Note: Descriptions are shown in the official language in which they were submitted.
CA 02276188 1999-06-25
WO 98/29528 PCT/US97/23771
LAUNDRY DETERGENT COMPOSITIONS WITH CELLULOSIC POLYMERS
TO PROVIDE APPEARANCE AND INTEGRITY BENEFITS TO FABRICS
LAUNDERED THEREWITH
TECHNICAL FIELD
The present invention relates to heavy duty laundry detergent compositions, in
either liquid or granular fonm, which contain certain types of modified
cellulose ether
materials to impart appearance and integrity benefits to fabrics and textiles
laundered
in washing solutions formed from such compositions.
BACKGROUND OF THE INVENTION
It is, of course, well known that alternating cycles of using and laundering
fabrics and textiles, such as articles of worn clothing and apparel, will
inevitably
adversely affect the appearance and integrity of the fabric and textile items
so used
and laundered. Fabrics and textiles simply wear out over time and with use.
Laundering of fabrics and textiles is necessary to remove soils and stains
which
accumulate therein and thereon during ordinary use. However, the laundering
operation itself, over many cycles, can accentuate and contribute to the
deterioration
of the integrity and the appearance of such fabrics and textiles.
. Deterioration of fabric integrity and appearance can manifest itself in
several
ways. Short fibers are dislodged from woven and knit fabric/textile structures
by the
mechanical action of laundering. These dislodged fibers may form Lint, fiizz
or "pills"
which are visible on the surface of fabrics and diminish the appearance of
newness of
the fabric. Further, repeated laundering of fabrics and textiles, especially
with bleach-
containing laundry products, can remove dye from fabrics and textiles
CA 02276188 2003-09-03
2
and impart a faded, worn out appearance as a result of diminished coloc
intensity, and
in many cases, as a result of changes in hues or shades of color.
Given the foregoing, there is clearly an ongoing need to identify materials
which
could be added to laundry detergent products that would associate themselves
with
the fibers of the fabrics and textiles laundered using such detergent products
and
thereby reduce or minimize the tendency of the laundered fabricltextiles to
deteriorate
in appearance. Any such detergent product additive material should, of course,
be
able to benefit fabric appearance and integrity without unduly interfering
with the
ability of the laundry detergent to perform its fabric cleaning function. The
present
invention is directed to detergent compositions containing certain types of
cellulosic
materials that perform in this desired manner.
SUMMARY OF TAE INVENTION
The laundry detergent compositions herein comprise from about 1% to 80% by
weight of a detersive surfactant, from about 1% to 80% by weight of an organic
oc
inorganic detergency builder and from about 0.1% to 8% by weight of certain
types
of modified cellulose ether fabric treatment agents. The detersive surfactant
and
detergency builder materials can be any of those useful in conventional
laundry
detergent products. The modified cellulose ether materials are those which
have a
molecular weight of from about 10,000 to 2,000,000 and are wmprised of
repeating
substituted anhydroglucose units corresponding to the general Structural
Formulas
Nos. I, II and III set forth hereinafter in the "Detailed Description of the
Invention"
section. (In the Structural Formulas hereinafter set forth, substituents are
shown in
specific positions on the anhydroglucose rings which repeat to form the
substituted
cellulose ether polymers. It should be understood that this is for
illustration purposes
only and that such substituents may be found on any of the carbon atoms of the
anhydroglucose rings.)
One useful type of ~ cxllulose ethers comprises hydrophobicallyr~wdified,
nonionic materials with anhydroglucose ring alkyl substitution ranging from
about
0.1% to 5% by weight of the cellulose ether. Ring substituents are alkoxylated
in
amounts ranging from about 1 to 20 moles.
A second useful type of cellulose ether comprises cationic cellulose ether
materials which may have anhydrogiucosa ring alkyl substitution ranging from
about
0.1% to 5% by weight of the cellulose ether. Anhydroglucose ring substituents
CA 02276188 2005-O1-20
3
contain from about 1 to 20 moles of alkoxylation and from about 0.005 to 0.5
moles of quaternary ammonium moieties.
A third type of cellulose ether comprises anionic cellulose ether materials
which may have anhydroglucose ring alkyl substitution ranging from about
0.1 % to 5 % by weight of the cellulose ether. The anhydroglucose rings in
such
anionic materials also have a degree of carboxymethyl substitution ranging
from
about 0.05 to 2.5. Combinations of the nonionic, cationic and anionic modified
cellulose ethers can also be employed.
In one particular embodiment there is provided a built, heavy duty
laundry detergent composition in liquid or granular form which imparts fabric
appearance benefits selected from pilUfuzz reduction, antifading, improved
abrasion resistance and enhanced softness to fabrics and textiles laundered in
aqueous washing solutions formed therefrom, which composition is
characterized by: A) from 1% to 80% by weight of a detersive surfactant; B)
from 1 % to 80 % by weight of an organic or inorganic detergency builder; C)
from 0.1 % to 8 % by weight of a modified cellulose ether fabric treatment
agent
selected from: hydrophobically-modified, nonionic cellulose ethers which have
a
molecular weight of from 10,000 to 2,000,000 and which have repeating
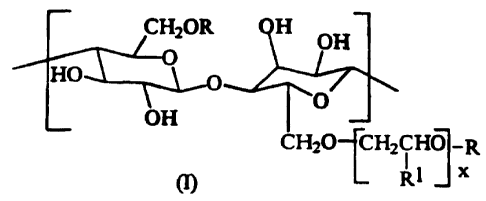
substituted anhydroglucose units corresponding to the general formula:
R
c
wherein:
R is independently selected from the group consisting of H and Cg-CZa
alkyl with alkyl substitution of the anhydroglucose rings ranging in an
amount of from 0.1 % to 5 % by weight of the cellulose ether material;
RI is H or methyl; and
x ranges from 1 to 20.
