Note: Descriptions are shown in the official language in which they were submitted.
CA 02275278 1999-06-17
DESCRIPTION
TITLE OF THE INVENTION
Novel Resin Curing Method Enabling the Energy Radiation
Curing of Resins Containing an Energy Radiation Screening
Substance, Compositions, Molded Articles and Molding Methods
TECHNICAL FIELD
The present invention relates to a resin curing method
for curing resins rapidly by means of energy radiation such
as ultraviolet radiation (UV), electron beam (EB), infrared
radiation, X-rays, visible light, light from lasers (e.g.,
argon, CO2 and excimer lasers), sunlight and radiated heat
rays, and other energy such as heat, and to polymerization
initiators and compositions which enable resins to be cured
by this method, molded articles made therefrom, and their
production methods and apparatus.
More particularly, the present invention relates to a
resin curing method for thick-walled resins in which energy
radiation is attenuated or absorbed by the resin to cause a
marked reduction in curing action and, therefore, the deep
part thereof cannot be cured, and for resins reinforced with
fillers (e.g., carbon fiber, metallic fibers and glass fiber)
or metallic inserts in which energy radiation is screened by
such reinforcing materials and, therefore, the shaded part of
the resin cannot be completely cured, and to polymerization
initiators and compositions which enable such resins to be
-1-
CA 02275278 1999-06-17
cured by this method, molded articles made therefrom, and
their production methods and apparatus.
The present invention also relates to a novel resin
curing method which enables the energy radiation curing of
resin systems containing substances highly capable of
screening energy radiation (e.g., carbon, carbon fiber (CF),
metals and other inorganic fillers), such as carbon
fiber-reinforced composite materials (CFRPs) and
carbon/metal/inorganic matter-containing resins, and to
compositions therefor, molded articles made therefrom, and
their production methods.
The present invention also relates to a method of making
a fiber-reinforced composite material (FRP) wherein a
composition capable of inducing the novel resin curing
mechanism in the aforesaid resin curing method is used as the
matrix resin, and a reinforcing fiber (e.g., CF) is
impregnated with this resin and then cured by means of energy
radiation typified by UV.
The aforesaid curing method, compositions, molded
articles and molding methods are effective without regard to
the UV screening properties of fibers or fillers, and the
length, size and shape thereof. Moreover, they can be
utilized not only in the field of composite materials, but
also in the fields of adhesives, sealers, varnishes, paints,
coating materials, inks, toners and the like.
-2-
CA 02275278 1999-06-17
BACKGROUND ART
In recent years, energy radiation-curable resins
typified by UV-curable resins are being used in various
fields and applications. These resins are characterized in
that they are cured only in regions exposed to more than a
certain amount of energy radiation. On the other hand,
energy radiation typified by UV radiation is characterized in
that it is attenuated while it passes through a resin. As a
result, it is difficult for energy radiation to reach the
deep part of a resin, or energy radiation is greatly
attenuated or absorbed, for example, in the presence of a
substance capable of absorbing a wavelength equal to that of
the energy radiation. Thus, photo-curable.resins are cured
only in a surface layer having a thickness of several
micrometers to several millimeters through which energy
radiation can pass, and the deep part thereof remain uncured.
Consequently, it is difficult or impossible to apply
photo-curable resins to thick-walled materials. Moreover, in
the case of resins containing fillers and other substances
hindering the passage of energy radiation, they tend to
undergo inhibition of their cure and hence become incurable.
Owing to these problems, their application has been chiefly
limited to the fields of photoresists, coatings, paints,
adhesives, varnishes and the like.
Typical solutions to these problems are offered by
-3-
CA 02275278 1999-06-17
highly UV-curable resins (Mitsubishi Rayon Co., Ltd.; active
energy radiation-curable compositions; Japanese Patent
Provisional Publication No. 8-283388/'96) and UV- and
heat-cocurable resins {Optomer KS series (Asahi Denka Kogyo
K.K.); Radicure (Hitachi Chemical Co., Ltd.); UE resin
(Toyobo Co., Ltd.); Japanese Patent Publication (JP-A) No. 6-
38023/'86; and the like}. However, highly UV-curable resins
still have the problem that they cannot be cured when energy
radiation is blocked by a filler or the like. Moreover, in
UV- and heat-cocurable resins which are cured by heating after exposure to UV
radiation, their energy radiation
curability is similar to that of conventional photo-curable
resins, and the problems associated with the curing of
thick-walled resins and filler-containing resins remain
entirely unsolved. Thus, under the existing circumstances,
these problems cannot be solved and are hence coped with by
heat curing subsequent to photo-curing (of a surface layer
alone).
If a technique by which the above-described thick-walled
resins containing an energy radiation screening substance or
highly capable of attenuating or absorbing energy radiation
can be cured rapidly is established, this technique may be
applied not only to conventional fields of application, but
also to various other fields in which the application of
energy radiation curing has been impossible owing to the
-4-
CA 02275278 2004-05-19
problems of photo-curable resins. One example thereof is
application to the matrix resins of FRPs, particularly
CFRPs.
Conventionally, a variety of processing techniques
and production processes are employed for FRPs, but the
matrix resin consists of a thermosetting or thermoplastic
resin in most cases. The problems associated with the
molding of FRPs, particularly CFRPs, are that high
processing costs are involved because a long curing time
results from complicated temperature control, a large-
sized heating oven is required for the curing of large-
sized FRPs, resins capable of being cured at room
temperature in a short period of time cannot be used for
large-sized FRPs requiring a long curing time, the
molding of FRPs is difficult because the resin-
impregnated state varies according to changes in resin
viscosity with temperature, and the formation of voids
arises from residual solvent during the curing of the
resin and causes a reduction in the quality of the molded
article.
Recently, as a solution to these problems, the
utilization of a photo-curable resin as the matrix resin
is attracting attention. A particular and typical
example of this matrix resin curing method is the
filament winding molding process of Loctite Corp. which
uses a combination of UV curing and heat curing (Loctite
Corp.; Fiber/resin composition and its preparation
method; Published PCT International Patent Application
No. WO 94/21455).
-5-
CA 02275278 2004-05-19
However, in the FRP molding process using such a
composition, an uncured resin-impregnated FRP is
irradiated with UV radiation so as to cure its surface
and so as thicken (or gelatinize) its inner part
extremely and thereby enable the maintenance of its shape
and resin-impregnated state to some extent, and then
heated to achieve a complete cure. Accordingly, as
compared with the conventional production process using a
thermoplastic or thermosetting resin, the change in resin
viscosity with temperature is very slight and the
handling of the FRP after resin impregnation is easy, but
a heat curing step is still required in order to achieve
a complete cure. Thus, the problem of high processing
costs arising from the fuel and light expenses and
operating time required for heat curing, the problem of a
long curing time, and the need for a large-sized heating
oven in the molding of large-sized FRPs remain unsolved.
DISCLOSURE OF THE INVENTION
In view of the above-described disadvantages of
conventional resins cured by energy radiation and the
above-described disadvantages of FRPs, particularly
CFRPs, the present inventors made intensive
investigations on the energy radiation curing of thick-
walled resins containing an energy radiation screening
substance (i.e., a substance capable of screening energy
radiation) and the energy radiation curing of FRPs,
particularly CFRPs, and have now
-6-
. CA 02275278 2002-09-20
attained the present invention. An object of an aspect
of the present invention is to provide a novel resin
curing method which enables the energy radiation curing
of resin systems containing substances highly capable of
screening energy radiation {e.g., carbon, carbon fiber
(CF), metals and other inorganic fillers}, such as carbon
fiber-reinforced composite materials (CFRPs) and
carbon/metal/inorganic matter-containing resins, as well
as compositions therefor, molded articles made therefrom,
and molding methods thereof.
Another object of an aspect of the present invention
is to incorporate a specific photopolymerization
initiator (reaction catalyst system) comprising at least
two components (i.e., a system comprising two or more
components) into a resin composition highly capable of
screening energy radiation, such as a carbon fiber-
reinforced composite material (CFRP), whereby even the
shaded part or deep part of the resin composition can be
completely cured solely by exposure to energy radiation
such as W or EB.
The above objects of aspects of the invention can be
effectively accomplished by various inventions summarized
below.
In accordance with one embodiment of the present
invention, there is provided a resin curing method
wherein, when a first type of energy is applied from an
external energy source to a resin composition, a second
-7-
CA 02275278 2002-09-20
type of energy other than the first type of energy from
the external energy source is autogenously generated
within the resin composition, so that the resin
composition is cured by means of the autogenously
generated energy, or by both the autogenously generated
energy and the first type of energy from the external
energy source, whether or not the resin composition
contains a substance capable of screening the first type
of energy from the external energy source.
In accordance with another embodiment of the present
invention, there is provided a resin curing method
wherein, when a first type of energy is applied from an
external energy source to a resin composition, a second
type of energy other than the first type of energy from
the external energy source is autogenously generated
within the resin composition, and a third type of energy
is successively generated by the autogenously generated
second type of energy, so that the resin composition is
cured by means of the second and third types of energies,
or by both the second and third types of energies and the
first type of energy from the external energy source,
whether or not the resin composition contains a substance
capable of screening the first type of energy from the
external energy source.
In accordance with another embodiment of the present
invention, there is provided a resin curing method
wherein, when a resin composition is exposed to a first
-7a-
CA 02275278 2002-09-20
type of energy from an external energy radiation source,
a second type of energy other than the first type of
energy from the external energy radiation source is
autogenously generated within the resin composition, so
that the resin composition is cured by means of the
autogenously generated energy, or by both the
autogenously generated energy and the first type of
energy from the external energy radiation source, whether
or not the resin composition contains a substance capable
of screening energy radiation.
In accordance with another embodiment of the present
invention, there is provided a resin curing method
wherein, when a resin composition is exposed to a first
type of energy from an external energy radiation source,
a second type of energy other than the first type of
energy from the external energy radiation source is
autogenously generated within the resin composition, and
a third type of energy is successively generated by the
autogenously generated second type of energy, so that the
resin composition is cured by means of the second and
third types of energies, or by both the second and third
types of energies and the first type of energy from the
external energy radiation source, whether or not the
resin composition contains a substance capable of
screening energy radiation.
In accordance with another embodiment of the present
invention, there is provided a photopolymerization
-7b-
CA 02275278 2002-09-20
initiator composition comprising at least two components
comprising a photopolymerization initiator and a photo-
and thermopolymerization initiator for initiating
polymerization by means of both light and heat, and
serving as the polymerization initiator making it
possible to carry out a resin curing method as described
above.
In accordance with another embodiment of the present
invention, there is provided a resin composition wherein,
when the resin composition is exposed to a first type of
energy from an external energy radiation source, a second
type of energy other than the first type of energy from
the external energy radiation source is autogenously
generated within the resin composition, so that the resin
composition is cured by means of the autogenously
generated energy, or by both the autogenously generated
energy and the first type of energy from the external
energy radiation source, whether or not the resin
composition contains a substance capable of screening
energy radiation, or wherein, when the resin composition
is exposed to a first type of energy from an external
energy radiation source, a second type of energy other
than the first type of energy from the external energy
radiation source is autogenously generated within the
resin composition, and a third type of energy is
successively generated by the autogenously generated
energy, so that the resin composition is cured by means
-7c-
CA 02275278 2002-09-20
of the second and third types of energies, or by both the
second and third types of energies and the first type of
energy from the external energy radiation source, whether
or not the resin composition contains a substance capable
of screening energy radiation.
In accordance with another embodiment of the present
invention, there is provided a resin composition
comprising a photopolymerization initiator composition
comprising at least two components comprising a
photopolymerization initiator and a photo- and
thermopolymerization initiator for initiating
polymerization by means of both light and heat, and
wherein, when the resin composition is exposed to a first
type of energy from an external energy radiation source,
a second type of energy other than the first type of
energy from the external energy radiation source is
autogenously generated within the resin composition, so
that the resin composition is cured by means of the
autogenously generated energy, or by both the
autogenously generated energy and the first type of
energy from the external energy radiation source, whether
or not the resin composition contains a substance capable
of screening energy radiation, or wherein, when the resin
composition is exposed to a first type of energy from an
external energy radiation source, a second type of energy
other than the first type of energy from the external
energy radiation source is autogenously generated within
-7d-
CA 02275278 2002-09-20
the resin composition, and a third type of energy is
successively generated by the autogenously generated
energy, so that the resin composition is cured by means
of the second and third types of energies, or by both the
second and third types.of energies and the first type of
energy from the external energy radiation source, whether
or not the resin composition contains a substance capable
of screening energy radiation.
In accordance with another embodiment of the present
invention, there is provided a cured product obtained by
a resin curing method as described above.
In accordance with another embodiment of the present
invention, there is provided a molded article made from a
resin composition containing a photopolymerization
initiator composition comprising at least two components
as described above.
In accordance with another embodiment of the present
invention, there is provided a method of making a fiber-
reinforced composite material wherein, when a resin
composition infiltrated into a three-dimensional textile
is exposed to a first type of energy from an external
energy radiation source, a second type of energy other
than the first type of energy from the external energy
radiation source is autogenously generated within the
resin composition, so that the resin composition is cured
by means of the autogenously generated energy, or by both
the autogenously generated energy and the first type of
-7e-
CA 02275278 2003-05-27
energy from the external energy radiation source, whether
or not the resin composition contains a substance capable
of screening energy radiation, or wherein, when the resin
composition is exposed to a first type of energy from an
external energy radiation source, a second type of energy
other than the first type of energy from the external
energy radiation source is autogenously generated within
the resin composition, and a third type of energy is
successively generated by the autogenously generated
energy, so that the resin composition is cured by means
of the second and third types of energies, or by means of
both the second and third types of energies and the first
type of energy from the external energy radiation source,
whether or not the resin composition contains a substance
capable of screening energy radiation.
In accordance with another embodiment of the present
invention, there is provided a method of repairing a
fiber-reinforced object selected from the group
consisting of a composite material, a building, a
structure and a product, wherein, when a resin
composition used to fill a part of the object to be
repaired is exposed to a first type of energy from an
external energy radiation source, a second type of energy
other than the first type of energy from the external
energy radiation source is autogenously generated within
the resin composition, so that the resin composition is
cured by means of the autogenously generated energy, or
by both the autogenously generated energy and the first
7f
CA 02275278 2005-04-13
type of energy from the external energy radiation source,
whether or not the resin composition contains a substance
capable of screening energy radiation, or wherein, when
the resin composition is exposed to a first type of
energy from an external energy radiation source, a second
type of energy other than the first type of energy from
the external energy radiation source is autogenously
generated within the resin composition, and a third type
of energy is successively generated by the autogenously
generated energy, so that the resin composition is cured
by means of the second and third types of energies, or by
both the second and third types of energies and the first
type of energy from the external energy radiation source,
whether or not the resin composition contains a substance
capable of screening energy radiation.
