Note: Descriptions are shown in the official language in which they were submitted.
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
SUBSTITUTED NAPHTHOPYRANS
DESCRIPTION OF THE INVENTION
The present invention relates to certain novel
naphthopyran compounds. More particularly, this invention
relates to novel photochromic naphthopyran compounds and to
compositions and articles containing such novel naphthopyran
compounds. When exposed to light radiation involving
ultraviolet rays, such as the ultraviolet radiation in
sunlight or the light of a mercury lamp, many photochromic
compounds exhibit a reversible change in color. When the
ultraviolet radiation is discontinued, such a photochromic
compound will return to its original color or colorless state.
Various classes of photochromic compounds have been
synthesized and suggested for use in applications in which a
sunlight-induced reversible color change or darkening is
desired. U.S. Patent 3,567,605 (Becker) describes a series of
pyran derivatives, including certain benzopyrans and
naphthopyrans. These compounds are described as derivatives
of chromene and are reported to undergo a color change, e.g.,
from colorless to yellow-orange, on irradiation by ultraviolet
light at temperatures below about -30°C. Irradiation of the
compounds with visible light or upon raising the temperature
to above about 0°C is reported to reverse the coloration to a
colorless state.
U.S. Patent 5,066,818 describes various 3,3-diaryl-
3H-naphtho[2,1-b]pyrans as having desirable photochromic
properties, i.e., high colorability and acceptable fade, for
ophthalmic and other applications. Also disclosed by way of
comparative example in the 818 patent are the isomeric 2,2-
" ,
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
-2-
diaryl-2H-naphtho[1,2-b]pyrans, which are reported to require
unacceptably long periods of time to fade after activation.
U.S. Patent 3,627,690 describes photochromic 2,2-di-
substituted-2H-naphtho[1,2-b]pyran compositions containing
minor amounts of either a base or weak-to-moderate strength
acid. The addition of either an acid or base to the
naphthopyran composition is reported to increase the fade-rate
of the colored naphthopyrans, thereby making them useful in
eye protection applications such as sunglasses. It is
reported therein further that the fade rate of 2H-naphtho-
[1,2-b]pyrans without the of orementioned additives ranges from
several hours to many days to reach complete reversion. U.S.
Patent 4,818,096 discloses a blue coloring photochromic benzo-
or naphthopyran having at the position alpha to the oxygen of
the pyran ring a phenyl group having a nitrogen containing
substituent in the ortho or para positions.
The present invention relates to novel substituted
2H-naphtho[1,2-b]pyran compounds which have been unexpectedly
found to have an acceptable fade rate in addition to a high
activated intensity and a high coloration rate. In
particular, the use of certain substituents at the 5-position
of the naphtho-portion of the naphthopyran compound increases
the fade rate without the addition of acids or bases. In
addition, these compounds have certain substituents at the 2-
position of the pyran ring. Certain substituents may also be
present at the number 6, 7, 8, 9 or 10 carbon atoms of the
naphtho portion of the naphthopyran.
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
-3-
DE'T'ATLED DESCRT 'rTON OF THE INVENTION
In recent years, photochromic plastic materials,
particularly plastic materials for optical applications, have
been the subject of considerable attention. In particular,
photochromic ophthalmic plastic lenses have been investigated
because of the weight advantage they offer, vis-a-vis, glass
lenses. Moreover, photochromic transparencies for vehicles,
such as cars and airplanes, have been of interest because of
the potential safety features that such transparencies offer.
In accordance with the present invention, it has now
been discovered that certain novel 2H-naphtho(1,2-b]pyran
compounds having an acceptable fade rate, high activated
intensity and a high coloration rate may be prepared. These
compounds may be described as naphthopyrans having certain
substituents at the 2 position of the pyran ring and at the
number 5 carbon atom of the naphtho- portion of the
naphthopyran ring. Certain substituents may also be present
at the 6, 7, 8, 9 or 10 carbon atoms of the naphtho portion of
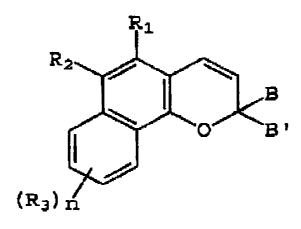
the naphthopyran ring. These compounds may be represented by
the following graphic formula:
( R3
I
In graphic formula I, R1 is the group, -C(O)W, W
being -OR4 or -N(R5)R6, wherein R,~ is hydrogen, allyl, C1-C6
alkyl, e.g., methyl, ethyl, propyl, butyl, pentyl, and hexyl,
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
-4-
phenyl, mono(C1-C6)alkyl substituted phenyl, mono(C1-C6)-
alkoxy-substituted phenyl, phenyl(C1-C3)alkyl,
mono(Cl-C6)alkyl substituted phenyl(C1-C3)alkyl,
mono(C1-C6)alkoxy substituted phenyl(C1-C3)alkyl,
C1-C6alkoxy(C2-C4)alkyl, or C1-C6 haloalkyl; and R5 and R6 may
each be selected from the group consisting of hydrogen, C1-C6
alkyl, CS-C~ cycloalkyl, phenyl and mono- or di-substituted
phenyl. The phenyl substituents may be C1-C6 alkyl and C1-C6
alkoxy and the halo substituent may be chloro or fluoro.
Preferably, R1 is the group, -C(O)W, W being the
groups -OR4 or -N(RS)R6, wherein R4 is hydrogen, C1-C4 alkyl,
phenyl, mono(C1-C4)alkyl substituted phenyl,
mono(C1-C4)alkoxy substituted phenyl, phenyl(C1-C2)alkyl,
mono(C1-C4)alkyl substituted phenyl(C1-C2)alkyl,
mono(C1-C4)alkoxy substituted phenyl(C1-C2)alkyl,
mono(Cl-C4)alkoxy(C2-C3)alkyl or C1-C4 haloalkyl; and R5 and
R6 may each be selected from the group consisting of hydrogen,
C1-C4 alkyl, C5-C~ cycloalkyl, phenyl and mono- or di-
substituted phenyl. The phenyl substituents may be selected
from C1-C4 alkyl and C1-C4 alkoxy, and the halo substituent
may be chloro or fluoro.
More preferably, R1 is the group -C(O)W, w being the
group -OR4 or -N(R5)R6, wherein R4 is hydrogen, Cl-C3 alkyl,
phenyl, mono(C1-C3)alkyl substituted phenyl, mono(C1-C3)alkoxy
substituted phenyl, mono(C1-C3)alkoxy(C2-C3)alkyl or C1-C3
haloalkyl, and wherein R5 and R6 are each selected from the
group consisting of hydrogen, C1-C3 alkyl, C5-C~ cycloalkyl,
phenyl and mono-substituted phenyl. The phenyl substituents
may be selected from the group consisting of C1-C3 alkyl and
___._ _._._...._._.._~..r_.._-.__-__...
CA 02293834 1999-12-03
PGTNS ~ l ~ 0 9 6 8
IPEAIUS p s J U L 1998
-5-
C1-C3 alkoxy, and the halo substituent is fluoro. Most
preferably, Rl is the group, -C(O)W, W being -OR4 or -N(R5)R6,
wherein R4 is hydrogen or C1-C3 alkyl, R5 and R6 are each
hydrogen, C1-C3 alkyl or phenyl.
