Note: Descriptions are shown in the official language in which they were submitted.
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
1
FIV Vaccine
Background
The present invention relates to a feline immunodeficiency
proviral (FIPV) polynucleotide fragment comprising a
dysfunctional pol gene region, a recombinant vector comprising
said FIVP polynucleotide: fragment, a host cell containing said
FIPV polynucleotide fragment, a feline immunodeficiency virus
(FIV) vaccine comprising said FIPV polynucleotide fragment, a
method of treating FIV-related disease, and pharmaceutical
compositions compr~~~sing raid FIPV polynucleotide fragment for use
as a prophylactic .and/or therapeutic agent in cats.
Feline immunodefic:iency virus (FIV) is a member of the
Retroviridae; it :is a lentivirus which is associated with a
debilitating immunodeficiency syndrome in cats (Pedersen N.C. et
al., Science (1987) Vol. 235, pp. 790-793).
Lentiviruses by nature do display a large degree of
molecular and biological variation. This natural variation is
thought to be in part ascribable to the low fidelity of the viral
enzyme reverse transcriptase in the process of copying the viral
genomic RNA to DtJA (Preston et al., Science 242: 1168-1171
(1988), Roberts et: al., Science 242: 1171-1173 (1988)). As a
result, several variant FIV-strains have been found.
To date, isolates of several variant FIV strains, some of
which have been subjected to molecular cloning, have been
described. Amongst these strains are two isolates from the
United States (Petaluma:-strains (Olmsted et al., Proc. Natl.
Acad. Sci USA 86: 8088-Ft092 (1989), Talbott et al., Proc. Natl.
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
2
Acad. Sci. USA 86: 5743-5747 (1989)) and San Diego strain
(Phillips et al., J. Virol. 64: 4605-4613 (1990)), one from the
United Kingdom (Harbour et al., Vet. Rec. 122: 84-86 (1988)} and
two from Japan (Ishida et al., J. Am. Vet. Med. Assoc. 194: 221-
225 (1989), Miyazawa et al., Arch. Virol. I08: 59-68 (1989)),
which were obtained from the DNA of in vitro propagated strains.
One strain, the F14 clone of Olmsted et al., supra has been
deposited in the Genbank data base under Accession No. M25381.
Molecular characterisation and determination of
heterogeneity between FIV isolates has been described by Maki et
al., (Arch. Virol. 123: 29-45 (1992)). The construction of DNA
clones from two FIV proteins, i.e. the envelope (ENV} protein and
the virion core (GAG) protein and their use for detecting and
preventing FIV has been described in WO 92/15684.
Sero-epidemiological surveys have revealed that the virus
occurs all over the world (Furuya et al., Jpn. J. Vet. Sci. 52:
891-893 (1990), Gruffydd-Jones et al., Vet. Rec. 123: 569-570,
(1988), Ishida et al., Jpn. J. Vet. Sci. 52: 453-454 (1990),
Ishida et ,al. , Japn. J. Vet. Sci. 50: 39-44 (1988) , Ishida et
al., J. AM. Vet. Med. Assoc. 194: 221-225 (1989), Swinney et al.,
N.Z. Vet. J. 37: 41-43 (1989)).
FIV has a complex genome structure comprising group antigen
proteins (GAG), which are the major structural proteins of the
virus; POL, proteins of the polymerase gene; and ENV, proteins
of the envelope gene. The gag gene encodes matrix, capsid and
nucleocapsid proteins, and the pot gene encodes protease, reverse
transcriptase, dUTPase and integrase. The env gene encodes
surface and transmembrane envelope glycoproteins. In addition
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
3
to the structural and enzymatic proteins, at least three more
genes (Vif, ORFA, Rev) are present in FIV (Miyazawa T., Arch.
- Virol. (1994) Vol. 134 pp. 221-234). As with other members of
the Retroviridae, the integrated genome of FIV is bordered by
long terminal repeats (LTRs) comprised of U5, R, and U3 domains.
Likewise, the basic structural elements gag, pot and env are
encoded in the apx~roxim~~te 9500 base pair genome. In addition
to these common elements, FIV encodes several short open reading
frames (sORFs). Details, of the genomic organisation of FIV may
be found in "Infectious Agents and Disease Vol. 2 pp. 361-374
(1994)" under the review paper by John H. Elder and Tom R.
Phillips.
Control by vaccination of FIV infection has been a long-
sought goal.
WO 94/20622 deascribes the provision of a vaccine against FIV
comprising a polype~ptide fragment of an FIV surface protein which
is capable of inducing neutralising antibodies against FIV.
There is no reference to the potential or actual use of proviral
FIV DNA in'the production of DNA vaccines against FIV infection.
Development of protective FIV vaccines has proven difficult
(Hosie M.J. and 'Yamamoto J.K. (1995) Feline Immunology and
Immunodeficiency (Willett B.J. and Jarrett O. Eds.) Oxford
University Press, rdew York, pp. 263-278) . An initial success was
reported with the dev~'lopment of a cell line (FL4) that
constitutively re leases large numbers of FIV particles (Yamamoto
J.K. et al. (19~~1) Inter-Virology Vol. 32, pp. 361-375).
Inactivated viral and whole cell vaccines based on this cell line
showed the first e:videnc:e of protection against FIV infection,
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
4
however, this protection has subsequently been shown to be of
limited spectrum (Hosie M.J. et al., (1995) J. Virol. 69 pp.
1253-1255), suggesting that the reported strategy will be less
useful for antigenically diverse natural isolates of FIV that are
not readily propagated in vitro. Subunit vaccines for FIV have
not been particularly successful to date. While viral load
reduction after challenge has been demonstrated in animals
immunised with glycoprotein purified from virions (Hosie M.J. et
al., (1996) Vaccine Vol. 14 pp. 405-411), studies using
recombinant proteins as immunogens led instead to enhancement of
early infection (Hosie M.J. et al., (1992) Vet. Immunol. Pathol.
Vol. 35, pp. 191-198; Siebelink K.H.J. et al., (1995) J. Virol.
Vol. 69, pp. 3704-3711).
Genetic immunisation for eliciting an immune response was
first reported by Tang D.C. et al., (1992) Nature (London) Vol.
356, pp. 152-154. A general review on genetic immunisation is
further reported by Hassett D.E. and Whitton J.L. in Trends.
Microbiol. (1996) Vol. 4, pp. 307-312. Protective immunisation
has been achieved in virus-host systems using inoculation of DNA
(Fynan E.F. et al., (1993) Proc. Natl. Acad. Sci. USA Vol. 90,
pp. 11478-11482; Webster R.G. et al., (1994) Vaccine Vol. 12, pp.
1495-1498). However, efforts so far have employed plasmids
containing individual viral genes or combinations of genes but
have been restricted to non-replicating vectors. Protection
against infection by lentiviruses such as FIV has been attempted
by expression of the ENV protein of FIV in cats (Cuisinier A-M
et al., (1996) 3rd International Feline Retrovirus Research
Symposium, Fort Collins, Colarado).
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
The above oul:lined problems emphasise the need to consider
alternative and innovative approaches to lentivirus vaccination
and in particular, FIV vaccination.
The prior ant does not teach the use of FIV pot region
deletion mutants c:ompri~;ing a dysfunctional reverse transcriptase
(RT) gene region in the manufacture and use of vaccines against
FIV related disease.
It is thought that. D~1A delivery may improve the prospects
for the use of attenuated viral vaccines, since it may be
possible to de7_iver more comprehensively disabled viral
derivatives that cannot be obtained as stable high-titer viruses.
The present invention seeks to mitigate against the
disadvantages associated with the prior art.
