Note: Descriptions are shown in the official language in which they were submitted.
CA 02278770 1999-07-23
WO 98/32853 PCT/tJS98/01396
10
SECRETED PROTEINS AND POLYNUCLEOTIDES ENCODING THEM
This application is a continuation-in-part of Ser. No. 60/XXX,XXX (converted
to
a provisional application from non-provisional application Ser. No.
08/788,789), filed
January 24, 1997, which is incorporated by reference herein.
2 p FIELD OF THE INVENTION
T'he present invention provides novel polynucleotides and proteins encoded by
such polynucleotides, along with therapeutic, diagnostic and research
utilities for these
polynucleotides and proteins.
2 5 BACKGROUND OF THE INVENTION
Technology aimed at the discovery of protein factors (including e.g.,
cytokines,
such as lymphokines, interferons, CSFs and interleukins) has matured rapidly
over the
past decade. The now routine hybridization cloning and expression cloning
techniques
clone novel polynudeotides "directly" in the sense that they rely on
information directly
3 0 related to the discovered protein (i.e., partial DNA / amino acid sequence
of the protein
in the case of hybridization cloning; activity of the protein in the case of
expression
cloning). More recent "indirect" cloning techniques such as signal sequence
cloning, which
isolates DNA sequences based on the presence of a now well-recognized
secretory leader
sequence motif, as well as various PCR-based or low stringency hybridization
cloning
3 5 techniques, have advanced the state of the art by making available large
numbers of
DNA / amino acid sequences for proteins that are known to have biological
activity by
virtue of their secreted nature in the case of leader sequence cloning, or by
virtue of the
cell or tissue source in the case of PCR-based techniques. It is to these
proteins and the
polynucleotides encoding them that the present invention is directed.
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
SUMMARY OF THE INVENTION
In one embodiment, the present invention provides a composition comprising an
isolated polynucleotide selected from the group consisting of:
(a} a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:1;
(b) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:1 from nucleotide 506 to nucleotide 643;
(c) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:1 from nucleotide 471 to nucleotide 765;
(d) a polynucleotide comprising the nucleotide sequence of the full-
length protein coding sequence of clone AA35 2 deposited under accession
number ATCC 98303;
(e) a polynucleotide encoding the full-length protein encoded by the
cDNA insert of clone AA35 2 deposited under accession number ATCC 98303;
(f) a polynucleotide comprising the nucleotide sequence of the mature
protein coding sequence of clone AA35_2 deposited under accession number
ATCC 98303;
(g) a polynucleotide encoding the mature protein encoded by the
cDNA insert of clone AA35_2 deposited under accession number ATCC 98303;
2 0 (h) a polynucleotide encoding a protein comprising the amino acid
sequence of SEQ ID N0:2;
(i) a polynucleotide encoding a protein comprising a fragment of the
amino acid sequence of SEQ ID N0:2 having biological activity;
(j) a polynucleotide which is an allelic variant of a polynucleotide of
2 5 (a)-(g) above;
(k) a polynucleotide which encodes a species homologue of the protein
of (h) or (i) above ; and
(1) a polynucleotide capable of hybridizing under stringent conditions
to any one of the polynucleotides specified in (a)-(i).
3 0 Preferably, such polynucleotide comprises the nucleotide sequence of SEQ
ID
N0:1 from nucleotide 506 to nucleotide 643; the nucleotide sequence of SEQ ID
N0:1
from nucleotide 471 to nucleotide 765; the nucleotide sequence of the full-
length protein
coding sequence of clone AA35_2 deposited under accession number ATCC 98303;
or the
nucleotide sequence of the mature protein coding sequence of clone AA35_2
deposited
2
r r .. _~~ _ .__ . , _._ . ___._.._.__.
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
under accession number ATCC 98303. In other preferred embodiments, the
polynucleotide encodes the full-length or mature protein encoded by the cDNA
insert of
clone AA35 2 deposited under accession number ATCC 98303. In yet other
preferred
embodiments, the present invention provides a polynucleotide encoding a
protein
comprising the amino acid sequence of SEQ ID N0:2 from amino acid 1 to amino
acid 32.
Other embodiments provide the gene corresponding to the cDNA sequence of SEQ
ID NO:1.
In other embodiments, the present invention provides a composition comprising
a protein, wherein said protein comprises an amino acid sequence selected from
the group
consisting of:
(a) the amino acid sequence of SEQ ID N0:2;
(b) the amino acid sequence of SEQ ID N0:2 from amino acid 1 to
amino acid 32;
(c) fragments of the amino acid sequence of SEQ ID N0:2; and
(d) the amino acid sequence encoded by the cDNA insert of clone
AA35_2 deposited under accession number ATCC 98303;
the protein being substantially free from other mammalian proteins. Preferably
such
protein comprises the amino acid sequence of SEQ ID N0:2 or the amino acid
sequence
of SEQ ID N0:2 from amino acid 1 to amino acid 32.
2 0 In one embodiment, the present invention provides a composition comprising
an
isolated polynucleotide selected from the group consisting of:
(a) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:3;
(b) a polynucleotide comprising the nucleotide sequence of SEQ ID
2 5 N0:3 from nucleotide 71 to nucleotide 736;
(c) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:3 from nucleotide 113 to nucleotide 736;
(d) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:3 from nucleotide 1 to nucleotide 343;
3 0 (e) a polynucleotide comprising the nucleotide sequence of the full-
length protein coding sequence of clone AM42_3 deposited under accession
number ATCC 98303;
(f) a polynucleotide encoding the full-length protein encoded by the
cDNA insert of clone AM42_3 deposited under accession number ATCC 98303;
3
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(g) a polynucleotide comprising the nucleotide sequence of the mature
protein coding sequence of clone AM42_3 deposited under accession number
ATCC 98303;
(h) a polynucleotide encoding the mature protein encoded by the
cDNA insert of clone AM42 3 deposited under accession number ATCC 98303;
(i) a polynucleotide encoding a protein comprising the amino acid
sequence of SEQ ID N0:4;
(j) a polynucleotide encoding a protein comprising a fragment of the
amino acid sequence of SEQ ID N0:4 having biological activity;
(k} a polynucleotide which is an allelic variant of a polynucleotide of
(a)-(h) above;
a polynucleotide which encodes a species homologue of the protein
of (i) or (j) above ; and
(m) a polynucleotide capable of hybridizing under stringent conditions
115 to any one of the polynucleotides specified in (a)-(j).
Preferably, such polynucleotide comprises the nucleotide sequence of SEQ ID
N0:3 from nucleotide 71 to nucleotide 736; the nucleotide sequence of SEQ ID
N0:3 from
nucleotide 113 to nucleotide 736; the nucleotide sequence of SEQ ID N0:3 from
nucleotide 1 to nucleotide 343; the nucleotide sequence of the full-length
protein coding
2 0 sequence of clone AM42 3 deposited under accession number ATCC 98303; or
the
nucleotide sequence of the mature protein coding sequence of clone AM42 3
deposited
under accession number ATCC 98303. In other preferred embodiments, the
polynucleotide encodes the full-length or mature protein encoded by the cDNA
insert of
clone AM42 3 deposited under accession number ATCC 98303. In yet other
preferred
2 5 embodiments, the present invention provides a polynucleotide encoding a
protein
comprising the amino acid sequence of SEQ ID N0:4 from amino acid 1 to amino
acid 91.
Other embodiments provide the gene corresponding to the cDNA sequence of SEQ
ID N0:3.
In other embodiments, the present invention provides a composition comprising
3 0 a protein, wherein said protein comprises an amino acid sequence selected
from the group
consisting of:
(a) the amino acid sequence of SEQ ID N0:4;
(b) the amino acid sequence of SEQ ID N0:4 from amino acid 1 to
amino acid 91;
4
,.. _'".. ~ ._ .._ ~.......... T.. ., ,. __.......,.r.__.. _..
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(c) fragments of the amino acid sequence of SEQ ID N0:4; and
(d) the amino acid sequence encoded by the cDNA insert of clone
AM42_3 deposited under accession number ATCC 98303;
the protein being substantially free from other mammalian proteins. Preferably
such
protein comprises the amino acid sequence of SEQ ID N0:4 or the amino acid
sequence
of SEQ ID N0:4 from amino acid 1 to amino acid 91.
In one embodiment, the present invention provides a composition comprising an
isolated polynucleotide selected from the group consisting of:
(a) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:5;
(b) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:5 from nucleotide 55 to nucleotide 423;
(c) a polynucleotide comprising the nucleotide sequence of the full-
length protein coding sequence of clone BG137 7 deposited under accession
number ATCC 98303;
(d) a polynucleotide encoding the full-length protein encoded by the
cDNA insert of clone BG 137 7 deposited under accession number ATCC 98303;
(e) a polynucleotide comprising the nucleotide sequence of the mature
protein coding sequence of clone BG137 7 deposited under accession number
2 0 ATCC 98303;
(f) a polynucleotide encoding the mature protein encoded by the
cDNA insert of clone BG137 7 deposited under accession number ATCC 98303;
(g) a polynucleotide encoding a protein comprising the amino acid
sequence of SEQ ID N0:6;
2 5 (h) a polynucleotide encoding a protein comprising a fragment of the
amino acid sequence of SEQ ID N0:6 having biological activity;
(i) a polynucleotide which is an allelic variant of a polynucleotide of
(a)-(f) above;
(j) a polynucleotide which encodes a species homologue of the protein
3 0 of (g) or (h) above ; and
{k) a polynucleotide capable of hybridizing under stringent conditions
to any one of the polynucleotides specified in (a)-(h).
Preferably, such polynucleotide comprises the nucleotide sequence of SEQ ID
N0:5 from nucleotide 55 to nucleotide 423; the nucleotide sequence of the full-
length
5
CA 02278770 1999-07-23
WO 98/32853 PCT/LTS98/01396
protein coding sequence of clone BG 137 7 deposited under accession number
ATCC
98303; or the nucleotide sequence of the mature protein coding sequence of
clone BG137_7
deposited under accession number ATCC 98303. In other preferred embodiments,
the
polynucleotide encodes the full-length or mature protein encoded by the cDNA
insert of
clone BG137_7 deposited under accession number ATCC 98303. In yet other
preferred
embodiments, .the present invention provides a polynucleotide encoding a
protein
comprising the amino acid sequence of SEQ ID N0:6 from amino acid 62 to amino
acid
123.
Other embodiments provide the gene corresponding to the cDNA sequence of SEQ
ID N0:5.
In other embodiments, the present invention provides a composition comprising
a protein, wherein said protein comprises an amino acid sequence selected from
the group
consisting of:
(a) the amino acid sequence of SEQ ID N0:6;
(b) the amino acid sequence of SEQ ID N0:6 from amino acid 62 to
amino acid 123;
(c) fragments of the amino acid sequence of SEQ ID N0:6; and
(d) the amino acid sequence encoded by the cDNA insert of clone
BG137_7 deposited under accession number ATCC 98303;
2 0 the protein being substantially free from other mammalian proteins.
Preferably such
protein comprises the amino acid sequence of SEQ ID N0:6 or the amino acid
sequence
of SEQ ID N0:6 from amino acid 62 to amino acid 123.
In one embodiment, the present invention provides a composition comprising an
isolated polynucleotide selected from the group consisting of:
2 5 (a) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:7;
(b) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:7 from nucleotide 186 to nucleotide 2030;
(c) a polynucleotide comprising the nucleotide sequence of SEQ ID
3 0 N0:7 from nucleotide 873 to nucleotide 2030;
(d) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:7 from nucleotide 802 to nucleotide 1173;
6
__........ ~ ..._..._..~.. ~ .._...,...,.T"~...~..»........
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(e) a polynucleoHde comprising the nucleotide sequence of the full-
length protein coding sequence of clone CH699_1 deposited under accession
number ATCC 98303;
(f) a polynucleotide encoding the full-length protein encoded by the
cDNA insert of clone CH699_1 deposited under accession number ATCC 98303;
(g) a polynucleotide comprising the nucleotide sequence of the mature
protein coding sequence of clone CH699_1 deposited under accession number
ATCC 98303;
(h) a polynucleotide encoding the mature protein encoded by the
cDNA insert of clone CH699_1 deposited under accession number ATCC 98303;
(i) a polynucleotide encoding a protein comprising the amino acid
sequence of SEQ ID N0:8;
(j) a polynucleotide encoding a protein comprising a fragment of the
amino acid sequence of SEQ ID N0:8 having biological activity;
(k) a polynucleotide which is an allelic variant of a polynucleotide of
(a)-(h) above;
(1) a polynucleotide which encodes a species homologue of the protein
of (i) or (j) above ; and
(m) a polynucleotide capable of hybridizing under stringent conditions
2 0 to any one of the polynucleotides specified in (a)-(j).
Preferably, such polynucleotide comprises the nucleotide sequence of SEQ ID
N0:7 from nucleotide 186 to nucleotide 2030; the nucleotide sequence of SEQ ID
N0:7
from nucleotide 873 to nucleotide 2030; the nucleotide sequence of SEQ ID N0:7
from
nucleotide 802 to nucleotide 1173; the nucleotide sequence of the full-length
protein
2 5 coding sequence of clone CH699_1 deposited under accession number ATCC
98303; or the
nucleotide sequence of the mature protein coding sequence of clone CH699_1
deposited
under accession number ATCC 98303. In other preferred embodiments, the
polynucleotide encodes the full-length or mature protein encoded by the cDNA
insert of
clone CH699_1 deposited under accession number ATCC 98303. In yet other
preferred
3 0 embodiments, the present invention provides a polynucleotide encoding a
protein
comprising the amino acid sequence of SEQ ID N0:8 from amino acid 218 to amino
acid
329.
Other embodiments provide the gene corresponding to the cDNA sequence of SEQ
ID N0:7.
7
CA 02278770 1999-07-23
WO 98/32853 PCT/LTS98/01396
In other embodiments, the present invention provides a composition comprising
a protein, wherein said protein comprises an amino acid sequence selected from
the group
consisting of:
(a) the amino acid sequence of SEQ ID NO:8;
(b) the amino acid sequence of SEQ ID N0:8 from amino acid 218 to
amino acid 329;
(c) fragments of the amino acid sequence of SEQ ID N0:8; and
(d) the amino acid sequence encoded by the cDNA insert of clone
CH699_1 deposited under accession number ATCC 98303;
the protein being substantially free from other mammalian proteins. Preferably
such
protein comprises the amino acid sequence of SEQ ID N0:8 or the amino acid
sequence
of SEQ ID N0:8 from amino acid 218 to amino acid 329.
In one embodiment, the present invention provides a composition comprising an
isolated polynucleotide selected from the group consisting of:
(a) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:10;
(b) a polynucleotide comprising the nucleotide sequence of SEQ ID
NO:10 from nucleotide 111 to nucleotide 677;
(c) a polynucleotide comprising the nucleotide sequence of SEQ ID
2 0 N0:10 from nucleotide 156 to nucleotide 677;
(d) a polynucleotide comprising the nucleotide sequence of the full-
length protein coding sequence of clone C0851 1 deposited under accession
number ATCC 98303;
(e) a~zolynucleotide encoding the full-length protein encoded by the
2 5 cDNA insert of clone C0851 1 deposited under accession number ATCC 98303;
(f) a polynucleotide comprising the nucleotide sequence of the mature
protein coding sequence of clone C0851 1 deposited under accession number
ATCC 98303;
(g) a polynucleotide encoding the mature protein encoded by the
3 0 cDNA insert of clone C0851 1 deposited under accession number ATCC 98303;
(h) a polynucleotide encoding a protein comprising the amino acid
sequence of SEQ ID N0:11;
(i) a polynucleotide encoding a protein comprising a fragment of the
amino acid sequence of SEQ ID N0:11 having biological activity;
8
E ..._.._ _.__....~ ...T._ .., ..._.
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(j) a polynucleotide which is an allelic variant of a polynucleotide of
(a)-(g) above;
(k) a polynucleotide which encodes a species homologue of the protein
of (h) or (i) above ; and
(1) a polynucleotide capable of hybridizing under stringent conditions
to anyone of the polynucleotides specified in (a)-(i).
Preferably, such polynucleotide comprises the nucleotide sequence of SEQ ID
NO:10 from nucleotide 111 to nucleotide 677; the nucleotide sequence of SEQ ID
N0:10
from nucleotide 156 to nucleotide 677; the nucleotide sequence of the full-
length protein
coding sequence of clone C0851 1 deposited under accession number ATCC 98303;
or the
nucleotide sequence of the mature protein coding sequence of clone C0851_1
deposited
under accession number ATCC 98303. In other preferred embodiments, the
polynucleotide encodes the full-length or mature protein encoded by the cDNA
insert of
clone C0851 1 deposited under accession number ATCC 98303. In yet other
preferred
embodiments, the present invention provides a polynucleotide encoding a
protein
comprising the amino acid sequence of SEQ ID N0:11 from amino acid 120 to
amino acid
189.
Other embodiments provide the gene corresponding to the cDNA sequence of SEQ
ID NO:10, SEQ ID N0:9 or SEQ ID N0:12 .
2 0 In other embodiments, the present invention provides a composition
comprising
a protein, wherein said protein comprises an amino acid sequence selected from
the group
consisting of:
(a) the amino acid sequence of SEQ ID N0:11;
(b) the amino acid sequence of SEQ ID N0:11 from amino acid 120 to
2 5 amino acid 189;
(c) fragments of the amino acid sequence of SEQ ID N0:11; and
(d) the amino acid sequence encoded by the cDNA insert of clone
C0851_1 deposited under accession number ATCC 98303;
the protein being substantially free from other mammalian proteins. Preferably
such
3 0 protein comprises the amino acid sequence of SEQ ID N0:11 or the amino
acid sequence
of SEQ ID N0:11 from amino acid 120 to amino acid 189.
In one embodiment, the present invention provides a composition comprising an
isolated polynucleotide selected from the group consisting of:
9
a ,
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(a) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:13;
(b) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:13 from nucleotide 123 to nucleotide 755;
(c) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:13 from nucleotide 279 to nucleotide 755;
(d) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:13 from nucleotide 1 to nucleotide 631;
(e) a polynucleotide comprising the nucleotide sequence of the full-
length protein coding sequence of clone CP111_1 deposited under accession
number ATCC 98303;
(f) a polynucleotide encoding the full-length protein encoded by the
cDNA insert of clone CP111_1 deposited under accession number ATCC 98303;
(g) a polynucleotide comprising the nucleotide sequence of the mature
protein coding sequence of clone CP111_1 deposited under accession number
ATCC 98303;
(h) a polynucleotide encoding the mature protein encoded by the
cDNA insert of clone CP111 1 deposited under accession n-umber ATCC 98303;
(i) a polynucleotide encoding a protein comprising the amino acid
2 0 sequence of SEQ ID N0:14;
(j) a polynucleotide encoding a protein comprising a fragment of the
amino acid sequence of SEQ ID N0:14 having biological activity;
(k) a polynucleotide which is an allelic variant of a polynucleotide of
(a)-(h) above;
2 5 (1) a polynucleotide which encodes a species homologue of the protein
of (i) or (j) above ; and
(m) a polynucleotide capable of hybridizing under stringent conditions
to any one of the polynucleoHdes specified in (a)-(j).
Preferably, such polynucleotide comprises the nucleotide sequence of SEQ ID
3 0 N0:13 from nucleotide 123 to nucleotide 755; the nucleotide sequence of
SEQ ID N0:13
from nucleotide 279 to nucleotide 755; the nucleotide sequence of SEQ ID N0:13
from
nucleotide 1 to nucleotide 631; the nucleotide sequence of the full-length
protein coding
sequence of clone CP111_1 deposited under accession number ATCC 98303; or the
nucleotide sequence of the mature protein coding sequence of clone CP111 1
deposited
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
under accession number ATCC 98303. In other preferred embodiments, the
polynucleotide encodes the full-length or mature protein encoded by the cDNA
insert of
clone CP111_1 deposited under accession number ATCC 98303. In yet other
preferred
embodiments, the present invention provides a polynucleotide encoding a
protein
comprising the amino acid sequence of SEQ ID N0:14 from amino acid 1 to amino
acid
171.
Other embodiments provide the gene corresponding to the cDNA sequence of SEQ
ID N0:13.
In other embodiments, the present invention provides a composition comprising
a protein, wherein said protein comprises an amino acid sequence selected from
the group
consisting of:
(a) the amino acid sequence of SEQ ID N0:14;
(b) the amino acid sequence of SEQ ID N0:14 from amino acid 1 to
amino acid 171;
(c) fragments of the amino acid sequence of SEQ ID N0:14; and
(d) the amino acid sequence encoded by the cDNA insert of clone
CP111_1 deposited under accession number ATCC 98303;
the protein being substantially free from other mammalian proteins. Preferably
such
protein comprises the amino acid sequence of SEQ ID N0:14 or the amino acid
sequence
2 0 of SEQ ID N0:14 from amino acid 1 to amino acid 171.
In one embodiment, the present invention provides a composition comprising an
isolated polynucleotide selected from the group consisting of:
(a) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:15;
2 5 (b) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:15 from nucleotide 214 to nucleotide 2760;
(c) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:15 from nucleotide 406 to nucleotide 2760;
(d) a polynucleotide comprising the nucleotide sequence of SEQ ID
3 0 N0:15 from nucleotide 2011 to nucleotide 2565;
(e) a polynucleotide comprising the nucleotide sequence of the full-
length protein coding sequence of clone CS278_1 deposited under accession
number ATCC 98303;
11
F ,
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(f) a polynucleotide encoding the full-length protein encoded by the
cDNA insert of clone CS278_1 deposited under accession number ATCC 98303;
(g) a polynucleotide comprising the nucleotide sequence of the mature
protein coding sequence of clone CS278 1 deposited under accession number
S ATCC 98303;
(h) a polynucleotide encoding the mature protein encoded by the
cDNA insert of clone CS278_1 deposited under accession number ATCC 98303;
(i) a polynucleotide encoding a protein comprising the amino acid
sequence of SEQ ID N0:16;
(j) a polynucleotide encoding a protein comprising a fragment of the
amino acid sequence of SEQ ID N0:16 having biological activity;
(k) a polynucleotide which is an allelic variant of a polynucleotide of
(a)-(h) above;
(1) a polynucleotide which encodes a species homologue of the protein
of (i) or (j) above ; and
(m) a polynucleotide capable of hybridizing under stringent conditions
to any one of the polynucleotides specified in (a)-(j).
Preferably, such polynucleotide comprises the nucleotide sequence of SEQ ID
N0:15 from nucleotide 214 to nucleotide 2760; the nucleotide sequence of SEQ
ID N0:15
2 0 from nucleotide 406 to nucleotide 2760; the nucleotide sequence of SEQ ID
N0:15 from
nucleotide 2011 to nucleotide 2565; the nucleotide sequence of the full-length
protein
coding sequence of clone CS278_1 deposited under accession number ATCC 98303;
or the
nucleotide sequence of the mature protein coding sequence of clone CS278_1
deposited
under accession number ATCC 98303. In other preferred embodiments, the
2 5 polynucleotide encodes the full-length or mature protein encoded by the
cDNA insert of
clone CS278 1 deposited under accession number'ATCC 98303. In yet other
preferred
embodiments, the present invention provides a polynucleotide encoding a
protein
comprising the amino acid sequence of SEQ ID N0:16 from amino acid 596 to
amino acid
784.
3 0 Other embodiments provide the gene corresponding to the cDNA sequence of
SEQ
ID N0:15.
In other embodiments, the present invention provides a composition comprising
a protein, wherein said protein comprises an amino acid sequence selected from
the group
consisting of:
12
,.. r_.. .._...._........ ~ ._...~,._.,_....,.,....... T.... . ..
CA 02278770 1999-07-23
WO 98/32853 PCT/L1S98/01396
(a) the amino acid sequence of SEQ ID N0:16;
(b) the amino acid sequence of SEQ ID N0:16 from amino acid 596 to
amino acid 784;
(c) fragments of the amino acid sequence of SEQ ID N0:16; and
(d) the amino acid sequence encoded by the cDNA insert of clone
CS278~1 deposited under accession number ATCC 98303;
the protein being substantially free from other mammalian proteins. Preferably
such
protein comprises the amino acid sequence of SEQ ID N0:16 or the amino acid
sequence
of SEQ ID N0:16 from amino acid 596 to amino acid 784.
In one embodiment, the present invention provides a composition comprising an
isolated polynucleotide selected from the group consisting of:
(a) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:17;
(b) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:17 from nucleotide 901 to nucleotide 1074;
(c) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:17 from nucleotide 970 to nucleotide 1074;
(d) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:17 from nucleotide 626 to nucleotide 1147;
2 0 (e) a polynucleotide comprising the nucleotide sequence of the full-
length protein coding sequence of clone DF968_3 deposited under accession
number ATCC 98303;
(f) a polynucleotide encoding the full-length protein encoded by the
cDNA insert of_clone DF968_3 deposited under accession number ATCC 98303;
2 5 (g) a polynucleotide comprising the nucleotide sequence of the mature
protein coding sequence of clone DF968_3 deposited under accession number
ATCC 98303;
(h) a polynucleotide encoding the mature protein encoded by the
cDNA insert of clone DF968_3 deposited under accession number ATCC 98303;
3 0 (i) a polynucleotide encoding a protein comprising the amino acid
sequence of SEQ ID N0:18;
(j) a polynucleotide encoding a protein comprising a fragment of the
amino acid sequence of SEQ ID N0:18 having biological activity;
13
CA 02278770 1999-07-23
WO 98132853 PCT/US9810I396
(k) a polynucleotide which is an allelic variant of a polynucleotide of
(a)-(h) above;
a polynucleotide which encodes a species homologue of the protein
of (i) or (j) above ; and
(m) a polynucleotide capable of hybridizing under stringent conditions
to any one of the polynucleotides specified in (a)-(j).
Preferably, such polynucleotide comprises the nucleotide sequence of SEQ ID
N0:17 from nucleotide 901 to nucleotide 1074; the nucleotide sequence of SEQ
ID N0:17
from nucleotide 970 to nucleotide 1074; the nucleotide sequence of SEQ ID
N0:17 from
nucleotide 626 to nucleotide 1147; the nucleotide sequence of the full-length
protein
coding sequence of clone DF968 3 deposited under accession number ATCC 98303;
or the
nucleotide sequence of the mature protein coding sequence of clone DF968 3
deposited
under accession number ATCC 98303. In other preferred embodiments, the
polynucleotide encodes the full-length or mature protein encoded by the cDNA
insert of
clone DF968 3 deposited under accession number ATCC 98303.
