Note: Descriptions are shown in the official language in which they were submitted.
CA 02292819 1999-12-22
PROCESS FOR THE PRODUCTION OF A PRECIPITATED SILICA SLURRY
WITH A CONTROLLED AGGREGATE PARTICLE SIZE DISTRIBUTION
In one of its aspects, the present invention relates to a process for the
production of
a precipitated silica slurry. In another of its aspects, the present invention
also provides a
means of controlling the average sizes of the silica particle clusters or
aggregates within this
slurry. In still another of its aspects, the present invention relates to a
monodisperse
distribution of silica aggregates and moreover yields clusters or aggregates
which have both
a fractal morphology and low mechanical strength. As used throughout this
specification,
"monodisperse" is intended to mean aggregate particles that have a narrow size
distribution
characterised by the standard deviation about the mean value of their radius.
As used
throughout this specification, the term "fractal morphology" is intended to
mean that each
of the particle clusters is a rough or fragmented geometric shape as opposed
to "spheroidal
morphology" wherein it is meant that the particles have a substantially
constant internal
radius. In yet another of its aspects, the present invention relates to
precipitated silica slurries
which can advantageously be used in the production of rubber/silica
masterbatches.
Precipitated silica is well known as a filler material useful in a variety of
products.
One of the applications in which precipitated silica has achieved particular
interest recently
is as a filler for polymers. It is particularly useful for the reinforcement
of articles made from
vulcanized elastomers (e.g., butadiene rubber, styrene-butadiene rubber,
natural rubber,
EPDM a.nd the like), including tire treads.
One requirement for the achievement of optimum reinforcing in a cured rubber
article is that the silica or other filler be homogeneously dispersed
throughout the elastomer
compound in the form of discreet small particles. Dispersed filler particle
sizes in the same
order as polymer chain molecular dimensions (i.e., from a few tens of
nanometers to a
hundred nanometers or so, nanometer = 10-9 meters) provide excellent
reinforcement
potential while larger particles are generally viewed as being less
advantageous in this
respect. Dispersed particles with diameters in excess of one micron (103
nanometers) are, as
-1-
CA 02292819 1999-12-22
a rule, non-reinforcing, and rubber articles containing such large particles
may even have less
utility than those without any added filler.
Many methods exist for the characterization of dry silica powders. The most
important silica properties for rubber reinforcement are the average particle
size (ASTM
C721 and D1366; ISO 787 Part XVII), the specific surface area (ASTM D1933 and
D5604;
ISO 5794 Part I), the mean projected area of the aggregates (ASTM D3849), and
the oil
absorption (ASTM D2414; ISO 787 Part V). In the case of wet (i.e., never-
dried) silicas, the
average particle size may be determined from the original surface area
measurement method
of Sears, (G.W. Sears Jr., Analytical Chemistry, Vol. 28, No. 12 (December,
1956) pages
1981-1983), or modifications thereof as described in Iler, pages 203-205 or by
an improved
test as set forth and detailed in United States patent 5,739,197, col. 7. The
CTAB surface
area is also an important parameter and may be used to further characterize
non-dried silicas.
The CTAB area is defined as the external surface area, as evaluated by
absorption of cetyl
trimethyl ammonium bromide with a pH of 9, following the method set forth by
Jay, Jansen
and Kraus in Rubber Chemistry and Technology, 44, pages 1287-1296 (1971). A
full
procedure for this test is set forth in United States patent 5,739,197, col. 5-
7.
Methods also exist for assessing the dispersibility of silica powders without
the
necessity of mixing them with rubber (see for instance US Patent 5,403,570 or
A. Blume,
"Analyti~~al Properties of Silica - a Key for Understanding Silica
Reinforcement", presented
at the Rubber Division Meeting, ACS, Chicago, April 13-16, 1999). If mixing is
used, then
optical or electron microscopy may be used to assess the resulting degree of
filler dispersion;
a full procedure is described in United States patent 5,739,197 col. 1-5.
