Note: Descriptions are shown in the official language in which they were submitted.
CA 02293358 2007-06-28
TRICYCLIC SULFONAMIDE INHIBITORS OF FARNESYL-PROTEIN TRANSFERASE
BACKGROUND
Patent application WO 95/00497 published 5 January 1995 under the
Patent Cooperation Treaty (PCT) describes compounds which inhibit the enzyme,
farnesyl-protein transferase (FTase) and the farnesylation of the oncogene
protein Ras. Oncogenes frequently encode proteih components of signal
transduction pathways which lead to stimulation of cell growth and
mitogenesis.
Oncogene expression in cultured cells leads to cellular transformation,
characterized by the ability of cells to grow in soft agar and the growth of
cells as
dense foci lacking the contact inhibition exhibited by non-transformed cells.
Mutation and/or overexpression of certain oncogenes is frequently associated
with human cancer.
To acquire transforming potential, the precursor of the Ras oncoprotein
must undergo farnesylation of the cysteine residue located in a carboxyl-
terminal
tetrapeptide. Inhibitors of the enzyme that catalyzes this modification,
farnesyl
protein transferase, have therefore been suggested as anticancer agents for
tumors in which Ras contributes to transformation. Mutated, oncogenic forms of
Ras are frequently found in many human cancers, most notably in more than 50%
of colon and pancreatic carcinomas (Kohl et al., Science, Vol. 260, 1834 to
1837,
1993).
In view of the current interest in inhibitors of farnesyl protein transferase,
a
welcome contribution to the art would be additional compounds useful for the
inhibition of farnesyl protein transferase. Such a contribution is provided by
this
invention.
SUMMARY OF THE INVENTION
Inhibition of farnesyl protein transferase by tricyclic compounds of this
invention has not been reported previously. Thus, this invention provides a
method for inhibiting farnesyl protein transferase using tricyclic compounds
of this
invention which: (i) potently inhibit farnesyl protein transferase, but not
geranylgeranyl protein transferase I, in vitro; (ii) block the phenotypic
change
induced by a form of transforming Ras which is a farnesyl acceptor but not by
a
form of transforming Ras engineered to be a geranylgeranyl acceptor; (iii)
block
intracellular processing of Ras which is a farnesyl acceptor but not of Ras
CA 02293358 1999-12-09
WO 98/57949 PCTIUS98/11508
engineered to be a geranylgeranyl acceptor; and (iv) block abnormal cell
growth
in culture induced by transforming Ras.
This invention provides a method for inhibiting the abnormal growth of
cells, including transformed cells, by administering an effective amount of a
compound of this invention. Abnormal growth of cells refers to cell growth
independent of normal regulatory mechanisms (e.g., loss of contact
inhibition).
This includes the abnormal growth of: (1) tumor cells (tumors) expressing an
activated Ras oncogene; (2) tumor cells in which the Ras protein is activated
as a
result of oncogenic mutation in another gene; and (3) benign and malignant
cells
of other proliferative diseases in which aberrant Ras activation occurs.
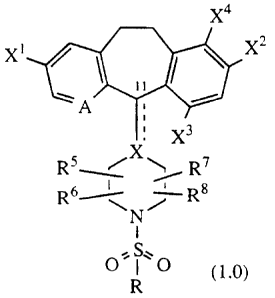
Compounds useful in the claimed methods are represented by Formula 1.0:
x4
x' x2
A
X3
Rs X
R7
R6~ Rs
Nj
I
OS~O
R (1.0)
or a pharmaceutically acceptable salt or solvate thereof, wherein:
A represents N or N-oxide;
X represents N, CH or C, such that when X is N or CH, there is a single bond
to
carbon atom 11 as represented by the solid line; or when X is C, there is a
double bond to carbon atom 11, as represented by the solid and dotted lines;
Xl and X2 are independently selected from bromo, iodo or chloro;
X3 and X4 are independently selected from bromo, iodo, chloro, fluro or
hydrogen provided only one of X3 or X4 is hydrogen;
R5, R6, R7 and R8 each independently represents hydrogen, alkyl, aryl, or
-CONR20R21 wherein R20 and R21 independently represent hydrogen, alkyl,
alkoxy, aryl, aralkyl, heteroaryl, heteroarylalkyl, cycloalkyl,
cycloalkylalkyl,
heterocycloalkyl and heterocycloalkylalkyl, and further wherein R5 may be
-2-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
combined with R6 to represent =0 or =S and/or R7 may be combined with R8
to represent =0 or =S;
R can represent alkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
cycloalkyl,
cycloalkylalkyl, heterocycloalkyl, heterocycloalkylalkyl or -NRi oR11,
wherein R10 and Rll can independently represent hydrogen, alkenyl, alkyl,
aryl, aralkyl, heteroaryl, heteroarylalkyl, cycloalkyl, cycloalkylalkyl,
heterocycloalkyl or heterocycloalkylalkyl.
Preferably in compound (1.0), there is a single bond at carbon atom 11; X
is CH; R5, R6, R7 and R8 are hydrogen; Xl, X2 and X3 are bromo or chloro and
X4 is hydrogen; and R is alkyl, trifluoromethyl, alkenyl, aryl, heteroaryl or
-NRtcRtj wherein R10 and R>> are independently selected from hydrogen and
alkyl. When R is alkyl, an optional substituent on the alkyl group may be
trifluoromethyl. When R is heteroaryl, optional substituents on the heteroaryl
group may include alkyl or heteroaryl. Preferred compounds include those of
Examples 1, 3, 4, 5, 6, 9, 10, 11 and 13.
In another embodiment, the present invention is directed toward a
pharmaceutical composition for inhibiting the abnormal growth of cells
comprising an effective amount of compound (1.0) in combination with a
pharmaceutically acceptable carrier.
In another embodiment, the present invention is directed toward a method
for inhibiting the abnormal growth of cells, including transformed cells,
comprising
administering an effective amount of compound (1.0) to a mammal (e.g., a
human) in need of such treatment. Abnormal growth of cells refers to cell
growth
independent of normal regulatory mechanisms (e.g., loss of contact
inhibition).
This includes the abnormal growth of: (1) tumor cells (tumors) expressing an
activated Ras oncogene; (2) tumor cells in which the Ras protein is activated
as a
result of oncogenic mutation in another gene; (3) benign and malignant cells
of
other proliferative diseases in which aberrant Ras activation occurs, and (4)
benign or malignant cells that are activated by mechanisms other than the Ras
protein. Without wishing to be bound by theory, it is believed that these
compounds may function either through the inhibition of G-protein function,
such
as ras p21, by blocking G-protein isoprenylation, thus making them useful in
the
treatment of proliferative diseases such as tumor growth and cancer, or
through
inhibition of ras farnesyl protein transferase, thus making them useful for
their
antiproliferative activity against ras transformed cells.
-3-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
The cells to be inhibited can be tumor cells expressing an activated ras
oncogene. For example, the types of cells that may be inhibited include
pancreatic tumor cells, lung cancer cells, myeloid leukemia tumor cells,
thyroid
follicular tumor cells, myelodyspiastic tumor cells, epidermal carcinoma tumor
cells, bladder carcinoma tumor cells, prostate tumor cells, breast tumor cells
or
colon tumors cells. Also, the inhibition of the abnormal growth of cells by
the
treatment with compound (1.0) may be by inhibiting ras farnesyl protein
transferase. The inhibition may be of tumor cells wherein the Ras protein is
activated as a result of oncogenic mutation in genes other than the Ras gene.
Alternatively, compounds (1.0) may inhibit tumor cells activated by a protein
other
than the Ras protein.
This invention also provides a method for inhibiting tumor growth by
administering an effective amount of compound (1.0) to a mammal (e.g., a
human) in need of such treatment. In particular, this invention provides a
method
for inhibiting the growth of tumors expressing an activated Ras oncogene by
the
administration of an effective amount of the above described compounds.
Examples of tumors which may be inhibited include, but are not limited to,
lung
cancer (e.g., lung adenocarcinoma), pancreatic cancers (e.g., pancreatic
carcinoma such as, for example, exocrine pancreatic carcinoma), colon cancers
(e.g., colorectal carcinomas, such as, for example, colon adenocarcinoma and
colon adenoma), myeloid leukemias (for example, acute myelogenous leukemia
(AML)), thyroid follicular cancer, myelodysplastic syndrome (MDS), bladder
carcinoma, prostate carcinoma and breast carcinoma and epidermal carcinoma.
It is believed that this invention also provides a method for inhibiting
proliferative diseases, both benign and malignant, wherein Ras proteins are
aberrantiy activated as a result of oncogenic mutation in other genes--i.e.,
the Ras
gene itself is not activated by mutation to an oncogenic form--with said
inhibition
being accomplished by the administration of an effective amount of the N-
substituted urea compounds (1.0) described herein, to a mammal (e.g., a human)
in need of such treatment. For example, the benign proliferative disorder
neurofibromatosis, or tumors in which Ras is activated due to mutation or
overexpression of tyrosine kinase oncogenes (e.g., neu, src, abl, Ick, and
fyn),
may be inhibited by the N-substituted urea compounds (1.0).
In another embodiment, the present invention is directed toward a method
for inhibiting ras farnesyl protein transferase and the farnesylation of the
oncogene protein Ras by administering an effective amount of compound (1.0) to
mammals, especially humans. The administration of the compounds of this
-4-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
invention to patients, to inhibit farnesyl protein transferase, is useful in
the
treatment of the cancers described above.
DETAILED DESCRIPTION OF THE INVENTION
As used herein, the following terms are used as defined below unless
otherwise indicated:
M+ -represents the molecular ion of the molecule in the mass spectrum;
MH+ -represents the molecular ion plus hydrogen of the molecule in the
mass spectrum;
Bu-represents butyl;
Et-represents ethyl;
Me-represents methyl;
Ph-represents phenyl;
benzotriazol-1-yloxy represents
N
.
flI'N
~
0- .
