Note: Descriptions are shown in the official language in which they were submitted.
CA 02284705 1999-09-23
WO 98!42379 PCT/US98105701
CHEMICALLY AND THERMALLY STABLE NORASTEMIZOLE FORMULATIONS
CROSS-REFERENCE TO RELATED APPLICATIONS
This application is a continuation-in-part of
pending Application No. 08/851,786, filed May 6, 1997, which
is a continuation-in-part of pending Application No.
08/824,477, filed March 26, 1997, which are both expressly
incorporated herein by reference thereto in their entirety.
FIELD OF THE INVENTION
The present invention relates to chemically and
thermally stable pharmaceutical compositions containing
norastemizole.
BACKGROUND OF THE INVENTION
Many factors affect the stability of a
pharmaceutical product, including the stability of the
therapeutic drug ingredient(s), the potential interaction
between the therapeutic drug ingredients) and the inactive
ingredient(s), the manufacturing process, the packaging, the
environmental conditions encountered during shipment, storage
and handling, the length of time between manufacture and
usage and the type of the dosage form. In addition to
physical stability, the chemical stability of the
pharmaceutical product should be considered. Knowledge of
the physical and chemical stability of a pharmaceutical
formulation is very important for at least three primary
reasons.
First, a pharmaceutical product, preferably, should
appear fresh, elegant and professional. Any changes in
physical appearance and color including fading, color
variation, appearance of haziness and the like can cause the
patient to lose confidence in the product. Second, since
some products are dispensed in multiple-dose containers,
uniform dosage of the therapeutic agents) over time must be
assured. For example, a non-uniform dosage pattern may be
indicated by a cloudy solution, a broken emulsion, a
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
discolored tablet, a discolored capsule or the like. Third,
the therapeutic drug ingredients) must be available to the
patient throughout the expected shelf life of the dosage
form. A breakdown in the physical or chemical integrity of
the dosage form can lead to a lack of bioavailability or
detrimentally altered bioavailability of the therapeutic drug
ingredient(s).
A variety of pharmaceutical dosage forms are
available for successfully administering many marketed drugs.
Common pharmaceutical dosage forms listed in the U.S.
Pharmacopeia/National Formulary (USP/NF) include, but are not
limited to, aerosols, capsules, cachets, collyria, creams,
emulsions, extracts, fluid extracts, gels, inhalations,
injections, lotions, magmas, milks, ointments, pastes,
pellets or implants, powders, solutions, ophthalmic
solutions, oral solutions, otic solutions, pastilles, topical
solutions, spirits, suppositories, suspensions, sublingual
lozenges, syrups, tablets, tinctures, troches, aromatic
waters and the like. For oral administration, syrups,
solutions, suspensions, troches, tablets and capsules are
preferred. However, for greater ease of administration, for
increased carrying convenience and for improved patient
compliance with a prescribed dosage regimen, troches,
tablets, and hard and soft gelatin capsules are most
preferred. In some cases, tablets are preferred over
capsules because tablets are sometimes easier to swallow.
Troches, tablets and capsules, typically, contain
the drug ingredient, a diluent and other excipients, such as
lubricants and the like, which are well known in the art.
well known excipients include, for example, coating agents,
colorants, desiccants, emulsifying agents, solubilizing
agents, flavors, anti-caking agents, plasticizers, suspending
agents, viscosity increasing agents, binders, diluents,
wetting agents and the like.
Lactose is a commonly used diluent or excipient.
Spray-dried lactose is a commonly available form of lactose
which is widely used as a direct compression excipient.
- 2 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
Since the advent of spray-dried lactose, its use as an
excipient has expanded. The rapid acceptance of spray-dried
lactose is, in part, due to its ease of incorporation in
. direct compression tablets. In this application, spray-dried
lactose is in its ready-to-use form and does not require
further granulation or introduction of complicated processing
steps. Spray-dried lactose can also be readily and
conveniently incorporated into a troche or a capsule dosage
form. Spray-dried lactose may be directly added to a drug to
yield a desired dilution ratio therewith. Thereafter, for
example, the combination of the lactose and the drug may be
dry compressed into a tablet or formulated into a troche or a
capsule with other excipients, as necessary.
Lactose, whether spray-dried or not, is typically
present in equilibrium between its alpha and beta forms,
wherein interconversion between these forms is ongoing.
Alpha-lactose is a disaccharide of beta-D-galactose and
alpha-D-glucose. Beta-lactose is a disaccharide of beta-D-
galactose and beta-D-glucose. Beta-lactose occurs only in
its anhydrous form, whereas alpha-lactose may be obtained
either in anhydrous form or as a monohydrate.
During interconversion between the alpha and beta
forms of lactose, an aldehyde intermediate is formed which is
known to be incompatible with most primary amines. Primary
amines add to the carbonyl carbon of aldehydes (and ketones)
to form imines:
H H
fizp
lan imue>
The incompatibility of most primary amines with lactose is
well-recognized. See, Castello et al., J. Pharm. Sci., 51
(2):106-108 (Feb. 1962). See also, Blaug et al., J. Pharm.
Sci., 61(11):1770-1775 (Nov. 1972); Hartauer et al., Drug
Dev. and Indust. Pharm., 17(4):617-630 (1991).
Castello et al. tested the compatibility of
amphetamine sulfate (a primary amine salt) with lactose.
- 3 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
They found that a mixture of lactose and amphetamine sulfate
became discolored, especially in the presence of alkaline
lubricants such as magnesium stearate. Blaug et al. tested
dextroamphetamine sulfate (a primary amine salt) with spray-
s dried lactose. They found that the lactose formed a Schiff
base (i.e., an imine) in the presence of dextroamphetamine
sulfate. Hartauer et al. tested aminophylline with lactose,
and found that some incompatibility, evidenced by
discoloration, between aminophylline and lactose occurred,
especially when heat, of about 60°C, was applied.
Aminophylline contains a ratio of two molecules of
theophylline (a secondary amine) for one molecule of ethylene
diamine (a primary amine). However, Hartauer et al. tested
these components and found that while theophylline alone (a
secondary amine) did not react with lactose in the presence
or absence of heating to 60°C, ethylene diamine did react
with the lactose, especially when heated to 60°C. Thus, the
incompatibility of aminophylline with lactose appeared to
result from incompatibility of the primary amine component of
aminophylline, ethylenediamine, with lactose.
The drug astemizole, a secondary amine, appears to
be compatible with lactose, as it is commercially available
as HISMANAL~ in a tablet dosage form containing lactose.
According to the Physician's Desk Reference, 50th Edition,
Medical Economics Co., Montvale, NJ, p. 1293 (1996), each
tablet of Hismanal~' contains 10 mg astemizole, lactose,
cornstarch, microcrystalline cellulose, pre-gelatinized
starch, povidone K90, magnesium stearate, colloidal silicon
dioxide and sodium lauryl sulfate.
Likewise, it is expected that norastemizole,
another secondary amine and the primary metabolite of
astemizole, should be compatible with lactose, especially in
the absence of applied heat. Norastemizole has been reported
to be both more potent and less toxic than astemizole. Thus,
norastemizole is an attractive alternative to astemizole for
the treatment of allergic disorders. It should be recognized
that both astemizole and norastemizole are antihistamines
- 4 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
containing secondary amine moieties; however, norastemizole
has two secondary amine moieties, whereas astemizole has one.
SZTMMARY OF THE INVENTION
The present invention relates to stable
pharmaceutical dosage forms of norastemizole that avoid the
incompatibility between norastemizole and lactose. In one
aspect, the present invention relates to a lactose-free
pharmaceutical composition which includes norastemizole, or a
pharmaceutically acceptable salt thereof, and at least one
non-lactose pharmaceutically acceptable excipient. In
another embodiment, the invention relates to a solid
pharmaceutical composition that includes norastemizole, or a
pharmaceutically acceptable salt thereof, and at least one
pharmaceutically acceptable excipient, wherein said excipient
is not lactose.
In one preferred embodiment, at least one non-
lactose pharmaceutically acceptable excipient is a binder, a
filler, or mixtures thereof. In another preferred
embodiment, at least one pharmaceutical excipient is a
binder, a filler, or mixtures thereof. In a more preferred
embodiment, the above excipients further include a lubricant,
a disintegrant, or mixtures thereof. In a more preferred
embodiment, the excipients are croscarmellose,
microcrystalline cellulose, pre-gelatinized starch, and
magnesium stearate. In a preferred embodiment, the
disintegrant is a super disintegrant. In another embodiment,
the pharmaceutical composition is substantially free of all
mono- or di-saccharide excipients.
The invention also relates to a thermally stable
solid pharmaceutical composition free of lactose comprising
norastemizole, or a pharmaceutically acceptable salt thereof,
and at least one pharmaceutically acceptable excipient. In
another embodiment, the invention relates to a chemically
stable solid pharmaceutical composition free of lactose which
includes about 1% to about 50% by weight of norastemizole, or
a pharmaceutically acceptable salt, and about 99% to about
- 5 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98I05701
50% by weight of at least one pharmaceutically acceptable
excipient.
In a second embodiment, the invention encompasses
non-hygroscopic pharmaceutical compositions comprising
norastemizole, or a pharmaceutically acceptable salt thereof,
and at least one pharmaceutically acceptable excipient. Non-
hygroscopic pharmaceutical compositions of this invention may
contain pharmaceutically acceptable excipients that are
substantially free of unbound water, i.e., water available to
participate in norastemizole/excipient interactions, such as,
but not limited to, any interactions between lactose and
norastemizole. The present invention also provides
chemically and thermally stable non-hygroscopic
pharmaceutical compositions comprising norastemizole and at
least one pharmaceutically acceptable excipient, wherein said
excipient can include lactose or other mono- or di-
saccharides.
In other words, the norastemizole compositions of
the present invention are (a) substantially free of lactose
(and preferably substantially free of mono- or di
saccharide), (b) include excipients substantially free of
unbound water, which excipients may include lactose, such as
alpha-lactose monohydrate or other mono- or di-saccharides,
or (c) contain large particles or particles coated with an
inert agent, along with excipients that may include lactose,
such as alpha-lactose monohydrate or other mono- or di-
saccharides. In any case, Applicants have discovered highly
stable norastemizole formulations. In addition, it should be
noted that the compositions of the invention which are non-
hygroscopic may nevertheless include some hygroscopic
ingredients; however, the composition overall must be
substantially non-hygroscopic. Further, the non-hygroscopic
pharmaceutical compositions of the present invention may also
utilize hydrated ingredients.
