Note: Descriptions are shown in the official language in which they were submitted.
CA 02276087 1999-06-25
w0 98/45457 PCTIUS97/23495
TRANSGENIC PLANTS WTfH MODIFIED STEROL BIOSYNTHETIC PATHWAYS
FIELD OF THE INVENTION
The present invention broadly relates to plant genetic engineering. More
particularly, it
concerns the manipulation of the levels andlor activities of endogenous plant
phytosterol
compositions as a strategy for minimizing crop damage due to plant insects and
other
pests, and/or for improving the nutritional value of plants.
BACKGROUND OF THE INVENTION
1 o Productivity in agricultural industries can be adversely affected by
various
environmental stresses, including drought, severe cold, weeds, and organisms
that feed
on crops. Conventional approaches for alleviating weeds and parasitic
organisms have
relied almost exclusively on chemical herbicides, pesticides and fungicides.
Widespread
use of these agrochemicals, however, has led to development of resistance. In
fact,
insect resistance has been reported against most major classes of insecticides
including
organophosphates, chlorinated hydrocarbons, and carbamates.
Sterols comprise a class of essential natural compounds required to some
extent by all
eukaryotic organisms. They have a common tetracyclic steroid nucleus and a
side
2 o chain, as shown in the diagram below. Some sterols serve a structural role
in cell
membranes, while others are required during development.
Plants produce more than 250 different phytosterols (Akisha et al., 1992). As
many as
60 sterols have been identified in the single species, Zea mat's (corn) (Guo
et ai., 1995).
2 5 However, insects, fungi and nematodes, as well as many other sterol-less
parasitic
organisms, do not synthesize all of their necessary sterols de novo. Rather,
they satisfy
. their nutritional requirements for sterols by feeding on plants. This fact
has been
utilized in the development of commercial agrochemicals such as triazoles,
pyrimidines
' and azasterols, which act by interfering with production of sterols within
parasitic
3 0 organisms.
-1-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
20R
"right-handed"
29
2s From
H C-2MVA
2t~
O Asymmetric center zs
is zo~~~~~
z ~~ H
nucleus ~ ~ 2~ '25S
is is
t ~ C3 D (25 ay
8
p a d aquitorial z to 4 5
lies i plan of molecule A g ~ F~
C-s(3m)MVA
HO 3 n lies to front of
ao'~, t s ~ side chain
a a a>oal
lies ck to molecule
V ~ 24 a.alkyl is R,
an a~a , but S when a a
a and equatorial) lies out of plane of molecule present
lies in plane of molecule
p lies to back of
24 ~i.alkyl is S,
but R when 4 ~
present
Recent advances in molecular biology have made it possible to introduce
advantageous
traits into plants via genetic engineering. Some forms of insect resistance
have been
introduced into plants by genetic approaches. For example, transgenic plants
expressing
foreign genes encoding endotoxins of Bacillus thuringiensis (Bt) can confer on
the plants
the ability to kill pests which feed on them. Unfortunately, approaches such
as this are
1 o effective only against the particular insects susceptible to the
endotoxin. There remains
in the agricultural industries a continual need for alternative pest control
strategies,
particularly those that could be broadly effective against numerous
pests/pathogens.
-2-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
SUMMARY OF THE INVENTION
'The present invention broadly relates to approaches for genetically
engineering plants to
have altered sterol compositions, levels and/or metabolism. Such approaches
can
increase the plants natural insect resistance, can increase the plants
resistance to drought
and cold, and/or can improve the nutritional/health value of the plants.
In accordance with one aspect of the invention, there are provided recombinant
DNA
molecules comprising:
a promoter which functions in plants to cause the production of an RNA
sequence,
operably linked to
1 o a DNA coding sequence encoding an enzyme which binds a first sterol and
produces a
second sterol, operably linked to
a 3' non-translated region which causes the polyadenylation of the 3' end of
the RNA
sequence; wherein the promoter is heterologous with respect to the DNA
sequence.
The DNA coding sequence encoding an enzyme which binds a first sterol and
produces
a second sterol can be in the sense or antisense orientation. Thus, the DNA
molecule of
the invention can encode a non-translatable RNA molecule (e.g., antisense or
cosuppression) or a protein molecule. The RNA or protein so produced
selectively
targets the expression and/or activity of a sterol biosynthetic enzyme to
affect a desired
2 o change in the phytosterol profile of the plant.
Therefore, in accordance with another aspect of the present invention, there
is provided
an approach for modifying the sterol composition of plants to increase their
resistance to
insects, nematodes, and pythiaceous fungi. This aspect of the invention
enhances the
2 5 plant's ability to resist pests and disease by modifying the composition
and/or
distribution profile of certain phytosterols. Such an approach overcomes many
of the
limitations inherent in the use of agrochemicals, or with transgenic plants
where the
foreign product introduced into the plant has the potential to eventually
select for new
mechanisms of resistance by the pest. The present invention retains the
benefits
-3-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
obtained through the use of agrochemicals, but avoids many of their
disadvantages. By
targeting an existing essential pathway in pests and pathogens, this invention
reduces the
likelihood of the evolution of mechanisms which circumvent this pathway.
Plant sterol composition is modified in this aspect by increasing the amount
of non-
utilizable sterols such as 4-methyl sterol, 9/3,19-cyclopropyl sterol, 07-
sterol, ~g-sterol,
14a-methyl sterol, ~23cza~-24-alkyl sterol, ~2~5~,24-alkyl sterol or
02sc2~>,24-alkyl sterol.
Alternatively, sterol compositions can be modified to contain lower levels of
sterols
having a DS group.
Another aspect of the present invention relates to producing sterols in plants
that confer
resistance to drought and cold in plants.
Another aspect of the invention relates to altering the sterol profile of
plants such that
levels of cholesterol-lowering sterols are increased.
The aspects of the invention described herein are typically achieved by
modifying the
expression andlor activities of sterolic enzymes, preferably S-adenosyi-L-
methionine-
02a-sterol methyl transferases (SMTI and SMT~I), C-4 demethylase,
cycloeucalenol to
2 0 obtusifoliol-isomerase, 14a-methyl demethylase, ~8 to o7-isomerase, D'-
sterol-C-5-
desaturase, or 24,25-reductase.
Another aspect of the invention is directed to transgenic plants having
altered levels of
selected sterols, produced by introducing recombinant DNA molecules of the
invention
into the genome of plant cells and selecting for cells expressing said
molecule.
Transgenic plants are regenerated from the transformed plant cells and plants
containing
the recombinant DNA are grown to maturity. Plants expressing the recombinant
DNA
are identified and those having a desired sterol profile in accordance with
the present
invention are selected and propagated.
._
CA 02276087 1999-06-25
WO 98/45457 PCTIUS97I23495
BRIEF DESCRIPTION OF THE DRAWINGS
The following drawings form part of the present specification and are included
to further
demonstrate certain aspects of the present invention. The invention may be
better
understood by reference to one or more of these drawings in combination with
the
detailed description of specific embodiments presented herein.
Fig. 1 shows HPLC radiocount (panel B) and mass spectrum (panel A) results of
testing
SMT enzyme with radiolabeled substrate co-factor;
Fig. 2 shows six inhibitors used to test the SMT enzyme;
Fig. 3 shows SMT activity during seedling development;
Fig. 4 shows the pathway of sterol end-products during development of
seedlings;
Fig. 5 shows the yeast SMT gene sequence (panel B; SEQ ID NO:I) and the
deduced
amino acid sequence (panel A; SEQ ID N0:2) with the predicted conserved
regions
highlighted;
2 o Fig. 6 shows the Arabidopsis SMT gene (panel B; SEQ ID N0:3) and deduced
amino
acid {panel A; SEQ ID N0:4) sequences;
Fig. 7 shows the ERG6 constructs prepared with pUCl8cpexp expression cassette;
2 5 Fig. 8 shows sequences of yeast SMT gene (SEQ ID NO:S). Underlined
sequences are
those used as primers for screening genomic DNA from transgenic tomato plants;
and
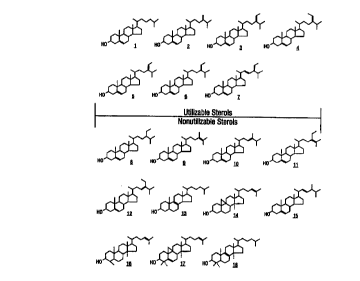
Fig. 9 shows structures of plant sterols tested on Heliothis zea and found to
be utilizable
or non-utilizable.
-5-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Figure 10 (SEQ ID N0:6) shows the nucleotide and amino acid sequences of the
corn
..SMT gene.
DESCRIPTION OF ILLUSTRATIVE EMBODIIVVIENTS
PHYTOSTEROLS
The phytosterol metabolic pathway consists of enzymes that act on the
tetracyclic ring
nucleus and the side chain. The major pathway in advanced vascular plants
starts from
cycloartenol (I):
(I)
HO
and ends with DS-24-alkyl sterols, predominantly sitosterol (II), stigmasterol
(IIn and
campesterol (IV):
(II)
HO
-6-
CA 02276087 1999-06-25
WO 98/45457 PCTIUS97I23495
(III)
HO
(IV)
HO
The number of alternate pathways is su~ciently great to produce as many as 60
or more
different sterols in a single plant. These alternate pathways vary according
to tissue-
1o and development-specific genetic programs.
Studies of sterol metabolism have utilized inhibitors of sterol biosynthesis.
These
inhibitors include several commercial fungicides which block sterol metabolic
pathways
in plant pathogenic fungi and thereby inhibit their growth. The following
steps of the
major metabolic pathway were determined using metabolic inhibitors. The major
pathway consists of the 12 chemical transformations as follows.
CA 02276087 1999-06-25
WO 98/45457 PCT/US9~123495
In reaction 1, the enzyme S-adenosyl-L-methionine-sterol-C-24 methyl
transferase
-(SMT~) catalyzes the transfer of a methyl group from a cofactor, S-adenosyl-L-
methionine, to the C-24 center of the sterol side chain. The circled sterol
feature is the
functional group undergoing transformation.
1
HO
HO
Cycloartenol 24(28)-Methylenecycloartanol
This is the first of two methyl transfer reactions, and is an obligatory and
rate-limiting
1 o step of the sterol-producing pathway in plants. A different SMT enzyme,
SMTI~,
catalyzes the conversion of cycloartenol to a X23(24)-24-alkyl sterol,
cyclosadol (Guo et
al . , 1996) .
Reaction 2 involves a demethylation at C-4. This is the first of several
demethylation
reactions in the nucleus.
2
HO
H
24(28)-Methylenecycloartanol Cycloeucalenol
_g_
CA 02276087 1999-06-25
WO 98/45457 PCTIUS97I23495
Reaction 3 involves opening the cyclopropyl ring at C-9( 10) by the enzyme
.cycloeucalenol-obtusifoliol isomerase (COI), which also creates a double bond
at C-8.
3
HO
i
Cycloeucalenol Obtusifoliol
Reaction 4 involves a demethylation at C-14 which removes the methyl group at
C-14
and creates a double bond at C-14.
4
HO
HO
4a-Methyl-Sa-ergosta-
Obtusifoliol 8,24(28)-trien-ø-of
to
Reaction 5 is catalyzed by a ~I4 reductase.
5
. HO , HO
4a-Methyl-Sa,-ergosta- 4a-Methyl-Sa,-ergosta-
8,24(28)-trien~i-of -9- 8,24(28}-dien-~3-of
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Reaction 6 involves a O8- to O'-isomerase reaction which produces a d' group.
6
Ho
HO
4a-Methyl-Sa-ergosta-
8,24(28)-dien-3(3-0l
24-Methylenelophenol
Reaction 7 is a second C- methylation of the sterol side chain. The reaction
is catalyzed
by SMTI, the same enzyme that initiated the major pathway.
io
7
HO , HO
24-Methylenelophenol 24-Ethylidenelophenol
(citrastadienol)
Reaction 8 involves a C-4. demethylase to generate a 4,4-desmethyl sterol.
8
24-Ethylidenelophenol -10-
(citrastadienol)
Avenasterol
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
' 5 Reaction 9 involves a OS desaturase, producing a double bond at C-5 in the
tetracyclic
ring.
9
HO
Stigmasta-5 , 7,24(L4' }-
Avenasterol trien-3~i-of
1 o The product of reaction 9 is then transformed in reaction 10 by a ~~-
reductase by
removing the double bond at C-7.
HO
Isofucosterol
Stigmasta-5,7,24(24')
trien-3~i-of
Reaction 11, involves a ~2'~28~- to 024~~~-isomerase which modifies the side
chain. (It is
believed that this reaction would have proceeded from the product of reaction
5 if the
kinetics were more favorable.)
-11-
- CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
HO
11
Stigmasta-5,24-dienol
Isofucosterol
Reaction 12: the ~2°~25) double bond at C-24 is reduced
stereoselectively to produce
sitosterol (li).
12
HO HO
Stigmasta-5,24-dienol
Sitosterol
In addition to this major pathway of sterol biosynthesis, it has been found
that a
developmental program regulates expression of the SMT enzymes. In corn,
enzymology
studies have shown that two different SMT enzymes exist (SMTI and SMT~I) whose
expression depends on the tissue and stage of differentiation. Blades mainly
contain 24-
ethyl sterols (resulting from the activity of SMTI), whereas the sheaths
contain mainly
24-methyl sterols (VI) (resulting from the activity of SMTII). These sterols
are the
products of the two different SMT enzymes that react with the same starting
material,
cycloartenoi.
The first enzyme, SMTI, produces p2aczs>-methylene and the second enzyme
produces
- 0~~2°~-methyl sterol (~. The first isoform leads to a utilizable
sterol (a sterol which can
-12-
CA 02276087 1999-06-25
WO 98145457 PCTIUS97/23495
be utilized by insects, pythiaceous fungi, and nematodes to complete their
life cycles).
The second isoform produces a non-utilizable sterol (a sterol which cannot be
utilized by
insects, pythiaceous fungi, and nematodes to complete their life cycles).
Therefore, one
could inhibit expression of the first isoform so as to cause accumulation of
the non-
utilizable ~23c24~-methyl sterols.
(V)
HO
Cyclosadol 15
As a result, the sterols that accumulate in the tissue contain a double bond
at C-23 (VI)
and a methyl at C-24.
H
24-methyl cholesta-5,23-dienol
-13-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
RECOMBINANT DNA MOLECULES:
In order to achieve a desired alteration in sterol composition, the invention
provides
recombinant DNA molecules for use in the production of transgenic plants. A
recombinant DNA molecule of the invention generally comprises a promoter
region
capable of causing the production of an RNA sequence in plants, a structural
DNA
sequence, and a 3' non-translated region.
Transcription of DNA into mRNA is regulated by the region of a gene referred
to as the
"promoter" . The promoter region contains a sequence of bases that signals RNA
polymerase to associate with the sense and antisense DNA strands and to use
the sense
strand as a template to make a corresponding strand of mRNA complimentary to
the
sense DNA strand. This process of mRNA production using a DNA template is
commonly referred to as gene "expression" or "transcription" .
In the recombinant DNA molecules of the invention, it is generally preferred
that the
promoter is heterologous with respect to the DNA coding sequence. The term
"heterologous" with respect to a promoter means that the DNA coding sequence
of a
recombinant DNA molecule of the invention is not derived from the same gene tc
which
the promoter is attached.
Promoters may be obtained from a variety of sources, such as plants and plant
viruses.
The particular promoters selected for use in embodiments of the present
invention
should preferably be capable of causing the production of sufficient
expression to affect
the desired change in the sterol distribution profile of the plant.
A number of promoters which are active in plant cells have been described in
the
literature, and are suitable for use in the DNA molecules of this invention.
