Note: Descriptions are shown in the official language in which they were submitted.
CA 02283251 2006-12-20
1
Device and method for carrying out fluorescence
immonoassays
The invention relates to a device for carrying out
quantitative fluorescence immunoassays by means of
evanescent field excitation. Various known biochemical
assays of general receptor-ligand systems, such an antigen-
antibody, lectin-carbohydrate, DNA or RNA-complementary
nucleic acid, DNA or RNA-protein, hormone receptor, enzyme-
enzyme cofactors, Protein G or Protein A-immunoglobin or
avidin-biotin can be used as a basis. However, antigen-
antibody systems are preferred. In particular, high-
molecular and low-molecular compounds (e.g. haptens) can be
detected according to the invention.
Fluorescence immunoassays or even fluorescence immunosensors
have already been generally used for a long time, and they
serve, mainly in a liquid sample matrix, to quantify an
unknown amount of a specific chemical or biochemical
substance. Antibodies are selectively bound to the
substance to be determined. The substance to be determined
is also referred to by the expert as an antigen. In the
fluorescence immunoassays, the analyte-specific antibodies
are marked with a marking substance which is optically
excited at a certain substance-specific wavelength AFX and
the fluorescent light with a different wave length, which is
generally greater, is used with a suitable detector with
evaluation of the intensity of the fluorescent light. The
exploitation of the evanescent field excitation in carrying
out such fluorescence immunoassays, or respectively in the
fluorescence immunosensors, is known in the prior art. Thus
different solutions have already been described in WO
94/27137, by R.A. Badlay, R.A.L. Drake, I.A. Shanks, F.R.S.,
A.M. Smith and P.R. Stephenson in "Optical biosensors for
CA 02283251 2006-12-20
2
immunoassays; fluorescence capillary-fill device", Phil.
Trans. R. soc. Lund. B 316, 143 to 160 (1987) and D.
Christensen, S. Dyer, D. Fowers and J. Herron, "Analysis of
Excitation and Collection Geometries for Planar Waveguide
Immunosensors", Proc. SPIE-Int. Soc. Opt. Eng. Vol. 1986,
Fiber Optic Sensors in Medical Diagnostics, 2 to 8(1993).
In addition, in WO 90/05295 Al, an optical biosensor system
is described. In this system, one or more samples are
guided, with the use of pumps and valves, through ducts to
one or more flow-through measuring cells. These flow-
through measuring cells are open upwards and biomolecules
can be quantitately detected by an optical structure
disposed above them. For measuring successive new samples,
considerable purification outlay is consequently required,
in order to avoid measuring errors. Necessary preparation
of such a sample generally has to be carried out externally
of this system, before the actual measuring, since no
elements or measures suitable for this purpose are provided.
In WO 90/06503, a sensor is described in which the
excitation light is directed at an appropriate angle through
an optically transparent substrate onto a boundary surface
to an optically transparent buffer layer. An additional
waveguide layer is provided to which the analytes to be
determined can be bound.
The refractive index of the buffer layer is smaller than
that of the substrate and of the waveguide. At the boundary
layer substrate/buffer, total reflection comes about through
appropriate choice of the angle of the excitation light, and
via the evanescent field product here, the excitation light
is coupled into the waveguide situated above the buffer
CA 02283251 2006-12-20
3
layer. The light coupled into the waveguide is guided via
total reflection in the waveguide and the evanescent field
formed during this process is used for fluorescence
excitation.
The sample can be received in one or more cavities. The
dimensions of such a cavity are only restricted to the
extent that its size permits the transport of the samples in
the cavities by means of capillary force. After the sample
has been received in the cavities, no further flow or
movement of the sample takes place.
The known solutions have, however, in general the
disadvantage that they are only suitable for specific assay
formats and an expensive structure with corresponding
process management is necessary.
It is therefore a feature of an embodiment of the invention
to create a means to carry out, with a very simply
constructed device, quantitative fluorescence immunoassays
with different biochemical assays.
