Note: Descriptions are shown in the official language in which they were submitted.
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-1-
TISSUE EXAMINATION
Background
This invention relates to tissue examination, and
in particular to detecting foreign structures in, e.g.,
breast tissue.
One traditional way of examining breast tissue to
detect foreign structures such as lumps is to palpate the
breast manually. For example, the patient firmly presses
on the breast with three fingers while moving the fingers
in a circular palpating motion. Typically, such manual
breast self-examinations can detect lumps as small as a
few centimeters in diameter.
Instruments for electronically examining the
breast are available. One such instrument includes an
array of pressure sensors which is pressed against the
breast. Each pressure sensor in the array generates an
electrical signal proportional to the local pressure
imposed on the sensor when the array is pressed against
the breast. When adjacent sensors are positioned across
the boundary of a lump within the breast tissue, the
sensor that lies over the lump generates an electrical
signal indicating the detection of greater local
pressure than the adjacent transducer element, which is
located over soft tissue alone. The instrument
determines whether a lump is present by analyzing the
differences between the electrical signals generated by
the sensors.
Summary
This invention concerns performing tissue
examination with a device that includes a sensor which
produces signals in response to pressure imposed on the
sensor as the sensor is pressed against the tissue. In
one general aspect, the tissue is vibrated, the response
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/003T7
-2-
of underlying tissue structures to the vibrating is
detected by a processor from the signals produced by the
sensor, and different types of underlying tissue
structures are discriminated from each other based on the
response.
Preferred embodiments may include one or more of
the following features.
The processor determines the extent to which the
underlying tissue structures are vibrated within the
tissue based on the signals produced by the sensor, and
performs the discrimination based on the extent of the
vibration. For example, ribs or other structures within
the tissue which are more connected to the tissue than
some other structures may not vibrate as freely as other
structures which are able to move more freely within the
tissue. Moreover, different structures within the tissue
may vibrate differently (e. g. have different vibrations
and/or amplitudes). The processor discriminates between
the different types of underlying tissue structures based
on these response characteristics.
More specifically, a normal tissue structure is
discriminated from a potentially foreign tissue structure
based on the response the structure to the vibration, and
a user is notified as to whether an underlying tissue
structure is a potentially foreign tissue structure. For
example, different indicators are actuated to indicate
whether the underlying tissue structure is a potentially
foreign tissue structure (such as non-breast tissue,
e.g., a carcinoma) or is a normal tissue structure.
Signals corresponding to the amplitudes of the signals
produced by the sensor may be displayed to allow the user
to visualize the pressure signature.
The tissue is vibrated over a selected frequency
range, which may be between 1 Hz. and 200 Hz. The
processor determines a resonant frequency of the response
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-3-
within the range based on the signals produced by the
sensor, and an amplitude of the response based on the
signals produced by the sensor. This amplitude may be
the amplitude of the response at the resonant frequency.
The processor discriminates between the different types
of underlying tissue structures based on the resonant
frequency or the amplitude of the response, or both. The
processor may discriminate based on whether the resonant
frequency is within a selected portion of the frequency
range, whether the amplitude of the resonant frequency
exceeds a threshold, or both. The processor may also
discriminate based on whether the resonant frequency is
outside of a selected portion of the frequency range or
whether the amplitude of the resonant frequency is below
a threshold.
Preferably, a plurality of sensors are used. The
signals produced by the plurality of sensors in response
to pressure imposed thereon comprise a frame. The frames
of signals from the sensors are successively acquired,
and the processor analyzes the frames of signals to
detect the response of the underlying tissue structures
to the applied vibrations. The processor determines an
amplitude of the response at each frequency based on
amplitudes of the signals in the frame, and designates
the frequency of the response having a highest amplitude
as the resonant frequency. The processor stores the
frames in a memory and identifies each of the stored
frames by the frequency to which the frame corresponds.
The processor determines the resonant frequency of
the response by comparing the amplitudes of the signals
in each frame to a threshold and determining an average
of the amplitudes of the signals that exceed the
threshold. The processor then determines the frame
having a highest average amplitude, and designates the
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/OU377
-4-
frequency to which that frame corresponds as the resonant
frequency of the response.
The processor employs so-called "fuzzy logic"
techniques to discriminate among normal tissue and
different structures. The processor evaluates the
resonant frequency with respect to a selected portion of
the frequency range, and evaluates the amplitude of the
resonant frequency with respect to a threshold amplitude.
The processor also determines a pressure profile of the
underlying tissue structure based on the amplitudes of
the signals in the frame having the highest average
amplitude, and evaluates the pressure profile with
respect to a base pressure profile. Based on any, some,
or all of these evaluations, the processor develops an
outcome that indicates a degree of membership of the
underlying tissue structure in a class of foreign tissue
structures.
In a preliminary mode of operation, the tissue is
not vibrated, and preliminary frames of the signals are
acquired from the sensors as the sensors are moved over
the tissue. The processor analyzes the preliminary
frames of signals to determine if the underlying tissue
structures are candidates for further analysis. If that
is the case, the processor activates a vibrator to apply
the vibrations to the tissue and causes additional frames
of signals to be successively acquired from the sensors.
The processor analyzes these additional frames of signals
to detect the response of the underlying tissue
structures to the applied vibrations. The processor
determines the amplitude of the resonant frequency of the
response by reducing the average amplitude of the frame
having the highest average amplitude by an amount that
corresponds to the response of the underlying tissue
detected by the plurality of sensors during the
preliminary mode.
CA 02277328 1999-07-08
WO 98/31283 PCT/LTS98/00377
-S-
Preferably, the vibrator includes an oscillator
for generating a driving signal at a selected frequency,
and a mechanism for converting the driving signal to
mechanical vibrations for vibrating the tissue. The
oscillator produces the driving signal over the selected
frequency range to cause the mechanism to produce the
vibrations over this range. The mechanism includes a
pair of plates each of which is configured to be applied
against a surface of the tissue. The plates are spaced
from each other and disposed at a selected angle with
respect to each other to direct the vibrations toward a
common point within the tissue.
The plates are mounted to a housing. The sensor
or the plurality of the sensors are disposed on a sensor
surface of the housing between the plates. The sensor
surface is convex. During operation of the vibrator,
plates move with respect to the sensor surface between an
extended position in which the plates are applied to the
surface of the tissue and a retracted position in which
the plates are spaced from the surface of the tissue.
Advantages of the invention may include one or
more of the following advantages. The device is non-
intrusive because the device examines the tissue from the
surface of the breast and does not require manipulating
the breast in an invasive or uncomfortable manner. The
device distinguishes possible carcinomas from other
structures. The device may also distinguish among
different types of foreign structures in the tissue based
on their response to vibration. The device requires
minimal training for proper operation by the user. The
device is inexpensive. The device is more accurate than
manual examination of the tissue by a person unskilled in
manual tissue examination.
Other advantages and features will become apparent
from the following description and from the claims.
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-6-
Brief Description of the Drawing
Fig. 1 is a block diagram of a tissue examination
device which includes an oscillating mechanism for
vibrating the tissue.
Fig. 2 is a diagram of a first embodiment of the
tissue examination device.
