Note: Descriptions are shown in the official language in which they were submitted.
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
TITLE OF THE INVENTION
THROMBIN INHIBITORS
BACKGROUND OF THE INVENTION
Thrombin is a serine protease present in blood plasma in
the form of a precursor, prothrombin. Thrombin plays a central role in
the mechanism of blood coagulation by converting the solution plasma
protein, fibrinogen, into insoluble fibrin.
Edwards et al., J. Amer. Chem. Soc. (1992) vol. 114, pp.
1854-63, describes peptidyl a-ketobenzoxazoles which are reversible
inhibitors of the serine proteases human leukocyte elastase and porcine
pancreatic elastase.
European Publication 363 284 describes analogs of peptidase
substrates in which the nitrogen atom of the scissile amide group of the
substrate peptide has been replaced by hydrogen or a substituted
carbonyl moiety.
Australian Publication 86245677 also describes peptidase
inhibitors having an activated electrophilic ketone moiety such as
fluoromethylene ketone or a-keto carboxyl derivatives.
2D Thrombin inhibitors described in prior publications contain
sidechains of arginine and lysine. These structures show low selectivity
for thrombin over other trypsin-like enzymes. Some of them show
toxicity of hypotension and liver toxicity.
European Publication 601 459 describes sulfonamido
heterocyclic thrombin inhibitors, such as N-[4-[(aminoimino-
methyl)amino]butyl]-1-[N-(2-naphthalenylsulfonyl )-L-phenylalanyl]-L-
prolinamide.
WO 94/29336 describes compounds which are useful as
thrombin inhibitors.
WO 96/18644 describes heterocyclic derivatives as thrombin
inhibitors.
-1-
CA 02277929 1999-07-14
WO 98/31670 PCTlUS98/00875
SUMMARY OF THE INVENTION
The invention relates to compounds of the formula:
R2~X \ Rs0
I
N~ NBA
H ~ H
wherein
W is
hydrogen,
Rl_
R10C(O)-,
R1C(O)-,
R1S02-,
(R1 )m(CH2)nNHqC(O)-,
where n is 0-4, m is 1 or 2, wherein R1 is same or different, and
q is 0 or 1, with the proviso that where n is 1-4, q is 1 and m is 1,
and where n is 0, m is 1 or 2, and q is 0 or 1, and where n is 0,
mis2andqis0;
R1 is -
R17(CH2)t-, where t is 0-4;
(R17)(OR17)CH(CH2~-) where p is 1-4,
(R17)2CH(CH2)r-, where r is l)-4 and each 117 can be the same or
different, and wherein (Its ~ rl can alac~ form a ring with CH
represented by C3-7 cvcloalkyl) C'.;_~1 bicylic alkyl, C10-16
tricylic alkyl, or a 5- to 7- membered mono- or bicyclic
heterocyclic ring which can be saturated or unsaturated, and
which contains from one to three heteroatoms selected from
the group consisting of N, O and S,
R170(CH2~-, wherein p is 1-4;
R2, R14 and R17 are independently selected from
-phenyl, unsubstituted or substituted with one or more of
-2-
CA 02277929 1999-07-14
WO 98/31670 PGT/US98/00875 _ -
C1_4 alkyl,
C1_4 alkoxy,
halogen,
hydroxy,
COON, or
CONH2~
naphthyl,
biphenyl,
a 5- to 7- membered mono- or a 9- to 10-membered bicyclic
heterocyclic ring which can be saturated or unsaturated, and
which contains from one to four heteroatoms selected from the
group consisting of N, O and S,
-C1-7 alkyl, unsubstituted or substituted with one or more of
hydroxy,
COOH,
amino,
aryl,
C3_7 cycloalkyl,
heteroaryl, or
heterocycloalkyl,
-CFg
C3_7 cycloalkyl,
C7-12 bicyclic alkyl, or
C 10-16 t~cyclic alkyl;
X is
CF2,
CR15~R16
wherein R15 and Rls are independently
- hydrogen,
C3_7 cycloalkyl,
C1_4 alkyl unsubstituted or substituted with one or more of
hydroxy,
COOH,
-3-
CA 02277929 1999-07-14
. WO 98/31670 PCT/US98/00875 _ ,
amino,
aryl,
heteroaryl, or
heterocycloalkyl,
aryl,
heteroaryl,
heterocycloalkyl, or
R15 and R16 are joined to form a four to seven
membered cycloalkyl ring unsubstituted or
substituted with hydroxy, amino or aryl, or
S(O)r, where r is 0-2;
R3 is
hydrogen,
C1_4 alkyl,
C3_7 cycloalkyl, or
trifluoromethyl;
A is chosen from one of the following radicals:
R~ 3 Ri 2 R~ a R12 F~
~~~~ NH2 ~~=~N \
~ ~N ~ i ~ i
NH2
II III ~ I V
with the proviso that when A is radical IV, R2-X is not C1_4 alkyl,
C3_7 cycloalkyl, or trifluoromethyl;
R4 is
hydrogen,
C 1_4 alkyl,
-4-
CA 02277929 1999-07-14
WO 98I3I670 PCT/US98/00875 _ r
C 1-4 alkoxy,
halogen,
-OCH2CF3,
-COOH,
-OH,
-COOR6, where R6 is C1_4alkyl,
-CONR7R8, where R7 and R8 are independently
hydrogen or C1-4alkyl,
-(CH2)1_40H,
-CH2NHC(O)CH3,
-CH2NHC(O)CF3,
-CH2NHS02CH3,
-S02NH2~
-(CH2 )1-4SO2NR7R8~
-(CH2>1-4S02R6>
a 5- to 7- membered mono- or a 9- to 10-membered bicyclic
heterocyclic ring which can be saturated or unsaturated, and
which contains from one to four heteroatoms selected from the
group consisting of N, O and S,
-ZCH2C02H,
-ZCH2C02CHg,
-ZCH2R14,
-ZCH2C02(CH2)1-3CH3~
-Z(CHR9)1-gC(O)NR.lORll~
wherein
R9 is H or C1-4 alkyl, .
R10 and R11 are independently
hydrogen,
C3_7 cycloalkyl,
aryl,
heteroaryl,
heterocycloalkyl,
C1_4 alkyl unsubstituted or substituted with one or more of
hydroxy,
COOH,
-5-
CA 02277929 1999-07-14
WO 98/31670 PCT/U598/00875
amino,
aryl,
heteroaryl, or
heterocycloalkyl, or
R10 and R11 are joined to form a four to seven
membered cycloalkyl ring unsubstituted or
substituted with hydroxy, amino or aryl,
wherein Z is O, S or CH2;
R5 is
hydrogen,
halogen,
C1_4 alkyl,
C1_4 alkoxy,
CN, or
C02NH2~ and
R12 and R13 are independently
hydrogen,
C1_4 linear or branched alkyl or alkoxy,
Cg_7 cycloalkyl,
halogen, or
trifluoromethyl;
and pharmaceutically acceptable salts thereof.
The invention includes a composition for inhibiting loss of
blood platelets, inhibiting formation of blood platelet aggregates,
inhibiting formation of fibrin, inhibiting thrombus formation, and
inhibiting embolus formation in a mammal, comprising a compound of
the invention in a pharmaceutically acceptable carrier. These
compositions may optionally include anticoagulants, antiplatelet agents,
and thrombolytic agents. The compositions can be added to blood, blood
-6-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
products, or mammalian organs in order to effect the desired
inhibitions.
The invention also includes a composition for preventing or
treating atrial fibrillation, deep venous thrombosis, pulmonary
embolism, cardiac thromoembolism and associated stroke in patients
with atrial fibrillation, mechanical heart valves, or recent myocardial
infarction with decreased left ventricular function, disseminated
intravascular coagulation, ocular build up of fibrin, unstable angina,
refractory angina, transient ischemic attacks, thrombotic stroke,
embolic stroke, and reocclusion or restenosis of recanalized vessels, in a
mammal, comprising a compound of the invention in a
pharmaceutically acceptable carrier. These compositions may
optionally include anticoagulants, antiplatelet agents, and thrombolytic
agents.
The invention also includes a method for reducing the
thrombogenicity of a surface in a mammal by attaching to the surface,
either covalently or noncovalently, a compound of the invention.
_7_
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875 . x
DETAILED DESCRIPTION OF THE INVENTION
A class of these compounds is
3
R2~X ~ \ R O
W.N N~LNi'A
H 0 H
wherein
W is
hydrogen,
-C 1_4 alkyl,
-C3_7cycloalkyl,
-S02C1_7 alkyl, or
-(CH2)nCOOH, where n is 1-4;
R2 is
-C 1_7 alkyl
-(CH2)u-Cg-7cycloalkyl, wherein a is 0, 1, or 2,
-(CH2)u-phenyl, wherein phenyl is unsubstituted or substituted
with one or more of the moieties selected from the group
consisting of
C1_4 alkyl,
C 1-4 alkoxy,
halogen,
hydroxy,
COOH, or
CONH2
wherein a is 0, 1, or 2~
-2-thienyl, or
-3-thienyl;
X is
-S-, -S02-, or CH2;
_g_
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
R3 is
C 1-4 linear alkyl;
A is chosen from one of the following radicals:
Ri2 Ris Rs
\~:/~ N H2 \
I ~N °r I /
R4
IV
with the proviso that when A is radical IV, R2-X- is not C1_4 alkyl,
and pharmaceutically acceptable salts thereof.
A group of this class of compounds is
3
R2~X I \ R O
W.N N~Ni'A
H O' H
wherein
W is
hydrogen,
-S02CHg, or
-CH3
-CH2COOH;
R2 is
-CH3
. -(CH2)u-C3-6cYcloalkyl, wherein a is 0 or 1;
-(CH2)u-phenyl, wherein a is 0 or 1,
. -CH2C(CH3)3,
-CH(CH3)2,
-9-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
-phenyl-CH3,
-2-thienyl, or
-3-thienyl;
X is
R3 is
-S-, -S02-, or CH2;
-CH3~
A is chosen from one of the following radicals:
CI
\ NH2 \ NH2
1' ~ ~ ~ \
,N ,N or
'~ ~, ~ o
o~J~ N-a
IV H
with the proviso that when A is radical IV, R2-X- is not C1_g. alkyl,
and pharmaceutically acceptable salts thereof.
Specific embodiments of this group are
O
H2N N~ N ~ N
O H \
NH2
-10-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
\ O
/ S2 \ O
N
H2N O H \ ,N
NH2
02 H
~S I \ O O~N
i
H2N N~ N \ O
O H
/ CI
S \ O
I
H2N N~ N ~ N
o H ~ /
I O NH2 ,
\ S2 \
I
H2N N H ~ ~ N
O / NH2 ,
S ( \ O
H2N N v _ N ~ N
o H
. NH2 ,
-11-
CA 02277929 1999-07-14
WO 98/31670 PCTIUS98100875
~2
SH2N N H ~ N
O I ~ NH
2
02
H2N N H ~ N
O I ~ NH
02 2
O
H2N N v N ~ N
H
NH2
-12-
CA 02277929 1999-07-14
WO 98/31670 PGT/US98100875
'~-~S \ O
I I
H2N N~ N ~ N
H
NH2 ,
02
~S \
O
H2N N~ N ~ N
H
O NH2
2 '
\
I N
H2N ~ H
NH2 ,
~~02
S \ O
I
H2N N ~ N ~ N
O H I /
NH2 ,
02
~S \ O
I
H2N Nv _N ~N
I
O H I / NH
2
02
S \ O
I N
H2N O H I /
NH2
-13-
CA 02277929 1999-07-14
WO 98131670 PCT/US98/00875
~~2
O
HN N v N ~ N
O H I /
NH2
O2 H
\ O O~N~
i u
H2N N~N \ O
H
CI
\ O
I
H2N N v N ~ N
H ~ /
NH2
~2
\ O
I
H2N N v N ~ N
H ~ /
NH2
-14-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
~S \ O
H2N Nv _N ~N
O H I /
NH2
H
S I \ O O~N
H2N N~ N \ O
0 H I /
CI
\ O
'
HN Nv _N ~N
' ~ H
~S02 O / NH2 , and
O
HN Nv _N ~N
H
H02C ~ O / NH2
Compounds of the present invention, which are thrombin
inhibitors, are useful in anticoagulant therapy. Anticoagulant therapy
is indicated for the treatment and prevention of a variety of thrombotic
conditions, particularly coronary artery and cerebrovascular disease.
Those experienced in this field are readily aware of the circumstances
requiring anticoagulant therapy. The term "patient" used herein is
taken to mean mammals such as primates, including humans, sheep,
horses, cattle, pigs, dogs, cats, rats, and mice.
Thrombin inhibition is useful not only in the anticoagulant
therapy of individuals having thrombotic conditions, but is useful
whenever inhibition of blood coagulation is required such as to prevent
coagulation of stored whole blood and to prevent coagulation in other
biological samples for testing or storage. Thus, thrombin inhibitors can
-15-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
be added to or contacted with any medium containing or suspected of
containing thrombin and in which it is desired that blood coagulation be
inhibited, e.g. when contacting the mammal's blood with material
selected from the group consisting of vascular grafts, stems, orthopedic
prothesis, cardiac prosthesis, and extracorporeal circulation systems
The compounds of the invention can be administered in
such oral forms as tablets, capsules (each of which includes sustained
release or timed release formulations), pills, powders, granules, elixers,
tinctures, suspensions, syrups, and emulsions. Likewise, they may be
administered in intravenous (bolus or infusion), intraperitoneal,
subcutaneous, or intramuscular form, all using forms well known to
those of ordinary skill in the pharmaceutical arts. An effective but non-
toxic amount of the compound desired can be employed as an anti-
aggregation agent. For treating ocular build up of fibrin, the compounds
may be administered intraocularly or topically as well as orally or
parenterally.
