Note: Descriptions are shown in the official language in which they were submitted.
a ~ '
CA 02288947 1999-11-04
Title: METHOD AND APPARATUS FOR MEASURING THE
DEGREE
OF TREATMENT OF A MEDIUM BY A GAS
FIELD OF THE INVENTION
The present invention relates to a method and
apparatus for measuring the degree of treatment of a medium by a
gas. In one particular embodiment, the present invention relates to
1 0 a method and apparatus for determining the degree of oxidative
treatment of a medium, such as water, by measuring the total
amount of oxidative agent, such as ozone, passing through the
water without undergoing a conversion to oxygen.
1 5 BACKGROUND TO THE INVENTION
In many areas, a gas is used as a processing agent to treat a
material. Examples of this include water treatment, waste water
treatment and chemical oxidation (i.e. bleaching).
Accordingly, various different sensors have been developed
2 0 to measure the level of a gas in a liquid. These include ORP sensors,
photometric devices and electrolytic devices.
Ozone is used in various applications in industry and
accordingly, sensors for detecting the concentration of ozone have
been developed. Typically, these operate by passing ultraviolet light
2 5 through a fluid stream and measuring the ultraviolet light which is
received on a detector. Another type of gas sensor is disclosed in
United States Patent No. 5,167,9256 to Karlson. Karlson discloses a
monitor which measures the heat energy which is released when a
gas, eg. ozone, is catalytically converted to a different compound (eg.
3 0 oxygen).
One example of the use of ozone is to purify water for
drinking by passing ozone through the water to kill microorganism
contaminants such as bacteria present in the water. Various
processes to treat water have been developed using combinations of
3 5 filtration and ozonation.
CA 02288947 1999-11-04
- 2 -
For example, United States Patent No. 5,683,576 to Olsen
describes an apparatus for treating contaminated water by passing
ozone through the water. In the system disclosed by Olsen, an
ozone containing gas is passed through the water to be treated, until
the instantaneous concentration of ozone in the head space above
the water being treated reaches a predetermined level. Then, the
flow of ozone through the water continues for a predetermined
period of time.
The amount of ozone which must be passed through the
1 0 water to purify it to any particular state will vary depending upon
the initial quality of water to be treated. For example, untreated well
or lake water may require a higher degree of purification than
treated city water which has previously been treated to some degree.
One disadvantage of Olsen is that it can not be reliable used
1 5 with such disparate types of water supply. Olsen does not monitor
the total amount of ozone which passes through the water
unreacted. Thus, the actual degree of treatment of the water is not
measured. The system is designed only to ensure that a
predetermined minimum amount of ozone passes through the
2 0 system unreacted. The system makes the assumption that once the
concentration of ozone reaches the predetermined level, that it does
not subsequently drop below that level, or rise above that level.
Further, it assumes that once the water to be treated has been
exposed to the preset ozone concentration for a predetermined time
2 5 that the water is suitable for use. However, depending on the degree
of contamination of the water to be treated, the time required to
treat the material will vary.
In Olsen, the amount of unreacted ozone passing through
the system prior to the time when the instantaneous concentration
3 0 reaches the predetermined level is not measured. Further, the
amount of unreacted ozone passing through the system measured
CA 02288947 1999-11-04
c
-3-
during the predetermined amount of time after the predetermined
instantaneous concentration is reached is not measured. Thus, the
actual degree of treatment of the water is not measured.
Another disadvantage of the method of Olsen is that, in
some applications, it is desirable to monitor the degree of treatment
of material as it is being oxidized.
Accordingly, there is a need for a method and apparatus to
accurately measure the amount of treatment to which water has
been subjected by passing ozone through the water. Further, there is
1 0 a need to do so on a cost effective scale.
SUMMARY OF THE INVENTION
In accordance with one aspect of the present invention,
there is provided a method of treating a material to a predetermined
1 5 state with a reactable gas comprising the steps of:
(a) contacting the reactable gas and the material and
obtaining treated material, a by-product produced from the
reactable gas and unreacted gas;
(b) comparing the unreacted gas having treated the material
2 0 without having being converted into the by-product with a
preset amount corresponding to a predetermined state of
treatment of the material; and,
(c) treating the material until the total amount of unreacted
gas is at least equal to the preset amount.
2 5 In one embodiment, the method additionally comprises the
steps of terminating the treatment of the material after a
predetermined time if the total amount of unreacted gas is less than
the preset amount at the end of the predetermined time.
In another embodiment, the method additionally comprises
3 0 the steps of:
CA 02288947 1999-11-04
r ,
-4-
(a) stopping the flow of the readable gas after the total
amount of unreacted gas is at least equal to the preset
amount; and,
(b) issuing a signal to a user that the material has been
treated to the predetermined state.
In another embodiment, the material to be treated comprises
water and the reactable gas is ozone and the method further
comprises introducing the water and the ozone to obtain potable
water.
1 0 In another embodiment, step (b) comprises treating at least a
portion of the unreacted gas to produce a signal and determining
the amount of unreacted gas based on the signal produced.
In another embodiment, step (b) comprises subjecting at
least a portion of the unreacted gas to a chemical reaction to produce
1 5 heat and determining the amount of unreacted gas based on the
heat produced. In a preferred embodiment, all of the unreacted gas
is subjected to the chemical reaction.