CA 02276188 2005-O1-20
3a
In its method aspect, ttar invention rotates to the ~aung or tresang
of fabrics and textiles in aqueous washing or treating solutions ~t~mal from
effective
amounts of the detergent compositions described heron, or formed from the
individual components of such compositions. Laundering of fltbrics and
textiles in
such washing solutions, followed by rinsing and drying, imparts fabric
appearance
benefits to the fabric and textile articles so ueated. gush be~refits can
include
improved overall appearance, pilUfuzz n~uction, antifading, in~rovad abrasion
resistance, and/or enhanced softness.
SLED DESCRIPTION OF THE INY~~ON
As noted, the laundry detergent compositions of the present imrention
essentially oontsin v~e s~rrfacxsnt, daagmt builder and certain nrodiged
cellulose ether fabric treatment agents which serve to enhance fabric
appearance and
integrity upon use of the detergent aompoaitions to launder fabrics and
textiles. Each
of these ~smttial detergent composition components, as well as optional units
for such compositions and metlada of using such compositions, are described in
ddail as follows: All peroeatages and ratios given are by weight unless other
speed.
A)
The det~rt compositions hendn eaaentia~lly comprise R~om about 1% t0 80%
by weight of a detersive surfactant. Prefeeabiy such ~mpos~ons comprise fl~om
about 5'X. to 50yG by w~aig~ of this Wit. Det~v~e surd 'ut>bmed can be
of the anionic, noniotric, zvvitterionic, smpholytic or cationic type or can
comprise
oompstible mixtures of these types. Detergent sur~tsnts uaegrl ha~in are
descn'bed
in U.S. Patent 3,664,961, Norria, issued May 23, 1972, U.S. Pstent 3,919,678,
Laughlin et al., iaa~red December 30, 1975, U.S. Patent 4,222,905,
CA 02276188 2003-09-03
4
Cockrell, issued September 16, 1980, and in U.S. Patent 4,239,659, Murphy,
issued
December 16, 1980. Of all the surfactants, anionics and nonionics are
preferred.
Useful anionic surfactants can themselves be of several different types. For
example, water-soluble salts of the higher fatty acids, i.e., "soaps", are
useful anionic
surfactants in the compositions herein. This includes alkali metal soaps such
as the
sodium, potassium, ammonium, and alkylolammonium salts of higher fatty acids
containing from about 8 to about 24 carbon atoms, and preferably from about 12
to
about 18 carbon atoms. Soaps can be made by direct saponification of fats and
oils
or by the neutralization of free fatty acids. Particularly useful are the
sodium and
potassium salts of the mixtures of fatty acids derived from coconut oil and
tallow,
i.e., sodium or potassium tallow and coconut soap.
Additional non-soap anionic surfactants which are suitable for use herein
include the water-soluble salts, preferably the alkali metal, and ammonium
salts, of
organic sulfuric reaction products having in their molecular structure an
alkyl group
containing from about 10 to about 20 carbon atoms and a sulfonic acid or
sulfuric
acid ester group. (Included in the term "alkyl" is the alkyl portion of aryl
groups.)
Examples of this group of synthetic surfactants are a) the sodium, potassium
and
ammonium alkyl sulfates, especially those obtained by sulfating the higher
alcohols
(Cg-C 1 g carbon atoms) such as those produced by reducing the glycerides of
tallow
or coconut oil; b) the sodium, potassium and ammonium alkyl polyethoxylate
sulfates, particularly those in which the alkyl group contains from 10 to 22,
preferably
from 12 to 18 carbon stoma, and wherein the poiyethoxylate chain contains from
1 to
15, preferably 1 to 6 ethoxylate moieties; and c) the sodium and potassium
alkylbenzene sulfonates in which the alkyl group contains from about 9 to
about 15
carbon atoms, in straight chain or branched chain configuration, e.g., those
of the _
type described in U.S. Patents 2,220,099 and 2,477,383. Especially valuable
are
linear straight chain alkylbenzene sulfonates in which the average number of
carbon
atoms in the alkyl group is from about 11 to 13, abbreviated as C 11-13 ~~
Preferred nonionic surfactants are those of the formula R1(OC2H4)nUH,
wherein Rl is a C l p-C 1 b ~ Si'~P or a Cg-C 12 alkyl phenyl group, and n is
from
3 to about 80. Particularly preferred are condensation products of C ~ 2-C 15
alcohols
with from about 5 to about 20 motes of ethylene oxide per mole of alcohol,
e.g.,
C 12'C 13 ~oohol °°~ensed with about 6.5 moles of ethylene
oxide per mole of
alcohol.
CA 02276188 2003-09-03
Additional suitable nonionic surfactants include polyhydroxy fatty acid amides
of the formula:
O R~
R-~-N-Z
wherein R is a Cg-17 alkyl or alkenyl, R1 is a methyl group and Z is glycityl
derived
from a reduced sugar or alkoxylated derivative thereof. Examples are N-methyl
N-
1-deoxyglucityl cocoamide and N-methyl N-1-deoxyglucityl oleamide. Processes
for
making polyhydroxy fatty acid amides are known and can be found in Wilson,
U.S.
