Note: Descriptions are shown in the official language in which they were submitted.
CA 02281371 1999-09-03
RECHARGEABLE NICKEL-ZINC CELL
BACKGROUND OF THE INVENTION
Alkaline nickel-zinc cells have been built as plate cells for many years, but
have not
achieved commercial importance to date, mainly due to the still limited life
of the zinc electrode
caused by electrode shape change, dendrite growth and zinc corrosion.
Electrolytes with low
alkalinity containing KF and KZC03 and Ca(OH)~ additions to the anode where
successfully
applied to suppress solubility of zinc species and electrode shape change of
flat cells. In plate
type sealed nickel-zinc cells dendrite formation is mostly eliminated, since
any dendrite
produced is quickly oxidized by the present oxygen.
General characteristics regarding the nickel-zinc system have been summarized
in detail
in the contribution of M.Klein and F.McLarnon:"Nickel-Zinc Batteries", in D.
Linden, ed.,
Handbook of Batteries, Chapter 29, McGraw-Hill, Inc., NY, 1995. The history
and the
development of Ni-Zn cells is reviewed by J. Jindra, J. Power Sources, 66, 15
(1997).
The overall cell reaction of the nickel-zinc system can be written in a
simplified form as
follows:
2 Ni00H + Zn + 2 H20 ~ 2 Ni(OH)2 + Zn(OH)2.
In addition to this main current-generating process several parasitic
reactions may occur.
At the end of charge (70-80 % state of charge) and during overcharging a cell,
which is necessary
for a better charge acceptance of the nickel electrode, oxygen evolution takes
place. In the case of
good access to the negative electrode oxygen can be directly recombined at the
zinc electrode or
an auxiliary electrode can be incorporated to enhance recombination. After
repeated cycling also
hydrogen evolution can take place at the zinc electrode. To minimize the
hydrogen amount a
sufficient Zn0 excess has to be provided. In general a Zn : Ni ratio between
between 2 and 3
should be established. Furthermore to avoid zinc corrosion in alkaline medium
corrosion
inhibitors (In, Pb, Hg, organic compounds) have to be added.
In nickel-zinc cells different types of nickel electrodes are used: sintered,
nonsintered and
lightweight substrates. Sintered nickel electrodes are prepared by sintering
carbonyl nickel
powder into a porous plaque containing a nickel screen and is then filled with
active nickel
hydroxide. Typically sintered nickel electrodes have a ratio of inactive to
active nickel between 1
to 1.4 :1 providing excellent cycle life and stability, but with the
disadvantage of being very
heavy. Nonsintered nickel electrodes are made by kneading and calendering an
electrode strip
consisting of nickel hydroxide, graphite and plastic binder laminated on both
sides of an
appropriate current collector. Applying lightweight substrates based on a
fiber structure filled
with active electrode mass has the advantage of reducing electrode weight as
well as material
costs.
Cylindrical cells with spirally rolled nickel electrode/separator/zinc
electrode assemblies,
quite similar to Ni-Cd cells, have been tentatively produced by some
manufacturers, but they
suffered from serious short circuit troubles due to zinc dendrites growing
during the charge
cycles across the narrow (open) spiral distances between cathodes and anodes.
The objectives of this invention are mainly to produce high current, high
capacity,
cylindrical consumer cells which could be hermetically sealed and showing an
acceptable cycle
life at deep discharge conditions.
CA 02281371 1999-09-03
BRIEF DESCRIPTION OF THE DRAWINGS
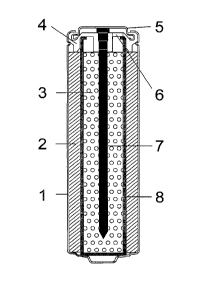
FIG. 1 shows a cut through a cylindrical AA-size Ni-Zn cell made according to
this invention.
FIG. 2 shows a nickel electrode with two layers from the top and a three-
dimensional view.
FIG. 3 shows multiple (three) sleeves of a nickel electrode from the top and a
three-dimensional
view.
FIG. 4 shows the discharge capacity of cell A75, A77 and A79 with one nickel
layer as a
function of cycles.
FIG. 5 shows the discharge capacity of cell A71 and A86 with two nickel layers
as a function of
cycles.
FIG. 6 shows the discharge capacity of cell A81 and A87 with one nickel layer
charged with
constant current as a function of cycles.
FIG. 7 shows the discharge capacity of cell A121 containing 2 % and cell A79
with 8.6 % Ni
powder / T-210 as a function of cycles.
FIG. 8 shows the discharge capacity of cell A128 containing 2 % and cell A131
with 0 % Co
extra-fine powder as a function of cycles.