In another embodiment, the step of determining the
amount of unreacted gas based on the heat produced comprises
2 0 measuring the instantaneous amount of heat produced; and,
determining the total amount of heat produced by the unreacted
gas, wherein the preset amount is determined based on the total
amount of heat released by the unreacted gas when the material has
been treated to the predetermined state of treatment.
2 5 In another embodiment, the step of measuring the amount
of heat produced comprises measuring the change in temperature
from subjecting the unreacted gas to the chemical reaction; and,
correlating the temperature differential to the amount of unreacted
gas.
3 0 In another embodiment, the step of measuring the amount
of heat produced comprises:
CA 02288947 1999-11-04
r
-5-
(a) measuring a first temperature of the unreacted gas;
(b) measuring a second temperature of the gas after
subjecting the unreacted gas to the chemical reaction;
(c) calculating the difference between the first temperature
and the second temperature; and,
(d) correlating the temperature differential to the amount of
unreacted gas.
In another embodiment, the material to be treated comprises
water and the reactable gas is ozone and the step of subjecting at
1 0 least a portion of the unreacted gas to a chemical reaction comprises
exposing the ozone to a catalyst to convert ozone into oxygen.
In another embodiment, the method further comprises the
step of forming a liquid solution containing microbubbles of the
reactable gas prior to contacting the reactable gas with the material.
In another embodiment, the method further comprises
passing a fluid and the reactable gas through a prandtl layer turbine
and, more preferably, then trough a reduced pressure zone
downstream of the prandtl layer turbine, prior to contacting the
reactable gas with the material.
2 0 In another embodiment, the method further comprises
passing the material to be treated and the reactable gas through a
prandtl layer turbine and a reduced pressure zone downstream of
the prandtl layer turbine.
In accordance with another aspect of the present invention,
2 5 there is provided an apparatus for measuring the degree to which a
material has been treated with a reactable fluid, the reactable fluid
capable of treating the material and in the process being converted
into a by-product, the apparatus comprising:
(a) a container for containing the material during treatment
3 0 with the reactable fluid;
CA 02288947 1999-11-04
T
-6-
(b) an inlet for introducing the reactable fluid to the
material;
(c) a sensor for sensing the amount of unreacted reactable
fluid in an unreacted state after exposure to the material;
and,
(d) a controller for comparing the amount of reactable fluid
in an unreacted state with a preset amount corresponding to
a predetermined state of treatment of the material and
treating the material until the total amount of unreacted
1 0 fluid is at least equal to the preset amount.
In another embodiment, the material to be treated comprises
water and the readable fluid is ozone and the apparatus further
comprises a member for dispersing the ozone in the water.
In another embodiment, the controller includes a timer and
1 5 the treatment of the material is terminated after a predetermined
time if the total amount of unreacted gas is less than the preset
amount at the end of the predetermined time.
In another embodiment, the reactable fluid is a gas, the
apparatus further comprising a prandtl layer turbine, the reactable
2 0 fluid being fed with a liquid through the prandtl layer turbine prior
to the reactable fluid being fed to the container.
In another embodiment, the apparatus further comprising a
passage to convey at least a portion of the fluid to the sensor.
In another embodiment, the unreacted fluid is conveyed to
2 5 the sensor and the sensor comprises a zone for treating the
unreacted fluid to produce a signal.
In another embodiment, the unreacted fluid is conveyed to
the sensor and the sensor comprises a reaction zone for subjecting
the unreacted fluid to a chemical reaction, the reaction zone having
3 0 an inlet end and an outlet end, and the reactable fluid exhibits a
CA 02288947 1999-11-04
-7-
detectable change in temperature after being subjected to the
chemical reaction.
In another embodiment, the sensor further comprises:
(a) a first temperature sensor for measuring the temperature
of unreacted fluid upstream of the reaction zone; and,
(b) a second temperature sensor for measuring the
temperature of the fluid after being subjected to the chemical
reaction.
In another embodiment, the controller comprises a
1 0 comparator for comparing the difference in temperature between
the measurements of the first and second temperature sensors, and
relating the difference in temperature to the preset amount.
In another embodiment, the reaction zone comprises a
catalyst for converting the unreacted fluid entering the reaction
1 5 zone into a waste by-product. The unreacted fluid may comprise
ozone, and the catalyst may be selected from the group of catalysts
consisting of manganese dioxide, titanium dioxide, iron oxide and
carbon. At least a portion of the second temperature sensor may be
provided with the catalyst.
2 0 In another embodiment, the first and second temperature
sensors are in the form of thermistors and measure the electrical
resistance across the sensors as a function of temperature.
In another embodiment, the apparatus further comprises a
signal to indicate that the material has been treated to the
2 5 predetermined state.
In another embodiment, the reactable fluid is a gas and the
apparatus further comprises a prandtl layer turbine and a reduced
pressure zone positioned downstream of the prandtl layer turbine,
the reactable fluid being fed with a liquid through the prandtl layer
3 0 turbine prior to the reactable fluid being fed to the container. The
liquid may comprise the material to be treated.
CA 02288947 1999-11-04
f ,
In accordance with another aspect of the present invention,
there is provided an apparatus for measuring the degree to which a
material has been treated with a reactable fluid, the readable fluid
capable of treating the material and in the process being converted
into a by-product, the apparatus comprising:
(a) means for retaining the material during treatment with
the reactable fluid;
(b) means for contacting the reactable fluid with the
material;
1 0 (c) means for determining the amount of reactable fluid
which does not react with the material; and,
(d) means for determining the degree of treatment based on
the amount of reactable fluid which does not react with the
material and treating the material until a prespecified
1 5 condition is met, the prespecified condition selected from
the group:
(i) until the total amount of readable fluid which does
not react with the material is at least equal to the preset
amount, or
2 0 (ii) for a predetermined time corresponding to an
estimate of the time required for material to be treated
to the predetermined state in the apparatus.
