Note: Descriptions are shown in the official language in which they were submitted.
CA 02287706 1999-10-26
SILICONE RUBBER COMPOSITION FOR COMPOSITE MOLDING
BACKGROUND OF INVENTION
The present invention relates to a silicone rubber composition for composite
molding, such as insert molding or mufti-color molding, and more particularly
relates
to a silicone rubber composition that provides superior adhesion to organic
resins and
superior mold release properties in such composite molding.
A method that has been adopted for bonding a silicone rubber to an organic
resin in composite molding such as insert molding or mufti-color molding
involves
prlmmg a pre-molded organic resin and then curing it while it is brought
together with
a silicone rubber composition. The problems with this method are that a
priming step
is required and the adhesion of the silicone rubber is decrease if this
priming treatment
is inadequate.
In addition, to bond a silicone rubber securely to an organic resin, there are
known methods involving the use of an organic resin containing aliphatic
unsaturated
groups (see Japanese Laid-Open Patent Applications 6-171021, 6-171022, and
6-171023), or methods featuring the use of a silicone rubber composition to
which
vinyltrimethoxysilane, 3-glycidoxypropyltrimethoxysilane, 3-methacryloxypropyl-
2o trimethoxysilane, or another such adhesion promoter has been added. A
limitation to
the former method, is that a special organic resin has to be used, while a
problem with
the latter method is that the mold release properties are decreased, and in
extreme
cases, the silicone rubber even adheres to the mold.
Specifically, it is an object of the present invention to provide a silicone
rubber
composition for composite molding that affords superior adhesion to organic
resins and
superior mold release properties in composite molding, such as insert molding
or multi-
color molding.
CA 02287706 1999-10-26
TSL1484
SUMMARY OF INVENTION
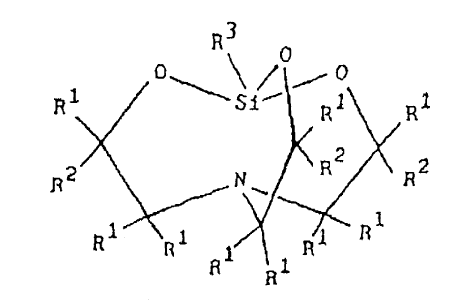
A silicone rubber composition for composite molding comprising (A) a
polydiorganosiloxane having at least two silicon atom-bonded alkenyl groups
per
molecule, (B) a polyorganosiloxane having at least two silicon atom-bonded
hydrogen
atoms per molecule, (C) 0.1 to 10 weight parts of a silatrane derivative
described by
general formula:
R3
i o'''~~ !o~-- o
s~- ~z Rz
1~2 N \ R2 R2
1 z
R !is R1 1 R:1 R
R
where each R' is independently selected from a hydrogen atom and an alkyl
group;
each RZ is independently selected from the group consisting of a hydrogen
atom, alkyl
groups, and alkenyloxyalkyl groups expressed by the general formula -R4-O-RS
where
R4 is an alkylene group and RS is an alkenyl group; at least one Rz group is
an
alkenyloxyalkyl group; and R' is selected from the group consisting of
substituted and
unsubstituted monovalent hydrocarbon groups, C, to C,o alkoxy groups,
glycidoxyalkyl
groups, oxiranylalkyl groups, acyloxyalkyl groups, and aminoalkyl groups, and
(D) a
platinum type catalyst in an amount sufficient to cure the composition.
' CA 02287706 1999-10-26
TSL1484
DESCRIPTION OF INVENTION
The present invention is a silicone rubber composition for composite molding
comprising
(A) 100 weight parts of a polydiorganosiloxane having at least two silicon
atom-
bonded alkenyl groups per molecule;
(B) a polyorganosiloxane having at least two silicon atom-bonded hydrogen
atoms per molecule in an amount such that the ratio of the number of moles of
hydrogen atoms bonded to silicon atoms in this component to the number of
moles of
alkenyl groups bonded to silicon atoms in component (A) is between 0.5:1 and
20:1;
(C) 0.1 to 10 weight parts of a silatrane derivative described by formula:
R3
o _ \ ~o~" o
R' 's~' R1 \ Rf
p2 N\~~R2~ R2
Rf
Ry 1~ R1
R R1
where each R' group is an independently selected hydrogen atom or alkyl group;
each
Rz is independently selected from the group consisting of a hydrogen atom,
alkyl
groups, and alkenyloxyalkyl groups described by formula
_Ra_O_Rs
where R4 is an alkylene group and RS is an alkenyl group; at least one RZ
group is an
alkenyloxyalkyl group; and R3 is selected from the group consisting of
substituted and
CA 02287706 1999-10-26
4 TSL1484
unsubstituted monovalent hydrocarbon groups, C, to C,o alkoxy groups,
glycidoxyalkyl
groups, oxiranylalkyl groups, acyloxyalkyl groups, and aminoalkyl groups; and
(D) a platinum type catalyst in an amount sufficient to cure the composition.