According to an aspect of the present invention,
there is provided a resin curing method wherein, when
light energy is applied from an external energy source to
a resin composition, heat energy is autogenously
generated within the resin composition, so that the resin
composition is cured by means of the autogenously
generated heat energy, or both the autogenously generated
heat energy and the light energy from the external energy
source, whether or not the resin composition contains a
substance capable of screening the light energy from the
external energy source, wherein the resin composition
contains a photopolymerizable oligomer or
photopolymerizable monomer, and a photopolymerization
initiator composition comprising at least two components
comprising a photopolymerization initiator and a photo-
and thermopolymerization initiator for initiating
polymerization by means of both light and heat, wherein
the photopolymerization initiator comprises at least one
7g
CA 02275278 2005-04-13
compound selected from the group consisting of a
diazonium salt type compound, an iodonium salt type
compound, a pyridinium salt type compound, a phosphonium
salt type compound, a sulfonium salt type compound, an
iron-arene complex type compound, and a sulfonate type
compound, and the photo- and thermopolymerization
initiator comprises at least one of the sulfonium salts
represented by the following general formulae (I) to
(VII)
R2 R4
~CHa -cr
A (z)
R3
where R1 represents hydrogen, methyl, acetyl or
methoxycarbonyl, R2 and R3 each independently represent
hydrogen, halogen or an alkyl group of 1 to 4 carbon
atoms, R4 represents hydrogen, halogen or methoxy, R5
represents an alkyl group of 1 to 4 carbon atoms, and A
represents SbF6, PF6, AsF6 or BF4
CH3
CHZ S /
Re Q
OR7
CH3
CH2 +S/ (II' )
=A-
Rs O
OR7
where R6 represents a hydrogen atom, a halogen atom, a
nitro group or a methyl group, R' represents a hydrogen
atom, CH3CO or CH30C0, and A represents SbF6, PF6, BF6 or
AsF6
7h
CA 02275278 2005-04-13
CH3
CH3--'S~ (III)
O A
oRB
where R8 represents a hydrogen atom, CH3CO or CH3OCO, and
A represents SbF6, PF6, BF6, AsF6 or CH3SO4
X Q S n Y. mZe ( Iv)
where X represents a sulfonio group of the general
formula
9
R (a)
in which R9 represents an aliphatic group of 1 to 18
carbon atoms, R10 represents an aliphatic group of 1 to 18
carbon atoms or a substituted or unsubstituted aromatic
group of 6 to 18 carbon atoms, and R9 and R10 may be
joined together to form a ring; Y represents a sulfonio
group of the general formula
Rit
Rt2/S (b)
in which R11 represents an aliphatic group of 1 to 18
carbon atoms, R12 represents an aliphatic group of 1 to 18
carbon atoms or a substituted or unsubstituted aromatic
group of 6 to 18 carbon atoms, and R11 and R12 may be
joined together to form a ring, or Y represents a
7i
CA 02275278 2005-04-13
hydrogen atom, a halogen atom, a nitro group, an alkoxy
group, an aliphatic group of 1 to 18 carbon atoms, or a
substituted or unsubstituted phenyl, phenoxy or
thiophenoxy group of 6 to 18 carbon atoms; n and m are
each independently 1 or 2; and Z is an anion represented
by the formula MQ1(e1) or MQl(el)-lOH in which M is B, P, As
or Sb, Q is a halogen atom, and l(el) is 4 or 6
R13 /CHZ-@
H O-'S+\CHZ~ = A (V)
R14
where R13 and R14 each independently represent hydrogen or
an alkyl group of 1 to 4 carbon atoms, and A represents
SbF6, PF6 or AsF6
a A~s Rte
R154 Q S+/CH2'1~
\ Rts q- (VI)
Rt~
where R15 represents ethoxy, phenyl, phenoxy, benzyloxy,
chloromethyl, dichloromethyl, trichloromethyl or
trifluoromethyl, R16 and R17 each independently represent
hydrogen, halogen or an alkyl group of 1 to 4 carbon
atoms, R18 represents hydrogen, methyl, methoxy or
halogen, R19 represents an alkyl group of 1 to 4 carbon
atoms, and A represents SbF6, PF6, BF4 or AsF6
R 20 22
Q~S+ R p A- (vzY)
'
R 2'
7j
CA 02275278 2005-04-13
where Q represents methoxycarbonyloxy, acetoxy,
benzyloxycarbonyloxy or dimethylamino, R20 and R21 each
independently represent hydrogen or an alkyl group of 1
to 4 carbon atoms, R22 and R23 each independently
represent an alkyl group of 1 to 4 carbon atoms, and A
represents SbF6, PF6, AsF6 or BF4.
According to another aspect of the present
invention, there is provided a resin curing method
wherein, when light energy is applied from an external
energy source to a resin composition, a first heat energy
is autogenously generated within the resin composition,
and a second heat energy is successively generated by the
autogenously generated first heat energy, so that the
resin composition is cured by means of the first and
second heat energies, or by both the first and second
heat energies and the light energy from the external
energy source, whether or not the resin composition
contains a substance capable of screening the light
energy from the external energy source, wherein the resin
composition contains a photopolymerizable oligomer or
photopolymerizable monomer, and a photopolymerization
initiator composition comprising at least two components
comprising a photopolymerization initiator and a photo-
and thermopolymerization initiator for initiating
polymerization by means of both light and heat, wherein
the photopolymerization initiator comprises at least one
compound selected from the group consisting of a
diazonium salt type compound, an iodonium salt type
compound, a pyridinium salt type compound, a phosphonium
salt type compound, a sulfonium salt type compound, an
iron-arene complex type compound, and a sulfonate type
compound, and the photo- and.thermopolymerization
initiator comprises at least one of the sulfonium salts
7k
CA 02275278 2005-04-13
represented by the following general formulae (I) to
(VII)
R2 R4
~.CH2
R~U
-0 g ~'R5 q- (I)
3
where R' represents hydrogen, methyl, acetyl or
methoxycarbonyl, R2 and R3 each independently represent
hydrogen, halogen or an alkyl group of 1 to 4 carbon
atoms, R 4 represents hydrogeri, halogen or methoxy, R5
represents an alkyl group of 1 to 4 carbon atoms, and A
represents SbF6, PF6, AsF6 or BF4
/ CH3
CHZ S = q- ( I I )
Re
OR7
CH3
CH2 +S/ (II' )
=A-
RB Q
OR~
where R6 represents a hydrogen atom, a halogen atom, a
nitro group or a methyl group, R7 represents a hydrogen
atom, CH3CO or CH3OCO, and A represents SbF6i PF6, BF6 or
As F6
CH3
CH3 'S/
A_ (III}
QRB
where R8 represents a hydrogen atom, CH3CO or CH3OCO, and
71
CA 02275278 2005-04-13
A represents SbF6, PF6, BF6, AsF6 or CH3SO4
X O S ---~ n 1r . mZe (IV)
where X represents a sulfonio group of the general
formula
R9 (a)
R10""S
in which R9 represents an aliphatic group of 1 to 18
carbon atoms, R10 represents an aliphatic group of 1 to 18
carbon atoms or a substituted or unsubstituted aromatic
group of 6 to 18 carbon atoms, and R9 and R10 may be
joined together to form a ring; Y represents a sulfonio
group of the general formula
Rit
Rt2/S (b)
in which R11 represents an aliphatic group of 1 to 18
carbon atoms, R12 represents an aliphatic group of 1 to 18
carbon atoms or a substituted or unsubstituted aromatic
group of 6 to 18 carbon atoms, and R" and R12 may be
joined together to form a ring, or Y represents a
hydrogen atom, a halogen atom, a nitro group, an alkoxy
group, an aliphatic group of 1 to 18 carbon atoms, or a
substituted or unsubstituted phenyl, phenoxy or
thiophenoxy group of 6 to 18 carbon atoms; n and m are
each independently 1 or 2; and Z is an anion represented
by the formula MQ1(el) or MQ1(ei)-10H in which M is B, P, As
or Sb, Q is a halogen atom, and l(el) is 4 or 6
7m
CA 02275278 2005-04-13
R13
/CHZ~
H O-~'S+
. A (V)
R14
where R13 and R 14 each independently represent hydrogen or
an alkyl group of 1 to 4 carbon atoms, and A represents
SbF6, PF6 or AsF6
ts R~e
R15~ 0 S+/CH2~
~ ts A- (VI)
R17 R
where R15 represents ethoxy, phenyl, phenoxy, benzyloxy,
chioromethyl, dichloromethyl, trichloromethyl or
trifluoromethyl, R16 and R17 each independently represent
hydrogen, halogen or an alkyl group of 1 to 4 carbon
atoms, R18 represents hydrogen, methyl, methoxy or
halogen, R19 represents an alkyl group of 1 to 4 carbon
atoms, and A represents SbF6, PF6, BF4 or AsF6
R20 R 22
0õf~S ~ Rz3 , A- (VII )
~'J
R21
where Q represents methoxycarbonyloxy, acetoxy,
benzyloxycarbonyloxy or dimethylamino, R20 and R21 each
independently represent hydrogen or an alkyl group of 1
to 4 carbon atoms, R22 and R23 each independently
represent an alkyl group of 1 to 4 carbon atoms, and A
represents SbF6r PF6, AsF6 or BF9.
7n
CA 02275278 2005-04-13
According to a further aspect of the present
invention, there is provided a photopolymerization
initiator composition comprising at least two components
comprising a photopolymerization initiator and a photo-
and thermopolymerization initiator for initiating
polymerization by means of both light and heat, wherein
the photopolymerization initiator comprises at least one
compound selected from the group consisting of a
diazonium salt type compound, an iodonium salt type
compound, a pyridinium salt type compound, a phosphonium
salt type compound, a sulfonium salt type compound, an
iron-arene complex type compound, and a sulfonate type
compound, and the photo- and thermopolymerization
initiator comprises at least,one of the sulfonium salts
represented by the following general formulae (I) to
(VII)
R2 R4
CHZ
R~O 5 ~fl$
3
where R' represents hydrogen, methyl, acetyl or
methoxycarbonyl, R2 and R3 each independently represent
hydrogen, halogen or an alkyl group of 1 to 4 carbon
atoms, R 4 represents hydrogen., halogen or methoxy, R5
represents an alkyl group of 1 to 4 carbon atoms, and A
represents SbF6, PF6, AsF6 or BF4
7o
CA 02275278 2005-04-13
/~CH3
CH2 S .q- (II)
Re Q
OR7
CH3
. A
CH2 +S/ (II' )
R O
Oa'
where R6 represents a hydrogen atom, a halogen atom, a
nitro group or a methyl group, R' represents a hydrogen
atom, CH3CO or CH3OCO, and A represents SbF6, PF6, BF6 or
AsF6
CH3
CH3--+S/ (III)
O A
ORB
where R 8 represents a hydrogen atom, CH3CO or CH30C0, and
A represents SbF6, PF6, BF6, AsF6 or CH3SO4
X O g O n Y. mZe (IV)
where X represents a sulfonio group of the general
formula
9
R~. (a)
RtO,.'-O* S
in which R9 represents an aliphatic group of 1 to 18
7p
CA 02275278 2005-04-13
carbon atoms, R10 represents an aliphatic group of 1 to 18
carbon atoms or a substituted or unsubstituted aromatic
group of 6 to 18 carbon atoms, and R9 and R10 may be
joined together to form a ring; Y represents a sulfonio
group of the general formula
Rit
R12/ (b)
in which R11 represents an aliphatic group of 1 to 18
carbon atoms, R12 represents an aliphatic group of 1 to 18
carbon atoms or a substituted or unsubstituted aromatic
group of 6 to 18 carbon atoms, and R" and R12 may be
joined together to form a ring, or Y represents a
hydrogen atom, a halogen atom, a nitro group, an alkoxy
group, an aliphatic group of 1 to 18 carbon atoms, or a
substituted or unsubstituted phenyl, phenoxy or
thiophenoxy group of 6 to 18 carbon atoms; n and m are
each independently 1 or 2; and Z is an anion represented
by the formula MQ1(el) or MQl(el)_10H in which M is B, P, As
or Sb, Q is a halogen atom, and l(el) is 4 or 6
_(q~1j3~.. /CH2~
H O '~ S+--'CH2-~ A (V)
04 20 where R13 and R19 each independently represent hydrogen or
an alkyl group of 1 to 4 carbon atoms, and A represents
SbF6, PF6 or AsF6
p R~s Rta
R15G~ Q--r-
'%-% A' (VI)
R17
7q
CA 02275278 2005-04-13
where R15 represents ethoxy, phenyl, phenoxy, benzyloxy,
chloromethyl, dichloromethyl, trichloromethyl or
trifluoromethyl, R16 and R17 each independently represent
hydrogen, halogen or an alkyl group of 1 to 4 carbon
atoms, R18 represents hydrogen, methyl, methoxy or
halogen, R19 represents an alkyl group of 1 to 4 carbon
atoms, and A represents SbF6, PF6, BF4 or AsF6
R20 22
Q ~S+~ A- (VII)
\RR21
where Q represents methoxycarbonyloxy, acetoxy,
benzyloxycarbonyloxy or dimethylamino, R20 and R21 each
independently represent hydrogen or an alkyl group of 1
to 4 carbon atoms, R22 and R23 each independently
represent an alkyl group of 1 to 4 carbon atoms, and A
represents SbF6, PF6, AsF6 or BF9.