In another embodiment, the material to be treated comprises
water and the reactable fluid is an oxidant and the reactable gas is
2 5 introduced into the container as finely dispersed bubbles.
In another embodiment, the means for determining the
amount of reactable fluid which does not react with the material
comprises means for subjecting the unreacted fluid to a reaction to
cause a temperature change in unreacted fluid due to the chemical
3 0 reaction.
CA 02288947 1999-11-04
_g_
In another embodiment, the means for determining the
degree of treatment comprises means for comparing the
temperature change and relating the temperature change to the
preset amount.
In another embodiment, the apparatus further comprises
means to signal the user when the prespecified condition is met.
In another embodiment, the apparatus further comprises
means to stop treatment of the material when the prespecified
condition is met.
1 0 In another embodiment, the reactable fluid is a gas and the
apparatus further comprises means for forming a liquid solution
containing microbubbles of the reactable gas prior to contacting the
reactable gas with the material.
In another embodiment, the readable fluid is a gas and the
1 5 apparatus further comprises a prandtl layer turbine and a reduced
pressure zone downstream of the prandtl layer turbine, the reactable
fluid being fed with a liquid through the prandtl layer turbine prior
to the reactable fluid being fed to the means for retaining the
material during treatment. The liquid may comprise the material
2 0 to be treated.
One advantage of the instant method and apparatus is that
the degree of treatment of a material is indirectly measured.
Provided a known quantity of material to be treated is placed in the
container, then the amount of reactable fluid required to treat the
2 5 material to a desired degree of treatment can be theoretically
determined. Based on the efficiency of the apparatus, the average
amount of reactable fluid required to treat the material to a desired
degree of treatment can be determined. As the amount of reactable
fluid introduced into the container to treat the material may be
3 0 determined (either for example, by knowing the input rate, the rate
of generation of the material) the amount of unreacted fluid
CA 02288947 1999-11-04
1 r
- 10 -
upstream of the treatment area may be correlated to the degree of
treatment of the material.
In particular, the unreacted fluid is treated to produce a
signal which may be easily measured by equipment known to those
skilled in the art. For example the unreacted fluid may be subjected
to a chemical reaction to produce a temperature change which may
easily be measured such as by a thermistor. By measuring the signal,
the amount of unreacted fluid may be tracked and the progress of
the treatment of the material may be determined by comparing the
1 0 amount of unreacted fluid measured via the signal with the degree
of treatment associated with the production of that amount of
unreacted fluid.
A further advantage is that a signal may be sent to a user if
one or more circumstances arise. For example, a treatment profile
1 5 may be determined for the apparatus comprising the degree of
treatment of the material against the amount of unreacted fluid
measured by the sensor. If the treatment profile is not matched in
use, then the material may require more treatment than anticipated
and the user may be so advised, the apparatus may be defective and
2 0 the user may be so advised of the use of the apparatus may match
the treatment profile in which case the user may be advised that the
treatment was successful.
A further advantage is that these results may be achieved on
a cost effective basis since a sensor measures not the unreacted fluid
2 5 itself, but a signal which may be correlated to the amount of
unreacted fluid.
BRIEF DESCRIPTION OF THE DRAWINGS
These and other features of the present invention will
3 0 be more fully and completely understood through a conjunction of
CA 02288947 1999-11-04
- 11 -
the following description taken together with the drawings of a
preferred embodiment of the invention in which:
Figure 1 is a schematic drawing of a water purification
apparatus in which an ozone sensor made in accordance with a
preferred embodiment of the present invention may be utilized;
Figure 2 is a perspective view of an ozone sensor made in
accordance with a preferred embodiment of the present invention;
Figure 3 is a cross sectional view of the ozone sensor of
Figure 2, taken along the line 3-3;
1 0 Figure 4 is an alternate embodiment of an ozone sensor;
Figure 5 is a graph showing a typical relationship between
the instantaneous concentration of unreacted ozone passing
through water being treated versus time; and,
Figure 6 is a schematic drawings of a gas/liquid mixing
1 5 apparatus which may be used with the ozone sensor of Figure 1.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENT
The method and apparatus of the present application may be
used in conjunction with the treatment of a material with a fluid.
2 0 The fluid may be any fluid which can be treated to provide a
detectable signal. Preferably, the fluid may be any fluid which will
under go a temperature change when treated. For example, the fluid
may be subjected to a chemical reaction pursuant to which there is a
detectable temperature change. Any such fluid is referred to as a
2 5 "reactable fluid". Preferred example of such readable fluids are
oxidants, eg. ozone and peroxide, which release heat when reacted
to form oxygen. It will be appreciated that if the reactable fluid reacts
to produce a by-product, the by-product is preferably
environmentally friendly.
3 0 In a particularly preferred embodiment, the readable fluid is
a gas. This method and apparatus is preferentially used in the
CA 02288947 1999-11-04
7
- 12 -
treatment of water to obtain, for example, potable water, and the
following description exemplifies the use of the method and
apparatus in conjunction with the treatment of water.