According to a first aspect of the invention there is
provided a vaccine formulation comprising a feline
immunodeficiency provi:rus (FIPV) polynucleotide comprising a
dysfunctional po:L genE~ which is substantially incapable of
encoding a functionally competent reverse transcriptase (RT) or
a functional RT fragment thereof.
A "FIPV" polynucleotide can be viewed as a polynucleotide
fragment of an fIV capable of integration into a host cell
genome. Host cells comprising FIPV of the invention are capable
of producing FIV proteins, except for functionally competent RT
.. or functionally cc>mpetent fragments thereof. As such, host cells
for the FIPV of the invention are able to release non-infectious
FIV viral particles i.Ea. FIV particles which are substantially
incapable of replication.
CA 02283490 1999-09-09
WO 98/40493 PCTlGB98/00715 -
6
A "dysfunctional pot gene" is one which is substantially
incapable of coding for a native RT or a functional equivalent
thereof. Thus a "dysfunctional pol gene" means that the pot gene
has been modified by an in-frame deletion, insertion or
substitution (or other change in the DNA sequence such as
rearrangement) such that the pot gene is generally unable to
express a functionally competent RT or a functionally competent
equivalent polypeptide product thereof.
pot genes of the invention which are substantially incapable
of . encoding a functionally competent RT may be rendered
dysfunctional by any one of several ways:
(i) A deletion of the entire in-frame RT coding domain of the
pot gene from a wild type FIPV genome. For example, depending
on the wild type of FIPV or FIV of concern, a deletion of the
nucleotide sequence from a wild type FIPV or FIV genome between
about nucleotide 2337 ~ 12 bases to about nucleotide 4013 ~ 12
bases can be made. An example of a FIV clone from which a
deletion can be made is the F14 clone of FIV. Using this clone
a deletion of the entire in-frame RT coding region can be made
between nucleotide 2337 and nucleotide 4013. The in-frame
deletion should be such so as not to substantially affect the
expression of other gene products from the FIV or FIPV genome.
(ii) A deletion of a portion of the in-frame RT coding domain
of the pot gene of a wild type FIPV genome. A "portion of the
in-frame RT coding domain" means a polynucleotide fragment which
by its deletion from the RT coding region is sufficient to render
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
7
any RT or fragment or fragments thereof encoded and/or
expressible thereJ~y, substantially incapable of a physiological
activity attribut~~ble to that of a functional RT produced by a
FIV or FIPV. The deletion portion of RT may comprise a dE:letion
of a small number of nucleotides, for example, 1, 2 or more
nucleotides. Such. deletions within the RT encoding domain of the
poI gene can be aclZieved using recombinant DNA technology. Thus,
the translational ORF f~~r an RT can be altered resulting in the
production of a protein which lacks the physiological
functionality or functional competence of an RT found under
native circumstances, for example, an RT derived from a pot gene
in a wild type F7;PV or FIV. The skilled addressee will also
appreciate that s,ach deletions in the translational ORF of the
RT domain of the pol gene may also give rise to a dysfunctional
pot gene which »s substantially incapable of coding for a
functionally competent RT, truncated RT even any RT or
polypeptide fragment thereof. Such proteins/polypeptides, if
produced, generally lack. the functional competence typical of the
enzyme, RT.
(iii) The deletion of the or a portion of the RT domain of the
pol gene as described in (i) or (ii) above will leave a "gap" in
the pot gene. A suitable polynucleotide fragment, such as a gene
_ or gene fragment or genes or fragments thereof may be inserted
into the "gap". Gene insertions can include genes which express
polypeptides capable of augmenting an immune response, such as
feline cytokines, for example, y feline interferon or other genes
such as marker genes. Suitable marker genes may include but are
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
8
not restricted to enzyme marker genes, for example the lac-Z gene
from E.coli, antibiotic marker genes such as hygromycin, neomycin
and the like. Generally, marker genes, if any, may be employed
in an RT deletion. FIPV or FIV mutants of the invention should
be such so as to not cause substantial deleterious or long
lasting side-effects to a recipient animal.
In a preferment, the "gap" made by the deletion of the or
a portion of the RT domain of the pot gene from a FIPV is not
filled with a polynucleotide insert, the cut ends of the deletion
site being ligated together using conventional recombinant DNA
technology. The skilled addressee will also appreciate that the
"gap" left by the partial or total deletion of the RT encoding
region of the pot gene may be filled with a polynucleotide
sequence which is a nonsense nucleotide sequence or an anti-sense
sequence: In both instances any defective RT which may be
produced from a polynucleotide fragment including such sequences
should be incapable of RT functionality.
(iv) Nucleotide insertions can also be made at suitable
restriction enzyme sites within the RT coding region using
recombinant DNA technology. Such insertions can give rise to a
dysfunctional RT or fragments) thereof which are substantially
incapable of an RT activity. For example, when using the FIV F14
clone, stop codons may be inserted into the RT region at suitable
insertion sites such as at the Pac 1 restriction site (nucleotide
3540 to 3547) of the RT encoding region of the pot gene, which
can result in the production of a non-functional fragments) of
RT.
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
9
A "function,~lly <:ompetent reverse transcriptase" is one
which is capable of R'.C functionality. That is to say, an RT
functionality permitting the copying of a ribose nucleic acid to
a deoxyribose nucleic acid form, for example, in a host cell or
in the genome of <~ host organism such as a feline. Thus, FIPV's
of the invention comprising dysfunctional pot genes are
substantially incapable of giving rise to infectious FIV
particles.
As a preferment, there is provided a vaccine formulation
wherein the FIPV polynucleotide comprises a deletion, still
preferably an in-i:rame deletion, within the RT domain of the pot
gene.
In a preferment there is provided a defective FIPV
polynucleotide fragmeni~ comprising an in-frame deletion and/or
insertion compri:~ing at least one nucleotide in the RT region
within the RT domain of the pot gene. The deletion should be
such that coding sequences for other gene products of the FIPV,
for example the p~~1 gene products and other FIPV gene products,
upstream _ and/or downstream from the RT domain are not
substantially affected. That is to say that other gene products
ordinarily having an immunogenic function and which are expressed
from the FIPV substantially retain their immunogenic function.
The deletion may tie made between about nucleotide 2337 ~ 12 bases
and 4013 ~ 12 bases of the RT domain of the pot gene depending
on the FIV isolatead. The deletion can be of any size so long as
any RT polypeptidEa product which may be generated, such as an RT
fragment thereof (or RT fragments thereof} does (do) not possess
RT functionality and any coding sequences upstream or downstream
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
thereof are not substantially affected. The deletion can be made
starting at any suitable restriction enzyme site located in the
RT region of the pot gene. However, it is preferred if the
deletion is made starting at a restriction site which is unique
to within the RT domain of the pot gene, if not the whole FIPV
such as Ncol, Pac 1 and Sph 1. A suitable example of a starting
restriction enzyme site, thought to be unique to at least within
the RT region of the FIV F14 clone is the Pac 1 site located at
nucleotides 3540-3547 thereof. The skilled addressee will
appreciate that other FIV or FIPV isolates comprising similar
enzyme restriction sites within the RT domain of the pot gene are
encompassed by the present invention.
In a preferment there is provided a defective FIPV
comprising a polynucleotide fragment deletion in the RT domain
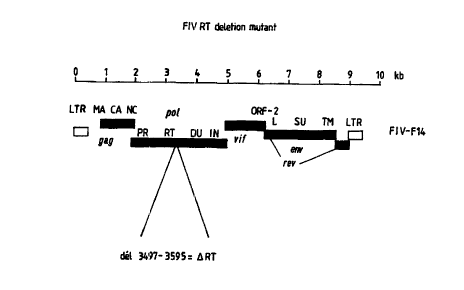
of the pot gene wherein the deletion is from nucleotide 3497 to
nucleotide 3595 of the RT domain.