Other embodiments provide the gene corresponding to the cDNA sequence of SEQ
ID N0:17.
In other embodiments, the present invention provides a composition comprising
a protein, wherein said protein comprises an amino acid sequence selected from
the group
2 0 consisting of:
(a) the amino acid sequence of SEQ ID N0:18;
(b) fragments of the amino acid sequence of SEQ ID N0:18; and
(c) the amino acid sequence encoded by the cDNA insert of clone
DF968 3 deposited under accession number ATCC 98303;
2 5 the protein being substantially free from other mammalian proteins.
Preferably such
protein comprises the amino acid sequence of SEQ ID N0:18.
In one embodiment, the present invention provides a composition comprising an
isolated polynucleotide selected from the group consisting of:
(a) a polynucleotide comprising the nucleotide sequence of SEQ ID
3 0 N0:19;
(b) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:19 from nucleotide 560 to nucleotide 820;
14
.. T... ~ ....___.-."....~......_.........
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(c) a polynucleotide comprising the nucleotide sequence of the full-
length protein coding sequence of clone DN1120_2 deposited under accession
number ATCC 98303;
(d) a polynucleotide encoding the full-length protein encoded by the
cDNA insert of clone DN1120 2 deposited under accession number ATCC 98303;
(e) a polynucleotide comprising the nucleotide sequence of the mature
protein coding sequence of clone DN1120 2 deposited under accession number
ATCC 98303;
(f) a polynucleotide encoding the mature protein encoded by the
cDNA insert of clone DN1120_2 deposited under accession number ATCC 98303;
(g) a polynucleotide encoding a protein comprising the amino acid
sequence of SEQ ID N0:20;
(h) a polynucleotide encoding a protein comprising a fragment of the
amino acid sequence of SEQ ID N0:20 having biological activity;
' (i) a polynucleotide which is an allelic variant of a polynucleotide of
(a)-(f) above;
(j) a polynucleoHde which encodes a species homologue of the protein
of (g) or (h) above ; and
(k) a polynucleotide capable of hybridizing under stringent conditions
2 0 to any one of the polynucleotides specified in (a)-(h).
Preferably, such polynucleotide comprises the nucleotide sequence of SEQ ID
N0:19 from nucleotide 560 to nucleotide 820; the nucleotide sequence of the
full-length
protein coding sequence of clone DN1120 2 deposited under accession number
ATCC
98303; or the nucleotide sequence of the mature protein coding sequence of
clone
2 5 DN1120_2 deposited under accession number ATCC 98303. In other preferred
embodiments, the polynucleotide encodes the full=length or mature protein
encoded by
the cDNA insert of clone DN1120 2 deposited under accession number ATCC 98303.
In
yet other preferred embodiments, the present invention provides a
polynucleotide
encoding a protein comprising the amino acid sequence of SEQ ID N0:20 from
amino acid
3 0 1 to amino acid 61.
Other embodiments provide the gene corresponding to the cDNA sequence of SEQ
ID N0:19.
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
In other embodiments, the present invention provides a composition comprising
a protein, wherein said protein comprises an amino acid sequence selected from
the group
consisting of:
(a) the amino acid sequence of SEQ ID N0:20;
(b) the amino acid sequence of SEQ ID N0:20 from amino acid 1 to
amino acid 61;
(c) fragments of the amino acid sequence of SEQ ID N0:20; and
(d) the amino acid sequence encoded by the cDNA insert of clone
DN1120_2 deposited under accession number ATCC 98303;
the protein being substantially free from other mammalian proteins. Preferably
such
protein comprises the amino acid sequence of SEQ ID N0:20 or the amino acid
sequence
of SEQ ID N0:20 from amino acid 1 to amino acid 61.
In one embodiment, the present invention provides a composition comprising an
isolated polynucleotide selected from the group consisting af:
(a) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:21;
(b) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:21 from nucleotide 649 to nucleotide 786;
(c) a polynucleotide comprising the nucleotide sequence of SEQ ID
2 0 N0:21 from nucleotide 736 to nucleotide 786;
(d) a polynucleotide comprising the nucleotide sequence of SEQ ID
N0:21 from nucleotide 525 to nucleotide 787;
(e) a polynucleotide comprising the nucleotide sequence of the full-
length protein coding sequence of clone D0589_1 deposited under accession
2 5 number ATCC 98303;
(f) a polynucleotide encoding the full-length protein encoded by the
cDNA insert of clone D0589_1 deposited under accession number ATCC 98303;
(g) a polynucleotide comprising the nucleotide sequence of the mature
protein coding sequence of clone D0589_1 deposited under accession number
3 0 ATCC 98303;
(h) a polynucleotide encoding the mature protein encoded by the
cDNA insert of clone D0589_1 deposited under accession number ATCC 98303;
(i) a polynucleotide encoding a protein comprising the amino acid
sequence of SEQ ID N0:22;
16
.. 1. r ........... _.........._. T.,._..__....~...,Y...-,_. ... _ _._
_.~..~..__v.~.,~____.._.......
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(j) a polynucleotide encoding a protein comprising a fragment of the
amino acid sequence of SEQ ID N0:22 having biological activity;
(k) a polynucleotide which is an allelic variant of a polynucleotide of
(a)-(h) above;
(1) a polynucleotide which encodes a species homologue of the protein
of (i) or (j) above ; and
(m) a polynucleotide capable of hybridizing under stringent conditions
to any one of the polynucleotides specified in (a)-(j).
Preferably, such polynucleotide comprises the nucleotide sequence of SEQ ID
N0:21 from nucleotide 649 to nucleotide 786; the nucleotide sequence of SEQ ID
N0:21
from nucleotide 736 to nucleotide 786; the nucleotide sequence of SEQ ID N0:21
from
nucleotide 525 to nucleotide 787; the nucleotide sequence of the full-length
protein coding
sequence of clone D0589_1 deposited under accession number ATCC 98303; or the
nucleotide sequence of the mature protein coding sequence of clone D0589_1
deposited
under accession number ATCC 98303. In other preferred embodiments, the
polynucleotide encodes the full-length or mature protein encoded by the cDNA
insert of
clone D0589_1 deposited under accession number ATCC 98303.
Other embodiments provide the gene corresponding to the cDNA sequence of SEQ
ID N0:21.
2 0 In other embodiments, the present invention provides a composition
comprising
a protein, wherein said protein comprises an amino acid sequence selected from
the group
consisting of:
(a) the amino acid sequence of SEQ ID N0:22;
(b) fragments of the amino acid sequence of SEQ ID N0:22; and
2 5 (c) the amino acid sequence encoded by the cDNA insert of clone
D0589_1 deposited under accession number ATCC 98303;
the protein being substantially free from other mammalian proteins. Preferably
such
protein comprises the amino acid sequence of SEQ ID N0:22.
In certain preferred embodiments, the polynucleotide is operably linked to an
3 0 expression control sequence. The invention also provides a host cell,
including bacterial,
yeast, insect and mammalian cells, transformed with such polynucleotide
compositions.
Aiso provided by the present invention are organisms that have enhanced,
reduced, or
modified expression of the genes) corresponding to the polynucleotide
sequences
disclosed herein.
17
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
Processes are also provided for producing a protein, which comprise:
(a) growing a culture of the host cell transformed with such
polynucleotide compositions in a suitable culture medium; and
(b) purifying the protein from the culture.
The protein produced according to such methods is also provided by the present
invention. Preferred embodiments include those in which the protein produced
by such
process is a mature form of the protein.
Protein compositions of the present invention may further comprise a
pharmaceutically acceptable carrier. Compositions comprising an antibody which
specifically reacts with such protein are also provided by the present
invention.
Methods are also provided for preventing, treating or ameliorating a medical
condition which comprises administering to a mammalian subject a
therapeutically
effective amount of a composition comprising a protein of the present
invention and a
pharmaceutically acceptable carrier.
BRIEF DESCRIPTION OF THE DRAWINGS
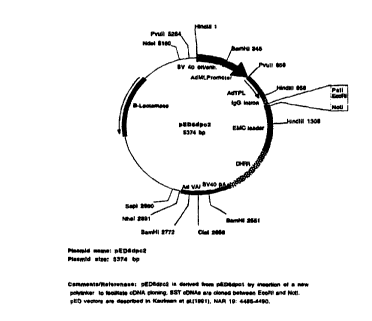
Figures 1A and 1B are schematic representations of the pED6 and pNOTs vectors,
respectively, used for deposit of clones disclosed herein.
2 0 DETAILED DESCRIPTION
ISOLATED PROTEINS AND POLYNUCLEOTIDES
Nucleotide and amino acid sequences, as presently determined, are reported
below for each clone and protein disclosed in the present application. The
nucleotide
sequence of each clone can readily be determined by sequencing of the
deposited clone
2 5 in accordance with known methods. The predicted amino acid sequence (both
full-length
and mature) can then be determined from such nucleotide sequence. The amino
acid
sequence of the protein encoded by a particular clone can also be determined
by
expression of the clone in a suitable host cell, collecting the protein and
determining its
sequence. For each disclosed protein applicants have identified what they have
3 0 determined to be the reading frame best identifiable with sequence
information available
at the time of filing.
As used herein a "secreted" protein is one which, when expressed in a suitable
host
cell, is transported across or through a membrane, including transport as a
result of signal
sequences in its amino acid sequence. "Secreted" proteins include without
limitation
18
1,. .__...._.... _..... ~. ...__.._ ._.......... T.
....._...._...._.._.____r___~_._______ _._..
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
proteins secreted wholly (e.g., soluble proteins) or partially (e.g. ,
receptors) from the cell
in which they are expressed. "Secreted" proteins also include without
limitation proteins
which are transported across the membrane of the endoplasmic reticulum.
Clone "AA35 2"
A polynucleotide of the present invention has been identified as clone "AA35
2".
AA35_2 was isolated from a human fetal kidney cDNA library using methods which
are
selective for cDNAs encoding secreted proteins {see U.S. Pat. No. 5,536,637),
or was
identified as encoding a secreted or transmembrane protein on the basis of
computer
analysis of the amino acid sequence of the encoded protein. AA35 2 is a full-
length clone,
including the entire coding sequence of a secreted protein (also referred to
herein as
"AA35_2 protein").
The nucleotide sequence of AA35_2 as presently determined is reported in SEQ
ID N0:1. What applicants presently believe to be the proper reading frame and
the
predicted amino acid sequence of the AA35_2 protein corresponding to the
foregoing
nucleotide sequence is reported in SEQ ID N0:2.
The EcoRI/NotI restriction fragment obtainable from the deposit containing
clone
AA35_2 should be approximately 1400 bp.
The nucleotide sequence disclosed herein for AA35 2 was searched against the
GenBank and GeneSeq nucleotide sequence databases using BLASTN/BLASTX and
FASTA search protocols. AA35_2 demonstrated at least some similarity with
sequences
identified as C16789 (Human placenta cDNA 5'-end GEN-529D11), H23653
(yn72e01.r1
Homo Sapiens cDNA clone 173976 5' similar to contains Alu repetitive element),
L31848
(Homo Sapiens serine/threonine kinase receptor 2 (SKR2) gene, 3 alternative
splices, 3'
2 5 ends), U40455 (Human chromosome X cosmid, clones 196B12, 9H11 and 43H9,
repeat
units and sequence tagged sites), and 282197 (Human DNA sequence from clone
J293L6).
The predicted amino acid sequence disclosed herein for AA35 2 was searched
against the
GenPept and GeneSeq amino acid sequence databases using the BLASTX search
protocol.
The predicted AA35 2 protein demonstrated at least some similarity to
sequences
3 0 identified as U58658 (unknown [Homo sapiens]) and X55777 (put. ORF [Homo
sapiens]).
Based upon sequence similarity, AA35 2 proteins and each similar protein or
peptide may
share at least some activity. The nucleotide sequence of AA35 2 indicates that
it may
contain an Alu repetitive element.
19
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/OI396
Clone "AM42 3"
A polynucleotide of the present invention has been identified as clone
"AM42_3".
AM42_3 was isolated from a human fetal kidney cDNA library using methods which
are
selective for cDNAs encoding secreted proteins (see U.S. Pat. No. 5,536,637),
or was
identified as encoding a secreted or transmembrane protein on the basis of
computer
analysis of the amino acid sequence of the encoded protein. AM42 3 is a full-
length clone,
including the entire coding sequence of a secreted protein (also referred to
herein as
"AM42_3 protein").
The nucleotide sequence of AM42_3 as presently determined is reported in SEQ
ID N0:3. What applicants presently believe to be the proper reading frame and
the
predicted amino acid sequence of the AM42_3 protein corresponding to the
foregoing
nucleotide sequence is reported in SEQ ID N0:4. Amino acids 2 to 14 are a
predicted
leader/signal sequence, with the predicted mature amino acid sequence
beginning at
amino acid 15, or are a transmembrane domain.
T'he EcoRI/NotI restriction fragment obtainable from the deposit containing
clone
AM42_3 should be approximately 1400 bp.
The nucleotide sequence disclosed herein for AM42 3 was searched against the
GenBank and GeneSeq nucleotide sequence databases using BLASTN/BLASTX and
FASTA search protocols. AM42_3 demonstrated at least some similarity with
sequences
2 0 identified as AA109637 (mm01f02.r1 Stratagene mouse kidney (#937315) Mus
musculus
cDNA clone 520251 5'), AA131170 (zo08e05.s1 Stratagene neuroepithelium NT2RAMI
937234 Homo Sapiens cDNA clone 567104 3'), AA131483 (zo08e05.r1 Stratagene
neuroepithelium NT2RAMI 937234 Homo sapiens cDNA clone 567104 5'), and
AA445683
(vf62h07.r1 Barstead 1V1_PLRB1 Mus musculus cDNA clone 848413 5'). Based upon
2 5 sequence similarity, AM42_3 proteins and each similar protein or peptide
may share at
least some activity. The TopPredII computer program predicts a potential
transmembrane
domain within the AM42_3 protein sequence centered around amino acid 152 of
SEQ ID
N0:4.
3 0 Clone "BG137 7"
A polynucleotide of the present invention has been identified as clone
"BG137_7".
BG137_7 was isolated from a human adult brain cDNA library using methods which
are
selective for cDNAs encoding secreted proteins (see U.S. Pat. No. 5,536,637);
or was
identified as encoding a secreted or transmembrane protein on the basis of
computer
T R ~ ,_._. T _. _.___ _.._....._ . .
CA 02278770 1999-07-23
WO 98132853 PCT/US98/01396
analysis of the amino acid sequence of the encoded protein. BG137_7 is a full-
length
clone, including the entire coding sequence of a secreted protein (also
referred to herein
as "BG137_7 protein'}.
The nucleotide sequence of BG137_7 as presently determined is reported in SEQ
ID N0:5. What applicants presently believe to be the proper reading frame and
the
predicted amino acid sequence of the BG137_7 protein corresponding to the
foregoing
nucleotide sequence is reported in SEQ ID N0:6.
The EcoRI/NotI restriction fragment obtainable from the deposit containing
clone
BG137_7 should be approximately 500 bp.
The nucleotide sequence disclosed herein for BG137_7 was searched against the
GenBank and GeneSeq nucleotide sequence databases using BLASTN/BLASTX and
FASTA search protocols. BG137_7 demonstrated at least some similarity with
sequences
identified as D87683 (Human mRNA for KIAA0243 gene, partial cds). Based upon
sequence similarity, BG137_7 proteins and each similar protein or peptide may
share at
least some activity.
Clone "CH699 1"
A polynucleotide of the present invention has been identified as clone
"CH699_1".
CH699_1 was isolated from a human fetal kidney cDNA library using methods
which are
2 0 selective for cDNAs encoding secreted proteins (see U.S. Pat. No.
5,536,637), or was
identified as encoding a secreted or transmembrane protein on the basis of
computer
analysis of the amino acid sequence of the encoded protein. CH699_1 is a full-
length
clone, including the entire coding sequence of a secreted protein (also
referred to herein
as "CH699_1 protein").
2 5 The nucleotide sequence of CH699_1 as presently determined is reported in
SEQ
ID N0:7. What applicants presently believe to be the proper reading frame and
the
predicted amino acid sequence of the CH699_1 protein corresponding to the
foregoing
nucleotide sequence is reported in SEQ ID N0:8. Amino acids 217 to 229 are a
predicted
leader/signal sequence, with the predicted mature amino acid sequence
beginning at
3 0 amino acid 230, or are a transmembrane domain.
The EcoRI/NotI restriction fragment obtainable from the deposit containing
clone
CH699_1 should be approximately 2000 bp.
The nucleotide sequence disclosed herein for CH699_1 was searched against the
GenBank and GeneSeq nucleotide sequence databases using BLASTN/BLASTX and
21
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/0139b
FASTA search protocols. CH699_1 demonstrated at least some similarity with
sequences
identified as AA155014 (mr99h05.r1 Stratagene mouse embryonic carcinoma
(#937317)
Mus musculus cDNA clone 605625 5'), AA423476 (ve76d07.r1 Soares mouse mammary
gland NbMMG Mus musculus cDNA clone 8321415'), U79271 (Human clones 23920 and
23921 mRNA sequence), and W72147 (zd70f08.s1 Soares fetal heart NbHHI9W Homo
Sapiens cDNA. clone 346023 3'). The predicted amino acid sequence disclosed
herein for
CH699_1 was searched against the GenPept and GeneSeq amino acid sequence
databases
using the BLASTX search protocol. The predicted CH699_1 protein demonstrated
at least
some similarity to sequences identified as X51591 (beta-myosin heavy chain
[Homo
sapiens]). Based upon sequence similarity, CH699_1 proteins and each similar
protein or
peptide may share at least some activity.
Clone "C0851 1"
A polynucleotide of the present invention has been identified as clone
"C0851_1".
C0851_1 was' isolated from a human adult brain cDNA library using methods
which are
selective for cDNAs encoding secreted proteins (see U.S. Pat. No. 5,536,637),
or was
identified as encoding a secreted or transmembrane protein on the basis of
computer
analysis of the amino acid sequence of the encoded protein. C0851_1 is a full-
length
clone, including the entire coding sequence of a secreted protein (also
referred to herein
2 0 as "C0851 1 protein").
The nucleotide sequence of the 5' portion of C0851 1 as presently determined
is
reported in SEQ ID N0:9. An additional internal nucleotide sequence from
C0851_1 as
presently determined is reported in SEQ ID N0:10. What applicants believe is
the proper
reading frame and the predicted amino acid sequence encoded by such internal
sequence
2 5 is reported in SEQ ID N0:11. Amino acids 3 to 15 of SEQ ID N0:11 are a
predicted
leader/signal sequence, with the predicted mature amino acid sequence
beginning at
amino acid 16, or are a transmembrane domain. Additional nucleotide sequence
from the
3' portion of C0851_l, including the polyA tail, is reported in SEQ ID N0:12.
The EcoRI/NotI restriction fragment obtainable from the deposit containing
clone
3 0 C0851_1 should be approximately 1800 bp.
The nucleotide sequence disclosed herein for C0851_1 was searched against the
GenBank and GeneSeq nucleotide sequence databases using BLASTN / BLASTX and
FASTA search protocols. C0851 1 demonstrated at least some similarity with
sequences
identified as AA132585 (zo20c04.r1 Stratagene colon (#937204) Homo sapiens
cDNA clone
22
r ~ __. _. _ _ ~. __._. __ .. e... . . _..__.__
CA 02278770 1999-07-23
WO 98132853 PCT/US98/01396
587430 5'), H51262 (yp83b07.s1 Homo Sapiens cDNA clone 194005 3'), W44070
(mc73a09.r1
Soares mouse embryo NbME13.514.5 Mus musculus cDNA clone 354136 5'), and
X92871
(X.laevis mRNA for an unknown transmembrane protein). The predicted amino acid
sequence disclosed herein for C0851 1 was searched against the GenPept and
GeneSeq
amino acid sequence databases using the BLASTX search protocol. The predicted
C0851 1 protein demonstrated at least some similarity to sequences identified
as X92871
(unknown transmembrane protein [Xenopus laevis]). Based upon sequence
similarity,
C0851_1 proteins and each similar protein or peptide may share at least some
activity.
The nucleotide sequence of C0851_1 indicates that it may contain an Alu
repetitive
element.
Clone "CP111 1"
A polynucleotide of the present invention has been identified as clone "CP111
1 ".
CP111_l was isolated from a human adult salivary gland cDNA library using
methods
which are selective for cDNAs encoding secreted proteins {see U.S. Pat. No.
5,536,637), or
was identified as encoding a secreted or transmembrane protein on the basis of
computer
analysis of the amino acid sequence of the encoded protein. CP111 1 is a full-
length clone,
including the entire coding sequence of a secreted protein (also referred to
herein as
"CP111_1 protein").
2 0 The nucleotide sequence of CP111_1 as presently determined is reported in
SEQ
ID N0:13. What applicants presently believe to be the proper reading frame and
the
predicted amino acid sequence of the CP111_1 protein corresponding to the
foregoing
nucleotide sequence is reported in SEQ ID N0:14. Amino acids 40 to 52 are a
predicted
leader/signal sequence, with the predicted mature amino acid sequence
beginning at
2 5 amino acid 53, or are a transmembrane domain.
The EcoRI/NotI restriction fragment obtainable from the deposit containing
clone
CP111_1 should be approximately 3200 bp.
The nucleotide sequence disclosed herein for CP111_1 was searched against the
GenBank and GeneSeq nucleotide sequence databases using BLASTN/BLASTX and
3 0 FASTA search protocols. CP111 1 demonstrated at least some similarity with
sequences
identified as T53688 (ya98g07.r1 Homo Sapiens cDNA clone 69756 5') and W70295
(zd58f03.s1 Soares fetal heart NbHHI9W Homo sapiens cDNA clone 344861 3'). The
predicted amino acid sequence disclosed herein for CP111_1 was searched
against the
GenPept and GeneSeq amino acid sequence databases using the BLASTX search
protocol.
23
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
The predicted CP111_1 protein demonstrated at least some similarity to
sequences
identified as X88852 (env protein [Primate T-cell lymphotropic]). Based upon
sequence
similarity, CP111 1 proteins and each similar protein or peptide may share at
least some
activity. The TopPredII computer program predicts a potential transmembrane
domain
within the CP111 1 protein sequence centered around amino acid 50 of SEQ ID
N0:14.
Clone "CS278 1"
A polynucleotide of the present invention has been identified as clone
"CS278_1 ".
CS278_1 was isolated from a human fetal brain cDNA library using methods which
are
selective for cDNAs encoding secreted proteins (see U.S. Pat. No. 5,536,637),
or was
identified as encoding a secreted or transmembrane protein on the basis of
computer
analysis of the amino acid sequence of the encoded protein. CS278_1 is a full-
length clone,
including the entire coding sequence of a secreted protein (also referred to
herein as
"CS278_1 protein").
The nucleotide sequence of CS278 1 as presently determined is reported in SEQ
ID N0:15. What applicants presently believe to be the proper reading frame and
the
predicted amino acid sequence of the CS278_l protein corresponding to the
foregoing
nucleotide sequence is reported in SEQ ID N0:16. Amino acids 52 to 64 are a
predicted
leader/signal sequence, with the predicted mature amino acid sequence
beginning at
2 0 amino acid 65, or are a transmembrane domain.
The EcoRI/NotI restriction fragment obtainable from the deposit containing
clone
CS278 1 should be approximately 4400 bp.
The nucleotide sequence disclosed herein for CS278_1 was searched against the
GenBank and GeneSeq nucleotide sequence databases using BLASTN/BLASTX and
2 5 FASTA search protocols. CS278_1 demonstrated at least some similarity with
sequences
identified as AA234319 (zr66c07.r1 Soares NhHMPu S1 Homo sapiens cDNA clone
668364
5'), H44192 (yo73f09.r1 Homo sapiens cDNA clone 183593 5'), W18258 (mb86a11.r1
Soares
mouse p3NMF19), X76589 (H.sapiens DNA 3' flanking simple sequence region clone
wg2c3), and 274652 (M.musculus mRNA; expressed sequence tag (tcc2)). The
predicted
3 0 amino acid sequence disclosed herein for CS278_1 was searched against the
GenPept and
GeneSeq amino acid sequence databases using the BLASTX search protocol. The
predicted CS278_1 protein demonstrated at least some similarity to sequences
identified
as M34651 {ORF-3 protein [Suid herpesvirus 1 ] ). The predicted CS278_1
protein also
demonstrated at least some similarity to a protein motif, cytochrome P450
cysteine heme-
24
~ ..._.._ _ . T _ .. t.. _ .
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
iron ligand signature. Based upon sequence similarity, CS278_1 proteins and
each similar
protein or peptide may share at least some activity. The TopPredII computer
program
predicts five potential transmembrane domains within the CS278 1 protein
sequence,
which are centered around amino acids 75) 160, 525, 610, and 700 of SEQ ID
N0:16,
respectively. The nucleotide sequence of CS278_1 may contain GAA simple repeat
elements.
Clone "DF968 3"
A polynucleotide of the present invention has been identified as clone "DF968
3".
DF968_3 was isolated from a human adult brain cDNA library using methods which
are
selective for cDNAs encoding secreted proteins (see U.S. Pat. No. 5,536,637),
or was
identified as encoding a secreted or transmembrane protein on the basis of
computer
analysis of the amino acid sequence of the encoded protein. DF968_3 is a full-
length
clone, including the entire coding sequence of a secreted protein (also
referred to herein
as "DF968_3 protein")
The nucleotide sequence of DF968 3 as presently determined is reported in SEQ
ID N0:27. What applicants presently believe to be the proper reading frame and
the
predicted amino acid sequence of the DF968 3 protein corresponding to the
foregoing
nucleotide sequence is reported in SEQ ID N0:18. Amino acids 11 to 23 are a
predicted
2 0 leader/signal sequence, with the predicted mature amino acid sequence
beginning at
amino acid 24, or are a transmembrane domain. Another possible DF968_3 reading
frame
and predicted amino acid sequence is encoded by basepairs 191 to 430 of SEQ ID
N0:17
and is reported in SEQ ID N0:33.
'The EcoRI/NotI restriction fragment obtainable from the deposit containing
clone
2 5 DF968_3 should be approximately 1010 bp.