According to another aspect of the present
invention, there is provided a resin composition
comprising a photopolymerizable oligomer or
photopolymerizable monomer, and a photopolymerization
initiator composition comprising at least two components
comprising a photopolymerization initiator and a photo-
and thermopolymerization initiator for initiating
polymerization by means of both light and heat, wherein
the photopolymerization initiator comprises at least one
compound selected from the group consisting of a
diazonium salt type compound, an iodonium salt type
compound, a pyridinium salt type compound, a phosphonium
salt type compound, a sulfonium salt type compound, an
iron-arene complex type compound, and a sulfonate type
compound, and the photo- and-thermopolymerization
7r
CA 02275278 2005-04-13
initiator comprises at least one of the sulfonium salts
represented by the following general formulae (I) to
(VII)
~ R4
CH2
R S =\'fls = A- (I)
3
where R1 represents hydrogen, methyl, acetyl or
methoxycarbonyl, R2 and R3 each independently represent
hydrogen, halogen or an alkyl group of 1 to 4 carbon
atoms, R 4 represents hydrogen, halogen or methoxy, R5
represents an alkyl group of 1 to 4 carbon atoms, and A
represents SbF6, PF6, AsF6 or BF4
/ CH3
CH2 S =A- (II)
RB
OR7
CH3
CH2 +S/ (II ~ )
=A
Rs 1O
oR'
where R6 represents a hydrogen atom, a halogen atom, a
nitro group or a methyl group, R' represents a hydrogen
atom, CH3CO or CH3OCO, and A represents SbF6, PF6, BF6 or
AsF6
CH3
CN3 ---+
=a_ (III~
ORB
where R8 represents a hydrogen atom, CH3CO or CH30CO, and
7s
CA 02275278 2005-04-13
A represents SbF6, PF6, BF6, AsF6 or CH3SO4
X O S n Y= mZe ( Iv)
where X represents a sulfonio group of the general
formula
R9
~= (a)
Rio/S
in which R9 represents an aliphatic group of 1 to 18
carbon atoms, R10 represents an aliphatic group of 1 to 18
carbon atoms or a substituted or unsubstituted aromatic
group of 6 to 18 carbon atoms, and R9 and R10 may be
joined together to form a ring; Y represents a sulfonio
group of the general formula
Rtt
R(b)
in which R" represents an aliphatic group of 1 to 18
carbon atoms, R12 represents an aliphatic group of 1 to 18
carbon atoms or a substituted or unsubstituted aromatic
group of 6 to 18 carbon atoms, and R" and R12 may be
joined together to form a ring, or Y represents a
hydrogen atom, a halogen atom, a nitro group, an alkoxy
group, an aliphatic group of 1 to 18 carbon atoms, or a
substituted or unsubstituted phenyl, phenoxy or
thiophenoxy group of 6 to 18 carbon atoms; n and m are
each independently 1 or 2; and Z is an anion represented
by the formula MQ1(e1) or MQ1(e1)-ZOH in which M is B, P, As
or Sb, Q is a halogen atom, and l(el) is 4 or 6
7t
CA 02275278 2005-04-13
R13 +~CHZ~
H O--S = A (V)
R14
where R13 and R19 each independently represent hydrogen or
an alkyl group of 1 to 4 carbon atoms, and A represents
SbF6, PF6 or AsF6
q R~e Rls
R~~~
~ R~~ = A' (vz )
Rt7
where R15 represents ethoxy, phenyl, phenoxy, benzyloxy,
chloromethyl, dichloromethyl, trichloromethyl or
trifluoromethyl, R16 and R17 each independently represent
hydrogen, halogen or an alkyl group of 1 to 4 carbon
atoms, Rla represents hydrogen, methyl, methoxy or
halogen, R19 represents an alkyl group of 1 to 4 carbon
atoms, and A represents SbF6, PF6, BF4 or AsF6
R20 22
-.-S+/ , A (VII)
~ Rx~
R2t
where Q represents methoxycarbonyloxy, acetoxy,
benzyloxycarbonyloxy or dimethylamino, R20 and R21 each
independently represent hydrogen or an alkyl group of 1
to 4 carbon atoms, R22 and R23 each independently
represent an alkyl group of 1 to 4 carbon atoms, and A
represents SbF6, PF6, AsF6 or BF4.
7u
CA 02275278 2005-04-13
(1) A resin curing method wherein, when energy is
applied to a resin composition, another kind of energy
than the energy from an external energy source is
autogenously generated within the resin, so that the
resin composition is cured by means of the autogenously
generated energy, or both
7v
CA 02275278 1999-06-17
the autogenously generated energy and the energy from the
external energy source.
(2) A resin curing method wherein, when energy is
applied to a resin composition, another kind of first energy
than the energy from an external energy source is
autogenously generated within the resin, and the same kind of
second energy is successively generated by the autogenously
generated energy, so that the resin composition is cured by
means of the first and second energies, or both the first and
second energies and the energy from the external energy
source.
(3) A resin curing method wherein, when a resin
composition is exposed to energy radiation, another kind of
energy than the energy from an external energy radiation
source is autogenously generated within the resin, so that
the resin composition is cured by means of the autogenously
generated energy, or both the autogenously generated energy
and the energy from the external energy radiation source.
(4) A resin curing method wherein, when a resin
composition is exposed to energy radiation, another kind of
first energy than the energy from an external energy
radiation source is autogenously generated within the resin,
and the same kind of second energy is successively generated
by the autogenously generated energy, so that the resin
composition is cured by means of the first and second
-8-
CA 02275278 1999-06-17
energies, or both the first and second energies and the
energy from the external energy radiation source.
(5) A resin curing method wherein heat energy is
autogenously generated within the resin as the generated
energy described above in (3), so that the resin composition
is cured by means of the heat energy, or both the heat energy
and the energy from the external energy radiation source,
whether or not the resin composition contains a substance
capable of screening energy radiation (hereinafter referred
to as "an energy radiation screening substance").
(6) A resin curing method wherein a first heat energy
is autogenously generated within the resin as the generated
energy described above in (4), and a second heat energy is
successively generated by the generated first heat energy, so
that the resin composition is cured by means of the first and
second heat energies, or both the first and second heat
energies and the energy from the external energy radiation
source, whether or not the resin composition contains an
energy radiation screening substance.
(7) A resin curing method wherein the heat of curing
reaction evolved during the cure of the resin composition by
exposure to external energy radiation is positively generated
as the heat energy autogenously generated within the resin as
described above in (5), so that the resin composition is
cured by means of the reaction heat energy, or both the
-9-
CA 02275278 1999-06-17
reaction heat energy and the energy from the external energy
radiation source, whether or not the resin composition
contains an energy radiation screening substance.
(8) A resin curing method wherein the heat of curing
reaction evolved during the cure of the resin composition by
exposure to energy radiation is positively generated as the
first heat energy autogenously generated within the resin as
described above in (6), and the curing reaction is further
effected, like a chain reaction, by the action of the heat of
curing reaction to generate additional heat of curing
reaction as the successively generated second heat energy, so
that the resin composition is cured by means of the first and
second reaction heat energies, or both the first and second
reaction heat energies and the energy from the energy
radiation source, whether or not the resin composition
contains an energy radiation screening substance.
(9) A resin curing method wherein, in the curing
reaction described above in any of (1) to (8), at least one
species selected from the group consisting of a cation, a
radical and an anion is utilized to induce curing or
facilitate the curing reaction by the action of the energy
from the external energy source, the energy radiation from
the energy radiation source, or the heat of reaction. (10) A resin curing
method as described above in (9)
wherein, when the resin composition is exposed to energy
-10-
CA 02275278 1999-06-17
radiation, a cation and a first heat of curing reaction
evolved during the cure of the resin composition by the
action of the energy radiation are positively generated
within the resin, and the curing reaction is further
effected, like a chain reaction, by the action of the cation
and the first heat of curing reaction to successively
generate an additional cation and a second heat of curing
reaction, so that the resin composition is cured by means of
the first and second reaction heat energies and the cation,
or the combination of the first and second reaction heat
energies, the cation, and the energy from the energy
radiation source, whether or not the resin composition
contains an energy radiation screening substance.
(11) A resin curing method as described above in any of
(1) to (10) wherein the cure of the resin composition is
facilitated by warming it previously in a temperature range
which does not cause its cure.
(12) A resin curing method as described above in any of
(1) to (11) wherein a polymerization initiator is used.
(13) A resin curing method as described above in any of
(1) to (12) wherein the cure of the composition is initiated
by means of heat or the composition is cured by means of
heat.
(14) A photopolymerization initiator comprising at
least two components including a photopolymerization
-11-
CA 02275278 1999-06-17
initiator and a photo- and thermopolymerization initiator for
initiating polymerization by means of both light and heat,
and serving as the polymerization initiator making it
possible to carry out a resin curing method as described
above in (13 ) .
(15) A photopolymerization initiator comprising at
least two components as described above in (14) wherein a
photo- and thermopolymerization initiator having a powerful
polymerization-initiating effect upon exposure to heat is
used as the photo- and thermopolymerization initiator.
(16) A photopolymerization initiator comprising at
least two components which includes at least one
photopolymerization initiator comprising at least two
components as described above in (14) or (15), and a
thermopolymerization initiator.
(17) A photopolymerization initiator comprising at
least two components as described above in any of (14) to
(16) which consists essentially or entirely of radical
polymerization initiators.
(18) A photopolymerization initiator comprising at
least two components as described above in any of (14) to
(16) which consists essentially or entirely of anionic
polymerization initiators.
(19) A photopolymerization initiator comprising at
least two components as described above in any of (14) to
-12-
CA 02275278 1999-06-17
(16) which consists essentially or entirely of cationic
polymerization initiators.
(20) A photopolymerization initiator comprising at
least two components as described above in (19) wherein the
photopolymerization initiator comprises at least one compound
selected from diazonium salt type compounds, iodonium salt
type compounds, pyridinium salt type compounds, phosphonium
salt type compounds, sulfonium salt type compounds,
iron-arene complex type compounds, and sulfonate type
compounds, and the photo- and thermopolymerization initiator
comprises at least one of the sulfonium salts represented by
the following general formulae (I), (II), (III), (IV), (V),
(VI) and (VII).
R2 R4
~ ~I)
R 0 S+ CHZ A-
R5
where R1 represents hydrogen, methyl, acetyl or
methoxycarbonyl, RZ and R3 each independently represents
hydrogen, halogen or an alkyl group of 1 to 4 carbon atoms,
R9 represents hydrogen, halogen or methoxy, RS represents an
alkyl group of 1 to 4 carbon atoms, and A represents SbF61
PF6, AsF6 or BF4. 25
-13-
CA 02275278 1999-06-17
CH3
CH2 +S
Re Q
OR7
/CH3
(Q CH2 -+S
R6 O ~A (II')
OR7
where R6 represents a hydrogen atom, a halogen atom, a nitro
group or a methyl group, R' represents a hydrogen atom, CH3CO
or CH3OCO, and A represents SbF6, PF6, BF6 or AsF6.
CH3
/
CH3-+S
a- (III~
):OD,
OR8
where R8 represents a hydrogen atom, CH3CO or CH3OCO, and A
represents SbF6, PF6, BF6, AsF6 or CH3SO4.
X O S O n y= mZe (N)
where X represents a sulfonio group of the general formula
R9
. je S (a)
-14-
CA 02275278 1999-06-17
in which R9 represents an aliphatic group of 1 to 18 carbon
atoms, R10 represents an aliphatic group of 1 to 18 carbon
atoms or a substituted or unsubstituted aromatic group of 6
to 18 carbon atoms, and R9 and R10 may be joined together to
form a ring; Y represents a sulfonio group of the general
formula
Rs (b)
R
in which R" represents an aliphatic group of 1 to 18 carbon
atoms, RlZ represents an aliphatic group of 1 to 18 carbon
atoms or a substituted or unsubstituted aromatic group of 6
to 18 carbon atoms, and R" and R12 may be joined together to
form a ring, or Y represents a hydrogen a hydrogen atom, a
halogen atom, a nitro group, an alkoxy group, an aliphatic
group of 1 to 18 carbon atoms, or a substituted or
unsubstituted phenyl, phenoxy or thiophenoxy group of 6 to 18
carbon atoms; n and m are each independently 1 or 2; and Z is
an anion represented by the formula MQ1 or MQ1_10H in which M
is B, P, As or Sb, Q is a halogen atom, and 1 is 4 or 6.
R13
+/HZ~ (V)
H O-~-S ~CH2 O . A-
R14 ~
where R13 and R14 independently represent hydrogen or alkyl
groups of 1 to 4 carbon atoms, and A represents SbF61 PF6 or
-15-
CA 02275278 1999-06-17
As F6 .
O R16 Rie
F~SC O S+',,eCH2-~YA_ (vi)
~'
W7
where R15 represents ethoxy, phenyl, phenoxy, benzyloxy,
chloromethyl, dichloromethyl, trichloromethyl or
trifluoromethyl, R16 and R17 each independently represents
hydrogen, halogen or an alkyl group of 1 to 4 carbon atoms,
R18 represents hydrogen, methyl, methoxy or halogen, R19
represents hydrogen, methyl, methoxy or halogen, R19
represents an alkyl group of 1 to 4 carbon atoms, and A
represents SbF61 PF6, BF4 or AsF6.
R20 22
Q-0-S+ R~ = A- (yII)
R21
where Q represents methoxycarbonyloxy, acetoxy,
benzyloxycarbonyloxy or dimethylamino, R20 and R21 each
independently represents hydrogen or an alkyl group of 1 to 4
carbon atoms, R22 and R23 each independently represents an
alkyl group of 1 to 4 carbon atoms, and A represents SbF6,
PF6, AsF6 or BF4.
(21) A photopolymerization initiator comprising at
least two components as described above in (20) wherein the
photopolymerization initiator comprises an arylsulfonium salt
type compound, and the photo- and thermopolymerization
-16-
CA 02275278 1999-06-17
initiator comprises at least one sulfonium salt represented
by the general formula (I), (II) or (III).
(22) A photopolymerization initiator comprising at
least two components which includes at least one of the
photopolymerization initiator comprising at least two
components as described above in any of (19) to (21), and a
thermopolymerization initiator comprising at least one of the
compounds represented by the following chemical formulae
(VIII) and (IX).
CH2 }S -S b Fs (W
'
S+-CH2-C =C- CH3=SbF6- (IX)
(23) A composition making it possible of carry out a
curing method as described above in any of (1) to (13)
wherein, when the composition is exposed to energy radiation,
another kind of energy than the energy from the energy
radiation source is autogenously generated within the
composition, or wherein, when the composition is exposed to
the energy radiation, another kind of first energy than the
energy from the energy radiation source is autogenously
generated within the composition, and the same kind of second
energy is successively generated by the generated first
-17-
CA 02275278 1999-06-17
energy.