In a further embodiment of the invention, the defective FIVP
can form part of a recombinant nucleic acid molecule comprising
a replication defective FIPV under the control of regulatory
sequences which enable expression of viral gene products in a
host cell genome and production of FIV proteins other than
functional RT or functional fragments thereof.
Regulatory sequences enabling integration and/or production
of FIV proteins other than functional RT or functional fragments
thereof can be promoter sequences which may or may not be
associated with appropriate enhancer sequences. Suitable
promoters include those as outlined by Norimine J. et al., (1992)
J. Vet. Med. Sci. 51(1) pp. 189-191, and may include promoters
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
11
obtained or derived from prokaryotic, eucaryotic and/or viral
origins. Examples of promoters include but are not limited to
the cytomegalovirus (CMV) promoter immediate early (IE) promoter
region, for example the' human cytomegalovirus (HCMV) immediate
early (IE) promot:er region, the Rous sarcoma virus (RSV) long
terminal repeat (hTR), feline leukaemia virus (FeLV) LTR, simian
immunodeficiency virus from African green monkey (SIV AGM) LTR,
and the SV40 early-promoter region.
The person sl~:illed in the art will also appreciate that the
natural promoter sequence of the defective FIPV carrying a
dysfunctional pol gene (i.e. located in the 5' LTR thereof) could
also form part o:E a recombinant nucleic acid molecule of the
invention.
Thus, FIPV oi: the invention can be obtained by taking cDNA
encompassing the genorne of an appropriate FIV isolate and
inserting it into a suitable vector, such as a pGEM vector or a
lambda vector. F, suitable FIV clone is the F14 clone of FIV-
Petaluma described by Olmsted R.A. et al. (1989) Proc. Natl.
Acad. Sci: (USA) Vol. 8~5 pp. 8088-8092. The FIV clone can then
be linearised using an appropriate restriction enzyme such as Nco
1, Sph 1, Bae 1 Pac 1 and the like, the linearised vector is then
purified, for example by precipitation followed by digestion with
a suitable exonuclea:ae such as Ba131 under appropriate
exonuclease digestion conditions for a desired period of time
(Maniatis et al. Molecular Cloning - a Laboratory Manual; Cold
Spring Harbor Laboratory Press First Edition (1989) p 135).
After further purification, suitably by organicsolvent extraction
and alcohol precipitation, appropriately exonuclease digested
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
12
nucleic acid molecules can be re-circularised by ligation and the
products thereof used to transform an appropriate host cell, such
as a bacterium host cell, e.g. E.coli. Clones thus obtained may
then be characterised by polymerase chain reaction (PCR)
amplification across the nucleic acid molecule in order to
ascertain the size and location of the deletion in the RT domain
of the pot gene (i.e. in-frame or otherwise).
A suitably sized deletion region has been found to be a 235
by region of the pot gene of the FIV Petaluma strain within which
is-found the Pac 1 restriction enzyme site.
The deletion generally has to be made in the RT domain of
the pot gene in a position such that any defective FIPV
incorporated into a host cell genome retains a sufficient
immunogenic function to elicit, on expression of protein or
polypeptides encoded by the FIPV, at least a cellular immune
response (such as a cytotoxic T-cell response) in a host animal,
such as a feline.
Suitable clones comprising deletion regions of the invention
can be further characterised using DNA sequence analysis using
primers of any acceptable length, such as primers of up to 60
nucleotide bases in length, preferably primers of about 20 to 60
nucleotide bases in length. More preferably such primers are
from 20 to 30 nucleotides in length.
The selection of vector is not critical provided that it is
able to carry the desired FIV clone into a suitable host cell.
The host cell can be one in which replication of the recombinant
vector molecule can occur. The host cell can be a cell in which
regulatory sequences of the or at least one other vector can also
CA 02283490 1999-09-09
WO 98/40A93 PCT/GB98/00715
13
be recognised such that at least a further polypeptide
fragment(s), such as a fragment capable of augmenting or
eliciting at least an immune response as described above, can be
expressed. For e~,:ample, if the prophylactic and/or therapeutic
effect of an appropriatEaly cloned FIPV of the present invention
is to be augmente=d, a further vector encoding an appropriate
adjuvant protein or polypeptide, such as a cytokine coding
vector, for example, a feline Y interferon (YIFN) coding vector,
can also be employed as a component of a vaccine or
pharmaceutical composit=ion of the invention. International
Patent Application WO 96/03435 describes the provision of a
feline Y interferon, and includes the provision of a
polynucleotide fragment encoding feline Y interferon and vectors
therefor. Such polynucleotide fragments as described in WO
96/03435 can be ad.minist:ered in conjunction with vectors coding
for defective FIPV' of the invention to animals in need thereof.
A wide range of vectors is currently known, including
vectors for use in bacteria, e.g. pBR322, 325 and 328, various
pUC-vectors a.o. PUC 8, 9, 18, 19, specific expression-vectors;
PGEM, pGEX, and Bluescr~ipt~F' , vectors based on bacteriophages;
lambda-gtWes, Charon 28, M13-derived phages, vectors containing
viral sequences on the basis of SV40, papilloma-virus, adenovirus
or polyomavirus (Rodriquez, R.L. and Denhardt, D.T., ed.;
Vectors: A survey of molecular cloning vectors and their uses,
Butterworths (1988), Le~nstra et al., Arch. Virol.; 110: 1-24
(1990)).
All recombinant molecules comprising the nucleic acid
molecule under the control of regulatory sequences enabling
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/08715
14
expression of the defective FIPV by said nucleic acid molecule
are considered to be part of the present invention.
Thus, as a further embodiment of the invention there is
provided a vector comprising a defective FIPV in recombinant form
under the control of regulatory sequences enabling expression of
viral proteins of the FIPV yet which is substantially unable to
express a functional RT or a functional fragment thereof.
In a further embodiment of the invention there is provided
a host cell comprising a dysfunctional FIVP or the present
invention under the control of a regulatory sequence enabling
expression of viral proteins of the FIPV yet which is
substantially unable to express a functional RT or a functional
fragment thereof.
A host cell may be a cell of bacterial origin, e.g.
Escherichia coli, Bacillus subtilus and Lactobacillus species,
in combination with bacteria-based vectors as PBR322, or
bacterial expression vectors as pGEX, or with bacteriophages.
The host cell may also be of eukaryotic origin, e.g. yeast-cells
in combination with yeast-specific vector molecules, or higher
eukaryotic cells such as insect cells (Luckow et al; Bio-
technology 6: 47-55 (1988)) in combination with vectors or
recombinant baculoviruses, plant cells in combination with e.g.
Ti-plasmid based vectors or plant viral vectors (Barton, K.A. et
al; Cell 32: 1033 (1983), cells of mammalian origin such as Hela
cells, Chinese Hamster Ovary cell (CHO) or Crandell Feline
Kidney-cells, also with appropriate vectors or recombinant
viruses.
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/007I5 -
The FIPV fragment according to the present invention may be
cloned under the control of a promoter sequence or not under the
control of a promoter sequence in a viral genome, as the case may
be. In such a manner, the virus may be used as a means of
transporting the FIPV fragment into a target cell. Such
recombinant viruses ar~~ called vector viruses. The site of
integration may beg a site in a gene not essential to the virus,
or a site in an intergenic region. Viruses often used as vectors
are Vaccinia viruses (Panicali et al; Proc. Natl. Acad. Sci. USA,
79: 4927 (1982), H:erpesviruses (E.P.A. 0473210A2), Retroviruses
(Valerio, D. et al; in Baum, S.J., Dicke K.A., Lotzova, E. and
Pluznik, D.H. (Eels.), Experimental Haematology today - 1988.
