Note: Descriptions are shown in the official language in which they were submitted.
CA 02284616 1999-09-23
WO 98/42892 PCT/NZ98/00040
-1-
ANODISING MAGNESIUM AND MAGNESIUM ALLOYS
This invention is a method for the anodisation of magnesium and its alloys
which,
at least in the preferred form, produces an even and corrosion resistant film
and which, at
least in the preferred form, is suitable as a pre-treatment for other
processes or as a final
treatment for magnesium articles.
Magnesium is a very light, yet strong metal and is finding increasing
acceptance
for metal die castings, particularly where weight savings are desired. In
addition, its
property of shielding electromagnetic radiation is causing it to be of
interest as a
replacement for plastics in applications such as computers and mobile
telephones.
However, it is a reactive metal and corrosion, whether general or by galvanic
effects, is
a major problem.
Traditionally, a number of methods for applying a protective anodic oxide film
on
magnesium metal have been available. These have sought to imitate the well
established
processes available for coating aluminium and its alloys, but achieving the
same result on
magnesium articles has been extremely difficult. In part this is due to the
fact that the
oxide formed from a given volume of magnesium metal occupies less space than
the
original metal and thus any film of the oxide formed on the surface is subject
to tensile
stress and before a significant layer can be built up, it cracks and spans
away from the
substrate.
The anodisation of aluminium and its alloys is often conducted in sulphuric
acid
in which the oxide layer formed is slightly soluble. As the film builds
outwards from the
metal substrate, its rate of build decreases, so ultimately there is a point
at which the rate
of dissolution is equal to that of further film growth. The dissolution of the
film causes
the formation of pores through which the ionic migration necessary to the
electrochemical
oxidation of the metal takes place. Without these pores only very thin films
would be
possible. After the electrochemical oxidation process is complete, the pores
are sealed.
Sealing of anodised aluminium can be achieved with hot water or simple
inorganic
chemical solutions.
Clearly an analogous process involving magnesium or alloys of magnesium would
attempt to simulate these features. However, because of the tendency of the
forming film
to crack and break due to the imposed tensile stresses, there are
complications. Also, the
use of an acidic solution to anodise magnesium is fraught with serious
difficulties as
magnesium is rapidly attacked by most common acids. Therefore, we believe
anodisation
of magnesium should take place in alkaline solutions. Nevertheless some prior
art
processes use hydrofluoric acid or acid fluoride salts in which magnesium is
not attacked
because of the formation of a protective layer of magnesium fluoride on the
metal surface.
CA 02284616 1999-09-23
WO 98/42892 PCT/NZ98100040
-2-
This is not soluble in water and thus prevents further attack.
A further method of anodising magnesium or alloys of magnesium relies on this
property to create a rough, very porous layer which forms an excellent base
for paint or
other surface coatings to be applied afterwards. Commonly, such an anodic film
may be
formed in an electrolyte of very high pH, containing alkali hydroxides. The
process
proceeds by means of sparking which sparking forms a sintered ceramic oxide
film as the
metal substrate is coated.
A number of proprietary methods for anodisation of magnesium or alloys of
magnesium exist which seek to avoid this problem and create a uniform film.
This can
only be done by incorporating other species into the film as it is formed.
Some processes
use silicates. Others use various ceramic materials. Some of these processes
involve the
use of hydrofluoric acid or acid fluoride salts, eg; ammonium bifluoride.
These are
extremely hazardous materials causing fume and safety problems to the plant
operators,
and disposal problems.
In PCT/NZ96/00016 (WO 96/28591 ) (Barton) there is disclosed a viable
procedure
for anodising magnesium or magnesium alloys. It involves anodising the
material in an
ammonia containing electrolyte solution. The presence of some phosphate
compounds
in the solution is disclosed.
The object of the invention is to provide as an alternative to or as a
refinement of
the process of WO 96/28591 a process which also can produce an even film, or
which at
least provides the public with a useful choice.
STATEMENTS OF INVENTION
In one aspect the present invention consists in a method of anodising
magnesium
or magnesium alloys comprising or including:
immersing said magnesium containing material in an electrolyte as an anode;
providing a cathode in said electrolyte; and
passing a current through said electrolyte;
and wherein the electrolyte, possessing a pH greater than 7, comprises or
includes
in water
(i) ammonia or an amine, or a mixture of the two, (preferably any ammonia
being present in a concentration substantially in the range of 0.4 to 12
molar); and
(ii) phosphoric acid or a water soluble phosphate salt.