These
include, for example, the cauliflower mosaic virus (CaMV) 35S promoter (Odell
et al.
1985), the Figwort mosaic virus (FMV) 35S (Sanger et al. 1990), the sugarcane
3 0 bacilliform virus promoter (Bouhida et al., 1993), the commelina yellow
mottle virus
-14-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
promoter (Medberry and Olsewski 1993), the light-inducible promoter from the
small
~ubunit of the ribulose-1,5-bis-phosphate carboxylase (ssRUBISCO) (Coruzzi et
al.,
1984), the rice cytosolic triosephosphate isomerase (TPI) promoter {Xu et al.
1994}, the
adenine phosphoribosyltransferase {APRT) promoter of Arabidopsis (Moffatt et
al.
1994), the rice actin 1 gene promoter (Zhong et al. 199b), the mannopine
synthase and
octopine synthase promoters (Ni et al. 1995). All of these promoters have been
used to
create various types of DNA constructs which have been expressed in plants.
Recombinant DNA molecules also typically contain a S' non-translated leader
sequence.
1 o This sequence can be derived from the promoter selected to express the
gene, and if
desired, can be specifically modified so as to increase translation of the
mRNA. The 5'
non-translated regions can also be obtained from viral RNAs, from suitable
eukaryotic
genes, or from synthetic gene sequences.
The structural DNA sequence of the recombinant DNA molecule of the invention
will
cause the desired alteration in the sterol profile of the plant, as discussed
further below.
The 3' non-translated region of a recombinant DNA molecule of the invention
can be
obtained from various genes which are expressed in plant cells. For example,
the
2 0 nopaline synthase 3' untranslated region (Fraley et al . 1983), the 3'
untranslated region
from pea ssRUBISCO (Coruzzi et al. 1994), and the 3' untranslated region from
soybean 7S seed storage protein gene (Schuler et al. 1982) are frequently
used. The 3'
non-translated region of a recombinant DNA molecules contains a
polyadenylation
signal which functions in plants to cause the addition of adenylate
nucleotides to the 3'
2 5 end of the RNA.
Other desired regulatory sequences known to the skilled individual, or
combinations
thereof, can be included in a recombinant DNA molecule of the invention. For
example, intron sequences are frequently included in recombinant DNA molecules
used
3 0 for producing transgenic plants in order to enhance expression levels.
Examples of
-15-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
plant introns suitable for expression in plants can include maize hsp70
iniron, rice actin
4 intron, maize ADH 1 intron, Arabidopsis SSU intron, Arabidopsis EPSPS
intron,
petunia EPSPS intron and others known to those skilled in the art.
PLANT TRANSFORMATION AND REGENERATION
A double stranded DNA molecule of the present invention can be inserted into
the
genome of a plant by any suitable method. Numerous plant transformation
methods
have been described, including Agrobacterium-mediated transformation, the use
of
liposomes, electroporation, chemicals that increase free DNA uptake, free DNA
1 o delivery via microprojectile bombardment, transformation using viruses or
pollen, etc.
After transformation of cells (or protoplasts), choice of methodology for the
regeneration step is not critical, with suitable protocols being available for
hosts from
Leguminosae (alfalfa, soybean, clover, etc. ), Umbelliferae {carrot, celery,
parsnip),
Cruciferae (cabbage, radish, rapeseed, etc. ), Cucurbitaceae (melons and
cucumber),
Graminae (wheat, rice, corn, etc.), and Solanaceae (potato, tobacco, tomato,
peppers).
Methods for transformation and regeneration of dicots, primarily by use of
Agrobacterium tumefaciens, and obtaining transgenic plants, have been
described for
numerous plant species, including cotton (U.S. Patent No. 5,004,863; U.S.
Patent No.
5,159,135; U.S. Patent No. 5,518,908), soybean (U.S. Patent No. 5,569,834;
U.S.
Patent No. 5,416,011; Christou et al. (1988)), Brassica (U.S. Patent No.
5,463,174),
peanut (Cheng et al. (1996); papaya {Yang et al. (1996), and pea (Schroeder et
al.
(1993); De Kathen and Jacobsen (1990)), and others.
2 5 Transformation of monocots using electroporation, particle bombardment,
and
Agrobacterium have also been reported. Transformation and plant regeneration
have
been achieved, for example, in asparagus (Bytebier et. (1987)), barley (Wan
and
Lemaux { 1994)}, maize (Rhodes et al . ( 1988); Gordon-Kamm et al. ( 1990);
Fromm et
al. (1990); Koziel et al. (1993); Armstrong et al. (1995)), oat (Somers et al.
(1992)),
3 o orchardgrass {Horn et al. (1988)), rice (Toriyama et al. (1988); Battraw
and Hail
-16-
CA 02276087 1999-06-25
WO 98145457 PCT/US97/23495
( 1990); Christou et al. ( 1991 )), rye (Bryant ( 1987)), sugar cane (Bower
and Birch
E1992)), tall fescue (Wang et al: (1992)), and wheat (Vasil et al. (1992);
Weeks et al.
(1993)).
For reviews of plant transformation and/or regeneration methodologies see, for
example,
Ritchie and Hodges ( 1993} or Hinchee et al. ( 1994}.
INSECT/PEST RESISTANCE VIA PHYTOSTEROL ALTERATIONS
A series of phytosterols were tested in insects and many were found to be
unable to
1o support insect growth, i.e., were non-utilizable. These sterols included
9,19-
cyclopropyl sterols. Furthermore, novel 0~23c2a>- and ~2a{2s>-alkene and
02scz'oalkyl
sterols were also determined to be unable to support insect growth and
maturation.
These were tested in vivo using Heliothis zea (a corn earworm), cultured on
synthetic
media that was sterol-free with the exception of added test sterols. It was
found that if
the ratio of utilizable to nonutilizable sterols was 1:9 or less, insects
could not undergo
normal develop: In fact, even at 1:1 ratios, insect development was adversely
affected.
The metabolism of insects; nematodes and pythiaceous fungi is limited by the
availability of major plant sterols. These pests cannot use a sterol with a C-
4 methyl
2 o group; a 9~3, 19-cyclopropyl group, or a O8 group. Furthermore, nematodes
and insects
cannot utilize 14-a methyl-sterols, and some insects, including lepidoptera,
diptera and
coleo sera, cannot utilize C-24 24(25) 23(24) zsc27>
p alkyl sterols with D , 0 , or O groups for
mechanistic reasons. Some insects cannot utilize sterols lacking a OS group.
Consequently, elevation of these sterols in plants would provide a detrimental
dietary
source of sterols for these pests.
The DNA molecule of the present invention, when expressed in transgenic
plants, will
cause alterations in the composition/distribution of the sterols present in
the plant. In
one preferred embodiment, the DNA molecule causes the accumulation of sterols
that
3 o are non-utilizable by insects and other pests, so as to increase the
plants resistance to the
-17-
CA 02276087 1999-06-25
WO 98/45457 PCT/ITS97/23495
organisms. This can be accomplished, for example, by a number of approaches,
including overexpression, antisense, cosuppression etc. The DNA molecule of
the
invention will typically target an endogenous gene encoding an enzyme selected
from
the kinetically favored pathways of sterol biosynthesis.
in this embodiment, it is preferred that gene expression and/or translation of
a sterol
biosynthetic enzyme is targeted for inhibition. This inhibition can be
achieved, for
example, by engineering a DNA molecule of the invention to produce an
antisense,
ribozyme or cosuppression RNA molecule complementary to an endogenous gene
being
targeted. Approaches for the targeted inhibition of gene expression are well
known to
the skilled individual (for reviews, see Bird et al. , 1991; Schuch, 1991;
Gibson et al . ,
1997)
A preferred target for inhibition is the S-adenosyl-L-methionine-~24~25~-
sterol methyl
transferase (SMT) enzyme. By targeting this gene with an antisense or
cosuppression
construct, expression of SMT can be effectively suppressed, thereby causing
the
accumulation of non-utilizable sterols.
Besides SMT, other genes in the phytosterol transformation pathway can also be
2 0 targeted in this and other embodiments of the invention in order to alter
the profile of
sterols in transgenic plants. The preferred target will depend on the
application,
however the approach is the same, i. e. , to express an RNA or protein
molecule capable
of modifying the sterol composition of the plant in a desirable manner.
2 5 Therefore, in addition to SMT, other preferred cellular targets for
causing sterol
modifications include:
(i) C-4 demethylase: This enzyme is involved in the removal of the two methyl
groups at C-4 and represents reactions 2 and 8 in the description section. A
single
3 o protein is responsible for both the reactions. Blocking this enzyme will
lead to
-18-
CA 02276087 1999-06-25
WO 98/45457 PCTfUS97/23495
accumulation of 4,4-dimethyl sterols such as cycloartenol, 24(28)-methylene
sycloartenol or a novel sterol such as 24-dihydrolanosterol (structure 18 in
Fig. 9). All
these are nonutilizable sterols. This may be achieved through suppression of
this gene
in plants.
(ii) Cycloeucalenol to obtusifoliol isomerase (COI) and Ag-to-O' isomerase:
These
enzymes represent reactions 3 and 6 in the pathway. Certain fungicides are
known to
block these two enzymes in plants leading to the accumulation of 9~i,19-
cyclopropyl
sterols. Locusts reared on these treated plants are known to have abnormal
development
1 o and levels of cholesterol and ecdysteroids in these insects are depleted.
This suggests
that if either of these enzymes are disrupted or suppressed, the plant sterols
can be
altered such that they will not support insect development (Coste et al. ,
1987).
(iii) C-14 demethylase: This is reaction 4 in the pathway. There are several
fungicides and plant growth regulators that block this step in fungi and
plants. In plants
this blockage leads to a depletion of the normal DS-sterols and an
accumulation of
9(3,19-cyclopropyl, 14a-methyl and D8-sterols that are intermediates of the
main
phytosterol pathway. These are also non-utiiizable sterols. Studies with
chemical
inhibitors have also shown that plants accumulating these intermediates are
tolerant to
2 o water and cold stress. Thus, suppression of this enzyme activity through
gene
manipulation is also a useful strategy.
(iv) D'-sterol-C-5-desaturase: This is reaction 9 in the pathway. Inhibition
of this
enryme leads to a depletion of DS-sterols and an increase in D'-sterols.
Certain insects
2 5 are known to be unable to metabolize ~'-sterols into ecdysteroids.
Therefore,
accumulation of D'-sterols in plants can also provide a way to form non-
utilizable
sterols. Further, 0'-sterols can replace DS-sterols in plant membranes without
any
morphological changes in plant development.
-19-
CA 02276087 1999-06-25
WO 98/45457 PCT/LTS97I23495
(v) C-24 reductase: This is the terminal step in phytosterol transformation
(reaction
-12) during the formation of sitosterol, the major OS-sterol in plants.
Disruption or
suppression of the gene encoding this enzyme would result in the accumulation
of
a~czs~-24-alkyl sterols which are also non-utilizable.
Many of the genes encoding these preferred sterol biosynthetic enzymes to be
targeted
by the present invention have been isolated from yeast (for review, see Lees
et ai. ,
1997). Some have been isolated from plants. For example, SMT genes have been
isolated from soybean (Shi et al., 1996), arabidopsis (Husselstein et al.,
1996; Bouvier-
Nave et al., 1997) tobacco and castor (Bouvier-Nave et al., 1997); and corn
(Grabenok
et al. , 1997). Other plant sterol biosynthetic genes that have been isolated
include
delta7-sterol-CS-desaturase from arabidopsis (Gachotte et al., 1996) and
cycloartenol
synthase from arabidopsis (Corey et al., 1993).
Where not available, the gene encoding a sterol biosynthetic enzyme can be
readily
isolated from a desired source by approaches known to the skilled individual.
For
example, an isolated gene or cDNA from one source can be used as a
hybridization
probe for the isolation of homolgous sequences from other sources. However, it
should
be noted that a DNA molecule of the invention should be active in numerous
plant
2 o types, regardless of the source of the sterol biosynthetic gene used in
the targeting
construct, given the successful demonstration provided herein of using a yeast
ERG6
antisense construct to alter the sterol profile in tomato.
Preferably, the following sterolic metabolic enzymes are targeted for
inhibition: S-
2 5 adenosyl-L-methionine-024-sterol methyl transferase, C-4 demethylase,
cycloeucalenol to
obtusifoliol-isomerase, 14a-methyl demethylase, e8- to ~'-isomerase, D'-sterol-
C-5-
desaturase, or a 24,25-reductase.
Plants produced according to this embodiment preferably have increased amounts
of
3 0 certain sterols that are non-utilizable, particularly 4-methyl sterol,
9(3,19-cyclopropyl
-20-
CA 02276087 1999-06-25
WO 98145457 PCT/US97/23495
sterol, O8-sterol, ~~-sterol, l4oc-methyl sterol, 023c2a>,24-alkyl sterol,
02'~~~-24-alkyl
.sterol or ~2s~27>-24-alkyl sterol, or decreased levels of sterols having a OS
group.
Preferred crops for use in providing insect resistance according to this
embodiment of
the invention include corn (European corn borer, corn earworm, fall
armyworrn), rice,
sorghum, forestry, potato, tomato (tomato hornworm), and vegetable brassicas.
Preferred crops for use in providing nematode resistance according to this
embodiment
of the invention include soybean (soybean cyst nematode), tomato (root knot
nematode),
sugarbeet and cucurbits.
to
Preferred crops for use in providing fungal resistance according to this
embodiment of
the invention include corn, rice, wheat, surghum, soybean (Phytophthora root
rot),
sunflower, forestry, fruits and berries, potato (late blight), tomato (late
blight),
sugarbeet, cucurbits, and vegetable brassicas.
PHYTOSTEROLS AS CHOLESTEROL-LOWERING AGENTS
Animal and human studies have demonstrated that phytosterols can reduce serum
and/or
plasma total cholesterol and low density lipoprotein (LDL) cholesterol (Ling
and Jones,
1995). In this regard, transgenic plants having altered sterol profiles could
be instrumental
2 0 in establishing a dietary approach to cholesterol management and
cardiovascular disease
prevention.
Structure-specific effects of individual phytosterols have recently been shown
where
saturated phytosterols, such as sitostanol, are more efficient compared to
unsaturated
compounds such as sitosterol in reducing cholesterol levels. Another
structural feature
2 5 that seems to play a role is esterification of the phytosterols. Some
studies suggest that the
femilate esters of sitosterol, sitostanol or cycloartenol have a more potent
effect on
" lowering serum cholesterol than the corresponding free sterols (Meittinen
and Vanhanen,
1994).
-21-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97123495
Some of the natural sources of phytosterols in the diet are rice bran oil,
corn f ber oil and
-soybean oil. Rice bran and corn fiber are by far the most enriched sources of
phytosterols.
Soybean phytosterols are a byproduct of the oil refining process. Technologies
that can
generate higher levels of these nutritionally useful phytosterols in these and
other plants
will assist in the development of new food products to improve human health
and
wellness.
Therefore, the present invention, in another embodiment, relates to increasing
cholesterol-
lowering sterols in transgenic plants. For example, with a recombinant DNA
molecule of
the invention, the conversion of cycloartenol in developing seeds can be
inhibited, for
1 o example by antisense, cosuppression, or ribozyme-mediated inhibition of
SMT expression,
thereby leading to an accumulation of this sterol in seed oils. Alternatively,
the SMT gene
can be overexpressed in order to increase the levels of sitosterol.
Preferred crops for use in accordance with this embodiment of the invention
include
sunflower, corn, soybean, oilseed brassicas and cotton.
