Note: Descriptions are shown in the official language in which they were submitted.
CA 02285072 1999-09-28
WO 98/43763 PCT/IB98100449
A METHOD FOR PRODUCING A NON-EVAPORABLE GETTER AND A GETTER PRODUCED BY SAID
METHOD
. The present invention relates to powder metallurgy and
more particularly to a process of producing nonevaporable
Better materials and to Betters manufactured therefrom,
featuring enhanced mechanical and sorption properties.
Nonevaporable Betters are well-known'in the field of
vacuum technology, and have been successfully used therein
for more than thirty years for the provision and maintenance
of a high vacuum level in different devices where vacuum is
required: kinescopes, thermal insulation vessels and
cathode-ray tubes, in elementary particle sources and
accelerators (the thermonuclear fusion reactor of the
TOKAMAK T-15 type) or the LEP (Large Electron-Positron)
accelerator at CERN in Geneva, where the use of NGs makes it
possible to reach a residual pressure below 10-1° Pa. Another
broad field of NG application is the purification of inert
gases. The best-known nonevaporable Betters are alloys: Zr-
A1, containing 84 weight o Zr, described in US Patent No.
3,203,901; a ternary alloy, having the composition 70 weight
o Zr, 24.6 weight a V, and 5.4 weight o Fe, described in US
Patent No. 4,312,69; and an intermetallic compound ZrNnFe
described in US Patent No. 5,180,568. Getter elements are -
CONFIRMATION COPY
CA 02285072 1999-09-28
WO 98143763 PCT/IB98/00449
manufactured mainly from powders whose particle size varies
from several microns to several hundreds of microns. Since
loose powders in most cases can be used as Better elements,
such powders are pressed into articles of different shapes
(tablets, washers, disks, etc.) or rolled into strips.
Porous Betters with high sorption properties are
manufactured as disclosed in US Patent No. 4,928,852; UK
Patent No. 2,077,987; and German Patent No. 2,209,714.
In the information sources cited above, the Better
material is produced by melting and subsequent crushing of
the ingot down to powder; Betters produced from these powder
materials possess low mechanical properties.
Known in the art are Betters made from powder alloys,
described in RF Patent No. 1,649,827 - a Zr-V-Ca
composition, in RF Patent No. 2,034,084 - a Ti-Cr-Ca
composition, and in RF Patent No. 1,750,256, which is the
closest in terms of the technical solution, the latter
comprising preparation of powders for Better materials
having the composition Ti-V-Ca by reducing a mixture of Ti
and V oxides with calcium hydride in accordance with the
main reaction
Me0 + CaH2 -~ Me + Ca0 + HZ T + Q kcal ( 1 ) .
The reaction product is a mixture of powders of metals
and CaO, sintered into a briquette ("sinter"). This "-sinter"
is then crushed and treated with hydrochloric acid to
2
CA 02285072 1999-09-28
WO 98!43763 PCT/IB98100449
separate the metal powder from CaO; after that the powder is
shaped. The reducing temperature is 1175°C with 6 h keeping,
and the resulting finished product is believed to be a
powder alloy. However, an in-depth study showed that the
abovesaid Ti-V-Ca composition is chemically heterogeneous
and comprises predominantly a mixture of almost pure
metallic particles which have not reacted with each other,
and owing to such a high and non-regulated degree of
chemical heterogeneity this Better material, though
displaying a sufficiently high level of chemical properties
with respect to all the above-mentioned materials, has
insufficiently high gas-sorption properties. In the prior-
art method, the reduction conditions, as well as non-
regulated conditions of shaping and sintering the metal
powder, do not allow to produce articles with equally high
mechanical and sorption properties. In the prior art no
information could be found on the interrelation of the
mechanical and sorption properties of the Better with its
chemical heterogeneity.
For the Better to meet all the requirements imposed on
it, it must have very good mechanical properties along with
high sorption characteristics with respect to such gases as
H2, O2, N2, C0, and the like. Low plasticity and strength do
. not provide sufficient resistance to mechanical loads- and
stresses caused by the processes of heat-cycling in the
3
,. _. . . . _ '~ ..., -Y _~ ~~ __~ .._~.,.._ ~CA o22sso~2 1999-o9-is - ~C; r
~~~' ~..~ ~ C"G .4.1 .