In accordance with one embodiment of the present invention
there is provided a device for carrying out fluorescence
immunoassays by evanescent field excitation comprising:
at least one light source, emitting substantially
monochromatic light; an optically transparent base plate
made of material having a refractive index nl; a cuvette-
shaped receiving region for a sample; a covering plate
covering the receiving region on a side disposed opposite
the base plate; at least one functional layer between the
base plate and covering plate or in an inflow region of
CA 02283251 2006-12-20
4
the sample in the receiving region, the functional layer
permitting lateral or transverse flow through suction,
pressure or capillary force; a detector on the same side of
the base plate as the light source to detect the fluorescent
light; wherein the at least one light source directs light
rays having a wavelength causing fluorescence of a marking
substance bound to a chemical or biochemical partner of a
general receptor-ligand system, at an angle a, onto the
boundary surface; and wherein the refractive index nl of the
material of the base plate is greater than a refractive
index n2 of a material above the boundary surface.
In accordance with another embodiment of the present
invention there is provided a method of carrying out
fluorescence immunoassays by evanescent field excitation,
the method comprising the steps of: providing a sample
volume; guiding the sample volume through at least one
functional layer; guiding the sample volume through a
cuvette-shaped receiving region; binding a marked chemical
or biochemical component to a surface in the receiving
region; emitting light with a wavelength causing
fluorescence of the marked chemical or biochemical component
bound to the surface of the receiving region; measuring,
through evanescent field excitation, the fluorescent light.
In the device light of at least one light source is directed
at an angle a on the boundary surface of two media with
differing refractive indices. Here a light source is
selected which emits practically monochromatic light with a
wavelength which is suitable for exciting the marking
substance, in this case the fluorophore. Particularly
suitable as the light source are laser diodes, since they
have a suitable beam profile and sufficient luminous
CA 02283251 2006-12-20
efficiency, with small constructional size and low energy
consumption.
However, other light sources which emit monochromatic light
can also be used.
The angle a, at which the emitted light is sent to the
boundary surface, determines, besides the refractive index
of the material disposed in the beam path before the
boundary surface, and the material adjoining same, together
with the wavelength of the light, the penetration depth d
for the evanescent field. The refractive index nl of the
material which is disposed in the beam path before the
boundary surface must render possible total reflection at
the boundary surface and should therefore be greater than
the refractive index n2 of the other material disposea
thereafter. The angle a is preferably chosen such that the
following is true: sin (a) > n2/nl. If this condition is
met, all the light is reflected at the boundary surface and
thus total reflection is achieved. However, when this
condition is met, a relatively small portion of the light
penetrates through the boundary surface into the material,
which is disposed in the beam path after the boundary
surface, and the evanescent field is produced. Through the
evanescent field, only those marking substances which are
located in the immediate proximity of the boundary surface
are optically excited. For carrying out the fluorescence
immunoassays, the result of this is that only the marking
substances of the antibodies or antigens which are bound to
the surface of the boundary surface are excited. The
fluorescence intensity of the light emitted by these
fluorophores is thus directly proportional to the
concentration of the marked antibodies or antigens bound to
CA 02283251 2006-12-20
6
the surface, and, according to the biochemical assay used,
proportional or inversely proportional to the antigen
concentration.
Now the device noted above uses at least one light source,
which emits practically monochromatic light and directs this
at an angle providing the penetration depth d for the
evanescent field, onto a base plate which is transparent to
this light. The refractive index nl of the base plate
should be greater than 1.33. On the other side of the base
plate, a cuvette-shaped receiving region is formed between a
covering plate. Between the base plate and the cuvette-
shaped receiving region is formed said boundary surface.
The evanescent field acts, with the given penetration depth
d, within the cuvette-shaped receiving region on marked
chemical or biochemical partners, binds to the surface of a
general receptor-ligand system and excites the fluorophores
used as the marking substance.
The fluorescence caused is measured at the corresponding
intensity with a detector. The detector is disposed on the
same side of the base plate as the light source.
A single light-sensitive detector, or a linear or a surface
arrangement of a plurality of light-sensitive detectors can
be used as the detector.
In the above-described arrangement it is advantageous to
direct polarised light onto the sample to be determined.
For this purpose, a polarizer can be arranged in the beam
path of the light, following the light source.
The spacer and possibly the separating layers to be used are
CA 02283251 2006-12-20
7
0.001 to 10 mm thick, preferably 50um. A recess in the
spacer forms the receiving region for the sample. Spacer
and separating layers are preferably a biocompatible
adhesive film, which is designed to adhere on both sides.
