Note: Descriptions are shown in the official language in which they were submitted.
CA 02280308 1999-08-17
-1-
RELEASE AGENT DONOR MEMBER WITH FLUOROSILICONE
INTERPENETRATING NETWORK
FIELD OF THE INVENTION
The present invention relates generally to an electrophotographic
printing apparatus and more particularly to a fusing system for fixing toner
material to support substrate. In particular the present invention relates to
a
release agent donor member for a toner fixing station in such apparatus.
BACKGROUND OF THE INVENTION
In the process of electrophotography, a light image of an original to
be copied is typically recorded in the form of an electrostatic latent image
upon a
photosensitive member with subsequent rendering of the latent image visible by
the application of electroscopic marking particles commonly referred to in the
art
as toner. By methods now well known in the art, the residual toner image can
be
either fixed directly upon the photosensitive member or transferred from the
member to another support, such as a sheet of plain paper, with subsequent
affixing of the image thereto.
Problems associated with transferring the latent image to a support,
especially the following problem referred to as "toner offset," have been
experienced in the field. In these fusing systems, since the toner image is
tackified by heat, it frequently happens that a part of the image carried on
the
supporting substrate will be retained by the heated fuser roller and not
penetrate
into the substrate surface. This tackified material will stick to the surface
of the
CA 02280308 1999-08-17
-2-
fusing roller and come in contact with the subsequent sheet of supporting
substrate
bearing a toner image to be fused. A tackified image which has been partially
removed from the first sheet, may transfer to the second sheet in non-image
portions of the second sheet. In addition, a portion of the tackified image of
the
second sheet may also adhere to the heated fuser roller. In this way and with
the
fusing of subsequent sheets of substrates bearing the toner images, the fuser
roller
may be thoroughly contaminated. In addition, since the fuser roller continues
to
rotate when there is no substrate bearing a toner image to be fused there
between,
toner may be transferred from the fuser roll to the pressure roll. These
conditions
are referred to in the copying art as "offset." Attempts have been made to
control
the heat transfer to the toner and thereby control the offset. However, even
with
the abhesive surfaces provided by the silicone elastomers, this has not been
entirely successful.
It has also been proposed to provide toner release agents such as
silicone oil, in particular, polydimethyl silicone oil, which is applied on
the fuser
roll to a thickness of the order of about 1 micron to act as a toner release
material.
These materials possess a relatively low surface energy and have been found to
be
materials that are suitable for use in the heated fuser roll environment. In
practice,
a thin layer of silicone oil is applied to the surface of the heated roll to
form an
interface between the roll surface and the toner image carried on the support
material. Thus, a low surface energy, easily parted layer is presented to the
toners
that pass through the fuser nip and thereby prevents toner from offsetting to
the
fuser roll surface. In cases where the toner release surface contains
appreciable
amounts of silicone to allow sufficient oil wetting, a nonfunctional
CA 02280308 1999-08-17
-3-
polydimethylsiloxane oil may be used as the toner release agent. The use of
nonfunctional silicone oil with silicone elastomers is known in the art.
According to prior art techniques the toner release agents may be
applied to the fuser roll by several delivery mechanisms including wicking,
impregnating webs and by way of a donor roll which may comprise a high
temperature vulcanized silicone rubber material.
While these silicone elastomer donor rolls have been commercially
successful in some commercial applications, they suffer from certain
difficulties
in that they tend to swell from being in contact with a silicone oil release
agent
which migrates or is absorbed into the silicone rubber. While a small degree
of
swelling may be acceptable if it is uniform, failure of such rolls has been
observed
by excessive swelling over a period of operation wherein the donor roll may
actually be twice the original size. Under such circumstances, the silicone
rubber
donor roll may no longer function in providing a uniform layer of release
fluid to
1 S the fuser roll.
Further, while donor rolls such as those described in U.S. Pat. No.
4,659,621 have attractive oil delivery capabilities in that they are capable
of
transporting sufficient quantities of functional release agent to the fuser
roll to
form the interfacial barner layer between the fuser roll and the toner, they
also
tend to swell with the oil penetrating the rubber whereby there may be an
interchange of the siloxane oil with the siloxane in the silicone rubber
network
leading to breakdown of the network and a lower crosslinked network. This
reduces the toughness of the silicone rubber barrier layer as more release
agent
penetrates the surface. This difficulty is particularly pronounced when
operating
CA 02280308 1999-08-17
-4-
at temperatures in excess of 300° F. Another failure mode is referred
to as
debonding wherein the swelling of the silicone rubber becomes so significant
that
it actually delaminates from the core of the donor roll.
Another recent development described in U.S. Pat. No. 5,061,965
to Ferguson et al. describes the use of a donor roll made of a base member, an
intermediate comformable silicone elastomer layer, and an elastomer release
layer
comprising poly(vinylidene fluoride-hexafluoropropylene-tetrafluoroethylene)
where the vinylidene fluoride is present in an amount <40 mole%, a metal oxide
present in an amount sufficient to interact with polymeric release agent
having
functional groups to transport a sufficient amount of polymeric release agent
to
provide an interfacial burner layer between the fusing surface and the toner.
This
donor roller suffers from the oil wetting capability between nonfunctional
PDMS
release agent and the nonreactive donor roller surface, since the invention
counts
on the polymeric release agent having functional groups to react with the
metal
oxide which is dispersed in the fluoroelastomer layer.
