Note: Descriptions are shown in the official language in which they were submitted.
CA 02289690 1999-11-15
WO 98154575 PCT/GB98/01520
1
Method for Assessing Wound Healing
The present invention relates to a method for assessing
the healing ;status of a wound in a mammal. In particular it
relates to a method for determining whether healing of a
wound is taking place or is likely to take place by
measuring t:he amount of at least one lymphocyte cell
membrane antigen present in a sample of body fluid or
tissue.
When a wound occurs the upper layer of skin, i.e. the
epidermis, which comprises keratinocytes, is punctured, so
too is the layer below the epidermis, i.e. the dermis which
comprises mainly fibroblast cells and other cell types.
Following wounding, an inflammatory response occurs which
involves lymphocytes migrating to the site of injury, or
"wound bed".
Lymphocyte cell or other cell surface or membrane
antigens have been characterized using monoclonal antibodies
and assigned. a CD (cluster of differentiation) number. For
example, CD:~ is a protein complex associated with the T-
lymphocyte receptor, and CD4 and CD8 are glycoprotein
adhesion mo7_ecules present on T-lymphocytes. CD25 is the
receptor fo:r interleukin-2. CD19 and CD20 are membrane
antigens found on H-lymphocytes.
T-lymphocytes are divided into at least three different
types or subsets; cytotoxic T cells (T~), suppressor T cells
(TS) and helper T cells (TH) . TH cells generally possess the
cell surface: marker CD4 whilst TS cells carry CD8. Several
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
2
of the cell surface markers that are present on T-
lymphocytes have been found in soluble form in, for example,
plasma and serum.
Simon M. et al in J Derm., 23, 305-309, 1996, examined
the expression of the thrombospondin receptor (CD36) by
keratinocytes in acute uninflamed wounds.
Greiling et al developed an in-vitro assay to assess
the role of the glycoprotein CD44, which is expressed by
human dermal fibroblastsin fibroblast migration during wound
repair (see Annual Meeting of 6th International Congress on
Cell Biology, San Francisco, U.S.A., Dec. 7-11 1996.
Molecular Biology of the Cell 7 (Suppl.) 1996.
Mertz, P.M. et al in J. Invest. Dermatol., 98(4), 634,
1992 examined the expression of CD44 by keratinocytes during
burn wound healing.
W097/11369 describes a method for quantifying an
inflammatory response by measuring the cellular infiltration
in a tissue biopsy of a warm-blooded animal.
W096/25670 relates to a cell enumeration immunoassay
for quantising the number of cells in a subpopulation of a
cell sample. The assay is described as an efficient
alternative to flow cytometry.
U.S. Patent No. 5 006 459 is directed to the
measurement of soluble T-lymphocyte cell differentiation
antigens and the use of such measurements in the diagnosis
and therapy of diseases and disorders. In specific
embodiments it describes the measurement of serum or plasma
interleukin-2 receptor levels to detect leukaemia or
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
3
lymphoma, as. well as the measurement of CD8 levels for the
differential diagnosis of renal allograft rejection, as
distinct from Cyclosporin A nephrotoxicity, and of
rheumatoid arthritis, as distinct from other joint diseases.
U. S . Pa.tent No . 5 292 636 is directed to methods' for
the measurement of soluble CD4 antigens, which measurements
can be used to diagnose a state of immune activation, to
diagnose rheumatoid arthritis, to monitor therapeutic
efficacy (e. g. of AIDS treatment) or to stage adult T cell
leukaemia.
U.S. Patent No. 5 426 029 describes the measurement of
soluble leuc:ocyte surface markers, soluble T cell growth
factor receptors, soluble complement receptors, soluble T
cell differentiation antigens and the use of such
measurements in the diagnosis or therapy of diseases and
disorders. In particular it is directed to the measurement
of soluble CD35 which can then be used in the diagnosis of
autoimmune disease such as systemic lupus erythematosus,
rheumatoid arthritis, glomerulonephritis, inflammation,
infectious disease such as AIDS, transplantation, blood
transfusion, haemodialysis, cardiopulmonary bypass thermal
injury, adult respiratory distress, sepsis and barotrauma.
Following tissue injury, an ordered sequence of
cellular events is initiated that in healthy subjects leads
to wound closure. These events can be divided into the
three phases of an initial inflammatory phase, followed by
a second phase of proliferation and a final phase of matrix
formation and remodelling [Clark, R.A.F: Wound Repair:
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
4
Overview and general considerations. In: The Molecular and
Cellular Biology of Wound Repair (RAF Clark, ed), 2nd edn.,
Plenum Press, NY and London, 1996; 3-50]. This process is
complex and many of the events may occur in parallel through
each phase. The acute inflammatory phase is characterised
by a neutrophil infiltrate which is rapidly replaced by
mononuclear cells [Dyson M., Young SR, Pendle CL, et al.