9
rangy from 300-7C0°C to t.-.he a.~ient temperatv~re. P:i 1 thzs
;ead,s to disin=egrGtie:: ~~f Better= into separate fragrZe:~~s
cr to the-r crumbling, wh~.ci~: cannot be tolerate d in vac~:~~m
systQms, e.g., 'n vacuum. tubes, in eyementary carticle
sources and accelerators, ~rhereas low sorption properr-~s
car:not provide ? ong-t'_me :nainver~ance of a residual pxessuxe
or. !~~re order cf ~ess tha:; 1C-i° Fw.
Theref<ore, tr:e~ prov:v.sioa of setters noted for r
com~inat~c:. c' improved r,~2c'anica:. ar.d eorpt~.cn proper tits
is an urgent probJ.er~. An ex=eraier. of the range e. rlaterials -
~.:5ed :.n t~;e product_on o:: get ;ors is a no less urgent
orob:.e:~.
In the pTopcse~~ group cf irvent_ons the First =~.:'cec~
=eves tpe problem. of providing Better material; the second
=object relates tc the ge'ter prodncec, wh_ch combi:~.es
a~hanced mECha.~.ica1 a.~.d :~crption property es. Tri're5tiaati~~:~s
whowad that a crnbiratior: of ~:nnanced mec :apical and
sorption r~rnpertiss is prcJided due to the def~.r.ite regree
s
of chemical. he4ercaeneit:~ of the Better mater..al, the zones
of re? ativeiy pure plast=.c metals which ester irate the
corposition of the mater~.aL and have poorly reacted ~~rith
eacr~ other be:~ng respons:.ble for the mechanicaw pxcpert-es,
and t'.-:e zones cf the=r iraeracticn be=ng responsible for the
sorption act_vity bevel.
AMENDED SHEET
4
CA 02285072 1999-09-28
WO 98143763 PCT/IB98/00449
This is achieved in the following manner. As concerns
the first subject of the invention - the method of producing
a nonevaporable Better comprises preparing of a metallic
powder by reducing the corresponding metal oxides entering
into its composition with calcium hydride, subsequent
shaping of the resulting powder and sintering thereof, the
starting materials (metal oxides) being selected so as to
obtain a metallic powder, whose first component comprises at
least one element from the group of Ti, Zr, and whose second
component comprises at least one element from the group of
V, Cr, Mn, Fe, Ni; reduction is carried out at a temperature
of 1180--1230°C for 7-15 hours, powders are shaped at a
pressure of 10-500 kg/cm2 and sintered at 800--1100°C. In
the second subject of the invention it is proposed to
provide a nonevaporable Better with an improved combination
of mechanical and sorption properties from a powder alloy,
whose first component comprises at least one element from
the group Ti, Zr, whose second component comprises at least
one element from the group V, Cr, Mn, Fe, Ni, and whose
third element is calcium oxide (Ca0), the weight ratio of
the first and second components being from 10:1 to 1:5,
preferably from S:1 to 1:2, the content of calcium content
not exceeding 1 weight o; the content of said elements in
the local zones of the Better is different, and the degree
of chemical heterogeneity is determined from the premise
CA 02285072 1999-09-28
WO 98/43763 PCT/IB98/00449
that the arithmetic mean of the concentration ratios of each .
of the elements of the first and second components at
arbitrarily selected several pairs of points should not
exceed 30.
The essence of the invention, as regards the method, is
in preparing a metallic powder of a prescribed chemical
composition by reduction with calcium hydride. To this end,
a mixture of metal oxides is prepared in a ratio
corresponding to the quantitative and qualitative
composition of the Better material, with CaH2 added in an
amount 1.1-1.2 times greater than the stoichiometrically
required amount for reducing the oxides.