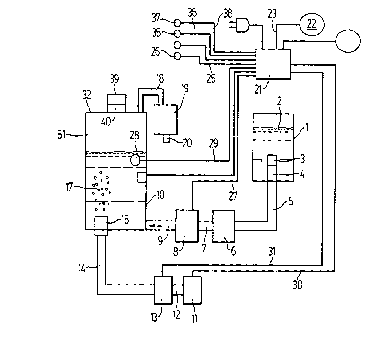
Referring to Figure 1, there is shown a schematic
representation of an apparatus for purifying water, which may be
used in connection with the present invention. The apparatus may
be used to treat water contaminated, eg., by microorganisms so that
it is fit for human consumption. The apparatus includes a water
vessel 1 into which water to be treated 2 is placed. The water vessel
1 0 1 has a valve 3 and a connector 4, separating the water vessel 1 from
a hose 5. The valve 3 and connector 4 are arranged such that when
the connector 4 is in communication with hose 5 the valve 3 is
open and allows contaminated water to flow from the vessel 1 into
the hose 5. When the valve 3 is closed, contaminated water cannot
1 5 flow from the vessel 1 to the hose 5. The water treatment cycle is
controlled by means of a microcontroller 21.
When the apparatus is supplied with power, the
microcontroller 21 provides power to a light 26 through wiring 25.
The light 26 indicates that the power to the water treatment system
2 0 is on and that the system is functioning correctly. A start button 22
is connected to the microcontroller 21 through wiring 23. The
momentary depression of the start button 22 signals the
microcontroller 21 to begin a water purification cycle and is
considered the beginning of the water treatment cycle. The
2 5 microcontroller 21 then supplies power to illuminate a light 35,
indicating that the water is being treated, through wiring 36.
The micro-controller also supplies power to a water
pump 8 through wiring 27. The contaminated water 2 is pumped
into the hose 5 by a water pump 8. The contaminated water 2 flows
3 0 from the water vessel 1 through the valve 3 and connector 4 into
the hose 5. The contaminated water then flows through a filter 6
CA 02288947 1999-11-04
- 13 -
and a hose 7. The contaminated water 2 then flows through the
water pump 8 and a hose 9 which may have a one way check valve,
into an ozone contacting chamber 10. A float switch 28 provides a
signal to the microcontroller 21 through wiring 29 when the water
level reaches the height of the float switch 28.
The signal from the float switch 28 causes the
microcontroller 21 to stop the water pump 8, and to start an air
pump 11. The air pump 11 is connected with the micro-controller
21 through wiring 30. Similarly, the micro-controller starts an
1 0 ozone generator 13 through wiring 31. The air pump 11 pumps air
containing oxygen through a hose 12 into the ozone generator 13.
The ozone generator 13 converts a portion of the oxygen in the air
containing oxygen into ozone. The ozone containing gas then
passes from the ozone generator 13 through a hose 14 and a sparger
1 5 15, causing the ozone containing gas to be dispersed into the
contaminated water 2 located in the ozone contacting chamber 10, in
the form of bubbles 17. Preferably, the bubbles are finely dispersed so
as to provided an extended contact surface with the water and, more
preferably they are microbubbles (i.e. they may have a diameter
2 0 from about 1 to about 250 microns, more preferably from about 1 to
about 50 and most preferably from about 1 to about 5). The ozone
containing gas may be introduced to ozone contacting chamber 10 in
any manner known in the art so as to provide contact between the
water and the ozone, such as passing the ozone containing gas and
2 5 the water counter current to each other (eg. in a packed tower) by
plate contacting techniques, and stirred reaction vessels.
The unreacted ozone is collected and at least a portion
is fed to ozone sensor 19. Preferably, all of the unreacted gas is fed to
ozone sensor 19. In the embodiment of Figure 1, the bubbles pass
3 0 through the contaminated water 2 into head space 32 of the ozone
contacting chamber 10 where they are off-gas 51. The off-gas 51
CA 02288947 1999-11-04
- 14 -
contains ozone which does not react with contaminants in the
contaminated water 2. The off-gas 51 then passes through hose 18
into an ozone sensor 19. After passing through ozone sensor 19
(where remaining ozone is preferably converted into an innocuous
by-product such as oxygen) the air may be discharged, eg. to the
room, by a hose 20. The ozone sensor 19 will be described in detail
later herein. It will be appreciated that a bleed stream of off-gas 51
may be passed to ozone sensor 19 and the remaining off-gas may be
passed, eg., to an ozone destructor prior to being vented from the
1 0 system (not shown).
The microcontroller 21 monitors the ozone sensor as
the basis for determining when the contaminated water has been
sufficiently treated. An advantage of the instant invention is that, if
after a predetermined period of time the required treatment
1 5 conditions are not achieved, the microcontroller 21 may cut off
power to the air pump 11 and the ozone generator 13 and supplies
power to illuminate a light 37 (through wiring 38), indicating that
the water has not been satisfactorily treated and purified. The
contaminated water 2 may then be disposed of by the user, by
2 0 removing a cap 39 from the ozone contact chamber 10, and pouring
the contaminated water 2 out through spout 40.
If the microcontroller 21 receives a signal from the float
switch 28 through wiring 29 that the water level is no longer at the
required level in the ozone contacting chamber 10, the start button
2 5 will cause the microcontroller 21 to turn off the light 26 and to begin
the next water treatment cycle.