Comparison of the effects of moist and dry conditions on
dermal repair. J. Inv Derm 1988; 91: 435-9] with the
monocyte component maturing to form wound macrophages.
These cells persist through the following phases of healing
and the macrophage, in particular, is considered to play a
key role in regulating the events that lead to successful
wound healing.
Based on data obtained from experimental animal models,
it has been postulated that cells of the immune system, such
as lymphocytes and macrophages play a central role in normal
wound healing. Depletion of T-lymphocytes from normal mice
by in vivo administration of a T-lymphocyte specific
monoclonal antibody impairs the healing of incisional
wounds.
Depletion of the T-helper subset had no inhibitory
effect on healing whilst depletion of the T-suppressor
subset enhanced the healing rate. This evidence suggests
that one role played by T-lymphocytes at the wound site is
a negative one exerted by suppressor cells. This hypothesis
is supported by the observation that CD8' cells present in
non-healing wounds within tumours have been demonstrated to
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
inhibit fibroblast proliferation.
Little direct evidence is available to define the
positive, oz- negative, functional role of leucocytes in
human wound healing. Both T-lymphocytes and macrophages are
5 associated with healing surgical wounds. This leucocyte
infiltration gradually decreases with time after wounding
except in hypertrophic and keloid scars where high numbers
of T-helper cells were observed suggesting that the presence
of this population may generate a positive signal for
fibroblast proliferation.
These data suggest that lymphocytes may play a role in
providing a positive signal for healing to proceed and that
as the healing process progresses toward completion it may
be switched off in part as a consequence of a counter-
regulatory signal provided by suppressor lymphocytes.
Although some work has been carried out to suggest that
T-lymphocytes do play an active role in skin healing (Barbul
A: Immune aspects of wound repair. Clin. Plast. Surg. 17:33-
442, 1990; Adolph VR. DiSanto SK, Bleacher JC, et al. The
potential role of the lymphocyte in fetal wound healing. J
Paediatric Sur 28:1316-20, 1993; Wojciak B, Crossan JF. The
effects of T cells and their products on the in vitro
healing of epitenon cell microwounds. Immunology 83: 83-98,
1994) most lzas been done on animal or in vitro models.
Martin CW and Muir IF: The role of lymphocytes in wound
healing. Br J Plast Surg. 43: 655-62 is the only reference
to present evidence that they may play such a role in human
tissue.
CA 02289690 1999-11-15
WO 98/54575 i'CT/GB98/OI520
6
U.S. Patent No. 5 270 168 describes a method for the
diagnosis of non-healing ulcers in humans by assaying for
certain cell adhesion-related proteins, such as fibronectin
and vitronectin, or their degradation products in ulcer
exudate.
It has now been found that by measuring the amount of
certain lymphocyte cell membrane antigens present in a
sample of body fluid or tissue the healing status of a wound
can be assessed. Furthermore, by monitoring the amount of
the cell membrane antigen over a period of time, the
clinician can determine the healing status of a wound that
is whether the wound is healing or likely to heal, or
whether the wound has become a chronic wound which is not
healing.
According to one aspect of the invention there is
provided a method for assessing the healing status of a
wound in a mammal comprising measuring or detecting the
amount of at least one lymphocyte cell membrane antigen
present in a sample of body fluid or tissue.
Preferably the method comprises measuring the amount of
at least one lymphocyte cell membrane antigen from each of
two different types or subsets of T-lymphocytes and
assessing the healing status of the wound by comparing the
relative proportion of the two types or subsets of T
lymphocytes.
Thus, for example, the method may be used to measure
firstly the proportion of T-suppressor lymphocytes present
in a sample by detecting the amount of CD8 antigen, and
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
7
secondly the proportion of T-helper lymphocytes present in
a sample by detecting the amount of CD4 antigen. The
healing status can then be determined by the ratio of
CD4:CD8.
The sample of body fluid may be blood or serum obtained
by venupuncture. Alternatively, the body fluid may be wound
fluid which is present on the surface of the wound, and
which rnay be sampled by soaking it into an absorbent
material such as filter paper. The wound fluid or exudate
is then eluted from the absorbent material prior to
measuring the amount of lymphocyte cell membrane antigen.
