Note: Descriptions are shown in the official language in which they were submitted.
CA 02277417 1999-07-09
- 1 -
STRIPPING LANTHANIDE-LOADED SOLUTIONS
FIELD OF THE INVENTION
This invention relates to a method for stripping
lanthanide-loadecL solvent.
BACKGROUND OF THE INVENTION
The lanthanides, also known as the rare earths,
comprise elements 57 to 71 of the Periodic Table. Depending
upon the atomic number of the element, this series is often
divided into three sub-groups i.e. the light, medium and heavy
rare earths. The lightest is lanthanum with atomic number 57.
In contrast, the four heavy rare earths are erbium, thullium,
ytterbium, and lutetium with atomic numbers of 68, 69, 70, and
71, respectively. Scandium, atomic number 21, and yttrium,
atomic number 39, are often found with, and discussed with,
the lanthanides. In nature, they are generally found in their
oxide form, and a.re commercially isolated from monazite,
xenotime, bauxite, and bastnasite, or similar ores. Their
isolation, wherein the elements are recovered together or
separated from one another, has become of importance in recent
years.
One source of lanthanides is red mud that is
produced as a by-product of the Bayer process for producing
alumina. The Bayer process for producing alumina comprises,
as its first step, leaching or digesting bauxite or similar
crude ores with a. solution of sodium hydroxide to extract
alumina minerals contained therein as a solution of sodium
75365-150
CA 02277417 1999-07-09
- 2 -
aluminate. At the same time, as much as one third to one half
of the total weight of the crude ore used is discharged as a
residual red mud. This red mud typically contains substantial
amounts of silica, alumina, iron oxide, titania and sodium
compounds. Many red muds also contain small but valuable
quantities of lanthanide elements, and in some cases include
significant quantities of scandium and yttrium. If the red
mud is digested with dilute mineral acid, the lanthanide
elements, and scandium and yttrium, selectively dissolve in
the acid, together with sodalite and calcium compounds,
leaving iron and titanium in the red mud substantially
undissolved. Examples of suitable acids include hydrochloric,
nitric, sulphuric', and sulphurous acids.
The first general separation procedures, introduced
in the 1950s, were based on complexation-enhanced ion exchange
processes. However, in the 1960s liquid-liquid extraction
processes were introduced and these types of processes are
generally used in large-scale commercial production today.
One procedure employed, especially for the
separation of lanthanides from aqueous hydrochloric and nitric
acid solutions, i.s as follows. The aqueous acidic lanthanide
solution is contacted counter-currently or co-currently with
an extractant solution comprising a water-immiscible organic
solvent containing the mono-2-ethylhexyl ester of mono-2-
ethylhexylphosphonic acid, known as MEPA, or di(2-ethylhexyl)
phosphoric acid, known as DEPA. The lanthanide is extracted
from the aqueous acidic solution into the water-immiscible
75365-150
CA 02277417 1999-07-09
- 3 -
organic solvent. The organic solution containing the
extracted lanthanide(s) is then stripped with an acid such as
hydrochloric, nitric, or sulphuric acid to remove the
lanthanide(s) from the organic solution. The lanthanide
passes into the acidic aqueous solution and is then
precipitated from the acidic solution, for example as a
hydroxide, oxalate or carbonate, by the addition of a
neutralizing or precipitating agent.
One problem, particularly in regard to the heavy
lanthanides, namely erbium, thulium, ytterbium, and lutetium,
is that they are very strongly complexed by the
organophosphorus extractant during the extraction process, and
as a result require a greater concentration of acid in the
stripping process. This is also true of scandium and yttrium.
The need for a greater concentration of acid results in
increased processing costs because large quantities of acid
have to be neutralized. In the case of stripping with
hydrochloric acid, it can also result in contamination of the
final product by chloride ions. In addition, use of highly
concentrated acid is undesirable for safety reasons.
There have been some attempts to improve the
stripping efficiency. U.S. Patent 5,639,433 teaches the use
of an extractant solution containing an organophosphonic acid,
such as MEPA, in combination with an organophosphinic acid,
such as bis(2,4,4-trimethylpentyl)phosphinic acid (BTPP) for
the extraction of: rare earths. An advantage obtained is that
the lanthanide(s) are then more readily stripped from the
75365-150
CA 02277417 1999-07-09
- 4 -
solution.