The method is based essentially on the fact that a defined
sample volume is guided through the cuvette-shaped receiving
region and is subjected to an evanescent field excitation,
as has been described already. The sample volume can be
guided through the cuvette-shaped receiving region and the
functional layer(s) by way of suction, pressure or capillary
forces.
In an advantageous embodiment, there is provided at least
one inlet aperture in a covering plate, into which a sample
container can be inserted or disposed. The aperture is
disposed in the covering plate such that a connection may be
produced between inlet aperture or sample container and
receiving region. In addition, there is a second aperture
which represents an outflow and which is also connected with
the cuvette-shaped receiving region.
The second aperture can also be provided in the covering
plate. An external pump can be connected to this second
aperture, or an internal pump may be inserted.
The invention is characterised by the fact that a relatively
simply constructed basic device according to the inverition
can be altered or used in various forms. Thus the essential
elements, base plate, covering plate and spacer with
cuvette-shaped receiving region, can be combined in the
various ways. They can be combined through functional
layers, the separating layers disposed if necessary in
CA 02283251 2006-12-20
8
between yet allowing the sample to flow through. One or
more of such functional layers can, however, also be
arranged in the inflow region for the sample into the
receiving region. An inlet aperture or a connection between
a sample container which may be inserted into the inlet
aperture, or the connection of inlet aperture and receiving
region forms part of this inflow region.
With the invention, the different assay formats may be
carried out and thereby high- and low-molecular compounds
can be equally detected. All known assay formats, such as
sandwich-titration/competition and displacement formats, can
be carried out.
Where separating layers are used between functional layers,
or enclosing the functional layers, the separating layers
must have corresponding openings, such that the sample
volume can flow through the entire device. As separating
layers, adhesive films having openings therein through
stamping can be used.
The invention will be described in more detail below, by way
of example.
The Figures show:
Fig. 1 illustrates a portion of the device according to
the invention for receiving a sample;
Fig. 2 is a schematic representation of an embodiment of
a device configured according to the invention, with two
light sources;
Fig. 3 illustrates a device with sample container;
Fig. 4 illustrates a device with cylindrical hollow body;
CA 02283251 2006-12-20
9
Fig. 5 illustrates a device with additional functional
layers, with lateral flow;
Fig. 6 illustrates a device with a plurality of
functional layers and transverse flow;
Figs. 7+8 illustrate time-dependent fluorescence intensity
patterns;
Fig. 9 illustrates a sandwich assay format;
Fig. 10 illustrates another sandwich assay format;
Fig. 11 illustrates a titration or competition assay
format;
Figs. 12+
13 illustrate a competition or displacement assay
format with directly proportional ratio of analyte
concentration and signal intensity;
Fig. 14 illustrates an assay format using an additional
solid phase;
Fig. 15 illustrates a displacement assay with additional
solid phase;
Fig. 16 illustrates a further displacement assay; and
Fig. 17 illustrates a general key to the assay formats
shown in Figs. 9 to 16.
Fig. 1 shows the basic structure of a portion of the device
according to the invention. The three parts shown, i.e.,
the base plate 1, the spacer 4 and the covering plate 3, can
be connected to one another before the fluorescence
immunoassay is carried out. Alternately, the three parts
can be in the form of an already completely finished unit
and resemble in their structure a flow-through cell and a
measuring cuvette.
The base plate 1 consists of a highly refractive transparent
material, such as, for example, glass or a plastics
CA 02283251 2006-12-20
material, such as a polymer (PMMA or PC) with a refractive
index nl > 1.33. The thickness of the base plate can be
within a range of 0.01 to 10 mm, preferably between 0.5 and
1 mm.
The spacer 4 is preferably a thin foil, which is provided on
both sides with an adhesive film. Alternately, a thin
adhesive film may be applied firstly to the base plate 1 and
secondly to the covering plate 3. The total thickness of
the spacer including the adhesive used should be in a range
between 0.001 and 10 mm, preferably between 0.01 and 0.2 mm.
A thickness of 50 pm is most particularly preferred. An
opening is worked into the spacer 4 and forms a cuvette-
shaped receiving region 2.
As noted in Fig. 1, the covering plate 3 can also have
continuous apertures 9 and 11 formed therein. The function
of these apertures or bores will be described hereinafter.
Apertures 9 and 11 are disposed to at least partially
overlap the area of the receiving region 2 of the spacer 4.