R2 and each R3 in graphic formula I may be hydrogen,
C1-C6 alkyl, C3-C~ cycloalkyl, substituted or unsubstituted
phenyl, the group -ORS, wherein R~ is hydrogen, C1-C6 alkyl,
phenyl(C1-C3)alkyl, mono(C1-C6)alkyl substituted
phenyl(C1-C3)alkyl, mono(C1-C6)alkoxy substituted
phenyl(C1-C3)alkyl, C1-C6 alkoxy(C2-C4)alkyl, C3-C~
cycloalkyl, mono(C1-C4)alkyl substituted C3-C~ cycloalkyl,
C1-C6 haloalkyl, allyl, and the group, -CH(Rg)X, wherein X is
-CN, -CF3, halogen or -C(O)W and Rg is hydrogen or C1-C6
alkyl; or R~ is the group, -C(O)Y, wherein Y is hydrogen,
C1-C6 alkyl, C1-C6 alkoxy, the substituted or unsubstituted
aryl groups phenyl or naphthyl, phenoxy, C1-CE mono- or di-
alkyl substituted phenoxy, C1-C6 mono- or di-alkoxy
substituted phenoxy, each of said phenyl and naphthyl
substituents being selected from C1-C6 alkyl or C1-C6 alkoxy,
said halogen or halo substituents are chloro or fluoro and n
is selected from the integers 0, 1, 2 and 3.
Preferably, R2 and each R3 are hydrogen, C1-C3
alkyl, C3-C5 cycloalkyl, substituted or unsubstituted phenyl
or -ORS, wherein R~ is hydrogen, C1-C3 alkyl, or the group, -
CH(R8)X, wherein X is -CN or -C(O)W and Rg is hydrogen or
methyl; or R~ is the group -C(O)Y wherein Y is C1-C3 alkyl or
AIV1ENUEa SHEEP
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
-6-
C1-C3 alkoxy, said phenyl substituents being C1-C3 alkyl or
C1-C3 alkoxy and n is selected from the integers 0 and 1.
More preferably, R2 and R3 are each selected from the group
consisting of hydrogen, C1-C3 alkyl, phenyl and -ORS, wherein
R~ is hydrogen or C1-C3 alkyl, or R~ is the group, -C(O)Y,
wherein Y is C1-C3 alkyl or CI-C3 alkoxy, and n is selected
from the integers 0 and 1. Most preferably, R2 and R3 are
each hydrogen or C1-C3 alkyl. In the definitions of R1, R2
and R3 in graphic formula I, like letters have the same
meaning unless stated otherwise.
B in graphic formula I may be the unsubstituted,
mono-, di- or tri-substituted aryl groups phenyl and naphthyl,
said aryl substituents may be selected from the group
consisting of C1-C6 alkyl, C1-C6 alkoxy, halogen, amino, C1-C6
monoalkylamino, C1-C6 dialkylamino, i.e., di-
(C1-C6)alkylamino, morpholino, piperidino, indolinyl, 1-
imidazolidyl, 2-imidazolin-1-yl, 2-pyrazolidyl, pyrazolinyl,
1-piperazinyl and pyrrolidyl, said halogen being fluoro or
chloro. Preferably, B is an unsubstituted, mono- or di-
substituted phenyl, the phenyl substituents being selected
from the group consisting of C1-C4 alkyl, C1-C4 alkoxy,
halogen, amino, C1-C4 monoalkylamino, C1-C4 dialkylamino,
morphoiino, piperidino, indolinyl and pyrrolidyl, said halogen
being fluoro or chloro. More preferably, B is an
unsubstituted, mono- or di-substituted phenyl, the phenyl
substituents being selected from the group consisting of C1-C3
alkyl, C1-Cg alkoxy, fluoro, amino, C1-C3 monoalkylamino,
C1-C3 dialkylamino, morpholino, and piperidino. Most
preferably, B is an unsubstituted, mono- or di-substituted
phenyl, the phenyl substituents being selected from the group
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
_7_
consisting of C1-C2 alkyl, C1-C2 alkoxy, fluoro, C1-C2
monoalkylamino, C1-C2 dialkylamino, morpholino and piperidino.
B' in graphic formula I may be selected from the
group consisting of:
(i) the mono-substituted aryl groups phenyl
and naphthyl, said aryl substituents may be selected from the
group consisting of amino, C1-C6 monoalkylamino, C1-C6
dialkylamino, morpholino, piperidino, indolinyl, 1-
imidazolidyl, 2-imidazolin-1-yl, 2-pyrazolidyl, pyrazolinyl,
1-piperazinyl and pyrrolidyl;
(ii) the group represented by the following
graphic formula II A:
H
C=C
i
U V
II A
wherein U may be hydrogen or C1-C4 alkyl, and V may be
selected from the unsubstituted, mono- and di-substituted
members of the group consisting of naphthyl, phenyl, furanyl
and thienyl, each of said substituents of said group being
Cl-C4 alkyl, C1-C4 alkoxy, fluoro or chloro; and
(iii) the group represented by the following
graphic formula II B:
(Rs) a
'M
(R9) a
II B
CA 02293834 2004-04-07
_$_
wherein M may be CH2, O, S, N-R14, wherein R14 may be
selected from the group consisting of hydrogen, C1-C6 alkyl,
CS-C7 cycloalkyl, phenyl, mono-substituted, and di-
substituted phenyl, said phenyl substituents being selected
from C1-C6 alkyl, C1-C6 alkoxy, chloro and fluoro; Rg is
C1-Cg alkyl, C1-C6 alkoxy, chloro or fluoro and a is
selected from the integers 0, 1, 2 and 3; or
(iv) B and B' taken together may form an
unsubstituted, mono- or di-substituted fluoren-9-ylidene or
form a member selected from the group consisting of
saturated C3-C12 spiro-monocyclic hydrocarbon rings, e.g.,
cyclopropylidene, cyclobutylidene, cyclopentylidene,
cyclohexylidene, cycloheptylidene, cyclooctylidene,
cyclononylidene, cyclodecylidene, cycloundecylidene,
cyclododecylidene; saturated C~-C12 spiro-bicyclic
hydrocarbon rings, e.g., bicyclo[3.2.1]octylidene,
bicyclo[3.3.1]nonan-9-ylidene and bicyclo[4.3.2]undecane,
and saturated C7-C12 spiro-tricyclic hydrocarbon rings,,
e.g., tricyclo[2.2.1.02'6]heptylidene and tricyclo-
[5.3.1.12'6]dodecylidene, provided that this group does not
include tricyclo[3.3.1.13'7]decylidene, i.e., adaman-
tylidene, 1,7,7-trimethyl bicyclo[2.2.1]heptylidene, i.e.;
bornylidene, and bicyclo[2.2.1]heptylidene, i.e.,
norbornylidene. The fluoren-9-ylidene substituents may be
selected from the group consisting of C1-C4 alkyl, C1-C4
alkoxy, fluoro and chloro; or
(v) B and B' are each selected from the
groups that are defined in (ii) and (iii) hereinbefore.