BTPP is commercially available from Cytec as
CYANEXTM 272, but: this product also contains tris(2,4,4-
trimethylpentyl)phosphine oxide, in an amount of approximately
12-14%, and mono-~2,4,4-trimethyl-pentylphosphonic acid, in an
amount of approximately 1%. Thus, there is present about
13.8% to about 1E~.5% of tris-(2,4,4-trimethylpentyl)phosphine
oxide, based on t:he amount of BTPP.
SUMMARY OF THE INVENTION
It has now surprisingly been found that the presence
of a phosphine oxide or an ester of phosphorus in the
extractant solution affects the efficiency of stripping by
hydrochloric and nitric acid.
In one aspect this invention provides a process for
stripping a lanthanide from a lanthanide-containing extractant
solution which process comprises:
(a) contacting the lanthanide-containing extractant
solution with aqueous hydrochloric acid to strip the
lanthanide into the aqueous hydrochloric acid, wherein the
extractant solution contains the lanthanide and further
comprises:
(i) a water-immiscible diluent,
(ii) an organophosphonic acid or an organo-
thiophosphonic acid of formula
75365-150
CA 02277417 1999-07-09
- 5 -
Z1
R1 ~~ Z2x
OR2
wherein
Rl and R2 may be the same or different and
each represents a C4_i2 substituted or unsubstituted
alkyl group or a C4_g substituted or unsubstituted
cycloalkyl group, or a substituted or unsubstituted
phenyl, group,
Zl and Z2 may be the same or different and
each represents oxygen or sulphur, and
X represents hydrogen or a salt-forming
radical,
that is soluble in the water-immiscible diluent,
optionally
(iii) an organophosphinic acid or an
organothiophosphinic acid of formula
Z3 Z3
R~ ~~ ~x or 3
R ~ ~ ~x
R4 R4
75365-150
CA 02277417 1999-07-09
- 6 -
wherein
R3 and R4 may be the same or different and
each represents a C4-12 substituted or unsubstituted
alkyl group or C4_g substituted or unsubstituted
cycloa.lkyl group, or a substituted or unsubstituted
phenyl group,
Z~ and Z4 may be the same or different and
each represents oxygen or sulphur, and
X represents hydrogen or a salt-forming
radical,
that is soluble in the water-immiscible diluent,
(iv) a phosphine oxide or an ester of phosphorus of
formula
O
RS P R~ or R5 ~~ R7
R6 \R6
wherein R5, R6, and R~ may be the same or different
and each represents a C4-12 substituted or
unsubstituted alkyl, alkoxy, cycloalkyl, cycloalkoxy
group, or a substituted or unsubstituted phenyl
group, provided that the number of carbons in R5,
R6, and R~ together total at least 18, and
that is soluble in the water-immiscible diluent, and
with the proviso that if component (ii) is the mono-2-
ethylhexyl ester of mono-2-ethylhexylphosphonic acid,
75365-150
CA 02277417 1999-07-09
_ 7 _
component (iii) i.s bis(2,4,4-trimethylpentyl)phosphinic acid,
and the sole component (iv) is tris(2,4,4-trimethyl-
pentyl)phosphine oxide, then the amount of tris(2,4,4-
trimethylpentyl)phosphine oxide is greater than 16.5%, based
on the amount of bis(2,4,4-trimethylpentyl)phosphinic acid; or
(b) contacting the lanthanide-containing extractant
solution with aqueous nitric acid to strip the lanthanide into
the aqueous nitric acid, wherein the extractant solution
contains the lanthanide and further comprises components (i),
(ii), and (iii) and optionally component (iv), with the
proviso that if component (ii) is the mono-2-ethylhexyl ester
of mono-2-ethylhexylphosphonic acid, and component (iii) is
bis(2,4,4-trimethylpentyl)phosphinic acid, then tris(2,4,4-
trimethylpentyl)phosphine oxide is present in an amount not
greater than 13.8%, based on the amount of bis(2,4,4-
trimethylpentyl)phosphinic acid, preferably not greater than
about 10% and, most preferably, is absent.
