Note: Descriptions are shown in the official language in which they were submitted.
CA 02285161 1999-10-06
IN VITRO CULTURED LIGAMENT TISSUE AND METHOD OF MAKING SAME
SCOPE OF THE INVENTION
The present invention relates to a ligament tissue construct and a method of
making a
ligament tissue construct in vitro for implantation in a human or other animal
subject.
BACKGROUND OF THE INVENTION
Ligament tissues perform bone-to-bone connection in humans and other animals
and
most typically comprise a dense band of connective tissue which is primarily
composed of the
protein collagen. Ligament injuries which occur when the connective tissues
tear or detach from
bone completely are common and frequently do not heal well. For example, the
injury to the
ligaments of the knee, and in particular the anterior cruciate ligament (ACL),
has been the
subject of considerable research as ACL injuries often result in joint
instability and the
subsequent onset of osteoarthritis.
Current methods of ACL reconstruction involve a surgical autograft procedure
in which a
portion of the patient's patellar tendon or quadriceps tendon is harvested and
implanted at the
knee joint. It has been found, however, that although initially strong, the
tendon autograft tends
to remodel over time and is replaced by weaker scar tissue which is subject to
fatigue failure and
creep, leading to laxity in the joint. Remodeled autografts are weaker than
the original ligament
and are therefore susceptible to reinjury.
The use of tendon or ligament xenografts and allografts avoids the healing
problems
associated with harvesting autograft tendons. Ligament replacement with
xenograft and allograft
implants, however, introduce the problems associated with the potential
rejection of the donated
graft as well as possible disease transmission. Often supplies of allogeneic
material may be
limited, and a significant antigenic response is associated with the
implantation of unprocessed
allogeneic material. Xenografts may be more readily available, however, it is
typically more
CA 02285161 1999-10-06
2
antigenic than allograft implants. Even if the antigenic problems and
viral/prion transmission
threat associated with allogeneic and xenogeneic implants are neutralized
through chemical
processing, the problem of in vivo remodeling remains and weakened scar
tissues may form in
the same manner as within autogenous implant material.
In an effort to produce better graft materials, biodegradable polymers,
acellular and
chemically-processed biological materials and reconstituted chemically-
crosslinked collagen
matrices have been utilized. Although all these materials exhibit good initial
strength, the cells
that repopulate these grafts typically are not ACL specific fibroblasts and as
a result, they suffer
the same loss of strength as autografts. Furthermore, as polymers degrade,
they can incite a giant
cell (macrophage) reaction.
SUMMARY OF THE INVENTION
It is an object of the present invention to provide an engineered tissue for
use in replacing
damaged ligament and/or tendon tissues which mirrors as closely as possible
the original tissues
in terms of function, structure and composition.
Another object of the invention is to provide a method of forming tendon-like
or
ligament-like tissues in vitro for later implantation into a human or animal
subject.
A further object of the invention is to provide an in vitro cultured tendon-
like or
ligament-like tissue from source allogeneic or xenogeneic material, and which
has been seeded
with fibroblasts from the site of intended use.
The present approach to the in vitro formation of an implantable tendon-like
or ligament-
like tissue construct involves the creation of a ligament from a composite of
a donor or source
scaffold derived from allogeneic, autogeneic or most preferably xenogeneic
collagen fibers and
directly seeded ligament or tendon fibroblasts. More preferably, the seeded
fibroblasts are host
derived, expanded in vitro, and are seeded directly on a scaffold, such as
detergent extracted
xenogeneic tendon collagen fibers, polymers or other suitable biodegradable
materials. Most
CA 02285161 1999-10-06
preferably, following initial seeding, secondary collagen seeding is performed
around the
scaffold. Secondary, collagen seeding allows the efficient delivery of high
numbers of host site
fibroblasts and sufficient collagen for these cells to rapidly produce a
ligament/tendon tissue-like
material in vitro. Optionally, endothelial cells (host derived), peptides,
growth factors, cytokines
are also added with the collagen and cells during secondary seeding. The
construct is cultured in
vitro for a sufficient period of time to allow cell mediated matrix
organization and integration,
after which the construct may be implanted at the desired host site.
