Note: Descriptions are shown in the official language in which they were submitted.
CA 02274832 1999-06-11
Formulation in the form of a matrix material-excipient
compound, matrix material-active substance compound
and/or matrix material-excipient-active substance
compound, and processes for the preparation thereof and
the use thereof for the preparation of tablets
and/or other larger matrix units
The invention relates to a formulation in the form of a
compound which has an excipient phase with at least one
excipient and/or an active substance phase with at least
one active substance and a phase of a matrix-forming
material (also called matrix material in the following)
chosen from polymer and/or lipid, i.e. a polymeric phase
and/or a lipid phase with at least one polymer or lipid,
and therefore to polymer- and/or lipid-containing
delayed/prolonged release dosage forms, processes for the
preparation thereof and the use thereof, in particular for
the preparation of tablets or other larger matrix units.
Such compounds are physical combi:~ations of at least two
starting substances and are employed in particular in the
pharmaceuticals sector.
To achieve a release of active substances from a formula-
tion which is controlled, delayed, prolonged or indepen-
dent of physiological parameters, it is known to process
the starting substances such that the resulting formula-
tions or the medicament forms prepared from these formula-
tions have a coating which controls the release (e.g. of
polymers, such as polymethacrylates, or organic molecules,
such as shellac or cellulose acetate phthalate) or alter-
natively have a matrix system comprising polymers.
Matrix units for controlled release which are prepared
using polymers are described in the literature:
1. polymer particles
(e. g. pellets, granule grains, microparticles)
2. larger matrix units
(e. g. tablets, coated tablet cores and implants).
CA 02274832 1999-06-11
- 2 -
The particles described in more detail in the following
are characterized in that the active substance is embedded
in the polymeric phase in a molecularly disperse or
particulate form.
The larger matrix units described in more detail in the
following must as a rule be prepared by the expensive
process of compression after prior granulation.
Medicament forms/drug formulations for controlled release
using polymers:
The release-controlling effect of such a formulation or
medicament form, also called "controlled release" formula-
tion (CR formulation) , is controlled on the one hand by
the properties of the polymeric phase itself, such as, for
example, the wettability, the swellability or the
crystallinity, and on the other hand by the structure of
the matrix formed by the polymeric phase. This matrix
structure, which can be homogeneous or heterogeneous in
construction, either is already present in the formulation
itself or forms during processing during formulation to
the medicament form.
The solubility properties may be mentioned here as pro-
perties of the polymeric phase which influence the
release. Thus, because of their insolubility and/or
swellability in aqueous solvents, polymers ormacromole-
cules are suitable for delayed release of active sub-
stances which are embedded in a matrix of such polymers or
macromolecules. Medicament forms with polymer substances
which, because of the solubility of the polymers in
gastric or intestinal juice, are a formulation which
controls the site of the release are furthermore known.
A distinction may be made in particular between two groups
in particular of these formulations which control the
CA 02274832 1999-06-11
- 3 -
release of active substances.
On the one hand, polymer-containing particles of an order
of size of approx. 0.01 to 2 mm, which are also called
microparticles (0.05 to 0.2 mm), granule grains or pel-
lets, are known. However, the microparticles or microsphe-
rules having a typical size of 50 to 200 Vim, nanoparti-
cles, nanopellets and nanospherules which have only been
known for a relatively short time are also assigned to the
group of polymer-containing particles if they have a
polymeric phase. The particles are present as an indepen-
dent release unit (single dosage unit) in the form of a
particulate matrix, the formulation then already having a
matrix structure.
On the other hand, the particles described in the present
application can be combined to larger release units or
larger matrix units. This further processing is described
in detail below.
Examples which may be mentioned of particulate matrices,
the particles of which form independent release units, are
the dispersion of microparticles for parenteral injection,
which allow a controlled release of LH-RH analogues, and
the filling of pellets into a gelatine capsule in the case
of commercial preparations, such as sympathomimetics.
These are described by Miiller, R.H., Hildebrand G.E.
(eds.) in "Pharmazeutische Technologies Moderne Arzneifor-
men [Pharmaceutical Technology: Modern Drug Dosage
Forms]", Wissenschaftliche Verlagsgesellschaft mbH Stutt-
gart, (1997), by Bauer, K.H., Fromming, K-H., Fiihrer, C.
in "Pharmazeutische Technologie [Pharmaceutical Technol-
ogy] " Georg Thieme Verlag Stuttgart, New York, ( 1991 ) , and
by List, P.H. "Arzneiformenlehre [Pharmaceutical Dosage
FormsJ" Wissenschaftliche Verlagsgesellschaft mbH Stutt-
gart, (1986).
CA 02274832 1999-06-11
- 4 -
EP 0 261 677 furthermore describes polymer-containing
compositions which are said to allow a delayed release of
the active substance. A spray drying process is disclosed
for the preparation of these compositions, so that by
applying the doctrine of this publication, particles
having a size of at least 30 ~m which contain the active
substance in a uniform distribution are obtained.
Several processes are described in the literature for the
preparation of such formulations with a particulate matrix
structure.
In the processes by the "solvent evaporation" or "in-
liquid drying" method, the polymer or the matrix-forming
agent is a substance (e. g. polymers, such as polylactides
or polylactide/glycolide) which is soluble in an organic
solvent. The polymer is dissolved in an organic solvent
and the active substance is also dissolved or - in the
case of insoluble active substances - dispersed in the
organic phase. The polymer or matrix-forming agent so-
lution comprising the active substance is then added to an
aqueous surfactant solution and an 0/W emulsion is pre-
pared by stirring. The organic solvent is then removed and
the matrix-forming agent precipitates. Solid pellets or
microparticles are formed. A distinction is made between
the "solvent evaporation" and the "in-liquid drying"
method, depending on the method of removal of the solvent.
These processes have been described by Speiser, P. in
Miiller, R.H., Hildebrand G.E. (eds.) in "Pharmazeutische
Technologies Moderne Arzneiformen [Pharmaceutical Technol-
ogy: Modern Drug Dosage Forms]", Wissenschaftliche Ver-
lagsgesellschaft mbH Stuttgart, (1997) by Beck, L.R.,
Pope, V.Z., Cowsar, D.R., Lewis, D.H., Tice, T.R., in
"Evaluation of a new three-month injectable contraceptive
microsphere system in primates (baboons)", Contracept.