Springer Verlag, New York: pp 92-99 (1989)) and baculoviruses
(Luckow et al; Bio-technology 6: 47-55 (1988)).
The invention also comprises a virus vector containing a
FIPV fragment or a recombinant nucleic acid molecule encoding the
FIPV fragment under the control of regulating sequences enabling
expression of the ~~rotein encoded by said nucleic acid sequence.
In an alternative, defective FIPV polynucleotides of the
invention may be ~~pplied directly to the cells of an animal in
vivo, or by in vitro transfection of cells taken from the said
animal , which cells are then introduced back into the animal .
Defective FIPV ma~~ be delivered to various cells of the animal
body including muscle, skin or blood cells thereof. The
defective FIPV may be loaded for example, into muscle or skin
using a suitable loading means such as a syringe. Methods of
applying naked dE:fective FIPV of the invention directly to
thebody are described in WO 90/11092, especially at pages 35 to
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
16
43 thereof.
As such, defective FIPV polynucleotides of the invention may
be administered as pharmaceutically acceptable salts to animals
in need thereof.
Polynucleotide salts: Administration of pharmaceutically
acceptable salts of the polynucleotides described herein is
included within the scope of the invention. Such salts may be
prepared from pharmaceutically acceptable non-toxic bases
including organic bases and inorganic bases. Salts derived from
inorganic bases include sodium, potassium, lithium, ammonium,
calcium, magnesium, and the like. Salts derived from
pharmaceutically acceptable organic non-toxic bases include salts
of primary, secondary, and tertiary amines, basic amino acids,
and the like. Further pharmaceutical salts are described in,
S.M. Berge et al., Journal of Pharmaceutical Sciences 66: 1-19
(1977).
Polynucleotides for injection, may be prepared in unit
dosage form in ampules, or in multidose containers. The
polynucleotides may be present in such forms as suspensions,
solutions, or emulsions in oily or preferably aqueous vehicles.
Alternatively, the polynucleotide salt may be in lyophilized form
for reconstitution, at the time of delivery, with a suitable
vehicle, such as sterile pyrogen-free water. Both liquid as well
as lyophilized forms that are to be reconstituted will comprise
agents, preferably buffers, in amounts necessary to suitably
adjust the pH of the injected solution. For any parenteral use,
particularly if the formulation is to be administered
intravenously, the total concentration of solutes should be
CA 02283490 1999-09-09
WO 98140493 PCT/GB98/00715 -
17
controlled to maks~ the preparation isotonic, hypotonic, or weakly
hypertonic. Nonionic materials, such as sugars, are preferred
for adjusting tonicity,. and sucrose is particularly preferred.
Any of these forms ma~~r further comprise suitable formulatory
agents, such as starch or sugar, glycerol or saline. The
compositions per unit dosage, whether liquid or solid, may
contain from 0.1% to 990 of polynucleotide material.
In a further embodiment of the invention there is provided
a vaccine against FIV comprising a defective FIPV polynucleotide
fragment of the invention. The FIPV fragment may take the form
of a naked FIPV polynucleotide fragment, that is, a FIPV
polynucleotide fragment not bound up in a vector form, such as
a plasmid form. The vaccine of the invention may optionally
include a further polynucleotide fragment encoding a further
compound having an immunogenic function such as a cytokine, for
example, feline y interferon. The additional polynucleotide
fragment may be ~.n the form of a further vector as described
herein, for example an additional plasmid vector. Alternatively,
the additional polynucleotide can be in the form of a naked DNA.
Such naked DNA may be adhered to a microprojectile or in an
appropriate holding solution, such as a saline solution.
Alternatively, the FIPV polynucleotide fragment can be available
in the form of a vector or of a host cell.
The vaccine may also comprise a dysfunctional FIPV
polynucleotide fragment as described hereinbefore in combination
with a further vector or further polynucleotide fragment encoding
a gene which when exprea>sed the gene product thereof retains an
immunogenic functi~~n. A suitable further polynucleotide fragment
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
18
for use in a vaccine of the invention can be selected fram those
described in WO 96/03435, such as vectors encoding feline Y
interferon.
In a preferred presentation, the vaccine can also comprise
an adjuvant. Adjuvants in general comprise substances that boost
the immune response of the host in a non-specific manner. A
number of different adjuvants are known in the art. Examples of
adjuvants may include Freund's Complete adjuvant, Freund's
Incomplete adjuvant, liposomes, and niosomes as described in WO
90-/11092, mineral and non-mineral oil-based water-in-oil emulsion
adjuvants, cytokines, short immunostimulatory polynucleotide
sequences, for example in plasmid DNA containing CpG
dinucleotides such as those described by Sato Y. et al. (1996)
Science Vol. 273 pp. 352-354; Krieg A.M. {1996) Trends in
Microbiol. 4 pp. 73-77. Further adjuvants of use in the
invention include encapsulators comprising agents capable of
forming microspheres (1-10 /cm) such as poly(lactide-coglycolide),
facilitating agents which are capable of interacting with
polynucleotides such that the said polynucleotide is protected
from degradation and which agents facilitate entry of
polynucleotides such as DNA into cells. Suitable facilitating
agents include cationic lipid vectors such as:
1,3-di-oleoyloxy-2-(6-carboxy-spermyl)-propylamid (DOSPER),
N - [ 1 - ( 2 , 3 - d i o 1 a o y 1 o x y ) p r o p y 1 ] - N , N , N
trimethylammoniummethylsulfate (DOTAP),
N-[1-(2,3-dioleoyloxy)propyl))-N,N,N-trimethylammonium
chloride (DOTMA),
(N,N,N',N'-tetramethyl-N, N'-bis(2-hydroxylethyl)-2,3-
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
19
dioleoyloxy-1,4-butaned.iammonium iodide,
bupivacaine-HC1,
non-ionic poi.yoxypropylene/polyoxyethylene block copolymers,
polyvinyl polymers and the like.
Such cationic lipid vectors can be combined with further
agents such as L--dioleoyl phosphatidyl ethanolamine {DOPE) to
form multilamella:r vesicles such as liposomes.
The vaccine may also comprise a so-called "vehicle". A
vehicle is a compound, or substrate to which the FIPV
po-lynucleotide fragmeni~ can adhere, without being covalently
bound thereto. Typical "vehicle" compounds include gold
particles, silica part~~~cles such as glass and the like. Thus
FIPV polynucleotides of the invention can be introduced into
appropriate cello using biolistic methods such as the high-
velocity bombardment method using polynucleotide coated gold
particles as described in the art (Williams R.S. et al. (1991)
Proc. Natl. Acad. Sci. USA 88 pp. 2726-2730; Fynan E.F. et al.
(1993) Proc. Natl. Acad Sci. USA Vol. 90 pp. 11478-11482).
In addition, the vaccine may comprise one or more suitable
surface-active compounds or emulsifiers, e.g. Span or Tween.
In a further aspect of the invention there is provided the
use of a FIPV po:Lynucleotide fragment as described herein for
producing at least a cell mediated immunity to FIV which
comprises a defective FIPV as described above for the manufacture
of a FIV vaccine for the prophylaxis and/or treatment of FIV-
related disease. PrefE~rably, there is provided use of a FIP~
polynucleotide fragment in naked or vector form for the
manufacture of a FIV vaccine for the prophylaxis and/or treatment
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
of FIV infection. Most preferably, the use is in felines.