It would be desirable to have further improvement in the field to
overcome the problems of toner offset and donor roll durability.
SUMMARY OF THE INVENTION
In accordance with the present invention, a long life, non-oil
swelling, composite release agent donor member is described. This donor roller
is
to be used in a fusing assembly of the type wherein a functional polymeric
release
agent is applied to the surface of fuser members which come into contact with
CA 02280308 1999-08-17
-5-
toner. This composite oiler donor roller has a fluorocarbon/silicone elastomer
interpenetrating network coating.
In one specific aspect of the present invention the release agent
donor member comprises a base member, an optional intermediate conformable
silicone elastomer layer and an elastomer release agent donor layer comprising
poly(vinylidenefluoride hexafluoropropylene tetrafluoroethylene) wherein the
vinylidenefluoride is present in an amount greater than 45 mole percent,
fluorocarbon-curing agent, fluorocarbon cure accelerator, and siloxane
polymers)
including one or more curable, silanol-terminated, polyfunctional poly(C1_6
alkyl)siloxane polymers, said siloxane polymer comprising at least two
different
functional siloxane units selected from the group consisting of
monofiulctional,
difunctional, trifimctional and tetrafunctional siloxane units or one or more
curable, silanol-terminated, polyfunctional poly(C~_6 alkyl)arylsiloxane
polymers,
said siloxane polymer comprising at least two different functional siloxane
units
selected from the group consisting of monofunctional, difilnctional,
trifimctional
and tetrafunctional siloxane units to form a fluorosilicone interpenetrating
network. The fluorosilicone interpenetrating network release agent donor layer
is
cured from a solvent solution thereof in the presence of more than S parts by
weight of inorganic base per 100 parts of polymer, said inorganic base being
effective to partially dehydrofluorinate the vinylidenefluoride.
In a further aspect of the present invention the intermediate silicone
elastomer layer comprises the crosslinked product of a mixture of crosslinking
agent and crosslinking catalyst and at least one polyorganosiloxane having the
formula:
CA 02280308 1999-08-17
-6-
A-[Si(CH3)R~ O]"[Si(CH3)R20]m-Si(CH3)2D
wherein:
R' and R2 may be any of hydrogen or unsubstituted alkyl, alkenyl or aryl
having less than 19 carbon atoms or fluorosubstituted alkyl having less than
19
S carbon atoms;
each of A & D may be any of hydrogen, methyl, hydroxyl or vinyl groups;
and
m and n are both integer numbers defining the number of repeat units and
independently range from 0 to 10,000.
In a further aspect of the present invention the intermediate layer is from
about 0.5
millimeters to about 7.5 millimeters thick and the release agent donor layer
is
from about 0.0125 to about 0.125 mm thick
The donor member has a hardness greater than 30 Shore A.
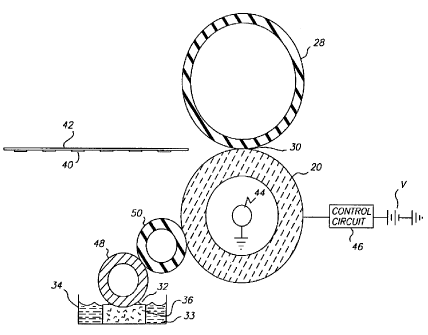
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a schematic front cross-sectional view of a fuser in accordance with
the
present invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Refernng now to FIG.1, a fuser is shown which includes a fuser
roller 20 and an elastomeric pressure roller 28 which form a nip 30. A supply
of
offset preventing oil 33 is shown provided in a oil reservior 34. The fuser
roller
20 can be made of zirconia ceramic and its composites as will be discussed
later.
CA 02280308 2002-O1-07
_7_
Particulate imaging material 40 disposed on a receiver 42 is fused into the
receiver
42 at the nip 30 by the application of heat and pressure. As shown a heating
lamp
44 is connected to a control circuit 46. The heating lamp 44 is well known to
those skilled in the art is provided inside the core of the fuser roller 20.
Alternatively, the fuser may be externally heated by a heated roller riding
along
the fuser roller. This external heated roller may replace or merely assist the
internal lamp. It will be understood depending on the particulate imaging
material
40 that is used that only pressure need be applied to fuse particulate imaging
material 40 into the receiver 42. A wicking device 32 shown in the form as a
wick
36, absorbs the offset preventing oil 33 and is contacted by a metering roller
48
intermediate between the fuser roller 20 and the metering roller 48 is a donor
roller 50. The donor roller 50 delivers offset preventing oil 33 to the
particulate
imaging material 40 to the receiver 42. A continuous supply of offset
preventing
oil 33 must be used which is approximately 1 to 20 mg per receiver 42, on
which
particulate imaging material is fixed. This offset preventing oil is
nonfunctional
polydimethylsiloxane in the viscosity range of 50 to 2000cts.
The release agent donor member according to the present invention
is made by the method described in US Patent No. 6,225,409 of Davis, Chen and
Boulatnikov, titled FLL10R()SILICONE INTERPENETRATING NETWORK: AND
METHODS OF PREPARINCr SAME, issued on May l, 2001.
