Note: Descriptions are shown in the official language in which they were submitted.
PC783
CA 02292853 1999-12-20
1
PROCESS AND APPARATUS FOR SYNTHESIZING
AND GROWING CRYSTALS
FIELD OF THE INVENTION
The present invention relates generally to a process and apparatus for
synthesizing polycrystalline compounds and for growing monocrystals from such
compounds. More particularly, the present invention relates to a process and
apparatus
for synthesizing Group II-VI and Group III-V polycrystalline compounds of high
purity and
correct stoichiometry, comprising one or more reactants or dopants which are
of high
volatility at a temperature below the melting point of the chosen encapsulant
and for
growing monocrystals from such compounds using known crystal growth
techniques.
BACKGROUND OF THE INVENTION
Many Group II-VI compounds, such as CdTe, are suitable for use in a wide
variety
of semiconductor applications, including optical applications, in the
production of detectors
for high-energy particles such as y and x particles, and as substrates in
structures with
HgCdTe for use in infra-red radiation detectors and solar cells. For example,
European
Patent Application EP 0886167, in the name of the Applicant, describes, inter
alia, an
optical switching device that uses an indium-doped CdTe crystal. Such
applications
require the monocrystalline material to be of both high purity and of high
crystallinity.
While the growth of high quality silicon-based monocrystals in bulk quantities
has
been possible for many years, this is not the case for Group II-VI and III-V
compounds.
The growth of monocrystals with the desired stoichiometry from various
compounds of
elements belonging to Groups II and VI, in particular from CdTe, is
particularly complex
because of the volatility of one or more of these elements and in some cases,
large
differences between the vapor pressures of the constituents of a compound at
elevated
temperatures and low thermal conductivity. Furthermore, in preparing the
alloy,
segregation of reactants can occur because of differences in composition of
the solid and
liquid phases in equilibrium at a given temperature, resulting in a non-
uniform distribution
of reactants, impurities, dopants or microphases in localized concentrations.
Moreover, at
the temperatures at which growth normally takes place, reaction of the
reactants or
compound with the container material can result in contamination of the
crystal. Crystal
growth can also be adversely affected by a high impurity content, dislocation
density, or
an excess of one of the constituents which is too high and/or non-uniform in
distribution
~PC783
CA 02292853 1999-12-20
2
resulting in a non-stoichiometric compound and/or a crystal with impaired
electronic
properties.
The relevant art teaches various methods for synthesizing Group II-VI
semiconductor compounds, among which are the solid-state reaction method and
the
vapor-phase synthesis method. N.N. Kolesnikov et al., "Growth and
Characterization of
P-Type Cd,_xZnxTe (x - 0.2, 0.3, 0.4)," J. Crystal Growth, Vol. 174, pp. 256-
262 (1997),
describe the synthesis of CdTe by solid-state reaction of Cd and excess Te in
closed silica
ampoules. The same article also describes the vapor-phase synthesis of ZnTe by
the
reaction at 1050°C of Zn and excess Te vapors, evaporated from separate
sources, in
closed silica ampoules.
F.P. Doty and J.F. Butler, "Properties of CdZnTe crystals grown by high
pressure
Bridgman method", J.Vac.Sci. Technol. B 10(4), pp. 1418-1422 (Jul/Aug 1992),
describe a
crystal growth process where the charge is heated on a programmed schedule for
reaction and homogenization of the melt in high purity graphite crucibles with
tight fitting
caps to reduce evaporative loss of the charge.
Table I, of the same article, shows the charge lost during growth of CdZnTe
crystals.
Various semiconductor monocrystal growth techniques for compounds of Group II-
VI are also known in the art, particularly for the compound CdTe. One known
growth
technique is the Bridgman method of vertical type, (VB), or horizontal type,
(HB), growth.
In the article "Crystal Growth of Large-Area Single-Crystal CdTe and CdZnTe by
the
Computer-Controlled Vertical Modified-Bridgman Process," J. Crystal Growth,
Vol. 86
(1988), pp. 111-117, S. Sen et al. describe a particular VB type growth method
used to
grow a CdTe crystal. The apparatus used includes a hot zone which is separate
from a
cold zone, the two zones being arranged vertically and being such as to allow
control of
the heat gradients between these zones. The polycrystal used for the growth is
obtained
by vapor-phase reaction of the components.
Yellin, N. and Szapiro, S., "Vapor Transport of Nonstoichiometric CdTe in
Closed
Ampoules," J. Crystal Growth, Vol. 69, No. pp. 555-60 (1984), describe a
method of
physical vapor transport ("PVT") growth. First, polycrystalline CdTe was
synthesized by
low temperature reaction of Cd and excess Te under high vacuum seal in a
constant
volume quartz ampoule. Next, the polycrystalline CdTe was transferred to
growth
ampoules and monocrystals were grown by the vertical unseeded vapor growth
(VUVG)
technique at a temperature of between 930-933°C.
Another known growth technique is the liquid encapsulant Czochralski (LEC)
process. In Crystal Growth Processes (John Wiley 8~ Sons, 1986) on pages 151-
54
\ PC783
CA 02292853 1999-12-20
3
relating to the growth of GaAs, J.C. Brice describes such a method, the basis
of which is
that it does not have a vapor phase. The method involves placing a certain
amount of the
material to be grown and the encapsulant in a crucible heated by a radio-
frequency field.
The encapsulant melts first, so as to separate the molten material from the
crucible and
coats the crystal and the surface of the molten material. The seed crystal is
brought to the
surface of the liquid and is grown by known processes under pressure, but it
is coated
with encapsulant that prevents it from decomposing. B203 is mentioned as a
suitable
encapsulant.
U.S. Patent No. 4,740,264 describes a method of growth using float zone
techniques in which a rod of crystalline material, such as GaAs, is placed in
a support
structure and an encapsulant, such as 8203, having a lower melting point than
the
crystalline material, is placed between the crystalline material and the
surrounding support
structure. Prior to melting the rod, the container is heated, by use of
resistance heating
coils, to melt the encapsulant surrounding the rod, with the result that the
support
structure, the encapsulant and the rod are all heated. The support structure
is moved
longitudinally through a heated zone to progressively melt sections of the rod
as in
conventional float zone processes.
U.S. Patent No. 5,057,287 describes a method of growth for Group III-V and
Group
II-VI crystals in which a seed crystal (e.g. GaAs), precompounded crystal
material (e.g.
polycrystalline GaAs), and an encapsulant are placed in a vessel in a furnace,
and are
passed sequentially through three furnace zones. The first furnace zone is
maintained at
a temperature above the melting point of the encapsulant but below the melting
point of
the crystal material, the second furnace zone is shorter in length than the
length of the
vessel and is maintained at a temperature at least equal to the melting
temperature of the
crystal material, and the third furnace zone is maintained at a temperature
above the
melting point of the encapsulant but below the melting point of the crystal
material.
Another method for growing Group III-V and Group II-VI crystals is described
in
U.S. Patent No. 5,131,975. According to that method, small pieces of boron
oxide
encapsulant are dispersed throughout a comminuted compound semiconductor
charge in
a crucible that also contains a seed crystal. The temperature of the crucible
is first raised
to a value above the melting point of the boron oxide, which allows the boron
oxide to form
a sufficiently uniform layer of liquid between the walls, the base of the
crucible and the
semiconductor still in the solid state. The temperature is then raised
approximately to the
melting point of the compound semiconductor material and the crystal growth
proceeds
according to the liquid encapsulant vertical cooling gradient growth process.
~PC783
CA 02292853 1999-12-20
4
Tomoki Inada et al., "Growth of Semi-Insulating GaAs Crystals with Low Carbon
Concentration Using Pyrolytic Boron Nitride Coated Graphite," Appl. Phys.
Lett. 50(3), pp.