The method and apparatus of the instant invention
comprises may advantageously use a prandtl layer turbine as a
device for mixing the readable gas with at least one liquid. Various
3 0 embodiments of prandtl layer turbines have been developed over
the years. Prandtl layer turbines comprise a plurality of rotatably
CA 02288947 1999-11-04
- 15 -
mounted members (generally in the form of flat discs which are
typically relatively thin) which are rotatably mounted in a housing.
These devices are described in the United States Patent No. 1,061,206
(Tesla).
The design described in Tesla may be used as a pump or as a
motor. Such devices take advantage of the properties of a fluid
when in contact with the rotating surface of the discs. If the discs are
driven by the fluid, then as the fluid passes through the housing
between the spaced discs, the movement of the fluid will cause the
1 0 discs to rotate thereby generating power which may be transmitted
via a shaft for use elsewhere. Accordingly, such devices function as
a motor. Conversely, if the fluid in the housing is initially static,
the rotation of the discs will cause the fluid in the housing to
commence rotating in the same direction as the discs thereby
1 5 causing the apparatus to function as a pump, drawing the fluid
through the housing. In this application, all such devices are
referred to herein as a "prandtl layer turbine".
Various designs for prandtl layer turbines have been
developed. These include those disclosed in the United States
2 0 Patent No. 4,402,647 (Effenberger), United States Patent No. 4,218,177
(Robel), United States Re-Issue Patent No. 28,742 (Rafferty et al.),
United States Patent No. 5,470,197 (Cafarelli) and United States
Patent No. 4,655,679 (Giacomel). The method and apparatus of the
instant invention is applicable to all designs of a prandtl layer
2 5 turbine.
Referring to Figure 6, fluid 2 and gas 50 are introduced into a
housing 52, for example, by being drawn through a venturi 54 by
means of a prandtl layer turbine 56. The prandtl layer turbine 56
consists of a series of plates (preferably discs) 58 which are non-
3 0 rotatably mounted to a shaft 60 which is itself rotatably mounted in
housing 52 such as by being connected to a motor 62 which provides
CA 02288947 1999-11-04
- 16 -
the motive force to rotate the plates 58. The rotation of the plates 58
causes the fluid to be drawn through the venturi 54 which in turn
causes a gas 50 to be drawn from the hose 14 into the venturi 54.
As shown in Figure 6, a single fluid stream is combined with
the single gas stream which are fed via venturi 54 into prandtl layer
turbine 56. It will be appreciated that gas 50 may comprise one or
more gases (which may be combined with one or more liquids) and,
similarly, fluid 2 may comprise one or more liquids (which may be
combined with one or more gases). It will further be appreciated
1 0 that the gases and the liquids may be separately introduced into
prandtl layer turbine 56 into prandtl layer turbine 56.
Gas 50 and fluid 2 are preferably mixed prior to their
introduction into prandtl layer turbine 56. More preferably, the gas
50 is preferably mixed with fluid 2 in such a manner as to form
1 5 small gas bubbles 64 in the fluid flow stream. The bubbles may vary
in size from about 50 to about 250 microns in diameter, more
preferably from about 50 to about 100 and, most preferably, 50 to
about ~5w. It will further be appreciated that various other devices
besides venturi 54 may be used to create bubbles 64, such as a
2 0 sparger. By creating a plurality of small gas bubbles 64 which are
introduced into prandtl layer turbine 56, the surface area of gas 50 in
fluid 2 which is introduced into prandtl layer turbine 56 is increased
thereby increasing the dissolution which may be achieved of gas 50
into fluid 2 in prandtl layer turbine 56.
2 5 The gas laden fluid stream 66 is drawn through venturi 54
and into the spaced apart plates 58 such as via openings 68 in plates
58. As the fluid is forced outwards on a radial serpentine path along
the rotating plates 58 the pressure of the fluid increases thereby
increasing the dissolution of the gas 50 into the liquid 4. This
3 0 increase in the pressure of the fluid is possible because, unlike
conventional vane or centrifugal pumps, plates 58 in prandtl layer
CA 02288947 1999-11-04
- 17 -
turbine 56 will not be cavitated by the presence of the gas. The
prandtl layer turbine may create a force of, for example, up to 100
psig and, more preferably up to 250 psig. The fluid with the gas
dissolved therein may be sent to other apparatus for further
processing.
The pressurized liquid mixture 70 is then subjected to a
reduced pressure. For example, the pressurized gas and liquid
mixture 70 may be passed into an expansion zone 72 wherein the
pressure to which the gas and liquid mixture 70 is subjected is
1 0 reduced and preferably rapidly reduced. The liquid/gas mixture in
the expansion zone may be at a pressure of, for example, 30-60 psig.
This depressurization may occur in under 2 seconds, preferably
under 1 second and, most preferably, is effectively instantaneous
upon the liquid/gas mixture entering expansion zone 72. This
1 5 depressurization allows the dissolved gas to come out of solution to
form a suspension of ultra-fine bubbles 17. The bubbles may vary in
size from about 1~, to about 20w in diameter, more preferably from
about 1 micron to about 5 microns and, most preferably, from 1~. to
about 3~. Due to the relatively fine nature of the bubbles, a large
2 0 increase in the surface area of the gas is achieved. If the pressure
reduction is conducted so as to achieve bubbles which are a few
microns in diameter, then the number of bubbles which are
achieved may be sufficiently high such that mixture 70 becomes
translucent and, preferably, opaque. By varying the rate of pressure
2 5 reduction and the amount of the pressure reduction, the size and
the number of the bubbles may be adjusted.