The silicone rubber composition for composite molding of the present invention
will now be described in detail.
Component A is the principal component of the present composition, and is a
polydiorganosiloxane having at least two silicon atom-bonded alkenyl groups
per
molecule. The molecular structure of component A is substantially linear, but
part of
the molecular chain may have some branching. Examples of the silicon atom-
hnnriPrl
1 o alkenyl groups include vinyl, allyl, butenyl, and propenyl. This alkenyl
group may be
bonded in any position, including the molecular chain terminals, pendant
position, or
both. It is preferable for these alkenyl groups to be at least at the
molecular chain
terminals because the mechanical properties of the resulting silicone rubber
will be
better. Examples of silicon atom-bonded groups other than alkenyl groups which
may
be present in component A include substituted and unsubstituted monovalent
hydrocarbon groups having no aliphatic unsaturated carbon-carbon bonds
including for
example alkyl groups such as methyl, ethyl, propyl, butyl, and octyl; aryl
groups such
as phenyl and tolyl; and substituted hydrocarbon groups such as chloromethyl
and
3,3,3-trifluoropropyl. There are no restrictions on the viscosity of component
A, but a
range of 10 to 1,000,000 mPa ~ s at 25°C is nreferahie
Examples of the polydiorganosiloxane comprising component A include a
dimethylvinylsiloxy end-capped polydimethylsiloxane capped at both ends of the
molecular chain, a trimethylsiloxy end-capped dimethylsiloxane ~
methylvinylsiloxarie
copolymer capped at both ends of the molecular chain, a dimethylvinylsiloxy
end-
capped dimethylsiloxane ~ methylvinylsiloxane copolymer capped at both ends of
the
molecular chain, a dimethylvinylsiloxy end-capped dimethylsiloxane
methylphenylsiloxane copolymer capped at both ends of the molecular chain, a
dimethylvinylsiloxy end-capped dimethylsiloxane ~ methyl(3,3,3,-
trifluoropropyl)-
CA 02287706 1999-10-26
TSL1484
siloxane copolymer capped at both ends of the molecular chain, a
dimethylhexenylsiloxy end-capped polydimethylsiloxane capped at both ends of
the
molecular chain, a trimethylsiloxy end-capped dimethylsiloxane
dimethylhexenylsiloxane copolymer capped at both ends of the molecular chain,
a
dimethylhexenyloxy end-capped dimethylsiloxane ~ dimethylhexenylsiloxane
copolymer
capped at both ends of the molecular chain, a dimethylhexenyloxy end-capped
dimethylsiloxane - methylphenylsiloxane copolymer capped at both ends of the
molecular chain, and a dimethylhexenylsiloxy end-capped dimethylsiloxane
methyl(3,3,3,-trifluoropropyl)-siloxane copolymer capped at both ends of the
molecular
chain.
Component B acts as a cross-linking agent in the present composition, and is a
polyorganosiloxane having at least two silicon atom-bonded hydrogen atoms per
molecule. There are no restrictions on the molecular structure of component B,
but
examples include linear, linear with some branches, branched, cyclic, and
resinous.
Examples of groups that can be bonded to the silicon atoms of component B,
besides
the silicon atom-bonded hydrogen atoms, include alkyl groups such as methyl,
ethyl,
and propyl; aryl groups such as phenyl and tolyl; and halogenated alkyl groups
such as
chloromethyl, 3-chloropropyl, and 3,3,3-trifluoropropyl group. There are no
restrictions on the viscosity of component B, but a range of 1 to 10,000 mPa ~
s at 25°
C is preferable. When component A is a polydiorganosiloxane having two alkenyl
groups per molecule, it is preferable for component B to include at least a
polyorganosiloxane having three or more silicon atom-bonded hydrogen atoms per
molecule, but when component A includes at least a polydiorganosiloxane having
three
or more silicon atom-bonded alkenyl groups per molecule, component B may be
composed of just a polyorganosiloxane having two silicon atom-bonded hydrogen
atoms per molecule.