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
-9-
Preferably, B' is selected from the group
consisting of: (i) mono-substituted phenyl, the phenyl
substituents being selected from the group consisting of
amino, C1-C4 monoalkylamino, Cl-C4 dialkylamino, morpholino,
piperidino, indolinyl and pyrrolidyl; (ii) the group
represented by the graphic formula II A wherein U may be
hydrogen or methyl, and V may be phenyl or mono-substituted
phenyl, the phenyl substituents being C1-C4 alkyl, C1-C4
alkoxy, fluoro, or chloro; and (iii) the group represented
by the graphic formula II B wherein M may be CH2, O, or N-
R14, and R14 may be selected from the group consisting of
hydrogen, C1-C4 alkyl, phenyl, and mono-substituted phenyl,
the phenyl substituents being selected from C1-C4 alkyl,
C1-C4 alkoxy, chloro and fluoro, R9 may be C1-C4 alkyl,
IS C1-C4 alkoxy, chloro or fluoro, and a is selected from the
integers 0, 1 and 2; or (iv) B and B' taken together form an
unsubstituted or mono-substituted fluoren-9-ylidene or a
member selected from the group consisting of saturated C3-Cg
spiro-monocyclic hydrocarbon rings saturated C~-Clp spiro-
bicyclic hydrocarbon rings and saturated C~-Clp spiro-
tricyclic hydrocarbon rings, said fluoren-9-ylidene
substituents being selected from the group consisting of
C1-C3 alkyl, Cl-C3 alkoxy, fluoro and chloro.
More preferably, B' is selected from the group
consisting of: (i) mono-substituted phenyl, the phenyl
substituents being selected from the group consisting of
amino, C1-C3 monoalkylamino, C1-C3 dialkylamino, morpholino
and piperidino; (ii) the group represented by the graphic
formula II B wherein M is CH2, O, or N-R14, and R14 is
selected from the group consisting of hydrogen, Cl-C4 alkyl
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
- 10-
or phenyl; Rg is selected from the group consisting of C1-C4
alkyl, C1-C4 alkoxy and fluoro, and a is selected from the
integers 0, 1 and 2; or (iv) B and B' taken together form
fluoren-9-ylidene or bicyclo[3.3.1]nonan-9-ylidene. Most
preferably, B' is mono-substituted phenyl, 9-ethyl
carbazolyl or 9-phenyl carbazolyl, said phenyl substituents
being C1-C2 monoalkylamino, C1-C2 dialkylamino, morpholino
or piperidino.
Compounds represented by graphic formula I may be
prepared by the following described methods. In Reaction A 1,
the benzoyl derivative of a carbazole, dibenzothiophene or
dibenzofuran is prepared by Friedel-Crafts methods. See the
publication Fr~e~Pi-rYafr~ and Related Reactions, George A.
Olah, Interscience Publishers, 1964, Vol. 3, Chapter XXXI
(Aromatic Ketone Synthesis), and "Regioselective Friedel-
Crafts Acylation of 1,2,3,4-Tetrahydroquinoline and Related
Nitrogen Heterocycles: Effect on NH Protective Groups and Ring
Size" by Ishihara, Yugi et al, J. Chem. Soc., Perkin Traps. 1,
pages 3401 to 3406, 1992; Heterocxclic Compounds, Robert C.
Elderfield, 1951, Vol. 2, Chapter 3 (Dibenzofuran) and Chapter
5 (Dibenzothiophene); ThP Chemistry of Heteroc~rclic Compounds,
H. D. Hartough and S. L. Meisel, 1954, Vol. 7, Chapter IV
(Dibenzothiophene and its Derivatives); Advances in
Heterocy~~~~ Chemistry, A. R. Katritzky and A. J. Boulton,
1974, Vol. 16, Chapter V (Recent Advances in the Chemistry of
Dibenzothiophenes); and H ocyslic Compounds, Robert C.
Elderfield, 1952, Vol. 3, Chapter 3 (The Chemistry of
Carbazole).
In Reaction A 1, the compounds represented by
graphic formulae III and IV are dissolved in a solvent, such
as carbon disulfide or methylene chloride, in the presence of
a Lewis acid, such as aluminum chloride, to form the
___.~.....__-..__ _ _ . .._ _._._____~.e_~~" _._._._..
CA 02293834 1999-12-03
WO 98!55457 PCT/US97/09687
-11-
corresponding substituted benzoyl derivative represented by
graphic formula V. R represents potential phenyl
substituents.
REACTION A 1
COCl
A1C13 (R9) a
+ -
'-- CHZC12 M R
(R9) a j"1~~(R9) a R ~R9) a
III IV
Compounds represented by graphic formula VB are
either purchased or prepared, as shown in Reaction A 2, by
direct amination of a commercially available fluoro
substituted benzophenone represented by graphic formula VA
with a primary or secondary amine compound, for example, ethyl
amine, morpholine, piperidine, etc. See the publication
"Nucleophilic Displacements of Activated Fluorine in Aromatic
Compounds" by Bader, Henry et al, J. Org. Chem., Vol. 31,
pages 2319-2321, 1966.
In Reaction A 2, the compounds represented by
graphic formula VA and the amino compound are dissolved in a
solvent, such as dimethyl sulfoxide (DMS), and refluxed to
form the corresponding amino substituted benzophenone
represented by graphic formula VB. R represents potential
phenyl substituents.
CA 02293834 1999-12-03
WO 98/55457 PCT/L1S97/09687
-12-
REACTION A 2
O O
H
/ ~ + /N\ D
~F Rs R6 \ N,Rs
R R \
Rs
VA VB
In Reaction B, the ketone represented by graphic
S formulae V, VB, or in more general terms VC, is reacted with
sodium acetylide in a suitable solvent, such as anhydrous
tetrahydrofuran (THF), to form the corresponding propargyl
alcohol represented by graphic formula VI. Propargyl alcohols
having B' groups represented by graphic formula II A may be
prepared by the methods described in U.S. Patent 5,274,132,
column 2, lines 40 to 68.