143-145 (January 19, 1987), describe a method for minimizing the incorporation
of carbon
into GaAs crystals grown by the in situ synthesis liquid encapsulated
Czochralski method
under an Ar gas ambient. The method involves the use of pyrolytic boron
nitride coated
graphite components in the large puller.
U.S. Patent No. 4,652,332 describes a method for synthesizing and growing
CuInSez (I-III-VI) monocrystals. The Applicant has noted an apparent error in
the text of
the above-mentioned US patent, which may indicate that temperatures indicated
in
degrees C in the text should in reality be expressed in degrees K. For
example, in column
5, lines 21-22, it is stated that "the 8203 begins to soften at approximately
700°C." The
Handbook of Chemistry and Physics, p. B-79 (66'" Edition, 1985-86) gives the
melting
point of B203 as 450°C. 450°C is 723° K. The method
described comprises placing
stoichiometric amounts of Cu, In and Se in a crucible, along with 8203. The
Cu, In, Se and
8203 are heated under pressure by means of an RF induction heating coil. The
increase
in temperature causes liquefaction of the 8203 and coats the Cu, In and Se
placed in the
crucible and also causes the Se to begin to evaporate significantly. However,
because
the 8203 is relatively soft and close to melting, most of the selenium is
confined in the
crucible. As the temperature is raised, the Cu, In and Se inside the crucible
form molten
encapsulated CuInSez, and the temperature is further raised to homogenize the
compound. Next, a crystal is grown by inserting a seed crystal through the
encapsulant in
contact with the molten CuInSe2, and then raising it again at a suitable rate.
The patent
discloses that despite the presence of the encapsulant in combination with an
excess
pressure of 55-70 atmospheres, there is still some small loss of Se from the
crucible
during the process, which can prevent the stoichiometric growth of the
crystal. To solve
this problem, the patent teaches adding an extra 1.8% to 2.0% of Se to the
crucible in
order to compensate for these losses and to obtain final conditions that are
correct for
stoichiometric growth.
From the account outlined above, relating to the known techniques for the
synthesis and growth of monocrystals which involve the use of a liquid
encapsulant, it is
clear that considerable efforts have been made to improve the quality of the
crystal grown.
These efforts include, among other things, modifying the growth apparatus,
appropriately
selecting the materials of apparatus construction, producing particular
temperature
gradients in the growing crystal or various temperature changes over time and
even by
adding excess amounts of reactants to compensate for their vaporization.
CA 02292853 1999-12-20
PC783
Applicants have found, however, no teaching in the prior art of using an
encapsulant method for the synthesis of Group II-VI polycrystalline compounds
from which
Group II-VI monocrystals are grown.
This is likely due to the fact that many of the Group II and Group VI elements
begin to
5 vaporize significantly at temperatures below the melting temperature of
encapsulants such
as 8203. Thus, in prior art processes where melting the encapsulant
necessarily resulted
in heating the reactants, before melting of the encapsulant and coating of the
reactants
was complete, one or more of the volatile reactants would begin to vaporize
unimpeded by
the encapsulant resulting in non-stoichiometric polycrystalline compounds.
Consequently,
because the starting materials for monocrystal growth were non-stoichiometric,
the above
described growth methods would produce Group II-VI monocrystals having
incorrect
stoichiometry and/or impaired electronic properties.
Inventors have determined in particular that the solid-state reaction method
does
not ensure the complete reaction of the elements. A polycrystal obtained by
this method
can be strongly non-stoichiometric. A non-correct stoichiometry can occur also
with the
closed ampoule syntheses method, such as the one described in the cited paper
by F.P.
Doty et al.
Inventors have observed that the vapor phase synthesis is, in general, used
for
small productions of compounds and requires a long synthesis time.
Furthermore, the
apparatus used to perform this method are complex due to the requirement of
avoiding
losses of reactants' vapors. Cd and Te vapors, for example, are dangerous in
that they
can cause cancer.
SUMMARY OF THE INVENTION
In accordance with the invention as embodied and broadly described herein,
the present invention in one aspect includes a process for producing Group II-
VI or Group
III-V polycrystalline compounds of high purity and correct stoichiometry. The
process
comprises heating an encapsulant to a first temperature sufficient to melt the
encapsulant;
surrounding stoichiometric amounts of at least two reactants with the
encapsulant in a
molten state wherein during encapsulation the reactants do not achieve a
temperature
sufficient to cause vaporization of one or more of the reactants; and forming
a
polycrystalline Group II-VI or Group III-V compound from the reactants.
Typically, the step of surrounding stoichiometric amounts of at least two
reactants with the encapsulant in a molten state further comprises heating
said reactants
surrounded by said encapsulant to a second temperature sufficient to melt said
reactants
CA 02292853 1999-12-20
PC783
6
and cause said reactants to react with each other and form a reaction product;
and
heating said reaction product to a third temperature sufficient to melt said
reaction product
and for a time sufficient to synthesize and homogenize said reaction product.
The present invention also includes a process for producing polycrystalline
compounds of high purity and correct stoichiometry, comprising the steps of:
placing
stoichiometric amounts of at least two reactants in a first region; placing an
amount of an
encapsulant in a second region sufficient to completely surround the reactants
when the
encapsulant is in a molten state, the second region being suitable for the
transfer of the
encapsulant in a molten state into the first region and the first and second
regions being
capable of being heated separately from each other; creating an environment of
gas
around the first and second regions and pressurizing the environment to a
pressure
substantially above the vapor pressure of the most volatile of the reactants
at a selected
maximum operating temperature; heating the second region to a first
temperature
sufficient to melt the encapsulant while not heating the first region, the
first region being
maintained at a temperature sufficient to avoid vaporization of the reactants
thus causing
the encapsulant, in a molten state, to move from the second region into the
first region and
surround the reactants; heating the reactants surrounded by the encapsulant to
a second
temperature sufficient to melt the reactants and cause the reactants to react
with each
other and form a reaction product; and heating the reaction product to a third
temperature
sufficient to melt the reaction product and for a time sufficient to
synthesize and
homogenize the reaction product.
The present invention further includes a process for producing polycrystalline
compounds of high purity and correct stoichiometry, comprising the steps of:
a) placing
stoichiometric amounts of at least two reactants in a first region of a
container; b) placing
an amount of an encapsulant in a second region of the container sufficient to
completely
surround the reactants when the encapsulant is in a molten state, the second
region being
suitable for the transfer of the encapsulant in a molten state into the first
region and the
second region being immediately above and adjacent to the first region and the
first and
second regions being capable of being heated separately from each other; c)
placing the
container in an enclosed vertically oriented chamber; d) creating an
environment of gas in
the container and pressurizing the environment to a pressure substantially
above the
vapor pressure of the most volatile of the reactants at a selected maximum
operating
temperature; e) heating the second region to a first temperature sufficient to
melt the
encapsulant while maintaining the first region at a temperature sufficient to
avoid
vaporization of the reactants thus causing the encapsulant, in a molten state,
to move
PC783
CA 02292853 1999-12-20
7
from the second region into the first region and surround the reactants; f)
heating the
reactants surrounded by the encapsulant to a second temperature sufficient to
melt the
reactants and cause the reactants to react with each other and form a reaction
product; g)
heating the reaction product to a third temperature sufficient to melt the
reaction product
and for a time sufficient to synthesize and homogenize the reaction product.
Additionally, the process comprises the steps after step (g) of: cooling said
reaction product surrounded by said encapsulant to a temperature below the
melting point
of said reaction product but above the melting point of said encapsulant; and
separating
said reaction product from said encapsulant.
Preferably, said reactants comprise at least one Group II element and at least
one
Group VI element or at least one Group III element and at least one Group V
element.
More preferably, said reactants are Cd and Te.
Preferably, the container into which the reactants are placed is associated
with at
least one other container. More preferably, a crucible is removably inserted
within said
container.
Advantageously, said encapsulant, employed in said processes for producing
polycrystalline compounds, is Bz03.
