Note: Descriptions are shown in the official language in which they were submitted.
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
PHOTOCATALYTIC REACTOR AND METHOD FOR DESTRUCTION OF
ORGANIC AIR-BORNE POLLUTANTS
Field of the Invention
The present invention relates to reactors for the control of pollutant
emissions
from manufacturing facilities and more particularly, to a novel photocatalytic
reactor
and method for the destruction of volatile organic air-bome pollutants.
to Background of the Invention
Increasing interest is steadily growing over the removal of the undesired
organic contaminants from air streams. This is partially due to the fact that
chemical
plants and manufacturing facilities, especially petrochemical plants,
increasingly emit
air-borne pollutants. Air pollutants of major concern belong to three main
classes:
metals, organic and inorganic substances. Organic emissions represent a class
of
chemicals that can be produced for example during the incomplete consumption
of
fuels used for heating and transportation. A specific class of organic
emissions are
named volatile organic compounds (VOCs). These are produced in various
industrial
operations such as paint drying, metal degreasing, printing and air striping
units.
VOC effluents cannot be vented directly from industrial and commercial sites
due to
their potential health hazards. The emissions must therefore be treated before
released to the environment. Organic chemical species can be either totally
mineralized (destroyed) or treated by absorption, adsorption, incineration,
and
condensation (Miller et al., 1993).
Adsorption processes involve contacting a polluted gaseous stream with
activated carbon granules. The carbon granules act to adsorb the organic
molecules in
the gaseous stream leaving a clean air effluent stream. However, this process
does not
provide the complete destruction of the pollutants and instead only acts to
transfer
pollutants from the gaseous phase to the solid phase thus creating a solid
disposal
problem. In addition, this method is limited to gaseous streams with
relatively low
concentrations of organic molecules (Miller et al., 1993), because of the
finite carbon
adsorption capacity. Carbon particles also require regeneration and eventual
disposal
1
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
which represents a significant extra cost and difficulty to the process.
Finally, this
method does not suit all potential organic pollutants since not all of them
have good
adsorbability properties on the activated carbon particles.
Condensation, is not considered as a possible treatment approach because its
potential use is well outside the limits set for organic pollutant
concentrations. While
incineration, whether direct or catalytic, has a very high operating cost
which presents
a serious burden on the users of this technology (Miller et al., 1993).
On the other hand, total or complete destruction or mineralization of the
organic pollutants may be achieved naturally or using an oxidation process.
Natural
organic degradation is initiated by sunlight and molecular oxygen which are
naturally
abundant. However, this process is very slow and may take years to come to
completion. As a result, new technologies are currently being considered to
speed up
these processes. One approach towards pollution abatement at chemical plant
sites is
to manage or control the "source of emission" by various mechanisms such as
using
Advanced Oxidation Processes (AOPs). The purification of water and air using
photocatalysts is one promising methodology of the so-called advanced
oxidation
processes.
Advanced oxidation processes are usually classified as homogeneous and
heterogeneous processes. In the heterogeneous processes the surface of an
illuminated semi-conductor acts, at ambient temperature, as a catalyst by
using band
gap light as a source of solid excitation (Peral et al., 1992). On the other
hand, the
homogeneous process involves the UV photolysis of chemicals such as H20Z and
03
to produce 'OH radicals which are directly involved in the reaction (Bolton et
al.,
1995). The main principle involved in the homogeneous process is the
generation of
hydroxyl radical (OH). As the -OH radicals are formed, they attack the organic
molecules and react with the pollutant in one of two ways. One possible path
is the
abstraction of a H atom forming a water molecule and another radical. Another
possibility is the addition reaction which requires the addition of an -OH
group to the
pollutant molecule forming a combined pollutant *OH radical. The process
continues
with a series of reaction steps giving water, carbon dioxide and inorganic
salts as end
products.
2
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Heterogeneous advanced oxidation treatment involves the accelerated
oxidation of the desired chemicals with the help of ultra-violet light and
semi-
conductors acting as catalysts. This process utilizes TiO2 (anatase) as the
photocatalyst due the fact that it is insoluble, non-toxic, has a powerful
oxidizing
ability, it can be excited with solar light and it is attachable to various
types of
supports. The possibilities for photocatalytic technology is very impressive
given the
minimum energy cost, or essentially zero energy cost when solar energy is
employed
for powering the photoreactors. Potential applications for photocatalytic
reactors
cover the degradation of a wide spectrum of impurity levels contained in the
air as
well as in industrial waste water and potable domestic water. Photocatalytic
processes
are also advantageous due to the fact that there is no chemical addition other
than the
catalyst. Also, catalyst recovery or regeneration is possible and energy is
relatively
inexpensive, renewable and environmentally friendly.
Carey et al (1976) were among the first to utilize Ti02 for the photocatalytic
degradation of pollutants and reported that by using a light beam with a wave
length
of 365 nm it was possible to achieve degradation of chloro-organic molecules
in
water. Near UV irradiated TiO2 can also be applied for the photoconversion of
organic air-borne pollutants (Holden et al, 1993). Various organic molecules
such as
alkanes, alkenes, alcohols, aldehydes and aromatics have all been found
susceptible to
this treatment. In the case of non-chlorinated compounds, no intermediate
products
have been observed with pollutants being completely converted to carbon
dioxide.
For chlorinated compounds, chlorine and phosgene intermediates have been
observed
(Holden et al, 1993). While results for photocatalytic degradation of
pollutants are
encouraging, several aspects of the technology, including catalyst activity,
activity
decay with time-on-stream and catalyst regeneration are not optimal (Luo and
Ollis,
1996, Jacoby et al, 1996) and therefore a desired high level of efficiency is
not
achieved.
While some of the basic principles for photocatalysis are relatively well
understood, suitable photoreactors for achieving high energy efficiency and
complete
photoconversion of intermediates have not yet been designed. Photocatalytic
reactors
designed for air borne pollutants involve different approaches for supporting
the
photocatalyst and for photoreactor configurations. The main choices reported
are:
3
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
a) entrapment of TiO2 in a glass mesh (Al-Ekabi et al, 1993), b) support of
the TiOZ in
coated tubes (lbushki et al, 1993) and in honeycombs (Suzuki, 1993) and c)
holding
Ti02 in a ceramic membrane (Anderson et al, 1993). While the designs in b) and
c)
are of limited applicability for large volumes of gases, the use of TiO2
embedded in a
fiber glass mesh is an option that offers considerable potential. In this
respect, a
photocatalytic reactor based on this principle was reported by Al-Ekabi et al,
(1993)
which utilized several layers of an Ti02 impregnated mesh "enwrapped" on an
emitting light source of the photoreactor. However, this method and the
reactor had
several intrinsic limitations such as a lack of a secure degree of Ti02
loading in the
1 o crystalline "anatase" form and the lack of intimate or uniform contact of
the evolving
fluid (i.e. polluted air) with the me . Finally, only a very limited fraction
of the
immobilized Ti02 was being irr: .ated.
There is therefore an apparent need to develop a photocatalysis system for
oxidizing impurities in a more efficient and effective system than could be
previously
accomplished by known prior art systems. It is therefore an object of the
present
invention to provide an advantageous and novel photocatalytic reactor suitable
to
process different types of air streams containing various amounts of volatile
organic
carbon pollutants in order to destroy the pollutants therein, which overcomes
at least
one of the problems and shortcomings encountered using known photocatalytic
systems.
Summary of the Invention
In accordance with the present invention, there has been developed a novel
photocatalytic reactor and method for the treatment and degradation of organic
air-
borne pollutants. The novel photocatalytic reactor, herein referred to as the
Photo-
CREC-Air Reactor, is useful for air purification and utilizes TiOZ/UV photo-
oxidation
technology. This reactor has been designed to provide a novel geometric
configuration with optimal Ti02 catalyst loading and directed light
distribution to
yield optimal catalytic destruction of air borne pollutants. The fundamentally
based
novel design provides for optimal physico-chemical reactions and engineering
aspects
of the technology.
4
CA 02286152 2006-07-26
The novel photocatalytic reactor of the present invention has a variety of
applications not only limited to the control of organic pollutant emission
from
manufacturing and commercial facilities, but also for the remediation of
contaminated
soils and groundwater for the improvement of indoor or closed system air
quality and
for the destruction of air borne microorganism contaminants. The novel
photoreactor
of the present invention has been named the Photo-CRECTM-Air reactor
incorporating
Ti02/UV technology in a highly energy efficient system which is able to
photoconvert
significant amounts of pollutants with minimum light power.
According to an object of the present invention is a photocatalytic reactor
for
the destruction of organic air-borne pollutants, the photoreactor comprising a
means
for admission of a gas stream carrying air-borne volatile organic pollutants
into the
photoreactor, a means for directing and increasing the velocity of the gas
stream while
simultaneously creating a suction effect, and a means for oxidizing and
degrading the
air-borne volatile organic pollutants within the gas stream. Preferably, the
oxidizing
means is positioned transversely with respect to the air stream flow.
In accordance with an aspect of the present invention, there is provided a
photocatalytic reactor for the destruction of organic air-borne pollutants,
the
photoreactor comprising;
means for admission of a gas stream carrying air-borne volatile organic
pollutants into a closed tubing system;
a Venturi section for constraining and increasing the velocity of the gas
stream
while simultaneously creating a suction effect to promote self-cleaning of
said Venturi section by the removal of dust and dirt from the air stream and
preventing dust and dirt to accumulate within said Venturi section; and
means for irradiating the air-borne volatile organic pollutants within the gas
stream;
wherein said Venturi section comprises an elongate pipe having a convergent
section, a straight section and a divergent section, said divergent section
having a UV light illuminating means and a reflective means to direct
reflected light onto the irradiating means.
In accordance with another aspect of the present invention, there is provided
a
photocatalytic reactor for the destruction of organic air-borne pollutants,
the
photoreactor comprising;
5
CA 02286152 2006-07-26
a system for containing and enclosing a gas stream;
inlet means for admission of said gas stream within said
system;
means for irradiating the air-borne volatile organic pollutants within said
gas
stream;
a Venturi section for constraining and increasing the velocity of said gas
stream
while simultaneously creating a suction effect to promote self-cleaning of
said
Venturi sections, said Venturi section comprising an elongate pipe having a
convergent section, a straight section and a divergent section, said divergent
section having a UV light illuminating means and a reflective means to direct
reflected light onto the irradiating means;
an outlet means located downstream of said irradiating means to release the
treated air stream from said reactor; and
a fan means located upstream of said irradiating means to circulate the gas
stream
towards the irradiating means.
In accordance with another aspect of the present invention, there is provided
a
method for the destruction of organic air-borne pollutants, said method
comprising the
steps of:
circulating a gas stream carrying volatile organic pollutants therein through
a
closed tubing system comprising a Venturi section for constraining and
increasing the velocity of the gas stream while simultaneously creating a
suction effect, the Venturi section comprising an elongate pipe having a
convergent section, a straight section and a divergent section, said divergent
section having a UV light illuminating means and a reflective means to direct
reflected light onto the irradiating means; and
directing said gas stream through said irradiating means for degradation of
the
pollutants.
The photoreacior may additionally include a mechanism to recirculate the
treated gas stream back through the reactor. Additionally, the photoreactor
may be
designed to allow air streams to pass through without any photocatalytic
treatment.
According to another object of the present invention is a method for the
preparation of a supported photocatalyst which method comprises applying a
desired
5a
CA 02286152 2006-07-26
catalyst to a fibrous transparent mesh and fixing the catalyst to the mesh
until
a desired amount of homogeneously loaded catalyst is achieved.
According to another object of the present invention is a supported
photocatalyst adapted for the photoxidation of organic pollutants in an air
stream, the
5b
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
supported photocatalyst comprising a transparent fibrous mesh having several
layers
of fixed catalyst and containing up to 50% catalyst per gram of fibrous mesh.
Preferably, the catalyst loaded mesh is supported by a perforated heated
plate.
To demonstrate the performance of the reactor developed, toluene was
employed as an example of a model pollutant. Performance evaluation also
involved
the qualitative and quantitative analysis of intermediate species and end
products
conducted at operating conditions representative of air treatment equipment.
The
photocatalytic reactor of the present invention was used to examine the
effects of
water vapor content, temperature, pollutant concentration on the
photocatalytic
oxidation rate which provided data to establish a photodegradation rate model
as an
aid for extrapolation and scaling up of the system for commercial
applications.
The photocatalytic reactor of the present invention provides excellent
oxidation and thus destruction of pollutants and thus the reactor can now be
used for
scale up and commercialization at industrial/residential sites.
