Note: Descriptions are shown in the official language in which they were submitted.
CA 02291363 1999-11-30
ARTICLE COATED WITH MULTILAYER COATING
Field of the Invention
This invention relates to decorative and protective
coatings.
Background of the Invention
It is currently the practice with various brass
articles such as lamps, trivets, faucets, door knobs, door
handles, door escutcheons and the like to first buff and
polish the surface of the article to a high gloss and to
then apply a protective organic coating, such as one
comprised of acrylics, urethanes, epoxies, and the like,
onto this polished surface. This system has the drawback
that the requisite buffing and polishing operation,
particularly if the article is of a complex shape, is labor
intensive. Also, the known organic coatings are not as
durable as desired and wear off.
These deficiencies are remedied by a coating
containing a nickel basecoat and a non-precious refractory
metal compound such as zirconium nitride, titanium nitride
and zirconium-titanium alloy nitride top coat. However, it
has been discovered that when titanium is present in the
coating, for example as titanium nitride or zirconium-
titanium alloy nitride, in corrosive environments the
coating may experience galvanic corrosion. This galvanic
corrosion renders the coating virtually useless. It has
been surprisingly discovered that the presence of a layer
comprised of zirconium compound, such as zirconium nitride,
or a zirconium alloy compound over the layers containing
the titanium compound or titanium alloy compound
significantly reduces or eliminates galvanic corrosion.
CA 02291363 1999-11-30
Summary of the Invention
The present invention is directed to a protective and
decorative coating for a substrate, particularly a metallic
substrate. More particularly, it is directed to a
substrate, particularly a metallic substrate such as brass,
having on at least a portion of its surface a coating
comprised of multiple superposed layers of certain specific
types of metals or metal compounds wherein at least one of
the layers contains titanium or a titanium alloy. The
coating is decorative and also provides corrosion, wear and
chemical resistance. In one embodiment the coating
provides the appearance of polished brass with a golden
hue, i.e. has a golden-brass color tone. Thus, an article
surface having the coating thereon simulates polished brass
with a gold hue.
A first layer deposited directly on the surface of the
substrate is comprised of nickel. The first layer may be
monolithic, i.e., a single nickel layer, or it may consist
of two different nickel layers such as a semi-bright nickel
layer deposited directly on the surface of the substrate
and a bright nickel layer superimposed over the semi-bright
nickel layer. Over the nickel layer is a layer comprised
of chrome. Over the chrome layer is a strike layer
comprised of titanium or titanium alloy. Over the titanium
or titanium alloy layer is a layer comprised of titanium
compound or titanium alloy compound.
Over the titanium compound or titanium alloy compound
layer is a thin layer comprised of zirconium compound or
zirconium alloy compound. This layer functions to reduce
or eliminate galvanic corrosion.
2
CA 02291363 1999-11-30
Brief Description of the Drawing
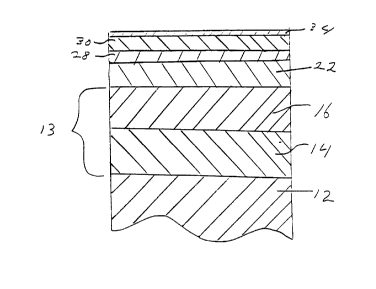
Fig. 1 is a cross-sectional view, not to scale, of the
mufti-layer coating on a substrate.
Description of the Preferred Embodiment
The substrate 12 can be any plastic, metal or metallic
alloy. Illustrative of metal and metal alloy substrates
are copper, steel, brass, tungsten, nickel alloys and the
like. In one embodiment the substrate is brass.
A nickel layer 13 is deposited on the surface of the
substrate 12 by conventional and well known electroplating
processes. These processes include using a conventional
electroplating bath such as, for example, a Watts bath as
the plating solution. Typically such baths contain nickel
sulfate, nickel chloride, and boric acid dissolved in
water. All chloride, sulfamate, and fluoroborate plating
solutions can also be used. These baths can optionally
include a number of well known and conventionally used
compounds such as leveling agents, brighteners, and the
like. To produce specularly bright nickel layer at least
one brightener from class I and at least one brightener
from class II is added to the plating solution. Class I
brighteners are organic compounds which contain sulfur.