(24) A composition as described above in (23) which
contains a photopolymerization initiator and a
photopolymerizable oligomer or photopolymerizable monomer.
(25) A composition as described above in (23) or (24)
which contains, as an essential ingredient, a
photopolymerization initiator comprising at least two
components as described above in any of (14) to (22).
(26) A resin composition as described above in (25)
which comprises, as essential ingredients, any of the
photopolymerization initiators comprising at least two
components as described above in any of (19) to (22), and a
cationic photopolymerizable oligomer or cationic
photopolymerizable monomer.
(27) A resin composition as described above in (26)
wherein a photbpolymerizable epoxy oligomer or
photopolymerizable epoxy monomer is used as the cationic
photopolymerizable oligomer or cationic photopolymerizable
monomer.
(28) A resin composition as described above in (27)
wherein a photopolymerizable alicyclic epoxy oligomer or
photopolymerizable alicyclic epoxy monomer is used as the
photopolymerizable epoxy oligomer or photopolymerizable epoxy
monomer.
(29) A resin composition as described above in (28)
-18-
CA 02275278 1999-06-17
wherein 3,4-epoxycyclohexylmethyl
3,4-epoxycyclohexanecarboxylate is used as the
photopolymerizable alicyclic epoxy monomer.
(30) A resin composition as described above in any of
(25) to (29) wherein the photopolymerization initiator
comprising at least two components is contained in an amount
of 0.5 to 6.0 parts by weight per 100 parts by weight of the
photopolymerizable resin component (photopolymerizable
oligomer or monomer), and the weight ratio of the photo- and
thermopolymerization initiator to the photopolymerization
initiator constituting the photopolymerization initiator
comprising at least two components is in the range of 1 to 4.
(31) A resin composition as described above in (30)
which comprises a photopolymerization initiator comprising at
least two components as described above in any of (20) to
(22), and a photopolymerizable resin component as described
above in any of (26) to (29).
(32) A composition as described above in any of (23) to
(31) which contains at least one additive selected from
energy radiation screening substances, various fillers and
organic components.
(33) A composition as described above in any of (23) to
(32) which additionally contains at least one additive
selected from photosensitizers, reactive diluents and
photosensitive compounds.
-19-
CA 02275278 1999-06-17
(34) A cured product obtained by a method as described
above in any of (1) to (13).
(35) A molded article made from a composition
containing a photopolymerization initiator comprising at
least two components as described above in any of (4) to
(22).
(36) A molded article made from a composition as
described above in any of (23) to (33). (37) A molding material, fiber-
reinforced composite
material, carbon fiber-reinforced composite material, other
composite material, adhesive, sealer, varnish, paint or
coating material, ink or toner which contains a composition
making it possible to carry out a resin curing method as
described above in any of (1) to (13).
(38) A molding material, fiber-reinforced composite
material, carbon fiber-reinforced composite material, other
composite material, adhesive, sealer, varnish, paint or
coating material, ink or toner which contains a
photopolymerization initiator comprising at least two
components as described above in any of (14) to (22).
(39) A molding material, fiber-reinforced composite
material, carbon fiber-reinforced composite material, other
composite material, adhesive, sealer, varnish, paint or
coating material, ink or toner which contains a composition
as described above in any of (23) to (33).
-20-
CA 02275278 1999-06-17
(40) A method of making a molded article of a molding
material, fiber-reinforced composite material, carbon
fiber-reinforced composite material or other composite
material, a cured product of an adhesive, sealer, varnish,
paint or coating material, or matter printed with ink or
toner, by utilizing a resin curing method as described above
in any of (1) to (13).
(41) A method of making a molded article of a molding
material, fiber-reinforced composite material, carbon
fiber-reinforced composite material or other composite
material, a cured product of an adhesive, sealer, varnish,
paint or coating material, or matter printed with ink or
toner, by utilizing a resin composition making it possible to
carry out a curing method as described above in any of (1) to
(13).
(42) A method of making a molded article of a molding material, fiber-
reinforced composite material, carbon
fiber-reinforced composite material or other composite
material, a cured product of an adhesive, sealer, varnish,
paint or coating material, or matter printed with ink or
toner, by utilizing a composition containing a
photopolymerization initiator comprising at least two
components as described above in any of (14) to (22).
(43) A method of making a molded article of a molding
material, fiber-reinforced composite material, carbon
-21-
CA 02275278 1999-06-17
fiber-reinforced composite material or other composite
material, a cured product of an adhesive, sealer, varnish,
paint or coating material, or matter printed with ink or
toner, by utilizing a resin composition as described above in
any of (23) to (33)
(44) A method of making a fiber-reinforced composite material or carbon fiber-
reinforced composite material as
described above in any of (40) to (43) wherein the
fiber-reinforced composite material or carbon
fiber-reinforced composite material is made by utilizing at
least one technique selected from hand lay-up, spray-up,
filament winding, tape winding, roll winding, draw molding
and continuous roll pressing.
(45) A method of making a prepreg which comprises
impregnating a reinforcing fiber or a reinforcing fiber cloth
material with a resin composition as described above in any
of (23) to (33).
(46) A method of making a fiber-reinforced composite
material which comprises stacking prepregs made by the method
described above in (45), and curing them according to a
curing method as described above in any of (1) to (13).
(47) A method of making a fiber-reinforced composite
material which comprises impregnating a three-dimensional
textile with a composition as described above in any of (23)
to (33), and curing it according to a curing method as
-22-
CA 02275278 1999-06-17
described above in any of (1) to (13).
(48) A method of repairing a fiber-reinforced composite
material, building, structure or product which comprises
filling a part to be repaired of a fiber-reinforced composite
material, building, structure or product with a composition
as described above in any of (23) to (33), or attaching a
prepreg made by the method described above in (45) to a part
to be repaired of a fiber-reinforced composite material,
building, structure or product; and curing the composition or
prepreg according to a curing method as described above in
any of (1) to (13).
(49) A method of reinforcing a fiber-reinforced
composite material, building, structure or product which
comprises attaching a prepreg made by the method described
above in (45) to a part to be reinforced of a
fiber-reinforced composite material, building, structure or
product, and curing the prepreg according to a curing method
as described above in any of (1) to (13), or which comprises
using a composition as described above in any of (23) to
(33), attaching a reinforcing fiber or reinforcing fiber
cloth material to a part to be reinforced of a
fiber-reinforced composite material, building, structure or
product by spray-up or brushing, and curing the composition
according to a curing method as described above in any of (1)
to (13).
-23-
CA 02275278 1999-06-17
(50) A method as described above in any of (40) to (49)
wherein carbon fiber is used as the fibrous material.
(51) A method as described above in any of (40) to (50)
wherein there is used a material in which the composition
contains a photopolymerization initiator comprising at least
two components as described above in any of (20) to (22).
(52) A method as described above in any of (40) to (50)
wherein there is used a material comprising a composition as
described above in (30).
(53) A method as described above in any of (40) to (50)
wherein there is used a composition as described above in
(31).
(54) A molded article made by a method as described
above in any of (40) to (43).
(55) A fiber-reinforced composite material or carbon
fiber-reinforced composite material as described above in any
of (37) to (39).
(56) A molded article made by the method described
above in (44).
(57) A prepreg made by the method described above in
(45).
(58) A fiber-reinforced composite material obtained by
curing a prepreg as described above in (57).
(59) A fiber-reinforced composite material obtained by
impregnating a three-dimensional textile with a composition
-24-
CA 02275278 1999-06-17
as described above in any of (23) to (33), and curing the
resin composition.
(60) A repair material for filling a part to be
repaired of a fiber-reinforced composite material, building,
structure or product which comprises a composition as
described above in any of (23) to (33).
(61) A reinforcing material for a fiber-reinforced
composite material, building, structure or product which
comprises a composition as described above in any of (23) to
(33). (62) A material or molded article as described above in
any of (37) to (39) or any of (54) to (61) wherein carbon
fiber is used as the fibrous material.
(63) A material or molded article as described above in
any of (37) to (39) or any of (54) to (62) wherein the
composition contains a photopolymerization initiator
comprising at least two components as described above in any
of (20) to (22).
(64) A material or molded article as described above in
any of (37) to (39) or any of (54) to (62) which comprises a
composition as described above in (30).
(65) A material or molded article as described above in
any of (36) to (38) or any of (54) to (62) which comprises a
composition as described above in (30).
In the above-described methods of the present invention,
-25-
CA 02275278 1999-06-17
particularly in the methods described in (1) to (13), the
resin composition can be cured by causing energy (e.g., heat
energy) to be autogenously generated within the resin and, in
some cases, causing energy to be successively generated by
the generated energy. Specifically, the above-described
methods can be carried out by using, as the polymerization
reaction catalyst, a photopolymerization initiator system
(reaction catalyst system) comprising at least two components
including a photopolymerization initiator and a photo- and
thermopolymerization initiator.
That is, to sum up more briefly, the present invention
is characterized by a novel resin curing mechanism enabling
the energy radiation curing of CFRPs and thick-walled resins
containing an energy radiation screening substance,
photopolymerization initiator systems (reaction catalyst
systems) comprising at least two components and capable of
inducing this curing mechanism, and compositions containing
them. It is preferable to use a photopolymerization
initiator system (reaction catalyst system) comprising at
least two components in which the photopolymerization
initiator comprises at least one compound selected from
diazonium salt type compounds, iodonium salt type compounds,
pyridinium salt type compounds, phosphonium salt type
compounds, sulfonium salt type compounds, iron-arene complex
type compounds and sulfonate type compounds, and the photo-
-26-
CA 02275278 1999-06-17
and thermopolymerization initiator comprises at least one of
the sulfonium salts represented by the general formulae (I)
to (VII). It is more preferable to use a photopolymerization
initiator system (reaction catalyst system) comprising at
least two components in which the photopolymerization
initiator comprises an arylsulfonium type compound (i.e., a
triarylsulfonium type compound) and the photo- and
thermopolymerization initiator comprises at least one of the
sulfonium salts represented by the general formulae (I), (II)
and (III).
Moreover, thermopolymerization initiators, typified by
those of chemical formulae (VIII) and (IX), may be added to
the aforesaid photopolymerization initiator systems
comprising at least two components. Furthermore, the present
invention also relates to the compositional range of specific
photopolymerization initiator systems comprising at least two
components; resin compositions capable of inducing the novel
resin curing mechanism, the formulations thereof, and molded
articles made therefrom; a method for utilizing the aforesaid
curing mechanism and resin compositions; and a method of
making FRPs using such a resin as the matrix resin, resin
compositions therefor, and molded articles made therefrom.
BRIEF DESCRIPTION OF DRAWINGS
FIG. 1 is a schematic view illustrating the manner in
which UV energy is attenuated while it passes through a resin
-27-
CA 02275278 1999-06-17
composition;
FIG. 2 is a schematic view illustrating the manner in
which UV energy is attenuated while it passes through a resin
composition containing a carbon cloth material;
FIG. 3 includes schematic views illustrating the
UV-cured state of the respective resin compositions shown in
FIGs. 1 and 2;
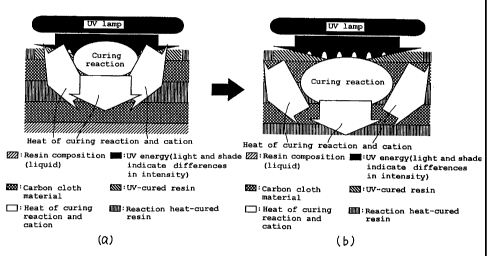
FIG. 4 includes schematic views for explaining the resin
curing mechanism of the present invention (i.e., a curing
system utilizing light plus heat of curing reaction and
cation);
FIG. 5 is an explanatory view of a curing model for
highly UV-curable resins;
FIG. 6 is an explanatory view of a curing model for UV-
and heat-curable resins known in the prior art;
FIG. 7 is a flow diagram of an exemplary FRP molding
process, illustrating O a lay-up process;
FIG. 8 is a flow diagram of exemplary FRP molding
processes, illustrating O a drawing process, O a
filament/tape/roll winding process, and a continuous roll
pressing process;
FIG. 9 is a graph showing a proper compositional range
for a photopolymerization initiator system in accordance with
the present invention;
FIG. 10 is a graph showing the relationship between the
-28-
CA 02275278 1999-06-17
time elapsed and the resin temperature after UV irradiation
for 60 seconds in the practice of the present invention;
FIG. 11 is a graph showing the relationship between the
UV irradiation distance and the resin temperature after UV
irradiation for 60 seconds in the practice of the present
invention; and
FIG. 12 is an explanatory view illustrating the manner
in which a prepreg laminate sample is made in accordance with
the present invention.
BEST MODE FOR CARRYING OUT THE INVENTION
First of all, the present inventors have paid attention
to the fact that resins containing an energy radiation
screening substance and thick-walled resins, and their
applications such as FRPs and CFRPs, cannot be cured with
energy radiation because OO energy typified by UV energy is
attenuated while it passes through a substance (resin) (FIG.
1), OO it is easily blocked by a substance capable of
absorbing the same wavelength (FIG. 2), and OO energy
radiation-curable resins typified by UV-curable resins are
cured only in regions through which more than a certain
amount of energy radiation has passed (FIG. 3). With
consideration for the fact that the features OO and are
based on fundamental principles and hence hard to modify, the
present inventors made intensive investigations on the
securement of energy required for curing purposes, the
-29-
CA 02275278 1999-06-17
prevention of energy required for curing purposes from being
blocked, and a novel resin curing mechanism enabling the cure
of regions not exposed to energy radiation. As a result, the
present inventors have revealed a novel resin curing
mechanism in which, when a resin composition is exposed to
energy radiation or energy is applied to a resin composition,
another ki-nd of energy is autogenously generated within the
resin, so that the resin composition is cured by means of
this energy, or both this energy and the energy from the
energy radiation source or energy source, and have developed
a resin curing method based on this mechanism.