It should be pointed out that due to the high
thermodynamic activity of the CaH~ interaction with the
oxides of such metals as iron and nickel, the reaction of
their reduction is accompanied by liberation of a large
quantity of thermal energy,. and this may render the reaction
difficult to control. Therefore, when preparing Better
compositions containing iron, nickel, or their~mixtures~, the
oxides of these metals in the composition of a charge
intended for their reduction may be partially replaced by
metallic powders of iron and nickel. The mixture of powders
is charged into a container, the container is closed ,
heated to 1180-1230°C, and kept from 7 to 15 hours. Said
temperature and process duration ranges in accordance with-
6
~ ~.
CA 02285072 1999-09-28
WO 98/43763 PCT/IB98/00449
the present invention ensure the preparation of a metallic _
powder, whose particles are heterogeneous in their chemical
composition: they differ in the ratio of the elements, i.e.,
the metallic powder of the Better material consists of
particles, wherein zones with relatively pure metals and
zones with different chemical composition are present, as a
result of different degree of interaction between different
metals.
At a temperature below 1180°C, complete reduction of
the oxides is not ensured, and the resulting powder consists
predominantly of strongly dispersed particles, while in the
sintered article the degree of chemical heterogeneity is so
high that the necessary level of sorption properties cannot
be attained, whereas reduction at a temperature above 1230°C
leads to almost complete interaction between the particles
of metals, yielding coarse conglomerates of particles (of 3
mm and over in diameter),. having an almost homogeneous
composition with Ca0 inclusions sintered in them. Depending
on the composition of the Better material, individual
particles of the resulting powder may undergo fusion. All
this leads to a sharp lowering of the mechanical and
sorption properties of Betters manufactured from such
powders.
The main object of the invention is to provide a
metallic powder with a definite degree of chemical
7
CA 02285072 1999-09-28
WO 98/43763 PCT/IB98/00449
heterogeneity of particles as a result of different degree
of interaction between the formed particles of pure metals.
The duration of the process which allows the provision of
the above-mentioned structure of the powder is a function of
several parameters, including the composition of the Better
material, the composition of the charge, and the reduction
temperature. With the reaction time less than 7 hours, a
powder is obtained, consisting of particles with a small
degree of cross-doping, the degree of chemical heterogeneity
of sintered Better material exceeds the permissible value,
whereby sufficiently high sorption properties of the
resulting Better are not ensured, whereas the reaction time
more than 15 hours leads to a high chemical homogeneity of
the metallic powder, where all the particles are closer in
the chemical composition to the prescribed overall
composition of the powder, the particles being conglomerates
of finer metal particles; the size of these conglomerates
may reach 1-3 mm. The Better manufactured from such
particles-conglomerates possesses low mechanical and
sorption properties.
The proposed reduction conditions, according to the
present invention, favor the formation, in the first place,
of chemical heterogeneity of the Better material, at which
the zones of relatively pure plastic metals, i.e., zones
with a low degree of interdiffusion of the metals entering.
8
..r t. ~. ............
_ CA 02285072 1999-09-28
WO 98/43763 PCTIIB98100449
into the composition of the alloys are responsible for the -
mechanical properties, while areas with a high degree of
their interaction are responsible for sorption of gases; in
the second place, the proposed reduction conditions favor
the formation of spongy structure of the powder particles,
where coalescence of metallic particles occurs by way of
"light linkages" owing to the formation of "necks" or
"bridges" between them, preserving thereby an open porous
structure of Betters, ensuring their high gas-sorption
properties along with good mechanical properties.
The product obtained as a result of reduction -
"sinter", comprising a mixture of a metallic powder and
calcium oxide (Ca0) is then crushed and treated with a
hydrochloric acid solution to remove the major part of CaO.
Crushing of the "sinter" is effected under sparing
conditions so as to preserve the internal porous structure
of particles, formed in the process of reduction, which
causes high sorption properties of the Better. In the
process of washing-off use is made of water and hydrochloric
acid (HC1), which, reacting with CaO, yield calcium chloride
(CaCl2). CaCl2 is readily soluble in water and can be easily
removed. However, it is reasonable not to remove CaCl2
completely, but leave it in an amount not over 1 weight o,
because this component behaves later on as an anti-sintering
agent.