In a preferred embodiment, a rat tail tendon is selected as the source
xenograft for the
collagen fiber scaffold. The primary function of the scaffold material is to
provide high strength
during the first six to twelve months after implantation, and in that regard
it is similar to a
patellar autograft. The rat tail tendon core is composed of groups of
substantially aligned,
elongated tendon fibers, and the seeding of the rat tail tendon with
fibroblasts from the
implantation site is performed so as to maximize fibroblast attachment
throughout the tendon
fibers. The fibroblast cells improve tendon-collagen integration.
Although not essential, the tendon or ligament construct may be contiguous at
one or
each end with a disc, plug and/or pin formed from an implantable material.
Suitable implantable
materials would include titanium or stainless steel, as well as biomaterials
such as porous
condensed calcium polyphosphate (CPP), as is disclosed in Canadian Patent
Application No.
2,252,860, filed 15 May 1997, and laid open to the public December 4, 1997.
Optionally, cyclic
or continuous loading can be used during the in vitro culturing of the
ligament-type tissue, to
stimulate cell mediated matrix organization and crosslinking.
BRIEF DESCRIPTION OF THE DRAWINGS
Reference is now made to the following detailed description, taken together
with the
accompanying drawings in which:
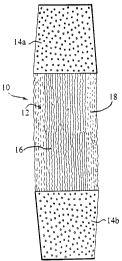
Fig. 1 is the appearance of a ligament-like tissue construct in culture and
formed in
accordance with a preferred embodiment of the invention;
CA 02285161 1999-10-06
4
Fig. 2 is a photomicrograph of an engineered ligament tissue grown in
accordance with
the present invention under four weeks of constant tension; and
Fig. 3 is a photomicrograph of an engineered ligament tissue grown in
accordance with
the present invention under two weeks of constant tension interrupted with
daily periods of
cyclic tension.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The invention as shown in Figure 1 relates to a ligament-like construct 10
which has been
prepared in vitro for later implantation into a human or animal host subject
as either a ligament
and/or tendon tissue.
The construct 10 consists of an engineered fibrous ligament-like tissue 12
which is
contiguous at each of its ends with an implantable plug 14a,14b. As will be
described hereafter,
the engineered ligament-like tissue 12 is formed in vitro by seeding and
culturing ligament or
tendon fibroblast cells in collagen material surrounding a suitable scaffold.
The plugs 14a,14b
are formed from a porous condensed calcium polyphosphate (CCP) material, such
as that
described in Canadian Patent Application No. 2,252,860 to facilitate bone
growth therein. The
engineered ligament tissues 12 may be mechanically attached to each plug
14a,14b or
alternately, may be cultured directly thereon. The design of the
biodegradablelbioabsorbable
CPP facilitates osteoblast ingrowth and it will become replaced with bone over
time.
To insert the construct 10 into a host patient, a bore corresponding in
diameter to each
plug l4a,l4b is formed in each of the patient's bones to be joined by
construct. The construct 10
is then positioned in place by fitting in each plug into the complementary
bores in a pressure fit
manner.
(a) Scaffold Preparation
In the in vitro preparation of the engineered ligament tissue 12, a source
xenogeneic
tendon is initially obtained to serve as the framework or scaffold for the
cultured ligament.
CA 02285161 1999-10-06
Preferably, the source xenograft is selected from tendon tissues characterized
by spaghetti-like
collagen fibers which can be extracted as an elongated bundle and readily
separated from each
other.
The tails of rats have been found to be particularly suitable as providing a
suitable three
dimensional scaffold material. The tendon in rat tail is composed of numerous
very long fibers
each with a diameter of approximately 100 ~m and, unlike most tendons, these
fibers are
physically distinct and easily separated. Bundles of rat tail fibers are very
strong with maximum
strength comparable to that of ligaments. In addition, mechanical testing on
rat tail tendon
samples reveals that their mechanical properties compare well with that of
human anterior
cruciate ligament. As previously indicated bundles of tendon fibers can be
combined to match
the dimensions and strength of the tissue it would replace. For example, to
match the properties
of the human ACL, which has an ultimate tensile load of 2195 N and stiffness
of 306 N (Am. J.
