Note: Descriptions are shown in the official language in which they were submitted.
CA 02291352 1999-12-O1
-1-
Case No.:
Pat. Appln.
D~ETHOD Oh PROVIDING COSD~ETIC / HHDICAL T8$R118Y
Field of the Iaveation
The present invention relates generally to cosmetic/
medical therapy and more particularly to a method of
io electrically preparing a substance with therapeutic qualities.
Backcround of the Invention
The use of transcutaneous electrotherapy to provide
therapy is well known. Transcutaneous electrotherapy involves
the passage of an electrical current from one electrode to
another, such that the therapeutic current is caused to pass
directly through a target tissue of the patient. Exemplary
devices used in the performance of transcutaneous
2o electrotherapy are provided in United States Patent Nos.
397,474; 3,794,022; 4,180,079; 4,446,870; 5,058,605; in French
Patent 2621-827-A; and European Patent Application EP-377-057-
A.
Although the use of transcutaneous electrotherapy has
proven beneficial for some conditions, primarily for use on
broken bones, such therapy suffers from inherent disadvantages.
For example, during transcutaneous electrotherapy, electrical
current passes through the target tissue of the patient. Many
patients may find this undesirable. The current tends to
3o concentrate where the electrodes are, making even distribution
difficult. The amount of current that can be and is in fact
generally used is quite low. Further, transcutaneous
electrotherapy is generally not self-administrable, and
therefore generally requires the presence of a skilled
CA 02291352 1999-12-O1
-2-
operator. The administration of transcutaneous electrotherapy
also tends to be costly, and generally does not offer a
technique for the improvement of the cosmetic appearance of
skin and soft tissue.
Other cosmetic procedures include such processes as
surgical cut and lift operations, laser treatments,
dermabrasion and chemical burns. These techniques have various
disadvantages, such as cost, complexity, requiring trained
operators or medical personnel, scarring, limited
so effectiveness, ect.
In view of the foregoing, it is desirable to provide an
effective alternative to transcutaneous electrotherapy wherein
electric current is not caused to flow through the treated
tissue of the patient, which is self-administrable, addresses
cosmetic conditions such as wrinkling of the skin as well as
medical conditions, and is comparatively easy to use.
~ummarv of the Iaveatfoa
2o The present invention specifically addresses and
alleviates the above-mentioned deficiencies associated with the
prior art. More particularly, the present invention comprises
a method with a different approach for providing
cosmetic/medical therapy, which is simple to administer, highly
effective, and relatively low in cost. The steps include a
process of preparing an electrically active substance which has
unique benefits when administered to a subject. Administering
the electrically activated substance to the subject provides a
cosmetic/medical benefit to the subject.
3o Although, the subject as described herein, is a human
being, the methodology of the present invention may be
practiced upon various different animals with similar results.
Thus, the use of a human being in this application is by way
of example only and not by way of limitation.
CA 02291352 1999-12-O1
-3-
The electrically activated substance is applied topically
to skin to treat skin problems such as wrinkles, cuts,
abrasions, etc. The electrically activated substance may also
be ingested, so as to treat internal medical conditions.
According to the preferred embodiment of the present
invention, the electrically activated substance is formed of a
water base, preferably distilled water. However, other
substances, particularly those comprised of simple molecules,
may likewise be utilized.
1o Certain parameters must be adhered to in order to obtain
the qualities required for effectiveness.
One step of preparing the electrically activated substance
comprises applying a particular electrical signal to the
substance. According to the preferred embodiment of the
present invention, an alternating current signal, preferably
having a generally symmetric waveform, is utilized. Thus, for
example, a sinusoidal waveform, a square waveform, or a
triangular waveform are suitable. Those skilled in electrical
arts will appreciate that various other generally symmetric
2o waveforms are likewise provide alternating current.
The electrical signal preferably comprises an alternating
current signal having a particular frequency of between
approximately 5 to 10 KHz and approximately 1 MHz, and
optimally between approximately 25 KHz and approximately 100
KHz. According to the preferred embodiment of the present
invention, the frequency of the electrical signal may also be
varied within a frequency range of approximately 50 KHz to 100
KHz.