C",ommercially available dry silica powders are composed of aggregates of
primary
particles. Larger structures termed "agglomerates" may also be present,
usually as a result
of the drying process used during the manufacture. In general, superior
reinforcing properties
in rubber compounds are obtained from the use of silicas which have both a
high specific
surface area (i.e. > 200 m2/gm) coupled with a small average aggregate
particle size.
However, such silica materials are difficult to disperse during rubber mixing
("dry mixing")
and also yield compounds with high viscosities that are difficult to process
further by
-2-
CA 02292819 1999-12-22
operations such as calendaring, extrusion or molding which are needed to form
the desired
end-products. Extended mixing times and high levels of ancillary agents are
normally
required to attain a good dispersion with such silica materials, making their
use
uneconomical or impractical for most rubber applications. Fine particle silica
materials
moreover present difficult in-plant material logistics due to poor material
flow characteristics
and concomitant dust generation.
The ease of dispersion of a dry silica powder during rubber mixing is
recognized as
being dependent on the porosity of the aggregate structure. The latter is in
turn governed to
a large extent by the size and packing of the primary silica particles (see
for example, Iler,
Chapter 5). The degree of aggregate porosity is usually measured by a form of
absorption
test. Oil is commonly employed for this purpose (ASTM D2414). Silica particle
aggregates
with high degree of oil absorption have a high internal void volume; i.e.,
they contain
numerous suitably-sized pores into which the oil molecules can migrate. Pores
of sizes
approaching molecular dimensions (2-200 nanometers) have been classified as
"mesopores"
by Unger (Klaus Unger, "Structure of Porous Adsorbents", Angew Chem. Int. Ed.,
11 (4) 267
(1972)); during the dry mixing process, the polymer component may also enter
these pores.
The shearing and elongational forces of dry mixing cause the rubber-entrained
silica particles
to fracture and disperse in the bulk polymer. A porous particle also exhibits
a diminished
fracture strength compared to a similar sized solid particle, and is thus more
easily fractured
by the forces inherent in the mixing process. However, practical limits are
imposed on the
particle porosity by the rigors of the dry silica production process itself,
as will be discussed
later. Considerable efforts have thus been made to develop processes which
yield silicas with
improved dispersibility (see for example, United States patent 5,929,156 and
United States
patent S,S87,514) by controlling the various manufacturing variables.
As a second requirement to achieve maximum development of reinforcing
properties
in elastomers, filler particles not only need to be well dispersed but their
surface also needs
to be substantially chemically compatible with the polymers in which they are
used as fillers.
Special chemical agents are normally employed to reduce the surface polarity
of the silica
particles when these are used with non-polar hydrocarbon rubbers. In standard
practice, these
-3-
CA 02292819 1999-12-22
surface-reactive agents (usually bifunctional sulfur-containing silanes) are
added during the
mixing cycle, i.e. during Banbury mixing. Chemical reactions take place
between these
added agents and the silanol groups (Si-OH) on the silica surface during the
mixing step and
later between the agent and the polymer at the compound curing step. This
allows the silica
particle to be linked to the polymer backbone. Special thermomechanical mixing
regimens
are required to complete and maintain separation of these two different
reactions on a
practical time scale, making the overall mixing process quite difficult to
control. In this
regard, recent efforts have been made to develop new techniques for dispersing
silica
aggregates (normally hydrophilic) into such elastomers (normally hydrophobic)
which
circumvent the problems associated with the dry mixing process. See, for
example, any of
the following references:
published International patent application WO 98/52954 [Koski];
published International patent application WO 98/53004 [Koski]; and
published International patent application WO 99/15583 [von Hellens];
hereinafter collectively referred to as "the Bayer patent applications".
As it is well known that primary particle size and surface area are directly
related,
silica materials composed of aggregates of very small primary particles have a
relatively high
surface area. To ensure maximum compatibility of such silica materials with
non-polar
polymers, as well as to prevent absorption of curative chemicals on the
surface, high ratios
of expensive surface treating agents per unit silica weight are therefore
necessary. Practical
limits thus exist on the maximum surface area that can be economically used.
There are two general approaches for the production of precipitated silicas
known in
the prior art.