The spacer 4 can preferably also consist of a biocompatible
adhesive film, which is preferably provided on both sides
with a detachable commercially available protective layer.
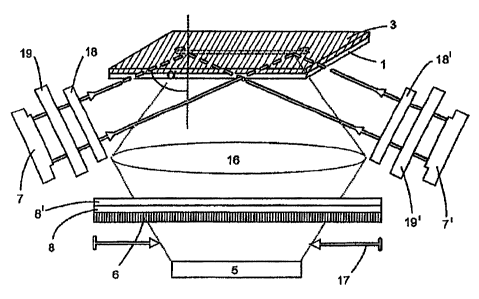
In the example represented in Fig. 2 the device according to
the invention uses two light sources 7, 7', filters 19,19'
and polarizers. The light source 7' emits light of a
wavelength which is different from the first light source 7.
In this example, polarised light is preferably used. The
device shown in Fig. 2 can be advantageously used when
differing marking substances, which can be excited at
different wavelengths, are used. Examples of these are the
fluorophores Cy5 and Cy7. To excite the fluorophore Cy5, a
CA 02283251 2006-12-20
11
laser diode is used with light having awavelength between
635 and 655 nm. To excite the fluorophore Cy7, a laser
diode is used which emits light having a wavelength between
730 and 780 nm.
In this embodiment, measuring takes place by way of the
diodes 7,7' being either switched in alternating manner or,
for example, correspondingly synchronised choppers cari be
used, to ensure that only light from one light source 7 or
7' can reach the sample to excite it and thus no
falsifications occur.
However, since in this arrangement two different
fluorescence signals have to pass the same filter, a
wideband filter 8 can no longer be used. Therefore, two
filters 8, 8' should be disposed in succession, which
selectively block the wavelengths of the exciting light
sources 7, 7'. Notch filters can, for example, be used for
this purpose.
With this arrangement, a reference signal can first be
obtained which renders an internal calibration of the
measuring signal possible. For reference measurement, a
reference antibody is used which is not directed against an
antigen from the sample. The reference antibody is first
quantified and made distinguishable, with a different
marking substance, from the analyte-specific antibody Ak to
be determined. The amount of reference antibody actually
bound to the surface can be determined with a second light
source 7', which causes light of a fluorescence of the
different marking substance, a second scattered light filter
8' and the detector 5. With this determination, the l.osses
of the marked analyte-specific antibodies Ak or antigens Ag,
CA 02283251 2006-12-20
12
not bound to the surface, can be taken into account.
Besides obtaining a reference signal, however, two
immunoassays, running independently of one another, can be
carried out, the difference coming about with the aid of the
different fluorophores.
Fig. 3 shows how a sample container 10 is disposed towards
aperture 9 in the covering plate 3 thus forming a connection
between sample container 10 and the receiving region 2 via
aperture 9. Here the sample container 10 forms the
container in which the known amount of biocomponent marked
with the marking substance fluorophore is mixed in the
sample to be determined. The sample container 10 can
clearly define the sample volume and thus, with a fixed and
known sample volume, a quantitative statement about the
antigen concentration can be obtained. The sample container
must, therefore, always be filled with the same amount in
order to be able to obtain reproducible results.
Advantageously it should always be filled to the maximum.
In some assay formats which may be carried out, the specific
biocomponent is respectively on the surface of the sample
container 10, and through contact with the liquid sample, it
detaches itself from the surface and gets into the sample.
Moreover the biocomponents can also be found on additional
solid.phases in the sample container 10. A simple and
already known method consists in applying lyophilized
antibodies to the surface of the sample container 10. In
this way, it becomes possible to store the whole for a
relatively long time before the immunoassays are actually
carried out. The receiving region 2 defines the surface on
the base plate on which, according to the assay format, the
respectively corresponding chemical or biochemical
CA 02283251 2006-12-20
13
substances are immobilised.
As illustrated in Fig. 4 a preferably cylindrical hollow
body 12, in which a piston 13 or some other suitable
covering is received serve together as a pump. If the
piston 13 moves out of the cylindrical hollow body 12, a
negative pressure is produced which sucks the sample
material out of the sample container 10 through the
receiving region 2 in a direction towards the cylindrical
hollow body 12. The flow is maintained by capillary forces
in the receiving region 2 and by an absorbent fleece, until
the entire sample volume is conveyed through the receiving
region 2. The cylindrical hollow body 12 is configured and
positioned such that a connection to the receiving region 2
is present. This connection can be provided through the
second aperture 11 in the covering plate 3. If no covering
plate 3 is used, the connection can also be configured in
another manner. The hollow body 12 may also have a hole in
its base to facilitate connection.