9
CA 02285072 1999-09-28
WO 98/43763 PCT/IB98/00449
Calcium oxide (Ca0) favors the preservation of the
porous structure of the Better under the conditions of its
operation at temperatures of 300--900°C and heat cycling in
the range of 20-700°C. Under these conditions calcium oxide
acts as an anti-sintering agent and preserves high sorption
properties of the Better.
To impart a prescribed shape to Better elements, the
powders are shaped. This operation must be carried out at
low pressures, preferably in the range of from 10 to 500
kg/cm2. At shaping pressures higher than the values
indicated herein (above 500 kg/cm2), the sorption properties
of Better elements are impaired because of a decrease in
their porosity, whereas at pressure values lower than 10
kg/cm2 the produced Better elements possess low mechanical
properties and disintegrate easily. Shaping can provide
either individual articles or a continuous strip. In the
first case powders are shaped in press molds; in the second
case powders are shaped by continuous rolling between two
rolls. Rolling can be performed, e.g., in a vertical
direction, so that powder supply occurs by powder falling
down. In this case pressure is controlled by varying the
distance between the rolls and the powder mass that gets
between the rolls per unit time. Articles obtained after
shaping are sintered in vacuum or in an inert atmosphere at
800-1100°C for 30-60 minutes. Sintering at temperatures
._
CA 02285072 1999-09-28
WO 98/43763 PCT/IB98/00449
lower than 800°C lowers the mechanical properties of the
Better, whereas a temperature increase to more than 1100°C
lowers the gas-sorption properties of Better elements
because of their increased shrinkage.
The second subject of the invention relates to a Better
element produced by the above-described method.
In accordance with the second subject of the present
invention, a nonevaporable Better is made from an alloy,
whose first component comprises at least one element from
the group Ti, Zr, whose second component comprises at least
one element of the group V, Cr, Mn, Fe, Ni, whose third
component is calcium oxide (Ca0), the weight ratio of the
first and second components being from 10:1 to 1:5,
preferably from 5:1 to 1:2, and the content of calcium oxide
being not over 1 weight %; the content of said elements in
local zones of the Better is different, i.e., the Better has
a heterogeneous chemical composition throughout its mass,
assuming the presence of local zones of relatively pure
metals and zones differing in the degree of interaction
between these metals. The degree of chemical heterogeneity
of the Better is controlled by the difference in the
concentration of each of the elements entering into the'
groups of the first and second components in the local zones
of the Better, at which concentration the arithmetic mean of
the concentration ratios of each cf the elements at
11
CA 02285072 1999-09-28
WO 98/43763 PCT/IB9$/00449
arbitrarily selected several pairs of points should not
exceed 30.
The choice of titanium (Ti), zirconium (Zr) or their
mixtures as one of the components of Better material is
dictated by the fact that these elements are highly active
gas absorbers, forming a continuous series of solid
solutions with each other. Vanadium (V), chromium (Cr), iron
(Fe), manganese (Mg), and nickel (Ni) or mixtures thereof
are used as components lowering the activation temperature
of the Better material. Said ratios of the elements of the
first and second components improve the sorption properties
of Betters. The content of said elements in quantities
beyond the scope of said ratios lowers the gas-sorption and
mechanical properties of the produced Betters. Calcium
oxide, as an anti-sintering agent, makes it possible to
obviate appreciable shrinkage in sintering; it also
preserves the porous internal structure during service, when
Better elements are heated repeatedly from the ambient
temperature to 300--700°C. The content of calcium oxide
higher than 1 weight o lowers the mechanical properties of
the Better and increases its crumbling. Ca0 content should
not exceed 1 weight o, preferably 0.5 weight o. The absence
of Ca0 impairs the quality of the Better, decreasing its
sorption properties, e.g., because of shrinkage in sintering
and heat cycling in service. -
12
T
.~ .__.y.,.-.rv_~.