REACTION B
O
HO\ ~C=CH
+ HC=CNa T~ /C~
B B' B B'
VC VI
Naphthols represented by graphic formula XIIIA may
be prepared by Reactions C or D. In Reaction C, substituted
or unsubstituted 1,4-dihydroxy-2-naphthoic acid, represented
by graphic formula VII, is reacted with methyl iodide (or
ethyl iodide, propyl iodide, benzyl bromide, etc.) in the
presence of ethyldiisopropyl amine in a suitable solvent such
as anhydrous dimethylformamide (DMF), to form the
corresponding methyl-1,4-dihydroxy-2-naphthoate, which is
represented by graphic formula VIII (or XIIIA in Reaction E).
This reaction is further described in J. of Org. Chem., Vol.
46{17), page 3477, 1981.
............ _._....._«....~,.._._.._...._...__....._.....m.~__.__..._._ ...
.T...
CA 02293834 2004-04-07
-13-
REACTION C
CIi3 CH2N ( CH ( CH3 ) 2 ) 2
iyl Iodide
DMF
(R3) (R3)
VII VIII
In Reaction D, a substituted or unsubstituted
acetophenone, benzophenone, or benzaldehyde represented by
graphic formula IX is reacted with dimethyl succinate (graphic
formula X) in the presence of a base such as sodium hydride or
a potassium t-butoxide in a suitable solvent such as toluene
to form the appropriate substituted monoester of an a-
arylidene succinic acid, represented by graphic formula XI.
Other ester substituents on the compound represented by
graphic'formula XI may be prepared by using different
succinate esters, such as diethyl succinate. Compound XI is
heated with acetic anhydride and anhydrous sodium acetate to
form the corresponding acetate derivative represented by the
graphic formula XII. Compound XII is reacted with
hydrochloric acid and an anhydrous alcohol such as anhydrous
methanol to form the corresponding naphthol, represented by.
graphic formula XIII (or XIIIA in Reaction E). Reaction D is
further described in the text ~-ganic Reactions, Vol. VI,
Chapter 1, pages 1-73, John Wiley & Sons, Inc., New York.
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
-14-
REACTION D
R2
O O
COOCH3
R2 OCH3
NaH, Toluene _
OCH3 HO
( Ra ) n O
(R3)n IX X XI
O
(CH3C0)20
CH3COONa
COOCH3
HC1
( R3 ) n C~--- ( Ft3 ) n
XIII XII
In Reaction E, the propargyl alcohol represented by
graphic formula VI is coupled with the naphthol represented by
graphic formula XIII A to form compounds represented by
graphic formula I.
_ __...____.r ..___.._.... . ._ _...._ ~__.._._ . ._.... T
CA 02293834 1999-12-03
WO 98/55457 PCT/US97109687
-15-
REACTION E
HO\ j ~CH H+
(R3) + C
Toluene
(R3)
XIIIA VI I
As shown in Reaction F, compounds represented by
graphic formula XIV (compounds of graphic formula I in which
R2 is a hydroxy) can be converted to a variety of different
groups by reaction with acylating or alkylating agents. For
example, the compound represented by graphic formula XIV may
be reacted with methyl iodide (or other alkylating agent) in
the presence of anhydrous potassium carbonate in a suitable
solvent such as anhydrous acetone to form compounds
represented by graphic formula XV, in which R2 is a methoxy
substitutent. Alkylating reactions are further described in
"Organic Synthesis', Vol. 31, pages 90-93, John Wiley & Sons,
Inc., New York, NY. Alternatively, Compound XIV may be
reacted with acetyl chloride (or other acylating agent) in the
presence of triethylamine (NEt3) in an appropriate solvent,
such as methylene chloride, to form compounds represented by
the graphic formula XVI, in which R2 is an acetoxy (Ac0)
substitutent. Acylating reactions are further described in
"Organic Synthesis," Vol. 32, pages 72-77, John Wiley & Sons,
Inc., New York, NY.
CA 02293834 1999-12-03
WO 98!55457 PCT/US97/09687
-16-
REACTION F
R,
HC
CH3I , K2C03
Acetone
(R3)n ___. (R3)
CH3COC1 CHZC12
NEt3
Ac(
(R3)
nvi
Compounds represented by graphic formula I may be
used in those applications in which organic photochromic
substances may be employed, such as optical lenses, e.g.,
vision correcting ophthalmic lenses and plano lenses, face
shields, goggles, visors, camera lenses, windows, automotive
windshields, aircraft and automotive transparencies, e.g.,
T-roofs, sidelights and backlights, plastic films and sheets,
textiles and coatings, e.g., coating compositions such as
paints, and verification marks on security documents, e.g.,
documents such as banknotes, passports and drivers licenses
for which authentication or verification of authenticity may
be desired. Naphthopyrans represented by graphic formula I
exhibit color changes from colorless to colors ranging from
orange to blue.
_._ _~._ ___._._______W.._.r__ . _ __ .._..... _ _.__._.~~_..~_.
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
- 17-
Examples of contemplated naphthopyrans within the
scope of the invention are the following:
(a) 2-(4-morpholinophenyl)-2-phenyl-5-methoxycarbonyl-6-
hydroxy-2H-naphtho[1,2-b]pyran;
(b) 2-(4-morpholinophenyl)-2-phenyl-5-methoxycarbonyl-6-
methoxy-2H-naphtho[1,2-b]pyran;
(c) 2-(4-morpholinophenyl)-2-phenyl-5-methoxycarbonyl.-6-
acetoxy-2H-naphtho[1,2-b]pyran;
(d) 2-(9-ethylcarbazol-2-yl)-2-phenyl-5-methoxycarbonyl-
6-acetoxy-2H-naphtho[1,2-b]pyran;
(e) 2-(9-phenylcarbazol-2-yl)-2-phenyl-5-methoxycarbonyl-
6-acetoxy-2H-naphtho[1,2-b]pyran;
(f) 2-(4-dimethylaminophenyl)-2-phenyl-5-methoxycarbonyl-
6-acetoxy-2H-naphtho[1,2-b]pyran;
(g) 2-(4-dimethylaminophenyl)-2-phenyl-5-methoxycarbonyl-
6-methyl-2H-naphtho[1,2-b]pyran; and
(h) 2,2-bis(4-dimethylaminophenyl)-5-methoxycarbonyl-6-
acetoxy-2H-naphtho[1,2-b]pyran.
It is contemplated that the organic photochromic
naphthopyrans of graphic formula I be used in combination with
other appropriate complementary organic photochromic materials
so that together they produce a near neutral gray or brown
color shade when the plastic lens containing such photochromic
materials are exposed to ultraviolet light. For example, a
compound which colors to yellow may be blended with a compound
that colors to an appropriate purple to produce a brown shade.
Similarly, a compound which is orange in its colored state
will produce a shade of gray when used in conjunction with an
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
- 18-
appropriate blue coloring compound. The aforesaid described
combination of photochromic materials may be used also in
applications other than ophthalmic lenses.