STRESS TOLERANCE THROUGH ALTERATIONS IN PHYTOSTEROLS
Another embodiment of this invention derives from the fact that certain
sterols are
associated with reducing water permeability of membranes. For this reason,
sterol
2 0 manipulation should provide an effective means for preventing or at least
minimizing
drought induced damage.
Several studies with chemical inhibitors of sterol biosynthesis have
documented that the
treated plants show secondary physiological responses that include tolerance
to
2 5 environmental stresses such as drought and frost (Fletcher, 1988). Such
responses are
primarily due to elevated levels of hormones such as abscisic acid. However,
changes
in membrane fluidity have also been recognized as being responsible for stress
tolerance
(Steponkus, 1984).
-22-
CA 02276087 1999-06-25
WO 98/45457 PCTNS97/23495
Membrane fluidity is controlled by several factors such as the type of sterols
and fatty
acids and the ratio between fatty acids and sterols in the membranes. Of these
factors,
the type of sterols is by far the most important factor. A principal function
of the
sterols is to buffer membranes against abrupt changes in fluidity. They also
may have
more specific influences on the activity of membrane-bound enzymes. An
impairment
of sterol biosynthesis, through the application of inhibitors, resulting in
depletion of
terminal sterols and accumulation of intermediates might therefore be expected
to alter
membrane function.
1 o There is evidence to show that inhibition of sterol biosynthesis in plants
leads to
elevated levels of abscisic acid and closure of stomata (Haeuser, C. et al
1990 J. Plant
Physiol. 137: 201-207). How this process is mediated is not clear. But what is
well
documented is that modification of phytosterols can lead to some forms of
stress
tolerance, which is most likely mediated by elevated levels of abscisic acid.
Further, in
all these studies with chemical inhibitors of sterol biosynthesis) the
accumulating sterols
are those recognized in this invention as nonutilizable. These are again,
9~i,19-cyclopropyl sterols, 14a-methyl sterols and D8-sterols. Thus, formation
of non-
utilizable sterols in plants through the various gene manipulation strategies
described in
this invention will not only protect the plants from pests and pathogens but
also from
2 o environmental stresses such as drought and cold. Preferred sterols to be
elevated in
this aspect include DS-24 alkyl sterols, such as 24-methyl cholesta-5,23-
dienol, and
cycloartenol.
Preferred crops for use in accordance with this embodiment of the invention
include
2 5 corn, wheat, rice, sorghum, soybean, oilseed brassicas (rapeseed, canola),
sunflower,
palm, peanut, cotton, forestry, fruits, berries, nuts, potato, tomato,
sugarbeet,
sugarcane, cucurbits (squash, melons, cucumbers, watermelons, pumpkins),
vegetable
brassicas, alfalfa, ornamental crops, turfgrass, peanut, tea and coffee.
The following examples are included to demonstrate preferred embodiments of
3 0 the invention. It should be appreciated by those of skill in the art that
the techniques
-23-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
disclosed in the examples which follow represent techniques discovered by the
inventor
-to function well in the practice of the invention, and thus can be considered
to constitute
preferred modes for its practice. However, those of skill in the art should,
in light of
the present disclosure, appreciate that many changes can be made in the
specific
embodiments which are disclosed and still obtain a like or similar result
without
departing from the spirit and scope of the invention. Unless specif cally
indicated, all
techniques discussed in the description above and used in the examples which
follow can
be performed by standard molecular biological and biochemical methodologies
well
known to the skilled individual (as described, for example, in Sambrook et
al., 1989).
EXAMPLES
Example 1. Plant Phytosterols
Sterol isomers were extracted from corn and were isolated to homogeneity using
chromatographic methods. Novel phytosterols were identified with side chains
that have
been found to be non-utilizable in insects.
The sterols were structurally characterized by mass spectroscopy and 1H and
t3C nuclear
2 0 magnetic resonance {NMR) (Table 1) (Guo et al, 1995).
The initial studies showed that 4-day corn shoots could produce mono- and di-
alkyiated
sterols at C-24. Corn could produce those sterols, since isolated 24(28)-
methylene and
24(28)ethylidene sterols were obtained from seedling tissue of corn and their
structures
were confirmed by mass and proton nuclear magnetic resonance spectroscopy.
Table 1
Sterol Composition of Zea mat's
Sterol ~ MSa TLCa Plant Source
(M+)
- Cycloartenol 426 0.29 st, c, g, r, sh, b, p
-24-
CA 02276087 1999-06-25
WO 98/45457
PCT/US97/23495
Sterol ~ MS$ TLCa Plant Source'
(M+) (
24(28)-Methylene-cycloartanol440 0.29 st, c, g, r, sh, b, p
Cyclosadol 440 0.29 st, g, sh
Cyclolaudenol 440 0.29 st
Cycloartanol* 428 0.29 sh
24-Methylcycloartanol 442 0.29 g
24(28)-Methyleneparkeol* 440 0.29 sh
a-Amyrin (triterpene) 426 0.29 st, c, g, r, sh, b
(3-Amyrin (triterpene) 426 0.29 st, c, g, r, sh, b
4a, 14a-Dimethylergosta-7,24(28)-424 0.25 st, c, g, r, sh, b
dienol
Lophenol 400 0.25 g, sh
24-Methylene-lophenol 412 0.25 c, g, r, sh, b, p, I
24-Methyl-lophenol 414 0.25 g, sh
24-Ethyl-lophenol 428 0.25 g
Cycloeucalenol 426 0.25 c, g, r, sh
Obtusifoliol 426 0.25 c, g, r, sh, b, p
Dihydroobtusifoliol* 428 0.25 sh
31-Norianosterol* 412 0.25 sh
4a-Methylergosta-8,24(28)-dienol*412 0.25 b
4a-Methylergosta-7(E)-23-dienol412 0.25 c, g, sh
4a-Methylergosta-7(Z)-23-dienol*412 0.25 sh
Citrastadienol 426 0.25 c, g, r, sh, b
Isocitrastadienol* 426 0.25 sh
4a,14a-Dimethyl-ergosta-8(E)-23-426 0.25 c, sh
dienol
4a,14a-Dimethyl-ergosta-8(Z)-23-426 0.25 sh
dienol*
4a,14a-Dimethyl-24-ethyl-cholest-8-442 0.25 sh
enol*
4a,14a-Dimethyl-9,19-cycloergost-426 0.25 c, sh
23-enol
4a-Methyl-cholesta- 410 0.25 sh
8(9),14(15),24(28)-trienol*
Cholesta-5,22-dienol* 384 0.18 sh
Cholest-7-enol* 386 0.16 b
Cholest-8(9)-enal* 386 0.18 b
-25-
CA 02276087 1999-06-25
WO 98/45457 PCTlUS97/23495
Sterol ~ MSa TLCa Plant Source
(M+)
Cholesterol 386 0.18 st, c, g, sh, b, p
Cholestanol 388 0.16 st
Brassicasterol 398 0.18 st, sh
24-Methylene-cholesterol 398 0.18 st, c, g, sh, b, r, t,
p
Ergosta-5(E)-23-dienol 398 0.18 st, c, g, sh, b, r
Codisterol 398 0.18 st, sh
Ergosta-7(E}-23-dienol 398 0.16 st, c, sh
24-Methylene-cholest-7-enol 398 0.16 st, c, sh, p
24-Methylene-zymosterol 398 0. p
i
8
Campesterol 400 0.18 st, c, g, sh, b, r, t,
p
24-Epicampesterol 400 0.18 st, c, g, sh, b, r, p
Ergost-(E)-23-enol** 400 0.16 sh
14a-Methyl-cholest-7-enol* 400 0.16 sh
Ergost-7-enol 400 0.16 st, c
Ergost-8(9)-enol* 400 0.18 sh
Ergostanol 402 0.16 st, c, sh
24(3-Ethylcholesta-5,22,25-trienol 410 0.18 sh
14a-Methylergosta-8,25-dienol* 412 0.18 sh
14a-Methylergosta-8,24(28)-dienol* 412 0.18 sh
Stigmasta-7,25-dienol 412 0. sh
i6
Stigmasta-8,25-dienol* 412 0.18 sh
24-ethyl-cholesta-5,25-dienol 412 0.18 st, sh
Stigmasta-5,23-dienol 412 0.18 sh
Fucosterol 412 0.18 st, g, sh
Isofucosterol 412 0.18 st, c, g, sh, b, r, t,
p
24-Ethylcholesta-5,24(25)-dienol 412 0.18 st, sh
Avensterol 412 0.16 st, c, sh
25-Methyl-24-methylene-cholesterol*412 0.18 sh
Stigmasterol 412 0.18 st, c, g, sh, b, r, t,
p
Stigmast-7-enol 412 0.16 c
Stigmast-22-enol 414 0.16 st, sh
l4oc-methylergost-8(9)-enol 4I4 0.18 sh
Sitosterol 414 0.18 st, c, g, sh, b, r, t,
p
Stigmastanol 416 0.16 st, sh
- a MS, mass spectrometry; TLC,
think-layer chromatography
-26-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Sterol ~ MS$ TLCa Plant Source'
(M+)
" new corn sterol; new natural sterol
' st, shoot; c, cloeoptile; g, germ oil; sh, sheath; b, blade; r, root; i,
inflorescence; t,
tassel; p, pollen
Either trivial or systematic name is given
Biosynthesis of the sterols was analyzed to determine sterol precursor-product
relationships. Developmental regulation of sterol metabolism was examined by
comparison of different corn tissues. The results show sterols in blades
contain mainly
24-ethyl sterols, e.g., sitosterol, while sheaths contained mainly 24-methyl
sterols, e.g.,
24-methyl-cholesta-5,23-dienol.
Feeding-trapping experiments with four [3 3H]24-methyl sterol isomers
incubated with
8-day etiolated sheath tissues indicated that ~24~2g~-methylene and ~2~2s~-24-
methyl
sterols were precursors of 24a- and 24(3-methyl sterols, whereas ~23t2a>-24-
methyl and
~ZSCZ7>-methyl sterols were end products of the sterol pathway.
The results showed that a single SMTI enzyme is responsible for the catalysis
of two
methylation steps and that a critical slow step between cycloartenol (start of
pathway)
and ~5-24-alkyl phytosterol (end of pathway) production is the methylation
step, which
is subject to feed back regulation from 24-ethyl sterols. The SMTI enzyme
regulates the
type and amount of phytosterols produced from cycloartenol during plant growth
and
maturation. This finding contradicts the generally accepted view of the role
of
2 0 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGR). This enzymatic
step
occurs very early in the isoprenoid pathway from which sterols are derived and
has been
considered as the rate-limiting step in phytosterol biosynthesis. The present
finding
shows that HMGR's role is limited merely to controlling carbon flow into the
sterol
pathway.
-27-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Expression studies of microsomal HMGR activity and microsomal SMT enzyme
activity
-during seedling development following seed imbibition (Figs. 3C and 3D) show:
(1) that
SMT activity is correlated with sterol synthesis and plant growth; (2) neither
sitosterol
nor 24(28)-methylene cycloartanol at 100 mM affected HMGR activity, suggesting
that
HMGR activity does not correlate to growth or sterol production; and (3) the
rate of
phytosteroi turnover correlates to the activities of the f rst and second
methylation of
SMTI enzyme and not HMGR activity.
These results demonstrate that during the initial shoot development following
seed
imbibition sterol biosynthesis is down-regulated. Sterol that accumulates in 3-
day shoots
is derived from translocation of sterol originating in the seed. Subsequent
corn seedling
development results in an up-regulation of phytosterol synthesis. Carbon flow
is
directed into the phytosterol pathway: es-24-alkylsterols are synthesized at
rates to meet
the increasing demands of membrane synthesis. Cycloartenol and related C-4
methylated sterols are turned over to ~5-end products. The critical slow step,
which is
the first transformation step in phytosterol synthesis, is methylation of
cycloartenol.
Fig. 4 summarizes the pathway to kinetically favored DS-24-alkyl sterol end
products in
corn during development of the seedling into blades and sheaths under dark-
grown
2 0 conditions. Expression of SMT enzyme activities during early blade and
sheath
formation, and sterol specificity data, show that corn synthesizes at least
two different
SMT enzymes: SMTI catalyzes the successive methyl transfer to produce
e2ac28~-methylene and ~z~z8~-ethylidene sterols; and SMTIi catalyzes the
methyl transfer
to ~z3cza~-24-methyl sterols.
Example 2. Identification of sterols required for growth of plants
The phytosterols identified in Example 1 were tested individually for their
ability to
support growth. In the absence of a plant sterol mutant for such studies the
yeast sterol
3 o auxotroph, GL-7, was cultured in the presence of sterols identified
according to
-28-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Example 1, above (Li, 1996) . This yeast mutant is used as a model system
because it
pan take up sterols from the culture medium and incorporate the test sterol
into the
membrane lipid bilayer and proliferate. The amount of proliferation of the
cells was
measured in the presence and absence of hormonal levels of ergosterol, the
major yeast
sterol.
Sterols were classified according to their effect on growth. Those sterols
sparking
growth included ergosterol. Those sterols that migrated to membrane and cell
structural
components without affecting the rate of growth of the cells included
cholesterol and
1o sitosterol (Nes et al., 1993).
Example 3. Enzvmologv of sterol-converting enzymes
The sterol specificity of the microsome-bound and soluble SMT enzyme from 4-
day
corn seedlings was determined in order to elucidate the enzymatic basis for
the plant
sterols identified in Example 1. Using a microsome-bound enzyme system, we
observed
that cycloartenol is the preferred sterol acceptor and that 24(28)-methylene
lophenol was
methylated to produce 24(28)-ethylidenelophenol. Table 2 summarizes the
specificities
to various sterol substrates using the soluble SMT enzyme from corn seedlings.
Table 2
Sterol specificity of the (S)-adenosyl-L-methionine:D24-sterol methyl
transferase
Substrate Enzyme Activity % Activity,
(dpm/min) relative to
cycloartenol
methylation
Cycloartenol 37,515 100 (C1)
Lanosterol 24,384 65 (C1)
Parkeol 6,002 lb (C1)
31-Norcycloartenol 18,757 50 (C1)
24-Dehydropollinstanol8,253 ~ 22 (C1)
Zymosterol 5,252 14 (C1)
4a-Methylzymosterol 10,504 28 (C1)
-29-
CA 02276087 1999-06-25
WO 98145457 PCT/US97/23495
14a-Methylzymosterol 3,376 9 (C1)
-3-Desoxyzymosterol BG 0 (CI)
Cholest-8-enol BG 0 (Cl)
24(28)-Methylenelophenol 3,800 10 (C2)
4a-Methylergosta-8,24(28)-dienol1,500 4 (C2)
Obtusifoliol BG 0 (C2)
Cycloeucalenol BG 0 (C2)
Ergosta-8,24(28)-dienol BG 0 (C2)
Ergosta-7,24(28)-dienol BG 0 (C2)
Ergosta-5,24(28)-dienol BG 0 (C2)
24(28)-Methylene cycloartanol BG 0 (C2)
There was little difference in the relative binding efficiencies (Km) of
sterols in the
microsome-bound and soluble enzyme systems studied. There was a difference in
the
apparent V~ for the substrates, but this was expected as the level of protein
and total
sterol endogenous sterol changes during enzyme solubilization. The properties
of the
soluble SMT enzyme from 4-day corn was similar to that of the microsome-bound
SMT
enzyme from sunflowers.
The first methyl transfer was demonstrated using cycloartenol and [methyl 3H]-
AdoMet
1 o incubated with a soluble enzyme preparation from 4-day shoots. In a study
on
methylation mechanisms operating in corn, [2713C]-lanosterol was used to
confirm the
methylation mechanism producing a 24(28)-methylene sterol in 4-day shoots (Guo
et al. ,
1996).