The composite donor member is an economical, highly reliable,
long life cylindrical roll which is conformable with a fuser roller in a fuser
assembly. The donor member uniformly delivers to the fuser roller a sufficient
amount of a polymeric release agent not having functional groups. This
provides
an interfacial burner layer between the fusing surface and the toner. By
selecting
the structure of the release agent donor member and materials of the composite
according to the present invention the positive properties of the individual
CA 02280308 1999-08-17
_g_
components are accentuated while the negative properties are minimized. Thus,
as previously described, although silicone elastomer rolls, as release agent
donor
members, on their own tend to swell and fail, with the donor member of the
invention, the release agent does not penetrate into the donor member and
bring
about early failure from swelling.
In particular, the donor member of the invention operates within a
fusing assembly for fixing toner images to a substrate, wherein a polymeric
release agent not having functional groups is applied to the surface of a
fuser
roller. The assembly comprises:
(A) a heated fuser roll;
(B) a pressure roller engaging said fuser roller to provide a nip therebetween
through which a copy sheet having an unfused toner image may be passed to fuse
said toner image by contact with said heated fuser roll
(C) means to apply a polymeric release agent not having functional groups to
the
surface of said fuser roll, said means including a release agent donor member
comprising a base member, an intermediate conformable silicone elastomer layer
and an elastomer release agent donor layer comprising poly(vinylidenefluoride-
hexafluoropropylene-tetrafluoroethylene) where the vinylidenefluoride is
present
in an amount greater than 45 mole percent, said elastomer release agent donor
layer having been cured from a solvent solution thereof with a nucleophilic
curing
agent soluble in said solution and in the presence of less than 4 parts by
weight of
inorganic base per 100 parts of polymer, said inorganic base being effective
to at
least partially dehydrofluorinate the vinylidenefluoride.
CA 02280308 1999-08-17
-9-
In operation the four rolls may be independently driven or
according to a preferred embodiment of the present invention, the drive input
is
directed to the fuser roll with the release agent donor roll 50 being driven
by
frictional contact with the surface of the fuser roll 20 and the oil metering
roll 48
being driven by frictional contact with the release agent donor roll 50 in the
direction indicated by the arrows in FIG.1. The pressure roll 28 may also be
driven by frictional control with the fuser roll thereby forming the fusing
nip there
between it and fuser roll 20. As the donor roll 50 rotates in contact with the
fuser
roll 20 the thin film of offset preventing release agent 33 on the donor roll
50 is
split with a portion about 50 percent being transferred to the fuser roll 20,
and a
portion being retained on the donor roll 50.
The release agent donor roll according to the present invention may
comprise a shaft with a solid or hollow cylinder about 8 millimeters to 22
millimeters in diameter and a conformable donor surface coating from 3 about
to
7 millimeters in thickness. The surface coating may be even thicker if desired
to
adjust for certain nip characteristics. Typically the rolls are from about 10
to 18
inches in length.
As used herein, the term "copolymer" refers to the product of
polymerization of two or more substances at the same time, for example
terpolymers which contain three distinct monomers.
The fluorosilicone interpenetrating network elastomers which may
be used with the release agent donor member of the present invention must be
elastomers which can withstand elevated temperatures generally from about
90°
CA 02280308 2002-O1-07
-10-
C. to about 200°C. or higher, depending on the temperature desired for
fusing or
fixing the thermoplastic resin powder to the substrate.
The coating composition is obtained by compounding the
fluorocarbon copolymer, metal oxide or hydroxides to act as acid acceptors,
fluorocarbon-curing agent with a fluorocarbon-curing accelerator and
optionally
other fillers to form a material suitable for dispersion in a solvent. The
accelerator
and fillers are optional; the curing agent may be omitted at this stage and
added
just before the composition is applied as a coating to a surface. The
accelerator
promotes crosslinking between the curing agent and the fluorocarbon copolymer.
Prior to coating this material, a curable polyfunctional poly(C~1-s~
alkyl)siloxane polymer and/or a curable polyfunctional poly(C~1_6>
alkyl)arylsiloxane polymer i.s added. The siloxane polymer is preferably heat-
curable and can comprise one or more polyfunctional poly(C~1_6~ alkyl)siloxane
polymers, copolymer, polyfunctional poly(C~l_6~ alkyl)arylsiloxane polymer or
reaction products of such materials. The siloxane polymer is cured
concurrently
with the fluorocarbon copolymer or terpolymer. 'Che resulting mixture is
solution
milled to form a homogeneous mixture suitable for coating in thin film
applications. Details of the method are described in US Patent No. 6,225,409
of
Davis, Chen and Boulatnikov, titled FLUOROSIL,ICONE INTERPENETRATING
NETWORK AND METHODS OF PREPARING SAME, issued on May 1, 2001.
While not wishing to be bound by any particular theory, it is
believed that the concurrent curing of the individual polymers of the mixture
results in an interpenetrating network of the separately crosslinked polymers.
CA 02280308 1999-08-17
-11-
That is, the network formed by crosslinking the fluorocarbon copolymer or
terpolymer with the fluorocarbon-curing agent and the network formed by
crosslinking of the polyfunctional siloxane polymer mesh together to create an
interpenetrating polymeric network. The cured polymeric mixture forms a
coating
with advantageous release properties attributable to the silicones and
mechanical
and chemical properties characteristic of the fluorocarbon copolymer or
terpolymer are retained.
Fluorocarbon copolymers and silicones tend to phase separate
because, on a molecular level, they are incompatible and will not readily mix.
Phase separation can be avoided by the methods of the instant invention.