Sports Med 25, 472-478, 1997), the diameter of and length of the tendon
construct would need to
be 7mm and 75mm respectively. A tendon bundle with a diameter of 3-4 mm would
provide
initial strength to that of traditional grafts used in the tendon and ligament
repair (381-678 N).
The rat tail fibers are composed primarily of type I collagen, a highly
conserved protein that
differs little from the type I collagen that makes up the bulk of human ACL.
Fibroblasts attach
rapidly to type I collagen and grow in contact with this protein. Although rat
tail tendon will be
implanted as a modified xenograft as will be described, the potential
immunogenicity of the graft
is first mitigated through detergent extraction and coating with allogeneic or
autogeneic collagen
and eventually autogeneic fibroblasts. Moreover, the rat-tail tendon scaffold
is advantageously
biodegradable and will eventually be replaced in vivo with ligamentous tissue.
In use, the rat tail is severed at its base following euthanasia of the rat.
The tail is then
frozen and thawed three times to kill and lyse the tendon cells, after which
the tail is immersed in
a 4°C bath consisting of 70% ethanol for a minimum of 20 minutes to
kill any bacteria. The
tendon xenograft is then physically extracted from the tail by pulling
longitudinally with
hemostats. The extracted tendon xenograft has a length of between about 2 and
15 cm, and most
preferably about 10 cm, possessing a spaghetti-like fiberous construction in
which each of the
tendon fibers are elongated and generally parallel to each other.
CA 02285161 1999-10-06
6
Following the physical extraction of the source tendon xenograft, the tendon
fibers are
again immersed in a 70% ethanol bath at room temperature for a period of
approximately 30
minutes, to kill most of the remaining contaminating bacteria and reduce the
lipid content of the
tendon.
The tendon fibers are thereafter cleaned by washing sequentially ten times in
a beaker
containing 100-250 ml of an aqueous buffered solution having a pH selected
between 6.5 and 8.
Preferred solutions include phosphate buffered saline solution (PBS) which is
magnesium and
calcium-free. The tendon fibers are maintained in the PBS bath for a period of
30 minutes and at
a temperature of between about 20 and 25°C. The immersion of the tendon
fibers in the
phosphate buffered saline solution (PBS) removes further cellular debris and
blood. The absence
of Mg2+/Ca2+ further inactivates some proteases.
Further cleaning is next performed by immersing the tendon fibers in a
cleansing bath of
250-500 ml of a water and non-ionic detergent solution. Suitable solutions
would therefore
include those containing 0.1 % Triton-X 100TM detergent, and the solution is
maintained at room
temperature (20-25°C) and changed three times over a 24 hour period. On
immersion in the
solution, the tendon fibers optionally may be gently agitated to remove non-
collagenous proteins,
some lipids, as well as antigenic elements from within the fiber bundle.
After cleansing in the 0.1 % Triton-X 100TM detergent solution, the tendon
fibers are
incubated for 72 hours in 250-500 ml of a 0.1 % ionic detergent solution of
sodium dodecyl
sulfate (SDS). The SDS solution is kept at room temperature and changed three
times over a 72
hour period. Incubation in the SDS solution removes any additional antigenic
elements.
The extracted tendon fibers are thereafter washed 10 times with 250-500 ml
water over 1
hour followed by 10 changes of 250-500 ml purified water over 24 hours at room
temperature to
remove any residual detergent. Histologically the tendon appears acellular
after this extraction
procedure. Strength is not significantly affected. Higher concentrations of
either detergent,
CA 02285161 1999-10-06
7
although possible, disadvantageously disrupt tendon collagen organization and
reduce the
strength of the tendon.