The nucleotide sequence disclosed herein for DF968_3 was searched against the
GenBank and GeneSeq nucleotide sequence databases using BLASTN/BLASTX and
FASTA search protocols. DF968 3 demonstrated at least some similarity with
sequences
identified as AA426010 (zw49e12.s1 Soares total fetus Nb2HF8 9w Homo Sapiens
cDNA
3 0 clone 773422 3' similar to contains element LTR5 repetitive element),
H28256 (yn48a04.r1
Homo sapiens cDNA clone 171630 5'), and T06820 (EST04709 Homo Sapiens cDNA
clone
HFBDZ29). The predicted amino acid sequence disclosed herein for DF968 3 was
searched against the GenPept and GeneSeq amino acid sequence databases using
the
BLASTX search protocol. The predicted DF968 3 protein demonstrated at least
some
CA 02278770 1999-07-23
WO 98/32853 PCT/ITS98/01396
similarity to sequences identified as 238125 (orf, len 112, CAI 0.07). Based
upon sequence
similarity, DF968_3 proteins and each similar protein or peptide may share at
least some
activity. The nucleotide sequence of DF968 3 indicates that it may contain
repeat
sequences.
Clone "DN1120 2"
A polynucleotide of the present invention has been identified as clone "DN1120
2".
DN1120 2 was isolated from a human fetal brain cDNA library using methods
which are
selective for cDNAs encoding secreted proteins (see U.S. Pat. No. 5,536,637),
or was
identified as encoding a secreted or transmembrane protein on the basis of
computer
analysis of the amino acid sequence of the encoded protein. DN1120_2 is a full-
length
clone, including the entire coding sequence of a secreted protein (also
referred to herein
as "DN1120 2 protein")
The nucleotide sequence of DN1120 2 as presently determined is reported in SEQ
ID N0:19. What applicants presently believe to be the proper reading frame and
the
predicted amino acid sequence of the DN1120 2 protein corresponding to the
foregoing
nucleotide sequence is reported in SEQ ID N0:20.
The EcoRI/NotI restriction fragment obtainable from the deposit containing
clone
DN1120_2 should be approximately 1000 bp.
2 0 The nucleotide sequence disclosed herein for DN1120 2 was searched against
the
GenBank and GeneSeq nucleotide sequence databases using BLASTN/BLASTX and
FASTA search protocols. DN1120 2 demonstrated at least some similarity with
sequences
identified as M62256 (EST00323 Homo Sapiens cDNA clone HHCH15 similar to Alu
repetitive element), M78991 (EST01139 Homo sapiens cDNA clone HHCPG39), Q59179
2 5 (Human brain Expressed Sequence Tag EST00323), and Q61084 (Human brain
Expressed
Sequence Tag EST01139). Based upon sequence similarity, DN1120_2 proteins and
each
similar protein or peptide may share at least some activity.
Clone "D0589 1"
3 0 A polynucleotide of the present invention has been identified as clone
"D0589_1".
D0589_l was isolated from a human adult testes cDNA library using methods
which are
selective for cDNAs encoding secreted proteins (see U.S. Pat. No. 5,536,637),
or was
identified as encoding a secreted or transmembrane protein on the basis of
computer
analysis of the amino acid sequence of the encoded protein. D0589_1 is a full-
length
26
'». ._...»._., T ,~~"~,--,v,".-.....
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
clone, including the entire coding sequence of a secreted protein (also
referred to herein
as "D0589_1 protein").
The nucleotide sequence of D0589_1 as presently determined is reported in SEQ
ID N0:21. What applicants presently believe to be the proper reading frame and
the
predicted amino acid sequence of the D0589_1 protein corresponding to the
foregoing
nucleotide sequence is reported in SEQ ID NO:22. Amino acids 17 to 29 are a
predicted
leader/signal sequence, with the predicted mature amino acid sequence
beginning at
amino acid 30, or are a transmembrane domain.
The EcoRI/NotI restriction fragment obtainable from the deposit containing
clone
D0589_1 should be approximately 1800 bp.
The nucleotide sequence disclosed herein for D0589_1 was searched against the
GenBank and GeneSeq nucleotide sequence databases using BLASTN/BLASTX and
FASTA search protocols. D0589_1 demonstrated at least some similarity with
sequences
identified as AA402420 (zu47e04.s1 Soares ovary tumor NbHOT Homo sapiens cDNA
clone 741150 3'), AA426621 (zw03a09.r1 Soares NhHMPu S1 Homo sapiens cDNA
clone
768184 5'), AA436749 (zv67c10.r1 Soares total fetus Nb2HF8 9w Homo sapiens
cDNA
clone 758706 5'), H12845 (yj14h06.r1 Homo Sapiens cDNA clone 148763 5'),
842350
(yg01b05.s1 Homo Sapiens cDNA clone 30909 3'), W02775 (zc65g07.s1 Soares fetal
heart
NbHHI9W Homo Sapiens cDNA clone 327228 3'), W24833 (zc65g07.r1 Soares fetal
heart),
2 0 W58173 (zd19f02.s1 Soares fetal heart NbHHI9W Homo sapiens cDNA clone
341115 3'
similar to contains Alu repetitive element;contains element Ll repetitive
element), and
282201 (Human DNA sequence from clone J345P10). Based upon sequence
similarity,
D0589_1 proteins and each similar protein or peptide may share at least some
activity.
2 S Deposit of Clones
Clones AA35 2, AM42_3, BG137_7, CH699_1, C0851 1, CP111 1, CS278_1,
DF968_3, DN1120_2, and D0589_1 were deposited on January 23,1997 with the
American
Type Culture Collection as an original deposit under the Budapest Treaty and
were given
the accession number ATCC 98303, from which each clone comprising a particular
3 0 polynucleotide is obtainable. All restrictions on the availability to the
public of the
deposited material will be irrevocably removed upon the granting of the
patent, except
for the requirements specified in 37 C.F.R. ~ 1.808(b).
Each clone has been transfected into separate bacterial cells (E. coli) in
this
composite deposit. Each clone can be removed from the vector in which it was
deposited
27
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
by performing an EcoRI/NotI digestion (5' site, EcoRI; 3' site, NotI) to
produce the
appropriate fragment for such clone. Each clone was deposited in either the
pED6 or
pNOTs vector depicted in Fig. 1. The pED6dpc2 vector ("pED6") was derived from
pED6dpc1 by insertion of a new polylinker to facilitate cDNA cloning (Kaufman
et al.,
1991, Nucleic Acids Res. 19: 4485-4490); the pNOTs vector was derived from
pMT2
(Kaufman et al., 1989, Mol. Cell. Biol. 9: 946-958) by deletion of the DHFR
sequences,
insertion of a new polylinker, and insertion of the M13 origin of replication
in the CIaI site.
In some instances, the deposited clone can become "flipped" (i.e., in the
reverse
orientation) in the deposited isolate. In such instances, the cDNA insert can
still be
isolated by digestion with EcoRI and NotI. However, NotI will then produce the
5' site
and EcoRI will produce the 3' site for placement of the cDNA in proper
orientation for
expression in a suitable vector. The cDNA may also be expressed from the
vectors in
which they were deposited.
Bacterial cells containing a particular clone can be obtained from the
composite
deposit as follows:
An oligonucleotide probe or probes should be designed to the sequence that is
known for that particular clone. This sequence can be derived from the
sequences
provided herein, or from a combination of those sequences. The sequence of the
oligonucleotide probe that was used to isolate each full-length clone is
identified below,
2 0 and should be most reliable in isolating the clone of interest.
Clone Probe Sequence
AA35_2 SEQ ID N0:23
AM42_3 SEQ ID N0:24
2 5 BG137_7 SEQ ID N0:25
CH699_1 ~ SEQ ID N0:26
C0851_l SEQ ID N0:27
CP111_1 SEQ ID N0:28
CS278_1 SEQ ID N0:29
3 0 DF968 3 SEQ ID N0:30
DN1120_2 SEQ ID N0:31
D0589_1 SEQ ID N0:32
28
T ~ _._.~_ T _ ._...... _....___ W_...r..___._..., i
CA 02278770 1999-07-23
WO 98/32853 PCT/LTS98/01396
In the sequences listed above which include an N at position 2, that position
is occupied
in preferred probes / primers by a biotinylated phosphoararnidite residue
rather than a
nucleotide (such as , for example, that produced by use of biotin
phosphoramidite (1-
dimethoxytrityloxy-2-(N-biotinyl-4-aminobutyl)-propyl-3-O-(2-cyanoethyl)-(N,N-
diisopropyl)-phosphoramadite) (Glen Research, cat. no. 10-1953)).
The design of the oligonucleotide probe should preferably follow these
parameters:
(a) It should be designed to an area of the sequence which has the fewest
ambiguous bases ("N's"), if any;
(b) It should be designed to have a Tm of approx. 80 ° C (assuming
2° for each
A or T and 4 degrees for each G or C).
The oligonucleotide should preferably be labeled with g-32P ATP (specific
activity 6000
Ci / mmole) and T4 polynucleotide kinase using commonly employed techniques
for
labeling oligonucleotides. Other labeling techniques can also be used.
Unincorporated
label should preferably be removed by gel filtration chromatography or other
established
methods. The amount of radioactivity incorporated into the probe should be
quantitated
by measurement in a scintillation counter. Preferably, specific activity of
the resulting
probe should be approximately 4e+6 dpm/pmole.
The bacterial culture containing the pool of full-length clones should
preferably
2 0 be thawed and 100 ul of the stock used to inoculate a sterile culture
flask containing 25 ml
of sterile L-broth containing ampicillin at 100 ug/ml. The culture should
preferably be
grown to saturation at 37°C, and the saturated culture should
preferably be diluted in
fresh L-broth. Aliquots of these dilutions should preferably be plated to
determine the
dilution and volume which will yield approximately 5000 distinct and well-
separated
2 5 colonies on solid bacteriological media containing L-broth containing
ampicillin at 100
pg/ml and agar at 1.5% in a 150 mm petri dish when grown overnight at
37°C. Other
known methods of obtaining distinct, well-separated colonies can also be
employed.
Standard colony hybridization procedures should then be used to transfer the
colonies to nitrocellulose filters and lyse, denature and bake them.
3 0 The filter is then preferably incubated at 65°C for 1 hour with
gentle agitation in
6X SSC (20X stock is 175.3 g NaCI / liter, 88.2 g Na citrate / liter, adjusted
to pH 7.0 with
NaOH) containing 0.5% SDS,100 lZg/ml of yeast RNA, and 10 mM EDTA
(approximately
10 mL per 150 mm filter). Preferably, the probe is then added to the
hybridization mix at
a concentration greater than or equal to 1e+6 dpm/mL. The filter is then
preferably
29
CA 02278770 1999-07-23
WO 98132853 PCT/US98/01396
incubated at 65°C with gentle agitation overnight. The filter is then
preferably washed in
500 mL of 2X SSC/0.5% SDS at room temperature without agitation, preferably
followed
by 500 mL of 2X SSC/0.1% SDS at room temperature with gentle shaking for 15
minutes.
A third wash with O.1X SSC/0.5% SDS at 65°C for 30 minutes to 1 hour is
optional. The
filter is then preferably dried and subjected to autoradiography for
sufficient time to
visualize the positives on the X-ray film. Other known hybridization methods
can also
be employed.
The positive colonies are picked, grown in culture, and plasmid DNA isolated
using standard procedures. The clones can then be verified by restriction
analysis,
I 0 hybridization analysis, or DNA sequencing.
Fragments of the proteins of the present invention which are capable of
exhibiting
biological activity are also encompassed by the present invention. Fragments
of the
protein may be in linear form or they may be cyclized using known methods, for
example,
as described in H.U. Saragovi, et al., Bio/Technology 10) 773-778 (1992) and
in R.S.
1'S McDowell, et al., J. Amer. Chem. Soc. 114) 9245-9253 (1992), both of which
are incorporated
herein by reference. Such fragments may be fused to carrier molecules such as
immunoglobulins for many purposes, including increasing the valency of protein
binding
sites. For example, fragments of the protein may be fused through "linker"
sequences to
the Fc portion of an immunoglobulin. For a bivalent form of the protein, such
a fusion
2 0 could be to the Fc portion of an IgG molecule. Other immunoglobulin
isotypes may also
be used to generate such fusions. For example, a protein - IgM fusion would
generate a
decavalent form of the protein of the invention.
The present invention also provides both full-length and mature forms of the
disclosed proteins. The full-length form of the such proteins is identified in
the sequence
2 5 listing by translation of the nucleotide sequence of each disclosed clone.
The mature form
of such protein may be obtained by expression of the disclosed full-length
polynucleotide
(preferably those deposited with ATCC) in a suitable mammalian cell or other
host cell.
The sequence of the mature form of the protein may also be determinable from
the amino
acid sequence of the full-length form.
3 0 The present invention also provides genes corresponding to the
polynucleotide
sequences disclosed herein. "Corresponding genes" are the regions of the
genome that
are transcribed to produce the mRNAs from which cDNA polynucleotide sequences
are
derived and may include contiguous regions of the genome necessary for the
regulated
expression of such genes. Corresponding genes may therefore include but are
not limited
? _..._.~_ ~._. T _....._._..,~_ . ... ._._.. _.
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
to coding sequences, 5' and 3' untranslated regions, alternatively spliced
exons, introns,
promoters, enhancers, and silencer or suppressor elements. The corresponding
genes can
be isolated in accordance with known methods using the sequence information
disclosed
herein. Such methods include the preparation of probes or primers from the
disclosed
sequence information for identification and/or amplification of genes in
appropriate
genomic libraries or other sources of genomic materials. An "isolated gene" is
a gene that
has been separated from the adjacent coding sequences, if any, present in the
genome of
the organism from which the gene was isolated.
Organisms that have enhanced, reduced, or modified expression of the genes)
corresponding to the polynucleotide sequences disclosed herein are provided.
The
desired change in gene expression can be achieved through the use of antisense
polynucleotides or ribozymes that bind and/or cleave the mRNA transcribed from
the
gene (Albert and Morris,1994, Trends Pharmacol. Sci. 15(7): 250-254; Lavarosky
et al., 1997,
Biochem. Mol. Med. 62(1): 11-22; and Hampel, 1998, Prog. Nucleic Acid Res.
Mol. Biol. 58: 1-
39; all of which are incorporated by reference herein). Transgenic animals
that have
multiple copies of the genes) corresponding to the polynucleotide sequences
disclosed
herein, preferably produced by transformation of cells with genetic constructs
that are
stably maintained within the transformed cells and their progeny, are
provided.
Transgenic animals that have modified genetic control regions that increase or
reduce
2 0 gene expression levels, or that change temporal or spatial patterns of
gene expression, are
also provided (see European Patent No. 0 649 464 B1, incorporated by reference
herein).
In addition, organisms are provided in which the genes) corresponding to the
polynucleotide sequences disclosed herein have been partially or completely
inactivated,
through insertion of extraneous sequences into the corresponding genes) or
through
2 5 deletion of all or part of the corresponding gene(s). Partial or complete
gene inactivation
can be accomplished through insertion, preferably followed by imprecise
excision, of
transposable elements (Plasterk,1992, Bioessays 14(9): 629-633; Zwaal et
al.,1993, Proc. Natl.
Acad. Sci. USA 90(16): 7431-7435; Clark et al.,1994, Proc. Natl. Acad. Sci.
USA 91(2): 719-722;
all of which are incorporated by reference herein), or through homologous
recombination,
3 0 preferably detected by positive/negative genetic selection strategies
(Mansour et al., 1988,
Nature 336: 348-352; U.S. Patent Nos. 5,464,764; 5,487,992; 5,627,059;
5,631,153; 5,614, 396;
5,616,491; and 5,679,523; all of which are incorporated by reference herein).
These
organisms with altered gene expression are preferably eukaryotes and more
preferably
are mammals. Such organisms are useful for the development of non-human models
for
31
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
the study of disorders involving the corresponding gene(s), and for the
development of
assay systems for the identification of molecules that interact with the
protein products)
of the corresponding gene(s).
Where the protein of the present invention is membrane-bound (e.g., is a
receptor),
the present invention also provides for soluble forms of such protein. In such
forms part
or all of the intracellular and transmembrane domains of the protein are
deleted such that
the protein is fully secreted from the cell in which it is expressed. The
intracellular and
transmembrane domains of proteins of the invention can be identified in
accordance with
known techniques for determination of such domains from sequence information.
Proteins and protein fragments of the present invention include proteins with
amino acid sequence lengths that are at least 25%(more preferably at least
50%, and most
preferably at least 75%) of the length of a disclosed protein and have at
least 60% sequence
identity (more preferably, at least 75% identity; most preferably at least 90%
or~ 95%
identity) with that disclosed protein, where sequence identity is determined
by comparing
the amino acid sequences of the proteins when aligned so as to maximize
overlap and
identity while minimizing sequence gaps. Also included in the present
invention are
proteins and protein fragments that contain a segment preferably comprising 8
or more
(more preferably 20 or more, most preferably 30 or more) contiguous amino
acids that
shares at least 75% sequence identity (more preferably, at least 85% identity;
most
2 0 preferably at least 95% identity) with any such segment of any of the
disclosed proteins.
Species homologs of the disclosed polynucleotides and proteins are also
provided
by the present invention. As used herein, a "species homologue" is a protein
or
polynucleotide with a different species of origin from that of a given protein
or
polynucleotide, but with significant sequence similarity to the given protein
or
2 5 polynucleotide, as determined by those of skill in the art. Species
homologs may be
isolated and identified by making suitable probes or primers from the
sequences provided
herein and screening a suitable nucleic acid source from the desired species.
The invention also encompasses allelic variants of the disclosed
polynucleotides
or proteins; that is, naturally-occurring alternative forms of the isolated
polynucleotide
3 0 which also encode proteins which are identical, homologous, or related to
that encoded
by the polynucleotides .
The invention also includes polynucleotides with sequences complementary to
those of the polynucleotides disclosed herein.
32
,.. T. E .._,._..r _...._...._........r.. ..
CA 02278770 1999-07-23
WO 98!32853 PCTIUS98/01396
The present invention also includes polynucleotides capable of hybridizing
under
reduced stringency conditions, more preferably stringent conditions, and most
preferably
highly stringent conditions, to polynucleotides described herein. Examples of
stringency
conditions are shown in the table below: highly stringent conditions are those
that are at
least as stringent as, for example, conditions A-F; stringent conditions are
at least as
stringent as, for example, conditions G-L; and reduced stringency conditions
are at least
as stringent as, for example, conditions M-R.
StringencyPolynucleotideHybridHybridization TemperatureWash
ConditionHybrid Lengthand Temperature
(bp)x Buffers and Buffer'
A DNA:DNA z 50 65C; lxSSC -or- 65C; 0.3xSSC
42C; lxSSC, 50% formamide
B DNA:DNA <50 TB*; lxSSC TB*; lxSSC
C DNA:RNA z 50 67C; lxSSC -or- 67C; 0.3xSSC
45C; lxSSC, 50% formamide
D DNA:RNA <50 T~*; lxSSC TD*; lxSSC
E RNA:RNA > 50 70C; lxSSC -or- 70C; 0.3xSSC
50C; lxSSC, 50% formamide
F RNA:RNA <50 TF*; lxSSC TF*; lxSSC
G DNA:DNA s 50 65C; 4xSSC -or- 65C; lxSSC
42C; 4xSSC, 50% formamide
H DNA:DNA <50 TH*; 4xSSC TH*; 4xSSC
I DNA:RNA s 50 67C; 4xSSC -or- 67C; lxSSC
45C; 4xSSC, 50% formamide
J DNA:RNA <50 T~*; 4xSSC T~*; 4xSSC
2 K RNA:RNA s 50 70C; 4xSSC -or- 67C; lxSSC
0 50C; 4xSSC, 50% formamide
L RNA:RNA <50 T, *; 2xSSC T~*; 2xSSC
M DNA:DNA s 50 50C; 4xSSC -or- 50C; 2xSSC
40C; 6xSSC, 50% formamide
N DNA:DNA <50 TN*; 6xSSC TN*; 6xSSC
O DNA:RNA s 50 55C; 4xSSC -or- 55C; 2xSSC
42C; 6xSSC, 50% formamide
2 P DNA:RNA <50 T,>*; 6xSSC T,,*; 6xSSC
5
Q RNA:RNA z 50 60C; 4xSSC -or- 60C; 2xSSC
45C; 6xSSC, 50% formamide
R RNA:RNA <50 Ta*; 4xSSC TR*; 4xSSC
~: The hybrid length is that anticipated for the hybridized regions) of the
hybridizing polynucleotides. When
3 0 hybridizing a polynucleotide to a target polynucleotide of unknown
sequence, the hybrid length is assumed
33
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
to be that of the hybridizing polynucleotide. When polynucleotides of known
sequence are hybridized, the
hybrid length can be determined by aligning the sequences of the
polynucleotides and identifying the region
or regions of optimal sequence complementarity.
': SSPE (lxSSPE is 0.15M NaCI, lOmM NaHzP04, and 1.25mM EDTA, pH 7.4) can be
substituted for SSC
(lxSSC is 0.15M NaCI and lSmM sodium citrate) in the hybridization and wash
buffers; washes are
performed for 15 minutes after hybridization is complete.
*TB - TR: The hybridization temperature for hybrids anticipated to be less
than 50 base pairs in length should
be 5-10 ° C less than the melting temperature (Tm) of the hybrid, where
Tm is determined according to the
following equations. For hybrids less than 18 base pairs in length,
Tm(°C) = 2(# of A + T bases) + 4(# of G +
C bases). For hybrids between 18 and 49 base pairs in length, Tm(°C) =
81.5 + 16.6(log,o[Na']) + 0.41(%G+C) -
(600/N), where N is the number of bases in the hybrid, and [Na '] is the
concentration of sodium ions in the
hybridization buffer ([Na'] for lxSSC = 0.165 M).
Additional examples of stringency conditions for polynucleotide hybridization
are
provided in Sambrook, J., E.F. Fritsch, and T. Maniatis, 1989, MolecLdar
Cloning: A
Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor,
NY,
chapters 9 and 11, and Current Protocols in Molecular Biology,1995, F.M.
Ausubel et al., eds.,
John Wiley & Sons, lnc., sections 2.10 and 6.3-6.4, incorporated herein by
reference.
Preferably, each such hybridizing polynucleotide has a length that is at least
2 0 25%(more preferably at least 50%, and most preferably at least 75%) of the
length of the
polynucleotide of the present invention to which it hybridizes, and has at
least 60%
sequence identity (more preferably, at least 75% identity; most preferably at
least 90% or
95% identity) with the polynucleotide of the present invention to which it
hybridizes,
where sequence identity is determined by comparing the sequences of the
hybridizing
2 5 polynucleotides when aligned so as to maximize overlap and identity while
minimizing
sequence gaps.
The isolated polynucleotide of the invention may be operably linked to an
expression control sequence such as the pMT2 or pED expression vectors
disclosed in
Kaufman et al., Nucleic Acids Res. 19, 4485-4490 (1991), in order to produce
the protein
3 0 recombinantly. Many suitable expression control sequences are known in the
art. General
methods of expressing recombinant proteins are also known and are exemplified
in R.
Kaufman, Methods in Enzymology 185, 537-566 (1990). As defined herein
"operably
linked" means that the isolated polynucleotide of the invention and an
expression control
sequence are situated within a vector or cell in such a way that the protein
is expressed
3 5 by a host cell which has been transformed (transfected) with the ligated
polynucleotide/expression control sequence.
A number of types of cells may act as suitable host cells for expression of
the
protein. Mammalian host cells include, for example, monkey COS cells, Chinese
Hamster
Ovary (CHO) cells, human kidney 293 cells, human epidermal A431 cells, human
Co1o205
34
...... s .......~ ..... .t _, ......._~ ..... .._.._.........,.._..
CA 02278770 1999-07-23
WO 98/32853 PCT/L1S98101396
cells, 3T3 cells, CV-1 cells, other transformed primate cell lines, normal
diploid cells, cell
strains derived from in vitro culture of primary tissue, primary explants,
HeLa cells,
mouse L cells, BHK, HL-60, U937, HaK or Jurkat cells.
Alternatively, it may be possible to produce the protein in lower eukaryotes
such
as yeast or in prokaryotes such as bacteria. Potentially suitable yeast
strains include
Saccharomyces cerevisiae, Schizosaccharomyces pombe, Khcyveromyces strains,
Candida, or any
yeast strain capable of expressing heterologous proteins. Potentially suitable
bacterial
strains include Escherichia coli, Bacilhcs subtilis, Salmonella typhimurium,
or any bacterial
strain capable of expressing heterologous proteins. If the protein is made in
yeast or
bacteria, it may be necessary to modify the protein produced therein, for
example by
phosphorylation or glycosylation of the appropriate sites, in order to obtain
the functional
protein. Such covalent attachments may be accomplished using known chemical or
enzymatic methods.
The protein may also be produced by operably linking the isolated
polynucleotide
of the invention to suitable control sequences in one or more insect
expression vectors,
and employing an insect expression system. Materials and methods for
baculovirus/insect cell expression systems are commercially available in kit
form from,
e.g., Invitrogen, San Diego, California, U.S.A. (the MaxBac~ kit), and such
methods are
well known in the art, as described in Summers and Smith, Texas Agricultural
Experiment
2 0 Station Bulletin No. 1555 (1987), incorporated herein by reference. As
used herein, an
insect cell capable of expressing a polynucleotide of the present invention is
"transformed."
The protein of the invention may be prepared by culturing transformed host
cells
under culture conditions suitable to express the recombinant protein. The
resulting
2 5 expressed protein may then be purified from such culture (i.e., from
culture medium or
cell extracts) using known purification processes, such as gel filtration and
ion exchange
chromatography. The purification of the protein may also include an affinity
column
containing agents which will bind to the protein; one or more column steps
over such
affinity resins as concanavalin A-agarose, heparin-toyopearl~ or Cibacrom blue
3GA
3 0 Sepharose~; one or more steps involving hydrophobic interaction
chromatography using
such resins as phenyl ether, butyl ether, or propyl ether; or immunoaffinity
chromatography.
Alternatively, the protein of the invention may also be expressed in a form
which
will facilitate purification. For example, it may be expressed as a fusion
protein, such as
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
those of maltose binding protein (MBP), glutathione-S-transferase (GST} or
thioredoxin
(TRX). Kits for expression and purification of such fusion proteins are
commercially
available from New England BioLab (Beverly, MA), Pharmacia (Piscataway, NJ)
and
InVitrogen, respectively. The protein can also be tagged with an epitope and
subsequently purified by using a specific antibody directed to such epitope.