1-methyl-tetrazol-5-ylthio represents
N-N
N~
%. N
i
CH3
alkyl-(including the alkyl portions of alkoxy, alkylamino and
dialkylamino)-represents straight and branched carbon chains and contains from
one to twenty carbon atoms, preferably one to six carbon atoms; for example
methyl, ethyl, propyl, iso-propyl, n-butyl, t-butyl, n-pentyl, isopentyl,
hexyl and the
like; wherein said alkyl group may be optionally and independently substituted
with one, two, three or more of the following: halo, alkyl, aryl, cycloalkyl,
cyano,
-CF3, oxy (=0), aryloxy, -OR10, -OCF3, heterocycloalkyl, heteroaryl, -NRlOR12,
-NHSO2R10, -SO2NH2, -SO2NHR10, -S02R10, -SOR10, -SR10, -NHSO2, -NO2,
-CONRloR12, -NR12COR10, -COR10, -OCOR10, -0C02R1c or -COOR10, wherein
RIo and R12 can independently represent hydrogen, alkyl, alkoxy, aryl,
aralkyl,
heteroaryl, heteroarylalkyl, cycloalkyl, cycloalkylalkyl, heterocycloalkyl or
heterocycloalkylalkyl;
-5-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
alkenyl-represents straight and branched carbon chains having at least
one carbon to carbon double bond and containing from 2 to 12 carbon atoms,
preferably from 2 to 6 carbon atoms and most preferably from 3 to 6 carbon
atoms; wherein said alkenyl group may be optionally and independently
substituted with one, two, three or more of the following: halo, alkyl, aryl,
alkoxy,
amino, alkylamino, cyano, -CF3, dialkylamino, hydroxy, oxy, phenoxy, -OCF3,
heterocycloalkyl, -SO2NH2, -NHSO2R1O, -SO2NHR10, -S02R10, -SOR10, -SR10,
-NHSO2, -NO2, -CONR10, -NCOR10 or -COOR10;
alkoxy-an alkyl moiety of one to 20 carbon atoms covalently bonded to
an adjacent structural element through an oxygen atom, for example, methoxy,
ethoxy, propoxy, butoxy, pentoxy, hexoxy and the like; wherein said alkoxy
group
may be optionally and independently substituted with one, two, three or more
of
the following: halo, alkyl, aryl, cycloalkyl, cyano, -CF3, oxy (=0), aryloxy, -
OR10,
-OCF3, heterocycloalkyl, heteroaryl, -NRlflR12, -NHSO2R1O, -SO2NH2,
-SO2NHR10, -S02R10, -SOR10, -SR10, -NHSO2, -NO2, -CONRlOR12,
-NR12COR10, -COR10, -OCOR10, -OC02R1c or -COOR10, wherein R10 and R12
are as defined hereinabove;
aryl (including the aryl portion of arylalkyl)-represents a carbocyclic
group containing from 6 to 15 carbon atoms and having at least one aromatic
ring
(e.g., aryl is phenyl), wherein said aryl group optionally can be fused with
aryl,
cycloalkyl, heteroaryl or heterocycloalkyl rings; and wherein any of the
available
substitutable carbon and nitrogen atoms in said aryl group and/or said fused
ring(s) may be optionally and independently substituted with one, two, three
or
more of the following: halo, alkyl, aryl, cycloalkyl, cyano, -CF3, oxy (=0),
aryloxy,
-OR10, -OCF3, heterocycloalkyl, heteroaryl, -NRiOR12, -NHSO2R10, -SO2NH2,
-SO2NHR10, -S02R10, -SOR10, -SR10, -NHSO2, -NO2, -CONRlOR12,
-NR12COR10, -COR10, -OCOR10, -OC02R10 or -COOR10, wherein R10 and R12
are as defined hereinabove;
arylalkyl - represents an alkyl group, as defined above, wherein one or
more hydrogen atoms of the alkyl moiety have been substituted with one or more
aryl groups; wherein said aralkyl group may be optionally and independently
substituted with one, two, three or more of the following: halo, alkyl, aryl,
cycloalkyl, cyano, -CF3, oxy (=0), aryloxy, -OR10, -OCF3, heterocycloalkyl,
heteroaryl, -NR10R12, -NHSO2R10, -SO2NH2, -SO2NHR10, -SO2R10, -SOR ,
-SR10, -NHSO2, -NO2, -CONR10R12, -NR12COR10, -COR10, -OCOR10,
-OCO2R1O or -COOR10, wherein R10 and R12 are as defined hereinabove;
-6-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
aryloxy - represents an aryl group, as defined above, wherein said aryl
group is covalently bonded to an adjacent structural element through an oxygen
atom, for example, phenoxy, wherein said aryl group optionally can be fused
with
aryl, cycloalkyl, heteroaryl or heterocycloalkyl rings; and wherein any of the
available substitutable carbon and nitrogen atoms in said aryloxy group and/or
said fused ring(s) may be optionally and independently substituted with one,
two,
three or more of the following: halo, alkyl, aryl, cycloalkyl, cyano, -CF3,
oxy (=0),
aryloxy, -OR10, -OCF3, heterocycloalkyl, heteroaryl, -NRlOR12, -NHS02R1O,
-SO2NH2, -SO2NHR10, -S02R10, -SOR10, -SR10, -NHSO2, -NO2, -CONRlOR12,
-NR12COR10, -COR10, -OCOR10, -OC02R1o or -COOR10, wherein R10 and R12
are as defined hereinabove;
cycloalkyl-represents saturated carbocyclic rings branched or
unbranched of from 3 to 20 carbon atoms, preferably 3 to 7 carbon atoms;
wherein said cycloalkyl group may be optionally and independently substituted
with one, two, three or more of the following: halo, alkyl, aryl, cycloalkyl,
cyano,
-CF3, oxy (=0), aryloxy, -OR10, -OCF3, heterocycloalkyl, heteroaryl, -NR1aR12,
-NHS02R1O, -SO2NH2, -SO2NHR10, -S02R10, -SOR10, -SR10, -NHSO2, -NO2,
-CONRloR12, -NR12COR10, -COR , -OCOR10, -OC02R10 or -COOR10, wherein
R 10 and Ri 2 are as defined hereinabove;
cycloalkylalkyl - represents an alkyl group, as defined above, wherein
one or more hydrogen atoms of the alkyl moiety have been substituted with one
or more cycloalkyl groups; wherein said cycloalkylalkyl group may be
optionally
and independently substituted with one, two, three or more of the following:
halo,
alkyl, aryl, cycloalkyl, cyano, -CF3, oxy (-0), aryloxy, -OR10, -OCF3,
heterocycloalkyl, heteroaryl, -NR1OR12, -NHS02R1O, -SO2NH2, -SO2NHR10,
-S02R10, -SOR10, -SR10, -NHSO2, -NO2, -CONRlOR12, -NR12COR10, -COR10,
-OCOR10, -OCO2R1Q or -COOR10, wherein R10 and R12 are as defined
hereinabove;
halo-represents fluoro, chloro, bromo and iodo;
heteroalkyl-represents straight and branched carbon chains containing
from one to twenty carbon atoms, preferably one to six carbon atoms
interrupted
by 1 to 3 heteroatoms selected from -0-, -S- and -N-; wherein any of the
available
substitutable carbon and nitrogen atoms in said heteroalkyl chain may be
optionally and independently substituted with one, two, three or more of the
following: halo, alkyl, aryl, cycloalkyl, cyano, -CF3, oxy (=0), aryloxy, -
OR10,
-OCF3, heterocycloalkyl, heteroaryl, -NR1OR12, -NHS02R1O, -SO2NH2,
-SO2NHRIO, -S02R10, -SOR10, -SR10, -NHSO2, -NO2, -CONRloR12,
-7-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
-NR12COR10, -COR10, -OCOR10, -OC02R1O or -COOR10, wherein R10 and R12
are as defined hereinabove;
heteroaryl-represents cyclic groups having at least one heteroatom
selected from 0, S and N, said heteroatom(s) interrupting a carbocyclic ring
structure and having a sufficient number of delocalized pi electrons to
provide
aromatic character, with the aromatic heterocyclic groups containing from 2 to
14
carbon atoms,wherein said heteroaryl group optionally can be fused with one or
more aryl, cycloalkyl, heteroaryl or heterocycloalkyl rings; and wherein any
of the
available substitutable carbon or nitrogen atoms in said heteroaryl group
and/or
said fused ring(s) may be optionally and independently substituted with one,
two,
three or more of the following: halo, alkyl, aryl, cycloalkyl, cyano, -CF3,
oxy (=0),
aryloxy, -OR10, -OCF3, heterocycloalkyl, heteroaryl, -NR1OR12, -NHSO2R10,
-S02NH2, -SO2NHR10, -S02R1o, -SOR10, -SR10, -NHSO2, -NO2, -CONRloR12,
-NR12COR10, -COR10, -OCOR10, -OC02R1c or -COORic, wherein R10 and R12
are as defined hereinabove.
Representative heteroaryl groups can include, for example, furanyl,
imidazoyl, pyrimidinyl, triazolyl, 2-, 3- or 4-pyridyl or 2-, 3- or 4-pyridyl
N-oxide
wherein pyridyl N-oxide can be represented as:
\ \ \ \ \ \
~. ~+. o<
N N N
I I ~
O O-
O
heteroarylalkyl - represents an alkyl group, as defined above, wherein
one or more hydrogen atoms have been replaced by one or more heteroaryl
groups; wherein said heteroarylalkyl group may be optionally and independently
substituted with one, two, three or more of the following: halo, alkyl, aryl,
cycloalkyl, cyano, -CF3, oxy (=0), aryloxy, -OR10, -OCF3, heterocycloalkyl,
heteroaryl, -NR1oR12, -NHSO2R1O, -SO2NH2, -SO2NHR10, -S02R1O, -SOR10,
-SR10, -NHSO2, -NO2, -CONRlcR12, -NR12COR10, -COR10, -OCOR10,
-OC02R1o or -COOR10, wherein R10 and R12 are as defined hereinabove;
heterocycloalkyl-represents a saturated, branched or unbranched
carbocylic ring containing from 3 to 15 carbon atoms, preferably from 4 to 6
carbon atoms, which carbocyclic ring is interrupted by 1 to 3 heteroatoms
selected from -0-, -S- and -N- , wherein optionally, said ring may contain one
or
two unsaturated bonds which do not impart aromatic character to the ring; and
wherein any of the available substitutable carbon and nitrogen atoms in the
ring
-8-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
may be optionally and independently substituted with one, two, three or more
of
the following: halo, alkyl, aryl, cycloalkyl, cyano, -CF3, oxy (=0), aryloxy, -
OR10,
-OCF3, heterocycloalkyl, heteroaryl, -NR10R12, -NHSO2R1o, -SO2NH2,
-SO2NHR10, -S02R10, -SOR10, -SR10, -NHSO2, -NO2, -CONR1OR12,
-NR12COR10, -COR10, -OCOR10, -OC02R10 or -COOR10, wherein R10 and R12
are as defined hereinabove. Representative heterocycloalkyl groups can include
2- or 3-tetrahydrofuranyl, 2- or 3- tetrahydrothienyl, 1-, 2-, 3- or 4-
piperidinyl, 2- or
- ~N_R~o
3 pyrrolidinyl, 1-, 2- or 3-piperizinyl, 2- or 4-dioxanyl, morpholinyl,
-N s(o~
or wherein R10 is defined hereinbefore and t is 0, 1 or 2.
heterocycloalkalkyl- represents an alkyl group, as defined above,
wherein one or more hydrogen atoms have been replaced by one or more
heterocycloalkyl groups; wherein optionally, said ring may contain one or two
unsaturated bonds which do not impart aromatic character to the ring; and
wherein said heterocycloalkylalkyl group may be optionally and independently
substituted with one, two, three or more of the following: halo, alkyl, aryl,
cycloalkyl, cyano, -CF3, oxy (-0), aryloxy, -OR10, -OCF3, heterocycloalkyl,
heteroaryl, -NRloR12, -NHSO2R1o, -SO2NH2, -SO2NHR10, -S02R10, -SOR10,
-SR10, -NHSO2, -NO2, -CONRloR12, -NR12COR10, -COR10, -OCOR10,
-OC02R'O or -COOR10, wherein R10 and R12 are as defined hereinabove.
