Note: Descriptions are shown in the official language in which they were submitted.
CA 02293760 1999-12-08
~1'O 98/59027
PC7 /EP9b/0369~
1
ADDITIVE FOR A DETERGENT FORMULATION, DETERGENT FORMULATION
COMPRISING SUCH AN ADDITIVE AND USE
OF SAID FORMULATLON FOR CLEANING BOTTLES
Field of the invention
The present invention relates to an additive for a
detergent formulation, a detergent formulation comprising
said additive, and to the use of said formulation for
cleaning bottles, in particular returnable plastic bottles
such as PET (polyethylene terephthalate) bottles,
polyethylene naphthalate (PEN), copolymers of PET/PEN
and/or returnable polycarbonate bottles.
Background of the invention
Many soft drinks are being sold in returnable bottles,
these bottles being made of either glass or plastic, in
particular PET or polycarbonate. In order to ensure product
quality it is very important that such bottles be
thoroughly cleaned and disinfected before being refilled
with the soft drink for example.
The use of glycine-N-N-diacetic acid (NTA) derivatives as
textile detergent builders is known from WO 94/29421.
An existing problem with such beverage bottles after they
have been emptied of their contents, is that fungal growth
can occur on any residue left behind.
Ari objective of the present invention is to provide an
additive suitable for a detergent formulation, for
effectively removing mould from beverage bottles.
It was now surprisingly found that a combination of an
nonionic and/or a hydrotrope with a specific group of
Gl a c~ -~o r /~ o ~ ~- ~c c ~e ~ h ~ it
AMENDED S'~EET
,r,cr.W~
CA 02293760 1999-12-08
WO 9/59027 PCT/EP98,'036~~
2
sequestrants, as specified below, provides particularly
good cleaning performance.
Definition of the invention
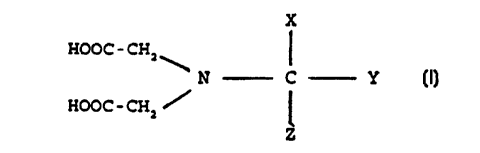
Consequently, according to a first embodiment of the
present invention there is provided an additive for a
detergent formulation, said additive ~TT~nri cir,rr n
s.~.i~_ _ ~G~ vn fi. Go rte.. ~ o !' n ~ r ~a n
h
X ' AS a(~1 Lr,'6c~G i"~.
HOOC-CH2 G ~0.rw~ S .
Y
HOOC-CHZ/
Z
wherein:
X - a C96H group or an alkyl chain comprising a COOH
g~P .
Y an H atom
Z /= an H atom or C1-C3o, CZ-C3o alkenyl group
an,~/ wherein the formulation further comprises one or more
In an other aspect of the invention, there is provided a
formulation comprising an additive according to the
invention wherein the sequestrant is present in a wt$ range
of 5-60, preferably 15-35, and the nonionic andj~r
hydrotrope in a wt$ range of 0.5-40, preferably 1-25.
The formulation of the invention can ~3dditionally contain
other common detergent components, including oxidizing
agents, caustic, H202, hypochlorite, anti-foaming agents,
alkali sources, threshold agents to prevent deposition of
hard water scale, structuring or emulsifying polymers, and
bleaches to degrade or decolorise oxidisable stains.
A;wEI~DED SHEET
CA 02293760 1999-12-08
WO 98/59017 T'C'TJ.h:P9a/0369~
3
According to a third aspect of the present invention, there
is provided the use of the formulation of the invention as
a detergent in cleaning bottles, particularly retunable
PET bottles, when the formulation comprises no hydrotrope,
and particularly returnable polycarbonate bottles when the
formulation comprises no nonionic surfactant.
According to a fourth aspect of the invention, there is
provided a process for washing bottles, comprising the
steps of exposing bottles to a formulation or additive
according to the invention, wherein the sequestrant,
nonionic surfactant ands hydrotrope and possibly any
further standard detergent formulation components, such as
TM
a defoamer, for example Dehypon LT109 pare collectively or
individually supplied into a bottle washing system
Detailed description of the invention
the sequestrant according to the present invention
preferably comprises one or more of the following:
- hydroxyl groups, preferably not more t five,
- formyl groups,
- C1- to Cq-alkoxy groups,
- phenoxy groups,
- C1-C4 alkoxycarbony groups,
and/or a phenylalkyl 'th 1-20 C atoms in the alkyl group,
and can comprise a or more of the following:
- a fiver or six-membered unsaturated or saturated
heterocy is ring preferably with up to 3 hetero-atoms,
most referably selected from the group nitrogen, oxygen,
AMENDED SHEET
IPEAIEP
CA 02293760 1999-12-08
WO 98159027 PCT/EP98/9~6°5
9
ring may comprise one or more of the follo g groups:
- a C1-C4 alkyl group,
- a hydroxyl group,
- a carboxyl group,
- a sulfo group
- a phospho group
- a sul ate ester
- a hosphate ester
~ a C,-C4 alkoxycarbonyl group.