T'he first, more recent approach relies on the controlled hydrolysis
("hydrolytic
polycondensation") of a hydrolysable silane (i.e., tetraethoxysilane, TEOS or
ethyl silicate)
hereafter called "the Stober processes" (W. Stober et al., J. Colloid Int.
Sci., 26, 62-69,
-4-
CA 02292819 1999-12-22
(1968)). The second, more classical approach entails the reaction of an acid
(e.g., HZS04)
with a water solution of alkali silicate (e.g., Na2O(S1O2)_2.5-3.34)
There are many patents which describe variations of the Stober process to
produce
silica materials of controlled particle size, narrow particle size
distribution and degree of
spheroidal morphology (see, for example any one of United States patent
4,775,520; United
States patent 4,861,572 and United States patent 5,425,930). While some of the
silaceous
materials produced by these Stober process variants may be technically
acceptable as
reinforcing rubber fillers, they are necessarily expensive owing to the
complexity of the
processes and above all the high cost of the raw materials. These products
have commercial
utility only in special applications where their cost/performance ratio is
acceptable, such as
in chromatography packings, electronic applications and as catalyst supports.
Further
references will thus not be made to silaceous materials produced by such
Stober-type
processes in the context of the present specification since these materials
are generally cost-
prohibitive as primary rubber fillers.
Process variants utilizing the second approach are manifold as evidenced by
the
large number of patents on precipitated silica materials. The classical
precipitation processes
are understood to consist of the following steps (Encyclopedia of Chemical
Technology,
Volume 21, p.1024):
~ formation of colloidal particles through nucleation and growth of these
particles
to form the primary particle;
~ coagulating the primary particles into aggregates to form a precipitate;
and
~ reinforcement of the aggregate particle
In practice, the processes are usually conducted by adding an acid at a
predetermined
rate to the heated alkali silicate solution contained in a first vessel. The
first vessel is agitated
throughout the reaction phase and relatively high reaction temperatures (i.e.,
60°C to 85°C)
are usually employed. Large vessel volumes are typically used. Addition of
acid may be
-S-
CA 02292819 1999-12-22
interrupted at a certain pH (ie. ~8-8.5) and additional silicate may also be
added then, or the
precipitation may be taken to completion and additional silicate and acid may
be added later
in a post-treatment (United States patent 4,336,245). These interruptions or
post-treatments
serve to improve particle aggregate strengths, and are usually referred to as
"building" steps.
Single or multiple building steps are possible (United States patent 4,243,428
and United
States patent 5,911,963). The amount of reinforcement thus conferred to the
aggregate is
termed the "build-up ratio" (Iler, p.557). Build-up ratio is further defined
as the ratio of the
final weight of silica in the system to the weight of the aggregated silica.
Build-up ratios of
4:1 or less are usually required to attain a silica that can be dispersed
during dry mixing (Iler,
p. 557). A certain amount of "build-up" is required to prevent collapse of the
particle
structure during the ensuing drying step as a result of capillary forces and
those of surface
tension as water is removed (Iler, p. 534-536). However, this build-up step
also decreases the
pore size and strengthens the particle thereby diminishing its intrinsic
utility as a filler. One
embodiment of a silica particle during "build-up" is illustrated in Figure 2b.
After a period of time to allow for the desired amount of build-up, acid is
again
added to complete the desired degree of neutralization. The contents of the
first vessel are
then transferred to a ripening tank (e.g., a second vessel equipped with an
agitator) wherein
particle growth is completed over the course of several hours or days (known
within the art
as "Ostwalt ripening'. During this stage, many of the smaller particles
dissolve and are
redeposited on the surfaces of, or at the contact points between larger
particles - see Figure
2a. The particle size distribution thus narrows and the average particle size
increases. This
ripening has the further effect of increasing particle strength thereby
improving filterability
by reducing the number of very small particles. It is these smaller particles
which generally
pass through the filter and are lost, or if their volume fraction becomes
excessive, may clog
the filter substrate. Filtering is still the most common process used to
separate the silica from
the mother liquor.