Preferably said phosphoric acid and said phosphoric acid is provided in the
range
of 0.05 to 0.2 molar.
Preferably said electrolyte contains a foaming agent.
__ __. T_ _._____ . _ T
CA 02284616 1999-09-23
WO 98/42892 PCT/NZ98/00040
-3-
Optionally said electrolyte contains a water soluble peroxide.
Preferably an amine is used alone for step (i) or as partial replacement for
ammonia
in step {i) and said amine is a water-soluble primary, secondary or tertiary
amine having
a pKa greater than 5.
S Preferably said amine has a pKa greater than 9.
Preferably the magnesium or magnesium alloy article is cleaned by a
pretreatment
step prior to anodisation.
Preferably the pre-treatment step includes at least one of the following:
{A) immersion of the article in a mixture of sodium tetraborate and sodium
pyrophosphate solution at 70 ° C to 90 ° C for approximately at
least five minutes;
or
(B) immersion of the article in 35% hydrofluoric acid (v/v) at ambient
temperature for at least approximately one minute; or
{C) immersion of the article in a one to one mixture of 35% hydrofluoric acid
{w/w) and 68% nitric acid (w/v) for at least approximately one minute.
Preferably the material is anodised using an electrical current having the
following
characteristics:
(I){a) (i) a DC voltage (where the electrolyte includes ammonia and no amine)
from 300 volts {and particularly 350 volts upwards) upwards; or
(ii) a DC voltage (where the electrolyte includes an amine or ammonia
and an amine) from 250 volts upwards; and
(b) optionally
(i) an AC voltage usually between zero to 40 volts, but under some
circumstances more; and/or
(ii) a pulsed (square wave form) voltage usually between zero and 40
volts, but sometimes greater; and
(II) a current density from 50 to 1000 amps per square metre.
Preferably the current density is from 200 to 350 amps per square metre.
In a further aspect the invention consists in a method of anodising magnesium
or a magnesium alloy (hereafter "the magnesium material") comprising or
including;
providing an electrolytic solution;
providing a cathode in or for said solution;
placing the magnesium based material as an anode in said solution and,
passing a current between the anode and cathode through said solution so that
an
anodised surface results,
and wherein the electrolyte, possessing a pH greater than 7, comprises or
includes
in water
___-
CA 02284616 1999-09-23
WO 98/42892 PCT/NZ98/00040
-4-
(a) (i) ammonia and an amine or (ii) an amine
and
(b} (i) at least one source of phosphate ions, and/or (ii) at least one source
of
aluminate anions and at least one source of fluoride ions; and wherein
the current applied during the anodisation is to a voltage limit
(A) (i) if no hydrogen peroxide and/or a soluble peroxide is present in the
electrolyte solution, greater than 220 Volts, and
(ii) if hydrogen peroxide and/or a soluble peroxide is present in the
electrolyte solution , greater than 210 Volts, and
(B) below that which provides any substantial degree of spark formation on the
magnesium material or its anodising surface as anode and/or plasma discharges
yet is
higher than would otherwise be possible without any substantial degree of
spark formation
on the magnesium material or its anodising surface and/or plasma discharges
were it not
for the ammonia and/or amine presence in the electrolyte solution.
Preferably said amine is capable in alkaline solution of expressing ammonia
gas
or a volatile amine moiety.
Preferably said electrolyte solution includes at least one source of phosphate
ions.
Preferably there is a source of phosphate ions and none of the optional
aluminate
and fluoride ions.
Preferably the anodisation is carned out whilst the electrolyte solution is
below
50°C.
Preferably the voltage limit is in the case of (A) (i) greater than 300 Volts
and
preferably less than 600 Volts, and in the case of (A) (ii) greater than 280
Volts and
preferably less than S50 Volts.
Preferably the aqueous electrolyte solution contains at least 3% w/v ammonia
(when expressed as ammonia gas).
Preferably the aqueous electrolyte solution contains 5% w/v ammonia or above
(when expressed as ammonia gas).
Preferably at least one source of phosphate ions is selected from the group of
phosphoric acid, soluble phosphate salts) and soluble ammonium phosphate(s).
Preferably the phosphate ions have been obtained by adding phosphoric acid to
the
bath thereby forming various phosphate anions by hydrolysis.
