Note: Descriptions are shown in the official language in which they were submitted.
PROCESS FOR RECOVERING VALUE METALS FROM
IRON-CONTAINING ALLOYS
FIELD OF THE INVENTION
The present invention relates to a process for recovering value metals such as
cobalt
and/or nickel from alloys and mattes containing substantial amounts of iron.
BACKGROUND OF THE INVENTION
Substantial reserves are known to exist of ores., both of the oxidic and
sulfidic types, as
well as slags, which contain relatively small amounts of value metals such as
cobalt, copper and/or
nickel and relatively large amounts of iron. The first step in recovering
value metals from such
ores and/or stags is a pyrometallurgical reduction process which converts
oxides of metals in the
ore or slag to metals having a zero oxidation state. This reduction process is
conducted at high
temperature in a reduction furnace, and the material produced by the reduction
is typically
referred to as a "matte" or "alloy".
Alloys typically contain about 35 to 70 percent iron, and less than about 10
to 60 percent
of value metals such as cobalt, copper and/or nickel. Mattes are similar in
composition to alloys,
but have a relatively high sulfur content, typically exceeding about 10
percent. The sulfur is
typically associated with copper, which can comprise up to about 30 percent of
the matte or alloy.
Metals are recovered from mattes and alloys by a hydrometallurgical process
involving an
CA 02273067 1999-OS-27
-2-
acidic leach process conducted under oxidative conditions, in which all the
metals present in the
matte or alloy are oxidized and dissolved in the form of soluble metal salts.
After separation of
the liquid fraction from the solid residue, iron is separated from the
remaining metals in the liquid
fraction by precipitation.
One disadvantage with conventional processes is that the hydrometallurgical
leaching step
involves dissolving all the metals to produce a liquid fraction in which the
value metals account
for only a small fraction of the total dissolved metals. Clearly, it would be
desirable from an
economic standpoint to reduce the volumes of liquid used in the leaching step
and thereby
improve its efficiency.
SUMMARY OF THE INVENTION
The present invention overcomes the above-mentioned problems of prior art
processes for
recovering value metals from a matte or alloy by providing a process in which
the iron in the
matte or alloy remains in a substantially insoluble state during the leaching
process, thereby
significantly reducing the volume of liquid which is required to dissolve the
metals in the matte or
alloy.
The process of the present invention is particularly adapted for recovery of
value metals
such as cobalt, copper and/or nickel from mattes and alloys in which the value
metals are
entrained with relatively large amounts of iron. The process of the present
invention involves a
CA 02273067 1999-OS-27
-3-
first hydrometallurgical leaching step in which a quantity of matte or alloy
is contacted with an
aqueous, acidic solution containing sulfate ion to form a soluble sulfate of
the value metal
contained in the matte or alloy. This first leach is conducted under
conditions in which the iron
contained in the matte or alloy is oxidized and transiently forms soluble
sulfates which are
immediately converted to insoluble compounds which precipitate out of the
solution. A
solid/liquid separation then separates the value-metal containing liquid
fraction from the iron-
containing solid residue, and the liquid fraction is purified, where
necessary, and further processed
to recover the value metals therefrom.
Therefore, in one aspect, the present invention provides a process for
selectively
extracting a value metal from a ferrous solid, said value metal being selected
from one or more
members of the group consisting of cobalt and nickel and said ferrous solid
being selected from
mattes and alloys containing iron and said value metal in metallic form,
wherein said process
comprises: (a) providing a first aqueous solution in contact with an oxygen-
containing
atmosphere, said first aqueous solution having an initial pH of less than
about 2.0 and containing
sufficient sulfate ion to form a soluble sulfate with said value metal
contained in a predetermined
quantity of said ferrous solid; (b) adding said predetermined quantity of said
ferrous solid to said
first aqueous solution over a period of at least one hour to increase the pH
of the first aqueous
solution to the range of from about pH 4 to about pH 6, wherein a temperature
of the first
aqueous solution during addition of at least a final 50 percent of said
ferrous solid is maintained at
less than about 100°C; (c) conducting a solid/liquid separation to
separate a first liquid fraction
containing soluble sulfates of said value metal from a first solid residue
containing substantially all
CA 02273067 1999-OS-27
-4-
of the iron in said predetermined quantity of said ferrous solid; and (d)
reducing and recovering
said value metal from said first liquid fraction.
In addition to containing large amounts of iron;, mattes and alloys may also
contain up to
about 30 percent copper. Copper is typically contained in a matte or alloy in
the form of insoluble
sulfides and passes through the first leach substantially unleached, being
present in the solid
residue recovered from the first leach. In circumstances where it is desired
to also recover copper
from the matte or alloy, the process of the present invention includes
optional steps for treating
the solid residue of the first leach under conditions in which the copper
sulfides in the solid
residue are oxidized to soluble copper sulfates. After a second solid/liquid
separation, the liquid
fraction containing copper sulfates is purified and treated to recover copper
therefrom.
Accordingly, in a second aspect, the present invention provides the process as
described
above, wherein said ferrous solid additionally contains up to about 10 percent
by weight sulfur in
the form of copper sulfides, said copper sulfides being substantially
unoxidized during said steps
(a) and (b) and being contained in said first solid residue, said copper being
extracted from the
first solid residue by a process comprising: (a) oxidizing said copper
sulfides in said first solid
residue to produce soluble copper sulfates by contacting said first solid
residue with a second
aqueous solution containing sulfate ion and having an initial pH of less than
about 3.0 in the
presence of a pressurized oxygen-containing atmosphere and at a temperature of
from about 120
to about 220°C; (b) conducting a liquid/solid separation to produce a
second liquid fraction
containing said soluble copper sulfates and a second solid residue; (c)
reducing and recovering
CA 02273067 1999-OS-27
-5-
said copper from said second liquid fraction.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will now be described, by way of example only, with reference to
the
accompanying drawings, in which:
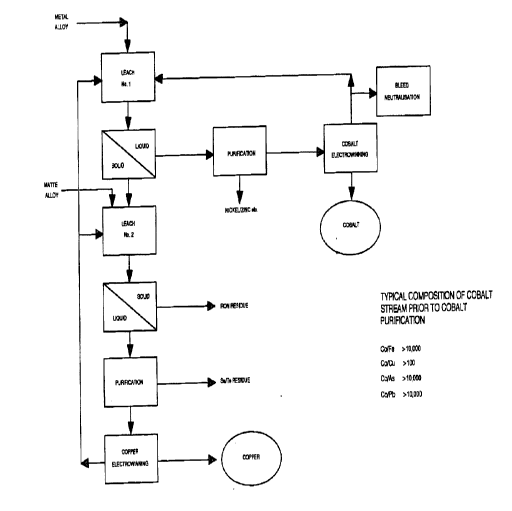
Figure 1 is a flow diagram showing a preferred two-stage leaching process
according to
the present invention; and
Figure 2 is a flow diagram showing an alternate two-stage leaching process
according to
the present invention.
DETAIL DESCRIPTION OF PREFERRED EMBODIMENTS
A preferred process for extracting value metals from a matte or alloy will now
be
described below with reference to the flow diagrams shown in Figures 1 and 2.
As discussed above, the present invention is useful for extracting value
metals from mattes
and alloys which are produced by reduction of iron-rich ores. These mattes or
alloys typically
contain for example about 3 5 to 70 percent by weight iron, less than about 10
up to about 60
percent cobalt and/or nickel and up to about 30 percent copper. Alloys
typically contain little or
CA 02273067 1999-OS-27
-6-
no sulfur, whereas mattes contain greater than about 10 percent sulfur. The
method of the
present invention is also useful for extracting value metals from materials
having a sulfur content
of up to about 10%, which are neither mattes nor alloys. These materials are
sometimes referred
to as "malloys". However, the terms "matte" and "alloy" as used herein are
intended to include
materials containing from 0 to 10 percent sulfur.
In a particularly preferred embodiment, the process of the present invention
is utilized to
recover cobalt and copper from a material which contains about 65 percent
iron, 6 to 7 percent
cobalt (this amount includes nickel which has similar chemistry), about 13 to
27 percent (average
19 percent) copper, relatively small amounts of zinc, selenium, tellurium,
manganese, chromium,
cadmium, arsenic and lead, which may or may not be recovered according to the
process of the
invention. The material also contains about 5 percent sulfur, and therefore,
strictly speaking, it
can neither be categorized as a matte nor an alloy, but is nevertheless
referred to herein as a matte
or alloy.
Most of the metals in the matte or alloy, including iron and value metals, are
present in
their zero oxidation state, having being reduced in the previous ore
processing step. However, at
least some of the copper in the matte or alloy is present in the form of the
copper sulfide Cu2S
and, if the sulfur content is relatively high, will also be present as CuS.
The first step in the preferred process of the present invention comprises
leaching cobalt
from the matte or alloy by an acidic leaching step conducted under oxidative
conditions. In a
CA 02273067 1999-OS-27
preferred embodiment, the matte or alloy is charged over a period of time into
a reactor
containing an acidic solution of sulfate ion under an oxygen-containing
atmosphere. The amount
of sulfate ion contained in the leaching liquid is preferably
stoichiometrically matched to the value
metal content ofthe matte or alloy. The adjustment of'sulfate content is
discussed more fully
below.
Under the conditions employed in the preferred leaching step, value metals
such as cobalt
and nickel are oxidized and form soluble sulfates, namely CoS04 and NiS04. The
metallic iron
present in the matte or alloy is oxidized and reacts with sulfuric acid in the
leaching solution to
form the soluble iron sulfates FeS04 and Fe2(S04)3. However, these iron
sulfates immediately
undergo disproportionation, primarily forming iron hydroxide Fe0(OH) and iron
hydroxysulfate
Fe(OH)504, both of which are insoluble and precipitate from the solution.
Sulfuric acid is
regenerated in the production of these insoluble iron compounds. Therefore,
iron is transiently
dissolved during the first leaching step, but is immediately precipitated as
insoluble compounds.
The transient dissolution of iron does not need to be accounted for in the
design of the leach step,
in terms of reactor volume, liquid volume or sulfixric acid concentration, and
therefore iron may
effectively be regarded as being insoluble during the first leach.
Under the conditions used in the first leaching step, copper which is present
in the matte
or alloy in the form of copper sulfides, is substantially unoxidized and
remains in the solid phase.
Therefore, the first leach effectively separates value metals such as cobalt
and/or nickel from iron
and copper. The preferred conditions for the first leach are now discussed
below.
CA 02273067 1999-OS-27
_g_
The solution which forms the liquid fraction of the first leach is acidic,
preferably
containing sulfuric acid and having an initial pH no greater than about 2Ø
More preferably, the
leaching solution is a recycled electrolyte from a cobalt and/or copper
electrowinning process,
discussed in greater detail below. Where the liquid fraction is a recycled
electrolyte from copper
or cobalt electrowinning, it will contain sulfate ion in the form of sulfi~ric
acid, and in the form of
soluble sulfates of metals such as copper. Sulfate ion in these forms is
referred to herein as
"exchangeable sulfate", since it is available for formation of soluble
sulfates of value metals such
as cobalt and/or nickel.