Preferably, the alternating current has approximately zero
3o direct current bias. In order to mitigate direct current bias,
the electrical signal is preferably applied to the substance
via a capacitor-resistor network. Alternatively, the
electrical signal is applied to the substance via an isolation
transformer.
CA 02291352 1999-12-O1
-4-
The electrical signal preferably has a voltage of between
approximately 50 volts rms and approximately 150 volts rms.
The electrical signal is applied to the substance to be
electrically activated via at least one pair of electrodes. As
those skilled in the art will appreciate, a plurality of pairs
of electrodes may be utilized, if desired. The electrodes are
preferably comprised of either a biologically inert, non-
reactive metal or a non-metallic material having a low atomic
number. For example, it has been found that gold, carbon 6,
1o and graphite-carbon loaded thermo-plastic material are
suitable.
When distilled water is to be electrically activated, then
a substance is added to the water to so as to form an
electrolyte therefrom. This increases the conductivity of the
water so as to facilitate adequate current flow therethrough.
According to the preferred embodiment of the present invention,
sodium chloride or potassium is utilized to form the
electrolyte.
According to the preferred embodiment of the present
2o invention, the substance, e.g., sodium chloride, is added to
the distilled water while monitoring current flow therethrough,
until the desired current is obtained.
According to a preferred embodiment of the present
invention, approximately 1000 milli-amps (1 amp) rms of current
is caused to flow through the substance being electrically
activated. Typically, a voltage of approximately 100 volts rms
is required to effect a current of 1 amp rms. It has been
found that currents as low as 1 milliamp may be used with
limited results. Preferably, at least 10 milliwatts of power
3o per milliliter of substance as utilized. The electrodes should
be shielded from accidental operator contact.
The electrodes preferably have a resistance below 500 ohms
per square centimeter, preferably below 50 ohms per square
centimeter.
CA 02291352 1999-12-O1
-5-
When fully prepared, the substance takes on new physical
properties. These properties exhibit stimulative effects when
applied to biological tissue.
v~lhen administered topically, approximately 0.05 ml of the
electrically activated substance is applied per square
centimeter of treatment area. The electrically activated
substance is preferably administered 3 to 6 times with
approximately 1 to 4 days between administrations.
Such topical application of the electrically activated
so substance of the present invention has been found to be
effective in mitigating wrinkles on human skin.
When taken orally, approximately 2 ml of the electrically
activated substance is preferably ingested per day for
approximately 6 weeks.
i5 These, as well as other advantages of the present
invention will be more apparent from the following description
and drawings. It is understood that changes in the specific
structure shown and described may be made within the scope of
the claims without departing from the spirit of the invention.
Brief Descrintioa of the DrawiaQs
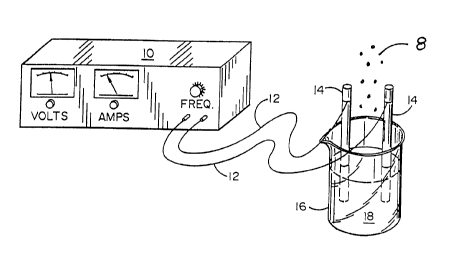
Figure 1 is a perspective view showing a variable
frequency current source connected to liquid contained within a
beaker;
Figure 2 is a block diagram showing a first alternative
configuration of the apparatus of Figure 1;
Figure 3 is a block diagram showing a second alternative
configuration of the apparatus of Figure 1;
Figure 4 is a flow chart showing the steps involved in the
practice of the method for forming an electrically charged
substance, according to the present invention.