Examples of the polyorganosiloxane of component B include a trimethylsiloxy
end-capped polymethylhydrogensiloxane capped at both ends of the molecular
chain, a
CA 02287706 1999-10-26
TSL1484
trimethylsiloxy end-capped dimethylsiloxane ~ methylhydrogensiloxane copolymer
capped at both ends of the molecular chain, a dimethylhydrogensiloxy end-
capped
dimethylsiloxane ~ methylhydrogensiloxane copolymer capped at both ends of the
molecular chain, a cyclic dimethylsiloxane ~ methylhydrogensiloxane copolymer,
a
cyclic polymethylhydrogensiloxane, an organosiloxane copolymer composed of
siloxane units described by formula R3Si0"2 , siloxane units described by
formula
RZHSiO,n , and siloxane units described by formula Si04,~; an organosiloxane
copolymer composed of siloxane units described by formula RZHSiO"z and
siloxane
units described by formula Si04,~ ; an organosiloxane copolymer composed of
siloxane
units described by formula RHSi02,~ and siloxane units described by formula
RSi03,2 or
siloxane units described by formula HSi03,~; and mixtures of two or more types
of
these polyorganosiloxanes. R in the above formulas is a substituted or
unsubstituted
monovalent hydrocarbon group with no aliphatic unsaturated carbon-carbon
bonds,
examples of which are the same groups as those listed above.
The amount in which component B is contained in this composition is such that
the ratio of the number of moles of hydrogen atoms bonded to silicon atoms in
this
component to the number of moles of alkenyl groups bonded to silicon atoms in
component A is between 0.5:1 and 20:1. This is because the resulting
composition will
tend not to cure sufficiently if the component B content is below the range
given above,
and because the mechanical strength of the resulting silicone rubber will tend
to
decrease if the above range is exceeded.
Component C serves to enhance the adhesion to organic resins, without
adversely affecting the mold release properties of the present composition,
and is a
silatrane derivative described by the general formula:
CA 02287706 1999-10-26
TSL1484
R3
0_ ~ ~0~ 0
R. .Si~~ R1 \ R1
R2 N~ ~\R2~ Rz
R1
R~ R1~ 1 R1
R
In the formula, each R' is an independently selected hydrogen atom or alkyl
group.
Examples of the alkyl group of R' include methyl, ethyl, propyl, butyl,
pentyl,
isopropyl, isobutyl, cyclopentyl, and cyclohexyl. It is particularly favorable
for R' to
be a hydrogen atom or a methyl group. Each RZ in the above formula is
independently
selected from the group consisting of a hydrogen atom, alkyl groups, and
alkenyloxyalkyl groups expressed by the general formula -R4-O-R5. At least one
Rz
group must be an alkenyloxyalkyl group. Examples of the alkyl group of RZ are
the
same as those given for R' above. With the alkenyloxyalkyl group of R2, R4 in
the
formula is an alkylene group, examples of which include methylene, ethylene,
methylmethylene, and propylene, with methylene being preferred. RS in the
above
formula is an alkenyl group, examples of which include vinyl, allyl, butenyl,
pentenyl,
and hexenyl, with a C3 to C,o alkenyl group being preferable, and the allyl
group being
particularly favorable. R3 in the above formula is selected from the group
consisting of
substituted and unsubstituted monovalent hydrocarbon groups, C, to C,o alkoxy
groups,
glycidoxyalkyl groups, oxiranylalkyl groups, acyloxyalkyl groups, and
aminoalkyl
groups. Examples of the monovalent hydrocarbon group of R3 include alkyl
groups.
such as methyl, ethyl, propyl, butyl, pentyl, isopropyl, cyclopentyl, and
cyclohexyl;
alkenyl groups such as vinyl, allyl, butenyl, pentenyl, and hexenyl; aryl
groups such as
phenyl, tolyl, and xylyl; aralkyl groups such as benzyl and phenethyl; and
halogenated
alkyl groups such as chloromethyl, 3-chloropropyl, and 3,3,3-trifluoropropyl.
Examples of the alkoxy group of R' include methoxy, ethoxy, and propoxy;
examples
CA 02287706 1999-10-26
8 TSL 1484
of the glycidoxyalkyl group of R3 include the 3-glycidoxypropyl group;
examples of the
oxiranylalkyl group of R' include 4-oxiranylbutyl and 8-oxiranylbutyl;
examples of the
acyloxyalkyl group of R' include acetoxypropyl and 3-methacryloxypropyl; and
examples of the aminoalkyl group of R' include 3-aminopropyl and N-(2-
aminoethyl)-
3-aminopropyl.
The following compounds are examples of the silatrane derivative comprising
component C.