The wet filter cake resulting from the filtering step may be further processed
by
pressing to remove entrained mother liquor, washing to remove soluble salts,
drying (i.e., in
a tunnel drier) and finally grinding (i.e., using a hammer mill, jet mixer,
ball mill or similar
-6-
CA 02292819 1999-12-22
apparatus). These steps yield the finished product as a powder having a broad
particle size
distribution and an average agglomerate particle size of from about 1 ~m to
about 100 Vim,
or in some cases even larger. Moreover, because the particles are shattered by
the final
grinding process, their shapes tend to be angulated rather than fractal or
spheroidal, and the
flow characteristics of the powders are thus poor.
As a known means of improving the powder flow characteristics, the washed
filter
cake may be reslurried and then finished by a spray drying process. This
process
modification yields a more free flowing powder composed of spheroidal particle
agglomerates; however because the so-produced dried particles are generally of
larger size
(i.e. > 50 microns) than those produced by grinding, longer mixing times are
generally
required to attain the same degree of filler dispersion when such spray-dried
powders are
used in the rubber compound. However, the spray drying process does produce a
silica with
much reduced tendency to dust, which is a desirable feature.
Several variations of the above general acid precipitation process are known
in the
1 S prior art:
For instance, an acidic gas may be employed as the precipitating agent. The
acidic
gas may be added directly to the heated silicate solution or it may be
generated in-situ, for
instance by the combustion of hydrocarbon (i.e., natural gas, in a submersible
burner as
described in United States patent 3,372,046) to provide COZ and heat.
Thereafter, the formed
silica particles are ripened and the slurry is then filtered to remove the
particles in the form
of a wet filter cake. The filter cake may be further washed free of soluble
salts, dried and
ground to provide an end product having an average agglomerate particle size
of from about
0.5 hum t:o about 100 ~M. Silicas produced by the above modified processes
share the same
disadvantages as those produced from direct addition of mineral acid to
soluble silicate.
In yet another modified approach to the above acid gas technique, Derleth et
al
(United States patent 5,232,883) describe a process whereby an
electrostatically charged
alkali silicate solution is sprayed into a chamber containing an acidic gas
(i.e. HCl). This
process is said to produce a silica comprised of microspheroidal particles of
narrow particle
size distribution of between 50 to 200 micrometers and with a specific surface
in excess of
_7_
CA 02292819 1999-12-22
200 m2/g, a pore volume of between 1 and 3.5 cm3/g, a roundness factor lower
than 1.40, and
a variation coefficient of the particle size distribution lower than 60%.
However, these silica
particles are designed specifically for use as catalyst supports for alpha-
olefin
polymerization. The accepted requirement of crush resistance in this
application would
suggest that these particles would be too hard for application as reinforcing
fillers since
reduction of the 50-200 micrometer agglomerates to the nanometer range
necessary for
reinforcement would be a lengthy and likely impractical process. The large
surface area of
these particles (i.e. up to 700 m2/g) would necessitate the use of large
quantities of expensive
surface-reactive agents to ensure polymer/filler compatibility. Finally, the
production of
such particles requires a special apparatus of complicated design and would
not be suitable
for the large volume production of fillers.
Spheroidal silica particles in the 0.5 to 20 micrometer diameter range can
also be
obtained by agglomeration of colloidal silica in a polymeric matrix of urea
and formaldehyde
(United States patent 4,010,242). The colloidal silica, in this case "Ludox
HS", is made by
a precipitation of an alkali silicate utilizing an acid-form ion exchange
resin. This patent
further directs that the organic matrix must be removed by burning; this
process thus suffers
from unnecessary complexity and is wasteful of organic materials.
»egardless which modification of the acid precipitation prior art approaches
is used,
(with the exception of United States patent 5,232,883), the silica at the wet
filter cake stage
typically has a wide particle size distribution (PSD) of aggregates. Since
each silica
aggregate is made up of a number of smaller primary (ultimate) particles of
varying sizes,
a wide PSD means that, for a given aggregate: there are a relatively large
number of contact
points (i.e., between adjacent agglomerated particles) resulting in a particle
aggregate with
relatively high mechanical strength - see Figure lb, for example. While this
strength is
detrimental to ease of processing, it is nevertheless necessary to prevent
collapse of the
particle and the formation of a solid sintered mass during the drying process.