The compounds can be administered in the form of a depot
injection or implant preparation which may be formulated in such a
manner as to permit a sustained release of the active ingredient. The
~0 active ingredient can be compressed into pellets or small cylinders and
implanted subcutaneously or intramuscularly as depot injections or
implants. Implants may employ inert materials such as biodegradable
polymers or synthetic silicones, for example, Silastic, silicone rubber or
other polymers manufactured by the Dow-Corning Corporation.
The compounds can also be administered in the form of
liposome delivery systems, such as small unilamellar vesicles, large
unilamellar vesicles and multilamellar vesicles. Liposomes can be
formed from a variety of phospholipids, such as cholesterol,
stearylamine or phosphatidylcholines.
The compounds may also be delivered by the use of
monoclonal antibodies as individual carriers to which the compound
molecules are coupled. The compounds may also be coupled with
soluble polymers as targetable drug carriers. Such polymers can
include polyvinlypyrrolidone, pyran copolymer, polyhydroxy-propyl-
methacrylamide-phenol, polyhydroxyethyl-aspartamide-phenol, or
-16-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
polyethyleneoxide-polylysine substituted with palmitoyl residues.
Furthermore, the compounds may be coupled to a class of biodegradable
polymers useful in achieving controlled release of a drug, for example,
polylactic acid, polyglycolic acid, copolymers of polylactic and
polygiycolic acid, polyepsilon caprolactone, polyhydroxy butyric acid,
polyorthoesters, polyacetals, polydihydropyrans, polycyanoacrylates and
cross linked or amphipathic block copolymers of hydrogels.
The dosage regimen utilizing the compounds is selected in
accordance with a variety of factors including type, species, age, weight,
sex and medical condition of the patient; the severity of the condition to
be treated; the route of administration; the renal and hepatic function of
the patient; and the particular compound or salt thereof employed. An
ordinarily skilled physician or veterinarian can readily determine and
prescribe the effective amount of the drug required to prevent, counter,
or arrest the progress of the condition.
Oral dosages of the compounds, when used for the indicated
effects, will range between about 0.001 mg per kg of body weight per day
(mg/kg/day) to about 100 mg/kg/day and preferably 0.01-100 mg/kg/day
and most preferably 0.1-20 mg/kg/day. Intravenously, the most
preferred doses will range from about 0.001 to about 10 mg/kg/minute
during a constant rate infusion. Advantageously, the thrombin
inhibitors may be administered in divided doses of two, three, or four
times daily. Furthermore, they can be administered in intranasal form
via topical use of suitable intranasal vehicles, or via transdermal routes)
using those forms of transdermal skin patches well known to those of
ordinary skill in that art. To be administered in the form of a
transdermal delivery system, the dosage administration will, or course,
be continuous rather than intermittent throughout the dosage regime.
For example, oral tablets can be prepared which contain an
amount of active compound of between 1 and 500 mg, e.g. 1, 10, 100, 200,
300, 400 or 500 mg. Typically, a patient in need of thrombin inhibitor
compound, depending on weight and metabolism of the patient, would be
administered between about 20 and 500 mg active compound per day.
For a patient requiring 500 mg per day, two tablets containing 125 mg of
active compound can be administered in the morning and two tablets
-17-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98l00875
containing 125 mg of active compound can again be administered in the
evening. For a patient requiring 200 mg per day, one tablet containing
100 mg of active compound can be administered in the morning and one
tablet containing 100 mg of active compound can again be administered
in the evening. For patient requiring 10 mg per day, one tablet
containing 5 mg of active compound can be administered in the morning
and one tablet containing 5 mg of active compound can again be
administered in the evening.
An i.v. formulation can be administered to patients
requiring parenteral administration, such as hospitalized patients who
are fasted (e.g. perioperative patients) and certain patients with
indwelling catheters. For example, an i.v. formulation having 100
mg/ml active ingredient, at pH 4, could be administered twice a day at a
dose of 0.5 ml/kg.
The compounds are typically administered as active
ingredients in admixture with suitable pharmaceutical diluents,
excipients or carriers (collectively referred to herein as "carrier"
materials) suitably selected with respect to the intended form of
administration, that is, oral tablets, capsules, elixers, syrups and the
like, and consistent with convention pharmaceutical practices.
For instance, for oral administration in the form of a tablet
or capsule, the active drug component can be combined with an oral,
non-toxic, pharmaceutically acceptable, inert carrier such as lactose,
starch, sucrose, glucose, methyl cellulose, magnesium stearate,
dicalcium phosphate, calcium sulfate, mannitol, sorbitol and the like;
for oral administration in liquid form, the oral drug components can be
combined with any oral, non-toxic, pharmaceutically acceptable inert
carrier such as ethanol, glycerol, water and the like. Moreover, when
desired or necessary, suitable binders, lubricants, disintegrating agents
and coloring agents can also be incorporated into the mixture. Suitable
binders include starch, gelatin, natural sugars such as glucose or beta-
lactose, corn-sweeteners, natural and synthetic gums such as acacia,
tragacanth or sodium alginate, carboxymethylcellulose, polyethylene
glycol, waxes and the like. Lubricants used in these dosage forms
include sodium oleate, sodium stearate, magnesium stearate, sodium
-18-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
benzoate, sodium acetate, sodium chloride and the like. Disintegrators
include, without limitation, starch methyl cellulose, agar, bentonite,
xanthan gum and the like.
The compounds can also be co-administered with suitable
anti-coagulation agents or thrombolytic agents such as plasminogen
activators or streptokinase to achieve synergistic effects in the treatment
of various ascular pathologies. For example, the compounds enhance
the efficiency of tissue plasminogen activator-mediated thrombolytic
reperfusion. The compounds may be administered first following
thrombus formation, and tissue plasminogen activator or other
plasminogen activator is administered thereafter. They may also be
combined with heparin, aspirin, or warfarin.
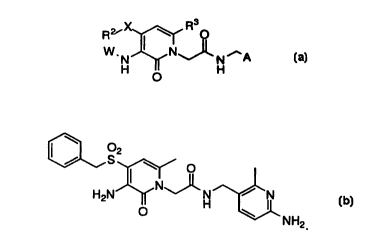
Compounds of the invention are shown in the table below.
These compounds inhibit thrombin with the following potency according
to ~ vitro measurements:
thrombin Ki (nM)
* >10
Structure ** < 10
\ O
/ S2 \ O
N
H2N O H \ ,N
NH2 **
H
S I \ O N
H2N N~N
O H
CI **
-19-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
0
HN Nv N ~N
~ S02 O H I ~ NH
2
In. vitro assay for determing nroteinase inhibition
Assays of human a-thrombin and human trypsin were
performed at 25°C in 0.05 M TRIS buffer pH 7.4, 0.15 M NaCl, 0.1% PEG.
Trypsin assays also contained 1 mM CaCl2.
In assays wherein rates of hydrolysis of a p-nitroanilide
(pna) substrate were determined, a Thermomax 96-well plate reader
was used to measure (at 405 nm) the time dependent appearance ofp
nitroaniline. sar-PR-pna (sarcosine-Pro-Arg-p-nitroanilide ) was used to
assay human a-thrombin (Km=125 ~.M) and human trypsin (Km=59
~.M). p-Nitroanilide substrate concentration was determined from
measurements of absorbance at 342 nm using an extinction coefficient of
8270 cm-1M-1.
In certain studies with potent inhibitors (Ki < 10 nM> where
the degree of inhibition of thrombin was high, a more sensitive activity
assay was employed. In this assay the rate of thrombin catalyzed
hydrolysis of the fluorogenic substrate ?,-GPIt-afc (Cbz-Gly-Pro-Arg-?-
amino-4-trifluoromethyl coumarin ~ ( Km=2 7 ~rlZ i w as determined from
the increase in fluorescence at 500 nm ~ excitation at 400 nm) associated
with production of 7-amino-4-trifluoromethy~1 coumarin. Concentrations
of stock solutions of Z-GPR-afc were determined from measurements of
absorbance at 380 nm of the 7-amino-4-trifluoromethyl coumarin
produced upon complete hydrolysis of an aliquot of the stock solution by
thrombin.
Activity assays were performed by diluting a stock solution
of substrate at least tenfold to a final concentration <_ 0.5 Km into a
solution containing enzyme or enzyme equilibrated with inhibitor.
Times required to achieve equilibration between enzyme and inhibitor
-20-
CA 02277929 1999-07-14
WO 98/31670 PCTIUS98/00875
were determined in control experiments. Initial velocities of product
formation in the absence (Vo) or presence of inhibitor (Vi) were
measured. Assuming competitive inhibition, and that unity is
negligible compared Km/[S], [I]/e, and [I]/e (where [S], [I], and a
respectively represent the total concentrations, of substrate, inhibitor
and enzyme), the equilibrium constant (Ki) for dissociation of the
inhibitor from the enzyme can be obtained from the dependence of Vo/Vi
on [I] shown in equation 1.
V o/Vi = 1 + [I]/Ki ( 1 )
The activities shown by this assay indicate that the
compounds of the invention are therapeutically useful for treating
various conditions in patients suffering from unstable angina,
refractory angina, myocardial infarction, transient ischemic attacks,
atrial fibrillation, thrombotic stroke, embolic stroke, deep vein
thrombosis, disseminated intravascular coagulation, and reocclusion or
restenosis of recanalized vessels.
Some abbreviations that may appear in this application are
as follows.
Designation
BOC (Boc) t-butyloxycarbonyl
HBT(HOBT or HOBt) 1-hydroxybenzotriazole hydrate
BBC reagent benzotriazolyloxy-bis(pyrrolidino)-
carbonium hexafluorophosphate
PyCIU 1,1,3,3-bis(tetramethylene)-
chlorouronium hexafluorophosphate
EDC 1-ethyl-3-( 3-dimethylaminopropyl
)
carbodiimide hydrochloride
(BOC)20 di-t-butyl dicarbonate
DMF dimethylformamide
Et3N or TEA triethylamine
EtOAc ethyl acetate
TFA trifluoroacetic acid
-21-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
DMAP dimethylaminopyridine
DME dimethoxyethane
BH3-THF Borane-tetrahydrofuran complex
D-Phe(3,4-C12) D-3,4-Dichlorophenylalanine
D-3,3-dicha D-3,3-Dicyclohexylalanine
Pro Proline
Ar g Arginine
Gly Glycine
D-3,3,-diphe D-3,3-Diphenylalanine
LAH lithium aluminum hydroxide
Cy cyclohexyl
POC13 phosphorous oxychloride
MeCN acetonitrile
BnEt3N+Cl- benzyl triethyl ammonium chloride
NaH sodium hydride
DMF dimethylformamide
BrCH2COOtBu tert butyl bromoacetate
EtOH ethyl alcohol
Pd(C) palladium on activated carbon catalyst
CF3COOH trifluoroacetic acid
DCM dichloromethane
DIPEA diisopropylethylamine
The compounds of the present invention may have chiral
centers and occur as racemates, racemic mixtures and as individual
diastereomers, or enantiomers with all isomeric forms being included
in the present invention.
The term "alkyl" means straight or branched alkane
containing 1 to about 10 carbon atoms, e.g., methyl, ethyl, n-propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, pentyl, iso-amyl, hexy,
octyl radicals and the like. The term "alkenyl" means straight or
branched alkene containing 2 to about 10 carbon atoms, e.g., propylenyl,
buten-1-yl, isobutenyl, pentenylen-1-yl, 2,2-methylbuten-1-yl, 3-
methylbuten-1-yl, hexen-1-yl, hepten-1-yl, and octen-1-yl radicals and the
Iike. The term "alkynyl" means straight or branched alkyne containing
-22-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
2 to about 10 carbon atoms, e.g., ethynyl, propynyl, butyn-1-yl, butyn-2-yl,
pentyn-1-yl, pentyn-2-yl, 3-methylbutyn-1-yl, hexyn-1-yl, hexyn-2-yl,
hexyn-3-yl, 3,3-dimethylbutyn-1-yl radicals and the like. Cycloalkyl
means a cyclic, saturated ring containing 3 to 8 carbon atoms, e.g.,
cyclopropyl, cyclohexyl, etc. Halogen means chloro, bromo, fluoro or
iodo. The term "aryl" means a 5- or 6-membered aromatic ring
containing 0, 1, or 2 heteroatoms selected from O, N, and S, e.g. phenyl,
pyridine, pyrimidine, imidazole, thiophene, oxazole, isoxazole, thiazole,
and amino- and halogen- substituted derivatives thereof.
The pharmaceutically-acceptable salts of the compounds of
the invention (in the form of water- or oil-soluble or dispersible products)
include the conventional non-toxic salts or the quaternary ammonium
salts which are formed, e.g., from inorganic or organic acids or bases.
Examples of such acid addition salts include acetate, adipate, alginate,
aspartate, benzoate, benzenesulfonate, bisulfate) butyrate, citrate,
camphorate, camphorsulfonate, cyclopentanepropionate, digluconate,
dodecylsulfate, ethanesulfonate, fumarate, glucoheptanoate,
glycerophosphate, hemisulfate, heptanoate, hexanoate, hydrochloride,
hydrobromide, hydroiodide, 2-hydroxyethanesulfonate, lactate, maleate,
2U methanesulfonate, 2-naphthalenesulfonate, nicotinate, oxalate,
pamoate, pectinate, persulfate, 3-phenylpropionate, picrate, pivalate,
propionate, succinate, tartrate, thiocyanate, tosylate, and undecanoate.
Base salts include ammonium salts, alkali metal salts such as sodium
and potassium salts, alkaline earth metal salts such as calcium and
magnesium salts, salts with organic bases such as dicyclohexylamine
salts, N-methyl-D-glucamine, and salts with amino acids such as
arginine, lysine, and so forth. Also, the basic nitrogen-containing
groups may be quaternized with such agents as lower alkyl halides,
such as methyl, ethyl, propyl, and butyl chloride, bromides and iodides;
dialkyl sulfates like dimethyl, diethyl, dibutyl and diamyl sulfates, long
chain halides such as decyl, lauryl, myristyl and stearyl chlorides,
bromides and iodides, aralkyl halides like benzyl and phenethyl
bromides and others.