In yet another embodiment, the present invention
encompasses anhydrous pharmaceutical compositions, said
compositions comprising norastemizole, or a pharmaceutically
- 6 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
acceptable salt thereof, and one or more pharmaceutically
acceptable carriers or excipients, which may include lactose.
Such compositions may be prepared using anhydrous or low
moisture containing ingredients using low moisture or low
humidity conditions such that the resulting pharmaceutical
composition is substantially anhydrous. Further, the present
invention provides chemically and thermally stable anhydrous
pharmaceutical compositions comprising norastemizole and at
least one pharmaceutically acceptable excipient, wherein said
excipient can include lactose or other mono- or di-
saccharides.
The invention also encompasses pharmaceutical
compositions for the treatment of histamine-induced disorders
comprising large particles of norastemizole, or a
pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable excipient. The present invention
also provides chemically and thermally stable pharmaceutical
compositions having large particles of norastemizole and at
least one pharmaceutically acceptable excipient, wherein said
excipient can include lactose or other mono- or di-
saccharides.
In a preferred embodiment, about 40 weight percent
or more of the large particles of norastemizole, or
pharmaceutically acceptable salt thereof, comprises particles
having a size of 200 ~Cm or larger. In one embodiment, the
large particle pharmaceutical composition may include lactose
as a pharmaceutically acceptable excipient.
The invention also encompasses solid pharmaceutical
compositions for the treatment of histamine-induced disorders
comprising a therapeutically effective amount of coated
norastemizole, or a pharmaceutically acceptable salt thereof,
which comprises norastemizole, or a pharmaceutically
acceptable salt thereof, coated with an inert coating agent,
and a pharmaceutically acceptable excipient. The present
invention further provides chemically and thermally stable
pharmaceutical formulations of coated norastemizole that
avoid the incompatibility between norastemizole and lactose,
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
wherein said excipient can include lactose or other mono- or
di-saccharides.
In one embodiment, the excipient comprises lactose.
In another embodiment, the coated norastemizole, or a
pharmaceutically acceptable salt thereof, further comprises a
granulated formulation of norastemizole, or a
pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable inert excipient, wherein said
granulated formulation is coated with an inert coating agent.
In a preferred embodiment, the inert coating agent comprises
an inert film-forming agent in a solvent. In a more
preferred embodiment, the inert film-forming agent is
selected from the group consisting of methylcellulose,
hydroxymethyl cellulose, carboxymethyl cellulose,
hydroxypropylmethylcellulose, hydroxypropyl cellulose,
hydroxyethylcellulose, methylhydroxyethylcelluose, sodium
carboxymethylcellulose, and mixtures thereof.
In one embodiment, norastemizole is present in an
amount from about 1 mg to about 200 mg. In a more preferred
embodiment, norastemizole is present in an amount of about 2
mg to about 100 mg. In another preferred embodiment,
norastemizole is present in a therapeutically effective
amount for treatment of an allergic disorder. In yet another
preferred embodiment, the therapeutically effective amount is
sufficient for the prophylaxis or treatment in humans of an
allergic disorder.
The invention also relates to a solid
pharmaceutical composition that includes norastemizole or a
pharmaceutically acceptable salt thereof, microcrystalline
cellulose, pre-gelatinized starch, magnesium stearate, and
croscarmellose sodium. In one embodiment, the solid
pharmaceutical composition is provided in a tablet or a
capsule dosage form.
The invention also relates to a method for treating
at least one allergic disorder in a mammal by administering a
therapeutically effective amount of one of the above
compositions. In a preferred embodiment, the mammal is a
_ g _
CA 02284705 1999-09-23
WO 98!42379 PCT/US98/05701
human. In a more preferred embodiment, the allergic disorder
is allergic rhinitis.
BRIEF DESCRIPTION OF THE DRAWINGS
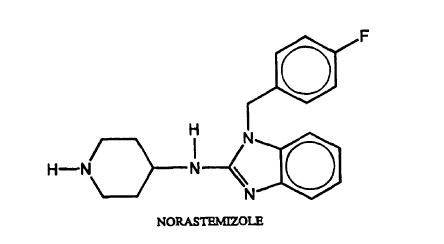
Figure 1 depicts the chemical structure of
norastemizole.
Figure 2 presents in bar-graph format the change in
initial potency of a dosage form of norastemizole and various
pharmaceutical excipients when the dosage form is exposed to
a temperature of 60°C at 75% relative humidity using non
hermetically sealed containers (i.e., screw-top vials).
DETAILED DESCRIPTION OF THE INVENTION
Applicants have discovered that, even in the
absence of applied heat, surprisingly, the discoloration
reaction found with primary amines and lactose is also found
with norastemizole. Thus, there appears to be an heretofore
unappreciated incompatibility between the secondary amine,
norastemizole, and lactose. It is, therefore, desirable to
formulate dosage forms of norastemizole that are lactose-
free. Further, Applicants have also discovered that the
instability of lactose and norastemizole may be initiated
and/or accelerated upon the exposure of a
norastemizole/lactose formulation to water, including
atmospheric moisture, e.g., humidity. The instability is
also initiated and/or accelerated upon exposure to heat at
temperatures of greater than about 60°C. Moreover,
Applicants have also discovered that the instability of
lactose and norastemizole may be initiated and/or accelerated
by the high surface area of the small particles of
norastemizole conventionally used in pharmaceutical
compositions upon the exposure of a norastemizole/lactose
formulation. Additionally, Applicants have also discovered
that the instability of lactose and norastemizole may be
inhibited or avoided by coating norastemizole particles prior
to formulation of the norastemizole with reactive excipients,
such as lactose.
_ g _
CA 02284705 1999-09-23
WO 98/42379 PCTIU598/05701
In PCT application PCT/US93/08349, published as WO
94/07495, a formulation of norastemizole is proposed in
Example 4, which happens to lack lactose. Formulas A, B and
C, of Example 4, each contain 1.0 weight percent of magnesium
stearate BP, 94.0, 89.0 and 79.0 weight percent of Starch
1500 (a pre-gelatinized starch commercially available from
Colorcon, Ltd.), respectively, and the remainder of the
composition is a metabolite of astemizole (e. g.,
norastemizole). However, in practice, one would not prepare
or utilize the lactose-free formulations of Example 4 because
the magnesium stearate BP and Starch 1500 are incompatible in
the weight percents described. In other words, the
formulations of Example 4 in this PCT publication are
unsuitable for actual pharmaceutical use. Moreover, this
publication neither discloses nor suggests that norastemizole
and lactose are incompatible, as evidenced by the lactose-
containing tablet formulation of norastemizole in Example 5
therein.
In view of the heretofore unappreciated problems
associated with pharmaceutical formulations including the
secondary amine, norastemizole, and lactose, it is desired to
prepare stable solid pharmaceutical formulations of
norastemizole that avoid the incompatibility between
norastemizole and lactose. The present invention
advantageously recognizes and provides lactose-free dosage
formulations of norastemizole.
Based upon the pharmacological benefits of
norastemizole over astemizole, there is a need for stable,
high performance dosage forms of norastemizole. To date,
there is no commercially available stable norastemizole
formulation. However, the inventors have found that by
eliminating lactose and using the alternative ingredients
described herein, lactose-free dosage forms of norastemizole
are surprisingly chemically, physically and thermally stable.
This stability may be achieved by the present invention
without loss of either manufacturing ease or dosage
performance.
- 10 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98105701
One feature of the present invention is thus
directed to chemically and thermally stable pharmaceutical
formulations that include norastemizole, or a
pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable carrier or excipient that does
not include or utilize any form of lactose. Lactose has been
widely accepted and used by the pharmaceutical industry,
inter alia, because of its ease of manufacture. However,
Applicants have advantageously found that formulations
containing norastemizole and lactose are unstable over time
and degrade more rapidly upon exposure to heat and moisture.
Secondary amines were previously considered to be
compatible with lactose, especially at ambient temperatures
or where exposure to heat (e. g., below about 60°C) is either
minimal or altogether avoided. As noted, for example, the
drug astemizole is available in a tablet dosage form
containing lactose and other excipients under the tradename
Hismanal~" .
It has now been discovered that physical and/or
chemical incompatibility exists between the secondary amine,
norastemizole, and lactose. Without being limited by theory,
it is believed that the incompatibility of norastemizole with
lactose results from the formation of enamines due to
reaction between the aldehyde intermediate of lactose and a
secondary amine:
c c H=o + t-lrr H ~ > c=c ~-H ~ + H 2 0
(an enamine)
It has also been discovered that the
incompatibility exists even at ambient temperatures (e. g.,
temperatures below about 60°C) and at ambient relative
humidity. Further, Applicants have also discovered highly
stable pharmaceutical compositions containing norastemizole
without the use of the widely accepted excipient lactose.
According to one feature of the present invention,
norastemizole is provided in lactose-free pharmaceutical
- L1 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98I05701
compositions. These compositions possess potent
antihistaminic activity and are useful in treating a variety
of conditions. Some of these conditions include, for
example, allergic rhinitis, asthma and other allergic
disorders, vertigo, motion sickness, vestibular disturbances
(e. g., Meniere's disease), diabetic retinopathy, other small
vessel disorders associated with diabetes melitis.
More importantly, these lactose-free compositions
provide a stable and convenient dosage form for delivering
norastemizole to humans. The lactose-free compositions of
the invention are stable, inter alia, in that they have
significant shelf-life. Further, the compositions of the
invention remain stable even when exposed to mild temperature
and humidity changes. Moreover, even though the compositions
of the invention are lactose-free, the compositions are still
easily manufactured, and the compositions have desirable
dosage performance properties. The compositions of the
invention include solid unit dose formulations comprising
norastemizole, or a pharmaceutically acceptable salt thereof,
and at least one non-lactose pharmaceutically acceptable
excipient. The compositions may also optionally include
other therapeutic ingredients, binders/fillers,
disintegrants, lubricants, anti-caking agents, preservatives,
film coating agents, sweetening agents, colorants, flavors,
desiccants, plasticizers, dyes, dispersing agents and/or
surface active agents. However, any such optional ingredient
must be compatible with norastemizole, a secondary amine, to
insure the stability of the formulation.