One such
epitope ("Flag'.') is commercially available from Kodak (New Haven, CT).
Finally, one or more reverse-phase high performance liquid chromatography (RP-
HPLC) steps employing hydrophobic RP-HPLC media, e.g., silica gel having
pendant
methyl or other aliphatic groups, can be employed to further purify the
protein. Some or
all of the foregoing purification steps, in various combinations, can also be
employed to
provide a substantially homogeneous isolated recombinant protein. The protein
thus
purified is substantially free of other mammalian proteins and is defined in
accordance
with the present invention as an "isolated protein."
The protein of the invention may also be expressed as a product of transgenic
animals, e.g., as a component of the milk of transgenic cows, goats, pigs, or
sheep which
are characterized by somatic or germ cells containing a nucleotide sequence
encoding the
protein.
The protein may also be produced by known conventional chemical synthesis.
Methods for constructing the proteins of the present invention by synthetic
means are
2 0 known to those skilled in the art. The synthetically-constructed protein
sequences, by
virtue of sharing primary, secondary or tertiary structural and/or
conformational
characteristics with proteins may possess biological properties in common
therewith,
including protein activity. Thus, they may be employed as biologically active
or
immunological substitutes for natural, purified proteins in screening of
therapeutic
2 5 compounds and in immunological processes for the development of
antibodies.
The proteins provided herein also include proteins characterized by amino acid
sequences similar to those of purified proteins but into which modification
are naturally
provided or deliberately engineered. For example, modifications in the peptide
or DNA
sequences can be made by those skilled in the art using known techniques.
Modifications
3 0 of interest in the protein sequences may include the alteration,
substitution, replacement,
insertion or deletion of a selected amino acid residue in the coding sequence.
For
example, one or more of the cysteine residues may be deleted or replaced with
another
amino acid to alter the conformation of the molecule. Techniques for such
alteration,
substitution, replacement, insertion or deletion are well known to those
skilled in the art
36
~ .......~....~..,.._. ... T .~ .__m..~ w.__..__.___~ .. _. i
CA 02278770 1999-07-23
WO 98/32853 PCT/t1S98/01396
(see, e.g., U.S. Patent No. 4,518,584). Preferably, such alteration,
substitution, replacement,
insertion or deletion retains the desired activity of the protein.
Other fragments and derivatives of the sequences of proteins which would be
expected to retain protein activity in whole or in part and may thus be useful
for screening
or other immunological methodologies may also be easily made by those skilled
in the art
given the disclosures herein. Such modifications are believed to be
encompassed by the
present invention.
USES AND BIOLOGICAL ACTIVITY
The polynucleotides and proteins of the present invention are expected to
exhibit
one or more of the uses or biological activities (including those associated
with assays
cited herein) identified below. Uses or activities described for proteins of
the present
invention may be provided by administration or use of such proteins or by
administration
or use of polynucleotides encoding such proteins (such as, for example, in
gene therapies
or vectors suitable for introduction of DNA).
Research Uses and Utilities
The polynucleotides provided by the present invention can be used by the
research
community for various purposes. The polynucleotides can be used to express
2 0 recombinant protein for analysis, characterization or therapeutic use; as
markers for
tissues in which the corresponding protein is preferentially expressed {either
constitutively or at a particular stage of tissue differentiation or
development or in disease
states); as molecular weight markers on Southern gels; as chromosome markers
or tags
(when labeled) to identify chromosomes or to map related gene positions; to
compare
2 5 with endogenous DNA sequences in patients to identify potential genetic
disorders; as
probes to hybridize and thus discover novel, related DNA sequences; as a
source of
information to derive PCR primers for genetic fingerprinting; as a probe to
"subtract-out"
known sequences in the process of discovering other novel polynucleotides; for
selecting
and making oligomers for attachment to a "gene chip" or other support,
including for
3 0 examination of expression patterns; to raise anti-protein antibodies using
DNA
immunization techniques; and as an antigen to raise anti-DNA antibodies or
elicit another
immune response. Where the polynucleotide encodes a protein which binds or
potentially
binds to another protein (such as, for example, in a receptor-ligand
interaction), the
polynucleotide can also be used in interaction trap assays (such as, for
example, that
37
CA 02278770 1999-07-23
WO 98/32853 PCT/LTS98/01396
described in Gyuris et al., Cell 75:791-803 (1993)) to identify
polynucleotides encoding the
other protein with which binding occurs or to identify inhibitors of the
binding
interaction.
The proteins provided by the present invention can similarly be used in assay
to
determine biological activity, including in a panel of multiple proteins for
high-
throughput screening; to raise antibodies or to elicit another immune
response; as a
reagent (including the labeled reagent) in assays designed to quantitatively
determine
levels of the protein (or its receptor) in biological fluids; as markers for
tissues in which
the corresponding protein is preferentially expressed (either constitutively
or at a
particular stage of tissue differentiation or development or in a disease
state); and, of
course, to isolate correlative receptors or ligands. Where the protein binds
or potentially
binds to another protein (such as, for example, in a receptor-ligand
interaction), the
protein can be used to identify the other protein with which binding occurs or
to identify
inhibitors of the binding interaction. Proteins involved in these binding
interactions can
also be used to screen for peptide or small molecule inhibitors or agonists of
the binding
interaction.
Any or all of these research utilities are capable of being developed into
reagent
grade or kit format for commercialization as research products.
Methods for performing the uses listed above are well known to those skilled
in
2 0 the art. References disclosing such methods include without limitation
"Molecular
Cloning: A Laboratory Manual", 2d ed., Cold Spring Harbor Laboratory Press,
Sambrook,
J., E.F. Fritsch and T. Maniatis eds., 1989, and "Methods in Enzymology: Guide
to
Molecular Cloning Techniques", Academic Press, Berger, S.L. and A.R. Kimmel
eds.,1987.
2 5 Nutritional Uses
Polynucleotides and proteins of the present invention can also be used as
nutritional sources or supplements. Such uses include without limitation use
as a protein
or amino acid supplement, use as a carbon source, use as a nitrogen source and
use as a
source of carbohydrate. In such cases the protein or polynucleotide of the
invention can
3 0 be added to the feed of a particular organism or can be administered as a
separate solid
or liquid preparation, such as in the form of powder, pills, solutions,
suspensions or
capsules. In the case of microorganisms, the protein or polynucleotide of the
invention
can be added to the medium in or on which the microorganism is cultured.
38
T _w_._..~.... .. _. ~.w._..._ ...e..r __.
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
Cytokine and Cell Proliferation/Differentiation Activity
A protein of the present invention may exhibit cytokine, cell proliferation
(either
inducing or inhibiting) or cell differentiation (either inducing or
inhibiting) activity or may
induce production of other cytokines in certain cell populations. Many protein
factors
discovered to date, including all known cytokines, have exhibited activity in
one or more
factor dependent cell proliferation assays, and hence the assays serve as a
convenient
confirmation of cytokine activity. The activity of a protein of the present
invention is
evidenced by any one of a number of routine factor dependent cell
proliferation assays
for cell lines including, without limitation, 32D, DA2, DA1G, T10, B9, B9/11,
BaF3,
MC9/G, M+ (preB M+), 2E8, RBS, DA1, 123, T1165, HT2, CTLL2, TF-1, Mo7e and
CMK.
The activity of a protein of the invention may, among other means, be measured
by the following methods:
Assays for T-cell or thymocyte proliferation include without limitation those
described in: Current Protocols in Immunology, Ed by J. E. Coligan, A.M.
Kruisbeek, D.H.
Margulies, E.M. Shevach, W Strober, Pub. Greene Publishing Associates and
Wiley-
Interscience (Chapter 3, In Vitro assays for Mouse Lymphocyte Function 3.1-
3.19; Chapter
7, Immunologic studies in Humans); Takai et al., J. Immunol. 137:3494-3500,
1986;
Bertagnolli et al., J. Immunol.145:1706-1712, 1990; Bertagnolli et al.,
Cellular Immunology
2 0 133:327-341, 1991; Bertagnolli, et al., J. Immunol. 149:3778-3783, 1992;
Bowman et al., J.
Immunol. 152: 1756-1761, 1994.
Assays for cytokine production and / or proliferation of spleen cells, lymph
node
cells or thymocytes include, without limitation, those described in:
Polyclonal T cell
stimulation, Kruisbeek, A.M. and Shevach, E.M. In Current Protocols in
Immunology. J.E.e.a.
2 5 Coligan eds. Vol 1 pp. 3.12.1-3.12.14, John Wiley and Sons, Toronto. 1994;
and
Measurement of mouse and human Interferon y, Schreiber, R.D. In Current
Protocols in
Immunology. J.E.e.a. Coligan eds. Vol 1 pp. 6.8.1-6.8.8, John Wiley and Sons,
Toronto.1994.
Assays for proliferation and differentiation of hematopoietic and
lymphopoietic
cells include, without limitation, those described in: Measurement of Human
and Murine
3 0 Interleukin 2 and Interleukin 4, Bottomly, K., Davis, L.S. and Lipsky,
P.E. In Current
Protocols in Immunology. J.E.e.a. Coligan eds. Vol 1 pp. 6.3.1-6.3.12, John
Wiley and Sons,
Toronto. 1991; deVries et al., J. Exp. Med. 173:1205-1211, 1991; Moreau et
al., Nature
336:690-692, 1988; Greenberger et al., Proc. Natl. Acad. Sci. U.S.A. 80:2931-
2938, 1983;
Measurement of mouse and human interleukin 6 - Nordan, R. In Current Protocols
in
39
CA 02278770 1999-07-23
WO 98/32853 PCTIiJS98/01396
Immunology. J.E.e.a. Coligan eds. Vol 1 pp. 6.6.1-6.6.5, John Wiley and Sons,
Toronto. 1991;
Smith et al., Proc. Natl. Acad. Sci. U.S.A. 83:1857-1861, 1986; Measurement of
human
Interleukin 11 - Bennett, F., Giannotti, J., Clark, S.C. and Turner, K. J. In
Current Protocols
in Immunology. J.E.e.a. Coligan eds. Vol 1 pp. 6.15.1 John Wiley and Sons,
Toronto. 1991;
Measurement of mouse and human Interleukin 9 - Ciarletta, A., Giannotti, J.,
Clark, S.C.
and Turner, K.J. In Current Protocols in Immunology. J.E.e.a. Coligan eds. Vol
1 pp. 6.13.1,
John Wiley and Sons, Toronto. 1991.
Assays for T-cell clone responses to antigens (which will identify, among
others,
proteins that affect APC-T cell interactions as well as direct T-cell effects
by measuring
proliferation and cytokine production) include, without limitation, those
described in:
Current Protocols in Immunology, Ed by J. E. Coligan, A.M. Kruisbeek, D.H.
Margulies,
E.M. Shevach, W Strober, Pub. Greene Publishing Associates and Wiley-
Interscience
(Chapter 3, In Vitro assays for Mouse Lymphocyte Function; Chapter 6,
Cytokines and
their cellular receptors; Chapter 7, Immunologic studies in Humans);
Weinberger et al.,
Proc. Natl. Acad. Sci. USA 77:6091-6095, 1980; Weinberger et al., Eur. J.
Immun.
11:405-412, 1981; Takai et al., J. Immunol. 137:3494-3500, 1986; Takai et al.,
J. Immunol.
140:508-512, 1988.
Immune Stimulating or Suppressing Activity
2 0 A protein of the present invention may also exhibit immune stimulating or
immune suppressing activity, including without limitation the activities for
which assays
are described herein. A protein may be useful in the treatment of various
immune
deficiencies and disorders (including severe combined immunodeficiency
(SCID)), e.g.,
in regulating (up or down) growth and proliferation of T and / or B
lymphocytes, as well
2 5 as effecting the cytolytic activity of NK cells and other cell
populations. These immune
deficiencies may be genetic or be caused by viral (e.g., HIV) as well as
bacterial or fungal
infections, or may result from autoimmune disorders. More specifically,
infectious
diseases causes by viral, bacterial, fungal or other infection may be
treatable using a
protein of the present invention, including infections by HIV, hepatitis
viruses,
3 0 herpesviruses, mycobacteria, Leishmania spp_, malaria spp. and various
fungal infections
such as candidiasis. Of course, in this regard, a protein of the present
invention may also
be useful where a boost to the immune system generally may be desirable, i.e.,
in the
treatment of cancer.
r ~ ___.~...~-.~ .... ,...__..
CA 02278770 1999-07-23
WO 98/32853 PCTlUS98/01396
Autoimmune disorders which may be treated using a protein of the present
invention include, for example, connective tissue disease, multiple sclerosis,
systemic
lupus erythematosus, rheumatoid arthritis, autoimmune pulmonary inflammation,
Guillain-Barre syndrome, autoimmune thyroiditis, insulin dependent diabetes
mellitis,
myasthenia gravis, graft-versus-host disease and autoimmune inflammatory eye
disease.
Such a protein of the present invention may also to be useful in the treatment
of allergic
reactions and conditions, such as asthma (particularly allergic asthma) or
other respiratory
problems. Other conditions, in which immune suppression is desired (including,
for
example, organ transplantation), may also be treatable using a protein of the
present
invention.
Using the proteins of the invention it may also be possible to immune
responses,
in a number of ways. Down regulation may be in the form of inhibiting or
blocking an
immune response already in progress or may involve preventing the induction of
an
immune response. The functions of activated T cells may be inhibited by
suppressing T
cell responses or by inducing specific tolerance in T cells, or both.
Immunosuppression
of T cell responses is generally an active, non-antigen-specific, process
which requires
continuous exposure of the T cells to the suppressive agent. Tolerance, which
involves
inducing non-responsiveness or anergy in T cells, is distinguishable from
immunosuppression in that it is generally antigen-specific and persists after
exposure to
2 0 the tolerizing agent has ceased. Operationally, tolerance can be
demonstrated by the lack
of a T cell response upon reexposure to specific antigen in the absence of the
tolerizing
agent.
Down regulating or preventing one or more antigen functions (including without
limitation B lymphocyte antigen functions (such as , for example, B7)), e.g.,
preventing
2 5 high level lymphokine synthesis by activated T cells, will be useful in
situations of tissue,
skin and organ transplantation and in graft-versus=host disease (GVHD). For
example,
blockage of T cell function should result in reduced tissue destruction in
tissue
transplantation. Typically, in tissue transplants, rejection of the transplant
is initiated
through its recognition as foreign by T cells, followed by an immune reaction
that destroys
3 0 the transplant. The administration of a molecule which inhibits or blocks
interaction of
a B7 lymphocyte antigen with its natural Iigand(s) on immune cells (such as a
soluble,
monomeric form of a peptide having B7-2 activity alone or in conjunction with
a
monomeric form of a peptide having an activity of another B lymphocyte antigen
(e.g., B7-
1, B7-3) or blocking antibody), prior to transplantation can lead to the
binding of the
41
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
molecule to the natural ligand(s) on the immune cells without transmitting the
corresponding costimulatory signal. Blocking B lymphocyte antigen function in
this
matter prevents cytokine synthesis by immune cells, such as T cells, and thus
acts as an
immunosuppressant. Moreover, the lack of costimulation may also be sufficient
to
anergize the T cells, thereby inducing tolerance in a subject. Induction of
long-term
tolerance by B lymphocyte antigen-blocking reagents may avoid the necessity of
repeated
administration of these blocking reagents. To achieve sufficient
immunosuppression or
tolerance in a subject, it may also be necessary to block the function of a
combination of
B lymphocyte antigens.
The efficacy of particular blocking reagents in preventing organ transplant
rejection or GVHD can be assessed using animal models that are predictive of
efficacy in
humans. Examples of appropriate systems which can be used include allogeneic
cardiac
grafts in rats and xenogeneic pancreatic islet cell grafts in mice, both of
which have been
used to examine the immunosuppressive effects of CTLA4Ig fusion proteins in
vivo as
described in Lenschow et al., Science 257:789-792 (1992) and Turka et al.,
Proc. Natl. Acad.
Sci USA, 89:11102-11105 (1992). In addition, murine models of GVHD (see Paul
ed.,
Fundamental Immunology, Raven Press, New York, 1989, pp. 846-847) can be used
to
determine the effect of blocking B lymphocyte antigen function in vivo on the
development
of that disease.
2 0 Blocking antigen function may also be therapeutically useful for treating
autoimmune diseases. Many autoimmune disorders are the result of inappropriate
activation of T cells that are reactive against self tissue and which promote
the production
of cytokines and autoantibodies involved in the pathology of the diseases.
Preventing the
activation of autoreactive T cells may reduce or eliminate disease symptoms.
2 5 Administration of reagents which block costimulation of T cells by
disrupting
receptor:ligand interactions of B lymphocyte antigens can be used to inhibit T
cell
activation and prevent production of autoantibodies or T cell-derived
cytokines which
may be involved in the disease process. Additionally, blocking reagents may
induce
antigen-specific tolerance of autoreactive T cells which could lead to long-
term relief from
3 0 the disease. The efficacy of blocking reagents in preventing or
alleviating autoimmune
disorders can be determined using a number of well-characterized animal models
of
human autoimmune diseases. Examples include murine experimental autoimmune
encephalitis, systemic lupus erythmatosis in MRL / Ipr/Ipr mice or NZB hybrid
mice,
murine autoimmune collagen arthritis, diabetes mellitus in NOD mice and BB
rats, and
42
r _ . r. _ _.._._.r.... . ~_ _ ~m. . _ ..._.__.
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
murine experimental myasthenia gravis (see Paul ed., Fundamental Immunology,
Raven
Press, New York, 1989, pp. 840-856).
Upregulation of an antigen function (preferably a B lymphocyte antigen
function),
as a means of up regulating immune responses, may also be useful in therapy.
Upregulation of immune responses may be in the form of enhancing an existing
immune
response or eliciting an initial immune response. For example, enhancing an
immune
response through stimulating B lymphocyte antigen function may be useful in
cases of
viral infection. In addition, systemic viral diseases such as influenza, the
common cold,
and encephalitis might be alleviated by the administration of stimulatory
forms of B
lymphocyte antigens systemically.
Alternatively, anti-viral immune responses may be enhanced in an infected
patient
by removing T cells from the patient, costimulating the T cells in vitro with
viral antigen-
pulsed APCs either expressing a peptide of the present invention or together
with a
stimulatory form of a soluble peptide of the present invention and
reintroducing the in
vitro activated T cells into the patient. Another method of enhancing anti-
viral immune
responses would be to isolate infected cells from a patient, transfect them
with a nucleic
acid encoding a protein of the present invention as described herein such that
the cells
express all or a portion of the protein on their surface, and reintroduce the
transfected
cells into the patient. The infected cells would now be capable of delivering
a
2 0 costimulatory signal to, and thereby activate, T cells in vivo.
In another application, up regulation or enhancement of antigen function
(preferably B lymphocyte antigen function) may be useful in the induction of
tumor
immunity. Tumor cells (e.g., sarcoma, melanoma, lymphoma, leukemia,
neuroblastoma,
carcinoma) transfected-with a nucleic acid encoding at least one peptide of
the present
2 5 invention can be administered to a subject to overcome tumor-specific
tolerance in the
subject. If desired, the tumor cell can be transfected' to express a
combination of peptides.
For example, tumor cells obtained from a patient can be transfected ex vivo
with an
expression vector directing the expression of a peptide having B7-2-like
activity alone, or
in conjunction with a peptide having B7-1-like activity and/or B7-3-like
activity. The
3 0 transfected tumor cells are returned to the patient to result in
expression of the peptides
on the surface of the transfected cell. Alternatively, gene therapy techniques
can be used
to target a tumor cell for transfection in vivo.
The presence of the peptide of the present invention having the activity of a
B
lymphocyte antigens) on the surface of the tumor cell provides the necessary
43
CA 02278770 1999-07-23
WO 98132853 PCT/US98/01396
costimulation signal to T cells to induce a T cell mediated immune response
against the
transfected tumor cells. In addition, tumor cells which lack MHC class I or
MHC class II
molecules, or which fail to reexpress sufficient amounts of MHC class I or MHC
class II
molecules, can be transfected with nucleic acid encoding all or a portion of
(e.g., a
cytoplasmic-domain truncated portion) of an MHC class I a chain protein and
biz
microglobulin. protein or an MHC class II a chain protein and an MHC class II
~i chain
protein to thereby express MHC class I or MHC class II proteins on the cell
surface.
Expression of the appropriate class I or class II MHC in conjunction with a
peptide having
the activity of a B lymphocyte antigen (e.g., B7-1, B7-2, B7-3) induces a T
cell mediated
immune response against the transfected tumor cell. Optionally, a gene
encoding an
antisense construct which blocks expression of an MHC class II associated
protein, such
as the invariant chain, can also be cotransfected with a DNA encoding a
peptide having
the activity of a B lymphocyte antigen to promote presentation of tumor
associated
antigens and induce tumor specific immunity. Thus, the induction of a T cell
mediated
immune response in a human subject may be sufficient to overcome tumor-
specific
tolerance in the subject.
The activity of a protein of the invention may, among other means, be measured
by the following methods:
Suitable assays for thymocyte or splenocyte cytotoxicity include, without
2 0 limitation, those described in: Current Protocols in Immunology, Ed by J.
E. Coligan, A.M.
Kruisbeek, D.H. Margulies, E.M. Shevach, W Strober, Pub. Greene Publishing
Associates
and Wiley-Interscience (Chapter 3, In Vitro assays for Mouse Lymphocyte
Function 3.1
3.19; Chapter 7, Immunologic studies in Humans); Herrmann et al., Proc. Natl.
Acad. Sci.
USA 78:2488-2492,1981; Herrmann et al., J. Immunol. 128:1968-1974, 1982; Handa
et al.,
2 5 J. Immunol. 135:1564-1572,1985; Takai et al., J. Immunol. 137:3494-3500,
1986; Takai et al.,
J. Immunol. 140:508-512, 1988; Herrmann et al., Proc. Natl. Acad. Sci. USA
78:2488-2492,
1981; Herrmann et al., J. Immunol. 128:1968-1974, 1982; Handa et al., J.
Immunol.
135:1564-1572, 1985; Takai et al., J. Immunol. 137:3494-3500, 1986; Bowmanet
al., J.
Virology 61:1992-1998; Takai et al., J. Immunol. 140:508-512, 1988;
Bertagnolli et al.,
3 0 Cellular Immunology 133:327-341,1991; Brown et al., J. Immunol. 153:3079-
3092, 1994.
Assays for T-cell-dependent immunoglobulin responses and isotype switching
(which will identify, among others, proteins that modulate T-cell dependent
antibody
responses and that affect Thl /Th2 profiles) include, without limitation,
those described
in: Maliszewski, J. Immunol. 144:3028-3033, 1990; and Assays for B cell
function: In vitro
44
__~~__._.____~ i
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
antibody production, Mond, J.J. and Brunswick, M. In Current Protocols in
Immunology.
J.E.e.a. Coligan eds. Vol 1 pp. 3.8.1-3.8.16, John Wiley and Sons, Toronto.
1994.
Mixed lymphocyte reaction (MLR) assays (which will identify, among others,
proteins that generate predominantly Th1 and CTL responses) include, without
limitation,
those described in: Current Protocols in Immunology, Ed by J. E. Coligan, A.M.
Kruisbeek,
D.H. Margulies, E.M. Shevach, W Strober, Pub. Greene Publishing Associates and
Wiley-
Interscience (Chapter 3, In Vitro assays for Mouse Lymphocyte Function 3.1-
3.19; Chapter
7, Immunologic studies in Humans); Takai et al., J. Immunol. 137:3494-3500,
1986; Takai
et al., J. Immunol. 140:508-512, 1988; Bertagnolli et al., J. Immunol.
149:3778-3783, 1992.
Dendritic cell-dependent assays (which will identify, among others, proteins
expressed by dendritic cells that activate naive T-cells) include, without
limitation, those
described in: Guery et al., J. Immunol. 134:536-544, 1995; Inaba et al.,
Journal of
Experimental Medicine 173:549-559, 1991; Macatonia et al., Journal of
Immunology
154:5071-5079, 1995; Porgador et al., Journal of Experimental Medicine 182:255-
260, 1995;
Nair et al., Journal of Virology 67:4062-4069, 1993; Huang et al., Science
264:961-965,
1994; Macatonia et al., Journal of Experimental Medicine 169:1255-1264,1989;
Bhardwaj
et al., Journal of Clinical Investigation 94:797-807, 1994; and Inaba et al.,
Journal of
Experimental Medicine 172:631-640, 1990.
Assays for lymphocyte survival/apoptosis (which will identify, among others,
2 0 proteins that prevent apoptosis after superantigen induction and proteins
that regulate
lymphocyte homeostasis) include, without limitation, those described in:
Darzynkiewicz
et al., Cytometry 13:795-808, 1992; Gorczyca et al., Leukemia 7:659-670, 1993;
Gorczyca et
al., Cancer Research 53:1945-1951, 1993; Itoh et al., Cell 66:233-243, 1991;
Zacharchuk,
Journal of Immunology 145:4037-4045, 1990; Zamai et al., Cytometry 14:891-897,
1993;
2 5 Gorczyca et al., International Journal of Oncology 1:639-648, 1992.
Assays for proteins that influence early steps of T-cell commitment and
development include, without limitation, those described in: Antica et al.,
Blood
84:111-117, 1994; Fine et al., Cellular Immunology 155:111-122, 1994; Galy et
al., Blood
85:2770-2778, 1995; Toki et al., Proc. Nat. Acad Sci. USA 88:7548-7551,1991.
Hematopoiesis Re ug latin~ Activity
A protein of the present invention may be useful in regulation of
hematopoiesis
and, consequently, in the treatment of myeloid or lymphoid cell deficiencies.