The following solvents and reagents are referred to herein by the
abbreviations indicated: tetrahydrofuran (THF); ethanol (EtOH); methanol
(MeOH); acetic acid (HOAc or AcOH); ethyl acetate (EtOAc); N,N-
dimethylformamide (DMF); trifluoroacetic acid (TFA); trifluoroacetic anhydride
(TFAA); 1-hydroxybenzotriazole (HOBT); m-chioroperbenzoic acid (MCPBA);
triethylamine (Et3N); diethyl ether (Et20); ethyl chloroformate (CICO2Et); and
1-
(3-dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride (DEC).
Reference to the position of the substituents Xl, X2, X3 and X4 is based on
the numbered ring structure:
-9-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
x4
x) 4 5 6 7 / x2
3
~A -
x3
R5-_õ,,,'x R7
6~~ / 8
R ~N~ R
I
S
O ' o
R (1.0)
Certain compounds of the invention may exist in different stereoisomeric
forms (e.g., enantiomers, diastereoisomers and atropisomers). The invention
contemplates all such stereoisomers both in pure form and in mixture,
including
racemic mixtures. For example, the carbon atom at the C-11 position can be in
the S or R stereoconfiguration.
Certain tricyclic compounds will be acidic in nature, e.g. those compounds
which possess a carboxyl or phenolic hydroxyl group. These compounds may
form pharmaceutically acceptable salts. Examples of such salts may include
sodium, potassium, calcium, aluminum, gold and silver salts. Also contemplated
are salts formed with pharmaceutically acceptable amines such as ammonia,
alkyl amines, hydroxyalkylamines, N-methylglucamine and the like.
Certain basic tricyclic compounds also form pharmaceutically acceptable
salts, e.g., acid addition salts. For example, the pyrido-nitrogen atoms may
form
salts with strong acid, while compounds having basic substituents such as
amino
groups also form salts with weaker acids. Examples of suitable acids for salt
formation are hydrochloric, sulfuric, phosphoric, acetic, citric, oxalic,
malonic,
salicylic, malic, fumaric, succinic, ascorbic, maleic, methanesulfonic and
other
mineral and carboxylic acids well known to those skilled in the art. The salts
are
prepared by contacting the free base form with a sufficient amount of the
desired
acid to produce a salt in the conventional manner. The free base forms may be
regenerated by treating the salt with a suitable dilute aqueous base solution
such
as dilute aqueous NaOH, potassium carbonate, ammonia and sodium
bicarbonate. The free base forms differ from their respective salt forms
somewhat
in certain physical properties, such as solubility in polar solvents, but the
acid and
base salts are otherwise equivalent to their respective free base forms for
purposes of the invention.
-10-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
All such acid and base salts are intended to be pharmaceutically
acceptable salts within the scope of the invention and all acid and base salts
are
considered equivalent to the free forms of the corresponding compounds for
purpopses of the invention.
Compounds of the present invention can be prepared according to the following
Scheme I:
4 Scheme I
x' / ' ~ ~ x2 x~ / ~ x2
p
N
~. ~ ..
CI-S--R N
X3 x3
R5X R7 (2.6) R5_-!x R7
R6~ ~ Rg --' R6._~ R8
(5.0, 5.01, 6.0 and 10.9) ~
O-~'- S" O (1.0)
R
wherein X, Xl, X2, X3, X4, R, R5, R6, R7, R8, and the solid and dotted lines
are as
defined hereinbefore.
Referring to the Scheme I, compounds of formula (1.0) can be prepared by
reacting the compound of formula (5.0, 5.01, 6.0 or 10.9) with the
corresponding
sulfonyl chloride reagent of formula (2.6) with a base and aprotic solvent
such as
THF, dioxane, toluene, methylene chloride (CH2CI2), acetonitrile, or DMF at
temperatures which can range from 0 to 100 C, or reflux of the reaction
mixture.
The amount of sulfonyl chloride (2.6) can range from 1 to about 10 moles per
mole of compound (5.0, 5.01, 6.0 or 10.9).
In an alternative procedure, the compounds of formula (1.0) wherein R is
-NR1oR11 can be prepared by reacting the compound of formula (5.0, 5.01, 6.0
or
10.9) with thionyl chloride in an aprotic solvent as described above, in the
presence of a base, followed by reaction with an amine of the formula HNR10R11
(2.8) in an aprotic solvent wherein R10 and Rll are defined hereinbefore, at a
temperatures from 00 to 100 C or reflux of the reaction mixture. The amount of
the thionyl chloride or amine (2.8) can range from about 1 to 10 moles per
mole of
compound (5.0, 5.01, 6.0 or 10.9).
-11-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
In another alternative procedure, the compounds of formula (1.0) wherein
R is -NH2 can be prepared by reacting compound (2.0) with the sulfonamide
SO(NH2)2 in a protic solvent such as water at temperatures ranging from 500 to
100 C.
Compounds of fomula (1.0) can be isolated from the reaction mixture using
conventional procedures, such as, for example, extraction of the reaction
mixture
from water with organic solvents, evaporation of the organic solvents,
followed by
chromatography on silica gel or other suitable chromatographic media.
Alternatively, compounds (1.0) can be dissolved in a water-miscible solvent,
such
as methanol, the methanol solution is added to water to precipitate the
compound, and the precipitate is isolated by filtration or centrifugation.
(+)-Isomers of compounds of formula (5.0, 6.0 and 10.9) wherein X is CH
can be prepared with high enantioselectivity by using a process comprising
enzyme catalyzed transesterification. Preferably, a racemic compound of
formula
(5.0, 6.0 and 10.9) , wherein X is C, the double bond is present and X3 is not
H, is
reacted with an enzyme such as Toyobo LIP-300 and an acylating agent such as
trifluoroethly isobutyrate; the resultant (+)-amide is then hydrolyzed, for
example
by refluxing with an acid such as H2SO4, to obtain the corresponding optically
enriched (+)-isomer wherein X is CH and R3 is not H. Alternatively, a racemic
compound of formula (5.0, 6.0 and 10.9), wherein X is C, the double bond is
present and R3 is not H, is first reduced to the corresponding racemic
compound
of formula (5.0, 6.0 and 10.9) wherein X is CH and then treated with the
enzyme
(Toyobo LIP-300) and acylating agent as described above to obtain the (+)-
amide, which is hydrolyzed to obtain the optically enriched (+)-isomer.
Compounds of the present invention and preparative starting materials
thereof, are exemplified by the following examples, which should not be
construed as limiting the scope of the disclosure.
Example 1 (+)-4-(3-Bromo-8,10-dichioro-6,11-dihydro-5H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11-yl)-1-(methylsulfonyl)pipiridine
-12-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br ' \ ci
Br ~ I\ Cl Q C1
N
N H3C'S~\O Cl
Cl
KC03
' N
I
N O= S= 0
CH3
Dry anhydrous potassium carbonate (0.23g, 1.7 mmol) was suspended in 6 mL
of anhydrous toluene. To this mixture was added the title compound of
Preparative Example 10 (0.2g, 0.47 mmol), methane sulfonyl chloride (0.055g,
40
L, 0.47 mmol) and stirred at room temperature for - 72h. The reaction mixture
was then filtered, and washed with CH2CI2. The filtrate was washed with
saturate
NaHCO3, dried over MgSO4, filtered and concentrated to dryness to afford 0.23g
of the title compound as a white solid: mp = 160-163 C, FAB-MS: MH+ = 505
(97% Yield), COS IC50 = 0.420 ( M).
Example 2 (+)-4-(3-Bromo-8,10-dichloro-6,11-dihydro-5H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11-y!)-N-N-dimethyl-l-piperidinesulfonamide
Br ci
Br CI
N Cl
Cl
N
N 0=S=0
H I
~N,
H3C CH3
The title compound is prepared following essentially the same procedure as
described in Example 1 except that N,N-dimethyl sulfonyl chloride was used
instead of methane sulfonyl chloride to obtain a solid, FAB-MS: MH+ = 534, mp=
202-203 C; (65% Yield).
Example 3 (+)-4-(3-Bromo-8,1 0-dichioro-6,1 1 -dihydro-5H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11-yl)-1-(aminosulfonyl)piperidine
-13-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br ~ r \ Cl
Br ~- I \ Cl N
C1
N
C1
N
i
H 0=S=0
NH,)
The title compound of Preparative Example 10 (0.2g, 0.47 mmol), and sulfamide
(0.45g, 4.7 mmol) are dissolved in 7 mL of H20 and the reaction mixture heated
to reflux for 72h. The reaction mixure was then cooled and filtered. The
filtrate
was extracted with CH2CI2, dried over MgSO4 and concentrated. Purification by
flash chromatography on silica gel eluting with 5% MeOH(sat. with ammonia)-
CH2CI2 afforded 0.035g (15% yield) of the title compound, as a white solid.
FAB-
MS: MH+ = 506. mp=133-134 C .