In FIG. 1, the manner in which the intensity of UV
energy is gradually attenuated while energy radiation from a
UV lamp passes through a resin composition is indicated by
light and shade in an arrow (i.e., a wavy pattern in this
figure). In FIG. 2, UV energy is easily blocked owing to the
presence of an energy radiation screening substance such as a
carbon cloth material. FIGs. 3(a) and 3(b) each illustrate
the manner in which, when a liquid resin is exposed to UV
energy, the resin is cured only in regions through which more
than a certain amount of energy radiation has passed (as
indicated by rightward inclined parallel lines in this
figure). (b) indicates the case in which an energy radiation
screening substance such as a carbon cloth material is
present, so that the cure of the resin is interrupted by the
-30-
CA 02275278 1999-06-17
screening substance.
Besides ultraviolet radiation, useful types of energy
radiation include electron rays, X-rays, infrared radiation,
sunlight, visible light, laser light (from excimer, 02 and
other lasers), radiated heat rays and other energy.
Moreover, the applied energy may comprise not only light or
electromagnetic radiation, but also heat or the like.
As a result of further intensive investigations based on
this concept, the present inventors have discovered the
successive production of autogenously generated energy, the
use of heat energy as the autogenously generated energy, the
successive production of heat energy, the use of heat of
curing reaction (curing exotherm) as the heat energy, the
utilization of a cation, radical or anion, the enhancement of
curability by preheating, the utilization of a polymerization
initiator, and the like, and have developed a novel resin
curing mechanism in which, when a resin composition is
exposed to energy radiation, a cation and heat of curing
reaction (curing exotherm) are positively generated within
the resin, and the resin is further cured, like a chain
reaction, by the action of the cation and the heat of curing
reaction to successively generate additional cation and heat
of curing reaction (curing exotherm), so that the resin
composition is cured by means of the reaction heat energy, or
both reaction heat energy and the energy from the energy
-31-
CA 02275278 1999-06-17
radiation source, whether or not the resin composition
contains an energy radiation screening substance (FIG. 4), as
well as a resin curing method based on it.
FIGs. 4(a) and 4(b) are schematic views for explaining
the resin curing mechanism of the present invention (i.e., a
curing system utilizing light plus heat of curing reaction
and cation), and illustrate the manner in which, when a resin
composition is exposed to energy radiation, a cation and heat
of curing reaction are positively generated within the resin,
and the resin is further cured, like a chain reaction, by the
action of the cation and the heat of curing reaction. (a)
indicates an initial stage and (b) indicates a stage in which
the reaction heat cure of the resin composition has
progressed to the lowermost layer thereof. In either case,
the curing reaction proceeds whether or not the resin
composition contains a carbon cloth material. Although a
combination of a cation and heat of curing reaction is utilized for purposes
of polymerization in this embodiment,
it is a matter of common knowledge that a radical or an anion
may also be utilized in the present invention as a species
participating in the polymerization of the resin.
Furthermore, the curing mechanism of the present invention
enables resins to be cured by means of not only light or
electromagnetic radiation, but also heat or other energy.
This novel resin curing mechanism, which has now been
-32-
CA 02275278 1999-06-17
developed, is quite different from the resin curing
mechanisms of highly UV-curable resins and combined UV- and
heat-curable resins that are typical of the prior art (FIGs.
and 6). Owing to this difference, the novel resin curing
5 mechanism of the present invention does not suffer from the
disadvantages of the prior art, such as the poor curability
of filler-containing resins and the need for heating after
exposure to energy radiation. FIGs. 5(a) and 5(b) each
illustrate the resin curing mechanism of a conventional
highly UV-curable resin. As shown in (a), this is
advantageous in that a thick cured film can be obtained when
no energy radiation screening substance is present. However,
when such a screening substance is present, the curing
reaction does not proceed as shown in (b).
FIG. 6(a) illustrates the resin curing mechanism of a
conventional combined UV- and heat-curable resin. When this
resin is exposed to UV energy as shown in (b), its cure does
not proceed owing to the presence of an energy radiation
screening substance (see the lower picture). Accordingly, in
order to cause its cure to proceed, the resin must be heated
after exposure to energy radiation as shown in (a). Where an
energy radiation screening substance such as a carbon cloth
material is present, the problems of conventional UV curing
cannot be solved without heating. In both (a) and (b), the
upper picture indicates the case in which such a screening
-33-
CA 02275278 1999-06-17
substance is not present, and the lower picture indicates the
case in which such a screening substance is present.
Next, as a result of intensive investigations on
polymerization initiators capable of inducing the
above-described novel resin curing mechanism and making it
possible to carry out the above-described resin curing
method, the present inventors have found that a
photopolymerization initiator system (reaction catalyst
system) comprising at least two components including a
photopolymerization initiator and a photo- and
thermopolymerization initiator which initiates polymerization
upon exposure to both light and heat is useful in
accomplishing the objects of the present invention.
In the present invention, it is preferable to use a
photopolymerization initiator comprising at least one
compound selected, for example, from diazonium salt type
compounds shown in Table A below, iodonium salt type
compounds shown in Table B, pyridinium salt type compounds
represented by the following general formula
R
CH N+ = X-
-34-
CA 02275278 1999-06-17
phosphonium salt type compounds described in Japanese
Patent Provisional Publication Nos. 6-157624/'94 and 7-
82283/'95, sulfonium salt type compounds shown in Table C
below (see Table 1 which will be given later in Example 1),
iron-arene complex type compounds such as initiator OO shown
in Table 1, and sulfonate type compounds, in combination with
a photo- and thermopolymerization initiator comprising at
least one of the compounds represented by the general
formulae (I) to (VII).
Table A Aryldiazonium salt photo-initiators
Cationic moiety (diazonium) Anionic moiety Maximum absorption
wavelength (nm)
2,5-Diethoxy-4-(p- BF4 355, 391
toluylmercapto)benzene
2,4-Dichlorobenzene SnCls 285
p-Nitrobenzene FeCI4 243, 257, 310, 360
p-Chlorobenzene PFB 273
p-(N-morpholino)benzene AsFe 257, 378
2,5-Dichlorobenzene SbFs 238, 358
o-Nitrobenzene BC18 285, 313
-35-
CA 02275278 1999-06-17
Table B Aromatic iodonium salt ohoto-initiators
Cationic moiety Anionic moiety A max (nm) s max
1. 1+ BF4 227 17,800
2. C H g O I+ O C H3 BF4 236 18,000
3. C H 3 O I+ O C H3 BF4- 237 18,200
4. C H 3 O I+ O CH3 AsF4 237 17,500
5. C H 3 O I~ C C H 3 BF4 238 20,800
6. C H 3 O I+ C H 3 PFe 238 20,000
7. C H 3 I+ C H3 AsFe 238 20,700
8. C H 3 I+ C H3 SbFe 238 21,200
-36-
CA 02275278 1999-06-17
Table C Triarylsulfonium salt photo-initiators
Cationic moiety Anionic moiety A max (nm) e max
1. ( -8+ BF2 230 17,500
2. (QS+ AsFe 230 17,500
3. O 5+ O PF6 237, 20,400,
240 19,700
2
225, 21,740,
4. C H 3 0 S+ AsFs 280 10,100
3
+ 243, 24,700,
-
5. C H 3 0 S BF2 278 4,900
3
C H 3
263 25,200
6. K (o)_+ AsFs 280 22,400
>)3 316 7,700
CH3
-37-
CA 02275278 1999-06-17
Moreover, there may also be used radical
photopolymerization initiators shown in the following Tables
D and E.
Table D P1 tvpe nhoto-initiators
0 R1
~~ 1R C - ~ - R3 (Acetophenone structure)
R2
Designation R R, R2 R3
Benzoin butyl ether H OC4H9 H C6H5
Benzyl dimethyl ketal H OCH3 OCH3 C6H5
Ethoxyacetophenone H OC2H5 OC2H5 H
Acyloxime ester H NOCO CH3 C6H5
H NOCO OC2H5 CH3
Chlorinated acetophenone CaHe CI CI CI
Hydroxyacetophenone H OH CH3 CH3
CH3
iii o 1 11
CH3 C - P C C-OOCqHg
CH3 0
Acylphosphine oxide
-38-
CA 02275278 1999-06-17
Table E P2 tvae ohoto-initiators
0
R Rl (Thioxanthone structure)
X
Designation X R R,
Benzophenone - R H
Michler's ketone - (CH3)2N (CH02N
Dibenzosuberone CH2 CH2 H H
2-Ethylanthraquinone C=O H 2-C2H5
Isobutylthioxanthone S H 2-i-C3H7
O 0
II Il
Benzil
Specific examples of the compounds represented by the
general formulae (I) to (III) include photopolymerization
initiators OO to OO used in the Examples which will be given
later {"Journal of Polymer Science", Part A: "Polymer
Chemistry", Vol. 29, 1675-1680 (1991); "Kobunshi", Vol. 40,
December 1991, 794-797}.
Specific examples of the compounds represented by the
general formulae (IV) include bis{4-(dimethylsulfonio)phenyl}
-39-
CA 02275278 1999-06-17
sulfide bis-hexafluorophosphate and
dimethyl-4-thiophenoxyphenylsulfonium hexafluoroantimonate.
A specific example of the compounds represented by the
general formulae (V) is dibenzyl-4-hydroxyphenylsulfonium
hexafluoroantimonate, a specific example of the compounds
represented by the general formulae (VI) is
benzyl-4-(ethoxycarbonyloxy)phenylmethylsulfonium
hexafluoroantimonate, and a specific example of the compounds
represented by the general formulae (VII) is
4-acetoxyphenyldimethylsulfonium hexafluoroantimonate.
Preferred examples of other combinations include
photopolymerization initiator systems (reaction catalyst
systems) comprising at least two components in which the
photopolymerization initiator comprises at least one
arylsulfonium salt type compound as shown in Table C (i.e., a
triarylsulfonium salt such as photo-initiator (D shown in
Table 1) and the photo- and thermopolymerization initiator
comprises at least one of the sulfonium salts represented by
the above general formulae (I) to (III).
The present inventors carried the aforesaid
investigations further and, as a result, have found that it
is preferable to use, as the photo- and thermopolymerization
initiator, a photo- and thermopolymerization initiator having
a powerful catalytic effect upon exposure to heat, such as
the compounds represented by the above general formulae (I)
-40-
CA 02275278 1999-06-17
to (III) (i.e., photo-initiators (D to OO shown in Table 1),
and it is preferable to use, as the thermopolymerization
initiator,.prenyltetramethylenesulfonium hexafluoroantimonate
represented by chemical formula (VIII) or
2-butynyltetramethylenesulfonium hexafluoroantimonate
represented by chemical formula (IX).
Finally, as a result of similar intensive investigations
on resin compositions which can induce the aforesaid novel
resin curing mechanism and make it possible to carry out the
aforesaid resin curing method, the present inventors have
obtained the following findings. Specifically, it has been
found that resin compositions comprising a
photopolymerization initiator comprising at least two
components and a photopolymerizable oligomer or
photopolymerizable monomer, and molded articles made
therefrom are useful. Among others, it is preferable to use
a cationic photopolymerizable oligomer or cationic
photopolymerizable monomer and, in particular, a
photopolymerizable epoxy oligomer or photopolymerizable epoxy
monomer. Examples of such photopolymerizable oligomers
include alicyclic epoxies, glycidyl ether type epoxies,
epoxidized polyolefins, epoxy (meth)acrylates, polyester
acrylates and vinyl ether compounds. Examples of such
photopolymerizable monomers include epoxy monomers, acrylic
monomers, vinyl ether and cyclic ethers. Among others,
-41-
CA 02275278 1999-06-17
photopolymerizable alicyclic epoxy oligomers and
photopolymerizable alicyclic epoxy monomers are preferred. A
particularly preferred example of such photopolymerizable
alicyclic epoxy oligomers is 3,4-epoxycyclohexylmethyl
3,4-epoxycyclohexanecarboxylate.
Among others, resin compositions comprising a
photopolymerization initiator system comprising at least two
components in which the photopolymerization initiator
comprises at least one arylsulfonium salt type compound as
shown in Table C (i.e., a triarylsulfonium salt such as
photo-initiator (D shown in Table 1) and the photo- and
thermopolymerization initiator comprises at least one of the
sulfonium salts represented by the above general formulae (I) to (III), and at
least one photopolymerizable epoxy monomer
or oligomer such as 3,4-epoxycyclohexylmethyl
3,4-epoxycyclohexanecarboxylate, and molded articles made
therefrom, are preferred.
In the present invention, the preferred formulation of
the aforesaid resin compositions is such that the
photopolymerization initiator system (reaction catalyst
system) comprising at least two components is contained in an
amount of 0.5 to 6.0 parts by weight, more preferably 1.5 to
3.5 parts by weight, per 100 parts by weight of the
photopolymerizable resin component (photopolymerizable
oligomer or monomer), and the weight ratio of the photo- and
-42-
CA 02275278 1999-06-17
thermopolymerization initiator to the photopolymerization
initiator constituting the photopolymerization initiator
system is in the range of 1 to.4, more preferably 1.3 to 2.8.
If the amount of the photopolymerization initiator system
comprising at least two components is less than 0.5 part by
weight, little effect will be produced. Since its amount is
too small for the whole resin composition, it will not
function properly. Even if its amount is greater than 6.0
parts by weight, its photo-curing function will remain
unchanged. If the weight ratio of the photo- and
thermopolymerization initiator to the photopolymerization
initiator is less than 1, a sufficient amount of heat will
not be generated at the initial stage of cure. This will
cause the resin to be cured only in the surface thereof,
because the curing function constituting a feature of the
present invention is not performed properly. If the weight
ratio is greater than 4, the resin will show an undue
enhancement in curing properties and, in particular, heat
generation properties, resulting in foaming of the resin due
to its rapid exothermic cure (the related data are shown in
Tables 3 and 4 and FIGs. 9 and 10).
Furthermore, one or more of various commonly used
additives, such as energy radiation screening substances
{e.g., carbon, carbon fibers (short fiber, long fiber,
continuous filament, carbon cloth, etc.), inorganic fillers
-43-
CA 02275278 1999-06-17
and metal powders}, various fillers, organic components,
photosensitizers, reactive diluents and photosensitive
compounds, may be added to the aforesaid resin compositions
in such proportions as permit the resin compositions to be
cured.