Even
marginal biological activity in support of colony forming cells or of factor-
dependent cell
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
lines indicates involvement in regulating hematopoiesis, e.g. in supporting
the growth and
proliferation of erythroid progenitor cells alone or in combination with other
cytokines,
thereby indicating utility, for example, in treating various anemias or for
use in
conjunction with irradiation/chemotherapy to stimulate the production of
erythroid
precursors and/or erythroid cells; in supporting the growth and proliferation
of myeloid
cells such as granulocytes and monocytes/macrophages (i.e., traditional CSF
activity)
useful, for example, in conjunction with chemotherapy to prevent or treat
consequent
myelo-suppression; in supporting the growth and proliferation of
megakaryocytes and
consequently of platelets thereby allowing prevention or treatment of various
platelet
disorders such as thrombocytopenia, and generally for use in place of or
complimentary
to platelet transfusions; and/or in supporting the growth and proliferation of
hematopoietic stem cells which are capable of maturing to any and all of the
above-
mentioned hematopoietic cells and therefore find therapeutic utility in
various stem cell
disorders (such as those usually treated with transplantation, including,
without
limitation, aplastic anemia and paroxysmal nocturnal hemoglobinuria), as well
as in
repopulating the stem cell compartment post irradiation/chemotherapy, either
in-vivo or
ex-vivo (i.e., in conjunction with bone marrow transplantation or with
peripheral
progenitor cell transplantation (homologous or heterologous)) as normal cells
or
genetically manipulated for gene therapy.
2 0 The activity of a protein of the invention may, among other means, be
measured
by the following methods:
Suitable assays for proliferation and differentiation of various hematopoietic
lines
are cited above.
Assays for embryonic stem cell differentiation (which will identify, among
others,
2 5 proteins that influence embryonic differentiation hematopoiesis) include,
without
limitation, those described in: Johansson et al. Cellular Biology 15:141-151,
1995; Keller et
al., Molecular and Cellular Biology 13:473-486, 1993; McClanahan et al., Blood
81:2903-2915, 1993.
Assays for stem cell survival and differentiation (which will identify, among
3 0 others, proteins that regulate lympho-hematopoiesis) include, without
limitation, those
described in: Methylcellulose colony forming assays, Freshney, M.G. In Culture
of
Hematopoietic Cells. R.I. Freshney, et al. eds. Vol pp. 265-268, Wiley-Liss,
Inc., New York,
NY. 1994; Hirayama et al., Proc. Natl. Acad. Sci. USA 89:5907-5911, 1992;
Primitive
hematopoietic colony forming cells with high proliferative potential, McNiece,
LK. and
46
..»~_T.._~~ ._. . i
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
Briddell, R.A. In Culture of Hematopoietic Cells. R.I. Freshney, et al. eds.
Vol pp. 23-39,
Wiley-Liss, lnc., New York, NY. 1994; Neben et al., Experimental Hematology
22:353-359,
1994; Cobblestone area forming cell assay, Ploemacher, R.E. In Ccdture of
Hematopoietic
Cells. R.I. Freshney, et al. eds. Vol pp. 1-21, Wiley-Liss, Inc.., New York,
NY. 1994; Long
term bone marrow cultures in the presence of stromal cells, Spooncer, E.,
Dexter, M. and
Allen, T. In Culture of Hematopoietic Cells. R.I. Freshney, et al. eds. Vol
pp. 163-179,
Wiley-Liss, Inc., New York, NY. 1994; Long term culture initiating cell assay,
Sutherland,
H.J. In Culture of Hematopoietic Cells. R.I. Freshney, et al. eds. Vol pp. 139-
162, Wiley-Liss,
Inc., New York, NY. 1994.
Tissue Growth Activity
A protein of the present invention also may have utility in compositions used
for
bone, cartilage, tendon, ligament and/or nerve tissue growth or regeneration,
as well as
for wound healing and tissue repair and replacement, and in the treatment of
burns,
incisions and ulcers.
A protein of the present invention, which induces cartilage and / or bone
growth
in circumstances where bone is not normally formed, has application in the
healing of
bone fractures and cartilage damage or defects in humans and other animals.
Such a
preparation employing a protein of the invention may have prophylactic use in
closed as
2 0 well as open fracture reduction and also in the improved fixation of
artificial joints. De
novo bone formation induced by an osteogenic agent contributes to the repair
of
congenital, trauma induced, or oncologic resection induced craniofacial
defects, and also
is useful in cosmetic plastic surgery.
A protein of this invention may also be used in the treatment of periodontal
2 5 disease, and in other tooth repair processes. Such agents may provide an
environment
to attract bone-forming cells, stimulate growth of bone-forming cells or
induce
differentiation of progenitors of bone-forming cells. A protein of the
invention may also
be useful in the treatment of osteoporosis or osteoarthritis, such as through
stimulation
of bone and / or cartilage repair or by blocking inflammation or processes of
tissue
3 0 destruction (collagenase activity, osteoclast activity, etc.) mediated by
inflammatory
processes.
Another category of tissue regeneration activity that may be attributable to
the
protein of the present invention is tendon/ligament formation. A protein of
the present
invention, which induces tendon/ligament-like tissue or other tissue formation
in
47
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
circumstances where such tissue is not normally formed, has application in the
healing of
tendon or ligament tears, deformities and other tendon or ligament defects in
humans and
other animals. Such a preparation employing a tendon/ligament-like tissue
inducing
protein may have prophylactic use in preventing damage to tendon or ligament
tissue, as
well as use in the improved fixation of tendon or ligament to bone or other
tissues, and
in repairing defects to tendon or ligament tissue. De novo tendon/ligament-
like tissue
formation induced by a composition of the present invention contributes to the
repair of
congenital, trauma induced, or other tendon or ligament defects of other
origin, and is also
useful in cosmetic plastic surgery for attachment or repair of tendons or
ligaments. The
compositions of the present invention may provide an environment to attract
tendon- or
ligament-forming cells, stimulate growth of tendon- or ligament-forming cells,
induce
differentiation of progenitors of tendon- or ligament-forming cells, or induce
growth of
tendon/Iigament cells or progenitors ex vivo for return in vivo to effect
tissue repair. The
compositions of the invention may also be useful in the treatment of
tendinitis, carpal
tunnel syndrome and other tendon or ligament defects. The compositions may
also
include an appropriate matrix and/or sequestering agent as a carrier as is
well known in
the art.
The protein of the present invention may also be useful for proliferation of
neural
cells and for regeneration of nerve and brain tissue, i.e. for the treatment
of central and
2 0 peripheral nervous system diseases and neuropathies, as well as mechanical
and
traumatic disorders, which involve degeneration, death or trauma to neural
cells or nerve
tissue. More specifically, a protein may be used in the treatment of diseases
of the
peripheral nervous system, such as peripheral nerve injuries, peripheral
neuropathy and
localized neuropathies, and central nervous system diseases, such as
Alzheimer's,
2 5 Parkinson's disease, Huntingtori s disease, amyotrophic lateral sclerosis,
and Shy-Drager
syndrome. Further conditions which may be treated in accordance with the
present
invention include mechanical and traumatic disorders, such as spinal cord
disorders, head
trauma and cerebrovascular diseases such as stroke. Peripheral neuropathies
resulting
from chemotherapy or other medical therapies may also be treatable using a
protein of the
3 0 invention.
Proteins of the invention may also be useful to promote better or faster
closure of
non-healing wounds, including without limitation pressure ulcers, ulcers
associated with
vascular insufficiency, surgical and traumatic wounds, and the like.
48
_ ~ ~.~..a_._...._ . ._ T w
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
It is expected that a protein of the present invention may also exhibit
activity for
generation or regeneration of other tissues, such as organs (including, for
example,
pancreas, liver, intestine, kidney, skin, endothelium), muscle (smooth,
skeletal or cardiac)
and vascular (including vascular endothelium) tissue, or for promoting the
growth of cells
comprising such tissues. Part of the desired effects may be by inhibition or
modulation
of fibrotic scarring to allow normal tissue to regenerate. A protein of the
invention may
also exhibit angiogenic activity.
A protein of the present invention may also be useful for gut protection or
regeneration and treatment of lung or liver fibrosis, reperfusion injury in
various tissues,
and conditions resulting from systemic cytokine damage.
A protein of the present invention may also be useful for promoting or
inhibiting
differentiation of tissues described above from precursor tissues or cells; or
for inhibiting
the growth of tissues described above.
The activity of a protein of the invention may, among other means, be measured
by the following methods:
Assays for tissue generation activity include, without limitation, those
described
in: International Patent Publication No. W095/16035 (bone, cartilage, tendon);
International Patent Publication No. W095/05846 (nerve, neuronal);
International Patent
Publication No. W091/07491 (skin, endothelium ).
2 0 Assays for wound healing activity include, without limitation, those
described in:
Winter, ~idermal Wound Healing, pps. 71-112 (Maibach, HI and Rovee, DT, eds.),
Year
Book Medical Publishers, Inc., Chicago, as modified by Eaglstein and Mertz, J.
Invest.
Dermatol 71:382-84 {1978).
Activin/Inhibin Activity
A protein of the present invention may arso exhibit activin- or inhibin-
related
activities. Inhibins are characterized by their ability to inhibit the release
of follicle
stimulating hormone (FSH), while activins and are characterized by their
ability to
stimulate the release of follicle stimulating hormone (FSH). Thus, a protein
of the present
3 0 invention, alone or in heterodimers with a member of the inhibin a family,
may be useful
as a contraceptive based on the ability of inhibins to decrease fertility in
female mammals
and decrease spermatogenesis in male mammals. Administration of sufficient
amounts
of other inhibins can induce infertility in these mammals. Alternatively, the
protein of the
invention, as a homodimer or as a heterodimer with other protein subunits of
the inhibin-
49
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
~3 group, may be useful as a fertility inducing therapeutic, based upon the
ability of activin
molecules in stimulating FSH release from cells of the anterior pituitary.
See, for example,
United States Patent 4,798,885. A protein of the invention may also be useful
for
advancement of the onset of fertility in sexually immature mammals, so as to
increase the
lifetime reproductive performance of domestic animals such as cows, sheep and
pigs.
The activity of a protein of the invention may, among other means, be measured
by the following methods:
Assays for activin/inhibin activity include, without limitation, those
described in:
Vale et al., Endocrinology 91:562-572, 1972; Ling et al., Nature 321:779-
782,1986; Vale et
al., Nature 321:776-779, 1986; Mason et al., Nature 318:659-663, 1985; Forage
et al., Proc.
Natl. Acad. Sci. USA 83:3091-3095, 1986.
Chemotactic/Chemokinetic Activity
A protein of the present invention may have chemotactic or chemokinetic
activity
(e.g., act as a chemokine) for mammalian cells, including, for example,
monocytes,
fibroblasts, neutrophils, T-cells, mast cells, eosinophils, epithelial and /
or endothelial cells.
Chemotactic and chemokinetic proteins can be used to mobilize or attract a
desired cell
population to a desired site of action. Chemotactic or chemokinetic proteins
provide
particular advantages in treatment of wounds and other trauma to tissues, as
well as in
2 0 treatment of localized infections. For example, attraction of lymphocytes,
monocytes or
neutrophils to tumors or sites of infection may result in improved immune
responses
against the tumor or infecting agent.
A protein or peptide has chemotactic activity for a particular cell population
if it
can stimulate, directly or indirectly, the directed orientation or movement of
such cell
2 5 population. Preferably, the protein or peptide has the ability to directly
stimulate directed
movement of cells. Whether a particular protein has chemotactic activity for a
population
of cells can be readily determined by employing such protein or peptide in any
known
assay for cell chemotaxis.
The activity of a protein of the invention may, among other means, be measured
3 0 by the following methods:
Assays for chemotactic activity (which will identify proteins that induce or
prevent
chemotaxis) consist of assays that measure the ability of a protein to induce
the migration
of cells across a membrane as well as the ability of a protein to induce the
adhesion of one
cell population to another cell population. Suitable assays for movement and
adhesion
..... .. ~ ..__.. ..._..._._...T ~ .......
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
include, without limitation, those described in: Current Protocols in
Immunology, Ed by
J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, W.Strober, Pub.
Greene
Publishing Associates and Wiley-Interscience {Chapter 6.12, Measurement of
alpha and
beta Chemokines 6.12.1-6.12.28; Taub et al. J. Clin. Invest. 95:1370-1376,
1995; Lind et al.
APMIS 103:140-146, 1995; Muller et al Eur. J. Immunol. 25: 1744-1748; Gruber
et al. J. of
Immunol. 152:.5860-5867, 1994; Johnston et al. J. of Immunol. 153: 1762-1768,
1994.
Hemostatic and Thrombolytic Activity
A protein of the invention may also exhibit hemostatic or thrombolytic
activity.
As a result, such a protein is expected to be useful in treatment of various
coagulation
disorders {including hereditary disorders, such as hemophilias} or to enhance
coagulation
and other hemostatic events in treating wounds resulting from trauma, surgery
or other
causes. A protein of the invention may also be useful for dissolving or
inhibiting
formation of thromboses and for treatment and prevention of conditions
resulting
therefrom (such as, for example, infarction of cardiac and central nervous
system vessels
(e.g., stroke).
The activity of a protein of the invention may, among other means, be measured
by the following methods:
Assay for hemostatic and thrombolytic activity include, without limitation,
those
2 0 described in: Linet et al., J. Clin. Pharmacol. 26:131-140, 1986; Burdick
et al., Thrombosis
Res. 45:413-419,1987; Humphrey et al., Fibrinolysis 5:71-79 (1991); Schaub,
Prostaglandins
35:467-474, 1988.
Receptor/Ligand Activity
2 5 A protein of the present invention may also demonstrate activity as
receptors,
receptor ligands or inhibitors or agonists of recepfor/ligand interactions.
Examples of
such receptors and ligands include, without limitation, cytokine receptors and
their
ligands, receptor kinases and their ligands, receptor phosphatases and their
ligands,
receptors involved in cell-cell interactions and their ligands (including
without limitation,
3 0 cellular adhesion molecules (such as selectins, integrins and their
ligands) and
receptor/ligand pairs involved in antigen presentation, antigen recognition
and
development of cellular and humoral immune responses). Receptors and ligands
are also
useful for screening of potential peptide or small molecule inhibitors of the
relevant
receptor/ligand interaction. A protein of the present invention (including,
without
51
CA 02278770 1999-07-23
WO 98/32853 PCT/iJS98/01396
limitation, fragments of receptors and ligands) may themselves be useful as
inhibitors of
receptor/ligand interactions.
The activity of a protein of the invention may, among other means, be measured
by the following methods:
Suitable assays for receptor-ligand activity include without limitation those
described in:Current Protocols in Immunology, Ed by J.E. Coligan, A.M.
Kruisbeek, D.H.
Margulies, E.M. Shevach, W.Strober, Pub. Greene Publishing Associates and
Wiley-Interscience {Chapter 7.28, Measurement of Cellular Adhesion under
static
conditions 7.28.1-7.28.22), Takai et al., Proc. Natl. Acad. Sci. USA 84:6864-
6868, 1987;
Bierer et al., ]. Exp. Med. 168:1145-1156, 1988; Rosenstein et al., j. Exp.
Med. 169:149-160
1989; Stoltenborg et al., J. Immunol. Methods 175:59-68,1994; Stitt et al.,
Cell 80:661-670,
1995.
Anti-Inflammatory Activity
Proteins of the present invention may also exhibit anti-inflammatory activity.
The
anti-inflammatory activity may be achieved by providing a stimulus to cells
involved in
the inflammatory response, by inhibiting or promoting cell-cell interactions
(such as, for
example, cell adhesion), by inhibiting or promoting chemotaxis of cells
involved in the
inflammatory process, inhibiting or promoting cell extravasation, or by
stimulating or
2 0 suppressing production of other factors which more directly inhibit or
promote an
inflammatory response. Proteins exhibiting such activities can be used to
treat
inflammatory conditions including chronic or acute conditions), including
without
limitation inflammation associated with infection (such as septic shock,
sepsis or systemic
inflammatory response syndrome (SIRS)), ischemia-reperfusion injury, endotoxin
2 5 lethality, arthritis, complement-mediated hyperacute rejection, nephritis,
cytokine or
chemokine-induced lung injury, inflammatory bowel disease, Crohn's disease or
resulting
from over production of cytokines such as TNF or IL-1. Proteins of the
invention may also
be useful to treat anaphylaxis and hypersensitivity to an antigenic substance
or material.
3 0 Cadherin/Tumor Invasion Su~pressor ActivitX
Cadherins are calcium-dependent adhesion molecules that appear to play major
roles during development, particularly in defining specific cell types. Loss
or alteration
of normal cadherin expression can lead to changes in cell adhesion properties
linked to
tumor growth and metastasis. Cadherin malfunction is also implicated in other
human
52
r ~ _._.m~_-... .. T _~__. ._. .
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
diseases, such as pemphigus vulgaris and pemphigus foliaceus (auto-immune
blistering
skin diseases), Crohn's disease, and some developmental abnormalities.
The cadherin superfamily includes well over forty members, each with a
distinct
pattern of expression. All members of the superfamily have in common conserved
extracellular repeats (cadherin domains), but structural differences are found
in other
parts of the molecule. The cadherin domains bind calcium to form their
tertiary structure
and thus calcium is required to mediate their adhesion. Only a few amino acids
in the
first cadherin domain provide the basis for homophilic adhesion; modification
of this
recognition site can change the specificity of a cadherin so that instead of
recognizing only
itself, the mutant molecule can now also bind to a different cadherin. In
addition, some
cadherins engage in heterophilic adhesion with other cadherins.
E-cadherin, one member of the cadherin superfamily, is expressed in epithelial
cell
types. Pathologically, if E-cadherin expression is lost in a tumor, the
malignant cells
become invasive and the cancer metastasizes. Transfection of cancer cell lines
with
polynucleotides expressing E-cadherin has reversed cancer-associated changes
by
returning altered cell shapes to normal, restoring cells' adhesiveness to each
other and to
their substrate, decreasing the cell growth rate, and drastically reducing
anchorage-
independent cell growth. Thus, reintroducing E-cadherin expression reverts
carcinomas
to a less advanced stage. It is likely that other cadherins have the same
invasion
2 0 suppressor role in carcinomas derived from other tissue types. Therefore,
proteins of the
present invention with cadherin activity, and polynucleotides of the present
invention
encoding such proteins, can be used to treat cancer. Introducing such proteins
or
polynucleotides into cancer cells can reduce or eliminate the cancerous
changes observed
in these cells by providing normal cadherin expression.
2 5 Cancer cells have also been shown to express cadherins of a different
tissue type
than their origin, thus allowing these cells to invade'and metastasize in a
different tissue
in the body. Proteins of the present invention with cadherin activity, and
polynucleotides
of the present invention encoding such proteins, can be substituted in these
cells for the
inappropriately expressed cadherins, restoring normal cell adhesive properties
and
3 0 reducing or eliminating the tendency of the cells to metastasize.
Additionally, proteins of the present invention with cadherin activity, and
polynucleotides of the present invention encoding such proteins, can used to
generate
antibodies recognizing and binding to cadherins. Such antibodies can be used
to block
the adhesion of inappropriately expressed tumor-cell cadherins, preventing the
cells from
53
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
forming a tumor elsewhere. Such an anti-cadherin antibody can also be used as
a marker
for the grade, pathological type, and prognosis of a cancer, i.e. the more
progressed the
cancer, the less cadherin expression there will be, and this decrease in
cadherin expression
can be detected by the use of a cadherin-binding antibody.
Fragments of proteins of the present invention with cadherin activity,
preferably
a polypepHde comprising a decapeptide of the cadherin recognition site, and
poly-
nucleotides of the present invention encoding such protein fragments, can also
be used
to block cadherin function by binding to cadherins and preventing them from
binding in
ways that produce undesirable effects. Additionally, fragments of proteins of
the present
invention with cadherin activity, preferably truncated soluble cadherin
fragments which
have been found to be stable in the circulation of cancer patients, and
polynucleotides
encoding such protein fragments, can be used to disturb proper cell-cell
adhesion.
Assays for cadherin adhesive and invasive suppressor activity include, without
limitation, those described in: Hortsch et al. J Biol Chem 270 (32): 18809-
18817, 1995;
Miyaki et al. Oncogene 11: 2547-2552, 1995; Ozawa et al. Cell 63: 1033-1038,
1990.
Tumor Inhibition Activity
In addition to the activities described above for immunological treatment or
prevention of tumors, a protein of the invention may exhibit other anti-tumor
activities.
2 0 A protein may inhibit tumor growth directly or indirectly (such as, for
example, via
ADCC). A protein may exhibit its tumor inhibitory activity by acting on tumor
tissue or
tumor precursor tissue, by inhibiting formation of tissues necessary to
support tumor
growth (such as, for example, by inhibiting angiogenesis), by causing
production of other
factors, agents or cell types which inhibit tumor growth, or by suppressing,
eliminating
2 5 or inhibiting factors, agents or cell types which promote tumor growth.
Other Activities
A protein of the invention may also exhibit one or more of the following
additional
activities or effects: inhibiting the growth, infection or function of, or
killing, infectious
3 0 agents, including, without limitation, bacteria, viruses, fungi and other
parasites; effecting
(suppressing or enhancing) bodily characteristics, including, without
limitation, height,
weight, hair color, eye color, skin, fat to lean ratio or other tissue
pigmentation, or organ
or body part size or shape (such as, for example, breast augmentation or
diminution,
change in bone form or shape); effecting biorhythms or caricadic cycles or
rhythms;
54
_.._ T ~, . ~ ._. .. ____~m.~.-...~~ .. 1
CA 02278770 1999-07-23
WO 98/32853 PCTIUS98/01396
effecting the fertility of male or female subjects; effecting the metabolism,
catabolism,
anabolism, processing, utilization, storage or elimination of dietary fat,
lipid, protein,
carbohydrate, vitamins, minerals, cofactors or other nutritional factors or
component(s);
effecting behavioral characteristics, including, without limitation, appetite,
libido, stress,
cognition (including cognitive disorders), depression (including depressive
disorders) and
violent behaviors; providing analgesic effects or other pain reducing effects;
promoting
differentiation and growth of embryonic stem cells in lineages other than
hematopoietic
lineages; hormonal or endocrine activity; in the case of enzymes, correcting
deficiencies
of the enzyme and treating deficiency-related diseases; treatment of
hyperproliferative
disorders (such as, for example, psoriasis); immunoglobulin-like activity
(such as, for
example, the ability to bind antigens or complement); and the ability to act
as an antigen
in a vaccine composition to raise an immune response against such protein or
another
material or entity which is cross-reactive with such protein.
ADMINISTRATION AND DOSING
A protein of the present invention (from whatever source derived, including
without limitation from recombinant and non-recombinant sources) may be used
in a
pharmaceutical composition when combined with a pharmaceutically acceptable
carrier.
Such a composition may also contain (in addition to protein and a carrier)
diluents, fillers,
2 0 salts, buffers, stabilizers, solubilizers, and other materials well known
in the art. The term
"pharmaceutically acceptable" means a non-toxic material that does not
interfere with the
effectiveness of the biological activity of the active ingredient(s). The
characteristics of the
carrier will depend on the route of administration. The pharmaceutical
composition of
the invention may also contain cytokines, lymphokines, or other hematopoietic
factors
2 5 such as M-CSF, GM-CSF, TNF, IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-
8, IL-9, IL-10, IL-11,
IL-12, IL-13, IL-14, IL-15, IFN, TNFO, TNFl, TNF2, G-CSF, Meg-CSF,
thrombopoietin, stem
cell factor, and erythropoietin. The pharmaceutical composition may further
contain other
agents which either enhance the activity of the protein or compliment its
activity or use
in treatment. Such additional factors and / or agents may be included in the
3 0 pharmaceutical composition to produce a synergistic effect with protein of
the invention,
or to minimize side effects. Conversely, protein of the present invention may
be included
in formulations of the particular cytokine, lymphokine, other hematopoietic
factor,
thrombolytic or anti-thrombotic factor, or anti-inflammatory agent to minimize
side effects
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
of the cytokine, lymphokine, other hematopoietic factor, thrombolytic or anti-
thrombotic
factor, or anti-inflammatory agent.
A protein of the present invention may be active in multimers (e.g.,
heterodimers
or homodimers) or complexes with itself or other proteins. As a result,
pharmaceutical
compositions of the invention may comprise a protein of the invention in such
multimeric
or complexed.form.
The pharmaceutical composition of the invention may be in the form of a
complex
of the proteins) of present invention along with protein or peptide antigens.
The protein
and / or peptide antigen will deliver a stimulatory signal to both B and T
lymphocytes. B
lymphocytes will respond to antigen through their surface immunoglobulin
receptor. T
lymphocytes will respond to antigen through the T cell receptor (TCR)
following
presentation of the antigen by MHC proteins. MHC and structurally related
proteins
including those encoded by class I and class II MHC genes on host cells will
serve to
present the peptide antigens) to T lymphocytes. The antigen components could
also be
supplied as purified MHC-peptide complexes alone or with co-stimulatory
molecules that
can directly signal T cells. Alternatively antibodies able to bind surface
immunolgobulin
and other molecules on B cells as well as antibodies able to bind the TCR and
other
molecules on T cells can be combined with the pharmaceutical composition of
the
invention.
2 0 The pharmaceutical composition of the invention may be in the form of a
liposome
in which protein of the present invention is combined, in addition to other
pharmaceutically acceptable carriers, with amphipathic agents such as lipids
which exist
in aggregated form as micelles, insoluble monolayers, liquid crystals, or
lamellar layers
in aqueous solution. Suitable lipids for liposomal formulation include,
without limitation,
2 5 monoglycerides, diglycerides, sulfatides, lysolecithin, phospholipids,
saponin, bile acids,
and the like. Preparation of such liposomal formulations is within the level
of skill in the
art, as disclosed, for example, in U.S. Patent No. 4,235,871; U.S. Patent No.
4,501,728; U.S.
Patent No. 4,837,028; and U.S. Patent No. 4,737,323, all of which are
incorporated herein
by reference.
3 0 As used herein, the term "therapeutically effective amount" means the
total
amount of each active component of the pharmaceutical composition or method
that is
sufficient to show a meaningful patient benefit, i.e., treatment, healing,
prevention or
amelioration of the relevant medical condition, or an increase in rate of
treatment, healing,
prevention or amelioration of such conditions. When applied to an individual
active
56
1.. ~ _._ (._.. . I ., ........__._..._.._~_ ........__T
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
ingredient, administered alone, the term refers to that ingredient alone. When
applied to
a combination, the term refers to combined amounts of the active ingredients
that result
in the therapeutic effect, whether administered in combination, serially or
simultaneously.