Figure 5 illustrates a waveform provided by the current
source of Figure 1;
CA 02291352 1999-12-O1
-6-
Figures 6a illustrates the electrically activated
substance being applied to a recipient;
Figure 6b illustrates the recipient after topical
application of the substance to treat facial wrinkles;
Figure 7a illustrates a recipient ingesting the substance;
Figure 7b illustrates the recipient after ingesting the
substance;
Figures 8a and 8b show a human recipient before and after
being dabbed with the electrically charged substance;
1o Figure 9 shows the electrically charged substance being
dabbed onto an animal; and
Figures 10 and 11 illustrate capillaries and blood vessels
in a cross section cutaway of the skin of the recipient.
s5 Detaile8 Descrintioa of the preferred 8mbodimeat
The electrically activated substance and method for making
the same of the present invention are illustrated in Figures 1
5 of the drawings which depict a presently preferred embodiment
2o thereof.
Referring now to Figure 1, according to the present
invention a variable current source 10 is electrically
connected, via wires 12 to probes or electrodes 14 which are at
least partially immersed within the substance 18 to be
25 electrically activated, which is contained within beaker 16.
The variable frequency current source 10 preferably
comprises the variable frequency current source having an
output frequency range from approximately 10 KHz to
approximately 1 MHz, and a voltage output from approximately 50
3o volts rms to 150 volts rms, and a maximum current output in
excess of 1 amp rms.
According to the preferred embodiment of the present
invention, the variable frequency current source 10 provides an
alternating current output waveform having substantially 0
CA 02291352 1999-12-O1
direct current bias.
The variable frequency current source 10 is capable of
providing a generally symmetric waveform, with low or zero DC
bias. This is illustrated in Figure 5. The centerline baseline
38 of Figure 5 represents time. It also indicates zero (o)
current flow between the electrodes. The (+) area 40 indicates
current flowing in one direction between the electrodes. The (-
area 42 indicates current flowing in the opposite direction
between electrodes. As shown the current flow between the
1o electrodes reverses direction periodically. The sum of both
portions of the waveform are preferably zero. Various other
generally symmetrical alternating current waveforms could
likewise also be used, such as square, triangular, or other odd
shaped waveforms that reverse the direction of current flow.
According to the preferred embodiment of the present
invention, the frequency output of the variable frequency
current source 10 is capable of being swept or automatically
varied between a minimum and maximum frequency. Alternatively,
the variable frequency current source 10 is capable of being
2o manually swept in frequency.
The wires 12 preferably comprise copper wires having a
current rating sufficient to carry the required current, e.g.,
1 amp rms, without excessive heating.
Each electrode 14 is preferably comprised of a chemically
and biologically inert material, preferably a non-reactive
metal such as gold, or alternatively, a non-metal having a low
atomic number. It is believed that it is not desirable to have
ions form from the substance of the electrodes. For example,
if lead electrodes are utilized, it has been found that lead
3o ions permeate the substance being electrically activated,
potentially resulting in undesirable contamination of the
medium and potential poisoning of the patient to whom the
electrically activated substance is administered. Although
aluminum and copper electrodes may be suitable in some
CA 02291352 1999-12-O1
_8-
applications, they are generally thought to be undesirable.
Carbon has been found to be suitable for the construction
of electrodes according to the present invention. Carbon
electrodes may be formed utilizing contemporary plastic
injection molding techniques wherein a graphite-carbon loaded
thermo-plastic material, such as nylon, is injected into the
molds, or with other processes.
Typical dimensions for the electrodes 14 are 3 mm thick,
20 mm wide, and 10 cm long. However, various different
1o dimensions and cross-sectional configurations, e.g., round,
oval, square, triangular, etc., are likewise useable. Large
flat electrodes with a large surface area generally work best.
According to the preferred embodiment of the present
invention, the electrical resistance of the finished electrodes
is preferably less than 500 ohms/cmz, preferably less than 50
ohms / cmz .
In one embodiment, the two electrodes are positioned
several centimeters apart in a 250 ml container, e.g., a
beaker. The container is formed of a non-metallic material,
2o such as glass. Thus, as described herein, the method for
electrically activating a substance is preferably practiced
utilizing approximately 200 ml of the substance at a time.