CA 02285072 1999-09-28
WO 98/43763 PCT/IB98/00449
The invention contemplates the use of a sufficiently
broad range of materials for the provision of Betters. This
becomes possible due to the experimentally established
influence of the chemical heterogeneity of an alloy from
which the Better is manufactured on the mechanical and
sorption properties of the Better. The degree of chemical
heterogeneity of the elements entering into the groups of
the first and second components recommended by the invention
for use, is controlled by the difference in the
concentration of each of the elements in the local zones, at
which the arithmetic mean of the of the concentration ratios
of each of the elements at arbitrarily selected several
pairs of points should not exceed 30. It is preferable, that
the lower limit of this particular parameter should be about
2. Investigations showed that the use of said materials
alone in the manufacture of Betters does not ensure the
provision of Betters possessing sufficiently high sorption
and mechanical properties. In the manufacture of Betters,
only the use of said elements in said proportions with the
stipulated degree of chemical heterogeneity in terms of the
Better mass leads to the above-stated desirable effect.
Broadening of the range of elements when choosing the
composition of Better materials allows one to make the
Better manufacturing process more economically advantageous,
ecologically and fire-safe. If the chemical heterogeneity of
13
CA 02285072 1999-09-28
WO 98/43763 PCT/IB98100449
the Better material exceeds the maximum permissible degree,
the sorption properties of the Better become impaired
drastically.
Examples illustrating the use of the invention are
presented below, and the results of investigations are shown
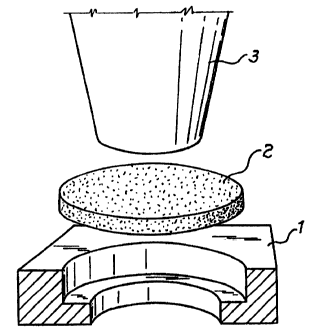
in Figures 1-3. Figure 1 is a sketch of an appliance for
determining the collapsing forces of Better materials.
Figure 2 shows the dependence of the gas sorption rate. on
the amount of absorbed gas for the compositions Ti-Zr-V and
Ti-Cr. Figure 3 shows the dependence of the gas sorption
rate on the amount of absorbed gas for the composition
TiV30, prepared in accordance with the invention: curve 1
corresponds to H2, and curve 3 corresponds to C0; for the
TiV30 composition prepared in accordance with the prior-art
method curve 2 in Figure 3 corresponds to HZ and curve 4
corresponds to CO.
The level of mechanical properties of Better samples is
estimated with the help of an appliance which is shown
diagrammatically in Figure 1. The appliance consists of
metallic die 1 with an annular shoulder serving to support
test sample 2 shaped as a tablet about 7.5 mm in diameter
and 0.7 mm thick, and punch 3 about 6 mm in diameter. Force
is imparted to the sample by means of the punch, and any
load at the moment of testing is recorded by a system of
sensors. A sharp drop of the load indicates destruction of_
14
t i . . ~.. ... . ...
CA 02285072 1999-09-28
WO 98143763 PCT/IB98100449
the sample, and the last value of the load is recorded as _
the collapsing force (P). Tests were carried out on three
samples, and the arithmetic mean of the collapsing force was
calculated.
The sorption properties of Betters produced in
accordance with the invention and of samples produced by the
prior-art method are determined in accordance with the
procedures ASTM F 798-82, using hydrogen and carbon monoxide
gas as the gases to be sorbed. The gas evacuation rate S
{m3/m2's) in Figures 2 and 3 is represented as a function of
the amount of sorbed gas Q (Pa/ m3/m2).
The degree of chemical heterogeneity is determined with
the help of an electron-scan microscope by measuring the
content of each of the elements of the first and second
components, i.e., of Ti, Zr, V, Cr, Mn, Fe, Ni, in
succession at several arbitrarily chosen pairs of points and
finding at these points the value of the ratio (spread) of
the concentrations of each of the elements by dividing the
greater value by the smaller one and then by determining the
mean arithmetic of the concentration ratios (spread) at the
points of several pairs (the number of pairs is at least 3.