CA 02274832 1999-06-11
- 5 -
Deliv. 1 Syst., 1, 79-80 (1980), by Beck, L.R., Flowers,
C.E., Pope, V.Z., Tice, T.R., Wilborn, W.H., in "Clinical
evaluation of an improved injectable microcapsule contra-
ceptive system" in Amer. J. Obstet. Gynecol. 147 (7), 815-
821 (1983) and by Beck, L.R., Pope, V.Z., Flowers, C.E.,
Cowsar, D.R., Tice, T.R., Lewis, D.H., Dunn, R.L., Moore,
A.B., Gilley, R.M., in "Poly(d,l-lactidecoglycolide)/nor-
ethisterone microcapsules: An injectable biodegradable
contraceptive" in Biol. Reprod. 28, 186-195 (1983a).
Very fine particles in the region of a few micrometres can
be obtained by these processes. However, the large outlay
with which the preparation method is associated and the
contamination of the particles with residual solvent are
disadvantages . For this reason, there is also as yet no
product in Germany which has been prepared by one of these
processes and meets the approval criteria for a medica-
ment. Alternatively, the polymer or matrix-forming agent
solution comprising the active substance can be spray
dried. Here also, a residual content of organic solvents
in the products cannot be avoided because of the prepara-
tion process. Products prepared by this process, such as
e.g. microparticles for parenteral administration of
bromocriptine, are described by Fahr, A., Kissel, T., in
Miiller, R.H., Hildebrand G.E. (eds.) in "Pharmazeutische
Technologies Moderne Arzneiformen [Pharmaceutical Technol-
ogy: Modern Drug Dosage Forms]", Wissenschaftliche Ver-
lagsgesellschaft mbH Stuttgart, (1997). They are available
on the pharmaceuticals market. However, the problem of
the residual solvent content has only been shifted in that
here also the release of the toxic solvent is delayed and
therefore occurs in a small daily amount. The amount
released from the matrix per day remains below the maximum
daily tolerated value.
All the processes mentioned so far are characterized in
CA 02274832 1999-06-11
- 6 -
that the polymeric phase or the matrix-forming agent is
present as a molecule in a dissolved form and is in an
organic solvent. Particulate formulations, the polymeric
phase of which comprises the active substance in a
molecularly disperse form or in the form of fine particles
are formed. These formulations have a so-called homogenous
matrix structure, as is also described by Fahr, A.,
Kissel, T. in Mi.iller R.H., Hildebrand G.E. (eds.) in
"Pharmazeutische Technologies Moderne Arzneiformen [Phar-
maceutical Technology: Modern Drug Dosage Forms]", Wissen-
schaftliche Verlagsgesellschaft mbH Stuttgart, (1997).
Another process for the preparation of a particulate
formulation with a polymeric phase avoiding the use of
organic solvents is described in EP 0 361 677. The matrix-
forming agent, which is water-soluble according to this
publication, or the polymeric phase is dissolved in water
(e. g. ethyl acrylate/methacrylate copolymer in ammoniacal
solution), the active substance is also dissolved or
dispersed and - in contrast to the "solvent evaporation"
and "in-liquid drying" method - instead of an 0/W a W/0
emulsion is now prepared. Dispersion media are water-
immiscible organic solvents, e.g. liquid paraffin or
methylene chloride. The matrix-forming agent can be
dissolved in water or also emulsified in the aqueous
phase. In the second case, an emulsion in a water-
immiscible organic solvent is dispersed. Polymer particles
which include the active substance in a molecularly
disperse or particulate distribution are precipitated by
expensive azeotropic distillation of water and the organic
solvent. The particles are obtained by separation by means
of filtration and subsequent washing.
US-A-5 043 280 describes a process for the preparation of
a particulate formulation by extraction in supercritical
gases. In this case, the matrix-forming agent - as in the
CA 02274832 1999-06-11
_ 7 _
"solvent evaporation" - is a substance which is soluble in
an organic solvent, such as e.g. a polymer. The polymer is
dissolved in an organic solvent and the active substance
is also dissolved or - in the case of insoluble active
substances - dispersed in the organic phase. The matrix-
forming agent solution comprising the active substance is
then finely sprayed into a supercritical gas phase. Fine
drops are distributed in the supercritical gas, which
extracts the organic solvent from the drops. Precipitation
of particles which comprise the active substance occurs as
a result.
These processes mentioned also lead to formulations which
contain the active substance in a molecularly disperse or
particulate form embedded in the polymeric phase. As a
result of this process-related inclusion of the active
substance into the polymeric phase, the external phase of
the formulation mostly comprises polymer, which also
determines the pharmaceutical properties, which are of
importance for any possible further processing. The
formulations mentioned furthermore have the disadvantage
that they can be prepared only with considerable expendi-
ture of cost and time.
The possibility of further processing of particulate,
polymer-containing formulations to medicament forms which
have larger matrix units, such as, for example, to tab-
lets, coated tablet cores or implants, is known. The
preparation of implants comprising LH-RH analogues is thus
described by Miiller, R.H., Hildebrand G.E. (eds.) in
"Pharmazeutische Technologies Moderne Arzneiformen [Phar-
maceutical Technology: Modern Drug Dosage Forms)", Wissen-
schaftliche Verlagsgesellschaft mbH Stuttgart, (1997). The
preparation of tablets is of particular importance here,
because this medicament form has many advantages, such as,
for example, the possibility of processing almost all
CA 02274832 1999-06-11
_ g _
solid active substances, the high dosage accuracy, the
easy taking and handling and the good storability and
transportability.
Medicament forms which are larger matrix units, and in
particular tablets, are usually prepared by compression.
Several process steps are necessary here for processing
conventional polymer-containing formulations in the form
of particulate matrices.