FIG. 9 shows the material utilization of cell C23 with one nickel layer and
cell C21 with two
nickel layers as a function of cycles. The highest discharge capacity value of
cell C23 (6.cycle) is
taken as 100 %.
DESCRIPTION OF PREFERRED EMBODIMENTS
FIG. 1 of the drawings shows a cut through a cylindrical AA-size Ni-Zn cell
embodying
the present invention. The cell comprises a Ni-plated steel can 1 housing a
porous nickel oxide
cathode 2, a zinc anode 3 and a separator 8 as the main components of a
rechargeable galvanic
element. The cathode 2 may comprise one or several layers of a porous nickel
substrate filled
with nickel hydroxide, additives and a binder, and is separated from anode 3,
which may
comprise zinc powder, zinc oxide and gelling agent, by an electrolyte
permeable separator 8. The
electrolyte, which may consist of aqueous potassium and lithium hydroxide
permeates the nickel
cathode 2 and zinc anode 3 through separator 8. A current collector nail 7,
that is connected to
the negative cap 5 and embedded into the plastic top seal 4, is located in the
center of the nickel-
zinc cell. For safety reasons the plastic top seal 4 is provided with a safety
vent break area 6.
FIG. 2 illustrates the embodiment of a nickel electrode made of two layers of
a nickel
foam, pasted with a mixture of nickel hydroxide, nickel powder, cobalt powder
and a binder
(PVA-solution), that is shaped into a very tight cylindrical arrangement.
The embodiment of FIG. 3 differs from that of FIG. 2 in that, the nickel
electrode is
prepared by three or multiple sleeves of a nickel foam filled with nickel
hydroxide mixture.
2
CA 02281371 1999-09-03
EXAMPLES OF THE INVENTION
Example 1
A cylindrical AA-size nickel zinc cell was fabricated which consisted of one
positive
nickel electrode layer and a negative zinc electrode assembled in an
arrangement as shown in
FIG. 1. The nickel electrode was prepared by blending a mixture of 8.6 % of
nickel T-210
powder (from Inco Technical Services Ltd., Missisauga, Ontario), 4.3 % of
cobalt extra-fme
powder (UNION MINIERE, INC. - Carolmet Cobalt Products, Laurinburg, N.C.),
30.0 % of
PVA-solution (1.17 % PVA in water/ethanol) and 57.1 % of nickel hydroxide
(from Inco
Technical Services Ltd., Missisauga, Ontario). Some water was added to obtain
a light
suspension. The slurry was pasted into a nickel foam of 38 mm x 36 mm provided
with a
spotwelded nickel foil current collector (36 mm x 4 mm, 0.125 mm thick, 99.98
%, from
Goodfellow Cambridge Ltd.,) at the longitudinal direction. The pasting
procedure was carried
out a few times on both sides of the nickel foam with a spatula to ensure that
the slurry
completely penetrates into the foam. Wet surplus material was removed from the
foam surface.
The nickel electrode was dried at 110°C for one hour. Three different
nickel foam types were
used to prepare a nickel electrode as described above: Retec 80 PPI, 1.6 mm
thick, Retec 110
PPI, 1.6 mm thick (both from RPM Ventures, ELTEC Systems Corp., Ohio) and Inco
foam, 2.7
mm thick (from Inco Technical Services Ltd., Missisauga, Ontario).
The zinc electrode was prepared by mixing up 59 % of zinc oxide (from Merck),
10 % of
zinc / type 004F (from Union Miniere S.A., Overpelt, Belgium), 0.50 % of
Carbopol 940 (from
Nacan, Toronto) and 30.5 % of 7 M KOH to a gelous paste. Two overlapping
layers of a
laminated product comprising one piece of regenerated high purity cellulose
bonded to a non-
woven polyamide synthetic fiber (from Berec Components Ltd., Co. Durham) were
used to
construct the separator bag. The nickel electrode was rolled up around the
separator bag, inserted
into the nickel plated steel can, filled with 27 % KOH - 10 g/1 LiOHxHzO
electrolyte and
allowed to soak for 24 hours. The zinc anode paste was filled into the
separator bag and the
cylindrical AA-size nickel zinc cell was closed with the negative cap unit as
shown in
FIG. 1.
Cell cycling was carried out with constant voltage taper charging at 1.90
Volts for
approximately 500 minutes followed by the discharge process at 3.9 Ohms to a
cut-off voltage of
800 mV. FIG. 4 shows the discharge capacity of each cycle of cylindrical AA-
size nickel zinc
cells A75, A77 and A79 containing one layer of nickel electrode consisting of
the above
mentioned nickel foam types and a pasted zinc electrode as a function of cycle
life. The results
obtained show a stable discharge behaviour for at least 100 cycles with a
relatively flat discharge
profile and a small capacity decline during cycling. The first few cycles are
formation cycles that
run under the cycling condition described above. Cell A77 contains less nickel
hydroxide and
therefore delivers lower capacity.