In practicing the method of treatment or use of the present invention, a
therapeutically effective amount of protein of the present invention is
administered to a
mammal having a condition to be treated. Protein of the present invention may
be
administered in accordance with the method of the invention either alone or in
combination with other therapies such as treatments employing cytokines,
lymphokines
or other hematopoietic factors. When co-administered with one or more
cytokines,
lymphokines or other hematopoietic factors, protein of the present invention
may be
administered either simultaneously with the cytokine(s), lymphokine(s), other
hematopoietic factor(s), thrombolytic or anti-thrombotic factors, or
sequentially. If
administered sequentially, the attending physician will decide on the
appropriate
sequence of administering protein of the present invention in combination with
cytokine(s), iymphokine{s), other hematopoietic factor(s), thrombolytic or
anti-thrombotic
factors.
Administration of protein of the present invention used in the pharmaceutical
composition or to practice the method of the present invention can be carried
out in a
variety of conventional ways, such as oral ingestion, inhalation, topical
application or
2 0 cutaneous, subcutaneous, intraperitoneal, parenteral or intravenous
injection.
Intravenous administration to the patient is preferred.
When a therapeutically effective amount of protein of the present invention is
administered orally, protein of the present invention will be in the form of a
tablet,
capsule, powder, solution or elixir. When administered in tablet form, the
pharmaceutical
2 S composition of the invention may additionally contain a solid carrier such
as a gelatin or
an adjuvant. The tablet, capsule, and powder contain from about 5 to 95%
protein of the
present invention, and preferably from about 25 to 90% protein of the present
invention.
When administered in liquid form, a liquid carrier such as water, petroleum,
oils of animal
or plant origin such as peanut oil, mineral oil, soybean oil, or sesame oil,
or synthetic oils
3 0 may be added. The liquid form of the pharmaceutical composition may
further contain
physiological saline solution, dextrose or other saccharide solution, or
glycols such as
ethylene glycol, propylene glycol or.polyethylene glycol. When administered in
liquid
form, the pharmaceutical composition contains from about 0.5 to 90% by weight
of protein
57
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
of the present invention, and preferably from about 1 to 50% protein of the
present
invention.
When a therapeutically effective amount of protein of the present invention is
administered by intravenous, cutaneous or subcutaneous injection, protein of
the present
invention will be in the form of a pyrogen-free, parenterally acceptable
aqueous solution.
The preparation of such parenterally acceptable protein solutions, having due
regard to
pH, isotonicity, stability, and the like, is within the skill in the art. A
preferred
pharmaceutical composition for intravenous, cutaneous, or subcutaneous
injection should
contain, in addition to protein of the present invention, an isotonic vehicle
such as Sodium
Chloride Injection, Ringer's Injection, Dextrose Injection, Dextrose and
Sodium Chloride
Injection, Lactated Ringer's Injection, or other vehicle as known in the art.
The
pharmaceutical composition of the present invention may also contain
stabilizers,
preservatives, buffers, antioxidants, or other additives known to those of
skill in the art.
The amount of protein of the present invention in the pharmaceutical
composition
of the presentinvention will depend upon the nature and severity of the
condition being
treated, and on the nature of prior treatments which the patient has
undergone.
Ultimately, the attending physician will decide the amount of protein of the
present
invention with which to treat each individual patient. Initially, the
attending physician
will administer low doses of protein of the present invention and observe the
patient's
2 0 response. Larger doses of protein of the present invention may be
administered until the
optimal therapeutic effect is obtained for the patient, and at that point the
dosage is not
increased further. It is contemplated that the various pharmaceutical
compositions used
to practice the method of the present invention should contain about 0.01 lZg
to about 100
mg (preferably about O.lng to about 10 mg, more preferably about 0.1 ug to
about 1 mg)
2 5 of protein of the present invention per kg body weight.
The duration of intravenous therapy using the pharmaceutical composition of
the
present invention will vary, depending on the severity of the disease being
treated and
the condition and potential idiosyncratic response of each individual patient.
It is
contemplated that the duration of each application of the protein of the
present invention
3 0 will be in the range of 12 to 24 hours of continuous intravenous
administration.
Ultimately the attending physician will decide on the appropriate duration of
intravenous
therapy using the pharmaceutical composition of the present invention.
Protein of the invention may also be used to immunize animals to obtain
polyclonal and monoclonal antibodies which specifically react with the
protein. Such
58
T. ~ ___ _. ,..... ._,v.___....__
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
antibodies may be obtained using either the entire protein or fragments
thereof as an
immunogen. The peptide immunogens additionally may contain a cysteine residue
at the
carboxyl terminus, and are conjugated to a hapten such as keyhole limpet
hemocyarun
(KLH). Methods for synthesizing such peptides are known in the art, for
example, as in
R.P. Merrifield, J. Amer.Chem.Soc. 85, 2149-2154 (1963); J.L. Krstenansky, et
al., FEBS Lett.
211, 10 (I987).. Monoclonal antibodies binding to the protein of the invention
may be
useful diagnostic agents for the immunodetection of the protein. Neutralizing
monoclonal
antibodies binding to the protein may also be useful therapeutics for both
conditions
associated with the protein and also in the treatment of some forms of cancer
where
abnormal expression of the protein is involved. In the case of cancerous cells
or leukemic
cells, neutralizing monoclonal antibodies against the protein may be useful in
detecting
and preventing the metastatic spread of the cancerous cells, which may be
mediated by
the protein.
For compositions of the present invention which are useful for bone,
cartilage,
tendon or ligament regeneration, the therapeutic method includes administering
the
composition topically, systematically, or locally as an implant or device.
When
administered, the therapeutic composition for use in this invention is, of
course, in a
pyrogen-free, physiologically acceptable form. Further, the composition may
desirably
be encapsulated or injected in a viscous form for delivery to the site of
bone, cartilage or
2 0 tissue damage. Topical administration may be suitable for wound healing
and tissue
repair. Therapeutically useful agents other than a protein of the invention
which may also
optionally be included in the composition as described above, may
alternatively or
additionally, be administered simultaneously or sequentially with the
composition in the
methods of the invention. Preferably for bone and/or cartilage formation, the
2 5 composition would include a matrix capable of delivering the protein-
containing
composition to the site of bone and/or cartilage damage, providing a structure
for the
developing bone and cartilage and optimally capable of being resorbed into the
body.
Such matrices may be formed of materials presently in use for other implanted
medical
applications.
3 0 The choice of matrix material is based on biocompatibility,
biodegradability,
mechanical properties, cosmetic appearance and interface properties. The
particular
application of the compositions will define the appropriate, formulation.
Potential
matrices for the compositions may be biodegradable and chemically defined
calcium
sulfate, tricalciumphosphate, hydroxyapatite, polylactic acid, polyglycolic
acid and
59
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
polyanhydrides. Other potential materials are biodegradable and biologically
well-
defined, such as bone or dermal collagen. Further matrices are comprised of
pure proteins
or extracellular matrix components. Other potential matrices are
nonbiodegradable and
chemically defined, such as sintered hydroxapatite, bioglass, aluminates, or
other
ceramics. Matrices may be comprised of combinations of any of the above
mentioned
types of material, such as polylactic acid and hydroxyapatite or collagen and
tricalciumphosphate. The bioceramics may be altered in composition, such as in
calcium-
aluminate-phosphate and processing to alter pore size, particle size, particle
shape, and
biodegradability.
Presently preferred is a 50:50 (mole weight) copolymer of lactic acid and
glycolic
acid in the form of porous particles having diameters ranging from 150 to 800
microns.
In some applications, it will be useful to utilize a sequestering agent, such
as
carboxymethyl cellulose or autologous blood clot, to prevent the protein
compositions
from disassociating from the matrix.
A preferred family of sequestering agents is cellulosic materials such as
alkylcelluloses (including hydroxyalkylcelluloses), including methylcellulose,
ethylcellulose, hydroxyethylcellulose, hydroxypropylcellulose, hydroxypropyl-
methylcellulose, and carboxymethylcellulose, the most preferred being cationic
salts of
carboxymethylcellulose (CMC). Other preferred sequestering agents include
hyaluronic
2 0 acid, sodium alginate, polyethylene glycol), polyoxyethylene oxide,
carboxyvinyl
polymer and polyvinyl alcohol). The amount of sequestering agent useful herein
is 0.5-20
wt%, preferably 1-10 wt% based on total formulation weight, which represents
the
amount necessary to prevent desorbtion of the protein from the polymer matrix
and to
provide appropriate handling of the composition, yet not so much that the
progenitor cells
2 5 are prevented from infiltrating the matrix, thereby providing the protein
the opportunity
to assist the osteogenic activity of the progenitor cells.
In further compositions, proteins of the invention may be combined with other
agents beneficial to the treatment of the bone and/or cartilage defect, wound,
or tissue in
question. These agents include various growth factors such as epidermal growth
factor
3 0 (EGF), platelet derived growth factor (PDGF), transforming growth factors
(TGF-a and
TGF-(3}, and insulin-like growth factor (IGF).
The therapeutic compositions are also presently valuable for veterinary
applications. Particularly domestic animals and thoroughbred horses, in
addition to
humans, are desired patients for such treatment with proteins of the present
invention.
.~. . . C. _.. T
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
The dosage regimen of a protein-containing pharmaceutical composition to be
used in tissue regeneration will be determined by the attending physician
considering
various factors which modify the action of the proteins, e.g., amount of
tissue weight
desired to be formed, the site of damage, the condition of the damaged tissue,
the size of
a wound, type of damaged tissue (e.g., bone), the patient's age, sex, and
diet, the severity
of any infection, time of administration and other clinical factors. The
dosage may vary
with the type of matrix used in the reconstitution and with inclusion of other
proteins in
the pharmaceutical composition. For example, the addition of other known
growth
factors, such as IGF I (insulin like growth factor I), to the final
composition, may also effect
the dosage. Progress can be monitored by periodic assessment of tissue/bone
growth
and/or repair, for example, X-rays, histomorphometric determinations and
tetracycline
labeling.
Polynucleotides of the present invention can also be used for gene therapy.
Such
polynucleotides can be introduced either in vivo or ex vivo into cells for
expression in a
mammalian subject. Polynucleotides of the invention may also be administered
by other
known methods for introduction of nucleic acid into a cell or organism
(including, without
limitation, in the form of viral vectors or naked DNA).
Cells may also be cultured ex vivo in the presence of proteins of the present
invention in order to proliferate or to produce a desired effect on or
activity in such cells.
2 0 Treated cells can then be introduced in vivo for therapeutic purposes.
Patent and literature references cited herein are incorporated by reference as
if
fully set forth.
61
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT: Jacobs, Kenneth
McCoy, John M.
LaVallie, Edward R.
Racie, Lisa A.
Merberg, David
Treacy, Maurice
Spaulding) Vikki
Agostino, Michael J.
(ii) TITLE OF INVENTION: SECRETED PROTEINS AND POLYNUCLEOTIDES
ENCODING THEM
(iii) NUMBER OF SEQUENCES: 33
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: Genetics Institute, Inc.
(B) STREET: 87 CambridgePark Drive
(C) CITY: Cambridge
(D) STATE: MA
(E) COUNTRY: U.S.A.
(F) ZIP: 02140
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release #1.0) Version #1.30
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER:
(B) FILING DATE:
(C) CLASSIFICATION:
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: Sprunger, Suzanne A.
(B) REGISTRATION NUMBER: 41,323
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: (617) 498-8284
(B) TELEFAX: (617) 876-5851
(2) INFORMATION FOR SEQ ID N0:1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 1433 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
62
1 _. r _ T . ........_
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(ii) MOLECULE TYPE: cDNA
(xi)
SEQUENCE
DESCRIPTION:
SEQ
ID N0:1:
GCCCGTGGTTACACAGCTAA TAGGTGGTGGAGATGGAGACAGAATTCAAACCCAGGCATT60
CTTGATCTACAGTATACACT CTTACCCACCATCCTACACAGCCTTTCTTATTCATAAAAT120
ATTTTCTACAGTGCAAGAAA ATTTTGATAGCTTGCTTATTTATTCAAGATTTAGACTATA180
TAGATTAACTAGACTATCAA GATTTTAAATTCTTGTGTTTTTTGTTTTTYYCCCCCTCTG240
TGGCATAACTATCTCTTAGT GATTTGAAGTTCTGATAGGCATTTATTTATGTTTTTGATT300
AATTAAAAAAAGGGAA.AAAA ATGGAACATAATTATTGAAGCTATCGTCTAGGTAAAAACC360
TTTCTAAATGTAAGGTTCAT TTAGATTGATGACCTGTAGAGTGTAACAGTATTGCCATAG420
GCATACAGCTTTTTAATCAC ATATCATACATAAACAAATTAGTAATACAGGTGGGTAGAT480
ACAGACCCTAACTTTGAGCT CTAAGATGAAATTTGTTTATAAATCCCTAGTTTCCATTCA540
GTTTTTTCAATATTTATCAA ACACCTACTGTGCCAGGCATTGTTTAGGCACAGGGGATAC600
AGCAGGAGAACAAAATGAAC AAAATTTTTTGCCTTCACAGAGCTAATTTTTTGTATTTTT660
TTGTAGAGATGGGGTTTTGC CATGTTTGCCAGTCTGGTCTCAACCTCCTAAGCTCAAGCA720
GCCCACCCTCCTTGGCTTCC CAAAGTGCTGAGATTACAGGCATGAGCCACCGCACTCTTC780
TTAGCTATTTTTCATAGAAA CTTTATGTATAAAAATAGAAGGGTAATGACACACCACCTT840
TCTACTGATCTCCCCACTTC AGTAGTTATCACATAACAGTCTTTTTTCACCTATCTCCTT900
CACTTTACCTCCTCTCCCTT AGTACTTTGAAGTAAATCTCAATGCAAGCTGGTATGTTTT960
TCAAAATGAAACATATAAAC ATGGACTAGAAAAAAATCTCTTCATACAGGATTTGGTTTT1020
GCAGAGAATTTACAAAGTGC GGTTAATGTATGCCAATGGTTTCTCAGTTTGGATATCGAG1080
ATCCTTAGATGGACCATGAA GCTGGTAATAATTTTATAGCTAACTTTTGTTAAGTGCTTA1140
CTATATGCCAGGCACTGTTC TAAGCATTTTACGTGTATTCATTCATTCAGTTCTCACAAC1200
TCTTTTAATTAGGTATTATT ATGATCTCCATCTCAAAACAAAACAAAACAAAAAAATTAG1260
CCTGGCATGGTGGCAGGCGC CTGTAATCCCAGTTACTTGAGAGGCTAAGGCAGGAGAATC1320
GCTTGAATCTGGGAGGCAGA GGTTGCAGTGAGCCGAGATTGCACTACTGCACTCCAGCCT1380
GGGTGACAGAATGAGACTCT GTCTCAAAAAAAAAAAAAAAAAAAAAAAA.AAAA 1433
63
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(2) INFORMATION FOR SEQ ID N0:2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 46 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:2:
Met Lys Phe Val Tyr Lys Ser Leu Val Ser Ile Gln Phe Phe Gln Tyr
1 5 10 15
Leu Ser Asn Thr Tyr Cys Ala Arg His Cys Leu Gly Thr Gly Asp Thr
20 25 30
Ala Gly Glu Gln Asn Glu Gln Asn Phe Leu Pro Ser Gln Ser
35 40 45
(2) INFORMATION FOR SEQ ID N0:3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 1401 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(xi) SEQUENCE
DESCRIPTION:
SEQ ID
N0:3:
TCGGGACAGATTTAAGTGCAGCGTGGATTTTTTTTTTCTC ACTTTGCCTTGTGTTTTCCA60
CCCTGAAAGAATGTTGTGGCTGCTCTTTTTTCTGGTGAOT GCCATTCATGCTGAACTCTG120
TCAACCAGGTGCAGAAAATGCTTTTAAAGTGAGACTTAGT ATCAGAACAGCTCTGGGAGA180
TAAAGCATATGCCTGGGATACCAATGAAGAATACCTCTTC AAAGCGATGGTAGCTTTCTC240
CATGAGAAAAGTTCCCAACAGAGAAGCAACAGAAATTTCC CATGTCCTACTTTGCAATGT300
AACCCAGAGGGTATCATTCTGGTTTGTGGTTACAGACCCT TCAAAAAATCACACCCTTCC360
TGCTGTTGAGGTGCAATCAGCCATAAGAATGAACAAGAAC CGGATCAACAATGCCTTCTT420
TGTAAATGACCAAACTCTGGAATTTTTAAAAATCCCTTCC ACACTTGCACCACCCATGGA'480
64
~. , .._.~. __ T _ _. _._.__ ._.. . _.
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
CCCATCTGTGCCCATCTGGA TTATTATATTTGGTGTGATATTTTGCATCA TCATAGTTGC540
AATTGCACTACTGATTTTAT CAGGGATCTGGCAACGTAGAAGAAAGAACA AAGAACCATC600
TGAAGTGGATGACGCTGAAG ATAAGTGTGAAAACATGATCACAATTGAAA ATGGCATCCC660
CTCTGATCCCCTGGACATGA AGGGAGGGCATATTAATGATGCCTTCATGA CAGAGGATGA720
GAGGCTCACCCCTCTCTGAA GGGCTGTTGTTCTGCTTCCTCAAGAAATTA AACATTTGTT780
TCTGTGTGACTGCTGAGCAT CCTGAAATACCAAGAGCAGATCATATATTT TGTTTCACCA840
TTCTTCTTTTGTAATAAATT TTGAATGTGCTTGAAAGTGAAAAGCAATCA ATTATACCCA900
CCAACACCACTGAAATCATA AGCTATTCACGACTCAAAATATTCTAAAAT ATTTTTCTGA960
CAGTATAGTGTATAAATGTG GTCATGTGGTATTTGTAGTTATTGATTTAA GCATTTTTAG1020
AAATAAGATCAGGCATATGT ATATATTTTCACACTTCAAAGACCTAAGGA AAAATAAATT1080
TTCCAGTGGAGAATACATAT AATATGGTGTAGAAATCATTGAAAATGGAT CCTTTTTGAC1140
GATCACTTATATCACTCTGT ATATGACTAAGTAAACAAAAGTGAGAAGTA ATTATTGTAA1200
ATGGATGGATAAAAATGGAA TTACTCATATACAGGGTGGAATTTTATCCT GTTATCACAC1260
CAACAGTTGATTATATATTT TCTGAATATCAGCCCCTAATAGGACAATTC TATTTGTTGA1320
CCATTTCTACAATTTGTAAA AGTCCAATCTGTGCTAACTTAATAAAGTAA TAATCATCTC1380
TTTTAAAAAAAA.AAAAAAAA A 1401
(2) INFORMATION
FOR SEQ
ID N0:4:
(i) S EQUENCE CHARACTERISTICS:
(A) LENGTH: 222 acids
amino
(B) TYPE: amino
acid
(C) STRANDEDNESS:
(D) TOPOLOGY: linear
(ii) MOLECULE
TYPE:
protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:4:
Met Leu Trp Leu Leu Phe Phe Leu Val Thr Ala Ile His Ala Glu Leu
1 5 10 15
Cys Gln Pro Gly Ala Glu Asn Ala Phe Lys Val Arg Leu Ser Ile Arg
20 25 30
Thr Ala Leu Gly Asp Lys Ala Tyr Ala Trp Asp Thr Asn Glu Glu Tyr
35 40 45
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
Leu Phe Lys Ala Met Val Ala Phe Ser Met Arg Lys Val Pro Asn Arg
50 55 60
Glu Ala Thr Glu Ile Ser His Val Leu Leu Cys Asn Val Thr Gln Arg
65 70 75 80
Val Ser Phe Trp Phe Val Val Thr Asp Pro Ser Lys Asn His Thr Leu
85 90 95
Pro Ala Val Glu Val Gln Ser Ala Ile Arg Met Asn Lys Asn Arg Ile
100 105 110
Asn Asn Ala Phe Phe Val Asn Asp Gln Thr Leu Glu Phe Leu Lys Ile
115 120 125
Pro Ser Thr Leu Ala Pro Pro Met Asp Pro Ser Val Pro Ile Trp Ile
130 135 140
Ile Ile Phe Gly Val Ile Phe Cys Ile Ile Ile Val Ala Ile Ala Leu
145 250 155 160
Leu Ile Leu Ser Gly Ile Trp Gln Arg Arg Arg Lys Asn Lys Glu Pro
165 170 175
Ser Glu Val Asp Asp Ala Glu Asp Lys Cys Glu Asn Met Ile Thr Ile
180 185 190
Glu Asn Gly Ile Pro Ser Asp Pro Leu Asp Met Lys Gly Gly His Ile
195 200 205
Asn Asp Ala Phe Met Thr Glu Asp Glu Arg Leu Thr Pro Leu
210 215 220
(2) INFORMATION FOR SEQ ID N0:5:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 441 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:5:
GCGGCCGCAG GTCTAGAATT CAATCGGCCA CAAGCTACTC TTTGGAGCCC ATCTATGGTT 60
TGTGGTATGA CCACTCCTCC AACTTCTCCT GGAAATGTCC CACCTGATCT GTCACACCCT 120
TACAGTAAAG TCTTTGGTAC AACTGCAGGT GGAAAAGGAA CTCCTCTGGG AACCCCAGCA 180
ACCTCTCCTC CTCCAGCCCC ACTCTGTCAT TCGGATGACT ACGTGCACAT TTCACTCCCC 240
66
r _.-.~..~_____.T_
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
CAGGCCACAG TCACACCCCC CAGGAAGGAA GAGAGAATGG ATTCTGCAAG ACCATGTCTA 300
CACAGACAAC ACCATCTTCT GAATGACAGA GGATCAGAAG AGCCACCTGG CAGCAAAGGT 360
TCTGTCACTC TAAGTGATCT TCCAGGGTTT TTAGGTGATC TGGCCTCTGA AGAAGATAGT 420
ATTGAAAAAA P~~,~AAAAAAA A 4 41
(2) INFORMATION FOR SEQ ID N0:6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 123 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:6:
Met Val Cys Gly Met Thr Thr Pro Pro Thr Ser Pro Gly Asn Val Pro
1 5 10 15
Pro Asp Leu Ser His Pro Tyr Ser Lys Val Phe Gly Thr Thr Ala Gly
20 25 30
Gly Lys Gly Thr Pro Leu Gly Thr Pro Ala Thr Ser Pro Pro Pro Ala
35 40 45
Pro Leu Cys His Ser Asp Asp Tyr Val His Ile Ser Leu Pro Gln Ala
50 55 60
Thr Val Thr Pro Pro Arg Lys Glu Glu Arg Met Asp Ser Ala Arg Pro
65 70 75 80
Cys Leu His Arg Gln His His Leu Leu Asn Asp Arg Gly Ser Glu Glu
85 90 95
Pro Pro Gly Ser Lys Gly Ser Val Thr Leu Ser Asp Leu Pro Gly Phe
100 105 110
Leu Gly Asp Leu Ala Ser Glu Glu Asp Ser Ile
115 120
(2) INFORMATION FOR SEQ ID N0:7:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 2353 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
67
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(ii) MOLECULE TYPE: cDNA
(xi) SEQUENCE
DESCRIPTION:
SEQ ID
N0:7:
AAGAAGGCGATGTCACTATT GGAGAAGATG CACCAAATCTTTCTTTTAGCACCAGTGTGG60
GAAATGAGGACGCCAGGACA GCCTGGCCCG AATTACAACAGAGCCATGCTGTTAATCAGC120
TCAAAGATTTGTTGCGCCAA CAAGCAGATA AGGAAAGTGAAGTATCTCCGTCAAGAAGAA180
GAAAAATGTCCCCCTTGAGG TCATTAGAAC ATGAGGAAACCAATATGCCTACTATGCACG240
ACCTTGTTCATACTATTAAT GACCAGTCTC AATATATTCATCATTTAGAGGCAGAAGTTA300
AGTTCTGCAAGGAGGAACTC TCTGGAATGA AAAATAAAATACAAGTAGTTGTGCTTGAAA360
ACGAAGGGCTCCAGCAACAG CTAAAATCTC AAAGACAAGAGGAGACACTGAGGGAACAAA420
CACTTCTGGATGCATCCGGA AACATGCACA ATTCTTGGATTACAACAGGTGAAGATTCTG480
GGGTGGGCGAAACCTCCAAA AGACCATTTT CCCATGACAATGCAGATTTTGGCAAAGCTG540
CATCTGCTGGTGAGCAGCTA GAACTGGAGA AGCTAAAACTTACTTATGAGGAAAAGTGTG600
AAATTGAGGAATCCCAATTG AAGTTTTTGA GGAACGACTTAGCTGAATATCAGAGAACTT660
GTGAAGATCTTAAAGAGCAA CTAAAGCATA AAGAATTTCTTCTGGCTGCTAATACTTGTA720
ACCGTGTTGGTGGTCTTTGT TTGAAATGTG CTCAGCATGAAGCTGTTCTTTCCCAAACCC780
ATACTAATGTTCATATGCAG ACCATCGAAA GACTGGTTAAAGAAAGAGATGACTTGATGT840
CTGCACTAGTTTCCGTAAGG AGCAGCTTGG CAGATACGCAGCAAAGAGAAGCAAGTGCTT900
ATGAACAGGTGAAACAAGTT TTGCAAATAT CTGAGGAAGCCAATTTTGAAAAAACCAAGG960
CTTTAATCCAGTGTGACCAG TTGAGGAAGG AGCTGGAGAGGCAGGCGGAGCGACTTGAAA1020
AAGAACTTGCATCTCAGCAA GAGAAAAGGG CCATTGAGAAAGACATGATGAAAAAGGAAA1080
TAACGAAAGAAAGGGAGTAC ATGGGATCAA AGATGTTGATCTTGTCTCAGAATATTGCCC1140
AACTGGAGGCCCAGGTGGAA AAGGTTACAA AGGAAAAGATTTCAGCTATTAATCAACTGG1200
AGGAAATTCAAAGCCAGCTG GCTTCTCGGG AAATGGATGTCACAAAGGTGTGTGGAGAAA1260
TGCGCTATCAGCTGAATAAA ACCAACATGG AGAAGGATGAGGCAGAAAAGGAGCACAGAG1320
AGTTCAGAGCAAAAACTAAC AGGGATCTTG AAATTAAAGATCAGGAAATAGAGAAATTGA1380
GAATAGAACTGGATGAAAGC AAACAACACT TGGAACAGGAGCAGCAGAAGGCAGCCCTGG1440
68
T ~ __._ ~...._._ .