The various constituents, such as, for example, various
active substances, excipients and polymers, are first
mixed homogeneously. The mixture is then subjected to wet
granulation by addition of binders, granulating fluid or
solvents. The resulting granules are dried to remove the
residual moisture. Compression to tablets, coated tablet
cores or implants is then carried out with the dry
granules with the addition of further excipients, such as
flow regulators, lubricants and mould release agents.
The fact that the active substance is exposed to the
moisture of the granulation fluid or solvent for a long
time during the wet granulation and unavoidably is exposed
to an elevated temperature during the necessary drying
process is a disadvantage. Furthermore, because of the
various individual steps and the devices and apparatuses
required for these, the process is associated with a
relatively high expenditure of time and is therefore cost-
intensive.
Direct compression of tablets from formulations with
polymeric constituents, which is already often used for
the preparation of tablets without a polymeric phase
because of the low costs and the rapid implementation, has
hitherto not been possible because of the following difficulties.
CA 02274832 1999-06-11
- 9 _
On the one hand, due to predominantly elastic deformation,
the polymers have poor compression properties, since
compression is usually achieved chiefly by plastic defor-
mation.
On the other hand, the mixture for direct compression
tends towards an undesirable demixing between powdered
active substances and/or excipients and polymers because
of the different nature of the surfaces and the resulting
different flow properties. In direct compression, highly
inhomogeneous tablets would therefore be obtained due to
the progressive demixing of the tablet-forming material.
The generally poor flow properties of the polymers are
another problem. As a result of this, a satisfactory
delayed/prolonged release effect is not achieved because
of the limited capacity for admixing of polymers to the
tablet-forming mixture. In the literature, an addition of
as a rule a maximum of 10-15 % polymer in a tablet recipe
for direct compression is described for acrylate polymers
by McGinity, J.W., Cameron, C.G., Cuff, G.W., in "Con-
trolled-release theophylline tablet formulations contain-
ing acrylic resins. I. Dissolution properties of tablets",
Drug Development and Industrial Pharmacy, 9 (162), 57-68
(1983) and by Cameron, C.G., McGinity, J.W., in "Con-
trolled-release theophylline tablet formulations contai-
ning acrylic resins, II. Combination resin formulations"
and "III. Influence of filler excipient", loc. cit. 13
(8), 1409-1427 (1987), loc. cit. 13 (2), 303-318 (1987).
However, delayed/prolonged release dosage forms in which
lipids are used are also known. Such dosage forms
described in the literature for controlled release using
lipids are substantially:
CA 02274832 1999-06-11
- 10 -
1. suppositories
2. vaginal globuli
3. pellets for peroral administration (e. g. Mucosolvan
retard).
In comparison with polymers, lipids offer the following
advantages:
1. good tolerability in vivo, especially if they are
composed of physiological fatty acids
2. no toxicologically unacceptable residues from the
production (e. g. catalyst residues)
3. control of the degradation rate in vivo via the
chemical structure of the lipids
4. inexpensive
They are therefore excipients which can be employed in
addition to polymers for the preparation of CR formula-
tion.
Medicament/drug dosage forms for controlled release using
lipid formulations from compounds:
Suppositories and vaginal globuli are as a rule prepared
by pouring out the medicament-containing mixture into
moulds (P. H. List, Arzneiformenlehre, [Pharmaceutical
Dosage Forms], Wissenschaftliche Verlagsgesellschaft
1976).
It is also possible to prepare suppositories by compres-
sion of a mixture of lipid particles and drug powder, but
preparation on a large industrial scale presents diffi-
culties because of the generally poor flow properties of
these mixtures when filling the compression moulds. This
method is therefore primarily described for small-scale
preparation on a recipe scale in the pharmacy (K. Munzel,
J. Buchi, 0.-E, Schultz, Galenisches Praktikum [Practical
Galenics], Wissenschaftliche Verlagsgesellschaft Stutt-
CA 02274832 1999-06-11
- 11 -
Bart, p. 652, 1959). Only lipids which melt or at least
soften at body temperature are employed here.
Pellets which are prepared on a large industrial scale by
extrusion of molten lipids with an extruder and a perfor-
ated disc are medicament forms for peroral administration
(Voigt, Lehrbuch der Pharmazeutischen Technologie, [Text-
book of Pharmaceutical Technology], Verlag Chemie, 1975).
Disadvantages here are e.g. the incorporation of the drugs
into the lipid (e.g. by dispersion or dissolving), the
exposure of the drugs to heat during extrusion and the
need for further processing of the pellets in an addi-
tional production step (e. g. introduction into hard
gelatine capsules).
The object of the present invention is thus to provide a
formulation in the form of a matrix material-containing
compound, as a retarded action medicament formulation,
which has an excipient and/or an active substance (drug)
phase and a matrix material phase. The formulation should
have a sufficiently high matrix material content so that
controlled release of the active substance contained
therein or added subsequently during processing to larger
matrix units is made possible. Furthermore, it should be
possible to process the formulation to larger matrix units
by means of direct compression (e. g. tabletting). More-
over, a process for the preparation of this formulation or
compound is to be provided.
The object according to the invention is achieved by a
matrix material-containing retarded action medicament form
which is in the form of a matrix material-excipient
compound, matrix material-active substance compound and/or
matrix material-excipient-active substance compound, the
matrix material being chosen from polymers and lipids such
that the compound has a polymeric phase and/or lipid phase
CA 02274832 1999-06-11
- 12 -
and an excipient and/or an active substance phase. Such a
compound can be converted into its final medicament form
by direct compression.
The invention therefore relates to polymer- or lipid-
containing formulations which
are in the form of a compound which has a polymeric
or lipid phase with at least one polymer or lipid, an
excipient phase with at least one excipient and/or an
active substance phase with at least one active
substance.
According to the invention, it has been recognized that
the object can be achieved by the formulation described in
claim 1, which
a) has an excipient phase with at least one excipient
and/or an active substance phase with at least one
active substance and a polymeric phase with at least
one polymer, the polymeric phase being incoherent and
the excipient and/or active substance phase being
coherent, or
b) has a lipid phase with at least one lipid, an
excipient phase with at least one excipient and/or an
active substance phase with at least one active
substance, the lipid phase being incoherent and the
excipient and/or active substance phase being cohe-
rent.