Alternatively, the wound fluid present at the surface of a
wound can be sampled by aspiration with a syringe and needle
or pipette.
The sample of body tissue may be a sample of wound
tissue or tissue peripheral to the wound, which may be
obtained by :biopsy.
In order to identify and quantify the lymphocytic
infiltration of the biopsied tissue, serial sections can be
stained using immunohistochemical staining with a panel of
anti-lymphocytic monoclonal antibodies. The types of
lymphocytes and proportions of subpopulations can then be
compared.
Alternatively, where the sample to be assessed is a
fluid, such as wound fluid, the types of lymphocytes and
proportions of subpopulations may be determined using an
immunological assay such as an enzyme-linked immunosorbent
assay (ELISA). ELISA assays are commercially available for
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
8
soluble lymphocyte receptors including CD4, CD8 and CD25.
An ELISA for the soluble B lymphocyte associated receptor
CD23 is also available. Other tests suitable for
determining the type and proportion of lymphocytes present
S in a fluid sample include radioimmunoassays, precipitin
reactions, gel diffusion reactions, immuno-diffusion assays,
agglutination assays, complement fixation assays,
immunoradiometric assays, fluorescent immunoassays, protein
A immunoassays and immunoelectrophoresis assays.
Thus, in order to determine, for example, the
proportion of soluble CD4 antigen present in a fluid sample,
the sample may be reacted with a first antibody which
specifically binds to CD4 or its degradation products.
After treatment with the first antibody, the sample may be
reacted with a second antibody specifically binding the
first antibody. The second antibody is conjugated to a
label such as an enzyme, peroxidase for example, which forms
a chromophoric product on contact with a substrate for the
enzyme. The chromophoric product can then be quantified by
reading the optical density at the appropriate wavelength.
In one embodiment, the method for assessing the healing
status of a wound in a mammal comprises monitoring
lymphocyte populations within a wound by sampling fluid from
the wound, detecting or measuring soluble CD4 T-helper
lymphocyte antigen in the sample, detecting or measuring
soluble CD8 T-suppressor lymphocyte antigen in the sample,
and determining the presence or absence of healing of the
wound by the relative proportion of CD4:CD8.
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
9
In another embodiment the method for assessing the
healing stat:us of a wound in a mammal comprises monitoring
lymphocyte populations within a wound by obtaining a sample
of tissue from the wound, detecting or measuring the
proportion of T-helper lymphocytes in the sample, detecting
or measuring the proportion of T-suppressor lymphocytes in
the sample, and determining the presence or absence of
healing of the wound by the relative proportion of T-helper
lymphocytes to T-suppressor lymphocytes.
Using t;he method of the present invention it has been
found that early in healing the CD4:CD8 ratio is high, that
is in the range of 3.0 to 8Ø As the wound closes, so the
CD4:CD8 ratio falls within a range of 0.5 to 2.5.
Concomitantly the expression of CD25 antigen increases as
the wound heals. Thus, for example, the amount of CD25 may
be about !3% shortly after wounding, rising to 18%
immediately prior to wound closure.
In another embodiment the method of the invention may
be used to measure or detect the amount of B lymphocytes in
a sample from a wound, by measuring the amount of CD19 or
CD20.
Using this method it has been found that initial levels
of B lymphocytes shortly after surgery are the same as that
found in non-healing chronic wounds. As the wound heals, so
the level of B lymphocytes rises.
In yet ~~ further embodiment the method of the invention
may be used to measure or detect the amount of CD3 or CD25
lymphocyte cell membrane antigen.
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
Using the method of the invention it has been found
that:
1. a high CD4:CD8 ratio occurs early in wound healing.
2. a low CD4:CD8 ratio occurs in non-healing chronic
5 wounds.
3. the CD4:CD8 ratio is elevated prior to healing of a
previously non-healing wound.
4. high amounts of CD25 occur in healing wounds prior to
closure.
10 5. low amounts of CD25 occur in non-healing chronic
wounds.
6. high amounts of B lymphocytes occur in healing wounds
prior to closure.
7. low amounts of B lymphocytes in non-healing chronic
wounds.
According to a further aspect of the invention there is
provided a wound healing assessment kit for carrying out the
method of the invention which comprises a first antibody
that binds to a lymphocyte cell membrane antigen and a
labelled antibody that binds to the first antibody.
Preferably the kit also contains a second antibody that
binds to a different lymphocyte cell membrane antigen and a
labelled antibody that binds to the second antibody.