Class II brighteners are organic compounds which do not
contain sulfur. Class II brighteners can also cause
leveling and, when added to the plating bath without the
sulfur-containing class I brighteners, result in semi-
bright nickel deposits. These class I brighteners include
alkyl naphthalene and benzene sulfonic acid. The benzene
and naphthalene di- and trisulfonic acids, benzene and
naphthalene sulfonamides, and sulfonamides such as
saccharin, vinyl and allyl sulfonamides and sulfonic acids.
The class II brighteners generally are unsaturated organic
3
CA 02291363 1999-11-30
materials such as, for example, acetylenic or ethylenic
alcohols, ethoxylated and propoxylated acetylenic alcohols,
coumarins, and aldehydes. These class I and class II
brighteners are well known to those skilled in the art and
are readily commercially available. They are described,
inter alia, in U.S. Patent No. 4,421,611 incorporated
herein by reference.
The nickel layer 13 can be comprised of a single
nickel layer such as, for example, bright nickel, or it can
be comprised of two different nickel layers such as a semi-
bright nickel layer and a bright nickel layer. In the
figure layer 14 is comprised of semi-bright nickel while
layer 16 is comprised of bright nickel. This duplex nickel
deposit provides improved corrosion protection to the
underlying substrate. The semi-bright, sulfur free plate
14 is deposited by conventional electroplating processes
directly on the surface of substrate 12. The substrate 12
containing the semi-bright nickel layer 14 is then plated
in a bright nickel plating bath and the bright nickel layer
16 is deposited on the semi-bright nickel layer 14, also by
conventional electroplating processes.
The thickness of the nickel layer 13 is generally in
the range of from about 100 millionths (0.0001) of an inch,
preferably from about 150 millionths (0.00015) of an inch
to about 3,500 millionths (0.0035) of an inch.
In the embodiment where a duplex nickel layer is used,
the thickness of the semi-bright nickel layer and the
bright nickel layer is a thickness effective to provide
improved corrosion protection. Generally, the thickness of
the semi-bright nickel layer 14 is at least about SO
millionths (0.00005) of an inch, preferably at least about
100 millionths (0.0001) of an inch, and more preferably at
4
CA 02291363 1999-11-30
least about 150 millionths (0.00015) of an inch. The upper
thickness limit is generally not critical and is governed
by secondary considerations such as cost and appearance.
Generally, however, a thickness of about 1,500 millionths
(0.0015) of an inch, preferably about 1,000 millionths
(0.001) of an inch, and more preferably about 750
millionths (0.0075) of an inch should not be exceeded. The
bright nickel layer 16 generally has a thickness of at
least about 50 millionths (0.00005) of an inch, preferably
at least about 125 millionths (0.000125) of an inch, and
more preferably at least about 250 millionths (0.00025) of
an inch. The upper thickness range of the bright nickel
layer is not critical and is generally controlled by
considerations such as cost. Generally, however, a
thickness of about 2,500 millionths (0.0025) of an inch,
preferably about 2,000 millionths (0.002) of an inch, and
more preferably about 1,500 millionths (0.0015) of an inch
should not be exceeded. The bright nickel layer 16 also
functions as a leveling layer which tends to cover or fill
in imperfections in the substrate.
Disposed over the nickel layer 13, particularly the
bright nickel layer, is a layer 22 comprised of chrome.
The chrome layer 22 may be deposited on layer 13 by
conventional and well known chromium electroplating
techniques. These techniques along with various chrome
plating baths are disclosed in Brassard, "Decorative
Electroplating - A Process in Transition", Metal Finishing,
pp. 105-108, June 1988; Zaki, "Chromium Plating", PF
Directory, pp. 146-160; and in U.S. Patent Nos. 4,460,438,
4,234,396 and 4,093,522, all of which are incorporated
herein by reference.
CA 02291363 1999-11-30
Chrome plating baths are well known and commercially
available. A typical chrome plating bath contains chromic
acid or sales thereof, and catalyst ion such as sulfate or
fluoride. The catalyst ions can be provided by sulfuric
acid or its salts and fluosilicic acid. The baths may be
operated at a temperature of about 112° - 116° F. Typically
in chrome plating a current density of about 150 amps per
square foot, at about 5 to 9 volts is utilized.
The chrome layer 22 serves to provide structural
integrity to the vapor deposited layers or reduce or
eliminate plastic deformation of the coating. The nickel
layer 13 is relatively soft compared to the titanium
compound or titanium alloy compound layer 30. Thus, an
object impinging on, striking or pressing on layer 30 will
not penetrate this relatively hard layer, but this force
will be transferred to the relatively soft underlying
nickel layer 13 causing plastic deformation of this layer.