In order to provide the oxidative conditions necessary to oxidize the value
metals and the
iron in the matte or alloy, the acidic leaching solution is preferably in
contact with an oxygen-
containing atmosphere, such as air (20 percent oxygen) or an atmosphere
containing higher
amounts of oxygen, for example 93 percent oxygen from a vacuum swing
absorption plant or
99.5 percent from a cryogenic plant. The oxidative atmosphere preferably has a
pressure at or
above atmospheric pressure. More preferably, the oxygen-containing atmosphere
is pressurized,
thereby increasing the speed of the leaching reaction b;y increasing the
amount of oxygen in
solution. Most preferably, the oxygen-containing atmosphere has a pressure of
up to about 1,000
kPa, and even more preferably from about 300 to about 700 kPa.
The temperature of the acidic leaching solution is preferably maintained at an
average
temperature of less than about 100°C during the leach in order to avoid
oxidizing copper sulfides
to soluble copper sulfates. More preferably, the temperature is maintained in
a range of from
CA 02273067 1999-OS-27
-9-
about 65 to about 100 ° C, and even more preferably from about 75 to 85
° C.
In the process of the present invention, the solid matte or alloy is added to
the acidic
leaching liquid gradually. The gradual addition of the matte or alloy results
in a gradual rise in the
pH level of the liquid from an initial pH of less than about 2.0, typically
about 1.6, to a pH in the
range of from about 4 to 6, and preferably from about 4.5 to about 5.5. This
rise in pH reflects
the consumption of HZS04 in the oxidation of value metals such as cobalt
and/or nickel to
produce soluble metal sulfates.
The addition of the matte or alloy may either be continuous or step-wise in
portions. The
size and number of portions is variable, with each portion preferably
comprising from about 2 to
about 50 percent of the total mass of the solid added. In general, continuous
addition or addition
of a relatively large number of small portions produces better results than
the addition of relatively
few large portions.
The matte or alloy is preferably added to the leaching liquid over a period of
time of from
about 1 to about 6 hours, and more preferably from about 2 to about 3 hours.
The matte or alloy
may be added at either a constant or variable rate over this period of time.
The reaction between the metals in the matte or alloy with the acidic leaching
liquid is
highly exothermic. In prior art leaching processes, all of the matte or alloy
is typically added to
the leaching liquid in one portion at the beginning of the leaching process,
resulting in a rapid
CA 02273067 1999-OS-27
-10-
increase in temperature to at least 130°C. Allowing the temperature to
increase into this range
causes the oxidation of copper sulfides present in the matte or alloy,
producing soluble copper
sulfates, some of which react further to produce additional sulfizric acid and
hydrolyzed copper
compounds. This prevents the pH from increasing to the above-mentioned range,
resulting in
copper contamination of cobalt rich liquors, and a more difficult separation
of the liquid and solid
fractions. In contrast, the step-wise addition of matte or alloy to the acidic
leaching liquid in the
process of the present invention removes heat from leach slurry residues and
reduces the extent of
sulfur oxidation, thereby improving the purity of the cobalt rich liquor.
Preferably, the matte or alloy is added to the liquid such that the
temperature is kept below
100 ° C. However, it is to be noted that some excursions in temperature
above 100 ° C may be
tolerated at the start of the leach. Since the matte or alloy is added
gradually, there is typically
only a small proportion of the total matte or alloy present in the leaching
liquid at the beginning of
the leach. The oxidation of the copper sulfides in this portion of the matte
or alloy will typically
not have a significant detrimental effect on the extraction of the value
metals or the purity of the
cobalt rich liquor.
For example, the addition of the initial portions of the matte or alloy to the
liquid may
result in an exotherm to the range of from 100 to 150°C. After this,
the temperature may be
reduced, for example by flashing off steam, to the preferred range of less
than about 100°C.
Preferably, the temperature then remains at less than about 100 ° C for
the remainder of the leach.
In the preferred embodiment of the present invention, the temperature is
brought down to the
CA 02273067 1999-OS-27
-11-
preferred range of less than 100°C for the addition of at least the
final 50 percent of the matte or
alloy. More preferably, the temperature remains at less than 100 ° C
for substantially the entire
addition of the matte or alloy to the acidic leaching liquid.
The inventors have found that performing a first leaching step according to
the preferred
method described above results in substantially all the copper and iron
remaining in the solid
phase with from about 70 percent to about 95 to 100 percent of the value
metals such as cobalt
and/or nickel being extracted into the liquid phase. After completion of the
leach, a solid/liquid
separation is conducted to separate a value metal-containing stream from the
solid residue. This
separation may preferably be accomplished by filtration or by counter current
decantation (CCD).
In leaches conducted with a "malloy" having the composition set out above, the
following ratios
of cobalt to other metals are typically observed in the value metal stream:
Co/Fe > 10,000
Co/Cu > 100
Co/As > 10,000
Co/Pb > 10,000.
Therefore, it can be seen that the first leach in the preferred process of the
present
invention provides a liquid stream which is predominantly comprised of soluble
salts, primarily
sulfates, of value metals such as cobalt and/or nickel. As shown in the flow
diagram of Figure 1,
this value metal stream may preferably be subjected to fizrther purification
by conventional means
CA 02273067 1999-OS-27
-12-
to remove impurities such as nickel or zinc from the solution. After
purification, cobalt is
recovered from the liquid stream, preferably by electrowinning in which cobalt
ions are reduced
and precipitated as cobalt metal in its zero oxidation state. The spent
electrolyte from the
electrowinning step contains sulfizric acid and residual amounts of metal
salts. Preferably, at least
a portion of this electrolyte is re-circulated for incorporation into the
acidic leaching liquid in the
first leach, with optional bleeding of a portion of the spent electrolyte or
neutralization of the acid
in the electrolyte to adjust the amount of sulfate ion contained therein.
Although a specific purification circuit is shown in Figure 1, it is to be
appreciated that
numerous other methods exist for purifying the value metal-containing stream
obtained from the
solid/liquid separation conducted at the end of the first leach. In another
preferred embodiment of
the present invention, illustrated in Figure 2, cobalt is precipitated from
the value metal-containing
stream in the form of cobalt carbonate by addition of sodium carbonate and
sodium hydroxide to
the value metal stream. The cobalt carbonate then undergoes a series of
additional steps by which
it is purified, culminating in a cobalt electrowinning step to produce cobalt
metal.
Where the matte or alloy contains significant amounts of copper, and where it
is desired to
recover the copper contained in the matte or alloy, the solid residue obtained
from the solid/liquid
separation in the first leach is subjected to fiarther processing in a second
leaching step. The
object of the second leach is to extract copper and the remaining cobalt
unextracted in the first
leach into the aqueous phase while iron remains in the solid phase. As in the
first leach, the solid
phase is contacted with an acidic leaching liquid which contains sulfuric
acid, the leaching liquid
CA 02273067 1999-OS-27
-13-
preferably comprising a spent electrolyte from either a cobalt or copper
electrowinning step
which, as discussed above, may contain residual amounts of soluble metal
salts. The initial pH of
the leaching liquid is typically less than about 2.0, more typically about
1.4, reflecting the sulfizric
acid content of the leaching liquid. After addition of the solid residue to
the leaching liquid, the
pH may increase to about 2. S to 5.0, but decreases during the second leach to
about 1.4 to 1.6,
reflecting the liberation of HZS04 during the formation of insoluble iron
compounds.
The second leaching step is also conducted under oxidative conditions in order
to facilitate
oxidation of the copper sulfides present in the solid phase. As in the first
leach, the oxygen-
containing atmosphere may be comprised of air or oxygen in varying states of
purity. In the
second leach, the oxygen-containing atmosphere is pressurized, preferably to a
pressure of from
about 400 to about 2,500 kPa, and more preferably from about 700 to about
1,200 kPa. As in the
first leach, pressurization of the oxygen-containing atmosphere ensures that
sufficient oxygen
enters the liquid phase to oxidize any remaining metal compounds, including
sulfides, in the matte
or alloy.
The temperature in the second leach is preferably higher than that of the
first leach in
order to provide more vigorous conditions for copper sulfide oxidation.
Preferably, the
temperature in the second leach is from about 120 to 220 ° C, and more
preferably from about 13 0
to 170 ° C. In general, the higher the pressure and temperature in the
second leach, the higher will
be the degree of copper extracted from the solid phase. However, it will be
appreciated that the
actual pressure and temperature conditions used in the second step are largely
determined by
CA 02273067 1999-OS-27
-14-
economic considerations, with the increased value of extracted copper being
weighed against the
costs associated with raising the pressure and/or temperature of the second
leach. For example,
the use of a temperature of 130 to 170°C and a pressure of from 700 to
1,200 kPa will typically
extract about 70 percent of the copper from the solid phase during the second
leach. However,
the application of more vigorous conditions can increase the level of copper
extraction to about
95 to 100 percent.
Under the above conditions, copper is leached into the liquid phase as soluble
salts while
substantially all the iron remains in the solid phase, primarily in the form
of Fe0(OH), with some
iron (III) oxide (Fez03) being formed, both of these compounds being
substantially insoluble in
the liquid phase. The relative proportions of the iron compounds formed in the
second leach is at
least partially dependent on the leach temperature and pressure. For example,
higher
temperatures and pressures favour the formation of iron III oxide over
Fe0(OH).
In the second leach, the solid residue from the first leach may preferably be
added in one
or more portions, the size and number of the portions being relatively
unimportant because
temperature control is relatively unimportant in the second leach, as long as
it is sufficient to
oxidize copper sulfides. Preferably, the second leach is continued for a
period of from about 1 to
8 hours, most preferably about 3 to 5 hours.
In addition to extraction of copper from the solid phase, the second leach
also is usefi~l for
removing any residual value metals remaining in the solid residue after the
first leach. These value
CA 02273067 1999-OS-27
-15-
metals may be entrained in the solid residue in the form of soluble sulfates,
or in the form of
insoluble compounds such as hydroxysulfates or metallics. These compounds are
converted in the
second leach to soluble sulfates, such as cobalt sulfate,. and are extracted
into the liquid phase
during the second leach. The amounts of value metals extracted during the
second leach may be
significant, depending on the amounts extracted during the first leach. For
example, the second
leach may extract up to about 30 percent of the cobalt originally contained in
the matte or alloy.
A fizrther variation of the preferred process of the present invention
involves the
exothermic nature of the second leach. In many cases, the unleached sulfur in
the form of sulfides
present in the solid residue from the first leach is sufficient to provide the
exothermic heat
required to sustain the leach temperature of the slurry during the second
leach. Where an excess
of heat is generated, this excess may be recovered as steam in a flash-down
process or other
cooling method. Where the sulfide content is insufficient to support an
autogenous second leach,
then a fizrther variation of the preferred process comprises diversion of some
of the matte/alloy
from the first leach directly to the second leach, thereby producing an energy
source for the
second leach step.