Preferably, current flow through the substance being
electrically conditioned 18 is monitored as an electrolytic
substance Figure 1, 8 is added thereto so as to form an
electrolyte. The electrolytic substance added improves the
flow of current through the substance, so that activation can
occur. As the sodium chloride in either crystal or liquid form
is added to the water, current flow through the water may be
3o monitored until sufficient current flow is achieved, thereby
indicating the proper level of sodium chloride has been added
to the water. Potassium or other electrolytic substances may
also be used.
According to the preferred embodiment of the present
CA 02291352 1999-12-O1
-9-
invention, approximately 1 amp rms of current is caused to flow
through the substance being electrically activated while a
voltage of approximately 100 volts rms is applied thereto.
This provides an optimum power density level for preparing 200
ml of substance at a time. The amount of power used is
preferably scaled in approximate proportion to the amount of
material being activated at a time.
Typically, current is caused to flow through the substance
being electrically activated 18 until small gas bubbles are
formed upon the electrodes. This takes approximately 4 to 8
hours. When small gas bubbles are observed on the electrodes,
an indication is that the substance has been fully electrically
activated and is ready to use. then properly prepared, the
electrical signals may then be removed from the substance, and
the substance will retain it's activated qualities after
removal of the current therethrough. The substance may then be
applied to the recipient.
The degree to which the substance becomes electrically
activated, and thus the effectiveness thereof in providing
2o cosmetic or medical therapy, is directly related to the voltage
applied to the electrodes, the spacing of the electrodes, the
current caused to flow between the electrodes, and, to some
extent, the length of time that the current is applied.
However, current must be caused to flow between the electrodes
for a minimum of at least 10 minutes before any usable results
are obtained. Generally, at least 4 hours are required, and at
least 8 hours are recommended. Testing indicates that the
application of current for a time period much in excess of 8
hours produces little additional effectiveness of the
3o electrically activated substance.
The electrically activated substance is typically active
for only a limited amount of time after current flow
therethrough has ceased. The electrically activated substance
is most effective if utilized within approximately 4 hours
CA 02291352 1999-12-O1
-10-
after application of the electrical signals. The electrically
activated substance is somewhat effective for up to 4 days
after its production. It is believed that the decay in the
effectiveness of the electrically activated substance is
logarithmic in nature, with more than half of the effectiveness
thereof lost within approximately 24 hours. After about 7 days,
all the active nature of the substance has decayed, and no
activated benefit is obtained by the application of the
substance to a recipient.
1o The specified values for the applied voltage, duration,
and conductivity of the medium may be varied to some degree.
Indeed, a reduction in the effectiveness of the electrically
activated substance may be compensated for by varying one or
the other of the production parameters.
s5 A lower voltage may be utilized if additional sodium
chloride is added to the solution. However, if too much sodium
chloride is added, then the solution may become less bio-
compatible. Conversely, if less sodium chloride is utilized,
then a higher voltage is necessary to obtain sufficient current
2o flow through the substance. If an excessively high voltage is
used as the result of an inadequate concentration of
electrolytic material in the substance, then the substance will
generate excessive heat without an increase in the
corresponding activation potential. This results in
25 substantially reduced effectiveness of the electrically
activated substance.
It is thought that the electrically activated substance of
the present invention provides beneficial cosmetic/medical
effects by stimulating a "current of injuryp associated with
3o tissue trauma. then tissue is injured, a small electric
current is generated. This current is a result of a voltage
producing charge imbalance which occurs when portions of
previously connected tissue are disconnected from one another,
such as occurs if a bone is broken or skin is torn. This
CA 02291352 1999-12-O1
-11-
voltage causes a small amount of current, e.g., electrons or
ions, to flow from one point to another within the tissue.
The tissue disconnection of an injury simulated by active
substance of this device possibly upsets the electrical balance
of the biological circuit, thereby resulting in the production
of a very small current, typically on the order of nanoamps or
less. The presence of this electric current is an instrumental
signal in the formation of a blastema, which is a collection of
primitive, unformed cells, which gather at an injury site and
so are later developed into replacement tissue.