CA 02287706 1999-10-26
TSL1484
CH3o
o ~~ / o/ o
5i'~
CH20CH2CH=CHZ
N
CH30
o~~ /o/ o
5 i'
CH2 CHCH20CHZ CN20CH2CH=CH2
N
C2Fi50
o~~ /o/ o
5i~
CH20CN2CH=CH2
N
Cla3
Si
CH2 CHCH20CH2 CHZOCH2CH=CH2
N
cH3o 0
O~~i~ ~ 0' CHZOCH2CH=CHz
CH2 CH CH?OCNZ CN20GIizCH=CH2
N
CA 02287706 1999-10-26
10 TSL1484
CHZ CH
0 ~~ ~ 0/ 0
Sip
CH20CH2CH=Cli2
N
CH3
\ 0
0~~ j~ / 0 CH20CH2CH=CN2
CH2 CHCH20CH2 CH20CH2Cli=CH2
N
0/ 0
Si''
-CHZOCHZCIi=C)-I2
N
CH2 CH 0
0~~ i~ / 0 CIi~OCH2CH=CH2
CH2 CH CH20CH2 CH20CHZCIi=CH 2
N
CN2 CH
/0
Sip
CH2 CHCH20CH2 CH20CH2CH=CH2
N
CA 02287706 1999-10-26
11 TSL1484
C\ 2~ HCN2CH2CIi2CH2
0 0~~ ~0/ 0
5 i'
-C1120CHZCH=CHZ
N
Fi2NCH2CH2CN2
0,\ \ '0/ 0
5 i'
CH20CH2CH=CH2
N
C\2~ HCH2CH2CH2CH2
0 0~~ ~ / 0
Sip
CH2 CHCH20CH2 CH20CH2CH=CH
N
w
CH3
l
CH2=Ci OCHZCF12CH2
0 0 ~~ ~ 0/ 0
5i~
CH20CH2CH=CH2
N
CA 02287706 1999-10-26
12 TSL1484
H2NCN2CH2NNCH2C1i2CH~
0
0 ~ / /0
\Si~
CH20CHZC1~=Cii2
N
cr3cH2cH2
o~~ / i o
si~
CN20CH2CH=CH2
N
CH3
i
cfr2=C~ ocH2ctr2cH2
0 0~~ /oi o
i'
CH? CliCN20CH2 C1f20CH2C1i~Cli2
N
C\ 2~ NCfi2CFf2CH2CN2 0
0 O~~i/ ~ 0' Cfia0Cfi2CFf=CN2
CN2 CHCH20CH~ CH~OCHZC)f=CN2
N
An example of the method for manufacturing the silatrane derivative comprising
component C is given below.
An epoxy compound described by the general formula:
\'~CR'-Rq-O-RS
O
CA 02287706 1999-10-26
13 TSL1484
where each R' is independently selected from a hydrogen atom and an alkyl
group, R'~
is an alkylene group, and RS is an alkenyl group and an alkoxysilane compound
described by the general formula
R6Si(OR')3
where R6 is selected from the group consisting of substituted and
unsubstituted
monovalent hydrocarbon groups, C, to C,o alkoxy groups, acyloxyalkyl groups,
and
aminoalkyl groups, and R' is a C, to C,o alkyl group; are allowed to react
with
ammonia or an amine compound described by the general formula:
NHY(CR'ZCR'ZOH)c3-r>
where each R' is independently selected from a hydrogen atom and an alkyl
group, and
yislor2.
The epoxy compound is a raw material for forming the skeleton of the silatrane
derivative comprising component C, and is also a raw material for introducing
alkenyloxyalkyl groups into the molecules of this silatrane derivative: The R'
groups
in the above formula are the same or different and are each a hydrogen atom or
an
alkyl group, with examples of R' being the same as the groups listed above. R'
in the
above formula is an alkylene group, with examples being the same as the groups
listed
above for R4. RS in the above formula is an alkenyl group, with examples being
the
same as the groups listed above, although a C3 to C,o alkenyl group is
preferred, with
an allyl group being particularly favorable. Examples of epoxy compounds
include
allyl glycidyl ether and butenyl glycidyl ether.
The alkoxysilane compound is also a raw material for forming the skeleton of
the silatrane derivative comprising component C. R6 in the above formula is
selected
from the group consisting of substituted and unsubstituted monovalent
hydrocarbon
groups, C, to C,o alkoxy groups, acyloxyalkyl groups, and aminoalkyl groups.
Examples of the monovalent hydrocarbon group of R6 are the same as the
monovalent
hydrocarbon groups listed above for R', examples of the alkoxy group of R6 are
the
CA 02287706 1999-10-26
14 TSL1484
same as the alkoxy groups listed above for R~, examples of the acyloxyalkyl
group of
R6 are the same as the acyloxyalkyl groups listed above for R', and examples
of the
aminoalkyl group of R6 are the same as the aminoalkyl groups listed above for
R3. R'
in the above formula is a C, to C,o alkyl group, examples of which include
methyl,
ethyl, propyl, and butyl. Examples of alkoxysilane compounds include
tetramethoxysilane, tetraethoxysilane, methyltrimethoxysilane,
methyltriethoxysilane,
vinyltrimethoxysilane, vinyltriethoxysilane, phenyltrimethoxysilane,
3-methacryloxypropyltrimethoxysilane, 3,3,3-trifluoropropyltrimethoxysilane,
nonafluorobutylethyltrimethoxysilane, 3-aminopropyltrimethoxysilane,
3-aminopropyltriethoxysilane, and N-(2-aminoethyl)-3-
aminopropyltrimethoxysilane.