An external pump can also be connected to aperture 11.
After application of the sample (with the sample container
10), there is a wait time so that the desired binding
between the partners of a general receptor-ligand system can
take place completely. Thereafter, the pump 12, 13 is
activated and one waits until all the liquid has been pumped
through the receiving region 2. After excitation with light
source 7 or light sources 7 and 7', the antigen
conceritration can then be determined. This structure
according to the invention, as represented in Fig. 2, is to
be used for determination of the antigen concentration.
CA 02283251 2006-12-20
14
The structure, as previously shown and described, can be
used for the most varied biochemical assays, and further
examples will be returned to.
As can be seen especially from Figs. 1, 5 and 6, the
essential part of the device according to the invention can
be designed in various ways. Thus the different elements
(plates, layers) can be composed of a kit in variable
configurations and can be made available for different assay
formats in situ, according to desired requirements.
Fig. 5 shows an example of a device according to the
invention, in which additional functional layers with
lateral flow are represented. In this arrangement,
functional layers 26 and 27 and separating layers 25, 25'
are incorporated in the structure as explained as explained
in the description of Fig. 1. In this example, two
functional layers 26 and 27 are disposed one above the other
and are enclosed on all sides by separating layers 25 and
25'. The separating layers can preferably be configured as
adhesive films, in which openings are formed, as previously
described. These openings serve to make a connection
possible between inlet aperture 9, the functional layers 26,
2'7, the receiving region 2 and the outflow aperture 11. The
arrows drawn in Fig. 5 show the direction of flow.
Adaptation to different assay formats can be achieved by
variation of the arrangement and/or selection of the
functional layers 26, 27. Thus the functional layers 26 and
27 can be, for example, a reagent reservoir or a pure
reaction layer.
CA 02283251 2006-12-20
There also exists the possibility of arranging at least two
different functional layers in one plane, such that they can
be flowed through in succession.
The structure shown in Fig. 6 of a portion of a device
accorcling to the invention differs from the example shown in
Fig. 5 in that a transverse flow can be achieved. In this
example, three functional layers 28, 28' and 29 are
disposed, one directly above the other, i.e. without
separating layers, directly on the base plate. Within the
stack of layers so formed from functional layers 28, 28' and
29, the spacer 4 with the cuvette-shaped receiving region 2
is disposed underneath the covering plate 3. The arrows
indicate the direction of flow.
Other arrangements to that shown in Fig. 6, which ensure
transverse flow can, of course, also be constructed. As
already noted the functional layers can vary in their
number, arrangement and choice of function. In an opposite
manner to the example shown, the arrangement can also be
designed above the spacer 4.
Separating layers can be used in this example too, however,
the transverse flow must not be hindered. The functional
layers can again serve as reagent reservoir or reaction
layer. The functional layers to be used according to the
invention have the advantage that a complete, integrated
measuring system is produced and only the sample has to be
led through the structure.
Combinations of transverse and lateral flow are also
possible. As an example, the combination of the
arrangements of Figs. 5 and 6 can be utilized.
CA 02283251 2006-12-20
16
The functional layers 26, 27, 28, 28' and 29 can be used for
the tasks of preparing the samples (buffering, filtration,
separation, elimination of interferences, amongst other
things), can be used as reagent carrier layer (e.g. for
conjugate release) or as a reaction layer (e.g. for
derivatization, for immobilisation of biocomponents or for
the course of chemical and/or immunochemical reactions).
Suitable material for the sample preparation are e.g.
membranes made of fibrous material to separate plasma and
red blood corpuscles, which are available, for example, from
the company Pall Biosupport as "Hemadyne-Membran". However,
filter papers made of cellulose or regenerated cellulose can
also be used for this function.
Suitable materials for the reagent carrier layer are paper
made from 100% cellulose or activated nylon 66. It is
possible for the surfaces of the materials to be activated
or modified in order to alter the flow properties
(commercially available from the company Pall Biosupport
under the trade name "Prodyne oder ACCUWIK-Membrane"). For
lateral flow systems, polyester carriers with a modified
surface and in which the flow properties may be controlled
are particularly preferred.