Fig. 3A is a cross-sectional view of the tissue
examination device showing the oscillating mechanism when
not in use.
Fig. 3B is a cross-sectional view of the tissue
examination device showing the oscillating mechanism in
use.
Fig. 3C is a cross-sectional view of the tissue
examination device showing the periodic force
displacements travelling through the breast tissue in
response to the oscillating mechanism.
Fig. 4 is a diagram of the polymer chains in
breast tissue.
Fig. 5 is a stress/strain graph of breast tissue
in comparison to a more viscous material and a more
elastic material.
Fig. 6 is a mechanical model representing breast
tissue.
Fig. 7 is a representation of a foreign structure
in breast tissue.
Fig. 8A is a graph of amplitude/frequency
responses of two dampened springs having different spring
coefficients.
Fig. 8B is a graph of amplitude/frequency
responses of two dampened springs having different
dampening factors.
Figs. 9A and 9B are mechanical models representing
of a foreign structure in breast tissue.
Fig. 10 is a representation of a rib underlying
breast tissue which is close to the skin.
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
_'7_
Fig. 11A is a mechanical model of a bony
prominence in breast tissue.
Fig. 11B is a mechanical model of a mass near a
bony prominence.
S Fig. 12 is a graph representing the expected
profile of harmonic frequency and harmonic amplitude
response of foreign structures to the vibrations produced
by the oscillating mechanisms of the tissue examination
devise of Fig. 1.
Fig. 13 is a graph representing the expected
thresholds and the expected area of frequency and
harmonic amplitude responses of carcinomas.
Fig. 14 is a flow chart of the first stage of the
analysis performed by the tissue examination device of
Fig. 1.
Figs. 15A and 15B represent the relation between
the response wave and the sampling instances.
Fig. 16 is a flow chart of the second stage of the
analysis performed by the tissue examination device of
Fig. 1.
Fig. 17 is a representation of an example of the
location of sensors on the sensor array indicating a
potentially suspicious structure.
Fig. 18 is a flow chart of the third stage of the
analysis performed by the tissue examination device of
Fig. 1.
Figs. 19A and 19B are graphical representations of
the static pressure response and the oscillating response
of a foreign structure in breast tissue to external
vibration.
Description
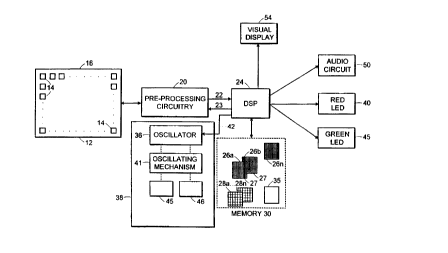
Referring to Fig. 1, a tissue examination device
10 includes an array 12 of pressure sensors 14 carried on
a thin, flexible membrane 16. Array 12 is, for example,
CA 02277328 1999-07-08
WO 98/31283 PCTIUS98/00377
_g_
a contact sensor such as that described in U.S. Patent
No. 4,856,993, entitled "Pressure and Contact Sensor
System for Measuring Dental Occlusion" (the '993 patent),
incorporated herein by reference, the individual pressure
sensors 14 of which are resistive elements. Pressure
sensors 14 are arranged in an orthogonal grid of rows and
columns in array 12. Pressure sensors 14 are relatively
small and are closely spaced to provide high resolution
capable of distinguishing between areas of underlying
tissue separated by 1 mm or less. Array 12 is
commercially available from Tekscan, Inc. (the assignee
of the '993 patent).
Referring also to Fig. 2, array 12 is mounted on a
sensor head 55 made from a rigid polymer such as
polycarbonate. Sensor head 55 is attached to a handle 60
which is grasped by a user to place array 12 against the
tissue to be examined (such as the user's breast). The
face of sensor head 55 on which array 12 is mounted is
convex, with a radius of curvature of approximately 1.5
inches to enhance the mechanical coupling between sensors
14 and the underlying tissue. The optimum range of the
array curvature for mechanical coupling between sensors
14 and the underlying tissue is a radius of curvature
between 1" - 2.5", although a radius as low as .5" or as
high as 3" may also be used.
The resistance of each pressure sensor 14 changes
in accordance with the amount of pressure applied to
sensor 14. The resistance change is inversely
proportional to the pressure imposed on sensor 14. Thus,
the resistance of each sensor 14 decreases as applied
pressure increases.
The individual resistances of pressure sensors 14
are read by preprocessing circuitry 20, the output 22 of
which is applied to a digital signal processor (DSP) 24.
Briefly, preprocessing circuitry 20 sequentially measures
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-9-
the resistance of pressure sensors 14 in response to row
and column address signals 23 provided by DSP 24 to
provide an indication of pressure applied to the location
in array 12 that corresponds to that sensor 14. During
S each resistance measurement, preprocessing circuitry 20
applies a reference potential (not shown) to the
addressed sensor 14, measures the voltage drop induced
across that sensor 14, and generates an output 22
corresponding to the voltage drop. Thus, each pressure
sensor 14 produces a signal (in this example, resistance
induced voltage) in response to the applied pressure.
The operation of preprocessing circuitry 20 is more fully
described in the '993 patent.
The preprocessor output signals 22 are digitized
(by A/D converters, not shown) and applied to DSP 24
(alternatively, an input stage of DSP 24 may perform the
A/D conversion). The set of sequentially produced output
signals 22 for all pressure sensors 14 in array 12 is
termed a "frame." DSP 24 stores frames obtained for
sensor array 12 in areas 26a-26n and 28a-28n of memory
30. Each memory area 26a-26n and 28a-28n contains
storage locations 27 which respectively correspond to the
locations of pressure sensors 14 in a frame.
A green LED 45 is illuminated when device 10 is
powered on. Green LED 45 remains illuminated throughout
the tissue examination procedure as a system self check.
A red LED 40 and an audio circuit 50 are driven by DSP 24
at various stages of the operation of device 10 to
indicate to the user whether the user is using device 10
properly, how the user should operate device 10 at
different stages of the examination, and whether
suspicious structures have been detected. Visual display
54 may show the user two-dimensional and three-
dimensional representations of the frames in real time
during the examination.
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
- 10-
A vibrator 38 vibrates.the underlying tissue by
applying a periodic force displacement to the tissue.
Vibrator 38 includes an oscillator 36 which drives an
oscillation mechanism 41 which in turn vibrates
oscillation plates 45 and 46 against the tissue.
Oscillation plates 45 and 46 apply the periodic force
displacement to the tissue to vibrate underlying tissue
structures. Referring also to Fig. 3A, in response to
signal 42 from DSP 24, oscillator 36 begins the rotation
of motor and motor gear 43 at a frequency selected by DSP
24. Motor gear 43 is cooperatively coupled to actuator
44 which is pivotally connected to sliders 47 and 48.
Each one of sliders 47 and 48 are pivotally connected to
a corresponding one of oscillation plates 45 and 46.
Oscillation plates 45 and 46 are also pivotally connected
to a housing 49 which also houses sensor array 12. The
rotation of motor gear 43 imparts an oscillating motion
to actuator 44 which is transferred to sliders 47 and 48
and finally to the oscillation plates 45 and 46. Fig. 3B
shows plates 45 and 46 in their lowest position in the
oscillation cycle.