BRIEF DESCRIPTION OF THE DRAWINGS
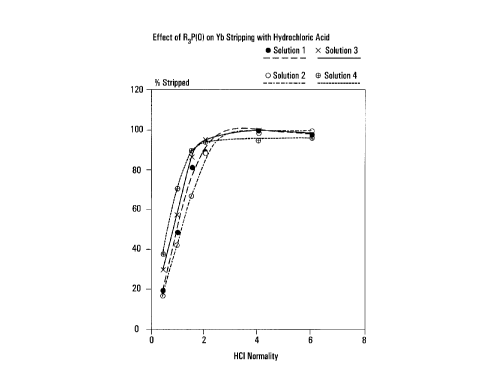
Figure 1 is a graph showing the effect of phosphine
oxide concentration on the stripping of Yb with hydrochloric
acid using 0.5 M of organophosphonic acid and 0.5 M of
organophosphinic acid.
Figure 2 is a graph showing the effect of phosphine
oxide concentration on the stripping of Yb with nitric acid
using 0.5 M of organophosphonic acid and 0.5 M of
organophosphinic acid.
Figure 3 is a graph showing the effect of phosphine
75365-150
CA 02277417 1999-07-09
oxide concentration on the stripping of Yb with hydrochloric
acid using 0.75 M of organophosphonic acid and 0.75 M of
organophosphinic acid.
Figure 4 is a graph showing the effect of phosphine
oxide concentration on the stripping of Yb with nitric acid
using 0.75 M of organophosphonic acid and 0.75 M of
organophosphinic acid.
DESCRIPTION OF PREFERRED EMBODIMENTS OF THE INVENTION
Using an organophosphonic/organothiophosphonic acid,
and, optionally, an organophosphinic/organothio-phosphinic
acid as the extractant, the Applicant has now demonstrated
that the efficiency of stripping can be improved by varying
the concentration of phosphine oxide or ester of phosphorus in
the extractant solution. Namely, the stripping efficiency of
hydrochloric acid is increased by adding or increasing the
concentration of phosphine oxide or ester of phosphorus in the
extractant solution; and the stripping efficiency of nitric
acid is increased by removing or decreasing the concentration
of phosphine oxide or ester of phosphorus in the extractant
solution.
The ext,ractant solution from which the lanthanide is
to be stripped is usually a solution that has been obtained by
subjecting an aqueous acid leachate of a lanthanide-containing
ore to extraction with an organophosphorus-containing organic
extractant. The leachate is usually a very weakly acidic
solution, say about pH 2 to about 5, that is contacted, co-
currently or countercurrently, with the extractant, causing
75365-150
CA 02277417 1999-07-09
_ g _
lanthanides to be extracted from the weakly acidic aqueous
solution into th.e organophosphorus-containing organic
extractant. The relative volume of the aqueous acidic
leachate containing the lanthanide to the organic extractant
can vary between wide limits; it may depend, for instance,
upon the particular arrangements at an extraction plant or
mine. Ratios from about 20:1 to about 1:20, particularly from
about 10:1 to about 1:10 are mentioned.
The extractant solution includes an organo-
phosphoric or organothiophosphonic acid of formula:
Z1
2
R P ZX
OR2
wherein R1 and R~2 may be the same or different and each
represents C4_12~ Preferably C6-10~ substituted or
unsubstituted alkyl group or a C4_g, preferably C5 or C6~
substituted or unsubstituted alkyl group, cycloalkyl group, or
a substituted or unsubstituted phenyl group, and Z1 and Z2 may
be the same or different and each represents oxygen or
sulphur. Preferably Z1 is oxygen; more preferably both Z1 and
Z2 are oxygen. ~ is hydrogen or a salt-forming radical;
preferably, X is hydrogen, an alkali metal, or ammonium ion.
Mixtures of such compounds can be used.
Examples of suitable organophosphonic and
75365-150
CA 02277417 1999-07-09
- 10 -
organothiophosphonic acids include the mono-2-ethylhexyl ester
of mono-2-ethylhexyl phosphonic acid; the cyclohexyl ester of
3,3,5-trimethylhexylphosphonic acid; the cyclopentyl ester of
2-ethylhexylphosphonic acid; the mono-2-ethylhexyl ester of
phenylphosphonic: acid; the mono-3-methyloctyl ester of n-
amylphosphonic acid; the 3,5,5-trimethylhexyl ester of 3,3,5-
trimethylhexylphosphonic acid; the monoisodecyl ester of 2-
ethylhexylphosphonic acid; the monoisodecyl ester of
isodecylphosphonic acid; the monoisodecyl ester of
isodecylphosphonic acid and the like, and also the
corresponding train compounds. Especially preferred is the
mono-2-ethylhexyl ester of mono-2-ethylhexylphosphonic acid.