Example 4 (+)-4-(3,10-Dibromo-8-chloro-11-dihydro-5H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11-y1)-1-(methyisulfonyi)pipiridine
Br C1
Br Cl
Br
N Br
N
H 0=S=0
CH3
The title compound is prepared following essentially the same procedure as
described in Example 1 except that the title compound of Preparative Example 3
(+)-4-(3,10-dibromo-8-chloro-6,11-dihydro-5H-benzo[5,6]cyclo-hepta[1,2-
b]pyridin-11-yl)-1-pipiridine) was used instead of (+)-4-(3-bromo-8,10-
dichloro-
6,11-dihydro-5H-benzo[5,6]cyclo-hepta[1,2-b]pyridin-11-yl)-1-pipiridine to
obtain
the title compound, a solid. FAB-MS: MH+ = 549, mp= 216-217 C, yield = 74%,
COS IC50 = 0.015 ( M).
Example 5 (+)-4-(3,10-Dibromo-8-chloro-11-dihydro-5H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11-yl)-1-(ethylsulfonyl)pipiridine
-14-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br Cl
Br ~ l \ Cl
N Br
Br
N
H 00
CH3
The title compound is prepared following essentially the same procedure as
described in Example 4 except that ethane sulfonyl chloride was used instead
of
methane sulfonyl chloride to obtain a solid FAB-MS: MH+ = 563. mp= 202-203 C
yield=90%
Example 6 (+)-4-(3,10-Dibromo-8-chloro-11-dihydro-5H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11-yl)-1-(propyisulfonyl)pipiridine
Br ~ 1 \ CI
Br ~ I \ Cl
1 ~
Br
Br
N
H 0'S'
CH3
The title compound is prepared following essentially the same procedure as
described in Example 4 except that propane sulfonyl chloride was used instead
of methane sulfonyl chloride to obtain a solid FAB-MS: MH+ = 577. mp= 97-98 C
yield = 95 %
Example 7 (+)-4-(3,10-Dibromo-8-chloro-11-dihydro-5H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11-y1)-1-(isopropylsulfonyl)pipiridine
-15-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br ~ I \ ci
Br ~ J \ CI N
N B r
Br
N
N O~ O
H3C CH3
The title compound is prepared following essentially the same procedure as
described in Example 4 except that isopropane sulfonyl chloride was used
instead of methane sulfonyl chloride to obtain a solid FAB-MS: MH+ = 577. mp=
203-205 C (yield = 65 %).
Example 8 (+)-4-(3,10-Dibromo-8-chloro-11-dihydro-5H-benzo[5,6]cyciohepta[1,2-
b]pyridin-1 1 -yl)- 1 -(butylsulfonyl)pipiridine
Br ~- I \ ci
Br ~ I \ Cl N
N ~ Br
Br
N
N O=S=O
H
CH3
The title compound is prepared following essentially the same procedure as
described in Example 4 except that butane sulfonyl chloride was used instead
of
methane sulfonyl chloride to obtain a solid FAB-MS: MH+ = 591. mp= 73-74 C
yield = 28 %
-16-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Example 9 (+)-4-(3,10-Dibromo-8-chloro-11-dihydro-5H-benzo[5,6]cyclohepta[1,2-
b]pyridin-11-yl)-1-(trifluoromethyl sulfonyl)pipiridine
Br ~ I \ ci
Br ~ I \ cl ~ N 1
N Br
Br
N
H 0=S=0
CF3
The title compound is prepared following essentially the same procedure as
described in Example 4 except that trifluoromethane sulfonyl chloride was used
instead of methane sulfonyl chloride to obtain a solid FAB-MS: MH+ = 603. mp=
111-112 C yield = 47 %
15 Example 10 (+)-4-(3,10-Dibromo-8-chloro-11-dihydro-5H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11-yl)-1-(trifluoroethyl
sulfonyl)pipiridine
Br cl Br cl
Br Br
N N
H I
0=5=0
CF3
The title compound is prepared following essentially the same procedure as
described in Example 4 except that trifluoroethane sulfonyl chloride was used
instead of methane sulfonyl chloride to obtain a solid FAB-MS: MH+ = 617. mp=
174-175 C yield = 46 %
Example 11 (+)-4-(3,10-Dibromo-8-chioro-11-dihydro-5H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11-yl)-1-(vinylsulfonyl)pipiridine
-17-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br '~ I\ Cl Br ~ I\ Cl
1 N
I
N Br Br
N N
H O , Sl- O
~ CH,
The title compound is prepared following essentially the same procedure as
described in Example 4 except that 2-chloro-ethane sulfonyl chloride was used
instead of methane sulfonyl chloride to obtain a solid FAB-MS: MH+ = 514. mp=
129-130 C yield = 35 %
Example 12 (+) -4-(3,10-Dibromo-8-chloro-6,11-dihydro-5H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11(R)-yl)-1-(phenylsulfonyl)pipiridine
~
Br Br.
CI I
N N CI
H- _
Br Br
N
H ~
0:::: S=
6
To the title compound of Preparative Example 3 (0.05 g, 0.11 mmol) and
triethylamine (0.015 mL, 1.5 eq) dissolved in anhydrous dichloromethane (10
mL)
was added benzenesulfonyl chloride (0.015 mL, 1.1 eq). After stirring at room
temperature overnight, the solution was diluted with dichloromethane, washed
with
1 M hydrochloric acid, then washed with 1 N aqueous sodium hydroxide and dried
over anhydrous magnesium sulfate. Filtration and concentration in vacuo
afforded
the title compound (0.064 g, 99% yield, mp=124.3-129 C).
-18-
--- - --- -----
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Example 13 (+) -4-(3,10-Dibromo-8-chloro-6,11-dihydro-5H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11(R)-yl)-1-[(1-methyl-1 H-imidazol-4-
yl)sulfonyl]pipiridine
Br BrI
cl 14' H. ci
N
.=
H.,=- -
Br Br
N
0=S=0
H
H3C
To the title compound of Preparative Example 3 (0.05 g, 0.11 mmol) and
triethylamine (0.015 mL, 1.5 eq) dissolved in anhydrous dichloromethane (10
mL)
was added 1 -methylimidazole-4-sulfonyl chloride (0.021 g, 1.1 eq). After
stirring
at room temperature overnight, the solution was diluted with dichioromethane,
washed with 1 M hydrochloric acid, then washed with 1 N aqueous sodium
hydroxide and dried over anhydrous magnesium sulfate. Filtration and
concentration in vacuo afforded the title compound (0.054 g, 82% yield, mp
157.5-161.2 C).
Example 14 (+) -4-(3,10-Dibromo-8-chloro-6,11-dihydro-5H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11(R)-yI)-1-[(5-(3-isoxazolyl)-2-
th ienyl]sulfonyl]pipiridine
Br
Br ci N
K Br ci
Ft= -
N
Br I
0.S=0
S
H
N
/0
To the title compound of Preparative Example 3(0.05 g, 0.11 mmol) and
triethylamine (0.015 mL, 1.5 eq) dissolved in anhydrous dichloromethane (10
mL)
was added 5-(isoxazol-3-yl)thiophen-2-sulfonyl chloride (0.029 g, 1.1 eq).
After
stirring at room temperature overnight, the solution was diluted with
-19-
--- --- - - - -------
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
dichloromethane, washed with 1 M hydrochloric acid, then washed with 1 N
aqueous sodium hydroxide and dried over anhydrous magnesium sulfate.
Filtration and concentration in vacuo afforded the title compound (0.069 g,
94%,
mp 131.7-134.8 C).
-20-
CA 02293358 1999-12-09
WO 98/57949 PCT/U898/11508
PREPARATION OF STARTING MATERIALS
Starting materials useful in preparing the compounds of the present invention
are
exemplified by the following preparative examples, which should not be
construed to limit the scope of the disclosure. The tricylic compounds used as
starting materials, such as compound (11.0), inorganic and organic bases, and
alcohols can be prepared using known methods in the art, such as taught in See
J. K. Wong et al., Bioorganic & Medicinal Chemistry Letters, Vol. 3, No. 6,
pp.
1073-1078, (1993); U.S. Patents 5,089,496; 5,151,423; 4,454,143; 4,355,036;
PCT /US94/11390 (W095/10514); PCT/US94/11391 (WO 95/10515);
PCT/US94/11392 (W095/10516); Stanley R. Sandler and Wolf Karo, Organic
Functional Group Preparations, 2nd Edition, Academic Press, Inc., San Diego,
California, Vol. 1-3, (1983), and in J. March, Advanced Organic Chemistry,
Reactions & Mechanisms, and Structure, 3rd Edition, John Wiley & Sons, New
York, 1346 pp. (1985). Alternative mechanistic pathways and analogous
structures within the scope of the invention may be apparent to those skilled
in
the art.
Starting materials used to prepare the compounds of the present invention
are depicted in Scheme IV:
-21-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Scheme IV
x' X' X' ' X2 x' \ X2
A A NOZ A NH~
Rs-_ R7
R5-~ R~ Rs ! R~ B
R6- 1 -11 % R8 A R6- R8 -~ R6-- \= R8
N (11.0) N (10.0) N (9.0)
OIli ORIS O14 OR15 p pRis
C
X) C X2 Xj X' X, X'
A\ x A A 3 NH2
3 X3 I x
D 5 _
R5-~_ R7 R5'j R7 'F- R6~- R8
R6"_~ R8 R6 N R8 R N (8.0)
N G (7.0)
(6.0) O ~ OR15 O~ pR1s
F
Xl X2 R
0=S=0
A
X3 CI (2.6)
RS-- X~' R7 G
~ Ra (1-0)
R6~ i
, R7
H (5.0) R5 N
~ 8
X=CH from (6.0) R6' N R }{i X2
X=N from (6.7) H (6.9) / ~ I \
C1 x3
Q
(6.7)
P
}{' / \ x' x, / x2 X! / 1 I \ X'
~ A ~ ~ --~ ~ A 0 A OH 3
O X3 z O X3 ---- x
]y[I (6.31) (6.3) (6.5)
x, \ X' L X' \ X' K xI /, I\ X2
7 ~- ,, .=-- ~ ~
' ' O (6.2) NH2 A O (6.1) N02 O
(5.9)
-22-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
wherein for Scheme IV,
A, X, Xl, X2, X3, R, Z, R5, R6, R7 and R8 the solid and dotted lines are as
defined
hereinbefore; and R15 can represent any of the values for R10 or R12 as
defined
hereinbefore.