The first antibody in the kit binds to the CD4 cell
membrane antigen from T-helper lymphocytes, whilst the
second antibody in the kit binds to the CD8 cell membrane
antigen from T-supressor lymphocytes.
The method of the invention will aid in the assessment
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
11
of the effect of wound treatment, both experimental and
routine, and. in the assessment of patients at presentation.
Using this method allows the clinician to obtain an early
indication of healing which will then assist them to
evaluate different wound dressings and select the best~type
to promote a.nd achieve complete healing.
Description of the drawings
Figure 1 shows the proportion of B lymphocytes in chronic
and acute wounds.
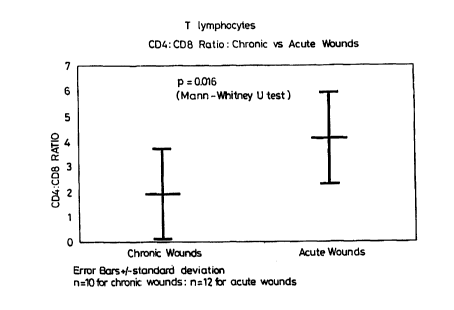
Figure 2 shows the decline in CD4:CD8 ratio in an acute
wound as it heals.
Figure 3 shows the ratio of CD4:CD8 in chronic and acute
wounds.
The invention will be described further with reference
to the following examples.
Example 1
Study of T-lymphocytes within chronic lea ulcers
Materials anal Methods
A 6mm punch biopsy was taken under local anaesthesia
from the margin of each patient's leg ulcer. Twelve
patients were selected with chronic leg ulcers due to venous
disease or whose general condition, immobility and leg
oedema prevented healing. These wounds had been present for
a minimum of 6 months and clinical records showed no
evidence of healing occurring in the 6 weeks prior to
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
12
biopsy.
Biopsies were snap frozen in liquid nitrogen and 6~e
cryostat sections mounted on poly-L-lysine treated
microscope slides. Slides were stored desiccated at -20°C
for up to 14 days prior to staining. Serial sections. were
fixed in dry acetone, washed in phosphate buffered saline
(PBS) 3 times and incubated in optimal dilutions of
monoclonal antibody (MAb) for 30 minutes. The antibody
panel, with antigen specificity and working dilutions in
parentheses, was applied to serial sections in the following
order: anti-CD45 (all leucocytes, 1:20), anti-CD19 (B-
lymphocytes, 1:25), anti-CD3 (T-lymphocytes, 1:50), anti-CD4
(T-helper/inducer lymphocytes, 1:10), anti-CD8 (T-
suppressor/cytotoxic lymphocytes, 1:30), anti-CD25
(interleukin-2 receptor expressed on activated T-lymphocytes
and macrophages, 1:20), anti-CD45RA (suppressor/inducer T-
lymphocytes and B-lymphocytes, 1:20), anti-CD45R0 (Memory T-
lymphocytes, 1:50), anti-CD68 (macrophages, monocytes,
1:40), anti-CD14 (LPS receptor on macrophages, monocytes and
neutrophils, 1:30), anti-CD16 (Fc~ylll receptor on activated
macrophages, neutrophils and NK cells, 1:30), anti-CD35 (C3b
receptor on macrophages, monocytes, B-lymphocytes and
neutrophils, 1:30) and anti-HLA Class 11 antigen (1:100).
They were then washed 3 times in PBS and antibody
localisation identified by a standard streptavidin-biotin
peroxidase technique (Vector Laboratories, Peterborough, UK)
with final reaction product developed using 3,3'-
diaminobenzidine (DAB). The sections were counterstained
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
13
with Ehrlich's haemotoxylin, dehydrated, cleared and mounted
in DPX mounting medium. Positive staining was seen as a
brown-black deposit and non-stained cells could be clearly
distinguished as blue counterstained nucleated cells with no
associated :brown DAB stain.
All monoclonal antibodies (MAbs) used were obtained
from Dako Lt: d, High Wycombe, UK except for 2H4 (anti-CD45RA)
which was obtained from Coulter Immunology Division,
Hialeah, F1., USA.
For each biopsy the area underlying the epidermis
adjacent to the wound margin was identified. Commencing at
the epidermal tip and moving distally to the wound using a
x20 objective six adjacent fields were counted in the
papillary dermis along with the six underlying fields
immediately below. This counting method included the area
of dermis at the wound margin that contained the highest
leucocyte density demonstrated by anti-CD45 staining. The
numbers of positive stained cells were counted per field and
the average number of stained cells/field calculated.