After the completion of the second leach, a solid/liquid separation is
conducted in order to
separate the copper containing liquid stream, which may also contain some
other value metals,
from the iron-containing solid residue. As in the first leach, the separation
is preferably
accomplished by filtration or counter current decantation (CCD) methods. In
order to remove
soluble copper and cobalt compounds from the solid residue as completely as
possible, the solid
CA 02273067 1999-OS-27
- I6-
residue is preferably washed at this point by addition of fresh water, and the
wash liquors are
added to the copper containing liquid stream. In preferred embodiments of the
invention in which
spent electrolytes from cobalt and/or copper electrowinning are used as the
leaching liquids, this
stage is a convenient location for intake of fresh water into the process.
After washing the solid
residue, the combined liquid phase is subjected to further purification in
order to recover copper
therefrom.
As a first purification step, shown in Figure 1, the copper containing liquid
phase may be
subjected to a conventional process in which selenium and/or tellurium are
precipitated from the
liquid phase. These metals are typically present in the :matte or alloy in
small amounts. After
recovery of selenium and/or tellurium, the purified liquid stream is then
preferably subjected to
copper electrowinning, during which copper ions are reduced to provide copper
metal in its zero
oxidation state.
The spent electrolyte from the copper electrowinning step primarily contains
sulfizric acid,
but typically also contains some amounts of soluble metal sulfates, such as
copper sulfate, iron
sulfates and cobalt sulfate. The amount of copper in the spent electrolyte can
be significant, on
the order of about 30 g/L. Similarly, as discussed above the spent electrolyte
may contain up to
about 30 percent of the cobalt originally present in the matte or alloy. In
order to recover these
residual metals, the spent electrolyte is preferably recycled for reuse as the
leaching liquid in the
first and/or second leach, and may optionally be combined with spent
electrolyte from the cobalt
electrowinning step discussed above. The recycling of spent electrolyte is
also preferred because
CA 02273067 1999-OS-27
-17-
sulfuric acid generated during electrowinning reduces the need to input fresh
sulfuric acid into the
process.
In the preferred process of the invention in which spent electrolyte is
recycled, the sulfur
contained in the matte or alloy forms the primary source of sulfate ion in the
leaching liquids for
the first and second leaches. As discussed above, mattes and alloys contain
varying amounts of
sulfur. Where the sulfi~r content is relatively low, the amount of sulfate
generated may be
insufficient to completely leach value metals from the matte or alloy. Under
these circumstances,
the sulfate content of the liquid phase is preferably supplemented by addition
of sulfizric acid or by
recycling cobalt and copper spent electrolytes.
Conversely, in mattes and alloys having a relatively high sulfur content, it
may be preferred
to reduce the sulfate content of the spent electrolyte in order to balance the
exchangeable sulfate
demand over the first and second leaches. Sulfate content may preferably be
reduced by bleeding
some of the spent electrolyte and replacing it with fresh water (preferably
during the washing step
discussed above) or by neutralizing some of the acid in the spent electrolyte
by addition of a base.
An alternate preferred method for reducing sulfate content in the liquid phase
is to add a
jarosite-forming salt to the second leach in order to form jarosite, a sulfate-
containing iron
mineral, having a formula such as NaFe3(S04)Z(OH)6, which is insoluble in the
leaching liquid,
thereby efi~ectively removing some of the sulfate from the liquid phase.
Preferred jarosite-forming
salts include alkaline metal salts such as sodium or potassium sulfate.
CA 02273067 1999-OS-27
-18-
The present invention is further illustrated by the following examples.
EXAMPLE 1
This Example comprised a two-step process according to the preferred
embodiment of the
present invention. The conditions for the first leach are shown in Table 1,
comprising a batch
leach log sheet. In this Example, 1,700 g of an alloy containing 65 percent
Fe, 6 to 7 percent Co,
19 percent Cu and 5 percent S was added step-wise in portions to 3,000 ml of a
leaching liquid
comprising the spent electrolyte from a copper electrowinning process. As
shown in Table 1,
most of the alloy was added to the leaching liquid during the first hour of
the leach, during which
time the pH was raised to 4.70. The leach was continued for over five hours,
at the end of which
time the pH had increased to 5.25. The leach was run at a canstant pressure of
500 lcPa and at a
temperature of approximately 80°C.
Table 2 shows the extraction data for the first leach of Example 1. As shown
in Table 2,
the copper content in the liquid phase, initially at 40.7 g/1, was reduced to
0.078 g/1 after one
hour. This reflects the conversion of soluble copper sulfate in the spent
electrolyte to insoluble
copper hydroxysulfate , 2 Cu(OH)Z.Cu 504. Also after one hour of leach time,
the amount of
cobalt in the liquid phase increased from 4.53 g/1 to 21.2 g/1, translating to
an extraction of 74.4
percent of the cobalt in the solid alloy. Table 2 also shows the content in
the liquid phase of a
number of other metals. Most notably, iron is present in the liquid phase in
an amount of less than
0.45 ppm.
CA 02273067 1999-OS-27
-19-
Following filtration of the leach mixture, the solid residue is treated by a
second leach
under the conditions shown in Table 3. In this leach, the liquid phase
comprised spent electrolyte
from a copper electrowinning process (Reagent 1 ), supplemented with
additional sulfuric acid
(Reagent 2). The composition of the copper spent electrolyte was Cu = 19.7
g/1, Co = 3.31 g/1,
HzS04 = 28.4 g/1. To this liquid phase was added 2259.8 g of the solid residue
obtained from the
first leach of Example 1, the residue being added in four portions. This leach
was conducted at a
pressure of 680 kPa and at a temperature of about 160°C. The pH of the
leach liquid decreased
from an initial pH of 4.69 to a final pH of 1.56 after a leach time of eight
hours.
As shown in Table 4, most of the copper was removed from the solid residue and
into the
liquid phase, with the extraction of copper from the solid residue after a
leach time of five hours
being 92.0 percent. Furthermore, after a leach time of five hours, 97.1
percent of residual cobalt
contained in the solid residue was extracted into the liquid phase. In
contrast, as shown in Table
4, the content of iron in the liquid phase remains fairly low, being 996 ppm
after a leach time of
nine hours.
EXAMPLE 4
As shown in Table 5, 850 g of the alloy of Example 1 was added into a solution
of spent
electrolyte from a copper electrowinning process, the alloy being added over a
period of one hour
at a temperature of about 80°C and a pressure of SOO 1cI'a. The pH of
the liquid phase increased
gradually from 2.45 to 5.37 over a period of four hours. The extraction data
for the first leach of
CA 02273067 1999-OS-27
-20-
Example 4 is shown in Table 6, and is generally consistent with that discussed
above in Example
The conditions for the second leach of Example 4 are shown in Table 7. In the
second
leach, 981.76 g of residue was added to a solution containing spent
electrolyte and sulfuric acid at
a pressure of 1,100 to 1,200 kPa and a temperature of 150°C, causing
the pH to be reduced to
1.36 after a leach time of eight hours. No fresh sulfuric acid was added, only
that recycled in the
spent electrolyte (Reagent 1) having the following composition: Cu = 0.55 g/l,
Co - 20.3 g/l,
HzS04 = 69.6 g/1. The extraction data for the second leach is shown in Table
8, showing excellent
extraction of cobalt and copper during the second leach.
EXAMPLE 5
This Example shows only conditions and extraction data for the first leach in
the process.
As shown in Table 9, 1,400 g of the alloy of Example 1 was added in small
portions to a spent
electrolyte solution at a pressure of SO kPa and a temperature of 85 to
90°C, causing the pH to
rise to 5.11 after about ten hours.
As shown in Table 10, the conditions used in the leach of Example 5 resulted
in poor
extraction of cobalt from the solid phase, whereas most of the copper in the
liquid phase was
transferred to the solid residue. Significant soluble iron remained in the
leach liquor (8.2 g/1).
CA 02273067 1999-OS-27
-21-
EXAMPLE 6
As shown in Table 11, 1,800 g of the alloy of Example 1 was added in small
portions over
a period of five to six hours to a solution containing spent electrolyte at a
pressure of 700 kPa and
a temperature of about 70 to 85 ° C, raising the pH to 5.23 after a
leach time of seven hours.
The results of the extraction in the first leach are shown in Table 12, with
the cobalt
extraction reaching a maximum of 84.7 percent after five hours, and the copper
content of the
liquid phase being reduced to 0.238 g/1 at the same time.
The conditions for the second leach of Example 6 are shown in Table 13, in
which the
solid residue from the first leach was added to a leach liquid at a pressure
of 1,200 kPa and a
temperature of about 160°C, decreasing the pH to 1.66 after eight
hours. These conditions
resulted in high extraction of both cobalt and copper fi~om the residue, with
cobalt extraction
reaching a maximum of 97.7 after five hours, and copper extraction reaching a
maximum of 95.4
after nine hours.
EXAMPLE 7
As shown in Table 15, 1,450 g of the alloy of 1?xample 1 was added in small
portions over
a period of six hours to a solution of spent electrolyte at a pressure of 300
kPa and a temperature
of about 80 ° C, increasing the pH from 1.22 to 5.08 after five hours.
As shown in Table 16, the
CA 02273067 1999-OS-27
-22-
copper content of the liquid phase was reduced to 0.07 g/1 after five hours,
whereas cobalt was
extracted to 74 percent after the same time.
In the second leach of Example 7, the conditions of which are shown in Table
17, 2173.33
g of the solid residue from the first leach was added to a solution of spent
electrolyte and sulfuric
acid at a pressure of 700 kPa and a temperature of about 130°C,
decreasing the pH to 1.40. As
shown in Table 18, copper extraction reached a maximum of 91.2 percent after
seven hours and
cobalt extraction reached a maximum of 94.8 percent after seven hours.
EXAMPLE 8
In the first leach of Example 8, the conditions of which are shown in Table
19, 1,250 g of
the alloy of Example 1 was added in small portions over a period of six to
seven hours at
atmospheric pressure and a temperature of about 85°C, increasing the pH
to 4.46 after six and
one half hours. As shown in Table 20, cobalt extraction was 92.9 percent after
three hours, and
the copper content of the liquid phase was reduced to a minimum of 1.2 ppm
after five hours.
In the second leach, as shown in Table 21, 2,618.4 g of the solid residue from
the first
leach was added to a solution of sulfizric acid at a pressure of 1,000 kPa and
a temperature of
about 170°C, lowering the pH of the liquid phase to 1.40 after seven
hours.
As shown in Table 22, copper extraction from the solid residue reached a
maximum of
CA 02273067 1999-OS-27
-23-
95.5 percent after eight hours, and cobalt extraction reached a maximum of
95.2 percent, also
after eight hours. It is to be noted that since the leach liquid in the second
leach comprised a
solution of sulfuric acid, and not a spent electrolyte solution, the initial
concentration of copper
and cobalt in the liquid phase was zero.
EXAMPLE 9
In the first leach of Example 9, shown in Table 23, 1,800 g of the alloy of
Example 1 was
added in small portions over a period of about six hour's to a spent
electrolyte solution at a
pressure of 500 kPa, bringing the pH from 1.42 to 5.06 after six hours. It is
to be noted that the
temperature of the leach was allowed to rise to a high of 150°C over
the first hour of the leach,
and was subsequently reduced to about 80 ° C for the remainder of the
leach time. About 28
percent (500 g) of the total mass of the alloy was added during the first hour
of the leach.