In neither incubation with cycloartenol or lanosterol was the sterol acceptor
molecule
methylated to the second methyl product (Nes et al., 1991; Venkatramesh et al,
1996).
If the SMT is a single protein species, then there may be two binding sites on
the
enzyme.
2 o The corn SMT protein is a tetramer with 4 subunits of 39 kDa. A
bifunctional sterol-
methylating (SMT) enzyme was partially purified from 4-day etiolated Zea mat's
(corn)
shoots by the following steps:
- (i) non-ionic detergent soiubilization of the microsome-bound SMT enzyme;
-30-
CA 02276087 1999-06-25
_ WO 98/45457 PCT/US97/23495
{ii) gel-filtration fractionation of the solubilized protein to produce active
fractions with an apparent native molecular weight of circa 156 kd; and
(iii) hydroxyapatite chromatography of active fractions.
Both methylation activities copurified approximately 200-fold.
Fig. 1 shows an HPLC-radiocount (Fig. 1B) and mass spectrum (Fig. lA) of the
reaction product from 50 pooled assays from a soluble SMT enzyme (4-day
seedlings)
assayed with 24(28)-methylene lophenol. The second methyl transfer from
24(28)-methylene Iophenoi to 24(28)-ethylidene lophenol is demonstrated in
this
incubation. Thus the SMT enzyme from 4-day corn shoots catalyzes the
successive first
and second methyl transfers of an appropriate sterol acceptor molecule.
Table 3 shows the effect of a series of substrate and transition state analogs
on the first
and second methyl transfer reactions.
Table 3
Effect of substrate and transition state analog inhibitors on
(S)-adenosyl-L-methionine: X24-sterol methyl transferase activity.
Inhibitor Entry K; relative to K; relative
the to the
no.' first methyl second methyl
transfer transfer
Campesterol 1 NA NA
24(28)-Methylene 2 20 p,M NA
cycloartanol
26,27-Cyclopropylidene 3 25 p,M NA
cycloartenol 4 55 nM 55 pM
. 24-(R,S)-25-Epimino- 5 NA 75 p,M
lanosterol 6 NA 100 p.M
Z-24(28)-Ethylidene
laphenol
-31-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
SItOSter01
* Numbers indicate structures in Fig. 2
Various inhibitors were tested with soluble SMT enzyme from 4-day seedlings
(Fig. 2).
The inability of some inhibitors to affect the methyiation activity of both
sterol
substrates suggested that the SMT enzyme has two binding sites.
SMT catalyzes two successive transmethylations from the coenzyme (S)-
adenosyl-L-methionine to different substrates: cycloartenol (a24-4,4-dimethyl
sterol)
with 20 mM Km and 4 pmol/minlmg protein Vmax; and 24(28)-methylene lophenol
(07,24(28)-4-monomethyl sterol) with 11 p,M Km and 1 pmol/minlmg protein Vmax.
Accordingly, cycloartenol was the preferred substrate for the first
methylation reaction
and 24(28)-methylene lophenol was the preferred sterol substrate for the
second
methylation reaction. Zymosterol (t18'24-4-desmethyl sterol), a preferred
sterol substrate
of yeast SMT enzyme, was a poor sterol substrate of the first methylation
reaction.
Substrate specificity and inhibition studies suggested two binding sites on
the SMT
enzyme: binding site I catalyzes a first methyl transfer to produce a 24(28)-
methylene
sterol; and binding site II catalyzes the second methyl transfer to produce a
24(28)-ethylidene sterol.
For Example, sitosterol (24a-ethyl cholesterol), the major end product of corn
sterol
production in blade tissue, inhibited the second methyl transfer (100 ~,M K;),
without
affecting the first methyl transfer; campesterol (24a-methyl cholesterol)
failed to inhibit
either the first or second methylation reaction; 24(28)-methylenecycloartanol,
a product
2 5 of cycloartenol transmethylation, was not methylated; and 24(28)-
methylenecycloartanol
inhibited the first methyl transfer (20 pM KI) whereas it failed to inhibit
the second
methyl transfer. 26,27-cyclopropylidene cycloartenol, which failed to bind to
the yeast
SMT enzyme, was a potent competitive inhibitor of the first metllylation
reaction (25
~M K~), while not affecting the second methyl transfer.
-32-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
-The second allrylation was inhibited by product inhibition from 24(28)-
ethylidene
lophenol (75 mM K;), while not affecting the first methyl transfer. A
transition state
analog, 24-(R,S)-25-epiminolanosterol inhibited the first and second
methylation
reactions with a similar K; value of 55 nM and to exhibit a non-competitive
type kinetic
pattern. The sterol features of the substrate in the initial enzyme-substrate
interaction
appears to be typical of other plant SMT enzymes, i.e., a requirement for
nucleophilic
groups at C-3 and C-24. The 5 p,M Km for the coenzyme was the same for the
first and
second methylation reactions.
to
Example 4 SMT genes from yeast
The yeast SMT gene, ERG6, was derived from a yeast ERG6 genomic fragment,
pRG458/erg6 (Fig. SB; SEQ ID NO:1).
The cloned ERG6 gene was expressed in E. colt. The recombinant protein was
shown
to be the sterol biomethylation enzyme by enzymatic study which proved that
the kinetic
properties were similar to that of the native enzyme in yeast. In contrast to
plant SMT
which prefer cycloartenoi, zymosterol, a 024-4-desmethyl sterol, is the
preferred
2 o substrate of the yeast SMT.
The molecular weight of the yeast SMT monomer was confirmed to be 43 kD after
successfully overexpressing the active protein in E. colt using a T7 promoter-
based
pET23a(+) vector. The overexpressed protein was visualized on SDS-PAGE gel
both
2 5 by Coomassie blue staining and Western blot using a yeast SMT polyclonal
antibody.
The recombinant protein has also been purified from this system.
From the deduced amino acid sequence of the yeast SMT (Fig. SA; SEQ ID N0:2)
the
potential AdoMet binding motif was predicted as the first conserved region
identified in
3 o Fig. SA (YEYGWGS) and based on mechanistic analysis of biomethylation
described in
-33-
CA 02276087 1999-06-25
WO 98/45457 PCTlUS97123495
Example 3, the amino acid tryptophan (W) was determined to be the binding
site. By
.site-directed mutagenesis of the ERG6 gene this amino acid was replaced with
alanine.
The mutated DNA was also overexpressed in E. coli by cloning into pET23a(+).
This
protein was not active under conditions where the wild-type protein was
active.
Such a strategy provides a means to alter phytosterols by introducing inactive
SMT
protein into plants. The introduction of non-functional SMT monomers can
result in the
suppression of SMT activity, for example by affecting the ability of the cell
to form a
functional SMT enzyme complex, thereby leading to the formation of
nonutilizable
t o sterols. For example, suppressing the activity of the first SMTI reaction
will lead to
formation of 023tza>-24-alkyl sterols, products of SMTa activity.
Alternatively,
suppressing the activity of the second SMTi reaction will lead to the
formation of 02a~2s>-
24-alkyl sterols.
Example 5 SMT Genes from Arabidopsis
The SMT gene from Arabidopsis was cloned and sequenced (Fig. 6; SEQ ID N0:3).
This gene was overexpressed in E. coli. Arabidopsis SMT was partially purified
and
characterized in stereochemical detail.
The Arabidopsis SMT gene was amplified by PCR from a cDNA library. The primers
used were designed from the full-length cDNA sequence retrieved from the
GeneBank
(Accession number X89867). The amplified product was the full-length
Arabidopsis
SMT gene which was sub-cloned into a T/A cloning vector and sequenced. From
the
2 5 sequence data the ORF was identified. A Nde I site was created at the ATG
start codon
through PCR mediated site-directed mutagenesis. The full-length ORF containing
a Nde
1 site at the start and a BamH I site at the stop was cloned into the pET23 a(
+ ) vector
just as the ERG6 gene was in Example 4. The recombinant protein was active in
transforming both cycloartenol and 24(28)-methylene lophenol to their
respective alkyl
3 o products (Tong et al, 1997). In the case of cycloartenol only one product
was formed,
-34-
CA 02276087 1999-06-25
WO 98!4545? PCTIUS97/23495
which is 24(28)-methylene cycloartanol, i.e., SMTI in Fig. 4. Since a single
gene
- product was able to metabolize both sterol substrates it further confirms
the
enzymological data in Example 3. Further, since cycloartenol metabolism by the
recombinant plant SMT gave rise to only one product which also is the product
of SMTI
it suggests that the alternate product, cyclosadoi (structure 6 in Fig. 4}, is
formed from
an isoform (SMTn) encoded by a different gene.
Example 6 SMT genes from corn
The corn sterol methyl transferase (SMT) gene was isolated from a commercial
corn
1 o cDNA library (Stratagene, La Jolla, CA). Five microliters of corn cDNA
(equivalent
to 5x10' pfu} were used as template in the amplification of the SMT gene by
polymerase
chain reaction (PCR). Because the cDNA library was constructed in the vector
Uni-Zap
XR (Stratagene), the T7 sequence in this vector was used as one of the two
primers for
PCR amplification (3'end primer). The 5' end primer (2650-1) was designed'
from
nucleotides 2-20 of a putative SMT fragment published in Gene Bank (T23297).
Thirty
cycles of PCR were conducted using five units of Taq polymerase from Promega
in a
total volume of 100 microliters, according to the manufacturer's instructions.
One
microliter of PCR product from this reaction was used as the template for a
second
round of PCR using the T7 primer and a primer designed from nucleotides 250-
268 of
2 0 T23297. When the resulting reaction products were analyzed on a 1 %
agarose gel, a
band of 1.3 kb was seen. This PCR band was subcloned into the plasmid pGEM-T
(Promega) and was sequenced.
To obtain the 5' end of the SMT gene, a pair of primers designed from
nucleotides 2-20
2 5 and 366-349 of sequence T23297, was used in the PCR amplification. A band
of 366
nucleotides was obtained and sequenced. The sequence of this 366-nucleotide
PCR
fragment overlapped with the 1.3 kb clone for 116 nucleotides. These two
fragments
were joined together by PCR, using a pair of primers, 2650-1 and 3082-2. The
latter
primer was designed from the 1.3 kb fragment 20 nucleotides before poly A
sequence.
-35-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Both of the 366 by and the 1.3 kb PCR fragments were used as the DNA
templates. The
-reconstructed SMT gene was ligated to the PCR cloning vector pGEM-T and was
sequenced bi-directionally using the ABI Prism Automatic DNA Sequencer (Model
310).
The cloned SMT cDNA was 1497 nucleotides, with a coding region of 1032
nucleotides, which encodes 344 amino acids (Figure 10; SEQ ID N0:6). The start
codon, ATG, was located at nucleotide 66-68. There was one stop codon
preceding the
start codon (ATG), located at position 42-4.4, suggesting that the
reconstructed SMT
l0 sequence contains the complete 5'end. A poly A tail of 28 nucleotides was
located 371
nucleotides downstream of the stop codon, indicating the cDNA fragment was
complete
at 3'end. Therefore, this cDNA clone is a full length cDNA clone.
The deduced amino acid sequence from this cDNA clone contains 344 amino acids,
encoding a polypeptide of 38.8 kiioDaltons. This deduced amino acid sequence
contains all three of the proposed conservative regions for methyl transferase
(Kagan
and Clarke, 1994. Arch. Biochem. Biophys. 310: 417-427): LDVGCGIGGP at
position
104-114 (amino acid sequence) and TLLDAVYA at position 167-174, and VLKPGQ at
position 194-199. In addition, another conserved region for sterol methyl
transferase,
2 0 proposed by Nes (SFYEYGWGESFHFA, Guo et al. ,1997. Antifungal sterol
biosynthesis inhibitors. In Subcellular Biochemistry Volume 28: Cholesterol:
Its
function and Metabolism in Biology and Medicine, edited by Robert Bittman.
Plenum
Press, New York), was seen at position 60-73.
2 5 The deduced corn SMT amino acids sequence was compared with amino acid
sequences
from other known SMT genes using GCG progams (Gap and Bestfit). The deduced
corn SMT amino acid sequence shared a 93. 6 % similarity with an independently
isolated corn SMT sequence (Genbank U79669), 88.1 % homology, 78. 8 % identity
with
soybean SMT (Genbank U43683), and a 93.9 % homology, 88.3 % identity with
partial
3 0 wheat SMT sequence (Genbank U60754 ), 58.8 % ~ homology, 39 % identity
with
-36-
CA 02276087 1999-06-25
WO 98145457 PCT/US97I23495
Arabidopsis thaliana (Genbank X89867), and a 66.5 % homology, 50.4 % identity
with
-yeast SMT (Genbank X74249). The high similarity between this cDNA clone and
SMT
genes from other plant species confirms that this cDNA clone is a full length
SMT
cDNA clone of Zea mays. Furthermore, since Grabenok et al. have functionally
expressed their corn SMT gene in a yeast expression system and found no 24-
alkyl
sterols other than ergosterol, this suggests that the corn SMT gene isolated
by my
laboratory catalyzes the same stereoselective C-methylation to 0228}, thereby
supporting
the view that corn synthesizes several different SMT enzymes.
1 o A similar strategy can be used for isolating the cDNA for the SMTQ
isoform. In fact,
cDNA fragments isolated by the described method should be representative of
both
SMTI and SMTII based on the conservation of the region from which the primers
were
derived.
Example 7 SMT Qenes from Prototheca wickerhamii
Another example of a preferred SMT gene is that from Prototheca wickerhamii .
This
yeast-like alga produces ~2scz7~-24-methyl sterol as the main product of
transmethylation.
The favored substrate is cycloartenol.
Studies from microsome preparations of P. wickerhamii have shown that the
preferred
substrate of the SMT is cycloartenol. However, the preferred product is not
24(28)-
methylene cycloartenol but cyclolaudenol (VII) which is a ~~~2~~-24-alkyl
sterol, a
nonutilizable sterol.
Cloning the gene of this SMT will facilitate the introduction of this gene
into plants in
order to transform the plant sterol, cycloartenol, into a product,
cyclolaudenol, which
will lead to the accumulation of nonutilizable sterols, viz., ~2sc27>-24-alkyl
sterols.
-37-
. CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
t
t
Cloning of Prototheca SMT
Prototheca wickerhamii cells are grown to mid log phase in YPD rich medium
(yeast
extract - peptone - dextrose). The pelleted cells are disrupted in the
presence of Tri
Reagent (MRC) using 0.5 mm glass beads and a mini-Beadbeater (both from
Biospec
Products, Bartlesville, OK). High quality total cellular RNA is isolated
according to the
manufacturer's instructions.
Total cellular RNA is subjected to 3' RACE (rapid amplification of cDNA ends)
and 5'
RACE using reagents and protocols found in kits obtained from GibcoBRL. For 3'
RACE, total cDNA is synthesized by the action of reverse transcriptase after
annealing
oligo(dT)-containing primers to the poly(A)-tailed RNAs present in the
unfractionated
total RNA. The RNA templates are degraded and the cDNA serves as template for
polymerise chain reaction (PCR) amplification. The user-supplied primer
"YEYGWG"
(see Rationale for primer design below) anneals to the cDNA and is extended
toward the
3' end of the gene under the direction of Taq polymerise. The kit-supplied
primer for
extension from the 3' end to the terminus defined by the "YEYGWG" primer
anneals to
a sequence composed of three restriction endonuclease recognition sites that
was part of
2 0 the original oligo-dT containing primer. A second PCR amplification in
which the
primer pair is a second "nested" primer ("GCGVGG") and the kit-supplied 3'
primer is
performed to enrich for cDNAs representing the 3' half of SMT. Another nested
primer
("ATCHAP") has been similarly used.