In addition, the present inventors paid attention to the
fact that, in the production of FRPs, particularly CFRPs, a
(long-time) heat curing step is considered to be responsible
for high processing costs, the size of the apparatus or
equipment cannot be reduced because a large-sized heating
oven is required for the curing of large-sized FRPs, a
short-time curable resin cannot be used for large-sized FRPs
because the starting time of cure cannot be controlled at
will, it is difficult to maintain the resin-impregnated state
and mold the FRP because heating in the production process
causes changes in resin viscosity, and the formation of voids
causing a reduction in quality arises from residual solvent,
and made intensive investigations on the development of a
method of making FRPs, particularly CFRPs, in which a heating
step is not required, the resin is cured in a short period of
time, the starting time of resin cure can be controlled at
will, and no solvent is needed. As a result, the present
inventors have developed a method of making FRPs and CFRPs
which comprises using a resin composition of the present
invention as the matrix resin, impregnating a fiber with this
-44-
CA 02275278 1999-06-17
matrix resin, and curing the FRP or CFRP by exposure to
energy radiation typified by UV radiation while utilizing the
novel resin curing mechanism and resin curing method of the
present invention, and products so made. The term "product"
as used herein means articles, other than buildings and
structures, which can be artificially made and fall within
the scope of the present invention.
In a filament winding process using a combination of UV
curing and heat curing, which is a typical example of
conventional molding techniques, UV curing participates only
in the cure of the surface of the resin and the thickening of
its inner part. After all, the whole resin is cured by the
application of heat as usual. In this conventional
technique, therefore, various problems associated with the
heat curing step (e.g., those with processing costs and
operating time) and other problems such as the need for a
large-sized heating oven in the molding of large-sized FRPs
remain unsolved. In contrast, the method of making FRPs and
CFRPs in accordance with the present invention does not
involve such problems.
In the FRPs made according to the present invention,
there may be used any of various fibers commonly used as
reinforcing fibers for FRPs, such as carbon fiber, glass
fiber and organic fibers. Moreover, these fibers may have
any desired form such as a unidirectionally aligned material,
-45-
CA 02275278 1999-06-17
a woven fabric or a knit fabric. Furthermore, no particular
limitation is placed on the combined use of fibers, and there
may be used a combination of carbon fiber and glass fiber or
of carbon.fiber and a hybrid therebetween. Furthermore, in
order to mold FRPs, there may employed any of various common
FRP-molding techniques including hand lay-up, spray-up,
filament winding, tape winding, roll winding, draw molding
and continuous roll pressing (FIGs. 7 and 8).
EXAMPLES
The present invention is further illustrated by the
following examples. However, these examples are not to be
construed to limit the scope of the invention.
Example 1
(A) 100 parts by weight of ERL-4221 (an alicyclic epoxy
resin manufactured by Union Carbide Japan K.K.;
3,4-cyclohexylmethyl 3,4-epoxycyclohexanecarboxylate) was
mixed with 1.75 parts by weight of San Aid SI-80L {a cationic
photo- and thermopolymerization initiator manufactured by
Sanshin Chemical Co., Ltd.; general formula (II)}, and 0.75
part by weight of DAICAT 11 (a cationic photopolymerization
initiator manufactured by Daicel Chemical Industries Ltd.; an
arylsulfonium salt).
(B) Then, a glass vessel {40 mm (diameter) x 80 mm
(height)} covered with black paper except its upper part was
filled with the above resin to the top of the glass vessel.
-46-
CA 02275278 1999-06-17
(C) This resin was irradiated with UV radiation for 60
seconds. The UV irradiation was carried out under the
following conditions.
Ultraviolet irradiator: UVL-1500 M2 (manufactured by Ushio
Inc.)
Type of lamp: Metal halide lamp.
Intensity of lamp: 120 W/cm.
Length of lamp: 125 mm.
Atmosphere, temperature and pressure: Air, room temperature
and atmospheric pressure.
Irradiation distance: 19 cm.
After UV irradiation, the resin within the glass vessel
was completely cured in several minutes. The wall thickness
of the resin was 80 mm (the greatest measurable value) which
was the limit defined by the glass vessel.
Examples 2 to 245 and Comparative Examples 1 to 187
Tests were carried out under the same conditions as
described in Example 1, except that the resin compositions
shown in Table 1 were used and tested according to the
formulations shown in Tables 2 and 3. The test results thus
obtained are shown in Tables 2, 3 and 4 and FIG. 9. The data
obtained by measuring the resin temperature due to curing
exotherm are shown in FIG. 10.
-47-
CA 02275278 1999-06-17
Table I List of resin compositions
Type Composition No. Product name or code Remarks Manufacturer
Oligomer (D Celoxide 2021 P 3,4-Epoxycyclohexylmethyl-3,4-
e ox c Icohexanecarbox late
Oligomer Celoxide 2081 Flexible alicyclic epoxy
Oli mer Celoxide 3000 Alicyclic e ox diluent
Oligomer Celoxide 2000 Alicyclic monoepoxy having a vinyl
group Daicel Chemical
Oligomer Epolead GT301 Polyfunctional alicyclic epoxy Industries Ltd.
(trifunctional)
Oligomer Epolead GT401 Polyfunctional alicyclic epoxy
(tetrafunctional)
E
Oligomer EHPE3150 Alicyclic solid epoxy
o Oligomer 8 PB3600 Containing e ox /vin I groups
E 3,4-Epoxycyclohexylmethyl-3,4-
m Oligomer Q9 ERL-4221
+, e ox c Icohexanecarbox late
~ Oli omer 1 ERL-4299 Flexible alicyclic epoxy Union Carbide
o m Oligomer 1 ERL-4206 Alicyclic e ox diluent Japan K.K.
E~ Alicyclic monoepoxy having a vinyl
~ Q Oligomer 1 VCMX group
40J
m s Oligomer Epicoat 828 Bisphenol A type epoxy
L a
m o Oligomer Epicoat 806 Bisphenol F type epoxy
Epicoat 815 Bisphenol A type e ox /BGE
N m Oligomer
E Oligomer 1 Epicoat 834 Bisphenol A type e ox (semisolid)
o Yuka-Shell
Epicoat 1004 Bisphenol A type epoxy (solid)
E Oli omer Epoxy Co., Ltd.
a m Oligomer 1 Epicoat 1001 B80 Bisphenol A type epoxy (solution)
.~ R Oli omer Epicoat 5046B80 Flame-retardant e ox (solution)
Oli mer Epicoat 152 Polyfunctional e ox
a m
E Oli mer (1) Epicoat 154 Polyfunctional e ox
z Oli mer (2) E icoat YX310 Tough epoxy
0
o Oligomer (3) 850 Bisphenol A type epoxy
o Dainippon Ink &
v Oligomer (4) 830 Bisphenol F type epoxy
Chemicals, Inc.
Oli mer (5) N-665 Cresol novolak type epoxy
Oligomer (6) N740 Phenol novolak type epoxy
Oligomer (7) ECON-1 02S Cresol novolak type epoxy Nippon Kayaku
Oligomer (8) ECON-1 020 Cresol novolak type epoxy Co., Ltd.
Oligomer (9) EPPN-201 Phenol novolak type epoxy
Oligomer (10) CY1 77 Alicyclic epoxy Ciba-Geigy
Oli mer (11) CY179 Alicyclic e ox (Japan) Ltd.
Oli mer (12) Rapicure CHVE Vinyl ether
-48-
CA 02275278 1999-06-17
Table 1(contd.) List of resin compositions
Type Composition No. Product name or code Remarks Manufacturer
Photo-initiator Q San Aid SI-60L
Sanshin
Photo-initiator San Aid SI-80L General formula (1), (II) or (III) Chemical
Co.,
~ Ltd.
~ Photo-initiator 3Q San Aid SI-100L
Bis (4-(dimethylsulfonio)phenyl)
c Photo-initiator Q - sulfide bis-hexafluorophosphate
o -
(general fomula (IV))
N
Dimethyl-4-
E _ Photo-initiator thiophenoxyphenylsulfonium
_a, Q -
o hexaflouoroantimonate (general
E formula (IV))
Dibenzyl-4-hydroxyphenylsulfonium
Photo-initiator 1 - hexafluoroantimonate (general -
~ formula (IV))
Benzyl-4-(ethoxycarbonyloxy)
0 _ phenylmethylsulfonium
-
Photo-initiator
o hexafluoroantimonate (general
a= formula (VI))
4-Acetoxyphenyldimethylsulfonium
Photo-initiator 1 - hexafluoroantimonate (general -
formula (VII))
Photo-initiator DAICAT 11 Arylsulfonium salt (hazardous Daicel Chemical
~ material 3-I11, water-insoluble) Industries Ltd.
m
o Photo-initiator CI-2734 Sulfonium salt type (containing y-
b rolactone) Nippon Soda Co.,
N Photo-initiator CI-2855 Sulfonium salt type (containing r- Ltd.
E but rolactone)
z 17 5-2,4-Cyclopentadien-l-yl)
0
o Photo-initiator Q9 IRGACURE 261 ((1.2,3,4,5,6- 77 )-(1- Ciba-Geigy
o methylethyl)benzene)-iron(1+)- (Japan) Ltd.
s hexafluoro hos hate 1-)
a
4,4'-Bis(di(fl -
Photo-initiator [13] - hydroxyethoxy)phenylsulfonio) phenyl -
sulfide bis-hexafluoroantimonate
c
0
4 Prenyltetramethylenesulfonium
~ Heat-Initiator[14] - hexafluoroantimonate (chemical -
0 E o formula (VIII))
=-
~ 2-Butynyltetramethylenesulfonium
m Heat-Initiator[15] - hexafluoroantimonate (chemical -
formula (IX))
F-
-49-
CA 02275278 1999-06-17
Table 2 List of the fonnulations of resin compositions
Photo- and Cured wall
Ex. or Photopolymerizable resin thermopolymerization Photopolymerization
initiator thickness
Com.Ex. No. initiator (mm)
Oligomer No. Proportion Photo-initiator Proportion Photo-initiator Proportion
(max 80
wt. arts No. (wt. arts No. (wt. arts mm)
Ex. 1 Oligomer Q 100
Ex. 2 Oli omer
Ex. 3 Oligomer 1/ 80/20
Oli omer
Ex. 4 Oligomer (1)/ 80/20
Oli omer 4
Oligomer U/
Ex.5 Oligomer Q/ 50/20/30
li omer
Oligomer U/
Ex. 6 Oligomer Q/ 50/20/30
Oligomer Oligomer U/
Ex. 7 Oligomer / 30/50/20
li omer
8
Ex. 8 Oligomer
Ex.9 Oligomer 9 100
Ex. 10 Oti omer 1
Ex. 11 Oligomer 9/ 80/20
Oli omer 1 Photo-initiator 1 75 Photo-initiator 0.75 80
Ex. 12 Oligomer 9/ 80/20
Oligomer (fb
Ex. 13 Oligomer
Ex. 14 Oligomer
Ex. 15 Oli omer 1
Ex. 16 Oli omer
Ex. 17 Oligomer 1
Ex. 18 Oli omer
Ex. 19 Oli omer
Ex. 20 Oligomer
Ex. 21 Oligomer (1)
Ex. 22 Oligomer (2) 100
Ex. 23 Oligomer 3
Ex. 24 Oligomer (4)
Ex. 25 Oligomer (5)
Ex. 26 Oligomer (6)
Ex. 27 Oligomer (7)
Ex. 28 Oli omer 8
Ex. 29 Oligomer 9
Ex. 30 Oli omer
Ex. 31 Oli omer
-50-
CA 02275278 1999-06-17
Table 2 (Contd.) List of the formulations of resin compositions
Photo- and Cured
Ex. or Photopolymerizable resin thermopolymerization initiator
Photopolymerization initiator wall
Com.Ex. No. thickness
Oligomer No. Proportion Photo-initiator No. Proportion Photo-initiator No.
