Note: Descriptions are shown in the official language in which they were submitted.
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
SUBSTTfUTED TETRAHYDROISOQUINOLINE DERIVATIVES AS MODULATORS OF DOPAMINE D3
RECEPTORS
The present invention relates to novel tetrahydroisoquinoline derivatives,
processes for their preparation, pharmaceutical compositions containing them
and their
use in therapy, as modulators of dopamine D3 receptors, in particular as
antipsychotic
agents.
US Patent No. 5,294,621 describes tetrahydropyridine derivatives of the
formula:
R'
R2
T
N~
XIAr'
R
Ar
wherein is an optionally substituted thienyl or optionally substituted
phenyl ring; R1, R2 and R3 are each inter alia hydrogen; X is inter alia
(CH2)mNR~CO;
m is 2-4; and Arl is an optionally substituted heterocyclic ring or an
optionally
substituted phenyl ring. The compounds are said to be useful as antiarrhythmic
agents.
European Patent Application 0 464 $46 A1 describes imide derivatives of the
formula:
O
R~ (CH2)n \
N-(CH2)p-A-(CH2)q- ~ -Ar
B
R3 R4
wherein B is a carbonyl group or a sulphonyl group, R1, R2, R3 and R4 are each
hydrogen or a lower alkyl group, or R 1 and R2 or R 1 and R3 may be combined
together
to make a non-aromatic hydrocarbon ring, or R1 and R3 may be combined together
to
make an aromatic ring, and n is 0 or 1; A is a non-aromatic hydrocarbon ring,
and p and
q are each 0, 1, or 2; Ar is an aromatic ring, a heteroaromatic group, a
benzoyl group, a
phenoxy group, or a phenylthio group and G is N, CH, or CHOH. The compounds
are
said to be useful as antipsychotic agents.
WO 95/10513 describes benzothiophene derivatives and related compounds as
estrogen agonists.
We have now found a class of tetrahydroisquinoline derivatives which have
affinity for dopamine receptors, in particular the D3 receptor, and thus
potential in the
treatment of conditions wherein modulation of the D3 receptor is beneficial,
eg as
antipsychotic agents.
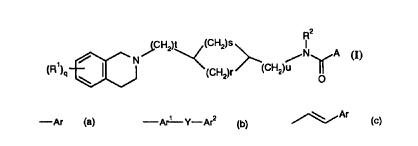
In a first aspect the present invention provides compounds of formula (I)
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
R2
(CHZ)t (CH2)S I
N / /N A
(R ) ~ CH a
(CH2}r ( 2)
0
Formula (I)
wherein:
R1 represents a substituent selected from: a hydrogen or halogen atom; a
hydroxy,
cyano, nitro, trifluoromethyl, trifluoromethoxy, trifluoromethanesulfonyloxy,
pentafluoroethyl, C 1 _4alkyl, C 1 _4alkoxy, arylC 1 _4alkoxy, C 1
_4alkylthio,
C 1 _4alkoxyC 1 _4alkyl, C3_6cycloalkylC 1 _4alkoxy, C 1 _4alkanoyl, C 1
_4allcoxycarbonyl,
C 1 _4allcylsulphonyl, C 1 _q,alkylsulphonyloxy, C 1 _4a1ky1sulphonylC 1
_4alkyl,
arylsulphonyl, arylsulphonyloxy, arylsulphonylC 1 _4alkyl, C 1
_4alkylsulphonamido, C 1 _
4alkylamido, C 1 _4alky1sulphonamidoC 1_4alkyl, C 1 _4alkylamidoC 1 _4alkyl,
arylsulphonamido, arylcarboxamido, arylsulphonamidoCl_4alkyl,
arylcarboxamidoCl_
4alkyl, aroyl, aroylCl_4alkyl, or arylCl_q.alkanoyl group; a group
R30C0(CH2)~p ,
R3CON(R4)(CH2)p, R3R4NC0(CH2)p or R3R4NS02(CH2)p where each of R3 and R4
independently represents a hydrogen atom or a C1_4alkyl group or R3R4 forms
part of a
C3_6azacyloalkane or C3_6(2-oxo)azacycloalkane ring and p represents zero or
an integer
from 1 to 4; or a group Ar3-Z, wherein Ar3 represents an optionally
substituted phenyl
ring or an optionally substituted 5- or 6- membered aromatic heterocyclic ring
and Z
represents a bond, O, S , or CH2;
R2 represents a hydrogen atom or a C1_4alkyl group;
q is 1 or 2;
s represents an integer from zero to 2 and r represents an integer from 1 to
4, such
that the sum of s + r is 1 to 4;
t represents an integer from zero to 1 and a represents an integer from zero
to 2;
A represents a group of the formula (a}, (b) or (c):
-Ar -Ar'--Y-Ar2 ~Ar
) (
wherein
Ar represents an optionally substituted phenyl ring or an optionally
substituted 5-
or 6- membered aromatic heterocyclic ring; or an optionally substituted
bicyclic system;
Arl and Ar2 each independently represent an optionally substituted phenyl ring
or
an optionally substituted 5- or 6- membered aromatic heterocyclic ring; and
Y represents a bond, -NHCO-, -CONH-, -CH2-, or -(CH2)mYl(CH2)n-, wherein
Yl represents O, S, S02, or CO and m and n each represent zero or 1 such that
the sum
of m+n is zero or 1; providing that when A represents a group of formula (a),
any
substituent present in Ar ortho to the carboxamide moiety is necessarily a
hydrogen or a
methoxy group;
2
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
and salts thereof.
In the compounds of formula (I) above an alkyl group or moiety may be straight
or branched. Alkyl groups which may be employed include methyl, ethyl, n-
propyl, n-
S butyl, n-pentyl, n-hexyl and any branched isomers thereof such as isopropyl,
t-butyl, sec-
pentyl, and the like.
Examples of compounds of formula (I) include those in which Ar is a bicyclic
aromatic or heteroaromatic ring system, and t and a are both 1 and in which R1
is other
than pentafluoroethyl.
When Rl represents an arylCl_4alkoxy, arylsulphonyl, arylsulphonyloxy,
arylsulphonylCl_4alkyl, arylsulphonamido, arylcarboxamido,
arylsulphonamidoC 1 _4alkyl, arylcarboxamidoC 1 _4alkyl, aroyl, aroylC 1
_4alkyl, or
arylCl_4alkanoyl group, the aryl moiety may be selected from an optionally
substituted
phenyl ring or an optionally substituted 5- or 6-membered heterocyclic ring.
In the group
R1 an aryl moiety may be optionally substituted by one or more substituents
selected
from hydrogen, halogen, amino, cyano, Cl_4alkyl, Cl_4alkylamino,
C1_4dialkylamino,
C1_4alkylamido, C1_4alkanoyl, or RSR6NC0 where each of RS and R6 independently
represents a hydrogen atom or C1_4alkyl group.
A halogen atom present in the compounds of formula (I) may be fluorine,
chlorine, bromine or iodine.
When q is 2, the substituents R1 may be the same or different.
An optionally substituted 5- or 6-membered heterocyclic aromatic ring, as
defined
for any of the groups Ar, Arl, Ar2 or Ar3 may contain from 1 to 4 heteroatoms
selected
from O, N or S. When the ring contains 2-4 heteroatoms, one is preferably
selected from
O, N and S and the remaining heteroatoms are preferably N. Examples of 5 and 6-
membered heterocyclic groups include furyl, thienyl, pyrroiyl, oxazolyl,
thiazolyl,
imidazolyl, .oxadiazolyl, thiadiazolyl, pyridyl, triazolyl, triazinyl,
pyridazyl, pyrimidinyl
and pyrazolyl.