The sequestrant is ~~ selected
from the group consisting of Nitrilo triacetic acid (NTA),
~i-Alanine diacetic acid (~3-ADA), Methyl glycine diacetic
acid (MGDA), Serine diacetic acid (SDA) and Ethyl glycine
diacetic acid (EGDA), wherein MGDA and SDA yield
particularly good cleaning results.
The non-ionic surfactant preferably comprises one or more
of the following:
- polyoxyethylene or polyoxypropylene condensates of
aliphatic carboxylic acids having 8 to 18
carbon atoms in an aliphatic chain and incorporating from
a.~a~t 2 to a~ae~ 50 ethylene oxide and/or propylene oxide
units,
polyoxyethylene or polyoxypropylene condensates of
aliphatic alcohols having afrom 6 to 24 carbon
atoms and incorporating from ahs~ 2 to 50 ethylene
oxide and/or propylene oxide units,
- a compound of formula:
R-(CHZCHzO)aH
ANfENDED SHEEN'
~pEAIEP
CA 02293760 1999-12-08
' WO 98/59027 PC7 /EP9b/03695
wherein R is a C6-C24 linear or branched alkyl group and a
is an integer from 2 to 50,
- a compound of formula:
R'- (CHzCHO) X (CHZCH20) y (CHzCHO) ZH
5
Rz R3
wherein R' is a linear alkyl group having an average of
about 6 to about 18 carbon atoms, Rz and R3 are each linear
alkyl groups of 1 to ~be~tt 9 carbon atoms, x is an
integer from 1 to 6, y is an integer from 4 to 20 and z is
an integer from 4 to 25,
- polyoxyethylene or polyoxypropylene condensates of
alkyl phenols having about 6 to 12 carbon atoms and
incorporating from about 2 to 25 moles of ethylene oxide
and/or propylene oxide,
- polyoxyethylene derivatives of sorbitan
mono-, di-and tri-fatty acid esters wherein the fatty acid
component has between 12 and 24 carbon atoms and the
polyethylene chains contain between about 9 and 30 ethylene
oxide units,
- polyoxyethylene-polyoxypropylene block copolymers
having the formula:
HO ( CHzCH20 ) a ( CH ( CH3 ) CH20 ) b ( CHZCHZO ) ~H or
HO (CH (CH3) CHZO) d (CHZCHZO) a (CH (CH3) CHZO) fH
wherein a, b, c, d, e, and f are integers from 1 to 350
reflecting the respective polyethylene oxide and
polypropylene oxide blocks of said polymer, wherein the
polyoxyethylene component of the block polymer is at least
about 10~ of the block polymer,
AMENDED SHEET
IPEAIEP i
CA 02293760 1999-12-08
WO 98/59027
PC'1'/EP9l~/03h9s
6
- alkyl glycosides having formula:
R90(R50)"(Z1) P
wherein R4 is a monovalent organic radical, for example a
monovalent saturated aliphatic, unsaturated aliphatic or
aromatic radical such as alkyl, hydroxyalkyl, alkenyl,
hydroxyalkenyl, aryl, alkylaryl, hydroxyalkylaryl,
arylalkyl, alkenylaryl, arylalkenyl and the like having
from aft 6 to 30 carbon atoms, wherein RS is a
divalent hydrocarbon radical containing from 2 to a~e~t 4
carbon atoms such as ethylene, propylene or butylene (most
preferably the unit (R50)" represents repeating units of
ethylene oxide, propylene oxide and/or random or block
combinations thereof); n is an integer from 0 to a~e~rt 12;
Z1 represents a moiety derived from a reducing saccharide
containing 5 or 6 carbon atoms (most preferably a glucose
unit); and p is a number from 0.5 to a~e~ 10,
- amine oxides having formula:
R6R'RBN=0
wherein R6, R' and Re are saturated aliphatic groups or
substituted saturated aliphatic groups. wherein preferably
R6 is an alkyl chain of .a~etrt 10 to 20 carbon atoms and R'
and R8 are methyl or ethyl groups or both R6 and R' are
alkyl chains of 6 to 14 carbon atoms and R8 is a
methyl or ethyl group.
A.n additive with such a non-ionic surfactant in combination
with the above sequesr4ant yielded goe:= cleaning results.
The non-ionic surfactant ~r~for~~,~~~ has a hydrophilic-
lipophilic balance (HLB) of
from abeQt 14-20.
AMENDED Sh~EET
IPE~IEP i
CA 02293760 1999-12-08
WO 98/59027 PCTI~P98/L369~
7
Non-ionic surfactants can be broadly defined as surface
active compounds with one or more uncharged hydrophilic
substituents. A major class of non-ionic surfactants are
those compounds produced by the condensation of alkylene
oxide groups with an organic hydrophobic material which may
be aliphatic or alkyl aromatic in nature. The length of the
hydrophilic or polyoxyalkylene radical which is condensed
with any particular hydrophobic group can be readily
adjusted to yield a water-soluble compound having a desired
degree of balance between hydrophilic and hydrophobic
elements.
