Note: Descriptions are shown in the official language in which they were submitted.
CA 02292393 1999-12-17
1
Rubber Mixtures Which Contain Organosilanepolysulfanes
The present invention provides rubber mixtures which
contain organosilanepolysulfanes.
The use of organosilanepolysulfanes as coupling agents or
reinforcing additives in oxide-filled rubber mixtures such
as, for example, the treads and other parts of car tires
has been disclosed (DE 2 141 159, DE 2 212 239, US 3 978
103, US 4 048 206). These types of organosilanepolysulfanes
such as, for example, bis-(3-[triethoxysilyl]-
propyl)tetrasulfane (TESPT), generally consist of a
polysulfane mixture, wherein the length of the sulfane
chains (SX) is generally in the range 2 to 10.
It is also known, when using these types of coupling agents
in oxide-filled rubber mixtures, that processing
temperatures higher than 130°C have to be maintained in
order to enable the reaction between silica and the
organosilane to take place. The plasticity of the mixture
is then reduced. The reaction of organosilanes with silica
and the emission of the alcohol which is released
accelerates with increasing temperature of the mixture.
Furthermore, it is known that the organosilanepolysulfanes
which are mainly used, such as bis-(3-[triethoxysilyl]-
propyl)tetrasulfane, require particular attention when
being incorporated into rubber in order to avoid pre-
vulcanisation when mixing the components. In particular,
reactive longer-chain polysulfanes with SX>4 tend to enter
into unwanted cross-linking reactions with the rubber at
temperatures higher than 140°C. This is made obvious, inter
alia, by an increase in the plasticity of the mixture
(Gorl, Munzenberg, ACS-Meeting Rubber Division, Anaheim,
California/USA, May 1997, 38).
The use of organosilanes with shorter polysulfane chains
has also been disclosed (WO-A 97/48264, D-A 197 02 046).
CA 02292393 1999-12-17
2
Known organosilanes with shorter polysulfane chains may be
obtained by the reaction (desulfurization by nucleophilic
reagents) of longer-chain organosiIanepolysulfanes with
trivalent phosphorus compounds, sulfites or cyanides (D 195
41 404 and EP-A 845 472). However, the preparation of these
organosilanepolysulfanes requires at least one additional
process step. It is complicated and expensive.
The invention provides rubber mixtures which contain at
least one organosilane and at least one desulfurizing
l0 reagent from the class of compounds which contains
trivalent phosphorus compounds.
According to the invention, it was found that, when
preparing the mixture, the tendency to pre-vulcanisation
when using longer-chain organosilanepolysulfanes in rubber
mixtures can be largely avoided by the direct addition of
trivalent phosphorus compounds, sulfites or cyanides which
are capable of reducing the proportion of longer-chain
polysulfanes via a desulfurization reaction.
Known organosilanepolysulfanes may be used as
organosilanepolysulfanes. In particular
organosilanepolysulfanes which correspond to formula I are
used.
(R1 Rz R3 SiR4) zSx I
wherein
R1, RZ , R3 may be identical or di f f erent and may be
H, (C1-C4) alkyl, CZ-C4 alkoxy or halogen, wherein the
halogen may be C1 or Br;
wherein, preferably R1 = RZ = R3 = methoxy or ethoxy.
R4 may be a (C1-C6) linear or branched alkylidene;
X = 2 to 10.
CA 02292393 1999-12-17
3
The following nucleophiles are particularly suitable for
desulfurizing the organosilanpolysulfanes in the mixture:
phosphines with the general structure: P(R1)3 and P(NRZR3)3,
where R1, Rz and R3, independently, represent H, alkyl or
aryl; in particular R1 - phenyl;
phosphites with the general structure: P(OR4)3 and HOP(OR4)z
where R4 - alkyl or aryl;
and dithiophosphites with the general structure:
,OR~ ,R60~
RSO Pv ~ ~P ORS n
OR6 R60
where RS and R6, independently, represent alkyl or aryl.