Unless otherwise stated, all NMR determinations were
made using 400 MHz field strength.
-23-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
The compounds of the present invention, where X is S(O)r,
may be prepared using METHODS 1-5:
METHOD 1 (as exemplified by Example II)
Starting 2,4-dihydroxy-3-nitropyridine is reacted with a
dehydrating chloride source, for example phosphorous oxychloride, in
Step A to give the 4-chloropyridine. This is reacted in Step B with a thiol
in the presence of a base such as triethylamine, and is then alkylated in
Step C with an acetate equivalent such as t-butylbromoacetate. The ester
may be deprotected by a strong acid such as TFA in Step D and the
resulting carboxylic acid coupled in Step E with the appropriate amine,
in this case cyclopropyl-(2-aminomethyl-4-chlorophenoxy)-acetamide (a
method for the preparation of this class of amines is shown below) and
the nitro group reduced in Step F by a reducing agent such as stannous
chloride to give the final product.
OH CI
02N I ~ OZN
HO N~ Step A HO N~ Step B
Ph, S
Ph~s 02N . /
02N ~ .-
O ~ Ste D
Step C p
HO N
O
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875 . -
Ph~S
02N ~ CI
Ph~S I
O N' \ /
02N / H I
N \
O N Step E O O
OH N
H
O
Step F
Ph~S
H2N / CI
O N' \ /
H
N \ O
O O~ N
H
Method for making et ~vl-(2-aminomethv~1-4-chlorophenoxv)-acetamide
4-Chlorosalicaldehyde is condensed with hydroxylamine
hydrochloride in ethanolic aqueous sodium carbonate solution in Step A.
The oxime is reduced by hydrogenation over a catalyst such as rhodium
and the amine is protected as its BOC derivative under standard
conditions. The phenol is alkylated in Step D with an acetate equivalent
such as ethyl bromoacetate and the resulting ester is hydrolysed with
lithium hydroxide. The product carboxylic acid is coupled to an amine
such as ethylamine or cyclopropylamine in Step F, and the BOC group is
removed by strong acid such as TFA in Step G.
-25-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98I00875 _ y
CI CI
\ NHzOH.HCI \ H2, Rh/C
Na2C03, EtOH/H20 I EtOH, H2S04
OHC ~ Step A I ~ Step B
OH HO.N OH
Cf CI
(BOC)2O
\ NMM, DMF I \ BrCH2C02Et Step D
Cs2C03, DMF
Step C
NH2 OH BOCNH OH
CI CI
\ LiOH I \
S~ tep E
BOCNH O~C02H BOCNH O~C02Et
Step F EDC) EtNH2.HCl
HOBT, NMM, DMF
CI CI
\ TFA, CH 2C12 . I \
Step G
BOCNH O~CONHEt NH2 O~CONHEt
Modifications of this method will allow different R4 and R5
groups contemplated by the scope of the broad claim below to be present
by the use of an appropriate reagent or appropriately substituted starting
material in the indicated synthetic step. For example, appropriate
choice of the amine in Step F will allow different values of Rl~ and R11 to
-26-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
be achieved. Obvious variations and modifications of the method to
produce similar and obvious variants thereof, will be apparent to one
skilled in the art.
Ste~A,: ~ 4-Chlorosalicaldehvde Oxime
A solution of hydroxylamine hydrochloride (16.7 g, 0.24 mol)
and sodium carbonate (12.7 g, 0.12 mol) in water (120 ml) was added to a
stirred solution of 4-chlorosalicaldehyde (25.0 g, 0.16 mol) in ethanol ( 160
ml) and the resulting solution was heated to reflux. After 1 h the
reaction was cooled, water (320 ml) was added and the resulting
crystalline precipitate was isolated by filtration. A second crop was
similarly collected and the combined solids were dried to give the title
compound:
1H NMR, (CDC13) d 6.92 (d, J=8.8Hz, 1 H), 7.15 (d, J=2.6Hz, 1H), 7.23 (dd,
J=2.6 and 8.8 Hz, 1H), 7.26 (s, 1H), 8.16 (s, 1H), 9.71 (s, 1H).
Step B: 2-Hvdrox~r-5-Chlorobenzvlamine
A mixture of 4-chlorosalicaldehyde oxime ( 10 g, 58.3 mmol >
and 5% Rh/C (2.0 g) in ethanol ( 100 ml ) containing concentrated sulfuric
acid ( 10 ml ) was shaken in a Parr apparatus under H2 ( 60 psi ) for 24 h.
Water (100 ml) was added and the mixture was filtered through celite.
The filtrate was concentrated until the product had crystallized out of
solution. The solid was collected by filtration and the filtrate was further
concentrated, adding water to give a second crop which was combined
with the first to give after drying the title compound:
1H NMR (CD30D) d 4.07 (s, 2 H), 6.88 (d, J=8.6Hz) 1 H)) 7.25 (dd, J=2.6
and 8.6Hz, 1H)) ?.31 (d, J=2.6Hz, 1 H).
-27-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875 . -
Step C: N-t-Butoxvcarbonvl-2-Hvdroxv-5-Chlorobenzvlamine
A mixture of 2-hydroxy-5-chlorobenzylamine ( 1.22 g, 4.77
mmol assuming the bisulfate salt), (BOC)20 ( 1.56 g, 7.16 mmol) and N-
methylmorpholine (1.05 ml, 9.54 mmol) in DMF (10 ml) was stirred for 5
h at r.t. The reaction was partitioned between water and ethyl acetate
and the organic layer was washed with 5% KHS04 solution (2 times),
sodium hydrogen carbonate solution and brine, dried (Na2S04) and
evaporated in uacuo to a solid. The crude product was recrystallized
from ethyl acetate/hexanes (1:5, 12 ml) to give the title compound:
1H NMR (CDC13) d 1.44 (s, 9 H, t-Bu), 4.17 (d, J=6.8Hz, 2H, CH2), 5.22 (br
t, 1H, NH), 6.87 (d, J=8.6Hz, 1H, H-3), 7.03 (d, J = 2.6Hz, 1H, H-6), 7.15
(dd, J=2.6 and 8.6 Hz, 1H, H-4).
to D: Ethyl-(2-t-Butoxycarbonylaminomethyl-4-Chlorophen-
oxv)-Acetate
A mixture of N-t-butoxycarbonyl-2-hydroxy-5-chloro-
benzylamine (730 mg, 2.83 mmol), Cs2C03 (923 mg, 2.83 mmol) and
ethylbromoacetate (0.314 ml, 2.83 mmol) in DMF (5 ml) was stirred for 2
h. The crude reaction mixture was partitioned between ethyl acetate
and water and the organic layer was washed with brine, dried (Na2S04 >
and evaporated i.n uacuo to an oil which was used for the next step.
Step E: 2-t-Butoxycarbonylaminomethyl-4-Chlorophenoxyacetic
Acid
The product from Step D was suspended in 1:1:1
methanol/THF/ water (9 ml) and lithium hydroxide hydrate (126 mg, 3.0
mmol) was added. After 16 h the volatiles were removed in uaccuo and
the solution was diluted with water and was washed with ethyl acetate,
adding sufficient brine to disperse the emulsion. The aqueous layer was
acidified with 5°l° KHS04 solution and was extracted with
methylene
chloride which was then dried (Na2S04) and evaporated in uacuo to give
the title compound as a solid:
1H NMR (CDC13) d 1.44 (s, 9H, t-Bu), 4.35 (br s, 2H, NCH2), 4.62 (s, 2H,
OCH2), 5.04 (br s, 1H, NH), 6.74 (d, J=7.9Hz, 1H, H-3), 7.20 (d, J=2.6Hz,
1H, H-6), 7.24 (d obscured, 1H, H-4).
-28-
CA 02277929 1999-07-14
WO 98131670 PCT/US98/00875
Steg F: Ethyl-(2-t-Butoxycarbonylaminomethyl-4-Chlorophen-
oxy)-Acetamide
EDC Hydrochloride (249 mg, 1.3 mmol) was added to a
stirred mixture of 2-t-butoxycarbonylaminomethyl-4-chloro-
phenoxyacetic acid (316 mg, 1.0 mmol), HOBT (176 mg, 1.3 mmol),
ethylamine hydrochloride (106 mg, 1.3 mmol) and N-methylmorpholine
(0.396 ml, 3.6 mmol) in DMF (4 ml) and the mixture was stirred for 16 h.
The reaction was partitioned between ethylacetate and 5% KHS04
solution and the organic layer was washed with 5% KHS04 solution,
water, NaHC03 solution and brine, dried (Na2S04) and evaporated in.
u~cuo to a solid (333 mg) which was used for the next step.
S~,g~: Ethyl-(2-Aminomgthyl-4-Chlorophenoxv)-Acetamide
Ethyl-(2-t-butoxycarbonylaminomethyl-4-chlorophenoxy >
acetamide from Step F was dissolved in 2:1 methylene chloride/TFA (3
ml) and after 15 min the solvent was evaporated in vacuo. The residue
was dissolved in water and the solution was washed with methylene
chloride (twice). The aqueous layer was then basified with saturated
sodium carbonate solution and NaCl was added to saturation. The
mixture was extracted with ethyl acetate, and the organic layer was
dried (Na2S04) and evaporated in vacuo to give the title compound as a
crystalline solid:
1H NMR (300 MHz, CDC13) d 1.12 (t, J=7.3 Hz, 3H, Me), 1.54 (s, 9H, t-Bu),
3.31 (quintet, J=7.3 Hz, 2H, CH2Me), 3.90 (s, 2H, NCH2), 4.58 (s, 2H,
OCH2), 6.80 (d, J=8.3 Hz, 1H, H-3), 7.19-7.23 (m, 2H, H-4, H-6)) 8.01 (br s,
1H, CONH).
-29-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
METHOD 2 (AS EXEMP~.IFIED BY EXAMPLE III)
The product of METHOD 1, Step A is alkylated with an
acetate equivalent such as ethyl bromoacetate in Step A which is then
reacted with the thiol in Step B. The ester is then hydrolysed in Step C
with lithium hydroxide and the resulting carboxylic acid is coupled with
the appropriate amine, in this case 5-aminomethyl-2-t-
butoxycarbonylamino-6-methylpyridine (a method for the preparation of
this amine is shown below) in Step D. The vitro group is reduced in Step
E with hydrogen in the presence of a catalyst such as palladium on
carbon and the BOC group removed by a strong acid such as HCl in Step
F to give the final product.
CI
CI 02N
O2N
Step A O N OEt Step B
HO N
O
S
02N / S
- 02N
O N Step C ~'
O N Step D
O Et
OH
O
O
-30-
CA 02277929 1999-07-14
WO 98131670 PCT/US98/00875
S
02N
~ NHBOC
O N' \
H
N \ N
O
Step E
S
H2N
NHBOC
O N H
N \ N
O
Step F
S
H2N /
~ NH2
O N'
f-I
N ~ N
O
Modifications of METHODS 1 and 2 will allow different W,
R2, R3 and A groups contemplated by the scope of the broad claim below
to be present by the use of an appropriate reagent or appropriately
-31-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
substituted starting material in the indicated synthetic step. For
example the starting pyridine in Step A can have as its side chain at the
6-position, ethyl, isopropyl, cyclopropyl, trifluoromethyl, and the like, to
achieve the different operable values of R3. Similarly the thiol in Step B
can have as its R2 group, benzyl, cyclopropylmethyl, and the like, to
achieve the different operable values of R2. An appropriate choice of the
amine in the coupling step will allow the different operable values of A to
be achieved. Different W groups may be introduced by such methods as
alkylation, acyiation or sulfonylation of the products of METHOD 1, Step
F and METHOD 2, Step E. Obvious variations and modifications of the
method to produce similar and obvious variants thereof, will be apparent
to one skilled in the art.
Method for making 5-aminomethvl-2-t-butoxvcarbonvlamino-6-
methvlpvridine
Preparation of 2-Amino-5-c3rano-6-methvlpvridine
A mixture of 2-amino-5-bromo-6-methylpyridine (20.0 g,
0.107 mol) (Maybridge) and copper (I) cyanide 11.0 g, 0.123 mol) in DMF
(25 ml) was heated to reflux for 4 h. The DMF was evaporated in. vacuo
and the residue was partitioned between ethyl acetate and 10°l~ sodium
cyanide solution. The organic layer w as washed w ith 10% sodium
cyanide solution and brine, dried (Na2SU4~ and evaporated in vacuo to a
brown solid. This was dissolved in a minimum amount of ethyl acetate
and the product was precipitated by adding hexanes. The mixture was
filtered to give the title compound as a brown powder:
1HNMR(CDC13)d2.56(s,3H),4.97(brs,2H),6.33(d,J=8.6 Hz, 1H),7.54
(d,J=8.6 Hz, 1H).
Preparation of 2-t-Butoxvcarbonvlamino-5-cvano-6-methvlpvridine
A mixture of 2-amino-5-cyano-6-methylpyridine ( 10.0 g, 75.1
mmol), (BOC)20 (16.39 g, 75.1 mmol), triethylamine (11.5 ml, 82.6 mmol)
and DMAP (0.92 g, 7.5 mmol) in methylene chloride (200 ml ) was stirred
for 3 h. More triethylamine (4.22 ml) and (BOC2)O (1.64 g) were added
and after 16 h the reaction was diluted with ethyl acetate and was
-32-
CA 02277929 1999-07-14
. WO 98/31670 PCT/US98/00875
washed with 1 M AcOH (3 times), dried (Na2S04) and evaporated in
uacuo to give dark brown solid. The crude product was purified by flash
column chromatography {10% ethylacetate/hexanes) to give the title
compound as a white solid:
1H NMR (CDCl3) d 1.52 (s, 9 H), 2.62 (s, 3 H), 7.46 (br s, 1 H), 7.80 (d, J =
8.8
Hz, 1 H), ?.88 (d, J = 8.8 Hz, 1 H).