Proportion (max 80
wt parts) wt arts (wt. arts mm)
Ex.32 Oligomer / 95/5
Oli mer 1
Oligomer /
Ex.33 75/25
Oli omer
Oligomer / Photo-initiator
Ex.34 li omer 12 80/20
Oligomer / Photo-initiator
Ex.35 Oligomer 12 50/50
Ex.36 Photo-initiato 1
Ex. 37 Photo-initiato
Ex. 38 Photo-initiato 1.75 0.75
Ex. 39 Oligomer 1Q Photo-initiato 80
Ex. 40 Photo-initiator
Ex. 51 Photo-initiator Photo-initiator
Ex. 52 Photo-initiator 9
Ex. 53 100 Photo-initiato 1
Ex. 54 Photo-initiato 3 Photo-initiator
Ex. 55 Photo-initiato 4
Ex. 56 Oligomer 1Q Photo-initiato 5
Ex. 57 Photo-initiato Photo-initiator
Ex. 58 Photo-initiato 2 Photo-initiator 8
Ex. 59 Photo-initiato Photo-initiator 9
Ex. 60 Oligomer 1Q Photo-initiato / 1,00/0.75 Photo-initiato 6/ 0.50/0.25
Photo-initiato Photo-initiator(7)
Com. Ex. 1 Photo-initiato 1
Com. Ex. 2" Photo-initiato 2
Com. Ex. 3 Photo-initiato 2.50 -
Com. Ex. 4 Photo-initiato 4 2
Com. Ex. 5 Oligomer 1Q Photo-initiato 5
Com. Ex. 6 Photo-initiator 2
Com. Ex. 7 - Photo-initiator 2.50
Com. Ex. 8 Photo-initiator 8
Com. Ex. 9 Photo-initiator 9 2
Com. Ex. 10 100 Photo-initiato 0.75 Photo-initiator 6 1.75
Com. Ex. 11 Photo-initiato 1
Com. Ex. 12 Photo-initiato 2
Com. Ex. 13 Photo-initiato 2.50 -
Com. Ex. 14 Photo-initiato 4
Com. Ex. 15 Oligomer Photo-initiato 5
Com. Ex. 16 Photo-initiator
Com. Ex. 17 - Photo-initiator 2.50
Com. Ex. 18 Photo-initiator 8
Com. Ex. 19 Photo-initiator 9
Com. Ex. 20 Photo-initiato 0.75 Photo-initiator 6 1.75
-51-
CA 02275278 1999-06-17
Table 3 List of the formulations of two-component
photo-initiators and ratings for the cured state
Photo-initiator 6 (wt parts)
0.4 0.5 0.6 0.7 0.8 0.9
0.4 Ex. 61 Ex. 75 Com. Ex. 36 Com. Ex. 43 Com. Ex. 50 Com. Ex. 57
Cured state = = x x x x
0.5 Ex. 62 Ex. 76 Ex. 90 Com. Ex. 44 Com. Ex. 51 Com. Ex. 58
Cured state = = = x x x
0.6 Ex. 63 Ex. 77 Ex. 91 Com. Ex. 105 Com. Ex. 52 Com. Ex. 59
Cured state = = = = x x
0.7 Ex. 64 Ex. 78 Ex. 92 Ex. 106 Ex. 120 Com. Ex. 60
Cured state o = = = = x
0.8 Ex.65 Ex.79 Ex.93 Ex.107 Ex.121 Ex.135
Cured state o0 0 Oo = = =
0.9 Ex.66 Ex.80 Ex.94 Ex. 108 Ex.122 Ex.136
Cured state 0 0 0 0 = =
1.0 Ex.67 Ex. 81 Ex. 95 Ex. 109 Ex.123 Ex.137
Cured state 0 0 0 0 a =
1.1 Ex.68 Ex.82 Ex.96 Ex.110 Ex.124 Ex.138
Cured state Do oQ oQ Qo Qo Qo
1.2 Ex.69 Ex.83 Ex.97 Ex. 111 Ex.125 Ex.139
Cured state @
co
Q- 1.3 Ex.70 Ex.84 Ex.98 Ex. 112 Ex.126 Ex.140
Cured state @
1.4 Ex.71 Ex.85 Ex.99 Ex. 113 Ex.127 Ex.141
Cured state Qo
0 1.5 Ex.72 Ex.86 Ex.100 Ex. 114 Ex.128 Ex.142
Cured state ~ @
1.6 Ex.73 Ex.87 Ex.101 Ex. 115 Ex.129 Ex.143
Cured state @ 0 0 a ~
0 1.7 Ex.74 Ex.88 Ex.102 Ex. 116 Ex.130 Ex.144
s
a Cured state 0 @ ~
1.8 Com. Ex. 21 Ex. 89 Ex. 103 Ex. 117 Ex. 131 Ex. 145
Cured state = 0 a Qo Qo 0
1.9 Com. Ex. 22 Com. Ex. 29 Ex. 104 Ex. 118 Ex. 132 Ex. 146
Cured state A A 0 0 0 a
2.0 Com. Ex. 23 Com. Ex. 30 Com. Ex. 37 Ex. 119 Ex. 133 Ex. 147
Cured state A AL A 0 0 @
2.2 Com. Ex. 24 Com. Ex. 31 Com. Ex. 38 Com. Ex. 45 Ex. 134 Ex. 148
Cured state A A A = 0 0
2.4 Com. Ex. 25 Com. Ex. 32 Com. Ex. 39 Com. Ex. 46 Com. Ex. 53 Com. Ex. 61
Cured state A I- ~ 1~ = I,
2.6 Com. Ex. 26 Com. Ex. 33 Com. Ex. 40 Com. Ex. 47 Com. Ex. 54 Com. Ex. 62
Cured state A A A A A A
2.8 Com. Ex. 27 Com. Ex. 34 Com. Ex. 41 Com. Ex. 48 Com. Ex. 55 Com. Ex. 63
Cured state A A A A A A
3.0 Com. Ex. 28 Com. Ex. 35 Com. Ex. 42 Com. Ex. 49 Com. Ex. 56 Com. Ex. 64
Cured state = A I A A A I A
Photopolymerizable resin component Oligomer D. 100 parts by weight
Rating system for the cured state: 0 Completely cured to 80 mm; 0 Completely
cured to 80
mm (but cracked); = Cured to 80 mm (but with a low hardness);
A Cured to 80 mm (but formed like millet cake); x The internal part remained
uncured (cured
only in an about 1 mm thick surface layer).
-52-
CA 02275278 1999-06-17
Table 3 (Contd.) List of the formulations of two-component
photo-initiators and ratings for the cured state
Photo-initiator 6 (wt parts)
1.0 1.1 1.2 1.3 1.4
0.4 Com. Ex. 65 Com. Ex. 74 Com. Ex. 83 Com. Ex. 93 Com. Ex. 104
Cured state x x x x x
0.5 Com. Ex. 66 Com. Ex. 75 Com. Ex. 84 Com. Ex. 94 Com. Ex. 105
Cured state x x x x x
0.6 Com. Ex. 67 Com. Ex. 76 Com. Ex. 85 Com. Ex. 95 Com. Ex. 106
Cured state x x x x x
0.7 Com. Ex. 68 Com. Ex. 77 Com. Ex. 86 Com. Ex. 96 Com. Ex. 107
Cured state x x x x x
0.8 Com. Ex. 69 Com. Ex. 78 Com. Ex. 87 Com. Ex. 97 Com. Ex. 108
Cured state x x x x x
0.9 Ex. 149 Com. Ex. 79 Com. Ex. 88 Com. Ex. 98 Com. Ex. 109
Cured state = x x x x
1.0 Ex. 150 Ex. 162 Com. Ex. 89 Com. Ex. 99 Com. Ex. 110
Cured state = = x x x
1.1 Ex. 151 Ex. 163 Ex. 175 Com. Ex. 100 Com. Ex. 111
Cured state = = = x x
1.2 Ex. 152 Ex. 164 Ex. 176 Ex. 187 Com. Ex. 112
co Cured state ~ @ = = x
a 1.3 Ex. 153 Ex.165 Ex.177 Ex. 188 Ex.198
" Cured state @ 0 @ = =
1.4 Ex. 154 Ex.166 Ex.178 Ex.189 Ex.199
Cured state ~ @ @ ~
0 1.5 Ex.155 Ex.167 Ex.179 Ex.190 Ex.200
Cured state Oo @ 0 @ @
1.6 Ex.156 Ex.168 Ex.180 Ex.191 Ex.201
o Cured state ~ @ @ @
0 1.7 Ex. 157 Ex.169 Ex.181 Ex.192 Ex.202
a Cured state @ @
1.8 Ex.158 Ex.170 Ex. 182 Ex.193 Ex.203
Cured state @ 0 @ 0 a
1.9 - Ex. 159 Ex. 171 Ex. 183 Ex. 194 Ex.204
Cured state @ @ a 0 @
2.0 Ex.160 Ex.172 Ex.184 Ex.195 Ex.205
Cured state a a 0 @ @
2.2 Ex. 161 Ex.173 Ex. 185 Ex.196 Ex.206
Cured state 0 a @ @ 0
2.4 ' Com. Ex. 70 Ex. 174 Ex. 186 Ex. 197 Ex. 207
Cured state = 0 0 O O
2.6 Com. Ex. 71 Com. Ex. 80 Com. Ex. 90 Com. Ex. 101 Com. Ex. 113
Cured state A A A A A
2.8 Com. Ex. 72 Com. Ex. 81 Com. Ex. 91 Com. Ex. 102 Com. Ex. 114
Cured state A A A A A
3.0 Com. Ex. 73 Com. Ex. 82 Com. Ex. 92 Com. Ex. 103 Com. Ex. 115
Cured state A A A A A
Photopolymerizable resin component Oligomer D. 100 parts by weight.
Rating system for the cured state: QO Completely cured to 80 mm; 0 Completely
cured to 80
mm (but cracked): = Cured to 80 mm (but with a low hardness);
A Cured to 80 mm (but formed like millet cake): x The internal part remained
uncured (cured
only in an about 1 mm thick surface layer).
-53-
CA 02275278 1999-06-17
Table 3 (Contd.) List of the formulations of two-component
photo-initiators and ratings for the cured state
Photo-initiator 6 (wt parts)
1.5 1.7 1.8 2.0 2.2
0.4 Com. Ex. 116 Com. Ex. 129 Com. Ex. 143 Com. Ex. 157 Com. Ex. 172
Cured state x x x x x
0.5 Com. Ex. 117 Com. Ex. 130 Com. Ex. 144 Com. Ex. 158 Com. Ex. 173
Cured state x x x x x
0.6 Com. Ex. 118 Com. Ex. 131 Com. Ex. 145 Com. Ex. 159 Com. Ex. 174
Cured state x x x x x
0.7 Com. Ex. 119 Com. Ex. 132 Com. Ex. 146 Com. Ex. 160 Com. Ex. 175
Cured state x x x x x
0.8 Com. Ex. 120 Com. Ex. 133 Com. Ex. 147 Com. Ex. 161 Com. Ex. 176
Cured state x x x x x
0.9 Com. Ex. 121 Com. Ex. 134 Com. Ex. 148 Com. Ex. 162 Com. Ex. 177
Cured state x x x x x
1.0 Com. Ex. 122 Com. Ex. 135 Com. Ex. 149 Com. Ex. 163 Com. Ex. 178
Cured state x x x x x
1.1 Com. Ex. 123 Com. Ex. 136 Com. Ex. 150 Com. Ex. 164 Com. Ex. 179
Cured state x x x x x
N 1.2 Com. Ex. 124 Com. Ex. 137 Com. Ex. 151 Com. Ex. 165 Com. Ex. 180
Cured state x x x x x
~- 1.3 Com. Ex. 125 Com. Ex. 138 Com. Ex. 152 Com. Ex. 166 Com. Ex. 181
Cured state x x x x x
1.4 Ex. 208 Com. Ex. 139 Com. Ex. 153 Com. Ex. 167 Com. Ex. 182
Cured state = x x x x
y 1.5 Ex. 209 Com. Ex. 140 Com. Ex. 154 Com. Ex. 168 Com. Ex. 183
.2 Cured state oQ x x x x
1.6 Ex. 210 Ex. 217 Com. Ex. 155 Com. Ex. 169 Com. Ex. 184
Cured state @ = x x x
0 1.7 Ex. 211 Ex. 218 Ex. 225 Com. Ex. 170 Com. Ex. 185
a Cured state oQ Qo = x x
1.8 Ex. 212 Ex. 219 Ex. 226 Ex. 233 Com. Ex. 186
Cured state oQ Qo oQ = x
1.9 Ex. 213 Ex. 220 Ex. 227 Ex. 234 Com. Ex. 187
Cured state oQ oQ oQ = x
2.0 Ex. 214 Ex. 221 Ex. 228 Ex. 235 Ex. 240
Cured state Oo Qo Qo 0 =
2.2 Ex. 215 Ex. 222 Ex. 229 Ex. 236 Ex. 241
Cured state Oo Qo oQ Qo Qo
2.4 Ex. 216 Ex. 223 Ex. 230 Ex. 237 Ex.242.
Cured state Qo oQ Qo oQ Qp
2.6 Com. Ex. 126 Ex. 224 Ex. 231 Ex. 238 Ex. 243
Cured state A 0 0 a QO
2.8 Com. Ex. 127 Com. Ex. 141 Ex. 232 Ex. 239 Ex. 244
Cured state A A 0 Q Q
3.0 Com. Ex. 128 Com. Ex. 142 Com. Ex. 156 Com. Ex. 171 Ex. 245
Cured state = A A A 0
Photopolymerizable resin component Oligomer 1, 100 parts by weight
Rating system for the cured state: 0 Completely cured to 80 mm; 0 Completely
cured to 80
mm (but cracked); = Cured to 80 mm (but with a low hardness);
A Cured to 80 mm (but formed like millet cake); x The internal part remained
uncured (cured
only in an about 1 mm thick surface layer).
-54-
CA 02275278 1999-06-17
Table 4 List of resin compositions and samples for measuring
the resin temperature during curing
Composition No.
Comparative Example 24 Composition No. Q
Comparative Example 37 Composition No.
Example 130 Composition No.
Example 143 Composition No.
Example 155 Composition No. 50
Example 166 Composition No.
Comparative Example 89 Composition No. Q
Comparative Example 59 Composition No.
Example 110 Composition No.
Example 125 Composition No.
Example 182 Composition No.
-55-
CA 02275278 1999-06-17
Example 246
The same resin composition as described in Example 1(A)
was prepared, and a sample was constructed in the same manner
as described in Example 1(B).
This sample was irradiated with UV radiation under the
same conditions as described in Example 1(C), except that the
irradiation distance was 25 cm.
After UV irradiation, the resin within the glass vessel
was completely cured in several minutes. The wall thickness
of the resin was 80 mm (the greatest measurable value) which
was the limit defined by the glass vessel (see FIG. 11).
Example 247
The same resin composition as described in Example 1(A)
was prepared, and a sample was constructed in the same manner
as described in Example 1(B).
This sample was irradiated with UV radiation under the
same conditions as described in Example 1(C), except that the
irradiation distance was 20 cm.
After UV irradiation, the resin within the glass vessel
was completely cured in several minutes. The wall thickness
of the resin was 80 mm (the greatest measurable value) which
was the limit defined by the glass vessel (see FIG. 11).
Example 248
The same resin composition as described in Example 1(A)
was prepared, and a sample was constructed in the same manner
-56-
CA 02275278 1999-06-17
as described in Example 1(B).
This sample was irradiated with UV radiation under the
same conditions as described in Example 1(C), except that the
irradiation distance was 15 cm.
After UV irradiation, the resin within the glass vessel
was completely cured in several minutes. The wall thickness
of the resin was 80 mm(the greatest measurable value) which
was the limit defined by the glass vessel (see FIG. 11).
Example 249
The same resin composition as described in Example 1(A)
was prepared, and a sample was constructed in the same manner
as described in Example 1(B).
(D) This sample was irradiated with UV radiation under
the following conditions.
Ultraviolet irradiator: UVL-3500 M2 (manufactured by Ushio
Inc.)
Type of lamp: Metal halide lamp.
Intensity of lamp: 120 W/cm.
Length of lamp: 250 mm.
Atmosphere, temperature and pressure: Air, room temperature
and atmospheric pressure.
Irradiation distance: 19 cm.
Irradiation time: 60 seconds.
After UV irradiation, the resin within the glass vessel
was completely cured in several minutes. The wall thickness
-57-
CA 02275278 1999-06-17
of the resin was 80 mm (the greatest measurable value) which
was the limit defined by the glass vessel.
Example 250
The same resin composition as described in Example 1(A)
was prepared, and a sample was constructed in the same manner
as described in Example 1(B).
This sample was irradiated with UV radiation under the
same conditions as described in Example 247(D), except that
the intensity of the lamp was 200 W/cm.
After UV irradiation, the resin within the glass vessel
was completely cured in several minutes. The wall thickness
of the resin was 80 mm (the greatest measurable value) which
was the limit defined by the glass vessel.