Thus, the
requirements of the silica for a useful manufacturing process and those which
provide for of
ease of processing during rubber mixing are somewhat at odds.
_g_
CA 02292819 1999-12-22
Further, for dried silica materials, a relatively high shear energy is needed
to de-
agglomerate the particle, and the particle porosity is low. Thus, it is
somewhat difficult to
conduct surface chemistry on all of the agglomerated particles in the sample.
The latter property is particularly problematic when employing a conventional
silica
preparation in the hydrophobicizing treatment set out in the Bayer patent
applications.
Specifically, when dispersing silica particles in an elastomer, it is
desirable to treat silica
particles prior to use to facilitate dispersion thereof into the elastomer.
While the treatment
of silica particles as described in the Bayer patent applications represents a
significant
advance in the art, there is still room for improvement.
for example, the Bayer patent applications refer to treatment of conventional
silica
preparations to facilitate dispersion thereof in the elastomer via a
masterbatch technique.
While the treatment technique is adequate on a small scale, it would be
beneficial to have an
improved silica preparation, inter alia, which would facilitate treatment of
the surface of the
silica particles and ultimately facilitate dispersion of the treated silica
particles in the
elastomer.
It is an obj ect of the present invention to obviate or mitigate at least one
of the above-
mentioned disadvantages of the prior art.
It is another object of the present in«ention to provide a novel process for
producing
a precipitated silica slurry.
It is yet another object of the present invention to provide a novel
precipitated silica
slurry.
Accordingly, in one of its aspects, the present invention provides a process
for
production of a precipitated silica slurry comprising the steps o~
(i) continuously feeding to a mixing zone an aqueous metal silicate compound,
an acid and an electrolyte to produce the reaction mixture;
(ii) maintaining the reaction mixture at a temperature of less than about
100°C
in a quiescent zone;
(iii) continuously removing the reaction mixture from the quiescent zone.
-9-
CA 02292819 1999-12-22
T'he present inventor has unexpectedly discovered that silica particles useful
as fillers
may be ~,~rown without the need to utilize a "building" step as described
hereinabove. It is
surprising then that easily dispersible silicas may be produced by this
invention. Preferably,
the silica particles produced in the process have a volume average particle
size of at least 5
pm, more preferably in the range of from about 10 ~m to about 40 pm.
Embodiments of the present invention will be described with reference to the
accompanying drawings, in which:
Figures 1 and 2 illustrate schematic views of various forms of silica
particulate
material;
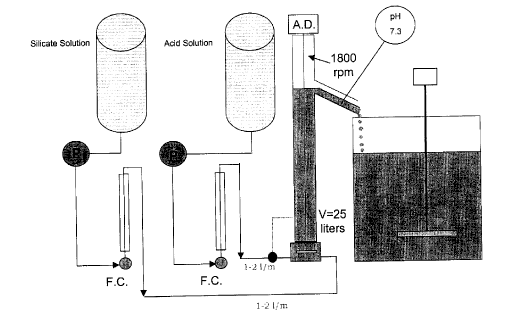
Figure 3 illustrates a schematic of a reactor used in the example described
herein
below and which is the subject of a co-pending patent application;
Figures 4 and 6 illustrate particle size distribution curves for a pair of
silica slurries
based on volume fraction;
Figures 5 and 7 illustrate particle size distribution curves for a pair of
silica slurnes
based on number fraction;
Figure 8 illustrates the result of an ultrasonic dispersion test on the number
average
particle size for a pair of silica slurries;
Figure 9 illustrates an electron microscope image of a commercially available
silica
material; and
Figure 10 illustrates an electron microscope image of a silica material made
in
accordance with the present process.
T'he precipitated silica slurry produced in accordance with the present
process has a
relatively narrow PSD and moreover the particle aggregates have a fractal
morphology.