Patent 2,965,576 and Schwartz, U.S. Patent 2,703,798 .
B) eraent Builder
The detergent compositions herein also essentially comprise from about 0.1%
to 80% by weight of a detergent builder. Preferably such compositions in
liquid
form will comprise from about 1 °l° to I O% by weight of the
builder component.
Preferably such compositions in granular form will comprise from about
1°/. to 50%
by weight of the builder component. Detergent builders are well known in the
art
and can comprise, for example, phosphate salts as well as various organic and
inorganic nonphosphorus builders.
Water-soluble, nonphosphorus organic builders useful herein include the
various alkali metal, ammonium and substituted ammonium polyacetates,
carboxylates, polycarboxylates and polyhydroxy sulfonates. Examples of
polyacetate
and polycarboxyiste builders are the sodium, potassium, lithium, ammonium and
substituted ammonium saps of ethylene diamine tetraacetic acid,
nitrilotriacetic acid,
oxydisuccinic acid, mellitic acid, benzene polycarboxylic acids, and citric
acid. Other
suitable polycarboxylates for use herein are the polyacetal carboxylates
descnbed in
U.S. Patent 4,144,226, issued March 13, 1979 to Crutchfield et al, and U.S.
Patent
4,246,495, issued March 27, 1979 to Crutchfield et al.
Particularly preferred polycarboxylate builders are
the oxydisuccinates and the ether carboxylate builder compositions comprising
a
combination of tartrate monosuccinate and tartrate disuccinate described in
U.S.
Patent 4,663,071, Bush et al., issued May 5, 1987.
Examples of suitable nonphosphorus, inorganic builders include the silicates,
aluminosilicates, borates and carbonates. Particularly preferred are sodium
and
potassium carbonate, bicarbonate, sesquicarbonate, tetrabotate decahydrate,
and
CA 02276188 2003-09-03
6
silicates having a weight ratio of Si02 to alkali metal oxide of from about
0.5 to
about 4.0, preferably from about 1.0 to . about 2.4. Also preferred ace
aluminosilicates including zeolites. Such materials and their use as detergent
builders
are more fully discussed in Corkill et al, U.S. Patent No. 4,605,509. Also,
crystalline layered silicates such as those discussed in Corkill et al, U.S.
Patent
No. 4,605,509, are suitable for use in the detergent compositions of this
invention.
C) Modified Cellulosic Polymers
The thi;d essential component of the detergent compositions herein comprises
one or more modified cellulosic polymers. Such materials have been found to
impart .
a number of appearance benefits to fabrics and textiles laundered in aqueous
washing
solutions formed from detergent compositions which contain such modified
cellulosic
materials. Such fabric appearance benefits can include, for example, improved
overall appearance of the laundered fab~cs, reduction of the formation of
pills and
fuzz, protection against ~ color fading, improved abrasion resistance, etc.
The
modified cellulosic polymers used in the compositions and methods herein can
provide such fabric appearance benefits with acceptably little or no loss in
cleaning
performance provided by the laundry detergent compositions into which such
materials are incorporated.
The modified cellulosic polymers useful herein may be of the nonionic,
cationic
or anionic types, or the modified cellulosic polymeric component of the
compositions
herein may comprise combinations of these cellulosic polymer types. The
modified
cetlulosic polymer component of the compositions herein will generally
comprise
from about 0.1% to 8% by the weight of the composition. More preferably, such
modified cellulosic materials will comprise from about 0.5% to 4% by weight of
the
compositionsy most preferably from about 1% to 3%.
One suitable type of modified cellulosic polymer for use herein comprises
hydrophobicaUy-modified, nonionic cellulose ethers having a molecular weight
of
from about 10,000 to 2,000,000, preferably from about 50,000 to 1,000,000.
'The
hydrophobically-modified nonionic materials have repeating, substituted
anhydroglucose units which correspond to the genera! Structural Formula No. I
as
follows:
CA 02276188 2003-09-03
7
-R
x
,_
Structural Formula No. I
In Structural Formula No. I, R is a combination of H and Cg-C24 alkyl,
preferably Cg - C 16 alkyl. Alkyl substitution on the anhydrogluwse rings of
the
polymer ranges from about 0.1% to 5% by weight, more preferably from about
0.2%
to 2% by weight, of the polymu material. Also, in Structural Formula No. I, R1
is H
or methyl, and x ranges from about 1 to 20, prefetably from about 1 to 10.
The hydrophobically-modified nonionic cellulose ethers of Structural Formula
No. I include those which ate commercially available and also include
materials which
can be prepared by conventional chemical modification of commercially
available
materials. Commercially available Mcellulose ethers of the Structural Formula
No. I
type include Polysurf 67, Natrosol Plus 430 and Natrosol Plus 330, all
marketed by
Hercules, Inc.
Another suitable type of modified celluiosic polymer for use herein comprises
certain cationic cellulose ethers, which may or may not be
hydrophobically~modified,
having a molecular weight of from about 10,000 to 2,000,000, more preferably
from
about 10,000 to 1,000,000. These cationic materials have repeating substituted
anhydroglucose units which correspond to the general Structural Formula No. II
as
follows:
R3
~+
N-R4]y ~
RS
CA 02276188 2003-09-03
Structure! Formula No. II
In Structural Formula No. II, R is H or Cg-C24 alkyl, preferably Cg - C16
alkyl. Alkyl substitution on the anhydroglucose rings of the polymer ranges
from
about 0.1 % to 5% by weight, more preferably from about 0.2% to 2% by weight,
of
the polymeric material. Also, in Structural Formula No. II, R2 is CH2CHOHCH2
or
Cg-C24 alkyl, preferably Cg - C 16 alkyl. R3, R4 and RS are each independently
methyl, ethyl or phenyl. R6 is H or methyl. Further, in Structural Formula No.