Manual counting with a microscope eyepiece grid was used as
it was not possible to enumerate the stained cells using a
computerised image analysis system (IBAS) because of the
asymmetric distribution of stained cells and the close
approximation of stained cell membranes within aggregates
particularly in the perivascular regions.
Positive cells were enumerated in the same way in
corresponding areas of each subsequent serial section
stained with the MAb panel. The CD4:CD8 ratio was
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
14
calculated by dividing the average number of CD4' cells/field
by the average number of CD8' cells/field and expression of
other antigens by use of the following formulae;
i) ~CD25' T-lymphocytes - CD25' cells/field x 100;
CD3' cells/field
ii) ~B-lymphocytes - CD19' cells/field x 100
(CD3'cells/field) + (CD19'cells/field)
iii} ~CD16(35) macrophages = CD16(35)' cells/field x 100;
CD68' cells/field
RESULTS
With respect to leucocytes infiltrating the wound
margin sections stained with the pan leucocyte marker CD45
allowed the tissue to be divided into four areas. These
were (i) the epidermis which by comparison to normal skin
was thickened both at the immediate wound edge and also in
adjacent areas distal to the wound, (ii) an area including
the papillary dermis and the upper region of the reticular
dermis which could be delineated by the high numbers of
blood vessels and associated leucocytes close to the wound
margin, (iii) the reticular dermis distal to the wound
margin characterised by the absence or low density of blood
vessels and few leucocytes and (iv) associated wound bed
tissue.
Some of the antigens under study, CD4, CD25 and CD45R0,
may be expressed by macrophages. Staining for these
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
antigens appeared either as a membrane type staining pattern
on cells of a small round morphology which were assumed to
be lymphocytes or as a cytoplasmic more diffuse staining
pattern where the antigen was expressed on larger cells of
5 asymmetric nnorphology. The latter staining pattern. was
identical to that found in sections stained for the
macrophage associated CD68 antigen and these cells were
therefore considered to be macrophages. These two
morphologies could be distinguished microscopically and only
10 those cells exhibiting a lymphocytic morphology were
enumerated for expression of CD4, CD25 and CD45R0.
Leucocyte populations present within the infiltrate
adjacent to the wound margin were enumerated. Cells of a
macrophage morphology were distributed in the intervascular
15 areas of the papillary dermis and throughout the reticular
dermis in close approximation to non-stained cells of
fibroblast morphology.
Cells of lymphocytic morphology, small round cells with
little cytoplasm and a typically round nucleus, were
identified in greatest numbers in perivascular areas.
Additional CD45' cells were identified throughout the
epidermis adjacent and distal to the wound margin. These
cells had a dendritic morphology and were CD3-/CD68-/HLA
Class 11' indicating that they were typical Langerhans cellslo
[Weber-Matthiesen K, & Wolfram S. Organisation of the
Monocyte/Macrophage system of normal human skin. J Invest
Dermatol 1990; 95: 83-B9]. Significant numbers of CD45'
cells were also identified within the wound bed tissue but
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
16
it was not possible to enumerate these with any degree of
accuracy because of non cell associated background staining
in this area.
The wound margin leucocyte population was comprised
essentially of CD3' T-lymphocytes and CD68' monocytes and
macrophages.
Lymphocyte Antigen Expression
The perivascular infiltrate in the biopsies examined
contained a significant number of CD3' T-lymphocytes. Few
CD3' cells were found in tissue remote from vessels and only
isolated CD3' cells were observed within the epidermis.
In the majority of chronic wounds tested the ratio of
CD4':CD8' lymphocytes was within the range 0.5 to 2.2 (mean
- 1.5~0.6)(Table 1).
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
17
TABLE 1 Lytnphocyte antigen expression at the margin of
Chronic Wounds
Patient No. %8-lymphocytesCD4:CD8 %CD25' T Lymphocytes
1 0 1.7 6
2 0 NT 0
5 2.2 2
4 4 1.8 5
5 0 1.4 NT
6 6 0.5 2
7 0 1.6 5
8 2 2.0 6
9 0 0.7 7
NT - Not tested
Stained cells were counted in serial sections in the
same area of each section adjacent to the wound margin
approximating to the area of leucocyte infiltrate. Total
CD68' macrophages were counted followed by cells expressing
each antigen in subsequent sections. The data shown
indicates the percentage of total macrophages expressing
each individual antigen.