Specifically, since each silica particle in the precipitated slurry produced
in accordance with
the present process is itself a fractal aggregate of a number of smaller
particles, a narrow
PSD means that, for a given silica particle: (i) there are a relatively small
number of contact
points (i.e., between adjacent particles within the aggregate), (ii) only a
relatively low shear
energy is needed to de-agglomerate the particle since only a few contact
points need to be
broken, and (iii) it is possible to conduct surface chemistry on substantially
all of the surfaces
-10-
CA 02292819 1999-12-22
of the aggregate since these are readily accessible (i.e., compared to a
slurry having a
relatively wide PSD of silica particles).
Thus, the precipitated silica slurry produced in accordance with the present
process
may be used advantageously in the hydrophobicizing treatment set out in the
Bayer patent
applications. In other words, in a preferred embodiment, the precipitated
silica slurry
produced in accordance with the present process is not filtered and dried;
rather it is subject
to further treatment whilst in the nascent slurry form. This allows for
production of
improved treated silica particles which leads to improved dispersion thereof
in an elastomer
via the masterbatch technique described in the Bayer patent applications.
Step (i) of the present process comprises continuously feeding to a mixing
zone an
aqueous metal silicate compound, an acid and an electrolyte to produce the
reaction mixture.
Preferably, the aqueous metal silicate comprises an alkali metal silicate.
More
preferably, the alkali metal silicate has the formula
MZO.(Si02)X
wherein M is an alkali metal and x is at least 2. Preferably, x is in the
range of from about
2 to about 4 (including fractional numbers). Non-limiting examples of useful
alkali metal
silicates may be selected from the group comprising sodium silicate, potassium
silicate,
ammonium silicate and mixtures thereof. Alkaline earth silicates such as
calcium silicate or
magnesium silicate may also be used. For economical reasons, the most
preferred alkali
metal silicate is sodium silicate, particular a sodium silicate comprising a
SiO2:Na20 ratio
in the range of from about 0.5 to about 4.0, also referred to as 'water
glass.'
The acid used in Step (i) is conventional. Non-limiting examples of such acids
may
be selected from the group comprising hydrochloric acid, phosphoric acid,
sulfuric acid,
nitric acid and mixtures thereof. Acidic gases such as CO2, HCI, SOZ and the
like may also
be used.
The electrolyte used in Step (i) is conventional. Preferably, the electrolyte
comprises
an alkali metal salt. More preferably it is a sodium salt. Non-limiting
examples of useful
-11-
CA 02292819 1999-12-22
sodium salts may be selected from the group comprising sodium chloride, sodium
nitrate,
sodium carbonate, sodium bicarbonate, sodium chlorate, sodium bromide and
mixtures
thereof.
The reaction mixture produced in Step (i) of the present process is retained
in the
mixing zone for a period of time sufficient to achieve a uniform distribution
of reactants.
Preferably, the residence time of the reaction mixture in the mixing zone is
less than about
minutes, more preferably less than about 10 minutes, even more preferably less
than about
8 minutes, even more preferably less than about 4 minutes, most preferably
less than about
1 minute.
10 The ratio of acid to alkali silicate is such that the pH of the reaction
mixture in the
mixing zone is maintained in the range of from about 6.0 to about 10Ø More
preferably,
the pH of the reaction mixture in the mixing zone is maintained in the range
of from about
7.0 to about 9Ø Most preferably, the pH of the reaction mixture in the
mixing zone is
maintained in the range of from about 8.0 to about 9Ø Appropriate pH control
can be
15 achieved by regulating the amount of acid used in Step (i) of the process
whilst keeping the
flow of alkali silicate constant.
Step (ii) of the present process comprises maintaining the reaction mixture in
a
quiescent zone at a temperature of less than about 100°C. Preferably,
Step (ii) comprises
maintaining the reaction mixture in the quiescent zone at a temperature in the
range of Hess
than about 90°C, and most preferably from about 30°C to about
SO°C.
Step (iii) of the present process comprises continuously removing the reaction
mixture from the quiescent zone. This allows continued introduction of
silicate, acid and
electrolyte in Step (i) thereby rendering the present process continuous.