Preferably a source of phosphate ions in the range of from 0.01 to 0.2 molar
is
present.
Preferably the source of phosphate ions are present at about 0.05 to about
0.15
molar.
Optionally hydrogen peroxide or a soluble peroxide is present.
T _.__._ ~- .~ __.
CA 02284616 1999-09-23
WO 98/42892 PCT/NZ98/00040
-5-
Optionally the electrolyte solution contains in addition at least one of the
group of
aluminates, silicates, borates, fluorides, phosphates, citrates and phenols.
Preferably the electrolyte solution is free of any substantial presence of
chromium
(III) and chromium (VI).
S Preferably the electrolyte solution contains no alkali salt yielding
hydroxide ions
upon hydrolysis.
In still another aspect the invention consists in a method of anodising
magnesium
based material (ie; magnesium or magnesium alloys) comprising or including
providing an electrolytic solution and wherein the electrolyte, possessing a
pH
greater than 7, comprises or includes in water
(a) {i) ammonia and an amine or (ii) an amine
and
(b) (i) at least one source of phosphate ions, and/or (ii) at least one source
of
aluminate anions and at least one source of fluoride ions;
providing a cathode in or for said solution;
placing magnesium based material as an anode in said solution; and
passing a current between the anode and cathode through said solution so that
an
anodised surface is formed on said material,
wherein
said ammonia and/or amine in said electrolytic solution is provided in
sufficient
quantity to avoid sparks and/or plasma-discharges during the anodisation
process
that cause partial melting or fusion of the anodised surface layer, and
wherein said electrolyte solution comprises or includes water and a source of
phosphate ions provided in the range of .O1 to 0.2 molar, and
wherein
said source of phosphate ions is selected from the group of phosphoric acid or
a
soluble phosphate salt (eg; a sodium hydrogen phosphate, ammonium sodium
hydrogen
phosphate, ammonium dihydrogen phosphate, and diammonium hydrogen phosphate).
Preferably said ammonia comprises at least 1 % w/v of the electrolytic
solution
when expressed as a gas.
Preferably a magnesium or magnesium alloy article anodised by a method of any
one of the preceding claims.
Preferably said magnesium containing article contains not less than 50%
magnesium by weight.
In still a further aspect the invention consists in a method of anodising
magnesium
or magnesium alloys comprising or including operating an anodising system of
an
electrolyte solution, a cathode and the magnesium or magnesium alloy material
as the or
CA 02284616 1999-09-23
WO 98/42892 PCT/NZ98/00040
-6-
an anode, wherein the electrolyte solution contains (i) an amine or amines or
(ii) (a) an
amine or amines and (b) ammonia in solution, in electrical input conditions
which but for
the inclusion of the amines) and any ammonia in the solution would not provide
a
coherent anodised coating (whether owing to so-called "sparking" or
otherwise).
As used herein reference to ammonia presence as the gas by w/v% is after any
neutralisation by any acid moieties.
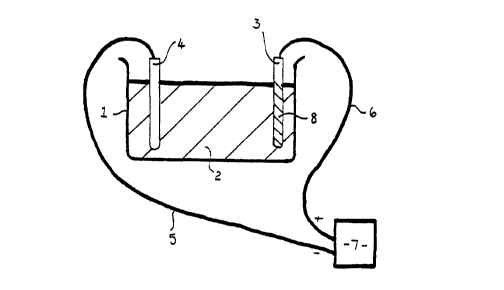
Preferred embodiments of the invention will now be described with reference to
the drawings in which Figure 1 shows a diagrammatic view of an anodisation
bath in
accordance with one embodiment of this invention.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
The method for the anodisation of magnesium containing material {such as
magnesium itself or its alloys) disclosed in WO 96/28591 has been further
investigated
by us. The process has been found to be useful on substantially pure magnesium
samples
as well as magnesium alloys such as AZ91 and AM60 which are common magnesium
alloys used in casting.
The process of WO 96/28591 and also of this invention utilised a bath 1 having
a
solution 2 into which the magnesium containing material 3 may be at least
partially
immersed.
Electrodes 3 and 4 are provided in the bath 1 and into the solution 2, the
solution
2 being an electrolytic solution.
Suitable connections such as cables 5 and 6 are provided from the electrodes 3
and
4 to a power supply 7.