Preparation of 5-aminomethyl-2-t-butoxycarbonylamino-6-
methvlnvridine
A mixture of 2-t-butoxycarbonylamino-5-cyano-6-
methylpyridine (14.68 g, 62.9 mmol) and 10°h Pd/C ( 1.5 g) in glacial
acetic
acid (150 ml) was shaken on a Parr apparatus at 60 psi for 88 h. The
reaction was filtered through celite and was evaporated in uacuo. The
residue was dissolved in water and the solution was washed with
methylene chloride (2 times), then was basified with sodium carbonate
and extracted with ethyl acetate (2 times). The combined ethyl acetate
layers were dried (Na2S04) and evaporated in uacuo to a solid. The
crude product was recrystallized (ethyl acetate/hexanes) to give the title
compound:
2D 1 H NMR ( CDC13 ) d 1.50 ( s, 9 H ), 2.43 ( s, 3 H ) ) 3.8 l ( s, 2 H ),
7.23 (br s, 1 H ),
7. 57 (d, J = 8.3 Hz, 1 H), ?.70 (d, J = 8.3 Hz, 1 H ).
Modifications of this method will allow different R12 and R13
groups contemplated by the scope of the broad claim below to be present by
Zr~ the use of an appropriate reagent or appropriately substituted starting
material in the indicated synthetic step. .Obvious variations and
modifications of the method to produce similar and obvious variants
thereof, will be apparent to one skilled in the art.
30 METHOD 3 (as exemplified by Example X)
The product of METHOD 2, Step B, in this case where R2 is
benzyl, is reduced in Step A with a reducing agent such as iron in acetic
acid to give the amine. The ester is then hydrolysed and coupled with
35 the appropriate amine, in this case 2-amino-5-aminomethyl-6-
-33-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
methylpyridine (a method for the preparation of this amine is shown
below) in Step B to give the final product.
Ph~S Ph~S
O2N / H2N
O N' \ ~ O N'
Step A
OEt OEt
O O
Step B
Ph~S
H2N /
~ NH2
O N' \ /
I I
N ~ N
O
Modifications of this Method will allow different W, R2, R3
and A groups contemplated by the scope of the broad claim below to be
present by the use of an appropriate reagent or appropriately substituted
starting material in the indicated synthetic step. For example the
starting pyridinone in Step A can have as its side chain at the 6-position,
ethyl, isopropyl, cyclopropyl, trifluoromethyl, and the like, to achieve the
different operable values of R3. Similarly it can have as its R2 group,
phenyl, cyclopropylmethyl, and the like, to achieve the different operable
values of R2. An appropriate choice of the amine in Step C will allow the
different operable values of A to be achieved. Different W groups may be
CA 02277929 1999-07-14
WO 98/31670 PCT/US98100875 _ -
introduced by such methods as alkylation, acylation or sulfonylation of
the product of Step A. Obvious variations and modifications of the
method to produce similar and obvious variants thereof, will be apparent
to one skilled in the art.
Preparation of 2-Amino-5-methylamino-6-methylpyridine
dihvdrochloride
A mixture of 2-amino-5-cyano-6-methyipyridine (4.0 g, 30.0
mmol) and 10°/~ Pd/C (3.08 g) in ethanol (80 mL), methanol (30 mL),
concentrated HCl (6 mL) and water ( 10 mL) was shaken on a Parr
apparatus at 60 psi for 25 h. The reaction was filtered through celite,
rinsing with 1:1 ethanol/methanol and was evaporated in uacuo to a
solid, which was triturated with 5:1 ethyl acetate/ethanol to give the title
compound (5.95 g, 94%):
1H NMR (CD30D ): d 2.58 ( s, 3 H ), 4.12 ( s, 2 H ), 6.92 ( d, J = 9.2 Hz, 1 H
), 7.93
(d,J=9.2 Hz, 1H).
Modifications of this method will allow different R12 and
R13 groups contemplated by the scope of the broad claim below to be
present by the use of an appropriate reagent or appropriately substituted
starting material in the indicated synthetic step. Obvious variations and
modifications of the method to produce similar and obvious variants
thereof, will be apparent to one skilled in the art.
METHOD 4 (as exemplified by Example IV)
The product of METHOD 2, Step A is reacted with a
sulfinate salt in this case were R2 is cyclopropylmethyl in a solvent such
as ethanol or DMF in Step A to give the sulfone. The nitro group may be
reduced in Step B with hydrogen in the presence of a catalyst such as
palladium on carbon, the ester hydrolysed in Step C and the resulting
carboxylic acid coupled to with the appropriate amine, in this case 2-
amino-5-aminomethyl-6-methylpyridine, in Step D to give the final
product.
-35-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
C1 S02
02N / 02N /
O N'
Step A O N Step B
OEt OEt
O O
S02 S02
H2N / H2N /
N'
O Step C O N
OEt OH
O O
Step D
-36-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
SO2
H2N /
NH2
O H
N ~ N
O
Sulfinate salts used in the preparation of compound of the
invention may be generated from the sulfinic acids. They may also be
generated by a number of other methods, including the reductive
cleavage of 2-sulfonylbenzothiazoles, the reaction of organometallics
with sulfur dioxide, the reduction of sulfonyl chlorides, the oxidation of
thiols and the cleavage of phthalimidomethyl sulfones.
Modifications of this method will allow different W, R2, R3
and A groups contemplated by the scope of the broad claim below to be
present by the use of an appropriate reagent or appropriately substituted
starting material in the indicated synthetic step. For example the
starting pyridinone in Step A can have as its side chain at the 6-position,
ethyl, isopropyl, cyclopropyl, trifluoromethyl) and the like, to achieve the
different operable values of R~~. Similarly the sulfinate in Step A can
have as its R2 group, benzyl) cyclopentyl, and the like) to achieve the
different operable values of R2. An appropriats~ choice of the amine in
Step D will allow the different operable values of A to be achieved.
Different W groups may be introduced by such methods as alkylation)
acylation or sulfonylation of the product of Step B. Obvious variations
and modifications of the method to produce similar and obvious variants
thereof, will be apparent to one skilled in the art.
-37-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
METHOD 5 (as exemplified by Example VIIb)
The product of METHOD 2, Step B, in this case where R2 is
benzyl) may be oxidized with a suitable oxidizing agent such as
OXONE~ or a peracid such as mCPBA to give the sulfone which may be
further manipulated, for example by the procedures of METHOD 4, Steps
B-D, to give the products of the invention.
Ph~S Phi S02
02N / 02N
N' \ O N'
O
O~ O~
O O
The compounds of the present invention, where X is
CR15R16, may be prepared using METHODS 6 and 7:
METHOD 6 (as exemplified by Example XI)
The starting 2-hydroxy-3-nitropyridine is alkylated with an
acetate equivalent such as t-butyl bromoacetate in Step A to give the
pyridinone. This is reacted with an organomagnesium reagent, in this
case cyclohexylmethyl magnesium bromide, in Step B. The resulting
lactam is oxidised in Step C with an oxidising agent such as DDf~I or
nickel peroxide to regenerate the pyridinone. The t-butyl group is
removed using a strong acid such as HCl in Step D and the resulting
carboxylic acid coupled with the appropriate amine, in this case 2-BOC-
amino-5-aminomethyl-6-methylpyridine in Step E. The nitro group is
reduced in Step F by hydrogenation using a catalyst such as palladium
on carbon and the the BOC group is removed in Step G with a strong acid
such as TFA to give the final product.
-38-
CA 02277929 1999-07-14
. WO 9$/31670 PCT/US98/00875 _ "
02N
02N / Step A ~ Step B
O N
HO \N O
O
Step C
Step D
02N
O~N~~
O
O
O
O
Step E
H
O
-39-
CA 02277929 1999-07-14
. WO 98131670 PCT/US98I00875 _ -
02N
N~ / NHBOC
H II
N \ N
O '
Step F
NHBOC
H I I
N \ N
O
~2
-40-
Step G
CA 02277929 1999-07-14
WO 98/31670 PCT/US9$100875 _ -
METHOD 7 (as exemplified by Example I)
The starting acid is esterified in Step A and is alkylated
with t-butyl bromoacetate in Step B. The resulting pyridinone is reacted
with an organomagnesium reagent, in this case benzyl magnesium
chloride, in Step C and the lactam is oxidised in Step D with an oxidising
agent such as DDQ or nickel peroxide to regenerate the pyridinone. The
t-butyl group is removed using a strong acid such as TFA in Step E and
the resulting carboxylic acid coupled with the appropriate amine, in this
case 2-BOC-amino-5-aminomethyl-6-methylpyridine in Step F. The ethyl
ester is hydrolysed with lithium hydroxide in Step G and the acid was
rearranged to the isocyanate via the acyl azide using DPPA and a base
such as triethylamine followed by heating in Step H. The intermediate
isocyanate is then reacted with benzyl alcohol in the presence of a base to
1<~~ give the CBZ derivative. The CBZ group was removed by hydrogenolysis
in Step I using a catalyst such as palladium and the amine is then
sulfonylated in Step J, in this case with methane sulfonyl chloride, in
the presence of a base such as pyridine. The BOC group is removed in
Step K with a strong acid such as TFA to give the final product.
-41-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
H02C / Step A Et02C / I Step B
HO \N' \ HO \N'
Ph
Et02C , ( Step C Et02C Ste D
I p
O N O N
O O
O
O
Ph Ph
Et02C / Step E Et02C , Step F
~I
O N'
O N
O OH
O O
-42-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
Ph
Et02C
~ NHBOC
O N'
H II
N \ N
Step G
O
Ph
H02C
~ NHBOC
O N'
H II
N ~ N Step H
O
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
Ph
CBZNH
~ NHBOC
O N' \
I I
Step I
O
Ph
H2N
~ NHBOC
O N-
H II
N ~ N Step J
O
Ph
MeS02NH
~ NHBOC
O N'
H II
N ~ N Step K
O '
Ph
MeS02NH
NH2
O N H
N ~ N
O
Modifications of METHODS 6 and 7 will allow different W,
R2, R3 and A groups contemplated by the scope of the broad claim below
to be present by the use of an appropriate reagent or appropriately
CA 02277929 1999-07-14
, WO 98/31670 PCT/US98/00875 _ -
substituted starting material in the indicated synthetic step. For
example the starting pyridine can have as its side chain at the 6-
position, ethyl, isopropyl, cyclopropyl, trifluoromethyl, and the like, to
achieve the different operable values of R3. Different operable values of
R2.may be acheived by the appropriate choice of the organometallic
reagent. An appropriate choice of the amine in the coupling step will
allow the different operable values of A to be achieved. Different W
groups may be introduced by such methods as alkylation, acylation or
sulfonylation of the products from METHOD 6, Step F, and METHOD 7,
Step I. Obvious variations and modifications of the method to produce
similar and obvious variants thereof, will be apparent to one skilled in
the art.
Amide couplings to form the compounds of this invention
can be performed by the carbodiimide method with reagents such as
dicyclohexylcarbodiimide, or 1-ethyl-3-(3-dimethyl-aminopropyl )
carbodiimide. Other methods of forming the amide or peptide bond
include, but are not limited to the synthetic routes via an acid chloride,
azide, mixed anhydride or activated ester. Typically, solution phase
amide couplings are performed, but solid-phase synthesis by classical
Merrifield techniques may be employed instead. The addition and
removal of one or more protecting groups is also twpical practice.
The following examples are illustrative of the invention as
contemplated by the inventors and should not be construed as being
'~ limits on the scope or spirit of the instant invention.
-45-
CA 02277929 1999-07-14
WO 98/31670 PCT/LTS98/00875 _ 4
EXAMPLE I
Preparation of 3-Methanesulfonylamino-4-benzyl-6-methyl-1-(2-amino-
6-methyl-5-meth3rlenecarboxamidomethvlpy~~ridinvl)-2-wridinone
NH2
Ph I ~ N
O I N _
O
_S_N
O H O H
I-11
St, ep A: Ethyl 6-methvlpvridinflHl-2-one-3-carboxvlate (I-1)
To a stirred slurry of 7.66 g (50 mmol) of 2-hydroxy-6-
methylpyridine-3-carboxylic acid, 3.52 mL (60 mmol) of anhydrous
ethanol, and 6I1 mg (5 mmol) of 4-(N,N-dimethylamino)pyridine
(DMAP) in 100 mL of CH2C12 was added 10.32 g (50 mmol) of
dicyclohexylcarbodiimide (DCC) and the mixture stirred overnight at
ambient temperature. The mixture was filtered and the precipitate was
washed with minimal CH2C12. The filtrate was concentrated and
crystallized from 100 mL of ethyl acetate to give I-1 as a colorless solid:
1H NMR (CDC13) c7 8.15 (d, 1H, 7.5 Hz), 6.3 (br s, 1H), 4.37 (q, 2H, 7.1 Hz),
2.46 (s, 3H), 1.38 (t, 3H, 7.1 Hz).
Step B: Ethyl 6-methyl-1-(t-butyl acetate)pyridin[1H]-2-one-3-
carboxvlate (I-2)
To a 0°C stirred suspension of 3.5 g (19.3 mmol) of ethyl 6-
methylpyridin-2-one-3-carboxylate in 50 mL anhydrous THF under Ar
was added 0.8 g (20 mmol) of NaH (60% in oil) in portions over a 2 min
period. After the mixture became homogeneous over 10 min, 3.55 mL
(22 mmol) of t-butyl bromoacetate was added dropwise. The cold bath
was removed and the reaction mixture stirred under Ar overnight.