Preferably, said inert gas, employed in said processes for producing
polycrystalline
compounds, is argon gas.
Preferably, pressure in said chamber is between about 10 atm and about 100
atm.
More preferably, the pressure in said chamber is about 20 atm.
Preferably, said first temperature, in step (e) is at least the melting point
of the
encapsulant.
Preferably, said second temperature, in step (f) is at least the melting point
of the
highest melting reactant.
Preferably, said third temperature, in step (g) is at least the melting point
of the
reaction product.
Typically, said reaction product is a polycrystalline compound. Preferably,
said
reaction product is CdTe.
In another aspect, the present invention includes the polycrystalline compound
produced in accordance with the said process for producing polycrystalline
compound.
Preferably, said polycrystalline compound has a purity of at least about 5N.
Preferably, said second region of said process for producing polycrystalline
compound, contains a heating element. Advantageously, said encapsulant is
melted in
step (e) by moving said container within said chamber relative to an
electrical source
CA 02292853 1999-12-20
PC783
8
within said chamber sufficient for an electric current from said electrical
source to induce
an electric current in said heating element without inducing a current in said
reactants in
said first region.
Preferably, in said process for producing polycrystalline compound said
reactants
are heated in step (f) by moving said container within said chamber relative
to an electrical
source within said chamber sufficient for an electric current from said
electrical source to
induce a current in said reactants.
Preferably, during step (g) said reaction product is rotated.
Preferably, said heating element is in contact with said encapsulant.
Preferably, said heating element is selected from the group consisting of
liquid
gallium or liquid indium. More preferably, said heating element is in a
receptacle within
said container.
Preferably, said heating element consists essentially of a graphite disc
coated with
pyrolytic boron nitride.
In another aspect, the present invention includes a process for producing a
monocrystal of high purity and correct stoichiometry from a polycrystalline
compound
produced by said process, wherein the process further comprises the steps of:
placing a
seed monocrystal in a housing in said first region of said container,
performing steps (a)
through (g); and gradually lowering said container, relative to said chamber,
such that the
temperature of said first region is gradually reduced and said monocrystal is
grown.
In another aspect, the present invention includes a process for producing an
encapsulated reactant composition comprising the steps of: heating an
encapsulant to a
first temperature sufficient to melt the encapsulant; surrounding
stoichiometric amounts of
at least two reactants with the encapsulant in a molten state before the
reactants achieve
a temperature sufficient to cause vaporization of one or more of the reactants
wherein the
reactants comprise at least one Group II element and at least one Group VI
element.
The present invention also includes an encapsulated reactant composition
comprising at least two reactants surrounded by an encapsulant wherein the
reactants
comprise at least one Group II element and at least one Group VI element or at
least one
Group III element and at least one Group V element.
In another aspect, the present invention includes a polycrystalline compound
comprising at least one Group II element and at least one Group VI element or
at least
one Group III element and at least one Group V element and having a purity of
at least
about 99.998% and wherein none of the elements comprising the compound
deviates
from stoichiometric composition by more than about 0.01 mol%.
CA 02292853 1999-12-20
PC783
9
In another aspect, the present invention includes a process for producing a
monocrystal of high purity and correct stoichiometry, comprising the steps of:
a) heating an encapsulant to a first temperature sufficient to melt the
encapsulant; b)
surrounding stoichiometric amounts of at least two reactants with the
encapsulant in a
molten state before the reactants achieve a temperature sufficient to cause
vaporization of
one or more of the reactants, wherein the reactants comprise at least one
Group II
element and at least one Group VI element; c) heating the reactants surrounded
by the
encapsulant to a second temperature sufficient to melt the reactants and cause
the
reactants to react with each other and form a reaction product; d) heating the
reaction
product to a third temperature sufficient to melt the reaction product and for
a time
sufficient to synthesize and homogenize the reaction product; e) forming a
monocrystal
from the reaction product. Preferably, said encapsulant in step (a) forms a
liquid.
Preferably, said monocrystal in step (e) is formed by growing.
In a further aspect, the present invention includes a process for producing a
monocrystal of high purity and correct stoichiometry, comprising the steps of:
producing
an encapsulated reaction product according to the said process for producing
an
encapsulant reaction product ; heating said reaction product to a molten
state; inserting a
seed monocrystal into said reaction product; and gradually raising said seed
monocrystal
out of said molten reaction product.
In another aspect, the present invention includes a monocrystal of high purity
and
correct stoichiometry comprising at least one Group II element and at least
one Group VI
element or at least one Group III element and at least one Group V element and
having a
purity of at least about 99.9998% .
In another aspect, the present invention includes an apparatus for producing
polycrystalline compounds having high purity and correct stoichiometry,
comprising: an
oven having at least one chamber; a container within the chamber having a
first region for
containing reactants and at least a second region for containing an
encapsulant and a
heating element; heater for the encapsulant; means for rotatably and
vertically supporting
and moving the container within the chamber; and a heater for the first and
second
regions of the container independently of each other. Preferably, said oven
contains at
least one window.
In a further aspect, the present invention includes an apparatus for producing
polycrystalline compounds having high purity and correct stoichiometry,
comprising: an
oven having at least one vertically oriented chamber; a container within the
chamber
having a first region for containing reactants and a second region immediately
above and
PC783
CA 02292853 1999-12-20
adjacent to the first region for containing an encapsulant and a heating
element for
heating the encapsulant; a member connected to a motor for rotatably and
vertically
supporting and moving the container within the chamber; and an electrically
powered
inductive heating coil for inducing an electric current in the heating element
when the
5 heating coil is encircling the second region of the container while not
heating the first
region of the container, and for inducing an electric current in the reactants
when the
heating coil is encircling the first region of the container. Preferably, said
container is
associated with at least one other container. Preferably, a crucible is
removably inserted
within said container.
BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings, which are incorporated in and constitute a part of
this specification, illustrate embodiments of the invention and, together with
the
description, explain the advantages and principles of the invention.
Fig. 1 is a cross-sectional view of an apparatus used to synthesize
polycrystalline
compounds in the stage prior to melting of the encapsulant consistent with an
embodiment
of the present invention.
Fig. 2 is a similar view to Fig. 1 in the stage after the encapsulant has
melted and
surrounded the reactants.
Fig. 3 is a similar view to Fig. 2 in the stage after the encapsulated
reactants have
melted.
Fig. 4 is a diagram showing power of the heating coil vs. time for CdTe during
practice of the process of the present invention.
Fig. 5A is a cross-sectional view of a conventional apparatus for closed
ampoule
PVT growth of monocrystals.
Fig. 5B shows a typical thermal profile of an oven for PVT growth of
monocrystals.
Fig. 6 is a similar view to that in Fig. 1 of another embodiment of the
present
invention showing a modified container having a seed pocket in the bottom
below the
reactants.
Fig. 7 is a similar view to that in Fig. 1 of another embodiment of the
present
invention showing a rod above the container for inserting a seed crystal into
the
encapsulated melt.
It is to be understood that both the foregoing general description and the
following
detailed description are exemplary and explanatory only and are not
restrictive of the
PC783
CA 02292853 1999-12-20
11
invention, as claimed. The following description, as well as the practice of
the invention,
set forth and suggest additional advantages and purposes of this invention.
DETAILED DESCRIPTION
The present invention provides a novel process and apparatus for producing
novel
polycrystalline compounds as well as novel monocrystals formed from such
compounds
for use in a wide variety of semiconductor applications. The present invention
further
provides novel encapsulated reactant compositions from which the above-
mentioned
novel polycrystalline compounds and monocrystals can be produced.
The present invention provides a process and apparatus for producing
crystalline
compounds of high purity and of correct stoichiometry.