Brief Description of the Drawings
A detailed description of the preferred embodiments is provided herein below
with reference to the following drawings in which:
Figure 1 shows a side elevational view of the catalytic photoreactor in
accordance with the preferred embodiment of the present invention;
Figure 2 shows a schematic representation of the Venturi section of Figure 1;
Figure 3 shows a sectional view through lines A-A of Figure 2;
Figure 4 shows the details of the Photo-CREC-Air reflector;
Figure 5 shows the perforated plate;
Figure 6 shows TPD of the 3M Blue Pleated Filter. The full line represents
the water desorption from the mesh. The dashed line is the adopted temperature
prograin;
Figure 7 shows a single treated strand of glass mesh with TiOZ firmly attached
to the strand;
Figure 8 shows the toluene/air ratio versus time, the internal standard used
in
the experimental runs;
6
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Figure 9 shows the velocity profile at 25 C, the average superficial velocity
being 2.83 m/s;
Figure 9B shows the velocity profile at 97 C, the average superficial velocity
being 3.0 m/s;
Figure l0A shows the UV intensity profile across the filter sectional area
with
r=0 representing the center of the filter. Position 1: 0 degrees; Position
2:90 degrees;
Position 3:180 degrees; Position 4:270 degrees;
Figure lOB shows the radial UV intensity decay profile across the mesh with
r=0 representing the center of the mesh;
Figure 11 A shows the results of blank runs using the Photo-CREC-Air reactor
lacking Ti02 mesh and with no UV irradiation at 20 C;
Figure 11B shows the results of blank runs using the Photo-CREC-Air reactor
lacking TiO2 mesh and with no W irradiation at 100 C;
Figure 12 shows experimental curves of changes of reactant and product
concentration as a function of time-on-stream with toluene concentration being
10.4
g/cm' and heating plate at 100 C;
Figure 13A shows an experimental run using the Photo-CREC-Air reactor
with an initial toluene concentration of 5.2 g/cm', temperature at 100 C,
water level
below 25 g/cm';
Figure 13B shows an experimental run using the Photo-CREC-Air reactor
with an initial toluene concentration of 7.78 g/cm', temperature at 100 C,
water
level below 25 g/cm';
Figure 13C shows an experimental run using the Photo-CREC-Air reactor
with an initial toluene concentration of 10.4 g/cm', temperature at 100 C,
water level
below 25 g/cm';
Figure 13D shows an experimental run using the Photo-CREC-Air reactor
with an initial toluene concentration of 13.0 g/cm', temperature at 100 C,
water level
below 25 g/cm';
Figure 14 shows the rate of toluene oxidation as a function of the initial
toluene concentration;
7
CA 02286152 1999-10-06
WO 98/46335 . PCT/CA98/00314
Figure 15A shows an experimental run using the Photo-CREC-Air reactor
with an initial toluene concentration of 10.4 g/cm3, temperature at 75 C,
water level
below 25 g/cm3;
Figure 15B shows an experimental run using the Photo-CREC-Air reactor
with an initial toluene concentration of 10.4 g/cm3, temperature at 50 C,
water level
below 25 g/cm';
Figure 15C shows an experimental run using the Photo-CREC-Air reactor
with an initial toluene concentration of 10.4 g/cm', temperature at 20 C,
water level
below 25 g/cm';
Figure 15D shows an experimental run using the Photo-CREC-Air reactor
with an initial toluene concentration of 10.4 g/cm', temperature at 100 C,
water level
about 30 }eg/cm';
Figure 16A the kinetic constants for the different initial toluene
concentration:
Figure 16B shows the quantum yields assessed for the different toluene initial
concentrations studied;
Figure 17A shows the simulated chemical species distribution for the
operating constants and conditions: k'=0.03(hr-'), k2=0.03(hr-'), and Co=18
g/cm3
(5000ppm);
Figure 17B shows the simulated chemical species distribution for the
operating constants and conditions: k'=0.03(hr''), k2=0.22(hf'), and Co=18
g/cm3
(5000ppm);
Figure 17C shows the simulated chemical species distribution for the
operating constants and conditions: k'=0.03(hr''), kz=2.2(hr''), and Co=18
g/cm3
(5000ppm); and
Figure 18 shows the estimated errors of the kinetic parameters associated with
the different measured variables.
In the drawings, preferred embodiments of the invention are illustrated by way
of example. It is to be expressly understood that the description and drawings
are only
for the purpose of illustration and as an aid to understanding and are not
intended as a
definition of the limits of the invention.
8
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Detailed Description of the Preferred Embodiments
Those skilled in the art of chemistry and in particular photochemistry and
physics will understand the various nomenclature used throughout this
application.
However, to facilitate a clear understanding of such nomenclature it is
clearly
described in Tablel.
Prior to the detailed description of the photocatalytic reactor of the present
invention, it is important to explain some of the fundamental aspects of
photocatalysis
using Ti02 as a catalyst in order that the numerous advantages of the
presently
developed novel photocatalytic reactor be fully understood and appreciated.
]0
Photocatalysts
Photocatalysts are usually semi-conductor materials that enhance the
photocatalytic reaction by lowering the required activation energy of the
reaction due
to the special electronic band structure they posses. A wide range of semi-
conductors
have been tested for photocatalytic processes such as: TiOZ, ZnO, FeZ031 W03,
CdS,
and ZnS. However, the desirable properties of TiO, in terms of catalytic
activity,
chemical stability, non toxicity, relative inexpensive cost and availability
make it the
most desirable photocatalyst.
Photocatalysts, and more specifically Ti021 possess a special electronic band
structure. As a semi-conductor, it contains equally spaced energy levels with
electrons termed "valence band". In addition, there is another set of equally
spaced
energy levels, at a higher state, which are electron deficient called the
"conduction
band". The separation between these two bands is termed the "band gap". When a
photocatalyst undergoes illumination by a light source emitting radiation at a
specific
wavelength with an energy equal or greater than that of the band gap, it
absorbs the
energy promoting an electron excitation from the valence band, to the
conduction
band. This leaves a fraction of the surface with electron deficiency forming a
hole
denoted as h. This process is illustrated in the following equation:
Ti02+h v --_.-> h+ + e' (2.1)
The above described step lowers the activation energy of the oxidation
reaction of pollutants with oxygen. The final products of this oxidation
reaction are
9
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
eventually, in most cases, carbon dioxide, water and several other mineral
salts.
Titanium dioxide is a semi-conductor with a chemical formula of TiOZ. It does
not
dissolve in water which makes it a very good candidate for water treatment
processes.
However, it dissolves in alcohol and organic solvents such as methanol and
acetone.
TiO2 powder is white in color, has no smell and it crystallizes in two forms:
anatase
and rutile. Ti02 in the rutile crystallographic form, is usually used as a
pigment in
white paints and as a base in cosmetic products.
Light absorption of Ti02 is suitable at the band gap light energy between 230-
390 nm. This region falls in the near ultraviolet spectrum. Anatase phase has
demonstrated great efficiency in terms of photocatalytic activity much more
than the
rutile phase. The band gap of the anatase crystal is 3.2 eV (Bolton et al.,
1995), and
this indicates that only photons that have a 290-390 nm wavelength may be
absorbed
by the crystals.
Photoreactor Design and Confieuration
The photocatalytic reactor of the present invention was designed to be highly
efficient and promote total pollutant mineralization. In order to achieve this
the
following factors were specifically developed and optimized: the LN source,
reactor
configuration, special lamp arrangement, catalyst type, size, distribution and
impregnation and efficient interaction between the light, the catalyst and the
reacting
fluid.
A significant number of factors were considered during the development of the
reactor configuration of the present invention such as the different classes
of
pollutants, pollutant concentrations and operating conditions. Operating
conditions
involve temperature, relative humidity, pressure, space time and irradiation
time.
With respect to the specific application of gaseous streams containing organic
pollutants solid-gas reactors with Ti02 particles, constituting the solid
phase are
considered. Ti02 particles may be held on a solid surface in many different
ways
including:
a) A thin film of Ti02 coated on the inner surface of the reactor wall (Jacoby
et a1.,1996).
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
b) Ti02 supported onto a porous fibrous mesh (Peral et al., 1992), or monolith
(Blanco et al., 1996, Obee et al., 1995, Honeycomb Suzuki 1993).
c) Ti02 entrapped in supporting particles (Dibble et al., 1992, Yamazaki-
Nishida et al., 1993, Yamazaki-Nishida et al., 1994 and Anderson et al.,
1993).
d) Ti02 coated on an optical fiber bundle. Optical fibers have the advantage
of direct fiber-photocatalyst radiation transfer and high activated surface
area to reactor volume. Care has to be taken given the potential catalyst
deactivation due to heat build up in the fiber optic bundle array (Peill et
al.,
1995 and Peill et al., 1996).
Regarding the above mentioned options, most of the work developed in the
past used either option a) or b). The above mentioned supports for TiO2 can be
configured in reactors of different geometry such as: fluidized beds, fixed
powder
layer reactors, annular reactors, and monolith reactors.
Light Absorption and Sources
Regarding the light absorbed, it is assumed that the light is absorbed by the
photocatalyst (Iabc.cat) with no light absorbed by fluid or substrate
molecules (Childs et
al., (1993). Iabs.can is influenced by many factors including: reactor
geometry,
wavelength, inhomogeneity of reaction mixture, absorption coefficients and
light
source. In agreement with Beer-Lambert law, the intensity profile of a light
source in
an absorbing medium can be related to the incident light intensity as follows:
Log(Ia/I)= x= Absorbance (2.2)
with being the absorbance coefficient of powdered solids, x the penetration
depth
into TiO2 layer, and Ip the incident intensity.
A typical value of UV light penetration into a Ti02 powder is about 2 m
(Childs et al., 1980). Thus, the range of light penetration for particle (for
example 100
m) may be limited to the 1-2 m of the Ti02 particle outer shell. Anderson et
al.
(1993) reported that in a packed bed, LN light is completely absorbed in the
first 10-
15 m of the Ti02 pellets. Thus, the use of particles bigger than 10 microns
is largely
unwarranted.
11
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
There are many different photoreactor types and light sources which are used
for the purpose of illuminating the photocatalyst. Besides the solar energy,
artificial
lamps of different kinds can be used. Bolton (1995) classified the various UV
sources
as follows:
a) Low pressure mercury lamps. They have the characteristic of having long
life (>5000 hr), low energy density (--1 W/cm), and approximately 80% of
the emission is in 254 nm range.
b) Medium pressure mercury lamps. This kind of iN lamps is known to
have a moderate life (>2000 hr). Besides their broad spectral output, not
much below 250nm, they provide a moderate energy density (t125 W/cm).
c) Advanced proprietary medium pressure mercury lamps. These lamps
provide high energy density (250W/cm), a strong output below 250 nm,
and have long life (>3000 hr).
Radiation modeling inside the reactor and its complexity can differ from one
reactor configuration to another and from one lamp to another. Modeling
usually
requires knowledge of the UV lamp radiation emittance intensity, and the
optical
characteristics of the catalytic thin film coating.
If homogeneous photoreactions are considered, the estimation of light
absorption may be done by actinometry. On the other hand, when heterogeneous
2o reactions are involved special procedures are required. Anderson et al.,
(1993)
reported that reaction rates showed a first order with respect to light
intensities. It was
found that higher intensity of UV light leads to higher reaction rates without
losing
efficiency "at constant quantum yield". It was also anticipated that the
linearity in the
relationship indicate that mass transfer is not limiting the TCE conversion
rate. In the
analysis the absorbed light intensity was the amount absorbed by the catalyst
particles
and not by the fluid molecules. Corrections for light scattering, and
reflection should
also be accounted for.
12
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Reaction Kinetics
Reaction Pathwav and the LimitinQ Sten
The photocatalytic reaction involves a number of physical and chemical
processes that take place before the formation of the end products (CO, and
H,O ). In
this respect, Jacoby et al., (1996) has proposed the following steps:
(1) Bulk mass transport of the reactants from the gas phase to the surface of
the
catalyst particle "interparticle diffusion".
(2) Mass transport of the reactants within the catalyst particles
"intraparticle
diffusion".
(3) Adsorption of the reactants onto the catalyst surface.
(4) Surface chemical reaction.
Following the surface chemical reaction two other steps, product desorption
and mass transport from catalyst surface to the bulk flowing stream, take
place.
Among these steps the slowest one in the whole process is usually considered
as the
limiting or the controlling step. The mass transfer coefficient for the
external
resistance, which covers the transfer of substrate from the mixing gas stream
in the
bulk to the exterior surface of the catalyst pellet (0.3-1.6 mm) in a packed
bed, was
estimated by Yamazaki et al., (1993) and Anderson et ai., (1993) using the
Petrovic-
Thodos correlation and Chilton-Colburn factor. Results indicated that the
calculated
values for the mass transfer coefficient were several orders of magnitude
higher than
the corresponding pseudo-order rate constant. Therefore, they concluded that
external
mass transfer is not controlling the photocatalytic reaction rate.
Yamazaki et al., (1993) investigated the effects of internal film resistance
using Ti02 particles supported on 0.3-1.6 mm pellets. The experiments were
conducted by changing the photocatalyst pellet size (0.3-1.6 mm) for a given
catalyst
weight. The results revealed no effect on the rate of TCE degradation. Given
UV light
is completely absorbed on the 10-15 m pellet outer region, it was concluded
that
diffusion effects are not significant.
In another report, Jacoby et al., (1996) developed additional research with
TiOz to investigate the relative influence of the different physical and
chemical steps
and to determine the limiting step. Adsorption was examined by conducting
experiments using benzene as a model pollutant. Experiments were performed by
13
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
feeding benzene, water vapor, and air to an annular photocatalytic reactor
with Ti02
coated on the inner surface of the outer cylinder under various parameters and
operating conditions. The results suggest that the interaction between both
the LJV
light and the Ti02 particles is important in the adsorption process. It was
also found
that photoreaction occurs at rates much slower than the adsorption process,
hence it
was concluded that surface chemical reaction is the controlling step.