The novel naphthopyran compounds of the present
invention, such as those heretofore described, may be used
alone or in combination with complementary photochromic
compounds, i.e., organic photochromic compounds having at
least one activated absorption maxima within the range of
between about 400 and 700 nanometers, or substances containing
same, and may be incorporated, e.g., dissolved or dispersed,
in a polymeric organic host material used to prepare
photochromic articles and which color when activated to an
appropriate hue.
A first group of complementary organic photochromic
substances contemplated for use with the organic photochromic
naphthopyrans of the present invention are those having an
activated absorption maximum within the visible range of
greater than 570 nanometers, e.g., between about greater than
570 to 700 nanometers. These materials typically exhibit a
blue, bluefish-green, or bluefish-purple color when exposed to
ultraviolet light in an appropriate solvent or matrix. Many
of such compounds are described in the open literature. For
example, spiro(indoline)naphthoxazines have been described,
among others, in U.S. Patent Nos. 3,562,172; 3,578,602;
4,215,010; and 4,342,668; spiro(indoline)naphthoxazines having
certain substituents on the 8' and 9' positions of the
naphthoxazine portion of the molecule are described in U.S.
Patent 5,405,958; spiro(indoline)pyridobenzoxazines are
described in U.S. Patent 4,637,698; spiro(benzindoline)pyrido-
benzoxazines and spiro(benzindoline)naphthoxazines are
described in U.S. Patent 4,931,219; spiro(benzindoline)-
naphthopyrans are described in Japanese Patent Publication
_?.._.___ _._....... _._. _.._.__~....-_.__._....._._.. __..~.....- ~.r.~_.
CA 02293834 2004-04-07
- 19-
62/195383; spiro(indoline)benzoxazines are described in U.S.
Patent 4,816,584; spiro(indoline)benzopyrans,
spiro(indoline)naphthopyrans, and spiro(indoline)quinopyrans
are described, for example, in U.S. 4,880,667; and
benzopyrans and naphthopyrans having a nitrogen-containing
substituent in the 2-position of the pyran ring are described
in U.S. Patent 4,818,096. Spiro(indoline)pyrans are also
described in the text, Techni~es in Chemistry, Volume III,
°Photochromism," Chapter 3, Glenn H. Brown, Editor, John Wiley
and Sons, Inc., New York, 1971.
A second group of complementary organic photochromic
substances contemplated for use with the organic.photochromic
naphthopyrans of the present invention are those having at
least one absorption maximum within the visible range of
between about 400 and less than 500 nanometers. These
materials typically exhibit a yellow-orange color when exposed
to ultraviolet light in an appropriate solvent or matrix.
Such compounds include certain chromenes, i.e., benzopyrans
and naphthopyrans. Many of such chromenes are described in
the open literature, e.g., U.S. Patents 3,567,605; 4,826,977;-
and 5,066,818. Other examples of complementary benzopyrans
and naphthopyrans that may be used with the naphthopyrans of
the present invention include: those having a spiro adamantane
group at the position alpha to the oxygen atom of the pyran
ring, which are described in U.S. Patent 4,826,977; 2H-
naphtho-[1,2-b]pyran compounds having certain substitutents at
the number 5 and 6 carbon atoms of the naphtho portion of the
naphthopyran and at the 2 position of the pyran, which are the
subject of co-pending U.S. Patent 5,458,814,
filed December 9, 1993; 3H-naphtho[2,1-b]pyrans
having at least one ortho-substituted phenyl substituent at
the 3-position of the pyran ring which are described in U.S.
CA 02293834 2004-04-07
v
-20-
Patent 5,066,818; 3H-naphtho(2,1-b]pyran compounds having
certain substituents at the number 8 carbon atom and certain
substituents at the number 7 or 9 carbon atom, all
substituents being on the naphtho portion of the naphthopyran,
which are the subject of co-pending U.S. Patent 5,466,398,
filed June 21, 1993; 3H-naphto[2,1-b]pyrans substituted at the
3 position of the pyran ring with (i) an aryl substituent and
(ii) a phenyl substituent having a 5- or 6-member heterocyclic
ring fused at the number 3 and 4 carbon atoms of the phenyl
substituent are described in U.S. Patent 5,384,077; diaryl-3H-
naphtho[2,1-b]pyran compounds having a substituted or
unsubstituted 5 or 6 member heterocyclic ring fused to the g,
i, or 1 side of the naphthopyran, which are the subject of co-
pending U.S. Patent 5,451,344, filed April 8, 1994;
naphthopyran compounds substituted at the number 8 carbon atom
on the naphtho portion of the naphthopyran ring, with for
example, a.methoxy group which are the subject of U.S. Patent
5,238,931; naphthopyran compounds, examples of which are 3-
aryl-3-arylalkenyl naphthopyrans, which are described in U.S.
Patent 5,274,132; and naphtho[2,1-b]pyrans substituted at the
number five carbon atom.with, for example, an acetoxy group,
which are the subject of U.S. Patent 5,244,602.
A third group of complementary organic photochromic
substances contemplated for use with the organic photochromic
naphthopyrans of the present invention are those having an
absorption maximum within the visible range of between about
400 to about 500 manometers and another absorption maximum
within the visible range of between about 500 to about 700
manometers. These materials typically exhibit colors)
ranging from yellow to purple and yellow/brown to purple/gray
when exposed to ultraviolet light in an appropriate solvent or
CA 02293834 2004-04-07
-21-
matrix. Examples of these compounds include certain
- substituted 2H-phenanthro[4,3-b]pyrans; substituted 3H-
phenanthro[1,2-b]pyrans; and benzopyran compounds, such as
those having substituents at the 2-position of the pyran ring
5' and a substituted or unsubstituted heterocyclic ring, such as
a benzothieno or benzofurano ring fused to the benz portion of
the benzopyran. Such later described compounds are the
subject of co-pending U.S. Patent 5,514,817 filed August 4, 1994
and U.S. Patent 5,411,679.
Photochromic articles of the present invention may
contain one photochromic compound or a mixture of photochromic
compounds, as desired or required. Individual photochromic
compounds or mixtures of photochromic compounds may be used to
attain certain activated colors such as neutral grays or
13 browns .
The compounds of the present invention (hereinafter
also referred to and included as a second group photochromic
compound) may be used also in combination with the organic
photochromic substances of the first complementary group of
photochromic compounds described herein, i.e., those that
color to blue, bluefish-green, or bluefish-purple or with other
organic photochromic substances in the aforesaid second group
of photochromic compounds. Either members of the first or
second group of photochromic compounds or mixtures of such
compounds may be combined with or used in conjunction with the
third group of photochromic compounds described herein that
exhibit colors ranging from yellow to purple and yellow/brown
to purple/gray.
- Each of the'photochromic substances described herein
may be used in amounts (or in a ratio) such that an organic
host material to which the photochromic compounds or mixture
of compounds is applied or in which they are incorporated
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
- 22 -
exhibits a desired resultant color, e.g., a substantially
neutral color when activated with unfiltered sunlight, i.e.,
as near a neutral color as possible given the colors of the
activated photochromic compounds.