In Step A (Scheme IV), compounds of formula (10.0) can be prepared by
reacting the compounds of formula (11.0) with a nitrating agent and/or
optional
protic or aprotic solvent such as those described hereinbefore. In a first
procedure, compound (11.0) is reacted with about an equimolar amount of a
nitrate salt, such as potassium nitrate, and acid, such as sulfuric acid at
temperatures ranging from about -200 to +50 C. In a second procedure,
compound (11.0) is treated with a mixture comprised of about two equivalents
of
trifiuoromethanesulfonic acid and about one equivalent nitric acid in a
solvent
such as trifluoromethanesulfonic acid. In a third procedure, compound (11.0)
is
treated with a mixture comprised of about one equivalent of fuming nitric acid
and
about ten equivalents of trifluoromethanesulfonic anhydride in a solvent such
as
nitromethane. In a fourth procedure, compound (11.0) is treated with a
nitronium
salt, such as nitronium tetrafluoroborate, in a solvent, such as sulfolane. In
a fifth
procedure, compound (11.0) is reacted with fuming nitric acid at temperatures
ranging from about -200 to +500 C.
In Step B(Scheme IV), compounds of formula (9.0) can be prepared by
reacting compounds of the formula (10.0) with a reducing agent. In a first
procedure, compound (10.0) can be reacted with about ten equivalents of a
metal, such as iron, in a solvent, such as ethanol, in the presence of a salt,
such
as calcium chloride, at temperatures ranging from about 00 to +800 C. In a
second procedure, compound (10.0) can be reacted with about ten equivalents of
a metal, such as zinc, in a solvent, such as ethanol, in the presence of an
acid,
such as acetic acid at temperatures ranging from about 00 to +800 C. In a
third
procedure, compound (10.0) can be reacted with about five equivalents of
stannous chloride hydrate in a solvent, such as ethyl acetate. In a fourth
procedure, compound (10.0) can be reacted with about ten equivalents of a
metal, such as tin, in a solvent, such as ethanol, in the presence of an acid,
such
as hydrochloric acid.
In Step C(Scheme IV), compounds of formula (8.0) can be prepared by
reacting compounds of the formula (9.0) with a halogenating agent. In a first
procedure, compound (9.0) can be reacted with an excess of an elemental
halogen, such as bromine, in a suitable solvent, such as acetic acid at
temperatures ranging from about 00 to 200 C. In a second procedure, compound
-23-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
(9.0) can be reacted with a salt, such as pyridinium bromide perbromide, in a
solvent, such as THF, at temperatures from about 00 to +400 C. In a third
procedure, compound (9.0) can be reacted with a halogen, such as chlorine, in
the presence of a Lewis acid, such as iron(III) chloride, in a suitable
solvent, such
as dichloromethane.
In Step D(Scheme IV), compounds of formula (7.0) can be prepared by
reacting compounds of the formula (8.0) with an oxidizing agent followed by a
reducing agent, or by reacting compounds of the formula (8.0) with an
oxidizing
agent in the presence of a hydrogen atom source. In a first procedure,
compound
(8.0) can be reacted with a diazotizing agent, such as t-butyl nitrite, in a
solvent
and hydrogen atom source, such as DMF at temperatures from about 00 to +1000
C. In a second procedure, compound (8.0) can be reacted with a diazotizing
agent, such as sodium nitrite, and an acid, such as hydrochloric acid, and a
reducing agent, such as hypophosphorous acid at temperatures from about -150
to +500 C. In a third procedure, compound (8.0) can be reacted with a
diazotizing
agent, such as sodium nitrite, and an acid, such as aqueous sulfuric acid,
followed by treatment with a metal, such as copper. In a fourth procedure,
compound (8.0) can be reacted with a diazotizing agent, such as sodium
nitrite,
and an acid, such as fluoboric acid, followed by treatment with a reducing
agent,
such as sodium borohydride.
In Step E(Scheme IV), compounds of formula (6.0) can be prepared by
reacting compounds of the formula (7.0) under hydrolysis conditions. In a
first
procedure, compound (7.0) can be reacted with an acid, such as hydrochloric
acid, at temperatures from about 200 to +900 C. In a second procedure,
compound (7.0) can be reacted with a base, such as aqueous sodium hydroxide,
in a suitable solvent, such as ethanol, at temperatures from about 200 to +900
C.
In a third procedure, compound (7.0) can be reacted with a nucleophile, such
as
hydrazine hydrate, in a solvent, such as ethanol, with an optional base, such
as
sodium hydroxide, at temperatures from about 200 to +900 C. In a fourth
procedure, compound (7.0) can be reacted with a silyl chloride, such as
trimethylsilyl chloride, in a solvent, such as THF or CH2C12 at temperatures
ranging from about 0 C to reflux. In a fifth procedure, compound (7.0) can be
reacted with an acid, such as trifluoroacetic acid, in an aprotic solvent,
such as
CH2CI2.
In Step F(Scheme IV), compounds of formula (5.0) wherein X- CH can be
prepared by reacting compounds of the formula (6.0) under reducing conditions.
Compound (6.0) can be reacted with an alkyl-metal hydride, such as diisobutyl
-24-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
aluminum hydride or lithium aluminum hydride (LAH), in a solvent, such as
toluene or THF, at temperatures from about 00 to +900 C.
In Step G(Scheme IV), compounds of formula (1.0) can be prepared
as described previously for Scheme I.
In Step K(Scheme IV), compounds of formula (6.1) can be prepared by
reacting the compound of formula (5.9) with a nitrating agent and/or optional
protic or aprotic solvent according to the procedures described in Step A
(Scheme IV).
In Step L (Scheme IV), compounds of formula (6.2) can be prepared by
reacting the compound of formula (6.1) with a reducing agent according to the
procedures described in Step B (Scheme IV).
In Step M (Scheme IV), compounds of formula (6.31) can be prepared by
reacting the compound of formula (6.2) with a halogenating agent according to
the procedures described in Step C (Scheme IV).
In Step N (Scheme IV), compounds of formula (6.3) can be prepared by
reacting the compound of formula (6.31) with an oxidizing agent followed by a
reducing agent, or by reacting compounds of the formula (6.31) with an
oxidizing
agent in the presence of a hydrogen atom source according to the procedures
described in Step D (Scheme IV).
In Step O(Scheme IV), compounds of formula (6.5) can be prepared by
reacting compounds of formula (6.3) with sodium borohydride (NaBH4) in a
solvent such as ethanol/toluene under reflux conditions for 10 minutes or at
25 C
for two hours or more.
In Step P (Scheme IV), compounds of formula (6.7) can be prepared by
reacting compounds of formula (6.5) with SOCI2 in a solvent such as CH2CI2 at
a
temperature of about 25 C for about 4 hours or more.
In Step Q(Scheme IV), compounds of formula (5.0) wherein X = N, can be
prepared by reacting compounds (6.7) with an excess amount of the piperazine
compound of formula (6.9) in a solvent such as THF at 25 C or reflux for one
hour
or more.
Additional starting materials which can be used to prepare the compounds
of the present invention are depicted in Scheme V.
-25-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Scheme V
2 Xa Xa
X1 X xl X2 X] X~
A 1 I i
NOZ A 1 I ' A
R5~ _. ~ R~ NOZ NH'
R6-"~ R8 AA - RS--- R7 BB RS--- R7
N R6~-= N= R8 --- R6N ~ R8
O/fJ OR15
(10.0) O OR15 (10.3) O)'OR15 (10.5)
~ A 1 cc
x4
x' 1 x2 x4 x 1 1 x2
A X1 xz A
s 7 Rs~ R7
R6~ % Rg ~A ~- _ EE R6~ j R8
N O N
01OR15 I 00 (10.8) ts
xa O-A OR (10.7)
(11.0) xi X'- ~ DD
X4
A OH xl x2
PP (6.51)
A
X4 Rs_. R7
_~ j R8
x1 X2 R6 N
A C1 H (10.9)
(6.71) FF
X4
R5_!N R7 X~ X2
R6---#,, N i Rg A
QQ H
(6.9)
R RS"_iX R7
1 R8
O=S=O R6fN
(1.0) Cl (2.6) H (5.01)
X=CH from (10.9)
GG X=N from (6.71, 6.9)
In Step A (Scheme V), compounds of fomula (10.0) can be prepared from
compound of formula (11.0) using the procedures described in Scheme IV, Step
A.
In Step AA(Scheme V), compounds of formula (10.3) can be prepared by
reacting compound of formula (10.0) with 1,3-dibromo-5,5-dimethylhydantoin in
-26-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
an acid, such as trifluoromethane sulfonic acid or sulfuric acid for about 24
h or
more at 25 C.
In Step BB (Scheme V), compounds of the formula (10.5) can be prepared
by treating the compounds of formula (10.3) with a reducing agent, using the
procedures taught in Scheme IV, Step B.
In Step CC (Scheme V), compounds of formula (10.7) can be prepared by
reacting compounds of formula (10.5) with sodium nitrite (NaNO2) in
concentrated aqueous HCI at temperatures ranging from about -10 C to 0 C for
about 2 h or more, then treating the reaction mixture with phosphorous acid
(H3P02) at 0 C for 4 h or more.
In Step DD(Scheme V), compounds of formula (10.9) can be prepared by
reacting compounds of formula (10.7) with concentrated aqueous HCI at about
85 C for about 18 h or more. Compound (10.9) can be reacted using the same
procedures described in Scheme IV for treating compound (5.0) and (6.0) and
subsequent intermediates therefrom, in order to obtain the desired compounds
of
formula (1.0).
In Step EE (Scheme V), compounds of formula (10.8) can be prepared by
reacting compound of formula (10.7) with NalO4 and Ru02 in acetonitrile and
water for about 18 to 24 h or more at 25 C.
In Step FF(Scheme V), compounds of formula (5.01) wherein X = CH can
be prepared by reacting compounds of the formula (10.9) under reducing
conditions. Compound (10.9) can be reacted with an alkyl-metal hydride, such
as
diisobutyl aluminum hydride, in a solvent, such as toluene, at temperatures
from
about 00 to +900 C.
In Step GG(Scheme V), compounds of formula (1.0) can be prepared
using the methods as described in Scheme I, hereinbefore.