-38-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Total cellular RNA is also subjected to 5' RACE. cDNA is synthesized by
reverse
xranscriptase using the antisense primer "EWVMTDas". cDNA is modified at the
3'
end by the addition of a polydeoxycytidine "tail" using terminal
deoxynucleotidyl
transferase (TdT). An initial PCR reaction is carried out using this C-tailed
cDNA as
template and the primers "EW VMTDas " and a kit-supplied poly-G containing
primer.
A second PCR reaction is carried out on this PCR product using the nested
primer
"ATCHAPas" and a kit-supplied primer that anneals to a part of the poly-G
primer that
contains restriction enzyme recognition sites. This second PCR reaction
enriches for 5'
SMT cDNA sequences.
to
The 3' RACE and 5' RACE PCR products are isolated from gels and ligated into
the
plasmid pPCRII (Invitrogen). Clones obtained after transformation into E. coli
are
characterized by sequencing. An Apa I restriction site is present in the DNA
of all
plants and yeast that have been sequenced in the GCGVGG motif and is present
in both
the 3' and 5' cDNA clones. This allows splicing of Ehe two 3' and 5' halves of
the
SMT gene together, completing the entire coding region.
Rationale for primer design
The first step in designing the user-supplied primers was to examine the
several very
2 o highly conserved peptide motifs in the SMTs of those plants and yeast that
have been
sequenced. Within these are found shorter stretches of amino acid sequences
that can be
encoded by a minimum number of DNA sequences, the codons of which usually only
vary at the third (degenerate) base. It was also desirable that the codon
preferred by 3
different yeast species according to codon usage tables found in Wada, et. al.
(Nucleic
2 5 Acids Res. , vol 19, p 198 I , 1991 ) be present in the mix of degenerate
codons for each
amino acid. Each user defined primer is thus a mixture of deoxynucleotides
that defines
an internal end of a PCR product. It was also reqiured that 4 or 5 of the 6 3'
deoxynucleotides of each primer be perfectly matched in all species and had
greater than
50% G andlor C.
-39-
CA 02276087 1999-06-25
WO 98/45457 PCT/IJS97/23495
The first three primers described below are sense orientation primers that
anneal to
-,antisense DNA (and the original cDNA). The fourth and fifth primers are
antisense
primers that anneal to the sense DNA strand of the SMT gene.
YE[Y/FIWJGWG (amino acids 81-86 of the yeast sequence; nonidentical residues
at a
position are in brackets) was the part of a larger conserved region of SMT
that was the
basis for the "YEYGWG" primer:
5' - TA[TIC]GA[A/G]T[AIGIT][T/G]GG[T/A/C]TGGGG - 3'
(Degenerate nucleotide positions are included in brackets)
The "GCGVGG" primer was suggested by the DNA sequence that encodes part of a
second conserved domain (GCG[V/I]GG) at yeast amino acid residues 129-134. The
sequence of primer "GCGVGG" is:
5' - GGATG[TIC]GG[TIA][G/A]T[T/C]GG[G/C]GG - 3'.
Primer "ATCHAP" is based on the DNA sequence encoding a third highly conserved
domain (yeast amino acids 196-203). The primer sequence is:
5' - GCCAC[A/G/T]TG[TIC]CA[C/T]GC[T/G/A]CC - 3'.
2 o Primer "EWVMTDas" is an antisense primer for first strand cDNA synthesis
in the 5'
RACE experiment. It is based on the small conserved domain at yeast amino acid
residues 225-23I. The sequence is:
5' - TC[A/C/G]GTC[GlA]T[T/AIG][CIA][CIA]CCA[C/T]TC - 3'.
2 5 Primer "ATCHAPas" is a nested antisense primer for the 5' RACE experiment
with the
sequence:
5' - GG[T/C/A]GC[AIG]TG[GlA]CA[A/ClT]GTGGC - 3'.
-40-
CA 02276087 1999-06-25
WO 98!45457 PCT/CTS97123495
_Example 8 SMT genes from other plants
Using the Arabidopsis cDNA or another plant derived SMT sequence as a probe,
cDNA
libraries from any crop of interest can be screened and corresponding clones
of
appropriate sizes can be isolated and sequenced. cDNA library construction a~
screening methodologies are well known in the art. As described in Example 6,
appropriate primer combinations can be readily determined using information of
the
conserved regions of known sequences for various SMT genes. To confirm the
identity
of sequences cloned by this method, they can be compared with known plant SMT
enzyme sequence and/or in vitro tranlsated and evaluated biochemically.
Example 9 Plant transformation with ERG6 DNA
To obtain transgenic plants with altered sterol profiles a DNA fragment
containing the
open reading frame of the SMT ERG6 gene of yeast isolated from a genomic clone
was
identified {Example 4). The ERG6 DNA was modified by PCR to include
restriction
sites for Nco I on either end of the open reading fame. This PCR procedure
gave ruse
to a mutaion which introduced a frameshift in the gene. This mutation made the
ERG6
2 0 gene introduced into the plant untranslatable, but capable of inhibiting
the endogenous
tomato SMT via antisense or co-suppression mechanisms, depending upon the
nature of
the construct.
The modified ERG6 DNA fragment was cloned into the pUCl8cpexp expression
2 5 cassette vector. Clones with the ERG6 DNA in the sense as well as the
antisense
orientations to the 35S promoter were generated (Fig. 7).
Hind III digestion of these clones gave rise to the ERG6 constructs that
included the 35S
promoter and termination sequences flanking the ERG6 open reading frame. These
3 o Hind III digested fragments were cloned to the binary vector pJTS246 that
contains
-4I-
CA 02276087 1999-06-25
WO 98/45457 PCT/L1S97/23495
T-DNA border recognition sequences and the NPTII gene conferring kanamycin
.resistance.
The cloned binaries with either the sense or antisense ERG6 constructs were
transformed
into Agrobacterium tumefaciens which were cocultivated with cotyledons of
tomato
(Solanum lycoperiscum) to obtain transformed plant cells. From calli formed on
selective medium containing kanamycin transgenic plants were produced.
The leaves from control (no inserts) and transgenic plants (with inserts) were
analyzed
1 o for the transgene. DNA was extracted from leaf samples of each of the
transformants
and an untransformed tomato plant. The DNA extracts were quantified by A260
absorbance.
Aliquots corresponding to 200 ng DNA from each sample were used in PCR
reactions
for amplifying ERGfi fragments using oligonucleotide primers corresponding to
the
ERG6 sequence (underlined in Fig. 8). Controls in the PCR included a sample
with no
template DNA and samples of the sense and antisense ERG6 containing binary
plasmids.
PCR was performed under non-stringent conditions (55 °C annealing
temperature for 2
min in each cycle) in 20 cycles and aliquots were electrophoresed on 0.8 %
agarose gels.
The primers were selected such that a 1100 by fragment of the ERG6 DNA would
be
amplified (Fig. 8). All the regenerated transgenic tomato plants (Ro) carried
this
fragment as did the plasmid controls. There also is some non-specific
amplification
because of the non-stringent conditions leading to other bands appearing in
the
2 5 transformed plants and in the untransformed control. However, the level of
these
amplifications is significantly less than that of the target fragment. This
confirms the
presence of the ERG6 DNA in the tomato genome.
-4.2-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Sterol analysis was performed on the nonsaponifiable lipid fraction of leaf
material frqm
-one regenerated plant transformed with the sense construct and one
regenerated plant
transformed with the antisense construct. The results are shown in Table 4.
Table 4
Sterol Composition of Tomato Plants
(as % total sterol)
Sterol Control ERG6 sense ERG6 antisense
insert insert
Cholesterol 29 18 20
Cholest-7-enol none 21 13
Stigmasterol 25 22 24
Sitosterol 26 27 24
Isofucosterol 20 12 19
mg sterol/g fr.wt.16 150 380
The result confirmed that the ERG6 gene was incorporated into the transgenic
plants and
that the sterol compositions of the transgenic plants were changed. A novel
sterol,
cholest-7-enol, which is not present in control tomato plant leaves, was
detected and
characterized by mass spectroscopy.
Z5
A scheme for the new pathway introduced into the tomato plants due to the
insertion of
the yeast ERG6 gene is predicted to be as follows:
Since both the sense and antisense inserts of the ERG6 gene lead to the
accumulation of
the cholest-7-enol (VIII), it is likely that in both cases there is a
suppression of
2 o endogenous SMT activities. This will lead to a shunt of carbon flow into
an alternate
minor pathway proposed for phytosterol metabolism where the first step in
cycloartenol
metabolism is a reduction of the C-24 double bond by a reductase enzyme. The
resulting sterol, which is cycloartanol (IX), will then undergo the usual
demethylation,
isomerization, desaturation and reduction just as in the main pathway leading
to the
-43-
CA 02276087 1999-06-25
WO 98/45457 PCTILTS97/23495
formation of cholest-7-enol. This is a D'-sterol and the double bond at C-5 is
absent,
_suggesting that some insects will not be able to utilize this sterol to
complete their Iife
cycles.
II)
HO
(IX)
HO
1 o The regenerant (Ro) plants were allowed to flower and set fruit. Seeds
were collected,
and the following generation (R~) was grown. Individual plants arising from
seeds were
assayed for the presence or absence of the selectable marker (NPT2) via ELISA
assay
for the NPT2 protein. Fifty-three plants from six Ri progeny and a
nontransgenic plant
were analyzed for sterol composition. The sterol profiles of these plants
could be
divided into four distinct groups, or phenotypes:
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Table 5
_ Means and standard deviations (Std) of sterols (as percent of total sterols)
of Rl plants in the four classes of progeny identified.
Phenotype 1 2 3 4
Mean Std Mean Std Mean Std Mean Std
Sterol
Cholesterol 7.62 2.54 6.20 2.77 4.93 1.148.60 2.97
Campesterol 4.17 3.15 16.60 11.244.50 1.956.60 4.83
Stigmasterol 13.14 3.13 12.80 5.26 8.86 1.4122.60 1.14
Sitosterol 11.48 2.86 11.60 2.19 9.57 1.8716.60 3.91
Isofucosterol 13.14 2.08 7.60 3.71 9.86 2.3214.40 4.98
b-Amyrin 12.52 3.90 9.75 5.91 10.36 3.958.80 1.79
Cycloartenol 31.76 5.67 31.60 4.72 49.36 4.9128.80 6.98
24(28)-methylene 1.14 1.46 6.80 6.61 2.17 2.122.00 2.00
cycloartanol
All of the R1 plants which tested negative for the NPT2 marker (and were
therefore
non-transgenic segregants) as well as the nontransgenic control plant
displayed the
normal phenotype (Phenotype 1 ). The Rl plants which tested positive for the
NPT2
marker (and were therefore transgenic) fell into all four classes. A
statistical
1 o comparison was conducted for each sterol (using the arcsin transformation
of the percent
sterol levels; Student-Neuman-Keuls Test, 5 % significance level), and a
qualitative
summary of the results is given below:
-45-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Table 6
- Comparison of sterol phenotypes (Phenotypes 2, 3 and 4 versus normal
Phenotype 1 )
Sterol Phenotype Phenotype Phenotype Phenotype
1 2 3 4
Cholesterol Normal Normal Low Normal
Campesterol Normal High Normal Normal
StigmasterolNormal Normal Low High
IsofucosterolNormal Low Low Normal
~3-amyrin Normal Normal Normal Normal
CycloartenolNormal Normal High Normal
24(28)- Normal Normal Normal Normal
methylene
cycloartanol
Sitosterol Normal Normal Normal High
The distribution of plants in the various categories (i.e. nontransgenic
controls in the
normal category only and the transgenics plants in all four categories) is
consistent with
the expectations of plants resulting from transformation with either an
antisense or co-
suppression constnlct. Varying levels of suppression can be expected between
and
within progenies, thus leading to varying levels of expression of an altered
sterol
1 o phenotype. Therefore, these results are consistent with the transformed
ERG6
gene having a suppressive effect. More specifically, phenotypes 2 and 3
accumulate
intermediates which are consistent with partial inhibition of the first or
second
methylation activities of sterol methyltransferase in the biosynthetic
pathway. The
elevated levels of sitosterol and stigmasterol (the normal endproducts) are
not consistent
with suppression, and cannot be explained without further study.
Independent analyses of a subset of these progeny further supports the
hypothesis than
suppression of the SMT gene is being observed in the transgenic lines. Table 7
below
gives the sterol compositions of nontransformed and nontransgenic segregants.
-46-
CA 02276087 1999-06-25
WO 98145457 PCT/US97/23495
Table 7
.Sterol composition of control plants (nontransformed plants and nontransgenic
segregants)
Plant NontransformedG55 (nontransgenicG62 (nontransgenicMean Std.
segregant) segregant) Dev.
Sterol
Cholesterol 18 13 13 14.7 2.9
D-Cholesterol - tr. I 1.0
14-a-CH3-07- - 5 5 5.0 0.0
Cholesterol
07-Cholesterol - - -
14-a-CH3-08- 3 1 1 1.7 1.2
cholesterol
Zymosterol 18 - - 18.0
~7,24_Zymosterol5 - - 5.0
24-CH2- - 19 1 10.0 12.7
Cholesterol
Campesterol 2 8 3 4.3 3.2
Desmosteroi 2 - 2.0
~-Campesterol - - 1 1.0
Stigmasterol 18 20 25 21.0 3.6
D-Stigmasterol - tr. I 1.0
Sitosterol 7 13 18 12.7 5.5
~-Sitosteroi - - tr.
Isofucosterol 4 2 2 2.7 1.2
Cycloartenoi ? 19 29 18.3 11.0
24-CH2- 14 - tr. 14.0
Cycloartenol
24-CH2-Lophenol1 - tr. 1.0
Obtusifoliol 1 - tr. 1.0
-dash lines .. NSF was
means not detected; chromatographed
tr. is trace; on TLC
N.D.-not determined
plates and bands , 4-monomethyl
matching 4-desmethyl- and 4,4-dimethyl
sterol standards
were eluted
from the plate chromatography it
and examined on 3 % SE-30 of
further by columns and
GC-MS. Lim
detection is sterol per
0.1 mg leaf sample.
These controls can be compared with transgenic plants, the sterol composition
of which
are given in tables 8, 9, and 10.
-47-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Table 8
Sterol composition of transgenic plants from line G3
Plant G31 G32 G34 G35 G37 G38 G39
Sterol
Cholesterol 12 10 8 10 8 11 8
DO-Cholesterol 1 tr. 1 1 1 tr. 1
14-a-CH3-~~-Cholesterol3 - - - - - 3
07-Cholesterol - 8 6 13 11 1 -
14-a-CH3-48-cholesterol1 2 2 - - - -
Zymosterol 10 5 12 - - - g
07,2a_Zymosterol 2 - 1 - - - 1
24-CH2-Cholesterol - - - _ _ _ _
Campesterol 4 2 3 - - 1 1
Desmosterol - - - _ _ -
~~-Campesterol - - - - - - -
Stigmasterol 16 14 12 20 16 16 6
DO-Stigmasterol 1 - - tr. - -
Sitosterol 15 9 12 10 8 16 6
Do-Sitosteroi 1 tr. - - - _ -
Isofucosterol 4 2 2 2 2 1 1
Cycloartenol 26 41 36 40 44 41 41
24-CH2-Cycioartenol I 3 3 4 4 4 4
24-CH2-Lophenol 2 3 2 tr. 4 6 tr.
Obtusifoliol 1 I 1 tr. 2 3 tr.