Specifically by:
---compounding the fluorocarbon copolymers and the optional addenda, such as
the curing agent, accelerators and fillers to form an intimate, homogeneous,
solid
mixture; and
---dispersing the solid mixture along with the curable polyfunctional
poly(C~1_6>
alkyl)siloxane polymer and/or curable polyfunctional poly(C~1_6>
alkyl)arylsiloxane polymer with a molecular weight sufficient to allow
dispersion.
Also, the solvent system must not hinder reaction of the silicon phase as such
hindered reaction would cause subsequent phase separation. By "suitable
solvent"
is meant a solvent that can dissolve both phases and will not restrict the
silicone
cure. One such appropriate solution is 2-butanone preferably containing less
then
5% by weight of methanol. Minimal methanol is needed in contrast to 3M
Processing Digest, Vol 17 (3), Oct. 1986 describing the use of methanol to
increase solution pot life. As the reaction rate slows in solution the
tendency for
CA 02280308 1999-08-17
-12-
phase separation increases. Other suitable solvents include methyl ethyl
ketone,
methyl isobutyl ketone, ethyl ethyl ketone and mixtures of the foregoing
containing less than 15% of cosolvents methanol, ethanol and acetone as well
as
similar solvents/ solvent systems as would be known by those skilled in the
art.
In a preferred embodiment of the invention, the fluorosilicon
interpenetrating network comprises a solid fluorocarbon copolymer and a
liquid,
curable polyfunctional poly(C~1_6~ alkyl)siloxane polymer, for example, a
polyfunctional hydroxy-functionalized poly(C~1_6~ alkyl)siloxane polymer.
The siloxane polymer preferably has a number average molecular
weight range of greater than 20,000 when measured, for example, by size-
exclusion chromatography (SEC). The polyfunctional poly(Cy-s>
alkyl)arylsiloxane polymer preferably has a number average molecular weight
range of greater than 2000 when measured, for example, by size exclusion
chromatography.
Such components do not readily form homogeneous mixtures due
to phase separation. However, the present invention teaches that by solution
dispersion in a media conducive to further polymerization of the
polyfunctional
hydroxy-functionalized poly(C~1_6~ alkyl)siloxane polymer with the
mechanically
compounded fluorocarbon copolymer or terpolymer and the optional addenda in
the designated sequence and under the conditions taught, suitable compositions
can be obtained.
Compounding (mechanical mixing) is preferably carried out in a
two-roll mill by compounding the fluorocarbon copolymer or terpolymer, the
accelerator and fillers (if present) until a uniform, dry, smooth sheet is
obtained.
CA 02280308 1999-08-17
-13-
This compounding process can be carned out at a temperature of, for example,
from 50° to 70° F. (approx. 10° to 21°C),
preferably from SS° to 65°F. (approx.
13° to 28°C). Compounding of the mixture prior to addition of
the siloxane oil
affords an even band in 30 to 60 minutes. The fluorocarbon-curing agent can
then
be added and compounded in until a uniform, dry, flexible composite sheet is
obtained. Variations to the order of addition of the components can be made by
those skilled in the art without causing disintegration of the composition.
Subsequently, the liquid, curable siloxane polymer is added along with the
compounded material (now in sheet form), into a suitable solvent so that the
siloxane oil is uniformly distributed and in intimate contact with the
fluorocarbon
copolymer.
The composition obtained by such a process can be reduced to
small particles for dispersing in a coating solvent without phase separation
occurring. The particles are small enough to effect solution of the soluble
1 S components in less than about 5 hours, thus minimizing gel formation for
compositions having a tendency to gel rapidly. Before the composition is
applied
as a coating, it must be degassed to remove all dissolved gasses.
In yet another aspect of the invention, for example when a solvent
transfer coating process is contemplated, the fluorocarbon-curing agent can be
withheld from the compounding mixture and added to the coating medium, thus
minimizing any tendency for premature curing of the composition.
Suitable fluorocarbon copolymers of the invention include the
vinylidene fluoride based fluoroelastomers containing hexafluoropropylene
known commercially as Viton~ A. Also suitable are the terpolymers of
vinylidene
CA 02280308 1999-08-17
-14-
fluoride, hexafluoropropylene and tetrafluoroethylene known commercially as
Viton~ B and Fluoore~" FX-9038. Viton~ A and Viton~ B and other Viton~
designations are trademarks of E.I. Dupont de Nemours and Company.
commercially available materials include, for example, vinylidene fluoride-
s hexafluoropropylene copolymer or terpolymers Fluoref~ FX-2530, FluorelTM FC
2174 and Fluorel~" FC 2176. Fluorel~" is a trademark of 3M Company. Other
vinylidene fluoride based polymers which can be used are disclosed in U.S.
Pat.
No. 5,035,950. Mixtures of the foregoing vinylidene fluoride-based
fluoroelastomers may also be suitable. Although it is not critical in the
practice of
this invention, the number-average molecular weight range of the fluorocarbon
copolymer or terpolymers may vary from a low of about 10,000 to a high of
about
200,000. In the more preferred embodiments, the vinylidene fluoride-based
fluoroelastomers have a number-average molecular weight range of about 50,000
to about 100,000.