Reaction Mechanism
The detailed mechanism of the semi-conductor assisted photoreactions is still
not fully understood. A suggested mechanism representing initial reaction
steps is the
one proposed by Peral et al., (1992):
Ti02+hv ---~ h ++ e (2.13)
h++ OH" --~ OH (2.14)
TiO'' + e ~ TiO3+ (2.15)
and
Ti3+ +Ozaal ---~ Ti"+ 02aa, (2.16)
or
02,& > 20,d, (2.17)
Ti3+ +Oads ) Ti4++ Oad., (2.18)
Thus, once the process is initiated by the promotion of electrons in the TiO2
catalyst by UV light to the higher energy band (conduction band) holes are
left behind
(eq. 2.13). Electrons are trapped by TiO+4 (eq. 2.15) or adsorbed oxygen
molecules
(Ozad) yielding either two adsorbed oxygen atoms Oad; (eqs. 2.17 and 2.18) or
an
adsorbed oxygen molecule 02a,,- (eq. 2.16). On the other hand, the generated
hole
adsorbs hydroxyl ions or water molecules creating hydroxyl radicals which
react with
an adsorbed pollutant molecules initiating the degradation process.
14
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
The main products of the Ti02 conduction band electrons are adsorbed O-ud, or
O",d,, and/or adsorbed hydroxyl radicals from the generated holes in the
valence band.
Anderson et al. (1993) demonstrated that O'2a6 radicals contributed to
additional OH
radical formation through the following reaction sequence:
Ti02 +hv ~ h ++ e" (2.19)
02ads+ e > O2aas (2.20)
02,as-+ H'aas > HO2,& (2.21)
2HO2,6 > H2O2,as+02ads (2.22)
H20zaas+ e' > 'OH,ds+ OHaas +028es (2.23)
These extra 'OH radicals can also be involved in the photoconversion reaction.
Influence of Water Vapor on Kinetics
Water vapor content has different effects on contaminant degradation rates and
this depends on its concentration and the pollutant's structure. It was found
that water
vapor strongly inhibits the oxidation of iso-propanol (Bickley et al, 1973),
TCE at
high concentration (Dibble et al., 1992, Bickley et al, 1973) and acetone
(Peral et
a1.,1992). However, vapor enhances toluene oxidation (Peral et al., 1992), has
no
effect on 1-butanol oxidation and increases m-xylene oxidation rate up to 1500
mg/m3
and decreases the rate thereafter (Peral et al., 1992). Furthermore, for low
TCE inlet
concentration (6 ppm) the reaction rate was not influenced by the water
concentration
(Dibble et al., 1992).
Differences in oxidation rates due to water content were explained by Peral
and Ollis (1992) as due to the relative "adsorption competition". Acetone
appeared to
be less strongly adsorbed into the Ti02 than 1-butanol thus, water could
displace
surface-adsorb acetone but not the latter. As a result, the variable role of
water in m-
xylene photo-oxidation may follow that of TCE, where traces of water are
required for
activity, but excess water is inhibitory (Peral and Ollis, 1992).
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Muradov et al., (1996) demonstrated different results and stated that water
vapor in the inlet air stream increases the oxidation yields of acetone and
ethanol by
5.6% and 13.3%, respectively. This was explained arguing that an increased
water
vapor enhances hydroxyl radical formation and this outweighs the inhibiting
effects
caused by water adsorption on the available active sites on the TiO, surface.
Moreover, in a recent study by Luo and Ollis (1996) it was found that toluene
oxidation rate was higher in the presence of water up to 23-40 % relative
humidity(2000-3000 mg/m'). Inhibition was significant at 60% relative humidity
(6100mg/m'). A formula applicable for water contaminants below 6000mg/m3 was
postulated as follows and relates the surface photochemical reaction rate of
toluene
with water concentration:
r=710.7[CH20]/[ 1+5.325 * 10" [CH2O]+1.9241 * 10"'([CH2o])'] (2.24)
where [C,.,Zo] is in mg/m' and r is in mg/(m3.min). These results are
consistent with
Ibusuki et al., (1986) who studied the photo-oxidation of toluene and found
that the
relative humidity in an air stream increases toluene photo-oxidation. Their
findings
were explained by the following sequence of steps as proposed by Bickley et
al.,
(1973):
TiOZ --~ h'+ e' (2.25)
OH'+h' )'OHj. (2.26)
02+e --~ 'O 2ad. (2.27)
As it has been pointed out earlier, the excitation of a TiO2 molecule by UV
light
produces a hole and an electron. Once the hole is trapped with OH-,ds, the
electron
promotes the 02 adsorption on the Ti02 surface. The more water molecules
available
the more OH-,d., will be available, allowing even further adsorption of oxygen
molecules. Since both =OH,, and =OZ",ds have the potential to oxide the
intermediate
products resulting from the oxidation of toluene, higher rates of oxidation
are
achieved with little or no intermediates being detected.
16
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
The Influence of Temperature on the Kinetics of the Reaction
Temperature is a significant parameter that influence the photocatalytic
reaction
rates. In general, temperature was found to have a minimal effect on the photo-
oxidation process. However, temperature had some influence on the oxidation on
ethanol, acetone and nitroglycerine (Muradov et al., 1996). In this case, as
temperature was increased, the rate of photodegradation was decreased. It has
to be
mentioned that the observed temperature effect was mainly due to the
adsorption-
desorption dynamic process. The pollutant adsorption is an exothermic process
overall. Thus, increasing the temperature shifts the overall adsorption
process
towards a dominant desorption. In the case of acetone, the reduction of the
photodegradation rate was clear since acetone was less adsorbed on Ti0 ,
surface than
the other compounds.
Anderson et al., (1993) found that when increasing the temperature between
23 C and 62 C, the reaction rate for TCE degradation remained essentially
constant
meaning that there was no significant energy barrier for the initial reaction
steps to
take place. As a result it was concluded that photocatalytic reactions were
not
dramatically influenced by temperature. Fox et al., (1993, 1988) argued that
the
excitation energy is generally much larger than the energy required to
overcome
ground state activation energy barriers.
Previous Toluene Studies
TCE photo-oxidation has been broadly evaluated (Wang et al., 1993; Dibble et
al., 1990; and Yamazaki et al. 1994). Toluene is frequently used given it is a
typical
pollutant from several chemical industries and the largest constituent of
aromatic
hydrocarbon anthropogenic emissions (Lonneman et al., 1974 and Heuss et al.,
1974).
Toluene oxidation has been studied as a single pollutant and in mixtures to
determine
the selectivity behavior of the photocatalytic reaction.
Blanco et al. (1996), investigated the oxidation of toluene (3000-6000ppm) on
monolithic catalysts. These monoliths were based on titania dispersed on a
fibrous
silicate and irradiated with 4000W Xenon lamp (average flux reaching the
surface=8W/cmZ) at temperatures of 130-450 C. Runs below 130 C were avoided
due to potential toluene condensation as well as runs above 500 C due to
catalyst
17
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
properties changes. This set-up did not have a very efficient lamp, since 0.2
% of the
actual supplied power reached the catalyst surface.
lbusuki et al., (1986) reported toluene photo-oxidation (80 ppm) and on the
activity of TiOZ with respect to Oõ NOZ and H,O concentration in the gas
stream.
These authors detected an insignificant level of benzaldehyde (<1 ppm) besides
carbon dioxide and noted that water plays a significant role in the formation
of the
active sites.
Initially Suzuki et al.(1993) studied the photo-oxidation deodorization of air
with low pollutants concentration including toluene (80 ppm) in a 8 x 6 x 2
cm' box
lo with a 500 W. A pseudo-first order reaction was proposed for all compounds
including toluene which degraded in 60 min achieving a 90% conversion with a
rate
constant of 0.059 miri'.
Luo and Ollis (1996) studied the kinetics inhibition and promotion, and time-
dependent catalyst activity for both individual and in-mixture oxidation of
toluene and
TCE. Toluene oxidation in the range of 80-550 mg/m' and relative humidity of
20%
were tested in a bed flow reactor designed by Peral and Ollis. This study
revealed
Langmuir-Hinshelwood rates with 8-20% conversion, no intermediate product
detection and reaction rate constant and adsorption constant of k=3.14 g/L.min
and
K,d,=0.00463 m'/mg, respectively.
Another study of toluene was conducted by Obee et al., (1995) who proposed
a Langmuir-Hinshelwood reaction rate for bimolecular surface reaction of the
following form:
r=kaFpFw (2.28)
FP K,CW(1+K,Cp+KZC,,,,) (2.29)
FW=K4Cõ/(1+K3Cp+K4CW) (2.30)
where r is the oxidation rate ( mol.crri Zh-'), ko is the constant of
proportionality
( mol. cm Z h-'), Kõ K2, K3, and K, are the Langmuir adsorption equilibrium
constants
(ppmv'), Cp, CW are the gas phase concentrations of the pollutant and water
vapor,
respectively, and Fp and FW are the competitive adsorption for the pollutant
and the
water on the same active sites. Experiments were conducted at room temperature
and
18
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
40 % relative humidity. Values for the Langmuir adsorption equilibrium
constants
were found to be: K,=2.02, KZ .000727, K3=2.02, K4 .000727, and ko 3.84. These
authors explain that in the case of high toluene concentration (>60 ppm), the
L-H rate
equation was first order in water concentration.
Novel Photocatalytic Reactor Design - Photo-CREC-Air Reactor
Although, there are several studies relating to reactors and catalytic
degradation of organic substances such as toluene, there still remains a lack
of proper
design and technical data for photocatalytic reactors in order to provide safe
and
completely efficient degradation of harmful organic pollutants that can be
used on a
wide commercial scale. The novel reactor of the present invention, Photo-CREC-
Air
reactor, is designed for optimal and safe operation taken into account the
need to
fulfill the requirements of good mixing, high mass and heat transfer, high
quantum
efficiency and optimum operation. Other factors that influence reactor
performance
and model pollutant conversion rate have now been found to include the
intensity of
the light source, air mixing and flow patterns, interaction between phases,
choice of
the material of construction, choice of photocatalyst, choice of photocatalyst
support,
choice of photocatalyst immobilization or impregnation method, and
illumination
arrangement. The following describes the Photo-CREC-Air reactor novel features
and its associated internal components.
The novel photocatalytic reactor of the present invention, Photo-CREC-Air,
was designed to optimize quantum efficiency, quantum yield and chemical yield.
A
Photo-CREC-Air reactor was designed, manufactured, and assembled. The novel
photocatalytic reactor of the present invention is illustrated in Figure 1 and
is
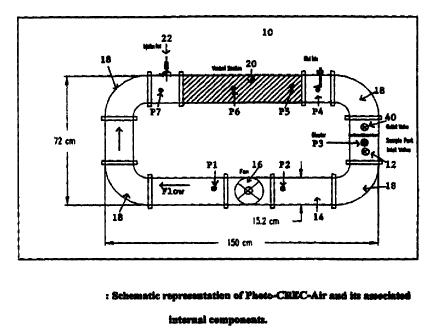
generally indicated as numeral 10. The reactor 10 comprises an inlet valve 12
for the
introduction of a gaseous stream carrying pollutants into the reactor and
which travel
through a continuous pipe 14 having a number of changes of diameter or cross-
section
through which gaseous flow occurs. The configuration of the pipe has four
elbows
18. A fan 16 is located within a section of the pipe to circulate and promote
gaseous
flow towards the Venturi section 20. An injection port 22 is provided into
which a
test sample may be introduced directly upstream of the Venturi section 20.
19
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
As better seen in Figure 2, the Venturi section 20 comprises a convergent
section 24, a straight section 26 and a divergent section 28. The Venturi
section is
designed to obtain smooth changes of gas flow without abnormal flow patterns
or
flow upset. The Venturi section constrains the gas stream progressively
towards a
smaller diameter with minimum disturbance and a significant increase in gas
velocity
and a reduction in the pressure such that a suction effect is created. The gas
stream
leaving the Venturi section is directed such that it impinges at high velocity
and with a
controlled and uniform pattern on an illuminated mesh section 30 which is
transversely located with respect to the air stream flow. As seen in Figure 3,
the
lo divergent section comprises windows 32 and light reflectors 34 (mirrors)
which help
to direct and focus the light on the mesh section 30.
The mesh section 30 is a transparent fibrous mesh impregnated with a catalyst,
preferably Ti02. The orientation of the mesh section in a transverse manner
with
respect to air flow is an important feature for the efficient destruction of
pollutants
within the air stream as this helps to increase the contact between pollutants
contained
within the air stream and the catalyst loaded onto the mesh. As seen in Figure
5, the
mesh is supported with a perforated plate 36 which has heaters 38 positioned
to
minimize or desorb any absorbed water. The UV light illuminated catalyst acts
to
photoconvert the contaminants present in the gas so that only non-toxic
reaction
products are left which move downstream of the Venturi section to an outlet
valve
section 40 of the reactor.