In a further aspect of the invention there is provided a
method of treating animals which comprises administering thereto
a vaccine composition comprising a defective FIPV polynucleotide
fragment as described herein to animals in need thereof.
Preferably, the animals are felines. Naturally, the vaccine
formulation may be formulated for administration by oral dosage
(e.g. as an enteric coated tablet), by parenteral injection or
otherwise.
- The invention also provides a process for preparing a FIV
virus vaccine, which process comprises admixing a defective FIVP
polynucleotide fragment in naked or vector form as herein
described with a suitable carrier or adjuvant.
The mode of administration of the vaccine of the invention
may be by any suitable route which delivers an immunoprotective
amount of the virus of the invention to the subject. However,
the vaccine is preferably administered parenterally via the
intramuscular or deep subcutaneous routes. Other modes of
administration may also be employed, where desired, such as oral
administration or via other parenteral routes, i.e.,
intradermally, intranasally, or intravenously.
Generally, the vaccine will usually be presented as a
pharmaceutical formulation including a carrier or excipient, for
example an injectable carrier such as saline or a pyrogenic
water. The formulation may be prepared by conventional means.
It will be understood, however, that the specific dose level
for any particular recipient animal will depend upon a variety
of factors including age, general health, and sex; the time of
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
21
administration; the route of administration; synergistic effects
with any other drugs being administered; and the degree of
protection being sought.. Of course, the administration can be
repeated at suitaJ~le intervals if necessary.
As a further aspect of the invention there is provided a
polynucleotide fragment encoding for an FIPV which is
substantially inc:apabl~~ of encoding a functional RT or a
functional RT fragment i:hereof for use as a medicament for FIV-
related disease. The skilled addressee will appreciate that a
deletion may be made in the RT domain of the pot gene which
deletion may be a:n in-frame deletion as described herein. The
skilled addressee: will also appreciate that insertions into
deletion sites may be made to FIPV of the invention as utilised
under this aspect of th~~ invention as described herein.
As a further aspect of the invention there is provided use
of an FIPV comprising a dysfunctional pot gene in the manufacture
of a vaccine for 'the prophylaxis and/or therapy of FIV-related
disease. In a preferment the pol gene comprises a deletion
within its RT domain, such as an in-frame deletion as described
herein. The skilled addressee will also appreciate that
insertions into deletion sites may be made to FIPV of the
invention as utilised under this aspect of the invention as
described herein.
Embodiments of the invention will now be illustrated by way
of the following l:'igure:~ and Examples.
Figure 1: Nucleotide sequence of FIV F14 (Petaluma strains)
showing CART site (3496 to 3595)(Sequence ID. No.
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
22
5) Pac I, Ncol and Sph I sites.
Figure 2: Feline Y-Interferon.
Figure 3: Construction of CMV~RT.
Figure 4: Sequence of Sst I fragment in CMVORT (Sequence
ID. No. 6) .
Ficxure 5: Genome Map of FIV RT deletion mutant.
Figure 6: Peripheral blood viral loads in a) trial-6(a) at
7 weeks post challenge and in b) trial-6(b) at 6
weeks post challenge, expressed as the mean (+/-
2SEM) of the log-transformed maximum likelihood
estimates of the initial number of infected cells
present in 2 x 10° PBMC.
Figure 7: Sequence of the Hind III - Not I fragment in
plasmid pRSV-Y-IFN (Sequence ID. No. 7).
EXAMPLES SECTION
a ac a t've y'
Summary
The F14 clone of FIV-Petaluma was modified by introducing
a deletion centred on a unique Pac1 restriction site in the RT
domain of the pot gene, in a region homologous to the
"connection" domain of human immunodeficiency virus RT. A clone
with a 33-codon, in-frame deletion was identified and designated
FIV-ART. This clone was characterised in vitro by transfection
into fibroblasts. Following transfection: 1, syncytia were
formed within 3 days; 2, cell lysates showed glycoprotein and Gag
protein expression by Western blot; 3, antigen was pelleted from
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
23
culture fluids b5~ cent:rifugation at 100,000 X g, suggesting it
is in particulate form; 4, no RT activity above background was
observed in ths~ culture fluids; and 5, unlike cultures
transfected with wild-type FIV-F14, no infectious virus was
detected in the culture fluids.
METHODS
1. Induction of FIV-Specific Cytotoxic T Cells
At 3, 6, 10,. 12, 16 and 20 weeks post vector delivery and
on~ the day of challenge, 5ml peripheral venous blood was
collected into an equal volume of Alsever's solution (Scottish
Antibody Production Unit, Carluke, UK), and PBMC were prepared
by centrifugation over Ficoll-Paque (Pharmacia LKB, Biotechnology
Inc., Piscataway, NJ) for the determination of virus-specific
lymphocytoxicity. Fibroblast cell lines were derived from skin
biopsy samples (~~mm in diameter) obtained from all cats under
general anaesthesia prior to immunisation or challenge, and
maintained in minimal essential medium (MEM) ALPHA medium with
ribonucleosides and deo:cyribonucleosides (Biological Industries,
Paisley, UK) supp~~.ementE~d with 10% foetal bovine serum (FBS), 2mM
L-glutamine, and 100IU of penicillin, 100~cg streptomycin, long
of human epidermal growth factor (Sigma, Poole, UK) per ml.
Virus-specific effector CTL present in the fresh PBMC were
detected using autologous or allogeneic skin fibroblast target
cells labelled wp.th 50 ~,Ci of sodium [5'Cr] chromate (Amersham
International, ASrlesbu:ry, UK) /10° cells for 18 hours at
37°C,
washed three times, and then infected with 5 to 10 plaque-forming
units/cell of recombinant vaccinia virus expressing either the
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98100715
24
gag or env gene product from FIV/Glasgow-14 or FIV/Petaluma,
respectively, or with wild-type vaccinia virus far 1 hour at
37°C. Unbound virus was washed away, and the cells were
incubated for an additional 2 hours to allow optimal expression
of the FIV Gag and Env products. Standard microcytotoxicity
assays were then performed in triplicate by adding appropriate
numbers of effector cells to 1x104 target cells to give effector:
target (E: T) ratios of 50, 25, 12.5 and 6.25:1 as described
previously (Flynn et al., (1996) supra).
2. Isolation of FIv
Peripheral blood mononuclear cells (PBMC) were isolated from
heparinized venous peripheral blood by centrifugation over
Ficoll-Hypaque (Pharmacia LKB, Biotechnology Inc., Piscataway,
NJ). Then 106 PMBC were co-cultivated as described in Hosie M.J.
and Flynn J.N. (1996) J. Virol. 70 pp. 7561-7568). Samples of
culture supernatant were tested at intervals for the presence of
FIV p24 by ELISA (IDEXX Laboratories, Portland, ME) and cultures
were maintained for 21 days before being scored as negative.