The ammonia or amine compound is also a raw material for forming the
skeleton of the silatrane derivative of component C. In the amine compound,
the R'
groups in the above formula are each independently selected from a hydrogen
atom and
an alkyl group, examples of which are the sameas the groups listed above. y in
the
~ 5 formula is 1 or 2. Examples of this amine compound include 2-
hydroxyethylamine,
2,2'-dihydroxyethylamine, and 2-hydroxy-2-methyl-ethylamine.
There are no restrictions on the amounts in which the epoxy compound and
alkoxysilane compound are added with respect to the ammonia in the above-
mentioned
manufacturing method, but in order to suppress by-products and obtain the
silatrane
derivative at a good yield, when the reaction is conducted under conditions
such that
the ammonia will not be lost during the reaction, it is preferable for the
epoxy
compound to be used in an amount of 2 to 20 mol per mole of ammonia, with a
range
of 3 to 15 mol being even better. It is also preferable for the amount in
which the
alkoxysilane compound is added to be from 0.5 to SO mol per mole of ammonia,
with a
range of 1 to 20 mol being even better. This means that it is recommended that
the
alkoxysilane compound be used in about a stoichiometric amount or an excess
amount
with respect to the ammonia in this manufacturing method. In general this
excess will
suppressed by-products, but an excess of alkoxysilane compound will remain
behind if
CA 02287706 1999-10-26
TSL1484
the alkoxysilane compound is used in an excess amount, but not so large an
amount
that the reaction will be slowed. This unreacted and remaining alkoxysilane
compound
can be separated and recovered from the silatrane derivative by distillation
or the like
as needed after the reaction. This reaction can also be conducted while
ammonia gas is
blown into the mixture of the epoxysilane compound and the alkoxy compound.
When
this reaction is conducted in an open system, part of the ammonia will not
react and
will be released outside the system, so the ammonia must be used in an excess
amount
large enough to compensate for this loss.
There are no restrictions on the amount in which the epoxy compound and
alkoxysilane compound are added with respect to the amine compound in this
manufacturing method, but in order to obtain the silatrane derivative at a
good yield,
when y in the amine compound is 1, the epoxy compound should be used in an
amount
of 0.5 to 10 mol per mole of the amine compound, with a range of 0.8 to 5 mol
being
even better. When y is 2 in the amine compound, the epoxy compound should be
used
in an amount of 1.5 to 20 mol, with a range of 1.8 to 10 mol being even
better, and an
amount of about 2 mol being particularly favorable. It is also preferable for
the
amount in which the alkoxysilane compound is added to be from 0.5 to SO mol
per
mole of the amine compound, with a range of 1 to 20 mol being even better.
This
means that it is recommended that the alkoxysilane compound be used in about a
2o stoichiometric amount or an excess amount with respect to the amine
compound in this
manufacturing method. In general, by-products will be suppressed, but an
excess of
alkoxysilane compound will remain behind if the alkoxysilane compound is used
in an
excess amount, but not so large an amount that the reaction will be slowed.
The .
unreacted and remaining alkoxysilane compound can be separated and recovered
from
25 the silatrane derivative by distillation or the like as needed after the
reaction.
In the above-mentioned manufacturing method, the reaction will proceed at
normal temperature or under heating, but heating to 100°C or lower is
preferred in
order to shorten the reaction time. The use of an organic solvent is optional
in the
CA 02287706 1999-10-26
16 TSL1484
manufacturing method of the present invention, and examples of organic
solvents that
can be used include hexane, heptane, octane, and other aliphatic hydrocarbons;
toluene,
xylene, and other aromatic hydrocarbons; methanol, ethanol, isopropanol, and
other
alcohols; acetone, methyl isobutyl ketone, and other ketones; diethyl ether,
tetrahydrofuran, and other ethers; ethyl acetate, isoamyl acetate, and other
esters; and
dimethylformamide, dimethylacetamide, and other amide compounds. In
particular,
the use of an alcohol such as methanol or ethanol allows the reaction time to
be
shortened and the targeted silatrane derivative to be obtained at a better
yield. In the
manufacturing method of the present invention, when an alcohol is added, it
should
t 0 preferable have the same number of carbon atoms as the silicon atom-bonded
alkoxy
groups in the raw material alkoxysilane compound in order to subject the
silicon atom-
bonded alkoxy groups to an alkoxy group exchange reaction. Also, when an
alcohol is
added in the above-mentioned manufacturing method, the reaction can be
markedly
shortened and the yield of the obtained silatrane derivative can be enhanced
by
t 5 conducting the reaction at the reflux temperature of this alcohol.