(b) Initial Collagen Seeding
Tendon fibers are organized into bundles of appropriate diameter to match that
of
ligament and provide sufficient strength. Preferably, the ends of the bundles
are tied at their ends
to maintain the generally longitudinal orientation. Preferably the tendon
fibers are held in
longitudinal tension and the ends of the tendon fibers are tied off, crimped
or otherwise secured
to maintain the generally longitudinal orientation of the individual fibers.
The bundles of tendon
fibers form a scaffold for ligament cell growth and serve as a very strong
stint following
implantation. The individual tendon fiber diameter ( 100 pm) and composition
are ideal for
ligament fibroblast attachment. It is expected that the rat tail tendon fibers
will remodel overtime
and will be replaced with new collagen over a period of time after
implantation.
The tendon scaffold is next again sterilized, as for example, by incubation in
70% ethanol
for 30 minutes with mixing (21°C) followed by incubation in lOx
antibiotic for 24 hours (37°C in
tissue culture incubator). The sterility of the scaffold is then confirmed by
swabbing or
incubation in medium without antibiotic for 48 hours (37°C in tissue
culture incubator
supplemented with 5% C02 and high humidity).
The scaffold tendon fibers are then positioned within a seeding trough and
directly
seeded with ligament fibroblasts. Although not essential, the initial seeding
is preferably
performed with the scaffold longitudinally stretched in a trough and medium
with seeding
fibroblast cells which have been harvested from the host site at which the
construct is to be
implanted is added. The medium transferred to the seeding trough contains
ligament fibroblasts
(0.5 to 10 x 106 cells) and is seeded directly onto a bundle of tendon fibers
in minimal volume of
medium, such as DME (Dulbecco's Modified Eagle's Medium). The spaghetti-like
fibers of the
scaffold are physically separated during initial collagen seeding to permit
the fibroblast cells to
penetrate throughout the scaffold interior. The result is that fibroblast
attachment is not merely
restricted to the outer periphery of the scaffold, but extends through its
entirety.
CA 02285161 1999-10-06
8
The fibroblasts are most preferably ligament specific and expanded in vitro.
Direct
seeding of tendon fibers ensures ligament fibroblast infiltration into the
tendon fiber bundle and
facilitates collagen seeded fibroblast layer integration. Cells are allowed to
attach and proliferate
for 2-3 days (37°C in tissue culture incubator, supplemented with 5%
COz and high humidity)
while periodically topping up the seeding medium.
The seeded scaffold 16 (Figure 1 ) is moved directly from the trough and
secured under
constant low tension in an axially vertical position within a vertically
oriented cylindrical tube.
The seeded scaffold is grown for a further 3 to 4 days to allow matrix
production and cell
proliferation prior to secondary fibroblast seeding in a collagen solution.
(c) Secondary Seeding
Following initial fibroblast seeding and growth on the seeded scaffold 16,
secondary
seeding is performed wherein the scaffold 16 is seeded with ligament specific
fibroblasts
combined with purified type I collagen. Sterile acid or pepsin purified
collagen dissolved in
acetic acid (pH 3.0) 1mM HC1 (4°C) is added to fibroblasts suspended in
a suitable medium
(37°C) Dulbecco's Modified Eagle's medium (DME) supplemented with 15%
fetal bovine serum
and ascorbic acid (10-100 ug/ml). The medium concentration is adjusted for the
diluting effect
of the collagen solution and then the pH is adjusted to 7.2 with NaOH. The
final collagen
concentration ranges from 0.6-1.0 mg/ml, and final cell concentration ranges
from 0.8-3 x 105
cells/ml, although the total amount of collagen and cell number depends on the
size of tissue to
be formed.
The tube is filled (20 to 90% by volume) with the hydrated collagen and cell
solution and
incubated at 37°C. The mixture polymerizes around the seeded scaffold
16 very rapidly at 37°C.
The collagenous matrix 18 is contracted by the fibroblasts around the seeded
tendon scaffold.
The cellular matrix shrinks radially and longitudinally towards the scaffold,
forming a construct
having a generally elongated cylindrical configuration. After 1 to 4 weeks of
culture in vitro
CA 02285161 1999-10-06
9
with feeding as needed, the cells have reorganized and remodeled the collagen
matrix into a
ligament-like tissue 12.