In particular, the polymer phase or the lipid phase can
comprise excipient and/or active substance or be free from
these.
In the formulation according to the invention, the content
of polymer phase or lipid phase can be between 1 and 98 %,
CA 02274832 1999-06-11
- 13 -
based on the total amount of excipient and/or active
substance phase and polymer phase or lipid phase.
In particular, the formulation can have a content of
polymer/lipid phase of 10 to 95 %.
The content of polymer/lipid phase in the formulation can
furthermore be more than 15 o and not more than 90 %.
However, for implementing the present invention, it is
particularly advantageous if the polymer/lipid phase has
a content of 40 to 70 %, based on the total amount of
excipient and/or active substance phase and polymer/lipid
phase.
In principle, the formulation according to the invention
can comprise any type of active substance or be free from
active substance. The active substance can furthermore be
added to the formulation subsequently, e.g. before a
further processing to larger matrix units. In general, the
formulation can comprise the following groups of active
substances:
- hydroxylated hydrocarbons
- carbonyl compounds, such as ketones (e. g.
haloperidol), monosaccharides, disaccharides and
amino sugars
- carboxylic acids, such as aliphatic carboxylic acids,
esters of aliphatic and aromatic carboxylic acids,
esters with basic substituents of aliphatic and
aromatic carboxylic acids (e. g. atropine
scopolamine), lactones (e. g. erythromycin), amides
and imides of aliphatic carboxylic acids, amino
acids, aliphatic aminocarboxylic acids, peptides
(e. g. ciclosporins), polypeptides, j3-lactam deriva-
tives, penicillins, cephalosporins, aromatic carboxy-
CA 02274832 1999-06-11
- 14 -
lic acids, (e.g. acetylsalicylic acid), amides of
aromatic carboxylic acids, vinylogous carboxylic
acids and vinylogous carboxylic acids esters
- carboxylic acid derivatives, such as urethanes and
thiourethanes, urea and urea derivatives, guanidine
derivatives, hydantoins, barbituric acid derivatives
and thiobarbituric acid derivatives
- nitro compounds, such as aromatic nitro compounds and
heteroaromatic nitro compounds
- amines, such as aliphatic amines, aminoglycosides,
phenylalkylamines, ephedrine derivatives, hydroxy-
phenylethanolamines, adrenaline derivatives, ampheta-
mine derivatives, aromatic amines and derivatives,
quaternary ammonium compounds
- sulphur-containing compounds, such as thiols and
disulphanes, sulphones, sulphonic acid esters and
sulphonic acid amides
- polycarbocyclic compounds, such as tetracyclines,
steroids with an aromatic ring A, steroids with an
alpha, beta-unsaturated carbonyl function in the ring
A and an alpha-ketol group (or methylketo group) on
C-17, steroids with a butenolide ring on C-17,
steroids with a pentadienolide ring on C-17 and seco-
steroids
- 0-containing heterocyclic compounds, such as chromane
derivatives (e. g. cromoglicic acid)
- N-containing heterocyclic compounds, such as pyrazole
derivatives (e. g. propyphenazone, phenylbutazone)
- imidazole derivatives (e. g. histamine, pilocarpine),
pyridine derivatives (e. g. pyridoxine, nicotinic
acid), pyrimidine derivatives (e. g. trimetoprim),
indole derivatives (e. g. indometacin), lysergic acid
derivatives (e. g. ergotamine), yohimban derivatives,
pyrrolidine derivatives, purine derivatives (e. g.
allopurinol), xanthine derivatives, 8-hydroxy-
quinoline derivatives, amino-hydroxy-alkylated
CA 02274832 1999-06-11
- 15 -
quinolines, aminoquinolines, isoquinoline derivatives
(e. g. morphine, codeine), quinazoline derivatives,
benzopyridazine derivatives, pteridine derivatives
(e. g. methotrexate), 1,4-benzodiazepine derivatives,
tricyclic N-containing heterocyclic compounds,
acridine derivatives (e.g. ethacridine) and
dibenzazepine derivatives (e. g. trimipramine)
- S-containing heterocyclic compounds, such as thioxan-
thene derivatives (e. g. chlorprothixene)
- N,0- and N,S-containing heterocyclic compounds, such
as monocyclic N,0-containing heterocyclic compounds,
monocyclic N,S-containing heterocyclic compounds,
thiadiazine derivatives, bicyclic N,S-containing
heterocyclic compounds, benzothiadiazine derivatives,
tricyclic N,S-containing heterocyclic compounds and
phenothiazine derivatives
- O,P,N-containing heterocyclic compounds (e. g.