Examples of bicyclic, for example, bicyclic aromatic or heteroaromatic, ring
systems for Ar include naphthyl, indazolyl, indolyl, benzofuranyl,
benzothienyl,
benzothiazoly), benzimidazolyl, benzoxazolyl, benzisoxazolyl,
benzisothiazolyl,
quinolinyl, quinoxolinyl, quinazolinyl, cinnolinyl, isoquinolinyl,
pyrazolo[1,5-
a]pyrimidyl, pyrrolo[3,2-bJpyridyl, pyrrolo[3,2-c]pyridyl, thieno[3,2-
b]thiophenyl , 1,2-
dihydro-2-oxo-quinolinyl, 2,3-dihydro-3-oxo-4H-benzoxazinyl, 1,2-dihydro-2-oxo-
3H-
indoiyl.
The rings Ar, Arl, or Ar2 may each independently be optionally substituted by
one or more substituents selected from: a hydrogen or halogen atom, or a
hydroxy,
cyano, nitro, C 1 _4alkyl, C 1 _4alkoxy, C 1 _4alkylenedioxy, C 1 _4alkanoyl,
C 1 _
4alkylsulphonyl, Cl_4alkylsulphinyl, Cl_4alkylthio, R~S02N(Rg)-, R~RgN-,
R~RgNCO-, R~RgNS02-, or R~CON(Rg)- group wherein each of R~ and Rg
independently represents a hydrogen atom or a C1_4 alkyl group, or R~Rg
together form
a C3_6 alkylene chain.
3
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
Alternatively, Ar and Ar2 may be optionally substituted by one or more S- or 6-
membered heterocyclic rings, as defined above, optionally substituted by a C 1-
2 alkyl or
R~RgN- group; wherein R~ and Rg are as defined above.
In the rings Ar and Ar2 substituents positioned ortho to one another may be
linked to form a S- or 6- membered ring.
It will be appreciated that for use in medicine the salts of formula (I)
should be
physiologically acceptable. Suitable physiologically acceptable salts will be
apparent to
those skilled in the art and include for example acid addition salts formed
with inorganic
acids eg. hydrochloric, hydrobromic, sulphuric, nitric or phosphoric acid; and
organic
acids eg. succinic, malefic, acetic, fumaric, citric, tartaric, benzoic, p-
toluenesulphonic,
methanesulphonic or naphthalenesulphonic acid. Other non-physiologically
acceptable
salts eg. oxalates, may be used, for example in the isolation of compounds of
formula (I)
and are included within the scope of this invention. Also included within the
scope of the
invention are solvates and hydrates of compounds of formula {I).
Certain of the compounds of formula (I) may form acid addition salts with one
or
more equivalents of the acid. The present invention includes within its scope
all possible
stoichiometric and non-stoichiometric forms.
It will be appreciated certain of the compounds of formula (I) contain two
asymmetric centres. Such compounds can exist in diastereomeric forms, namely
cis- and
traps- isomers; both forms and all mixtures thereof are included within the
scope of this
invention. Furthermore, each diastereoisomer can exist as optical isomers
(enantiomers).
Both the pure enantiomers, racemic mixtures (50% of each enantiomer) and
unequal
mixtures of the two are included within the scope of the invention. In
accordance with
convention the (+) and {-) designations used herein indicate the direction of
rotation of
plane-polarised light by the compounds. The prefix (+) indicates that the
isomer is
dextrorotatory (which can also be designated d) and the prefix (-) indicates
the
levorotatory isomer (which can also be designated 1). It will thus be
appreciated that the
invention extends to the individual diastereoisomers, individual enantiomers
and any and
all mixtures of these forms.
Certain of the other compounds of formula (I) can also exist in the form of
cis-
and traps- isomers. The present invention includes within its scope all such
isomers,
including mixtures.
In compounds of formula (I), it is preferred that either t and a are both zero
or
that t and a are both 1.
Certain of the substituted heteroaromatic ring systems included in compounds
of
formula (I) may exist in one or more tautomeric forms. The present invention
includes
within its scope all such tautomeric forms, including mixtures.
Particular compounds according to the invention include those specifically
exemplified and named hereinafter.
The present invention also provides a processs for preparing compounds of
formula (I) which process comprises:
(a) reacting a compound of formula {V):
4
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
Rz
(,CHz)t (CHz)s I
/ N ~ /NCH
(R' ~ CH a
(CHz)r ( z)
Formula (V)
with a compound of formula (VI):
A-COX
15
Formula (VI)
wherein A is as hereinbefore defined and X is a halogen atom or the residue of
an
activated ester;
(b) to prepare a compound of formula (I) wherein R 1 is Ar3-Z and Z is a bond,
reacting a compound of formula (VII):
Rz
(,CHz)t (CHz)s I
N A
(R'~ / N ~ ~ CH a
(CHz)r ( z)
O
Formula (VIi)
wherein one Rla represents a group W wherein W is a halogen atom or a
trifluoromethylsulphonyloxy group, or W is a group M selected from a boron
derivative
e.g. a boronic acid function B(OH)2 or a metal function such as
trialkylstannyl e.g.
SnBu3, zinc halide or magnesium halide, and when q is 2 the other R 1 a is R
1; with a
compound Ar3-W 1, wherein W 1 is a halogen atom or a
trifluoromethylsulphonyloxy
group when W is a group M or W 1 is a group M when W is a halogen atom or a
trifluoromethylsulphonyloxy group;
(c) to prepare a compound of formula (I) wherein R 1 is Ar3-Z and Z is O or S,
reacting a compound of formula (VIII):
R2
(,CHz)t (CHz)s I
/ N ~ ~ /N A
(R1b)a ~ (CHz)r (CHz)u
O
Formula (VIII)
wherein one Rlb represents a group ZH and when q is 2 the other Rlb represents
Rl;
with a reagent serving to introduce the group Ar3;
5
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
(d) to prepare a compound of formula (I) where Y is a bond, reaction of a
compound of formula (IX):
R2
' CH2)t (CH2)S /N Ar'-W
(R') / N ~ ~ CH a
(CH2)r ( 2)
q ~ I
O
Formula (IX)
wherein R1, R2, Arl and W are as hereinbefore defined, with a compound Ar2-Wl,
wherein W 1 is a halogen atom or a trifluoromethylsulphonyloxy group when W is
a
group M, or W 1 is a group M when W is a halogen atom or a
trifluoromethylsulphonyloxy group.
(e) interconversion of one compound of formula (I) to a different compound of
formula (n e.g. (i) alkylation of a compound (I) wherein R2 represents
hydrogen, (ii)
conversion of one R1 from alkoxy (e.g.methoxy) to hydroxy, or (iii) conversion
of R1
from hydroxy to sulphonyloxy, eg alkylsulphonyloxy or
trifluoromethanesulphonyloxy;
(iv) conversion of a compound wherein Y represents S to a compound wherein Y
is S02
or (v) conversion of Y from CO to CH2;
(f) where appropriate, separation of enantiomers, diastereoisomers, or cis-
and
traps- isomers of compounds of formula (I), or intermediates thereto, by
conventional
methods, e.g. chromatography or crystallisation;
and optionally thereafter forming a salt of formula (I).