It is preferred that the lactose-free dosage form
of norastemizole made in accordance with the present
invention comprise norastemizole and at least one non-lactose
excipient. Examples of such excipients are well known in the
art and are listed in the USP (XXI)/NF (XVI), incorporated
herein in its entirety by reference thereto. It is further
preferred that the lactose-free norastemizole dosage forms
made in accordance with the present invention comprise
norastemizole, a binder/filler and a lubricant in
- 12 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
pharmaceutically compatible and pharmaceutically acceptable
amounts. It is even further preferred that the lactose-free
norastemizole dosage forms made in accordance with the
present invention comprise norastemizole, microcrystalline
cellulose, pre-gelatinized starch, and magnesium stearate.
It has also been discovered that other sugars, such
as fructose and sucrose, cause similar, although not as
severe, degradation to that caused by lactose when used in
combination with norastemizole containing formulations.
Thus, in another embodiment, the lactose-free pharmaceutical
compositions comprise norastemizole, or a pharmaceutically
acceptable salt thereof, and at least one non-lactose
pharmaceutically acceptable excipient, and do not contain any
mono- or di-saccharide excipients, including, but not limited
to, glucose, sucrose, and fructose.
As mentioned above, norastemizole formulations
containing lactose that are exposed to unbound water, e.g.,
moisture or humidity, degrade more rapidly. The addition of
water (e. g., 5%) is widely accepted in the pharmaceutical
arts as a means of simulating long-term storage in order to
determine characteristics such as shelf-life or the stability
of formulations over time. See, e.g., Jens T. Carstensen,
Drug Stability: Principles & Practice, 2d. Ed., Marcel
Dekker, NY, NY, 1995, pp. 379-80. In effect, water and
temperature accelerate the study.
Further, the effect of water on a formulation is of
great significance since conditions favorable for
hygroscopicity, e.g., moisture and/or humidity, are commonly
encountered during manufacture, handling, packaging, storage,
shipment and use of the formulation. Thus, it is clear that
the use of lactose in pharmaceutical compositions or
formulations containing norastemizole should be avoided due
to the substantial contact with moisture and/or humidity that
the compositions have under normal manufacturing, packaging
and storage conditions.
Moreover, although excipients other than lactose
may be readily used to manufacture the disclosed lactose-free
- 13 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
pharmaceutical compositions of norastemizole without
impacting on the manufacturability and therapeutic
performance of the compositions, spray-dried lactose
continues to be an excipient of choice. In the spray-dried
form, lactose is among the best of all direct compression
fillers in fluidity and is very effective for low dose
formulations (e.g., < 50 mg per dose) where the
compactibility of the active ingredient does not play a major
role in the formulation. See, e.g., R. Shangraw, Selection
of Manufacturing Process and Excipients with an Emphasis on
Direct Compression, Course material from Granulation,
Tableting, and Capsule Technology, Center for Professional
Advancement, East Brunswick, NJ, 1996. Therefore, when
possible, it is desirable to include lactose among the
available possible excipients for the solid dosage forms or
pharmaceutical composition of norastemizole.
Therefore, as an alternative, the present invention
encompasses thermally and chemically stable pharmaceutical
compositions, particularly, solid pharmaceutical
formulations, which comprise norastemizole, or a
pharmaceutically acceptable salt thereof, and optionally one
or more pharmaceutically acceptable excipients, including but
not limited to lactose, wherein the lactose containing
formulations are anhydrous, i.e., substantially free of
unbound water.
The invention further encompasses thermally and
chemically stable non-hygroscopic pharmaceutical compositions
which comprise norastemizole, or a pharmaceutically
acceptable salt thereof, and one or more excipients or
ingredients including, but not limited to, lactose. Without
being limited by any theory, these stable anhydrous or non-
hygroscopic pharmaceutical compositions are based, in part,
on Applicants' discovery that the incompatibility between
norastemizole and lactose, or other mono-or di-saccharides,
is accelerated and/or possibly initiated by exposure of such
formulations to unbound water. Thus, preparing
pharmaceutical compositions that are substantially free of
- I4 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
unbound water will prevent the accelerated degradation of
norastemizole that occurs when a reactive excipient is used
and unbound water is present.
Thus, if lactose is a desired excipient, another
aspect of the invention relates to non-hygroscopic or
anhydrous pharmaceutical compositions comprising
norastemizole, lactose and optionally one or more additional
excipients or ingredients wherein the resulting
pharmaceutical compositions are substantially free of unbound
water. It should be recognized that the non-hygroscopic or
anhydrous formulations can be made by standard methods,
provided that suitable excipients are selected such that the
resulting pharmaceutical compositions are substantially free
of unbound water, and processing is conducted using
conditions of low humidity.
Anhydrous norastemizole pharmaceutical composition
prepared in accordance with the present invention should be
prepared and stored such that the anhydrous nature is
maintained. Accordingly, these compositions will be packaged
using materials well known in the art for preventing exposure
of the pharmaceutical composition to water, allowing them to
be included in suitable formulary kits. Such packaging will
include, but not be limited to, hermetically sealed foils,
plastic or the like, unit dose containers, blister packs, or
strip packs.
Accordingly, a second alternative aspect of the
invention encompasses a method of preparing a solid
pharmaceutical formulation comprising norastemizole and
lactose which method comprises admixing under anhydrous or
low moisture/humidity conditions, norastemizole, or a
pharmaceutically acceptable salt thereof, and lactose wherein
said ingredients are substantially free of unbound water.
The method may optionally further comprise packaging said
anhydrous or non-hygroscopic solid norastemizole formulation
under low moisture conditions. By using such conditions, the
risk of contact with water is reduced and the degradation of
norastemizole is prevented or substantially reduced during
- 15 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
processing and storage. Further, the final packaged product
has little or no unbound water present which substantially
improves stability and prevents degradation. Such
compositions can be provided in hermetically sealed packages
such as vials, sealed packets, blister packs and other vacuum
sealed and moisture free containers well known to the skilled
artisan.
Traditionally, when pharmaceutical compositions or
formulations are prepared, the active ingredient or
therapeutic agent (e. g., norastemizole) is milled and/or
screened to decrease the particle size and/or narrow the
particle size distribution. Most often, this is done in
order to optimize various physicochemical characteristics of
the formulation, such as dissolution, content uniformity,
bioavailability of the active ingredient, and the like.
Dissolution is of particular concern with norastemizole,
since the solubility is relatively low (approximately 10
mg/mL) at pH 3-4 and lower above pH 4. Without being limited
by any particular theory, however, Applicants believe that
the interaction between norastemizole and reactive
excipients, such as lactose, may be affected by the surface
area of the norastemizole particles in the pharmaceutical
composition or formulation.
Accordingly, another embodiment of the present
invention encompasses pharmaceutical compositions for the
treatment of histamine-induced disorders which comprise large
particles of norastemizole, or pharmaceutically acceptable
salts thereof, and a pharmaceutically acceptable carrier.
Pharmaceutically acceptable carriers suitable for use in
these compositions include carriers that may comprise one or
more excipients selected from the group consisting of inert
excipients and reactive excipients, such as lactose or other
mono- or di-saccharides. These "large particle"
pharmaceutical compositions of norastemizole have suitable
physicochemical characteristics (in terms of dissolution,
content uniformity, bioavailability, and the like), but do
- 16 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/057a1
not exhibit incompatibility with reactive recipients, such as
lactose.
In a preferred embodiment, the norastemizole, or a
pharmaceutically acceptable salt thereof, present in the
composition has a particle size distribution in which about
40% by weight or more of norastemizole, or a pharmaceutically
acceptable salt thereof, comprises particles having a size of
200 ~m or larger, preferably greater than about 250 ~Cm.
Another means for inhibiting or preventing the
interaction between norastemizole and reactive excipients,
such as lactose, in a pharmaceutical composition is to
prevent norastemizole from coming into contact with any
reactive excipients in the composition. One manner in which
this may be achieved is to coat the norastemizole particles
with an inert or non-reactive coating prior to formulation
with reactive excipients. Preferably, the inert coating
should not significantly influence the pharmacodynamic
characteristics (e.g., time to onset of efficacy, and
absorption in vivo) of the composition.
Accordingly, another embodiment of the present
invention relates to solid pharmaceutical compositions for
the treatment of histamine-induced disorders comprising a
therapeutically effective amount of coated norastemizole, or
a pharmaceutically acceptable salt thereof, which comprises
norastemizole, or a pharmaceutically acceptable salt thereof,
coated with an inert coating agent, and a pharmaceutically
acceptable carrier. In a preferred embodiment, the
norastemizole, or a pharmaceutically acceptable salt thereof,
is first granulated with an inert excipient (e. g., starch),
and then the resulting granules are coated with an inert or
non-reactive coating agent. Thereafter, the resulting coated
norastemizole may be blended with other excipients, including
reactive excipients.
Suitable inert coating agents, and methods for
coating particles or granules, are well known in the art.
Inert coating agents typically comprise an inert film-forming
agent dispersed in a suitable solvent, and may further
- 17 -
CA 02284705 1999-09-23
WO 98/42379 PCTIUS98/05701
comprise other pharmaceutically acceptable adjuvants, such as
colorants and plasticizers. Preferably, the particles or
granules of norastemizole are coated using aqueous or non-
aqueous film coating techniques or microencapsulation.
Suitable inert film-forming agents include, but are not
limited to, celluloses, such as methylcellulose,
hydroxymethyl cellulose, carboxymethycellulose, hydroxypropyl
methylcellulose, hydroxypropyl cellulose,
hydroxyethylcellulose, methylhydroxyethylcellulose, and
sodium carboxymethyl cellulose; vinyls, such as polyvinyl
pyrrolidione; glycols, such as polyethylene glycols;
acrylics, such as dimethylaminoethyl methacrylate-
methacrylate acid ester copolymer, and ethylacrylate-
methylmethacrylate copolymer; and other carbohydrate
polymers, such as maltodextrins, and polydextrose.
Preferably, the inert coating agent contains a hydrophilic
film-forming agent, such as hydroxypropyl methylcellulose, so
that absorption in vivo is not significantly delayed.
Once the particles or granulated formulations of
norastemizole are coated with the inert coating agent, the
coated norastemizole may be formulated using standard
techniques, including, but not limited to, blending,
granulation, compression, or combinations thereof, with other
inert and/or reactive excipients, such as lactose, to make
various dosage forms, such as tablets, caplets, capsules,
troches, and the like.