In Step OO(Scheme V), compounds of formula (6.51) can be prepared by
reacting compounds of formula (10.8) with sodium borohydride (NaBH4) in a
solvent such as ethanol/toluene under reflux conditions for 10 minutes or at
25 C
for two hours or more.
In Step PP (Scheme V), compounds of formula (6.71) can be prepared by
reacting compounds of formula (6.51) with SOCI2 in a solvent such as CH2CI2 at
a temperature of about 25 C for about 4 hours or more.
In Step QQ (Scheme V), compounds of formula (5.01) wherein X = N, can
be prepared by reacting compounds (6.71) with an excess amount of the
piperazine compound of formula (6.9) in a solvent such as THF at 25 C or
reflux
for one hour or more.
-27-
CA 02293358 2007-06-28
Referring to the Schemes IV and V, except as noted otherwise,
temperatures can range from 00 to 100 C, or reflux of the reaction mixture and
amounts of the reagents (e.g. compound 2.6) can range from 1 to about 10 moles
per mole of reactant (e.g. compound 5.0 or 6.0).
The following preparative examples are intended to exemplify selected
starting materials for preparing compounds of the present invention.
Preparative Example 1
Br , C!
N I
Br
N
H
Step A:
Br 1~ Ci Br ~ CI
N~ N
NOZ
~
0 ;~' OCH2CH3 0 OCH2CH3
Combine 15 g (38.5 mmol) of 4-(8-chloro-3-bromo-5,6-dihydro-1 1 H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidine-1-carboxyfic acid
ethyl ester and 150
mL of concentrated H2SO4 at -5 C, then add 3.89 g (38.5 mmol) of KN03 and stir
for 4 hours. Pour the mixture into 3 L of ice and basify with 50% NaOH
(aqueous). Extract with CH2CI2, dry over MgSO4, then filter and concentrate in
vacuo to a residue. Recrystallize the residue from acetone to give 6.69 g of
the
product.
Step B:
-28-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br Cl Br 1~ I \ CI
N NpZ N NH2
0 OCHZCH3 0 4J-1 OCHZCH3
Combine 6.69 g (13.1 mmol) of the product of Step A and 100 mL of 85%
EtOH/water, then add 0.66 g (5.9 mmol) of CaC12 and 6.56 g (117.9 mmol) of Fe
and heat the mixture at reflux overnight. Filter the hot reaction mixture
through
Celite and rinse the filter cake with hot EtOH. Concentrate the filtrate in
vacuo to
give 7.72 g of the product.
Step C:
B r ,~ I\ C I B r 1~"~ I\ C I
N NHZ N NHZ
Br
~
0 OCHZCH3 0 OCHZCH3
Combine 7.70 g of the product of Step B and 35 mL of HOAc, then add 45 mL of a
solution of Br2 in HOAc and stir the mixture at room temperature overnight.
Add
300 mL of 1 N NaOH (aqueous) , then 75 mL of 50% NaOH (aqueous) and
extract with EtOAc. Dry the extract over MgSO4 and concentrate in vacuo to a
residue. Chromatograph the residue (silica gel, 20%-30% EtOAc/hexane) to give
3.47 g of the product (along with another 1.28 g of partiaily purified
product).
Step D:
Br ~ I\ CI Br ~ I\ CI
N~ 1 N
NH2
Br Br
~
0 OCHZCH3 0 OCHZCH3
Combine 0.557 g (5.4 mmol) of t-butyinitrite and 3 mL of DMF, and heat the
mixture at to 60 -70 C. Slowly add (dropwise) a mixture of 2.00 g (3.6 mmol)
of
-29-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
the product of Step C and 4 mL of DMF, then cool the mixture to room
temperature. Add another 0.64 mL of t-butylnitrite at 40 C and reheat the
mixture
to 60 -70 C for 0.5 hrs. Cool to room temperature and pour the mixture into
150
mL of water. Extract with CH2CI2, dry the extract over MgSO4 and concentrate
in
vacuo to a residue. Chromatograph the residue (silica gel, 10%-20%
EtOAc/hexane) to give 0.74 g of the product.
Step E:
Br 1~ I\ ci Br 1~ I\ CI
N~ I i N I i
Br Br
N N
0 OCHZCH3
Combine 0.70 g (1.4 mmol) of the product of Step D and 8 mL of
concentrated HCI (aqueous) and heat the mixture at reflux overnight. Add 30 mL
of 1 N NaOH (aqueous), then 5 mL of 50% NaOH (aqueous) and extract with
CH2CI2. Dry the extract over MgSO4 and concentrate in vacuo to give 0.59 g of
the title compound.
Preparative Example 2
Br 7 / \ CI Br CI
N
I N
Br Br
N N
H
[racemic as well as (+)- and (-)-isomers]
Prepare a solution of 8.1 g of the title compound from Preparative Example 7
in
toluene and add 17.3 mL of a 1 M solution of DIBAL (diisobutyl aluminum
hydride)
in toluene. Heat the mixture at reflux and slowly add (dropwise) another 21 mL
of
1 M DIBAL/toluene solution over a period of 40 min. Cool the reaction mixture
to
about 0 C and add 700 mL of 1 M HCI (aqueous). Separate and discard the
organic phase. Wash the aqueous phase with CH2CI2, discard the extract, then
basify the aqueous phase by adding 50% NaOH (aqueous). Extract with CH2CI2,
dry the extract over MgSO4 and concentrate in vacuo to give 7.30 g of the
title
compound, which is a racemic mixture of enantiomers.
-30-
CA 02293358 2007-06-28
Preparative Example 3- Separation of Enantiomers:
Br k ' \ CI
N
Br , I \ CI
Br
N
Bt H (+)
N
H Br H CI
N
Br
N
H 0
The racemic title compound of Preparative Example 1 is separated by
preparative
chiral chromatography (Chiralpack*AD, 5 cm X 50 cm column, using 20%
iPrOH/hexane + 0.2% diethylamine), to give the (+)-isomer and the (-)-isomer
of
the title compound. Altenatively, the enantiomers can also be separated by
crystallization with an amino acid such as N-acetylphenylatanine.
Preparative Example 6
Br 1 ~ ' \ CI
/
N
N Br
N
H
[racemic as well as (+)- and (-)-enantiomerJ
Step A:
-31-
Trademark *
CA 02293358 2007-06-28
NO2
Br C!
N
CI
Br CN
/ 0 Br a
N ~
O 2
Combine 40.0 g (0.124 mole) of the starting ketone
and 200 mL of H2SO4 and cooi to 0 C. Slowly
add 13.78 g (0.136 mole) of KN03 over a period of 1.5 hrs., then warm to room
temperature and stir overnight. Work up the reaction using substantially the
same
procedure as described for Preparative Example 4, Step A. Chromatograph
(silica gel, 20%, 30%, 40%, 50% EtOAc/hexane, then 100% EtOAc) to give 28 g
of the 9-nitro product, along with a smaller quantity of the 7-nitro product
and 19 g
of a mixture of the 7-nitro and 9-nitro compounds. MH+ (9-nitro) = 367.
Step B:
Br \ CI Br ci
/ -- -----~- 1 / I .~
:NX
N O Np2 O NH2
React 28 g (76.2 mmol) of the 9-nitro product of Step A, 400 mL of 85%
EtOH/water, 3.8 g (34.3 mmol) of CaCI2 and 38.28 g (0.685 mole) of Fe at 50 C.
Heat the mixture at reflux overnight, filter through Celite and wash the
filter cake
with 2 X 200 mL of hot EtOH. Combine the filtrate and washes, and concentrate
in vacuo to a residue. Extract the residue with 600 mL of CH2CI2, wash with
300
mL of water and dry over MgSO4. Filter and concentrate in vacuo to a residue,
then chromatograph (silica gel, 30% EtOAc/CH2CI2) to give 24 g of the product.
Step C:
Br Z ~ \ a Br \ p
N :N/
0 NH2 0 Br NH
2
-32-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Combine 13 g (38.5 mmol) of the product of Step B, 140 mL of HOAc and slowly
add a solution of 2.95 mL (57.8 mmol) of Br2 in 10 mL of HOAc over a period of
20 min. Stir the reaction mixture at room temperature, then concentrate in
vacuo
to a residue. Add CH2C12 and water, then adjust to pH = 8-9 with 50% NaOH
(aqueous). Wash the organic phase with water, then brine and dry over Na2SO4.
Concentrate in vacuo to give 11.3 g of the product.
Steg :
Br Br a
~ ~ ~ ~ ---~- ~ ~ ~ .~
N
0 Br NH2 0 Br
Cool 100 mL of concentrated HCI (aqueous) to 0 C, then add 5.61 g (81.4 mmol)
of NaNO2 and stir for 10 min. Slowly add (in portions) 11.3 g (27.1 mmol) of
the
product of Step C and stir the mixture at 0 -3 C for 2.25 hrs. Slowly add
(dropwise) 180 mL of 50% H3P02 (aqueous) and allow the mixture to stand at
0 C overnight. Slowly add (dropwise) 150 mL of 50% NaOH over 30 min., to
adjust to pH = 9, then extract with CH2CI2. Wash the extract with water, then
brine and dry over Na2SO4. Concentrate in vacuo to a residue and
chromatograph (silica gel, 2% EtOAc/ CH2CI2) to give 8.6 g of the product.
Step E.
Br ' N a Br a
~
N - N
0 Br C)H Br
Combine 8.6 g (21.4 mmol) of the product of Step D and 300 mL of MeOH and
cool to 0 -2 C. Add 1.21 g (32.1 mmol) of NaBH4 and stir the mixture at -0 C
for
1 hr. Add another 0.121 g (3.21 mmol) of NaBH4, stir for 2 hr. at 0 C, then
let
stand overnight at 0 C. Concentrate in vacuo to a residue then partition the
residue between CH2CI2 and water. Separate the organic phase and
concentrate in vacuo (50 C) to give 8.2 g of the product.