The organophosphonic or organothiophosphonic acid
component is suitably present in an amount of 0.1 - 1M,
preferably 0.25 - 0.75M, more preferably about 0.4 - 0.6M in
the solution t~ be stripped.
The ex;tractant solvent further, optionally, includes
an organophosphinic or organothiophosphinic acid of formula:
2 0 Z3 Z3
R3 -~~ ~x or 3
R ~ ~ ~x
R4 R4
wherein R3 and R4 may be the same or different and each
represents a C4_12~ Preferably C6_10, substituted or
unsubstituted alkyl group or a C4_g, preferably C5 or C
substituted or unsubstituted cycloalkyl group, or a .
75365-150
CA 02277417 1999-07-09
- 11 -
substituted or unsubstituted phenyl group, and Z3 and Z4 may
be the same or different and each represents oxygen or
sulphur, X represents hydrogen or a salt-forming radical,
preferably hydrogen alkali metal, or ammonium ion. Mixtures
of such compounds can be used.
U.S. Pat. Nos. 4,348,367 and 4,353,883 disclose
suitable phosphinic acids and salts and are incorporated
herein by reference. Examples of suitable organophosphinic
acids include the following: di-n-butylphosphinic acid; di-
isobutylphosphinic acid; di-n-pentylphosphinic acid; di-n-
hexylphosphinic acid; di-heptylphosphinic acid; di-n-
octylphosphinic acid; bis(2-ethylhexyl)phosphinic acid; di-n-
nonylphosphinic acid; di-n-decylphosphinic acid; di-n-
dodecylphosphinic acid; bis(2,4,4-trimethylpentyl)phosphinic
acid; (2,4,4-trimethylpentyl)cyclohexylphosphinic acid;
(2,4,4-trimethylpentyl)octylphosphinic acid; dicyclo-
pentylphosphinic acid; dicyclohexylphosphinic acid;
dicyclooctylphosphinic acid; cyclohexyl-n-butylphosphinic
acid; cyclopentyl.-n-dodecylphosphinic acid; cyclooctyl-
ethylphosphinic acid; 2,4,6-triisopropyl-1,3,5,-
dioxaphosphodane-5-hydroxy-5-oxide phosphinic acid;
cyclohexyl-1-hydroxycyclohexylphosphinic acid; bis(2-methyl-1-
hydroxypentyl)phosphinic acid; cyclohexyl-1-hydroxycyclo-
pentylphosphinic acid; (1-methylpentyl)-(1-hydroxy-1-
methylpentyl)phosphinic acid; (1-hydroxy-1-methyl-
ethyl)isopropylphosphinic acid, and the like, and the
corresponding thi,o compounds.
75365-150
CA 02277417 1999-07-09
- 12 -
Especially preferred is bis(2,4,4-trimethylpentyl)-
phosphinic acid.
When using hydrochloric acid to strip, the
extractant solution may contain no phosphinic acid derivative,
i.e., no component (iii). It is preferred, however, that a
phosphinic acid derivative shall be present. A suitable
amount is from about 0.1 to about 1M, preferably 0.25 - 0.75M,
more preferably about 0.4 - 0.6M in the extractant solution.
The extractant solution may optionally contain a
component (iv), a phosphine oxide or ester of phosphorus of
formula
O
p R~ or RS
R6 \R5
t
wherein R5, R6, and R~ may be the same or different and each
represents C4_12~ Preferably C6_12. substituted or
unsubstituted alkyl, alkoxy, C4_g, preferably C5_6,
cycloalkyl, cycloalkoxy group, or a substituted or
unsubstituted phenyl or phenoxy group provided that the number
of carbon atoms present in R5, R6, and R~ together total at
least 18. Mixtures of phosphine oxides or phosphorus esters
can be used.
When none of R5, R6, and R~ is alkoxy, cycloalkoxy
or phenoxy, then the component (iv) is a phosphine oxide.
When one of R5, R6 and R~ is alkoxy, cycloalkoxy or phenoxy
75365-150
CA 02277417 1999-07-09
- 13 -
then the component (iv) is a phosphinate ester. When two of R
5, R6 and R7 are alkoxy, cycloalkoxy or phenoxy then the
component (iv) is a phosphonate ester. When three of R5, R6
and R7 are alkoxy, cycloalkoxy or phenoxy then the component
(iv) is a phosphate ester. Any of these, or mixtures of
these, can be used as component (iv), but it is preferred that
component (iv) is a phosphine oxide. Particularly preferred
as component (iv) are tri-n-octylphosphine oxide (TOPO),
tris(2,4,4-trimet.hylpentyl)phosphine oxide (TTMPP) and
trihexylphosphine oxide.