The preferred amount of norastemizole in all the
dosage forms made in accordance with the present invention
should be a therapeutically effective amount thereof, which
is also a medically acceptable amount thereof. Actual dosage
levels of norastemizole in the pharmaceutical compositions of
the present invention may be varied so as to obtain an amount
of norastemizole which is effective to achieve the desired
therapeutic response for a particular patient, pharmaceutical
composition of norastemizole, and mode of administration,
without being toxic to the patient.
- 18 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
The selected dosage level and frequency of
administration of the pharmaceutical compositions of the
invention will depend upon a variety of factors including the
route of administration, the time of administration, the rate
of excretion of the therapeutic agents) including
norastemizole, the duration of the treatment, other drugs,
compounds and/or materials used in combination with
norastemizole, the age, sex, weight, condition, general
health and prior medical history of the patient being
treated, and like factors well known in the medical arts.
For example, the dosage regimen is likely to vary with
pregnant women, nursing mothers and children relative to
healthy adults.
A physician having ordinary skill in the art can
readily determine and prescribe the therapeutically effective
amount of the pharmaceutical composition required. For
example, the physician could start doses of norastemizole
employed in the pharmaceutical composition of the present
invention at levels lower than that required to achieve the
desired therapeutic effect and gradually increase the dosage
until the desired effect is achieved.
A suitable daily dose of norastemizole will be that
amount of norastemizole which is the lowest effective dose to
produce a desired therapeutic effect. Such a therapeutically
effective dose will generally depend upon the factors
described above. For example, the unit dose of lactose-free
norastemizole may contain from about 1 mg to about 200 mg and
preferably about 2 mg to about 100 mg. For example, unit
dosages may be formulated with 2.5 mg, 5 mg, 10 mg, 12.5 mg,
15 mg, 20 mg, 25 mg, 30 mg, 35 mg, 40 mg, 45 mg, 50 mg, or
62.5 mg of norastemizole. If desired, the effective daily
dose of norastemizole may be administered separately at
appropriate intervals throughout the day, optionally, in unit
dosage forms as two, three, four, five, six or more sub-
doses. As previously noted, the preferred dosage forms are
tablets, caplets, troches, pastilles, pills, lozenges,
syrups, capsules and the like. However, other
- 19 -
CA 02284705 1999-09-23
WO 98142379 PCT/US98/05701
pharmaceutically acceptable dosage forms such as powders,
granules, dragees and the like may be used.
It is noted that all components comprising the
dosage forms of norastemizole made in accordance with the
present invention preferably meet or exceed the standards for
pharmaceutical ingredients and combinations thereof in the
USP/NF. The purpose of the USP/NF is to provide
authoritative standards and specifications for materials and
substances and their preparations that are used in the
practice of the healing arts. The USP/NF establish titles,
definitions, descriptions, and standards for identity,
quality, strength, purity, packaging and labeling, and also,
where practicable provide bioavailability, stability,
procedures for proper handling and storage and methods for
their examination and formulas for their manufacture or
preparation.
The lactose-free, non-hygroscopic, anhydrous, large
particle, and coated dosage forms of norastemizole described
herein and claimed meet the pharmaceutical standards set
forth in the USP/NF (e.g., USP XXI/NF XVI) for each of the
ingredients as well as the lactose-free, non-hygroscopic,
anhydrous, large particle, or coated norastemizole dosage
forms made with such ingredients. In effect, the lactose-
free, non-hygroscopic, anhydrous, large particle, or coated
dosage forms of norastemizole are said to be pharmaceutically
acceptable dosage forms made of pharmaceutically acceptable
ingredients in pharmaceutically acceptable combinations and
pharmaceutically acceptable amounts to at least meet the
standards set forth in the USP XXI/NF XVI, incorporated
herein in its entirety by reference thereto. In addition, it
should be noted that norastemizole can be made according to
methods known in the art, including those disclosed in
copending U.S. Application No. 08/182,685, filed January 18,
1994, which is incorporated herein by reference thereto for
the express purpose of teaching methods to prepare
norastemizole.
- 20 -
CA 02284705 1999-09-23
WO 98/42379 PCTIUS98105701
Stability of a pharmaceutical product may be
defined as the capability of a particular formulation, in a
specific container, to remain within its physical, chemical,
microbiological, therapeutic and toxicological specification,
although there are exceptions, and to maintain at least about
90% of labeled potency level. Thus, for example, expiration
dating is defined as the time in which the pharmaceutical
product will remain stable when stored under recommended
conditions.
Many factors affect the stability of a
pharmaceutical product, including the stability of the
therapeutic ingredient(s), the potential interaction between
therapeutic and inactive ingredients) (e. g., norastemizole
and excipients) and the like. Physical factors such as heat,
light and moisture may initiate or accelerate chemical
reactions.
For convenience, certain terms employed herein are
defined as follows. The term "carrier" as used herein is
synonymous with the term "vehicle." The term "lactose-free"
as used herein is intended to mean that the amount of lactose
present, if any, in the dosage form of norastemizole is
insufficient to cause the incompatibility between
norastemizole and lactose discovered by the inventors to
detrimentally affect the potency of the norastemizole below
about 90% of initial potency over the shelf life of the
dosage form. The term "unbound water" as used herein means
water that is not present in the form of a stable hydrate of
one or more components of the pharmaceutical composition,
e.g., alpha lactose monohydrate. Similarly, the term
"anhydrous" as used herein means the amount of unbound water
present, if any, in the dosage form of norastemizole is
insufficient to initiate and/or accelerate the
incompatibility between norastemizole and lactose. Further,
"anhydrous," "anhydrous conditions" or "anhydrous nature~~ as
used herein means substantially free of unbound water
including moisture. The term "non-hygroscopic~~ as used
herein means the overall formulation is substantially non-
- 21 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98105701
hygroscopic, i.e., does not provide unbound water sufficient
to initiate and/or accelerate the incompatibility between
norastemizole and reactive excipients, such as lactose. The
term "additives" is synonymous with the term "excipients" as
used herein. The term "substantially free" means less than
about 5 weight percent, preferably less than about 1 weight
percent, and more preferably less than about 0.1 weight
percent. The term "large particle" as used herein means a
composition wherein the norastemizole includes about 40
weight percent or more of particles of norastemizole, or a
pharmaceutically acceptable salt thereof, having a size of
200 um or larger, preferably greater than about 250 ~Cm. The
terms "coated," "inert coating," or "inertly coated" as used
herein preferably means an inert coating agent used to coat
norastemizole particles and inhibit the interaction of the
particles with reactive excipients, such as lactose.
Although non-inert coatings suitable for use in conventional
pharmaceutical applications are also suitable for use with
the lactose-free, non-hygroscopic, anhydrous, and large
particle formulations of the invention, it is preferred that
any coating used be inert and inhibit the interaction of
norastemizole with any reactive excipients.
The term "pharmaceutically acceptable" is used
herein to refer to those compounds, materials, compositions
and/or dosage forms which are, within the scope of sound
medical judgment, suitable for administration to and for use
in contact with the tissues and fluids of human beings and
animals without excessive toxicity, irritation, allergic
response, or other problem or complication, commensurate with
a reasonable medically sound benefit/risk ratio.
Further, the term "pharmaceutically acceptable"
excipient is employed to mean that there are no untoward
chemical or physical incompatibilities between norastemizole
(or a salt thereof) and any of the excipient components of a
given dosage form. For example, an untoward chemical
reaction is one wherein the potency of the norastemizole (or
salt thereof) is detrimentally reduced or increased due to
- 22 -
CA 02284705 1999-09-23
WO 98!42379 PCT/US98/05701
the addition of one or more excipients. Another example of
an untoward chemical reaction is one wherein the taste of the
norastemizole (or salt thereof) dosage form becomes
excessively sweet, sour or the like to the extent that the
S dosage form becomes unpalatable. Each excipient must be
"acceptable~~ in the sense of being compatible with the other
ingredients of the norastemizole formulation and not
injurious to the patient.
Physical incompatibility refers to incompatibility
among the various components of the dosage form such as
norastemizole (or salt thereof) and any of the excipient(s)
thereof. For example, the combination of the excipient(s)
and norastemizole may form an excessively hygroscopic mixture
or an excessively segregated mixture to the degree that the
desired shape of the dosage form (e. g., tablet, troche etc.),
its stability or the like cannot be sufficiently maintained
to be able to administer the dosage form in compliance with a
prescribed dosage regimen as desired.
Most often, antihistamines, such as astemizole or
norastemizole, are administered orally by means of solid
dosage forms such as tablets, capsules, troches, caplets and
the like. Further, capsule dosage forms such as hard gelatin
capsules, soft gelatin capsules and the like may also be
used. However, tablets remain a preferred dosage form
because of the advantages afforded both to the patient (e. g.,
accuracy of dosage, compactness, portability, blandness of
taste as well as ease of administration) and to the
manufacturer (e. g., simplicity and economy of preparation,
stability as well as convenience in packaging, shipping and
dispensing). Tablets are solid pharmaceutical dosage forms
containing therapeutic drug substances with or without
suitable additives.
In order for medicinal substances or therapeutic
ingredients of the present invention (i.e., lactose-free,
non-hygroscopic, anhydrous, large particle, or coated
norastemizole dosage forms), with or without diluents, to be
made into solid dosage forms (e. g., tablets) with pressure,
- 23 -
CA 02284705 1999-09-23
WO 98/42379 PCTIUS98/05701
using available equipment, it is necessary that the material,
either in crystalline or powdered form, possess a number of
physical characteristics. These characteristics include, for
example, the ability to flow freely, as a powder to cohere
upon compaction, and to be easily released from tooling.
Since most materials have none or only some of these
properties, methods of tablet formulation and preparation
have been developed to impart these desirable characteristics
to the material which is to be compressed into a tablet or
similar dosage form.
As noted, in addition to the drug or therapeutic
ingredient, tablets and similar dosage forms may contain a
number of materials referred to as additives. These
additives are classified according to the role they play in
the formulation of the dosage form such as a tablet, a
caplet, a capsule, a troche or the like. One group of
additives include, but are not limited to, binders, diluents
(fillers), disintegrants and lubricants.
While the discussion below of various additives for
use in the present invention specifically refers to lactose-
free dosage forms, the skilled artisan will readily
understand that a subset of each category includes additives
suitable for use in non-hygroscopic, anhydrous, large
particle, or coated pharmaceutical compositions of the
present invention. In addition, the non-hygroscopic,
anhydrous, large particle, or coated pharmaceutical
compositions of the present invention may also include
lactose or other mono- or di-saccharides as excipients. In
another embodiment, inorganic bisulfites may be used to
improve the stability of any of the norastemizole
compositions herein.