In this context, "high purity" is defined as a purity of at least about
99.999%, preferably
at least about 99.9997% and most preferably about 99.9999% for polycrystalline
compounds. In the context of monocrystals, high purity is defined as a purity
of at least
about 99.999, preferably at least about 99.99998%, and most preferably about
99.99999%. In general, the degree of impurity of a sample of a substance is
calculated by
evaluating the ratio, expressed as a percentage, between the weight of the
impurity,
contained in the sample, having the largest weight and the total weight of the
sample. The
degrees of purity is defined as 1 minus the degree of impurity. In addition,
it is common in
this art to express the purity of a sample by indicating how many of the first
figures are
equal to "9" in the degree of purity defined above. Thus, for a degree of
purity equal, for
example, to 99.99%, a purity of 4N is indicated. Consequently, a purity of at
least
99.998% is equal to a purity of at least about 5N. "Stoichiometry" deals with
combining
weights of elements and compounds using ratios that permit the calculation of
the moles
of one substance as related to the moles of another substance.
For the purposes of the present invention the term "charge" signifies the
material
introduced in a suitable container, comprising elements, compounds, dopants,
and which,
at the end of the process, form the synthesis or growth process product.
By the term "compound" it is meant a substance composed of atoms or ions of
two
or more elements in chemical combination having definite proportions by
weight.
By the term "alloy" it is meant a mixture (liquid or solid) of two or more
elements
which can have various proportions by weight.
By the term crystal it is meant a homogeneous solid made up of an element,
chemical compound or isomorphous mixture throughout which the atoms or
molecules are
arranged in a regularly repeating pattern.
PC783
CA 02292853 1999-12-20
12
The terms "monocrystal" and "single crystal" signify a crystal in which all
parts
have the same crystallographic orientation.
The term "polycrystal" signifies a material composed of aggregates of
individual
crystals.
Further, in the context of the present invention the terms "polycrystalline
compound" and "monocrystalline compound" signify a compound or an alloy, which
can
comprise dopants, having respectively polycrystalline and monocrystalline
structure.
The process of the present invention comprises several steps. First,
stoichiometric
amounts of at least two reactants are placed in a first region of a container.
The process
is especially well suited for preparing compounds from one or more reactants
which are of
high volatility below the melting point of the chosen encapsulant and/or which
are volatile
at a temperature close to the encapsulant melting point.
Applicant notes that, in accordance with the invention, it is avoided that
before the
encapsulant completely melts and covers the charge large amounts of these
elements
undergo sublimation and then are lost.
Suitable elements include Cd, Zn, Hg, Te, Se, Ga, As, In, TI, Mg, Sb, Ge, Ag,
Cu,
P, Se, S. Preferably, at least one reactant is a Group II element and at least
one reactant
is a Group VI element or at least one reactant is a Group III element and at
least one
reactant is a Group V element.
The process is particularly suitable for CdTe, CdZnTe, and compounds having at
least
one of Cd, Hg, Se, T e, Zn, S, Sb, As, P, that are relatively more volatile,
while the
advantage is more limited for Bi, In, Ga, Te, that are less volatile.
Most preferably, the reactants are Cd and Te, alone, or in combination with
one or
more other elements or dopants. Suitable dopants include In, CI, I, B, AI, Ga,
Fe, Cu, Ag,
Hg, Se, Zn, S, Se, Sb, As, P.
As an example, dopants can be added to the charge in order to obtain specific
opto-electronic or structural properties. If dopants are used in a process
according with
the present invention they are added with the reactants at the beginning of
the process.
The container into which the reactants are placed can itself be associated
with one
or more other containers. Preferably, the container is associated with a first
associated
container which, for purposes of clarity, but not by way of limitation, will
hereinafter be
referred to as a crucible. Most preferably, the container holds a crucible
which itself
contains a second associated container which, for purposes of clarity, but not
by way of
limitation, will hereinafter be referred to as a receptacle. One skilled in
the art can easily
determine suitable dimensions for the container, crucible and receptacle.
CA 02292853 1999-12-20
PC783
13
Advantageously, the ratio of the diameter to the height of the container is
from
about 1:5 to about 5:1. Preferably, the ratio of the diameter to the height of
the container
is from about 1:2 to about 2:1. Most preferably, the ratio of the diameter to
the height of
the container is about 1. Advantageously, the ratio of the diameter to the
height of the
crucible is from about 1:5 to about 5:1. Preferably, the ratio of the diameter
to the height
of the crucible is from about 1:3 to about 3:1. Most preferably, the crucible
has a diameter
to height ratio of about 0.7:1. Advantageously, the ratio of the diameter to
the height of
the receptacle is from about 10:1 to about 1:5. Preferably, the ratio of the
diameter to the
height of the receptacle is from about 5:1 to about 1:2. Most preferably, the
receptacle
has a diameter to height ratio of about 1.2:1.
Preferably, the container (or the crucible, if one is present) has two regions
which
are capable of being heated separately from each other and may be, but
preferably are
not, separated by a discrete barrier. The container may also have more than
two regions.
The container is supported by a member, preferably a shaft attached to a
motor, that
provides for vertical, horizontal, or rotational movement, or a combination
thereof, of the
container. Preferably, the member provides for vertical and rotational
movement of the
container. If synthesis and/or growth are to take place horizontally, the
member should
provide for horizontal and rotational movement.
In the context of the present invention, the terms "means for rotatably and
vertically
supportingu and "means for moving" comprise any device, mechanic, magnetic,
elastic,
electric, pneumatic, etc. that are suitable for the above described purposes.
The containers and shaft can be constructed from any suitable material, or
combination of materials, known to those skilled in the art for such purposes.
Suitable
materials of construction include quartz, graphite, graphite coated quartz ,
AI203 and
pyrolytic boron nitride (pBN) coated graphite. The use of pBN coated graphite
or AI203 for
any components of the apparatus that may come in contact with the encapsulant,
reactants, polycrystalline compound or monocrystal is advantageous because it
avoids
the potential for silicon contamination inherent in the use of quartz.
Consequently, the use
of such materials would be expected to contribute to the production of high
purity
compounds.
Next, an amount of an encapsulant sufficient to completely surround the
reactants
when the encapsulant is in a molten state is placed in the container.
Advantageously, the
encapsulant is inert with respect to the containers, the reactants and the
reaction product,
will adhere to the reactants, the reaction product and the growing crystal
without
damaging the crystal, has relatively low melting point, suitable density and
is easy to
CA 02292853 1999-12-20
PC783
14
remove from the various components when the synthesis and/or growth is
complete.
Suitable encapsulants include for example KCI-NaCI 1:1 and 8203. Preferably,
the
encapsulant is 8203. Preferably, the molten state is a liquid. The encapsulant
may be
placed in the same region as the reactants or in a different region than the
reactants.
Preferably, the encapsulant is in a second region, different to the region
containing
the reactants, and suitable for the transfer of the encapsulant in a molten
state into the first
region containing the reactants. Preferably the encapsulant is above and
directly adjacent
to the reactants. Most preferably, the encapsulant is in contact with the
reactants.
Next, a heating element is placed in the second region, adjacent to the
encapsulant, for heating the encapsulant. The heating element need not be, but
preferably is, in contact with the encapsulant. Most preferably, the heating
element is
above and in contact with the encapsulant. The heating element should be
capable of
having a current induced in it to generate heat. Such a heating element is
desirable
because the encapsulant 8203 is electrically insulating, thus it is not
possible to induce a
current in it directly to heat and melt it. Suitable heating elements include
liquid gallium,
liquid indium, other element or compounds with suitable low vapor pressure or
combinations thereof, which are preferably placed in the receptacle within the
crucible, as
well as graphite, pBN-coated graphite, SiC (silicon carbide) discs that need
not be placed
in the receptacle. Most preferably, the heating element is a graphite disc
coated with pBN
to avoid solubilization of the graphite in the encapsulant and subsequent
carbon
contamination of the synthesized polycrystalline compound or contamination of
the same
by impurities contained in the graphite.