EXAMPLE 1
To prepare 1 kg of metallic powder, containing, in
weight %: zirconium (Zr), 40; titanium (Ti), 30; vanadium
CA 02285072 1999-09-28
WO 98/43763 PCTIIB98/00449
(V), 30: oxides of said metals are taken in the following
amounts, kg: zirconium dioxide (Zr02), 0.296; titanium
dioxide (Ti02), 0.497; vanadium trioxide (V203), 0.490; 1.31
kg of calcium hydride is added, i.e., the amount 1.2 times
greater than the stoichiometric quantity necessary for
reducing said quantity of the oxides. Said materials are
mixed together and charged into a metallic container, heated
to 1190°C, and kept for 9 hours. During the heating period,
the hydrogen formed in accordance with reduction reaction
(1) is removed from the container by combustion.
When the evolution of hydrogen ceases, argon is
supplied to the container, and a pressure of about 0.2 atm
is maintained therein till cooling is completed. In 9 hours
the container is cooled down to room temperature, and its
contents comprising a sintered mass ("sinter"), consisting
of metallic particles and calcium oxide (Ca0), are
discharged. The "sinter" is crushed under a press into lumps
about 10-50 mm in size, and the lumps are gradually, in
small portions, transferred to a tank with water, where
"liming" takes place in accordance with the reaction Ca0 +
+ H20 -~ Ca (OH) 2 + Q kcal. The contents of the tank are
treated further with hydrochloric acid (HC1) at pH 4-5 and
washed with water to remove CaCl2. The preservation of
residual Ca0 in the finished metallic powder is controlled
16
..
CA 02285072 1999-09-28
WO 98143763 PCT/IB98/00449
by the reaction of a wet powder sample with phenolphthalein;
slight coloring is permissible.
After drying, the powder contains, in weight %: Ti,
29.6; V, 28.4; CaO, 0.21; Zr being the balance. The powder
is rolled into 0.7x30x120 mm plates under a pressure of
about 80 kg/cm2 and sintered in vacuum at 880°C for 1 hour.
X-ray diffraction analysis showed the presence in the
resulting Better material of several phases having different
compositions, as well as zones whose composition is close to
pure metals, this being an indication that the Better
material is chemically heterogeneous. The degree of chemical
heterogeneity is determined as follows: the content of the
elements is determined under an electron-scan microscope in
five pairs (10 points? of arbitrarily chosen local zones. In
the case discussed the chemical composition of the material
at the 1St point proved to be, in weight o: Zr, 18.1; V,
21.0; Ti, 61.1; at the 2nd point: Zr, 64.0; V, 16.1; Ti,
21.9. The ratio of Zr concentration in the 1St pair of
points is determined by dividing the greater value of Zr
content by the smaller value, i.e., by dividing the result
of Zr determination at the 2nd point by the result at the 1st
point: 64.0:18.1 - 3.5.
- the ratio of V concentrations in the first pair is
determined by dividing the result at the 1St point by the
result at the 2°d point: 21.0:16.1 = 1.3: -
17
CA 02285072 1999-09-28
WO 98/43763 PCT/IB98/00449
- the ratio of Ti concentrations in the first pair is
determined by division: 61.1:21.9 = 2.7.
The ratio of concentrations of the elements at the 2"d,
3rd 4th and 5th pairs of the arbitrarily chosen zones is
determined in a similar manner: points 3-4, 5-6, 7-8, and
9-10.
The results of measurements are presented in Table 1.
18
T , . . .
CA 02285072 1999-09-28
WO 98/43763 PCT/IB98I00449
~ -
U
' _0 ~ a~ ov~ ~
~ C m
-.~ v
r.
-ri b w o . M M
~ d m
ro E
r-~ ~ N O U ~1
~ U H W
~ b
O
-rl I~O
N
ro .n . .Wo
N b ~D.-IM
m
v N
yN O
N -~ rl l0N N
~
N p.v
O Ln ~ ' ~
rlN OD
U
0
N
~--I iJ 00r o
ro a
N b ODN N
ro
r ~o
a ~
PI '~ Q'7 1nT M
Q.