Rubber mixtures which contain a combination of an
organosilanepolysulfane and a nucleophile according to the
invention for desulfurization and the moulded articles
resulting after a vulcanization step, in particular
pneumatic tires or tire treads, in addition to increased
scorch resistance, surprisingly also have a higher
300~/100~ modulus which points to the higher coupling
effectiveness of the coupling agent. This is also reflected
in a lower tan 8 (60°C) value which correlates with a lower
rolling resistance.
In accordance with one embodiment of the invention, the
rubber mixtures may contain an organosilanepolysulfane in
amounts of 0.1 to 15 wt.~, in particular 5 to 10 wt.~, with
respect to the amount of filler used, and at least one
reagent for desulfurization which is capable of reducing
the proportion of longer-chain polysulfanes via a
desulfurization reaction in amounts of 5 to 80 wt.~, in
particular 10 to 40 wt.~, with respect to the amount of
organosilanepolysulfane used.
CA 02292393 1999-12-17
4
In a preferred embodiment of the invention, the mixtures
may contain a synthetic rubber and a silica as filler. The
rubber mixtures according to the invention may be prepared
by blending the rubber, at least one filler, an
organosilanepolysulfane and a desulfurization reagent with
each other.
Addition of the organosilanes, the nucleophiles to
desulfurize the same, and the addition of fillers,
preferably takes place in a common procedure in a
thermomechanical mixing step at bulk temperatures of 80 to
200°C, in particular 140 to 180°C.
The nucleophiles may preferably be added at the start of
mixture preparation, in order to ensure the increased
thermal stability of the mixture according to the invention
at the earliest possible stage of mixture preparation.
Fillers which may be used for rubber mixtures according to
the invention are:
- carbon blacks, which may be prepared by the flame,
furnace or gas carbon black process and have BET
surface areas of 20 to 200 m2/g.
- highly dispersable silicas prepared, for example, by
precipitation from solutions of silicates or by flame
hydrolysis of silicon halides with specific surface
areas of 5 to 1000, preferably 20 to 400 m2/g (BET
surface area) and with primary particle sizes of 10 to
400 nm. The silicas may optionally also be present as
mixed oxides with other metal oxides such as A1, Mg,
Ca, Ba, Zn and titanium oxides.
synthetic silicates such as aluminium silicate,
alkaline earth silicates such as magnesium silicate or
calcium silicate with BET surface areas of 20 to 400
mz/g and primary particle diameters of 10 to 400 nm.
CA 02292393 1999-12-17
- aluminium oxides with a proportion of -OH
functionalities.
- natural silicates such as kaolin and other naturally
occurring silicas.
5 - glass fibres and glass fibre products (mats, ropes) or
glass microbeads.
Carbon blacks with BET surface areas of 20 to 400 m2/g or
highly dispersed silicas, prepared by precipitation from
solutions of silicates, with BET surface areas of 20 to
400 mz/g may preferably be used in amounts of 5 to 150
parts by wt., each with respect to 100 parts of rubber.
The fillers mentioned above may be used individually or as
a mixture. In a particularly preferred embodiment of the
process, 10 to 150 parts by wt. of pale filler, optionally
together with 0 to 100 parts by wt. of carbon black, and
0.1 to 15 parts by wt., preferably 5 to 10 parts by wt., of
an organosilanepolysulfane, each with respect to 100 parts
by wt. of the filler used, and at least one nucleophile
which is capable of reducing the proportion of longer-chain
polysulfanes via a desulfurizing reaction, may be used in
amounts of 5 to 80 wt.~, in particular 10 to 40 wt.~, with
respect to the amount of organosilanepolysulfane used, may
be used to prepare the mixtures.
The organosilane may be a pure compound or may be combined
with a support, preferably carbon black.
The nucleophile may be added directly to the mixture as
such or else mixed with another constituent of the mixture,
preferably the silane or the rubber auxiliary substances.