II, x
ranges from about 1 to 20, preferably from about 1 to 10; and y ranges from
about
0.005 to 0.5, preferably from about 0.005 to 0.1; and Z is Ci- or Br-.
The cationic cellulose ethers of Structural Formula No. II likewise include
those
which are commercially available and further include materials which can be
prepared
by corrventional chemical modification of commercially available materials.
Commercially available cellulose ethers of the Structural Fo~rmuls No. II type
include
the JR 30M, JR 400, JR 125, LR 400 and LK 400 UCARlr polymers, all marketed by
Union Carbide Corporation.
A third type of suitable modified cellulose polymers for use herein comprises
certain anionic cellulose ethers, which also may or may not be hydrophobically
modified, having a molecular weight of from about 10,000 to 2,000,000, more
preferably from about 50,000 to 1,000,000. These anionic materials have
repeating
substituted anhydroglucose units which correspond to general Structural
Formula
No. III as follows:
CHiOR RO
OR
RO
'J
R HiOR
In Stnrctural Formula No. III, R is a combination of H and a) CH2COOA and,
optionally, b) C2-C24~ Prefe~ly C2 - C 16, alkyl, ~"'ith A being Na or K.
Alkyl
substitution on the anhydroglucose rings of the polymer ranges from about 0.1%
to
5% by weight, more preferably from about 0.2% to 2% by weight, of the polymer
material. The anionic cellulose ethers also have a degree of carboxymethyl
CA 02276188 1999-06-25
WO 98/29528 PCT/US97/23771
9
substitution which ranges from about 0.05 to 2.5, more preferably from about
0.1 to
1Ø
The anionic cellulose ethers of Structural Formula No. III also include those
materials which are commercially available and further include those which can
be
' prepared by conventional chemical modification of commercially available
materials.
Commercially available cellulose ethers of the Structural Formula No. III
include
CMC 7H, CMC 99-7M and CMC 99-7L, all marketed by Hercules, Inc. and CMC
D72, CMC D65 and CMC DHT, all marketed by Penn Carbose.
The commercially available cellulose ether materials useful herein are
themselves derived from suitable natural sources of cellulose. Such sources
include,
for example, cotton linters and other vegetable tissues. The modified
cellulose ethers
used in this invention are generally all water-soluble materials. They can
therefore be
utilized for detergent composition preparation in the form of aqueous
solutions of the
such cellulosic polymers if desired.
D) Optional Detergent In redients
In addition to the essential surfactants, builders and modified cellose ethers
hereinbefore described, the detergent composition of the present invention can
also
include any number of additional optional ingredients. These include
conventional
detergent composition components such as bleaches and bleach activators,
enzymes
and enzyme stabilizing agents, suds boosters or suds suppressers, anti-tarnish
and
anticorrosion agents, soil suspending agents, soil release agents, germicides,
pH
adjusting agents, non-builder alkalinity sources, chelating agents, organic
and
inorganic fillers, solvents, hydrotropes, optical brighteners, dyes and
perfumes.
A preferred optional ingredients for incorporation into the detergent
compositions herein comprises a bleaching agent, e.g., a peroxygen bleach.
Such
peroxygen bleaching agents may be organic or inorganic in nature. Inorganic
peroxygen bleaching agents are frequently utilized in combination with a
bleach
activator.
' Useful organic peroxygen bleaching agents include percarboxylic acid
bleaching
agents and salts thereof. Suitable examples of this class of agents include
magnesium
' monoperoxyphthalate hexahydrate, the magnesium salt of metachloro perbenzoic
acid, 4-nonylamino-4-oxoperoxybutyric acid and diperoxydodecanedioic acid.
Such
bleaching agents are disclosed in U.S. Patent 4,483,781, Hartman, Issued
November
20, 1984; European Patent Application EP-A-133,354, Banks et al., Published
CA 02276188 2003-09-03
lU
February 20, 1985; and U.S. Patent 4,412,934, Chung et al., Issued November 1,
1983. Highly preferred bleaching agents also include 6-nonylamino-6-
oxoperoxycaproic acid (NAPAA) .as described in U.S. Patent 4,634,551, Issued
January 6, i 98? to Burns et al.
Inocganic peroxygen bleaching agents may also be used, generally in
particulate
form, in the detergent compositions herein. Inorganic bleaching agents are in
fact
preferred. Such inorganic peroxygen compounds include alkali metal perborate
and
percarbonate materials. For example, sodium perborate (e.g. mono- or tetra-
hydrate)
can be used. Suitable inorganic bleaching agents can also include sodium or
potassium carbonate peroxyl~ydrate and equivalent "percarbonate" bleaches,
sodium
pyrophosphate peroxyhydrate, urea peroxyhydrate, and sodium peroxide.
Persulfate
bleach (e.g., OXONE,T manufactured commercially by DuPont) can also be used.
Frequently inorganic peroxygen bleaches will be coated with silicate, borate,
sulfate
or water-soluble surfactants. For example, coated percarbanate particles are
available from various commercial sources such as FMC, Solvay Interox, Tokai
Denka and Degussa.