A neutral gray color exhibits a spectrum that has
relatively equal absorption in the visible range between 400
and 700 nanometers. A neutral brown color exhibits a spectrum
in which the absorption in the 400-550 nanometer range is
moderately larger than in the 550-700 nanometer range. An
alternative way of describing color is in terms of its
chromaticity coordinates, which describe the qualities of a
color in addition to its luminance factor, i.e., its
chromaticity. In the CIE system, the chromaticity coordinates
are obtained by taking the ratios of the tristimulus values to
their sum, e.g., x=X/(X+Y+Z) and y=Y/(X+Y+Z). Color as
described in the CIE system can be plotted on a chromaticity
diagram, usually a plot of the chromaticity coordinates x and
y. See pages 47-52 of ~rinc?plPs of Color Tech_noloav, by F.
W. Billmeyer, Jr., and Max Saltzman, Second Edition, John
Wiley and Sons, N.Y. (1981). As used herein, a near neutral
color is one in which the chromaticity coordinate values of
"x" and "y" for the color are within the following ranges (D65
illuminant): x = 0.260 to 0.400, y = 0.280 to 0.400 following
activation to 40 percent luminous transmission by exposure to
solar radiation (Air Mass 1 or 2).
The amount of photochromic substance or composition
containing same applied to or incorporated into a host
material is not critical provided that a sufficient amount is
used to produce a photochromic effect discernible to the naked
eye upon activation. .Generally such amount can be described
as a photochromic amount. The particular amount used depends
often upon the intensity of color desired upon irradiation
?.. . _..... . ....... _...,... . . ,. ._ ....
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
- 23 -
thereof and upon the method used to incorporate or apply the
photochromic substances. Typically, the more photochromic
substance applied or incorporated, the greater is the color
intensity up to a certain limit.
The relative amounts of the aforesaid photochromic
compounds used will vary and depend in part upon the relative
intensities of the color of the activated species of such
compounds, and the ultimate color desired. Generally, the
amount of 'total photochromic substance incorporated into or
applied to a photochromic optical host material may range from
about 0.05 to about 1.0, e.g., from 0.1 to about 0.45,
milligrams per square centimeter of surface to which the
photochromic substances) is incorporated or applied. When
mixtures of the aforedescribed organic photochromic
IS complementary groups are used, the weight ratio of such
materials, i.e., (first to second), (second to third), and
(naphthopyran of the present invention to other second group
compounds) will vary from about 1:3 to about 3:1, e.g.,
between about 0.75:1 and about 2:1. The combination of the
first, second, and third described organic photochromic
complementary groups may have a weight ratio that will vary
from about 1:3:1 to 3:1:3.
The photochromic substances of the presenC invention
may be applied to or incorporated into a host material such as
a polymeric organic host material by various methods described
in the art. Such methods include dissolving or dispersing the
photochromic substance within the host material, e.g., casting
it in place by adding the photochromic substance to the
monomeric host material prior to polymerization; imbibition of
the photochromic substance into the host material by immersion
of the host material in a hot solution of the photochromic
substance or by thermal transfer; providing the photochromic
CA 02293834 1999-12-03
WO 98!55457 PCT/US97/09687
-24-
substance as a separate layer between adjacent layers of the
host material, e.g., as a part of a polymeric film; and
applying the photochromic substance as part of a coating
placed on the surface of the host material. The term
"imbibition" or "imbibe" is intended to mean and include
permeation of the photochromic substance alone into the host
material, solvent assisted transfer of the photochromic
substance into a porous polymer, vapor phase transfer, and
other such transfer mechanisms.
Compatible (chemically and color-wise) tints, i.e.,
dyes, may be applied to the host material to achieve a more
aesthetic result, for medical reasons, or for reasons of
fashion. The particular dye selected will vary and depend on
the aforesaid need and result to be achieved. In one
embodiment, the dye may be selected to complement the color
resulting from the activated photochromic substances, e.g.,
to achieve a more neutral color or absorb a particular
wavelength of incident light. In another embodiment, the dye
may be selected to provide a desired hue to the host matrix
when the photochromic substances is in an unactivated state.
Adjuvant materials may also be incorporated into
the host material with the photochromic substances prior to,
simultaneously with or subsequent to application or
incorporation of the photochromic substances in the host
material. For example, ultraviolet light absorbers may be
admixed with photochromic substances before their application
to the host material or such absorbers may be superposed,
e.g., superimposed, as a layer between the photochromic
substance and the incident light. Further, stabilizers may
be admixed with the photochromic substances prior to their
application to the host material to improve the light fatigue
resistance of the photochromic substances. Stabilizers, such
r
_ ......_._...~__ ~... _. .. _ . _ .
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
- 25 -
as hindered amine light stabilizers and singlet oxygen
quenchers, e.g., a nickel ion complex with an organic ligand,
are contemplated. They may be used alone or in combination.
Such stabilizers are described in U.S. Patent 4,720,356.
Finally, appropriate protective coatings) may be applied to
the surface of the host material. These may be abrasion
resistant coatings and/or coatings that serve as oxygen
barriers. Such coatings are known in the art.
The host material will usually be transparent, but
may be translucent or even opaque. The host material need
only be transparent to that portion of the electromagnetic
spectrum, which activates the photochromic substance, i.e.,
that wavelength of ultraviolet (W) light that produces the
open form of the substance arid that portion of the visible
spectrum that includes the absorption maximum wavelength of
the substance in its UV activated form, i.e., the open form.
Preferably, the host color should not be such that it masks
the color of the activated form of the photochromic substance,
i.e., so the change in color is readily apparent to the
observer. More preferably, the host material article is a
solid transparent or optically clear material, e.g., materials
suitable for optical applications, such as plano and
ophthalmic lenses, windows, automotive transparencies, e.g.,
windshields, aircraft transparencies, plastic sheeting,
polymeric films, etc.
Examples of polymeric organic host materials which
may be used with the photochromic substances or compositions
described herein include: polymers, i.e., homopolymers and
copolymers, of polyol(allyl carbonate) monomers, diethylene
glycol dimethacrylate monomers, diisopropenyl benzene
monomers, and alkoxylated polyhydric alcohol acrylate monomers
such as ethoxylated trimethylol propane triacrylate monomers;
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
-26-
polymers, i.e., homopolymers and copolymers, of
polyfunctional, i.e., mono-, di-, tri-, tetra, or multi-
functional, acrylate and/or methacrylate monomers,
polyacrylates, polymethacrylates, poly( C1-C12 alkyl
methacrylates) such as poly(methyl methacrylate),
polyoxy(alkylene methacrylates) such as polyethylene glycol
bis methacrylates), poly(alkoxylated phenol methacrylates)
such as poly(ethoxylated bisphenol A dimethacrylate),
cellulose acetate, cellulose triacetate, cellulose acetate
propionate, cellulose acetate butyrate, polyvinyl acetate),
polyvinyl alcohol), polyvinyl chloride), poly(vinylidene
chloride), polyurethanes, thermoplastic polycarbonates,
polyesters, polyethylene terephthalate), polystyrene, poly
(alpha methylstyrene), copoly(styrene-methyl methacrylate),
copoly(styrene-acrylonitrile), polyvinylbutyral and polymers,
i.e., homopolymers and copolymers, of diallylidene
pentaerythritol, particularly copolymers with polyol (allyl
carbonate) monomers, e.g., diethylene glycol bis(allyl
carbonate), and acrylate.monomers.