The HLB value represents the hydrophilic-lipophilic balance
of the molecule. The lower the HLB value the more
hydrophobic the material is, and vice versa. For simple
alcohol ethoxylates, the HLB value may be calculated from
HLH = E/5 (I)
where E is the weight percentage of ethylene oxide in the
molecule.
The simple straight chain alcohol ethoxylates are usually
well defined materials. The inventors have been able to
find a relationship between HLB-value (as calculated using
equation (I)) and cleaning performance (as measured by
mould removal). The straight chain alcohol ethoxylates
according to~yhe present invention possess an HLB-value
from about .~~to 20.
For fatty esters of polyalcohols and their alkoxylates, the
HLB value is given by:
HLB = 20 x (1-S/A) (II)
where
S = saponification number of the ester, ie. the number of
mg of potassium hydroxide needed to neutralise the
free and bonded acid in 1 g of the substance;
AMENDED SHEET
tp~~/FP
CA 02293760 1999-12-08
WO 98159027 PCTiEP98~~364s'
8
A = acid value of the esterified fatty acid, ie. the
number of mg of potassium hydroxide to neutralise the
free acid in 1 g of the substance.
The inventors have found that an additive with such a non-
ionic surfactant, exhibiting the above HLB' values, yields
particularly good cleaning results for PET bottles.
The additive most preferably comprise a non-ionic
surfactant selected from the group consisting essentially
ThJ Tly TM
of Synperonic A7, Synperonic L11, Lutensol A20, Lutensol
A030,M Lutensol AT80T~Neodol 45-4ETMDehypon G2084TM Poly-
TM ?M TiLJ
Tergent SLF18B45, Surfynol 504 and Plurafac LF231, wherein
the non-ionic surfactant preferably has a cleaning score of
about 3 or above, preferably about 4 or above and most
preferably about 5 or above.
Cleaning score
The cleaning results are obtained by measuring
the absorbance at ~max between 662.0 en 664.0 nm, which is a
measure for the amount of methylene blue in solution. To
compare the results of different batches of mould soiled
strips, an internal standard was measured for every new
batch of strips. This internal standard was a product
TM
containing 63.5$ Trilon ES9644 (35$ MGDA, ex. BASF), 7$
TM TM
Dissolvine NG (ex. Akzo Nobel), 1$ Bayhibit AM (ex. Bayer),
4$ Triton H-66T~ex. Union Carbide), 0.8$ Sodium Cumene
TM
Sulfonate (40$, ex Huls), 2$ Dehypon LT104 (ex. Henkel).
The difference in cleaning performance between
the internal st_:_dard and another observation is
significant when the calculated difference is larger than
the confidence intervals around the measured averages. This
can be transformed into a formula where every significant
difference will result in an increase or decrease of the
AMENDED SHEET
IPE~EP
CA 02293760 1999-12-08
W'O 98/59027 PCT/EP98/03695
9
cleaning score, compared to the internal standard. The
cleaning score can be calculated according to equation
(III) .
( Xi - XS )
Cleaning score = + Vi
Q X Tea-o.~;u~
Where:
Xi - Measured absorbance of the internal standard
(corrected for the blanc; i.e. measured
absorbance of an unsoiled strip);
XS - Measured absorbance of the sample (corrected for
the blanc; i.e. measured absorbance of an
unsoiled strip);
Q - Average deviation of the measurement. Following
the procedure described above, the average
deviation was found to be 0.18;
Tca=o.~;u>= Students value for a two tail test at 80g
confidence level with v degrees of freedom;
Vi - Value of internal standard (equals 4 for the
internal standard described above).
This definition of the cleaning score leads in this study
to a range from 0 to 11 depending on Q, in which the higher
the value the better the mould removal performance.
Table 1 shows a selection of preferred non-Tonics.