To characterise the T-lymphocyte population further
their expression of CD45RA and CD45R0 antigen was also
determined. To eliminate interference by CD45RA expressing
B-lymphocyteFi only biopsies which contained Lew (<4~) B-
lymphocytes were evaluated for expression of CD45 antigen
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
18
isotypes. CD45R0' T-lymphocytes predominated in the 4
biopsies examined (Table 2) with the proportion varying from
62~ to 94~. The majority of positive cells were located
within the T-lymphocyte populations in the perivascular
areas of the biopsies.
TABLE 2 Expression of CD45RA and CD45R0 by T-lymphocvtes at
."~,-.-.;,-, of r-hrnni c~ wounds
Patient No. %CD3+ loCD45RA' %CD45R0'
4 g6 26 43
1 9 98 8 94
o
11 gg 14 92
12 100 5 81
NT - Not tested
Stained cells were counted in serial sections in the
same area of each section adjacent to the wound margin
approximating to the area of leucocyte infiltrate. Total
lymphocyte counts were derived by summation of the counts of
CD3' T lymphocytes and CD19' B lymphocytes in adjacent
sections. B lymphocyte data are expressed as a percentage
of this total lymphocyte count. CD25' lymphocytes are
expressed as a percentage of the total CD3' lymphocyte count
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
19
only.
Macrophage antigen expression
CD68' cells were distributed throughout the entire
dermis with particularly dense aggregations of positive
cells in the: perivascular areas. These compromised either
smaller round cells, presumably recently emigrated
monocytes, and larger elongated cells which formed a
perivascular sheath. CD68' positive macrophages were also
identified within the intervascular areas of the dermis.
Processes extending from these cells interdigitated with
non-stained cells.
In all biopsies evaluated the expression of CD16 and
CD35 antigen was essentially identical with both being
elevated in 4/10 wounds tested and the remaining wounds
demonstrating little expression (<12~ of cells
positive)(Table 3). In all the biopsies except one (Patient
10) the greatest number of cells stained for these antigens
was restricted to those cells within the perivascular areas .
In the one biopsy (Patient 10) where the majority of
macrophages were positive for these two antigens clear
staining of cells throughout the dermis was observed
although stronger staining was observed in perivascular
areas.
In contrast to CD16 and CD35, CD14 antigen expression
was more uniformly distributed throughout the dermis.
Significant numbers of CD 14' cells were identified within
all biopsies and in only one biopsy (Patient 1) did markedly
less than 50~~ of macrophages express the CD14 antigen.
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
TABLE 3 Macrophage antigen expression at the mar4in of
chronic wounds
Patient No. CD16+ CD35' CD14'
as 96 of CD68'
cells
1 3 0 34
5 2 4 0 67
3 5 8 49
4 7 12 49
5 9 7 71
6 12 0 60
10 7 20 32 70
8 35 32 54
9 40 39 NT
10 97 79 58
NT - Not tested
15 Stained cells were counted in serial sections in the
same area of each section adjacent to the wound margin
approximating to the area of leucocyte infiltrate. Total
CD68' macrophages were counted followed by cells expressing
each antigen in subsequent sections. The data shown
20 indicates the percentage of total macrophages expressing
each individual antigen.
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
21
Example 2
Studv of change in lymphocyte subpopulations associated with
human wound healin4
Materials and Methods
Biopsies
6mm punch biopsies were obtained under 1% lignocaine
local anaestlnetic from the edge of surgically excised human
pilonidal sinus excision sites. Rates of healing of the
wounds were evaluated using the method of Marks J, Hughs LE
& Harding KG. Prediction of healing time as an aid to the
management o:E open granulating wounds. World J Surgery 7:
64I-645 (1983) Serial biopsies were taken from twelve
patients at day 0 (within 5 days of surgery) day 7, day 21,
and day 42 if the wound had not healed. In comparison,
single biopsies were taken from the wound edge of 10 chronic
venous leg u~'.cers .
Processina oi: biopsies
Biopsies were snap frozen in liquid nitrogen and 6~m
cryostat sections mounted on poly-L-lysine treated
microscope slides. Slides were stored desiccated at -20°C
for up to 14 days prior to staining. Serial sections were
fixed in dry acetone, washed in phosphate buffered saline
(PBS) 3 times and incubated with monoclonal antibody (MAB)
(Dako Ltd, High Wycombe, UK) for 30 minutes. The antibody
panel and specificities are described in Table 3 and were
applied to serial sections in the order shown. They were
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
22
then washed 3 times in PBS and antibody localisation
identified by a standard streptavidin-biotin peroxidase
technique (Vector Laboratories, Peterborough, UK) with final
reaction product developed using 3,3'-diaminobenzidine
(DAB). The sections were counterstained with Ehrlich's
Haematoxylin, dehydrated, cleared and mounted in DPX
mounting medium.