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
CCAGAGAGGA GTGCCTGAGA CTAACAGAACTGCTGGGCGAATCTGAGCAC CAACTGCACC1500
TCACCAGACA GGAAAAAGAT AGCATTCAGCAGAGCTTTAGCAAGGAAGCA AAGGCCCAAG1560
CCCTTCAGGC CCAGCAAAGA GAGCAGGAGCTGACACAGAAGATACAGCAA ATGGAAGCCC1620
AGCATGACAA AACTGAAAAT GAACAGTATTTGTTGCTGACCTCCCAGAAT ACATTTTTGA1680
CAAAGTTAAA GGAAGAATGC TGTACATTAGCCAAGAAACTGGAACAAATC TCTCAAAAAA1740
CCAGATCTGA AATAGCTCAA CTCAGTCAAGAAAAAAGGTATACATATGAT AAATTGGGAA1800
AGTTACAGAG AAGAAATGAA GAATTGGAGGAACAGTGTGTCCAGCATGGG AGAGTACATG1860
AGACGATGAA GCAAAGGCTA AGGCAGCTGGATAAGCACAGCCAGGCCACA GCCCAGCAGC1920
TGGTGCAGCT CCTCAGCAAG CAGAACCAGCTTCTCCTGGAGAGGCAGAGC CTGTCGGAAG1980
AGGTGGACCG GCTGCGGACC CAGTTACCCAGCATGCCACAATCTGATTGC TGACCTGGAT2040
GGAACAGAGT GAAATAAATG ATTTACAAAGAGATATTTACATTCATCTGG TTTAGACTTA2100
ATATGCCACA ACGCACCACG ACCTTCCCAGGGTGACACCGCCTCAGCCTG CAGTGGGGCT2160
GGTCCTCATC AACGCGGGCG CTGTCCCCGCACGCAGTCGGGCTGGAGCTG GAGTCTGACT2220
CTAGCTGAGC AGAGCTCCTG GTGTATGTTTTCAGAAATGGCTTGAAGTTA TGTGTTTAAA2280
TCTGCTCATT CGTATGCTAG GTTATACATATGATTTTCAATAAATGAACT TTTTAAAGAA2340
AAAAAAAAAA AAA 2353
(2) INFORMATION FOR SEQ
ID N0:8:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 615 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:8:
Met Ser Pro Leu Arg Ser Leu Glu His Glu Glu Thr Asn Met Pro Thr
1 5 10 15
Met His Asp Leu Val His Thr Ile Asn Asp Gln Ser Gln Tyr Ile His
20 25 30
His Leu Glu Ala Glu Val Lys Phe Cys Lys Glu Glu Leu Ser Gly Met
35 40 45
69
CA 02278770 1999-07-23
-WO 98/32853 PCT/US98/01396
Lys Asn Lys Ile Gln Val Val Val Leu Glu Asn Glu Gly Leu Gln Gln
50 55 60
Gln Leu Lys Ser Gln Arg Gln Glu Glu Thr Leu Arg Glu Gln Thr Leu
65 70 75 80
Leu Asp Ala Ser Gly Asn Met His Asn Ser Trp Ile Thr Thr Gly Glu
85 90 95
Asp Ser Gly Val Gly Glu Thr Ser Lys Arg Pro Phe Ser His Asp Asn
100 105 110
Ala Asp Phe Gly Lys Ala Ala Ser Ala Gly Glu Gln Leu Glu Leu Glu
115 120 125
Lys Leu Lys Leu Thr Tyr Glu Glu Lys Cys Glu Ile Glu Glu Ser Gln
130 135 140
Leu Lys Phe Leu Arg Asn Asp Leu Ala Glu Tyr Gln Arg Thr Cys Glu
145 150 155 160
Asp Leu Lys Glu Gln Leu Lys His Lys Glu Phe Leu Leu Ala Ala Asn
165 170 175
Thr Cys Asn Arg Val Gly Gly Leu Cys Leu Lys Cys Ala Gln His Glu
180 185 190
Ala Val Leu Ser Gln Thr His Thr Asn Val His Met Gln Thr Ile Glu
195 200 205
Arg Leu Val Lys Glu Arg Asp Asp Leu Met Ser Ala Leu Val Ser Val
210 215 220
Arg Ser Ser Leu Ala Asp Thr Gln Gln Arg Glu Ala Ser Ala Tyr Glu
225 230 235 240
Gln Val Lys Gln Val Leu Gln Ile Ser Glu Glu Ala Asn Phe Glu Lys
245 250 255
Thr Lys Ala Leu Ile Gln Cys Asp Gln Leu Arg Lys Glu Leu Glu Arg
260 265 270
Gln Ala Glu Arg Leu Glu Lys Glu Leu Ala Ser Gln Gln Glu Lys Arg
275 280 285
Ala Ile Glu Lys Asp Met Met Lys Lys Glu Ile Thr Lys Glu Arg Glu
290 295 300
Tyr Met Gly Ser Lys Met Leu Ile Leu Ser Gln Asn Ile Ala Gln Leu
305 310 315 320
Glu Ala Gln Val Glu Lys Val Thr Lys Glu Lys Ile Ser Ala Ile Asn
325 330 335
Gln Leu Glu Glu Ile Gln Ser Gln Leu Ala Ser Arg Glu Met Asp Val
_ -.____.r.~..~......~._.~....__.___...._.
CA 02278770 1999-07-23
WO 98/32853 PCT/fJS98/01396
340 345 350
Thr Lys Val Cys Gly Glu Met Arg Tyr Gln Leu Asn Lys Thr Asn Met
355 360 365
Glu Lys Asp Glu Ala Glu Lys Glu His Arg Glu Phe Arg Ala Lys Thr
370 375 380
Asn Arg Asp Leu Glu Ile Lys Asp Gln Glu Ile Glu Lys Leu Arg Ile
385 390 395 400
Glu Leu Asp Glu Ser Lys Gln His Leu Glu Gln Glu Gln Gln Lys Ala
405 410 415
Ala Leu Ala Arg Glu Glu Cys Leu Arg Leu Thr Glu Leu Leu Gly Glu
420 425 430
Ser Glu His Gln Leu His Leu Thr Arg Gln Glu Lys Asp Ser Ile Gln
435 440 445
Gln Ser Phe Ser Lys Glu Ala Lys Ala Gln Ala Leu Gln Ala Gln Gln
450 455 460
Arg Glu Gln Glu Leu Thr Gln Lys Ile Gln Gln Met Glu Ala Gln His
465 470 475 480
Asp Lys Thr Glu Asn Glu Gln Tyr Leu Leu Leu Thr Ser Gln Asn Thr
485 490 495
Phe Leu Thr Lys Leu Lys Glu Glu Cys Cys Thr Leu Ala Lys Lys Leu
500 505 510
Glu Gln Ile Ser Gln Lys Thr Arg Ser Glu Ile Ala Gln Leu Ser Gln
515 520 525
Glu Lys Arg Tyr Thr Tyr Asp Lys Leu Gly Lys Leu Gln Arg Arg Asn
530 535 540
Glu Glu Leu Glu Glu Gln Cys Val Gln His Gly Arg Val His Glu Thr
545 550 555 560
Met Lys Gln Arg Leu Arg Gln Leu Asp~Lys His Ser Gln Ala Thr Ala
565 570 575
Gln Gln Leu Val Gln Leu Leu Ser Lys Gln Asn Gln Leu Leu Leu Glu
580 585 590
Arg Gln Ser Leu Ser Glu Glu Val Asp Arg Leu Arg Thr Gln Leu Pro
595 600 605
Ser Met Pro Gln Ser Asp Cys
610 615
(2) INFORMATION FOR SEQ ID N0:9:
71
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 313 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:9:
GCGACCTCTT CTGCGGCCGG CCTGGGCAGG TGTCTTCCTC GAGAGGCAGG CAGGGGATCC 60
CGGACACTAG CTTTATCGTC ATCTGGGAAA TTGTTAAAAA TGCAAATTCG CAAGTTTGAG 120
AGCCATGGTT CCAAGAAACT GCATAAGCAT ACGAAATAAG TTGCAGCCTC CCGACTTATA 180
CCCTGGTACT TCTAGTCTAA AACAGGATTT GACTCTACTA ATCCAGCCTT ATACAGGATG 240
CTGTGTTCTT TGCTCCTTTG TGAATGTCTG TTGCTGGTAG CTGGTTATGC TCATGATGAT 300
GACTGGATTG ACC 313
(2) INFORMATION FOR SEQ ID N0:10:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 677 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(xi)
SEQUENCE
DESCRIPTION:
SEQ
ID N0:10:
CCTTGGATGATGCATTAAGTGATATTTTAATTAATTTTAAGTTTCATGATTTTGAAACAT 60
GGAAGTGGCGATTCGAAGATTCCTTTGGAGTGGATCCATATAATGTGTTAATGGTAATTC 120
TTTGTCTGCTCTGCATCGTGGTTTTAGTGGCTACTGAGCTGTGGACATATGTATGTTGGT 180
ACACTCAGTTGAGACGTGTTTTAATCATCAGCTTTCTGTTCAGTTTGGGATGGAATTGGA 240
TGTATTTATATAAGCTAGCTTTTGCACAGCATCAGGCTGAAGTCGCCAAGATGGAGCCAT 300
TAAACAATGTGTGTGCCAAAAAGATGGACTGGACTGGAAGTATCTGGGAATGGTTTAGAA 360
GTTCATGGACCTATAAGGATGACCCATGCCAAAAATACTATGAGCTCTTACTAGTCAACC 420
CTATTTGGTTGGTCCCACCAACAAAGGCACTTGCAGTTACATTCACCACATTTGTAACGG 480
72
_._. _. r ~ ......._..~.__....~.__~.~..~._~.~.... _..._......
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
AGCCATTGAA GCATATTGGA AAAGGAACTG GGGAATTTAT TAAAGCACTC ATGAAGGAAA 540
TTCCAGCGCT GCTTCATCTT CCAGTGCTGA TAATTATGGC ATTAGCCATC CTGAGTTTCT 600
GCTATGGTGC TGGAAAATCA GTTCATGTGC TGAGACATAT AGGCGGTCCT GAGAGCGAAC 660
CTCCCCAGGC ACTTCGG 677
(2) INFORMATION FOR SEQ ID N0:11:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 189 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:11:
Met Val Ile Leu Cys Leu Leu Cys Ile Val Val Leu Val Ala Thr Glu
1 5 10 15
Leu Trp Thr Tyr Val Cys Trp Tyr Thr Gln Leu Arg Arg Val Leu Ile
20 25 30
Ile Ser Phe Leu Phe Ser Leu Gly Trp Asn Trp Met Tyr Leu Tyr Lys
35 40 45
Leu Ala Phe Ala Gln His Gln Ala Glu Val Ala Lys Met Glu Pro Leu
50 55 60
Asn Asn Val Cys Ala Lys Lys Met Asp Trp Thr Gly Ser Ile Trp Glu
65 70 75 80
Trp Phe Arg Ser Ser Trp Thr Tyr Lys Asp Asp Pro Cys Gln Lys Tyr
85 90 95
Tyr Glu Leu Leu Leu Val Asn Pro Ile Trp Leu Val Pro Pro Thr Lys
100 105 110
Ala Leu Ala Val Thr Phe Thr Thr Phe Val Thr Glu Pro Leu Lys His
115 120 125
Ile Gly Lys Gly Thr Gly Glu Phe Ile Lys Ala Leu Met Lys Glu Ile
130 135 140
Pro Ala Leu Leu His Leu Pro Val Leu Ile Ile Met Ala Leu Ala Ile
145 150 155 160
Leu Ser Phe Cys Tyr Gly Ala Gly Lys Ser Val His Val Leu Arg His
165 170 175
73
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
Ile Gly Gly Pro Glu Ser Glu Pro Pro Gln Ala Leu Arg
180 185
(2) INFORMATION FOR SEQ ID N0:12:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 470 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
{ii) MOLECULE TYPE: cDNA
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:12:
AGACGGCAGG AGGAATTGAT TATAGACCTG ATGGTGGAGC AGGTGATGCCGATTTCCATT 60
ATAGGGGCCA AATGGGCCCC ATTGAGCAAG GCCCTTATGC CAAAATGTATGAGGGTAGAA 120
GAGAGATTTT GAGAGAGAGA GATGTTGACT TGAGATTTCA GGCTGGTCTCGAACTCCTGA 180
CCTCAAGTGA CCCGCCCTTG TCGGCCTCCC AAAGTGCTGG GATTACAGGCATGAGCCATT 240
GTGCCCAGCC TATATAGTGT GAAGCTTTTA GGAAAATCAG AACAGGGTAGACAGTTGTTA 300
AAAACAATGT TTAAATGGAA TAATGTTGAA TGTTTACAGG CTGTAAGAATTATTGTATAC 360
ACAAAATAAT ACACAAAGTT TGTACTTTGT GTACAAATAC AAATTTGTACTTTGTGTACA 420
AATAATACAA AAAGTTTGTA TACACAAAAA P,~~4AAAA.AAA 470
P~~,T~AAAAAAA
(2) INFORMATION FOR SEQ ID N0:13:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 2702 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:13:
CTGGAGTCCA CGCGGATTTT CGAAGCTGGG GCTGGCAAGA GGCCGCTGGA CACCACGCTC 60
CAGTCGTCAG CCCACTTCCT AGCTGAACAG CGCGAGGCGG CGGCAGCGAG CCGGGTCCCA 120
CCATGGCCGC GAATTATTCC AGTACCAGTA CCCGGAGAGA ACATGTCAAA GTTAAAACCA 180
74
T _ , .._.~ _~_ ____._...._~_ ___._.._._w.._...1...
CA 02278770 1999-07-23
WO 98/32853 PCT/I1S98/01396
GCTCCCAGCCAGGCTTCCTGGAACGGCTGAGCGAGACCTCGGGTGGGATGTTTGTGGGGC240
TCATGGCCTTCCTGCTCTCCTTCTACCTAATTTTCACCAATGAGGGCCGCGCATTGAAGA300
CGGCAACCTCATTGGCTGAGGGGCTCTCGCTTGTGGTGTCTCCTGACAGCATCCACAGTG360
TGGCTCCGGAGAATGAAGGAAGGCTGGTGCACATCATTGGCGCCTTACGGACATCCAAGC420
TTTTGTCTGATCCAAACTATGGGGTCCATCTTCCGGCTGTGAAACTGCGGAGGCACGTGG480
AGATGTACCAATGGGTAGAAACTGAGGAGTCCAGGGAGTACACCGAGGATGGGCAGGTGA540
AGAAGGAGACGAGGTATTCCTACAACACTGAATGGAGGTCAGAAATCATCAACAGCAAAA600
ACTTCGACCGAGAGATTGGCCACAAAAACCCCAGCTTCCTCTCTCCCACAGTGCCATGGC660
AGTGGAGTCATTCATGGCAACAGCCCCCTTTGTCCAAATTGGCAGGTTTTTCCTCTCGTC720
AGGCCTCATCGACAAAGTCGACAACTTCAAGTCCCTGAGCCTATCCAAGCTGGAGGACCC780
TCATGTGGACATCATTCGCCGTGGAGACTTTTTCTACCACAGCGAAAATCCCAAGTATCC840
AGAGGTGGGAGACTTGCGTGTCTCCTTTTCCTATGCTGGACTGAGCGGCGATGACCCTGA900
CCTGGGCCCAGCTCACGTGGTAACCTGGCTTCCCAGGGGCAGACACTAAGTCAGAGCCTC960
ACGACTTTCCTGGACACAGACACCTTGGTCAATGTCAGGAGCGCTTGGACCCCCTTTTCC1020
CTGGGGAAAGGCACACTCTCGCACACACTCTCAGCCAGGCACGCTTCTGAGCAGTTTCAG1080
AGCTCCCATGTCCCCACAGCCATCCATGGACCCCACGTTAAGAAGGGCAGCTCAAAAGGG1140
GTCTCATAGTCGCACCTTATGACAGGTGTTCCAGTCACACACAGACCCTCTCCCCAAGCC1200
CGTTTTGATCTGTCAATAATTGGTCTTGCGTTCCTGGCCTATGTGCAGTCCTGCCCCATC1260
CCCTGCTCTGCGCACTGCCCAAGAGCTTTGAATGCCTGGAGCTTTGAATGGAGCAGCTCA1320
GCCAGAGCTGCAGAGGTGGATGCATCCCAGATGGATGTATAGAGAGAGAAGCCCCAGGGT1380
CTCTGTGCTCACTTCCCCAGCCGGCACCCAGTCCCGGGAGGGTGGGCCATGGCTCTCATG1440
GGCGTGTCTCCCGCTGGTCACCCCTCAGCTCTAACACCAGGTCCTCTGACCAGGTCACTG1500
TGATTGCCCGGCAGCGGGGTGACCAGCTAGTCCCATTCTCCACCAAGTCTGGGGATACCT1560
TACTGCTCCTGCACCACGGGGACTTCTCAGCAGAGGAGGTGTTTCATAGAGAACTAAGGA1620
GCAACTCCATGAAGACCTGGGGCCTGCGGGCAGCTGGCTGGATGGCCATGTTCATGGGCC1680
TCAACCTTATGACACGGATCCTCTACACCTTGGTGGACTGGTTTCCTGTTTTCCGAGACC1740
TGGTCAACATTGGCCTGAAAGCCTTTGCCTTCTGTGTGGCCACCTCGCTGACCCTGCTGA1800
CCGTGGCGGCTGGCTGGCTCTTCTACCGACCCCTGTGGGCCCTCCTCATTGCCGGCCTGG1860
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
CCCTTGTGCCCATCCTTGTTGCTCGGACACGGGTGCCAGCCAAAAAGTTG GAGTGAAAAG1920
ACCCTGGCACCCGCCCGACACCTGCGTGAGCCCTAGGATCCAGGTCCTCT CTCACCTCTG1980
ACCCAGCTCCATGCCAGAGCAGGAGCCCCGGTCAATTTTGGACTCTGCAC CCCCTCTCCT2040
CTTCAGGGGCCAGACTTGGCAGCATGTGCACCAGGTTGGTGTTCACCAGC TCATGTCTTC2100
CCCACATCTCTTCTTGCCAGTAAGCAGCTTTGGTGGGCAGCAGCAGCTCA TGAATGGCAA2160
GCTGACAGCTTCTCCTGCTGTTTCCTTCCTCTCTTGGACTGAGTGGGTAC GGCCAGCCAC2220
TCAGCCCATTGGCAGCTGACAACGCAGACACGCTCTACGGAGGCCTGCTG ATAAAGGGCT2280
CAGCCTTGCCGTGTGCTGCTTCTCATCACTGCACACAAGTGCCATGCTTT GCCACCACCA2340
CCAAGCACATCTGTGATCCTGAAGGGCGGCCGTTAGTCATTACTGCTGAG TCCTGGGTCA2400
CCAGCAGACACACTGGGCATGGACCCCTCAAAGCAGGCACACCCAAAACA CAAGTCTGTG2460
GCTAGAACCTGATGTGGTGTTTAAAAGAGAAGAAACACTGAAGATGTCCT GAGGAGAAAA2520
GCTGGACATATACTGGGCTTCACACTTATCTTATGGCTTGGCAGAATCTT TGTAGTGTGT2580
GGGATCTCTGAAGGCCCTATTTAAGTTTTTCTTCGTTACTTTGCTGCTTC ATGTGTACTT2640
TCCTACCCCAAGAGGAAGTTTTCTGAAATAAGATTTAAAAACAAAACAAA P~~,AAAAAAAA2700
AA 2702
(2) INFORMATION EQ ID
FOR S N0:14:
(i) S EQUENCE RACTERISTICS:
CHA
(A) LENGTH:211 aminoacids
(B) TYPE:mino acid
a
(C} STRANDEDNESS:
(D) TOPOLOGY: linear
(ii) M OLECULE E: protein
TYP
(xi)SEQUENCE DESCRIPTION: N0:14:
SEQ
ID
Met AlaAla AsnTyrSer SerThr SerThrArgArg GluHisVal Lys
1 5 10 15
Val LysThr SerSerGln ProGly PheLeuGluArg LeuSerGlu Thr
20 25 30
Ser GlyGly MetPheVal GlyLeu MetAlaPheLeu LeuSerPhe Tyr
.
35 40 45
Leu IlePhe ThrAsnGIu GlyArg AlaLeuLysThr AlaThrSer Leu
76
~ _~~. _ _ _........_.__~.._.~___. ~
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
50 55 60
Ala Glu Gly Leu Ser Leu Val Val Ser Pro Asp Ser Ile His Ser Val
65 70 75 80
Ala Pro Glu Asn Glu Gly Arg Leu Val His Ile Ile Gly Ala Leu Arg
85 90 95
Thr Ser Lys Leu Leu Ser Asp Pro Asn Tyr Gly Val His Leu Pro Ala
100 105 110
Val Lys Leu Arg Arg His Val Glu Met Tyr Gln Trp Val Glu Thr Glu
115 120 125
Glu Ser Arg Glu Tyr Thr Glu Asp Gly Gln Val Lys Lys Glu Thr Arg
130 135 140
Tyr Ser Tyr Asn Thr Glu Trp Arg Ser Glu Ile Ile Asn Ser Lys Asn
145 150 155 160
Phe Asp Arg Glu Ile Gly His Lys Asn Pro Ser Phe Leu Ser Pro Thr
165 170 175
Val Pro Trp Gln Trp Ser His Ser Trp Gln Gln Pro Pro Leu Ser Lys
180 185 190
Leu Ala Gly Phe Ser Ser Arg Gln Ala Ser Ser Thr Lys Ser Thr Thr
195 200 205
Ser Ser Pro
210
(2) INFORMATION FOR SEQ ID N0:15:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 3395 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(xi)
SEQUENCE
DESCRIPTION:
SEQ
ID N0:15:
ATCTTCCTGCCCTTCACCTG CATTGGCTACACGGCCACCAATCAGGACTTCATCCAGCGC 60
CTGAGCACACTGATCCGGCA GGCCATCGAGCGGCAGCTGCCTGCCTGGATCGAGGCTGCC 120
AACCAGCGGGAGGAGGGCCA GGGTGAACAGGGCGAGGAGGAGGATGAGGAGGAGGAAGAA 180
GAGGAGGACGTGGCTGAGAA CCGCTACTTTGAAATGGGGCCCCCAGACGTGGAGGAGGAG 240
77
CA 02278770 1999-07-23
WO 98132853 PCT/US98/01396
GAGGGAGGAGGCCAGGGGGAGGAAGAGGAGGAGGAAGAGG RGCCGAGGAG300
ARGATGAAGA
GAGCGCCTGGCTCTGGAATGGGCCCTGGGCGCGGACGAAGACTTCCTGCTGGAGCACATC360
CGCATCCTCAAGGTGCTGTGGTGCTTCCTGATCCATGTGCAGGGCAGTATCCGCCAGTTC420
GCCGCCTGCCTTGTGCTCACCGACTTCGGCATCGCAGTCTTCGAGATCCCGCACCAGGAG480
TCTCGGGGCAGCAGCCAGCACATCCTCTCCTCCCTGCGCTTTGTCTTTTGCTTCCCGCAT540
GGCGACCTCACCGAGTTTGGCTTCCTCATGCCGGAGCTGTGTCTGGTGCTCAAGGTACGG600
CACAGTGAGAACACGCTCTTCATTATCTCGGACGCCGCCAACCTGCACGAGTTCCACGCG660
GACCTGCGCTCATGCTTTGCACCCCAGCACATGGCCATGCTGTGTAGCCCCATCCTCTAC720
GGCAGCCACACCAGCCTGCAGGAGTTCCTGCGCCAGCTGCTCACCTTCTACAAGGTGGCT780
GGCGGCTGCCAGGAGCGCAGCCAGGGCTGCTTCCCCGTCTACCTGGTCTACAGTGACAAG840
CGCATGGTGCAGACGGCCGCCGGGGACTACTCAGGCAACATCGAGTGGGCCAGCTGCACA900
CTCTGTTCAGCCGTGCGGCGCTCCTGCTGCGCGCCCTCTGAGGCCGTCAAGTCCGCCGCC960
ATCCCCTACTGGCTGTTGCTCACGCCCCAGCACCTCAACGTCATCAAGGCCGACTTCAAC1020
CCCATGCCCAACCGTGGCACCCACAACTGTCGCAACCGCAACAGCTTCAAGCTCAGCCGT1080
GTGCCGCTCTCCACCGTGCTGCTGGACCCCACACGCAGCTGTACCCAGCCTCGGGGCGCC1140
TTTGCTGATGGCCACGTGCTAGAGCTGCTCGTGGGGTACCGCTTTGTCACTGCCATCTTC1200
GTGCTGCCCCACGAGAAGTTCCACTTCCTGCGCGTCTACAACCAGCTGCGGGCCTCGCTG1260
CAGGACCTGAAGACTGTGGTCATCGCCAAGACCCCCGGGACGGGAGGCAGCCCCCAGGGC1320
TCCTTTGCGGATGGCCAGCCTGCCGAGCGCAGGGCCAGCAATGACCAGCGTCCCCAGGAG1380
GTCCCAGCAGAGGCTCTGGCCCCGGCCCCAGTGGAAGTCCCAGCTCCAGCCCCTGCAGCA1440
GCCTCAGCCTCAGGCCCAGCGAAGACTCCGGCCCCAGCAGAGGCCTCAACTTCAGCTTTG1500
GTCCCAGAGGAGACGCCAGTGGAAGCTCCAGCCCCACCCCCAGCCGAGGCCCCTGCCCAG1560
TACCCGAGTGAGCACCTCATCCAGGCCACCTCGGAGGAGAATCAGATCCCCTCGCACTTG1620
CCTGCCTGCCCGTCGCTCCGGCACGTCGCCAGCCTGCGGGGCAGCGCCATCATCGAGCTC1680
TTCCACAGCAGCATTGCTGAGGTTGAAAACGAGGAGCTGAGGCACCTCATGTGGTCCTCG1740
GTGGTGTTCTACCAGACCCCAGGGCTGGAGGTGACTGCCTGCGTGCTGCTCTCCACCAAG1800
GCTGTGTACTTTGTGCTCCACGACGGCCTCCGCCGCTACTTCTCAGAGCCACTGCAGGAT1860
TTCTGGMATCAGAAAAACACSGACTACAACAACAGCCCTTTCCACATCTCCCAGTGCTTC1920
78
__ ~_.___..~ _.._~.,...__..
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
GTGCTAAAGCTTAGTGACCTGCAGTCAGTC TTTTCGACCA GCATTTCCGG1980
AATGTGGGGC
CTGACGGGTTCCACCCCGATGCAGGTGGTMACGTGCTTGACGCGGGACAG CTACCTGACG2040
CACTGCTTCCTCCAGCACCTCATGGTCGTGCTGTCCTCTCTGGAACGCAC GCCCTCGCCG2100
GAGCCTGTTGACAAGGACTTCTACTCCGAGTTTGGGAACAAGACCACAGG GAAGATGGAG2160
AACTACGAGCTGATCCACTCTAGTCGCGTCAAGTTTACCTACCCCAGTGA GGAGGAGATT2220
GGGGACCTGACGTTCACTGTGGCCCAAAAGATGGCTGAGCCAGAGAAGGC CCCAGCCCTC2280
AGCATCCTGCTGTACGTGCAGGCCTTCCAGGTGGGCATGCCACCCCCTGG GTGCTGCAGG2340
GGCCCCCTGCGCCCCAAGACACTCCTGCTCACCAGCTCCGAGATCTTCCT CCTGGATGAG2400
GACTGTGTCCACTACCCACTGCCCGAGTTTGCCAAAGAGCCGCCGCAGAG AGACAGGTAC2460
CGGCTGGACGATGGCCGCCGCGTCCGGGACCTGGACCGAGTGCTCATGGG CTACCAGACC2520
TACCCGCAGGCCCTCACCCTCGTCTTCGATGACGTGCAAGGTCATGACCT CATGGGCAGT2580
GTCACCCTGGACCACTTTGGGGAGGTGCCAGGTGGCCCGGCTAGAGCCAG CCAGGGCCGT2640
GAAGTCCAGTGGCAGGTGTTTGTCCCCAGTGCTGAGAGCAGAGAGAAGCT CATCTCGCTG2700
TTGGCTCGCCAGTGGGAGGCCCTGTGTGGCCGTGAGCTGCCTGTCGAGCT CACCGGCTAG2760
CCCAGGCCACAGCCAGCCTGTCGTGTCCAGCCTGACGCCTACTGGGGCAG GGCAGCAGGC2820
TTTTGTGTTCTCTAAAAATGTTTTATCCTCCCTTTGGTACCTTAATTTGA CTGTCCTCGC2880
AGAGAATGTGAACATGTGTGTGTGTTGTGTTAATTCTTTCTCATGTTGGG AGTGAGAATG2940
CCGGGCCCCTCAGGGCTGTCGGTGTGCTGTCAGCCTCCCACAGGTGGTAC AGCCGTGCAC3000
ACCAGTGTCGTGTCTGCTGTTGTGGGACCGTTGTTAACACGTGACACTGT GGGTCTGACT3060
TTCTCTTCTACACGTCCTTTCCTGAAGTGTCGAGTCCAGTCCTTTGTTGC TGTTGCTGTT3120
GCTGTTGCTGTTGCTGTTGGCATCTTGCTGCTAATCCTGAGGCTGGTAGC AGAATGCACA3180
TTGGAAGCTCCCACCCCATATTGTTCTTCAAAGTGGAGGTCTCCCCTGAT CCAGACAAGT3240
GGGAGAGCCCGTGGGGGCAGGGGACCTGGAGCTGCCAGCACCAAGCGTGA TTCCTGCTGC3300
CTGTATTCTCTATTCCAATAAAGCAGAGTTTGACACCGTCAAAAAAAAAA P,~~AAAP.AAAA3360
P~~?~AAAAAAAp~~P.AAAAAAAF,~~AAAAAAAAAAAAA 3
3
9
5
(2) INFORMATION EQ ID
FOR N0:16:
S
(i) SEQUENCE
CHARACTERISTICS:
(A) LENGTH:848 aminoacids
(B) TYPE:
amino
acid
79
CA 02278770 1999-07-23
~WO 98/32853 PCT/US98101396
(C) STRANDEDNESS:
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:16:
Met Gly Pro Pro Asp Val Glu Glu Glu Glu Gly Gly Gly Gln Gly Glu
1 5 10 15
Glu Glu Glu Glu Glu Glu Glu Asp Glu Glu Ala Glu Glu Glu Arg Leu
20 25 30
Ala Leu Glu Trp Ala Leu Gly Ala Asp Glu Asp Phe Leu Leu Glu His
35 40 45
Ile Arg Ile Leu Lys Val Leu Trp Cys Phe Leu Ile His Val Gln Gly
50 55 60
Ser Ile Arg Gln Phe Ala Ala Cys Leu Val Leu Thr Asp Phe Gly Ile
65 70 75 80
Ala Val Phe Glu Ile Pro His Gln Glu Ser Arg Gly Ser Ser Gln His
85 90 95
Ile Leu Ser Ser Leu Arg Phe Val Phe Cys Phe Pro His Gly Asp Leu
100 105 110
Thr Glu Phe Gly Phe Leu Met Pro Glu Leu Cys Leu Val Leu Lys Val
115 120 125
Arg His Ser Glu Asn Thr Leu Phe Ile Ile Ser Asp Ala Ala Asn Leu
130 135 140
His Glu Phe His Ala Asp Leu Arg Ser Cys Phe Ala Pro Gln His Met
145 150 155 160
Ala Met Leu Cys Ser Pro Ile Leu Tyr Gly Ser His Thr Ser Leu Gln
165 ' 170 175
Glu Phe Leu Arg Gln Leu Leu Thr Phe Tyr Lys Val Ala Gly Gly Cys
180 185 190
Gln Glu Arg Ser Gln Gly Cys Phe Pro Val Tyr Leu Val Tyr Ser Asp
195 200 205
Lys Arg Met Val Gln Thr Ala Ala Gly Asp Tyr Ser Gly Asn Ile Glu
210 215 220
Trp Ala Ser Cys Thr Leu Cys Ser Ala Val Arg Arg Ser Cys Cys Ala
225 230 235 240
__..__~._...~._.. _ .... ..._.._.___ .. ..
CA 02278770 1999-07-23
WO 98/32853 PCT/US98I01396
Pro Ser Glu Ala Val Lys Ser Ala Ala Ile Pro Tyr Trp Leu Leu Leu
245 250 255
Thr Pro Gln His Leu Asn Val Ile Lys Ala Asp Phe Asn Pro Met Pro
260 265 270
Asn Arg Gly Thr His Asn Cys Arg Asn Arg Asn Ser Phe Lys Leu Ser
275 280 285
Arg Val Pro Leu Ser Thr Val Leu Leu Asp Pro Thr Arg Ser Cys Thr
290 295 300
Gln Pro Arg Gly Ala Phe Ala Asp Gly His Val Leu Glu Leu Leu Val
305 310 315 320
Gly Tyr Arg Phe Val Thr Ala Ile Phe Val Leu Pro His Glu Lys Phe
325 330 335
His Phe Leu Arg Val Tyr Asn Gln Leu Arg Ala Ser Leu Gln Asp Leu
340 345 350
Lys Thr Val Val Ile Ala Lys Thr Pro Gly Thr Gly Gly Ser Pro Gln
355 360 365
Gly Ser Phe Ala Asp Gly Gln Pro Ala Glu Arg Arg Ala Ser Asn Asp
370 375 380
Gln Arg Pro Gln Glu Val Pro Ala Glu Ala Leu Ala Pro Ala Pro Val
385 390 395 400
Glu Val Pro Ala Pro Ala Pro Ala Ala Ala Ser Ala Ser Gly Pro Ala
405 410 415
Lys Thr Pro Ala Pro Ala Glu Ala Ser Thr Ser Ala Leu Val Pro Glu
420 425 430
Glu Thr Pro Val Glu Ala Pro Ala Pro Pro Pro Ala Glu Ala Pro Ala
435 440 445
Gln Tyr Pro Ser Glu His Leu Ile Gln Ala Thr Ser Glu Glu Asn Gln
450 455 460
Ile Pro Ser His Leu Pro Ala Cys Pro Ser Leu Arg His Val Ala Ser
465 470 475 480
Leu Arg Gly Ser Ala Ile Ile Glu Leu Phe His 5er Ser Ile Ala Glu
485 490 495
Val Glu Asn Glu Glu Leu Arg His Leu Met Trp Ser Ser Val Val Phe
500 505 510
Tyr Gln Thr Pro Gly Leu Glu Val Thr Ala Cys Val Leu Leu Ser Thr
515 520 525
Lys Ala Val Tyr Phe Val Leu His Asp Gly Leu Arg Arg Tyr Phe Ser
81
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
530 535 540
Glu Pro Leu Gln Asp Phe Trp Xaa Gln Lys Asn Thr Asp Tyr Asn Asn
545 550 555 560
Ser Pro Phe His Ile Ser Gln Cys Phe VaI Leu Lys Leu Ser Asp Leu
565 570 575
Gln Ser Val Asn Val Gly Leu Phe Asp Gln His Phe Arg Leu Thr Gly
580 585 590
Ser Thr Pro Met Gln Val Val Thr Cys Leu Thr Arg Asp Ser Tyr Leu
595 600 605
Thr His Cys Phe Leu Gln His Leu Met Val Val Leu Ser Ser Leu Glu
610 615 620
Arg Thr Pro Ser Pro Glu Pro Val Asp Lys Asp Phe Tyr Ser Glu Phe
625 630 635 640
Gly Asn Lys Thr Thr Gly Lys Met Glu Asn Tyr Glu Leu Ile His Ser
645 650 655
Ser Arg Val Lys Phe Thr Tyr Pro Ser Glu Glu Glu Ile Gly Asp Leu
660 665 670
Thr Phe Thr Val Ala Gln Lys Met Ala Glu Pro Glu Lys Ala Pro Ala
675 680 685
Leu Ser Ile Leu Leu Tyr Val Gln Ala Phe GIn Val Gly Met Pro Pro
690 695 700
Pro Gly Cys Cys Arg Gly Pro Leu Arg Pro Lys Thr Leu Leu Leu Thr
705 710 715 720
Ser Ser Glu Ile Phe Leu Leu Asp Glu Asp Cys Val His Tyr Pro Leu
725 730 735
Pro Glu Phe Ala Lys Glu Pro Pro Gln Arg Asp Arg Tyr Arg Leu Asp
740 745 750
Asp Gly Arg Arg Val Arg Asp Leu Asp Arg Val Leu Met Gly Tyr Gln
755 760 765
Thr Tyr Pro Gln Ala Leu Thr Leu Val Phe Asp Asp Val Gln Gly His
770 775 780
Asp Leu Met Gly Ser Val Thr Leu Asp His Phe Gly Glu Val Pro Gly
785 790 795 800
Gly Pro Ala Arg Ala Ser Gln Gly Arg Glu Val Gln Trp Gln Val Phe
805 810 815
Val Pro Ser Ala Glu Ser Arg Glu Lys Leu Ile Ser Leu Leu Ala Arg
820 825 830
82
T ~ ___.~_.~__.~.~__._..~..
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
Gln Trp Glu Ala Leu Cys Gly Arg Glu Leu Pro Val Glu Leu Thr Gly
835 840 845
(2) INFORMATION FOR SEQ ID N0:17:
(i) SEQUENCE CHARACTERISTICS:.
(A) LENGTH: 1147 base pairs
{B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(xi)
SEQUENCE
DESCRIPTION:
SEQ
ID N0:17:
GGAAGGAGTTCTGGAATTGG AAAACCATCA TTTTTCAACCATCACAGTAA ATATGGCTCA60
GGCAAGAATTATCAATCAAT GCTAAAGCTA GGGGGAAATTTCGCTTAGGA GCAGGATATT120
AGGGTATTAGTCTGGGCTTA AAGTATCTCC TCACAGATTGTTGTTAGTTT CTGGGGAAAG180
AATAGTAACCATGCAATGGA AAAHAATGGA CAACCTCTTGACTAGGTTAT CAAAATTAAC240
CTCACCAATAAAGGGTGGAT GTTCAACATG TGCCTTCAAATGTGACCCAC TGAGAAGGAA300
ACAACATCACTGTAACAACA ACAACCAGAA ACGACAGGGGGTTTTGACTG AATTCTTCAA360
AAATGTCAATGTCATAGAAG ACAAAGAAAG GTTGTGGAAATGTTTCAGAT TAAATGATAG420
TAAAAACACCTGACAACTAA ACATAGTAAG TAATACTAGACTGGATTCTG TACCAGAGGT480
AACATAAGTGCTCCAAAGGA CAATGTTAGG TCAACTGGCAAATTGGAATA TAGACAGTCA540
ATCAGATAAGAAGTATACTT TGATTAAGTA AAAAAAATCCCTATTCTTGG AAAATACACA600
ATAAAGTATTTTGAGGTAAA GGGCCATAAT GTATGCAATCTACTCTCAAA AAATTCAGAA660
ACATATATTTGTGTGCATTT GCATGTGCAA CAGTACACACAAACATACAT AAAGAGAGCA720
ATTGATAAGGCAAATAAGGT AACATTTAAC AATAATCTGATACACATAAA TAGAGAAAGA780
GCAATTGATAAAGTAAATGA GGTAAAATTT AACAATAATCTGAGCAAAAG GTATATGTGT840
TTTCTTTGAGACAGTCTGAT TCTTGCAACT TATTCTGTAAGTTGGAACTT ATTTCCAAAC900
ATGATTGAAAAAAAACCCCG CACTTGGCAA CTTCTTCTCTTTTTCAGCCT AGAAATGTCT960
GTGTTAAGTGGTTTTTTATT TATTGTTGTT GTTTGTTGTTATTGTTGTTT TGTTGCCAGG1020
CTCCAACTCACAAAATACGA GTTTAAAAAC TGCGTTGTTATTTTTAGAGA TTTGTGATAA1080
83
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
TACAACTTGT TATAAAATTT ATTCCTCAAT AAATATAATT TCTCTACTAT GCP.AAAAAAA 1140
Ap,Ap p,Ap, 114 7
(2) INFORMATION FOR SEQ ID N0:18:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 58 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:18:
Met Ile Glu Lys Lys Pro Arg Thr Trp Gln Leu Leu Leu Phe Phe Ser
1 5 10 15
Leu Glu Met Ser Val Leu Ser Gly Phe Leu Phe'Ile Val Val Val Cys
20 25 30
Cys Tyr Cys Cys Phe Val Ala Arg Leu Gln Leu Thr Lys Tyr Glu Phe
35 40 45
Lys Asn Cys Val Val Ile Phe Arg Asp Leu
50 55
(2) INFORMATION FOR SEQ ID N0:19:
(i} SEQUENCE CHARACTERISTICS:
(A) LENGTH: 1013 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(xi) SEQUENCE DESCRIPTION: SEQ ID
N0:19:
CCTTTTTAAA AAATATCTGA AAAAAGCTTC AAACTCATAAAATAGCTGAT 60
ATATCTTTAC
TGGGCCATGG AGGAGATGAG GCTGTTTAGA TTTCAAGTTTGTCAATTTTC 120
ACTGGTTTTG
CCTGTATGAG AACTTGGGTA AAGCACAAAG TGCTAGTAACAGGTCTCCTG 180
AAACATACAG
CGCCCTGGAA CTAAGTGTTT GGAGGAAGGA GGGGAGGTGAGTATAAAATA 240
CTAAACCCCG
ATTCCACTAA GATCACCTCC TCAGTCCCCA GGTGGATCCTCTGGCCATCT 300
GAAGGCTGAT
84
T. , m-.~.. .T ._ , . . ._ ....n . 1
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
CCTGTGGGGTCTTACTGCTC CTCTGCCATTTCTCTATGCCTGAAGACACG AAGATGATAT360
CAAGGCAGAGCTACCATATC GCAGCCAGTCTCTAGGCTACTGCTGTGCAG TGGCTCCCAC420
TTTCTAATGCTTTTTTGTTT TTGCTTTTTCTAACAAAACAATCTTTTTTC AAAATGAATT480
CCAACCCCTGCTAGTTCCTT CGCTGCCTCCATACTGTTTTAGGCAGCACC GTTTATGTGA540
CAGAGTCCGTGTTTCTCAAA TGCATGGTGTTCCTCAGGTGGAGAGTGGGC AGAAGTTTTT600
GCAACACTTTTTTTTTAAGT TATTGGGTGCAAAATCCCAAACCAGGATAT GTGTATGTCT660
GTGTGTTTATGTTTTTTATT TGACCCTCCCCTCTTTCAACCTACCCCCTT TTATATCTAA720
TGTAGAAAAAGCGAAATTGA ATCTGGAAAGCAAACTGTTGTATATAGTTG CGGTAACAAT780
CATGAAGAGAGAGCCGGGCT GTCCCCTCAGTAATTCATTTTAAATAACAA ATTATTTAAA840
AATAAAATTCATGCCAGAGC CAGCTGAAGAGGCCTTCCTTCATCACCACT GAGGCCACCC900
CCAATCTGGGCCCTCTGTCC ATCTGGCATGTCTCCTCCCAGCAAGATTCA TCTGTTCAAT960
GCCATTTGCGTTTCAATAAA GTTATCTCCTGTACTGTCAAP,~~,~1AAAAAAA 1013
AAA
(2) INFORMATION
FOR SEQ
ID N0:20:
(i) S EQUENCE CHARACTERISTICS:
(A) LENGTH: 87
amino acids
(B) TYPE: amino
acid
(C) STRANDEDNESS:
(D) TOPOLOGY:
linear
(ii) MOLECULE
TYPE:
protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:20:
Met His Gly Val Pro Gln Val Glu Ser Gly Gln Lys Phe Leu Gln His
1 5 10 15
Phe Phe Phe Lys Leu Leu Gly Ala Lys Ser Gln Thr Arg Ile Cys Val
20 25 30
Cys Leu Cys Val Tyr Val Phe Tyr Leu Thr Leu Pro Ser Phe Asn Leu
35 40 45
Pro Pro Phe Ile Ser Asn Val Glu Lys Ala Lys Leu Asn Leu Glu Ser
50 55 60
Lys Leu Leu Tyr Ile Val Ala Val Thr Ile Met Lys Arg Glu Pro Gly
65 70 75 80
Cys Pro Leu Ser Asn Ser Phe
g$
CA 02278770 1999-07-23
WO 98/32853 PCTIUS98/41396
(2)~INFORMATION FOR SEQ ID N0:21:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 1763 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOL~:CULE TYPE: cDNA
(xi) SEQUENCE DESCRIPTION: SEQ ID
N0:21:
TCGGGATAAA AAGCAAGAAA AGAAAGAGAA AAGAAGATCTCTTTGAAAAT60
GACTGAGAAT
AAAATAAGAC TGCTAAAAGT ATTTGGTATA AATAAAGTTGAGGGAATCTC120
CAGTCTGGAA
TCCAGATAAA GAGCAAAAAG AAATAGATAG AGAAAGAAAAAAGACATAGA280
AAAAATATAA
CAATCAATAT GTAATGTTAG GAGTTCCTGG AGAGACAGTGTAGGTGAAGA240
AAGAGAGAAC
AATAAAAAGA AAAAGAATTG AAGAACAGAG CTCCAGATTGAGAGGGCCCA300
CAAGCTAAGT
ATACAATCTA CATCTAGACA CAATATTGTA ATATTAAGGATAGAAGGAAG360
AAATTTTGGA
ATATTAAAGT GGCCAGGGAG AAAACAAATG GATTAGCTCAACACAAAAAT420
AGGTCATCAC
GGATGAGAAA TAGACTGCTA ACAGATTTGT ACTGAATGCCAGAAGTCAAT480
CATCAGCAAC
GGATCAACAT CTTCAGAGCT TAAGGAAAAT AGAATTTCATAGTAAGGCAG540
TTTTGTACCT
ACTGTCAAGA AGAACATCAA AGTGAAGACA GGCAAATTTTCAGAAAGTCT600
TTTTCTGTCA
CCTTTGCACC CTTACTGAGG AAGTATCTTG CCAGCAAAATGAGGATGAAA660
AGGAAATTCT
ACCAGGAAAG AAGAAGAAAT GGGATCCATA CCTTACTTAGGATGTCTCAT720
AAACAGTGGA
TCTAGAGTGA CAGCCAAAAG GGTATCTCAC CAGCTATCCAGCAGACTAAT780
CCTAGAGTGA
TTCAGATGAG AGCATACTGT CTCGGGCTTT TGTGCATTCAGTGCCATAGA840
CTGGGAAGAA
TAGTATCACT GAAGAGCTGG GATGCTTGAG AGTCAAGAAAAAAGAAAGAC900
AAGATTATTT
AAATCAACAA TATGTCAAAA AATTCAGGTC GCAAAATAAAATGAGGCATG960
CAATTATAGA
ATTTTGAGTT ATTCATGAAG AATAAGAAGA GTACATTTCCTTTTCTATGG1020
GGCTTGATAG
CACAGGCATG ATGATATTGG GTGTGTAGGG CCTAGCTTATACTAGGCTCC1080
AAGAAAATAT
CAGTAAGAAG TATTTAAATA GCCAAAATAA ATTTATTAGTATTCAATGTT1140
TGTGGATATC
86
__~~.._.__ .T .. _.._ ~ _.:..__~._
r
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
CAGATCAGCC TATTAACAAA GTGTGAAAGGTTTCATTTTTTATTCAGAAC TGAAGTTGAA1200
AGTAATTAAT GCTGACAAAG GGAAAGAAAGCAGAAAGAGATTGAGAATTA GAGGAAGAGA1260
AGTGGAATCA AAGGTAGAGA TACTTATATATTCAAAGTGGGGATGAAAAG ATCTTCAGTT1320
AATGGAACAA GAACTAGAGG ATTAGTGTATTGTTCAAAGCTATAAAATCA AACCAATAGA1380
TGTATTAAAA AGTGATGTAA CTATCAGACATTTGGAGAGAGATGGACAAA GGAAAGTGGC1440
GATAGTGTPA GTTAAATCCT TATCTTTTGTAATGGGGAATTATTAAAGAT GTTGTAAAGT'.i00
CAGTAAGTCA AGAAATTATT GCTCAAACATATTATTTAAAGTTAGAAAGT TACCAGACGA1560
TCTAAAATAA ATATTGTTAA AAGCATTACCTCTAGGGAATGGGATTTAGA TTTAAAAAGG1620
GTGGGATGGG AAACTGTGTT TTTCATTTTAAGTCCTTCTGTACTATTTAA TTTTTTACCT1680
TGTGCATGTA TTACTTTGAA AAAATTTTTAATAAACCCAAATAAAAATCT P,~~,AAAAAAAA1740
1',~1AAAAAAAA p,~~~,AAAAAAA 17
AAA 6
3
(2) INFORMATION FOR SEQ
ID N0:22:
{i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 46 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:22:
Met Arg Met Lys Thr Arg Lys Glu Glu Glu Met Gly Ser Ile Lys Gln
1 _5 10 15
Trp Thr Leu Leu Arg Met Ser His Ser Arg Val Thr Ala Lys Arg Val
20 25 ~ 30
Ser His Pro Arg Val Thr Ala Ile Gln Gln Thr Asn Phe Arg
35 40 45
(2) INFORMATION FOR SEQ ID N0:23:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 28 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
{ii) MOLECULE TYPE: other nucleic acid
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(A) DESCRIPTION: /desc = "oligonucleotide"
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:23:
CNATCTTAGAG CTCAAAGTTA GGGTCTG 28
(2) INFORMATION FOR SEQ ID N0:24:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: other nucleic acid
(A) DESCRIPTION: /desc = "oligonucleotide"
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:24:
CNCAGAGCTGT TCTGATACTA AGTCTCAC 29
(2) INFORMATION FOR SEQ ID N0:25:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: other nucleic acid
(A) DESCRIPTION: /desc = "oligonucleotide"
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:25:
ANACTATCTTC TTCAGAGGCC AGATCACC 29
(2) INFORMATION FOR SEQ ID N0:26:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: other nucleic acid
(A) DESCRIPTION: /desc = "oligonucleotide"
g8
t _.. T. _, _._.~~._.__ _ ~
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:26:
CNAGAAGCCAG CTGGCTTTGA ATTTCCTC 29
(2) INFORMATION FOR SEQ ID N0:27:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: other nucleic acid
(A) DESCRIPTION: /desc = "oligonucleotide"
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:27:
CNTTTTCCAAT ATGCTTCAAT GGCTCCGT 29
(2) INFORMATION FOR SEQ ID N0:28:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: other nucleic acid
(A) DESCRIPTION: /desc = "oligonucleotide"
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:28:
TNGGTAGAAGG AGAGCAGGAA GGCCATGA ~ 29
(2) INFORMATION FOR SEQ ID N0:29:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: other nucleic acid
(A) DESCRIPTION: /desc = "oligonucleotide"
89
CA 02278770 1999-07-23
WO 98/32853 PCT/iJS98/01396
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:29:
GNCTTCTCTGG CTCAGCCATC TTTTGGGC 29
(2) INFORMATION FOR SEQ ID N0:30:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: other nucleic acid
(A) DESCRIPTION: /desc = "oligonucleotide"
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:30:
CNGTACACACA AACATACATA AAGAGAGC 29
(2) INFORMATION FOR SEQ ID N0:31:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: other nucleic acid
(A) DESCRIPTION: /desc = "oligonucleotide"
{xi) SEQUENCE DESCRIPTION: SEQ ID N0:31:
ANACGGACTCT GTCACATAAA CGGTGCTG 29
(2) INFORMATION FOR SEQ ID N0:32:
(i) SEQUENCE CHARACTERISTICS:
{A) LENGTH: 29 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: other nucleic acid
(Aj DESCRIPTION: /desc = "oligonucleotide"
_. t . ._.~....... T _. __..__~_
CA 02278770 1999-07-23
WO 98/32853 PCT/US98/01396
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:32:
GNTGAGATACC CTTTTGGCTG TCACTCTA 29
(2) INFORMATION FOR SEQ ID N0:33:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 80 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID N0:33:
Met Gln Trp Lys Lys Met Asp Asn Leu Leu Thr Arg Leu Ser Lys Leu
1 5 10 15
Thr Ser Pro Ile Lys Gly Gly Cys Ser Thr Cys Ala Phe Lys Cys Asp
20 25 30
Pro Leu Arg Arg Lys Gln His His Cys Asn Asn Asn Asn Gln Lys Arg
35 40 45
Gln Gly Val Leu Thr Glu Phe Phe Lys Asn Val Asn Val Ile Glu Asp
50 55 60
Lys Glu Arg Leu Trp Lys Cys Phe Arg Leu Asn Asp Ser Lys Asn Thr
65 70 75 80
91