Chrome layer 22, being relatively harder than the nickel
layer, will generally resist the plastic deformation that
the nickel layer 13 undergoes.
Chrome layer 22 has a thickness at least effective to
provide structural integrity to and reduce plastic
deformation of the coating. This thickness is at least
about 2 millionths (0.000002) of an inch, preferably at
least about 5 millionths (0.000005) of an inch, and more
preferably at least about 8 millionths (0.000008) of an
inch. Generally, the upper range of thickness is not
critical and is determined by secondary considerations such
as cost. However, the thickness of the chrome layer should
generally not exceed about 60 millionths (0.00006) of an
inch, preferably about 50 millionths (0.00005) of an inch,
6
CA 02291363 1999-11-30
and more preferably about 40 millionths (0.00004) of an
inch.
Disposed over chrome layer 22 is a strike layer 28
comprised of titanium or titanium alloy.
The strike layer 28 functions, inter alia, to improve
the adhesion of layer 30, comprised of titanium compound or
titanium alloy compound, to the chrome layer 22.
Generally, this thickness is at least about 0.25 millionths
(0.00000025) of an inch, preferably at least about 0.5
millionths (0.0000005) of an inch, and more preferably at
least about one millionth ( 0 . 000001 ) of an inch . The upper
thickness range is not critical and is generally dependent
upon considerations such as cost and appearance.
Generally, however, layer 28 should not be thicker than
about 50 millionths (0.00005) of an inch, preferably about
15 millionths (0.000015) of an inch, and more preferably
about 10 millionths (0.000010) of an inch.
Over the strike layer 28 is layer 30 comprised of
titanium compound or titanium alloy compound. Layer 30
provides wear and abrasion resistance and the desired color
or appearance, such as for example a brass color with a
golden hue. Layer 30 has a thickness effective to provide
abrasion and wear resistance and to provide the requisite
color. The color depends on the composition of layer 30.
Thus, titanium-zirconium nitride will provide a brass color
with a golden hue.
Generally layer 30 has a thickness of at least about 2
millionths (0.000002) of an inch, preferably at least 4
millionths (0.000004) of an inch, and more preferably at
least 6 millionths (0.000006) of an inch. The upper
thickness range is generally not critical and is dependent
upon considerations such as cost. Generally a thickness of
7
CA 02291363 1999-11-30
about 100 millionths (0.0001) of an inch, preferably about
5Q millionths (0.00005) of an inch, and more preferably
about 30 millionths (0.00003) of an inch should not be
exceeded.
The metals that are alloyed with the titanium to form
the titanium alloy or titanium alloy compound are the non-
precious refractory metals. These include zirconium,
hafnium, tantalum, and tungsten. The titanium alloys
generally comprise from about 10 to about 90 weight percent
titanium and from about 90 to about 10 weight percent of
another non-precious refractory metal, preferably from
about 20 to about 80 weight percent titanium and from about
80 to about 20 weight percent of another refractory metal.
The titanium compounds or titanium alloy compounds include
the oxides, nitrides, carbides and carbonitrides.
In one embodiment layer 30 is comprised of titanium-
zirconium alloy nitride and layer 28 is comprised of
titanium-zirconium alloy. In this embodiment the titanium-
zirconium alloy nitride layer has a brass color with a
golden hue.
A method of forming layers 28 and 30 is by utilizing
well known and conventional vapor deposition techniques
such as physical vapor deposition or chemical vapor
deposition. Physical vapor deposition processes include
sputtering and cathodic arc evaporation. In one process of
the instant invention sputtering or cathodic arc
evaporation is used to deposit a layer 28 of titanium alloy
or titanium followed by reactive sputtering or reactive
cathodic arc evaporation to deposit a layer 30 of titanium
alloy compound such as titanium-zirconium nitride or
titanium compound such as titanium nitride.
8
CA 02291363 1999-11-30
To form layer 30 wherein the titanium compound or the
t.~tanium alloy compound are the nitrides, nitrogen gas is
introduced during vapor deposition such as reactive
sputtering or reactive cathodic arc evaporation at a
desired value or flow rate to form titanium nitride or
titanium alloy nitride.