The mechanism whereby the substance becomes active may be
related to electrolytic reactions occurring in the substance.
This may be the result of partial separation and recombination
of the hydrogen and oxygen atoms. For example, when the
waveform is positive Figure 5, 40 oxygen may be formed at one
electrode, and hydrogen at the other. The waveform stays
positive just long enough to break through double layer effects
and begin molecular separation of the gasses. However, before
the gasses can fully dissociate and escape the liquid into the
2o atmosphere, the signal reverses direction Figure 5, 42 which
"jams" the atoms back together again.
The activating frequency used is between lOKHz to lMhz.
The recommended frequency is preferably between lOKHz and
100KHz, Figure 5b, 44.
It is thought that the active properties of the substance
of the present invention effects in biological tissue a
disruptive electrical imbalance, similar to that which occurs
when a mechanical strain to the tissue has occurred. This
electrical imbalance then triggers accelerated metabolic
3o activity in the treatment area. Blood flow accelerates while
cellular metabolic activity and interactions increase.
Capillaries dilate and there is increased activity toward
restored homeostasis. This is shown in Figures 10 and 11.
In Figure 10, capillaries and small blood vessels 50 are
CA 02291352 1999-12-O1
-12-
in a relaxed and normal state. In figure 11, beginning a few
minutes after application of the electrically active substance,
and continuing to a peak about 45 minutes after the application
of the substance, the capillaries and small blood vessels 50
swell up and increase the volume and velocity of blood flow
through them. This acceleration in blood flow is the first step
in the tissue repair and tightening process. On the skin this
takes the appearance of a pronounced red blushing or flushing.
Toxins, free radicals, metabolic waste products, and
1o unused remnant material may be re-formed or flushed away. Thus
the device and method provide a means to effective treatment
that does not rely on the passage of electrical current through
the tissue of the recipient.
It is thought that the electrically activated substance of
the present invention should not be applied to fresh injury
sites, since it may interfere with the timing and development
of the natural current of injury, thereby disrupting the
healing process. However, once the injury has stabilized, the
electrically activated substance of the present invention may
2o be applied thereto so as to enhance or re-activate the healing
process.
One exemplary use of the electrically activated substance
of the present invention is the removal of skin wrinkles. For
example, facial wrinkles may be treated with the electrically
activated water. This is shown in figure 8. In Figure 8a, the
active substance 18 is dabbed on to the skin using a sponge or
similar applicator. It may also be spritzed or dabbed onto the
skin for topical application thereto. Both wrinkled 46 and
unwrinkled areas may be treated. The typical dose rate is
3o approximately 0.05 ml/cm2 of treatment area. The tightening
effect that occurs on the non-wrinkled areas will still help
tighten the wrinkled areas. Mitigation of skin wrinkles
typically occurs beginning within approximately 30 minutes,
with the results being clearly visible.
CA 02291352 1999-12-O1
-13-
Preferably, the water is activated with a frequency of
between approximately 50 KHz and 100 KHz. It has been found
that such electrically activated water is particularly
beneficial to the skin and other soft tissue.
Blood flow and metabolic activity accelerates and has been
found to peak within approximately 45 minutes after the
application of the electrically activated substance of the
present invention. After this period of increased blood flow
and accelerated metabolism, the skin and tissue enters a
so recovery phase wherein the cellular structure thereof is
rebuilt.
This recovery phase typically has a duration of
approximately one to four days. Skin begins to draw together,
getting tighter, thicker, and causing wrinkles to shrink.
s5 Additional collagen forms at the site of treatment. There may
be heavy itching, but no pain. After four days, approximately
all such recovery has occurred. At the end of the recovery
phase, another treatment may be applied. It has been found
that the recovery phase must be complete before a subsequent
2o treatment, so as to avoid overwhelming the response mechanism.
It is believed that the improvements obtained via the use
of the present invention are essentially permanent in nature.
That is, it is believed that new wrinkles will take almost as
long to form as the original ones did.