If the reduced pressure mixture is used for water treatment,
then gas 50 may comprise or consists of ozone and fluid 2 includes
or consists essentially of water. In such a case, prandtl layer turbine
3 0 56 and expansion zone 72 may comprise an ozone chamber for
treating (eg. disinfecting) water. It will be appreciated that the
CA 02288947 1999-11-04
.
reduced pressure mixture may be used for various other treatment
applications using a reactable gas. For example, the reduced
pressure mixture may be used for treating another material (in such
a case, fluid 2 may be an inert carrier). If gas 50 was an oxidation
agent (eg. ozone or peroxide), then the reduced pressure mixture
may be fed to a tank containing a material (eg. a chemical
compound such as a pesticide or a herbicide, metal or mineral)
which is to be oxidized.
It will further be appreciated that a catalyst may be added to
1 0 the system. The catalyst may be added to the system with fluid
stream 66 or added separately to the apparatus. For example, the
catalyst may be in the form of a solid, liquid or a gas and accordingly
introduced with either or both of gas 50 or fluid 2. Preferably, the
catalyst is in the form of a liquid or a solid.
1 5 It will be appreciated that if fluid stream 66 is under a
sufficiently great pressure as it enters parental layer turbine 56, that
the fluid may assist motor 62 in rotating discs 58 or, alternately,
turbine 56 may not include a motor 62 and, instead, fluid stream 66
may comprise the necessary motive force to cause plates 58 to rotate.
2 0 Preferably, plates 58 rotate at an rpm from about 3000 to about 8000,
more preferably from about 3000 to about 5000 and, most preferably,
about 4000 rpm.
Referring now to Figures 2 and 3, there is shown an ozone
sensor, referred to generally as reference numeral 100, which may be
2 5 used in the previously described water purification system. The
sensor 100 may be used to monitor the degree of treatment to a
medium such as water, based upon the total amount of ozone
which passes through the medium without being converted to
oxygen. This may be achieved by monitoring the change in
3 0 temperature between two sensors over time.
CA 02288947 1999-11-04
- 19 -
While the sensor 100 will be described herein for use with
the water purification system previously described, it will be
appreciated that the sensor may be used during treatment of various
media other than water. In particular, it may be used in any
application where the degree of treatment of a material with a
reactable fluid, and preferably a reactable gas, may be related to the
concentration of the unreacted fluid upstream of the apparatus
where the treatment is conducted. For example, with reference to
Figure 6, the material to be treated may be the liquid in which the
1 0 reactable gas 50 is dissolved in turbine 56. In such a case expansion
zone 72 may be tank 10. Alternately, the material to be treated is
positioned downstream from expansion zone 12 (eg. in tank 10). In
such a case, the fluid in which the reactable gas is dissolved may be
inert.
Furthermore, while the embodiment of the sensor
described herein relates to detection of ozone, it will also be
appreciated that it may be modified to detect any fluid and,
preferably, any gas which when reacted exhibits a detectable change
in temperature. The following description is based upon the use the
2 0 sensor to monitor the degree of treatment of water with ozone.
The off gasses 51 pass through passage 18 to ozone sensor 19.
The off gasses 51 then pass through sensor 100. In sensor 100, at least
a portion of the unreacted gas is treated to produce a signal which
may then be read by sensor 100. The sensor may be in the form of a
2 5 temperature change in the off-gas stream, or the volume resistivity
of the material, or to emit light. This may be achieved, for example,
by subjecting the unreacted gas to a chemical reaction in a reaction
zone containing a catalyst to produce, for example heat or light.
As shown in Figure 3, the sensor contains a catalyst for
3 0 converting ozone to oxygen. The conversion of ozone into oxygen
may be represented by the following equation (1):
CA 02288947 1999-11-04
- 20 -
(1) 2 03 catal,~~ 3 02 + heat
Thus, the amount of heat released during conversion of
ozone to oxygen is directly related to the amount of ozone
converted to oxygen and may therefore be used as a measure of the
amount of ozone in the off-gas stream.
The sensor 100 includes a vessel 105 in which ozone passing
through the water being treated without being converted to oxygen
1 0 is then converted into oxygen. The vessel 105 may be of any suitable
size, shape or construction, depending upon its designed use. For
example, the vessel 105 may be a thin walled generally hollow
cylinder made from stainless steel, when designed for use in a home
water purification system.
1 5 The vessel 105 has an inlet end 110 and an outlet end 115 to
allow an ozone containing gas to flow through the vessel 105. The
ozone containing gas enters the inlet end 110 of the vessel 105 via
inlet tube 120. The inlet tube 120 fluidly connects the head space
above the water being purified (eg. via passage 18) with the vessel
2 0 105, to allow the flow of off-gas 51 from head space 32 into the
vessel. As with the vessel, the construction of the inlet tube 120
may be of any suitable design, and in one embodiment for use in a
home water purification system, is a stainless steel tube of a
relatively small interior diameter. It will be appreciated that all of
2 5 the off gasses may be passed through sensor 100 so as to essentially
react all of the ozone to oxygen. The amount of heat produced may
be correlated with the amount of unreacted ozone in the off-gas.
Based on the flow rate of gas into the treatment vessel and the
amount of heat produced, this may be used to calculate the total
3 0 amount of ozone to which the material treated was exposed.