Strictly, the term lanthanide includes elements 57
to 71 of the Periodic Table. The process of the invention is
more effective with lanthanides of atomic numbers 65 to 71
inclusive, and especially with those of atomic numbers 69 to
71. These are the lanthanides that complex most strongly with
the organophosphorus extractant, as stated above. As also
stated above, scandium and yttrium are often found with
lanthanides and the process of the invention is effective with
scandium and yttrium; in their stripping properties, these two
elements are similar to the heavy lanthanides. In the
description of the invention the term "lanthanide" should be
understood to include scandium and yttrium unless the context
requires otherwise. The concentration of the lanthanide in
the extractant solution may vary between wide limits, but is
suitably about 0.005M to about 0.2M, preferably about 0.05M to
about 0.15M.
When th.e extractant solution is to be stripped with
75365-150
CA 02277417 1999-07-09
- 14 -
hydrochloric acid, the concentration of component (iv) is
preferably about 0.05M to about 0.6M, more preferably about
O.iM to about 0.4M, most preferably about 0.15M to about
0.25M, and especially 0.2M. Component (iv) is preferably a
phosphine oxide, but it is in accordance with the invention
that some or all of component (iv) is an ester of phosphorus.
It has been found that the use of phosphine oxide or ester of
phosphorus at these concentrations permits use of lower
concentrations of hydrochloric acid for the stripping than
typically used in the prior art. Consequently, the
concentration, of hydrochloric acid used for the stripping is
preferably about 0.5 N to about 4 N, more preferably about
1 N to about 3 N, most preferably about 1.2 N to about 1.8 N.
When using hydrochloric acid for stripping, good
results can be obtained from an extractant solution that does
not contain a phosphinic acid derivative, i.e., does not
contain a component (iii). It is preferred that the
phosphinic acid derivative is present, however.
When the extractant solution is to be stripped with
nitric acid, component (iv) is preferably absent, or is
present in an amount not exceeding about 0.1 M. This permits
use of lower concentrations of nitric acid for the stripping
than typically used in the prior art. Consequently, the
concentration of nitric acid used for the stripping is
preferably about 0.5 N to about 4 N, more preferably about
1 N to about 3 N, most preferably about 1.7 N to about 2.3 N.
The extractant solution contains a water-immiscible
75365-150
CA 02277417 1999-07-09
- 15 -
diluent, as component (i). Examples of useful diluents
include halogenat;ed and non-halogenated aliphatic and aromatic
hydrocarbons such as, for example, hexane, heptane, octane,
dodecane, benzene, toluene, xylenes, ethylbenzene, and the
corresponding chlorinated compounds and petroleum cuts such as
kerosene, fuel oi.l, JP-1, aliphatic hydrocarbons available
under the trade-mark EXXSOL D-80, and the like. Components
(ii), (iii) and (iv) are soluble in the diluent.
The temperature at which the lanthanide is stripped
by the acid is not critical. It may range from about 10°C to
about 80°C, preferably from about 15°C to about 70°C,
most
preferably from about 20°C to about 60°C.
The ratio of acid to extractant solution can vary
between wide limits and, for example, may range from 20:1 to
1:20, particularly 10:1 to 1:10.
The aqueous leachate from which the lanthanide is
extracted into the extractant solution should be acidic, i.e.
the pH should be under about 6.5, preferably from about 1 to
4. If necessary or desirable the pH can be adjusted by
addition of a suitable reagent, for example aqueous sodium
hydroxide or ammonia.
After stripping, the lanthanide may be isolated by
precipitation, for example, using a base to precipitate a
hydroxide or carbonate, from the aqueous hydrochloric or
nitric acid used to strip. Alternatively, the lanthanide can
be precipitated by addition of oxalic acid or an oxalate salt.