25 Approximately six such treatment sessions are typically
required for the optimum removal of wrinkles. After the first
treatment session, at least some, occasionally most or all of
the fine line facial wrinkles of 1 mm or less in size are
either reduced in size or eliminated altogether. After three
3o to four treatment sessions, wrinkles of up to four mm in size
are typically substantially reduced. After approximately six
treatment sessions, many, if not most or all of the facial
wrinkles, including large wrinkles of the forehead are
typically substantially eliminated. Typically, six treatment
CA 02291352 1999-12-O1
-14-
sessions, at one session per week for six weeks, are utilized.
The more degenerated the skin, the more dramatic the results
are. Acne is frequently cleared in the process due to
increased blood flow. Thus, the general result is renewed skin
or tissue, without surgery, grafting, patchwork, dermabrasion,
laser vaporization, or other invasive or mechanical techniques.
As a result of such treatments, enhanced elastin and
collagen support is provided and typical age sagging is reduced
due to the shrinking of the skin by as much as ten to twenty
1o percent. This is illustrated in Figure 8b. The skin is
tighter, thicker, and wrinkles 46 are diminished quite
noticeably.
It has also been found that therapeutic medical benefits
may be obtained if the medium is ingested. In Figure 7a the
substance 18 is ingested orally. Wrinkles 46 are gradually
diminished over a period of time. In Figure 7b the skin becomes
much more smooth overall, with wrinkles 46 diminished. When
taken orally, the dose rate is preferably comparatively small,
approximately 2 ml per day for 6 weeks. It is thought that
2o such therapy is also beneficial for heart, digestion, and
circulatory problems. The substance may also be applied or
ingested to treat a variety of other conditions, not just
wrinkles.
To further appreciate the unique advantages of this
process, consider that this technique has been also found at
least partially effective in the conversion of surgical scar
tissue from a typically necrid white fibrous collagen material
back into normal healthy pink skin with nerve sensitivity,
blood vessels, and even hair follicles. These results may be
obtained with administration of a daily to semi-daily topical
application, typically over a 6 month period. As the collagen
is initially resistant to absorbing the medium, a vasodilator
additive is effective in speeding results by increasing
penetration of the medium.
CA 02291352 1999-12-O1
-15-
Moreover, the techniques described herein are also
applicable to animals. Figure 9 shows an example where the
substance 18 is applied to a canine 48. Health benefits may be
obtained from the enhanced circulation.
Because the electrically activated substance of the
present invention functions as a transfer agent or medium, at
no time is there any current flow from the variable frequency
current source through biological tissue. Thus, there is no
chance of burns, thereby enhancing the safety of such treatment
1o compared to traditional transcutaneous electrotherapy or laser.
Referring now to Figures 2 and 3, if the variable
frequency current source 10 does not provide approximately 0
direct current bias, then the output thereof can be processed
so as to mitigate direct current bias. Zero DC bias is
important in the activation signal. Without a zero or low DC
bias in the activation signal, the substance will not take on
it's therapeutic properties.
With particular reference to Figure 2, a resistor
capacitor network 22 filters the output of the variable
2o frequency current source 10, so as to mitigate direct current
bias. Such a resistor-capacitor network comprises at least one
capacitor 26 in series with the substance being electrically
activated and at least one resistor 28 in parallel therewith.
The resistor-capacitor network 22 functions according to known
electrical principles to mitigate the presence of DC bias in
the substance being electrically charged. Those skilled in the
art will appreciate that various other types of filters may be
utilized. For example, a capacitor inductor network may be
utilized. Also, the resistor 28 may often be deleted if the
3o resistance of the substance 18 between the two electrodes is
sufficiently small so as to form the resistor portion of the
circuit.
With particular reference to Figure 3, an isolation
transformer 24 isolates the substance 18 to be electrically
CA 02291352 1999-12-O1
-16-
charged from direct current bias present in the output of the
variable frequency current source 10.