Inorganic peroxygen bleaching agents, e.g., the perborates, the percarbonates,
etc., are preferably combined with bleach activators, which lead to the irr
situ
production in aqueous solution ~.e., during use of the compositions herein for
fabric
laundering~bleacwng) of the peroxy acrid corresponding to the bleach
activator.
Various non-limiting examples of activators are disclosed in U.S. Patent
4,915,854,
Issued April 10, 1990 to Mao d al.; and U.S. Patent 4,412,934 Issued November
1,
1983 to Chung et al. The nonanoyloxybenzene sulfonate (HOBS) and tetraacetyl
ethylene diamine (TAED) activators arc typical and preferred. ores thereof can
also be usexl: See also the hereinbefore referenced U.S. 4,634,551 for other
typical
bleaches and activators useful herein.
Other useful amido-derived bleach activators are those of the formulae:
R 11V(R5~(O)R2C(O)L or R i C(O)N(RS)R2C(O)L.
wherein Rl is an alkyl group containing from about 6 to about 12 carbon atoms,
R2
is an aikylene containing from I to about 6 carbon atoms, RS is H or alkyl,
aryl, or
alkaryl cornaining from about 1 to about l0 carbon atoms, and L is any
suitable
leaving group. A (caving group is any group that is displaced from the bleach
activator as a consequence of the nucleophilic attack on the bleach activator
by the
perhydrolysis anion. A preferred leaving group is phenol sulfonate.
CA 02276188 2003-09-03
11
Preferred examples of bleach activators of the above formulae include (6-
octanamido-caproyl)oxybenzenesulfonate, (6-nonanamidocaproyl)
oxybenzenesulfonate, (6-decanamido-caproyl~xybenzenesulfonate and mixtures
thereof as described in U.S. Patent 4,634,551.
Another class of useful bleach activators comprises the benzoxazin-type
activators disclosed by Hodge et al. in U.S. Pat~t 4,966, 723, Issued October
30,
1994. A highly preferred activator of the benzoxazin-type is:
O
'O
I
yC
N
Still another class of useful bleach activators includes the acyt lactam
activators,
especially aryl caprolactams and aryl valerolactams of the formulae:
O O
O C--CHZ-CHZ O ~--CHZ-CHH2
R -C-N . ~ ~ -C N
~CH2--CH2~ ~CHZ---CHz
wherein R6 is H or an alkyl, aryl, alkoxyaryl, or alkaryl group containing
from 1 to
about 12 carbon atoms. Highly preferred lactam activators include bennoyl
caprolactam, octanoyl caprolactarn, 3,5,5-trimethylhexanoyl caprolactam,
nonanoyl
caprolactam, decanoyl caprolaetam, undecenoyl caprolactam, benzoyl
valerolactam,
oetanoyl vaterolactam, nonanoyl valerolactam, decanoyl valerolactam,
undecenoyl
valerolactant, 3,5,5-trimethylhexanayl valerolactam and mixtures thereof. .
See also
U.S. Patent 4,545,784, Issued to Sanderson, October 8, 1985, which discloses
acyl
caprolactams, including benzoyl eaprolactam, adsorbed into sodium perborate.
If utilized, peroxygen bleaching agent will generally comprise from about 2%
to 30% by weight of the detergent compositions herein. More preferably,
peroxygen
bleaching agent will comprise from about 2% to 20'/e by weight of the
compositions.
Most preferably, pemxygen bleaching agent will be present to the axtent of
from
about 3°I° to 15% by weight of the compositions herein. If
utilized, bleach activators
can comprise from about 2% to 10% by weight of the detergent compositions
herein.
CA 02276188 1999-06-25
WO 98/29528 PCT/US97/23771
12
Frequently, activators are employed such that the molar ratio of bleaching
agent to
activator ranges from about 1;1 to 10:1, more preferably from about 1.5:1 to
5:1.
Another highly preferred optional ingredient in the detergent compositions
herein is a detersive enzymes component. Enzymes can be included in the
present
detergent compositions for a variety of purposes, including removal of protein-
based, carbohydrate-based, or triglyceride-based stains from substrates, for
the
prevention of refugee dye transfer in fabric laundering, and for fabric
restoration.
Suitable enzymes include proteases, amylases, lipases, ceIlulases,
peroxidases, and
mixtures thereof of any suitable origin, such as vegetable, animal, bacterial,
fungal
and yeast origin. Preferred selections are influenced by factors such as pH-
activity
andlor stability optima, thermostability, and stability to active detergents,
builders
and the like. In this respect bacterial or fungal enzymes are preferred, such
as
bacterial amylases and proteases, and fungal cellulases.
"Detersive enzyme", as used herein, means any enzyme having a cleaning, stain
removing or otherwise beneficial effect in a laundry detergent composition.
Preferred enzymes for laundry purposes include, but are not limited to,
proteases,
cellulases, lipases, amylases and peroxidases.
Enzymes are normally incorporated into detergent compositions at levels
sufficient to provide a "cleaning-effective amount". The term "cleaning-
effective
amount" refers to any amount capable of producing a cleaning, stain removal,
soil
removal, whitening, deodorizing, or freshness improving effect on substrates
such as
fabrics. In practical terms for current commercial preparations, typical
amounts are
up to about 5 mg by weight, more typically 0.01 mg to 3 mg, of active enzyme
per
gram of the detergent composition. Stated otherwise, the compositions herein
will
typically comprise from 0.001% to 5%, preferably 0.01%-1% by weight of a
commercial enzyme preparation. Protease enzymes are usually present in such
commercial preparations at levels sufficient to provide from 0.005 to 0.1
Anson units
(ALn of activity per gram of composition. Higher active levels may be
desirable in
highly concentrated detergent formulations.