-dash lines means not detected; tr. is trace; N.D.-not determined.. NSF was
chromatographed on TLC plates and bands matching 4-desmethyl-) 4-
monomethyl and 4 4-dimethyl sterol standards were eluted from the plate
and examined further by chromatography on 3 ~ SE-30 columns and GC-
MS. Limit of detection is 0.1 mg sterol per leaf sample.
-48-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Table 9
Sterol composition of plants from line GS
. Plant G51 G52 G53 G54G56 G57 G58 G596510
Sterol
Cholesterol 13 5 6 11 16 11 4 15 5
0~-Cholesterol 1 1 1 1 1 tr. tr. 1 1
14-a-CH3-~7-Cholesterol 1 3 1 2 6 5 2 4 1
07-Cholesterol - - - - - - _ - _
14-a-CH3-~8-cholesterol - 1 tr. tr.1 tr. tr. 1 1
Zymosterol - - - - _ - _ _ -
07,24-Zymosterol - - - - - - - _ _
24-CH2-Cholesterol - - 3 4 - I 6 6 -
Campesterol 8 15 4 2 1 2 2 3 19
Desmosterol - _ - _ 2 _ _ _ -
~o-Campesterol - 1 - - - - - - 1
Stigmasterol 20 6 10 13 20 17 4 11 6
0~-Stigmasterol - - tr. tr.- tr. - - 1
Sitosterol 21 11 7 9 9 8 3 11 1
~a-Sitosterol - tr. 1 tr.- tr. tr. I 1
Isofucosterol 1 1 1 1 1 8 1 2 1
Cycloartenol 34 48 58 52 41 47 49 35 43
24-CH2-Cycloartenol 1 4 6 S 1 1 28 10 14
24-CHZ-Lophenol 1 3 i tr.- tr. 1 tr.4
Obtusifoliol tr. 1 1 tr.- tr. tr. tr.I
-dash lines means not tr. N.D.-not
detected; is determined..
trace; NSF
was
chromatographed on TLC plates bandsmatching and
and 4-desmethyl-) 4,4-
4-monomethyl
dimethyl sterol standards wereelutedfromtheplateand examined
further
by
chromatography on 3 % SE-30 GC-MS. Limit 0.1
columns and of mg
detection sterol
is
per leaf sample.
-49-
CA 02276087 1999-06-25
WO 98/45457 PCTIUS97/23495
Table IO
Sterol composition of plants from line G6
Plant G63 G65 G66 G67 G68 G69 6610
Sterol
Cholesterol 7 7 9 8 5 6 7
~~-Cholesterol tr. 1 1 1 tr. tr. 1
14-a-CHg-~7-Cholesterol2 2 5 1 1 3 1
07-Cholesterol - - - - - _ _
14-a-CH3-O8-cholesterol1 1 1 1 tr. 1 1
Zymosterol - _ _ _ _ _ _
X7,24-Zymosterol - - - - - _ _
24-CH2-Cholesterol 2 tr. tr. - - - -
Campesterol 18 3 1 3 20 1 3
Desmosterol - - _ _ _ _ _
a~-Campesteroi tr. - tr. tr. 1 - -
Stigmasterol 10 7 11 8 5 6 7
0~-Stigmasterol tr. tr. I tr. tr. tr. tr.
Sitosterol 13 7 7 9 8 4 7
0~-Sitosterol tr. 1 tr. 1 tr. Tr tr.
Isofucosterol 2 2 1 1 1 tr. 2
Cycloartenol 30 61 61 61 39 72 70
24-CH2-Cycloartenol 12 8 3 6 20 7 1
24-CH2-Lophenol 2 - - - - _ _
Obtusifoliol 1 - - - _ _ _
-dash lines means not detected; tr. is trace; N.D.-not determined.. NSF was
chromatographed on TLC plates and bands matching 4-desmethyl-) 4-
monomethyl and 4,4-dimethyl sterol standards were eluted from the piate and
examined further by chromatography on 3 % SE-30 columns and GC-MS.
Limit of detection is 0.1 mg sterol per leaf sample.
These analyses indicate that cycloartenol levels of many of the transgenic
plants are
significantly elevated compared to controls. The cycloartenol levels
achievable by this
approach are at or above the level of nonutilizable sterol necessary to have a
detrimental
effect on insects, as demonstrated in Example 10 below. In addition, the
results are
consistent with successful in vivo suppression of the first methylation
catalyzed by SMT.
-50-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97J23495
Example 10 Sterol utilization and metabolism by Heliothis tea
-Several sterols were isolated from nature or prepared synthetically to feed
to the insects.
An in vivo model was used involving Heliothis zea, cultured on a synthetic
medium that
was devoid of sterol, except for the test sterol added to the diet.
Cycloartenol and
several 24-methyl and -ethyl sterol isomers were found to inhibit insect
growth in this in
vivo model.
Two important sterols from corn, 24-methyl cholesta-5,23-dienol and 24-methyl
cholesta-5,25(27)-dienol, were found to be non-utilizable. The 9,19-
cyclopropyl sterol
was also non-utilizable, as were the ~23c2a~- and O2sc27>-24-alkene sterol
isomers.
Heliothis zea (corn earworm) was reared on an artificial diet treated with
different sterol
supplements to study the relation between sterol structure and utilization in
insects. H.
zea eggs were used to establish a disease-free stock colony.
The stock insects were reared using sterile procedures on a pinto bean-based
diet.
Moths were fed 10 % sucrose. Cultures were maintained at 27 t 1 °C, at
40 t 10
relative humidity on a 14:10 light-dark photoperiod and an artificial diet was
used to
rear the insects on different sterol supplements. The experimental diet
contained agar,
2 0 which is known to contain trace contamination of cholesterol, otherwise
the
experimental diet was sterol-free.
Sterols were solubilized in acetone. Aliquots of the solutions were added to
the sterol-
free diet in a mortar, the material mixed thoroughly with the diet, and the
organic
solvent allowed to evaporate. Sterols were supplied to the medium at 200 ppm
(equivalent to 1 mg of sterol per experimental vessel containing one insect).
By day 20, H, zea larva are in the final stage of larval development (sixth
instar), after
which the insects may pupate. A single neonate larva was placed in an
experimental
-51-
CA 02276087 1999-06-25
WO 98/45457 PCTlUS97/23495
culture vial and allowed to grow for 20 days. The fresh weight, length and
instar stage
-of 20-day larva were recorded.
In some treatments, the larvae were allowed to grow for another 4 days to
determine
whether they could pupate properly and develop into moth forms. Neonate larvae
of H.
zea failed to molt to the second instar when sterol was absent from the diet.
Some of
these insects survived for more than 15 days.
Sterols isolated from the nonsaponifiable lipid fraction extracted from larvae
contain
long chain fatty alcohols. These fatty alcohols may comigrate with sterols
during some
forms of chromatography and interfere with sterol quantitation, particularly
of
cholesterol. Therefore, in order to confirm the identity and amount of
cholesterol in the
insect an aliquot of the NSF was injected into a HPLC column and the fraction
corresponding to cholesterol was examined by GC-MS.
Larvae did not develop on a sterol-less medium. DS-sterols substituted at C-24
in the
side chain with hydrogen, methylene, E- or Z ethylidene, or a- or b-ethyl
groups,
cholesterol, 24(28)-methylenecholesterol, s~rosterol, isofucosterol,
fucosterol,
clinonasterol, and stigmasterol supported larval growth to late-sixth instar.
These
2 o sterols are referred to as "utilizable" sterols (Table 11 and Fig. 9). In
each of the
incubations, the major sterol recovered from the larvae was cholesterol,
showing that H.
zea operates a typical insect 24-dealkylation sterol pathway.
In contrast, the sterol requirement of H. zea could not be met satisfactorily
by
2 5 derivatives of 3 ~i-cholestanol with a 93,19-cyclopropyl group, geminal
dimethyl group
at C-4 (e.g., cycloartenol and lanosterol), O8-bond, or by side chain modified
derivatives
that contained the following structural features: 023«x-24-methyl or 24-ethyl
group,
~2~~~-24-methyl or 24-ethyl group, or a2s~27~-24(3-ethyl group. These are
referred to as
"nonutilizable" sterols (Table 11 and Fig. 9). ,
-52-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
The major sterol recovered from larvae which developed on nonutilizable
sterols was the
-test sterol added to the medium. Competition experiments using different
proportions of
cholesterol and 24, 25-dihydrolanosterol (from 9/ 1 to 1/9 sterol mixtures)
indicated that
abnormal development of H. zea may be induced on < 1 to 1 sterol mixtures of
utilizable and nonutilizable compounds (Table 12). Sterol absorption was
related to the
degree of sterol utilization and metabolism.
Table 11
1 o Effect of
sterols on growth
and metabolism
by Heliothis
zea
Sterol Entry Growth Instar Total Sterol
supplement No.l response2reached sterol composition3
by
day 20 mglinsect(as % total
sterol)
Utilizable
sterols
Cholesterol 1 100 6 56 cholesterol
24(28)- 2 100 6 59 ts/cholesterol
Methylene- (16!84)
cholesterol
Fucosterol 4 100 6 71 ts/cholesterol
(10/90)
Isofucosterol 3 100 6 52 ts/desmosterol/
cholesterol
(8114/78)
Sitosterol 5 100 6 66 ts/cholesterol
(20/80)
Clionasterol 6 100 6 43 tslcholesterol
(50/50) { 14/84)
(75/25)
Stigmasterol 7 100 6 27 tsldesmosterol/
cholesterol
(15/1/84)
Nonutilizable
-53-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97I23495
Sterol Entry Growth Instar Total Sterol
- supplement No. t response2 reached by sterol composition3
day 20 mg/insect (as % total
sterol)
sterols
Cholest-8-enol 13 5 3 ND ND
24-Dehydro- I4 5 3 0.6 tslcholesterol
pollinastanol (8b/14)
24-Methyl- 10 SO 5 6 ts/cholesterol
cholesta- (80/20)
5,23-dienol
24-Ethyl 12 20 3 3 ts/cholesterol
cholesta- (86/ 14)
5,23-dienol
24-Methyl 9 5 3 1 ts/cholesterol
cholesta- (65/35)
5,24-dienol
24-Ethyl 11 10 3 ND ND
cholesta-
5,24-dienol
Clereosterol 8 20 3 3 ts/cholesterol
(80!20)
Ergosterol 15 30 3 5 ts/7-dehydro-
cholesterol/
cholesterol
(36141/23)
Cycloartenol I7 5 3 ND ND
Lanosterol 16 5 3 ND ND
24-Dihydro- 18 5 3 ND ND
ianosterol
' Structures of sterols are reported in Fig. 9.
Growth on cholesterol produced larvae that at 20 days
weighed, on average) 323 mg and were 30
mm long. Generally 16 to 20 insects survived on cholesterol
to pupate and develop into adult
moths. The growth responses on test sterols are relative
to the growth on cholesterol which is
normalized to 100 % . 24-methyl cholesta-5,23-dienol was
found to support pupations and adult
moth formation. However, these insects exhibited congenital
deformities. Insects in the
nonutilizable category generally weighed less than 100
mg per insect and their length ranged from
2 to 15 mm, with 6 to 12 insects alive at day 20.
3 Sterols isolated from insect tissues, after the gut
was purged of its contents, were analyzed by RP-
HPLC and GC-MS.
-54-
CA 02276087 1999-06-25
WO 98145457 PCT/US97/23495
ND Not determined.
is Total sterol.
The most effective sterols were absorbed and incorporated into tissues from 27
to 66 mg
per insect, whereas the least effective sterols were absorbed and incorporated
into
tissues from 0.6 to 6 mg per insect. These studies demonstrate that: {i) H.
zea
discriminates structural modifications in the sterol nucleus and side chain,
(ii) the
pathway of phytosterol dealkylation to cholesterol involves a high degree of
regio- and
stern-selectivity, and (iii) corn produces several of the nonutilizable
sterols described
herein.
Table 12
Utilization of 24-dihydrolanosterol (nonutilizable)
sparred with cholesterol {utilizable) by Heliotnis zea
Sterol mixtureEntry Growth Instar Total Sterol composition
{ratio) No.* response reached sterol (As % total
sterol)
by day mglinsect
20
Cholesterol 1 100 6 56 cholesterol
(100%) (100%)
Cholestero1/24,25-1/18 100 6 45 cholestero1/24,25-
dihydrolanosterol dihydrolanosterol
sterol (90:10) (93:7)
Cholesteroll24,25-1118 100 6 36 cholesteroll24,25-
dihydrolanosterol dihydrolanosterol
sterol (70:30) (88:12)
Cholesteroll24,25-1/18 70 6 25 cholestero1124,25-
dihydrolanosterol dihydrolanosterol
sterol (50:50) (75:25)
Cholesterol/24,25-1/18 30 3 12 cholestero1/24,25-
dihydrolanosterol dihydrolanosterol
sterol (30:70) (50:50)
Cholesteroll24,25-1/18 10 3 ND ND
dihydrolanosterol
(10:90)
*
Indicates the structures in Fig. 9.
-55-
CA 02276087 1999-06-25
WO 98145457 PCT/US97/Z3495
~'he minimal dietary concentration of cholesterol necessary for larvae to grow
and
pupate is 0.01 % of the experimental diet. This level of cholesterol does not
support a
rapid rate of molting as did higher levels of cholesterol. However, diets of
0.015
cholesterol or more enhanced the rate of development of larvae. Therefore, a
slightly
higher amount of dietary sterol (0.02 % ) was used to insure that a non-
limiting amount
of sterol (alone or as a mixture) was available in the experimental diet, or
no sterol was
added to the diet to act as a control.
l0 In all larvae treated with non-utilizable sterols, there were trace amounts
of cholesterol
that ranged from 80 to 350 nanograms of cholesterol per insect depending on
the
treatment. This source of cholesterol most likely results from carryover of
cholesterol
in the egg (we detected ca. 80 ng of cholesterol per egg) and from absorption
of trace
levels of cholesterol originally present in the agar.
I5
As the insect increases in size, the insect may accumulate increasing amounts
of
cholesterol from the agar diet. Cholesterol obtained in this manner may serve
as a
precursor for ecdysteroid synthesis. The different effectiveness of the pair
of isomers
sitosterollclionasteroi and isofucosterol/fucosterol, in growth support and in
their active
2 0 metabolism to cholesterol indicates that the 24-dealkylation pathway may
operate
stereoselectively.
Developmental outcomes of H, zea larva that proceeded into moths were
compared.
One insect was reared on a utilizable (cholesterol treatment) sterol and the
other
2 5 insects) was reared on a non-utilizable (24-methyl cholesta-5,23-dienol
treatment)
sterol.
Most of the insects reared on non-utilizable sterols failed to develop beyond
the third
instar (Table 11), indicating they were ineffective cholesterol surrogates and
harmful to
growth and development. Some of the non-utilizable sterol treatments were
found to
-56-
CA 02276087 1999-06-25
WO 98!45457 PCTIUS97/23495
pupate and develop into moths. However, these moths possessed incompletely
.developed wings and legs.
Table 11 and Fig. 9 show that the position of the double bond in the sterol
side chain
and nucleus is critical to sterol-controlled growth. The inability of cholest-
$-enol to
support growth suggests that H. zea cannot transform 9~i,19-cyclopropyl
sterols to
OS-sterols. Cyclopropyl sterols must pass through an D8-sterol intermediate to
give rise
to a OS-sterol. Blocking this process will lead to the formation of non-
utilizable sterols.
These results indicate for the first time that several sterols synthesized by
corn should
1 o be unsuitable as sterol replacements of cholesterol.
AlI of the compositions and methods disclosed and claimed herein can be made
and executed without undue experimentation in light of the present disclosure.