The solution 2 is provided to include ammonia to a suitable concentration. The
concentration of the ammonia in the electrolytic solution 2 may vary. However,
a
preferred range of between 1% and 33% w/v, ammonia (when expressed as the gas)
is
desirable.
As disclosed in WO 96/28591 it has been found that solutions in which the
concentration of ammonia is below 1% w/v tend to cause some sparks to form
with the
method of formation of the coating tending more towards a coating formed
through spark
formation similar to prior art methods of anodisation. A 33% maximum
concentration of
ammonia acts as an upper limit.
We have found the ammonia concentration to work suitably in the region of 5 to
10% w/v or, more preferably, 5 to 7% w/v.
We have found it best when a current from the power supply 7 is passed through
suitable connections (such as cables S and 6) to the electrodes 3 and 4
immersed within
the electrolytic solution 2 when the voltage is in the approximate range of
220 to 250 V
_ __ -_ T _ ___ -_-_ .-___.__ T
CA 02284616 1999-09-23
WO 98/42892 PCT/NZ98/00040
_7_
DC. It should be noted that the prior art anodisation processes prior to the
Barton process
occur between 50 and 150 V DC and, therefore, a reduction of the concentration
of
ammonia below the desired level tends to allow sparks to form through the
process taking
up the properties of the prior art alkaline hydroxide anodisation processes
before the
voltage can reach a level suitable to form the coating in accordance with the
present
invention. Other embodiments can allow the process to operate within the
approximate
range of 170 to 350 V DC.
In a process such as this embodiment, the formation of sparks can occur for a
number of reasons. The ammonia acts to repress sparks generally, but the
concentration
of salts in the bath also has an effect. If the ammonia gets too low, sparks
may form. If
the concentration of phosphate is increased greatly, sparks may occur at
higher voltages,
though the coating may form completely before the voltages are increased to
such a
voltage.
In a further embodiment, peroxide may be added to the electrolytic solution.
The
addition of peroxide has been observed to decrease the voltage at which the
coating forms
without spark formation. For example, a solution of 5% w/v ammonia (expressed
as the
gas), O.OSM sodium ammonium hydrogen phosphate and O.1M sodium peroxide or
hydrogen peroxide produces a coating at 210 V DC very similar to a 300 V DC
coating
formed in the absence of the peroxide. This may be advantageous in
circumstances where
a lower operating voltage is desired.
It has been further observed that decreasing the level of peroxide to O.OSM
produces no significant difference to the coating than the example with no
peroxide.
Further, increasing the peroxide to 0.2M appears to prevent any reasonable
coating being
formed due to the presence of damaging sparks.
On this basis, a further preferred embodiment in which peroxide is added at,
approximately, O.1M may allow lower operating voltages if desired.
Upon application of the current to the electrolytic solution 2, a coating
forms on
the material 3 forming the anode on that portion 8 of the material 3 which is
immersed
within the solution 2. The process itself is, to a large degree, self
terminating with the
current drawn by the anodising bath 1 falling off as the depth of coating on
the portion 8
increases. In this manner, the placement of an article 3 as an anode within
the anodising
bath 1 tends to draw current until the coating is formed and when sufficient
coating exists
to substantially isolate the magnesium in the material 3 from the electrolytic
solution 2,
the current drawn falls and can act as an indicator that the coating has been
applied.
A number of additives may be provided in the solution 2 to alter the final
coating
and its appearance. For example, phosphate compounds may be used to provide a
finish
similar to anodised aluminium and it has been found that phosphate compounds
provided
CA 02284616 1999-09-23
WO 98/42892 PCT/NZ98/00040
_g_
in the range of 0.01 to 0.2 molar can be suitable. Generally a concentration
less than 0.01
molar tends to provide a finish which is somewhat transparent. Concentrations
greater
than 0.2 molar lead to an opaque finish which again alters the appearance of
finished
product. A preferred range of 0.05 to 0.15 molar of a phosphate compound such
as
ammonium sodium hydrogen phosphate has been found to be suitable if it is
desired to
provide a finish similar in appearance to anodised aluminium. The ammonium
phosphate
has been found particularly useful and other ammonium phosphate compounds
could act
as direct substitutes.
Anodisation using the ammonium phosphate compounds give significant corrosion
resistance to the coating. Also the coating is particularly suited to further
coating with
paint or other organic sealers.