Suitable fluorocarbon-curing agents or crosslinking agents for use
in the process of the invention include the nucleophilic addition curing
agents as
disclosed, for example, in the patent to Seanor, U.S. Pat. No. 4,272,179. The
nucleophilic addition cure system is well known in the prior art. Exemplary of
this cure system is one comprising a bisphenol crosslinking agent and an
organophosphonium salt as accelerator. Suitable bisphenols include 2,2-bis(4-
hydroxyphenyl) hexafluoropropane, 4,4-isopropylidenediphenol and the like.
Although other conventional cure or crosslinking systems may be used to cure
the
fluoroelastomers useful in the present invention, for example, free radical
initiators, such as an organic peroxide, for example, dicumyl peroxide and
CA 02280308 1999-08-17
-15-
dichlorobenzoyl peroxide, or 2,S-dimethyl-2,5-di-t-butylperoxyhexane with
triallyl cyanurate, the nucleophilic addition system is preferred.
Suitable accelerators for the bisphenol curing method include
organophosphonium salts, e.g., halides such as benzyl triphenylphosphonium
chloride, as disclosed in U.S. Pat. No. 4,272,179 cited above.
Suitable fillers for producing these composites include mineral
oxides, such as alumina, silicate or titanate, and carbon of various grades.
Nucleophilic addition-cure systems used in conjunction with fluorocarbon
copolymer or terpolymers can generate hydrogen fluoride and thus acid
acceptors
are added as fillers. Suitable acid acceptors include metal oxides or
hydroxides
such as magnesium oxide, calcium hydroxide, lead oxide, copper oxide and the
like, which can be used as mixtures with the aforementioned fillers in various
proportions.
The preferred curable polyfunctional poly(C~~_6~ alkyl)siloxane
and/or a curable polyfunctional poly(C~~_6~ alkyl)arylsiloxane polymers,
useful in
the practice of this invention, when cured concurrently with the fluoro-
elastomers,
produce a coating suitable for use as the surface coating of a fusing member.
Such coated fusing members have low energy surfaces which release toner images
with minimal offset. These coatings can also be advantageously used with small
amounts of externally added polymeric release agents, for example
nonfunctional
polydimethylsiloxanes, to further minimize offset.
Preferred curable polyfunctional poly(C~~_6~ alkyl)siloxane
polymers and/or a curable polyfunctional poly(C~1_6~ alkyl)arylsiloxane
polymer
are heat-curable silicones; however peroxide-curable silicones can also be
used
CA 02280308 1999-08-17
-16-
with conventional initiators. Heat-curable silicones include the hydroxy-
functionalized polyfunctional organopolysiloxanes belonging to the class of
silicones known as "soft" silicones. Preferred soft silicones are silanol-
terminated
polyfunctional organopolysiloxanes containing repeating units of the formula,
S (R')a S1O(a_a~2
wherein R' is C~1_6~ alkyl and a is 0 to 3.
Alkyl groups which R' can represent include methyl, ethyl, propyl,
isopropyl, butyl, sec.butyl, pentyl and hexyl. Preferred soft silicones are
those in
which R' is methyl.
Preferred curable poly(C~~_6~ alkyl)arylsiloxane polymers are heat-
curable siloxanes, however peroxide-curable siloxanes can also be used with
conventional initiators. Heat curable siloxane polymers include the hydroxy-
functionalized organopolysiloxanes belonging to the classes of silicones known
as
"hard" and "soft" silicones. Preferred hard and soft silicones are silanol-
terminated polyfunctional organopolysiloxanes containing repeating units of
the
formula, R' a R2bS1O~4 _~a+b))
Wherein:R' and R2 are independently (C~~_6~ alkyl) or aryl; and a and b are
independently 0 to 3.
Alkyl groups which R' and R2 can represent include methyl, ethyl,
propyl, isopropyl, butyl, sec.butyl, pentyl and hexyl. Preferred hard and oft
silicones are those in which R' and R2 are independently methyl or phenyl.
Both hard and soft silicones can contain various proportions of
mono-, di-, tri- and tetra-functional siloxane repeating units. The degree of
functionality influences the hardness of the silicone. In general, the greater
the
CA 02280308 1999-08-17
- 17-
functionality, the harder is the silicone. However, the predominant influence
on
hardness is the ratio of aryl to alkyl groups present. Preferred hard
silicones are
characterized by having a ratio of phenyl to methyl groups greater than 0.5
and are
nonflowable, preferably between about 2 to 1. Soft silicones have a ratio of
aryl
to methyl groups less than 0.5, preferably no phenyl groups are present and
are
flowable. Hard silicones generally have a number-average molecular weight of
less than about 10,000, preferably less than about 4,000. Polyfunctional hard
silicones of such molecular weights have a high level of crosslinking on
curing
which contributes to the hardness. Soft silicones generally have a number-
average
molecular weight of greater than 20,000, preferably greater than 100,000 which
results in a low level of crosslinking on curing hard and soft silicones can
be used
singly or as mixtures of silicones and, in addition, can contain minor amounts
of
one or more polyfunctional silicones having number-average molecular weights
in
the range of 1,000 to 300,000.
1 S Particularly suitable silicones are the heat-curable silanol-
terminated hard silicone copolymers comprising difunctional and trifunctional
siloxane repeating units of the formulae, R32Si0 and R4SiOl.s~
wherein R3 and R4 are independently methyl or phenyl provided that the ratio
of
phenyl to methyl groups is at least about 1 to 1.