-46-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98100875 ,
Concentration of the reaction mixture at reduced pressure gave a
residue that was partitioned between CHC13 and 1M citric acid. The aq.
layer was extracted with CHC13, and the combined organic layers
washed with 10% Na2C03, dried over Na2S04 and the solvents removed
to give an oil that was chromatographed on 75 g of Si02 using 98.5:1.5 to
95:5 CHCl3-CH30H which gave ~ as a colorless solid: 1H NMR
(CDCl3) 7 8.1 (d, 1, 7.3 Hz), 6.13 (d, 1H, 7.3 Hz), 4.78 (s, 2H), 4.35 (q, 2H,
7.1 Hz), 2.34 (s, 3H), 1.48 (s, 9H), 1.37 (t, 3H, 7.1 Hz).
Ste~C: Ethyl4-benzyl-3,4-dihydro-6-methyl-1-(t-butyl
acetate)R3iridinflHl-2-one-3-carboxvlate (I-3>
To a stirred -78° solution of 1.0 g (3.39 mmol) of ethyl 6-me-
thyl-1-(t-butyl acetate )pyridin-2-one-3-carboxylate in 20 mL of anhydrous
THF was added a 2M THF solution of benzylmagnesium chloride
dropwise. After stirring for 15 min at -78, the reaction was stored in the
freezer (approx. -15 °C) overnight. After quenching with 200 ~tL (3.5
mmol ) of acetic acid, the reaction was concentrated at reduced pressure,
diluted with cold 5% HCl and extracted with EtOAc. The organic layer
was washed with sat. NaHC03) brine, dried over Na2S04 and
concentrated at reduced pressure to give an oil that was
chromatographed on 50 g Si02 using 11:29 EtOAc-hexane.
Concentration of the fractions at reduced pressure provided ~ as a
colorless oil: 1H NMR (CDC13) 7 7.35-7.15 (m, 5H), 4.94 (d, 1H, 4.4 Hz),
4.54 (d, 1H) 17.5 Hz), 4.3-4.1 (m, 2H), 4.08 (d, 1H, 17.6 Hz), 3.35 (d, 1H,
7.3
Hz), 3.15-3.05 (m, 1H), 2.8-2.65 (m, 2H), 1.83 (t, 3H, 1.3 Hz)) 1.47 (s, 9H),
1.25 (t, 3H, 7.1 Hz).
Step D: Ethyl 4-benzyl-6-methyl-1-(t-butyl acetate)pyridin[ 1H]-2-
one-3-carboxvlate (I-4)
To a stirred solution of 690 mg (1.78 mmol) of ethyl 4-benzyl-
3,4-dihydro-6-methyl-1-(t-butyl acetate)pyridin[1H]-2-one-3-carboxylate in
7 mL of dioxane was added 445 mg ( 1.95 mmol) of 2,5-dichloro-3,6-
-4?-
CA 02277929 1999-07-14
WO 98131670 PCTlUS98/00875
dicyano-1,4-benzoquinone (DDIa). After heating at 70°C for 24 h under
Ar, the cooled reaction mixture was diluted with 21 mL of benzene and
the precipitate removed by filtration. Concentration at reduced
pressure, followed by chromatography of the residue on 15 g Si02 using
1:2 to 2:3 EtOAc-hexane provided I-4 as a pale yellow solid: 1H NMR
(CDCl3) d 7.35-7.2 (m, 5H), 5.81 (s, 1H), 4.69 (s, 2H), 4.34 (q, 2H, 7.1 Hz),
3.82 (s, 2H), 2.19 (s, 3H), 1.46 (s, 9H), 1.31 (t, 3H, 7.1 Hz).
Ste : Ethyl4-benzyl-6-methyl-1-carboxymethylpyridin[IH]-2-
one-3-carboxy~ate (I-5)
To a stirred solution of 456 mg ( 1.18 mmol) of ethyl 4-benzyl-
6-methyl-1-(t-butyl acetate)pyridin[1H]-2-one-3-carboxylate in 30 mL of
CH2C12 was added 15 mL of trifluoroacetic acid (TFA). After stirring for
4 h under Ar, the reaction mixture was concentrated, dissolved in 30 mL
of DMF and re-concentrated to give I_-5 a sticky yellow solid which was
used directly in the following reaction.
Ste~F_: 4-Benzyl-3-(ethoxycarbonyl )-6-methyl-1-(2-t-
butyloxycarbonylamino-6-methyl-5-
methylenecarboxamidomethylpyridinyl) pyridin[1H)-2-one
(I-6)
To a stirred solution of 200 mg (0.61 mmol ) of ethyl 4-benzyl-6-methyl-1-
carboxymethylpyridin( 1H]-2-one-3-carboxylate and 144 mg ( 0.61 mmol ) of
2-t-butyloxycarbonylamino-5-aminomethyl-6-methylpyridine in 10 mL of
Z~ CH2Cl2 was added 126 mg (0.61 mmol) of dicyclohexylcarbodiimide
(DCC ) under Ar. After stirring for 3 h, tie precipitate that had formed
was removed by filtration, washed with minimal CH2C12, and the
filtrate was diluted and washed with 1M citric acid, 5°lo NaHC03, dried
over Na2S04 and concentrated at reduced pressure to give I-6 as a pale
yellow solid: IH NMR, (CDC13) 7 7.64 (d, 1H, 8.4 Hz), 7.41 (d, 1H, 8.4 Hz),
7 .35-7.2 (m, 5H ), 7. 09 ( s, 1H ), 5.90 ( s, 1H ), 4.65 (br s, 2H ), 4.4-4.
3 ( m, 4H ),
3.83(s,2H),2.41(s,3H),2.36(s,3H),1.51(s,9H),1.32(t,3H,7.1Hz).
-48-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875 . -
Sten GG: 4-Benzyl-3-carboxy-6-methyl-1-(2-t-butyloxycarbonylamino-
6-methyl-5-methylenecarboxamidomethylpyridinyl )
gvridinf 1H1-2-one (I-7)
A 348 mg (0.63 mmol) sample of 4-benzyl-3-(ethoxycarbonyl)-
6-methyl-1-(2-t-butyloxycarbonylamino-6-methyl-5-
methylenecarboxamidomethylpyridinyl) pyridin[1H]-2-one was dissolved
in 10 mL of 1,2-dimethoxyethane and treated with 2.5 mL of 1M LiOH
under Ar for 16 h at 40°C. The reaction mixture was concentrated at
reduced pressure and partitioned between EtOAc and 1M citric acid.
The organic layer was washed with water, brine, dried over Na2S04 and
concentrated at reduced pressure to give I-7 as a yellow solid which was
used directly in the following reaction.
Step H: 4-Benzyl-3-(benzyloxycarbonylamino)-6-methyl-1-(2-t-
butyloxycarbonylamino-6-methyl-5-
methylenecarboxamidomethylpyridinyl) pyridin(1H]-2-one
(I-8)
A solution of 281 mg (0.54 mmol) of 4-benzyl-3-carboxy-6-
methyl-1-(2-t-butyloxycarbonylamino-6-methyl-5-
methylenecarboxamidomethylpyridinyl ) pyridin[1H]-2-one, 117 ~L (0.54
mmol) of diphenylphosphoryl azide, and 75 ~L (0.54 mmol) of
triethylamine (TEA) were heated to 50-60°C in 3 mL of dioxane under Ar
for 20 h. Benzyl alcohol (61~,L, 0.59 mmol) and a second portion of
triethylamine were added and heating continued for 20 h. The reaction
mixture was concentrated at reduced pressure and partitioned between
CHC13 and 1M citric acid. The aqueous layer was extracted with CHC13
and the combined organic layers were washed with 10% Na2C03, dried
over Na2S04 and the solvents removed to give an oil that was
chromatographed on 20 g Si02 using 95:5 CHC13/CH30H to give ~$ as a
green tinted oil: 1H NMR (CDCl3) 9 7.64 (d, 1H, 8.3 Hz), 7.42-7.2 (m,
10H), 6.68 (br s, 1H), 5.85 (s, 1H), 5.16 {s, 2H), 4.64 (s, 2H), 4.29 (d, 2H,
5.6
Hz), 3.88 (s, 2H), 2.34 (s, 3H), 2.33 (s, 3H), 1.50 (s, 9H).
-49-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
Step I: 4-Benzyl-3-amino-6-methyl-1-(2-t-butyloxycarbonylamino-6-
methyl-5-methylenecarboxamidomethylpyridinyl )
gvridinf 1Hl-2-one (I-9)
A stirred solution of 185 mg (0.3 mmol) of 4-benzyl-3-
(benzyloxycarbonylamino)-6-methyl-1-(2-t-butyloxycarbonylamino-6-
methyl-5-methylenecarboxamidomethylpyridinyl) pyridin[1H]-2-one in
11 mL of ethanol was hydrogenated with 41 mg of 20% Pd(OH)2 on
carbon using a balloon overnight. The catalyst was removed by
filtration, washed with ethanol, and the filtrate concentrated at reduced
pressure to give I-~ as an oily residue which was carried forward to the
next step.
Step J: 4-Benzyl-3-(methanesulfonylamino)-6-methyl-1-(2-t-
butyloxycarbonylamino-6-methyl-5-
1,5 methylenecarboxamidomethylpyridinyl) pyridin[1H]-2-one
(I-10)
The product from Step I was dissolved in 3 mL of pyridine,
cooled in an ice bath and treated with 28 ~.L (0.36 mmol) of
methanesulfonyl chloride dropwise. The cold bath was allowed to expire
over a 2.5 h period, the reaction concentrated at reduced pressure,
partitioned between CHC13 and 1M citric acid. The organic layer was
washed with 5°lo NaHC03, dried over Na2S04, treated with activated
carbon and concentrated to give a yellow oil that was chromatographed
on 14 g of Si02 using 99:1 to 97:3 CHC13-CH30H to afford ,I-~0 as a
colorless solid: 1H NMR (CDC13) 7 7.69 (d, 1H, 8.1 Hz), 7.58 (br s, 1H),
7.45 (d, 1H, 8.4 Hz), ?.35-7.23 (m, 3H), 7.17 (d, 2H, 6.8 Hz), 6.94 (br t,
1H),
6.56 (s, 1H), 5.91 (s, 1H), 4.64 (s, 2H), 4.37 (d, 2H, 5.5 Hz), 4.13 (s, 2H),
2.82
(s, 3H), 2.39 (s, 3H), 2.35 (s, 3H), 1.51 (s, 9H).
Step K: 4-Benzyl-3-methanesulfonylamino-6-methyl-1-(2-amino-
6-methyl-5-methylenecarboxamidomethylpyridinyl)
pvridinf 1Hl-2-one (I-11)
To a stirred solution of 60 mg (0.1 mmol) of 4-benzyl-3-
( methane sulfonylamino )-6-methyl-1-( 2-t-butyl oxycarbonylamino- 6-
methyl-5-methylenecarboxamidomethylpyridinyl) pyridin[1H]-2-one in 8
-50-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
mL of CH2C12 was added 4 mL of TFA under Ar. After stirring for 45
min, the reaction was concentrated at reduced pressure and retreated
with TFA as above for an additional 30 min. The reaction was
concentrated at reduced pressure and purified by gradient elution
preparative HPLC using a C-18 stationary phase and 0.1°/~ aqueous
TFA/CH3CN as the mobile phase. The fractions were assayed by
analytical HPLC) combined and concentrated at reduced pressure to
give a residue that was triturated with 1:4 EtOAc-hexane to provide I-11
as a colorless solid: 1H NMR (D6-DMSO) 7 13.55 (br s, 1H)) 8.75 (br s,
2H), 7.74 (d, 1H, 7 Hz), 7.55 (br s, 2H), 7.4-7.15 (m, 5H)) 6.76 (d, 1H, 7
Hz),
5.90 ( s, 1H ), 4.63 ( s, 2H ), 4.15 (br d, 2H ), 3.95 ( s, 2H >, 2.98 ( s, 3H
), 2.40 ( s,
3H), 2.18 (s, 3H).
3-Amino-4-Phenylthio-6-Methyl-1-[Cyclopropyl-( 2-Methylene-
carboxamidomethvl-4-Chloro h~ enoxv)-Acetamidol-2-Pyridinone
O
NH Nv 'N ~ CI
2
O H
H O
~N
O
I I-6
2d Steu A:A: 4-Chloro-2-hvdroxv-6-methyl-3-nitrogvridine (II-1)
POC13 (7.6 mL, 81.4 mmol) was added to a solution of 2,4-
dihydroxy-6-methyl-3-nitropyridine (Fluka, 3.15 g, 18.5 mmol) and
-51-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/008?5
BnEt3NC1 (16.8 g, 74 mmol) in MeCN (65 mL). The resulting solution
was stirred at 40° C for 30 min then was heated at reflux for 1 h.
After
evaporation of the solvent, water (70 mL) was added and the mixture was
stirred at room temperature for 16 h. The precipitate which formed was
filtered and washed with hexane to afford II-1 as a yellow solid.
1H NMR (DMSO-d6) d 2.25 (s, 3H), 6.45 (s, 1H).
to B: 2-Hvdroxv-6-Methyl-3-Nitro-4-Thiophenvlnyridine (II-2)
To a solution of 4-chloro-2-hydroxy-6-methyl-3-nitropyridine
( 188 mg, 1.0 mmol) in 2 mL of EtOH and 0.2 mL (1.4 mmol) of Et3N was
added 0.11 mL (1.0 mmol) of PhSH. Precipitation occurred immediately
and the solution was allowed to stir at room temperature for 16 h. The
solid was filtered and washed with EtOH to afford II-2.
St : 1-(t-Butyl-Methylenecarboxy)-6-Methyl-3-Nitro-4
Thiophenvl-2-Pvridinone (II-3)
To a 0° C solution of 2-hydroxy-6-methyl-3-nitro-4-
thiophenylpyridine (171 mg, 0.654 mmol) in 2 mL of DMF was added 17
mg (0.72 mmol) of NaH. The solution was stirred at 0° C for 10 min
before the addition of 0.106 mL (0.72 mmol) of tert-butyl bromoacetate.