cyclophosphamide)
The following drugs (as the salt, ester or ether or in the
free form) are suitable, for example, for incorporation:
Analgesics/antirheumatics
narcotic substances, such as morphine, codeine,
piritramide, fentanyl and fentanyl derivatives,
levomethadone, tramadol, diclofenac, ibuprofen,
indometacin, naproxen, piroxicam, penicillamine
Antiallergics
pheniramine, dimetindene, terfenadine, astemizole,
loratidine, doxylamine, meclozine, bamipine,
clemastine
Antibiotics/chemotherapeutics
of these: polypeptide antibiotics, such as colistin,
polymyxin B, teicplanin, vancomycin; malaria agents,
CA 02274832 1999-06-11
- 16 -
such as quinine, halofantrine, mefloquine,
chloroquine, virustatic agents, such as ganciclovir,
foscarnet, zidovudine, aciclovir and others, such as
dapsone, fosfomycin, fusafungine, trimetoprim
Antiepileptics
phenytoin, mesuximide, ethosuximide, primidone,
Phenobarbital, valproic acid, carbamazepine,
clonazepam
Antimycotics
a) internal:
nystatin, natamycin, amphotericin B, flucytosin,
miconazole, fluconazole, itraconazole
b) external furthermore:
clotrimazole, econazole, tioconazole,
fenticonazole,
bifonazole, oxiconazole, ketoconazole,
isoconazole, tolnaftate
Corticoids (internal agents)
aldosterone, fludrocortisone, betametasone,
dexametasone, triamcinolone, fluocortolon, hydroxy
cortisone, prednisolone, prednylidene, cloprednol,
methylprednisolone
Dermatological agents
a) Antibiotics:
tetracycline, erythromycin, neomycin, genta
mycin, clindamycin, framycetin, tyrothricin,
chlortetracycline, mipirocin, fusidic acid
b) Virustatic agents as above, and furthermore:
podophyllotoxin, vidarabine, tromantadine
c) Corticoids as above, and furthermore:
amcinonide, fluprednidene, alclometasone, clobe-
tasol, diflorasone, halcinonide, fluocinolone,
CA 02274832 1999-06-11
- 17 -
clocortolone, flumetasone, diflucortolone,
fludroxycortide, halometasone, desoximetasone,
fluocinolide, fluocortin butyl, fluprednidene,
prednicarbate, desonide
Diagnostics
a) radioactive isotopes, such as Te99m, Inlll or
I131, covalently bonded to lipids or lipoids or
other molecules or in complexes
b) highly substituted iodine-containing compounds,
such as e.g. lipids
Haemostyptics/antihaemorrhagics
blood clotting factors VIII, IX
Hypnotics, sedatives
cyclobarbital, pentobarbital, phenobarbital,
methaqualone (BTM), benzodiazepines, (flurazepam,
midazolam, nitrazepam, lormetazepam, flunitrazepam,
triazolam, brotizolam, temazepam, loprazolam)
Hypophysis and hypothalamus hormones, regulatory peptides
and their inhibitors
corticotrophin, tetracosactide, chorionic
gonadotrophin, urofollitrophin, urogonadotrophin,
somatrophin, metergoline, bromocriptine, terli
pressin, desmopressin, oxytocin, argipressin, orni
pressin, leuprorelin, triptorelin, gonadorelin,
buserelin, nafarelin, goselerin, somatostatin
Immunotherapeutics and cytokines
dimepranol 4-acetamidobenzoate, thymopentin,
a,-interferon,(3-interferon, y-interferon, filgrastim,
interleukins, azathioprine, ciclosporin
CA 02274832 1999-06-11
- 18 -
Local anaesthetics
internal:
butanilicaine, mepivacaine, bupivacaine, etidocaine,
lidocaine, articaine, prilocaine
external furthermore:
propipocaine, oxybuprocaine, tetracaine, benzocaine
Migraine agents
proxibarbal, lisuride, methysergide, dihydroer-
gotamine, clonidine, ergotamine, pizotifen
Narcotic agents
methohexital, propofol, etomidate, ketamine,
alfentanil, thiopental, droperidol, fentanyl
Parathyroid hormones, calcium metabolism regulators
dihydrotachysterol, calcitonin, clodronic acid,
etidronic acid
Ophthalmological agents
atropine, cyclodrine, cyclopentolate, homatropine,
tropicamide, scopolamine, pholedrine, edoxudine,
idoxuridine, tromantadine, aciclovir, acetazolamide,
diclofenamide, carteolol, timolol, metipranolol,
betaxolol, pindolol, befunolol, bupranolol,
levobununol, carbachol, pilocarpine, clonidine,
neostimgine
Psychotropic agents
benzodiazepines (lorazepam, diazepam), clomethiazole
Thyroid therapeutics
1-thyroxine, carbimazole, thiamazole,
propylthiouracil
CA 02274832 1999-06-11
- 19 -
Sera, immunoglobulins, vaccines
a) general and specific immunoglobulins, such as
hepatitis types, rubella, cytomegaly, rabies,
early summer meningoencephalitis, varicella-
zoster, tetanus, rhesus factors
b) immune sera, such as botulism antitoxin, diph-
theria, gas gangrene, snake poison, scorpion
poison
c) vaccines, such as influenza, tuberculosis,
cholera, diphtheria, hepatitis types, early
summer meningoencephalitis, rubella, Haemophilus
influenzae, measles, Neisseria, mumps, polio-
myelitis, tetanus, rabies, typhus
Sex hormones and their inhibitors
anabolics, androgens, antiandrogens, gestagens,
oestrogens, antioestrogens (tamoxifen etc.)
Cystostatics and metastases inhibitors
a) alkylating agents, such asnimustine, melphalan,
carmustine, lomustine, cyclophosphamide,
ifosfamide, trofosfamide, chlorambucil,
busulfan, treosulfan, prednimustine, thiotepa
b) antimetabolites, such as cytarabine,
fluorouracil, methotrexate, mercaptopurine,
tioguanine
c) alkaloids, such as vinblastine, vincristine,
vindesine
d) antibiotics, such as aclarubicin, bleomycin,
dactinomycin, daunorubicin, doxorubicin,
epirubicin, idarubicin, mitomycin, plicamycin
a ) complexes of sub-group elements ( a . g. Ti, Zr, V,
Nb, Ta, Mo, W, Ru, Pt), such as carboplatin,
cisplatin and metallocene compounds, such as
titanocene dichloride
CA 02274832 1999-06-11
- 20 -
f) amsacrine, dacarbazine, estramustine, etoposide,
hydroxycarbamide, mitoxantrone, procarbazine,
temiposide
g) alkylamidophospholipids (described in J.M.
Zeidler, F. Emling, W. Zimmermann and H.J. Roth,
Archiv der Pharmazie, 324 (1991), 687)
h) ether lipids, such as hexadecylphosphocholine,
ilmofosine and analogues, described in R.
Zeisig, D. Arndt and H. Brachwitz, Pharmazie 45
(1990), 809-818.
There may be mentioned in particular: cyclosporins, such
as cyclosporin A, and cyclosporin derivatives, as well as
paclitaxel.