Exemplary hard and soft silicones are commercially available or
can be prepared by conventional methods. For example, DC6-2230 silicone and
DC-806A silicone (sold by Dow Corning Corp.), are hard silicone polymers, and
SFR-100 silicone (sold by General Electric Co.) and EC 4952 silicone (sold by
Emerson Cummings Co.), are soft silicone polymers. DC6-2230 silicone is
CA 02280308 1999-08-17
-18-
characterized as a silanol-terminated polymethylphenylsiloxane copolymer
containing phenyl to methyl groups in a ratio of about 1 to 1, difunctional to
trifunctional siloxane units in a ratio of about 0.1 to 1 and having a number-
average molecular weight between 2,000 and 4,000. DC 806A silicone is
characterized as a silanol-terminated polymethylphenylsiloxane copolymer
containing phenyl to methyl groups in a ratio of about 1 to 1 and having
difunctional to trifunctional siloxane units in a ratio of about 0.5 to 1. SFR
100
silicone is characterized as a silanol- or trimethylsilyl-terminated
polymethylsiloxane and is a liquid blend comprising about 60-80 weight percent
of a difunctional polydimethylsiloxane having a number-average molecular
weight of about 150,000 and 20-40 weight percent of a polymethylsilyl silicate
resin having monofunctional (i.e. trimethylsiloxane) and tetrafunctional (i.e.
5i02)
repeating units in an average ratio of between about 0.8 and 1 to 1, and
having a
number-average molecular weight of about 2,500. EC 4952 silicone is
characterized as a silanol-terminated polymethylsiloxane having about 85 mole
percent of difunctional dimethylsiloxane repeating units, about 15 mole
percent of
trifunctional methylsiloxane repeating units and having a number-average
molecular weight of about 21,000. Other polyfunctional poly(C~~_6~
alkyl)siloxane
polymers which can be used are disclosed in U.S. Pat. Nos. 4,387,176 and
4,536,529.
Preferred compositions of the invention have a ratio of siloxane
polymer to fluorocarbon copolymer or terpolymer between about 0.1 and 3 to 1
by
weight, preferably between about 0.2 and 0.5 to 1. The composite is preferably
obtained by curing a mixture comprising from about SO-70 weight percent of a
CA 02280308 1999-08-17
- 19-
fluorocarbon copolymer or terpolymer, 10-30 weight percent of a curable
polyfunctional polymethylsiloxane polymer, most preferably about 20-30 weight
percent. 1-10 weight percent of a fluorocarbon-curing agent, 1-10 weight
percent
of a fluorocarbon-curing accelerator, 9-30 weight percent of an acid acceptor
type
filler, and 0-30 weight percent of an inert filler.
Curing of the composite is carried out according to the well known
conditions for curing vinylidene fluoride based copolymer or terpolymers
ranging,
for example, from about 12-48 hours at temperatures of between 50° C to
250° C.
Preferably the coated composition is dried until solvent free at room
temperature,
then gradually heated to about 230° C. over 24 hours, then maintained
at that
temperature for 24 hours.
In accordance with the present invention, the coated article can be a
fusing member in the form of a roll, belt or any surface having a suitable
configuration for fixing or fusing a thermoplastic toner image to a receiver
such as
a paper sheet. The underlying structure onto which the coating is applied is
called
the substrate. When used with fusing rolls, substrate onto which the composite
of
the invention can be coated directly on is the fusing roll core preferably the
coating is applied on an underlying intermediate layer which is bonded
directly or
indirectly to the core. This intermediate layer is preferably a silicone
elastomer,
for example, EC 4952 silicone (sold by Emerson Cummings Co.). When the
fusing member is in the form of a belt, the belt comprises a continuous
flexible
substrate made of metal or polymeric material onto which the composite of the
invention can be coated. The fusing members can be coated by conventional
techniques, however, solvent transfer coating techniques are preferred.
CA 02280308 1999-08-17
-20-
Coating solvents which can be used include polar solvents, for
example, ketones, acetates and the like. Preferred solvents for the
fluoroelastomer
based composites are the ketones, especially methyl ethyl ketone and methyl
isobutyl ketone. The composites of the invention are dispersed in the coating
solvent at a concentration of between about 10 to SO weight percent,
preferably
between about 20 to 30 weight percent and coated on the fusing member to give
a
to 100~m thick sheet on drying. The coated article is cured under the
conditions described above.
The cured coatings of the invention have low surface energies and
10 exhibit good adhesion to underlying layers and substrates. Such coatings
have
excellent resistance to abrasion as measured on a Norman Abrader apparatus and
retain the advantageous mechanical and chemical properties characteristic of
fluoroelastomers, such as hardness, elongation, tensile and tear strength and
resistance to releasing oils. In addition, when evaluated as image-fixing
media,
the coatings have shown minimal reactivity with thermoplastic toner powders
while showing desirable release properties with minimal or no offsettings
under
simulated fusing conditions.
The rolls and belts produced in accordance with the present
invention are thus useful in electro-photographic copying machines to fuse
heat-
softenable toner to an image carrying receiver sheet. This can be accomplished
by
contacting a receiver, such as a sheet of paper, to which toner particles are
electrostatically attracted in an imagewise fashion with such a fusing member.
Such contact is maintained at a temperature and pressure sufficient to fuse
the
toner to the receiver.
CA 02280308 1999-08-17
-21-
The following examples illustrate the compounding, coating,
curing and testing of fluorocarbon/silicone elastomer polymeric compositions.