Over layer 30 is layer 34. Layer 34 is comprised of a
zirconium compound or a zirconium alloy compound. The
zirconium compounds or zirconium alloy compounds are the
oxides, nitrides, carbides and carbonitrides. The metals
that are alloyed with zirconium to form the zirconium alloy
compounds are the non-precious refractory metal compounds
excluding titanium. The zirconium alloy comprises from
about 30 to about 90 weight percent zirconium, the
remainder being non-precious refractory metal other than
titanium; preferably from about 40 to about 90 weight
percent zirconium, the remainder being non-precious
refractory metal other than titanium; and more preferably
from about 50 to about 90 weight percent zirconium, the
remainder being non-precious refractory metal other than
titanium.
Layer 34 may be, for example, zirconium nitride when
layer 30 is zirconium-titanium alloy nitride.
Layer 34 is very thin. It is thin enough so that it
is non-opaque, translucent or transparent in order to allow
the color of layer 30 to be seen. It is, however, thick
enough to significantly reduce or eliminate galvanic
corrosion. Generally layer 34 has a thickness from about
0.07 millionth to about 0.7 millionth, preferably from
about 0.2 millionth to about 0.3 millionth of an inch.
Layer 34 can be deposited by well known and
conventional vapor deposition techniques, including
9
CA 02291363 1999-11-30
physical vapor deposition and chemical vapor deposition
such ~as, for example, reactive sputtering and reactive
cathodic arc evaporation.
Sputtering techniques and equipment are disclosed,
inter alia, in J. Vossen and W. Kern "Thin Film Processes
II", Academic Press, 1991; R. Boxman et al, "Handbook of
Vacuum Arc Science and Technology", Noyes Pub., 1995; and
U.S. patent Nos. 4,162,954 and 4,591,418, all of which are
incorporated herein by reference.
Briefly, in the sputtering deposition process a
refractory metal (such as titanium or zirconium) target,
which is the cathode, and the substrate are placed in a
vacuum chamber. The air in the chamber is evacuated to
produce vacuum conditions in the chamber. An inert gas,
such as Argon, is introduced into the chamber. The gas
particles are ionized and are accelerated to the target to
dislodge titanium or zirconium atoms. The dislodged target
material is then typically deposited as a coating film on
the substrate.
In cathodic arc evaporation, an electric arc of
typically several hundred amperes is struck on the surface
of a metal cathode such as zirconium or titanium. The arc
vaporizes the cathode material, which then condenses on the
substrates forming a coating.
Reactive cathodic arc evaporation and reactive
sputtering are generally similar to ordinary sputtering and
cathodic arc evaporation except that a reactive gas is
introduced into the chamber which reacts with the dislodged
target material. Thus, in the case where zirconium nitride
is the layer 32, the cathode is comprised of zirconium and
nitrogen is the reactive gas introduced into the chamber.
By controlling the amount of nitrogen available to react
CA 02291363 1999-11-30
with the zirconium, the color of the zirconium nitride can
be adjusted to be similar to that of brass of various hues.
In order that the invention may be more readily
understood the following example is provided. The example
is illustrative and does not limit the invention thereto.
EXAMPLE 1
Brass faucets are placed in a conventional soak
cleaner bath containing the standard and well known soaps,
detergents, defloculants and the like which is maintained
at a pH of 8.9 - 9.2 and a temperature of about 145 - 200°F
for 10 minutes. The brass faucets are then placed in a
conventional ultrasonic alkaline cleaner bath. The
ultrasonic cleaner bath has a pH of 8.9 - 9.2, is
maintained at a temperature of about 160 - 180°F, and
contains the conventional and well known soaps, detergents,
defloculants and the like. After the ultrasonic cleaning
the faucets are rinsed and placed in a conventional
alkaline electro cleaner bath for about 50 seconds. The
electro cleaner bath is maintained at a temperature of
about 140° - 180°F, a pH of about 10.5 - 11.5, and contains
standard and conventional detergents. The faucets are then
rinsed and placed in a conventional acid activator bath for
about 20 seconds. The acid activator bath has a pH of
about 2.0 - 3.0, is at an ambient temperature, and contains
a sodium fluoride based acid salt.
The faucets are then placed in a conventional and
standard bright nickel plating bath for about 12 minutes.