The formulation according to the invention can comprise
customary polymers as the polymer, such as, for example,
polyacrylates or polymethacrylates (Eudragit E, L, F),
celluloses and cellulose derivatives (methylhydroxy-
propylcellulose, ethylcellulose, hydroxy-propylcellulose
acetate succinate (Aquoat') or naturally occurring polymers
(shellac, waxes, beeswax, polish waxes). The release
property of the formulation or of the larger matrix units
prepared therefrom can be controlled by the choice of the
polymer. A release of the active substance which is
delayed only slightly in comparison with non-retarded
tablets can thus be achieved by using methylhydroxy-
propylcellulose. The use of Eudragit E as the polymer
already leads to a delayed release of the active substance
in the stomach. If the formulation comprises Eudragit L or
F as the polymer, a controlled release of the active
substance initially in the intestinal region is possible.
The formulation according to the invention can comprise
customary lipids as the lipid, such as, for example,
naturally occurring, semi-synthetic and synthetic
CA 02274832 1999-06-11
- 21 -
triglycerides or mixtures thereof, mono- and diglycerides
by themselves or as a mixture with one another or with
e.g. triglycerides, naturally occurring and synthetic
waxes, fatty alcohols, including their esters and ethers,
and lipid peptides. Synthetic mono-, di- and
triglycerides, as individual substances or in a mixture
(e. g. hydrogenated fat), glycerol tri-fatty acid esters
(e.g. glycerol trilaurate, - myristate, -palmitate, -
stearate and -behenate) and waxes, such as e.g. cetyl
palmitate and cera alba (bleached wax, German Pharma-
copeia, 9th edition) and beeswax (e.g. Apifil, Apifac) are
particularly suitable.
Further lipids, in some cases with additionally emulsify-
ing (SE - self-emulsifying) properties are glycerol
behenate (e. g. Compritol 888 ATO), glycerol tribehenate
(Compritol 888), palmitostearate, such as e.g. glycerol
palmitostearate (e. g. Biogapress Vegetal ATO BM 297,
Precirol Ato 5, Geleol), diethylene glycol, propylene
glycol, ethylene glycol, polyglycol and propylene glycol
palmitostearate, stearates, such as glycerol stearate
(e. g. Precirol WL 2155 Ato) and polyglycol stearate,
isostearates, polyalcohol fatty acid esters (e. g. Compri-
tol WL 3284), PEG behenate (e. g. Compritol HD5 ATO), cetyl
palmitate (e.g. Precifac Ato), sucrose esters, such as
sucrose monodistearate and monopalmitate (e. g. Sucro-Ester
W.E. 15), sucrose distearate (e.g. Sucro-Ester W.E. 7),
polyglycerol esters, such as polyglycerol iso-
stearostearate (Lafil WL 3254) and palmitostearate,
polyglycolized glycerides (e. g. Gelucire, Labrafil,
Suppocire), self-emulsifying polyglycol stearate (e. g.
Superpolystate), self-emulsifying polyglycol
palmitostearate (e.g. Tefose series), glycerides of C12-Cls
fatty acids (e.g. Lipocire) and mixtures thereof of two or
more lipids.
CA 02274832 1999-06-11
- 22 -
The release property of the formulation or of the larger
matrix unit prepared therefrom can be controlled by the
choice of the lipid. The release can thus be accelerated
by using lipids which are fast degraded in the intestine,
since in addition to the release on the basis of diffusion
from the matrix, release on the basis of matrix erosion
also takes place. The release is more delayed with lipids
which are degraded more slowly or lipids which cannot be
degraded in the gastrointestinal tract. Dynasan 114 is
described as a lipid which can be degraded relatively
rapidly by pancreatic lipase/colipase, and the degradation
of Dynasan 118 takes place more slowly (C. Olbrich, R.H.
Miiller, Proceed. Int. Symp. Controlled Rel. Bioact.
Mater., Stockholm, 921-922, 1997).
The following groups of substances can be used in particu-
lar as excipients:
Fillers from the area of sugars, such as, for example,
disaccharides (lactose, sucrose), monosaccharides (glu
cose, fructose) or polysaccharides (starches, maize or
potato starch, cellulose, naturally occurring cellulose
powder, microcrystalline cellulose), sugar alcohols, such
as, for example, sorbitol or mannitol, or calcium phos
phates.
Binders, such as polyvinylpyrrolidone (PVP, Kollidon CL),
gelatine, starch glue, celluloses, cellulose ethers or
sugars.
According to the invention, it has been found that a
formulation in the form of a polymer-containing/lipid-
containing compound which has an excipient phase with at
least one excipient and/or an active substance phase with
at least one active substance and a polymeric phase/lipid
phase with at least one polymer/lipid, the polymer
CA 02274832 1999-06-11
- 23 -
phase/lipid phase of the formulation being incoherent and
the excipient and/or active substance phase being cohe-
rent, is obtained when the various phases of the formula-
tion are suspended or suspended and dissolved together in
a liquid, the polymer phase/liquid phase being insoluble
in the liquid, and this suspension is then spray dried.
A formulation in which the polymer phase/lipid phase is
free from the excipient and/or active substance phase is
obtained in particular by this procedure.
It is also possible to dry the suspension in a moving bed
or fluidized bed drier. In this procedure, the phases of
the formulation are again suspended or suspended and
dissolved together in a liquid, the polymer phase/lipid
phase being insoluble in the liquid, and this suspension
is then dried in a moving bed or fluidized bed drier.
For carrying out the process according to the invention,
the corresponding amounts of polymer/lipid and excipient
and/or active substance are suspended or suspended and
dissolved in a liquid with the aid of a high-speed stirrer
or a disperser, the polymer/lipid being insoluble in the
liquid but being present as solid particles, in contrast
to the known process with polymer processing. Depending on
the polymer/lipid to be suspended, it should be ensured
that shearing forces and temperatures which are too high
and lead to aggregation or merging of polymer
particles/lipid particles do not arise during the dispers
ing.
The liquid used is, in particular, demineralized water or
an aqueous or organic dispersing or suspending agent.
The particular desired viscosity of the suspension to be
sprayed in the spray drier, moving bed drier or fluidized
CA 02274832 1999-06-11
- 24 -
bed drier is controlled via the percentage solids content.
In the case of water-soluble excipients, additional
possibilities of regulation exist via tra concentration
and chemical nature thereof (e. g. lactose, excipients with
a pronounced viscosity-increasing effect).