Step F:
-33-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br ~ J \ a
Br a N I i
/ ~ -~ N
N Br
CH Br )
H
Combine 8.2 g (20.3 mmol) of the product of Step E and 160 mL of CH2CI2, cool
to
0 C, then slowly add (dropwise) 14.8 mL (203 mmol) of SOCI2 over a 30 min.
period. Warm the mixture to room temperature and stir for 4.5 hrs., then
concentrate in vacuo to a residue, add CH2CI2 and wash with 1 N NaOH
(aqueous) then brine and dry over Na2SO4. Concentrate in vacuo to a residue,
then add dry THF and 8.7 g (101 mmol) of piperazine and stir at room
temperature
overnight. Concentrate in vacuo to a residue, add CH2CI2, and wash with 0.25 N
NaOH (aqueous), water, then brine. Dry over Na2SO4 and concentrate in vacuo to
give 9.46 g of the crude product. Chromatograph (silica gel, 5% MeOH/CH2CI2 +
NH3) to give 3.59 g of the title compound, as a racemate.
Step G - Separation of Enantiomers:
Br H ~ \ a
N~
N Br
Br '~ \ a ) R-(+)
N
N H
N Br
~ Br ~ \ a
N H ~
H 1 N~
N Br
J
N S+)
H
The racemic title compound from Step F (5.7 g) is chromatographed by
preparative chiral chromatography (Chiralpack AD, 5 cm X 50 cm column, flow
rate 100 mUmin) using 30% iPrOH/hexane + 0.2% diethylamine, to give 2.88 g of
the R-(+)-enantiomer and 2.77 g of the S-(-)-enantiomer of the title compound.
-34-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Preparative Example 7
Br
Br ~ I \ l a
N
N
H
Step A:
Br ~ 1\ CI Br ~"' I\ CI
N 2
~
N N
0_11~_ OCH 2CH 3 0 OCt-I 2CH 3
Combine 25.86 g (55.9 mmol) of 4-(8-chloro-3-bromo-5,6-dihydro-1 1 H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11-yiidene)-1-piperidine-1-carboxylic acid
ethyl ester and 250 mL of concentrated H2SO4 at -5 C, then add 4.8 g (56.4
mmol) of NaNO3 and stir for 2 hours. Pour the mixture into 600 g of ice and
basify
with concentrated NH4OH (aqueous). Filter the mixture, wash with 300 mL of
water, then extract with 500 mL of CH2CI2. Wash the extract with 200 mL of
water, dry over MgSO4, then filter and concentrate in vacuo to a residue.
Chromatograph the residue (silica gel, 10% EtOAc/ CH2CI2) to give 24.4 g (86%
yield) of the product. m.p. = 165-167 C.
Step B:
-35-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br
Br ~ I\ a Br ~ r\ a
N N I i
N02 No2
N N
O-~- OCH 2CH 3 0 OaHCH
2 3
Combine 20 g (40.5 mmol) of the product of Step A and 200 mL of concentrated
H2SO4 at 20 C, then cool the mixture to 0 C. Add 7.12 g (24.89 mmol) of 1,3-
dibromo-5,5-dimethyl-hydantoin to the mixture and stir for 3 hours at 20 C.
Cool
to 0 C, add an additional 1.0 g (3.5 mmol) of the dibromohydantoin and stir at
20 C for 2 hours. Pour the mixture into 400 g of ice, basify with concentrated
NH4OH (aqueous) at 0 C, and collect the resulting solid by filtration. Wash
the
solid with 300 mL of water, slurry in 200 mL of acetone and filter to provide
19.79
g (85.6% yield) of the product.
StepC:
Br Br
Br ~ '\ a Br ~ I\ a
N N N
02 NNH2
N N
O OaH2CH 3 O-~' Oa-i 2CH 3
Combine 25 g (447 mmol) of Fe filings, 10 g (90 mmol) of CaC12 and a
suspension of 20 g (34.19 mmol) of the product of Step B in 700 mL of 90:10
EtOH/water at 50 C. Heat the mixture at reflux overnight, filter through
Celite
and wash the filter cake with 2 X 200 mL of hot EtOH. Combine the filtrate and
washes, and concentrate in vacuo to a residue. Extract the residue with 600 mL
of CH2CI2, wash with 300 mL of water and dry over MgSO4. Filter and
concentrate in vacuo to a residue, then chromatograph (silica gel, 30%
EtOAc/CH2CI2) to give 11.4 g (60% yield) of the product.
S tep D:
-36-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br Br
Br ~ ! \ a Br
a
1 N /
NH 2 :
N
~ N
O OCH 2CH 3 0 OCf-I 2CH 3
Slowly add (in portions) 20 g (35.9 mmol) of the product of Step C to a
solution of
8 g (116 mmol) of NaNO2 in 120 mL of concentrated HCl (aqueous) at -10 C. Stir
the resulting mixture at 0 C for 2 hours, then slowly add (dropwise) 150 mL
(1.44
mole) of 50% H3P02 at 0 C over a 1 hour period. Stir at 0 C for 3 hours, then
pour into 600 g of ice and basify with concentrated NH4OH (aqueous). Extract
with 2 X 300 mL of CH2CI2, dry the extracts over MgSO4, then filter and
concentrate in vacuo to a residue. Chromatograph the residue (silica gel, 25%
EtOAc/ hexanes) to give 13.67 g (70% yield) of the product.
Step E:
Br Br
Br i ~ \ CI
' Br ~ I\ a
N I ~ 1 N
---:
N
N
01~1 OCH 2CH 3
Combine 6.8 g (12.59 mmol) of the product of Step D and 100 mL of concentrated
HCI (aqueous) and stir at 85 C overnight. Cool the mixture, pour it into 300 g
of
ice and basify with concentrated NH4OH (aqueous). Extract with 2 x 300 mL of
CH2CI2, then dry the extracts over MgSO4. Filter, concentrate in vacuo to a
residue, then chromatograph (silica gel, 10% MeOH/EtOAc + 2% NH4OH
(aqueous)) to give 5.4 g (92% yield) of the title compound.
Preparative Example 8
37 -
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br
Br - N\ a
, /
N
(N)
N
H [racemic as well as (+)- and (-)-enantiomers]
Step A:
Br Br
Br
1 ~ I Br ~ I~ CI
N N
--~ 0
N
OCH 2CH 3
Combine 16.6 g (0.03 mole) of the product of Preparative Example 7, Step D,
with
a 3:1 solution of CH3CN and water (212.65 mL CH3CN and 70.8 mL of water)
and stir the resulting slurry overnight at room temperature. Add 32.833 g
(0.153
mole) of Na104 and then 0.31 g (2.30 mmol) of Ru02 and stir at room
temperature
(the addition of Ru02 is accompanied by an exothermic reaction and the
temperature climbs from 20 to 30 C). Stir the mixture for 1.3 hrs.
(temperature
returned to 25 C after about 30 min.), then filter to remove the solids and
wash
the solids with CH2CI2. Concentrate the filtrate in vacuo to a residue and
dissolve the residue in CH2Cf2. Filter to remove insoluble solids and wash the
solids with CH2CI2. Wash the filtrate with water, concentrate to a volume of
about
200 mL and wash with bleach, then with water. Extract with 6 N HCI (aqueous).
Cool the aqueous extract to 0 C and slowly add 50% NaOH (aqueous) to adjust
to pH = 4 while keeping the temperature <30 C. Extract twice with CH2CI2, dry
over MgSO4 and concentrate in vacuo to a residue. Slurry the residue in 20 mL
of EtOH and cool to 0 C. Collect the resulting solids by filtration and dry
the
solids in vacuo to give 7.95 g of the product.
Step B:
-38-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br Br
Br ~ 1\ a Br '~ I\ a
N N
0 OH
Combine 21.58 g (53.75 mmol) of the product of Step A and 500 mL of an
anhydrous 1:1 mixture of EtOH and toluene, add 1.43 g (37.8 mmol) of NaBH4
and heat the mixture at reflux for 10 min. Cool the mixture to 0 C, add 100 mL
of
water, then adjust to pH= 4-5 with 1 M HCI (aqueous) while keeping the
temperature <10 C. Add 250 mL of EtOAc and separate the layers. Wash the
organic layer with brine (3 X 50 mL) then dry over Na2SO4. Concentrate in
vacuo
to a residue (24.01 g) and chromatograph the residue (silica gel, 30 %
hexane/CH2CI2) to give the product. Impure fractions were purified by
rechromatography. A total of 18.57 g of the product is obtained.
Step C:
Br
Br Br ~ 1 \ a
Br ~ / \ a N
1 ~ --~ N
N OH
NJ
H
Combine 18.57 g (46.02 mmol) of the product of Step B and 500 mL of CHCI3,
then add 6.70 mL (91.2 mmol) of SOCI2, and stir the mixture at room
temperature
for 4 hrs. Add a solution of 35.6 g (0.413 mole) of piperazine in 800 mL of
THF
over a period of 5 min. and stir the mixture for 1 hr. at room temperature.
Heat the
mixture at reflux overnight, then cool to room temperature and dilute the
mixture
with 1 L of CH2C12. Wash with water (5 X 200 mL), and extract the aqueous wash
with CHCI3 (3 X 100 mL). Combine all of the organic solutions, wash with brine
(3 X 200 mL) and dry over MgSO4. Concentrate in vacuo to a residue and
chromatograph (silica gel, gradient of 5%, 7.5%, 10% MeOH/CH2CI2 + NH4OH)
to give 18.49 g of the title compound as a racemic mixture.
Step D - Separation of Enantiomers:
-39-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br
Br ,-~H a
Br
Br a N
N
N (+)-enantiomer
(N) N
H
Br
N
H Br H a
I:N
N
) (-)-enantiomer
H
The racemic title compound of Step C is separated by preparative chiral
chromatography (Chiralpack AD, 5 cm X 50 cm column, flow rate 100 mUmin.,
20% iPrOH/hexane + 0.2% diethylamine), to give 9.14 g of the (+)-enantiomer
and 9.30 g of the (-)-enantiomer.
Preparative Examale 9
Br
Br ' ~ a
N
N
H
[racemic as well as (+)- and (-)-enantiomer]
Step A:
Br Br
Br a Br ~ CI
1 N I i , /
I -i N
N N
H H
-40-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Combine 13 g (33.3 mmol) of the title compound from Preparative Example 7,
and 300 mL of toluene at 20 C, then add 32.5 mL (32.5 mmol) of a 1 M solution
of
DIBAL in toluene. Heat the mixture at reflux for 1 hr., cool to 20 C, add
another
32.5 mL of 1 M DIBAL solution and heat at reflux for 1 hr. Cool the mixture to
20 C and pour it into a mixture of 400 g of ice, 500 mL of EtOAc and 300 mL of
10% NaOH (aqueous). Extract the aqueous layer with CH2CI2 (3 x 200 mL), dry
the organic layers over MgSO4, then concentrate in vacuo to a residue.