Example 2
A cell was assembled as described above with the exception that the positive
electrode
was made of two nickel layers and the appropriate dimension of the nickel foam
was 38 mm x 70
mm. In the case of cylindrical cell design the assembly is volume limited and
therefore cells with
3
CA 02281371 1999-09-03
two layers contain less zinc. Two different foam types according with example
1 were taken to
prepare the nickel electrode. Inco foam, 2.7 mm thick, could not be used in a
douple layered
arrangement because of its high thickness resulting in a deficiency of
positive zinc electrode. In
FIG. 5 the discharge capacity of each cycle of cylindrical AA-size nickel zinc
cells A71 and A86
with 2 layers of nickel electrode and a pasted zinc electrode is shown. It
turned out that the cells
had high values of discharge capacity (600-500 mAh) for the first twenty
cycles but due to the
Zn/Ni ratio of only 1.2 the discharge capacity decreased with increasing
cycles.
Example 3
A cell was constructed as described in Example 1. Two different foam types
were used to
build a cylindrical AA-size nickel zinc cell: Retec 110 PPI, 1.6 mm thick and
Inco foam, 2.7 mm
thick. FIG. 6 shows the dependence of discharge capacity of each cycle of cell
A81 and A87 on
cycle life. Both cells were charged with constant current of 66 mA (for 320
min) respectively of
81 mA (for 425 min). The cells were tested up to 60 cycles and the results are
comparable to
FIG. 4 with a rather flat profile of discharge curve. Cell A81 contains 42 %
less nickel hydroxide
than cell A87 and delivers therefore only 300 mAh.
Example 4
A cell was assembled as described in Example 1 with 2 % of nickel T-210 powder
and
63.7 % of nickel hydroxide. The other components of nickel hydroxide slurry
were the same as
in Example 1. In that case it was not necessary to add water to this light
suspension that easy
penetrates into the Inco foam, 2.2 mm thick. In FIG. 7 the discharge capacity
of each cycle of
cylindrical AA-size nickel zinc cell A121 and A79 (from Example 1) can be
seen. In comparison
with cell A79, that is also constructed with one nickel layer, cell A121
delivers a 150-50 mAh
higher discharge capacity up to 50 cycles due to its composition with more
active nickel
hydroxide (63.7 % instead of 57.1 %) and to a bigger amount of pasted cathode
mass as indicated
in the following table:
Cell No. Nickel Foam T a Nickel Cathode
A79 Inco / 2.7 mm 2.88
A121 Inco / 2.2 mm 4.44
Example 5
Two cells were built as decribed in Example 1 with 2% and 0 % of cobalt extra
fine
powder and with 59.4 % and 61.4 % of nickel hydroxide. The other components of
nickel
hydroxide slurry were the same as in Example 1 and Inco foam, 2.2 mm thick was
used as foam
material. FIG. 8 shows the discharge capacity of each cycle of cylindrical AA-
size nickel zinc
cell A128 and A131. The discharge capacity of cell A128 with 2 % cobalt is
approximately 200
mAh higher than that of cell A131 containing 0 % cobalt since the addition of
cobalt increases
4
CA 02281371 1999-09-03
electronic conductivity of nickel electrode mass. The following table
summarizes foam type and
nickel cathode mass of both cells:
Cell No. Nickel Foam T a Nickel Cathode
A128 Inco / 2.2 mm 3.44
A 131 Inco / 2.2 mm 3.51
Example 6
Cylindrical C-size nickel zinc cells were assembled as described in Example 1
with Inco
foam, 2.2 mm thick. In cell C23 with one nickel layer the size of nickel foam
was 38 mm x 70
mm and for cell C21 containing two nickel layers a 38 mm x 130 mm foam was
used. A nickel
foil current collector (70 and 130 mm respectively x 4 mm, 0.125 mm thick) was
spotwelded at
the longitudinal direction of both nickel foams.
Cell cycling was earned out as described in Example 1 with the exception that
charging
time was 760 minutes. In FIG. 9 the material utilization of each cycle of
cylindrical C-size nickel
zinc cell C23 and C21 can be seen. The highest discharge capacity value of
cell C23 (6.cycle) is
taken arbitrary as 100 %. The discharge capacity of cell C23 with one nickel
layer is
approximately 20 % higher compared to cell C21 with two nickel layers although
both cells are
pasted with the same amount of nickel hydroxide. From this experiment it is
obvious that cells
with thinner nickel electrodes (one layer) deliver much better utilization of
active mass than cells
with thicker electrodes (two layers).