In further preferred forms of the invention, the electrolytic solution 2 may
contain
compounds such as ammonium dihydrogen phosphate, or alternatively or
additionally,
diammonium hydrogen phosphate. Both of these compounds may be more readily
available in commercial quantities for the anodisation process compared with
compounds
such as ammonium sodium hydrogen phosphate.
An alternative additive to provide a finish similar to anodised aluminium has
been
found to be the use of fluoride and aluminate in similar concentrations to the
phosphate
compounds. Typical concentrations of compounds such as sodium aluminate and
sodium
fluoride are 0.05 molar of each of these compounds. As the concentration of
sodium
aluminate and sodium fluoride is increased towards 0.1 molar, the finish
changes to a
pearl coloured finish. Although this may be aesthetically pleasing in itself,
it is not
directly comparable with the anodised aluminium finish and, therefore, may be
less
suitable if it is desired to manufacture components for the same product from
the different
materials and be able to provide matching finishes on both aluminium and
magnesium
products.
The process itself is conducted at relatively low currents compared with the
previous anodisation of magnesium processes. The current drawn is in the order
of 100
amps per square metre of magnesium surface. The low current and lack of spark
formation lead to a decrease in the temperature rise within the bath 1 to form
an equivalent
depth of coating compared with the alkaline hydroxide baths used previously.
This
reduction in the temperature rise of the bath leads to a significant decrease
in the cooling
equipment necessary to conduct the process.
Current preferred forms of the invention have been conducted at room
temperature
and it is preferred, although not essential, to conduct the anodisation
process at less than
40°C.
It should be noted that the choice of additives includes a phosphate additive
and/or
__- r __ _ _ ____ ___ ___ __T
CA 02284616 1999-09-23
WO 98/42892 PCT/NZ98/00040
-9-
a fluoride additive. If the fluoride additive is used in substitution for the
phosphate
additive, this leads to greater problems with the disposal of the solution.
Fluoride
compounds are environmentally costly owing to stringent environmental
regulation of
their effluent and disposal. By comparison, the phosphate compounds are less
damaging
to the environment and may be preferred for this reason alone.
The additives may also include sealants or other compounds and many of the
additives used in the previous anodisation processes such as aluminates,
silicates, borates,
fluorides, phosphates, citrates and phenol may be used.
The coating formed on the magnesium may be a mixed coating of magnesium
oxide and magnesium hydroxide with further constituents according to any
particular
additives used in the process. For example, the embodiment in which sodium
ammonium
hydrogen phosphate is provided leads to a magnesium phosphate component in the
coating. Further, the embodiment in which fluoride and aluminate compounds are
provided may lead to the presence of magnesium fluoride and magnesium
aluminate in
1 S the finished coating.
It should further be noted that the use of ammonia in the solution may
necessitate
the use of ventilation in the area about the anodisation bath 1.
The process as defined also tends to provide the coating somewhat faster than
the
prior use of alkaline hydroxide solutions.
A most preferred electrolyte composition where ammonia alone is used is:
ammonia - 3.0-3.3 molar* {usually made up from 25% aqueous solution);
phosphoric acid - 0.1-0.2 molar (alternatively a phosphate salt may be used);
and
a foaming agent - O.lmi per litre of a non-ionic foaming agent.
This bath has a pH of approximately 11.6.
*The ammonia concentration is 3.0 to 3.3 molar after the addition of the
phosphoric acid, hence the ammonia added initially to the bath is slightly
more than this.
The foaming agent ideally has the effect of reducing ammonia loss to the
atmosphere.
The most preferred electrochemical conditions for anodisation with such a
composition comprise:
(I)(i) DC Voltage endpoint- 350V to SOOV depending on desired film thickness;
and optionally:
(ii)(a) AC Voltage set point - zero to 40V; and/or
(ii)(b) Pulsed Voltage set point - zero to 40V; and
(II) Bulk DC current density - 150-400 amps per square metre.
The temperature is in the range from 0°C to 35°C (most
preferably 10-30°C).
The present invention recognises that partial or complete substitution of the
ammonia
CA 02284616 1999-09-23
WO 98/42892 PCT/NZ98/00040
- 10-
by an amine may be made whilst otherwise operating a process as disclosed in
WO
96/28591 but under the conditions previously disclosed for the present
invention.