The bright nickel bath is generally a conventional bath
which is maintained at a temperature of about 130 - 150°F, a
pH of about 4.0 - 4.8, contains NiS09, NiCL2, boric acid, and
brighteners. A bright nickel layer of an average thickness
11
CA 02291363 1999-11-30
of about 400 millionths (0.0004) of an inch is deposited on
the faucets. The bright nickel plated faucets are rinsed
three times and then placed in a conventional, commercially
available hexavalent chromium plating bath using
conventional chromium plating equipment for about seven
minutes. The hexavalent chromium bath is a conventional
and well known bath which contains about 32 ounces/gallon
of chromic acid. The bath also contains the conventional
and well known chromium plating additives. The bath is
maintained at a temperature of about 112°-116°F, and
utilizes a mixed sulfate/fluoride catalyst. The chromic
acid to sulfate ratio is about 200:1. A chromium layer of
about 10 millionths of an inch is deposited on the surface
of the bright-nickel layer. The faucets are thoroughly
rinsed in deionized water than then dried. The chromium
plated faucets are placed in a cathodic arc evaporation
plating vessel. The vessel is generally a cylindrical
enclosure containing a vacuum chamber, which is adapted to
be evacuated by means of pumps. A source of argon gas is
connected to the chamber by an adjustable valve for varying
the rate of flow of gas.
A cylindrical zirconium-titanium alloy cathode is
mounted in the center of the chamber and connected to
negative outputs of a variable D.C. power supply. The
positive side of the power supply is connected to the
chamber wall. The cathode material comprises zirconium and
titanium.
The plated faucets are mounted on spindles, 16 of
which are mounted on a ring around the outside of the
cathode. The entire ring rotates around the cathode while
each spindle also rotates around its own axis, resulting in
12
CA 02291363 1999-11-30
a so-called planetary motion which provides uniform
exposure to the cathode for the multiple faucets mounted
around each spindle. The ring typically rotates at several
rpm, while each spindle makes several revolutions per ring
revolution. The spindles are electrically isolated from
the chamber and provided with rotatable contacts so that a
bias voltage may be applied to the substrates during
coating.
The vacuum chamber is evacuated to a pressure of about
5x10-3 millibar and heated to about 150°C.
The electroplated faucets are then subjected to a
high-bias arc plasma cleaning in which a (negative) bias
voltage of about 500 volts is applied to the electroplated
faucets while an arc of approximately 500 amperes is struck
and sustained on the cathode. The duration of the cleaning
is approximately five minutes. Argon gas is introduced at
a rate sufficient to maintain a pressure of about 3x10-2
millibars. A layer of zirconium-titanium alloy having an
average thickness of about 4 millionths of an inch is
deposited on the chrome plated faucets during a three
minute period. The cathodic arc deposition process
comprises applying D.C. power to the cathode to achieve a
current flow of about 500 amps, introducing argon gas into
the vessel to maintain the pressure in the vessel at about
1x10-2 millibar, and rotating the faucets in a planetary
fashion described above.
After the zirconium-titanium alloy layer is deposited
a thicker zirconium-titanium nitride compound "color layer"
is deposited over it. A flow of nitrogen is introduced
into the vacuum chamber while the arc discharge continues
at approximately 500 amperes. The nitrogen flow rate is
13
CA 02291363 1999-11-30
set sufficiently high to fully react the zirconium and
titanium alloy atoms arriving at the substrate to form
zirconium-titanium nitride compound. The total time for
deposition is about 30 minutes. The arc is extinguished at
the end of this deposition period, the vacuum chamber is
vented and the coated substrates removed.
After the zirconium-titanium nitride compound layer is
deposited a final thin non-optically dense flash layer of
zirconium nitride is deposited to provide increased
corrosion resistance and to achieve the desired final
color. The coated substrate parts are placed into another
chamber fitted with cylindrical cathode target composed
primarily of zirconium metal. The chamber is evacuated to
pressures previously described as well as the parts cleaned
again by subjecting them to high-bias arc plasma as
described earlier. After the cleaning process is complete
the cathodic arc deposition process is repeated with
nitrogen and argon gas flows set sufficiently high to
provide full or nearly full reaction of the zirconium metal
to zirconium nitride compound. This flash process is
carried out for a one to three minute period. Finally the
arc is extinguished the chamber vented and the coated
substrates removed.
While certain embodiments of the invention have been
described for purposes of illustration, it is to be
understood that there may be various embodiments and
modifications within the general scope of the invention.
14