CA 02293760 1999-12-08
' WO 98159027 PCTIGP98/u369~
Table 1 Straight chain primary alkanol ethoxylates: H-
( CHZ ) "- ( OCHzCHz ) m OH
n 10 12 14 16/18(avg:l7)
m
5
3 aq: not solubleaq: not solubleaq: not solubleaq: not soluble
HLB: 9.1 (theoryHLB: 8.3 (theoryHLB: 7.6 (theo~HLB: 6.8 (theory
TM
Dehydol D3 Dobanol 23-3 Dobanol 25-3 Atlas G-70140
TM H TM
Dobanol 91-2.5Elfapur LT30SLTMElfapur LP Volpo N3 T'~
rM 25 S~
Ethylan CD Ethylan CD123TMElfapur LP
103 T~ 25 Sirs
Ethylan CD Genapol LA-030 Ethylan D253
913 TM TM TM
Genapol Z030XRemcopal 121 Renex 703
TM TM Trr
10 Synperonic Rolfor LA 3 Synperonic
91-2.5 TM A3T~
Synperonic L3
T M
Volpo L3 Special
TM
7 d.pnt cl.pnt: cl.pnt:48C cl.pnt:
HLB: 13.2(theor)HLB: 12.5 (theoryHLB: 11.8 HLB: 10.9 (theory
(theory
Ethylan CD Ethytan CD 127 Dobanol 25-7 Eumulgin WM7
107 rM T~ T~ TH
Ethylan CD Dobanol 23-6.5 Dobanol 45-7 Rolfor CO 7
916 TM rH TM rM
Sy,~,peronic Elfapur LT65SLNT~Renex 711
91-7 T~ Tn
Genapol LA-070 Synperonic
T~ A7 T~
Genapol LA-079
rH
Synperonic L7
T~
Volpo T7 TH
11 cl.pt approx cl.pnt: cl.pnt > 100Ccl.pnt > 100C
95C
2 0 HLB: 15.1 HLB: 14.4 (theory(5% aq) HLB: 13.1 (theor)
(theory r
h
Sellig LA11 ' HLB: 13.9 Britex CS 11
100M8 (the TO
Synperonic L11TMDobanol 45-11Genapol T-110TM
Etfapur LP110SWTLutensol AT11
TH
Ethylan CD Mariipal 1618111
4511T''~ T'~
Renex 720 Rotfor HT 11
rM T ~
Synperonic Simulsol 56
A11 TH T M
Synperonic TAE11T
.
20 cl.pnt > 100Ccl.pnt >100C ci.pnt >100C cl.pnt 90C
I
HLe 17.0 (theoryHL3: 16.5 (5% aq) (1 ~ in 5% NaCI)
T TM HLB: 16.1 HL3 15.5 (theory
Synperonic Seilig LA11 (theory
91-20 100
Seltig LA11 Renex 720 Atia~ G-4938
50 ~ T'~ T~'
Synperonic Brij 58 (C16)
A20 T~ TM
Bnj 78 (C18)TH
Britex S 200tH
Ethylan CS20TM
Genapol T-200TH
Sellig SU 25
100 ~
Volpo CS 20
T~
A~~i~~,'~~D S~~~T
~~'=A/~p ,
CA 02293760 1999-12-08
W O 98159027
PCT/I:P98/G3695
11
There are many more primary alkanol ethoxylates
e.g. n=10, m=4; n=10, m=5; n=10, m=6; n=10, m=8 ...
n=11, m=3, 9, 5, 6, 7, 8, 9, 10, 11, . . . , 80
n=20, m=3, . . . , 80
The hydrotrope ~~r--Ea~~~~s~--a ~~s~~eiah.~ l is .~ ~ '
U
,Y~ a+-s ~ h«~r~~'r~,~ °-~ is -.off re.~or~~., .. selected from
the group consisting l.l-~ of: sodium benzoate,
sodium 3-hydroxy-2-naphtoate, sodium xylene sulphonate,
phosphate esters, sodium decyl diphenyl oxide, sodium
dimethyl naphthalene sulphonate, sodium salts of linear
alkyl benzene sulphonate, having from about C8 to C12 in the
alkyl portion, as well as mixtures thereof, wherein the
h drotro a is most TM
y p preferably Triton H66 also being a
solubilizing agent. An additive comprising this hydrotrope
yielded particularly good cleaning results.
An hydrotrope is preferable to a non-ionic surfactant when
cleaning polycarbonate bottles, since non-ionic surfactants
are thought to damage these.
The invention will now be described by way of the following
examples and results, referring to figures 1-2 and the
tables.
Experimental
Standard soiling
The standard soiling was made by mixing
Tomato juice and a solution of Aspergillus niger.
AMENDED SHEET
IPEA/EP
CA 02293760 1999-12-08
WO 98/59027 PCT/EP98/03695
12
Preparation of standard soiling
Mntcri~lc
- Aspergillus niger ATC 6275
- Czapek Dox agar (code CM97 ex Oxoid)
- D-Glucose monohydrate
- l0o Lactic acid solution SR 021 K
- Bacto Pepton L37 (ex Oxoid)
- Sodium chloride
Procedures
Aspergillus niger was grown on 2 plates
containing Czapek Dox agar with 2~ glucose and 10 ml lactic
acid (10$) per litre agar. The mould was grown for 5 days
at 25°C. Subsequently, the moulds were taken from the plate
with two times 9 ml of a sterile solution containing 1 g/1
Pepton and 8.5 g/1 NaCl per plate. The mould solution was
added (=18 ml) to 300 gr of tomato juice (Zontomaatje ex
Riedel) and homogenised.
Preparation of ref-PET strips
M ~ t c r ; W c
- New ref-PET bottles
- Sodium hydroxide
Procedure
The bottom and the neck were cut from a 1.5 ltr
ref-PET bottle. The middle ,of thA bottle was used to cut
pieces of 70 x 25 mm from the length direction of the
bottle. The strips were then treated with 1.5~ caustic of
59°C for 15 minutes. The strips were rinsed with water to
remove the caustic and the strips were dried.