Identification and interpretation of lymt~hocytic infiltrate
The lymphocytic infiltrate of all biopsies was quantified by
counting X40 magnification views. Significant numbers of
positively staining cells were noted within the wound bed,
but it was impossible to quantify these accurately because
of the great amount of background staining and particulate
manner which also stained in this region. The leucocyte
infiltrate was therefore examined by counting numbers of
positive cells in an orderly manner consisting of 6 fields
commencing directly under the migrating epithelial tip and
progressing distally in both upper and lower dermis
achieving 12 fields in total for each biopsy. Subpopulations
are expressed in the following way: B-lymphocytes as
proportion of total lymphocytes (B and T lymphocytes),
T-lymphocytes as proportion of total lymphocytes, CD27' as
~ of CD3' T lymphocytes, CD25' as ~ of CD3' T lymphocytes,
and CD4':CDS' T lymphocytes are expressed as a ratio (Table
2).
RESULTS
Healing of pilonidal sinus excisional wounds progressed
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
23
over a pe~_-iod of seven weeks. CD45 staining, which
identified all leucocytes was generally concentrated under
the migrating epithelial tip and towards the wound edge.
Cells of lymphocytic morphology, small round cells with
little cytoplasm and a typically round nucleus, were found
preferentially in the perivascular areas. Macrophages were
found diffusely distributed within the dermis with no
distinct pattern of organisation.Further analysis of the
lymphocyte population with subset specific MAbs showed
statistically significant changes as healing of the wounds
progressed.
B Lymphoc r~te~s
B-lymphocytes were identified in greatest numbers at
the wound edge, but were also congregated in small clusters
within the upper and lower dermis. There was a consistently
low proportion of B lymphocytes of 2.9(~1.3)% in chronic
wounds. In the acute wounds, there was a significant rise
in the proportion of CD19' cells as healing progressed from
3.7 (~I.2) % t:o 17.7 (~4.1) % at day 7, 21. 1 (~4 .0) % at day 21,
and 27.2 (~4.2) % at day 42. The difference between day 0 and
day 7 was significant to a level of p=0.016, and the
difference between day 0 and biopsies taken on days 21 and
42 significant to levels of p=0.0014 and p<0.001
respectively (Fig 1). To confirm that the cells identified
as CD19' were B lymphocytes, sections were stained Lor the
separate B :Lymphocyte associated antigen CD20. Expression
of CD20 correlated well with CD19 staining, both spatially
CA 02289690 1999-11-15
WO 98!54575 PCT/GB98/01520
24
and numerically (r~=0.94, P<O.OOl,n=17).
T Lvmphocytes:
As with B lymphocytes, T lymphocytes were identified in
their greatest numbers directly at the wound edge, but
preferentially in the perivascular areas.
CD4:CD8 ratio:
In acute wounds, the CD4:CD8 ratio initially observed within
the T lymphocyte population at the wound margin was
4.0(~0.5). As healing progressed, the CD4:CD8 ratio
significantly decreased so that prior to wound closure it
was 1.3(~0.7-1.9) (p<0.01) (Fig 2). This was a result of an
increase in the absolute numbers of CD8' lymphocytes from
6.7(~0.9) to 11.6(~1.7) per/field (p=0.041), and a decrease
in the number of CD4' lymphocytes from 22.6(~3.2) to
16.15(~2.5) per/field (p=0.045). When data for all biopsies
taken throughout the healing process was pooled, there was
an overall ratio of 3.0(~0.3). In chronic wounds there was
a consistently low CD4:CD8 ratio of 1.9(~0.6)(fig. 3)). This
was significantly lower than that of the day 0 acute wounds
(p=0.016) .
CD27' & CD25' T lymphocytes
The lymphocyte associated expression of the CD25 antigen
(Interleukin-2 [IL-2] receptor) was generally lower than
CD27. However, expression of both antigens increased as
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
normal healing progressed. Numbers of CD25' cells increased
from 9.4(~1.0)% of T lymphocytes at day 0 to 17.9(~3.5)% at
day 42 (p=0.056). The corresponding increase in numbers of
CD27' lymphocytes was from 26.2 (~4.0) % to 46. 9 (~6.0)
S (p=0.046).