Suitable examples of proteases are the subtilisins which are obtained from
particular strains of B. subtilis and B. lichen formis. One suitable protease
is
obtained from a strain of Bacillus, having maximum activity throughout the pH
range
of 8-12, developed and sold as ESPERASE~ by Novo Industries A/S of Denmark,
hereinafter "Novo". The preparation of this enzyme and analogous enzymes is
described in GB 1,243,784 to Novo. Other suitable proteases include ALCALASE~
and SAVINASE~ from Novo and MA,XATASE~ from International Bio-
CA 02276188 2003-09-03
l3
Synthetics, Inc., The Netherlands; as weU as Protease A as disclosed in EP
130,756
A, January 9, 1985 and Protease B as disclosed in EP 303,761 A, April 28, 1987
and
EP 130,756 A, January 9, .1985. See also a high pH protease from Bacillus sp.
NCI1VIB 40338 described in WO 9318140 A to Novo. Enzymatic detergents
comprising protease, one or more other enzymes, and a reversible protease
inhibitor
are described in WO 9203529 A to Novo. Other preferred professes include those
of
WO 9510591 A to Procter & Gamble . When desired, a protease having decreased
adsorption and increased hydrolysis is available as described in WO 9507791 to
Procter & Gamble. A recombinant trypsin-like protease for detergents suitable
herein
is described in WO 9425583 to Novo.
Cellulases usable herein include both bacteria! and fungal types, preferably
having a pH optimum between 5 and 10. U.S: 4,435,307, Barbesgoard et al, March
6, 1984, discloses suitable fungal ceilulases from Humicola irrsolens or
Humicola
strain DSM1800 or a cellulase 212-producing fungus belonging to the genus
Aeromorras, and cellulase extracted from the hepatopancreas of a marine
mollusk,
Dotabella Auricula Solur~der. Suitable cellulases are also disclosed in GB-A-
2.075.028; GB-A-2.095.275 and DE-OS-2.247.832. CARFZYME~ and
CELLUZYME~ (Novo) are especially useful. See also WO 9117243 to Novo.
Suitable lipase enzymes for detergent usage include those producad by
microorganisms of the Pseudomoras group, such as Pseudomonas srrrtzeri ATCC
19.154, as disclosed in GB 1,372,034. See also lipases in Japanese Patent
Application 53/20487, laid open Feb. 24, 1978. This lipase is available from
Amano
Pharmaceutical Co. Ltd., Nagoya, Japan, under the trade mark Lipase P "Amano,"
or
"Arnano-P." Other suitable commercial lipsses include Amano-CES, lipases ex
Chromobacter viscosum, e.g. Chromobacter viscasum ~: lipolyticum NRRLB
3673 from Toyo Jozo Co., Tagata, Japan; Chromobckter viscasum lipases from
U.S.
BiocJr~ical Corp., U.S.A. and Disoynth Co., The Netherlands, and lipases ex
Pser~domo~ros gladioli. LIPOLASE~ enzyme derived from Humicala larrugiaasa
and commercially available from Novo, see also EP 341,947, is a preferred
lipase for
use herein.
The enzyme-containing compositions herein rnay optionally also comprise from
about 0.001 % to about 10'/0, preferably from about 0.005% to about 8%, most
preferably from about 0.01% to about 6%, by weight of an enzyme stabilizing
system. The enzyme stabilizing system can be any stabilizing system which is
compatible with the detersive enzyme. Such a system may be inherently provided
by
other formulation actives, or be added separately, e.g., by the formulator or
by a
CA 02276188 1999-06-25
WO 98/29528 PCT/US97/23771
I4
manufacturer of detergent-ready enzymes. Such stabilizing systems can, for
example,
comprise calcium ion, boric acid, propylene glycol, short chain carboxylic
acids,
boronic acids, and mixtures thereof, and are designed to address different
stabilization problems depending on the type and physical form of the
detergent
composition.
E) Detergent Composition PreQaration
The detergent compositions according to the present invention can be in
liquid,
paste or granular forms. Such compositions can be prepared by combining the
essential and optional components in the requisite concentrations in any
suitable
order and by any conventional means.
Granular compositions, for example, are generally made by combining base
granule ingredients (e.g. surfactants, builders, water, etc.) as a slurry, and
spray
drying the resulting slurry to a low level of residual moisture (5-12%). The
remaining dry ingredients can be admixed in granular powder form with the
spray
dried granules in a rotary mixing drum and the liquid ingredients (e.g.
organic
solutions of the essential cellulosic polymers, enzymes, binders and perfumes)
can be
sprayed onto the resulting ganules to form the finished detergent composition.
Granular compositions according to the present invention can also be in
"compact
form", i.e. they may have a relatively higher density than conventional
granular
detergents, i.e. from 550 to 950 g/1. In such case, the granular detergent
compositions according to the present invention will contain a lower amount of
"inorganic filler salt", compared to conventional granular detergents; typical
filler
salts are alkaline earth metal salts of sulphates and chlorides, typically
sodium
sulphate; "compact" detergents typically comprise not more than 10% filler
salt.