After 3 h, the solvent was removed in vacuo and the residue was
redissolved in 25 mL of EtOAc and washed with water (3x5 mL). The
organic phase was dried (MgS04), concentrated and chromatographed
(2/3 EtOAc / Hexane) to afford II-3.
~ 1H NMR (CDC13) d 7.50 (m, 5H), 5.45 (s, 1H), 4.65 (s, 2H), 2.15 (s, 3H),
1.46
(s, 9H).
Step D: 1- Methylenecarboxy-6-Methyl-3-Nitro-4-Thiophenyl-2-
Pvridinone (II-4)
To a 0° C solution of 1-(t-butyl-methylenecarboxy)-6-methyl-
3-nitro-4-thiophenyl-2-pyridinone (77 mg, 0.20 mmol) in 4 mL of DCM
was added 1 mL of TFA. The solution was stirred at 0° C for 3 h, before
the solvent was removed in vacuo and the residue was azeotroped with
benzene, then EtOAc, then ether to afford II-4.
1H NMR (CDC13) d 7.50 (m, 5H), 5.45 (s, 1H), 4.75 (s, 2H), 2.10 (s, 3H).
-52-
CA 02277929 1999-07-14
WO 98131670 PCT/US98/00875
~Ste~ 6-Methyl-3-Nitro-4-Thiophenyl-1-[Cyclopropyl-(2-
Methylenecarboxamidomethyl-4-Chlorophenoxy)-
~,cetamidol-2-P~~ridinone (II-5)
To a solution of 1-methylenecarboxy-6-methyl-3-vitro-4-
thiophenyl-2-pyridinone (65.2 mg, 0.204 mmol) and N-cyclopropyl(2-
aminomethyl-5-chlorophenoxy)acetamide (59 mg, 0.204 mmol) in 2 mL of
DMF was added 39.0 mg (0.204 mmol) of EDCI and 28 mg (0.204 mmol) of
HOBT followed by 0.177 mL (1.02 mmol) of DIPEA. The homogeneous
mixture was stirred at room temperature for 16h after which time the
solvent was removed under reduced pressure. The residue was disolved
in EtOAc and washed with sat'd NaHC03 then water. The organic
phase was dried (MgS04) and concentrated to afford II-5.
1H NMR (CDCl3) d 8.85 (bt, 1H), 8.00 (bs, 1H), ?.60 (m, 5H), 7.20 (m, 2H),
6.90 ( d, J=8.8 Hz, 1H ), 5.45 ( s, 1H ), 4.74 ( s, 2H ), 4.45 ( s, 2H ), 4.30
( d, J=4
Hz, 2H), 2.68 (m, 1H), 2.15 (s, 3H), 0.60 (m, 2H), 0.40 (m, 2H).
Ste,R F: 3-Amino-6-Methyl-4-Thiophenyl-1-[Cyclopropyl-(2-
Methylenecarboxamidomethyl-4-Chlorophenoxy)-
Acetamidol-2-Pvridinone (II-6)
To a solution of 6-methyl-3-vitro-4-thiophenyl-1-[cyclopropyl-
(2-methylenecarboxamidomethyl-4-chlorophenoxy)-acetamido]-2-
pyridinone (55.2 mg, 0.093 mmol) in 5 mL of EtOAc was added 107 mg
( 0.475 mmol ) of SnC12.H20. The mixture was stirred at reflux for 16h,
cooled and made basic by the addition of saturated NaHC03. The
mixture was extracted with saturated NaHC03 then water. The organic
phase was dried (MgS04) and concentrated to afford jI-66 as a white
solid.
1H NMR (CDCl3) d 7.65 (bs, 1H), 7.60 (m, 7H), 7.10 (bt, 1H), 6.75 (d, J=8.8
Hz, 1H), 6.00 (s, 1H), 4.68 (s, 2H), 4.55 (s, 1H), 4.40 (m, 3H), 3.50 (d, J=3
Hz, 1H), 2.88 (m, 1H), 2.30 (s, 3H), 0.70 (m, 4H).
-53-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
~',-XAMPLE III
Preparation of 3-Amino-4-isopropylthio-6-methyl-1-(2-amino-6-methyl-5-
methvlenecarboxamidomethvluvridinvl)-2-gvridinone
ys ~ O
H2N N v N ~ N
OI H
NH2
Ste : 4-Chloro-6-methyl-3-nitro-1-(ethyl-methylenecarboxy)-2-
pvridinone (III-1 )
Sodium hydride (0.90 g of a 60% dispersion in mineral oil,
22.6 mmol) was added to a stirred solution of 4-chloro-2-hydroxy-6-
methyl-3-nitropyridine (4.74 g, 25.1 mmol) in dry DMF (35 mL) at 0° C
under nitrogen. After 20 min ethyl bromoacetate (3.35 mL, 30.2 mmol)
was added and the mixture was warmed to room temperature. After 16
h, the solvent was evaporated in vacuo and the residue was partitioned
between ethyl acetate and 10°lo citric acid solution. The organic layer
was washed with water and brine, dried (Na2S04) and evaporated to a
heavy oil which was purified by flash column chromatography on silica
(60% ethyl acetate/hexanes) to give IIL-1 as an orange solid.
1H NMR (DMSO-d6) d 1.22 (t, J = 7.1 Hz, 3H), 2.41 (s, 3H), 4.18 (q, J = 7.1
Hz, 2H), 4.90 (s, 2H), 6.76 (s, 1H).
Stew B: 4-Isopropylthio-6-methyl-3-nitro-1-(ethyl-
methvlenecarboxv)-2-Rvridinone (III-2)
To a solution of of 4-chloro-6-methyl-3-nitro-1-(ethyl-
methylenecarboxy)-2-pyridinone (500 mg, 1.82 mmol) in 10 mL of EtOH
was added 0.22 mL (2.36 mmol) of isopropylthiol followed by 0.37 mL (2.7
mmol) of Et3N. The solution was stirred at 82° C for 15 h, cooled and
evaporated to an oil. The residue was partitioned between EtOAc and
water and the organic phase was washed with brine, dried (MgS04) and
-54-
CA 02277929 1999-07-14
WO 98/31670 PCTlUS98/00875
concentrated. Column chromatography (3:7 EtOAc / Hexanes) provided
,~ as a white solid.
1H NMR (CDCl3) d 6.13 (s, 1H), 4.79 (s, 2H), 4.25 (q, J=7.4 Hz, 2H), 3.53
(m, 1H), 2.36 (s, 3H), 1.40 (d, J=6.8 Hz, 6H), 1.25 (t, J=7.4 Hz, 3H).
St. en C: 4-Isopropylthio-6-methyl-3-vitro-1-methylenecarboxy-2-
gyridinone (III-3)
A solution of 4-isopropylthio-6-methyl-3-vitro-1-(ethyl-
methylenecarboxy)-2-pyridinone (330 mg, 1.04 mmol) in 5 mL of dioxane
and 2 mL of water was treated with 132 mg (3.14 mmol) of LiOH.H20 and
the whole was stirred for 15h. The solvent was removed and the residue
was treated with 4 mL of 1N HCl then washed with EtOAc (3 x 10 mL).
The combined organic washings were dried over MgS04 and evaporated
to leave ,.
1H NMR (CD30D > d 6.54 ( s, 1H ), 4.84 ( s, 2H ), 3.78 ( q, J=7.4 Hz, 1 H ),
2.40
(s, 3H), 1.35 (t, J=7.4 Hz, 3H).
Step D: 4-Isopropylthio-6-methyl-3-vitro-1-(2-t-
butyloxycarbonylamino-6-methyl-5-
2D methylenecarboxamidomethylpyridinyl )-2-pyridinone
(III-4)
To a solution of 4-isoprnpylthiu-f-methyl-3-vitro-1-
methylenecarboxy-2-pyridinone 1150 mg. U. il:l mmol ) and 2-amino-5-
aminomethyl-6-methylpyridine I 1H6 mfi, 0.7H4 mmol ) in 2 mL of DMr
was added 200 mg ( 1.04 mmol ~ of EI)('. I and 141 mg ( 1.04 mmol ) of HOBT
followed by 0.36 mL (2.09 mmol i of I)IPEA. The homogeneous mixture
was stirred at room temperature for 16h after which time the solvent
was removed under reduced pressure. The residue was disolved in
EtOAc and washed with sat'd NaHC03 then water. The organic phase
was dried (MgS04) and concentrated to afford ~4_ as a white solid.
1H NMR (CDCl3) d 7.85 (bt, 1H), 7.44 (d, J=8 Hz, 1H), 7.30 (d, J=8Hz, 1H),
6.14 (s, 1H), 4.64 (bs, 2H), 4.25 (bs, 2H), 3.68 (q, J=7.4 Hz, 1H ), 2.40 ( s,
3H ),
2.31 (s, 3H), 1.50 (s, 9H), 1.35 (t, J=7.4 Hz, 3H).
-55-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
Step E: 3-Amino-4-isopropylthio-6-methyl-1-(2-t-
butyloxycarbonylamino-6-methyl-5-
methylenecarboxamidomethylpyridinyl)-2-pyridinone (III-
5)
A solution of 4-isopropylthio-6-methyl-3-nitro-1-(2-t-
butyloxycarbonyl amino-6-methyl-5-
methylenecarboxamidomethylpyridinyl)-2-pyridinone in 10 mL of THF
was treated with 62 mg of 10% Pd on carbon and the whole was stirred
under an atmosphere of H2 for 16 h. The reaction mixture was filtered
through Celite and concentrated to afford III-5 as a tan colored foam
which was used without further purification.
1H NMR (CDC13) d 7.44 (d, J=8 Hz, 1H), 7.55 (s, 1H), 7.50 (bs, 1H), 7.40 (d,
J=8Hz, 1H ), 6.11 (s, 1H ), 4.70 (bs, 1H ), 4.54 (bs, 2H), 4.29 (bs, 2H ),
3.39 !m,
1H), 2.30 (s, 3H), 2.25 (s, 3H), 1.50 (s, 9H), 1.35 (d, J=?.4 Hz, 6H).
Step F: 3-Amino-4-isopropylthio-6-methyl-1-(2-amino-6-methyl-5-
methylenecarboxamidomethylpyridinyl )-2-pyridinone
(III-6)
A solution of 3-Amino-4-isopropylthio-6-methyl-1-(2-t-
butyloxycarbonylamino-6-methyl-5-
methylenecarboxamidomethylpyridinyl)-2-pyridinone (140 mg, 0.294
mmol ) in 10 mL of EtOAc and 5 mL of MeOH was treated with HCl gas
for 2 minutes. The flask was capped and the reaction mixture was
allowed to stir for lh. The solvent was removed and the resulting solid
was triturated with ether/EtOAc. The tacky tan solid was filtered to
afford III-6 as its bis-HCl salt. .
1H NMR (CD30D) d 8.85 (bt, 1H), 7.85 (d, J=9.5Hz, 1H), 6.95 (s, 1H), 6.83
(d, J=9.5Hz, 1H), 6.54 (s, 1H), 4.80 (s, 2H), 4.30 (bs, 2H), 3.80 (m, 1H),
2.50
(s, 3H), 2.40 (s, 3H), 1.40 (d, J=7.4 Hz, 6H).
Anal. Calc'd for C18H25N502S.2.55 CH30H.1.65HC1: C; 47.77, H; 7.19,
N; 13.56. Found: C; 47.84, H; 7.18, N; 13.53.
-56-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
Preparation of 3-Amino-4-cyclopropylmethylsulfonyl-6-methyl-1-(2-
amino-6-methyl-5-methylenecarboxamidomethvlnvridinvl)-2-gvridinone
~Oz
''''''~ S ~ O
I
H2N N v N ~ N
H
O I ~ NH
2
Sten AA: $ Cyclogropylmethvlthiobenzothiazole (IV-1)
A stirred mixture of 2-mercaptobenzothiazole ( 1.67 g, 10.0
mmol), bromomethylcyclopropane (0.97 mL, 10.0 mmol) and sodium
hydrogencarbonate ( 0.84 g, 10.0 mmol ) in absolute ethanol ( 10 ml ) was
heated to reflux. After 8 h, the reaction was diluted with ethyl acetate
and was washed with water, sodium carbonate solution and brine, dried
(Na2S04) and evaporated in Uacuo to give IV-1 as an oil which was used
without purification in the next step:
1H NMR (300 Mz, CDC13) selected signals d 0.39 (m, 2H), 0.65 (m, 2H),
1.26 (m, 1H), 3.32 (d, J = 7.3 Hz, 2H).
Step B: ;~-Cv~c ~pvlmethvlsulfonvlbenzothiazole (IV-2)
A solution of potassium permanganate ( 1.90 g, 12.0 mmol )
in water ( 100 mL) was added to a stirred. solution of 2-
cyclopropylmethylthiobenzothiazole ( 1.90 g) in acetic acid ( 150 mL ).
After 2 h, the dark brown mixture was decolorized with 10% sodium
sulfite solution and water (500 mL) was added. The resulting precipitate
was collected by filtration, washing with water, and dried at 0.5 mm Hg,
to give ~V-~ as a white crystalline solid.
1H NMR (300 Mz, CDC13) selected signals d 0.28 (m, 2H), 0.64 (m, 2H),
1.21 (m, 1H), 3.46 (d, J = 7.3 Hz, 2H).