The Photo-CREC-Air reactor of the present invention has a capacity of 0.065
m3. This unit may handle an air flow of 0.066 m'/s and a maximum gauge
pressure of
44.4 kPa. Changes in the reactor cross section was calculated to provide air
velocity
of 14 m/s at the throat and 3.6 m/s elsewhere. Air is introduced to the system
through
an inlet port 12 and its recirculation is driven by a 0.152 m diameter in-line
fan. As
the gaseous stream reaches the Venturi 20, its velocity is increased to a
maximum at
the throat. It is then decreased in a progressive way through the divergent
section,
where the mesh is illuminated by two Pen Ray LN lamps. It is at this
divergent
section where most of the pressure drop is recovered. At the exit of the
Venturi, air
passes through a perforated heated plate, before it recirculates in the loop
of the
reacting vessel.
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
The reactor is illuminated externally through transparent windows 32 which
allow maximum utilization of the provided light energy. Rubber gaskets are
used
between flanges and are very effective in preventing air leakage to the
surroundings.
In order to assess the photocatalytic oxidative capacity of the reactor, the
desired
amount of pollutants is injected through the injection port which is a heated
block
with a septum. Organic compounds are being introduced to the system by means
of a
syringe. In terms of safety, the reactor may be contained in an plywood
enclosure
with a fan in the upper section to achieve good ventilation.
The Venturi section, the windows with focused illumination, the mesh section,
lo and the heating plate are only some of the features of the Photo-CREC Air
reactor of
the present invention which act to increase the photooxidation of air borne
pollutants.
While these features along with others are reviewed in more detail in the
following
sections, main achievements with Photo-CREC-Air reactor in addition to
providing
excellent gas-Ti02-light interaction are summarized as follows:
* Minimum particles adhesion to windows;
* Maximum light transmission;
* Excellent support of Ti02 on a fibrous mesh;
* Minimum water sorption on the mesh;
* Uniform gas distribution contacting the mesh;
* Minimum radiation losses; and
* Focused illumination of the mesh.
Materials of Construction
The main body of Photo-CREC-Air reactor was made of straight exhaust pipe
0.152 cm in diameter connected by four zinc plated elbows. This choice was
made as
to provide a material which would be able to withstand the operating
conditions in
terms of temperature and pressure and at the same time, provide a good
resistance to
corrosion to prevent rust particle formation. Therefore, any material having
such
characteristics is suitable for use in the present invention.
The Venturi section was constructed out of stainless steel tubing given it is
easier to weld and it has better thermal resistance than the exhaust tubing.
However,
the Venturi section can be fabricated from any suitable non-corrosive
material. The
21
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
windows in the divergent section of the Venturi were made of plexiglass. The
edges
and the different parts of the photoreactor were sealed using welds and white
silicon
closed cell sponge gaskets. Clear transparent silicon sealant, was also used
to
properly seal the fan and some other sections of the reactors.
Catalyst and Catalyst Support
For the purposes of the photocatalytic reactor of the present invention, the
Ti02 used was "Ti02 P25" manufactured and supplied by Degussa Corporation. The
Ti02 batch used had a BET surface area of 35-65 m'/g , average primary
particle size
1o 21 nm, and a specific gravity of 3.7. In order to choose the support that
more
efficiently and tightly held the Ti02 particles, three different supports were
initially
tested: a) Filtrete TM, b) 3M Blue Pleated Filter, and c) Bionaire Filter. The
3M Blue
Pleated Filter mesh was found to be a good candidate for this type of
application due
to its cheapness, light weight, convenience of handling, transparency to light
in the
desired range, inertness to gases, possession of a fibrous porous structure
and
sufficient thermal resistance.
The porous structure of the 3M Blue Pleated Filter was desirable since it
minimized pressure drop, provided high surface area, good light transmittance,
did not
plug, and allowed maximum catalyst loading. The 3M Blue Pleated Filter also
developed electrical charges as air was flowing through it, inducing in this
way,
strong bonding of catalyst and pollutant particles.
Venturi Section
A Venturi section was incorporated into the Photo-CREC-Air reactor design to
provide good mass transfer and to establish a "self-cleansing" system. Such a
"self-
cleansing system" provides a vacuum condition or a suction effect in which
dirt/pollutants are aspirated from the air stream and thus do not accumulate
on the
windows or reflectors. This is especially important when treating polluted
dusty
gases containing suspended particles. Such dust and particles are sucked
through the
mesh and consequently, are not deposited on the windows or reflectors creating
a self-
cleaning system. The air flow pattern in the Venturi section shows a
combination of
high fluid velocity and vacuum pressure (suction) sufficient to prevent the
particles
22
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
from sticking to the windows which may affect light transmission to the
targeted
filter. The use of the Venturi also eliminated the need for window cleaning.
As the
air stream approaches the throat of the Venturi, it increases its velocity
reducing the
pressure and smoothly changing its flow direction, with minimum flow
disturbance.
The stream exiting the Venturi impinges on the transversely positioned mesh
with the
high velocity required for the high mass transfer condition. The position of
the mesh,
transverse to the air flow, provides for increased reaction of the air stream
with the
Ti02 loaded mesh and thus increased photocatalytic reaction and increased
destruction
of air borne pollutants within the air stream.
The incorporation of the Venturi section allowed the placing of lamps in such
an orientation that close to 100% illumination of the TiO, loaded in the mesh
was
achieved. Dimensions of the Venturi section were chosen as to meet specific
pressure
drops, thus allowing for good gas-mesh contacting. Figure 2 illustrates the
dimensions of the constructed Venturi section. Figures 3 and 4 show different
sections of the Venturi for visualization assistance. -The Venturi was 62 cm
in length
and was made up of three parts: convergent section (21cm), straight section
(6.8cm),
and divergent section (34.2cm). The upstream cone angle was I 1 and the
downstream cone angle was 7 . The latter was constructed of rectangular design
out
of four flat surfaces to assist in placing the windows, through which the LN
radiation
illuminated the mesh.
The pressure drop across the Venturi was calculated using the following
equation as noted in McCabe et al., (1993):
V 2= CY 2g~~Pt - P2)
P~~1-fl )
where:
V2: average fluid velocity at the throat of the Venturi (m/s).
C,: Venturi coefficient which is empirically determined and is about 0.98 for
a well
designed Venturi of pipe diameter 2-8 in (-).
Y: dimensionless expansion factor, for the flow of compressible fluid (-).
g,: dimensional constant (32.17 lb ft/1bf s2). If SI units are used then gc=1.
p,: fluid pressure under upstream conditions (Pa).
23
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
P2: fluid pressure at the throat conditions (Pa).
p,: density of the fluid under upstream conditions (kg/m3).
P: ratio of diameter of the Venturi throat to diameter of pipe (-).
Note that the dimensionless factor, Y is equal to unity for a non compressible
fluid
flow and it can be evaluated for the compressible fluid by eq. (4.2), [Perry's
et al.,
1984]:
~ 1-~
Y= rk (_k r k
k-1 1-r 2 (4.2)
1-,fl4rk
with k parameter being the specific heat ratio Cp/C,, and r the ratio of the
pressure at
the throat to the inlet pressure.
Windows
Windows for use in the reactor may be cut of any chemically stable transparent
solid media. It is important that the windows be completely sealed with a
suitable
sealant to prevent any air leakage. Different types of glass could be used
including:
plexiglass, quartz glass, Pyrex glass or stove glass. With an increasing cost
as well as
light transmission efficiency, they were ranked as follows: stove glass
provided 40%,
plexiglass 50%, PyrexTM 80%, and quartz 90 %. Plexiglass was good from a
safety
point of view, since in the case of high pressure or eventual pressure changes
it would
not shatter it would only crack. In the present invention, windows were
manufactured
from plexiglass which was efficient in transmitting the required light near UV
wave
length (365 nm). Moreover, the shape of the windows conformed, to the shape of
the
metallic frame of the reactor to provide uniform illumination of the coated
filter
placed transverse to the flow. Windows may be cut from PyrexTM glass having an
absorption of 20 % of the incident radiation instead of 50 % for the
plexiglass.
Selected plexiglass windows were 5 mm thick and had a parallelogram shape
with the parallel sides being of 4.5 and 8.5 cm in length. Initially, four
plexiglass
windows were used, but to minimize light loses and maximize its transmission
two of
them were replaced by mirrors of the same shape and size to directly focus the
incident and scattered light rays on the impregnated mesh (the target).
24
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Light Sources
UV sources for the reactor of the present invention can in principle be any
conventional lamp as long as it provides the required energy for the
photoconversion
reaction. Different types of W light sources exist including: low pressure
mercury
lamp, medium pressure mercury lamp and advanced proprietary medium pressure
mercury lamp. Each of these sources has specific characteristics and features
suitable
for the different applications. These lamps differ in terms of energy density,
emission
range, life and electrical to photon energy efficiency.
Irradiation specification including: wavelength, intensity and operating life
are
crucial factors in the photocatalytic processes. These factors depend mainly
on the
catalyst used, concentration of the pollutants, and lamp employed. As far as
TiO, is
concerned, ultraviolet light with wavelength >_350nm (Sauer et al., 1994) and
<385nm (Sczechowski et al., 1995, Zhang et al., 1994), is necessary to provide
the
band gap energy required to yield an electron-hole combination for the
initiation of
the photoconversion process.
As for the light intensity, this is a major factor that influences the
photoconversion rate. In the Photo-CREC-Air reactor of the present invention
two
ultra violet lamps with an output in the range of 365 nm and an electrical
output of
4W/lamp were used. These lamps were supplied by UVP and they were termed the
Pen-Ray lamps. These portable miniature Pen-Ray lamps were utilized due to
their small size that can be fitted within the Photo-CREC-Air reflectors.
These lamps
are low pressure, mercury gaseous discharge lamps that were constructed of
double
bore quartz with a tubular filter. These lamps are designed for stable, low
noise
operation, and have a rated lamp life of 5000 hours with an exponential
intensity
decay of 20% in the first 1000 hours and another 20% over the following 1000
operational hours. A decay curve was prepared to estimate the lamp power decay
with time of utilization. Corrections were introduced to the calculated
kinetic
constants and quantum yields according to this decay curve.
The Pen Ray Field lamps were supplied with DC power supplies (9V battery)
and were 12.07cm in length, with a lighted length of 5.72 cm and an outside
tube
diameter of 0.95 cm. Their peak transmission was at 365 nm. The intensity of
the
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
UV light was measured using UVX radiometer. The UVX radiometer was a digital
radiometer that works in conjunction with a specific sensor that measure the
wavelength in the desired range up to 20 mW/cm2 with an accuracy of 5%( LNP
manufacturer and supplier). The radiometer is calibrated using standards of
the
National Institute of Standards and Technology (NIST) and UVP 's published
standards. The radiometer provided three ranges of readings: 0-200 W/cm', 0-
2000
W/cmZ, and 0-20 mW/cmZ.
The treated filter mesh was illuminated externally from outside the windows.
Lamps in the Photo-CREC-Air reactor were not immersed into the fluid to avoid
particle deposition, flow disturbance, and condensation of product vapor.
Reflectors
Photo-CREC-Air reflectors were designed to optimize the utilization of the
emitted light. The reflectors were parabolic in shape with an elliptical cross
section
(Figure 4). These reflectors were manufactured from aluminum with the
following
dimensions: length of 7.7 cm, width: 7.2 cm from the bottom and 5.5 cm from
top.
Reflectors were equipped with side slots to hold the UV lamps. The Photo-CREC-
Air
reflector fnnction was coniplemented with mirrors, covering the section of the
windows not covered by the reflector, and helped focusing most of the light on
the
target mesh.
Light flux entering the reactor and reaching the filter area was measured with
the radiometer. Average radiation received was 40 W/cmZ. While received
radiation
tended to change across the filter surface, most of the filter was illuminated
with close
to 50 m/cm2. There was a relatively small outer annulus with radiation levels
below
40 m/cm2 (Figure l0A).
Perforated Plate
A perforated plate was designed as an extra support for the impregnated mesh
and to secure uniform distribution of fluid while contacting the mesh. The
plate was
heated in order to ensure that the mesh was free of water since it was
reported that
water may have a potential affect on the photoreaction rate. The perforated
plate can
be made from any suitable non corrosive material. One such material is
stainless steel
26
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
which has a very high resistance against corrosion and thus avoids any rust
particles
entering the gas stream. The plate has a 22 cm diameter and 0.83 cm thickness
with
144 holes each of them having a 0.83 cm diameter (Figure 5). It is understood
however, that the perforated plate can be made of any suitable size depending
on the
size of the impregnated mesh, the size of the Venturi section and the general
size of
the reactor itself.
Four 150 W cartridge heaters were symmetrically inserted in the plate and
connected to a variable voltage power supply. This supplied the required
energy to
maintain the plate at the desired temperature. The heaters were placed
equidistant
1o from each other and this to ensure uniform heating of the plate. The
heaters were 3.8
cm in length and 0.9 cm in diameter. Temperatures were measured by a type K
thermocouples connected to a digital thennometer. The thermocouples were
inserted
in opposite position of the plate as to confirm uniform temperatures level.
Thermocouples were 15.5 cm in length and 1.5 mm in diameter. Again, while four
heaters were utilized, it is understood by those skilled in the art that fewer
or a greater
number of heaters can be provided so long as the heat source provides for
sufficient
heat to desorb any absorbed water from the mesh.