Liquid detergent compositions can be prepared by admixing the essential and
optional ingredients thereof in any desired order to provide compositions
containing
components in the requisite concentrations. Liquid compositions according to
the
present invention can also be in "compact form", in such case, the liquid
detergent
compositions according to the present invention will contain a lower amount of
water, compared to conventional liquid detergents.
Addition of the cellulose ether component to liquid detergent compositions of
this invention may be accomplished by simply mixing into the liquid dertergent
aqueous solutions of the desired cellulose ethers. Cellulose ethers can alter
the
viscosity or other rheologica) characteristics of liquid detergent products.
It may
CA 02276188 1999-06-25
WO 98/29528 PCT/US97/23771
therefore be necessary to compensate for any rheological changes in the liquid
detergent product brought about by cellulose ether addition by altering the
type and
amount of hydrotropes and/or solvents that are used.
F) Fabric Laundering Method
The present invention also provides a method for laundering fabrics in a
manner
which imparts fabric appearance benefits provided by the cellulosic polymers
used
herein. Such a method employs contacting these fabrics with an aqueous washing
solution foamed from an effective amount of the detergent compositions
hereinbefore
described or formed from the individual components of such compositions.
Contacting of fabrics with washing solution will generally occur under
conditions of
agitation although the compositions of the present invention may also be used
to
form aqueous unagitated soaking solutions for fabric cleaning and treatment.
Agitation is preferably provided in a washing machine for good cleaning.
Washing is preferably followed by drying the wet fabric in a conventional
clothes
dryer. An effective amount of the liquid or granular detergent composition in
the
aqueous wash solution in the washing machine is preferably from about 500 to
about
7000 ppm, more preferably from about 1000 to about 3000 ppm.
G) Fabric Conditio Ana
The modified cellulose ethers hereinbefore described as components of the
laundry detergent compositions herein may also be used to treat and condition
fabrics
and textiles in the absence of the surfactant and builder components of the
detergent
composition embodiments of this invention. Thus, for example, a fabric
conditioning
composition comprising only the modified cellulose ethers themselves, or
comprising
an aqueous solution of the modified cellulose ethers, may be added during the
rinse
cycle of a conventional home laundering operation in order to impart the
desired
fabric appearance and integrity benefits hereinbefore described.
EXAMPLES
The following examples illustrate the compositions and methods of the present
invention, but are not necessarily meant to limit or otherwise define the
scope of the
invention.
CA 02276188 1999-06-25
WO 98/29528 PCT/L1S97/23771
16
EXAMPLE I
Liauid Detergent Test Composition Preparation
Several heavy duty liquid detergent compositions are prepared containing
various
modified ceDulosic polymers. Such liquid detergent compositions all have the
following basic formula:
Table A
Component Wt. /u
C 12-15 ~kYl ether {2.5) sulfate 3 g
C 12 glucose amide ~ 6. 86
Citric Acid 4.75
C12-14 Fatty Acid 2.00
Enzymes 1.02
MEA 1.0
Propanediol 0.36
Borax 6.58
Dispersant 1.48
Na Toluene Sulfonate 6.25
Modified Celluiosic Polymer (if present) 2.0
Dye, Perfume, Brighteners, Preservatives, B lanc
Suds Suppressor,
Other Minors, Water
100%
CA 02276188 1999-06-25
WO 98/29528 PCT/US97/23771
17
EXAMPLE II
Granular Detergent Test Composition Preparation
Several heavy duty granular detergent compositions are prepared containing
various
modified cellulosic polymers. Such granular detergent compositions all have
the
following basic formula:
Table B
Component
Wt.
C 12 Linear alkyl benzene sulfonate
9.31
C 14- I 5 alkyl ether (0.3 5 EO) sulfate
I 2.
74
Zeolite Builder
27.79
Sodium Carbonate
27.31
PEG 4000 1.60
Dispersant
2.26
C12-I3 ~~ohol Ethoxylate (9 EO) 1.5
Sodium Perborate 1.03
Soil Release Polymer 0.41
Enzymes 0.59
Modified Cellulosic Polymer (if present) 2.5
Perfume, Brightener, Suds Suppressor, Other Bal
Minors, Moisture, nc
Sulfate
100%
EXAMPLE IlI
Cellulosic Polymers Used in Test Compositions
The representative modified cellulosic polymers used in the liquid and
granular
detergent compositions described in Examples I and II are characterized in
Table C.
. The various substituents listed are those from Structural Formulas Nos. I,
Ii and III
described hereinbefore.
CA 02276188 1999-06-25
WO 98/29528 PCT/US97/23771
18
T le C
Cellulosic Polymers Used in Test Deter ent Compositions
Polymer m
Polymer DescriptionA B ~ D
Polymer TradenamePolysurf LK-400 CMC Modified LK-400
67
(D72)
Polymer ManufacturerHercules Union CarbidePenn Union Carbide
Carbose
Polymer Type Nonionic Cationic AnionicCationic
Molecular Weight700-750M -..400I~I ~30pM ,"400M
Structure No. I II ~ III II
'
R Cetyl H CH2C00 H
(C 16) A
Amount of Ring
Alkyl
Substitution 0.4%' 0 0 0
0.6%
Degree of Ring
Carboxymethyl - ' 0.5 -
Substitution
R1 H _ _
R2 - -CH2CH(OH)CH2-- -CH2CH(OH)CH2-
R3 _ -CH3 - -CH3
R4 _
-CH3 _ -CH3
RS _
-CH3 _ -CH3
R6 - H _
H
1-3 1-3 - 1-3
Y - --0.1 - -0.006
Z _ Cl- , C1_
A - - Na -
Test compositions prepared as described in Examples I and II are evaluated for
the
effects that the various cellulosic polymers of Example III provide when
samples of
fabrics or garments are washed using the test compositions as described, all
under
identical conditions. A control sample with no polymer is usually compared to
one
composition with a test polymer to be evaluated. Testing conditions are also
carefully monitored. Examples of controlled conditions include: wash time,
wash
CA 02276188 2003-09-03
1
water temperature and hardness; washer agitation; rinse time, rinse water
temperature
and hardness; dryer time and temperature; wash load fiber content and weight.