The nucleophile, the organosilane and/or the rubber
auxiliary substances may be used as pure substances or
mixed/combined with a support, preferably carbon black.
CA 02292393 1999-12-17
6
In addition to natural rubber, synthetic rubbers may also
be used to prepare rubber mixtures according to the
invention. Preferred synthetic rubbers are, for example,
described in W. Hofmann, Kautschuktechnologie, Genter
Verlag, Stuttgart 1980. They include, inter alia,
- polybutadiene (BR)
- polyisoprene (IR)
- styrene/butadiene copolymers with styrene contents of 1
to 60, preferably 5 to 50 wt.~ (SBR)
- isobutylene/isoprene copolymers (IIR)
- butadiene/acrylonitrile copolymers with acrylonitrile
contents of 5 to 60, preferably 10 to 50 wt.~ (NBR)
- partly hydrogenated or fully hydrogenated NBR rubbers
(~R)
- ethylene/propylene/diene copolymers (EPDM)
and mixtures of these rubbers. Anionic polymerised S-SBR
rubbers with a glass transition temperature above
-50 °C and their mixtures with diene rubbers are used in
particular for the production of vehicle tires.
Rubber vulcanisates according to the invention may contain
further rubber auxiliary substances such as reaction
accelerators, anti-ageing agents, heat stabilisers, light
stabilisers, anti-ozonants, processing aids, plasticisers,
tackifiers, blowing agents, colorants, waxes, extenders,
organic acids, retarding agents, metal oxides and
activators such as triethanolamine, polyethylene glycol or
hexanetriol.
The rubber auxiliary agents may be used in conventional
amounts which depend, inter alia, on the ultimate use.
Conventional amounts may be, for example, amounts of 0.1 to
CA 02292393 1999-12-17
7
50 wt.~, with respect to the rubber. The
organosilanepolysulfanes may be used on their own as cross-
linking agents. The addition of other cross-linking agents
is generally recommended. Sulfur or peroxides may be used
as other known cross-linking agents. In addition, rubber
mixtures according to the invention may also contain
vulcanization accelerators. Examples of suitable
vulcanization accelerators are mercaptobenzthiazoles,
sulfenamides, guanidines, thiurams, dithiocarbamates,
l0 thiourea and thiocarbonate. The vulcanization accelerator
and sulfur or peroxides are used in amounts of 0.1 to 10
wt.~, preferably 0.1 to 5 wt.~, with respect to the rubber.
Rubber mixtures according to the invention may be
vulcanised at temperatures of 80 to 200°C, preferably 130
to 180°C, optionally under a pressure of 10 to
200 bar. Mixing the rubber with the filler, optional rubber
auxiliary substances, the organosilanes and the
nucleophiles according to the invention may be performed in
conventional mixing equipment such as rollers, internal
mixers and mixer-extruders. Rubber vulcanisates according
to the invention are suitable for producing moulded
articles, for instance for the production of pneumatic
tires, tire treads, cable sheathing, hoses, drive belts,
conveyer belts, roller coatings, tires, soles of shoes,
sealing rings and damping elements.
Examples
Examples 2 and 3 demonstrate the advantages of the use
according to the invention of a combination of an
organosilanepolysulfide and a nucleophile for
desulfurizing, as compared with the prior axt (comparison
example 1).
CA 02292393 1999-12-17
8
General method used in the examples
The formulation used for the rubber mixtures is given in
table 1. The unit phr means proportion by weight, with
respect to 100 parts of the crude rubber used.