The platinum type catalyst of component D is a catalyst for curing the present
composition through a hydrosilylation reaction. Examples of this platinum type
catalyst of component D include platinum microparticles, chloroplatinic acid,
an
alcohol-modified chloroplatinic acid, a diketone complex of platinum, an
olefin
20 complex of platinum, platinum carried on a powder such as alumina, silica,
or carbon
black, and thermoplastic resin microparticles containing these platinum type
catalysts in
an amount of at least 0.01 Wt. % as platinum metal atoms. The thermoplastic
resin can
have a softening point of 50 to 150°C and an average diameter of the
microparticles
thereof of 0.01 to 10 ~.m. Examples of the thermoplastic resin include
thermoplastic
25 silicone resins, thermoplastic acrylic resins, thermoplastic polysilane
resins,
thermoplastic polystyrene resins, and thermoplastic methyl cellulose resins. A
feature
is that when thermoplastic resin microparticles containing at least 0.01 wt %
platinum
metal atoms, having a softening point of SO to 150°C, and having an
average diameter
CA 02287706 1999-10-26
TSL 1484
of 0.01 to 10 ~.m are used as the platinum type catalyst of component D, the
storage
stability of the obtained silicone rubber composition will be better and there
will be
very little change in viscosity even when this composition is left at room
temperature
for an extended period.
The amount in which component D is contained in the present composition is an
amount sufficient to cure the composition, and is preferably 0.1 to 1000
weight parts of
platinum metal atoms per million weight parts of component A, with a range of
0.1 to
S00 weight parts being more preferable, and a range of 1 to 100 weight parts
being
even better. This is because the curing of the obtained silicone rubber
composition will
tend to be markedly slower if the component D content is below the above
range, but
on the other hand, there will be no pronounced increase in curing rate if the
above
range is exceeded, and on the contrary, there is the danger of encountering
problems
such as the coloration of the silicone rubber during curing.
The present composition is composed of the above-mentioned components A to
D, but other components can also be added as needed, such as fumed silica, wet
silica,
and other such reinforcing fillers; iron oxide, rare earth compounds, and
other such
heat stabilizers; manganese carbonate, titanium dioxide, and other such flame
retardants; quartz powder, diatomaceous earth, calcium carbonate, carbon
black, and
other such extending fillers; and pigments.
2o In order to enhance the storage stability of the present composition and
make it
easier to handle and work with, it can also contain an acetylene compound such
as
2-methyl-3-butyn-2-ol, 2-phenyl-3-butyn-2-ol, 3-methyl-1-hexyn-3-ol, 1,5-
hexadiene,
or 1,6-heptadiene; an ene-ine compound such as 1-ethynyl-1-cyclohexanol, an
alkenylsiloxane oligomer such as 1,3-divinyltetramethyldisiloxane,
1,3,5,7-tetravinyltetramethylcyclotetrasiloxane, or 1,3-divinyl-1,3-
diphenyldimethyldisiloxane; a nitrogen-containing compound such as
tributylamine,
tetramethylethylenediamine, or benzotriazole; a phosphorus-containing compound
such
as triphenylphosphine; or a sulfur-containing compound, a hydroperoxy
compound, a
CA 02287706 1999-10-26
18 TSL1484
malefic acid derivative, or another such curing inhibitor. The amounts in
which these
curing inhibitors are contained should be from 0.005 to 10 weight parts per
million
weight parts of component A and component B combined.
The present composition is prepared by uniformly mixing the above-mentioned
components A to D, but in order to enhance the storage stability of this
composition
near room temperature and to maintain excellent curability when the
composition is
used in mufti-color molding or the like after storage, it is preferable for
the
composition to be a two-liquid type of silicone rubber composition divided
into a
silicone composition containing at least component A and component D but not
o containing component B, and a silicone composition containing at least
component B
but not containing component D. Component C in this case may be contained in
either
composition.
Because it contains component C, the present composition has excellent
adhesion to polyethylene resins, polypropylene resins, saturated polyesters
such as PET
and PBT, polystyrene resins, AS resins, ABS resins, polyamides,
polycarbonates,
acrylic resins, methacrylic resins, and other such organic resins, and since
the mold
release properties are excellent, mufti-color (such as two-color) molding,
insert
molding with a silicone rubber composition and an organic resin in injection
molding,
and other types of composite molding can be carried out more efficiently.