Compounds of formula (V) may be prepared by :-
(g) conversion of a compound of formula (IV):
R2
(CH2)t (CH2)S I
/ N / ~ ~ ~N~P
(R~~q ~ I (CH2)r
Formula (IV)
wherein R 1, R2, r, s, t and a are as hereinbefore defined and P is a
protecting group such
as t-butoxycarbonyl or trifluoroacetyl , to a compound of fomula (V).
Compounds of formula (IV) in which t is 1 may be prepared by:-
(h) by reacting a compound of formula (II):
6
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
,H
(R~)4 ~ N
\.
Formula (In
wherein R1 and q are as hereinbefore defined; with a compound of formula
(IIIa):
H (CH2)s
N
CH a
O (CH2)r (
Formula (IIIa)
wherein P, R2, r, s, and a are as hereinbefore defined;
Compounds of formula (IV) where t is zero may be prepared by: -
(i) reacting a compound of formula (II), wherein R 1 and q are hereinbefore
defined, with a compound of formula (IIIb):
(CH2)s R2
N
O ~ CH a
(CH2)r ( 2)
Formula (IIIb)
wherein P, R2, r, s, and a are as hereinbefore defined.
Compounds of formula (V), where t and a are both zero may be prepared by :-
(j) conversion of a compound of formula (X):-
(CH2)s O
~(CH2)v
'O
(R,)a N
(CH2)r
Formula (X)
wherein R1, q, r and s are as hereinbefore defined and v is 1 or 2, into a
corresponding
ketone, followed by reductive amination. This may be effected by methods well
known
7
CA 02288850 1999-11-08
WO 98/51671 PCTlEP98/02584
in the art for (i) conversion of a ketal to a ketone in the presence of
aqueous acid;
followed by (ii) reductive amination of the ketone with R2NH2 or ammonium
acetate in
the presence of a reducing agent. Suitable reducing agents which may be
employed
include sodium borohydride, cyanoborohydride or triacetoxyborohydride under
acidic
S conditions, or catalytic hydrogenation. The reaction may conveniently be
effected in a
solvent such as methanol, ethanol or dichloroethane..
Compounds of formula (X) wherein R1 and q are as hereinbefore defined,
may be prepared by:-
(k) reacting a compound of formula (XI):-
(CH2)v
O
Formula (XI)
wherein v, r and s are as hereinbefore defined, with a compound of formula
(II), wherein
R1 and q are as hereinbefore defined .
Processes (h), (i) and (k) require the presence of a reducing agent. Suitable
reducing agents which may be employed include sodium borohydride,
cyanoborohydride
or triacetoxyborohydride under acidic conditions, or catalytic hydrogenation.
The
reaction may conveniently be effected in a solvent such as ethanol.
Process (g) may be effected by standard methods well known in the art for (i)
removal of a t-butoxycarbonyl group, e.g., using acidic conditions; (ii)
removal of a
trifluoroacetyl group, e.g., using basic conditions.
Reaction of a compound of formula (VII) with Ar3W l, according to process (b)
or a compound of formula (IX) with Ar2-W 1 according to process (d) may be
effected in
the presence of a transition metal eg palladium catalyst such as bis-
triphenylphosphinepalladium dichloride or tetrakis-triphenylphosphinepalladium
(0).
When M represents a boronic acid function such as B(OH)2the reaction may be
carried
out under basic conditions, for example using aqueous sodium carbonate in a
suitable
solvent such as dioxane. When M is trialkylstannyl the reaction may be carried
out in an
inert solvent, such as xylene or dioxane optionally in the presence of LiCI.
When M is a
zinc or magnesium halide the reaction may be effected in an aprotic solvent
such as
tetrahydrofuran. The substituent W is preferably a halogen atom such as
bromine, or a
sulphonyloxy group such as trifluoromethylsulphonyloxy; and W 1 is preferably
a goup
M, such as triaIkylstannyl or B(OH)2.
In process (c) the reagent serving to introduce the group Ar3 is preferably a
compound of formula Ar3-Hal, wherein Hal is a halogen atom. The reaction may
be
effected in the presence of a base, such as potassium carbonate, in a solvent
such as
dimethylformamide.
8
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
Interconversion reactions according to process (e) may be effected using
methods
well known in the art.
Compounds of formula (II) may be prepared by methods known in the art.
Compounds of formula (IIIa) and (IIIb) are known or may be prepared using
standard procedures.
Compounds of formula (VII), (VIII) or {IX) may be prepared by processes
analogous to (a), {g), (h) and (i) described above. Compounds Ar2W 1, Ar3W 1
and
Ar3Ha1 are commercially available or may be prepared by standard methods.
Compounds of formula (XI) are commercially available or may be prepared using
standard procedures.
Compounds of formula (I) have been found to exhibit affinity for dopamine
receptors, in particular the D3 receptor, and are expected to be useful in the
treatment of
disease states which require modulation of such receptors, such as psychotic
conditions.
Compounds of formula (I) have also been found to have greater affinity for
dopamine D3
than for D2 receptors. The therapeutic effect of currently available
antipsychotic agents
(neuroleptics) is generally believed to be exerted via blockade of D2
receptors; however
this mechanism is also thought to be responsible for undesirable
extrapyramidal side
effects (eps) associated with many neuroleptic agents. Without wishing to be
bound by
theory, it has been suggested that blockade of the recently characterised
dopamine D3
receptor may give rise to beneficial antipsychotic activity without
significant eps. (see
for example Sokoloff et al, Nature, 1990; 347: 146-151; and Schwartz et al,
Clinical
Neuropharmacology, Vol 16, No. 4, 295-314, 1993). Preferred compounds of the
present invention are therefore those which have higher affinity for dopamine
D3 than
dopamine D2 receptors (such affinity can be measured using standard
methodology for
example using cloned dopamine receptors). Said compounds may advantageously be
used as selective modulators of D3 receptors.
We have found that certain compounds of formula (I) are dopamine D3 receptor
antagonists, others may be agonists or partial agonists. The functional
activity of
compounds of the invention (i.e. whether they are antagonists, agonists or
partial
agonists) can be readily determined using the test method described
hereinafter, which
does not require undue experimentation. D3 antagonists are of potential use as
antipsychotic agents for example in the treatment of schizophrenia, schizo-
affective
disorders, psychotic depression, mania, paranoid and delusional disorders.
Conditions
which may be treated by dopamine D3 receptor agonists include dyskinetic
disorders
such as Parkinson's disease, neuroleptic-induced parkinsonism and tardive
dyskinesias;
depression; anxiety, memory disorders, sexual dysfunction and drug (eg.
cocaine)
dependency.
In a further aspect therefore the present invention provides a method of
treating
conditions which require modulation of dopamine D3 receptors, for example
psychoses
such as schizophrenia, which comprises administering to a subject in need
thereof an
effective amount of a compound of formula (I) or a physiologically acceptable
salt
thereof.
9
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
The invention also provides the use of a compound of formula (I) or a
physiologically acceptable salt thereof in the manufacture of a medicament for
the
treatment of conditions which require modulation of dopamine D3 receptors, for
example
psychoses such as schizophrenia.
A preferred use for D3 antagonists according to the present invention is in
the
treatment of psychoses such as schizophrenia.
A preferred use for D3 agonists according to the present invention is in the
treatment of dyskinetic disorders such as Parkinson's disease.
For use in medicine, the compounds of the present invention are usually
administered as a standard pharmaceutical composition. The present invention
therefore
provides in a further aspect pharmaceutical compositions comprising a novel
compound
of formula (I} or a physiologically acceptable salt thereof and a
physiologically
acceptable carrier.