While
the compositions and methods of this invention have been described in terms of
preferred embodiments, it will be apparent to those of skill in the art that
variations may
be applied without departing from the concept, spirit and scope of the
invention. More
specifically, it will be apparent that certain agents which are both
chemically and
physiologically related may be substituted for the agents described herein
while the same
or similar results would be achieved. All such similar substitutes and
modifications
2 o apparent to those skilled in the art are deemed to be within the spirit,
scope and concept
of the invention as defined by the appended claims.
-57-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
BIBLIOGRAPHY
Akisha et al. in: "Physiology and Biochemistry of Sterols" (G.W. Patterson and
W. David
Nes, Eds.) (1992) American Oil Chemists' Society Press, pp. 172-228.
Armstrong et al. Field evaluation of European corn borer control in progeny of
173
transgenic corn events expressing the insecticidal protein from Bacillus
thuringinesis.
Crop Science (I995) vol. 35 pp. 550-557
Battraw and Hall. Histochemical analysis of CaMV 35S promoter-beta-
glucuronidase
gene expression in transgenic rice plants. Plant Mol. Biol. (1990) vol. 15 pp.
527-538
Bird and Ray. Manipulation of plant gene expression by antisense RNA.
Biotechnology & Genetic Engineering Reviews 9:207-27, 1991
Bouhida et al.. An analysis of the complete sequence of a sugarcane
bacilliform virus
genome infectious to banana and rice. Journal General Virology (1993) vol. 74
pp. 15-
22
Bouvier-Nave et al. Eur. J. Biochem 246: 518-529 (1997)
Bower and Birch. Transgenic sugarcane plants via microprojectile bombardment.
Plant J.
( 1992) vol. 2 pp. 409-416
Bryant. Successful genetic engineering of a cereal crop species transgenic rye
obtained by
plasmid injection into floral tiller. Trends Biotechnol. vol. 5 pp. 60-61
2 0 Bytebier et al. T-DNA organization in tumor cultures and transgenic plants
of the
monocotyledon Asparagus officinalis. Proc. Natl. Acad. Sci. ( 1987) vol. 84
pp. 5345-
5349
Christou et al. Stable transformation of soybean callus by DNA-coated gold
particles.
Plant Physiol. (1988) vol. 87 pp. 671-674
-58-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Christou et al. Production of transgenic rice (Oryza staiva L.) plants from
agronomically
- important indica and japonica varieties via electric discharge particle
acceleration of
exogenous DNA into immature zygotic embryos. ( I991 ) vol. 9 pp. 957-962
Cheng et aI. Efficient transformation of papaya by coat protein gene of papaya
ringspot
virus mediated by Agrobacterium following liquid-phase wounding of embryogenic
tissues with carborundum. Plant Cell Reports (1996) vol. 16 pp. 127-132
Corey et al. Proc. Nat. Acad. Sci. 90: 11628-11632 (1993)
Coruzzi et al. Tissue-specific and light-regulated expression of a pea nuclear
gene
encoding the small subunit of ribulose-1,5-bisphosphate. EMBO J. ( 1984) vol.
3 pp.
1671-1679
Coste et al. Proc. Nat. Acad. Sci 84, pp. 643-647 (1987)
De Kathen and Jacobsen. Agrobacterium tumefaciens mediated transformation of
pisum
sativum L. using binary and cointegrate vectors. Plant Cell Reports (1990)
vol. 9 pp.
276-279
Fletcher, R. A. et al. , 1988, in: D. Berg et al. (Eds . ) STEROL BIOSYNTHESIS
INHIBITORS-PHARMACEUTICAL AND AGROCHEMICAL ASPECTS, Ellis
Horwood, pp 321-331
Fraley et al. Expression of bacterial genes in plant cells. Proc. Natl. Acad.
Sci. (1983)
vol. 80 pp. 4803-4807.
2 0 Fromm et al. UCLA Symposium on Molecular Strategies for Crop Improvement,
Apr. 16-
22. Keystone, CO. { 1990)
Gachotte et al. The Plant Journal 9: 391-398 {1996)
Gibson and Shillitoe. Ribozymes. Their fucntions and strategies for their use.
Molecular Biotechnology 7(2): 125-37 {1997)
-59-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Gordon-Kamm et al. Transformation of maize cells and regeneration of fertile
transgenic
- plants. Plant Cell, Vol. 2, pp. 603-618 (1990)
Guo et al. Developmental Regulation of Sterol Biosynthesis in Zea mat's.
Lipids 30: 3, p.
203-219 (1995)
Guo et al. Stereochemistry of Hydrogen Migration from C-24 to C-2S during
Phytosterol
Biomethylation. J. Am. Chem. Soc. 118, pp. 8507-8508 (1996)
Guo et al. Phytosterol Biosynthesis: Isotope Effects Associated with
Biomethylation
Formation to 24-Alkene Sterol Isomers. Tetrahedron Letters 37: 38, pp. 6823-
6826
( 1996)
l0 Grabenok et al. Plant Mol. Biol. 34: 891-896 (I997)
Haeuser, C. et al 1990 J. Plant Physiol. 137: 201-207
Hinchee et al, in PLANT CELL AND TISSUE CULTURE (1994), pp.231-271, Vasil and
Thorpe {eds.), Kluwer Academic Publishers
Hom et al. Transgenic plants of orchardgrass Dactylis glomerata L. from
protoplasts.
Plant Cell Reports (1988) vol. 7 pp. 469-472
Husselstein et al. FEBS Letters 381: 87-92 (1996)
Koziel et al. Transgenic maize plants for the control of European corn borer.
Abstract,
ACS annual meeting, Chicago, Il, Aug 22-27, 1993. (1993)
Li, S. Stereochemical Studies on the Metabolism of Sterols by S. Cervesiae.
M.S. Thesis,
Texas Tech University (1996) pp. 1-123.
Medberry and Olszewski. Identification of cis elements involved in commelina
yellow
mottle virus promoter activity. Plant Journal (1993) vol. 3 pp. 619-626
-60-
- CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Moffatt et al. The adenine phosphoribosyltransferase-encoding gene of
Arabidopsis
~~i~. Gene ( 1994) vol I 43 pp. 211-216
Nes et al. Structural Requirements for Transformation of Substrates by the (S)-
Adenosyl-
L-methionine: 024~25)_Sterol Methyl Transferase. J. Bio. Chem. 266: 23, pp.
15202-
15212 (1991)
Nes et al. Arch. Biochem Biophys. 300, pp. 724-733 (1993)
Nes et al. Sterol Utilization and Metabolism by Heliothis zea. Lipids ( 1997)
vol 32, no 11
Ni et al. Strength and tissue specificity of chimeric promoters derived from
the octopine
and mannopine synthase genes. Plant J. (1995) vol. 7 pp. 661-676
1 o Odell et al. Identification of DNA sequences required for activity of the
cauliflower
mosaic virus 35S promoter. Nature (1985) vol. 313 pp. 810-812
Ritchie and Hodges, in: TRANSGENIC PLANTS (1993), Vol.l, pp.147-178, Shain-dow
Kung (Ed.), Academic Press Inc.
Rhodes et al. Science (1988) vol. 240 p. 204
Sanger et al. Characteristics of a strong promoter from figwort mosaic virus:
comparison
with the analogous 35S promoter from cauliflower mosaic virus and the
regulated
mannopine synthase promoter. Plant Mol. Biol. ( 1990) vol. 14 pp. 433-4.4.3
Sambrook, J., E. F. Fritsch, and T. Maniatis (1989) Molecular Cloning: A
Laboratory
Manual, Second Edition, Cold Spring Harbor Laboratory, Cold Spring Harbor, New
2 o York
Schroeder et al. Transformation and regeneration of two cultivars of pea Pisum
sativum L.
Plant Physiol. (1993) vol. 101 pp. 751-757
Schuch, W. Using antisense RNA to study gene function. Symposia of the Society
for
Experimental Biology 45:117-27, 1991
-61-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
Schuier et al. Nucleic Acids Res. ( 1982} vol. 10 pp. 8225-8244
Shi et al. J.Bio.Chem. (1996) 271: 9384-9389
Somers et al. Bio/Technology (1992) vol. 10 pp. 1589-1594
Steponkus, P.L. 1984, Annu. Rev. Plant Physiol. 35: 543-584
Tong et al. Tetrahedron Letters 38: 35, pp. 6115-6118 (1997)
Toriyama et al. Bio/Technology ( 1988) vol. 6 p. 10
Vasil et al. Bio/Technology (1992) vol. 10 pp. 667-674
Venkatramesh et al. Biochimica Biophysica Acta 1299 (1996) 3I3-324
Wan and Lemaux Plant Physiol. {1994) vol. 104 pp. 37-48
l o Wang et al. Bio/Technology ( I992) vol. 10 p. 691
Weeks et al. Plant Physiol (1993) vol. 102 pp. 1077-1084
Xu et al. Rice triosephosphate isomerase gene 5' sequence directs beta-
glucuronidase
activity in transgenic tobacco but requires an intron for expression in rice.
Plant
Physiology ( 1994) vol. 106 pp. 459-467
Yang et al., Plant Cell Reports 15:459-464 (1996)
Zhong et al. Analysis of the functional activity of the 1.4 kb 5' region of
the rice actin 1
gene in stable transgenic plants of maize (Zea mays L.). Plant Science (1996)
vol. 116
PP~ 73-84.
-62-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT: NES, DAVID W.
(ii) TITLE OF INVENTION: TRANSGENIC PLANTS WITH MODIFIED STEROL
COMPOSITIONS
(iii) NUMBER OF SEQUENCES: 6
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: ARNOLD) WHITE & DURKEE
I5 (B) STREET: P.O. BOX 4433
(C) CITY: HOUSTON
(D) STATE: TX
(E) COUNTRY: USA
(F) ZIP: 77210-4433
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release #1.0, Version
#1.30
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER: US 60/033,923
(B) FILING DATE: 26-DEC-1996
3 (C) CLASSIFICATION:
O
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: KAMMERER, PATRICIA A.
(B) REGISTRATION NUMBER: 29,775
(C) REFERENCE/DOCKET NUMBER: MOBT148
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: 713/787-1400
(B) TELEFAX: 713/787-1440
(2) INFORMATION FOR SEQ ID NO: l:
(i) SEQUENCE CHARACTERISTICS:
4 5 (A) LENGTH: 1320 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
55
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: l:
-63-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97I23495
TTACTTTCGA TTTAAGTTTT ACATAATTTA AAAAAACAAG AATAAAATAA TAATATAGTA
60
GGCAGCATAA GATGAGTGAA ACAGAATTGAGAAAAAGACA GGCCCAATTC ACTAGGGAGT
120
TACATGGTGA TGATATTGGT AAAAAGACAGGTTTGAGTGC ATTGATGTCG AAGAACAACT
180
CTGCCCAAAA GGAAGCCGTT CAGAAGTACTTGAGAAATTG GGATGGTAGA ACCGATAAAG
240
ATGCCGAAGA ACGTCGTCTT GAGGATTATAATGAAGCCAC ACATTCCTAC TATAACGTCG
300
TTACAGATTT CTATGAATAT GGTTGGGGTTCCTCTTTCCA TTTCAGCAGA TTTTATAAAG
360
GTGAGAGTTT CGCTGCCTCG ATAGCAAGACATGAACATTA TTTAGCTTAC AAGGCTGGTA
420
TTCAAAGAGG CGATTTAGTT CTCGACGTTGGTTGTGGTGT TGGGGGCCCA GCAAGAGAGA
480
TTGCAAGATT TACCGGTTGT AACGTCATCGGTCTAAACAA TAACGATTAC CAAATTGCCA
540
AGGCAAAATA TTACGCTAAA AAATACAATTTGAGTGACCA AATGGACTTT GTAAAGGGTG
600
ATTTCATGAA AATGGATTTC GAAGAAAACACTTTCGACAA AGTTTATGCA ATTGAGGCCA
660
CATGTCACGC TCCAAAATTA GAAGGTGTATACAGCGAAAT CTACAAGGTT TTGAAACCGG
720
GTGGTACCTT TGCTGTTTAC GAATGGGTAATGACTGATAA ATATGACGAA AACAATCCTG
780
4 AACATAGAAA GATCGCTTAT GAAATTGAACTAGGTGATGG TATCCCAAAG ATGTTCCATG
0
840
TCGACGTGGC TAGGAAAGCA TTGAAGAACTGTGGTTTCGA AGTCCTCGTT AGCGAAGACC
900
TGGCGGACAA TGATGATGAA ATCCCTTGGTATTACCCATT AACTGGTGAG TGGAAGTACG
960
TTCAAAACTT AGCTAATTTG GCCACATTTTTCAGAACTTC TTACTTGGGT AGACAATTTA
1020