In use, device 10 operates and analyses frames of
signals 22 in two modes. Briefly, in the first mode of
operation, device 10 operates in accordance with the
method disclosed in applicant's copending U.S. patent
application Serial no. 08/757,466, filed on November 27,
1996 (the "'466 application"), incorporated by reference
herein in its entirety. In a second mode of operation,
described more fully below, the underlying tissue is
excited by vibrating it with vibrator 38, and the
vibration response of the tissue and the structures
within the tissue are examined by DSP 24.
In the first mode of operation, head 55 is
manually translated across the skin by the user applying
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-11-
pressure with her hand placed on handle 60 (Fig. 2). The
translation technique is essentially a series of
stationary palpations which allow the user to increase
breast area coverage with less exam time.
Generally, the pressure imposed on the sensors 14
increases when the sensors 14 are pressed against
localized areas of stiffer tissue on, within, or below
the softer breast tissue. Examples of such stiffer
tissue include normal breast tissue structures -- such as
the nipple, the inframammary ligament, and underlying
ribs -- and foreign bodies such as cysts and solid masses
(whether or not pathogenic). Consequently, as array 12
is pressed and moved against the breast, the pressure
imposed on sensors 14 and, thus the resistance of sensors
14, varies in accordance with the properties of the
underlying tissue structures.
In the first mode of operation, DSP 24 addresses
preprocessing circuitry 20 at a rate sufficient to read
frames or more of output signals 22 per second. DSP
20 24 stores each frame of signals 22 in areas 26a-26n of
memory 30. Thus, each memory area 26a-26n contains a
"map" of the pressures detected by pressure sensors 14 in
a frame. This map can be viewed as a "pressure
signature" of the tissue structures beneath array 12.
Accordingly, memory areas 26a-26n contain a time sequence
of pressure signatures of the underlying tissue as array
12 is palpated across the breast. When the user is
applying the correct amount of pressure, DSP 24 performs
various processing tests defined by an operating program
35 stored in memory 30 on the pressure signatures stored
in memory areas 26a-26n. The tests enable DSP 24 to
discriminate normal underlying tissue structures from
potentially foreign structures. These tests are
described in detail in the '466 application. In one of
these tests, DSP 24 analyzes the amplitude of each signal
CA 02277328 1999-07-08
WO 98/31283 PCT/LTS98/00377
- 12-
22 to determine whether it is above or below a pressure
threshold that is dynamically determined for that frame.
Those signals 22 in the frame that exceed the pressure
threshold are termed "suspicious signals" 22 or "red
signals" 22. Signals in the frame that do not exceed the
pressure threshold are called "blue signals."
When breast examination device 10 operates in the
first mode of operation, it identifies certain suspicious
foreign structures within the breast. These suspicious
foreign structures may include soft lumps, fluid filled
cysts, hard lumps, carcinomas, or bony prominences which
have curved tops and are located close to the skin (these
bony prominences may appear as lumps to device 10).
Device 10 generally filters out small structures,
nipples, inframammary ligaments, flat bony prominences,
and possibly other non-suspicious elements based on the
pressure signatures of these structures and the
processing tests performed by DSP 24.
If DSP 24 determines a potentially foreign
structure is present, DSP 24 notifies the user by
illuminating a red LED 40. In addition, audio circuit
50, such as a buzzer, a tone generator, or both may be
actuated by DSP 24 in conjunction with red LED 40, as
discussed below. Handle 60 also includes a communication
port 52 for coupling the maps of signals 22 to a visual
display 54, thereby allowing the user to observe the
pressure signatures directly.
Following detection of a foreign structure in the
first mode of analysis, device 10 then begins to operate
in the second mode. Generally, in the second mode, the
user holds device 10 stationary over the underlying
tissue where device 10 identified a suspicious foreign
structure. In contrast with the first mode in which the
underlying tissue is static during examination, the
tissue is actively excited by vibrating it through a
CA 02277328 1999-07-08
WO 98/31283 PCT/LTS98/00377
-13-
range of frequencies using vibrator 38. DSP 24 then
examines frames of signals 22 corresponding to those
frequencies and analyzes the response of the foreign
structure detected in the first mode of operation.
Vibrating the underlying tissue allows DSP 24 to
better discriminate between carcinomas and benign
structures. For example, whether a foreign structure
moves in response to the applied vibrations may enable
DSP 24 to determine whether a structure is a rib, which
does not vibrate. Device 10 examines the response of the
structure through range of frequencies to determine its
harmonic or resonant frequency and amplitude. It is
expected that different structures will have different
harmonic responses because of a variety of factors
including degree of viscoelasticity of breast tissue,
characteristics of various foreign structures, and the
degree of connectedness of the structures to the
surrounding tissue. If DSP 24 determines that the
foreign structure may be a carcinoma, it notifies the
user through red LED 40 and audio circuit 50.
In the second mode of operation as the tissue and
the structures within it vibrate, they press with varying
pressure on sensors 14 in sensor array 12, just as in the
first mode of operation of device 10. Pressure sensors
14 on sensor array 12 detect the force of the vibration
of the breast tissue and any foreign structure in
response to the applied vibration. This data is acquired
and then analyzed by DSP 24 in three stages.
In the first stage, device. 10 vibrates the
underlying tissue at a frequency that is initially set at
1 Hz and gradually increased to 200 Hz. The periodic
force displacements applied to the breast by oscillation
plates 45, 46 travel in breast tissue and vibrate the
tissue and any foreign structure underneath the sensor
array 12. DSP 24 addresses preprocessing circuit 20 to
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
- 14-
obtain frames of signals 22 at a sampling rate (e.g. 20
frames per oscillation cycle) sufficient to determine the
peak amplitude of the harmonic frequency of structures
within the tissue. Prior to storing the frame, DSP 24
performs a preliminary test on each frame of signals 22
to determine that the user is applying the proper amount
of pressure on device 10. If so, DSP 24 stores the
acquired frames in memory areas 28a-28n in memory 30.
Memory areas 28a-28n are identical to memory areas 26a-
26n in the manner the signals 22 from prepossessing
circuit 20 are stored.
In contrast to the frames stored while performing
the first mode of analysis, the frames stored in areas
28a-28n are indexed by frequency of the periodic force
displacement frequency of oscillator 36 and the order in
which they were obtained. They are also linked to the
last frame 26a-26n (the "base frame") obtained in the
first mode of analysis. The linkage may be for example,
a pointer in each frame identifying the memory address of
the next frame. The base frame contains the pressure
profile for the area being currently examined prior to
the area being vibrated. In order to determine the
absolute amplitude response of this area, the value of
the static pressure must be subtracted from the data
obtained from the sensor array 12.
At the start of vibrating the tissue at the new
frequency, a transitional period exists when breast
tissue and the underlying structures which adjust from
their previous condition to vibrating at a new frequency.
During this time, DSP 24 does not request any frames of
signals 22 from pre-processing circuit 20 because the
frames during the transitional period can not be
accurately used for determining the harmonic frequency.