CA 02288947 1999-11-04
- 21 -
Alternately, passage 18 may convey only a bleed stream of
the off gasses 51 to sensor 100. Sensor 100 will provide a reading of
the ozone concentration in the head space by measuring the
concentration in the bleed stream and, based on the flow rate into
the treatment vessel and the flow rate of the bleed stream, this may
be used to calculate the total amount of ozone to which the material
treated was exposed.
A first temperature sensor 125 is located in the inlet tube 120,
preferably near the inlet end 110 of the vessel. The first temperature
1 0 sensor measures the temperature of the ozone containing gas
entering the vessel 105. The temperature sensor may be any
standard sensor which is known to those skilled in the art. In a
preferred embodiment, the first temperature sensor 125 is in the
form of a thermistor which measures temperature by measuring the
1 5 electrical resistance across the sensor. As the temperature of the
sensor increases, the resistance of the thermistor decreases. The
temperature of the ozone containing inlet gas is thus measured by
the electrical resistance of the sensor 125.
The vessel 105 contains a catalyst 130 to catalytically convert
2 0 any ozone present in the gas entering the vessel 105 into oxygen.
The catalyst may be any catalyst which efficiently converts ozone gas
to oxygen gas. Preferably, the catalyst is selected from one or more of
manganese dioxide, titanium dioxide, iron oxide, or carbon. Most
preferably, the catalyst is manganese dioxide. Of course, it will be
2 5 appreciated that if the fluid being detected in the vessel is other than
ozone, a catalyst appropriate to that fluid will be selected. The
catalyst 130 may be present in the vessel 105 in any manner, and is
preferably located on the interior surface 135 of the vessel 105. As
previously discussed, heat is released during conversion of the
3 0 ozone into oxygen. Accordingly, as ozone is converted to oxygen by
the catalyst 130, the temperature in the vessel 105 will rise relative
CA 02288947 1999-11-04
- 22 -
to the temperature of the ozone containing gas as it enters the inlet
end 110 of the vessel 105.
A second temperature sensor 135 is located within the vessel
105, to measure the temperature of the gas after conversion of
ozone in the gas to oxygen. The second temperature sensor 135, like
the first temperature sensor 125, may be any known device for
measuring temperature. In the preferred embodiment, the second
temperature sensor 135 is in the form of a thermistor which
measures the electrical resistance across the sensor 135. The second
1 0 temperature sensor 135 may be located anywhere within the catalyst
containing vessel, and is preferably embedded in the catalyst. This
ensures an accurate reading by the second temperature sensor.
However, if the vessel is thin walled or has good thermal
conductivity, a second sensor 137 may be positioned on the outer
1 5 wall of vessel 105. It will be appreciated that the second sensor may
be positioned downstream of vessel 105. In such a case the accuracy
of sensor 100 may decrease due to heat losses of the treated off-gasses
between vessel 105 and the position of the second sensor.
In the alternative embodiment shown in Figure 4, the
2 0 temperature sensor 135 may be in the form of a number of wings
140, positioned in tube 120. In this case, at least a portion of the
surface area of the wings 140 is preferably coated in the catalyst 130 to
define the reaction area. This embodiment may have an increased
surface area so as to produce a more accurate temperature reading.
2 5 Similarly, it will be appreciated that the accuracy of the temperature
sensor 125 may be increased by increasing the corresponding surface
area of the sensing area of the sensors.
After any ozone in the gas entering the vessel 105 at inlet
end 110 has been converted to oxygen, the gas exits the vessel 105
3 0 through the outlet end 115 of the vessel, by means of outlet tube 150.
CA 02288947 1999-11-04
- 23 -
Outlet tube 150 may be of any suitable construction, for example, a
stainless steel tube.
The first and second temperature sensors 125 and 135 may
take continuous readings of the temperature of the gas in the inlet
tube and in the vessel respectively, and transmit temperature
readings to a controller 21 which measures the difference in
temperature between the readings of the first and second
temperature sensors. If the first and second temperature sensors are
in the form of thermistors, then the controller 21 measures the
1 0 difference in potential resistance between the two sensors. It will be
appreciated that this is a measure of the difference in temperature
between the ozone containing gas contacting the first temperature
sensor 125 in the inlet tube 120 and the gas contacting the second
temperature sensor 135 in the vessel 105 after conversion of ozone
1 5 present to oxygen. Since the temperature reading of the reacted gas
stream is corrected for the temperature of the incoming gas stream
by comparing the readings from sensors 125 and 135, the controller
may correlate this information to the amount of ozone which has
been converted to oxygen in the vessel 105 during any particular
2 0 period of time.
If all of the off gases are fed to vessel 105 and if all of the
ozone is converted to oxygen, then in a plot of the instantaneous
difference in resistance between the first and second temperature
sensors versus elapsed time, the total amount of ozone converted to
2 5 oxygen during that time period is represented by the area under the
plotted curve. Figure 5 is a graph showing the difference in
resistance between the first and second temperature sensors plotted
as a function of time for the water treatment apparatus of Figure 1.
The area under the curve represents the total amount of ozone
3 0 which has been converted into oxygen in the vessel 105. It will be
appreciated that controller 21 may correct for sensor 100 converting
CA 02288947 1999-11-04
- 24 -
only a portion of the ozone to oxygen and if only a bleed stream of
the off gasses are fed to sensor 100.