Suitable materials for the reaction layers are nitroflow
membranes made of nitrocellulose, PVDF (polyvinyl
difluoride) membrane (commercially available from the
company Millipor with the trade name "Immobilon"). Here too,
if desired, the surface can be modified.
In general, fibrous materials, cellulose, nitrocellulose,
polypropylene, polycarbonate, polyvinyl difluoride,
CA 02283251 2006-12-20
17
hydrogels (e.g. dextran, acrylamide, agar-agar, carrageenan,
alginic acid) polyelectrolytes (e.g. acrylic acid, poly-L-
lysine, poly-L-glutamic acid) or nuclear track membranes or
glass-fibre membranes can be used.
Methods of evaluating the measurement signals are
represented in Figs. 7 and 8.
In Fig. 7, the intensity of the measured fluorescence signal
is shown dependent on time. With the linear rise in the
intensity of the fluorescence signal, it is sufficient to
determine the signal rise by differentiation, since the rise
can be correlated with the temporal alteration in the amount
of fluorophore, which can be measured with the device
according to the invention. In this way, the measuring time
can be kept very short, since the rise in the intensity of
the fluorescence only has to be determined over a short
period of time, independently of whether this takes place at
the beginning or a later point in time, in carrying out the
chemical or biochemical assay. Only the saturation range
has to be borne in mind, and care taken that the measurement
is only carried out in a time domain in which a temporal
alteration of the fluorescence intensity signal can be
detected.
Differing from this, another possibility is represented in
principle in Fig. 8. Here the difference between an initial
and a final value is formed and used for evaluation. A
basic signal S1 is first received before the addition of the
analyte to be determined at time tl and, following the
addition of the analyte, at a point of time t2, which can be
predetermined, a final value S2 of the measured fluorescence
CA 02283251 2006-12-20
18
intensity is determined. The analyte concentration can then
be determined through the difference of the values S2 and Sl.
The difference between the time t2 and tl, must be so great
that an equilibrium has formed.
Possible assay formats are represented in Figs. 9 to 16
which can be carried out with the invention.
Fig. 9 shows a sandwich assay format which is suitable for
high-molecular compounds (proteins, amongst other things).
This sandwich format can be carried out in principle in a
device, such as represented in Fig. 5 or Fig. 6, in which at
least one functional layer is to be used.
The analyte is incubated with the marked antibody first and
then led into the detection region of the base plate 3 for
evanescent field excitation and corresponding fluorescence.
Another possible way of carrying out a sandwich assay format
in sequential form, is to form the sandwich step by step
utilizing the analyte and then the marked antibody.
Further possible ways of immobilising the antibody in the
base plate region are:
1. adsorption
2. covalent bonding
3. affinity bonding (e.g. A-protein A/G or after
biotinylation to avidin)
4. by hybridisation of a nucleic acid marker located on
the antibody (single-strand RNA or DNA) to an
immobilised single-strand nucleic acid (RNA or DNA)
CA 02283251 2006-12-20
19
with complementary sequence.
Coating the base plate region, for the evanescent field
excitation, with protein A/G, avidin, amongst other things,
offers the possibility of producing a universal element (for
the most varied of analytes).
A particularly advantageous embodiment provides the pre-
incubation of the analyte with a biotinylised (collector)
antibody and a fluorescence-marked (detector) antibody. The
two antibodies can, for example, be released simultaneously
or in sequence from functionalised layers. The whole
immunocomplex is then bound by binding to a sensor surface
coated with avidin (alternatively streptavidin or
neutravidin). Critical for signal formation is the very
high affinity between biotin and avidin; this leads to an
improvement in the sensitivity of the assay.
In this embodiment, a device according to Fig. 2 can also be
used in conjunction with two different marking substances.
Thus the determination of concentration for two different
analytes can be carried out quasi simultaneously also
independently of the respective binding sites in --he
receiving region, such that the binding of the marked
biocomponents does not have to take place locally
selectively.
However, an assay format can also be carried out in which an
antibody and a marked antibody fragment (e.g. a Fc-part or
an ScFv-fragment) are incubated simultaneously with the
analyte, as is shown in Fig. 10. In this arrangement, only
the complete antibody binds (to protein A/G or, after
biotinylation, also to avidin), and thus the necessity for
CA 02283251 2006-12-20
an incubation disappears. With this format there is the
basic possibility of regenerating the structure used.