Conclusion
A high CD4:CD8 was found in the initial stages of wound
healing whi~~h declines as healing progresses. Such a
decline is the result of both a significant rise in the
10 number of T~; lymphocytes and a decline in the number of Th
lymphocytes. This proliferation of CD8" Ts lymphocytes is
consistent with an increase in expression of CD25 and CD27,
which are markers of lymphocyte activation and
proliferation. The low levels of potentially
15 'downregulatory' Ts cells in the initial stages of healing
may therefore represent the initial attempt of the wounded
tissue to close the acute defect . The high levels in the
final stages of normal healing may contribute to the
'switching off' of this process. This would also be
20 consistent with the findings in chronic wound tissue: where
a consistently low CD4:CD8 ratio is seen in the non-healing
wounds, reflecting high levels of these 'downregulatory' Ts
lymphocytes, whose presence may contribute to the chronicity
of the wound.
25 It was a surprising finding that CD19" B-lymphocytes
comprised a large proportion of the lymphocytic infiltrate
of normally healing wounds. In addition, they were
significantly more numerous in acute wounds when compared to
CA 02289690 1999-11-15
WO 98/54575 PCT1GB98/01520
26
those which were chronic and non-healing.
TABLE 3 Immunocytochemistry Primary Monoclonal Antibody
Panel
Antigen: Cellular Distribution:
CD45 Allleucocytes
CD19 B lymphocytes
CD20 B lymphocytes
CD3 T lymphocytes
CD4 T hefperlinducer lymphocytes
l0 CD8 T suppressorfcytotoxic lymphocytes
CD25 Activated T lymphocyteslmacrophages
(Interleukin-2 receptorl
CD27 Activated T lymphocytes
CD68 Macrophages. monocytes
Example 3
Using the method described in Example 1 biopsies were
taken from patients with venous leg ulcers and the CD4:CD8
ratios and CD25 levels were determined using the method
described in Example 1.
RESULTS
Table 4 shows the CD4:CD8 ratios and CD25 levels
CA 02289690 1999-11-15
WO 98/54575 YCT/GB98/01520
27
detected, together with the observed healing status of the
ulcers.
ri,TnT Z, ,, r.r,~l .r~r,ii ,-nr; na anr3 rn7~, 1 PVE!~ s and healincr status
Patient CD4:CD8 CD25 Status
A 5.7 19.4 Healed
B 7.4 17,9 Healed
C 6.1 NT Healed
D 3.7 t 7.1 Healed
E 1.1 10.5 No Improvement
1 F 2.6 13.8 No improvement
o
G 1.3 11.3 No Improvement
H 1.3 12.9 No Improvement
I 1,g NT No Improvement
i
1,6 5,1 No Improvement
I
Example 4
Detection of CD4 and CDS in Chronic Wound Fluid
Method
Wound fluid was collected from non-healing chronic
wounds by absorption into sterile chromatography paper.
Proteins were eluted from the chromatography paper into
sterile saline and the saline analysed.
CA 02289690 1999-11-15
WO 98/54575 PCT/GB98/01520
28
The levels of CD4 and CD8 were determined using
Cellf ree (RTM) ELISA kits obtained from T Cell Diagnostics.
The ELISAs were performed in duplicate with a full
calibration curve.
S Results
Of B samples collected free CD4 could be detected in 6
out of 7 samples and CD8 in 4 out of 8 samples as shown in
Table 5 below.
DISCUSSION
These analyses indicate that it is possible to detect
the presence of soluble CD4 and CD8 but not CD23 within
chronic wound fluid. This is consistent with our
immunohistological analysis of non-healing chronic wounds in
that CD4' and CD8'T lymphocytes are present in wound tissue
but that in the majority of wounds analysed B-lymphocytes,
the source of soluble CD23 antigen, are absent.
A wide range of values for CD4 and CD8 were obtained
but these were not crrelated to clinical course because they
were single time point samples. However the objective of
the study was to determine whether detectable levels of
lymphocyte derived antigen were present within wound fluid
and this has been confirmed.
CA 02289690 1999-11-15
. - r. . , .. ,.
n n. .. ,
r : r - ,
29
TABLE 5: CD4 and CD8 Detection
Sample Protein Activity Activity
No. Conc mg/ml ilnits/mg/ml Units/mg/ml
1 1 ..5 55 p
2 21.5 20 75
3 18.5 15 <detection level
4 22 0 100
i
5 12 100
6 1:5 17.5 p
7 15 15 0
8 1 5 15 100
AM~MD~D SHEET