The compounds of formula (I) may be administered by any convenient method,
for example by oral, parenteral, buccal, sublingual, nasal, rectal or
transdermal
administration and the pharmaceutical compositions adapted accordingly.
The compounds of formula (I) and their physiologically acceptable salts which
are active when given orally can be formulated as liquids or solids, for
example syrups,
suspensions or emulsions, tablets, capsules and lozenges.
A liquid formulation will generally consist of a suspension or solution of the
compound or physiologically acceptable salt in a suitable liquid carriers) for
example an
aqueous solvent such as water, ethanol or glycerine, or a non-aqueous solvent,
such as
polyethylene glycol or an oil. The formulation may also contain a suspending
agent,
preservative, flavouring or colouring agent.
A composition in the form of a tablet can be prepared using any suitable
pharmaceutical carriers) routinely used for preparing solid formulations.
Examples of
such carriers include magnesium stearate, starch, lactose, sucrose and
cellulose.
A composition in the form of a capsule can be prepared using routine
encapsulation procedures. For example, pellets containing the active
ingredient can be
prepared using standard carriers and then filled into a hard gelatin capsule;
alternatively,
a dispersion or suspension can be prepared using any suitable pharmaceutical
carrier(s),
for example aqueous gums, celluloses, silicates or oils and the dispersion or
suspension
then filled into a soft gelatin capsule.
Typical parenteral compositions consist of a solution or suspension of the
compound or physiologically acceptable salt in a sterile aqueous carrier or
parenterally
acceptable oil, for example polyethylene glycol, polyvinyl pyrrolidone,
lecithin, arachis
oil or sesame oil. Alternatively, the solution cari be lyophilised and then
reconstituted
with a suitable solvent just prior to administration.
Compositions for nasal administration may conveniently be formulated as
aerosols, drops, gels and. powders. Aerosol formulations typically comprise a
solution or
fine suspension of the active substance in a physiologically acceptable
aqueous or non-
aqueous solvent and are usually presented in single or muitidose quantities in
sterile form
in a sealed container, which can take the form of a cartridge or refill for
use with an
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
atomising device. Alternatively the sealed container may be a unitary
dispensing device
such as a single dose nasal inhaler or an aerosol dispenser fitted with a
metering valve
which is intended for disposal once the contents of the container have been
exhausted.
Where the dosage form comprises an aerosol dispenser, it will contain a
propellant which
can be a compressed gas such as compressed air or an organic propellant such
as a fluoro-
chlorohydrocarbon. The aerosol dosage forms can also take the form of a pump-
atomiser.
Compositions suitable for buccal or sublingual administration include tablets,
lozenges and pastilles, wherein the active ingredient is formulated with a
carrier such as
sugar and acacia, tragacanth, or gelatin and glycerin.
Compositions for rectal administration are conveniently in the form of
suppositories containing a conventional suppository base such as cocoa butter.
Compositions suitable for transdermal administration include ointments, gels
and
patches.
Preferably the composition is in unit dose form such as a tablet, capsule or
ampoule.
Each dosage unit for oral administration contains preferably from 1 to 250 mg
(and for parenteral administration contains preferably from 0.1 to 25 mg) of a
compound
of the formula (I) or a physiologically acceptable salt thereof calculated as
the free base.
The physiologically acceptable compounds of the invention will normally be
administered in a daily dosage regimen (for an adult~patient) of, for example,
an oral
dose of between 1 mg and 500 mg, preferably between 10 mg and 400 mg,e.g.
between
10 and 250 mg or an intravenous, subcutaneous, or intramuscular dose of
between 0.1 mg
and 100 mg, preferably between 0.1 mg and 50 mg, e.g. between 1 and 25 mg of
the
compound of the formula (I) or a physiologically acceptable salt thereof
calculated as the
free base, the compound being administered 1 to 4 times per day. Suitably the
compounds will be administered for a period of continuous therapy, for example
for a
week or more.
Biological Test Methods
The ability of the compounds to bind selectively to human D3 dopamine
receptors can be
demonstrated by measuring their binding to cloned receptors. The inhibition
constants
(Ki) of test compounds for displacement of [ 125n iodosulpride binding to
human D3
dopamine receptors expressed in CHO cells were determined as follows. The cell
lines
were shown to be free from bacterial, fungal and mycoplasmal contaminants, and
stocks
of each were stored frozen in liquid nitrogen. Cultures were grown as
monolayers or in
suspension in standard cell culture media. Cells were recovered by scraping
(from
monolayers) or by centrifugation (from suspension cultures), and were washed
two or
three times by suspension in phosphate buffered saline followed by collection
by
centrifugation. Cell pellets were stored frozen at -40°C. Crude cell
membranes were
prepared by homogenisation followed by high-speed centrifugation, and
characterisation
of cloned receptors achieved by radioligand binding.
11
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
Preparation of CHO cell membranes
Cell pellets were gently thawed at room temperature, and resuspended in about
20
volumes of ice-cold 50 mM Tris salts (pH 7.4 @ 37°C), 20mM EDTA, 0.2 M
sucrose.
The suspension was homogenised using an Ultra-Turrax at full speed for IS sec.
The
homogenate was centrifuged at 18,000 r.p.m for 20 min at 4°C in a
Sorvall RCSC
centrifuge. The membrane pellet was resuspended in ice-cold 50 mM Tris salts
(pH 7.4
@ 37°C), using an Ultra-Turrax, and recentrifuged at 18,000 r.p.m for
IS min at 4°C in a
Sorvall RCSC. The membranes were washed two more times with ice-cold 50 mM
Tris
salts (pH 7.4 @ 37°C). The final pellet was resuspended in 50 mM Tris
salts (pH 7.4 @
37°C), and the protein content determined using bovine serum albumin as
a standard
(Bradford, M. M. (1976) Anal. Biochem. 72, 248-254).
Binding experiments on cloned dopamine receptors
Crude cell membranes were incubated with 0.1 nM [I25n iodosulpride (--2000
Ci/mmol;
Amersham, U. K.), and the test compound in a buffer containing 50 mM Tris
salts (pH
7.4 @ 37°C), 120 mM NaCl, 5 mM KCI, 2 mM CaCl2, 1 mM MgCl2, 0.1% (w/v)
bovine
serum albumin, in a total volume of 1 ml for 30 min at 37°C. Following
incubation,
samples were filtered using a Brandel Cell Harvester, and washed three times
with ice-
cold 50 mM Tris salts (pH 7.4 @ 37°C), 120 mM NaCI, S mM KCI, 2 mM
CaCI2, 1 mM
MgCl2. The radioactivity on the filters was measured using a Cobra gamma
counter
(Canberra Packard). Non-specific binding was defined as the radioligand
binding
remaining after incubation in the presence of 100 IrM iodosulpride. For
competition
curves, 14 concentrations (half-log dilutions) of competing cold drug were
used.
Competition curves were analysed simultaneously whenever possible using non-
linear
least-squares fitting procedures, capable of fitting one, two or three site
models.
Compounds of Examples tested according to this method had pKi values in the
range 7.0 -
8.5 at the human cloned dopamine D3 receptor.