3. Quantitative Virus Isolation
The infectious virus burden was measured in peripheral blood
mononuclear cells (PBMC) that had been isolated from heparinized
peripheral blood by Ficoll-Paque separation (Pharmacia), frozen
and stored under liquid nitrogen. Decreasing numbers of PBMC (2
x 106, 2 x 105, 2 x 104, 2 x 10', 2 x 10-, 20 and 2) were co-
cultivated in duplicate in 24-well plates with 5 x 105 Miyazawa-1
cells in 1.5m1 RPMI-1640 medium (Gibco) supplemented with 10%
CA 02283490 1999-09-09
WO 98140493 PCT/GB98/00715 -
foetal bovine serum (Imperial Laboratories), 2 mmol/1 glutamine,
100 IU penicillin, 100mg/ml streptomycin (all from Gibco BRL) and
5 x 105 mol/1 2-mercaptoethanol (Sigma Chemical Co.). Twice
weekly, 0.5m1 of tine culture supernatant was removed and replaced
with fresh medium. The culture supernatant collected on day 14
was tested by ELIS~~ for :FIV p24 production (FIV antigen detection
kit, IDEXX).
example 1: ~s uci~ion of the Deletion in R
The F14 clone' of FIV/Petaluma (Olmsted et al. 1989 supra)
which includes approximately 9 kb of uncharacterised feline
genomic DNA flanking the proviral sequence within the vector
pGEM-7Zf + (Promega) includes a unique Pac 1 site within the RT
region of the po.Z gene: (nucleotides 3540-3547). Linearised
plasmid was purif:ued by precipitation then digested with Ba131
exonuclease under conditions calculated to allow a rate of 30
bp/minute (Maniatis T. et al. supra). After purification by
phenol/chloroform extraction and ethanol precipitation,
exonuclease digested DNA was recircularised by ligation and the
products were used to transform E.coli DS941 (Meaden et al. Gene
(1994) Vol. 41 pp. 97-101). Clones were examined by polymerase
chain reaction (PCR) amplification across a 235 by region of pot
encompassing the P~3c 1 site. One clone (aRT) (Sequence ID. No.
5) with a large in~-frame deletion 99bp was characterised by DNA
sequencing using the PCF; primers:
(1) TGTGATAT~~GCCTTAAGAGC (3429-3448) (Sequence ID. No. 1)
and
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
26
(2) TACCATGTTTCTGCTCCTGG (3645-3664) (Sequence ID No. 2)
This clone was designated FIV-ORT (Figure 1) (Sequence ID
No. 5).
~xamt~le 2: Characterisation of FIV-ART
FIV-ART (50 ~g plasmid DNA) was transfected into CrFK cells
by calcium phosphate co-precipitation. The parental F14 plasmid
served as positive control. After 3 days, syncytia were observed
in-the transfected cultures but not in mock-transfected cells (no
DNA). This result implied that cells expressing the deleted
provirus were able to fuse with neighbouring cells, presumably
because they elaborated functional envelope glycoprotein.
Syncytia were readily stained by immunofluorescence using serum
pooled from FIV-infected cats.
Production of viral proteins was also investigated by
enzyme-linked immunosorbance assay (ELISA) and immunoblotting.
Large amounts of Gag capsid protein (p24) were detected in
culture supernatants 6 days after transfection with F14 or ART
(Table 1) commercial antigen ELISA ("Petcheck"; IDEXX
Laboratories, USA). Other viral proteins in cell lysates were
analysed by SDS PAGE and immunoblotting using serum pooled from
FIV-infected cats. Gag precursor and mature (capsid) proteins,
and also envelope surface glycoprotein, were observed.
The capsid antigen could be pelleted from cell supernatants
by ultracentrifugation, as detected by ELISA and immunoblotting.~
Thus the defective provirus was still capable of directing
synthesis of antigenic particles.
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
27
RT activity was me<~sured in culture supernatants. Cultures
corresponding to wild i~ype F14 were strongly positive, whereas
cells transfected wit:h FIV-ART showed no activity above
background levels (Table 1).
The absence of infectious virus in the ORT cultures was
confirmed by passage of cells or supernatant fluids to fresh CrFK
cell monolayers. AftE:r 7 days, no syncytium formation, p24
antigen or RT acaivit~~ was observed in cultures seeded with
supernatant from ART-transfected cells, whereas supernatant from
cells transfected with wild-type FIV established infection
rapidly. Occasional syncytia were observed in cultures seeded
with ART - transfect:ed cells, presumably centred around
individual trans~:ected cells carried over from the initial
exposure to DNA.
Example 3: instruction of CMV-ART
A region from the ~~~LTR to the primer binding site in F14ART
was replaced by the immediate early promoter from human
cytomegalovirus. This procedure was designed both to enhance
expression of FIV antigens, and to reduce the risk of reversion
to a replicating provirus, in tissues after inoculation of DNA.
The construct way; designated CMVORT, and its construction was
achieved as follo~~rs:
Restriction sites for endonucleases Sal I and Sst I were
mapped. F14ART wa.s rearranged as in Figure 3 to an intermediate
(designated ART--Sal/Sat) having a unique Sst I site.
Accordingly, Sal I and S;st I were used to digest plasmid F14ART,
the resulting mi};ture of fragments was religated and used to
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
28
transform E.coli (DS941), and a clone with the structure expected
of ORT-Sal/Sst was identified. CMV sequences could then be
introduced upstream of the Sst I site.
A PCR product encompassing FIV sequences from the primer
binding site to a point downstream of the Sst I site was derived
from the F14 plasmid using Taq polymerase (Perkin Elmer) and the
method of Saiki et al (1985) Science 230 pp. 1350-1354; The
primers used (corresponding to co-ordinates 356-376 (Sequence ID
No. 3) and 1963-1980 (Sequence ID No. 4) of the F14 provirus)
we-re constructed with additional Sal I "tails", and had the
sequences: GATCGTCGACGTTGGCGCCCGAACAGGACT (5') and
GATCGTCGACTTATAAATCCAATAGTTT (3'). This PCR product was cloned
into the Hinc II site of plasmid vector pICl9R (Marsh et al.
(1984) Gene 32 pp 481-485) to yield pPBSGAG. FIV sequence from
pPBSGAG was then released as a Sal I fragment and cloned into the
Sal I site of pIC20H (Marsh et al. supra) to give pPBSSal. The
CMV IE promoter was cloned infront of these FIV sequences as a
Bgl II-Kpn I fragment from expression vector pcDNA3 (Invitrogen),
yielding pCMVPBS. An Sst I fragment from this clone, including
the IE promoter and FIV sequences from the primer binding site
to the proviral Sst I site, was then cloned into the Sst I site
in ART-Sal/Sst. The resulting DNA sequence from within the CMV
IE promoter to a point downstream of the FIV proviral Sst I site
was confirmed by direct sequencing.
The sequence of the Sst I fragment in CMV~RT is shown in
Figure 4 (Sequence ID. No. 6). FIV sequences downstream of the
Sst I site are identical to those in F14~RT.
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
29
Example 4: ~. ruction of pRSV-~-IFN
Feline Y-interferon cDNA was available as a cDNA clone in
pCR-ScriptSK(+) (~~tratac~ene) as described in Argyle D.J. et al.
(1995) (DNA Sequence 5, 169-171). The cDNA sequence was excised
with restriction en2ymes HindIII and NOtI (Sequence ID No. 7) and
inserted into pRc/RSV expression vector (Invitrogen) to produce
the pRSV-YIFN plasmid.
Example 5 ~7 pNA, Immunisation Trial~ Protection of
yac:cinated Cats
Procedure
The efficacy of DNA immunisation to protect cats from
infection with feline immunodeficiency virus (FIV) was
determined. Twenrty 12 week old kittens were randomised into 4
groups of 5. The DNA used in the inoculations comprised a
plasmid ART, either alone or in conjunction with feline Y-IFN
DNA, as shown below:
Group No. Cat No. Plasmid
Group 1 A481-485 100ug ART
Group 2 A4 8 6-4 9 0 100~Cg ART + 100,~g pRSV-Y-
IFN
Group 3 A491-495 100~,g pRSV-Y-IFN
Group 4 A496-500 no DNA
The cats were inoculated intramuscularly with test DNA at
each of 4 sites with 100ug DNA in 200,1 PBS on weeks 0, 10 and
23. The cats were: challenged intraperitoneally on week 26 with
25 cat infectious doses 50% (CIDSO) of FIV-Petaluma derived from
the F-14 molecular clone, propagated in Q201 cells (Willett et
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
al. (1991) AIDS Vol. 5 pp. 1469-1475).