1~ .-rW
N N . .
Ci 'C' o~a
lW n .-~r
O
-ri N l0
0 ~
, .n
,~ ro r, .-a.
N b ODM .1
l0O
~ ~
.~ -r/ ~ fDM
, COlDN
0
U
N
I d M
r N N
0
o
-.-i w
a~ a o
ro N . 6
V N b .-t--iM
b1
~Do v~
.~ovo~
M c .-i
r~
c .-a
C) ~ . ,n
N ~ m o~
M N u7
h l0tD~O
ro
N b a N t
O
r-1~
-rl N m oor
m
.--ao .-a
N
r-I ~-I riN 1D
u~ uo
J-~ JJ ow -
N C E; a~
C
rn ..a y-I -r-i
N N G
row ow o om
o o~ N ~ H
w ;r. o
o a w a.
a 3
19
CA 02285072 1999-09-28
WO 98143763 PCT/IB98/00449
The arithmetic mean values of the degree of chemical
heterogeneity of each of said elements were as follows: Zr,
5.9; V, 13.5; and Ti, 13.6. Hence, the arithmetic mean
values of the concentration ratios for each of the elements
entering into the Better composition proved to be smaller
than 30, and the resulting Better possesses a high sorption
activity. The sorption properties of the produced Better,
expressed as a dependence of the sorption rate on the
quantity of absorbed gases at room temperature are shown in
Figure 2 curve 1 for H2 and curve 3 for CO).
EXAMPhE 2
To prepare a powder containing, in weight o: chromium
(Cr), 25; calcium oxide (Ca0), less than 1; the balance
being titanium (Ti) , use is made of oxides Ti02, Cr~03, and
calcium hydride. Their quantities are calculated in
accordance with the reaction of reduction as in Example 1.
The charge obtained after mixing the components together is
heated to 1200°C, kept for 10 hours, and cooled down.
Crushing and hydrometallurgical treatment are carried out as
in Example 1. The resulting powder contains, in weight o:
chromium (Cr), 23.6; calcium oxide (Ca0), 0.24; titanium
(Ti) being the balance. The prepared powder is rolled under
a pressure of about 60 kg/cm2 to produce a 0.7x20x120_mm
plate, the latter being then sintered in vacuum at 900°C for
.._...~....~....._... . ~ , .,
CA 02285072 1999-09-28
WO 98/43763 PCT/IB98/00449
0.5 hour. Investigations showed that the titanium to _
chromium weight ratio both in the powder and in the Better
after sintering is different.
The degree of chemical heterogeneity in the Better is
determined as described in Example 1 in five pairs of
arbitrarily chosen points, at which the Ti and Cr content is
measured under with the help of electron-scan microscope.
The mean arithmetic values of the Ti and Cr concentration
ratios proved to be smaller than 30 and were 4.8 and 11.7,
respectively.
The gas sorption rate (S) as a function of the quantity
of absorbed gas (Q) is shown in Figure 2 (curve 2 for HZ and
curve 4 for CO).
EXAMPLE 3
To prepare 1 kg of a powder containing, in weight o: V,
30: Ca0 < l; Zr being the balance, a mixture is used,
consisting of (in kg) : V203, 0.440; ZrOz, 0.945; CaH2, 1.219.
Further the preparation is carried out as in Example 1.
Reduction is performed at 1200°C for 10 hours. Unloading and
further treatment of the powder are effected as in Example
1. The powder thus prepared contains, in weight %: vanadium
(V), 29.1; CaO, 0.31; the balance being zirconium (Zr).
Press-molding of the powder at a pressure of about 100
kg/cm2 and subsequent sintering thereof at 900°C for 1 hour_
21
CA 02285072 2000-03-31
22
gave Better eleme:zts in the form of tablets QS 20 mm, h 10
mm; rolling of thf~ powder gave 0.7x20x120 mm plates. An x-
ray spectrum anal~~rsis showed that the phases present in the
Better sample are mainly an intermetallic compound ZrV2 and
zones of differeni~ degree of interdiffusion of Zr and V. Ca0
is present as seo<irate inclusions.