As shown in Table 24, the copper content of th.e liquid phase was not reduced
during the
first hour of the leach, but was subsequently decreased to a minimum of 53 ppm
after a leach time
of seven hours. The cobalt extraction on the other hand reached a maximum of
94.1 percent after
one hour. The leach obtained after five hours, at which time copper was
reduced to 0.57 g/1 and
zinc was reduced to 0.29 ppm, while about 75 percent of cobalt was extracted,
represents highly
preferred reaction conditions to obtain a pure cobalt-containing liquid
stream. The lixiviant in the
leach had a composition of Cu = 25.3 g/1, Co = 9.73 gfl, HZS04 = 17.6 g/1.
CA 02273067 1999-OS-27
-24-
In the second leach of Example 9, shown in Table 25, a total of 2471.93 g of
the residue
from the first leach was added to a leaching liquid containing spent
electrolyte and sulfuric acid at
a pressure of 1,000 kPa and a temperature of 160°C, the pH being
reduced from 2.50 to 1.40
after a leach time of eight hours. It can be seen from Table 26 that copper
extraction in the
second leach reached a maximum of 92 percent after a leach time of nine hours,
and cobalt
extraction reached a maximum of 96.8 percent after nine hours. However, it
will also be seen that
the copper and cobalt extractions were also high after a leach time of only
three hours.
EXAMPLE 10
In the first leach of this Example, the conditions of which are shown in Table
27, 2,800 g
of the alloy of Example 1 was added in small portions over a period of about
three hours to a
solution of spent electrolyte at a temperature of about 140 ° C and a
pressure of 600 lcPa. In this
Example, the pH rose from 1.03 to 3.84 after 195 minutes, reaching a maximum
of 4.45 after 150
minutes.
In this Example, the temperature in the first leach is substantially higher
than that in any of
the other Examples and, as discussed above, oxidation of copper sulfides
present in the alloy
occurs, resulting in production of sulfuric acid and therefore the increase in
the pH is less than
that in the other Examples. The lixiviant in this leach had a composition of
Cu = 24.5 g/1, Co =
8.69 g/1, HZS04 = 16.7 g/1.
CA 02273067 1999-OS-27
-25-
The erect on the increased temperature on the copper and cobalt content of the
liquid
phase is significant. As shown in Table 28, the copper content of the liquid
phase could not be
reduced below 0.6 g/1, iron was not completely reduced and cobalt extractions
were lower
considering the feed alloy to lixiviant ratio. The lixiviant composition was
Cu = 24.5 g/1, Co =
8.69 g/1, HZS04 = 16.7 g/1.
In the second leach, shown in Table 29, a total of about 120 g of the solid
residue from the
first leach was extracted with a mixture of spent electrolyte and sulfuric
acid at a pressure of
1,000 kPa and a temperature of about 160°C, decreasing the pH to 1.41
after a leach time of five
hours. However, Table 30 shows that the copper extraction reached a maximum of
94.7 after six
hours and cobalt extraction reached a maximum of 88.3 percent after six hours.
EXAMPLE 16
As shown in Table 31, 3986 g of alloy was added in portions over a period of
135 minutes
to a synthetic solution containing 16.9 g/1 copper, 10 g/1 cobalt and 40 g/1
free acid. The leach
was conducted over a period of five hours at a temperature of about
85°C and a pressure of 500
kPa. The test extraction data for the first leach is shown in Table 32, with
the copper being
reduced from 16.9 g/1 to 0.35 g/1 after five hours, and cobalt extraction
reaching 68.97 percent
after the same time frame.
In the second leach, shown in Table 33,1708 g of the solid residue obtained
from the first
CA 02273067 1999-OS-27
-26-
leach was added to a solution of sulfuric acid in portions over a period of
ten hours, and a
pressure of 1,000 to 1,200 kPa and a temperature of about 170°C. As
shown in Table 34, copper
extraction reached a maximum of 93.5 percent after five hours and cobalt
extraction reached a
maximum of 96.5 percent (also after five hours) of the unleached cobalt
fraction.
Although the invention has been described in connection with certain preferred
embodiments, it is to be understood that it is not limited thereto. Rather,
the invention includes
within its scope all embodiments which may fall within the scope of the
following claims.
CA 02273067 1999-OS-27
s
y X
10 N
O
O Q
CI
Q _O
E-
vL' ~ a
a ~
= '~
c
~
y ~ , '
~ 2
~ ~
' '
O p fL a n
N
' 7 ~o~ h
O Q
~ Q
O
Q
o a o _
~
o
_ J J 2'
~ J
Q"
J
d'
H
h
M
_ r ~
O
_
~ O ~ ~ n
A O O r
O 1~~ 0i M M
N ~t'
'J ~
C
J
..
a
r l3 cc E " E mE
"
' E E ~ ! o
E ~.
~
m
~ ~ O O
~ O O ~o m
O O O
O w mn
O
O
~
O
o ~ ~u, m
E
wo
.n
ua
u
3 d
.-
V
a E=
w ,. o ~
N M ~ ~.
M
O O ~ A ~
m ~
~
O
~
d ~ ~ ~
S II
N
~,
"
d d A 7
K a
U
c d d d
m
> =
~
o0
_ r r
r r r
r r r
r
>-
r
~
r ~
r II
t
LL
c
~ a
LL1 ~ o ca 0 a m
a $ o ~
a 0 $
a h
$ $ ~n
$
o
~
n d
n
~
~
qi
Y
~n
~m
C ~
~
(/f n. U u
..
o
x
m o,m
Z ~ ~ ~ s A~ ~ ~ o
~ N ~ I~
Till N N N N~ (~ N
N '-
O
J
0
' o $~
' Io N 4
- o N N ui E ~
~ N
. i
~i
ri InIriIti u
~i Iti i
~ w Ic
v
0 0
Q Q
i
'c"' ~ a~o~o~om
9N maN
E
~
~ o O
~ a
a
o
d
~
~N
~ a0o
Q y O N
O
M
("' O
C
U
O~~N 100aOD
~N'
NN~
O N t
7
m
H
E
~
m o f9 E~ E
c cn E
~ o
cw
,~o h
o ~n
~ it
o ~
In
o
_ J ~ ~f i~ I O y n
O Cf N
~ OY
C7
O
~
O ~ ( a
0
3
0
d
~ E
v o~
> >> m ~.,
a~
H-~. y7 ~ '~ t~
H
E ~ H O
i ~ ~ ~ a ~ ~ x
~
i Z ~ Q ~ ~ ~
i E
d
d m (0 A '
10
~ a ' L
vl ~ G
'E
M N ~ a c ii i
41 a i~ l.
u-
V o ~ ~ tna o
J ~
----z ..~,.r.....-VnT\T.TV PT 7 TTn
lln:Qn ur as n
TTnTVn nO77TTO VN.1 n cnio
CA 02273067 1999-OS- 27
a
0
X
0
W ~u
w
Q o~
.J
n ~ N
d'
C
r ~ ~?~O tD
,
E ~ O N p
W
N iri ci
N r
0
7 _ .-
.
flx 1'O
tGcVr M
V
m
C
O M
I ~ t%x
L O ,~j~ 7 ai
M
~ a a v
d ~ ' ~ 0 0 0 '
ro
L ~ _ u~~c~n o~ E cNn
.
o IAIf7 In 9 O
(,~
~ r ~ ~ N ~
1 = O ID E
E ~ f N N D h M
~ ~
C ~ O s
C
_C
m
M ~ ~ a'~ E
A
a0 N a O
N ~" o o ~ = a
~ y n o o v vn~ G,
u~u w
"
a tn
ab
0
E a w
o o ,-c~v u~~n N "-0 0
L G V .
w
C
QI N
C a
E V OfN N 1~M d ~ p
w ~ aoaoaouo 'Z
0 1m- .
y
0
J fn
v
N v1
_
C
E d .. J ~ O
(~O
E
,~_,
N Z J J J J ~ ~ ~ J v N
m Q J p .- U
a a a ~aa ;~m a Q Q a
'-' t ~ a ~
n Q o
mWam. o g o o o o W e E g ~ ~ _
n' r~~n
a 4 'aa a a d n a < a m
...... /~ t~c~~1T\T TO_v~rvac~ rr~TAA oT7 n077TTQ TTf1 QV.1 TA : an nTI.T. RF
C(1/4(1
CA 02273067 1999-OS-27
L
V
J
X
O ~ _u
d ~ e~
E
z c m
1
4 3
~ t1 N ~
v ~ ~ H
N E
E
m 5 $ ~ $ $ $
a m o m ~ m
m
.IC' J 2'' J 2' J
w
~
A~ ~g H ~ ~ ~ P~f r0
0 r ~
mN ~ ~ 1
1
J
.e E
C o
E E E EE N
w g e
E i
C
d' U
'o
o ~ m
m N
m $ ,f7
~ t9 ,'
m ~ Li O h 7
~ E O yn m ,r~
m
H H m
it c o
_ o
,
aim
N,oc "
o c
d m_ s
~ E ~
"
o,o,
~
~ a ~ m '
C
C N
~ r r r rr r r rr r r rr r r rr
~ N
Q
,~e O O OO O O O OO O O
~ ~ ~ ,~mm m m n~ ~ ~ mm $ ~u ~ ~ E
o
7 o ~o , ~
t' n
a ~ ~ a
a
r Nm ~ ~pO N N ~p~7aN0 r~ d d
~ ~
N N (~f f If1 ,AunfN of~ ff
z
O m N
ZJ J O:
p~ ~ 41NON 1~0,0h ~ 1~ffN (pn 'm m m m
~ ~ ~ ~
f p,1P!1J r " " ' n ~ A
m
Q N
Ev ,$$ m m~i,or'~,~'i0,nm .u
m m ,om m
m '
~ a ~
aiZ.~ ~ o o N r
n ~ S,~a c N o ae,d
~ N m a
~ a ~~
~ ~ ~N ~ m _f 1~,~ $ ~ ~ ~
e7 m
NN N M v7PJ1~f f'
?
m a' O C a
aaC~ , h A~ ~f,~ M~ ~ O e~O O n o0 0 0 m$ C C N
,e o ,n,~ano ,no
~
0 0 ~ ~ :r~ "iisioi~i~o o iriofo o " :.i ~E E E
-
Q' N N N NN
o
a ~
~ ~3
z m
a .~ '"~ ~ ~ ~ _
Fa m m m m ~'~ ~'C~
C
~ 9 ~ Q w ,~
.n
~ ~ ~~ ~ ~ ~
o ~
~ a s ~ t ~L
CA 02273067 1999-OS-27
06/05 ' 99 THU OS : O1 FAX 011 8112280 213 HA'1'l;H A1~1(ll:A-~YltlN(~5 yJ uue
_ c
a m
~ r.