CTACAGCAAT GGTTACTGTA ATGGAAAAATTAGGTCTAGC CCCAGAAGGT TCCAAGGAAG
1080
CA 02276087 1999-06-25
WO 98145457 PCT/US97123495
TTACTGCTGC TCTAGAAAAT GCTGCGGTTG GTTTAGTTGC CGGTGGTAAG TCCAAGTTAT
1140
TCACTCCAAT GATGCTTTTC GTCGCTAGGA AGCCAGAAAA CGCCGAAACC CCCTCCCAAA
1200
CTTCCCAAGA AGCAACTCAA TAAATTCACT AGATCAATAA GATTCAAATA AAGCGCACGA
1260
TATATACCTA TTTTCCTATA TATGCAGATA AAAAGATAGC ACGTTCATTG CTAGCAGGCC
1320
(2) INFORMATION FOR SEQ ID N0:2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 383 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
2 0 ( D ) TOPOLOGY : 1 inear
30
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:2:
Met Ser Glu Thr Glu Leu Arg Lys Arg Gln Ala Gln Phe Thr Arg Glu
1 5 10 15
Leu His Gly Asp Asp IIe Gly Lys Lys Thr Gly Leu Ser Ala Leu Met
20 25 30
Ser Lys Asn Asn Ser Ala Gln Lys Glu Ala Val Gln Lys Tyr Leu Arg
35 40 45
Asn Trp Asp Gly Arg Thr Asp Lys Asp Ala Glu Glu Arg Arg Leu Glu
50 55 60
4 0 Asp Tyr Asn Glu Ala Thr His Ser Tyr Tyr Asn Val Val Thr Asp Phe
65 70 75 80
Tyr Glu Tyr Gly Trp Gly Ser Ser Phe His Phe Ser Arg Phe Tyr Lys
85 90 95
Gly-Glu Ser Phe Ala Ala Ser Ile Ala Arg His Glu His Tyr Leu Ala
100 105 110
Tyr._Lys Ala Gly Ile Gln Arg Gly Asp Leu Val Leu Asp Val Gly Cys
5 0 115 120 12 5
G1;~ Val Gly Gly Pro Ala Arg Glu Ile Ala Arg Phe Thr Gly Cys Asn
I30 135 140
- 5 5 Val Ile Gly Leu Asn Asn Asn Asp Tyr Gln Ile Ala Lys Ala Lys Tyr
-SS-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
145 150 155 160
Tyr Ala Lys Lys Tyr Asn Leu Ser Asp Gln Met Asp Phe Val Lys Gly
165 170 175
Asp PheMet MetAsp PheGlu GluAsnThr PheAsp LysVal Tyr
Lys
180 185 190
Ala IleGlu ThrCys HisAla ProLysLeu GluGly ValTyr Ser
Ala
195 200 205
Glu IleTyr ValLeu LysPro GlyGlyThr PheAla ValTyr Glu
Lys
210 215 220
Trp ValMet AspLys TyrAsp GluAsnAsn ProGlu HisArg Lys
Thr
225 230 235 240
Ile AlaTyr IleGlu LeuGly AspGlyIle ProLys MetPhe His
Glu
245 250 255
Val AspVal ArgLys AlaLeu LysAsnCys GlyPhe GluVal Leu
Ala
260 265 270
Val SerGlu LeuAla AspAsn AspAspGlu IlePro TrpTyr Tyr
Asp
275 280 285
Pro LeuThr GluTrp LysTyr ValGlnAsn LeuAla AsnLeu Ala
Gly
290 295 300
3 Thr PhePhe ThrSer TyrLeu GlyArgGln PheThr ThrAla Met
0 Arg
305 310 315 320
Val ThrVal GluLys LeuGly LeuAlaPro GluGly SerLys Glu
Met
325 330 335
Val ThrAla LeuGlu AsnAla AlaValGly LeuVal AlaGly Gly
Ala
340 34S 350
Lys SerLys PheThr ProMet MetLeuPhe ValAla ArgLys Pro
Leu
355 360 365
Glu AsnAla ThrPro SerGln ThrSerGln GluAla ThrGin
Glu
370 375 380
4 (2) INFORMATION
5 FOR
SEQ
ID N0:3:
(i} SEQUENCE
CHARACTERISTICS:
(A)LENGTH:1420
base
pairs
(B)TYPE:
nucleic
acid
5 (C)STRANDEDNESS:
0 double
(D)TOPOLOGY:
linear
-66-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97I23495
(xi) SEQUENCE DESCRIPTION:
SEQ ID N0:3:
CTCTCTCTCT CTCTCTCTTGGTCTTCCTCACTCTTAACGAAAATGGACTCTTTAACACTC
60
TTCTTCACCG GTGCACTCGTCGCCGTCGGTATCTACTGGTTCCTCTGCGTTCTCGGTCCA
120
GCAGAGCGTA AAGGCAAACGAGCCGTAGATCTCTCTGGTGGCTCAATCTCCGCCGAGAAA
180
GTCCAAGACA ACTACAAACAGTACTGGTCTTTCTTCCGCCGTCCAAAAGAAATCGAAACC
240
GCCGAGAAAG TTCCAGACTTCGTCGACACATTCTACAATCTCGTCACCGACATATACGAG
300
TGGGGATGGG GACAATCCTTCCACTTCTCACCATCAATCCCCGGAAAATCTCACAAAGAC
360
GCCACGCGCC TCCACGAAGAGATGGCGGTA~GATCTGATCCAAGTCAAACCTGGTCAAAAG
420
ATCCTAGACG TCGGATGCGGTGTCGGCGGTCCGATGCGAGCGATTGCATCTCACTCGCGA
480
GCAACGTAGT CGGGATTACAATAAACGAGTATCAGGTGAACAGAGCTCGTCTCCACAATA
540
AGAAAGCTGG TCTCGACGCGCTTTGCGAGGTCGTGTGTGGTAACTTCCTCCAGATGCCGT
600
TCGATGACAA CAGTTTCGACGGAGCTTATTCCATCGAAGCCACGTGTCACGCGCCGAAGC
660
TGGAAGAAGT GTACGCAGAGATCTACAGGGTGTTGAAACCCGGATCTATGTATGTGTCGT
720
4 ACGAGTGGGT TACGACGGAGAAATTTAAGGCGGAGGATGACGAACACGTGGAGGTAATCC
O
780
AAGGGATTGA GAGAGGCGATGCGTTACCAGGGCTTAGGGCTTACGTGGATATAGCTGAGA
840
CGGCTAAAAA GGTTGGGTTTGAGATAGTGAAGGAGAAGGATCTGGCGAGTCCACCGGCTG
900
AGCCGTGGTG GACTAGGCTTAAGATGGGTAGGCTTGCTTATTGGAGGAATCACATTGTGG
960
TTCAGATTTT GTCAGCGGTTGGAGTTGCTCCTAAAGGAACTGTTGATGTTCATGAGATGT
1020
-67-
CA 02276087 1999-06-25
WO 98/45457 PCT/EJS97123495
TGTTTAAGAC TGCTGATTGT TTGACCAGAG GAGGTGAAAC CGGAATATTC TCTCCGATGC
1080
ATATGATTCT CTGCAGAAAA CCGGAGTCAC CGGAGGAGAG TTCTTGAGAA AGGTAGAAAG
S 1140
GAAACATCAC CGGAAAAAGT ATGGAGAATT TTCTCAATTT GTTTTTATTT TTAAGTTAAA
1200
TCAACTTGGT TATTGTACTA TTTTTGTGTT TTAATTTGGT TTGTGTTTCA AGAATTATTA
1260
GTTTTTTTTT GTTTTGTTGC ATATGAGAAT CTTACTCTTG ATTTCTCCGC CGTAGAGCCG
1320
GCGAGACATA GGGGATTATT AGTATTTTTA AGTGTGTTTA AGATTGATTA ACAAGTTAGT
1380
AAAATAAAAT GTACTTAGGT GTCGAAAAAA AAAGGAATTC
1420
(2) INFORMATION FOR SEQ ID N0:4:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 361 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:4:
Met Asp Ser Leu Thr Leu Phe Phe Thr Gly Ala Leu Val Ala Val Gly
1 5 10 15
Ile Tyr Trp Phe Leu Cys Val Leu Gly Pro Ala Glu Arg Lys Gly Lys
20 25 30
Arg Ala Val Asp Leu Ser Gly Gly Ser Ile Ser Ala Glu Lys Val Gln
35 40 45
4 5 Asp Asn Tyr Lys Gln Tyr Trp Ser Phe Phe Arg Arg Pro Lys Glu IIe
55 60
Glu Thr Ala Glu Lys Val Pro Asp Phe Val Asp Thr Phe Tyr Asn Leu
65 70 75 80
Val Thr Asp Ile Tyr Glu Trp Gly Trp Gly Gln Ser Phe His Phe Ser
85 90 95
Pro Ser Ile Pro Gly Lys Ser His Lys Asp Ala Thr Arg Leu His Glu
- 55 loo 105 llo
-b8-
CA 02276087 1999-06-25
WO 98145457 PCT/US97/23495
Glu Met Ala Val Asp Leu Ile Gln Val Lys Pro Gly Gln Lys Ile Leu
- 115 120 125
Asp Val Gly Cys Gly Val Gly Gly Pro Met Arg Ala Ile Ala Ser His
130 135 140
Ser Arg Ala Asn Val Val Gly Ile Thr Ile Asn Glu Tyr Gln Val Asn
145 150 155 160
Arg Ala Arg Leu His Asn Lys Lys Ala Gly Leu Asp Ala Leu Cys Glu
165 170 175
Val VaI Cys Gly Asn Phe Leu Gln Met Pro Phe Asp Asp Asn Ser Phe
180 185 190
Asp Gly Ala Tyr Ser Ile Glu Ala Thr Cys His Ala Pro Lys Leu Glu
195 200 205
Glu Val Tyr Ala Glu Ile Tyr Arg Val Leu Lys Pro Gly Ser Met Tyr
210 215 220
Val Ser Tyr Glu Trp Val Thr Thr Glu Lys Phe Lys Ala Glu Asp Asp
225 230 235 240
Glu His Val Glu Val Ile Gln Gly Ile Glu Arg Gly Asp Ala Leu Pro
245 250 255
Gly Leu Arg Ala Tyr Val Asp Ile Ala Glu Thr Ala Lys Lys Val Gly
260 265 270
Phe Glu Ile Val Lys Glu Lys Asp Leu Ala Ser Pro Pro Ala Glu Pro
275 280 285
3 5 Trp Trp Thr Arg Leu Lys Met Gly Arg Leu Ala Tyr Trp Arg Asn His
290 295 300
Ile Val Val Gln Ile Leu Ser Ala Val Gly Val Ala Pro Lys Gly Thr
305 310 315 320
Val Asp Val His Glu Met Leu Phe Lys Thr AIa Asp Cys Leu Thr Arg
325 330 335
Gly Gly Glu Thr Gly Ile Phe Ser Pro Met His Met Ile Leu Cys Arg
340 345 350
Lys Pro Glu Ser Pro Glu Glu Ser Ser
355 360
(2) INFORMATION FOR SEQ ID N0:5:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 1320 base pairs
(B) TYPE: nucleic acid
5 5 (C) STRANDEDNESS: double
-69-
CA 02276087 1999-06-25
WO 98/45457 PCTIUS97/23495
(D) TOPOLOGY: linear
(xi) SEQUENCE DESCRIPTION:
SEQ ID N0:5:
TTACTTTCGA TTTAAGTTTT ACATAATTTAAAAAAACAAG AATAAAATAATAATATAGTA
60
GGCAGCATAA GATGAGTGAA ACAGAATTGAGAAAAAGACA GGCCCAATTCACTAGGGAGT
120
1 TACATGGTGA TGATATTGGT AAAAAGACAGGTTTGAGTGC ATTGATGTCGAAGAACAACT
5
180
CTGCCCAAAA GGAAGCCGTT CAGAAGTACTTGAGAAATTG GGATGGTAGAACCGATAAAG
240
ATGCCGAAGA ACGTCGTCTT GAGGATTATAATGAAGCCAC ACATTCCTACTATAACGTCG
300
TTACAGATTT CTATGAATAT GGTTGGGGTTCCTCTTTCCA TTTCAGCAGATTTTATAAAG
360
GTGAGAGTTT CGCTGCCTCG ATAGCAAGACATGAACATTA TTTAGCTTACAAGGCTGGTA
420
3 TTCAAAGAGG CGATTTAGTT CTCGACGTTGGTTGTGGTGT TGGGGGCCCAGCAAGAGAGA
O
480
TTGCAAGATT TACCGGTTGT AACGTCATCGGTCTAAACAA TAACGATTACCAAATTGCCA
540
AGGCAAAATA TTACGCTAAA AAATACAATTTGAGTGACCA AATGGACTTTGTAAAGGGTG
600
ATTTCATGAA AATGGATTTC GAAGAAAACACTTTCGACAA AGTTTATGCAATTGAGGCCA
660
CATGTCACGC TCCAAAATTA GAAGGTGTATACAGCGAAAT CTACAAGGTTTTGAAACCGG
720
4 GTGGTACCTT TGCTGTTTAC GAATGGGTAATGACTGATAA ATATGACGAAAACAATCCTG
5
780
AACATAGAAA GATCGCTTAT GAAATTGAACTAGGTGATGG TATCCCAAAGATGTTCCATG
84 0
TCGACGTGGC TAGGAAAGCA TTGAAGAACTGTGGTTTCGA AGTCCTCGTTAGCGAAGACC
900
TGGCGGACAA TGATGATGAA ATCCCTTGGTATTACCCATT AACTGGTGAGTGGAAGTACG
960
-70-
CA 02276087 1999-06-25
WO 98/45457 PCT/US97/23495
TTCAAAACTT AGCTAATTTG GCCACATTTT TCAGAACTTC TTACTTGGGT AGACAATTTA
1.020
CTACAGCAAT GGTTACTGTA ATGGAAAAAT TAGGTCTAGC CCCAGAAGGT TCCAAGGAAG
1080
TTACTGCTGC TCTAGAAAAT GCTGCGGTTG GTTTAGTTGC CGGTGGTAAG TCCAAGTTAT
1140
TCACTCCAAT GATGCTTTTC GTCGCTAGGA AGCCAGAAAA CGCCGAAACC CCCTCCCAAA
1200
CTTCCCAAGA AGCAACTCAA TAAATTCACT AGATCAATAA GATTCAAATA AAGCGCACGA
1260
TATATACCTA TTTTCCTATA TATGCAGATA AAAAGATAGC ACGTTCATTG CTAGCAGGCC
1320
(2) INFORMATION FOR SEQ ID N0:6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 1497 base pairs
(s) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ix) FEATURE:
(A) NAME/KEY: modified_base
(B) LOCATION: 1419
(D) OTHER INFORMATION: /mod base= OTHER
/note= ~~A or C or G or T~~
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:6:
4 0 AGACTCTGGT TCTGACATGC AGCAATTATT GCAGGTGCAT TTGATCCGTC CCGGCCGCCT
45
ACACGATGTC CAAGTCGGGA GCGCTGGATC TTGCTTCTGG CCTCGGAGGG AAGATCAACA
120
AGGTGGAAGT CAAGTCGGCC GTCGATGAGT ATGAGAAATA TCATGGATAC TATGGAGGGA
180
AGGAGGAAGC AAGGAAGTCC AACTATACTG ATATGGTTAA TAAATACTAT GATCTTGCCA
50 240
CTAGCTTCTA TGAGTATGGT TGGGGTGAAT CCTTCCACTT TGCTCACAGA TGGAATGGAG
300
-71-
CA 02276087 1999-06-25
WO 98/45457 PCT/CTS97/23495
AATCCTTACG TGAAAGCATC AAGCGACATG AGCATTTTCT TGCCCTGCAA CTTGGTTTGA
360
AACCAGGAATGAAGGTTTTAGATGTGGGCTGTGGAATAGGTGGACCACTG AGAGAAATTG
420
CAAGATTTAGCTCAACTTCAGTTACCGGATTGAATAACCACGAATACCAG ATAACCAGGG
480
GAAAGGAGCTCAACCGTTTAGCAGGAATTAGTGGAACATGTGATTTTGTC AAGGCGGACT
540
TCATGAAGATGCCGTTCGATGACACACTTTTGGATGCTGTTTACGCCATT GAGGCAACAT
600
GTCATGCACCTGATCCAGTTGGTTGCTACAAGGAGATATATCGTGTGTTG AAGCCTGGCC
660
AGTGCTTTGCCGTGTACGAGTGGTGCGTTACGGATCACTATGATCCTAAC AATGCAACCC
720
ACAAAAGGATCAAGGATGAAATTGAGCTTGGCAATGGCCTGCCAGATATC AGAAGCACTC
780
CGCAATGTCTCCGGGCTCTAAAAGACGCCGGGTTTGACGTTGTTTGGGAT AAGGATCTTG
840
CTGAAGATTCTCCCTTGCCTTGGTACTTGCCCTTGGACTCCAGCCGATGC TCACTGAGTA
900
GCTTCCGTCGACCTCCTGTCGGGACGCATGATACCCGCACAATGGTCAAG GCCCTGGAGT
960
ACGTTGGTCTTGCTCCGCAGGGCAGTGAGAGGTCTCTAGTTTTCCTGGAG AAGGCTGCAG
1020
AAGGGCTGGTAGAGGGCGGAAAGAAGGAGATCTTCACGCCAATGTACTTC TTTTTTGTTC
1080
4 GGAAGCCTCTTCTGGAATGAGCTCTTGGATCACCTTTTCAGAGAGAGAAG GCAAGTGGTC
O
1140
ATTTCGAAGAAGCCGAGGAGAGGGAACCTGGAATCAAGAAAACCTTCAGC TCTCCTGTGT
1200
AGGAGGAP':.GTTAACGAACAGTGTAGTAACTGTTCAGCTCTGTGTTTATT CAGTTGTTTT
1260
GCTGCT GTTATTCGTTTCTAGGTGGsTTGGAATC CTTTTCGCCA TAAACCTCTC
:3
1320
AGTGGC._rAAATAAGATGGTTTGCAT.' GTACTTCATGGATACCGTAA GGGCTACTAC
1380
-72-
CA 02276087 1999-06-25
WO 98/45457 PCT/CTS97I23495
TGAAAGAGAA ATGTTTAAGC AGCATGGTAT GTGAGCAANT AGTGATAATT ATTCCATCCT
1440
TTTTTTTAAT ATAAAGCAGG AGTTTTGTCA AAAAAAAAAA pu~~iAAAAAAAA AAAAAAA
1497
-73-