The container is placed in a chamber containing a heating system. Preferably,
the
chamber is in an oven, which can have a window through which the contents of
the
chamber can be viewed. The oven walls can be surrounded by fluid cooling
tubing,
operating in association with a conventional fluid circulation and
refrigeration system. The
fluid can be, e.g., water.
The chamber can be horizontally oriented, but, preferably, the chamber is
vertically
oriented. The heating system used can be any suitable heating system known in
the art
and may be stationary or movable vertically or horizontally. Preferably, the
heating
system is stationary. Suitable heating systems include graphite cylindrical
heating
elements, inductive heating coils and resistance heating elements. Resistance
heating
elements are electric elements that generate heat under an applied current.
Preferably, the heating system is an electrically powered inductive heating
coil.
PC783
CA 02292853 1999-12-20
' 15
Preferably, the inductive heating coils are associated with a fluid cooling
system.
The cooling fluid can be, e.g., water. The cooling fluid circulates inside the
coils in order to
avoid their overheating and the breakage during operation. Furthermore, the
fluid cooling
system quickly lowers the coils' temperature when the heating apparatus is
switched off.
Most preferably, the heating coil comprises copper coil windings powered by a
radio
frequency generator. Suitable dimensions for the heating system can be easily
determined by one skilled in the art. Advantageously, if copper coil windings
are used,
they should have a diameter to height ratio from about 1:5 to about 10:1.
Preferably the
diameter to height ratio is from about 1:1 to about 2:1. Most preferably, the
diameter to
height ratio is about 1.7:1.
Applicants observe that the heating coils have to be such as to heat the
element
32 without heating the reactants during the first heating step. Then, the
height upper limit
of the heating coils is not critical.
An environment of inert gas is created in the chamber. Suitable inert gases
can
easily be determined by one of skill in the art and include argon and
nitrogen. In general a
gas of one of the components can be used. An inert gas, however, is preferred
in order to
avoid corrosion and safety problems.
The environment is then pressurized. Preferably, the environment is
pressurized
to a pressure substantially above the vapor pressure of the most volatile of
the reactants
at a selected maximum operating pressure. Preferably, the maximum operating
temperature is higher than the melting temperature of the polycrystalline
compound. More
preferably, the environment is pressurized to a pressure of between about 10
atm and
about 100 atm.
Most preferably, the environment is pressurized to a pressure of between about
20
atm and about 40 atm. If the reactants are Cd and Te the most preferred
pressure is 20
atm.
Next, the heating element is heated to a temperature sufficient to melt the
encapsulant. The heating element can be heated by any suitable means known to
those
skilled in the art that will allow the region containing the heating element
to be heated
without heating the region containing the reactants. Preferably, the heating
element is
heated by means of the heating system. Most preferably, the container is
arranged within
the chamber such that the region containing the heating element is within the
ambit of the
inductive heating coils, while the first region containing the reactants is
outside the ambit
of the inductive heating coils. Power is then supplied to the inductive
heating coils
sufficient to induce an electric current in the heating element which in turn
heats and melts
CA 02292853 1999-12-20
PC783
16
the encapsulant without inducing a current in the reactants in the first
region. Preferably,
the encapsulant is heated to a temperature which is at least the melting point
of the
encapsulant. As the heating of the encapsulant does not induce any current in
the region
in which the reactants are placed, vaporization of the reactants is minimized,
what aids in
the conservation of stoichiometric ratios.
As the encapsulant melts, it moves into the region containing the reactants
and
surrounds the reactants. The region containing the reactants is not heated
during the
melting of the encapsulant and is thus maintained at a temperature sufficient
to avoid
vaporization of the reactants. This does not mean that no vaporization of the
reactants
occurs, rather it means that any vaporization that does occur is negligible
and does not
significantly impact the stoichiometry of the polycrystalline compound formed.
Because
the lower region containing the reactants is only heated after the reactants
have been
coated with the molten encapsulant, vaporization is minimized, further
contributing to the
synthesis of products having correct stoichiometry. Furthermore, because the
reactants
are not heated during the heating of the encapsulant, the length of time
during which the
polycrystalline compound experiences high temperatures is shortened and
contamination
by the materials which make up the crucible, or container, is reduced.
Moreover, the
production of polycrystalline compounds having correct stoichiometry and low
impurity
content improves the quality of the monocrystals grown from such compounds.
According to one aspect of the present invention, the reactants surrounded by
the
molten encapsulant constitutes an inventive product. The reactants surrounded
by molten
encapsulant can be cooled to a temperature below the melting point of the
encapsulant
such that the encapsulant solidifies around the reactants. The inventive
product can then
be removed from the container for polycrystalline compound synthesis at some
later time
according to the further process of the present invention or according to
another process.
After the reactants are surrounded by molten encapsulant, they are heated by
bringing the region containing the reactants surrounded by molten encapsulant
within the
ambit of the heating system. To accomplish this, either the container can be
moved so
that the region containing the reactants is in the position previously
occupied by the region
containing the heating element, or the heating system can be moved so that
instead of
being positioned around the region containing the heating element, it is
positioned around
the region containing the reactants. Preferably the container is moved to
bring the
reactants within the ambit of the heating system. Most preferably the
container is moved
vertically to a position where the heating coils encircle the region
containing the reactants.
CA 02292853 1999-12-20
PC783
17
The reactants are heated by the heating system to a temperature sufficient to
melt
the reactants and cause the reactants to react with each other and form a
reaction
product. Preferably, the reactants are heated to a temperature which is at
least the
melting point of the highest melting reactant. Preferably, the reactants are
heated by
direct induction of electrical current from heating coils, thereby avoiding
the use of heating
materials, such as graphite, which can contaminate the compound and degrade
the purity
of the resulting product. The reaction product is then heated to a temperature
sufficient to
melt the reaction product and for a time sufficient to synthesize and
homogenize the
reaction product. Preferably, the reaction product is heated to a temperature
which is at
least the melting point of the reaction product. Preferably, the reaction
product is rotated,
by rotation of the container, during melting, synthesis and homogenization.
Next, the reaction product is cooled to a temperature below the melting point
of the
reaction product but above the melting point of the encapsulant such that the
reaction
product solidifies and the encapsulant remains molten.
Then, the heating system is switched off and the encapsulant cools and
solidifies.
Furthermore, the cooling phase is made quicker by the fluid cooling tubing
system
associated with the oven.
The encapsulant can at this point be removed by plunging the container of the
reaction product in water, H20, or in alcohol (ethylic or methylic). The
encapsulant then
softens and, since the compound remains solid, their separation takes place.
In water the container may break because of the encapsulant expansion while in
alcohol the expansion is less rapid and the container can be preserved.
Quartz containers are less expensive than pBN ones.
The encapsulant can be dissolved also by means of ultrasonic waves softening
the
encapsulant and having no effect on the compound.
The reaction product can be cooled by any suitable cooling means known to
those
skilled in the art.
The apparatus comprising a water-cooled heating system and a water-cooled oven
has low heating inertia.
Such a system offers the possibility of producing rapid cooling ramps which
makes
it possible to synthesize even alloys with a relatively large number of
reactants or with
dopants while avoiding segregation and thus makes possible the following
growth of
crystals from such alloys. The polycrystalline compound reaction products of
the present
invention can be used to grow monocrystals of high purity and correct
stoichiometry and
can be used in any growth process and apparatus as further discussed below.
CA 02292853 1999-12-20
PC783
18
The following further detailed description refers to the accompanying
drawings.