Pressure drop across the plate was considered as a major aspect in its design,
since proper values should ensure uniform contacting between the gas stream
and the
supported mesh, as well as to avoid any gas flow maldistributions. The
pressure drop
was provided in part by the perforations provided in the plate. The pressure
drop
across the perforated plate was approximated using Van Winkle's equation
(Perry et
al., 1984). This equation applies for the flow of gases through perforated
plates with
square-edged holes on an equilateral triangular spacing for hole Reynolds
number
range of 400-20,000 and hole pitch/hole diameter ratio of 2-5:
W = CaAfY Fgp (4.3)
with W b
eing the mass flow rate, Co the orifice coefficient, Af the total free area of
holes, Ap the total sectional area of the perforated plate, Y is a
dimensionless
expansion factor for the flow of a compressible fluid, g, a gravity
dimensional
27
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
constant, p, the fluid density at the upstream conditions, and Ap the pressure
drop
across the plate.
The perforated plate designed for use in the Photo-CREC-Air reactor, had
punched holes slightly rounded. Thus expected Co coefficients should be
slightly
larger than the ones for the square-edged holes (Perry et al., pg5-37, 1984).
The
perforated plate orifice coefficient is a function of the hole Reynolds number
and the
physical characteristics of the plate. Y was approximated using the following
correlation:
Y= l- 1 k r(041+.3 5 J34 ) (4.4)
Regarding Y, it was found to be approximately equal to unity. This is expected
since
the pressure drop through the plate is moderate (124 Pa) and still adequate to
secure
uniform flow distribution.
Iniection Port
The injection port was made from a stainless steel block. A cartridge heater
of
120 W power was inserted into the block to help raising the temperature and
keep it at
around 100 C. At this condition the model pollutant (e.g. toluene) was
injected and
evaporated in the air stream.
The heater was 3.8 cm length and 0.6 cm in diameter and it was powered with
about 60 V. A thermocouple was introduced in injector block to monitor the
temperature. This thermocouple was 15.5 cm in length and 1.5 mm in diameter
and
was connected to a digital thermometer. The injection port was equipped with
septum
through which the desired amount of toluene, supplied by Caledon, was fed to
the
reacting vessel. As to prevent septum damage or back flow of the injected
sample out
of the system, an on-off valve was used.
Mode of Operation
The determination of the Photo-CREC-Air reactor performance is an
important aspect of the present invention. While it is understood by those
skilled in
the art that different modes of operation of the reactor may include batch,
semi batch
or a continuous system, the current testing was developed in a batch system
with high
28
CA 02286152 2006-07-26
gas recirculation given various safety issues. Moreover, this mode of
operation may
simulate the treatment of a confined volume of gas (building, painting shop)
being
continuously treated in Photo-CREC-Air reactor. Regarding reactor simulation,
this
mode of operation can be modeled as an ideal continuous stirred tank reactor
(CSTR)
with complete air recirculation and this given the various characteristics
provided to
the present prototype.
Testing of the Photo-CREC-Air system performance was developed in terms
of pressure profiles, velocity profiles at both room and elevated
temperatures, UV
intensity profiles across the filter surface at several positions, and UV
intensity decay
profiles.
Pressure Profile of the Photocatalvtic Reactor
Pressure was measured at several points along the reactor length using a water
manometer. The position of the seven pressure taps (P 1 to P7) are shown in
Figure 1.
Positive gauge pressure was observed after the fan (tap 1) and negative
pressures were
obtained elsewhere. Given air can only flow from high to low pressure, samples
were
exclusively taken from Tap I which provided a sufficient gas flow rate to fill
the
sampling bags in a reasonable time. It is worth noting that this profile was
taken at
room temperature, and as temperature was increased the positive gauge in Tap 1
was
also increased. Another interesting observation was given by the fact that the
lowest
pressure in the system was observed at P6 which corresponds to the neck of the
Venturi and this was expected given the Venturi fluid-dynamics where velocity
increases considerably and pressure is reduced at this particular section.
Velocity Profile
Velocity profiles in the Photo-CREC-Air reactor were measured immediately
after the perforated plate (P4). Measurements were made using a Pilot tube
supported
with a holder allowing measurements at different radial positions. The two
profiles
were recorded (Figures 9A and 9B) at room and elevated temperatures (~970C),
respectively.
From this data, it is apparent that there is a sharp velocity change in a
small
outer area of the mesh (r>6 and r<-6) with velocity changes becoming less
29
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
pronounced in the main central core of the stream (-6<r<6). While this trend
of
relatively uniform gas velocity was observed at both room temperature and 97
C,. in
the case of the higher temperature the gas velocities were more uniform and
provided
more symmetric velocity profile.
iN Intensity Profile
The intensity of the ultra violet light was measured at the exact location
where
the impregnated supported mesh was placed. As well UV was measured at
different
circumferential positions: 0 degree, 90 degrees, 180 degrees, and 270 degrees.
A
radiometer equipped with 365nm sensor and a specially designed rotating plate
were
used for this purpose. The observed irradiated profile was almost symmetric in
shape,
flat at the middle bending somewhat at the edges (Figure l0A). Regarding the
UV
lamp power decay with time of utilization, it was observed that there was a
consistent
power decay (Figure lOB). For instance, after 190 minutes at the radial
position of
0.5 the irradiation observed was 42.4 w/cm2 instead of 44.4 w/cm2 and this
represents 5% decay in about 3 hours..
Photocatalytic Conversiori
Photocatalytic degradation of pollutants carried in a gas stream involve three
main components: catalyst, radiation and contaminant. In order to assess the
various
effects in addition to the actual reaction runs, blank runs were developed.
Results of
both the blank runs and the reaction experiments are presented herein.
Toluene was chosen as an example of a model pollutant for several reasons:
(a) it is a compound that can be used safely given its relatively high
threshold
concentration value (50 ppm). Toluene has been studied already by other
researchers
which provides a useful basis for comparison, and (c) toluene photo-oxidation
does
not reveal formation of any significant amount of harmful intermediates (e.g.
phosgene typical intermediate of the photo-oxidation of chlorinated
compounds).
As stated above, blank nms were also conducted and are important to establish
the potential effect of the various factors outside the photocatalytic
reaction. Blank
runs experiments were performed with no UV illumination and with no TiO,
catalysts
particles or mesh at two temperatures: 20 C and 100 C. Results of the blank
runs at
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
the low (20 C) and the high (100 C) temperatures are illustrated in Figures 11
A and
11 B, respectively.
Figure 11A shows that at low temperature without W irradiation, there is
about 22% concentration drop with respect to the initial toluene concentration
over a
period of 9 hours. This toluene gas phase concentration drop was similar to
the one
observed during a reaction run (LN lamp on and Ti02 particles in mesh) for the
same
reactor time-on-stream. This showed that no or very little toluene photo-
oxidation
took place at 20 C. Therefore, given the above mentioned uncertainty, reaction
runs
at 20 C were not considered further when assessing kinetic constants, quantum
yields
and reaction rates.
Regarding toluene concentration changes at 20 C, these toluene concentration
drops were assigned to condensation. Even if initial calculations using HYSIM
thermodynamic package suggest no toluene condensation at the concentrations
and
temperatures under study, condensation appears to take place at 20 C.
Condensation
is a phenomenon also reported for concentrations in the same range by Blanco
et al.
(1996). Note that adsorption does not seem to occur with any significant
degree, since
11 % toluene concentration drop was observed over a period of 4 hours in both
cases:
with and without TiOZ mesh.
Regarding potential influence of toluene condensation at higher temperatures,
it can be stated that condensation was steadily reduced while increasing the
temperature of the heating plate supporting the mesh from 20 C to 100 C (Table
2).
Given 9% drop (blank runs) is relatively a small concentration drop versus the
20-32
% toluene conversions observed at 100 C under reaction conditions, it was
concluded
that at higher temperatures the photocatalytic oxidation was the dominant
phenomenon. Therefore, these data were the one considered to have relevance
for
kinetic parameter estimation.
Once adequate conditions were developed for the reaction tests using the blank
runs, a number of reaction runs were developed. Figure 12A shows a typical
plot of
the observed changes of model pollutant and product concentration as a
function of
time-on-stream and with mesh temperature held at 100 C. This figure shows a
consistent toluene concentration decrease, water and carbon dioxide increase.
An
interesting observation was given by the fact that change in toluene as well
as carbon
31
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
dioxide concentrations were quite regular while there was some higher
dispersion on
the water concentration measured. This higher dispersion on water
concentration
measurements was linked to the higher inaccuracies related to water
measurements.
Regarding these experimental results, it should be stated that the toluene
concentration drop during the first four hours (light off) was very mild and
this was a
potential indication of condensation and/or adsorption. However, given
condensation
was already estimated as 9%, it was concluded that adsorption in the context
of Photo-
CREC-Air reaction runs was not significant. Thus on this basis, the potential
applicability of a pseudo homogeneous model (no adsorption of pollutants on
the TiO,
mesh) for kinetics modeling is strongly envisioned.
Experiments were performed with the light source "off' during the first period
of the experiment (< 4 hours). This first period of the experiment allowed the
determination of the condensed/adsorbed amount of toluene on the Ti02 mesh
surface.
Following this, the lamp was turned "on" and a steeper decrease in the toluene
level
was noticed. Typical runs lasted 22-24 hours having the mesh-Ti02 about 18-20
hours under UV radiation.
Typical chromatograms of injected samples for both the TCD and the FID
along with Figure 12 revealed the following:
1. Main products from toluene photo-oxidation are water and carbon dioxide,
2. No intermediate products are observed,
3. There is a substantial increase in both carbon dioxide and water while the
run is
progressing.
It has to be pointed out that the first observation was consistent throughout
all
the carried experiments developed. While both detectors (FID and TCD) were
used
for product quantification, the FID detector was used specifically to help
identifying
potential intermediate species. Results suggest that there are no chemical
species
other than water and carbon dioxide formed or at least if individual
intermediate
species exist in the gas phase they are in negligible amounts (below the
detectable
limits). This observation is a very important one given intermediate species
can
represent a potential hazard in some cases with toxicity larger than the
original
pollutant. This experimental observation of negligible amount of intermediate
species
was consistent throughout all the experimental runs.
32
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
In order to clarify the influence of the different operating parameters, an
experimental program was developed with systematic changes of toluene
concentrations, temperatures, and humidity levels. The different experimental
conditions are given in Table 3.
Experiments with four different concentrations ranging between 5.2 g/cm'-13
g/cm' were carried out. The results are plotted in Figures 13A, 13B, 13C and
13D.
The straight lines through the data points represent the best linear fit for
each chemical
species during the reaction time. Model pollutant concentrations were obtained
by
injecting toluene samples of a volume between 0.4 and 1.0 ml into the reactor
vessel.
lo Data obtained illustrated that higher toluene concentration enhanced the
rate of photo-
oxidation.
Temperature effect on the operation of Photo-CREC-Air reactor over the 20-
100 C range was also investigated. Initially, it appeared that increasing the
temperature did not cause a significant enhancement of toluene photo-
oxidation.
However, once the problem of toluene condensation on the reactor walls was
well
established, it was realized that a detailed analysis of the effect of
temperature was of
particular importance. Therefore, runs with the heating plate at 20 , 50 , 75
, and
100 C were conducted (Figures 15A, 15B, 15C and 15D). It was observed that
only
the runs at 100 C provided adequate conditions to minimize toluene
condensation
and kinetic constant calculations.
In summary, the experiments developed with the heating plate at 100 C
provided conditions of minimum toluene condensation as well as good removal of
water adsorbed on the mesh surface.
When similar experiments were repeated in the presence of higher water
content (0.003 vol.%), the value of the kinetic constant calculated, as it
will be
discussed in the next section, was found to be essentially the same at 100 C
within
experimental errors for the tested lower water level. This indicates the
importance of
the heating plate in removing the water off the Ti02 mesh surface and hence
reducing
its effect on the photocatalytic reaction. Data are summarized in Figures 13C
and
15D.
After 18-20 hours of UV irradiation, typical conversions achieved were in the
17-50 % range, representing relatively low-moderate conversions. Conversion
values
33
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
are summarized in Table 4. Pollutant concentrations used in the present study
(5.2-13
g/cm') were one order of magnitude larger than typical levels in indoor air
pollution
or considered by other researchers (Luo et al., 1996, Obee et al., 1995 and
Ibusuki et
al., 1986). For example, Luo et al. (1996) obtained conversions of 8-20% for
0.550-
0.080 g/cm3 toluene concentration, respectively.
The kinetics of toluene photo-oxidation under the UV illumination in the novel
photocatalytic reactor of the present invention were also investigated. Photo-
oxidation
reaction rates were measured and values obtained were in the 0.0058-0.049
mole/gm.s range as illustrated in Table 3 and Figure 14.
While several kinetic models have been proposed in the literature (Luo et al.,
1996, Obee et al 1995), an attempt was undertaken to model the experimental
data
with a first order pseudo homogeneous model. This approach was chosen given
the
negligible adsorption effect observed. Adsorption effects normally translate
in the
need for a Hinshelwood-Langmuir model with an overall order between 0 and 1.