For non-hygroscopic formulations, special
precautions must be exercised in choosing excipients and
additives, such that overall, there is no propensity for
moisture sorption (absorption or adsorption) in the absence
of suitable environmental controls. For example, excipients
- 24 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
for use in such formulations include, but are not limited to,
alpha lactose monohydrate, mannitol and the like.
For anhydrous formulations, suitable anhydrous or
low moisture forms of the below identified excipients or
additives should be used, for example, AVICEL-PH-103'"" and
Starch 1500 LM.
A binder is used to provide a free-flowing powder
from the mix of tablet ingredients so that the material will
flow when used on a tablet machine. The binder also provides
a cohesiveness to the norastemizole tablet. Too little
binder will give flow problems and yield tablets that do not
maintain their integrity. Too much may adversely affect the
release tdissolution rate) of the drug from the tablet.
Thus, a sufficient amount of binder should be incorporated
into the tablet to provide a free-flowing mix of the tablet
ingredients without adversely affecting the dissolution rate
of the drug ingredients from the tablet. With lower dose
tablets, the need for good compressibility can be eliminated
to a certain extent by the use of suitable diluting
excipients called compression aids. The amount of binder
used varies upon the type of formulation and mode of
administration, and is readily discernible to those of
ordinary skill in the art.
Binders suitable for use with the lactose-free,
non-hygroscopic, anhydrous, large particle, or coated dosage
formulations of norastemizole made in accordance with the
present invention include, but are not limited to, corn
starch, potato starch, or other starches, gelatin, natural
and synthetic gums such as acacia, sodium alginate, alginic
acid, other alginates, powdered tragacanth, guar gum,
cellulose and its derivatives (e. g., ethyl cellulose,
cellulose acetate, carboxymethyl cellulose calcium, sodium
carboxymethyl cellulose), polyvinyl pyrrolidone, methyl
cellulose, pre-gelatinized starch, hydroxypropyl methyl
cellulose, (e. g., Nos. 2208, 2906, 2910), microcrystalline
cellulose or mixtures thereof.
- 25 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
Suitable forms of microcrystalline cellulose are,
for example, the materials sold as AVICEL-PH-101, AVICEL-PH-
103 and AVICEL-PH-105 (available from FMC Corporation,
American Viscose Division, Avicel Sales, Marcus Hook, PA.,
U.S.A.). An exemplary suitable binder is a mixture of
microcrystalline cellulose and sodium carboxymethyl cellulose
sold as AVICEL RC-581 by FMC Corporation.
Most commercial tablets weigh from about 100 mg to
about 500 mg. Thus, for many potent drugs including dosage
forms of norastemizole, a filler comprises a large portion of
the tablet. Fillers (e.g., diluents) are used to give the
powder (e.g., in the tablet or capsule) bulk so that an
acceptable size tablet, capsule or other desirable dosage
form is produced. Typically, therapeutic ingredients are
formed in a convenient dosage form of suitable size by the
incorporation of a diluent therewith. As with the binder,
binding of the drug to the filler may occur and affect
bioavailability. Consequently, a sufficient amount of filler
should be used to achieve a desired dilution ratio without
detrimentally affecting release of the drug ingredients)
from the dosage form containing the filler. Further, a
filler that is physically and chemically compatible with the
therapeutic ingredients) of the dosage form should be used.
Thus, as noted, lactose should not be used with norastemizole
to form the dosage forms of norastemizole made in accordance
with the present invention if precautions have not been taken
to eliminate unbound water. It is also preferable that the
lactose-free dosage forms of norastemizole according to the
present invention do not include mono- or di-saccharides,
such as, but not limited to, glucose, sucrose and fructose.
The amount of filler used varies upon the type of formulation
and mode of administration, and is readily discernible to
those of ordinary skill in the art.
Examples of suitable fillers for use with the
lactose-free dosage forms of norastemizole made in accordance
with the present invention include, but are not limited to,
talc, calcium carbonate (e. g., granules or powder),
- 26 -
r ,.
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
microcrystalline cellulose, powdered cellulose, dextrates,
kaolin, mannitol, silicic acid, sorbitol, starch, pre-
gelatinized starch, or mixtures thereof.
The binder/filler in pharmaceutical compositions of
the present invention is typically present in about 50 to
about 99 weight percent of the pharmaceutical composition.
Disintegrants are used to cause the tablet to
disintegrate when exposed to an aqueous environment. Too
much of a disintegrant will produce tablets which may
disintegrate in the bottle due to atmospheric moisture and
provide unbound water sufficient to initiate and/or
accelerate norastemizole lactose interaction. Too little may
be insufficient for disintegration to occur and may thus
alter the rate and extent of release of the drug
ingredients) from the dosage form. Thus, a sufficient
amount of disintegrant that is neither too little nor too
much to detrimentally alter the release of the drug
ingredients) should be used to form the dosage forms of
norastemizole made according to the present invention. The
amount of disintegrant used varies based upon the type of
formulation and mode of administration, and is readily
discernible to those of ordinary skill in the art.
Typically, about 0.5 to about 15 weight percent of
disintegrant, preferably about 1 to about 5 weight percent of
disintegrant, may be used in the pharmaceutical composition.
Suitable disintegrants that may be used to form the
lactose-free dosage forms of norastemizole made in accordance
with the present invention include, but are not limited to,
agar-agar, alginic acid, calcium carbonate, microcrystalline
cellulose, croscarmellose sodium, crospovidone, polacrilin
potassium, sodium starch glycolate, potato or tapioca starch,
other starches, pre-gelatinized starch, other starches,
clays, other algins, other celluloses, gums or mixtures
thereof .
Based on the physicochemical properties of
norastemizole, it is typically desirable to formulate the
lactose-free, non-hygroscopic, anhydrous, large particle, or
- 27 -
CA 02284705 1999-09-23
WO 98142379 PCT/US98/05701
coated pharmaceutical compositions of norastemizole such that
they dissolve fairly rapidly upon administration to the
subject, e.g., in the subject's stomach. Thus, in a
preferred embodiment, the lactose-free, non-hygroscopic,
anhydrous, large particle, or coated pharmaceutical
compositions of the present invention include a super
disintegrant, such as, but not limited to, croscarmellose
sodium or sodium starch glycolate.
Whatever the dose, adhesion of the dosage form
ingredients to the punches of the tableting machine must be
avoided. For example, when drug (e. g., norastemizole)
accumulates on the punch surfaces, it causes the tablet
surface to become pitted and therefore unacceptable. Also,
sticking of drug or other dosage form ingredients in this way
requires unnecessarily high ejection forces when removing the
tablet from the die. Excessive ejection forces may lead to a
high breakage rate and increase the cost of production not to
mention excessive wear and tear on the dies. In practice, it
is possible to reduce sticking by wet-massing or by the use
of high levels of lubricants, e.g., magnesium stearate.
However, selection of a drug salt with good anti-adhesion
properties also minimizes these problems.
As noted, the lubricant is used to enhance the flow
of the lactose-free norastemizole tableting powder mix to the
tablet machine and to prevent sticking of the tablet in the
die after the tablet is compressed. Too little lubricant
will not permit satisfactory tablets to be made and too much
may produce a tablet with a water-impervious hydrophobic
coating. Because lubricants are usually hydrophobic
materials such as stearic acid, magnesium stearate, calcium
stearate and the like, a water-impervious hydrophobic coating
may be formed by the use of too much lubricant. Further, a
water-impervious hydrophobic coating can inhibit
disintegration of the tablet and dissolution of the drug
ingredient(s). Thus, a sufficient amount of lubricant should
be used that readily allows release of the compressed tablet
from the die without forming a water-impervious hydrophobic
- 28 -
Y. ~
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
coating that detrimentally interferes with the desired
disintegration and/or dissolution of the drug ingredient(s).
Suitable lubricants for use with the lactose-free
dosage forms of norastemizole made in accordance with the
present invention include, but are not limited to, calcium
stearate, magnesium stearate, mineral oil, light mineral oil,
glycerin, sorbitol, mannitol, polyethylene glycol, other
glycols, stearic acid, sodium lauryl sulfate, talc,
hydrogenated vegetable oil (e. g., peanut oil, cottonseed oil,
sunflower oil, sesame oil, olive oil, corn oil, and soybean
oil), zinc stearate, ethyl oleate, ethyl laurate, agar, or
mixtures thereof. Additional lubricants include, for
example, a syloid silica gel (AEROSIL 200, manufactured by
W.R. Grace Co. of Baltimore MD), a coagulated aerosol of
synthetic silica (marketed by Deaussa Co. of Plano, Texas),
CAB-O-SIL (a pyrogenic silicon dioxide product sold by Cabot
Co. of Boston, Mass) or mixtures thereof. A lubricant may
optionally be added, typically in an amount of less than
about 1 weight percent of the pharmaceutical composition.
Another class of additives for use with the dosage
forms of norastemizole include, but are not limited to, anti-
caking agents, antimicrobial preservatives, coating agents,
colorants, desiccants, flavors and perfumes, plasticizers,
viscosity increasing agents, sweeteners, buffering agents,
humectants and the like.
Suitable anti-caking agents for use with the
lactose-free dosage forms of norastemizole made in accordance
with the present invention include, but are not limited to,
calcium silicate, magnesium silicate, silicon dioxide,
colloidal silicon dioxide, talc or mixtures thereof.
Suitable antimicrobial preservatives for use with
the lactose-free dosage forms of norastemizole made in
accordance with the present invention include, but are not
limited to, benzalkonium chloride solution, benzethonium
chloride, benzoic acid, benzyl alcohol, butyl paraben,
cetylpyridinium chloride, chlorobutanol, cresol,
dehydroacetic acid, ethylparaben, methylparaben, phenol,
- 29 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
phenylethyl alcohol, phenylmercuric acetate, phenylmercuric
nitrate, potassium sorbate, propylparaben, sodium benzoate,
sodium dehydroacetate, sodium propionate, sorbic acid,
thimersol, thymol or mixtures thereof.