Preferably, the apparatus for use in the present process comprises a
substantially
vertical reactor assembly equipped with at least one reactant inlet disposed
below a reaction
mixture outlet. The preferred reactor assembly is equipped with two reactant
inlets disposed
substantially near the bottom of the reactor assembly and a single reaction
mixture outlet
disposed near the top of the reactor. Preferably, the reactor is equipped with
an intensive
-12-
CA 02292819 1999-12-22
mixing zone and an intermediate substantially quiescent zone between the
mixing zone and
the outlet.
The preferred reactor assembly further comprises a means to achieve turbulent
mixing (e.g., an impeller or the like) disposed in the mixing zone at or near
the reactant
inlets) to achieve essentially complete axial mixing thereof, and a means to
reduce any
backmixing from the quiescent zone into the mixing zone.
In this preferred form of the process, Step (i) preferably comprises feeding
to the
mixing zone:
a first feed comprising an aqueous solution of the metal silicate and the
electrolyte;
and
a second feed comprising an aqueous solution of the acid and the electrolyte.
Practically, it is preferred that the aqueous solution in at least one,
preferably both of, the
first feed and the second feed comprises a brine solution.
'Chus, in this preferred embodiment, the first feed and the second feed are
continuously pumped into the reactor via their respective reactant inlets to
provide a reaction
mixture in the mixing zone. In the mixing zone, the reaction mixture is
subject to intense
axial mixing. The occurrence of vertical mixing in the reaction zone is
preferably minimized
or most preferably avoided by means as described in co-pending Canadian
application S.N.
* (Bayer Ref: POS-1075), filed on even date herewith. With continued pumping
of the first
feed and the second feed, the reaction mixture exits the mixing zone and rises
toward the top
of the reactor, into a substantially quiescent zone in a plug-flow manner.
Once the reaction mixture reaches the outlet, it exits the reactor assembly.
Preferably, the reaction mixture, now in the form of a precipitated silica
slurry, may then be
transferred to a settling tank wherein the pH of the slurry is further
adjusted, as necessary.
If desired, the silica particles may be allowed to settle to the bottom of the
settling tank after
which the mother liquor may be decanted. The silica particles may be further
filtered, washed
and dried in a conventional manner. Alternatively, the decanted aqueous silica
slurry may
be used directly in the above-mentioned hydrophobicizing reactions described
in the Bayer
-13-
CA 02292819 1999-12-22
patent applications - i.e., without the need to filter and dry the silica
particles. The decantate,
which consists of the electrolyte and by-product salts may be recycled.
F?mbodiments of the present invention will be illustrated with reference to
the
following Example, which should not be use to construe or limit the scope of
the present
invention.
EXAMPLE
A silicate solution was prepared by dissolving sodium silicate
[Na20(Si02)3.zs]
(28.93 kg) in water (163.5 kg). The ingredients were stirred and heated to
40°C, as required,
to produce an aqueous solution of sodium silicate. Thereafter, rock salt (5.13
kg) was added
with stirring to the aqueous solution of sodium silicate until all solids had
dissolved to
produce a brine solution of sodium silicate.
An acid solution was prepared by slowly adding with stirring concentrated
hydrochloric acid (17.2 kg; 36-38wt% HCl) to tap water (152.8 kg). Thereafter,
rock salt
(5.13 kg) was added with continued stirring until all solids had dissolved.
The silicate solution and the acid solution were independently fed by means of
pumps to a reactor shown schematically in Figure 3. The feed rate for each
solution was
adjusted by means of flow controllers and metered so as to provide a feed rate
of 1 to 2 litres
per minute for each solution.
T he solutions were independently fed to the bottom of the reactor assembly
where
they were intensively axially mixed in a mixing zone. With continued pumping
of each
solution to the mixing zone, the reaction mixture flowed into a quiescent zone
and thence
though an outlet into an overflow tank. The pH at the outlet was maintained at
7.3 by
adjusting the flow of acid to the acid inlet at the bottom of the reactor
assembly. The product
exiting the reactor assembly via the overflow was stored in a collection tank.
Total residence
time in the reactor assembly was between 10 and 25 minutes.
A sample of the silica slurry produced in this Example was filtered, washed
and then
adjusted with water to give a 2.5 wt. % slurry. For comparative purposes, a
2.5 wt. % slurry
-14-
CA 02292819 1999-12-22
of HiSiITM 233 ( a commercially available particulate reinforcing silica
material) was
prepared by adding the dry material to water and shaking manually to disperse.