Functional Activity at cloned dopamine receptors
The functional activity of compounds at human D2 and human D3 receptors (ie
agonism
or antagonism) may be determined using a Cytosensor Microphysiometer
(McConnell
HM et al Science 1992 257 1906-1912) In Microphysiometer experiments, cells
(hD2_CHO or hD3_CHO) were seeded into I2mm Transwell inserts (Costar) at
300000
cclis/cup in foetal calf serum (FCS)-containing medium. The cells were
incubated for 6h
at 37oC in 5%C02, before changing to FCS-free medium. After a further 16-18h,
cups
were loaded into the sensor chambers of the Cytosensor Microphysiometer
(Molecular
Devices) and the chambers perfused with running medium (bicarbonate-free
Dulbecco's
modified Eagles medium containing 2 mM glutamine and 44 mM NaCI) at a flow
rate of
100 ul/min. Each pump cycle lasted 90s. The pump was on for the first 60s and
the
acidification rate determined between 68 and 88s, using the Cytosoft
programme. Test
compounds were diluted in running medium. In experiments to determine agonise
12
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
activity, cells were exposed (4.5 min for hD2, 7.5 min for hD3) to increasing
concentrations of putative agonist at half hour intervals. Seven
concentrations of the
putative agonist were used. Peak acidification rate to each putative agonist
concentration
was determined and concentration-response curves fitted using Robofit
[Tilford, N.S.,
Bowen, W.P. & Baxter, G.S. Br. J. Pharmacol. (1995) in press]. In experiments
to
determine antagonist potency, cells were treated at 30 min intervals with five
pulses of a
submaximal concentration of quinpirole ( 100 nM for hD2 cells, 30 nM for hD3
cells),
before exposure to the lowest concentration of putative antagonist. At the end
of the next
30 min interval, cells were pulsed again with quinpirole (in the continued
presence of the
antagonist) before exposure to the next highest antagonist concentration. In
all, five
concentrations of antagonist were used in each experiment. Peak acidification
rate to each
agonist concentration was determined and concentration-inhibition curves
fitted using
Robofit.
Pharmaceutical Formulations
The following represent typical pharmaceutical formulations according to the
present
invention, which may be prepared using standard methods.
IV Infusion
Compound of formula (I) 1-40 mg
Buffer to pH ca 7
Solventlcomplexing agent to 100 ml
Bolus Injection
Compound of formula (I) 1-40 mg
Buffer to pH ca 7
Co-Solvent to 5 ml
Buffer : Suitable buffers include citrate, phosphate, sodium
hydroxide/hydrochloric
acid.
Solvent : Typically water but may also include cyclodextrins (1-100 mg) and co-
solvents such as propylene glycol, polyethylene glycol and alcohol.
Tablet
Compound 1 - 40 mg
Diluent/Filler * 50 - 250 mg
Binder 5 - 25 mg
Disentegrant * 5 - 50 mg
Lubricant 1 - 5 mg
Cyclodextrin 1 - 100 mg
* may also include cyclodextrins
13
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
Diluent : e.g. Microcrysta.lline cellulose, lactose, starch
Binder : e.g. Polyvinylpyrrolidone, hydroxypropymethylcellulose
Disintegrant : e.g. Sodium starch glycollate, crospovidone
Lubricant : e.g. Magnesium stearate, sodium stearyl fumarate.
Oral Suspension
Compound 1 - 40 mg
Suspending Agent 0.1 - 10 mg
Diluent 20 - 60 mg
Preservative 0.01 - 1.0 mg
Buffer to pH ca 5 - 8
Co-solvent 0 - 40 mg
Flavour 0.01 - 1.0 mg
Colourant 0.001 - 0.1 mg
Suspending agent :e.g. Xanthan gum, microcrystalline cellulose
Diluent : e.g. sorbitol solution, typically water
Preservative : e.g. sodium benzoate
Buffer : e.g. citrate
Co-solvent : e.g. alcohol, propylene glycol, polyethylene glycol, cyclodextrin
The invention is further illustrated by the following non-limiting examples
Description 1
7-Bromo-1,2,3,4-tetrahydroisoquinoline
A mixture of 7-bromo-2-trifluoroacetyl-1,2,3,4-tetrahydoisoquinoline (G.E.
Stokker, Tetrahedron Letters 1996, 37, 5453) (43.48, 0.14 mot), potassium
carbonate (104.38, 0.75 mot), methanol (1L) and water (150m1) was heated at
reflux for lh, then cooled and evaporated in vacuo. Residue was partitioned
between water (1L) and dichloromethane (4 x 200m1). Combined extracts were
dried (Na~SO,) and evaporated in vacuo to give an oil which was dissolved in
hexane. The mixture was filtered and the filtrate evaporated in vacuo to give
the
title compound as an oil ( 17.78, 60%).
'H NMR (CDCl3) 8: 1.77 (1H, br s), 2.73 (2H, t, J = 7 Hz), 3.13 (2H, t, J = 7
Hz), 3.98 (2H, s), 6.96 {1H, d, J = 9 Hz), 7.16 (1H, d, J = 2 Hz), 7.26 (1H,
dd, J =
9, 2 Hz).
14
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
The following compounds were prepared in a similar manner to Description 1
(a) 7-Cyano-1,2,3,4-tetrahydroisoquinoline
Mass spectrum (API'): Found 159 (MH'). C,oH,oN2 requires 158.
Description 2
IO 7-Cyano-2-tritluoroacetyl-1,2,3,4-tetrahydroisoquinoline
A mixture of 7-bromo-2- trifluoroacetyl -1,2,3,4-tetrahydroisoquinoline (51.7
g,
0.168 mol), copper (I} cyanide (31.8 g, 0.35 mol) and N-methyl-2-pyrrolidinone
(620 ml) was heated at reflux for 4h, cooled, then partitioned between dilute
aqueous ammonia (1.5 L) and dichloromethane (5 x 300m1). The combined
organic extracts were dried (NazSO,) and evaporated in vacuo to give the title
compound (42.6 g, 100 %) as an oil.
Mass spectrum {APr): Found 253 {M-H)-. C,zH9F,N~0 requires 254.
Description 3
(~)-traps-2-((N-(ten-Butyloxycarbonyl)amino)methyl)cyclopropane-1-
carboxaldehyde
To a solution of (t)-traps-1-((N-(tert-butyloxycarbonyl)amino)methyl)-2-((tert-
butyldiphenylsilyloxy)methyl)cyclopropane [T. Morikawa et al, J. Org.
Chem.,1994. 59,
97] (0.33g, 0.75 mmol) in dry THF (lOml) at 0°C, was added a 1M
solution of tetra-n-
butylammonium fluoride in THF (2.3m1, 2.3 mmol}. The mixture was stirred at
room
temperature for 3 hours, then partitioned between diethyl ether (25m1) and
water (25m1).
Aqueous phase was further extracted with diethyl ether {25m1 x 2) and the
combined
organic extracts were washed with brine (40m1), dried {NaZSOo) then evaporated
in vacuo
to give an oil. To a solution of oxalyl chloride (0.08g, 0.6 mmol) in dry
dichloromethane
{3m1) at -80°C under argon, was added dropwise a solution of dimethyl
sulfoxide (0.09g,
1.2 mmol) in dichloromethane (0.5m1). The resulting mixture was stirred at -
78°C for
0.75h, then a solution of the above oil in dry dichloromethane (3m1) was
added. The
mixture was stirred for lh then triethylamine (lml) was added and the mixture
warmed to
room temperature. The mixture was partitioned between dichloromethane ( 100m1)
and
water (SOmI). The organic layer was washed with water (30rn1) and brine
(30m1), then
dried (Na2S04) and evaporated in vacuo to give the title compound as an oil
(0.12g, 98%)
15
CA 02288850 1999-11-08
WO 98151671 PCTIEP98/02584
'H NMR (CDC13) S: 1.07 (1H, m), 1.30 (1H, m), 1.45 (9H, s), 1.69 - 1.90 (2H,
m), 2.95
- 3.30 (2H, m), 4.75 (1H, br s), 9.09 (1H, d, J = 5 Hz).