Transparent copolymers and blends of transparent
polymers are also suitable as host materials. Preferably, the
host material is an optically clear polymerized organic
material prepared from a thermoplastic polycarbonate resin,
such as the carbonate-linked resin derived from bisphenol A
and phosgene, which is sold under the trademark, LEXAN; a
polyester, such as the material sold under the trademark,
MYLAR; a poly(methyl methacrylate), such as the material sold
under the trademark, PLEXIGLAS; polymerizates of .a
polyol(allyl carbonate) monomer, especially diethylene glycol
bis(allyl carbonate), which monomer is sold under the
trademark CR-39, and polymerizates of copolymers of a polyol
(allyl carbonate), e.g., diethylene glycol bis(allyl
T _...~ ._... _. ...
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
-27-
carbonate), with other copolymerizable monomeric materials,
such as copolymers with vinyl acetate, e.g., copolymers o.f
from 80-90 percent diethylene glycol bis(allyl carbonate) and
10-20 percent vinyl acetate, particularly 80-85 percent of the
bis(allyl carbonate) and 15-20 percent vinyl acetate, and
copolymers with a polyurethane having terminal diacrylate
functionality, as described in U.S. Patents 4,360,653 and
4,994,208; and copolymers with aliphatic urethanes, the
terminal portion of which contain allyl or acrylyl functional
groups as described in U.S. Patent 5,200,483; polyvinyl
acetate), polyvinylbutyral, polyurethane, polymers of members
of the group consisting of diethylene glycol dimethacrylate
monomers, diisopropenyl benzene monomers, and ethoxylated
trimethylol propane triacrylate monomers; cellulose acetate,
cellulose propionate, cellulose butyrate, cellulose acetate
butyrate, polystyrene and copolymers of styrene with methyl
methacrylate, vinyl acetate and acrylonitrile. More
particularly, contemplated is use of the photochromic
naphthopyrans of the present invention with optical organic
resin monomers used to produce optically clear polymerizates,
i.e., materials suitable for optical applications, such as for
example plano and ophthalmic lenses, windows, and automotive
transparencies. Such optically clear polymerizates may have a
refractive index that may range from about 1.48 to about 1.75,
e.g., from about 1.495 to about 1.66.
The present invention is more particularly described
in the following examples which are intended as illustrative
only, since numerous modifications and variations therein will
be apparent to those skilled in the art.
CA 02293834 1999-12-03
WO 98/55457 PCT/iJS97/09687
_28_
4-Fluorobenzophenone (25 grams (gm), 0.12 moles) and
morpholine (15 gm) were added to a reaction flask containing
100 milliliters of anhydrous dimethylsulfoxide, stirred and
refluxed under an argon atmosphere for a hours. Afterwards,
the contents of the reaction flask mixture was added to 500 ml
of an ice-water mixture and stirred for 2 hours. The
resulting solid product was filtered, washed in sequence with
water followed by hexane and then air dried. The yield of the
isolated product was 30 gm. A nuclear magnetic resonance
(NMR) spectrum showed the product to have a structure
consistent with 4-morpholinobenzophenone, which was not
purified further but used directly in the next step.
STEP 2
4-Morpholinobenzophenone (10 gm, 0.037 moles) was
dissolved in a reaction flask containing 50 milliliters (ml)
of anhydrous dimethylformamide saturated with acetylene and
stirred at room temperature. An 18 weight percent suspension
of sodium acetylide in xylene/mineral oil (0.3 mole of sodium
acetylide) was added to the reaction flask and the mixture was
stirred. After stirring 3 hours at room temperature under a
nitrogen atmosphere, the contents of the reaction flask
mixture were added to 250 ml of an ice-water mixture and
stirred for one hour. The resulting solid product was
filtered, washed with water followed by hexane and air dried.
The yield of the isolated product was 8 gm. A nuclear
magnetic resonance (NMR) spectrum showed the product to have a
structure consistent with 1-(4-morpholinophenyl)-1-phenyl-2-
_ _.___r _ .-~. .
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
-29-
propyn-1-ol, which was not purified further but used directly
in the next step.
1-(4-Morpholinophenyl)-1-phenyl-2-propyn-1-of (about
0.025. mole) from Step 1 and methyl-1,4-dihydroxy-2-naphthoate
(5 gm, 0.022 mole) were added to a reaction flask containing
200 ml of toluene and stirred. A catalytic amount of p-
toluene sulfonic acid (about 100 milligrams) was added, and
the mixture was stirred for 4 hours. Afterwards, the reaction
mixture was poured into a l0 weight percent sodium hydroxide
solution. The organic layer was separated, washed with water,
and dried over anhydrous sodium sulfate. The remaining
solvent, toluene, was removed under vacuum. The resulting oil
was purified using a silica gel column and a 1:3 mixture of
chloroform: hexane as the eluant. The photochromic fractions
were combined and the eluent was removed under vacuum. The
resulting product was induced to crystallize from hexane. The
recovered product had a melting point of 178-179° C. A
nuclear magnetic resonance (NMR) spectrum showed the product
to have a structure consistent with 2-(4-morpholinophenyl)-2-
phenyl-5-methoxycarbonyl-6-hydroxy-2H-naphtho[1,2-b]pyran.
2-(4-Morpholinophenyl)-2-phenyl-5-methoxycarbonyl-6-
hydroxy-2H-naphtho[1,2-b]pyran (2 grams) prepared as described
in Example 1, anhydrous potassium carbonate (2 grams), and
methyliodide (2 grams) were added to a reaction flask
containing 40 milliliters of anhydrous acetone, stirred and
refluxed under an argon atmosphere. Afterwards, the acetone
was removed under vacuum and 25 milliliters each of water and
methylene chloride were added to the reaction mixture. The
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
-30-
mixture was stirred for 30 minutes and the organic layer was
separated, washed and dried. The remaining solvent, methylene
chloride, was removed under vacuum. The resulting oily
concentrate was crystallized from a 1:1 hexane: diethyl ether
mixture. The solid obtained was suction filtered, washed with
hexane and air dried. The resulting product had a melting
point of 175-176° C. A nuclear magnetic resonance (NMR)
spectrum showed the product to have a structure consistent
with 2-(4-morpholinophenyl)-2-phenyl-5-methoxycarbonyl-6-
methoxy-2H-naphtho(1,2-b]pyran.