CA 02293760 1999-12-08
WO 98/59027 PCT/EP98/03695
13
Applying the soiling to the strips
Strips were taken and submerged for 75~
vertically in the standard soiling (tomato juice with
Aspergillus niger). The strips were then taken out of the
solution and held 5-10 sec. vertically. In this way the
excess soiling can drip off the strips. The strips were
placed with the bold side up in a petri dish and put
together with other strips in a plastic bag in an incubator
at 25°C. After 5 days the plastic bag was removed and the
strips, in closed petri-dishes, were restored in the
incubator for 2 more days. After this the strips were ready
for a quality check before use.
Quality check and cleaning procedure
The standard soiled strips were taken and washed
for 10 minutes in 1.5o caustic to check the quality of the
soiling.
~r~+-o,-; ~ ~
- Beaker broad model 2000 ml
- Magnetic stirrer (25 mm X 5 mm triangle shaped)
- Temperature and rotation controlled stirrer
- Stripholder
- Chronograph
r-1, o,., ; ~- ~ i
- Methylene blue solution (0.10$), TNO procedure COP for
ref-PET, V18 method A
- Acetic acid (1.00$)
- Caustic solution (1.500 + additive (0.2~)
CA 02293760 1999-12-08
WO 98/59027 PCT/EP98/03695
14
Conditioning procedure
- Take the petri dishes with the standard soiled strips
out of the incubator.
- Take 4 soiled strips from the petri dishes and 2
unsoiled and place them in the stripholder.
- Heat a 1000 ml 1.50$ caustic solution up to 59°Ct 1°C
and set the stirring speed to 600 rpm. Place the
stripholder with the 9 soiled ref-PET strips and 2
clean ref-PET strips for exactly 10.0 minutes in the
caustic solution.
- Rinse the strips (3 times 5 sec) in 1000 ml of
demineralised water.
- Wait 1 minute.
- After this the strips are dyed for 20 seconds in a
0.10$ methylene blue solution.
- Wait 1 minute.
- The strips are again rinsed for 3 times 5 sec. in 1000
ml of fresh demineralised water.
- Wait 1 minute.
- Put the 4 dyed strips in a closable can with 25 g of
1.00 acetic acid. Repeat this for the two blanc
strips. Shake the dye from the strips.
- Filtrate 10 ml of the solution ( within 24 hrs) over a
0.45 /.cm filter and discard the first 5 ml. Put the
other 5 ml in a 1 cm cuvet.
- Measure the absorption at JlmaX (between 662.0 and 664.0
nm) against 1.00 ~ acetic acid.
- Carry out the above steps with another 3 x 6 strips in
the same cleaning solution.
CA 02293760 1999-12-08
WO 98/59027 1'CT/EP98/~365'S
Investigation of additives and formulations according to
the present invention
To assess the mould removal performance of
various bottlewashing additives, PET strips standardly
5 soiled with a tomato juice contaminated with a common mould
species (Aspergillus niger) were used. After conditioning,
as above, these strips were soaked in an alkaline medium
(1.5$ caustic in 8.5 DH water) for 10 minutes. The method
to quantify the soil residue, as detailed above was used to
10 determining the cleaning score for the various additives
and formulations, results of which are shown in the
following tables.
Table 2
Sequestrant Supplier Level in Cleaning
(Trade or development formulation Score
name)
(w~)
EDTA, tetra sodium salt B~F 80.0 3
TM
lTrilon E39, 39% solution)
Potassium tripolyphosphategudenhelm 27.2 1
(50% solution)
m salt B~F 5 4
u 63
MGDA, tri sod
T .
H
lTrilon ES9964, 35%
solution)
SDA, tri sodium salt g~cF 24.2 4
The results shown in table 2 were obtained using
formulations that, in addition to the sequestrant listed,
AMENDED SHEET
lPEA/EP
CA 02293760 1999-12-08
' WO 98/59027 PC ~ /EP9~/03G9~
16
TM
contained sodium gluconate (70), Bayhibit AM (1~ of a 500
TM
solution), Triton H66 (4$ of a 50o solution), sodium cumene
TM
sulfonate (0.80 of a 40~ solution), and Dehypon LT104 (20).
These formulations were dosed at a 0.2$ level in water of
8.5DH hardness, containing 1.5$ NaOH. The mould soiled
strips were soaked in these solutions for 10 min at 59°C.
The results shown in tables 3-8 were obtained
using formulations that, in addition to the surfactant,
TM
contained Trilon ES9644 (63.5$ of a 35$ solution), sodium
gluconate (7$), Bayhibit AM M1~ of a 50$ solution), Triton
H66TM4$ of a 50$ solution), and sodium cumene sulfonate
(0.8~ of a 40$ solution). These formulations were dosed at
a 0.2$ level in water of 8.5DH hardness, containing 1.5~
NaOH. The mould soiled strips were soaked in these
solutions for 10 min at 59°C (see above).
The formulations in table 3 exhibited good
cleaning scores.