However, this is not possible with avidin/biotin.
In the simultaneous incubation of analyte, antibody and
marked antibody fragment in a sandwich assay, as is shown in
Fig. 10, the marked antibody fragment and the antibody can
be contained, for example, in function layer 27, of the
example shown in Fig. 5.
Instead of immobilising a collector antibody, other
biocomponents, binding the analyte, can also be immobilised
(e.g. protein A/G in the case of a sandwich assay for
determining antibodies).
In all the sandwich assay formats, a directly proportional
correlation between the signal and the concentration of the
analyte occurs.
One or more components of the immunochemical reaction can,
moreover, be prepared on functional layers, such as is the
case for conjugate release.
In Fig. 11 possibilities for titration/competition formats
are represented which differ from one another through
sequential or simultaneous incubation of the immuno-
components. These two assay formats are suitable in
particular for determining low-molecular compounds which
cannot form a sandwich.
Moreover, the assay formats shown in Fig. 11 have no
directly proportional correlation between the analyte
CA 02283251 2006-12-20
21
concentration and the intensity of the measured fluorescence
signal. There is thus an inversely proportional
correlation.
Thus, in the upper example, shown in Fig. 11, the marked
antibody can be present for example in the functional layer
27, in the example shown in Fig. 5.
The middle example of Fig. 11 can be configured such that a
marked analyte can be contained, e.g. also in the functional
layer: The lower representation of Fig. 11 can be so
implemented that a marked analyte is contained, for example,
in functional layer 26 and an antibody in layer 27 of the
example shown in Fig. 5. However, the implementation of the
lower example, which is shown in Fig. 11, can also be
carried out in such a way that an antibody is contained in
functional layer 26 and the marked analyte in layer 27 as in
the example shown in Fig. 5.
From this it follows that, in the assay formats shown in
Fig. 11, either the analyte or the antibody can be
immobilised (cf. upper and middle examples of Fig. 11).
Therefore the methods described for the sandwich assay
formats can also be used, at least partially. Thus a
generic antibody (cf. lower example in Fig. 11) or, however,
also protein A/G (after biotinylation of the specific
antibody also avidin) can be immobilised. In this case, the
immobilised biocomponent serves exclusively to enrich the
added specific antibody and can therefore be immobilised in
excess.
Further assay formats having directly proportional
correlation between analyte concentration and fluorescence
CA 02283251 2006-12-20
22
signal intensity will be described below.
For this there are basically two possibilities. It is
possible to carry out the respective assay with an
additional solid phase or in solution.
For example, all the components can be incubated in
solution. The assay format shown in Fig. 12 provides for a
pre-incubation of the reactants and the forming of a binding
equilibrium. A free analyte competes with the marked
analyte for binding to the antibody, the same body being
immobilised on the base plate 3 in the detection region also
contained in the solution.
The immobilisation can be carried out as in sandwich assay
formats.
Since only free, i.e. not antibody-bound, marked analyte is
determined, a directly proportional correlation between the
analyte concentration and the fluorescence signal results.
Moreover, the immobilised biocomponent serves exclusively to
enrich the hapten-fluorophore conjugate and can thus be
immobilised in excess. Through immobilisation of a specific
antibody, a corresponding structure of the device according
to the invention can, however, only be used for respectively
one analyte.
The assay format shown in Fig. 12 can be carried out with a
device such as is shown in Fig. 5, if a marked analyte is
contained in functional layer 26 and antibody in functional
layer 27.
CA 02283251 2006-12-20
23
For the case where, instead of the marked analyte, a marked
analyte analogue is used, which has a clearly reduced
affinity to the antibody, a replacement assay, already
described, can be carried out. This is shown in Fig. 13. A
marked analyte or an analyte analogue can be contained for
example in functional layer 28, of the example shown in Fig.
6.
However, an additional solid phase can also be exploited,
which can be accommodated either in a separate reaction
space or as a functional layer directly on the detection
region of the base plate 3.
The additional solid phase can in principle exercise the
same functions as the functional layers.
The use of a separate reaction space (e.g. an incubation
test tube) such as the sample container 10, which is shown
in Figs. 3 and 4, has the advantage that generic structures,
i.e. structures utilizable for all the analytes, can be
used.