-57-
CA 02277929 1999-07-14
( WO 98/31670 PCT/US98/00875 . -
Step C: 4-Cyclopropylmethylsulfonyl-6-methyl-3-nitro-1-(ethyl-
methvlenecarboxv)-2-wridinone (IV-3)
Sodium borohydride (113 mg, 3.0 mmol) was added in
portions to a stirred mixture of 2-
cyclopropylmethylsulfonylbenzothiazole (0.38 g, 1.50 mmol) in absolute
ethanol (3 mL) with cooling. After 2 h, glacial acetic acid was added
dropwise to dissolve the suspension, giving a solution pH 4-5 (moist pH
paper) and 4-chloro-6-methyl-3-nitro-1-(ethyl-methylenecarboxy)-2-
pyridinone (275 mg, 1.0 mmol) was added. The solid quickly dissolves
and then a thick precipitate forms. After 2 h the solids were collected by
filtration, washing with ethanol, and dried at 0.5 mm Hg to give IV-3 as
a bright yellow powder.
1H NMR, (CDC13) d 0.40 (m, 2H), 0.70 (m, 2H), 1.13 (m, 1H), 1.32 (t, J = 7.1
Hz, 3H), 2.46 (s, 3H), 3.33 (d, J = 7.3 Hz) 2H), 4.28 (q, J = 7.1 Hz, 2H),
4.88
(s, 2H), 6.62 (s, 1H)
Sten DD: 3-Amino-4-cyclopropylmethylsulfonyl-6-methyl-1-(ethyl-
methvlenecarboxv)-2-pvridinone (1V-4 )
A mixture of 4-cyclopropylmethylsulfonyl-6-methyl-3-nitro-
1-( ethyl-methylenecarboxy)-2-pyridinone ( 365 mg ) and 10°l°
palladium on
carbon (0.30 g) in ethyl acetate ( 100 mL ~ was stirred under an
atmosphere of hydrogen (balloon I for 3 h. The reaction mixture was
filtered through celite, washing with ethyl acetate, and evaporated in
vacuo to give ~ as a colorless crvst,alline ~;olid which was used
2~5 without purification in the next step.
1H NMR. (CDC13 ) d 0.27 (m, 2H I) O.fi31 m, 2H I) I .Ofi l m) 1H ), 1.30 (t, J
= 7.1
Hz, 3H), 2.23 (d, J = 0.9 Hz, 3H)) 3.02 (d, J = 7.1 Hz, 2H), 4.25 (q) J = ?.1
Hz,
2H), 4.80 (s, 2H), 5.88 (br s, 2H), 6.28 (d, J = 0.9 Hz, 1H).
Step E: 3-Amino-4-cyclopropylmethylsulfonyl-6-methyl-1-
methvlenecarboxv-2-pvridinone (IV-5)
Lithium hydroxide hydrate (84 mg, 2.0 mmol) was added to
a stirred mixture of 3-amino-4-cyclopropylmethylsulfonyl-6-methyl-1-
(ethyl-methylenecarboxy)-2-pyridinone (the product from Step D) in 2:2:1
methanol/THF/water ( 10 mL). After 2 h a thick white precipitate
_58_
CA 02277929 1999-07-14
WO 98131670 PCT/US98/00875 _ (
formed. The mixture was acidified with 1 M HCl to give a clear solution
which was partitioned between methylene chloride and brine. The brine
was re-extracted with methylene chloride and the combined organic
layers were dried (Na2S04) and evaporated in vacuo to give a crystalline
solid. This was heated to reflux as a suspension in methylene chloride
( 10 mL), cooled and the solids collected by filtration to give IV-5 as a
colorless crystalline solid.
1H NMR (d6 DMSO) d 0.25 (m, 2H), 0.50 (m, 2H), 0.92 (m, 1H), 2.20 (s,
3H), 3.18 (d, J = 7.1 Hz, 2H), 4.73 (s, 2H), 6.16 (br s, 2H), 6.20 (s, 1H).
Step F: 3-Amino-4-cyclopropylmethylsulfonyl-6-methyl-1-(2-amino-
6-methyl-5-methylenecarboxamidomethylpyridinyl )-2-
pvridinone (IV-6)
N-Methylmorpholine (0.187 mL, 1.70 mmol) was added to a
stirred mixture of 3-amino-4-cyclopropylmethylsulfonyl-6-methyl-1-
methylenecarboxy-2-pyridinone (120 mg, 0.40 mmol), 2-amino-5-
aminomethyl-6-methylpyrinine dihydrochloride (84 mg, 0.40 mmol),
EDC.HCI (96 mg, 0.50 mmol) and HOBT.H20 (68 mg, 0.50 mmol) in DMF
(2 mL). After 16 h, water (20 mL) was added to give a white precipitate
and after allowing to stand for 30 min, the solids were collected by
filtration, washing with water, ethanol and ethyl acetate and air dried.
9.9 M HCl in absolute ethanol (0.1 mL) was added to a stirred fine
suspension of the resulting white solid in absolute ethanol (5 mL) to give
a solution. Over 1 h a pale yellow crystalline precipitate formed which
was collected by filtration, washing with ethanol and dried at 0.5 mm
Hg to give V~- as a pale peach colored crystalline solid.
1H NMR (d6 DMSO) d 0.25 (m, 2H), 0.50 (m, 2H), 0.91 (m, 1H), 2.17 (s,
3H),2.44(s,3H),3.17(d,J=7.1Hz,2H),4.16(d,J=5.6Hz,2H),4.68(s,
2H),6.12(brs,2H),6.18(d,J=0.7Hz, 1H),6.80(d,J=9.O Hz, 1H),7.63
(br s, 2H), 7.76 (d, J = 9.0 Hz, 1H), 8.72 (br t, J = 5.6 Hz, 1H); Anal. Calc.
for C19H25N5O4S.HC1.2H20: C 46.38, H 6.15, N 14.24. Found: C 46.51, H
6.08, N 13.89.
-59-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
EXAMPLE V
Preparation of 3-Amino-4-cyclopropylsulfonyl-6-methyl-1-(2-amino-6-
methvl-5-methvlenecarboxamidomethvlpvridinvl)-2-pvridinone
~2
0
d '~
H2N N v N ~ N
O H
NH2
Step A: Lithium cvclo~ropvlsulfinate (V-1)
V-1 was prepared by a modification of the method of Crowell
et. ail. (J. Med. Chem. 1989, 32, 2436). A solution of cyclopropyl bromide
( 1.73 mL, 21.6 mmol ) in dry diethyl ether (4 mL) was added dropwise to a
stirred slurry of 30% lithium dispersion in mineral oil ( 1.0 g) in dry
ether (8 mL) at 0° C (internal T<5° C) under nitrogen. After 3
h, an
excess of sulfur dioxide was blown on to the surface of the mixture to
give a thick white precipitate. The solvent was evaporated in vacuo and
absolute ethanol (50 mL) was added cautiously. The resulting mixture
was stirred thoroughly and filtered, washing with ethanol. The filtrate
was evaporated in vacuo to give V-1 which was used without purification
in the next step:
1H NMR (CD30D) d 0.61 (m, 2H), 0.75 (m, 2H), 1.87 (m, 1H).
Step B: 4-Cyclopropylsulfonyl-6-methyl-3-nitro-1-(ethyl-
methvlenecarboxv)-2 pvridinone (V-2)
Glacial acetic acid was added dropwise to a solution of the
crude lithium cyclopropylsulfinate from Step A (448 mg) in absolute
ethanol (2 mL) to give a solution pH 4-5 (moist pH paper). 4-Chloro-6-
methyl-3-nitro-1-(ethyl-methylenecarboxy)-2-pyridinone (137 mg, 0.50
mmol) was added to give solution. Over 1 h a thick precipitate forms
which was collected by filtration, washing with ethanol, and dried at 0.5
-60-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
mm Hg to give 1~-2 as a yellow solid which was used without purification
in the next step.
Sten CC: 3-Amino-4-cyclopropylsulfonyl-6-methyl-1-(2-amino-6-
methyl-5-methylenecarboxamidomethylpyridinyl)-2-
~~rridinone (V-3)
V-3 was prepared from 4-cyclopropylsulfonyl-6-methyl-3-
nitro-1-(ethyl-methylenecarboxy)-2-pyridinone using the procedures of
Example IV, Steps D - F.
1H NMR (d6 DMSO) d 1.04 (m, 2H), 1.11 (m, 2H), 2.17 (s, 3H), 2.44 (s, 3H),
2.88 (m, 1H), 4.16 (d, J = 5.5 Hz, 2H), 4.67 (s, 2H), 6.09 (br s, 2H), 6.18
(d, J
= 0.6 Hz, 1H), 6.80 (d, J = 9.0 Hz, 1H), 7.65 (br s, 2H), 7.77 (d, J = 9.0 Hz,
1H ), 8.75 (br t, J = 5.5 Hz, 1H ); Anal. Calc. for
C 18H23N5O4S.HC1Ø3H20: C 48.32, H 5.54, N 15.66. Found: C 48.41, H
5.38, N 15.28.
EXAMPLE VI
Preparation of 3-Amino-4-cyclobutylmethylsulfonyl-6-methyl-1-(2-amino-
6-methyl-5-methvlenecarboxamidomethvlp ' invl )-2-nvridinone
S ( \ O
H2N Nv _N ~N
O H I ~ NH
2
VI-1
~_1 was prepared from bromomethylcyclobutane using the
procedures of Example IV, Steps A - F.
1H NMR (d6 DMSO) d 1.76-2.01 (m, 6H), 2.16 (s, 3H), 2.44 (s, 3H), 2.60 (m,
1H), 3.35 (d, J = 7.1 Hz, 2H), 4.16 (d, J = 5.1 Hz, 2H), 4.67 (s, 2H), 6.10
(br s,
2H), 6.15 (s, 1H), 6.80 (d, J = 9.0 Hz, 1H), 7.66 (br s, 2H), 7.76 (d, J = 9.0
Hz,
1H), 8.73 (br t,1H).
-61-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875 _ Y
EXAMPLE VII
Preparation of 3-Amino-4-benzylsulfonyl-6-methyl-1-(2-amino-6-methyl-
5-methvlenecarboxamidomethvlRvridinvl)-2-pvridinone
O
\ ~ S2 \ O
I
H2N Nv N ~N
OI H
NH2
a) VII-2 was prepared from benzyl bromide using the
procedures of Example IV, Steps A - F.
1HNMR(d6DMS0)d2.14(s,3H),2.24(d,J=l.lHz,3H>,4.12(d,J=4.8
Hz, 2H), 4.54 (s, 2H)) 4.67 (s, 2H), 5.72 (s, 2H), 6.05 (br s, 3H), 6.23 (d, J
=
8.2 Hz, 1H), 7.21 (d, J = 8.2 Hz, 1H), 7.27-7.34 (m, 5H), 8.43 (br t, 1H).
b) Alternatively, VII-2 may be prepared from 4-benzylthio-6-
methyl-3-nitro-1-(ethyl-methylenecarboxy)-2-pyridinone by oxidation
with OXONEO to give 4-benzylsulfonyl-6-methyl-3-nitro-1-(ethyl-
methylenecarboxy)-2-pyridinone (VII-1), followed by the use of the
procedures of Example IV, Steps D-F:
Preparation of 4-Benzylsulfonyl-6-methyl-3-nitro-1-(ethyl-
methvlenecarbox~p~nidinone (VII-1 >
A mixture of 4-benzylthio-6-methyl-3-nitro-1-(ethyl-
methylenecarboxy)-2-pyridinone (135 mg, 0.373 mmol) and OXONEOO
(688 mg, 1.12 mmol) in 1:1 methanol/water (3 mL) was stirrred for 9
days. The mixture was partitioned between ethyl acetate and water and
the organic layer was washed with 5% sodium metabisulfite solution
and brine, dried (Na2S04) and evaporated in vacuo to an oil. The crude
product was purified by flash column chromatography on silica (70%
ethyl acetate/hexanes) to give VII-1.
-62-
CA 02277929 1999-07-14
WO 98/31670 PCTIUS98100875 -
1H NMR (CDC13) d 1.32 (t, J = 7.1 Hz, 3H), 2.24 (s, 3H), 4.27 (q, J = 7.1 Hz,
2H), 4.62 (s, 2H), 4.81 (s, 2H), 6.00 (s, 1H), 7.36 (s, 5H).
EXAMPLE VIII
Preparation of 3-Amino-4-phenylsulfonyl-6-methyl-1-(2-amino-6-methyl-
5-methylenecarboxamid~omethylpvridinyl >-2-pvridinone
/ \ S I w O
H2N N v N ~ N
H
NH2
VIII-2
Step A: 6-Methyl-3-nitro-4-phenylsulfonyl-1-(ethyl-
methylenecarboxy)-2-gvridinone (VIII-1 )
4-Chloro-6-methyl-3-nitro-1-(ethyl-methylenecarboxy)-2-
pyridinone (100 mg, 0.40 mmol) was added to a stirred solution of sodium
1,5 benzene sulfinate (66 mg, 0.40 mmol ) in DMF ( 0.40 ml ). After 2 h the
mixture was partitioned between water and ethyl acetate. The organic
layer was dried (Na2S04) and evaporated in vacuo to a yellow gum
which was purified by flash column chromatography (ethyl
acetate/hexanes gradient, 40-70% ethyl acetate ) to give V, II1-1 as a yellow
gum.
1H NMR (CD30D) d 1.26 (t, J = 7.1 Hz, 3H), 2.46 (s, 3H), 4.22 (q, J = ?.1 Hz,
2H ), 4.90 ( s, 2H ), 6.80 ( s, 1H ), 7.66-8.05 ( m, 5H ).
Step 3-Amino-4-phenylsulfonyl-6-methyl-1-(2-amino-6-methyl-
5-methylenecarboxamidomethylpyridinyl)-2-pyridinone
(VIII-2)
VIII-2 was prepared, as the free base, from 6-methyl-3-
nitro-4-phenylsulfonyl-1-(ethyl-methylenecarboxy)-2-pyridinone using
the procedures of Example IV, Steps D - F.