~
s
N
X
N
C
O
O V
X
a
CA~ n
_
0
e
; o
W
0 IffN (L1
7 ~
C? a) a0t0N
O J
_
m
N r
N
O ~ ~ P7~ ~ N
O D O
O
O p
a O M O
V
W
d
J v
7 ~ v7f
C
V a1Z ( lC
Ep0.~
O J
V
d a
O p~ ~ N
2~~ ~ ~.-lV
O
O
g
O ~ E '~~~ ~~!'
> ".l
7
O
C
o
r
c b o ~ r~~- E
a
v ~ ~ N o~~ a~ u
.
T 'f~
d, ~ ~ ~ ~ d ~ ~ ~ c
c
d N
u7
O ea
~
I_ ~ o ~ a r ~ E U
E ~ o r n rn ,Vn
N H ~ , a U
c
..,v i ~ s o c a ~
~ ~
~ ~ n n 0
E n
o L"
a
t
Q
Z E E '
o
m '~ H Z ~ ~ ~ J Z J V O
h o
r aia, m m m m ~ m
~ 0 0 o w E o
m W ~ 3 a a a a'a a m
v ; ~ ,~~ a ~
CA 02273067 1999-OS-27
06/05 ' 99 THU 08: 02 FAX 011 8112280 21's nnruH nl~ltic:n-arxlru'
a
a
~ h
w F
X
v
Z O
'c~
2 ~
" ~,c
Ie c '~
T~' c
~
.
E 2 m D ~ O O
o
D p
U
J ~ Q'
~ J
N N
V
01 ~ Ip ~~I7
~
O
p cf Or II
0f
N ~ ... D
O
N
~ ~
ce
J n
a
M V
S' c E E EE ' E
E
a
m
u~
p p N
g o g a''a' v $
'
a - "
o o
~ s o ~ ~
a a N
a
0
a
NP1m R O O m v
N N ~
~ d Nd
C
.J
_1
D ~ ~ r Y Y T~ )-Y
C
o~
H j
d
_
d o 0 o p p ~ o
(/~ N A ~ u~u~~a~ u~uwn
m d To
_ 7
a
0
= A ~ ~ a~P N ~ n V A
Ij/ ~
J
z
,n ~c,
V
i '~' - ~ ~ ~ ~i~ a a a
-
a v ~n~n
N Y V'~cJ1n Il7N lf7
t/7_O O
Q Q
1
p
_
p CDN
J E ~ n 0 0 o r- 1-~co
~ , '
II
. .. ~g
a;v
cV'~ N a
I_' c
U
p p ~ N p r
p ~ t N m
0
01
E
m ~ ~ '~' ~ ' ~ E6 E E
~ ~ '
0 3 ~ e,D 0 f p ~ -
n 4 I1O) -
a '
N
a
m
O _
a O
N
d ~E m m
y E oo ~ ~ a
o
~ ~ v
Z a c a Z _mt v o
~
H d ~ ~ ~~ J J W vi W
J Ov
x
o
zL a a a ~ ~
~
~ ~ E ~ ~ _
~
r ~ 7 dH Lp N C G
L N N
C
d N a' S Ii ii
A i~ ii
at ~ a v a W~ _ cd 1-
r ~ Q
V O OC2 ~ ~ O
J
CA 02273067 1999-OS-27
06/05 '99 THU 08:02 FAX 011 8112280 213 HATCH AHltlc;A-SYK1NVS ~ uuo
Q
a
p° 'n o °°
a~'~ m °l
ui o
~a
~n ~ ~~
c$ v
~ M ~ a0
~ Leiv
o:
~ ~ ~
' ~
~ ~c
w
CO
tl7
M
d t~~
a,
m
_
fl.
m
c~
tD
N
et
N
N
j
~-
(V,
N
C
S ~
~
a
'~
as
''
T
d1
J
r
7 ~ ~E
~ N
~
~
N
N
J D' " N
V a
~
J
O
O
4
IL7 ,p
N
(D
~
O IV C.
~C'
1V
N r-
~
V pN GMDf~O~ V da
7 V
0: E ~ d'
N V
N
M
y C
7
G M m
u
'
C1 V ~ a. . o
~ rn
H
'~ ~
~
o 0 0
0 z '
g
d ~ ' g ~ y u~ a
~
a .
"
. a ,a
0
U U
c ~ E o
j
o o u~ o .- ~ ~ o
M
m
~ s a
tn M ~ v
~o
0
m N ' ~ ~ v
~ o '~'
' m a
c, E ; a .
. O e
.
. o
~ r o-
_O fn J
Q 07
L
a. .
. O~
O J
Ii
r m ~ m
$
o
.
A ~ J Z J W
7
J
H J
Z
~ ~
~v
v' E ~
o T 7v ?vW E O tn Z a1
T
d A w IA ~ O O ~ C l1
O
o ~ , a a Q
a
CA 02273067 1999-OS-27
06/05 ' 99 THU 08: 03 FAX 011 8112280 213 HA'll:ri Hl~K1VA-~Yltlrih~
U
_o m
J
m ~ =
~
a o m x
~
m
Z ~ ~ .g ~ ~ y
~ ~ ~ ~ c
a
o a E
a
~
v '
' E
~ ~ -' - EL . A
~
~ 2 a =
y a .
J -J
J J
0 t~0 ~ O M W
f '7 N N
N ~ ~ Q
~ p o n _
L
0 0 ~ o Z'
o '~ ~ E
yo $ N m ~ m
J m N ~ O .O t0 Lei
0t te
-
a o -H ~ ~ r ~ ~ y
,
m
n
" g ~ o o ~a
~
' E N E
c E E E
E
~
E
_
V ~ m ~e
m
N O
N Q
y
!- c o rnL
m
U
V1
tn
~ Nf'7 ~ ~ O ~ H O
'~,
c cc ~ rn ~' w
E N
m m a c~
~ ' c m mm
J a' d'fY
G
o r r r r r r rr r r r
~ r r r r r r
~ O
U' ~ ~
n o ~ ~ ~ ~ o~ o O
a ~ ~ ~ ~ ~ N ~ N N
N ~
A ~ N N N N N ~
~ N N N
v
J m
' -'
U ao n asv u~N sc~ r.o ao - ~'am
u mn v Wnm ~ ~ ~n r
~o ~o ~ '~
u ~ ~
W a v ~ ~ ~ .r ~ a r o a ~a~
N ~ r .,r . V
v
J2'
1
N O
J '
na o u'~K~f ~ ~~ ~O 0i~ ~ m Jd
~ ~ ~ v,q ~ ~
eo
a N , , ,
d J O
~
Q _Q
Q
00 0 0 0 0 0
u~ v W u~ nn y 0 n ~ o
~n n ~ ~ nn
~
a o m ~
~ r
O ON
O O, ~ +
~ OYN~
aOO ~ T G
O Q v ~e.~~pam noo
V f0 07N O
r.NN NCJC~~~'7~Y d'
E
f= g _
O) H 7 ~ Y ~ O O ~O O at !?~ E N H
m V .r. < O : rnE
v' (~' a ~ , ~ iV!'~P7V' ~ ui ~on ~ _
~ 6i b~ o :- (V V' is v
~
_o Q
Q O y
~ D
w m
d~ H H .U
~
H ~ E -3 ~ ~ Q
'0
z ~ ~' H ' ~> > >
z _
~ ~ m
O J J J ~H0~
I
m fly ~ '~ . ~ m r my l i
~
E
c a m .'Q'g ~ ! o ~ a c' c c
~ H
! ~~ z ~ a $ a a ~ a
o
v
CA 02273067 1999-OS-27
06/05 ' 99 '1'HU 08: 04 1~AX U11 811LLifU L1:S HA'1'l:H Al!1(ll:A-JY1t11v1W ~
uvo
Q
R
G vi
N ~ O
~
1 r
W X
W
fA ~ v
Q OI
J
m
cO
.
~ ivi
L c
u
a
W o~~
a
rn~'~
O.
td
V Q~IO~ c~~7N
J
m
7 OO m Of
_
~p
O O O
J
~
C 0 0
a
V
d
O O Of m~ NN
~
V O
N~ ~ ~N ~Q
~
_
m
t~OW N ~ ~n NN
E CC
T
~ i0C C o . vv
li
L ' ' o ao
~ g ~ ~
! p ' ~ ~ v~ o
i E
uJ o
+., a
fp a
0
v c n ~r m ~ a~ yie~
~ ...
~ ~ ~ c0m ~ ~ IL~ pN
'~
( V 07dD t0
d
~
N N N
H M ~ Y
~ a
U
0
U
'
00 CI V=.,Z m Of ~ '7 ~~ N~ .~
~ L U m O
N ~
~
'
N
a
,. ~ ~a ~ ~
~
n w ~
I O
o~
N J
d a.
V
r y
Z C~ ~ ~ Q O
O tW
E J J ~J J Z JJ UJ
E
~ dr N ~ ~ ~~ m ha ~O N S m
Z l
r t ~ ~
m !~6 ~ M ~ O O O ~.A O ,
~ O a~ a' v~
m
a a <a a a
CA 02273067 1999-OS-27
06/05 ' 99 THU 08: 04 FAX 011 8112280 213 HA'1'LH Ak'ltl(:A-SY1Z1NGS I~r. uua
a
a
~
H N ~ t
o E ~ ~
N
~f7 n
o m R ~ " II a
.
x
Z C m ~ ~ ~
s ~ ~ ~ ii
' ~ ~ ~ ~
ol ~ ~ x -'
ai p ~ X
R ~ ~ tii R .GII
d CI H ~.i 4!O .Vt = C Cc7
a ~ l O
C N
~iI!N 7 E
y a
i ~ > v cv o ~ ~ z ~ cv Q
N
R N 01N O1 cp N Q
!~ W N N O
O O ~ ~ N II c
R O O ~ ~ N S O
O O V ~ ~ _ _
~
N ~ ~ L1 D O ~ ~ a~
~
V~ u.
II r
0
g
m G'mm ~r
v
E E E E EE o
B
a
w
a
O N
O p p p p p p
S O
m fa ~ O O O O O O O O ~ O
~i ~ ~
T T d
Q ~ a a
~
..
111e-N f~m g S S S
~
C C ~O1 ~ S ~
7
~ R A R
LL ~ ~ ~ z ~ '_'
O o ~-r r r r r r r r r r r
Z
H-
a
'o '
N ~ S N 0 0 ~ ~ O S ~ ~ N
A 4 1 1 1
~ 7 f1
d
J
Z
5
W
J
Z
air .t.'Viaiof W ~ciui E
ri
m
H ~' o
a
Qs
ae
a _
J E m eoaoo ~o~ ~oIreIn~
~j
m a~m m Q7CDCG0fm m aD (O
o .
.