The SFR-100 silicone used on the examples described below was
obtained from General Electric Co. and was determined by size exclusion
chromatography and NMR to consist essentially of a mixture of about 70 weight
percent of a polydimethylsiloxane having a number-average molecular weight of
about 150,000, and about 30 weight percent of a polytrimethylsilyl silicate
resin
having monofunctional and tetrafunctional repeating units in an average ratio
of
about 0.9 to 1 and having a number-average molecular weight of about 2,480.
The optional intermediate silicone elastomer layer is a polyorganosiloxane
curable
to a silicone elastomer and may be selected from the commercially available
condensation curable, addition curable and peroxide curable materials.
Typically
the silicone elastomer layer comprises the crosslinked product of a mixture of
crosslinking agent and crosslinking catalyst and at least one
polyorganosiloxane
1 S having the formula:
A-[Si(CH3)R'O]n[Si(CH3)R20]m Si(CH3)2D,
wherein
R' and R2 may be any of hydrogen or unsubstituted alkyl, alkenyl or aryl
having less than 19 carbon atoms or fluorosubstituted alkyl having less than
19
carbon atoms;
each of A & D may be any of hydrogen, methyl, hydroxyl or vinyl groups;
and
CA 02280308 1999-08-17
-22-
m and n are both integer numbers defining the number of repeat units and
independently range from 0 to 10,000). Typically, R' and R2 are hydrogen,
methyl, vinyl, phenyl or trifluoropropyl.
The substrate for the release agent donor member according to the
present invention may be of any suitable material. Typically, it takes the
form of
a cylindrical tube of aluminum steel or certain plastic materials chosen to
maintain
rigidity, in structural integrity, as well as being capable of having the
silicone
elastomer coated thereon and adhered firmly thereto. Typically the release
agent
donor rolls may be made by injection, compression or transfer molding, or they
may be extruded. In a typical procedure the core which may be a steel cylinder
is
degreased with a solvent and cleaned with an abrasive cleaner prior to being
primed with a primer such as Dow Corning 1200 which may be sprayed, brushed
or dipped followed by air drying under ambient conditions for thirty minutes
and
then baked at 150° C. for 30 minutes. The silicone elastomer may be
applied
according to conventional techniques such as injection molding and casting
after
which it is cured for up to 15 minutes and at 120 to 180 degrees centigrade to
provide a complete cure without a significant post cure operation. This curing
operation should be substantially complete to prevent debonding of the
silicone
elastomer from the core when it is removed from the mold. Thereafter the
surface
of the silicone elastomer is sanded to remove the mold release agent and it is
wiped clean with a solvent such as isopropyl alcohol to remove all debris.
The following Examples further define and describe donor rolls
prepared by the present invention and illustrate preferred embodiments of the
CA 02280308 1999-08-17
- 23 -
present invention. Unless otherwise indicated, all parts and percentages are
by
weight.
EXAMPLES
EXAMPLE 1
Viton~ A fluoropolymer (500 g), benzyl triphenylphosphonium chloride
(30 g), Magnesium oxide (Maglite Y) (60 g), Magnesium oxide (Maglite D)
(15g), and 2,2-bis(4-hydroxyphenyl) hexafluoropropane (12.5 g) were thoroughly
compounded for 60 minutes in a two-roll mill at 63° F. (approx.
17° C.) with
water cooling until a uniform, dry composite sheet was obtained. The uniform,
dry, flexible composite sheet obtained was divided into small pieces. SFR-100
silicone (20 g) was added to 117.5 g of the composite sheet and both were
suspended in a 85% methyl ethyl ketone and 15% methanol solution to form a 30
weight percent coating dispersion. Dispersion was formed by roll milling for
approximately 3 hours. A testing sample was made according to the following
procedure. An aluminum core was cleaned and then primed with a thin layer of
silicone primer and dried in ambient air before application of the base
cushion.
The base cushion, a 230 mil thick polydimethylsiloxane was injection molded to
a
dry thickness of 0.230 inches and cured for 2 hours at 80°C. After
demolding, the
base cushion was corona treated for 1 minute at 750 watts, at 25 revolutions
per
minute. The above described dispersion was degassed for 2 minutes under 25 mm
Hg before it was ring coated onto the base cushion layer. This donor roller
was
cured by air drying for 1 hour followed by 24 hours ramp to 230°C. and
then 24
hours at 230°C. The dry thickness of the coating on the roller was 1
mil.
EXAMPLE 2
Viton~ A fluoropolymer (500 g), benzyl triphenylphosphonium chloride
(30 g), Magnesium oxide (Maglite Y) (60 g), Magnesium oxide (Maglite D)
(15g), and 2,2-bis(4-hydroxyphenyl) hexafluoropropane (12.5 g) were thoroughly
CA 02280308 1999-08-17
-24-
compounded for 60 minutes in a two-roll mill at 63° F. (approx.
17° C.) with
water cooling until a uniform, dry composite sheet was obtained. The uniform,
dry, flexible composite sheet obtained was divided into small pieces. SFR-100
silicone (20 g) was added to 117.5 g of the composite sheet and both were
suspended in a 85% methyl ethyl ketone and 15% methanol solution to form a 30
weight percent coating dispersion. Dispersion was formed by roll milling for
approximately 3 hours. A testing sample was made according to the following
procedure. An aluminum core was cleaned and then primed with a thin layer of
silicone primer and dried in ambient air before application of the base
cushion.