In the second stage, DSP 24 determines the peak
amplitude of the response obtained. DSP 24 iterates
CA 02277328 1999-07-08
WO 98/31283 PCT/US98100377
-15-
through all the stored frames 28a-28n for this area, and
calculates the average pressure value for the area of
suspicion on the frame. This area consists of sensors 14
which have pressure value above a threshold which is
dynamically determined for that frame. DSP 24 then
determines which frame has the highest average pressure
value among all the frames acquired for all examined
frequencies. This value will be the raw harmonic
amplitude and the frequency index of the frame will be
the harmonic frequency. However, the raw harmonic
amplitude includes the pressure on sensors 14 resulting
from the pressure the user applies to device 10 while
holding it. Therefore, to obtain the absolute harmonic
amplitude, a base value is subtracted from the raw
harmonic amplitude to determine the absolute harmonic
amplitude. This base value is calculated by averaging
the pressure signature of the suspicious area in the base
frame.
In the third stage, DSP 24 uses fuzzy logic
analysis to determine from the absolute harmonic
amplitude and the harmonic frequency the nature of the
structure being examined.
In order to better understand the invention, the
nature of breast tissue and its properties will be
discussed. Breast tissue is a mixture of elastin,
collagen, and fat. Referring to Fig. 4, elastin and
collagen are long spiral polymer chains 85. In between
layers of a polymer chain, cis bonds 80 are formed. As a
polymer chain uncoils in response to an external force F,
these cis bonds, together with other chemical bonds in
the chain, stretch 82, 86 and apply an opposite force to
urge the bonds to return to their original form. The
spiral shape of these chains and the resistance of cis
bonds and other chemical bonds to being stretched (or
compressed) result in the chains returning to their
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
- 16-
original shape. In other words, these polymer chains
have a spring-like response with a specific spring
constant to any force displacement applied along their
length.
The elastin and collagen polymer chains also form
weak interchain links 81 with one another. As these
chains uncoil or slide past one another in response to an
external force F, the interchain links resist being
stretched or broken. Also, as the chains slide past one
another, some links are broken 83 and new links 84 are
formed. New links 84 resist the chains returning to
their original positions. Therefore, these links dampen
the spring response of the polymer chains both when they
uncoil and when they recoil. In essence, the polymer
chains act as dampened springs giving breast tissue a
viscoelastic characteristic.
Fat also affects viscoelasticity of breast tissue.
Fat in breast tissue generally exists as globules of
polymer chains. Generally, increased fat content of
breast tissue results in increased viscosity or dampening
factor of breast tissue and a Lower spring constant.
Fig. 5 shows a strain/stress graph 90 for a
viscoelastic material in comparison to a more elastic
material and a more viscous material. The lower graph
92a shows the strain response, shown by the y-axis, of a
very viscous material in response to external stress,
shown by the x-axis. The strain response of a viscous
material rises at a low slope and levels off at a low
level when there is a viscosity breakdown. The upper
graph 92b shows the strain response of a very elastic
material. The strain response rises steeply, as the
material continues to stretch, and levels off at a very
high level. The strain response of a viscoelastic
material 91 falls somewhere in between the more elastic
and viscous materials.
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-17-
Before the strain response levels~off, i.e. before
the elasticity of the material reaches its limit, strain
is responsive to the applied stress. In this area 90,
viscoelastic materials exhibit a different behavior than
that of either elastic or viscous materials in response
to applied periodic force displacement (i.e. vibration).
In response to externally applied periodic force
displacement, the response of elastic materials will be
directly proportional to the applied periodic force
displacement. Viscous materials, in contrast, absorb
most of the applied force and do not displace in response
to a periodic force displacement. In viscoelastic
materials, the viscous characteristics dampen the
frequency response of the elastic characteristics. This
results generally in a response with a lower amplitude to
an externally applied periodic force displacement than an
elastic material with the same spring constant. However,
at a certain frequency, a viscoelastic material vibrates
with an amplitude disproportionally high when compared to
the input amplitude. This is the harmonic frequency of
the material, and the amplitude of the response is its
harmonic amplitude.
The harmonic response of a viscoelastic material
may be demonstrated by the response of a dampened spring.
At the harmonic frequency, the rate of energy absorption
and release of the dampener and the spring are equal.
Therefore, they reflect off one another, multiplying the
amplitude of the response at that frequency.
Referring to Fig. 6, the mechanical response of
breast tissue to external vibration can be demonstrated
by a mechanical model of several dampened springs 100.
Each of the dampened springs may have different spring
and dampening coefficients, because the mixture of fat,
elastin and collagen is not consistent throughout the
breast. There may be areas of higher or lower viscosity
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-18-
or elasticity throughout the breast. However, these
values will be generally within a narrow range of values.
The system of several dampened springs will
respond harmonically to vibrations at a certain
frequency. For an externally applied periodic
displacement force P(t), the displacement of the system
X(t) will reach a maximum when P(t) is applied at the
harmonic frequency, where t is the period of the periodic
force .
Referring to Fig. 7, when a foreign structure 112
is present in breast tissue, it changes the harmonic
response of the tissue affecting either the harmonic
frequency or the amplitude of that response, or both.
Based on the harmonic frequency response of breast tissue
and possible structures embedded within it, it is
possible to detect the type of these structures by
applying an external periodic force (e.g., vibrations by
oscillation plates 45, 46) and measuring the resultant
amplitude at the harmonic frequency.
The change in harmonic response of breast tissue
that contains a foreign structure is a function of a
number of factors relating to the foreign structure,
including the density of the structure, the extent to
which it is connected to breast tissue or anchored to
ribs 114, and the depth in which it is embedded 116. We
will discuss the expected amplitude and frequency of
various foreign structures in detail below, based on
analysis of these factors.
Referring to Figs. 8A and 8B, generally, the
frequency and the amplitude of the harmonic response of a
viscoelastic system change with variations in spring
coefficient and dampening factor. In Fig. 8A, the effect
of varying spring coefficient on the frequency of the
harmonic system is shown. The harmonic frequency of a
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
- 19-
dampened spring is directly proportional to the square
root of the spring coefficient over the mass of the
system, i.e.,
fa k
m
Therefore, for the same mass, an increase in the spring
coefficient (i.e. stiffness of the spring? results mainly
in an increase in the harmonic frequency. It will also
result in a decrease in the harmonic amplitude. Graph 65
shows the response of dampened spring having a lower
stiffness than the system represented in graph 66.
In contrast, variations in dampening factor mainly
affect the amplitude of the response. As the dampening
factor increases, the amplitude of the response
decreases. Graph 67 shows the response of a system
having a dampening factor lower than the one represented
by graph 68.
A discrete and unanchored stiff structure
suspended within breast tissue replaces a mass of tissue
equivalent in volume (Fig. 7). This replacement is
equivalent to replacement of one of the dampened springs
in the mechanical model in Fig. 6 with a mass. We do not
expect the density of a lump to significantly affect the
harmonic response of a lump, because the density of
various types of lumps and breast tissue are
approximately the same or at least within a narrow range
of values. Therefore, for a given volume, all these
structures have approximately the same mass as the breast
tissue they displace. Therefore, the effect of varying
densities of lumps on harmonic frequency and amplitude
and response should not be significant.