Suitable coating agents for use with the lactose-
free dosage forms of norastemizole made in accordance with
the present invention include, but are not limited to, sodium
carboxymethyl cellulose, cellulose acetate phthalate,
ethylcellulose, gelatin, pharmaceutical glaze, hydroxypropyl
cellulose, hydroxypropyl methylcellulose (e. g., Nos.. 2208,
2906, 2910), hydroxypropyl methyl cellulose phthalate (e. g.,
Nos.: 200731, 220824), methylcellulose, polyethylene glycol,
polyvinyl acetate phthalate, shellac, sucrose, titanium
dioxide, carnauba wax, microcrystalline wax or mixtures
thereof. The amount of coating agent and the carrier vehicle
(aqueous or non-aqueous) used varies upon the type of
formulation and mode of administration, and is readily
discernible to those of ordinary skill in the art.
A coating of a film forming polymer may optionally
be applied to the norastemizole tablet (e. g., a capsule
shaped tablet often referred to as a caplet) in accordance
with the present invention by using one of several types of
equipment such as a conventional coating pan, Accelacota,
High-Cola or Worster air suspension column. Such equipment
typically has an exhaust-system to remove dust and solvent or
water vapors to facilitate quick drying. Spray guns or other
suitable atomizing equipment may be introduced into the
coating pans to provide spray patterns conducive to rapid and
uniform coverage of the tablet bed. Normally, heated or cold
drying air is introduced over the tablet bed in a continuous
or alternate fashion with a spray cycle to expedite drying of
the film coating solution. For non-hygroscopic, anhydrous,
large particle, or coated pharmaceutical compositions of the
invention containing reactive excipients, such as lactose,
non-aqueous operations are preferred, e.g., non-aqueous
coating should be used.
- 30 -
r i
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
The coating solution may be sprayed by using
positive pneumatic displacement or peristaltic pump systems
in a continuous or intermittent spray-dry cycle. The
particular type of spray application is selected depending
upon the drying efficiency of the coating pan.
In most cases, the coating material is sprayed
until the lactose-free, non-hygroscopic, large particle,
anhydrous, or coated norastemizole tablets are uniformly
coated to the desired thickness and the desired appearance of
the tablet is achieved. Many different types of coatings may
be applied such as enteric, slow release coatings or rapidly
dissolving type coatings for fast acting tablets.
Preferably, rapidly dissolving type coatings are used to
permit more rapid release of the active ingredients,
resulting in hastened onset. The thickness of the coating of
the film forming polymer applied to a tablet, for example,
may vary. However, it is preferred that the thickness
simulate the appearance, feel (tactile and mouth feel) and
function of a gelatin capsule. Where more rapid or delayed
release of the therapeutic agents) is desired, one skilled
in the art would easily recognize the film type and
thickness, if any, to use based on characteristics such as
desired blood levels of active ingredient, rate of release,
solubility of active ingredient, and desired performance of
the dosage form.
A number of suitable film forming agents for use in
coating a final dosage form, such as tablets comprising the
present lactose-free, non-hygroscopic, anhydrous, large
particle or coated formulations of norastemizole include, for
example, methylcellulose, hydroxypropyl methyl cellulose
(PHARMACOAT 606 6 cps), polyvinylpyrrolidone (povidone),
ethylcellulose (ETHOCEL 10 cps), various derivatives of
methacrylic acids and methacrylic acid esters, cellulose
acetate phthalate or mixtures thereof.
Suitable colorants for use with the lactose-free
dosage forms of norastemizole made in accordance with the
present invention include, but are not limited to,
- 31 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
pharmaceutically acceptable dyes and lakes, caramel, red
ferric oxide, yellow ferric oxide or mixtures thereof.
Suitable desiccants for use with the lactose-free, anhydrous,
large particle, or coated norastemizole dosage formulations
made in accordance with the present invention include, but
are not limited to, calcium chloride, calcium sulfate, silica
gel or mixtures thereof.
Suitable flavors for use with the lactose-free
dosage forms of norastemizole made in accordance with the
present invention include, but are not limited to, acacia,
tragacanth, almond oil, anethole, anise oil, benzaldehyde,
caraway, caraway oil, cardamom oil, cardamom seed, compound
cardamom tincture, cherry juice, cinnamon, cinnamon oil,
clove oil, cocoa, coriander oil, eriodictyon, eriodictyon
fluidextract, ethyl acetate, ethyl vanillin, eucalyptus oil,
fennel oil, glycyrrhiza, pure glycyrrhiza extract,
glycyrrhiza fluidextract, lavender oil, lemon oil, menthol,
methyl salicylate, monosodium glutamate, nutmeg oil, orange
flower oil, orange flower water, orange oil, sweet orange
peel tincture, compound orange spirit, peppermint, peppermint
oil, peppermint spirit, pine needle oil, rose oil, stronger
rose water, spearmint, spearmint oil, thymol, tolu balsam
tincture, vanilla, vanilla tincture, and vanillin or mixture
thereof .
Suitable plasticizers for use with the lactose-free
dosage forms of norastemizole made in accordance with the
present invention include, but are not limited to, castor
oil, diacetylated monoglycerides, diethyl phthalate,
glycerin, mono-and di-acetylated monoglycerides, polyethylene
glycol, propylene glycol, and triacetin or mixtures thereof.
Suitable viscosity increasing agents for use with
the lactose-free dosage forms of norastemizole made in
accordance with the present invention include, but are not
limited to, acacia, agar, alamic acid, aluminum monostearate,
bentonite, bentonite magma, carbomer 934,
carboxymethylcellulose calcium, carboxymethylcellulose
sodium, carboxymethylcellulose sodium 12, carrageenan,
- 32 -
T. ~
CA 02284705 1999-09-23
WO 98142379 PCT/US98/05701
cellulose, microcrystalline cellulose, gelatin, guar gum,
hydroxyethyl cellulose, hydroxypropyl cellulose,
hydroxypropyl methylcellulose (Nos. 2208; 2906; 2910),
magnesium aluminum silicate, methylcellulose, pectin,
polyvinyl alcohol, povidone, silica gel, colloidal silicon
dioxide, sodium alginate, tragacanth and xanthan gum or
mixtures thereof.
Suitable sweetening agents for use with the
lactose-free dosage forms of norastemizole made in accordance
with the present invention include, but are not limited to,
aspartame, dextrates, mannitol, saccharin, saccharin calcium,
saccharin sodium, sorbitol, sorbitol solution, or mixtures
thereof.
Suitable buffering agents for use with the lactose-
free dosage forms of norastemizole made in accordance with
the present invention include, but are not limited to,
magnesium hydroxide, aluminum hydroxide and the like, or
mixtures thereof. Suitable humectants include, but are not
limited to, glycerol, other humectants or mixtures thereof.
The dosage forms of norastemizole of the present invention
may further include one or more of the following: (1)
dissolution retarding agents, such as paraffin; (2)
absorption accelerators, such as quaternary ammonium
compounds; (3) wetting agents, such as, for example, cetyl
alcohol and glycerol monostearate; (4) absorbents, such as
kaolin and bentonite clay; (5) antioxidants, such as water
soluble antioxidants (e. g., ascorbic acid, cysteine
hydrochloride, sodium bisulfate, sodium metabisulfate, sodium
sulfite and the like), oil soluble antioxidants (e. g.,
ascorbyl palmitate, hydroxyanisole (BHA), butylated hydroxy
toluene (BHT), lecithin, propyl gallate, alpha-tocopherol and
the like); and (6) metal chelating agents, such as citric
acid, ethylenediamine tetracetic acid (EDTA), sorbitol,
tartaric acid, phosphoric acid and the like.
The lactose-free, non-hygroscopic, anhydrous, large
particle, or coated norastemizole dosage forms of the present
invention may also be provided in the form of hard or soft
- 33 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
capsules, for example, of gelatin or other suitable materials
together with various excipients previously noted with regard
to tablets. For the formation of tablets, the norastemizole
is combined with one or more excipients (e. g., diluents,
binders, disintegrants, dispersing agents, surface-active
agents, lubricants, coating materials, flavoring agents,
coloring agents, solvents, viscosity increasing agents,
suspending agents, sweeteners, colorants, dyes and the like)
in various proportions using traditional tableting equipment
such as twin shell or "v" blenders by known procedures to
manufacture chemically and thermally stable dosage forms
(e. g., tablets, caplets and the like) containing a uniform
distribution and blending of therapeutic agents. The exact
amounts of each of the various excipients may be readily
determined by those of ordinary skill in the pharmaceutical
art.
Large-scale production of lactose-free, non-
hygroscopic, anhydrous, large particle, or coated dosage
forms of norastemizole made in accordance with the present
invention may require, in addition to the therapeutic drug
ingredient(s), additives including, but not limited to,
diluents, binders, lubricants, disintegrants, colorants,
flavors, sweetening agents and the like or mixtures thereof.
By the incorporation of these and other additives, a variety
of dosage forms (e. g., tablets, capsules, caplets, troches
and the like) may be made. These include, for example, hard
gelatin capsules, caplets, sugar-coated tablets, enteric-
coated tablets to delay action, multiple compressed tablets,
prolonged-action tablets, tablets for solution, effervescent
tablets, buccal and sublingual tablets, troches and the like.
Sugar-coating preferably does not include lactose or mono- or
di-saccharides, except in norastemizole formulations
substantially free of unbound water.
Tablets of the lactose-free, non-hygroscopic,
anhydrous, large particle, or coated dosage forms of
norastemizole of the present invention are typically made by
molding, by compression or by generally accepted tablet
- 34 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
forming methods. Accordingly, compressed tablets are usually
prepared by large-scale production methods while molded
tablets often involve small-scale operations. For example,
- there are three general methods of tablet preparation for
making the dosage forms of norastemizole: (1) the wet-
granulation method; (2) the dry-granulation method; and
(3) direct compression. These methods are well known to
those skilled in the art. See Remington's Pharmaceutical
Sciences, 16th and 18th Eds., Mack Publishing Co., Easton,
Pennsylvania (1980 and 1990). See also U.S. Pharmacopeia
XXI, U.S. Pharmacopeial Convention, Inc., Rockville, Maryland
(1985). Preferably for non-hygroscopic or anhydrous dosage
forms, wet granulation is not used.