Table 3
Trade name Supplier Alkyl EO HLB Cleaning
(Nonionic chain calculated Score
surfactant) using (I)
TM
synperonic ICI C10 7 11.8 4
A7
T
synperonic ICI C12-14 11 19 5
Lil
ZOTM
Lutensol .~ BASF C13-15 20 16.2 5
Lutenso11~o30 BASF C13-15 30 17 5
TM
Lutensol AT80 BASF C13-15 80 18.5 6
~~'~~~~,n'_v Sr"~'~w
". ~1 ;~,
CA 02293760 1999-12-08
WO 98759027 f~CT~~P98/J3693
17
On the other hand, straight chain alcohol
ethoxylates with lower HLB-values exhibited a lesser
cleaning score, as shown by table 4:
Table 4
Trade name Supplie Alkyl EO 8LB Cleaning
(Nonionic r chain calculated Score
surfactant) using (I)
TM
Neodol 45-4E Shell C14-C15 4 8.9 1
Also some capped non-ionic surfactants showed
good cleaning scores, as shown by table 5:
Table 5
Trade name Supplie Alkyl EO PO Cap Cleanin
(Nonionic r chain g Score
surfactant) (s / b)
TM
Dehypon 62084 Henkel C16-20 8 - Butyl 4
(b)
Dehypon LT104 Henkel C12-C18 10 - Butyl 9
T M
(s)
Poly-Tergent Olin C20-C30 20 1 Octyl 5
ShF18B45 TM (s)
Additional nonionics that showed good performance are shown
in table 6:
AMENDED SHEET
IPEA/EP
CA 02293760 1999-12-08
WO 98/59027 PCT,'EPSB/33655
18
Table 6
Trade name Supplier Technical information Cleaning
by
(Nonionic
supplier Score
surf actant)
TM per Ethoxylated 9
Surfynol 509
Products tetramethyldecynediol
TM
AG 6202 Akzo-NobelLow-foaming 9
alkylpolyglucoside with
C12 alkyl chain
~
Plurafac LF231 BASF Alkali-stable, low-foaming9
fatty alcohol alkoxylate
Many nonionic surfactants however did not exhibit good
cleaning scores in combination with sequestrant types, as
shown by table 7:
Table 7
Trade name Supplier Alkyl EO PO Cleaning
(Nonionic
chain Score
surfactant)
TM
Dehypon LS24 Henkel C12-14 2 4 2
TM
Dehypon LS36 Henkel C12-14 3 6 1
TM
Dehypon LS45 Henkel C12-14 4 5 2
Dehypon LS54T~ Henkel C12-14 5 4 2
TM
Dynol 604 Air ethoxylated 2
Products acetylenic
n , diols
AMENDED SHEE'~
IPEA/EP
CA 02293760 1999-12-08
WO 98/59027 YL f ~EP98~U3~-'>
19
The following formulations were investigated
according to the cleaning procedure and yielded the
following results, Table 8:
H
f r r1 1' N V tf1
NI O H
m a
rv yn
~o r .r r o
H
N m V
H
r .. ~ N v ~~
a n o 11
,0
1I1 m
r ., ~ N H
p
i n o H
m a
r wn f N a 1!1
V
PI O 11
,D
N ~ V
O a
V
~n m
a
w . r .n r . ,
.,
~o
a
N m H
r .a a N a
1 a
N
a
H
Y r .~ r . N a
H
a O
v
w
C a
D a
a
" T
' ' o m
a ~
- r. m
w - 'r .
a
N H h ~ '
Tm -n H ., ~ a
~
i ~' c ' t
'
., t~ c ~ . o I a
a -
o ~ ~ ~ ~ o ~ ~ ~ a
H v. c w a ~ a o~ 1- C r .~ w
4 4 o
V
c t0 ~ ~ a .Z a JC w 2 o . . ..
4. Z a O -~ c c N ~ . . .~
r .. C a o o o
> .~
47a ~ m > . ~ V ~
o ~ ~
0 L ~ ~ = i ~~ P um u v
~ ~ ~
... < , a. ..~ t . . a a ..~ a a
x x , w w ~ ..r ~ ~ v i a
o a x x ~ a ~ W
a -. a
x
r-1 H H o m t~ H ~ ~ ~ . e
r. - a a a a a a ~
a
.L7
E
~t,r9EI~DED SHEE t
IPFA/EP
CA 02293760 1999-12-08
WO S3/59027 PCT/EP9FIU3E9~
The cleaning score (as given in the last row of this table)
is a measure for the cleaning performance of these
formulations. The higher the score the better the
performance.
5
Functionality of components:
T
Dissolvine NG (Na gluconate) - transition metal ion
sequestrant
T1~1
Bayhibit AM - threshold agent, prevents
10 deposition of inorganic
scales
Triton H-66 T~ - hydrotrope or
solubilising agent
Sodium Cumene Sulfonate - hydrotrope or
15 solubilising agent
Keltrol F T~ - structurant that
(Xanthon polysaccharide...) stabilises nonionic in
water emulsions
(suspends-non-ionic)
For reference purposes, the mould removal
performance of a few current commercial formulations were
tested under the same conditions. The results are shown in
table 9.