Simple amines, such as methyl or ethyl amine are volatile so it is recommended
that
any substitution involve a longer chain or more complex amine. Suitable amines
must be
S water soluble at least to a level of 3.0 molar and should feature basicity
similar to that of
ammonia (ability to form hydroxyl, OH- ions in solution). Some examples of
amines that
may be used are diethylene triamine and ethanolamine.
As mentioned above, a different anodising voltage may be used, and this will
most
preferably be from 250V DC upwards, with AC voltage imposed additionally as
may be
required.
The present invention in some preferred forms will now be described by
reference to
examples.
Example 1
An AZ91D magnesium plate was pre-cleaned in a solution containing 0.2 molar
sodium tetraborate and 0.07 molar sodium pyrophosphate. This was then anodised
in an
electrolyte comprising 4.9% ammonia (expressed as w/v NH3) and 0.2 molar
diammonium
hydrogen phosphate at a voltage that peaked at 400V DC at a bulk current
density of 200
amps per square metre. After attainment of 400V, which took just over seven
minutes, the
power supply was cut off and an anodic film of 9 microns was observed on the
sample.
Total cycle time was 7 minutes.
Example 2
An AM50 magnesium component was anodised at 100 amps per square metre, up to
an endpoint voltage of 350V DC. The electrolyte composition was 3% ammonia
(expressed as w/v ammonia gas) and 0.2 molar diammonium hydrogen phosphate.
The
component received a rinse prior to anodisation but no other pretreatment.
Upon
attainment of the endpoint voltage, the power was maintained to the sample and
held at
350V DC for approximately ten minutes. Upon rinsing the sample was found to
have an
anodic film of approximately 17 microns. The cycle time was approximately 30
minutes.
Example 3
An AZ91D magnesium plate was anodised in an electrolyte comprising ammonia at
8% concentration (w/v as ammonia gas) and phosphoric acid at 0.1 molar. The
sample
was pre-cleaned in a bath comprising 0.2 molar sodium tetraborate and 0.07
molar sodium
pyrophosphate at 60°C for five minutes, then it was activated in a bath
comprising 35%
hydrofluoric acid (v/v) for one minute prior to anodisation. The anodisation
was
CA 02284616 1999-09-23
WO 98/42892 PCT/NZ98/00040
-11-
conducted at 200 amps per square metre, using a DC power supply that attained
465V
which was then held for five minutes. A coating of 21.8 microns resulted. The
anodising
cycle required a total of 26 minutes.
Example 4
An AZ91D magnesium plate was anodised in an electrolyte comprising ammonia at
S.0% (expressed w/v as ammonia gas), 0.1 molar phosphoric acid and 0.03 molar
hydrogen peroxide. The plate was pre-cleaned as per example #3 above and
activated as
per example #3 above. It was then anodised using a power supply comprising a
DC
voltage that reached 385V, and an AC voltage which reached 52V. The DC current
density was 280 amps per square metre. while the AC current density peaked at
90 amps
per square metre. The DC endpoint voltage was held for five minutes, then the
sample was
post-treated for two minutes in a bath containing 1.0 molar sodium dihydrogen
phosphate
at 60 ° C. The sample was found to have an anodic coating of 19.7
microns. The anodising
cycle required a total time of 15 minutes.
Example 5
An AZ91D test plate was pre-cleaned in a bath comprising 0.2 molar sodium
tetraborate and 0.07 molar sodium pyrophosphate as in example #3 above. It was
then
anodised in an electrolyte comprising 2.5% ammonia (expressed as ammonia gas)
and 0.5
molar diethylene triamine (DETA), together with phosphoric acid at 0.1 molar,
at a DC
voltage that attained 360V which was held for five minutes. The current
density was 200
amps per square metre. The plate was found to have an anodic coating of 28.2
microns.
The total cycle time was 21 minutes for the anodising process.
Example 6
An AZ91 D test plate was precleaned in the mixture described in example #3
(but not
activated). It was then anodised in a solution comprising 19.8%
monoethanolamine (w/v)
and 0.2 molar sodium dihydrogen phosphate at a DC voltage that attained 350V
which
was held for five minutes. The current density was 200 amps per square metres.
The
sample was found to have an anodic coating of 20.2 microns. The total
anodising cycle
time was 16 minutes 30 seconds.
Note: in the above examples, process times quoted represent anodising times,
not
including pre-cleaning or activation where these are specified, nor any post-
anodisation
treatments.
__ _. ___._._