In the case where the lanthanide-containing
75365-150
CA 02277417 1999-07-09
- 16 -
extractant solution is obtained by extracting the lanthanide
from an aqueous acidic leachate, it is possible to include in
the process a scrubbing step before the extractant solution is
stripped by treatment with hydrochloric or nitric acid. The
scrubbing can be carried out by washing with a dilute aqueous
solution of a mineral acid, for example hydrochloric, nitric,
phosphoric or sulphuric acid. The solution is only weakly
acidic, so the lanthanide is not stripped from the organic
phase into the aqueous phase during this scrubbing step. As a
further aid to preventing loss of lanthanide into the
scrubbing solutian, the scrubbing solution may contain a
lanthanide so that partition between the organic and aqueous
solutions does not cause lanthanide from the organic solution
to enter the aqueous scrubbing solution.
After the extractant solution has been stripped of
lanthanide, it can be recycled for re-use to extract more
lanthanide from an aqueous acidic leachate from a lanthanide-
containing ore. Before recycling the stripped solution, it
may be desirable to wash the solution. For this purpose
strong aqueous mineral acid, for instance hydrochloric,
nitric, or sulphuric acid of pH less than about 1, preferably
less than about 0~.5 is suitable. The acid used for this
washing should be stronger than the acid used for stripping.
The invention is further illustrated in the
following non-limiting examples.
Example 1
Solutions from which a lanthanide was stripped were
75365-150
CA 02277417 1999-07-09
- 17 -
prepared using cammercial grade CYANEXTM 272, purified CYANEX
272, IONQUESTTM 801, and tri-n-octylphosphine oxide (TOPO).
EXXSOLTM D-80 was used as diluent.
Commercial grade CYANEX 272 contains 85-87%
bis(2,4,4-trimethylpentyl)phosphinic acid (BTPP):
and 12-14% tris(2,4,4-trimethylpentyl)phosphine oxide (TTMPP),
i.e. the amount of tris(2,4,4-trimethyl-pentyl)phosphine oxide
is about 13.8 to 16.5%, based on the amount of BTPP.
Purified CYANEX 272 contains greater than 99%
bis(2,4,4-trimethylpentyl)phosphinic acid (BTPP).
IONQUEST 801 contains 97% mono-2-ethylhexyl ester of
mono-2-ethylhexylphosphonic acid (MEPA).
EXXSOL D-80 is composed of a mixture of Clp-C12
hydrocarbons.
Nine different extractant solutions were prepared,
the compositions of which are shown in Table 1. The solutions
were washed with hydrochloric acid (100 g/L HC1) (ratio of
aqueous to organic phase (A/O) is 1, at 24°C) and then with
deionised water (A/O=1, at 24°C) and then centrifuged to remove
entrained water before use.
A lanthanide-loaded solution was prepared by
contacting the washed extractant solution with an aqueous
solution of O.1M Yb(3+) chloride at pH = 3 and 24°C for 10
minutes. Sodium hydroxide (100 g/L NaOH) was used for pH
control. The loaded extractant solution was centrifuged and
the concentration of Yb loaded was determined, by Inductively
75365-150
CA 02277417 1999-07-09
- 18 -
Coupled Plasma (ICP) analysis, to be 0.09 to O.1M.
Aliquots of the loaded solution were contacted with
aliquots of HC1 (0.5 to 6N) for 25 minutes at room temperature
and A/O=1, to investigate strippability. After phase
separation, the aqueous solutions (strip liquors) were
centrifuged to remove entrained organic material and were
analysed for Yb by ICP. The percentage of Yb stripped was
calculated by mass balance, using the known concentration of
Yb in the loaded solution.
Example 1(a)s Solutions 1 to 4 contained 0.5M of
BTPP (an organophosphinic acid), 0.5M MEPA (an organo-
phosphonic acid) and phosphine oxide in concentrations of
0.08M, OM, 0.2M and 0.4M, respectively. Using hydrochloric
acid to strip Yb from the loaded solution 3 or 4 is in
accordance with the invention. Using hydrochloric acid to
strip solution 1 or 2 is not in accordance with the invention;
these are comparative examples.
For solutions 1 to 4, the effect of HC1
concentration on Yb stripping is shown in Table 2 and plotted
in Figure 1. As is shown clearly in Figure 1, when using
hydrochloric acid at lower concentration up to about 2N, Yb is
more readily stripped from solutions 3 and 4 than from
solutions 1 and 2.
Example 1(b)s Solutions 5 to 7 contained 0.75M of
BTPP, 0.75M of MEPA and concentrations of phosphine oxide of
OM, O.11M and 0.2M, respectively. Use of solutions 5 and 6 is
not in accordance with the invention when stripped with HCl.