In any instance, when the variable frequency current
source 10 does not include a means for monitoring current flow
through the substance 18 being electrically activated, then
such means is preferably included in the electrical path of the
electrodes 14. For example, an amp meter 20 may be inserted in
line or applied inductively to one of the wires 12 which
provide an electrical pathway for the current which travels
1o between the electrodes 14. Alternatively, an oscilloscope may
be utilized to monitor current flow between the electrodes 14.
Referring now to Figure 4, the method for forming an
electrically activated substance of the present invention
generally comprises providing distilled water 30, adding sodium
chloride to the distilled water to form an electrolyte 32,
monitoring current flow between the electrodes if desired 32,
applying the alternating current to the electrodes 34, and,
removing the current and administering the electrically
activated substance 36, preferably within seven days after the
2o electrical activation thereof. The application of
alternating current 34 to the substance to be electrically
charged preferably takes place for a duration of approximately
at least 4 to 8 hours.
The electric current is applied to the intermediate
material, (i.e., the electrically activated substance), rather
than directly to a person. Thus, a substantial amount of more
power may be applied to the electrically activated substance
rather than directly to a person. Indeed, according to the
preferred embodiment of the present invention, much more power,
(e.g., approximately 100 watts), can be applied to the
electrically activated substance than could be tolerated by a
human being.
The minimum amount of electrical power applied to the
substance during activation thereof must be sufficient to
CA 02291352 1999-12-O1
-17-
overcome the activation decay rate of the substance. It has
been found that the approximate minimum amount of power that
can be used and still obtain any result is approximately 10
milliwatts per milliliter of substance. The application of
approximately 100 watts of electrical power to 200 ml of water
results in an acceptable decay rate.
The actual voltage and power used should be of a level
sufficient to raise the temperature of the substance being
electrically activated by at least three, and preferably thirty
1o to fifty degrees centigrade above ambient. If the substance
temperature should rise excessively, to about the boiling point
of the substance, excessive power is being used, and the
activation qualities dissipate. (The substance losses it's
activation effectiveness if boiled.)
i5 Due to the relatively large amount of power used to
electrically activate the water, a relatively high frequency is
used. In this way, premature electrolysis (gassing) of the
water is avoided. The high frequency also accelerates electron
agitation in the medium. The pH balance of the medium is
2o essentially unchanged.
Non-distilled or tap water or other bio-compatible
compounds, including tissue and fluids, may be possibly be
utilized instead of distilled water. However, the types and
amounts of impurities found in tap water vary considerably from
25 one location to another. Most tap water has been found to not
contain sufficient impurities to support adequate current flow
therethrough. Best results are obtained with an electrolytic
additive to the water.
The electrically activated substance is created using the
3o power levels, frequencies, current densities, and dosage
quantities described herein, or parameters similar to those
described herein. 4~hen the substance is produced in this
manner, it takes on unique properties (possibly on an atomic
level), which make it particularly well suited for the practice
CA 02291352 1999-12-O1
-18-
of the present invention.
Those skilled in the art will appreciate that various
other electrolyte forming substances, other than sodium
chloride are likewise suitable. The substance may be applied
to treat a variety of conditions, not just wrinkles.
It is understood that the exemplary electrically activated
substance and method for making the same described herein and
shown in the drawings represents only a preferred embodiment of
the invention. Indeed, various modifications and additions may
1o be made to such embodiment without departing from the spirit
and scope of the invention. For example, various different
sizes, shapes, and configurations of the container, the
electrodes, and the source and waveform of alternating current
are contemplated. Further, as those skilled in the art will
appreciate, the use of water as the electrically activated
substance is by way of example only, not by way of limitation.
Thus the method provides therapeutic medical and cosmetic
benefits without relying on surgical or laser or dermabrasion
techniques, and without relying on the passage of electrical
2o signal current through the tissue of the recipient.
The description sets forth the steps for constructing and
operating the invention in connection with the illustrated
embodiment. It is to be understood, however, that the same or
equivalent functions and sequences may be accomplished by
different embodiments that are also intended to be encompassed
within the spirit and scope of the invention.