Example 251
The same resin composition as described in Example 1(A)
was prepared, and a sample was constructed in the same manner
as described in Example 1(B).
This sample was irradiated with UV radiation under the
same conditions as described in Example 247(D), except that
the intensity of the lamp was 280 W/cm.
After UV irradiation, the resin within the glass vessel
was completely cured in several minutes. The wall thickness
of the resin was 80 mm (the greatest measurable value) which
was the limit defined by the glass vessel.
Example 252
-58-
CA 02275278 1999-06-17
(E) The same resin composition as described in Example
1(A) was prepared and used as a matrix resin. Then, prepregs
were made by impregnating 18 cm x 18 pieces of CF cloth with
this matrix resin.
(E) A prepreg laminate sample was made by stacking 40
such prepregs (to a thickness of about 8 mm), interposing
them between glass plates through the medium of bag films,
and applying weight thereto from above (FIG. 12).
This sample was irradiated with UV radiation under the
same conditions as described in Example 1(C), except that the
irradiation time was 3 minutes and the irradiation distance
was 15 cm.
After UV irradiation, the laminate was completely cured
to give a satisfactory CFRP (the related data are shown in
Table 5).
Table 5 Data on Properties of FRPs
Item GFRP CFRP
Tensile strength (kgf/cm2) 3100 7100
Bending strength (kgf/cm2) 3400 3000
Fiber content (wt. %) 59.2 52.3
Fiber content (vol. %) 38.0 41.7
Specific gravity (g/cm3) 1.71 1.43
Void fraction (vol. %) 1.99 0.73
Heat resistance (Tr, C) 150 150
Torsional elastic modulus (GPa) 2.2 2.2
-59-
CA 02275278 1999-06-17
Example 253
A prepreg laminate sample (with a thickness of about 8
mm) was made in the same manner as described in Example
252(E), except that 18 x 18 of GF cloth were used as the
reinforcing fibrous material.
This sample was irradiated with UV radiation under the
same conditions as described in Example 252.
After UV irradiation, the laminate was completely cured
to give a satisfactory GFRP (the related data are shown in
Table 5).
Example 254
A prepreg laminate sample was made in the same manner as
described in Example 252(E), except that 100 prepregs were
stacked (to a thickness of about 20 mm)
This sample was irradiated with UV radiation under the
same conditions as described in Example 252.
After UV irradiation, the laminate was completely cured
to give a satisfactory CFRP.
Example 255
A prepreg laminate sample was made in the same manner as
described in Example 252(E), except that the resin
composition of Example 13 was used as the matrix resin.
This sample was irradiated with UV radiation under the
same conditions as described in Example 252.
After UV irradiation, the laminate was completely cured
-60-
CA 02275278 1999-06-17
to give a satisfactory CFRP.
Example 256
A prepreg laminate sample was made in the same manner as
described in Example 252(E).
An electron beam (EB) was used as the energy radiation.
The EB irradiation was carried out under the following
conditions.
Irradiator: Linac (High Voltage Alco, Ltd.).
Beam energy: 10 MeV.
Scanning frequency: 4 Hz.
Pulse repetition rate: 60 Hz.
Scanning width: 20 cm.
Pulse width: 4 usec.
Radiation dose: 50 kGy.
After UV irradiation, the laminate was completely cured
to give a satisfactory CFRP.
ExamDle 257
(F) A matrix resin was prepared in the same manner as
described in Example 252(E). Carbon fiber was impregnated
with this matrix resin and then wound at a winding speed of
cm/sec (according to a filament winding technique) to form
a cylindrical laminate material made of CFRP (with a wall
thickness of 3 mm).
After completion of the winding, the cylindrical
25 laminate material was irradiated with UV radiation from all
-61-
CA 02275278 1999-06-17
directions (under the same conditions as described in Example
252).
After UV irradiation, the laminate material was
completely cured to give a satisfactory filament-wound CFRP.
Example 258
A cylindrical laminate material made of CFRP (with a
wall thickness of 3 mm) was formed in the same manner as
described in Example 257(F), except that glass fiber was used
as the reinforcing fiber.
After completion of the winding, the cylindrical
laminate material was irradiated with UV radiation from all
directions (under the same conditions as described in Example
252).
After UV irradiation, the laminate material was
completely cured to give a satisfactory filament-wound GFRP.
Example 259
Using a resin composition prepared by mixing 100 parts
by weight of Celoxide 2021P (oligomer 0; an alicyclic epoxy
resin manufactured by Daicel Chemical Industries Ltd.;
3,4-cyclohexylmethyl 3,4-epoxycyclohexanecarboxylate) with
1.50 parts by weight of San Aid SI-80L {photo-initiator OO; a
cationic photo- and thermopolymerization initiator
manufactured by Sanshin Chemical Co., Ltd.; general formula
(II)}, 0.50 part by weight of DAICAT 11 (photo-initiator ;
a cationic photopolymerization initiator manufactured by
-62-
CA 02275278 1999-06-17
Daicel Chemical Industries Ltd.; an arylsulfonium salt), 0.50
part by weight of
4,4'-bis(di(R-hydroxyethoxy)phenylsulfonio)phenyl sulfide
bis-hexafluoroantimonate (photo-initiator Q3 ), and 0.50 part
by weight of 2-butynyltetramethylenesulfonium
hexafluoroantimonate {photo-initiator ; general formula
(IX)}, a test was carried out under the same conditions as
described in Example 1.
After UV irradiation, the resin within the glass vessel
was completely cured in several minutes. The wall thickness
of the resin was 80 mm (the greatest measurable value) which
was the limit defined by the glass vessel.
Example 260
Using a resin composition prepared by mixing 100 parts
by weight of Celoxide 2021P (oligomer (1); an alicyclic epoxy
resin manufactured by Daicel Chemical Industries Ltd.;
3,4-cyclohexylmethyl 3,4-epoxycyclohexanecarboxylate) with
1.50 parts by weight of San Aid SI-80L {photo-initiator OO; a
cationic photo- and thermopolymerization initiator
manufactured by Sanshin Chemical Co., Ltd.; general formula
(II)}, 1.00 part by weight of DAICAT 11 (photo-initiator ;
a cationic photopolymerization initiator manufactured by
Daicel Chemical Industries Ltd.; an arylsulfonium salt), and
0.50 part by weight of prenyltetramethylenesulfonium
hexafluoroantimonate {photo-initiator Q; general formula
-63-
CA 02275278 1999-06-17
(VIII)], a test was carried out under the same conditions as
described in Example 1.
After UV irradiation, the resin within the glass vessel
was completely cured in several minutes. The wall thickness
of the resin was 80 mm (the greatest measurable value) which
was the limit defined by the glass vessel.
Comparative Examples 188 to 190
Tests were carried out in all the same manner as
described in Examples 246 to 248, except that the composition
of Comparative Example 1 was used as the resin composition.
After UV irradiation, the wall thickness of the resin
was about 1 mm, and its inner part remained uncured (see FIG.
11).
Comparative Example 191
A prepreg laminate sample was made in the same manner as
described in Example 252(E), except that the resin
composition of Comparative Example 1 was used as the matrix
resin.
This sample was irradiated with UV radiation under the
same conditions as described in Example 252.
After UV irradiation, the CFRP was cured only in the
first surface layer on the irradiated side, and the inner
part of the resin remained entirely uncured.
Comparative Example 192
A prepreg laminate sample was made in the same manner as
-64-
CA 02275278 1999-06-17
described in Example 253, except that the resin composition
of Comparative Example 1 was used as the matrix resin.
This sample was irradiated with UV radiation under the
same conditions as described in Example 252.
After UV irradiation, the GFRP was cured only up to the
second or third layer on the irradiated side, and the inner
part of the resin remained entirely uncured.
Examples 261 to 282
Tests were carried out under the same conditions as
described in Example 1, except that the resin compositions
shown in Table 1 were used and tested according to the
formulations shown in Tables 6 (continued from Table 2). The
test results thus obtained are shown in Table 6.
-65-
CA 02275278 1999-06-17
aLblq_B,, Ust of the formulations of resin compositions (continued from Table
2)
Photo- and Photo-and Cured wall
Ex. or Photopolymerizable resin thermopolymerization initiator
thermopolymerization thickness
Com.Ex. initiator (mm)
No.
Proportion Photo-initiator Proportion Photo-initiator Proportion (max 80
Oligomer No. ~ a~s No. (wt. arts No. (wt. arts mm)
Ex.261 Oligomer /
Oligomer Q
Ex.262 Oligomer /
Oli omer l
Ex.263 Oligomer /
Oli omer 1
Ex. 264 Oligomer 1 /
Oli mer 1
Ex. 265 Oligomer 1 /
Oligomer 1
Ex. 266 Oligomer 1 /
Oligomer 1
Ex. 267 Oligomer 1 /
Oli omer 1
Ex.268 Oligomer /
Oli omer (1)
Ex. 269 Oligomer [t /
Oligomer 1
Ex. 270 Oligomer [2]/ 50/50 Photo-initiator
Oli omer 1 1 75 Photo-initiator 0.75 80
Ex. 271 Oligomer 3 /
Oli omer 1
Ex. 272 Oligomer [4]/
Oligomer 1
Ex. 273 Oligomer [5 /
Oligomer 1
Ex.274 Oligomer 6]/
Oligomer 1
Ex.275 Oligomer 7]/
Oligomer 1
Ex. 276 Oligomer 8 /
Oligomer
Ex. 277 Oligomer [91/
Oligomer 1
Ex. 278 Oligomer 10 /
li omer
Ex. 279 Oligomer 11 /
li omer
Ex. 280 Photo-initiator t
Ex. 281 Oligomer Q 100 Photo-initiator Ex. 282 Photo-initiator
-66-
CA 02275278 1999-06-17
Example 283
The same resin composition as described in Example 1(A)
was prepared, and a sample was constructed in the same manner
as described in Example 1(B).
The constructed sample was heated in an oven kept at
150 C, instead of being irradiated with energy radiation.
The resin within the glass vessel was completely cured
in a little less than 10 minutes after the start.of heating.
The wall thickness of the resin was 80 mm (the greatest
measurable value) which was the limit defined by the glass
vessel.
Example 284
The same resin composition as described in Example 1(A)
was prepared, and a sample was constructed in the same manner
as described in Example 1(B).
The constructed sample was placed in an oven adjusted to
a temperature in a range which did not cause its cure (60 C
in this example), and held therein until the resin
temperature became equal to the temperature of the atmosphere
within the oven. Thereafter, the sample was taken out of the
oven and tested under the same conditions as described in
Example 1.
After UV irradiation, the resin within the glass vessel
was completely cured in several minutes (in a shorter time
than in Example 1). The wall thickness of the resin was 80
-67-
CA 02275278 1999-06-17
mm (the greatest measurable value) which was the limit
defined by the glass vessel.
INDUSTRIAL APPLICABILITY
Resin composition capable of inducing the novel resin curing
mechanism
It can be seen from the test results of Examples 1-60,
Examples 259-282 and Comparative Examples 1-20 shown in
Tables 1-3 and Table 6 that the compositions of the present
invention involving the novel resin curing mechanism are
excellent in energy radiation curability, particularly
thick-wall curability. Moreover, it can be seen from Example
284 that it is more effective for curing purposes to warm a
composition of the present invention previously (in a
temperature range which does not cause its cure) and then
expose it to energy radiation. Furthermore, it has been
confirmed by Example 283 that a composition of the present
invention containing a photopolymerization initiator
comprising at least two components can also be cured in a
short period of time by the application of heat.
Photopolymerization initiator systems comprising at least two
components capable of inducing the novel resin curinq
mechanism and their proper compositional ranae
The effectiveness of photopolymerization initiator
systems comprising at least two components capable of
inducing the novel resin curing mechanism and their proper
-68-
CA 02275278 1999-06-17
compositional range are evident from the test results of
Examples 1-245 and Comparative Examples 1-187 shown in Tables
1-3 and FIG. 9.
Verification of the novel resin curing mechanism
Among the results of Examples 1-245 and Comparative
Examples 1-187, curves showing a rise in resin temperature
due to the curing heat exotherm of the resin when each of the
compositions shown in Table 4 was exposed to energy radiation
are depicted in FIG. 10. Moreover, curves showing a rise in
resin temperature due to the curing exotherm of the resin
when each of the compositions of Examples 246-248 and
Comparative Examples 188-190 was exposed to energy radiation
are depicted in FIG. 11. It is evident from FIGs. 10 and 11
that the resin compositions of the present invention are
cured on the basis of the novel resin curing mechanism in
which energy other than the energy from the energy radiation
source, i.e. heat energy arising from the heat of curing
reaction (curing exotherm) in this case, is autogenously
generated within the resin, so that the resin composition is
cured by means of both the heat energy arising from the
curing reaction and the energy from the energy radiation
source.
Moreover, it can be confirmed by the test results of
Examples 246-251 that the novel resin curing mechanism of the
present invention is effective even if the conditions of
-69-
CA 02275278 1999-06-17
irradiation with energy radiation are varied.
Verification of the curability of CFRPs (thick-walled resins
containing an energy radiation screening substance) and GFRPs
It is evident from the results of Examples 252-258 and
Comparative Examples 191-192 that the photo-curing (energy
radiation curing) of CFRPs (thick-walled resins containing an
energy radiation screening substance) and the photo-curing
(energy radiation curing) of GFRPs and the like, which have
been impossible with conventional photo-curable resins, can
be achieved by the novel resin curing mechanism of the
present invention and the photopolymerization initiator
systems comprising at least two components and resin
compositions which are capable of inducing this mechanism.
Moreover, it can be confirmed by Example 256 that the
novel resin curing mechanism of the present invention, the
photopolymerization initiator systems comprising at least two
components and resin compositions which are capable of
inducing this mechanism, and the method of making FRPs
(CFRPs) in accordance with the present invention may also be
applied to the EB curing of FRPs (CFRPs).
Furthermore, it is evident from Example 257 that the
method of making FRPs (CFRPs) in accordance with the present
invention may be applied not only to a lay-up process, but
also to other FRP molding processes such as a filament
winding process.
-70-
CA 02275278 1999-06-17
Molded articles of CFRP and GFRP made according to the
present invention
Basic properties of the CFRP and GFRP made in Examples
252 and 253 were measured, and the results are shown in Table
5. It can be seen from Table 5 that they were satisfactory
samples.
-71-