75365-150
CA 02277417 1999-07-09
- 19 -
Use of solution 7 is in accordance with the invention when
stripped with HC1..
For solutions 5 to 7, the effect of HC1
concentration on Yb stripping is shown in Table 2 and plotted
in Figure 3. These results show, for stripping with HC1, an
increase in stripping efficiency for concentrations up to 2N
HC1 as the amount. of phosphine oxide in the solution
increases.
Example 1(c)a Solutions 8 and 9 contain 0.75M of
MEPA, no phosphinic acid, and concentrations of phosphine
oxide of OM and 0.2M, respectively. As seen from Table 2,
stripping efficiency improves at low concentrations of HC1,
even in the absence of phosphinic acid. However, the
stripping of Yb from solutions 8 and 9 with HC1 is more
difficult in the absence of phosphinic acid. Use of solution
9 is in accordance with the invention when stripped with HC1.
Thus, the invention permits use of hydrochloric acid
of lower concentration, realizing the advantages and avoiding
the disadvantages discussed above.
Example 2
Similar experiments were carried out using solvents
1 to 7 described in Example 1 and nitric acid to strip Yb from
the loaded solution. The results are given in Table 3.
Example 2(a) For solutions 1 to 4, the effect of HNO
g concentration on Yb stripping is plotted in Figure 2. Using
nitric acid to strip Yb from the loaded solution 2 is in
accordance with the invention. Using nitric acid to strip
75365-150
CA 02277417 1999-07-09
- 20 -
solutions 1, 3 and 4 is not in accordance with the invention;
these solutions are comparative examples. As is shown clearly
in Figure 2, with nitric acid Yb is more readily stripped from
solution 2, especially at lower concentrations of nitric acid,
up to about 2N.
Example 2(b) For solutions 5 to 7, the effect of
HN03 concentration on Yb stripping is plotted in Figure 4.
Use of solutions 6 and 7 with HN03 is not in accordance with
the invention. Use of solution 5 with HN03 is in accordance
with the invention.
Example 3
Stripping experiments similar to those reported in
Examples 1(a) and 2(a) were performed with another lanthanide
element, lutetium, with essentially equivalent results.
75365-150
CA 02277417 1999-07-09
- 21 -
W O O N ~ O O N O N
0
I
N o 0 0 0
O
H
b ri
0
O
NW ~ u~o W ~n u7 ~ ~ u~7u~'~
O O O O O O O O O
rl H
r~
~
r
O
N
N
O O O O O rl O O O
~ H O O
U
4a H
O b
~U
~
pa
rl ~ ~"p4.,'n o 0 0 o r o 0 0
~
U W o 0
O
N
r
N
bw
o 'n 'n 'n ~ o ~ 0 0
l
p4 0 0 0
H ~ 0 0
w
0
0
I ~-I N M d ~ lfl l~ 0001
ri
N
75365-150
CA 02277417 1999-07-09
- 22 -
o~ ~i
I
N
-p 001
N
Q
V M ~ 00 ~ N l~
~t' ~ l~ 01 G1
s
a
O O ~D ~ M d: I~ l~ d:
I M N t~ C1 01
O
o N o o ~n o 0
m ~, N ~ ~ ~ o
c
o
I M ~ 00 C1 01
O
00 N N 00 ~' N
I N ~ 0~0 0~1 O
.Q
L
0o N o0 ~O l~
e~~ ~ ~ ~ oNO v O
I
N
~t I~ N d: ~ O
00 0~0
H
z z z z z z
v) o ~ O c O
O -i -~ N ~ \O
75365-150
CA 02277417 1999-07-09
- 23 -
M N O~
~ I N ...-~ ~i
~-a N M
V d: 00 00 \C
~ M r-i ~ ~ N
V N ~ ~ I~ t~
Z p M M 00 l~ .-~
'.,~.,~ ~. N ~ G1 00
I N d' 00 ~n
O N ~ l~
C1 01 M O
N V1 v1
L ,i
'Lr M ~ ~ M .~ ~ 00
'~ I Cv N ~i o0 00
v'W ~ l~
0
O ~O C~ -~ M d:
G~ .-.-MrM lp 00 G1 00
.. ~ ~p l~ 'O O l~
M ~ ri ~ .--~.-: yo
I
.-a M l0 l~ 00 00
'' o' z z z z z z
o ~ O c c
O .-~ ,~ N ~ ~C
75365-150