The same reference numbers identify the same or similar elements. The
description
includes exemplary embodiments, however, other embodiments are possible, and
changes may be made to the embodiments described without departing from the
spirit and
scope of the invention. Rather, the scope of the invention is defined only by
the appended
claims. In the description, reference will be made, for simplicity, to the
synthesis and
growth of CdTe. However, it will be clear to those skilled in the art that the
inventive
concepts described are also applicable to the synthesis and growth of other
compounds
and alloys of the family II-VI such as, for example, binary, ternary or
quaternary
compounds and alloys such as CdSe, ZnTe, ZnSe, and ZnCdTe and semi-magnetic
compounds and alloys such as HgMnTe and CdMnTe, as well as materials doped
with
suitable elements, e.g., n or p type dopants.
The same inventive process and apparatus can also be extended to the synthesis
of certain Group III-V compounds and alloys, antimonides, arsenides and
ternary
compounds. The process and apparatus of the present invention can be employed
effectively even in the synthesis of polycrystalline compounds other than
Group II-VI or III-
V compounds and alloys including, for example, CuInSe2, AgGaSe2, AgGaTe2,
CdGeAs2,
InS with minor variations in the choice of the characteristic parameters that
will be readily
determinable by those skilled in the art.
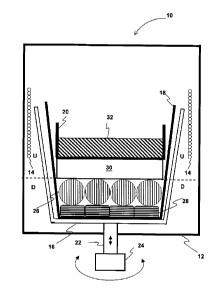
Fig. 1 is a diagram of a preferred embodiment of an apparatus consistent with
the
principles of the present invention which is appropriate for the synthesis of,
for example, a
CdTe compound suitable for growing a monocrystal. The apparatus includes an
oven 10
within which is a vertically oriented chamber 12 inside which is an inductive
heating coil
14, including a container 16 which preferably contains a crucible 18. A
receptacle 20 can
be inserted into the crucible 18. The container 16 is mounted onto a shaft 22
solidly
connected to a motor 24 so as to allow, with respect to the heating coil 14,
the rotation and
vertical translation of the container 16 and, with it, of the crucible 18 and
of the receptacle
20. Preferably, the container and receptacle 16 and 20, the crucible 18 and
the shaft 22
are made of quartz. Preferably, the oven 10 also has windows, which allow the
inside of
the apparatus to be seen.
More preferably, the oven 10 walls are surrounded by water cooling tubing
operating in association with a conventional fluid circulation and
refrigeration system (not
shown in the figure). The fluid can be, e.g., water.
A preferred process for synthesizing compounds, in accordance with the
principles
of the present invention, will become clear from the description of a process
carried out by
PC783
CA 02292853 1999-12-20
19
the Applicant for the synthesis of CdTe using the above-described novel
apparatus of Fig.
1.
The synthesis process was monitored and controlled by viewing the contents of
the chamber through the above-mentioned window. In particular, the state
changes
(melting, solidification) of the encapsulant and the charge and the chemical
reactions of
the charge was directly observed and/or deduced by color changes of the
charge, in a
way apparent to the skilled in the art.
The melting point of the boron oxide encapsulant is Tm = 450°C or
Tm=723°K.
Te has a melting point Tm = 430°C. The vapor pressure of Te,
P°TB(in bar), can be
calculated, for any temperature T (in °K), from: log P°Te=
4.7191-5960.2/T. The tellurium
vapor mainly (95%) consists of diatomic molecules, i.e., Te2. The vapor
pressure of Te2
at the melting point of the B2O3 is P°Tez (723 °K) = 3~10~' bar.
Cd has a melting point Tm = 320°C. The vapor pressure of Cd,
P°cd (in bar), can
be calculated, for any temperature T in (°K), from the following
relation: In P°cd= 26.15-
13859/T-1.8415 InT.
The vapor pressure of Cd, P°cd, at the melting point of the B2O3 is
P°cd(723°K) _
5.810-3 bar. Thus, one can see that Cd is more volatile than Te (because it
has a higher
vapor pressure) and that the melting points for both Cd and Te are below the
melting point
of 8203.
Applicant observes that according to conventional methods the complete melting
of the BZO3 is reached when the reactants have temperature greater than
450°C, then
their actual vapor pressures are even higher than the above mentioned values.
Consequently, prior to the present invention, the use of an encapsulant such
as
8203 in the synthesis of CdTe or similar compounds starting from at least one
low fusing
and/or high volatility reactant was not feasible because prior art methods
could not avoid
heating the reactants during heating of the encapsulant to its melting point.
Example 1
In a first stage of a preferred process, that of preparation of the charge,
the
reactants Cd 26 and Te 28 were placed in the crucible 18 in stoichiometric
amounts:
200.03g of Cd and 227.08g of Te.
A 30g pellet of Bz03 encapsulant 30 approximately 3 cm in height was placed on
the reactants 26 and 28. The melting point of the boron oxide encapsulant is
Tm = 450°C.
The charge (reactants 26 and 28) occupied a first, lower region D of the
crucible
18. A receptacle 20 containing a heating element 32 was placed on the pellet
of
encapsulant 30. The heating element 32 consisted of about 60 g of liquid
gallium.
CA 02292853 1999-12-20
PC783
Encapsulant 30 and receptacle 20 occupied a second, upper region U of the
crucible 18.
The heating element 32 was used to heat the encapsulant 30. Because Bz03 is
electrically insulating it is not possible to induce a current directly in it
by the heating coil
5 14.
The crucible 18 thus assembled was placed in the container 16. The chamber 12
was pressurized with argon gas, of high purity (99.999%) at 20 atm. This value
is greater
than the vapor pressure of Cd and Te2 in equilibrium with CdTe at
1110°C, i. e., the
highest temperature envisaged in the crucible.
10 The container 16 was then vertically positioned such that the heating coil
14
encircled only the upper region U of the crucible 18, corresponding to the
region occupied
by the heating element 32, as shown in Fig. 1.
Next, power was supplied to the heating coil 14 so as to induce heat in the
heating
element 32 which transmitted it to the pellet of encapsulant 30. The
encapsulant 30 was
15 softened and melted, initially in its top part, i.e. the part closest to
the heating element32,
and then a first layer of liquid encapsulant covered the contents of the
crucible18. Finally,
the encapsulant 30 melted completely as illustrated in Fig. 2.
Fig. 4 is an approximate power-time diagram of the synthesis process in
accordance with the present invention. In this diagram the power has been
expressed in
20 arbitrary units. The first line f-g corresponds to melting of the 8203
encapsulant30. The
liquefaction had a duration of about 30 minutes. The melting of the
encapsulant took
place without heating the region D of the crucible 18, thus maintaining the
temperature of
this region at a value which did not cause volatilization of the reactants.
The line g-h
corresponds, approximately, to a waiting time necessary for the covering of
the charge.
Applicants have been ensured of the completely covering of the charge by
observing the contents of the chamber through the above-mentioned window.
The quantity of gallium inside the receptacle 20 was such to let the
receptacle 20
float on the molten encapsulant 30. Alternatively the receptacle 20 can be
fixed at the
container 16 walls.
Next, the container 16 was moved vertically so as to bring the lower region D
of
the crucible 18 containing the reactants 26 and 28 and the molten encapsulant
30 within
the ambit of heating coil 14 as shown in Fig. 2. The supply power to the
heating coil 14
was then gradually raised, causing the reactants Cd 26 and Te 28 to melt and
then react
together exothermically, this reaction being accompanied by a flash of light.
In Fig. 4, the
line h-i shows the power-time relationship for the reaction phase. The
reactants 26 and28
CA 02292853 1999-12-20
PC783
21
were heated only after they had been completely coated with the molten B2O3
encapsulant
30, thereby preventing them from being lost by vaporization during the
reaction phase.
Importantly, the heating of the reactants 26 and 28 took place by exploiting
their intrinsic
electrical conductivity, thus directly inducing a current in them and avoiding
the use of
heating elements, made of graphite or other materials, to support the crucible
18.
Line i-I corresponds to a pause between the reaction phase and the following
melting phase (I-m). Its duration is not critical and this phase can be even
omitted.