The base cushion, a 230 mil thick polydimethylsiloxane was injection molded to
a
dry thickness of 0.230 inches and cured for 2 hours at 80°C. After
demolding, the
base cushion was corona treated for 1 minute at 750 watts, at 25 revolutions
per
minute. A solution of Emerson & Cummings resin EC4952 25 wt% solids in
MEK was ring coated onto the base cushion layer and cured by air drying for 12
hours to form a dry coated base cushion. The dry coated base cushion was
corona
treated for 1 minute at 750 watts, at 25 revolutions per minute. The above
described dispersion was degassed for 2 minutes under 25 mm Hg before it was
ring coated onto the dry coated base cushion layer. This donor roller was
cured by
air drying for 1 hour followed by 24 hours ramp to 230°C. and then 24
hours at
230°C. The dry thickness of the coating on the roller was 1 mil.
EXAMPLE 3
A second roller was prepared as described in Example 2 for Machine
testing.
Comparative Example 1
Several commercially available Xerox 5090 donor roller most likely
manufactured according to U.S.P 5,166,031 were obtained for comparative
testing.
CA 02280308 1999-08-17
-25-
Testing of IPN oiler donor rollers
Surface Energy Measurement and Wear Rate
The surface energy (S.E.) of the rollers was determined from
contact angle measurements of distilled water and diiodomethane using Rame-
Hart Inc., NRL model A-100 contact angle Goniometer.
The wear rate test of compression-molded slabs was performed
using a Norman Abrader Device (Norman Tool Inc., Ind.). For this test, the
Abrader Device was modified by replacing the standard grommet wheel with an
aluminum rod (1.1 inch in length and 0.625 inch in diameter), placing a
renewable
paper strip on the samples, and running the tests at about 350° F.
Cycles were
accumulated until coating failure.
The Surface Roughness Ra was measured on a Federal 2000
surfanalyzer with a chisel stylus.
Oil swell was measured by immersing a weighed sample in 350cs
Dow Corning DC200 polydimethylsiloxane for 7 days at 175°C and
calculating
the weight gain.
Sample wear (cycles/mil) Ra (winch) oil swell wt% S.E. (dyne/cm)
EX 1 200 24 31.8
EX 2 ~ 200 20 31.8
CE 1 90 54 49
Toner Release Test:
The test samples are employed to evaluate the toner offset
and release force characteristics of the fuser member coating. Two samples are
cut approximately 1-inch square of each example. One of these squares is left
untreated by release agent (the dry sample). To the surface of the other
sample is
applied in unmeasured amount of Xerox amino-functionalized PDMS 8879.
Each sample is incubated overnight at a temperature of 175°C.
Following this treatment, the surface of each sample is wiped with
CA 02280308 1999-08-17
-26-
dichloromethane. Each sample is then soaked in dichloromethane for one hour
and allowed to dry before off line testing for toner offset and release
properties.
Each sample is tested in the following manner:
A 1-inch (2.56-cm) square of paper covered with unfused
polyester toner is placed in contact with a sample on a bed heated to
175°C, and a
pressure roller set for 80 psi is locked in place over the laminate to form a
nip.
After 20 minutes the roller is released from the laminate.
The extent of offset for each sample is determined by microscopic
examination of the sample surface following delamination. The following
numerical evaluation, corresponding to the amount of toner remaining on the
surface, is employed.
1 0% offset
2 1-20% offset
3 21 -50% offset
4 51-90% offset
5 91 -100% offset
Qualitative assessment of the force required for delamination of the
paper from the sample is as follows:
1 low release force
2 moderate release force
3 high release force
The following examples further illustrate the test results.
Sample Release/Offset - Non Release/Offset
- NH3
EX 1 1 /2 1 /2
EX 2 1/2 1/2
CE 1 1/1-2 1/1
Machine Testing
CA 02280308 1999-08-17
-27-
Two rolls (Example 3 and Comparative Example 1) were used as release agent
donor rollers for supplying conventional nonfunctional silicone oil in a
Eastman
Kodak prototype test fixture. Results are shown below under identical testing
conditions of 320°F fuser roller temperature and a stainless steel
metering roller.
Both rollers showed long life and adequate oil deliver
Oil rate on Media (mg/A4 page)
Sample ~ Laserprint 90g Finch 90g Lustro laser 118g transparency
4 5.4 4.1
CE1 I 5 4 5.4 4.1
Thus according to the present invention a new and improved
release agent donor member and fusing assembly have been provided. In
particular, a release agent donor member having greatly improved wear
resistance
has been provided. This is achieved with a interpenetrating polymer network
donor roll coating capable of transporting nonfunctional release agent in
sufficient
quantities to the fuser roller while at the same time preventing penetration
of the
release agent into the intermediate silicone layer.
The release agent donor of this invention, particularly the fuser
rollers, possess extremely desirable physical and mechanical characteristics
as
indicated in the tests results above. The fuser rollers have excellent toner
release
properties, without sacrificing toughness and abrasion resistance. The coating
materials exhibit these desirable properties when they are prepared according
to
the process of this invention.
CA 02280308 1999-08-17
-28-
PARTS LIST
20 fusing roller
28 pressure roller
30 nip
33 offset prevention
oil
34 oil reservoir
36 wick
40 particulate imaging
material
42 receiver
44 heating lamp
46 control circuit
48 metering roller
50 donor roller