Chromatograph (silica gel, 12% MeOH/CH2CI2 + 4% NH4OH) to give 10.4 g of
the title compound as a racemate.
Step B - Separation of Enantiomers:
Br
Br H I \ q
N
Br v' N Br
H
Br a Br H I\ a
N N
N ON
H H
The racemic title compound of Step A is separated by preparative chiral
chromatography (Chiralpack AD, 5 cm X 50 cm column, using 5% iPrOH/hexane
+ 0.2% diethylamine), to give the (+)-enantiomer and the (-)-enantiomer of the
title
compound.
Preparative Example 10
Br 1 ~ CI
N
CI
(+)-enantiomer
N
I
H
Step A:
-41-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br ~ I\ a Br CI
N
I I N02
N N
O-~- OCH 2CH 3 O)~ OCH 2CH 3
Combine 15 g (38.5 mmol) of 4-(8-chloro-3-bromo-5,6-dihydro-1 1 H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11-ylidene)-1-piperidine-l-carboxylic acid
ethyl ester and 150 mL of concentrated H2SO4 at -5 C, then add 3.89 g (38.5
mmol) of KNO3 and stir for 4 hours. Pour the mixture into 3 L of ice and
basify
with 50% NaOH (aqueous). Extract with CH2CI2, dry over MgSO4, then filter and
concentrate in vacuo to a residue. Recrystallize the residue from acetone to
give
6.69 g of the product.
Step B:
Br a Br CI
N02 NH2
N N
O)-~- OCH 2CH 3 O)-- OCH 2CH 3
Combine 6.69 g (13.1 mmol) of the product of Step A and 100 mL of 85%
EtOH/water, then add 0.66 g (5.9 mmol) of CaCI2 and 6.56 g (117.9 mmol) of Fe
and heat the mixture at reflux overnight. Filter the hot reaction mixture
through
Celite@ and rinse the filter cake with hot EtOH. Concentrate the filtrate in
vacuo to
give 7.72 g of the product.
Step C:
-42-
CA 02293358 1999-12-09
WO 98/57949 PCT/US9S/11508
Br ~ I \ CI Br CI
N 1 N/
NH2 CI NH2
c----~-
N N
0 ~OCH2CH3 0 )",' OCH2CH3
Dissolve 9.90 g (18.9 mmol) of the product of Step B, in 150 mL CH2CI2 and 200
mL of CH3CN and heat to 60 C. Add 2.77 g (20.8 mmol) N-chlorosuccinimide
and heat to reflux for 3 h., monitoring the reaction by TCL (30%EtOAc/H20).
Add
an additional 2.35 g (10.4 mmol) of N-chlorosuccinimide and reflux an
additional
45 min. Cool the reaction mixture to room temperature and extract with 1 N
NaOH
and CH2CI2. Dry the CH2CI2 layer over MgSO4, filter and purify by flash
chromatography (1200 mL normal phase silica gel, eluting with 30% EtOAc/H20)
to obtain 6.24 g of the desired product. M.p. 193-195.4 C.
Step D:
Br CI Br CI
/
N CI NH2 N CI
N N
O)"', OCH2CH3 OJ~ OCH2CH3
To 160 mL of conc. HCI at -10 C add 2.07 g (30.1 mmol) NaNO2 and stir for 10
min. Add 5.18 g(10.1 mmol) of the product of Step A and warm the reaction
mixture from -10 C to 0 C for 2 h. Cool the reaction to -10 C, add 100 mL
H3P02
and let stand overnight. To extract the reaction mixture, pour over crushed
ice
and basify with 50% NaOH/ CH2CI2. Dry the organic layer over MgSO4, filter and
concentrate to dryness. Purify by flash chromatography (600 mL normal phase
silica gel, eluting with 20% EtOAc/hexane) to obtain 3.98 g of product.
Step E:
-43-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Br ~ I \ CI Br ~ I \ ci
N~ N
CI CI
--
N N
0 OCH2CH3
Dissolve 3.9 g of the product of Step D in 100 mL conc. HC1 and reflux
overnight. Cool the mixture, basify with 50 % w/w NaOH and extract
the resultant mixture with CH2Cl?. Dry the CH2C12 layer over MgSO4,
evaporate the solvent and dry under vacuum to obtain 3.09 g of the
desired product.
Step F:
Br CI Br 1 CI
CI CI
(+)-enantiomer
N N
I
H H
Using a procedure similar to that described in Preparative Example 8, obtain
1.73
g of the desired product, m.p. 169.6-170.1 C; [a]p =+48.2 (c=1, MeOH). MH+ _
425.
-44-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
A SAY S
1. In vitro enzyme assavs: FPT IC50 (inhibition of farnesyl protein
transferase, in vitro enzyme assay) are determined by the methods disclosed in
WO/1 0515 or WO 95/10516. The data demonstrate that the compounds of the
invention are inhibitors of Ras-CVLS farnesylation by partially purified rat
brain
farnesyl protein transferase (FPT). The data also show that there are
compounds
of the invention which can be considered as potent (IC50 <10 M) inhibitors of
Ras-CVLS farnesylation by partially purified rat brain FPT.
2. Cell-based assay. COS fC50 values refer to the COS cells activity
inhibition of Ras processing, are determined by the methods disclosed in
WO/1 0515 or WO 95/10516.
Example No. H-ras FPT
IC50 ( M)
1 0.0080
2 0.1640
3 0.0260
4 0.0070
5 0.0154
6 0.0320
7 0.1100
8 0.3200
9 0.0340
10 0.0890
11 0.0320
12 0.1790
13 0.0850
14 0.2760
For preparing pharmaceutical compositions from the compounds
described by this invention, inert, pharmaceutically acceptable carriers can
be
either solid or liquid. Solid form preparations include powders, tablets,
dispersible granules, capsules, cachets and suppositories. The powders and
tablets may be comprised of from about 5 to about 70 percent active
ingredient.
Suitable solid carriers are known in the art, e.g. magnesium carbonate,
-45-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
magnesium stearate, talc, sugar, lactose. Tablets, powders, cachets and
capsules can be used as solid dosage forms suitable for oral administration.
For preparing suppositories, a low melting wax such as a mixture of fatty
acid glycerides or cocoa butter is first melted, and the active ingredient is
dispersed homogeneously therein as by stirring. The molten homogeneous
mixture is then poured into convenient sized molds, allowed to cool and
thereby
solidify.
Liquid form preparations include solutions, suspensions and emulsions.
As an example may be mentioned water or water-propylene glycol solutions for
parenteral injection.
Liquid form preparations may also include solutions for intranasal
administration.
Aerosol preparations suitable for inhalation may include solutions and
solids in powder form, which may be in combination with a pharmaceutically
acceptable carrier, such as an inert compressed gas.
Also included are solid form preparations which are intended to be
converted, shortly before use, to liquid form preparations for either oral or
parenteral administration. Such liquid forms include solutions, suspensions
and
emulsions.
The compounds of the invention may also be deliverable transdermally.
The transdermal compositions can take the form of creams, lotions, aerosols
and/or emulsions and can be included in a transdermal patch of the matrix or
reservoir type as are conventional in the art for this purpose.
Preferably the compound is administered orally.
Preferably, the pharmaceutical preparation is in unit dosage form. In such
form, the preparation is subdivided into unit doses containing appropriate
quantities of the active component, e.g., an effective amount to achieve the
desired purpose.
The quantity of active compound in a unit dose of preparation may be
varied or adjusted from about 0.1 mg to 1000 mg, more preferably from about 1
mg. to 300 mg, according to the particular application.
The actual dosage employed may be varied depending upon the
requirements of the patient and the severity of the condition being treated.
Determination of the proper dosage for a particular situation is within the
skill of
the art. Generally, treatment is initiated with smaller dosages which are less
than
the optimum dose of the compound. Thereafter, the dosage is increased by small
increments until the optimum effect under the circumstances is reached. For
-46-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
convenience, the total daily dosage may be divided and administered in
portions
during the day if desired.
The amount and frequency of administration of the compounds of the
invention and the pharmaceutically acceptable salts thereof will be regulated
according to the judgment of the attending clinician considering such factors
as
age, condition and size of the patient as well as severity of the symptoms
being
treated. A typical recommended dosage regimen is oral administration of from
10
mg to 2000 mg/day preferably 10 to 1000 mg/day, in two to four divided doses
to
block tumor growth. The compounds are non-toxic when administered within this
dosage range.
The following are examples of pharmaceutical dosage forms which contain
a compound of the invention. The scope of the invention in its pharmaceutical
composition aspect is not to be limited by the examples provided.
-47-
CA 02293358 1999-12-09
WO 98/57949 PCT/US98/11508
Pharmaceutical Dosage Form Examples
EXAMPLE A-Tablets
No. Ingredients mg/tablet m/tablet
1. Active compound 100 500
2. Lactose USP 122 113
3. Corn Starch, Food Grade, 30 40
as a 10% paste in
Purified Water
4. Corn Starch, Food Grade 45 40
5. Magnesium Stearate 3 7
Total 300 700
Method of Manufacture
Mix Item Nos. 1 and 2 in a suitable mixer for 10-15 minutes. Granulate the
mixture with Item No. 3. Mill the damp granules through a coarse screen (e.g.,
1/4", 0.63 cm) if necessary. Dry the damp granules. Screen the dried granules
if
necessary and mix with Item No. 4 and mix for 10-15 minutes. Add Item No. 5
and mix for 1-3 minutes. Compress the mixture to appropriate size and weigh on
a suitable tablet machine.
EXAMPLE B-Ca suies
No. In redient mg/capsule mg/capsule
1. Active compound 100 500
2. Lactose USP 106 123
3. Corn Starch, Food Grade 40 70
4. Magnesium Stearate NF 7 7
Total 253 700
Method of Manufacture
Mix Item Nos. 1, 2 and 3 in a suitable blender for 10-15 minutes. Add Item
No. 4 and mix for 1-3 minutes. Fill the mixture into suitable two-piece hard
gelatin capsules on a suitable encapsulating machine.
While the present invention has been described in conjunction with the
specific embodiments set forth above, many alternatives, modifications and
variations thereof will be apparent to those of ordinary skill in the art. AIl
such
alternatives, modifications and variations are intended to fall within the
spirit and
scope of the present invention.
-48-