The degree o1. chemical heterogeneity in the Better is
determined as described in Example 1 in 5 pairs of
arbitrarily chosen points, where the content of Zr and V was
measured. The arithmetic mean values of the Zr and V
concentration ratios proved to be smaller than 30 and equal
to 6. 1- and 17 . 3, respecl=.ively.
The initial sorption rate (S) with the quantity of
absorbed gas Q to 133 Pa m3/m2 was about 4 m3/m2 s.
EXAMPLE 4
To prepare 1 kg of a metallir_ powder containing, in
weight %: titanium (Ti), 70; vanadium (V), 30; and Ca0 no
more than 1, in accordance with calculations, use is made of
(kg-) : Ti02, 1. 160; V203, 0.440; and calcium hydride (CaH2) ,
1.990. Carrying out the operations as described in Example
1, the mixture is reduced at 1190°C for 12 hours. The
resulting powder contains, in weight o: V, 28.9; CaO, 0.29,
the balance being 'ri. A 0.7x20x150 mm sample was produced by
CA 02285072 1999-09-28
WO 98143763 PCT/IB98100449
rolling the powder in rolls at a pressure of about 40 It~/cm2
and subsequent sintering in vacuum at 850°C for 1 hour.
Control carried out using an electron-scan microscope
showed that the weight content of the elements entering into
the composition of the Better material is different. The
degree of chemical heterogeneity in the Better was
determined as described in Example 1 in 6 pairs of
arbitrarily chosen points, where the content of Ti and V was
measured. The mean arithmetic values of the Ti and V
concentration ratios proved to be smaller than 30, equal to
2.4 and 9.8, respectively.
Figure 3 shows sorption curves for hydrogen (curve 1)
and for carbon monoxide (curve 3). The collapsing force P
for a sample of 6 mm in diameter and 0.7 mm thick was 37 N.
EXAMPLE 5
Metallic powder TiV30 is prepared as described in
Example 4, and reduction of the oxides is performed as
described in the prior-art method: the reduction temperature
was 1175°C and keeping time was 6 hours. The metallic powder
thus prepared contains, in weight o: V, 29.45; CaO, 0.91; Ti
being the balance. Getter plates are produced by shaping
powders in rolls at a pressure of about 50 kg/cm2 with
subsequent sintering in vacuum at 850°C for 0.5 hour..__
23
CA 02285072 1999-09-28
WO 98/43763 PCT/IB98/00449
The results of investigations showed that in the
material thus produced the chemical heterogeneity compared
with the material produced by the method of and in
accordance with the invention (Example 4) is more
pronounced.
The degree of chemical heterogeneity in the getter is
determined as described in Example 1 in 8 pairs of
arbitrarily chosen points, in which the content of Ti and V
is measured. The arithmetic mean ratios of the Ti and V
concentrations proved to be 24.6 and 34.1, respectively. It
is apparent that while the nonuniformity of Ti distribution
is higher than in Example 4 but does not exceed the maximum
permissible value, the degree of nonuniformity of V
distribution exceeded the regulated level, equal to 30. The
obtained material possesses high mechanical properties. The
collapsing force P for a 6 mm-diameter and 0.7 mm thick
sample was 74 N, but its sorption properties are appreciably
inferior to those of the material produced by the method of
the present invention (see Figure 3, curves 2°and 4), so
that the getter cannot be used under conditions requiring a
high vacuum with large gas flows.
Nonevaporable getters produced according to the
invention posses high sorption properties for such gases as
H2, CO, O2, N2, and the like, in combination with
sufficiently high mechanical properties. This makes such
24
._._.~ w«...__... ..,.. , . . . ...
CA 02285072 1999-09-28
WO 98/43763 PCT/IB98/00449
Betters suitable for use in vacuum devices for establishing -
and maintaining a high vacuum level, e.g., in kinescopes,
cathode-ray tubes, particle accelerators, etc., where their
application contributes the attainment of residual pressures
lower than 10-1° Pa.