Given the condition of negligible adsorption and the assumption of uniform
concentration in the Photo-CREC-Air reactor as a result of the intense air
recirculation, the following balance equation applies for toluene:
V dCT/dt=W r.r (7.1)
with V being the total gas hold up, W the weight of the catalyst utilized, CT
toluene
concentration at time t, and rT is the rate of toluene photo-oxidation. The
latter can be
formulated using the following first order equation:
rT= -k=CT (7.2)
with ks is the intrinsic rate constant. Substituting eq. (7.2) into eq. (7.1),
V dC.r/dt=W k,CT (7.3)
An integral form of eq. (7.3) yields the following:
ln(CT/C,.o)=-(k,W/V)t (7.4)
or
ln(C,/C,.o)=-kt (7.5)
34
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Kinetic constants (k) were evaluated in a two step process. In the first step,
In C,./CTo
was plotted versus time to produce a straight line with a slope equal in value
to the
kinetic constant k (eq. 7.5). Kinetic constants obtained were found to be in
the range
of 0.009-0.0274 (hr''). Results are reported in Table 4. Once the k values
were
established, they were con:ected to bring all of the them to the same lamp
time of
utilization (t'= 40 hour with an estimated light power reaching the mesh of 50
mW/cmZ) using the following correlation suggested by Ollis (1993), Luo et
al.(1996)
and Peral et al. (1992).
kcarmctea = k (Mo)a (7.6)
with 52=0.7, as reconunended by Peral et al. (1992) for acetone oxidation for
a
moderate light intensity. With the only exception of the runs at the lower
toluene
initial concentration (5.2 mg/cm3), the ko.,d values are in the range of 0.02-
0.047 hr-'
(Figure 16A). Kinetic constants at the high humidity level (30 mg/cm') were
also in
the same range. On this basis, the following can be postulated: a) the first
order is
adequate for the reaction of toluene photoconversion and this is consistent
with the
applicability of a pseudo-homogeneous model at 100 C, b) the humidity level
does
not appear to influence either positively or negatively the performance of the
Photo-
CREC-Air reactor and this is in agreement with the designed conditions of the
unit: a
heating plate preventing water adsorption on the mesh.
The quantum yield is a parameter that needs to be determined in photocatalytic
reactions to establish the overall photon utilization efficiency. The quantum
yield is
frequently defined as the number of moles of pollutant degraded per photon
being
provided to the system. In the present invention, it was preferred to define
the
quantum yield on the basis of photons absorbed by the TiO2 on the mesh. Thus,
the
following equation was employed for the quantum yield ((p) calculation:
cp=-av*[rmPjmaz''NAhC/P1,Qm,.b.I (7.7)
where:
a: number of photon required for the fonmation of an 'OH.
v*= Stiochiometric number for.OH reacting with the model compound
Stiochiometric number for the pol u tant c emica species reacting with H
[rPõ],,,,x: rate of model pollutant destruction.
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
V: total gas hold up.
NA: Avogadro number (6.023 x 1023 molecule/mole).
h: Plank's constant (6.62 x 10'34 J.s).
C: speed of light in vacuum (2.997 x 1010cm/s).
%: wavelength (nm).
Q m,,bs: rate of light energy absorbed by the TiO, mesh in the photocatalytic
reactor
(J.s).
As presented in Table 3, the quantum yields obtained were in the range of the
60-700 % range. Figure 16B depicts the increase in the quantum yield with the
initial
toluene concentration. It should be noted that when calculating the quantum
yields
corrections were introduced for the UV intensity decay and the average
k,,o,,ud value
was also used in this calculation.
Quantum yields obtained were consistently, except to the lowest toluene
concentration, bigger than 100%, and this confirm the special character of the
photocatalytic oxidation reaction where one photon appears to be involved in
more
than one photocatalytic event. As well, these levels of quantum yields points
towards
the excellent photocatalytic performance of the Photo-CREC-Air reactor of the
present invention under the conditions selected for its design and operation.
Kinetic modeling and the insights on the reaction network was another
important aspect of the present invention. The basis of the analysis is a
series reaction
mechanism where toluene is oxidized to intermediates (oxidized species) and
these
intermediates species are later on converted to carbon dioxide and water:
Toluene k I > Oxidized Intermediates k2 > COz +H20
(7.8)
Given intermediates could not be detected, were below detectable limits, a
possible quantification of the phenomena was achieved by increasing the
kinetic
constant k2, and leaving the kinetic constant k, unchanged for the following
reaction
network:
rT k,CT (7.9)
roi kiCT-k:Coj (7.10)
36
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
rco: k:Co1 (7.11)
It is shown in Figure 17A that having k, of a similar order of magnitude as k2
yields oxidized intermediates concentrations above the detectable limits.
Thus, in
order that the oxidized intermediates be just at detectable limits k, has to
be increased
to about 7 times k, (Figure 17B). Therefore, under these conditions it can be
estimated that the first step is the lowest step controlling the photo-
oxidation process.
Given that the actual experiments intermediates were not detectable it is
likely that
k, k2 and that the first oxidation step is the controlling for the complete
photo-
oxidation process.
In the case of desiring to achieve a faster photoconversion, a higher Qabr
(absorbed light power) may be provided. For instance, with 10 times bigger
Q,bs
(Figure 17C), 60% conversion is achieved in 3 hours only. It is assumed in
this
calculation that both constants k, and k, are equally affected by the light
power
increase. As well, this calculation assumes that the increase of the kinetic
constants is
directly proportional to the power increase.
Note that this Q,bs increase can be achieved in the Photo-CREC-Air reactor by
increasing the number of LN lamps used, the power of each lamp, using Pyrex
glass
to maximize light transmittance through windows, or by a concurrent change of
the
factors.
With respect to catalyst activity and eventual changes with time-on-stream,
catalyst activity was examined by repeating experiments using a new filter
mesh in
each experiment. This was done for the first 16 experiments of the series at
100 C
(Table 5). However, to monitor catalyst activity decay, runs 29 to 32 were
developed
with the same mesh which amounted to a filter being used for 90 hours.
Conversion
remained at a steady level (23-29%) and on this basis it was concluded that
there was
no deactivation of the Ti02 particles during the 90 hours of utilization.
As well, the water effect on the catalytic activity in the context of the
present
study was tested and it was found that the values of the kinetic constant for
toluene
photo-oxidation at the high water concentration (runs 20-32) remained
essentially at
the same level as the ones for the runs at low water concentration (runs 1-
16). Thus, it
was demonstrated that in the Photo-CREC-Air reactor, with a plate heating the
mesh
minimizing water adsorption, there was no effect of water on the catalytic
activity.
37
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
An error analysis was also developed and this included the propagation of
errors and the effect of ineasurable variables on the kinetic parameters.
Errors
calculations associated with the kinetic constant were performed assuming a
maximum of 5% error associated with toluene concentration measurements and 1%
on the experimental time. A Qbasic program helped in analyzing 35 samples with
errors randomized with respect to both toluene concentration measurements and
experimental time. This yielded an average error( ) of 0.26% with a standard
deviation(a) of 6% (Figure 18). Thus typical standard errors in the kinetic
constants
as calculated in the present study is 6%.
Note that the potential sources of errors as identified in the experimental
runs
were associated with the following: (i) imperfect injection of samples into
the GC, (ii)
diffusion of the sampling bags contents to the atmosphere, and (iii) eventual
toluene
condensation when warm samples were cooled to room temperature after being
stored
in the bags for a couple of hours.
Summary
Systematic runs of the Photo-CREC-Air reactor demonstrated excellent
performance of the reactor with the heating plate at 100 C. The heating plate
supporting the Ti02 mesh, minimizes condensation/adsorption on the TiO, mesh
surface and eliminates the potential influence of water content on catalytic
activity.
Under these conditions it was found that the reaction was first order kinetics
based on a pseudo homogeneous model of the photoreaction with low/minimum
adsorption of pollutants. Using the kinetic constants and the light absorbed
by the
mesh, quantum yields in the 60-700 % range were obtained and this confirmed
the
highly efficient and special character of the photoconversion in the Photo-
CREC-Air
reactor, where every photon is involved in more than one photoconversion
event.
To summarize, the present invention provides a superior photocatalytic reactor
in terms of its unique features, geometrical configuration and operation in
order to
efficiently and safely destroy organic pollutants and emit only non-toxic by-
products
back to the atmosphere without any intermediate by-products. The Photo-CREC-
Air
reactor of the present invention allows optimal photocatalytic performance and
high
photoconversion yields in the oxidation of air-borne organic pollutants. The
unit has
38
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
an optimum configuration in terms of flow patterns, as it provides for
intimate and
controlled contact of the flowing air stream with Ti02 by the provision of a
transversely positioned mesh. The photoreactor also provides for high loading
of
TiOZ particles on the supported mesh and a maximum use of light energy
resulting in
an optimum illumination of the mesh. Together, the unique design features of
the
present photocatalytic reactor such as the Venturi Section, the heating plate
supporting
the Ti02 mesh, and the focused illumination section optimize the unit
performance in
terms of fluid dynamic characteristics, TiOZ mesh loading, illumination and
quantum
yield. Also the self-cleaning provision of the reactor provided by the created
suction
in the Venturi section prevents the buildup of dust and dirt particles on the
windows
which subsequently no longer require cleaning.
This photocatalytic reactor can be brought on stream for emergency situations
in a matter of minutes. It is specially suited to deal with undesirable
conditions
caused by chemical leaks into air streams in chemical plants. The system is
also
conceived as a rugged design and is able to deal with hot and dusty gases. It
is
understood by those skilled in the art that the reactor is configured not only
to recycle
gas streams through an photocatalytic process to destroy pollutants contained
therein,
but may also have additional design features providing for the recycling of
the treated
gas stream back through the reactor in order to be treated again.
Alternatively, the
reactor can be configured to provide for the recycling of gas streams within
the reactor
without passing of the gas stream through the photocatalytic process.
Although,
specific dimensions of the present reactor are provided herein, it is
understood by
those skilled in the art, that the reactor and its individual components can
be
manufactured in various dimensions so long as the proportions provide for the
same
catalytic activity of pollutants.
The Photo-CREC-Air reactor displays high energy efficiency (high quantum
yields) being able to photoconvert significant amount of pollutants with
minimum
light power. The Photo-CREC-Air reactor is also considered to be a self
cleansing
system. Experimentation using the Photo-CREC-Air reactor covered a significant
range of operating conditions including: water vapor content, temperature, and
pollutant concentration.
39
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
On the basis of the data reported herein, it is demonstrated that Photo-CREC-
Air provides valuable performance in terms of toluene photodegradation in the
range
of concentration studied using minimum light power. Carbon dioxide and water
were
the only products observed from the photodegradation of toluene under the
conditions
studied. Intermediate species were below the detectable limits. It is appatent
to one
skilled in the art that the reactor of the present invention can be used not
only to
destroy toluene but also several different types of VOCs emitted from a
variety of
industrial and commercial sources.
It was demonstrated that a perforated heating plate supporting the TiO, mesh,
lo a special Photo-CREC-Air feature, was very effective in minimizing water
adsorption
and condensation at 100 C. Under these conditions no catalyst activity decay
was
observed. It was also found that pollutant adsorption at 100 C was mild, and
on this
basis a first order pseudo homogeneous model rate was considered. This model
was
adequate for representing the photo-oxidation rate of toluene under the
experimental
conditions tested. Quantum yields assessed, in the course of study, were very
high.
Values obtained were as high as 700% indicating the involvement of one photon
in
more than one photocatalytic event.
Examples
The examples are described for the purposes of illustration and are not
intended to limit the scope of the invention.
Methods of physical, organic and inorganic biochemistry referred to but not
explicitly described in this disclosure and examples are reported in the
scientific
literature and are well known to those skilled in the art.
ExammRle One-Characterization of Photo-CREC-Air Reactor
One prototype of the Photo-CREC-Air reactor (15.2 cm diameter) was
manufactured and debugged at the Mechanical Shop, UWO, assembled and tested at
the CRE-UWO. A filter impregnation technique was established and an
experimental
program was initiated to test the performance of the unit resulting in the
characterization of the system. In this unit, experiments with model
pollutants at
various concentration levels were conducted. Reactants and products including
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
various intermediates were measured and identified. This novel unit with the
configuration proposed is uniquely suited for destruction of organic
pollutants in
gaseous streams. In particular, the influence of the different operational
parameters
on the photo-oxidation rate of toluene was studied.
Examvle Two- Photocatalvst Impregnation Techniques
A number of different impregnation methods were developed so as to compare
attachment strength and catalyst loading. None of the considered methods
required
treatment of the mesh prior to the TiOZ loading process. These methods were
classified as wet, in which a suspension of 21 nm TiOZ particles is prepared,
and dry,
in which the catalyst in utilized as a dry powder.
A first method (Method 1) involved preparing a suspension of TiO, in a water-
methanol mixture. It is worth noting that the mixture contained 30% ethanol to
70%
water and 5 g per liter of TiO2. Ethanol addition was found to enhance the
attachment
of TiO2 particles to the filter as reported in Valladares (1995). A piece of
filter was
fixed by a hose clamp, to prevent its movement, on a special designed
plexiglass ring
with four opening from the bottom, as to allow the circulation of the water.
The solution was placed in a glass container along with a stirrer that
provided
adequate mixing keeping the TiO2 particles in suspension. Mixing was allowed
for 5
minutes before inserting the plexiglass ring with the filter, and the
suspension was
forced through the mesh for about 20 minutes. Unfortunately, low and non
uniform
Ti02 loading was achieved.