There are no
2o restrictions on the conditions under which the composite molding is carried
out with the
present composition, but it is preferable, for example, to heat the material
to a
temperature below the softening point of the organic resin to be composite
molded,
such as 150°C or lower. Nor are there any restrictions on the heating
time, but a
treatment lasting from a few seconds to a few minutes is favorable.
The silicone rubber composition for composite molding of the present invention
will now be described in detail through working examples. The viscosity
mentioned in
these working examples is the value at 25°C.
CA 02287706 1999-10-26
19 TSL1484
Reference Example. 12.2 g (0.2 mol) of 2-hydroxyethylamine, 81.7 g (0.6
mol) of methyltrimethoxysilane, 57.1 g (0.5 mol) of allyl glycidyl ether, and
32 g of
methanol were put in a S00 mL four-neck flask equipped with an agitator, a
thermometer, and a reflux condenser. This system was heated and agitated for 8
hours
at the reflux temperature of methanol. The total amount of the reactive
mixture thus
obtained was then transferred to a flask, and the low-boiling component was
distilled
off by rotary evaporator, which yielded 63.3 g of a faintly yellow transparent
liquid.
This transparent liquid was subjected to 29Si-nuclear magnetic resonance
analysis and
'3C-nuclear magnetic resonance analysis, which confirmed that the silatrane
derivative
t 0 described by the following formula was produced. This silatrane derivative
was
contained in a proportion of at least 90 wt% .
C
0 0
~~ ~ /0
5~'~
CH2 CH CH20CH2 CH20CH2CH=CH 2
N
Working Example 1. 100 weight parts of a dimethylvinylsiloxy end-capped
polydimethylsiloxane capped at both ends of the molecular chain and having a
viscosity
of 10,000 mPa ~ s, 30 weight parts of fumed silica with a specific surface
area of 200
m2/g, 5 weight parts hexamethyldisilazane (used as a surface treatment agent
for the
silica), and 2 weight parts water were uniformly mixed, and were then further
mixed
under a vacuum while being heated for 2 hours at 170°C. After cooling,
1 weight part
of the silatrane derivative prepared in Reference Example 1, 2.6 weight parts
of a
trimethylsiloxy end-capped dimethylsiloxane ~ methylhydrogensiloxane copolymer
capped at both ends of the molecular chain and having five silicon atom-bonded
hydrogen atoms per molecule and a viscosity of S mPa ~ s (the amount of this
CA 02287706 1999-10-26
20 TSL1484
copolymer was such that the ratio of the number of moles of silicon atom-
bonded
hydrogen atoms in the copolymer to the number of moles of silicon atom-bonded
vinyl
groups in the above-mentioned polydimethylsiloxane was 4.7:1), 0.06 weight
part 3-
methyl-1-hexyn-3-ol, and a 1,3-divinyltetramethyldisiloxane solution of a
1,3-divinyltetramethyldisiloxane complex of platinum, used in an amount such
that
there were 7 weight parts of platinum metal atoms per million weight parts of
the
above-mentioned polydimethylsiloxane, were mixed with 100 weight parts of the
base
compound obtained above to prepare a silicone rubber composition.
An organic resin test piece of the composition described in Table 1 was then
placed in
a chrome-plated mold, the above-mentioned silicone rubber composition was
injected
from above, and the material was heated for 10 minutes at 120°C to cure
the
composition. The adhesion of the material to the organic resins and its mold
release
properties were observed, the results of which are given in Table 1. To
evaluate the
adhesion of the silicone rubber to organic resins; the silicone rubber was
peeled away
from the organic resin, and a "O" was given if the silicone rubber underwent
cohesive
failure, whereas an "x" was given if the peeling occurred at the interface of
the silicone
rubber. In the evaluation of the mold release properties of the silicone
rubber, a "o"
was given if the silicone rubber came away from the mold easily, with no
cohesive
failure, whereas an "x" was given if the silicone rubber underwent cohesive
failure and
partially adhered to the mold. The silicone rubber composition was allowed to
stand
for 3 days at room temperature, and the change in viscosity was checked. These
results are all given in Table 1.
Comparative Example 1. Other than not adding the silatrane derivative used ~in
Working Example 1, a silicone rubber composition was prepared in the same
manner
as in Working Example 1. The adhesion of this silicone rubber composition to
organic
resins and its mold release properties were evaluated in the same manner as in
Working
Example 1. This silicone rubber composition was allowed to stand for 3 days at
room
CA 02287706 1999-10-26
21 TSL1484
temperature, and the change in viscosity was checked. These results are all
given in
Table 1.