EXAMPLE IV
Overall t~ppearance
In an Overall Appearance test, fabrics are washed using various test
compositions
containing either no cellulosic polymers or one of the Example III cellulosic
polymers. The fabrics so washed after ten cycles are then comparatively graded
by
three judges who evaluate the overall appearance of the washed fabrics. It is
the
decision of the judge as to what is to be evaluated unless specific direction
is given to
evaluate one attribute such as color, pilling, fuu, etc.
In the Overall Appearance test, the visual preference of the judge is
expressed using
the Scheff scale.
That is: 0 ø No difference
1 = I think this one is better (unsure).
2 = I know this one is a little better.
3 = I know this one is a lot better.
4 = I know this one is a whole lot better.
For the Overall Appearsnce test, laundering conditions are as follows:
Washer Type: Katcnofe ( 17 gallons)
Wash Time: 12 min
Wash Tempecatura: 90°F (32.2°C)
Wash Water Hardness: 6 grains per gallon
Washer Agitation: normal
Rinse Time: 2 min
Rinse Tanperatura: 60°F ( 15.6°C)
Rinse Water Hardness: 6 grains per gallon
I Wash Load Fabric Content: various colored and white garments and
fabrics
Wash Load Weight: 5.5 Ibs (2.5 kg)
The average overall appearance test results are shown in Tables D and E.
CA 02276188 1999-06-25
WO 98/29528 PCT/ITS97123771
Table D
Overall Appearance Test Results
Liauid Test Composition ID Poivmer Tested Overall Appearance Grade
Control None
0
A Polysurf 67 1.5
1.8
C CMC (D72) 1.0
Modified LK-400 1.2
Ta le E
Overall Appearance Test Results
Granular Test CompositionPolymer Tested Overall A
ppearance
Grade
Control None 0
A Polysurf 6? 1.4
LK-400 1.0
C CMC (D72) 1.0
D Modified LK-400 1.1
EXAMPLE V
Pill Reduction
In a Pill Reduction test, fabrics are washed using the various test
compositions
containing either no cellulosic polymers or one of the Example III cellulosic
polymers. The fabrics so washed are then graded for Pill Reduction using a
computer-assisted pilling image analysis system which employs image analysis
to
measure the number of pills on tested garments and fabrics. Pill reduction is
calculated as:
Pill reduction(%) _ { [# pills (control) - # pills (polymers)] / # pills
(control) } z
1fl0%
CA 02276188 1999-06-25
WO 98/29528 PCT/ITS97/23771
21
For the Pill Reduction test, laundering conditions are the same as used for
the Overall
Appearance test described hereinbefore in ExampleIV.
The average % Pill Reduction test results are shown in Tables F and G.
Ta I F
Pill Reduction Test Results i uids
Liauid Test Composition ID Polymer Tested PilUFuzz Reduction ((%1
Control None 0
A Polysurf 67 2 i .5
LK-400 42.4
C CMC(D72) 26.8
Modified LK-400 25.9
Table G
Pill Reduction Test Results Granular
Granular Test Composition Polymer Tested Pill/Fuzz Reduction (%1
ID
Control None
A Polysurf 67 33.3
LK-400 51.6
C CMC{D72) 7.6
Modified LK-400 16.6
CA 02276188 1999-06-25
WO 98/29528 PCT/US97/23771
22
EXAMPLE VI
Color Protection
In a Color Protection test, fabrics are washed using various test compositions
containing either no cellulosic polymers or one of the Example III cellulosic
polymers. The fabrics so washed are then tested with a Hunter colorimeter in
order
to determine a Delta E* value for each fabric tested. Delta E* is defined as
the color
difference (reflectance intensity, hue shift, etc.) between washed fabrics and
unwashed fabrics.
For the Color Protection test, laundering conditions are the same as used for
the
Overall Appearance test described hereinbefore in Example IV.
The extent of Color Protection provided is based on percent of Delta E*
difference
compared to an unwashed sample. Color protection is calculated as:
~/° Color Protection = { [dE*(control) - dE*(polymers)] / dE*(control)
} a 100%
The average color protection test results are shown in Tables H and I.
Table H
Color Protection Test Results - Liquids
Liquid Test Composition ID Polymer Tested Color Protection (%)
Control None p
A Polysurf 67 24.2
LK-400 36.5
C CMC (D72) 26.6
D Modified LK-400 27.2
CA 02276188 1999-06-25
WO 98/29528 PCT/US97/23771
23
Ta 1 I
Col9r Protection Test~.~ular
Resulrs
Granular Test Comp sition Polymer TestedColor Protection
(%1
m
Control None 0
Polysurf 67 33.9
LK-400 39.2
C CMC (D72) 15.5
Modified LK-400 24.7