An-important factor in determining the harmonic
response of a foreign structure in breast tissue is how
CA 02277328 1999-07-08
WO 98131283 PCT/US98/00377
-20-
connected it is in the tissue, for example, to the
polymer chains. Generally, the more connected a
structure is, the higher the spring coefficient of the
system is. Referring to Fig. 9A, in a mechanical model
S of such a system, the structure is suspended by two
dampened springs 96 and is laterally connected to two
other dampened springs 95. When vibrating vertically,
the dampened springs 95 and 96 provide additional
stiffness and dampening to the overall system. The more
connected the tissue is, the higher is the added
stiffness of dampening. If the model in Fig. 9A is
collapsed into the model in Fig. 9B, the effect of
lateral springs 95 is adding to the spring coefficient
and dampening factor of dampening springs 97 and 98.
Consequently, the amplitude of harmonic response is
reduced while the harmonic frequency is increased.
Therefore, the degree of connectedness affects both the
harmonic frequency and the harmonic amplitude.
The internal spring coefficient and dampening
factor of a structure also affects the harmonic response
of a lump. The mechanical model 93 of a lump may be
shown as a mass connected to four dampened springs 94
(Fig. 9A). The spring coefficients of dampened springs
94 is higher for hard lumps than for soft lumps and hard
shelled cysts whose spring coefficients are low. The
dampening factors of dampened springs 94, however, are
higher in soft lumps than in hard lumps. Hard shelled
cysts, because of their hard outer shell, would also have
a lower dampening factor than soft lumps (and soft
shelled cysts). Therefore, the internal structure of a
lump may significantly affect the harmonic response of
the lump. We also expect the internal structure to
influence the pressure profile of the lump during
oscillation.
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-21 -
. we will now discuss the expected harmonic
frequency and amplitude response of various types of
structures which may be present in the breast. In the
case of hard shelled fluid filled cysts, the outside
shell of the cysts is hard and forms few connections to
the polymer chains in breast tissue. Therefore, the
cysts can move more freely than other structures. Also,
because there are few connections between cysts and
breast tissue, the resulting spring coefficient and the
dampening factor are low. Therefore, we expect that the
harmonic amplitude of cysts will be higher than all other
structures because of this lack of connectedness to
breast tissue. However, because hard shelled cysts are
hard compared to soft lumps, they will likely have a
somewhat higher harmonic frequency than soft lumps.
The fluid inside cysts vibrates almost
independently of the cyst itself, creating a second
harmonic response in addition to the primary harmonic
response of the cyst. The pressure profile of cysts may
also be influenced by the vibration of the fluid inside
cysts. DSP 24 may be able to detect the second order of
vibration of cysts and use the data to distinguish fluid
filled cysts from other structures.
Hard lumps have a higher harmonic frequency than
soft lumps because hard lumps are not only connected to
surrounding tissue but are also internally stiff. They,
therefore, create a very stiff system with a high
harmonic frequency. Soft lumps are well connected to the
tissue, but their softness introduces a low spring
coefficient and a higher dampening factor than other
structures. Soft lumps absorb more of the vibration
energy than harder lumps. Soft lumps therefore will have
a low harmonic frequency and an amplitude response
similar to that of hard lumps. Therefore, breast tissue
having a hard lump appears as more stiff (i.e., with a
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-22-
lower spring coefficient) to a.periodic force
displacement than breast tissue that contains a soft
lump. Therefore, hard lumps vibrate at a higher harmonic
frequency than soft lumps.
Referring to Fig. 10, another structure which may
appear as a suspicious area when device 10 operates in
the first mode of operation is a rib 118 having a curved
profile and being located close to the skin. In order to
discuss the response of ribs, it is important to discuss
the effect of depth of structures within the breast on
their examination by vibrating them. Tissue examination
device 10 applies a periodic displacement force at the
surface of the breast and obtains the response of breast
tissue to the displacement force also at the surface of
the breast. Therefore, the response that device 10
obtains from the breast is affected by the depth of the
structure within the breast tissue. Generally, the
applied force attenuates as it travels further into the
breast tissue. The viscoelasticity of breast tissue
results in the tissue absorbing and dispersing the energy
of the force displacement applied at the surface as the
displacement wave travels through the tissue. For the
same reason, the harmonic response of a structure
attenuates as the response vibrations travel to the
surface. Therefore, the expected amplitude of the
response changes depending on the depth of the structure
being investigated. This attenuation has the advantage
of attenuating the response of ribs underlying the
breast, thereby eliminating effect of ribs on the
examination of the breast unless a rib is close to the
skin. Referring to Fig. 11A, the mechanical model
of a rib close to the surface of the skin is represented
by a short dampened spring 120 connected to an almost
immovable mass (i.e., the rib). A rib is anchored and
has a high density. In response to a periodic force
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-23-
displacement, a rib vibrates at a higher harmonic
frequency than all other structures because it is hard,
has a higher density compared to all other possible
structures in the breast, and is very well anchored.
However, it also has a low harmonic amplitude because it
is heavily anchored and therefore can not move as freely
in response to the vibrations.
Referring to Fig. 11B, a lump 124 that is so close
to the rib that it essentially rests on top of the rib
acts in a similar manner to the rib. Because of its
proximity to the rib, it cannot vibrate freely, and
therefore has a low harmonic amplitude response.
Carcinomas generally spread to the surrounding
tissue and anchor themselves within the breast. They
IS usually have a number of tentacle-like protrusions into
the surrounding tissue. The surface of these protrusions
and carcinoma form connections to many polymer chains in
the tissue. Hence, carcinomas may be considered to be
better connected to breast tissue than all other
structures. They cannot move as freely as other lumps in
the breast because of these connections; thus a system
containing carcinomas will appear as very stiff compared
to systems containing other structures. Carcinomas are
also very dense, often denser than hard lumps, and as a
result carcinomas introduce a high spring coefficient.
Therefore, a carcinoma will likely have a lower harmonic
amplitude than all other structures except ribs.
Moreover, a carcinoma will also likely have a higher
harmonic frequency than other lumps.
Referring to Fig. 12, based on the above
discussion, it may be concluded that the harmonic
responses of various structures within breast tissue,
which are identified as suspicious by breast examination
device 10 when operating in the first mode of operation,
are different from one another. Fig. 12 shows the
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-24-
expected regions of response of soft lumps 101, fluid
filled cysts 102, hard lumps 103, carcinomas 104, and
ribs 105, in comparison to one another. Based on the
above analysis, we expect the responses of these various
types of structure to fall within the ranges shown in
Fig. 12. Ribs 105 should have the lowest harmonic
amplitude, while fluid filled cysts 102 should have the
highest harmonic amplitude. The amplitude responses of
soft lumps 101, hard lumps 103, and carcinomas 104 are
expected to fall in between fluid filled cysts 102 and
ribs 105. Soft lumps 101 should have a lower frequency
than either hard lumps 103 or carcinomas 104. We expect
hard lumps 103 to have a higher amplitude response than
carcinomas 104.