Results
Antibody responses were measured by immunoblotting according
to the method of Hosie M.J., O. Jarrett (1990) AIDS 4 pp. 215-220
and to peptides representing two immunodominant epitopes from the
viral envelope proteins (V3 and TM) by enzyme linked
immunosorbent assay (ELISA) (Hosie M.J, and Flynn J.N., (1996)
J. Virol. 70 pp. 7561-7568) 3 weeks after each vaccination and
3, 6, 9, and 12 weeks following challenge.
Assays for cytotoxic T cell (CTL) activity against FIV Env
and Gag proteins were conducted during the immunisation schedule
and at the day of challenge (Hosie M.J. and Flynn J.N. (1996)
supra ) .
Antibody Responses
No antibodies were detected by peptide ELISA (as above)
prior to the day of challenge. Following challenge, any antibody
responses could therefore be equated With infection. The results
are included in Table 2.
Cytotoxic T Cell Response (CTL Responses)
FIV Gag- and Env-specific effector CTL activity was detected
following the method of Hosie M.J. and Flynn J.N. (1996) supra,
in the fresh peripheral blood of all cats immunised with the ORT
plasmid (A481-A485) three weeks following vector delivery. The
response was only observed on autologous target cells, suggesting
that the response was MHC-restricted. Furthermore, there was no
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
31
recognition of target ce:Lls infected with the wild-type vaccinia
virus confirming t:he specificity of the response. The FIF Gag-
specific response: appeared higher than (A481 and A482) or
similar to the levels of Env- specific lysis observed at an E:T
ratio 50:1 and levels ranged between 20 and 540. This pattern
of responses is similar 1.o that observed in the peripheral blood
of cats immunised with inactivated whole virus vaccine based on
the FL4 cell line. However, the levels of specific lysis
observed with WIV inactivated virus vaccines are generally
slightly lower than those: detected in the present study with the
ORT plasmid, and the predominant CTL response is directed towards
Env rather than Gac~ (Flynn et al., (1995) Aids Res. Human Retro.
11 pp. 1107-1113, l3osie and Flynn, (1996) supra).
Co-immunisation with the ORT plasmid and a feline y-IFN
plasmid induced very high levels (up to 73 o specific lysis) of
Gag-specific lysis in 3 out of 5 vaccinated cats (A486, A488 and
A490) , and Env-specific l.ysis in 2 out of 5 cats (A487 and A489) .
However, this response did not appear to be entirely MHC-
restricted, since c~~nsiderable lysis of allogeneic target cells
was also observed. The non-specific nature of the cytolytic
responses observed was further confirmed by the recognition of
autologous target cells :infected with wild-type vaccinia virus,
in 3 out of 5 cats. Immunisation with the y-IFN plasmid alone
resulted in the induction of FIV-specific cytolytic responses in
3 out of 5 cats (A491. to A493), in either autologous or
allogeneic target cells. In addition, high levels of lysis were
observed in 2 cats (A492 and A493) using target cells infected
with wild-type vaccinia virus. These results suggest that in
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
32
vivo delivery of the feline y-IFN plasmid to cats may elicit non-
specific cellular immune responses such as NK-type activity.
No FIV-specific immune responses were detected in control
cats immunised with PBS alone.
By 6 weeks after vector delivery, significant levels (>10%
specific lysis) of FIV Gag-specific CTL activity was detectable
in 4 out of 5 cats immunised with the ORT plasmid, and 3 of these
cats also had significant levels of Env-specific CTL activity.
However, the levels detected were lower than those observed at
3 weeks post immunisation. In the group immunised with ORT and
y-IFN plasmids, no FIV-specific CTL activity was detected.
Likewise no CTL activity was detected in the control groups
immunised with y-IFN alone or with PBS, the one exception being
A491 which displayed a response to FIV Gag and Env.
At 10 weeks post immunisation the CTL responses detected in
the group immunised with ART had declined still further, with FIV
Gag-specific activity detectable in one cat (A484) and Env-
specific activity in another (A482). At this time Gag-specific
lysis was observed in 2 cats immunised with ART together with y-
IFN and Env-specific activity was observed in A490. However the
levels observed were rather low compared to those at the 3 week
time point. Again no activity was observed in control cats. The
cats were re-boosted at this time and the FIV-specific CTL
responses induced the peripheral blood analysed 2 weeks later.
The boost at week 10 had the effect of raising the FIV Gag-
specific CTL activity in 3 out of 5 cats immunised with the ART
construct, in addition non-specific responses were detected in
2 cats. A similar effect was noted in cats immunised with ART
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
33
and y-IFN, with Gag-specific CTL activity boosted in 2 cats.
A490 maintained similar levels of Env-specific lysis to that
observed at week 10. Negligible FIV-specific lysis was recorded
in control cats.
Assays performed at: weeks 16 and 20 were unremarkable, and
assays performed on the day of challenge with 25 CID$o of F14
FIV/Petaluma, revealed low levels (12-15% specific lysis) of Gag-
specific CTL activity in 2/5 ART immunised cats and negligible
activity in the cats immunised with ART and y-IFN.
Results of Virus Detection
Virus isolation from PBMC was attempted following
immunisation but was neg<jtive at all times prior to and including
the day of challenge, indicating that there was no reversion to
virulence of the mutant provirus during this period. Following
challenge, cats were monitored for infection by virus isolation.
By 9 weeks post challenge, 5/5 control cats receiving no DNA had
become infected, t.ogeths:r with 5/5 cats inoculated with feline
y-IFN DNA... In coni:rast, there was evidence of protection in the
groups inoculated with ORT DNA (Table 3). No virus could be
isolated from one of the 5 cats in group 1 or from 3/5 cats in
group 2. Furthermore, the viral loads measured by quantitative
co-culture of PBMC with MYA cells in the infected cats that had
been inoculated wii:.h ART were lower than those of the cats in the
two control group (Table 4).
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
34
Since several parameters that were measured gave an
indication of infection and viral load following challenge, a
clinical scoring system was adopted in order to compare the
outcomes between groups (Table 5a). Clinical scores were
significantly lower in the groups immunised with ART and ART +
Y-IFN compared to their appropriate control (p < 0.05 and 0.005
respectively, Table 5b), providing further evidence that FIV DNA
immunisation induced protective immunity that was augmented by
feline Y-IFN DNA.
Example 6 Shortened FIV~RT Immunisation sch~du~P
To investigate whether the earlier described immunisation
schedule could be reduced without compromising protection, a
second experiment was conducted in which 2 groups of 5 cats
received either FIVORT + IFN-y or IFN-Y alone at 0,4 and 8 weeks.
As in the first trial, this regimen induced broad spectrum
cytolytic activity but no detectable antibody responses using the
same series of assays. After challenge at 12 weeks, 2/5
vaccinates remained seronegative and virus could not be isolated
at any of the times tested (Table 6(a) and 6(b)) whereas all of
the IFN-Y alone controls became seropositive and positive by
virus isolation, consistent with the results of the first trial.
Again, immunoblot analysis corroborated these findings fully.
Quantitative measurements of virus in the second trial (Figure
6) revealed that at 6 weeks post challenge, the FIV~RT+ IFN-Y
vaccinates developed significantly lower viral loads compared to
the IFN-Y vaccinates (P=0.027).