Next, in order to melt and homogenize the liquid and to ensure complete
reaction
of the reactants, the power of the heating coil 14 was further increased, line
I-m, so as to
melt the reacted CdTe (the melting point of CdTe is T~dTe = 1092°C).
During this state, the
crucible 18 (by means of the shaft 22 and the container 16) was rotated at a
speed of 5-10
rpm (revolutions per minute) for about 30 minutes. Line m-n corresponds to the
phase
needed to reach homogenization of the molten compound.
Applicants observed trough the suitable window and the molten encapsulant,
that
is transparent, the melting of the reacted compound, producing a bright yellow
color.
The compound was cooled by reducing the power of the heating coil 14 as shown
in Fig. 4 (line n-p) over about 120 minutes while at the same time moving the
first
container 16 downwards as in Fig. 3. In this situation, the reacted CdTe 34,
solidified
while the layer of encapsulant 30 was maintained in a liquid state.
Since 8203 has a weight density less than the one of CdTe the layer of
encapsulant 30 floated on the reacted compound of CdTe 34.
The synthesis process was monitored and controlled by viewing the contents of
the chamber through the above-mentioned window.
The apparatus described allowed a rapid cooling, also because the pressurized
environment of the oven had high thermal conductivity that ensured a
relatively high heat
loss from the apparatus walls.
Then, the radio-frequency power feeding the heating system was switched off
and
the encapsulant cooled.
The water-cooling in the heating system and the oven allowed a quick cooling
of
the encapsulant.
The crucible 18 was removed from the chamber 12 when the system had returned
to room temperature. Applicant observed no condensation of reactants on the
walls of the
chamber 12. This demonstrates that, during the process, there was no escape of
gaseous reactants through the encapsulant 30. Further proof of this was
obtained by
CA 02292853 1999-12-20
PC783
22
weighing the synthesized CdTe compound, which revealed no significant decrease
in
- weight relative to the initial amounts of reactants 26 and 28.
Applicant observes that, in the case of synthesis of doped charges or of
alloys the
cooling speed can be appropriately increased in order to avoid segregation of
the
reactants or the dopants. This is made possible by the apparatus of the
present invention
which has a low heat inertia.
Other temperature ramps different from the one shown are possible, these being
easily adapted by those skilled in the art, and are particularly useful in the
synthesis of
ternary and quaternary compounds or alloys or when dopants are used, to avoid
segregation of the reactants or of the dopants. The pressure to be exerted in
the chamber
12 for the synthesis of compounds different from CdTe can also be easily
determined by a
person skilled in the art.
The polycrystalline compound obtained was compared with a market available
polycrystalline compound acquired from ESPI, Italy. These polycrystalline
compounds
were analyzed, to evaluate the content in parts per million by weight (ppmw)
for each
impurity detected (ppmw = weight of impurity [pg] / weight of compound [g]).
Table 1 gives the results of the analyses performed. The first column gives
the
chemical symbols of the possible impurities, while the second and third
columns give the
corresponding ppmw values for the comparison sample of the polycrystalline
compound
and for the one produced according to the process described in Example 1,
respectively.
An empty position in the table corresponds to the absence of measurable amount
of an
impurity.
Table 1
CdTe CdTe
ComparisonExample
sample 1
Element ppmw ppmw
Li 0.003 0.003
Be
B 0.027 3.4
F
Na 0.045 0.007
Mg 0.16 0.13
AI 1.7 0.011
' PC783
CA 02292853 1999-12-20
23
Si 0.83 0.25
P 0.013 0.003
S 0.69 0.018
CI 200 0.047
K 0.023 0.007
Ca 0.1 0.019
Sc
Ti 0.038
V 0.003
Cr 0.017 0.004
Mn 0.021 0.008
Fe 0.59 0.09
Co 0.18 0.001
N i 0.64 0.002
Cu 0.3 0.018
Zn 0.076 0.007
Ga
Ge
As 0.64 0.03
Se 0.45 3.8
Br
Rb
Sr
Y
Zr
Nb
Mo 0.037 0.003
Ag 0.017
In 1.3
Sn 0.27
Sb 3.1
I
TI 0.055
Pb 9.5
PC783
CA 02292853 1999-12-20
24
Bi 0.083
Th
PURITY 99.98% ~ 99.9997%
It is clear from Table 1 that many of the impurities which are found in the
comparison polycrystalline compound (in particular the elements Ti, V, Ag, In,
Sn, Sb, TI,
Pb, Bi) are not present in the compound produced by the process and apparatus
of the
present invention. Although higher amounts of B and Se are present in the
inventive
polycrystalline compound, as discussed below, these levels are not of concern
in the
growth and performance of monocrystals. Specifically, Applicant assumes that
the Se
comes from the impurities thereof present in the Te reactant, and thus its
presence is
independent of the synthesis process.
Furthermore, because the Se is isoelectric with Te, its electronic properties
are
such that it does not adversely affect, at these concentrations, the behavior
of CdTe in
optoelectronic applications.
Applicant has performed another syntheses of a CdTe polycrystal by means the
method according to the invention and above described using a Te sample
acquired from
a different provider (Japan Energy). In this case, Applicant has found, in the
CdTe
polycrystal, a very low quantity of Se, i.e., 0.052 ppmw.
Moreover, B, as shown below, is not incorporated in vapor-phase growth
processes.
G.W. Blackmore et al. "Boron segregation in Czochralski-Grown CdTe" J. of
Crystal
Growth, pp. 335-340, vol. 8 (1987) describe that chemical analysis of CdTe
ingots grown
by the liquid encapsulated Czochralski technique using Bz03 encapsulant show
that the
material contains up to 90 pptua boron and that boron distribution is non-
uniform. Authors
of the article note that the observation of low carrier concentration and high
electrical
mobility in this material also supports the thesis that most of the boron is
not electrically
active. In this article the concentrations are expressed in "pptua", atomic
part per million.
The polycrystalline CdTe obtained in example 1 according to the present
invention
has a boron concentration of 3.4 ppmw, which corresponds to 75.48 pptua. The
conversion formula is: 1 pptua = M(CdTe)/M(B) x 1 ppmw = 240/10.81 ppmw = 22.2
ppmw; where M(CdTe) is the formula weight of CdTe and M(B) is the boron atomic
weight.
CA 02292853 1999-12-20
PC783
Applicants observes that the boron concentration of the material analyzed in
the
above mentioned article is greater than the boron concentration of the sample
synthesized
in accordance with the invention.
As for the other impurities detected (Na, Mg, AI, Si, P, S, CI, K, Ca, Cr, Mn,
Fe, Co,
5 Ni, Cu, Zn, As, Mo), the amounts found in the compound produced by the
process and
apparatus of the present invention are all substantially lower than the
amounts in the
commercially available ESPI product.
According to the results of the analyses shown in Table 1, the polycrystalline
compound acquired from ESPI has a degree of purity equal to 99.98%, i.e. a
purity of
10 about 4N. However, the polycrystalline compound produced by the process and
apparatus of the present invention has a degree of purity equal to 99.9997%,
i.e. a purity
of more than 5N.
The method of synthesis described is compatible with any method of growth. The
apparatus and growth methods described in the following examples are
illustrative and are
15 particularly advantageous for use in the practice of the present invention.
Example 1A
Applicant has synthesized a Cd2nTe alloy (Group II-II-VI) having a Zn quantity
percentage of 20%. Zn has melting point T,"=419.5°C.
In a first stage of a preferred process, that of preparation of the charge,
the
20 reactants Cd, Zn, Te, were placed in the crucible 18 in stoichiometric
amount: 139.37 g of
Cd, 20.27 g of Zn, 197.78 g of Te.
The charge has a total weight of 357.42 g.
A pellet 30 of about 30 g of 8203 encapsulant was placed on the reactants.
The reactants occupied a lower region D of the crucible 18.
25 A receptacle 20 containing a heating element 32 was placed on the pellet of
encapsulant 30. The heating element 32 consisted of about 60g of liquid
gallium.