The exact values of the range of responses may be
determined in clinical studies. The range of responses
may then be reduced to a number of amplitude and
frequency thresholds which define a number of windows
corresponding to the various types of structures which
may exist within breast tissue. Tissue examination
device 10 differentiates between carcinomas and all other
structures. Therefore, the frequency and amplitude
responses are examined to determine whether they are
within a window that corresponds to carcinomas.
Referring to Fig. 13, carcinoma window 106 may be
derived from analysis of clinical studies. Carcinoma
window 106 may have different borders than the range of
carcinoma responses derived from the clinical studies
(104 in Fig. 12) because, for example, it may be
desirable for device 10 to have more false positive
detections rather than fewer false negative detections.
The thresholds of carcinoma window 106 are not precisely
defined, but are instead statistically based. That is,
as the response moves away from the core area of clinical
responses, the likelihood that a response is that of a
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-25-
carcinoma decreases. Tissue examination device 10 uses
so-called "fuzzy logic" to determine the degree of
membership of a response and to decide whether the
structure is a carcinoma.
S The method of operation of tissue examination
device 10 will now be described in detail.
Preliminarily, note that the pressure signatures
are a function of the amount of average pressure applied
to sensors 14 when the user presses array 12 against the
body. The pressure applied by the user should be within
a selected range in order for the pressure signatures to
accurately correspond to the various tissue structure
types. The limits of the pressure range are a function
of size and sensitivity of array 12. For array 12
discussed above, the acceptable pressure range is 0.2 psi
to 2 psi.
Because the proper amount of user-applied pressure
is important, in the first mode of operation, a
preliminary pressure test is performed on the frame to
determine whether the average amount of pressure applied
to all sensors 14 is within the acceptable range. This
preliminary pressure test also determines if a minimum
number of sensors 14 are obtaining a reading across width
of array 12 such that DSP 24 recognizes that entire array
12 is in contact with the skin. In the first mode of
operation, if the frame fails the preliminary pressure
test (e.g., if the average applied pressure is below or
above the acceptable range), the frame is considered
invalid and is not tested any further in the initial test
procedure, and DSP 24 proceeds to the next frame stored
in memory 30.
In use, in the first mode of operation the user
translates the sensor head 55 over the skin. Sensor
array 12 can be moved across a section of the breast
vertically or horizontally while the user listens to the
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-2G-
low pitched humming tone 50 which indicates to the user
that she is applying proper downward pressure to device
10. If red LED 40 is illuminated and audio circuit 50
generates an alarm tone during any portion of the scan,
the user should scan that area of the breast again
(either in the same direction or in another direction,
e.g., horizontally).
If red LED 40 and audio circuit 50 generates the
alarm tone again, the user stops translating breast
examination device 10. DSP 24 enters the second mode and
performs a three stage analysis at this point. In the
first stage, it vibrates the tissue through a range of
frequencies, samples the tissue response, and stores the
data. In the second stage, DSP 24 examines the stored
data to determine the harmonic frequency and amplitude.
In the third stage, DSP 24 examines the values obtained
in the second stage to determine if the structure being
examined is a carcinoma. These three stages will now be
described in detail in reference to Figs. 14-19B.
Referring to Fig. 14, DSP 24 signals 200
oscillator 36 to oscillate oscillation plates 45 and 46.
Referring also to Fig. 3A, oscillation plates 45 and 46
remain in their raised position while device 10 operates
in the first mode, upon notifying the user of detection
of a suspicious mass, DSP 24 provides signals 42 to
oscillator 36 to vibrate oscillation plates 45 and 46.
Referring also to Fig. 3C, two periodic force
displacements 70 and 73 generated by vibrating plates 45,
46, travel towards each other at an angle. Horizontal
vectors 71, 74 of two applied forces 70, 73 are directly
opposed to each other. Any horizontal movement of a mass
76 directly underneath sensor array 12 due to one applied
force is cancelled out by an opposite but equal
horizontal displacement due to the other force.
Therefore, the tissue and any structure within it vibrate
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-27-
perpendicularly 77a, 77b to the surface of sensor array
12 in response to the vertical vectors 72, 75.
DSP 24 begins oscillating the underlying tissue
starting at 1 Hz and increases the oscillation frequency
gradually to a maximum of 200 Hz. The frequency is
increased in increments of 5 Hz. DSP 24 at all times
during the examination keeps track of the frequency at
which oscillation plates 45 and 46 are oscillating.
Oscillation plates 45 and 46 in turn vibrate the area of
the breast directly underneath sensor array 12. The
input amplitude of the vibration (i.e., the periodic
force displacement) is constant throughout the procedure
because the amplitude of the response is proportional to
the input amplitude. If the input amplitude is
significantly varied, comparison of amplitude responses
at different frequencies may not yield accurate results.
Moreover, the amplitude must be high enough to
mechanically oscillate the tissue and any foreign
structure within the tissue. Low amplitude displacement
waves may merely reflect from the structure or attenuate
to an extent that the response wave could not be detected
by sensor array 12.
In step 205, DSP 24 supplies oscillator 36 with
the frequency at which the tissue should be vibrated.
DSP 24 stores this frequency as the current vibration
frequency. DSP 24 allows one to two seconds for the
tissue to adjust to the new frequency (step 210).
Because prior to application of the new frequency the
tissue was either static or vibrating at a different
frequency, it will take one to two seconds before the
tissue and any structure within it are fully excited at
the new frequency and are responding at their peak
amplitude to the new frequency.
In step 215, DSP 24 calculates the sampling rate
for sampling the frequency response (i.e., the response
CA 02277328 1999-07-08
WO 98/31283 PCT/C1S98/00377
-28-
wave) of the area being examined. Referring also to Fig.
15A, in order to detect the amplitude of the response,
the response from the tissue must be sampled at a rate
fast enough for an amplitude at or close to the peak
amplitude to be sampled. In the preferred embodiment,
this sampling rate is 20 samples per each cycle of the
wave. For example, for a vibration frequency of 200 Hz,
the required sampling rate would be 4000 Hz.
However, sensor array 12 can only provide data at
a frequency of 20 Hz or less. Referring to Fig. 15A, the
response wave will be similar or identical in each cycle
because the applied periodic displacement force has a
constant frequency and amplitude at each frequency step.
Therefore, the frequency of the response (which is the
same as the oscillation frequency) and the amplitude of
the response will be constant. Therefore, for waves
requiring a sampling rate above the maximum data output
rate of sensor array 12, the 20 required samples may be
taken from different cycles of the response wave. During
each cycle when sensor array 12 can provide data (that
is, a cycle at least 1/20th of a second after the
previous cycle at which a sample was taken), the sampling
point is moved by 1/20th of the oscillation period from
the position of the previous sampling instance relative
to the wave cycle. The time period between each sampling
point (Tacq) is therefore determined according to the
following formula:
Tacq = Tacq max + 1/20(Tosc),
where Tosc is the period of the response wave and
Tacq max the upper limit of the rate at which data may be
obtained from sensor array 12 (i.e. 1/20 sec.). Hence,
referring to Figure 15B, for response waves with
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-29-
frequencies above the 1/20th of 1/Tacq max, samples 150
are taken from different cycles of the response wave 151.
From the twenty samples taken for each cycle, ten
of them will be from the trough of the response wave.