A second impregnation technique (Method 2) utilized an ethanol-water
solution of the same composition as in Method 1 and a special unit, made
mainly of
plexiglass and a stainless steel mesh supporting the filter. A submersed pump
was
used to pump the TiO2 suspension from one end to the other. In a typical
experimental run, the solution was pumped through the mesh for about 40
minutes.
This technique gave quite non uniform distribution of Ti02 particles on the
mesh.
Particles tended to settle and concentrate on the center while there were
little particles
on the filter sides (edges).
Another approach (Method 3) considered supporting the filter by a hose clamp
on a metallic ring, spraying the top surface with an ethanol-water solution of
the same
41
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
composition as in Method 1 and leave it to dry for 30 minutes before spraying
a
second coating. Analysis did not show any significant Ti02 loading.
The next attempt (Method 4) was a dry method involved using a small
fluidized bed. Ti02 was poured into the bed and the filter was fixed in the
upper
section. Air was introduced from the bottom allowing the TiO, to fluidize and
reach
the surface of the filter. Unfortunately, the TiO, powder being very fine did
not
fluidize very well and particles tended to fly through the porous structure of
the filter.
Finally, a process (Method 5) which involved applying a pre-weighted amount
of Ti02 powder, 2-2.5g, on a pre-weighted mesh, 6-8.5g, using a soft painting
brush,
was practiced and adopted. Care was taken to proceed gently in order not to
affect the
filter surface structure. Acetone was sprayed to attach the particles to the
upper
surface and transport them into the fibrous mesh. About five coatings were
performed
before the desired Ti02 amount was loaded on the mesh. This exercise gave
significant loading of the filter up to 50% (g of catalyst/g of fibrous mesh).
Example Three- Light Transmittance Measurement
Light transmittance through the filter before and after treatment is a very
important property of the inesh to ensure efficient light penetration through
out the
filter layer. Light transmittance of the specific wavelength (365 nm) was
measured
using a Spectrophotometer model 546A. In a typical measurement, a small piece
of
the filter was cut and placed in a specially designed cell made of plexiglass.
This cell
was inserted in the spectrophotometer after re-zeroing it with respect to the
bare
untreated filter. A reading was taken which corresponded to the amount of
light
absorbed by the mesh support. Then using Beer-Lambert law, the fraction of
focused
light beam that made its way through the filter piece was approximated as:
A = log (I/Io)=1og T=-abc (5.1)
with A being the absorbance, "a" the absorptivity (characteristic of the
substance), "b"
the path length, "c" the concentration and T the transmittance.
Analysis revealed that light transmittance of the FiltreteTM and 3M Blue
Pleated Filter were approximately (4-1)% and (2.5-0.2)%, respectively after
treatment
with TiO2 and acetone. These readings were not much larger than the ones for
the
bare untreated filters (12 %) and (1.3-2.8)%. Thus, it was concluded that the
addition
42
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
of TiOZ in the 3M Blue Pleated Filter did not affect the desired near UV light
(365
nm) transmission properties.
Example Four- Temperature Programmed Desorption (TPD)
This analysis was performed in order to estimate the temperature at which the
filter will be essentially water free. Temperature programmed desorption was
performed in a Micromeritics TPD/TPR 2900 machine. A small wet piece of the
filter
was inserted into a U tube and was treated continuously with helium as a
carrier gas.
Approximately 10 minutes were required to stabilize the system, after which
the
lo furnace temperature was taken up to a temperature of 140 C with a rate of
15 C/min.
According to the results, all three filters had good water desorption
properties, with
the water being fully desorbed from the filter at around 100 C, the
temperature at
which a peak was observed. Hence, the temperature of the plate was raised as
high as
100 C during the experiments and this to ensure that the filter operated free
of water.
-
Example Five- Particle Attachment to the Filter
TiOZ loaded filters were studied under Scanning Electron Microscopy (SEM,
Figure 7). This helped in the assessment of Ti02 distribution and it also
proved that
the particles were actually attached to the fibrous strands and not loosely
held in the
spaces of the porous structure. SEM photos of different regions revealed that
3M
Blue Pleated Filter is good in retaining the TiOZ particles. SEM photos show,
however, the presence of multiple uniformly distributed aggregates of well
anchored
Ti02 throughout the mesh fibers. Figure 7 shows the mesh fibers having
typically 10
m holding particles of about 1 m. Note that SEM analysis were limited to 3M
Blue
Pleated Filter and this given Bionaire filter was not able to hold Ti02
particles very
strongly.
Examnle Six- Electrostatic Charges
The bonding of the Ti02 particles to the surface of the fibrous mesh is
influenced to a significant degree by electrostatic forces. Electrostatic
filters have
inherent electrostatic charges generated by the "electret" fibers from which
they are
made. As a stream of fluid is circulating through the filter, the amount of
charges
43
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
increases and holds the particles more firmly and strongly. This property was
used to
assess the strength of particles bonding to the fibrous mesh.
The electrostatic charges induced or found on the different tested supports
were measured using a Faraday Pail. This test was carried out to test the
strength of
bonding between the catalyst particles and the mesh. A Faraday Pail consists
of two
pails placed one inside the other and connected by a wire to an electrometer.
Using
this technique it was possible to show that both FiltreteTM and the Bionaire
filters were
electrostaticlly charged compared to the neutral 3M Blue Pieated Filter.
Measurements revealed that the white FiltreteTM possessed negative charges,
about one
order of magnitude larger than that of the 3M Blue Pleated Filter. The
utilized set-up
from AERC provided measurements with a some uncertainty thus, measurements
with
the Faraday Pail were used for relative charge readings only.
Example Seven- Analysis of Reactor Samples
Gaseous samples containing unreacted chemicals along with the end products
and intermediates were analyzed quantitatively and qualitatively using 5890
Hewlett
Packard gas chromatograph (GC). This unit is equipped with a Hewlett Packard
3393A integrator which allow the reporting of the different peaks along with
their
retention time and integrated areas which are proportional to their respective
amount
in the injected sample volume.
To complete the various analytical tasks, two GCs were used. One was
equipped with a Porapak Q packing column 1.83 m long and 0.318 cm in diameter
in
conjunction with a thermal conductivety detector (TCD). The other one was
operated
with a 25m-0.33 m HP-1 capillary column associated with a flame ionizable
detector
(FID). Both GCs used helium as the carrier gas. In the case of the packed
column, a
2.5 ml sample was injected at an oven temperature of 30 C and was held there
for
about 0.5 min before its temperature was raised to 200 C at a rate of 45 C/min
and
remained there for 8 minutes. This allowed good separation and identification
of the
collected mixture components. Identification of intermediate products was
carried out
by injecting a 2.5 ml sample into the capillary column at 30 C. Temperature
was held
at 30 C for 3 minutes, ramped up to 90 C at a rate of 15 C/min and held there
for 2
minutes before it was brought back to 30 C.
44
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Example 8- Photo-CREC-Air Reactor. Experimental Procedure
Experiments were carried out in Photo-CREC-Air.
Several runs were carried out for each experimental condition to ensure the
reproducibility of the data. In addition to this, repeats were also carried
with the same
catalyst to record potential catalyst deactivation and to be able to predict
the eventual
influence of the catalyst deactivation on the reaction rate.
Dry air gas supplied from BOC, was used during the experimental program.
The reactor was filled with air bringing the final gauge pressure up to 13.78
kPa. This
over pressure provided an adequate internal pressure as for taking enough
samples
over the experimental time. For each run, at least half an hour was waited to
ensure
that the system was leak free. Following this, the heaters were turned "on"
until the
desired temperature (50-100 C) was attained. At the same time the heater of
the
injection port was turned "on" to reach --120 C, a temperature high enough to
completely vaporize the toluene sample. The reactor contents were allowed to
circulate and were recycled for about half an hour before any samples were
taken. In
the case of high humidity level experiments, water was also injected into the
reactor
for about half an hour before the toluene injection.
Note that during the first four hours the UV light was not turned on allowing
the toluene to be adsorbed onto the Ti02 particles. After that, light was
turned on for
20-22 hours. Samples were taken every 0.5-1 hour. Note also that, samples were
not
injected on-line in the GC. Reaction samples were collected in a vinyl tubing
from
the sampling port to a 1L Tedlar bag. Later on, gas samples were injected
manually
into the GC using a 2.5m1 syringe. Figure 14 provides a detailed drawing of
the
Tedlar sampling bag port. After the experimental time was elapsed, the system
was
purged with air for an hour. Eventually, a new mesh was set before the new
experiment was initiated.
Toluene concentrations of 5.2-13 g/cm' were used during the testing. These
concentrations were achieved by injected samples of 0.4 -1.0 ml of toluene
into the
reactor volume. In addition, four temperatures over the range of 20-100 C,
were used
in the heating plate supporting the mesh. The investigation also included two
humidity levels: high and low. The low concentration level corresponded to the
one
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
with no water addition and the high concentration level one was equivalent to
0.003
vol.%( 30 g/cm').
An internal standard procedure was implemented to reduce the uncertainty
related to variable gas samples injected in the GC. Given air was essentially
unaffected by the photoconversion, oxygen consumption was negligible, hence
the air
peak was taken as a reference value. Thus, the ratio of toluene/air was used
to
monitor the rate of toluene disappearance with time on-stream and this to
account for
the potential combined effects of leaking, adsorption, condensation and
reaction.
46
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Table 1: Nomenclature
A absorbance
a absorptivity (characteristic of the substance)
Af total free area of holes
AP total sectional area of the perforated plate
b path length
c concentration
C gas phase reactant concentration, and speed of light in vacuum
(2.997 x 1010cm/s)
C. orifice coefficient
Co1 concentration of the oxidized intermediates
CP is the gas phase concentration of the pollutant
C, venturi coefficient, empirically determined and is about 0.98 for
well designed venturi of pipe diameter 2 to 8 inches
CN, is the gas phase concentration of the water vapour
CT toluene concentration at time t
CTa the initial toluene concentration
f(C) concentration function of the rate equation
F. and FW factors for the competitive adsorption between the pollutant and
the water for the same active site
g. gravity dimensional constant (32.17 lb ft/obfsZ). If SI units are
used then g,=1
h Plank's constant, 6.62 x 10'14 J.s
I light intensity at distance
Io incident intensity
KNads) adsorption equilibrium constant which can be meansured at the
solid-gas
k(I) intensity dependent apparent rate constant
k reaction rate constant, also the ratio of the specific heats Cp/Cv
k.ted reaction rate constant corrected for LN decay
ko reaction rate constant at the surface and also the constant of
47
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
proportionality in the Langmuir-Hinshelwood bimolecular
reaction form ( mol.cm Zh'')
ks intrinsic reaction rate constant
K1, K2, K3, K4 are the Langmuir adsorption equilibrium constants
(PPmv' )
NA Avogadro number (6.023 x 1023 molecule/mole)
Pi fluid pressure under upstream conditions
P2 fluid pressure at the throat conditions
Q..ebs rate of light energy absorbed by the Ti02 in the photocatalytic
reactor (J.s)
r rate of reaction, ratio of p2/p, in the venturi calculations, also is
the oxidation rate ( mol.cm'-h')
roT the initial toluene photo-oxidation rate
[r,,,P.o],õax rate of model pollutant destruction
r.r rate of toluence photo-oxidation
T transmittance
V total gas hold up
V2 average fluid velocity at the throat of the venturi
W mass flow rate, also the weight of the catalyst involved in the
chemical reaction
x penetration depth into Ti02 layer
Y dimensionless expansion factor, for the flow of compressible
fluid
z the axial distance through the Ti02 layer
v* stoichiometric number for -OH reactinQ with the model compound
Stoichiometric number for the pollutant chemical species reacting with = OH
v gas superficial velocity
a required for the formation of an -OH
ratio of diameter of the venturi throat to diameter of pipe
the effective extinction coefficient of the photocatalyst
x wavelength (nm)
p, density of the fluid under upstream conditions
48
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Op pressure drop across the plate
S2 constant
0 surface coverage of the reactant
the absorbance coefficient of powdered solids
OC the finite difference between the pollutant concentration at
length CL and its intial concentration C.
49
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Table 2: Summary of toluene concentration drop in both blank and
reaction runs at 20 C and 100 C and at the different experimental time
Temperature (20 'C) (100 'lr')
Experimental time, (hr) 4 9 4 9
Toluene concentration drop, blank run 11% 22% 7% 9%
Toluene concentration drop, reaction run 11% 15% 9% 20-32%
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Table 3: Summary of the calculated parameters of the different
experimental runs
EJtperiment k kw..W.r Quantum Yiald Comrvrcion rT
~ (hr ) (hr ) (!b) ('!6) ( meN,l(9oatsl)
1 0.0189 0.016872 58.38985025 30.8 0.01033
2 0.0104 0.011005 58.31937936 28.2 0.00582
3 0.011 0.012008 80.33633219 28.5 0.007418
4 0.0085 0.012609 02.44800186 25.1 0.006688
0.0198 0.022135 175.3572829 37.4 0.02324
B 0.0196 0.023034 188.3350167 37.7 0.018184
7 0.038 0.040733 184.8491298 50 -77W3526
0.0142 0.03Z495 407.9301004 Z4.0 0.02437
0.02Z 0.027115 284.1293000 39.7 0.022783
0.0202 0.026406 282.9185214 39.2 0.020778
0.010 0.02.t669 272.0114651 36.2 0.02578
0.01 0.020707 533.2767015 17 0.021748
r1312
01 605 49.01Q4048 94 0.0Z310e
0.0133 0.