C ~ O rl
G~ O O C')
-
N S e N
O C~ C
O W O O O N m QN7N fODN S
eht0Of
~ N e9ehN N m
O
E ~
a ' mo ano o c c c ~ ano 0 0 = _
U7 7N L h N 1(7N N P7 O O ~ YlN
~ ~ ~ _ ~ ~ O E E
(' ~ N N l'~!'7Q tfiI~CoN ~ E E O V
O a N N
Q d
~ 0
'
d ~ 3 a .~
E I H rna
''~' y ~ ~ c o ~ ~ Q
o
Z ~ m N .c1- Z o 7 7 ~Id
U
d ~ O ~ ~ ~ a ~ ~ ~ J J ~ A '~yi~
'~
a
a a x'
Ov
~ ~ ~ If! l l ~
ilf)
C V 3? O ~N ~ !~1~1~~
d R ~ d ~ ~ C C
o a a a a ~ ~ ' iEi '
' ~
c ~ ~ rr~ a u ~ u
. .
CA 02273067 1999-OS-27
06/05 '99 THU 08:05 FAX 011 8112280 213 HATCH AFRICA-SPRINGS tp~utu
Q
c
Q
N
N ~ X
G
O
U
'
v
Q ~ W
J
m
7 x ~ N
-~tn~ r5
C
O
e~ao
~
niN
X
W
~ N N
O
_ V G ~1~ ~
Q J
E
N N
'~'(aV d'
V
Z N 07C~'1
,C ~rj
r
W N N m
LL V ~ ~ 0 ~ ~ x cVcVWit'
V J O
li: y n e~ra.- ~ E o
x N o o cc d a
p
O '
8
V m ~ ~ g c~g 'oa c~
N c~u~ V n o
.o
~
u ~ m
.
~ .
O E
W z
~ c~ ,
G1 ~c
0 r a
13
J ~ L
'Q O
r.
a
g ~ '~''> o
~ Z - a a a ~ v
o o o ~
o Q
a o
a
c
E m ' ~ o o~
a a
0 0
d V Z J J ~ J ~ J ~ J
1 ~ a ~ N a ~
1 4 7 h d l O
n Cf N ,n
~
m rt := o ' o R E o 0 0 o
o' 'm
v ~ o a a ~ a < v~a a a m
CA 02273067 1999-OS-27
osi05 ' 99 TIIU o8: os FAX oii 8112280 213 Hnn~H nrxic;n-5rmrv~~
m ~ e~
m N O
m N
O
~D
'
~
n
y D _ o
of D e
.. i
O
D
D
.a
a a
J
a
E
c
_
N
w
~f
N
~~8888$ m
'
Z ~E u 1
n$ i
u
m
U
N
J ~ Wt
_ g$
S
$
S
O
O
S
N
~
N
N
N
d ~ . c
~
a
'-'
z
J IG
~ ~-
2'
~
C
II
~ r Y
> r a
r r v
>
r
>-
>
r
r
~-
T r
W $ ~
C $ $
~ $
$ o
o
~
8
~
O
o
n
n
~ n n
n n
n n
n n
h
n
n
n
z ~
~
a u
N
h Y
~
~
~
PI
M
~
N
1p
~
!f ~
N 0
Q
w
J
Z
0
z
s m
~n
m
o
r~
~
~
d ~ ~t ~ am
e- ~' ~
eV U1
!V 1(1
m
Cj
!i
~
1
r rn
r
m r
n r m
N m N
.= II
~
n
'
W
m
O
N ~ r
~
t
O
~
W
0
~
N
~
m
a d N
?v ~ H
O
a rn V
E_ w
' W ge~an$itg ~CC'
o . E
o E
o ~
_ , ,
~. ,
01 .r,
' .~
v
m
o
~
~
Y
Q
K1
IfJ
(O
m
IA
n
m
Cp
CI
0~
, a
a
a
H
a
> >
>
H Z'd E -' ~ ~ ~ m n
a ~ ~ ~ ~ u, tr
~
K
o o ' B c
~ c
'~
E ~x
t ~
t~
t~
~ ~~ a ~ ~
o ao
'
~
a a ~ ~ ~ m O ~ i
J
t m
G O fY J ~' p,'~ ~ O
11 V ~ Y G. is
w to
w d ~ .ZeO O c
a ' J
d t m - O Q
7 J Y J
~'o
Q
J W
m
a
CA 02273067 1999-OS-27
06/05 ' 99 THU U8: 11 NAX U11 tillLLtsU Lts HAtctt airrcme-~rxrm~~
~a o N ~ ~O
W
_ X
C
O
_
W
to
X
H ~ X1.1
v
O'
Ol
J
n c~ ~ ~ r-~ ~
~ c c
ofo m uiw ci
~ ~
0
o
r
r. N~ O fi~ K711
', X
L r-~ ~W- i
W
0 N W1
d V 7 O :p 0
~"
Q. g w dNia
~' .- c o 0 0
p NN ~?ehN
OC~i.r-
c
r-.-
w- ac
O
a
V ~ pm .-~ p e.~p n L
a u~~ ~ ~ ~ ~ ~
o c
o n
J w
.ir
LN~. O ~ N ~Om N N ~DI~
~ r N
~ N N N N' V
N f N d
~7
J
L
N
' ~ ~"rnin ~ ~ ~ E
~~ '
ea . .~ c V O
c
(G(VN r O N N N V
i ~ ~ ~ d'O O ~ of01N c'1 -
~ ~ ~ ~ ~ p
N ~~7
'
~ ~ N fVN V c
7
L
m
_C
aDN tG
I
O O~ ~oN d O
~ v
! ~ N ~ c0 O.
O N N
C
V
~ O ~~ ~ O ~ ~ Z ~
C N ~ a ~
t
L
I
N 0 .
'~
U
U
a ~ ~ o ~ ,Q
u ~ ~ c ~
v u i t _
n
v
0
0
~ o
a ~ a sr ~ m N ~ v c E
~
c o~ m o~ - - s o m ~ a N .
a :~ r r a a
0 r ~ c
o
a
_N
L O
d .. 4l
O N
m
w
a
a a m
' > rc
., y a O~ N tt47t0~ CD . - v N r' o 1lJV
> 1 J Z J ~ J cD o
J J
J J
3 JJ J J J J J
~ " aa a a ~ ~ ~ ~ n ' ~ ~ ~ ~ ~ ao
~ ~ ~
_ 3 ~o~ococo a
~ a T E ~ O O
C ~ T T . ~ a d O ~ O ~
~' =~ o s sz? ' a O
A
m v o , a r a a a a m i
a a -ass a a a ~
aa a
CA 02273067 1999-OS-27
Image
06/05 '99 1HU Ulf:lL I'~A~ U11 fillLLbU Ll:S HA1'l:H A~KIIJA-.~Yltllvla.~ ~uuo
m
Q
N
X
H
C
O
~U
l0
X
W
~ 0~7~ OfO7D
1 c f
Cn ~~ N~0~ _
1 ~
E
d
N r'rd'~
V Q.'
O
C
V ~ ~ ~ ~v o n E ~ '!
~ ~ ~~ m ~ as
d
J ~'
C ~ O~ ~O~stch1~c0h V N
O
<O'QNc~7~'~ ~ Q V
J
N1~N I~07
~ t0t~7N NO1N ~ ~E
(GG O OC G O
~ O If7O N ~ < ~N 0D
E t~tnN ~ a.,~ejaic~io0
v ~
E c~c~~ M ~ --m om n
O
y i
E
V .
n oo ~ ~c~~ ~n
1 ~ v
~ ~ O
. ~ N c t0 ~
O f
' D~ NN N N
O
it V
d
~ g ~ n 0
Y g N .N z
c
a"
r
~ ui$ E o~ c.~u~o0oeo ~ o, m W
~
a oa
N
O
U
... i ~ " ~~
n
E ~a o ~o ~
c
0
0
a
0
d
d
0
E N
J m
s E
Z o> ~~ o
J ~J
J J J JJ J Z J
61 m m m mm m N mC~mm In~ Q O
it m 0D 0O ~ T fD1Dl0(OCD
p tf ~l 0
f
as t ~E 0 d ~
d IC Y n~ O O OO O O ~ OO O O N N
C O '~
j o ~y a a aQ aa ~ n aa aa a a
_
CA 02273067 1999-OS-27
Image
Uti/US !iy '1'HU U0:lb rHA u11 ollGGOU cto nny.n t~stw.ta-Jrnll~VJ .t,",...
Q
O Q
~ ~ O N
... CD ( x
O,
N ~l1cV
W
U
O'of
J
m
7 ~ M
d v
47(OCGV'~ IN
d'
G
0
a 1~N I~~c7
A C M
W
U 7 v N c~7O 01N Of
~ OlOf~ r ~
Q. j
~ 1~N N f~~ m
V N
' tCC C e-f
V 0.
p c
V O r ~ ~ N p N7
O Q ~ N N
W
J
t~1! v ~ .~v ~o o o ~ a E o
a'N ~ ~ o o c a a v
p y ~n~ aocy~oao 9 E cp
n x c v .i~ <.ici V a a
~ .~N ~-~- e~~- d V
J p
.. o Ino Ino
C o ~ ~ O ~ ~ x ~ N C 7
,~u7~ " ~ v v 0
U c~ tn
~ ' ~ ~n~ c~c.~c~~
C
lad
.
N V'tA1~O
N
~
V N
d
d Z a N
6.
O O
U
o
F = o .-enIn<oi- ,~. Z o
a
-
~o
E C~ cn N r~ ~ E co> o
aom m co ~ a ca
o
Q rn
cn
..
c
d
al~ ~ ~? a
E E E ~ o
h m ~ O _ crYW fp1~ O _ t W tn1~x a
Cl iAZ J n J J 111 r1
~
~ ~ . . , .J. ~J . . . m
d A '" $ Q J -J~.JO ~ ~ O O O O . .
$ $ O O H t
i ~ v a a a a a m
o
~ ~ a a a a d a ~
=o
CA 02273067 1999-OS-27
Image
06/05 ~ 99 TIiU U8: 18 HAX U11 811YL8U L13 HA'1'(:H Al~'ltl(:A-'YKlIVh' ~ uu4
m
S
Q
N
X
C
O_
TJ
X
d7 0 ~ " o~m n .u~r
0 eO I ~'~l)
V P ) ~ N
J
m
3
~
LL~ ~ NC O CO O
4
C
A L ~ m al'D01 ~ V
J
'
O
C O ~ 1I7N _ Nefm V N
V ~ ~ O ~~ ~ ~ O
w
J tp
.mQ.~N N II~ N '
f/J
/.- ~ '~~i0N Nr-N ' O
o s
off~ ~~ .N t~~OmfD
~ ' ' ~f fW ri
C ~ E w~ cn I :o o ~
M gr~ -'m nu~u~
....MM
c
m
b
L
N ~ tp10
YC C A 0)I'CADO~fNN
"
N07r f0
it
go o oo o E
~ a a
N,~ Y t'~ ~ nr ~ a
.a i.