However, pressure sensors 14 can sense only positive
pressure. Therefore, the frames corresponding to the
wave trough do not contain any useful information. They
will likely sense zero pressure. These frames are
automatically filtered out when DSP 24 examines all the
frames stored in memory 30 to determine the harmonic
amplitude, because their values will be less than the
frames from positive amplitude of the response wave.
In step 220, DSP 24 signals preprocessing circuit
to obtain a frame at the appropriate sampling period.
15 A preliminary pressure test similar to the preliminary
test in the first mode of operation is first performed on
the frame. The values from pressure sensors 14 are
averaged and compared to a minimum and a maximum
threshold pressure value. This preliminary pressure test
20 also determines if a minimum number of sensors 14 are
obtaining a reading across width of array 12 such that
DSP 24 recognizes that entire array 12 is in contact with
the skin (step 225). If the frame fails the pressure
threshold test, DSP 24 stops the humming tone, to
indicate that the user should adjust the pressure on
device 10 (step 230). A new frame at the same sampling
period relative to the response wave as the sampling
point of the previous frame is then obtained by not
moving the sampling point by 1/20 Tosc (step 220).
If the pressure exerted by the user is within the
appropriate range, DSP 24 reactivates the humming tone in
step 240 (if it was previously deactivated in step 230).
The frame is then stored in memory 30, indexed with the
frequency of the applied periodic force displacement and
the order in which it was obtained (step 245). The frame
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-30-
is also linked to the base frame by, for example, having
a word in the base frame pointing to the memory address
of the linked frame.
DSP 24 continues to obtain frames at the
appropriate sampling points until the required number of
frames for this frequency is obtained (step 250). In the
preferred embodiment, 20 frames for each vibrating
frequency are obtained. When the 20 frames for the
current frequency are obtained, DSP 24 then begins
vibrating the tissue at the next frequency (step 256).
Referring to Fig. 16, in the second stage, DSP 24
examines stored frames 28a-28n to determine the harmonic
frequency and amplitude of the underlying tissue. DSP 24
retrieves each of the frames and its frequency (step
305). DSP 24 next calculates the average of the
values from the "red" sensors for the area of suspicion
(step 310). A threshold test is used to determine which
sensors are "red". DSP 24 derives the threshold
dynamically for each frame by determining the average
pressure detected by all sensors 14 in array 12, and
multiplying the average by an empirical value (the
"red/blue factor"). (The average pressure for a frame is
obtained by adding the pressure values detected by
sensors 14 and dividing the result by the number of
sensors 14 in array 12.)
DSP 24 compares the pressure value of each
location in the frame (i.e., the amplitude of signal 22
produced by each sensor 14 in array 12) with the dynamic
threshold. If the pressure value produced by a sensor 14
is above the dynamic threshold, the location of the
sensor 14 is marked "red". If the pressure value is
below the dynamic threshold, the location of the sensor
14 is marked "blue". The area of suspicion is the area
of red sensors which was identified in the threshold
test .
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-31-
Referring also to Fig. 17, for example, pressure
sensors 170 which are located in d4-5, e3-6, f2-7, g3-6,
and h4 are the red sensors in this frame. When DSP 24
examines frequency indexed frames 28a-28n linked to the
base frame in memory 30, only the values corresponding to
red sensors 170 will be examined to determine the
harmonic frequency and amplitude, because these sensors
contain the pressure values corresponding to the response
of the structure under examination. The location of red
sensors relative to the frame of course may change
because of lateral movements of the lump or human error
in keeping device 10 in one location over the breast.
Therefore, red sensors must be identified in each frame.
Referring to Fig. 16, in step 310, DSP 24
calculates an average of the values from the red sensors.
If this value is greater than the value stored as the
greatest average up to this point (step 315), the
average, the frame, and the indexed frequency are stored
as those of the frame with the greatest average (step
320). (Of course, this value is reset prior to examining
the first frame.) Steps 305-320 are repeated until all
frames 28a-28n corresponding to the range of examined
frequencies for this suspicious area are examined (step
325). At the end of this stage, DSP 24 has determined
the frame having the greatest average. This average will
be the closest sampled average pressure value
proportional to the harmonic amplitude. The frequency of
this frame will be the harmonic frequency or the closest
examined frequency to the harmonic frequency of the
structure being examined.
Referring to Fig. 18, in the third stage, DSP 24
determines whether the structure being examined is a
carcinoma or a benign structure. In step 400, DSP 24
subtracts from the average of the red sensors in the
harmonic frequency frame, the average of the values from
CA 02277328 1999-07-08
WO 98/31283 PCT/LTS98/003'77
-32-
the red sensors in the base frame. This subtraction
yields the absolute value of the harmonic amplitude.
Referring to Fig. 19A, during the first mode of operation
of device 10, a static pressure profile 183 was obtained
S for the area under examination. Referring to Fig. 19B,
when the area is vibrated at the harmonic frequency, the
pressure imposed on the sensors is increased above that
of the static pressure. The data from sensor array 12 is
raw amplitude 180 which is the sum of absolute amplitude
181 and static pressure 182. In order to obtain absolute
amplitude 181, static pressure 182 obtained from the base
frame must be subtracted from raw amplitude 180. This is
done in step 400 by subtracting the average value of red
sensors in the base from the average value of red sensors
in the harmonic frequency frame.
Referring again to Fig. 18, following adjusting
for the static pressure, the harmonic frequency and the
harmonic amplitude are then compared to the threshold
values of carcinoma window (106 in Fig. 13) determined
from clinical studies (steps 405 and 410). The result of
these comparisons are stored so that together with the
result of the pressure profile test (step 415), described
below, can be analyzed by fuzzy logic techniques (step
420), described below.
In step 415, a pressure profile test, similar to
the one used in the first mode of operation and described
in the '466 application, is performed on the frame for
the harmonic frequency. The pressure profile test in
step 415 is a 3-D (three-dimensional) test in which DSP
24 analyzes the amplitudes of values from the red sensors
to determine whether the pressure signature of the tissue
structure is approximately lump-like in three dimensions.
For example, the pressure profile test enables DSP 24 to
determine whether the central region of the pressure
signature is relatively large (like that of a soft cyst),
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-33-
or is small (like that of a solid mass). Due to their
somewhat spherical shapes, foreign structures (such as
cysts, benign solid masses, or carcinomas) induce
pressure signals 22 with amplitudes that increase
progressively as pressure is sampled from the periphery
of the structures to their center. Accordingly, in the
pressure profile test DSP 24 determines the edge profile,
the relative stiffness, and the relative curvature of the
underlying structure based on how the amplitudes of
suspicious signals 22 change from the periphery of the
structure toward the center of the structure. DSP 24
evaluates the edge profile to determine whether it is
extremely sharp (which indicates that the structure may
be a rib, rather than a lump) or is more moderate. DSP
24 also determines whether the structure's stiffness and
curvature are more indicative of a lump than of a normal
tissue structure.
This test is performed during the first mode of
analysis. However, we expect that because of the
increased amplitudes at harmonic frequencies, some of the
characteristics of the pressure profiles for various
structures will be further exaggerated and therefore more
easily identifiable. Because during harmonic oscillation
lumps exert more pressure on sensor array 12 than in the
first mode of analysis, we expect the curvature of the
pressure profile to be steeper than the curvature of the
pressure profiles in the first mode analysis. The result
of clinical studies will further enhance the pressure
profile test for oscillating lumps.