Table 1
Substance Amount
[phr]
1st stage
Buna VSL 5025-1T"' 96.0
Buna CB 2 4~' 3 0 . 0
Ultrasil VN3~'~' 80.0
Zn0 3.0
Stearic acid 2.0
Naftolene ZD 10.0
Vulkanox 4020" 1.5
Protector G35P~" 1.0
TESPT 6.4
Triphenylphosphine 0 to 4
2nd stage
Batch stage 1
3rd stage
Batch Stage 2
Vulkacit DT'' 2.0
Vulkacit CZT' 1.5
Sulfur 1.5
CA 02292393 1999-12-17
9
The polymer VSL 5025-1 is a solution polymerised SBR
copolymer from Bayer AG, with a styrene content of 25 wt.~
and a butadiene content of 75 wt.~. 73 ~ of the butadiene
is 1,2 linked, 10 ~ is cis-1,4 linked and 17 ~ is trans-1,4
linked. The copolymer contains 37.5 phr of oil and has a
Mooney viscosity (ML 1+4/100°C) of 50 ~ 4.
The polymer Buna CB 24 is a 1,4-cis polybutadiene (Neodyme
type) from Bayer AG with a cis-1,4 content of 97 ~, a
trans-1,4 content of 2 ~, a 1,2 content of 1 ~ and a Mooney
viscosity of 44 ~ 5.
The silica VN3 from Degussa AG has a BET surface area of
175 m2/g.
Bis-(3-[triethoxysilyl]-propyl)tetrasulfane (TESPT) is sold
by Degussa AG under the tradename Si 69 and has an average
sulfane chain length of 4 and a polysulfane proportion
S(x>4) > 25~.
Triphenylphosphine in accordance with examples 2 and 3 was
purchased from the Merck Co.
Naftolen ZD from Chemetall is used as an aromatic oil.
Vulkanox 4020 is a PPD from Bayer AG. Protektor G35P is an
anti-ozonant wax from HB-Fuller GmbH. Vulkacit D (DPG) and
Vulkacit CZ (CBS) are commercial products from Bayer AG.
The rubber mixture is prepared in three stages in an
internal mixer in accordance with table 2:
CA 02292393 1999-12-17
Table 2:
Stage 1
Settings
Mixing unit Werner & Pfleiderer E-Typ
Friction 1:1.11
Speed 70 min 1
Core pressure 5.5 bar
Void volume 1.6 L
Filling extent 0.55
Thru'put temp. 80 C
Mixing process
0 to 1 min Buna VSL 5025-1 + Buna CB 24
1 to 3 min 1/2 Ultrasil VN3, ZnO, stearic acid,
Naftolen ZD, silane, optional nucleophile
3 to 4 min 1/2 Ultrasil VN3, Vulkanox 4020,
Protector G35P
4 min clean
4 to 5 min mix
5 min clean
5 to 6 min mix and discharge
Batch temp. 140-150C
Storage 24 h at room temperature
CA 02292393 1999-12-17
11
Stage 2
Settings
Mixing unit same as stage 1 down to:
Speed 80 min 1
Filling extent 0.53
Thru'put temp. 80 C
Mixing process
0 to 2 min stage 1 batch broken up
2 to 6 min Batch temperature 150C by varying the
speed
6 min discharge
Batch temp. 150-155C
Storage 4 h at room temperature
CA 02292393 1999-12-17
12
Stage 3
Settings
Mixing unit same as stage 1 down to
Speed 40 min 1
Filling extent 0.51
Thru'put temp. 50 C
Mixing process
0 to 2 min stage 2 batch + Vulkacit CZ + Vulkazit D
+ sulfur
2 min discharge and form a sheet on a
laboratory mixing roller
(diameter 200 mm, length 450 mm,
throughput temperature 50C)
Homogenise:
cut into and rotate 3* left, 3* right and
then
compress 8* with narrow roller gap (1 mm)
and
3* with wide roller gap (3.5 mm) and
then draw out as a sheet
Batch-Temp. 85-95C
CA 02292393 1999-12-17
13
The general procedure for preparing rubber mixtures and
their vulcanisates is described in the following book:
"Rubber Technology Handbook", W. Hofmann, Hanser Verlag
1994.
The vulcanisation time for the test specimen was 60 minutes
at 165°C.