A further advantageous embodiment is the addition of
wetting agents and/or binders and/or plasticizers (e. g.
triethyl citrate, propylene glycol and the like) to the
suspension. Particularly suitable binders are
polyvinylpyrrolidone, gelatine, starch glue, cellulose,
cellulose ethers or sugars. They increase the mechanical
resistance of the formulation. The plasticizes allows an
influence, which can be validated, on the plasticity,
deformability and film-forming properties of the poly-
mer/lipid and therefore allows the release of the active
substance to be controlled, in addition to the delayed
action effect of the polymer/lipid per se. Plasticizers
which can be employed are, above all, triethyl citrate and
propylene glycol. However, other internal and external
plasticizers which are known as customary additives to
polymers/lipids are suitable for controlling the release
of the active substance.
The suspension is subsequently spray dried under spray
pressures of usually above 20 bar with the aid of suitable
one- and multicomponent nozzles in a spraying tower at
suitable outlet air temperatures, depending on the sensi-
tivity of the active substances and excipients and on the
apparatus design of the spraying tower and its periphery,
or is dried in a moving bed or fluidized bed drier.
If necessary, the resulting formulation can then also
undergo secondary drying. Secondary drying and/or an
additional agglomeration of the formulation on moving bed
or fluidized bed driers is possible here.
CA 02274832 1999-06-11
- 25 -
On the basis of the drying operation in the spray drier or
moving bed or fluidized bed drier, the resulting formula-
tion has an approximately spherical form.
It has been recognized according to the invention that the
formulation described, which comprises an incoherent
polymer phase/lipid phase and a coherent excipient and/or
active substance phase, is suitable for use in the pre-
paration of relatively large matrix units with controlled
release properties. All the known process can be used for
this, so that larger matrix units of any desired shape are
obtained, such as, for example, tablets, pellets or
cylindrical rods. The known processes for the preparation
of extruded or spheronized pellets or for introducing the
formulation into capsules can also be carried out with the
formulation according to the invention.
It has furthermore been recognized that the formulation
according to the invention is particularly suitable for
the preparation of larger matrix units and/or tablets with
controlled release properties by means of direct compres-
sion. This is possible, in spite of the high polymer
content/lipid content of the formulation, since, inter
alia, a very good flow property and improved compression
properties of the formulation are achieved by the process
according to the invention.
The preparation of tablets by means of direct compression
from a formulation which is free from active substance and
is mixed with at least one active substance and, if
required, with further excipients, and from a formulation
which comprises active substance and, under certain
circumstances, can additionally also be mixed with at
least one active substance and, if necessary, with further
excipients is particularly advantageous.
CA 02274832 1999-06-11
- 26 -
Coated tablets cores, film-coated or jacketed tablets
cores or cylindrical rods in particular are also obtain-
able, in addition to the customary tablets, by direct
tablet-making or direct compression.
The formulation according to the invention can furthermore
be used for the preparation of larger matrix units which
comprise various active substances or the same active
substance in different doses (e. g. layered tablets), each
active substance or each dose having its own point in time
of release which is independent of the other active
substances or doses. For this, a formulation according to
the invention comprising active substance, which can also
additionally comprise at least one excipient, is mixed
with at least one further or the same active substance, if
necessary with the addition of auxiliaries, such as, for
example, fillers, mould release agents or binders. The
mixture is then processed to larger matrix units by means
of direct compression or by other known processes. This is
particularly advantageous in the case of incompatible
active substances, since this procedure leads to a spatial
separation of the active substances in the medicament
form.
By using the formulation according to the invention in a
process for the preparation of larger matrix units,
modifications of the release profile are rendered pos-
sible, since the active substance or substances in the
larger matrix unit are enclosed to different degrees, as
a function of the amount of polymer/amount of lipid, and
are thus released at different speeds.
The use according to the invention of the formulation for
direct compression has the advantage, in particular, that
the active substances and/or excipients are exposed to
moisture for only a very short time due to the drying
CA 02274832 1999-06-11
- 27 -
operation used, compared with the conventional wet granu-
lation, which has previously been necessary as a precursor
to the compression of polymer-containing formulations. In
the drying processes mentioned, the exposure to heat can
be controlled and can even be eliminated if drying is
carried out in a stream of air at room temperature.
The preparation of pellets may be mentioned as an example
of the preparation of larger matrix units by known pro-
cesses. For this, the formulation according to the
invention is extruded with an extruder customary for the
preparation of pellets, with the addition of adequate
excipients, and is converted into beads of pellet size via
a subsequent spheronization. Alternatively, the prepara-
tion can be carried out via a perforated roll compactor
connected to a pelleting container. Possible apparatuses
are a Spheronizer~ and Marumizer'. These pellets can also
be prepared from the formulation described using a pelle-
ting dish.
These pellets, like the formulation itself, can be intro-
duced, for example, into capsules or pressed to larger
units.
The invention is explained in more detail below with the
aid of embodiment examples and figures. All the percentage
data relate to the weight.
Examples
1. Preparation of a lactose-ethvlcellulose formulation
( 50 : 50 ~,:
The two components are dispersed in demineralized water
with the aid of a stirrer. The dispersion is sprayed at a
solids content of up to 40 per cent and a spray pump
pressure of 30-50 bar in a laboratory spraying tower at
CA 02274832 1999-06-11
- 28 -
outlet air temperatures of between 70 and 100 degrees
Celsius.
The result is a readily free-flowing sprayed agglomerate
comprising lactose and ethylcellulose in a particle size
distribution of between 1 and 630 Vim, the main content of
50-80 ~ being between 63 and 400 Vim.
The formulation prepared in this way is distinguished in
particular as being very readily miscible and loadable
with active substance on the basis of its feature that the
polymeric phase is incoherent and the excipient and/or
active substance phase is coherent, and its approximately
spherical form and the nature of its surface (cavities,
hollows).
In the case of lipophilic active substances, the duration
of release of the active substances can be prolonged by a
factor of up to three compared with non-retarded tablets
by direct mixing with the formulation. The duration of the
release can in turn be varied by changing the polymer
content in the tablet-making composition, e.g. by admixing
a filler of the starch and lactose type.