Description 4
(~)-traps-1-(N-(-ten-Butyloxycarbonyl)amino)methyl-2-(2-(7-cyano-1,2,3,4-
tetrahydro)isoquinolyl)methylcyclopropane
A mixture of (t}-traps-2-((N (tert-butyloxycarbonyl)amino)cyclopropane-1-
carboxaldehyde (0.12g, 0.6 mmol}, 7-cyano-1,2,3,4-tetrahydroisoquinoline (0.1
lg, 0.66
mmol) and sodium triacetoxyborohydride {0.19g, 0.9 mmol) in 1,2-
dichloromethane
(15m1) was allowed to stir at roam temperature for 20h, then partitioned
between
dichloromethane (120m1) and satwated aqueous NaHC03 (40m1). Organic phase was
washed with saturated NaHC03 (40m1), brine (40m1), dried (Na2S04) and
evaporated in
vacuo to an oil. Chromatography on silica with ethylacetate-hexane 20 - 40%
gradient
elution gave the title compound as an amber oil (0.16g, 78°l0).
Mass spectrum (API+): Found 342 (MH+). CZOH2~N302 requires 341.
'H NMR (CDC13) $: 0.40 - 0.55 (2H, m), 0.85 - 0.91 (2H, m), 1.47 (9H, s), 2.34
- 2.60
(2H, m), 2.75 - 2.85 (2H, m), 2.90 - 3.00 (2H, m), 3.02 - 3.10 (2H, m}, 3.55 -
3.80 (2H,
m), 4.68 (1H, br s), 7.20 (1H, d, J = 8 Hz), 7.35 {1H, s), 7.40 (1H, d, J = 8
Hz).
The following compound was prepared in a similar manner to Description 4.
(a) traps-2-(1-(4-(t-Butyloxycarbonyl)aminomethyl)cyclohexylmethyl)-7-cyano-
1,2,3,4-tetrahydroisoquinoline
Mass spectrum (API+): Found 384 (MH~}. C23Hs3N3O2 requires 383.
Description 5
(~)-traps-1-Aminomethyl-2-(2-(7-cyano-1,2,3,4-tetrahydro)isoquinolyl)-
methylcyclopropane
To a solution of {t)-traps-1-(N (tert-butyloxycarbonyl)methyl-2-(2-{7-cyano-
1,2,3,4-
tetrahydro)isoquinolyl)methylcyclopropane (0.16g, 0.47 mmol) in dry
dichloromethane
(lOml) at 0°C, was added trifluoroacetic acid (0.36m1). The mixture was
stirred at 0°C for
lh, then more trifluoroacetic acid (0.4m1) was added. The mixture was stirred
at room
16
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
temperature for 5h, then partitioned between dichloromethane (100m1) and
saturated
aqueous NaHC03 (50m1). Organic phase was washed with brine (50m1},
dried(NazS04)
and evaporated in vacuo to give the title compound as an amber oil (O.lg,
89%).
Mass spectrum (APIA: Found 242 (MH+). CISH19N3 requires 241.
'H NMR (CDC13) 8: 0.30 - 0.50 (2H, m), 0.70 - 0.90 {2H, m), 1.45 (2H, br s),
2.40 -
3.00 {8H, m), 3.68 (2H, s), 7.17 ( 1 H, d, J = 8 Hz), 7.32 ( 1 H, s), 7.37 ( 1
H, d, J = 8 Hz).
The following compound was prepared in a similar manner to Description 5.
(a) traps-2-(1-{4-Aminomethyl)cyclohexylmethyl)-7-cyano-1,2,3,4-
tetrahydroisoquinoline
Mass spectrum (APf'): Found 284 (MH+). C,8Hz5N3 requires 283.
Description 6
6-Cyano-1,2,3,4-tetrahydroisoquinoline
Prepared in a similar manner to that described in H.G. Selnick et al.,
Synthetic
Communications 25 (20) 3255 {1995).
Mass spectrum (API+): Found 159 (MH+). C~oH,oNz requires 158.
Description 7
4-(2-(7-Cyano-1,2,3,4-tetrahydro)isoquinolinyl)cyclohexanone
A mixture of 7-cyano-1,2,3,4-tetrahydroisoquinoline (2.37g, 15 mmol), 1,4-
dioxaspiro-
[4.5]decan-8-one (2.348, 15 mmol) and sodium triacetoxyborohydride (4.73g,
22.5 mmol)
in dichloroethane (50m1) was stirred at 20°C for 18h. Mixture was
partitioned between
saturated aqueous NaHC03 (250m1) and dichloromethane (3 x 50m1) and the
combined
organic extracts dried (NazS04) and evaporated in vacuo to give an oil.
Chromatography
on silica with 25 - 100% ethyl acetate - hexane gradient elution gave a solid
(3.53g). The
latter was dissolved in water containing concentrated H2S04 ( 1.35g, 13.5
mmol) and
heated at 65°C for 18h. Mixture was cooled, then partitioned between
saturated aqueous
NaHC03 {300m1) and dichloromethane (3 x 100m1). Combined organic extracts were
17
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
dried (Na2S04) and evaporated in vacuo to give the title compound (3.14g,
82%a) as an
oil.
Mass spectrum (API+): Found 255 (MH+). C16H18N20 requires 254.
The following compound was prepared in a similar manner to Description 7
(a) 4-(2-(6-Cyano-1,2,3,4-tetrahydro)isoquinolylkyclohexanone
Mass spectrum (API+}: Found 255 (MH+}. Cl6H,gN20 requires 254.
Description 8
cis- and traps-7-Cyano-2-(1-(4-trifluoroacetamidokyclohexyl)-1,2,3,4-
tetrahydroisoquinoline
A mixture of 4-(2-(6-cyano-1,2,3,4-tetrahydro)isoquinolyl}cyclohexanone 2.90g,
11.4
mmol), ammonium acetate (8.7g, 0.11 mol) and sodium triacetoxyborohydride
(16.6g,
79.4 mmol) in ethanol (250m1) was heated at reflux for 3h, cooled then
evaporated in
vacuo. Residue was partitioned between saturated aqueous NaHC03 (300m1) and
dichloromethane (3 x 100m1). Combined organic extracts were dried (Na2S04) and
evaporated in vacuo to give an oil (2.78g). A mixture of the latter with
triethylamine
(2m1; 14.3 mmol) in dichloromethane ( 100m1) at 0°C was treated
dropwise with
trifluoroacetic anhydride (1.9m1, 13.5 mmol). Resulting solution was stirred
at 20°C for
4h, then partitioned between saturated aqueous NaHC03 (300m1) and
dichloromethane (3
x 100 ml). Combined organic extracts were dried (NaZS04) and evaporated in
vacuo to
give an oil. Chromatography on silica with 10 - 100% ethyl acetate - hexane
gradient
elution gave, as the first-eluting component, the cis-isomer (1.58g, 38%),
'H NMR (CDCl3) 8: 1.50 - 2.00 (8H, m), 2.48 (1H, m}, 2.87 (2H, m), 2.98 (2H,
m), 3.78
(2H, s), 4.09 (1H, m), 6.29 (1H, m), 7.22 (1H, m), 7.29 - 7.49 (2H, m),
and, as the second-eluting component, the traps-isomer (0.63g, 15%)
'H NMR {CDC13) 8: 1.22 - 1.44 (2H, m), 1.45 - 1.b4 (2H, m), 2.05 (2H, m), 2.17
(2H,
m), 2.55 (1H, tt, J = 9, 2 Hz), 2.84 (2H, m), 2.95 (2H, m), 3.78 (2H, s), 3.80
(1H, m),
6.15 ( 1 H, m ), 7.19 ( 1 H, d, J = 8 Hz), 7.32 ( 1 H, d, J = 1 Hz}, 7.40 ( 1
H, dd, J = 8, 1 Hz).