Rubber-engineering tests were performed in accordance with
the test methods given in table 3.
Table 3
Physical tests Standard/
Conditions
ML 1+4, 100C DIN 53523/3, ISO 667
Vulcameter test, 165C DIN 53529/3, ISO 6502
Tensile test on a ring, 23C DIN 53504, ISO 37
Tensile strength
Modulus
Elongation at break
Shore A hardness, 23C DIN 53 505
Visco-elastic properties, 0 and DIN 53 513, ISO 2856
60C, 16 Hz, 50 N preliminary
force and 25 N Amplitude force
Complex modulus E*,
Loss factor tan 8
DIN abrasion, 10 N force DIN 53 516
Dispersion ISO/DIS
11345
CA 02292393 1999-12-17
14
Examples 1, 2 and 3: Triphenylphosphine as nucleophile
Examples 1 (comparison example), 2 and 3 are performed in
accordance with the general instructions given above,
wherein no triphenylphosphine is added to the mixture in
comparison example 1.
Differently from example 1, in the 1st mixing stage an
addition 2 phr of triphenylphosphine is incorporated into
the mixture in example 2 and an additional 4 phr of
triphenylphosphine is incorporated into the mixture in
example 3.
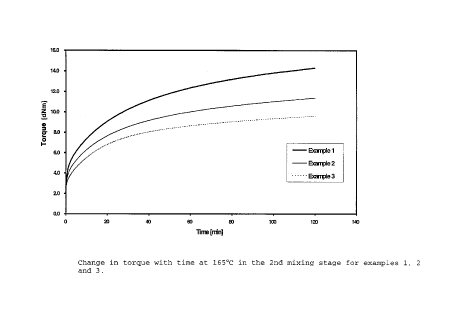
In figure 1, the changes in torque with time at 165°C in
the 2nd mixing stage, for examples 1, 2,and 3, are plotted,
wherein the increase in torque corresponds to the tendency
to pre-vulcanisation at the stated temperature.
It can be seen, from figure 1, that the increase in torque
for examples 2 and 3 which are in accordance with the
invention is much less than for comparison example 1 in
accordance with the prior art.
The rubber-engineering data for the crude mixture and the
vulcanisate are given in table 4.
CA 02292393 1999-12-17
Table 4:
Crude mixture re;aults
Feature: Units: - 1 - - 2 - 3 -
-
ML(1+4) at 100C (3rd stage)[ME] 71 71 69
Vulcameter test 165C
Dmax-Dmin [dNm] 18.7 16.21 16.29
t 10~ [min] 1.41 1.49 1.41
t 90~ [min] 27.1 24.8 19.9
Vulcanisate resu:Lts
Feature: Units: - 1 - - 2 - 3 -
-
Tensile test
Tensile strength [MPa] 16.1 15.5 16.6
Modulus 100 [MPa] 2.4 1.9 2.1
Modulus 300 [MPa] 10.9 9.3 10.4
Modulus 300~/100~ [ ] 4.5 4.9 5.0
Elongation at [~] 380 400 400
break
Fracture energy [J] 84.6 80.1 85.9
Shore A hardness [SH] 67 61 60
DIN abrasion [mm'] 74 67 58
Visco-elastic Properties
Complex modulus E* (0C) [MPa] 31.2 19.5 16.3
Complex modulus E* (60C) [MPa] 11.8 8.8 7.8
Loss factor tan b (0C) [-] 0.348 0.415 0.406
Loss factor tan 8 (60C) (-] 0.108 0.102 0.098
Dispersion [-] 6 6 6
CA 02292393 1999-12-17
16
It can be seen from table 4 that a generally balanced
effective rubber-engineering set of values is produced for
examples 2 and 3. In particular the modulus 300~/100~,
which points to increased coupling effectiveness, and a low
tan b (60°C) value, which correlates with a low rolling
resistance, appear to be positive features.