14 0.0274 0.038038 521.2886749 47.2 0.049924
1 0.023 0.032699 539.3172387 42.2 0.04768
16 0.02 0.04720 1038.987722 50 0.049565
29 0.0124 0.02642 740.0734646 23.7 0.02522
30 0.0152 .033 .6686143 24.6 0.03166
31 0.0148 0.033295 792.2-25479- ZZ 29.55 0.03179
32 0.0104 0.037529 81 D.022Z138 27.3 0.035833
51
O
o\o
Table 4: Summary of the average values of the calculated parameters.
c Experimental set [Toluene] Temperature Humidity kCOfected Quantum rT y
yield
cn
ug/cm' (C) ug/cm' (hr') (%) (pmole/(gcat.s)) m 1 5.2 100 <25 0.01382 66.8
0.00754
N vn 2 7.78 100 <25 0.02589 186.2 0.02545
m 3 10.4 100 <25 0.02447 263 0.02512
m
1 4 13 100 <25 0.0339 515.8 0.04020
8 10.4 100 >30 0.0326 780 0.0311
C
m
Of
b
00
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Table 5: Summary of the results under different experimental conditions.
Experiment [T] Plate Temp. Humidity Mesh # Time
# ml g/cm3 (C) g/cm3 (hr)
1 0.4 5.2 100 <25 14 24
2 0.4 5.2 100 <25 15 23
3 0.4 5.2 100 <25 16 22
4 0.4 5.2 100 <25 21 24
0.6 7.78 100 <25 12 24
6 0.6 7.78 100 <25 13 21
7 0.6 7.78 100 <25 19 22
8 0.6 7.78 100 <25 31 23.5
9 0.8 10.4 100 <25 26 21
0.8 10.4 100 <25 20 24
11 0.8 10.4 100 <25 25 23
12 0.8 10.4 100 <25 30 22.5
13 1 13 100 <25 24 27
14 1 13 100 <25 23 24
1 13 100 <25 22 22
16 1 13 100 <25 32 26
17 0.8 10.4 75 <25 27 23
18 0.8 10.4 75 <25 27 24
19 0.8 10.4 75 <25 27 23
0.8 10.4 75 <25 27 23
21 0.8 10.4 50 <25 28 22
22 0.8 10.4 50 <25 28 24
23 0.8 10.4 50 <25 28 24
24 0.8 10.4 50 <25 28 23
0.8 10.4 20 <25 29 22
26 0.8 10.4 20 <25 29 25
27 0.8 10.4 20 <25 29 24
28 0.8 10.4 20 <25 29 23
29 0.8 10.4 100 >30 30 22.5
0.8 10.4 100 >30 30 22
31 0.8 10.4 100 >30 30 24.5
32 0.8 10.4 100 >30 30 22.5
53
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
References
Akimoto, H., Hoshino, M., Inove, G., Okuda, M., and Washida, N., "Reaction
Mechanism of the Photo-oxidation of the Toluene -N02-02-N2 System in the Gas
Phase", Bulletin of the Chemical Society of Japan, v51(9), 2496-2502, 1978.
Anderson, M., Yamazaki-Nishida, S., and Cervera-March, S.,
"Photodegradation
of Trichloroethylene in the Gas Phase Using TIO, Porous Ceramic Membrane", in
Photocatalytic Purification and Treatment of Water and Air- Proceedings of the
15' International Conference on TiO~, 405-420, D. Ollis and H. Al-Ekabi
(eds.), 1993.
Atkinson, R., Carter, W., Darnall, K., Winer, A., and Pitts, J., "A Smog
Chamber and
Modeling Study of the Gas Phase NOx Air Photo-Oxidation of Toluene and the
Cresols", International Journal of Chemical Kinetics, v7, 779-836, 1980.
Besemer, A., "Formation of Chemical Compounds from Irradiated Mixtures of
Aromatic Hydrocarbons and Nitrogen Oxides", Atmospheric Environment, v16(6),
1599-1602,1982.
Bickley, G., Munuera, and Stone, F., "Photo Adsorption and Photocatalysis of
Rutile
Surfaces at Photocatalytic Oxidation of Iso-propanol", J. of Catalysis, v31,
398-407,
1973.
Blanco, J., Avila, P., Bahamonde, A., Alvarez, E., Sanchez, B., and Romero,
M.,
"Photocatalytic of Toluene and Xylene at Gas Phase on a Titania Based
Monolithic
Catalyst", Catalysis today, v29, 437-442, 1996.
54
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Bolton, J., Safarzadeh-Amiri, A., and Cater, S., "The Detoxification of Waste
Water
Streams Using Solar and Artificial UV Light Sources", in Alternative Fuels and
the
Environmental, 187-192, F. S. Sterret (ed.), 1995.
Carey et al (1976) - see provisional application
Childs, L., and Ollis, D., "Is Photocatalysis Catalytic?", Journal of
Catalysis, v66,
383-390, 1980.
Dibble, L., and Raupp, G., "Fluidized-Bed Photocatalytic Oxidation of
Tricholoethylene in Contaminated Air streams", Environ. Sci. and Technol.,
v26(3),
492-495, 1992.
Formenti, M., Juillet, F., Meriaude, P., and Teicher, S., "Heterogeneous
Photocatalysis for Partial Oxidation of Paraffins.", Chem. Technol., vl, 680,
1971.
Formenti, M., Juillet, F., and Teicher, S, "Photocatalytic Oxidation Mechanism
of
Alkanes over Titanium Dioxide: 2. Reaction Mechanism", Bull. Soc. Chem. Fr, v9-
10, 1315-1320,1976.
Formenti, M., and Teicher, S., in "Catalysis v.2. Specialist Periodical
Report", p87,
Chemical Society of London Edition, London, 1979.
Fujishim, A., and Honda, K., "Electrochemical Photolysis of Water at a Semi--
conductor Electrode", Nature, v238(5358), 37, 1972.
Heuss, J., Nebel, G., and D'Alleva, B., "Effects of Gasoline Aromatic and Lead
Content on Exhaust Hydrocarbon Reactivitv", Environs Sci. Technol., v8, 641-
647,
1974.
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
lbusuki, T., and Takeuchi, K., "Toluene Oxidation on UV Irradiated Titanium
Dioxide
With and Without OZ, NOZ, or H20 at Ambient Temperature", Atmospheric
Environment,
v 20(9), 1711-1715,1986.
Ibusuki, T., Kutsuna, S., and Takeuchi, K., "Removal of Low Concentration Air
Pollutants through Photoassisted Heterogeneous Catalysis", in Photocatalvtic
Purification and Treatment of Water and Air: Proceedings of the 1 st
International
Conference on TiO,, 375-386, D. Ollis and H. Al-Ekabi (eds.), 1993.
Jacoby, W., Blake, D., Fennell, D., Boulter, J., Vargo, L., George, M., and
Dolberg, S.,
"Heterogeneous Photocatalysis for Control of Volatile Organic Compounds in
Indoor
Air", Journal of Air and Waste Management Association, v46, 891-898, 1996.
Lonneman, W., Kopczynski, S., Darley, P., and SuterField,. F., " HydrocarbonY
Composition of Urban Air Pollution", Environ. Sci. Technol., v8, 229-236,1974.
Luo, Y., and Ollis, D., "Heterogeneous Photocatalytic Oxidation of
Trichloroethylene
and Toluene Mixtures in Air: Kinetic Promotion and Inhibition, Time-Dependent
Catalyst Activity", Joumal of Catalysis, v163, 1-11, 1996.
McCabe, S., Smith, J., and Harriott, P., Unit Operations of Chemical
Enizineering.
McGraw-Hill, Inc., Toronto, 1993.
Miller, R., and Fox, R., "Treatment of Organic Contaminants in Air by
Photocatalytic
Oxidation: A Commercialization Perspective", in Photocatalvtic Purification
and
Treatment of Water and Air: Proceedings of the 1 st International Conference
on TiO,
573-578, D. Ollis and H. Al-Ekabi (eds.), 1993.
Milne, T., and Nimlos, M., "Chemical Safety: Incomplete Photocatalytic
Oxidation of
TCE", Chem. Eng. News, v70(14-26), 2, (June,22) 1992.
56
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Muradov, N., T-Raissi, A., Muzzey, D., Painter, C., and Kemme, M., "Selective
Photocatalytic Destruction of Airborne VOCs", Solar Energv, v56(5), 445-
453,1996.
Muriel, M., Rueda, A., and Milow, B.(eds.), Plataforma Solar de Al-meria.
Annual
Technical Report., Spain, 1996.
Obee, T., and Brown, R., "TiO2 Photocatalysis Indoor Air Applications: Effects
of
Humidity and Trace Contaminant Levels on the Oxidation Rates of Formaldehyde,
Toluene, and 1,3-Butadiene", Environmental Science and Technology, v29(5),
1223-
to 1231, 1995.
Peill, N., and Hoffmann, M., "Development and Optimization of a Ti02 Coated
Fiber
Optic Cable Reactor: Photocatalytic Degradation of 4-Chlorophenol", Environs
Sci.Technol., v29, 2974-2981, 1995.
Peill, N., and Hoffinann, M., "Chemical and Physical Characten'zation of a
TiO, Coated
Fiber Optic Cable Reactor", Environs Sci. Technol., v30, 2806-2812,1996.
Peral, J., and Ollis, D., "Heterogeneous Photocatalytic Oxidation of Gas-Phase
Organics
for Air Purification: Acetone, 1-Butanol, Butyraldehyde, Formaldehyde, and m-
Xylene
Oxidation", Journal of Catalysis v136, 554-565, 1992.
Perr, R., and Green, D., Perry's Chemical Engineering Handbook. McGraw-Hill,
Inc.,
Toronto, 1984.
Raupp, G., Nico, J., Annangi, S., Changrani, R., and Annapragada, R., "Two
Flux
Radiation-Field Model for an Annular Packed Bed Photocatalytic Oxidation
Reactor",
AI hE, v43 (3), 792-801, 1997.
Sauer, M., and Ollis, D., "Acetone Oxidation in a Photocatalytic Monolith
Reactor",
Journal of Catalysis, v149, 81-91, 1994.
57
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Sczechowski, J., Koval, C., and Noble, R., "A Taylor Vortex Reactor for
Heterogeneous Photocatalysis", Chemical Engineering Science, v50(20), 3163-
3173,
1995.
Serrano, B., and de Lasa, H., "Photocatalytic Degradation of Water Organic
Pollutants. Kinetics Modeling and Energy Efficiency", Ind. Eng. Chem. Res.,
v36,
4705-4711, 1997.
Shepson, P., Edney, E., and Corse, E., "Ring Fragmentation Reactions on the
Photo-
io Oxidation of Toluene and O-xylene", The Journal of Physical Chemistl,y, v
88(18),4122-4126, 1984.
Suzuki, Ken-ichirou., "Photocatalytic Air Purification on TiO, Coated
Honeycomb
Support", in Photocatalytic Purification and Treatment of Water and Air:
Proceedings of the 151
International Conference on Ti02, 421-434, D. Ollis and H. Al-Ekabi (eds.),
1993.
Valladares, J., A New Photocatalytic Reactor for the Photode~radation of
Organic Contaminants
Water, PhD. Thesis, University of Western Ontario, 1995.
Van Vlack, Lawrence. Materials for Enaineering: Concents and Anplications.
Addison. Wesley, 1982.
Wang, K., and Marinas, B., "Control of VOC Emissions from Air Stripping
Towers: Development of Gas-Phase Photocatalytic Process", in Photocatalvtic
Purification and Treatment of Water and Air: Proceedines of the 1 st
International
Conference on Ti0,z,733-739, D. Ollis and H. Al-Ekabi (eds.), 1993.
Yamazaki-Nishida, S., Nagano, J., Phillips, L., Cervera-March, S., and
Anderson, M.,
"Photocatalytic Degradation of Trichloroethylene in the Gas Phase Using
Titanium
Dioxide Pellets", Journal of Photochemistrv and Photobiology A: Chemistcv,
v70, 95-
99, (1993).
59
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Yamazaki-Nishida, S., Read, H., Nagano, J., Jarosch, T., Eddy, C., Cervera-
March, S.,
and Anderson, M., "Gas Phase Photocatalytic Degradation on TiO2 Pellets of
Volatile
Chlorinated Compounds from a Soil Vapor Extraction Well", Journal of Soil
Contamination on TiO2, v3(4), 363-378, 1994.
Yue, P., "Modeling, Scale-up and Design of Multiphasic Photoreactors", in
Photocatalytic Purification and Treatment of Water and Air: Proceedings of the
1 S'
International Conference on TiO, 495-5 10-, D. Ollis and H. Al-Ekabi (eds.),
1993.
io Zhang, N., Crittenden, J., Hand, D., and Pen-am, D., "Fixed-Bed
Photocatalysts for Solar
Decontamination of Water", Environ. Sci. Technol., v28, 435-442,1994.
59
CA 02286152 1999-10-06
WO 98/46335 PCT/CA98/00314
Although preferred embodiments of the invention have been described herein
in detail, it is understood by those skilled in the art that variations may be
made
thereto without departing from the spirit of the invention of the scope of the
appended
claims.