18
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
The following compounds were prepared in a similar manner to Description 8.
(a) cis-6-Cyano-2-{1-(4-trifluoroacetamidokyclohexyl)-1,2,3,4-
tetrahydroisoquinoline
'H NMR (CDC13) S: 1.65 - 1.95 (8H, m), 2.47 (1H, m), 2.83 (2H, m}, 1.92 (2H,
m), 3.77
(2H, s), 4.05 {1H, m), 6.28 (1H, br s), 7.13 (1H, d, J = 8 Hz), 7.39 (2H, m).
(b) trar~s-G-Cyano-2-(1-(4-trifluoroacetamidokyclohexyl)-1,2,3,4-
tetrahydroisoquinoline
1H NMR (CDC13) 8: 1.24 - 1.62 (4H, m), 2.03 (2H, m), 2.15 (2H, m), 2.53 (1H,
tt, J = 9,
2 Hz), 2.82 {2H, m), 1.86 (2H, m), 3.76 (1H, m), 3.80 (2H, s), 6.I2 (1H, m),
7.09 (1H, d,
J = 8 Hz), 7.35 (2H, m).
Description 9
traps-2-{1-(4-Amino)cyclohexyl)-7-cyano-1,2,3,4-tetrahydroisoquinoline
A mixture of traps-7-cyano-2-(1-(4-trifluoroacetamido)cyclohexyl)-1,2,3,4-
tetrahydroisoquinoline (0.68g, 1.9 mmol), methanol (30m1), water (3.Sml) and
anhydrous
potassium carbonate (1.3g, 9.6 mmol) was heated at reflux for 3h, cooled then
evaporated
in vacuo. Residue was partitioned between saturated aqueous KZC03 (50m1) and
dichloromethane (3 x 50 ml), and the combined extracts were dried (Na2S04) and
evaporated in vacuo to give the title compound (0.48g, 96%) as an oil.
Mass spectrum (API+): Found 256 (MH''). C,6H2,N3 requires 255.
The following compounds were prepared in a similar manner to Description 9.
(a) traps-2-(1-{4-Amino)cyclohexyl)-6-cyano-1,2,3,4-tetrahydroisoquinoline
Mass spectrum (APIA: Found 256 (MH+). C,6HZ,N3 requires 255.
(b) traps-2-(1-(4-(2-Amino~thylkyclohexyl)-7-cyano-1,2,3,4-
tetrahydroisoquinoline
Mass spectrum (APf"): Found 284 (MH+). C,sH25N3 requires 283.
19
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
Description 10
4-(2-Trifluoroacetamidoethylkyclohexanone
To a mixture of 8-(2-hydroxyethyl)-1,4-dioxaspiro[4.5]decane (lS.Sg, 83 mmol)
and
triethylamine (15.2m1; 0.108 mol) in dichloromethane (300m1) under argon at
0°C was
added dropwise a solution of methylsulfonyl chloride (7.4m1; 96 mmol) in
dichloromethane
(lOml}. Resulting solution was stirred at 20°C for 2h, then partitioned
between saturated
aqueous NaHC03 (SOOmI) and dichloromethane (3 x SOmI). Combined organic
extracts
were dried (NazS04) and evaporated in vacuo to give an oil (21.8g). The latter
was
dissolved in toluene (SOmI} and added to a solution of trifluoroacetamide
anion prepared
by portionwise addition of trifluoroacetamide (7.91g, 70 mmol} to a stirred
suspension of
sodium hydride (60°l0; 2.6g, 65 mmol) in dimethylformamide (SOmI). The
resulting
mixture was stirred at 20°C for 18h, then evaporated in vacuo. Residue
was partitioned
between ether (SOOmI) and water (350m1). Organic phase was washed with water(2
x
200m1), dried (NazS04) and evaporated in vacuo to give an oil (15g).
Chromatography on
silica with 10 - 100°lo ethyl acetate - hexane gradient elution gave an
oil (4.96g). A
solution of the latter in tetrahydrofuran (200m1) was treated with water
(400m1) and
concentrated HZS04 (50 drops), then heated at reflux for 3h. The mixture was
cooled,
concentrated in vacuo to 200m1, then extracted with dichloromethane (3 x
200m1).
Combined extracts were dried (NazS04) and evaporated in vacuo to give the
title
compound (3.72g, 19%} as a colourless solid.
Mass spectrum (API-): Found 236 (M-H)'. C1oH14F3NOz requires 237.
Description 11
cis- and traps-7-Cyano-2-(1-(4-(2-trilluoroacetamido)ethylkyclohexyl)-1,2,3,4-
tetrahydroisoquinoline
A mixture of 7-cyano-1,2,3,4-tetrahydroisoquinoline (l.Sg, 9.5 mmol}, 4-(2-
trifluoroacetamidoethyl)cyclohexane (2.25g, 9.5 mmol) and sodium
triacetoxyborohydride
(3.Og, 14.3 mmol) in dichloromethane (100m1) was treated with glacial acetic
acid (10
drops) and stirred at 20°C for 18h. Mixture was partitioned between
saturated aqueous
NaHC03 (300m1) and dichloromethane (4 x SOmI), and the combined extracts were
dried
(NazSO.,) and evaporated in vacuo to give an oil (4.Og). Chromatography on
silica with
10 - 100% ethyl acetate - hexane gradient elution gave, as the first-eluting
component, the
cis-isomer (1.98g, SSolo)
'H NMR (CDCl3) 8: 1.44 - 1.85 (11H, m), 2.45 {1H, m}, 2.81 (2H, m), 2.92 (2H,
m}, 3.40
(2H, m), 3.72 (2H, s), 6.31 ( 1 H, br s), 7.20 ( 1 H, d, J = 8 Hz), 7.35 ( 1
H, d, J = 1 Hz), 7.40
( 1 H, dd, J = 8, 1 Hz),
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
and, as the second-eluting component, the traps-isomer (0.92g, 26%).
'H NMR (CDC13) 8: 0.95 - 1.17 (2H, m), 1.20 - 1.68 (5H, m}, 1.84 - 2.07 (4H,
m), 2.50
(1H, tt, J = 9, 2 Hz), 2.85 (2H, m), 2.93 (2H, m), 3.42 (2H, q, J = 7 Hz),
3.78 (2H, s),
6.32 (1H, br s), 7.19 (IH, d, J = 8 Hz), 7.33 (1H, d, J = 1 Hz), 7.40 (1H, d,
J = 8, 1 Hz).