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98100715
Table 1: Production of p24 but not RT by oRT DNA
Post transfection Post supernatant
transfer
DNA p24 RT p24 RT
( OD4os )
( OD4os )
F14 >3.00 255 >3.00 2329
ART 1.07 98 0 86
Control 0.11 87 0 91
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
~6
N ,O
> I 1 t ! I I I + I t t + ~
N ri I I I t t -f t
rl
,L1 + t t I I + 1 t t + + t t
H + t I t t t I
I tm n ,n
~
~S N N ~ tf7 U7
O O O O O O O U'7 N
N .-1 O .-1 N N u1
N N tn r-1
tn tn
'O 'O 'D 'b b 'L7 'b 'O '
> C 'b '>~ 'CJ
'C! 27 b
1 >~ I I C I C C C C ~ G
C G I C C C C
3~
O
.-I t t t I 1 + I t + t t t t
t t I t t t +
U 3
ri o>
O >
r~
I
25 0 0 0 o 0 0 o ~n o 0 0 0
u~ u~ o 0 0 0 0
0
U
r~ E' u~ u u~ u~ n uW
um n
N N N N N N N N N
G~ y O ~ O O N tn N tI7
3 -1
. . O N O ~O ~O .-1 N
.-1 O r1 r1 .-1
N rl N
O
U
a'J H
Q > t t t I l t I t t + + + t
N t t I + t t t
O
,,i
U O
W Cl
'~
R .
O ca
W H + + + I 1 + I + + + + + +
3 + + I + + + +
>
H c~
x ~,
~ o +++ I + * + I +++++ +++++
3 + I
N ~ C!
O r-~
b
W
o ~--I
O
.i
.L1 I I I I i I + + + + + + b
+ I + + + +
al f6 ri
id O
b N
W ;J .-1
O
O ~ ui td
' ~ U7 ~f7 ro
N ~t1 u'7
u1 V7
(II O tf ~ .~ u'1 N N u7
7 O O r1 O O tf7 N N N N
O ~
~
~i
O o GI N
ro
Qi .-i N fh ~ I~ OD r-i N t0 !~
l C' tf7 01 O M V' 00 01
tn
i. 00 OD O O m OD 01 a1 01 01
ro Q7 OJ OD 01 Q1 01 Q1 01
c 01
c c a c c cr c a c ~
U c c c c c a c c u, ~
aaaa a aaaaa c
aa
a aaaaa ~a ~
aa
N ~.1
a3
Y Y
H > > .~ L'..
.C',
GI
a~
~ ~
H a A. n. c A a
H N
SUBS'T'ITUTE SHEET (RULE 26)
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
37
Table 3: Protecaion against FIV infection induced by DNA
immunisation
Group Inoculum Proportion
protected
~I' 1/5
ORT + y-IFN 3/5
y-IFN 0/5
PBS
0/5
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
38
Table 4: Results of Quantiative Virus Isolation
Number of
PBMC Plated
DNA Cat No. 2x106 2x105 2x10'
RT A481' 1/2 0/2 0/2
A482' 1/2 0/2 0/2
A483' 0/2' 0/2 0/2
A484' 0/1 0/2 0/2
A485' 0/2 0/2 0/2
RT+YIFN A486' 0/2 0/2 0/2
A487 1/2 0/2 0/2
A488 0/2 0/2 0/2
A489' 0/2 0/2 0/2
A490- 0/2 0/2 0/2
Y-IFN A491 2/2 1/2 0/2
A492' 0/2 0/2 0/2
A493' 2/2 1/2 0/2
A494' 0/1 0/2 0/2
A495 2/2 1/2 0/2
None (PBS) A496' 2/2 1/2 0/2
A497 2/2 1/2 0/2
A498 2/2 0/2 0/2
A499' 2/2 0/2 0/2
I
A500 nd 0/1 0/2
x 106 cells available for test
z1/2 wells near cut off OD
nd = not done
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715 -
39
Table 5: Ranking of results by clinical score
a. Clinical Score Ratings
Virus isolation
positive at 3 weeks pc 1
positive at 6 weeks pc 1
Immunoblot analys:Ls of ;plasma pc
positive at 6 weeks pc 1
positive at 9 weeks pc 1
Viral load quantiation
virus isolated from 2 x 106 PBMC 1
virus isolated fr~~m 2 x 105 PBMC 1
virus isolat=ed from 2 x 10'' PBMC 1
Possible maximum score 7
b. _ Clinical ScorEa of Cats following challenge
~TuP Group 3
1
yIFN
A481 3 A491 6
A482 4 A492 4
A483 2 A493 5
A484 0 A494 3
A485 4 A495 6
mean 2.6' 4,8
SEM 0.75 0.58
Grou 2 p
ART pyIFN PBSu 4
A4860 A496 5
A4874 A497 6
A4880 A498 4
A489~~ A499 4
A4900 A500 4
mean1.4Z 4.6
SEM 0.87 0.4
'P 0.0462
=
ZP 0.0103
=
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98/00715
tf1 N N ltl In lf1 tl1 N
O O O O N O O O r-1 O '"I N N N In N Ill In r-1 tll
N
I i + I I I I 1 1 I -~ I + -~ ~- + -t- -I- + +
+ + + I + I + 1 + I + + + + + + + + + +
O1
+ ~- + I + I -t- ~- -t- + -~ +
+ i 1 + + + + -1-
+ + -F + I + I + ~- ~- + -I- + +
I 1 + + + +
~O
ZT
+ + + + I + I + + ~- ~- + + +
I I + +
r-~
r-I
f~
U
1 1 1 ~- I -~ I -i- -f- + + + -F
1 + I + + + +
O M N
f~ 't~
1 ~- + 1 I I I + + I I 1 + i I 1~
I 1 i + ~-
x u.
v v
v a~
I I I 1 1 t 1 I I 1 I I 1 I I I
I 1 I I
E~
0
N
O O O O O O O O O O O O O O O -rl
O O O O O
!~
I 1 1 1 I I I 1 1 I I I I I 1 I U
I I I I
N
t<r
I I I 1 1 t I 1 I I 1 I I I I !
I I I I
O
O ~
ri N '-I N f"7 ri N f'1 ri N C7 fl1
c'1 V' In V' lf1 d' lf1
d' lf1
1~
ri
f0
O O
r-i
UI
W
b o -~I
o
~ N
v
..
z -~
>
.~
a al 1 0
E O ~ wH ~
- ! 1- ~ ~DH
a
SUBSTITUTE SHEET (RULE 26)
CA 02283490 1999-09-09
WO 98/40493 PCT/GB98100715
41
In Lf~ lf1 N In ltl
N O O fV O N e-1 lfl N N
t I I I i ~- I t t t
N
t 1 t '!- I + t t t t
t I t '~ I + t t t t
'b 'Ll 'LS 't3 't3 'LS T7 'L7 't~ 'O
J; C ~ f, ~ C ~ G >~ s;
O
t I t -~ t t t t t
I
'~ t I I ;I- t t I 1 f
U I
N
O U
f3. 't3
t I I -I'- t t t I 1
1
M
b ~3 't7 't? '~ 'L~
Z7 b 't7 b
r~ ~ >~ s~ >~ ~ c
t. >; >~
H
ZT
0o I o0 00000 ,N-,
0
s~
U
I I 1 I I 1 1 I I
I
N
I i ! 0 I I I f I tv
I
'O
>~ O
O ,CZ
r-1 N C'1 v-1 N f'1 f0 !~
sr lf1 ~!' lf1
.~J
r~
Id
O O
rl U1
W ~
_ .rl
4 O
. O
O
t ro
~ ~
~l
~ --ri
-r-I
O
-.
r1 ~
~..1
.1
.
.. ..
..
E A w H
. ~DH~
SiJBSTITUTE
SHEET (RULE
26)