Table 9
Detergent trade name Supplier Cleaning score
M
Stabilon Flussig Henkel-Ecolab 1
SU860 - DiverseyLever 2
AMENDED SHEET
IPEA/EP
CA 02293760 1999-12-08
WO 98/59027 PCT/EP98/03695
21
Investigation of mould removal from PET bottles
Procedure for pre-washing PET bottles
New 1.5 litre PET bottles were washed/rinsed
according to the following procedure:
- 30 min soaking in 1.5o NaOH at 58-60°C; empty the
bottles
- 5 min delay
- 15 min rinsing / soaking in fresh tapwater (rinse 1)
- 5 min delay
- 15 min rinsing / soaking in fresh tapwater (rinse 2)
- 5 min delay
- 15 min rinsing / soaking in fresh tapwater (rinse 3)
- store bottles bottom-up in crates for 1 - 2 hours
- post - rinse with 0.5 litre demineralised water per
bottle
- storage of the bottles bottom-up in crates for 30 - 60
min
- wipe outside of the bottles until dry with absorbing
paper
- store the bottles, in upright position and allow the
remaining bit of water to evaporate (at least 24
hours) before further use of the bottle.
Procedure for mould-soiling of pre-washed PET bottles
The pre-washed PET bottles were "mould-soiled" as
follows:
- grow Aspergillns niger on 2 plates containing Czapek
Dox Agar with 2$ glucose and 10 ml lactic acid (10~)
per litre agar.
- grow the mould for at least 7 days at 25°C.
CA 02293760 1999-12-08
WO 98/59027 PCT/EP98/03695
22
- take the moulds from the plate with 9 ml of a sterile
solution containing 1 g/L Peptone and 8.5 g/L NaCl per
plate.
- add twice 9 ml of the mould suspension to 300 g of
tomato juice (Zontomaatje ex Riedel, the Netherlands)
and homogenate with glass pearls.
Application of this soil onto the inside surface
of the (1.5 litre, transparent colourless) pre-washed ref-
PET bottle was carried out within 6 hours after preparation
of the soil. 5 g of soil was used to cover the inner
wall/bottom up to 1/3 of the height of each bottle.
The soiled bottles stoppered by a wad of cotton
wool were stored for 6 weeks at room temperature in order
to allow the fungi to grow/sporulate and to allow the
tomato juice to dry.
Test procedure for cleaning PET bottles
The performance of a series of (n) cleaning
solutions, 70 litres, was compared by taking n sets of
m standard mould-soiled bottles.
A method, mimicking the conditions and mechanical
action in an industrial bottlewasher was used. Each
detergent solution was used to clean several sets of
(maximum 4) bottles:
t = 0 min: immerse (soak) 4 bottles in (70 1) detergent
solution (58-60°C).
t = 3 min: take bottles out of s~'_ution and empty them
t = 4 min: immerse bottles a fi + (fresh) water bath
t = 6 min: take bottles out of first water bath and
empty them
t = 7 min: immerse bottles in second (fresh) water bath
CA 02293760 1999-12-08
WO 98/59027 PCT/EP98/03695
23
t = 9 min: take bottles out of second water bath and
empty them
After this cleaning procedure, the bottles were
stored, overnight, bottom-up in crates for further
evaluation.
Per cleaning solution, the n (e. g. 12) cleaned
bottles were coded according to their ranking (1 =
cleanest, n = dirtiest. The result is m (e. g. 4) crates
containing n (e. g. 12) ranked bottles each.
Table 10
1-1 1-2 1-3 - - etc = - 4-1 4-2 4-3
>
1-4 1-5 1-6 4-4 4-5 4-6
1-7 1-8 1-9 4-7 4-8 4-9
1-10 1-11 1-12 - - etc = - 4-10 4-11 4-12
>
Procedure for evaluation of the cleaning result
After cleaning (procedure see: ), the (nxm)
bottles (coded 1-1, 1-2, ..1-m, 2-1, .., n-m) were ranked
1, 2, .., nxm-1, nxm (1: best cleaning result; nxm
(e.g.48): worst cleaning result).
CA 02293760 1999-12-08
WO 98/59027 PCT/EP98/03695
24
The sum of all rankings is: S (= e.g.
1+2+,..,.+47+48 = 1176).
Per cleaning solution: T = sum (total) of
rankings
The best possible result for T is: B (e. g.
1+2+...+11+12 = 78)
The worst possible result for T is: W(e.g.
37+38+...+47+48 = 510)
In some cases, the average ranking was taken such
that "S" remained constant (e.g. 2x13.5 instead of 13 and
14, or 3x46 instead of 45, 46, and 47),
The relative cleaning performance, P, is given
as:
P = 10 x (W - T) / (W - B)
(10 = best relative performance, 0 = worst relative
performance)
Table 11 below shows the formulation tested,
whilst figure 1 shows the relative performance of a number
of formulations according to the present invention.