Various tablet formulations of the lactose-free,
non-hygroscopic, anhydrous, large particle, or coated dosage
forms of norastemizole may be made in accordance with the
present invention. These include tablet dosage forms such as
sugar-coated tablets, film-coated tablets, enteric-coated
tablets, multiple-compressed tablets, prolonged action
tablets and the like. Lactose-free, non-hygroscopic,
anhydrous, large particle, or inert coated norastemizole
sugar-coated tablets (SCT) are compressed tablets containing
a sugar coating. Such coatings may be colored and are
beneficial in covering up drug substances possessing
objectionable tastes or odors and in protecting materials
sensitive to oxidation. Lactose-free, non-hygroscopic,
anhydrous, large particle, or coated norastemizole film-
coated tablets (FCT) are compressed tablets which are covered
with a thin layer or film of a water-soluble material. A
number of polymeric substances with film-forming properties
may be used. The film coating imparts the same general
characteristics as sugar coating with the added advantage of
a greatly reduced time period required for the coating
operation. Enteric-coated tablets are also suitable for use
in the present invention. Lactose-free, non-hygroscopic,
anhydrous, large particle or coated norastemizole enteric-
coated tablets (ECT) are compressed tablets coated with
- 35 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
substances that resist dissolution in gastric fluid but
disintegrate in the intestine. Enteric coating can be used
for tablets containing drug substances which are inactivated
or destroyed in the stomach, for those which irritate the
mucosa or as a means of delayed release of the medication.
Lactose-free, non-hygroscopic, anhydrous, large
particle, or coated norastemizole multiple compressed tablets
(MCT? are compressed tablets made by more than one
compression cycle, such as layered tablets or press-coated
tablets. Layered tablets are prepared by compressing
additional tablet granulation on a previously compressed
granulation. The operation may be repeated to produce
multilayered tablets of two, three or more layers.
Typically, special tablet presses are required to make
layered tablets. See, for example, U.S. Pat. No. 5,213,738,
incorporated herein in its entirety by reference thereto.
Press coated tablets are another form of multiple
compressed tablets. Such tablets, also referred to as dry-
coated tablets, are prepared by feeding previously compressed
tablets into a tableting machine and compressing another
granulation layer around the preformed tablets. These
lactose-free, non-hygroscopic, or anhydrous norastemizole
tablets have all the advantages of compressed tablets, i.e.,
slotting, monogramming, speed of disintegration, etc., while
retaining the attributes of sugar coated tablets in masking
the taste of the drug substance in the core tablet. Press-
coated tablets can also be used to separate incompatible drug
substances. Further, they can be used to provide an enteric
coating to the core tablets. Both types of norastemizole
tablets (i.e., layered tablets and press-coated tablets) may
be used, for example, in the design of prolonged-action
dosage forms.
Lactose-free, non-hygroscopic, anhydrous, large
particle, or coated norastemizole prolonged-action tablets
may comprise compressed tablets formulated to release the
drug substance in a manner to provide medication over a
period of time. There are a number of tablet types that
- 36 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
include delayed-action tablets in which the release of the
drug substance is prevented for an interval of time after
administration or until certain physiological conditions
exist. Repeat action tablets may be formed that periodically
release a complete dose of the drug substance to the
gastrointestinal fluids. Also, extended release tablets that
continuously release increments of the contained drug
substance to the gastrointestinal fluids may be formed.
The method of preparation and the additives to be
incorporated into a lactose-free, non-hygroscopic, anhydrous,
large particle, or coated norastemizole tablet are selected
in order to give the tablet formulation the desirable
physical characteristics while allowing the rapid compression
of tablets. After compression, the tablets preferably should
have a number of additional attributes such as appearance,
hardness, disintegration ability and uniformity which are
influenced both by the method of preparation and by the
additives present in the tablet formulation.
The basic unit in all tablet compression equipment
includes a lower punch which fits into a die from the bottom
and an upper punch, having a head of generally the same shape
and dimensions as that of the lower punch, which enters the
die cavity from the top after the tableting material fills
the die cavity. The tablet is formed by pressure applied on
the punches. Subsequently, the tablet is ejected from the
die. The weight of the tablet is determined by the volume of
the material which fills the die cavity.
The ability of the lactose-free, non-hygroscopic,
anhydrous, large particle, oz coated norastemizole tablet or
dosage form granulation to flow freely into the die cavity is
important in insuring an uniform fill. The flowability of
the granulation is also important to insure continuous
movement of the granulation from the source of supply or feed
hopper. Further, if the tablet granulation does not possess
cohesive properties, after compression the tablet will
crumble and fall apart on handling. Even further, as the
punches must move freely within the die and the tablet must
- 37 -
CA 02284705 1999-09-23
WO 98/42379 PCTIUS98105701
be readily ejected from the punch faces, the tableting
material must have a degree of lubrication to minimize
friction and to allow for the removal of the compressed
tablet. A granulating agent may be added to facilitate
granulation. The amount of granulating agent used varies
upon the type of formulation and mode of administration, and
is readily discernible to those of ordinary skill in the art.
Typically, about 5 to about 15 weight percent of granulating
agent is used in the pharmaceutical formulation. Preferably,
when lactose is present in the anhydrous or non-hygroscopic
compositions of the present invention, the granulating agent
should be non-aqueous.
Further, it is noted that stable lactose-free, non-
hygroscopic, anhydrous, large particle, or coated
norastemizole tablets or other dosage forms thereof retain
their original size, shape, weight and color under normal
handling and storage conditions throughout their shelf life.
Thus, for example, excessive powder or solid particles at the
bottom of the container, cracks or chips on the face of a
tablet, or appearance of crystals on the surface of tablets
or on container walls are indicative of physical instability
of uncoated tablets. Hence, the effect of mild, uniform and
reproducible shaking and tumbling of tablets should be
undertaken to insure that the tablets have sufficient
physical stability. Tablet hardness can be determined by
commercially available hardness testers. In addition, the in
vitro availability of the active ingredient should not change
appreciably with time.
The lactose-free pharmaceutical compositions of the
present invention may also be formulated in a soft elastic
gelatin capsule unit dosage form by using conventional
methods, well-known in the art (see, e.g., Ebert, Pharm.
Tech., 1(5):44-50 (1977)). Soft elastic gelatin capsules
have a soft, globular, gelatin shell somewhat thicker than
that of hard gelatin capsules, wherein a gelatin is
plasticized by the addition of glycerin, sorbitol, or a
similar polyol. The hardness of the capsule shell may be
- 38 -
CA 02284705 1999-09-23
WO 98/42379 PCT'/US98/05701
changed by varying the type of gelatin and the amounts of
plasticizer and water. The soft gelatin shells may contain a
preservative (such as methyl-and propylparabens and sorbic
acid) to prevent the growth of fungi. The active ingredient
may be dissolved or suspended in a liquid vehicle or carrier,
such as vegetable or mineral oils, glycols such as
polyethylene glycol and propylene glycol, triglycerides,
surfactants such as polysorbates, or a combination thereof.
The tablets, and other dosage forms of the
pharmaceutical compositions of the present invention, such as
dragees, capsules, pills and granules, may optionally be
scored or prepared with coatings and shells, such as enteric
coatings and other coatings well known in the pharmaceutical
formulating art.
The pharmaceutical compositions of the present
invention may also be formulated so as to provide slow or
controlled release of the active ingredient therein using,
for example, hydroxypropylmethyl cellulose in varying
proportions to provide the desired release profile, other
polymer matrices, liposomes and/or microspheres.
Unless indicated otherwise, all percentages noted
herein are percentages by weight based on the total weight of
all the components of a particular dosage form.
The lactose-free, non-hygroscopic, anhydrous, large
particle, or coated norastemizole compositions of the present
invention may further contain, for example, an analgesic, a
decongestant, a cough suppressant, or an expectorant.
The incompatibility of norastemizole with lactose
is illustrated in Table I below. The effect of lactose on
norastemizole at various temperatures (e.g., 25°C, 40°C and
60°C), at various relative humidity levels (e.g., 60% and 75%
relative humidity) and at various times (e. g., zero, 1 week,
1 month, 2 months, 3 months, 6 months and 9 months) was
evaluated. The results of such evaluation are presented in
Table I. The level of impurities within the capsules tested
was measured using high pressure liquid chromatography
(HPLC), and is presented in Table I as a percentage of the
- 39 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
dosage form tested. Note that discoloration from the initial
white opaque appearance is an indication of incompatibility
between norastemizole and lactose, which is corroborated by
increased percentages of impurities detected by HPLC.
10
20
30
- 40 -
,,
CA 02284705 1999-09-23
WO 98/42379 PCT/US98105701
-n
R
U
C_
v
3
c
c a
_ r~,r a oo r v~o. _ N v-,r o o v
Q c r a vi a ~ v o00o v;00 00 0 M ' y O
a. a a a o a c c a oo r r o0
U c ~
c ~n
E
A
a
U c
0
v,
N G
~0
A U
C
v O
V
L _c
e
v C
~, N
v
' G
a 3
a A
c r. m.-,c T .., '..n m c o p' ~ r -
o o i,
L ~ ~ v~. -rr .r-r c c
v O C r ~
fl
~
R
d ,
C
u
_
w
L
L G4
N
R
i v v
~ v
L L V y U L _ y L L L y V ~C
7
~ 7 7 ~ G ~ r 7 7 7
V ~ G G L L J T p f L L L G t
C U V V U r y U V ~ ~ ~ J ~
_
? L V ~ C
7 7 A 7 L ~ > j
7
t ~' ~' ~ ~ T " L ~'~ ? , ? " 7 h
G.
L L G G C. L L -' G L L ~ L 4 4 L N O'
~
L f G _ G L L In ..C _ ~ .. ' ~ _..-'J
G
= V V y U S . y V V L V y U ~ L
A L L ;f _ - - _ 4 4 0l/ = P!
f f
_ s s s s a s i s ,.
s s c .c
G ~ x C
G G
G
C ~D
r ea
4. v
J
~c ~ L L L s L L L t .~crJtU
C:
L C C C C C ~,'C ~ C C J y C n
C O R
~ r
c- s ~ ~ c E E f c ~ E ~ E s E R T
'
-, .-,.o c .-,.., ., c v
.
s v
y Q
U
v
a
e' ~
a r
c
v r r r. v, v, v, W V
i ~ ~ r r r-'r
r r r r OU
G
y ~ l
eC
r
A
L
C
a
v
L
c 7
C
L
_
R
3
a
a a
U o
v, ~n .nv, .n~n ~n o o c o 0 0 0 0
N N N N N (V N ~ Q Q er vy ~ ~p ~p
C
a
w-
x
0
T ~n
_ v
.D
~4.