CA 02277929 1999-07-14
WO 98/31670 PCT/US98I00875
1H NMR (d6 DMSO) d 2.15 (s, 3H), 2.42 (s, 3H), 4.13 (d, J = 5.7 Hz, 2H),
4.62 (s, 2H), 6.29 (s, 1H), 6.32 (s, 2H), 6.79 (d, J = 9.0 Hz, 1H), 7.24 (t, J
= 4.4
Hz, 1H), 7.67 (br s, 2H), 7.74 (d, J = 9.0 Hz, 1H), 7.94 (dd, J = 0.6 and 3.7
Hz, 1H), 8.0? (dd, J = 0.6 and 4.9 Hz, 1H), 8.41 (br t, 1H).
EXAMPLE IX
Preparation of 3-Amino-4-(2-thienylsulfonyl)-6-methyl-1-(2-amino-6-
meth~-5-methvlenecarboxamidomethvlgvridinyl)-2 pvridinone
~2
~ \ S ~ ~ O
SH2N Nv _N ~N
H
NH2
1~
IX-1 was prepared as the HCl salt from 4-chloro-6-methyl-3-
nitro-1-(ethyl-methylenecarboxy)-2-pyridinone and sodium
thienylsufinate [which was prepared by the method of Crowell et. al. (J.
Med. Chem. 1989, 32, 2436)] using the procedure of Example VIII.
1H NMR (d6 DMSO) d 2.14 (s, 3H), 2.20 (s, 3H), 4.08 (d, J = 4.9 Hz, 2H),
4.60 ( s, 2H ), 5.70 ( s, 2H ), 6.21 (d, J = 8.2 Hz, 1H ), 6.29 ( s, 1H ),
6.30 ( s, 2H ),
7.1? (d, J = 8.2 Hz, 1H), 7.62-7.99 (m, 5H), 8.41 (br t, 1H).
CA 02277929 1999-07-14
. WO 98/31670 PCT/US98/00875 _ -
EXAMPLE X
Preparation of 3-Amino-4-benzylthio-6-methyl-1-(2-amino-6-methyl-5-
methvlenecarboxamidomethvlnvridinvl)-2-~dinone
O
H2N N v N ~ N
O H I /
NH2
X-2
Step A: 3-Amino-4-benzylthio-6-methyl-1-(ethyl-methylenecarboxy)-
~~rridinone (X-1)
Iron powder (39 mg) was added to a stirred solution of 4-
benzylthio-6-methyl-3-nitro-1-(ethyl-methylenecarboxy)-2-pyridinone
[prepared from benzyl mercaptan and 4-chloro-6-methyl-3-nitro-1-(ethyl-
methylenecarboxy)-2-pyridinone using the procedure of Example III,
Step B] in a mixture of acetic acid (1.1 mL) and water (0.2 mL) and the
resulting mixture was heated to 90° C. After 4 h, the mixture was
cooled
and filtered, washing with acetic acid. The filtrate was partitioned
between ethyl acetate and water and the organic layer was washed with
water, dried (Na2S04) and evaporated in vacuo to an oil. The crude
product was purified by flash column chromatography on silica (30°/~
ethyl acetate/hexanes) to give X-1 as an oil.
1H NMR (CDCl3) d 1.28 (t, J = 7.3 Hz, 3H), 2.16 (s, 3H), 4.00 (s, 2H), 4.24
(q, J = 7.3 Hz, 2H), 4.46 ( br s, 2H), 4.78 (s, 2H), 5.98 (s, 1H), 7.26 (m,
5H).
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
Step B: 3-Amino-4-benzylthio-6-methyl-1-(2-amino-6-methyl-5-
methvlenecarboxamidomethy~p3rridin~l)-2-nvridinone (X-2)
X-2 was prepared, as the free base, from 3-amino-4-
benzylthio-6-methyl-1-(ethyl-methylenecarboxy)-2-pyridinone using the
procedures of Example IV, Steps E and F.
MS (FAB) 424 (M+1)+.
EXAMPLE XI
Preparation of 3-Carboxymethylamino-4-cyclohexylmethyl-6-methyl-1-(2-
amino-6-methyl-5-methvlenecarboxamidomethvlpvridinvl )-2 pvridinone
I y' o
H2 ~ N ~ N
H I
NH2
XI-7
1,5 to A: 1-(t-Butyl methylenecarboxy)-6-Methyl-3-nitro-
pvridinf 1H1-2-one (XI-1 )
To a 0°C stirred suspension of 1.0 g ( 6.49 mmol ) of 6-
methyl-3-nitropyridin-2-one in 15 mL anhydrous DMF under Ar was
added 262 mg (6.55 mmol) of NaH (60% in mineral oil) in 3 portions.
After the mixture became homogeneous over 15 min, 1.06 mL (6.55
mmol ) of t-butyl bromoacetate was in two portions. The cold bath was
removed and the reaction mixture stirred under Ar overnight. An
additional portion of 0.2 mL of t-butyl bromoacetate was added, and
the reaction heated to 50°C for 17 h. Concentration of the reaction
mixture at reduced pressure gave a residue that was partitioned
between ethyl acetate and 1M citric acid. The aq. layer was extracted
with ethyl acetate, and the combined organic layers washed with 10%
Na2COg, brine, treated with activated carbon, dried over Na2S04,
and the solvents removed to give an oil that was chromatographed on
-66-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
12 g of Si02 using 1:2 to 1:1 EtOAc-hexanes which gave Ice,- as a
yellow solid:
1H NMR (CDC13) a 8.34 (d, 1H, 8.0 Hz), 6.20 (d, 1H, 7.8 Hz), 4.81 (s,
2H), 2.43 (s, 3H), 1.49 (s, 9H).
1-(t-Butyl methylenecarboxy)-4-cyclohexylmethyl-3,4-
dihydro-6-methvl-3-nitro-Rvridinf 1Hl-2-one (XI-2)
To a stirred suspension of 73 mg (3.0 mmol) of Mg
turnings and a small crystal of I2 in 3 mL of dry THF was added 349
~L (2.5 mmol) of brornomethylcyclohexane, and the mixture heated to
reflux for 2h as the Mg slowly dissolved. After cooling to ambient
temperature, the resulting solution was added dropwise via syringe
to a -78°C stirred solution of 537 mg (2.0 mmol) 1-(t-butyl
methylenecarboxy)-6-methyl-3-nitro-pyridin[ 1H]-2-one in 6 mL of dry
THF. After stirring in the cold for 45 min, the cold bath was removed
and, as the reaction reached -15°C, it was quenched by the dropwise
addition of 286 ~.L (5 mmol) of acetic acid. After removal of the
solvents at reduced pressure, the residue was partitioned between
EtOAc and cold 5% HCI. The organic layer was washed with 5%
2U NaHC03, brine, dried over Na2S04 and the solvents removed to give a
yellow oil that was chromatographed on 50 g of Si02 using 1:3 EtOAc-
hexane to give I~-2 as a yellow semi-solid:
1H NMR (CDC13) a 4.97-5.0 (m, 2H), 4.42 (d, 1H, 17.6 Hz), 4.21 (d, 2H,
17.7 Hz), 3.25-3.35 (m, 1H), 1.89 (t, 3H, 1.5 Hz), 1.60-1.80 (m, 6H), 1.49
(s, 9H), 1.03-1.48 (m, 11 H), 0.8-1.02 (m, 2H).
te~C: 1-(t-Butyl methylenecarboxy)-4-cyclohexylmethyl-6
methvl-3-nitro-Ryridinf 1H1-2-one (XI-3)
To a stirred suspension of 104 mg of nickel peroxide
hydrate in 5 mL of CHCl3 was added 126 mg (0.34 mmol) of 1-(t-butyl
methylenecarboxy)-4-cyclohexylmethyl-3,4-dihydro-6-methyl-3-nitro-
pyridin[1H]-2-one. After 2h, an additional 127 mg of nickel peroxide
hydrate was added, and the mixture stirred for another 2h.
Filtration and concentration of the filtrate at reduced pressure gave
-67-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875 .
XI-3 as a yellow oil: 1H NMR (CDC13) a 5.95 (s, 1H), 4.74 (s, 2H), 2.37
(d, 1H, 7.1 Hz), 2.31 (s, 3H), 1.52-1.80 (m, 6H), 1.48 (s, 9H), 1.03-1.30 (m,
8 H), 0.85-1.02 (m, 3H).
Step D: 1-Methylenecarboxy-4-cyclohexylmethyl-f-methyl-3-
vitro-pvridinf 1Hl-2-one (XI-4)
To a stirred solution of 120 mg (0.33 mmol) of 1-(t-butyl
methylenecarboxy)-4-cyclohexylmethyl-6-methyl-3-vitro-pyridin[ 1H]-
2-one in 7.5 mL of CH2C12 was added 7.5 mL of TFA and stirred
under Ar for lh. The solvents were removed at reduced pressure,
and the residue partitioned between EtOAc and water. The aqueous
layer was extracted with EtOAc and the combined organic layers
washed with brine, treated with activated carbon, dried over Na2S04
and solvents removed to give XI-4 as a light tan solid which was
carried forward to the next step.
Step E: 4-Cyclohexylmethyl-6-methyl-3-vitro-1-(2-t-
butyloxycarbonylamino-6-methyl-5-
methylenecarboxamidomethylpyridinyl )-pyridin[ 1H)-2-
one (XI-5 )
The product from Step C was dissolved in 3 mL of DMF
along with 44 mg ( 0.32 mmol) of 1-hydroxybenzotriazole and 77 mg
(0.32 mmol) of 2-t-butyloxycarbonylamino-5-aminomethyl-6-
methylpyridine. To this stirred solution was added 62 mg (0.32
mmol ) of EDC and 90 ~tL (0.65 mmol ) of triethylamine, and stirred
under Ar overnight. The solvents were removed at reduced pressure,
and the residue partitioned between EtOAc and 1M citric acid. The
aqueous layer was extracted with EtOAc, and the combined organic
layers washed with 10% Na2C03, brine, treated with activated
carbon, dried over Na2S04 and the solvents removed at reduced
pressure to give X~ as a yellow oil which was carried forward to the
next step.
-68-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
Step F: 3-Amino-4-cyclohexylmethyl-6-methyl-1-(2-t-
butyloxycarbonylamino-6-methyl-5-
methylenecarboxamidomethylpyridinyl)-pyridin[1H]-2-
one (XI-6)
The product from Step D was dissolved in 18 mL of
ethanol, 2 mL of water, 1 mL of acetic acid and hydrogenated at 50 psi
using 32 mg of 20% Pd(OH)2 on carbon overnight. The catalyst was
removed by filtration, concentrated at reduced pressure and the
residue partitioned between CHC13 and 10% Na2C03. The organic
layer was dried over Na2S04 and the solvents removed at reduced
pressure to give I~-6 as a yellow oil:
1H NMR (CDC13) 0 7.64 (d, 1H, 8.3 Hz), 7.38 (d, 1H, 8.4 Hz), 7.36 (br t,
1H), 7.12 (br s) 1H), 5.88 (s, 1H), 4.73 (s, 2H), 4.33 (d, 2H, 5.7 Hz), 3.93
(s, 2H), 2.37 (s, 3H), 2.34 (s, 3H), 2.24 (d, 7.1 Hz), 1.51-1.75 (m, 7H), 1.50
(s, 9H), 1.05-1.28 (m, 4H), 0.92-1.05 (m, 2H).
3-Amino-4-cyclohexylmethyl-6-methyl-1-(2-amino-6-
methyl-5-methylenecarboxamidomethylpyridinyl)-2-
gvridinone (XI-7)
The product from Step F was dissolved in 5 mL of CH2C12
and treated with 5 mL of TFA under Ar for lh, concentrated at reduced
pressure and purified by gradient elution preparative HPLC using a C-
18 stationary phase and 0.1% aqueous TFA/CH3CN as the mobile phase.
The fractions were assayed by analytical HPLC, combined and
concentrated at reduced pressure and lyophilized to provide ~ as a
colorless solid: 1H NMR (D6-DMSO) a 8.68 (br t, 1H), 7.78 (d) 1H, 9.1 Hz),
7.74 (br s, 2H ), 6.80 (d, 1H, 8.8 Hz), 5.91 (s, 1H), 4.66 (s, 2H ), 4.15 (br
d,
2H ), 2.43 ( s, 3H ), 2.20-2.40 (m, 5 H ), 2.16 ( s, 3H ), 1. 50-1.7 0 ( m, 6
H ), 1. 05-
1.25 (m, 3H), 0.9-1.05 (m, 2H).
-69-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98100875
EXAMPLE XII
Tablet Preparation
Tablets containing 10.0, 25.0, and 50.0 mg, respectively, of
compound VII-2 are prepared as illustrated below:
Ingredient Amount-m~
VII-2 10.0 25.0 50.0
Microcrystalline cellulose 80.0 100.0 150.0
Modified food corn starch 10.0 15.0 20.0
Magnesium stearate 0.75 1.0 1.5
All of the active compound, cellulose, and a portion of the
corn starch are mixed and granulated to 10% corn starch paste. The
resulting granulation is sieved, dried and blended with the remainder of
the corn starch and the magnesium stearate. The resulting granulation
is then compressed into tablets containing 10Ø 25.0) and 50.0 mg,
respectively, of active ingredient per tahlet.
-70-
CA 02277929 1999-07-14
WO 98/31670 PCT/US98/00875
~~ ~~II
An intravenous dosage form of the above-indicated active
compound is prepared as follows:
VII-2 0.5-lO.Omg
Sodium Citrate 5-50mg
Citric Acid 1-l5mg
Sodium Chloride 1-8mg
Water for Injection (USP) q.s. to 1 L
Utilizing the above quantities, the active compound is
dissolved at room temperature in a previously prepared solution of
sodium chloride, citric acid, and sodium citrate in Water for Injection
(USP, see page 1636 of United States Pharmacopeia/National Formulary
for 1995, published by United States Pharmacopeial Convention, Inc.,
Rockville, Maryland, copyright 1994.
-71-