Encapsulant 30 and receptacle 20 occupied an upper region U of the crucible
18.
The synthesis process has been carried out in a manner analogous to the one
described in detail in the Example 1 with reference to figures 1-4. The
process parameters
(temperatures, times, pressures, etc.) were adapted according to known
techniques.
In order to avoid Zn segregation the cooling was effected by suitably reducing
the
power of the heating coils 14 and at the same time moving the first container
16
downwards as in Fig. 3.
The crucible 18 was removed from the chamber 12 when the system had returned
to room temperature. Also in this case, Applicant observed no condensation of
reactants
CA 02292853 1999-12-20
PC783
26
on the walls of the chamber 12. This demonstrates that, during the process,
there was no
escape of gaseous reactants through the encapsulant 30. Further proof of this
was
obtained by weighing the synthesized CdZnTe compound, which revealed no
significant
decrease in weight relative to the initial amounts of reactants.
Example 2
Applicants grew two CdTe monocrystals from polycrystalline compounds
synthesized by the process and apparatus of the present invention. Both
crystals (Crystal
1 and Crystal 2) were grown by the PVT (Physical Vapor Transport) method, as
described
in the above-mentioned article by Yellin, N. and Szapiro, S., "Vapor Transport
of
Nonstoichiometric CdTe in Closed Ampoules", J. of Crystal Growth, Vol. 9, No.
2-3, pp.
555-60. With reference to Fig. 5A, 10-20 g of polycrystalline CdTe 34 prepared
according
to the process and apparatus described in Example 1, were placed inside a
quartz
ampoule 36 connected to a pulling rod 38 and closed with a vacuum of greater
than 10-5
mbar. The quartz ampoule 36 containing the polycrystalline CdTe 34 was then
placed
inside a vertical oven (not shown in figure) having a temperature profile of
the type shown
in Fig. 5B. The quartz ampoule 36, initially placed relative to the
temperature profile of the
oven as shown in the figure, was then moved, by means of the pulling rod 38,
upwards
relative to this oven. Supersaturation of the Cd and Te vapors thus causes the
condensation of a solid phase only when the pressure inside the ampoule is
such that it
does not inhibit mass transport. Table 2 gives the boron concentration values
determined in the synthesized polycrystalline sample and in two crystals grown
by the
described vapor phase technique. The value for B (main impurity in the
polycrystal) is
reduced by one/two orders of magnitude in the crystal grown in the vapor
phase,
indicating that B is not incorporated in vapor phase growth. The crystals thus
obtained
had purities of 6/7N.
Table 2
Sample Boron concentration
(ppmw)
Polycrystalline CdTe 3.4
CdTe Crystal 1 0.36
CdTe Crystal 2 0.19
As noted in the Yellin and Szapiro article on p. 556, mass transport growth is
completely inhibited if the stoichiometry of the polycrystalline charge is
shifted towards Cd
by a fraction greater than 0.01 mol%, and the rate of growth is reduced
considerably if the
f PC783
CA 02292853 1999-12-20
27
stoichiometry of the polycrystalline charge is shifted towards tellurium by a
fraction greater
than 0.04 mol%. Repeated laboratory tests demonstrate that the polycrystalline
CdTe
compounds synthesized according to the process and apparatus of the present
invention
allow mass transport growth in a closed ampoule, thus indicating that the
polycrystalline
CdTe compound has a stoichiometric shift which is appreciably lower than these
levels.
Applicants have determined that also other polycrystalline compounds
synthesized
according to the process and apparatus of the present invention have low
stoichiometric
shift (e.g. correct stoichiometry) due to the fact that the reactants can be
encapsulated
before reaching a temperature at which any meaningful vaporization occurs.
Example 3
Fig. 6 shows apparatus which allows the synthesis of a polycrystalline
compound
in a manner similar to that described with reference to Fig. 1, and also
allows the
monocrystal to be grown in a subsequent stage according to the Vertical
Bridgman
method.
The container 16 and flat-bottomed quartz crucible 18 of Fig. 1 can be
replaced
with a container 42 and a crucible 40 which has at the center a housing or
pocket 46 in
which a seed monocrystal 44 of the material which is to be synthesized and
grown (e.g.
CdTe) can be placed.
Once the encapsulant (e.g., B203) 30 is molten, the crucible 40 is raised as
shown
in Fig. 2 in order to melt reactants (e.g., Cd 26 and Te 28) and to obtain the
reaction
product as described in Example 1. Up to this point, the CdTe seed 44 remains
solid,
since the melting point for CdTe is higher than the reaction temperature.
Next, the
temperature is increased and the CdTe is melted. In this stage, the heating
should be
carried out such as not to completely melt the CdTe seed 44 introduced. This
is possible
by using an appropriately sized and/or positioned induction heating coil 14
and by
controlling the heating ramp during the stage of melting of the polycrystal
(Fig. 4 I-m, m-n).
Growth can then take place as in a typical Vertical Bridgman method.
The seed crystal 44 produces a lattice structure on which the molten compound
can grow during a suitable cooling realized by lowering the crucible 40 by
means of the
movement of the container 42. During the cooling, the seed crystal 44 and the
grown
crystal are at a temperature lower then the corresponding melting point while
the upper
growing compound is kept in the molten state.
The suitable thermal gradient and the growing times are apparent to those
skilled
in the art.
PC783
CA 02292853 1999-12-20
28
In order to obtain an adequate temperature profile, additional heating
elements of
suitable shape can be introduced around the crucible 40.
Example 4
Fig. 7 shows apparatus which allows the synthesis of a polycrystalline
compound
according to another embodiment of the present invention in a manner similar
to that
described in Example 1, and which also allows a monocrystal to be grown
according to
the Czochralski method. To achieve this end, the receptacle20 of Fig. 1 can be
replaced
with a graphite ring 32 with an outside diameter similar to that of the
receptacle 20, i.e.,
3.5 cm, and an inside diameter greater than or equal to the diameter of the
desired
monocrystal (for example 2.5 cm). The ring can be mounted so as to float on
the surface
of the 8203, but not to rotate with respect to the crucible 18.
The synthesis then proceeds according to the method outlined in Example 1,
except that heat is induced in the Bz03 30 by means of the graphite ring 32.
Once the
synthesis has taken place and the CdTe in the crucible has melted, the growth
can take
place immediately according to the Czochralski technique, for example, by
solidly
attaching a seed monocrystal 44 to a moveable rod 48 located in the top of the
oven 10,
with the liquid CdTe maintained at a suitable temperature. The growth proceeds
by
plunging the rod in the liquid compound, trough the hole in the graphite ring
32, and
gradually raising the seed out of the molten mixture.
The apparatus of Figs. 6 and 7 can include a suitable intercepting member
arranged in the crucible in order to modify the temperature gradient in the
region of the
growth interface. A suitable intercepting member for use in the apparatus is
described, for
example, in European patent EP 509,312 with reference to figures 5 and 6. This
member,
for example ring-shaped, intercepts the radiation generated from the heating
coils
providing to the charge the right thermal gradient.
Over all, the present invention provides numerous advantages for the syntheses
and the growth of crystals.
These advantages include that the method and the apparatus described ensure a
complete reaction of the elements and a correct stoichiometry. In addition,
this method
has no quantity product limits, no vapor losses, safety (Cd and Te can cause
cancer),
short synthesis time and employs a simple apparatus.
The foregoing description of preferred embodiments of the present invention
provides illustration and description, but is not intended to be exhaustive or
to limit the
invention to the precise form disclosed. Modifications and variations are
possible in light
of the above teachings or may be acquired from practice of the invention. The
foregoing
PC783
CA 02292853 1999-12-20
29
description includes specific data values obtained through experimentation.
These values
serve as examples only and the true scope of the invention is defined only by
the claims
and their equivalents.