In step 420, DSP 24 applies so-called "fuzzy
logic" techniques (also known as "soft thresholding") to
weigh the results of steps 405, 410, 415. This technique
is a neural network concept that develops parameters of
imprecise measurements. DSP 24 weighs the differences
between the harmonic frequency and amplitude, and
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-34-
thresholds determined in steps 405 and 410 and the edge
profile, stiffness, and curvature determined in step 415.
It then develops a "degree of membership" outcome ranging
from 0 to 1 of the characteristics of the suspicious
region as a carcinoma (i.e., the degree to which, based
on the weighed results of steps 405, 410, 415 the
suspicious region resembles a carcinoma). DSP 24 weighs
the results of steps 405, 410, and 415 equally, but
alternatively could assign different weights to these
results. In determining the degree of membership of a
structure, DSP 24 may use results from only one, or a
combination, of the results from the tests in steps 405,
410, and 415. The results of the clinical studies will
provide better indication of which tests in combination
with the fuzzy logic technique yield the best results.
If in step 420 DSP 24 determines that the
structure may be a carcinoma, then DSP 24 causes audio
circuit 50 to generate a high pitch alarm tone to notify
the user (step 425). The user then should consult a
physician at the end of examination. The user should now
continue with the examination in accordance with the
first mode of analysis. If DSP 24 determines that the
structure is not a carcinoma, it provides a mid-pitch hum
to indicate to the user to continue with the examination
according to the first mode of operation of device 10.
Other embodiments are within the scope of the
claims.
Factors such as age, fitness level, percent body
fat, life style, child bearing and breast feeding
history, smoking habit, and so on may affect the ratio of
the mixture of fat, collagen and elastin in breast
tissue. The ratio of the mixture in turn determines the
viscoelastic characteristics of the breast tissue.
Therefore, these factors may in effect change the spring
coefficient and damping factor for the system, affecting
CA 02277328 1999-07-08
WO 98/31283 PCT/US98100377
-35-
harmonic response of breast tissue and structures within
it. Clinical studies for determining the threshold,
therefore, may also provide data to create a variety of
thresholds for different population groups based on these
S factors. The preferred embodiment may then be calibrated
to take into account these factors based on each user's
characteristics.
In one embodiment, device 10 would be configured
for different populations of women. For example, one
embodiment may be configured for women between 35-45
years of age who are non-smokers and are moderately
active. Another embodiment may be configured for women
between 25-35 years of age and who smoke and are
moderately active. Various embodiments may be provided
for the complete range of population. The devices may
then be prescribed by physicians, or bought off the shelf
or over the counter. In another embodiment, a clinician
may enter a woman's characteristics into an expert system
which then calibrates the device (using an EPROM for
example) for that woman.
In the first embodiment described herein, the
average of the red sensors are examined in the second and
the third stages of the second mode. In alternative
embodiments, the median value or the sensor having the
highest pressure value may be examined. In yet another
alternative embodiment, the response wave may be
reconstructed fully in 3D using the sample data and then
most relevant values for analysis chosen on a frame by
frame basis.
In an alternative embodiment, a Fourier transform
may be used to determine the peak amplitude response.
The Fourier transform may be performed on the pressure
value of one of pressure sensors 14 having the highest
output for a given area, the average the pressure values
of the red sensors, or all of the red sensors.
CA 02277328 1999-07-08
WO 98/31283 PCT/CTS98/00377
-36-
In alternative embodiments, other oscillating
mechanisms may be used, including piezo-electric or
magnetic devices.
In the first embodiment, all samples are stored
and then examined for determining the harmonic amplitude
and frequency. In an alternative embodiment, the frames
can be examined as they are received from preprocessing
circuit 20.
In an alternative embodiment, a single oscillation
plate may be used to vibrate the tissue. Alternatively,
more than two plates may be used. These plates may be
configured such that the response would be perpendicular
or parallel to the sensor array.
In an alternative embodiment, oscillation plates
45, 46 vibrate the breast tissue out of synch.
Therefore, a lump within the breast, would appear to
array 12 to be moving laterally or in a pendulum swing as
well as possibly vertically. DSP 24 then would analyze
the lateral movement of the lump to determine the nature
of the lump .
In an alternative embodiment, rather than
determining the harmonic frequency and amplitude, only
the fact that a structure is able to move or oscillate
within the breast is examined. The tissue is vibrated
through a range of frequencies and DSP 24 determines
whether the structure vibrates or moves in response to
the applied force. Based on whether the structure moves.
DSP 24 then can determine the structure type. For
example, a bony prominence, a very soft mass, or an area
of thickening in breast tissue, may not oscillate or move
at all in response to an applied force or it may not
oscillate or move in response to a particular range of
frequencies. Therefore, they can be distinguished from
other structures based on the fact they do not move in
response to an applied force.
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-37-
In an alternative embodiment, the phase difference
between the response wave and the vibrating wave may also
be analyzed to determine the characteristics of the
foreign structure.
In an alternative embodiment, multiple suspicious
areas under the array may be examined. In one
embodiment, the frames may be divided into halves or
quarters corresponding to a virtual division of the array
surface into halves or quarters. The red areas in each
section may then be examined separately by limiting the
analysis in the second and third stage only to those
sensors within the particular section being examined.
Alternatively, a mapping technique may be used to
examine each red area in the base frame. The mapping
technique comprises matching red areas in each of the
obtained frames by size, characteristic, and location to
the red area in the base frame under examination.
Generally, the structure under investigation may move
with respect to the array because of its oscillation.
The user may also move the array during the second mode
of analysis. Therefore, the red sensors in the base
frame will not necessarily be the red sensors of the
frames obtained in the second mode operation. Therefore,
the red sensors of a frame must be mapped onto the red
sensors of the base frame, i.e. DSP 24 must determine
whether red sensors in a frame correspond to the same
structure as the red sensors in the base frame. DSP 24
implements this on the basis of the assumption that
generally the red sensors in a frame will have the same
approximate area and will be in the same vicinity as the
red sensors in the base frame.
DSP 24 first finds the center of red areas in both
frames by drawing a hypothetical rectangle around the red
areas. The borders of the rectangles will coincide with
the outer most sensors of each red area. DSP 24 then
CA 02277328 1999-07-08
WO 98/31283 PCT/US98/00377
-38-
calculates the intersection of the two diagonals of the
rectangle which is taken to be center of the red area.
Alternatively, the center of red areas may be obtained
using other standard techniques, such as center of mass
or Centroid Weighted Technique.
If the distance between the centers of two red
areas in two frames is within a radius of tolerance (e. g.
1 cm) and the area of red sensors in the two frames are
similar in size, shape, and/or pressure profile, the two
areas are considered to be the same. Each frame for each
frequency is mapped in this manner, in order to determine
which signals correspond to the same structure in various
frames. For each red area in the base frame, the second
and third stages are repeated while using this mapping
technique to ensure examination of the same area.
Still other embodiments are within the scope of
the claims.