CA 02293760 1999-12-08
WO 98/59027 PCT/EP98/03695
W
a
r
m m o .-i rv m m
H wn ~o r
3 o
d ~ N N N N N r
N N N
N
N
W
N
'i
d
p o
N o ,
.1 r1 p U1 ~D ~
0W ~i rl N c0
G7
x
N
p a
H
N .o r u~ r ... b
r., n
N r .-1 r-1 ,..p,
N .-1 N N
a
-
n
N
r1
O O ~D m N
amn ~o r r
x m
r1 .H e1 N N -n
N N N ..i
.
W
m C
H
N
N
E" .r N ~ a mo amn
r m av
W
N
.i
\ O O O O O O
1I7
O O O m O u1
N n1
N rl O A
W
Q
0 0 o a o 0
y n o 0 o ao 0
N
rv n
n7 r .~ v~ c
N
H O A
a
a
G
a
Y,
~a
ro
x
~ A
E
E
x
C
O
.a
a x
"~
o
o~
x N
an a u, a
a ~o a a m a
~~ x a a
O
x x o
~
c a
f
~
au
~
C x ~a cap '_
y C
~
L
a .a p 9 .
p c
p p,
H ~ ~ ~
2
u
' a
4 F t
n Z
x w p,
CA 02293760 1999-12-08
WO 98/59027 PCT/EP98l03695
26
Procedure for mould-soiling of pre-washed PC bottles
Grow Aspergillus niger (ATCC 16404) on 2 plates
containing Malt Extract Agar. Grow the mould for 5 days at
25°C . Take the moulds form the plate with 10 ml of milk
per plate. Add the 2x10 ml mould contaminated milk to 200 g
yoghurt/milk mixture (150 g yoghurt Campina "halfvol" and
50 g milk Campina "halfvol") and homogenate with glass
pearls.
Application of this soil onto the inside surface
of the (1.0 litre, transparent colourless) pre-washed PC
bottle should occur within 6 hours after preparation of the
soil. An amount of 10 g mould contaminated milk/yoghurt is
used to cover the inner wall/bottom up to 1/2 of the height
of the bottle. The bottles are, loosely, closed by means of
a wad of cotton wool.
The soiled bottles are incubated for at least 6
weeks at 30°C in order to allow the fungi to grow/sporulate
and to allow the milk/yoghurt to dry.
Cleaning procedure for mould-soiled PC bottles
The performance of a series of (n) cleaning
solutions, 70 litres, is compared by taking n sets of m
standard mould-soiled bottles. Soiling of the bottles must
have taken place at least 6 weeks before, according to the
procedure for mould-soiling of pre-washed PC bottles.
The cleaning procedure for polycarbonate (PC)
be ..les comprises a combination of soaking in a 70 litre
ba~:h filled with detergent solution during 10 min at 79-
81°C, and spraying with the same cleaning solution into the
bottle according to the spraying program given below.
1. 15 sec spraying, followed by 10 sec delay
CA 02293760 1999-12-08
WO 98/59027 PCT/EP98/03695
27
2. 15 sec spraying, followed 10 sec delay
by
3. 15 sec spraying, followed 10 sec delay
by
4. 15 sec spraying, followed 10 sec delay
by
5. 15 sec spraying
For this detergent rinse, the bottles (one by
one) are placed, bottom-up, vertically over a nozzle.
Through this nozzle, a jet of cleaning liquid is directed
exactly to the middle of the bottom of the bottle. The flow
IO is adjusted to 1750-2000 ml per five pulses of 15 seconds
each. Nozzle: NF2000 (BSP BETE), supplied by Spraybest,
angle=)°, max flow = 20 1/min
Total contact time = 5 x 15 s = 75 s = 1 min 15 s
Flow rate = 1.4-1.6 1/min
After the cleaning procedure, the bottles are
rinsed by immersing them in cold tapwater directly followed
by emptying. The bottles are stored, bottom-up in crates
for further evaluation.
Per cleaning solution, the n (e. g. 12) cleaned
bottles are coded according to their ranking in the same
way as described in the procedure for PET bottles.
Table, l2 below shows the formulation tested,
whilst figure 2 shows the results thereof.
CA 02293760 1999-12-08
WO 98/59027 PCT/EP98/03695
28
w
a
Ei
H
h.' M cr tn 00 l0
~ lD l0
3 '~ ~ .~ mn M
~ ,- M
M
~f1 In N
d' rl N M
M ~
ri '-I
ri
~O
H N M tl1
01 O
M
1J
1:1
~ H
ll'1 lD 0p lD
W 01
N N
U
I~
I~ N d~ 01
lD
rl ri d~ ri
r-I
(~
H
U E-'
C~
W ri N M In ll1
O d~
~ ~
Tf 01
O O
r1 O O
,
3 . .
O ~r 04 ~ ~r
b ~n o00
r-.i ~ ~n o 0
3 '"'i ~r
M G ~
0
x
~-i N O O
e-( lD L(1 O
M v-i
W N p4 l0
O
tr1 00
O
O
rt~ p0
N
_
U
w
o ..,
- u~ x
x - o
N
ri v
~o
~~x
zoa .
m H