2. Preparation of a lactose-ethylcellulose formulation
comprising acetylsalicylic acid ~ASA~~:
The preparation is carried out as described in 1., and the
components lactose:ethy1ce11ulose:ASA are mixed in a
weight ratio of 45:45:10.
CA 02274832 1999-06-11
- 29 -
3. Preparation of a tablet from a formulation comprising
acetylsalicylic acid (ASA):
The lactose-ethylcellulose formulation prepared under 1.,
which is free from active substance, is mixed with ASA in
ratio of 90:10, 0.5 % Aerosil and 1 o magnesium stearate
are added to the mixture and the mixture is subjected to
direct compression.
4. Preparation of a tablet from a formulation comprising
acetylsalicylic acid (ASA)~:
0.5 % Aerosil and 1 % magnesium stearate are added to the
ASA-loaded lactose-ethylcellulose formulation prepared
under 2. and the mixture is subjected to direct compres-
sion.
5. Preparation of a paracetamol-lactose-ethylcellulose
formulation (20:40:40:
All the components are dispersed in demineralized water
and the dispersion is adjusted to a desired viscosity,
depending on the pumping and pressure. Spraying is carried
out by the processes described above. On the basis of
their powder properties, the formulation prepared in this
way is suitable directly for direct compression, it being
possible for the release of the active substance to be
delayed to the desired extent by the variably adjustable
percentage content of polymer - by admixing further
excipients, variable tablet hardness.
6. Preparation of a Compritol-trehalose compound:
Compritol 888 ATO (glycerol tribehenate) was melted,
poured into hot water, after addition of 1.2 o Poloxamer
188, and dispersed therein by means of a high-speed Ultra-
CA 02274832 1999-06-11
- 30 -
Turrax. After cooling, trehalose was dissolved in the
aqueous lipid particle dispersion, so that 10 % lipid and
3 o trehalose resulted as the final concentration. This
mixture was spray dried in a Mini-Buchi ( inlet temperature
. 110°C, outlet temperature: 50°C; spray flow: 600 normal
litres). A free-flowing lipid-excipient compound was
obtained.
7. Preparation of a tablet from a compound with 1 ~
paracetamol:
9 parts of the lipid-trehalose compound described in
example 6 were subjected to direct compression on a Korsch
eccentric press with the addition of 0.1 part paracetamol
and with admixing of 0.5 o Aerosil 200 and 0.5 % magnesium
stearate. Tablet nominal weight 505 mg.
8. Preparation of a tablet from a compound with 10 $
~aracetamol:
13 parts of the lipid-trehalose compound described in
example 6 were mixed with 3 parts of trehalose, 10
paracetamol was added to this mixture and the mixture was
subjected to direct compression on a Korsch eccentric
press with admixing of 0.5 o Aerosil 200 and 0.5 % mag
nesium stearate. Tablet nominal weight 505 mg.
9. Release from a tablet from a compound with 10 ~
paracetamol:
The release of paracetamol from the tablet prepared in
example 8 was determined with the paddle method in accor-
dance with the United States Pharmacopoeia, release
medium: water, temperature 37°C. The resulting release
curves are shown in figures 5 and 6.
CA 02274832 1999-06-11
- 31 -
Brief explanation of the figures:
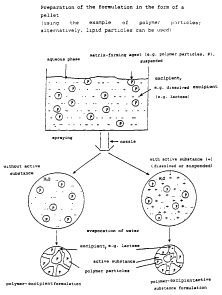
Figure 1:
Figure 1 shows the preparation of a formulation according
to the invention via a compound by the process according
to the invention: The matrix-forming agent (e. g. polymer
particles/lipid particles) is dispersed in water, the
excipient and/or active substance is likewise dissolved or
dispersed in the aqueous phase and the suspension is
sprayed, the water being removed by drying. A formulation
which itself is composed of small polymer particles/lipid
particles is formed, the intermediate spaces being filled
with the excipient (left) or with excipient and active
substance (right). The formulation has an incoherent
polymeric/lipid phase and a coherent excipient and/or
active substance phase.
Figure 2:
Figure 2 shows an example of the use of the formulation
according to the invention for the preparation of larger
matrix units. The formulation (e.g. of polymer and lactose
or of lipid and Flowlac 100 - spray-dried lactose, Meggle,
Germany), which is free from active substance, is mixed
with the active substance (in powder form), if necessary
tablet compression excipients are added and the mixture is
subjected to direct compression, e.g. to a tablet.
Figure 3a:
0/W emulsion process known from the prior art: Here, a
drop of an organic solvent with matrix-forming agent (e. g.
polymer) dissolved therein is dispersed in an aqueous
phase (O/W emulsion), the active substance being dissolved
(left) or, in the case of insoluble active substance,
dispersed (right) in the organic phase. For further
explanation, see the text.
CA 02274832 1999-06-11
- 32 -
Figure 3b:
W/0 emulsion process known from the prior art: Here, a
drop of water with matrix-forming agent (e. g. water-
soluble polymer) dissolved therein is dispersed in an
organic phase (0/W emulsion), the active substance being
dissolved (left) or, in the case of insoluble active
substance, dispersed (right) in the aqueous phase. For
further explanation, see the text.
Figure 4:
Figure 4 shows the process according to the invention for
the preparation of the formulation according to the
invention. The polymeric phase/lipid phase is not dis-
solved, but dispersed or suspended in drops of water,
which are distributed in a gas phase by spraying. An
excipient (e.g. lactose, left) or an excipient and an
active substance (right) are also dissolved or dispersed
or suspended in the drop of water. After removal of the
water, an excipient-polymer/lipid formulation which is
free from active substance (left) or an active substance-
excipient-polymer/lipid formulation (right) is formed, the
polymeric phase/lipid phase being incoherent in both
cases.
Figures 5 and 6:
Release of paracetamol from a tablet using the formulation
according to the invention (example 4). Plot of the amount
released as a function of time (figure 5) and as a func-
tion of the square root of the time (figure 6).