On this universal structure, not a specific but a generic
anti-antibody or protein A/G, avidin (after biotinylation of
the antibody), amongst other things is immobilised. Since
only one biocomponent above the base plate 3 is enriched,
the immobilised components can be applied in excess.
The procedure can, in general, be such that one of the
immunocomponents (the marked antibody or marked analyte) is
kept back on a solid phase, for example a functional layer
with hapten-protein conjugate. Only in the presence of free
analytes is a portion of the marked components not bound to
CA 02283251 2006-12-20
24
the solid phase and can then be measured above the base
plate-3 in the detection region. These circumstances are
represented schematically in the example shown in Fig. 14.
Here free analyte and analyte immobilised on the solid phase
complete for binding to the specific antibody, as is shown
in a first step in Fig. 14, at the top.
The solid phase is only passed by antibodies which have
bound beforehand to analyte, as is represented in the lower
part of Fig. 14. Consequently, only analyte-bound antibody
can be detected, for example by a generic anti-antibody.
Here, too, there is a directly proportional correlation
between the analyte concentration and the intensity of the
measured fluorescence.
If, in the concrete case of Fig. 14, a membrane is used as
the additional solid phase, which is integrated into the
structure, functional layer 26 (reservoir for the marked
antibody) and the solid phase layer 27, of the example shown
in Fig. 5, can be used in the example shown in Fig. 14.
Various material can serve as solid phases, and of these,
membranes can be easily integrated as functional layers.
Such membranes can be nitrocellulose, immuno-dyne, conjugate
release membranes, regenerated cellulose, amongst other
things. Here the respective biocomponent can be immobilised
by adsorption, covalent bonding or by affinity bonding.
Haptens can be immobilised as hapten-protein conjugate.
As opposed to membranes with transverse flow, membranes with
lateral flow and packed columns offer advantages through
repeated establishment of equilibrium and render a
CA 02283251 2006-12-20
quantitative binding of the biocomponents possible.
Suitable materials for packed columns are: sepharose, porous
media, amongst other things.
The wall of a suitable vessel, for example the wall of the
sample container 10, or the supply pipes can also serve as
the solid phase and be, for example, polystyrene vessels or
glass capillaries. Particle suspensions, in which the
sample can be a suspension with solid particles (magnetic
particles, latex, amongst other things) can also be
utilized. These particles can be separated through the
application of a magnetic field or through subsequent
filtration.
With the invention it is also possible to carry out so-
called displacement assay formats, two variations of this
are possible. The displacement can take place on an
additional solid phase in a functional layer or externally,
i.e. not in an integrated functional layer, or directly on
the base plate 3 in the detection region.
In Fig. 15, an example of a displacement assay with
additional solid phase is represented. The solid phase can
be either the sample container 10, a supply pipe or a
functional layer. A marked antibody or analyte is bound
through specific ligand/receptor action. Through the
addition of a free analyte, the displacement of the
biocomponents can be achieved.
The solid phase can be, for example, functional layer 26, in
the example according to Fig. 5.
CA 02283251 2006-12-20
26
In the example shown in Fig. 15, the marked antibody is
bound on the base plate 3 in the detection region by a
generic anti-antibody or, protein A/G, avidin, amongst other
things.
If however, the opposite procedure is carried out and a
marked analyte is bound to an immobilised antibody and then
displaced, in the detection region on the base plate 3, a
specific antibody, directed against the analyte, is
immobilised. Since in every case the displaced component
always detected, there is a direct proportional correlation
between the concentration of the respective analyte and the
fluorescence signal intensity.
However, the displacement can be carried out directly in the
detection region on the base plate 3 as a very simple assay
configuration, since only one sample is guided through the
element.
No pre-incubations or similar steps take place. Conditional
of the sample can be achieved through integration of
corresponding functional layers. Here, two different
possible ways of immobilising the analyte or the specific
antibody present themselves, as is shown in Fig. 16.
The decrease in the fluorescence intensity signal is
measured, such that an inversely proportional correlation
between the analyte concentration and the fluorescence
signal intensity occurs. The absolute value of the rise in
the fluorescence intensity signal is directly proportional
to the analyte concentration and can be evaluated in the
form previously described.
CA 02283251 2006-12-20
27
Fig. 17 serves as a general key for the different assay
formats shown in Figs. 9 to 16.