C
Lf7 O L!1 O
N
41
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
Thus, the results show that when norastemizole was
formulated with lactose, filled into hard gelatin capsules,
and stored in a non-hermetically sealed container, the
formulation was not chemically stable at elevated
temperatures and humidity. Moreover, even at 25°C with 60%
relative humidity, the norastemizole/lactose capsules showed
increased impurities and reduced in vitro potency after six
(6) and nine (9) months, indicating incompatibility between
norastemizole and lactose.
l0 In an effort to identify excipients, other than
lactose, suitable for use with norastemizole, an excipient
study was performed using a variety of classes of other
excipients. Examples of excipients tested include corn
starch, calcium sulfate dihydrate, calcium stearate, sucrose,
fructose, calcium carbonate, microcrystalline cellulose,
maltodextrin, CaHPO,~2H20, CaHPO,, magnesium stearate, starch
1500, croscarmellose sodium or mixtures thereof.
The effect of various excipients on the degradation
of norastemizole is depicted in Figure 2, wherein the
norastemizole/excipient combination was exposed to a
temperature of 60°C and relative humidity of 75%, and stored
in a non-hermetically sealed container, which are typical
conditions for excipient compatibility studies.
As is clear from Figure 2, norastemizole and
lactose are clearly incompatible because of the sharp drop in
the potency of the drug. However, no such drastic drop in
potency is seen between norastemizole and the other
excipients tested. However, the daily dosage may need to be
adjusted to take into account the potency variations among
the excipients seen and illustrated in Figure 2.
Figure 2 also provides some indication that mono-
and di-saccharide excipients should also preferably be
avoided in norastemizole formulations, e.g., as shown by the
degradation observed with norastemizole/sucrose combinations.
The above results were obtained using screw-cap
containers, high temperature and humidity which is a widely-
accepted means for determining the interactions of compounds
- 42 -
CA 02284705 1999-09-23
WO 98/42379 PCTIUS98/05701
with excipients under accelerated conditions. Applicants
have also found that norastemizole alone, when stored under
high humidity conditions (thus exposed to significant unbound
water), is extremely stable over long periods of time.
Another study was conducted to assess the effects of moisture
. changes on the lactose/norastemizole interactions.
In 20-ml amber glass crimp-top vials, the following
samples were prepared:
Norastemizole Neat
Norastemizole 20%/Lactose 80%
Norastemizole 20%/Lactose 80% with H20 5%
Norastemizole 1%/Lactose 99%
Norastemizole 1%/Lactose 99% with H,O 5%
The crimped vials were held at 60°C for 14 days and assayed
for norastemizole.
The results show that the incompatibility of
norastemizole and lactose is greatly reduced when unbound
water is not present and the container is hermetically
sealed. Indeed, the moisture effect on the reaction rate is
significant. Where unbound water was not purposely added to
the well sealed containers, the differences were not
substantially different than the control, i.e., neat
norastemizole. Reduced potency in the presence of unbound
water was observed, whereas reductions in potency comparable
to lactose-free or neat norastemizole were observed in the
absence of unbound water.
% Norastemizole
(Assay Results)
Norastemizole Neat 96.90
Norastemizole 20%/Lactose 80% 98.33
Norastemizole 20%/Lactose 80% with H20 5% 65.16
Norastemizole 1%/Lactose 99% 92.59
Norastemizole 1%/Lactose 99% with H20 5% 77.22
- 43 -
CA 02284705 1999-09-23
WO 98/42379 PCT/IJS98/05701
A lactose-free, non-hygroscopic, anhydrous, large
particle, or coated norastemizole dosage formulation such as
a troche, a tablet or a capsule may be formed by combining
norastemizole, or a pharmaceutically acceptable salt thereof,
with one or more pharmaceutically compatible excipients, as
described above, in pharmaceutically compatible amounts to
yield a unit dose norastemizole dosage formulation containing
from about 1 mg to about 200 mg of norastemizole, and
preferably containing from about 2 mg to about 100 mg of
norastemizole. The tablet, troche or capsule dosage
formulation may be formed, for example, by methods well known
in the art including wet granulation, dry granulation or
compression molding. Again, wet granulation is not useful
for non-hygroscopic or anhydrous formulations. Other methods
for forming tablets, troches and capsules, well known in the
art, may be used. However, compression molding is preferred
for the formulation of tablets and troches. For capsules,
hard gelatin capsule shells are preferred which are filled
with norastemizole and one or more excipients.
STARCH 1500' is a pre-gelatinized starch
manufactured by Colorcon Ltd. that is not recommended for use
in amounts exceeding 75 weight percent. In addition, when
magnesium stearate is used as a lubricant with STARCH 1500',
amounts greater than 0.25 weight percent of magnesium
stearate should not be used, as this may have an adverse
effect on dissolution. This adverse effect on dissolution in
formulations of STARCH 1500~'~ and greater than 0.25 weight
percent of magnesium stearate is particularly important for
compounds having relatively low water-solubility, such as
norastemizole.
Having described the invention, the following
examples illustrate preferred embodiments in accordance with
the presently claimed invention. It is understood that the
examples are illustrative and do not limit the scope or
breadth of the appended claims.
- 44 -
r
CA 02284705 1999-09-23
WO 98142379 PCT/US98/o5701
Example l: Hard Gelatin Capsule Unit Dosage Forms (Lactose-Free)
Component 2.5 mg capsule 5 mg capsule 20 mg capsule
. (amount in mg) (amount in mg) (amount in mg)
Norastemizole 2.5 5.0 20.0
- Microcrystalline90.0 90.0 90.0
Cellulose
Pre-gelatinized 100.3 97.8 82.8
Starch
Croscarmellose 7.0 7.0 7.0
Magnesium 0.2 0.2 0.2
Stearate
Component 2.~ mg capsule 5 mg capsule 20 mg capsule
(amount in mgt (amount in mg) (amount in mg)
Norastemizole 2.5 5.0 20.0
a-lactose 197.3 144.8 179.8
2 monohydrate
0
Magnesium 0.2 0.2 0.2
Stearate
Example 3: Hard Gelatin Capsulr Unit Dosage Forms (Anhydrous)
Component 2.5 mg capsule 5 mg capsule 20 mg capsule
(amount m mgi (amount in mg) (amount in mg)
Norastrmizolr '_ . 5 5.0 20.0
A VICEL-PH-103 50.0 50.0 50.0
Starch 1500 LM 97.3 94.8 79, g
a-lactose 50.0 50.0 50.0
(anhydrous)
Magnesium 0.2 0.2 0.2
Stearate
The active ingredient is sieved and blended with
the excipients listed. The mixture is filled into suitably
Example 2: Hard Gelatin Capsule Unit Dosage Forms (Mon-Hygroscopic)
- 45 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
sized two-piece hard gelatin capsules using suitable
machinery and methods well known in the art. See Remington's
Pharmaceutical Sciences, 16th or 18th Editions, each
incorporated herein in its entirety by reference thereto.
Other doses may be prepared by altering the fill weight and,
if necessary, changing the capsule size to suit. Any of the
stable, non-lactose, non-hygroscopic, and anhydrous hard
gelatin capsule formulations above may be formed.
1 o Example 4: Compressed Tablet Formulations (Lactose-Free)
Component 2.5 mg tablet 5 mg tablet 20 mg tablet
(amount in mg) (amount in mg) (amount in mg)
Norastemizole 2.5 5.0 20.0
Microcrvstalline90.0 90.0 90.0
Cellulose
Pregelatinized 100.3 97.8 82.8
Starch
Croscarmellose 7.0 7.0 7.0
2 Magnesium 0.2 0.2 0.2
0
Stearate
The active ingredient is sieved through a suitable
sieve and blended with the non-lactose excipients until a
uniform blend is formed. The dry blend is screened and
blended with the magnesium stearate. The resulting powder
blend is then compressed into tablets of desired shape and
size. Tablets of other strengths may be prepared by altering
the ratio of the active ingredient (i.e., norastemizole) to
the excipient(s) or modifying the tablet weight.
- 46 -
~.
CA 02284705 1999-09-23
WO 98/42379 PCT/US98/05701
Example 5: Wet Granulation (Lactose-Free)
Component Qu antity per mg)
Tablet (
' Formulation Formulation Formulation
A B C
Norastemizole 25 50 100
Pre-gelatinized starch100-150 100-125 50-100
Microcrystalline cellulose0-75 0-50 0-50
Povidone 7.5 -- 7.5
to polyethylene glycol -- 10-30 --
Croscarmellose 10 -- 10
Sodium starch glycolate-- 5-15 --
Magnesium stearate 1.5 1.5 1.5
1 S FDC Yellow #~2 lake 1.25 1.25 1.25
The active ingredient is sieved through a suitable
screen and blended with the non-lactose excipients (excluding
half of the croscarmellose (or sodium starch glycolate) and
20 all of the microcrystalline cellulose? until a uniform blend
is formed. Suitable volumes of water are added and the
powder granulated. After drying, the granules are screened
and blended with the microcrystalline cellulose, the
remainder of croscarmellose or sodium starch glycolate, and
briefly with the magnesium stearate. The resulting free-
flowing powder is then compressed into tablets of desired
shape and size. Tablets of other strengths may be prepared
by altering the ratio of the active ingredient (i.e.,
norastemizole) to the excipients or modifying the tablet
weight.
- 47 -
CA 02284705 1999-09-23
WO 98/42379 PCT/US98105701
Example 6: Direct Compression
Component Quantity
per Tablet
(mg)
Formulation Formulation
A B
Norastemizole 25 50
Pre-gelatinized starch12.5 12.5
Microcrystalline cellulose205 180
Silicon dioxide 0.625 0.625
Sodium lauryl sulfate 1.25 1.25
Croscarmellose 2.5 2.5
Magnesium stearate 2 2
FDC Yellow #? lake 1.25 1.25
The active ingredient is passed through a suitable
sieve and blended with the non-lactose excipients (except
magnesium stearate) until a uniform blend is formed. The dry
blend is screened and blended briefly with magnesium
stearate. The resulting powder blend is then compressed into
tablets of desired shape and size. Tablets of other
strengths may be prepared by altering the ratio of the active
ingredient (i.e., norastemizole) to the excipients or
modifying the tablet weight.
While the present invention has been described with
respect to the particular embodiments, it will be apparent to
those skilled in the art that various changes and
modifications may be made without departing from the spirit
and scope of the invention as defined in the claims.
- 48 -