'Che particle size distribution (PSD) for each slurry was determined by means
of laser
light scattering using a Malvern MastersizerTM instrument and the
manufacturer's directions.
S The results are illustrated in Figure S in terms of the volume average PSD
and in Figure 4
as the nLUnber average PSD. Figure 5 shows that the particle size distribution
of the Example
slurry is substantially narrower than that of the HiSiITM 233 slurry. Figure 4
shows that the
number average particle size of the Example slurry (1.45 Vim) is much smaller
than that of
the HiSiITM 233 slurry (4.17 Vim).
The ease of dispersion of the Example silica and HiSiITM 233 slurnes were
determined by the following protocol:
Exactly 80 milliters of the slurry was placed into a 100 ml. beaker and a
small stirnng bar
was introduced. The beaker was then placed on a magnetic stir plate and the
sounding horn
of a Branson Sonifier Model W-350 was immersed into the slurry to a depth of 4
cm. The
magnetic stirrer was activated at a medium setting and the slurry was stirred
for exactly 2
minutes.. The sonifier was then activated at a setting of 140 Watts/cm on the
dial over a
period of 28 minutes. Small samples of slurry were periodically withdrawn
during
sonification and the PSDs of each slurry were then measured as previously
noted using the
Malvern MastersizerTM. The analysis results for the slurry after 22 minutes of
sonification
are shown in Figure 6 (number average PSD) and Figure 7 (volume average PSD).
The
results in Figure 6 show that the number average particle size distribution
for the Example
slurry is substantially smaller (1.12 Vim) than that of the comparative
material (2.54 Vim)
indicating that the Example silica will give a dispersion of finer particles
and hence better
reinforcement. The Example silica also develops a distinct bimodality whereas
the only
indication of such that is evident in the comparative silica is a small
shoulder on the left side
of the main peak. In accordance with the interpretation offered by Blaume, (
see "Analytical
Properties of Silica - a Key for Understanding Silica Reinforcement",
Presented at the
Rubber Division Meeting, ACS, Chicago, April 13-16, 1999) the ratio of the
peak height of
the original agglomerate peak to the peak height of the decomposed
agglomerates may be
-15-
CA 02292819 1999-12-22
taken as an indication of dispersibility in rubber. This ratio, termed the WF
coefficient, is 1.1
for the Example silica and 3.4 for the comparative silica, indicating that the
agglomerates in
the Example silica are much easier to break down mechanically than those in
the
comparative silica and the Example silica should thus disperse more readily
during rubber
S mixing.
The change of the number average particle size with time of sonification for
the
Example silica and the comparative silica are both shown in Figure 8. While
the initial rate
of change for the comparative silica is initially quite fast (as indicated by
the slope of the
line) at C1.13 ~m/min, it slows to 0.035 ~m/min during the last minute or so
of sonification
and by graphical extrapolation it appears that it will eventually reach zero
at around 40
minutes. On the other hand, while the slope of the curve for the Example
silica is flatter at
0.021 ~m/min, there is no indication that it changes during the entire 28
minute course of
sonification; the Example silica should thus continue to disperse at the same
rate as mixing
is continued.
Figure 9 illustrates an electron microscope image of the HiSil TM 233 silica
material
and Figure 10 illustrates an electron microscope image of the Example silica
material. A
comparison of these two Figures clearly illustrates that the Example silica
material is made
up of a number of similarly sized aggregate particles (i.e., a narrow PSD)
while the
comparative silica is made up of particles having a larger variety of particle
sizes (i.e., a
broad PSD). The smaller average particle size for the Example silica is easily
seen when
compared to the HiSiITM 233. The fractal nature of the aggregates in the
Example silica are
distinct from the angulated particles of HiSiITM 233.
All publications, patents and patent applications referred to herein are
incorporated by reference in their entirety to the same extent as if each
individual
publication, patent or patent application was specifically and individually
indicated to be
incorporated by reference in its entirety.
-16-