Description 12
traps-4-(t-Butyloxycarbonyl)aminomethylcyclohexanecarboxaldehyde
A mixture of traps-4-aminomethylcyclohexanecarboxylic acid (20g, 0.127 mol),
methanol
(250m1) and concentrated sulfuric acid (7.5m1; 0.14 mmol) was heated at reflux
for 5h
then evaporated in vacuo to give a solid. The latter was mixed with
dichloromethane
(250m1), triethylamine {64.5m1, 0.463 mol) and di-t-butyl Bicarbonate (34g,
0.155 mol),
and the resulting solution stirred at 20°C for 18h. Mixture was
partitioned between
saturated aqueous NaHC03 {1L) and dichloromethane (3 x 200m1), and the
combined
organic extracts were dried (Na2S04) and evaporated in vacuo to give a solid
(36.6g).
The latter was dissolved in toluene (500m1} and cooled to -78°C under
argon. A solution
of diisobutylaluminium hydride in toluene (1M; 270m1) was added dropwise over
0.75h,
and stirring at-78°C was continued for lh. Methanol (54.Sm1) was added
dropwise over
O.Sh and mixture stirred at -70°C for 0.25h. The resulting solution was
then poured into
saturated aqueous potassium sodium tartrate (1L), and the mixture stirred
vigorously for
3h. The resultant was extracted with ether (3 x 200m1) and the combined
organic extracts
were dried (Na2S04) and evaporated in vacuo to give an oil (35.5g).
Chromatography on
silica with 10 - 100% ethyl acetate - hexane gradient elution gave the title
compound
(20.9g, 64%) as an oil.
'H NMR (CDCl3) 8: 0.92 - 1.09 (2H, m), 1.18 - 1.50 (3H, m), 1.46 (9H, s), 1.89
(2H, m),
2.04 (2H, m ), 2.19 ( 1 H, m ), 3.00 (2H, t, J = 7 Hz), 4.60 ( 1 H, br s),
9.61 ( 1 H, s).
Example 1
(t)-traps-1-((E)-3-(5-Indolyl)propenamido)methyl-2-(2-(7-cyano-1,2,3,4-
tetrahydro)isoquinolyl)methylcyclopropane
A mixture of (~)-traps-1-aminornethyl-2-(2-(7-cyano-1,2,3,4-
tetrahydro)isoquinolyl)methylcyclopropane (O.lg, 0.4 mmol), (E)-3-(5-
indolyl}propenoic
acid (0.09g, 0.5 mmol) 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
hydrochloride
(0.13g) and 1-hydroxybenzotriazole (0.06g) in dimethylformamide (lml) and
dichloromethane (7m1) was shaken for 20h, then washed with water (7ml).
Chromatography of the organic phase on silica,eluting with ethyl acetate in
hexane 20% -
100%, gave the title compound as a colourless solid (0.llg, 66%).
21
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
Mass spectrum (APIA: Found 411 (MH+). CZ6H26N40 requires 410.
IH NMR (CDCI3) 8: 0.44 - 0.64 (2H, m), 0.90 - 1.00 (2H, m}, 2.35 - 2.55 (2H,
m), 2.75
- 3.00 (4H, m), 3.20 - 3.50 (2H, m), 3.69 (2H, s), 2.75 - 2.80 (1H, m), 6.35
(1H, d, J = 15
Hz), 6.57 (IH, m), 7.10 - 7.40 (6H, m), 7.70 - 7.85 (2H, m), 8.40 (1H, br s).
The following compounds were prepared in a similar manner to Example 1
(a) traps-(E)-6-Cyano-2-(1-{4-(3-(4-fluoro)phenylpropenoyl)amino)cyclohexyl)-
1,2,3,4-tetrahydroisoquinoline
Mass spectrum (API''): Found 404 (MH+). C25H26FN3O requires 403.
NMR (CDC13) 8: 1.25 (2H, m), 1.42 - 1.64 (2H, m), 2.00 (2H, m), 2.19 (2H, m},
2.54
(1H, m), 2.85 (4H, m), 3.81 (2H, s), 3.90 (1H, m), 4.47 (1H, d, J = 8 Hz),
6.30 (1H, d, J
= 16 Hz), 7.09 (3H, m), 7.34 - 7.55 (4H, m), 7.60 (1H, d, J = 16 Hz).
(b) traps-{E~7-Cyano-2-(1-(4-{3-phenylpropenoyl)amino)cyclohexyl)-1,2,3,4-
tetrahydroisoquinoline
Mass spectrum (API+): Found 386 (MH''}. CZSHZ,N30 requires 385.
'H NMR (CDCI3) 8: 1.25 (2H, m), 1.44 - 1.66 (2H, m), 2.00 (2H, m), 2.16 (2H,
m), 2.44
(1H, m), 2.81 (2H, m), 2.93 (2H, m), 3.76 (2H, m), 3.90 (1H, m), 5.45 (1H, d,
J = 8 Hz),
6.35 (1H, d, J = 16 Hz}, 7.19 (1H, d, J = 8 Hz), 7.34 (SH, m), 7.49 (2H, m),
7.62 (1H, d,
J = 16 Hz).
(c) traps-7-Cyano-2-(1-(4-{2-(2-indolyl)carboxamido)ethyl)cyclohexyl)-1,2,3,4-
tetrahydroisoquinoline
Mass spectrum (API+}: Found 427 (MH+). CZ~H3oN40 requires 426.
'H NMR (CDCI3 + CD30D} 8: 0.95 - 1.18 (2H, m), 1.25 - 1.48 (3H, m), 1.58 (2H,
q, J =
7 Hz), 1.96 (4H, m), 2.50 (1H, m), 2.85 (2H, m), 2.94 (2H, m), 3.50 (2H, m),
3.79 (2H,
s), 6.59 (1H, m), 6.89 (1H, s), 7.09 - 7.24 (2H, m}, 7.29 (2H, m), 7.37 - 7.50
(2H, m),
7.65 ( I H, d, J = 8 Hz), 9.84 ( 1 H, br s).
(d) traps-(E)-7-Cyano-2-(1-(4-(3-phenylpropenoyl)aminomethyl)cyclohexylmethyl)-
1,2,3,4-tetrahydroisoquinoline
Mass spectrum {API+): Found 414 (MH'). C27H3,N3O requires 413.
22
CA 02288850 1999-11-08
WO 98/51671 PCT/EP98/02584
'H NMR (CDC13) 8: 0.78 - 1.15 (4H, m), 1.56 (2H, m), 1.86 (4H, m), 2.31 (2H,
d, J =.'7~
Hz), 2.69 (2H, t, J = 6 Hz), 2.93 (2H, t, J = 6 Hz), 3.27 (2H, t, J = 7 Hz),
3.59 (2H, s),
5.74 (1H, m), 6.40 (1H, d, J = 16 Hz), 7.19 (1H, d, J = 8 Hz), 7.33 (1H, s),
7.38 (4H, m),
7.51 (2H, m), 7.64 (1H, d, J = I6 Hz).
(e) traps-7-Cyano-2.-(1-(4-(2-
indolyl)carboxamidomethyl)cyciohexylmethyi~1,2,3,4-
~ tetrahydroisoquinoline
Mass spectrum (APT"): Found 427 (MH+). CZ,H3oN40 requires 426.
'H NMR (CDC13 + CD30D) 8: 0.85 - 1.17 (4H, m), 1.60 (2H, m), 1.90 (4H, m},
2.34
(2H, d, J = 7 Hz), 2.7I (2H, t, J = 6 Hz), 2.95 (2H, t, J = 6 Hz), 3.32 (2H,
t, J = 7 Hz),
3.60 (2H, s), 6.75 (1H, m), 6.91 (1H, s), 7.07 - 7.36 (4H, m), 7.42 (2H, m),
7.64 (1H, d, J
= 8 Hz), 9.95 ( 1 H, br s).
23