00 d "
r
~/j
~ M t ~ ea
o
,~,~$ A o O~ c mn~ ao ~ ~ z U
6
VJNM J t
~
U
w
'D
N~M~N N~ C n ~
...E m Cc fn
1 U . f1 ~ N
-
~
O O
O
O
O
N . C N
10
E ~ w
3
Z o~ a ' ~ ~ o
a
_ R O p 11~1D ~ Mvf'ff~a~rK
d 7 1n
JJ J JJ J E $J JJ JJ UJ
y mm m mm m IDm mm mm p O
" t r~r r~r~n r. ~~ n r~ ~~ Q
p
m ~E . Z m
O a
( 16~ OQ O O O C AO O
p r O
c~ ~ o ~a aa a aa a a ma aa aa zm
CA 02273067 1999-OS-27
Image
Ub/Ub ',1~ lllU UO:1'J t'HA V11 O11GGOV G1J 11t11v11 tlu~lW .n-J11\111VJ
.t,..v,.
Q
e p p h M
V'0 C~~~N 1
t
W a
U
V A
W
0
M ~
uiuiv v v '~v
c
0
N 0707 0147
N p O ~
O f
7 0
W
n ~ ~ ~ O N O
d V ~ ~1Of~ ~ 07O O~00
a
E
stN e-47
N N,~ N ~ ~ a0
tCO O N fVcVCV
G
s --
v
'.' CV~ i ;
J
_ _ E E ~ E o
r
y ~ Q 'em ~''.N N a n a v
b W
J r-t0~
V
N
. y n N m w u~~ 01 ~ E M
cvi~ std'' C7a O
C
O O O O of 4'!.-~O
~ ~ ~ ~ ~ v
~ o E 'r~~ ~ ~ m < v ~
c
. d
N
!C o
LLJ ~ ccN o~~ o ao
~ Qirnaom o1 ~ ~V
w r-c wn ~ ,
.. N
e~N N N N
1
v
N d ~
7
U U
r ~ o a ~ ~ o ~ r 1 cc~ ao ~ a z Q
~
o ~ = .
J
O
c E " '
p E m ' r ~ ' m a~'o ~ ai
:~ " n ~ -oo
a o o m a a
c a ,
~
a t - o Q_
Q ~ VJ J
_~
..
.CI c t
o .o ,n
E E ~ v
E
d W ~ p ~ ~ t D ~ 0 O r-t'! ~Ot~ao7C a V
~ N t ~ ( a J Z J J u7J J J UJ Ca
Z J J
Q1 J J J J ~ ~
E- ' ' Q ~ ~ ~ a ~ a a a a ~ a ~ a o 0
m ~ s ' . ~ T ~ . .h z t ~n
Q o o o o = c ~ = Q o , 0 0
0 ~
~ a a _ a a a v ~ a Q Q Q Q m
S a a x m
U "j ~ a a
CA 02273067 1999-OS-27
Image
06/05 ' 99 TI1U U1S: LU H'Ad U11 ll11LL1fU L13 riA-ll:ri A.bKlI;A-.~Yl<llVli.7
~uvu
m
7
a
O Q
N X
m ~ ~ ~i
C
O
_
W
N
v
X
111
Q Q1
J
m
~ ~~ ~ N
9 ~
C
O
N
m~
K
W
d '
~ Ofp C1QI0
0 a rc
~r
U 7
Q
J
cD~ ~ N
m ~ NG C~ O
C
~Of ~ ~' V
J u~
.a
C O ~ 1i7~(7~Oc0 C!N d
~ N
N N
<O~ t vt7O)
D
d
m
tND ~ ~ '
~C
y,.. ~ .r ~ ~. r
r-
E v mN ~''~n cnsornv
v
E $ ~ ~ ~ Wi ~~ ~n
~ ~c ~
~
t.. C
0
y ~ u~O~M~ ~u~a~ M
~ ~ ~ ~~ ~~ 3'
W o Nc Nr- ~
o
N
H aMOu> > 9 p p E m
c ~
g og Z a o0
o ~ a
N , '
~
vi
p ~ E a ~'
o
c~ p~ rmncor ao ~ U
(f1 M ~ ~
t
N U
r r~ ~O
EmV P-(O~P . ~ ~ U
F-
O O 7
a~
.
o
m
0
L d ~ ~ ~ V
Z Ol ~ J
N E
.~. d _
y ~ ~ Z J JJ JJ J ~~ ZJ JJ JLO U
~
F- CIm G1 Dm mm m m N NInmCDCO
oC op oo o ao ~~pW coaoeC
o aa aa o ~ Q o
a
.~ t ~E T TT .a
C
(~ ~ O T a T A EO OO OO - V
G1 QO O OO O
lE ~ 41 ~ ~C aa a Qa C lCa ao aA
O a a v~ Q am
v ~
o ~> a<
CA 02273067 1999-OS-27
Image
U(i/U5 ' 99 '1'HU ull: LL t'AA utt tfllCCtlu C1J nnW .m nn~t~m.e~-~racmvao
.r;,..~..
a
a
a
O ~ ~ ?f
X O O f C
I
r ~.~ O
W 10
X
O
~
J
m
~ M ~ D ~ 7
4
_
K ~
C
t~~ N C
n v
cneo~ .r~ r.
~ a ~
J ~
(~ m
~ M
~~~ 9 x C O C ~ fYN
1ia : t
C
O _ 07N X17C~ O
~ ~ ~ N N N f/1~ N
L: i
J
N r
~ " y . ' a Q ~ G
r'
ci, .
N a aDCi ~ V
N
lt~.. 9 tCJc~ N N 'r ~ a
O O
N7~ lp~ 'O V
N G. V
O m
~ E V $ f0N 07
Q ~ ~ ~ r O RJN !V
~ '
+r ~ E can~ e c r~cn ...st~ ~ ~ v
~ :i
c
a
c
-
m N ~o- E u~
a ' g ~ N
~
o a rn N U7 d
o
r-fVN N
d
C n a g ~ S Z a ch
N
W ~ = U7
W C o o L m ~L" E .-.-N O p o 0
<o~ ~
ao~ ~ E o - wn a ofaoo z Z
a v
~ ~ i = _
~
-a
o
d
a ~ > o
~ w d W N c E
~
C,E y a o a O ?ea N ~ S
;
'o o-
f!J J
Q
L
N
C y
w ~ ~ N
.
t m ~ U ::
~ ~
a v~
~ Z ~ J J ~ J J Z J J ~ J J lL G1
m 3r a ~
m ~ ~ ~ ~ ~ ~ ~ d ~ ~ ~ o 3~N Z W
. n
~ Q
m ~ :: 'm? o a o'o A E o 0 0
0 0 z
0 0 ' a m
v~v ~ > ~ ~ ~ ~ a a a v iaa a a
=o
CA 02273067 1999-OS-27
UEi/US ' 99 '1~HU Utf : LL H'AI U11 tlllLLtSU L1J riA11.t1 Al~ltm.H-aYtCitvlaa
m
m
J
O ~ n
X
a a ~ o
~
N
z W O a
~
'
m E
- n
8
c E ~ ~ >
m 3 ~ ~ ''~ a' a
m n ~ a m m m
~
D ~ ~ ~_D ~ ~ a~ m
JQ' JlY ~IL'~ ~ J~ JK
m
m A NOn
O~ 0 ~ O S ~L mY e7a~ N a!f ~ ~1'~O
19 H .fi
~ 1 ~ c
r, Y ~tPl 9 nO1 t~ 1(7~ ~ en C
N PO'fNM ~ 1~'7n ~N 11
0 0 ; 03 s ~o ~o
E ~ ~ E o W n ~ ~ mn 1~ S, n
~
w $ 6~
J "
U oc ~ ~+
~ 'N
~
LL &
~ m N
1~ 1 ~ r
G on g 0 ~ g",n
U = _ ~ ~ n m
> . ff ~ ~m~
O '
t/7 J ,Y
~ ' ~
J 11
O
N n c g ~ N I n m
...
~ ~ ~ N N
E
O E ~ m
~ rc
>-r >> > r> > > > >> > >> >
c > c
O >
W 'm~. o o ~~ o ~o ~ ~~ ~o'~'o c
W ~ ,t
'
a
o ~ o ~~ 2
~nono~ ~~d~ Y ~ n~ V ~n ~
f~ ' o~ A ~ f f
'
~
W ~ ~ f r
a
n
J O
IV
O ~f ~ ~~ f ~ , ~r ~ rf ~ ~ JJ
'NfV f
t U V
l ~ E mm m
~ mw o
m
m
m
~
t3 romo mto~omm ~eio'ol~i o~om~ o
n
L ' ~ ~
o
VN n
Ø.
N ~ ~ ~ +
~ O ~~ OO $ O O OO O ~O OO O
m C E ~ NH O n 7f 0 N NW
CO ~ N C H1 Pf'1~ fl
~ N 'f9 1 ' ~
N ! 1
m _ r
1
Q o~ E
0
a E ~ 3
~ ~
> m ~
'u1 ,~,E j m ~ ',
~ E ,n
Z o d ~ 4 0
m' ~ E~ ~ v m n J ~
m N ~ J J J J ~ ~
~
mm a ~ m m a m m ~ ~ ,t z
~ u m
"c a ~ :Q m m~ a _
m ~
c ~
~ ~~ a ~~ o ~ ~ v_ a
~ ~ ~
~ o o a
CA 02273067 1999-OS-27
Ub/US ~ ~~J '1'liU UO: LJ 1'HA U11 OlILLBU L1J riHl .11 e1n~1cW .H-JY1t11VtaJ
07
.- v c0 Q
uxi
0
.U
f0
~~~~a~ ~ ~ ~ ~ ~ w
d
o ~ ~ ~~ o~-
al~ NN r
r
st
J
~ O
_ N OO O
C'
s
cl N
J v m001O
IL
r.
O ,yc'nNI~~tl)~ N
00
d ti ofcva'o~ 'r~~ ~
g
N
~ '' ~ ~ ~' N
~, ~ y O
..
