CO e0 CO A
U
N0% e CO CO Nt~en 00 -~0,I' N0
c~ O QO t~ 'd, \0 N %0 00 M 1, 00 ~D t- M
w C
11 >00OO II >00mr r- en II >ooor-
.+ CO O OC Z Z V M M M N N U M M M N N
E- U F U o
-34-
CA 02272849 1999-02-26
WO 98/08991 PCT/US97/15109
eta cc ego m 0
oco~ II
M I~r %n It ST
4..
en hr,'nr
Md"CYlOc~
h N N
V
4r
'L7
M c
SO n N ^ yf.
V'1 O\ N e"1 M
~O V1 N M
00
..r
cc M cc .0 .0 r-
1O C o P- 0
M t- N 00 %0
00 M N N M
N
en r- r-
VN N N
ca eb t ~ to +~~.
~~pp b
N et ~D d O
N M N'f ^ U
lb
m to cc
~~pp V
V1 00 M O
CCP et 0%
^^ooo H
A
.a .G ea e0 V
n K1 M
^0~00e!'
C C C C O a
V
O
II >o,o,ooo
moocht- M
Z m M M M N N
U
-35-