Working Example 2. 1 weight part of the silatrane derivative prepared in
Reference Example 1, 2.6 weight parts of a trimethylsiloxy end-capped
dimethylsiloxane ~ methylhydrogensiloxane copolymer capped at both ends of the
molecular chain and having five silicon atom-bonded hydrogen atoms per
molecule and
a viscosity of 5 mPa ~ s (the amount of this copolymer was such that the ratio
of the
number of moles of silicon atom-bonded hydrogen atoms in the copolymer to the
,
number of moles of silicon atom-bonded vinyl groups in the above-mentioned
t0 polydimethylsiloxane was 4.7:1), 0.06 weight part 3-methyl-1-hexyn-3-ol,
and
thermoplastic silicone resin microparticles (the softening point of the
silicone resin was
85°C, and the average diameter of the microparticles was 1 Vim)
containing a
1,3-divinyltetramethyldisiloxane complex of platinum, used in an amount such
that
there were 7 weight parts of platinum metal atoms per million weight parts of
the
~ 5 above-mentioned polydimethylsiloxane, were mixed with 100 weight parts of
the base
compound prepared in Working Example 1 to prepare a silicone rubber
composition.
The adhesion of this silicone rubber composition to organic resins and its
mold release
properties were evaluated in the same manner as in Working Example 1. This
silicone
rubber composition was allowed to stand for 3 days at room temperature, and
the
2o change in viscosity was checked. These results are all given in Table 1.
Comparative Example 2. Other than adding vinyltrimethoxysilane instead of
the silatrane derivative used in Working Example 1, a silicone rubber
composition was
prepared in the same manner as in Working Example 1. The adhesion of this
silicone
rubber composition to organic resins and its mold release properties were
evaluated in
25 the same manner as in Working Example 1. This silicone rubber composition
was
allowed to stand for 3 days at room temperature, and the change in viscosity
was
checked. These results are all given in Table 1.
CA 02287706 1999-10-26
22 TS L 1484
Working Example 3. 90 weight parts of a dimethylvinylsiloxy end-capped
polydimethylsiloxane capped at both ends of the molecular chain and having a
viscosity
of 10,000 mPa ~ s, 10 weight parts of a dimethylvinylsiloxy end-capped
polydimethylsiloxane ~ methylvinylsiloxane copolymer capped at both ends of
the
molecular chain and having a viscosity of 340 mPa ~ s, 30 weight parts of
fumed silica
with a specific surface area of 200 mz/g, 5 weight parts hexamethyldisilazane
(used as a
surface treatment agent for the silica), and 2 weight parts water were
uniformly mixed,
and were then further mixed under a vacuum while being heated for 2 hours at
170°C.
After cooling, 1 weight part of the silatrane derivative prepared in Reference
Example
t0 1, 8 weight parts of a dimethylhydrogensiloxy end-capped
polydimethylsiloxane capped
at both ends of the molecular chain and having a viscosity of 10 mPa ~ s (the
amount of
this polymer was such that the ratio of the number of moles of silicon atom-
bonded
hydrogen atoms in the polydimethylsiloxane to the number of moles of silicon
atom-
bonded vinyl groups in the above-mentioned dimethylhydrogensiloxy group-capped
t 5 polydimethylsiloxane copolymer capped at both ends of the molecular chain
was
1.7:1), 0.06 weight part 3-methyl-1-hexyn-3-ol, and a 1,3-
divinyltetramethyldisiloxane
solution of a 1,3-divinyltetramethyldisiloxane complex of platinum, used in an
amount
such that there were 7 weight parts of platinum metal atoms per combined
million
weight parts of the above-mentioned dimethylhydrogensiloxy end-capped
20 polydimethylsiloxane copolymer capped at both ends of the molecular chain
and the
dimethylvinylsiloxy group-capped polydimethylsiloxane ~ methylvinylsiloxane
copolymer capped at both ends of the molecular chain, were mixed with 100
weight
parts of the base compound obtained above to prepare a silicone rubber
composition:
The adhesion of this silicone rubber composition to organic resins and its
mold release
25 properties were evaluated in the same manner as in Working Example 1. This
silicone
rubber composition was allowed to stand for 3 days at room temperature, and
the
change in viscosity was checked. These results are all given in Table 1.
CA 02287706 1999-10-26
23 TSL1484
Table 1
WorkingWorkingWorkingComp. Comp.
Ex. Ex.2 Ex.3 Ex. Ex
1 1 2
Adhesion to organic resins .
Nylon-6 resin O O O x O
Polypropylene resin O O O x O
O O O x O
Polybutylene terephthalate
resin
O p O x O
Polycarbonate resin
Mold release properties O O O O x
Change in viscosity of siliconeCured not cured cured cured
rubber comp. cured