Remodeling of the collagen matrix by ligament fibroblasts is preferably
achieved with the
tissue grown under tension. Constant tension is sufficient to induce matrix
reorganization. This
organization is, however, more rapid when the tissue is subjected to periods
of cyclic tension
with or without constant tension (lHz, 1800-36000 cycles/day). Figures 2 and 3
show the effect
of the application of cyclic tension. Figure 2 represents the ligament-like
tissue following
culturing under constant tension for 4 weeks. Figure 3 shows the ligament-like
tissue following
culturing for 2 weeks under constant tension with daily periods of cyclic
tension at 1 Hz, 1800
cycles/day. Figure 3 shows enhanced organization of the fibroblasts and
increased alignment of
collagen fibers achieved with cyclic tension over a shorter culturing time.
Some crimping of the
collagen fibers, more closely resembling undamaged ligaments, may also be
seen.
The shape of this tissue can be modified by changing the shape of the chamber
used
during secondary collagen seeding and/or by the use of internal anchors or
structures within the
chamber. In particular, the tube shape may be altered to form a ligament
construct having a
desired profile, or the anchors could be employed within the tube to secure
the engineered tissue
into a desired shape. For example, where a generally cylindrical ligament
implant is desired,
secondary seeding of the scaffold is performed in a generally cylindrical
tube. Where a flatter
ligament construct is desired, the secondary seeding may occur in an elongated
thin rectangular
tube. Similarly, the overall dimensions of the tube may be adjusted depending
upon the amount
of collagen and number of cells which are to be delivered and on the size of
the ligament to be
constructed.
Secondary collagen seeding of the tendon scaffold ensures a known number of
ligament
fibroblasts are delivered to the engineered tissue, as contrasted with direct
seeding which fails to
ensure that all seeded cells attach to the scaffold and where a substantial
proportion may fall
through the spaces between the fibers. The presence of sufficient cell numbers
around the
scaffold ensures adequate in vitro and in vivo remodeling. Where a larger
number of cells are to
CA 02285161 1999-10-06
be seeded, larger tubes, a larger volume of hydrated collagen material, and
longer incubation
times are used to perform secondary seeding functions.
In addition to acting as an efficient way to deliver a large and known number
of cells to
the scaffold, collagen seeding also provides the engineered tissue with a
substantial amount of
collagen which can be reorganized into ligament-like matrix. This protein
makes up
approximately 80% of the dry weight of a ligament. The number of fibroblasts
normally used to
collagen seed would not be able to synthesize the equivalent amount of
collagen during the same
culture period in vitro.
While the preferred embodiment discloses the use of CCP plugs 14a,14b, it is
to be
appreciated that the plugs could be omitted in their entirety and the
engineered ligament 12
implanted directly. Alternatively, other implantable structures including by
way of example
staples, pins, screws and the like could also be used. The plugs 14a,14b may
be formed from any
biologically suitable materials, including by way of non-limiting examples,
metals, resins,
minerals and plastics, which will now become apparent to persons skilled in
the art.
Although the preferred embodiment of the invention describes and illustrates
rat tail
tendons as being a suitable scaffolding structure, it is to be appreciated
that other types of
tendons and tissues may also be used with the present invention including by
way of non-
limiting example, tail tendons from other animals of the order R ti or
Marsupialia.
Although not essential, source tendons would preferably also have a similar
fibrous structure to
permit cell attachment and/or movement into the interior of the tendon tissue
bundle.
Reconstituted collagen fibers may also be potentially suitable but are not as
strong and highly
cross-linked.
Although the detailed description describes the preferred embodiment in the
formation of
a ligament-like tissue, it is to be appreciated that tendon-like tissues could
be formed in a similar
manner.
CA 02285161 1999-10-06
11
While the detailed description describes various preferred embodiments, the
invention is
not so limited. Many modifications and variations will now occur to persons
skilled in the art.
For a more precise definition of the invention, reference may be had to the
appended claims.