0
~o~ m
'~ g =~
'
E u ~ n ~n~
~
V ~ ....~,~~,v
>
5 m
y , a
, ~
a~ ~ r E u~
~
o ~ c m v a ~ 10
L1 N N
r m _
o 9 g 0 E
C
CC c '"a o 0$ $$
"
a
p ~ m~ ~ cn
~ o o cm n~ ~ a ~~ .-riU
a~
~ m ~ _ o
~ ~
v7 .d ~ U
N
.Q
v
v o
c '
o
Q ~ o
N
O d
~
1.. L
t
,t7 ~ a O
J
d ~ d ~
m
Z o~ a ~ , m
a
0
y ~ ~ JJ JJ Z JJ J JUJ Q O
Z J
~ _
m ~~ ~ m~ W ~O
m
6 ~~ 70 _ a Of O1
f . ",
O
Q1 (Q y ~ ~ ~O OO A E.O O O
a
o ~ > ~a aaa a n a~ a a
c
CA 02273067 1999-OS-27
06/05 ' 99 '1'HU urs : Z4 NAA U11 bllLLbU L1J IlAll:li Ht'lfll,H-aricm~~ ~r:~
~~~
'"
a
0
s
a
~
J
m
O
G Q O
W
o
a
a
.,
~
v E We z
~ i
i r m a
Z m
m
a
x ~ ~ i~
Y ~
Y
Y
x D ~
fa O ~ O _S
O
o _
s V K J 2'
e ~
v
1 t!7
L W
<
Q 1~- N Z
'
m
, 1_n~ ~ y N..g r
o a
n
o
'~
~
C V N a a
N 1~ G9
O 9
Z.
v
v
'!~ ~ N
D
K O~
J ~ a
a
0
s o
C
o
~n
E E
m 3
LL o~ N
O
m ~ g , I In ~ J
I~ I~'~
~mn u>,
~r
u
~
a
E o
' b' ~
3
V .~ a~
0
z g
LJJ
z N
N ~ ~ ~ N~ ~ ~R ~ N~ N
G E ~
U
~ U
x ~
3
o r >> r rr > >r r r >
>. >
~
c
O
Y
ppppg opmm pp
~~ ~ ~~
1 01
o 0
l
x
D
1
0
J
Z ~ ~~ n!1Q~1v~lr 'fv1 N h N
N b ~
V
a
J
a a
Z
~a o ~' r'o ~ v n
~ o n
x o 4 eA , ~'t f V' N
i n1 ~ Y ~
NIV1 I
h
f
t II
0 00 ~a ~ ~ ~ a o N
;r
E V .r~ .r. ~ , v - -
,~ .-
'
s
x
a
c
m ~ a ~ ~ f
~ o
Q _' ~ E In~o,~~~pnn S ~N ~
~
v
N
C
a m E
F
N O NO ~ ON
x L' $_ _ _= Z =_ ~ yn I~trl~
' Z vZ
o yR ~ ' Mp7p 7C Y 'CP
N W
d
3 t
o
8'
E a'~ ~ W
~
'~ ~ ' ~ ~ > a o
>
z
a
O r ~ E " ~ ~ m m
n
2
t ~ ~ ~ x m
~ ~ a
N
a i ~ e
~ 't
V J D d' t O
CA 02273067 1999-OS-27
U6/US ' 99 '1HU Utl: L4 H'A1 U11 tflILLKU L1:S HA'1'LH Amicm.ef-at-icim~~
- a
0
a
?C
~ r7< l(1
a
N 'C7
X
W
O w
N Q al
7
O1
O
d u'fd ~
~
c
O ~ 1~
N
v
~M
W
!~ O p~7N ~0
V w C N tni
Q m c ~
~
~
Q.
O01~YO1
T ~ N~ ~Of
1
v tCv-N NN
O o
'V ~N ~d p
~
d
o ~n.-rn~an s,E
~ i '
f c ~ ~~~o ~ ~-a
/7 ~ "
w
I O e ~ socc~r~. aE cN,
y.. ~ ~ ~i~ of.~ C1a o
3
R
a Er rn~ a~oao Nu~cD_
O o ~ '~''W ir~ x cv a~~
E
~ cnc v
~
V a y
~
n
0
r ~~ ~m
N aD
~
CO ~ ~ ~ ". ~ g~oo~g za ~a
$ n
N ~ ~ d Y
O
o
~ E a
o
~ op$ o .-N~v ~j V o
a t~7~ ~ w
N t
47
:O
'
~ O
v d
_ _ d ~ ~ ~ g ~ >
V
~
O
N
a
d
t
N
m O
C J
E ~ -
s o a ~
a _
Z o~ JJ ~~ J ~ J~ JJ
r.. a1 ~ '~ Q O
~
d 3 ~z Q a a ~;zaa aaa w
~ OO N
~O O O .~y ~T Z ~
'~ .C 'OE ~ os o C~ p ~ ~
m A ~ A
~ ~ O <a aQ Q ~C r~ =a
y a 4
V p~> t
p
CA 02273067 1999-OS-27
W vv JJ 111V W a.y 1'(>A Vii vita.a.JV ~iJ aaaalvaa aaaa\ivaa uaavavwu ,u~y~
m
O O
J O
O n
o g Q
a $ 1i1'~ ,d
E a ~ w x
Z .ame ~" J ~
O O
J
II C
E .~ ~ ~ m ~m 3,m ~ ~S
E ~c o
N
E ~ n ~ ~~ a ~ a
. ~ Wm A~ '~'
U m .F tn
Oo Op ofm m
J fll vV7 Jfn
m Q
_ U Y
a
--o o o ~ $ x
0 0~
_
V PN7 N 9 N ~ N~ N~ b 1~ O 7 t
01 V
~
O ~ pir 01m t~ n N ~ a V
m PfN_ PIN f ~ a W
N
o ~ rp a' p f'N i
a
D C CC C a o ~ ,,~t
a E ~ a
H
E EE _ ~ a
o
e C
o n E
LL ~ L"
b~ N
aE ~:3' 08 8 8
'
h ~ p~ ~ ~ ~4N c.nmtwi
'~
am ~ ~~ E
0 0
i
n
.- N ta7 s
E N $ 3
f ~
CD ~ N m
O
V
-0's
v > >> > >r >> > > >- N
~ 'Y 7
' m
> $3 >
0~ 5~g
w 0 ~ ~ 8
00 0 0
z
N
1
O
,J s m oN o In 'e~
~~ Y ~ Y V
W N ~~ Y Yf d
m
Q
O
O t
V
JJ ~ _
V ~ ~~ f ~ af V ~ Y 2
a N r- , F~OO O O
H
1
N
V o fm ~ o0 m~ 0 m 0
tA~ ~W b t0 N
0
f ~ 11
m
1 N
OO ~ a
1nN D
Q mM
Q a' ~ C N t
' E HU~m v ~ o N
.m o u ~N N N M
~ a no
0 0 N
_ N
Q QI
a
A o ofo ~ o o~ o ~ o o C~
g E
o o~ ~ iJ i.:i ~ ui p E
i~' v
m
_
V n
a o E
m
~ f=
0
>' E
Z
~ F- Z 1H ! 7 7 y
~ m J '~ ~ T3
L ' ~ t J ~$ , ~,
m a o ~ ~ ~ o 0 o L.'
a
i~a $ ~ c ~ c m x m
~ v! ~
o ~ ~ $ ~
~ rn
CA 02273067 1999-OS-27
UEi/US '99 '1HU Uti:G(i b~Al U11 ifllLLtlU L13 HA'1'l:H Al~ltll:A-JYl(11VGJ
auto
m
o
a
o ?
:y t ~ o~
ao Q
tCN N ~
t
W O_
O~ N
, _
O
G
01
J
m
7 r ~ Q
m
d'
C
O
F_. N to cn
~ N O CODO~D
O lu
r
~ N '1~O stN
f~1~
Q VJ ~ ~ N ~N
~' N
E
m
~ ~ ~ 4m7
N
t
O
N O O O
O
d"
O
C
~ f~ 1~ N
M
V N ~ m O
r
O E O O
~ f
J W
>> ~,1~O N rM m N,
t0
r 0 ~ ~~ H
~ 1
a
0
V
_ _.
Q C~~ O
O
c~N N ~ ~ O
~o M ~ o ~v vr
u 'n
E~ x ~~
'
C ~o ~ ~ m mi
u
~D O c~
m
a v-
V o
'73 ~ O 1~
N
L 'Om M m 1!') v N
_ ~ ~ ~
M ~ N N lV
N
~I.G.
.
G
' a
O
M
.cm l
d ~'E ~o .rn cc~
r
~_ r a U
t'
~f o
U
0
..-
N
N ~ 0
mV <OD ~ Cue. In
c~ r" ! t
0
~, m
o
_ o
Q rn
~
o
m L _u1
O
E 3 . G
o
m~ . o
~
J J J JJ ~ J~ ~J X J
J
m m mm mm h ue'mm mm ~
0 0 00 00 n m OO OO p
_
O O O OO C ~ OO OO ~
O
V O
v ~ o a a a aa a ~a aa m
a a
x a~
CA 02273067 1999-OS-27
Image
uoiua as triu vo.c~ rns uli omc~o~ cm ue~m.u nmm~.n-J11\11~VV "G"i"
O
U
C
X
' r r-' ~' o
~ E
o
~ ~ ' '''g~ ,
~ ,
n
.r a ~ x
o o~ ci
W
O O~ N N V:O~ O
'
_ N N r N
Nc Q
!
J U U t~ f '
a~
O t D CDd'N
C7COG7M f~
d\ ~' N N Or
O
C
O
r O W ~ ~O N
1 ~ O ~~ r
D
E
~m
~ ~tco 0
~,
ch
O J
~
O
_
.-.(p O1CPW1~CO
~f747~GLCIV7 d
. - - -- -
.
O p~ N r . a~ r
J
+~
J
v
LL V p ~ O W NN O
d ~ ~
L ~ ~ c~ ~ NN N
~
O
C '
W
O O O
D = ~ OO ~
~ M O N W Ino0C~3~ r f~
calph ~
C O ~ ~ ~ ~ NN 0 N
N N 0
N
O U ~
t~
-_
a O
~ ~ c~g oo oo U U
m n ~nu~~nua~n
,4", d a~
Z
H
o>ccw r~oa mn
1~aDo~00aDN W
U U
d
Q o d
'
~ h
tD C -
O~ -n
v
H m c~ v~7
H
MW O O O y
t p O ~ ~ ~ (,
O
V O r-e-N
t
'
J ~ b0
a
J O
w
O
U
U
Q O
a a J ',J~ ~
. J ~ .c
h ~ t0O ~OCOO
U p
N p x ~.
di ~N
.
V J D
CA 02273067 1999-OS-27
Image
Uti/U5 ' 99 '1'HU Utf : Ytl h'Aa U11 tfllGLtsU Ll:f HA'1'l:H A1~iclLA-'YltlNVS
~ uGu
C
O
.... M u7GOGOn
t
L n CDN COC p
O N m C O '
OD
U
N (OCDDON
Vv' ~ ~ ~ ~ ~ Sri
J o
U
d
- CO~ 1~,-01 "
O
. ~ fsN ~ c~1M O
W
m
- C O O G?O
' C
'
t0 .
r C
O
' ~
V o p ODvtT M fl
N~~ CDN !~1
m ~ ~ O O
etCOM I~-00
O r-p T N
N
1~I~N cOd'
V d
O M ~ ~D O
~ M ~ M
J d --r-cvi~ ~ riT
J
0 O O
Vm O X N N
0'
~ chi7 ~ ~ _ ~v
sl'
L O
V
t, O...
00O M o0N v7
~ ~ O ~ ~ N r
I C C 1 (
G
= O~ ~ ~ T .-r-
o
C~ o O o c
(p 0 O O O ,ON
0 ~ '
V
!'~
a" r T T r r
,
0
d
~
~ o ~ i ~
v
~n ~~ c - - -
o
-
,d, T , e r ~ ~ e ~ V
ch m a~
d ~ ~ H ~
d
H ~ '
N >
p a
0
o O a c o o
o c o
VO ~-M 'c1"Ci1
t
O
J
O
r.~
.O O
U
_
~
Ow V1
m Y
.-N C7wttf! ~
J
J J J J J N .~! v~
~ t0COcDCDc0 p
U
O x U
Z
,~
O ~ ~6
O
V D
J
CA 02273067 1999-OS-27