From the foregoing, it will be appreciated that the degree to
which water through which ozone is passed has been treated may be
measured by calculating the area under the curve of a graph of the
change in resistance plotted versus elapsed time. If finely divided
bubbles of ozone are initially passed through contaminated water,
essentially all of the ozone will be used in the treatment process,
and very little if any will escape into the head space above the water.
1 0 As a result, there will be little to no ozone to be converted into
oxygen in the vessel 105, and the difference of the resistance of the
first and second temperature sensors will be essentially zero.
As the water is treated and becomes purer (ie. with less
active biological contaminants present), less ozone will be
1 5 consumed as it passes through the water, and more will enter the
vessel 105, thus causing more ozone to be converted to oxygen in
the vessel. This raises the temperature in the vessel 105, and results
in a greater difference between the resistance of the first temperature
sensor and the second temperature sensor. When this difference in
2 0 resistance between the temperature sensors is plotted against
elapsed time, the area under the resulting curve increases with
time.
By measuring the conversion of ozone to oxygen in vessel
105, great flexibility is obtained in the operation of the treatment
2 5 cycle. For example, as the volume of water and the anticipated level
of contaminants in the water can be predetermined, it is possible to
calculate the amount of ozone that will be required to treat the
water and to program this information into controller 21. The
treatment of the water by ozonation may continue until a
3 0 predetermined total amount of ozone passes through the water
being treated and is then converted to oxygen in the vessel 105. The
CA 02288947 1999-11-04
- 25 -
predetermined amount of ozone is represented by a predefined area
under the curve of the difference in resistance between the two
temperature sensors, versus time.
The controller 21 may optionally have a signal indicating
apparatus (eg. light 41). The signal indicating apparatus may issue a
signal to the user when the water is treated to the desired level (ie.
when the area under the graph reaches a predetermined level) and
is ready for use. The controller 21 also preferably controls the flow
of ozone through the water being treated. When the water has been
1 0 treated to the desired level, the flow of ozone through the water is
stopped (eg. ozone generator 13 may be deactivated).
The signal indicating apparatus may also be used to indicate
when the water being treated should not be used, eg. if it should not
be consumed. For example, the amount of ozone flowing into the
1 5 vessel may not reach the requisite level of treatment within a
predetermined period of time, possibly indicating that the water is
too contaminated and should not be consumed. In such a case, the
signal indicating apparatus may indicate to a user that the water
should not be used, for example by illuminating a warning light 37.
2 0 For example, the controller may be programmed with a
predetermined amount of ozone which should be measured by
sensor 100 (eg. a specified amount of heat must be read by sensor
100) to indicated that the water has been treated to a predetermined
level. The amount of ozone may be based on trial runs in the
2 5 apparatus which evidence the level of ozone in the off-gas for
treating water of a known contaminant level to a specified purity. It
will be appreciated that additional trial runs may be conducted for
varying levels of purity and the controller may be programmed
with each such level and the apparatus may have a dial or the like
3 0 for adjusting the controller to a different program if the user can
determine the level of contamination of the water to be treated.
CA 02288947 1999-11-04
- 26 -
The apparatus may run until the predetermined amount of
ozone is measured. Alternately, or in addition, the apparatus may
shut down if the predetermined level of purity is not achieved in a
predetermined time. This may occur if the amount of ozone
5 converted to oxygen in sensor 100 does not achieve the
predetermined level in the predetermined period of time.
Alternatively, or in addition, if the amount of ozone
flowing into the vessel reaches the requisite level more quickly than
expected, a problem may exist with the dispersion of the ozone
1 0 containing gas throughout the contaminated water. That is, the
ozone may be passing through the water in a localized area (eg. the
sparger may be broken and releasing larger bubbles into the water to
be treated). In such a circumstance, the signal indicating apparatus
may illuminate a warning light, indicating to the user that the
1 5 apparatus should be checked to ensure it is in proper working order.
Finally, if after a predetermined length of time no ozone has
been detected in the vessel, the signal means may also be
programmed to issue an indication to the user to check that the
ozone generator to ensure that it is working properly.
2 0 It will be appreciated that various changes may be made
within the spirit of the described invention, and all such changes
are within the scope of the appended claims. In particular, it will be
appreciated that the method and apparatus described herein may be
modified to detect various gases used to treat a various media.
2 5 Further, different signalling devices may be used to alert the user
instead of the use of lights, or in addition thereto. For example, an
auditory signal may be used to alert the user, if, for example, the
predetermined state of treatment of the material is not achieved in
the predetermined time.
3 0 In one preferred embodiment, the apparatus is for use
in a domestic (i.e. residential) environment, eg. a house or a cottage,
CA 02288947 1999-11-04
- 27 -
and the water to be treated may be from a municipal water supply
which is fed to a house through supply pipes. It may also be water
which is obtained from a well maintained by the individual or any
other source that the individual has for their house or cottage. For
example, the water treatment apparatus may be a countertop water
purifier which is designed to treat small quantities of water (eg. 2
41). The apparatus may also be a point of entry water purifier which
is connected to a domestic water feed pipe to treat all or a portion of
the water which is supplied to a house by the water pipe. The
1 0 apparatus may also be a point of use water purifier which is
connected to a water feed pipe (eg. the cold water feed pipe) to a sink
to treat all or a portion of the water which is supplied by the water
pipe to the sink. In this embodiment, the treated water is preferably
dispensed to the sink through a supplemental faucet which is
1 5 connected in flow communication with the treated water exit of the
water purifier.