2-(4-Morpholinophenyl)-2-phenyl-5-methoxycarbonyl-6-
hydroxy-2H-naphtho[1,2-b]pyran (2 grams), prepared as
described in Example 1, and triethylamine (2 grams) were added
to a reaction flask containing 50 milliliters of anhydrous
methylene chloride and stirred. Acetyl chloride (2 grams) was
added to the reaction flask and the reaction mixture was
stirred for 1 hour. Distilled water (50 milliliters) was added
to the reaction flask and the reaction mixture was stirred for
another half hour. Afterwards, the organic layer was
separated, washed and dried over anhydrous sodium sulfate.
Evaporation of solvent resulted in an oily residue that was
crystallized from a 1:1 hexane:diethyl ether mixture. The
solid was suction filtered, washed with hexane, and air dried.
The resulting product had a melting point of 143-145° C. A
nuclear magnetic resonance (NMR) spectrum showed the product
to have a structure consistent with 2-(4-morpholinophenyl)-2-
phenyl-5-methoxycarbonyl-6-acetoxy-2H-naphtho[1,2-b]pyran.
_..~__.__ .. _. _ .. . ...
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
-31 -
N-Ethylcarbazole (50 grams, 0.25 mole) and benzoyl
chloride (0.28 mole) were added to a reaction flask containing
500 milliliters of anhydrous methylene chloride and stirred at
room temperature. Anhydrous aluminum chloride (0.30 mole) was
added slowly to the mixture. The reaction mixture was stirred
one hour and then poured into a 5 weight percent aqueous
hydrochloric acid and ice mixture. The resulting mixture was
stirred 15 minutes and extracted with methylene chloride. The
organic layer was separated and washed first with 10 weight
percent aqueous sodium hydroxide followed by distilled water.
The organic layer was separated and dried over anhydrous
magnesium sulfate. The methylene chloride solvent was removed
under vacuum. The resulting oily product crystallized from
hexane. The recovered product, 35 grams, was filtered and air
dried. A nuclear magnetic resonance (NMR) spectrum showed the
desired product to have a structure consistent with 2-benzoyl-
9-ethylcarbazole.
STEP 2
The procedure of Step 2 of Example 1 was followed
except that 2-benzoyl-9-ethylcarbazole was used in place of 4-
morpholinobenzophenone to produce 1-(9-ethylcarbazol-2-yl)-1-
phenyl-2-propyn-1-ol.
STEP 3
The procedure of Step 3 of Example 1 was followed
except that 1-(9-ethylcarbazol-2-yl)-1-phenyl-2-propyn-1-of
was used in place of 1-(4-morpholinophenyl)-1-phenyl-2-propyn-
CA 02293834 1999-12-03
WO_98/55457 PCT/US97/09687
-32-
1-0l to produce 2-(9-ethylcarbazol-2-yl)-2-phenyl-5-
methoxycarbonyl-6-hydroxy-2H-naphtho[1,2-b]pyran.
The procedure of Example 3 was followed except that
2-(9-ethylcarbazol-2-yl)-2-phenyl-5-methoxycarbonyl-6-hydroxy-
2H-naphtho[1,2-b]pyran was used in place of 2-(4-
morpholinophenyl)-2-phenyl-5-methoxycarbonyl-6-hydroxy-[2H]-
naphtho[1,2-b]pyran. The resulting product was an oil. A
nuclear magnetic resonance (NMR) spectrum showed the product
to have a structure consistent with 2-(9-ethylcarbazol-2-yl)-
2-phenyl-5-methoxycarbonyl-6-acetoxy-2H-naphtho[1,2-b]pyran.
The procedure of Example 4 was followed except that
in step 1, N-phenylcarbazole was used in place of N-
ethylcarbazole. The resulting product had a melting point of
188-189° C. A nuclear magnetic resonance (NMR) spectrum
showed the product to have a structure consistent with 2-(9-
phenylcarbazol-2-yl)-2-phenyl-5-methoxycarbonyl-6-acetoxy-2H-
naphtho[1,2-b]pyran.
RXAMP , . 6
The procedure of Steps 2 and 3 of Example 1 were
followed except that in Step 2, 4-dimethylaminobenzophenone
was used in place of 4-morpholinobenzophenone to produce 1-(4-
dimethylaminophenyl)-1-phenyl-2-propyn-1-ol, which was used in
Step 3 to produce 2-(4-dimethylaminophenyl)-2-phenyl-5-
methoxycarbonyl-6-hydroxy-2H-naphtho[Z,2-b]pyran.
_ . T __.____ .,___ ___ ___...__. ._..._~_._._._-._...___ ... _ __
CA 02293834 1999-12-03
WO 98!55457 PCT/US97/09687
- 33 -
The procedure of Example 3 was followed except that 2-(4-
dimethylaminophenyl)-2-phenyl-5-methoxycarbonyl-6-hydroxy-2H-
naphtho[1,2-b]pyran was used in place of 2-(4-
morpholinophenyl)-2-phenyl-5-methoxycarbonyl-6-hydroxy-2H-
naphtho[1,2-b]pyran. The resulting product had a melting point
of 156°C. A nuclear magnetic resonance (NMR) spectrum showed
the product to have a structure consistent with 2-(4-
dimethylaminophenyl)-2-phenyl-5-methoxycarbonyl-6-acetoxy-2H-
naphtho [ 1, 2 -b] pyran .
The procedure of Step 3 of Example 1 was followed
except that 1-(4-dimethylaminophenyl)-1-phenyl-2-propyn-1-of
was used in place of 1-(4-morpholinophenyl)-1-phenyl-2-propyn-
1-0l and methyl-4-hydroxy-1-methyl-2-naphthoate was used in
place of methyl-1,4-dihydroxy-2-naphthoate. The resulting
product had a melting point of 182-184°C. A nuclear magnetic
resonance (NMR) spectrum showed the product to have a
structure consistent with 2-(4-dimethylaminophenyl)-2-phenyl-
5-methoxycarbonyl-6-methyl-2H-naphtho[1,2-b]pyran.
The procedure of Example 6 was followed except that
in Step 1, 4,4'-bis(dimethylamino)benzophenone was used in
place of 4-dimethylaminobenzophenone. The resulting product
had a melting point of 222-224°C. A nuclear magnetic
resonance (NMR) spectrum showed the product to have a
CA 02293834 1999-12-03
WO 98/55457 PCT/US97109687
-34-
structure consistent with 2,2-bis(4-dimethylaminophenyl)-5-
methoxycarbonyl-6-acetoxy-2H-naphtho[1,2-b]pyran.
$ PART A
Testing was done with the photochromic naphthopyrans
of the Examples incorporated or imbibed into test square
polymerizates by one of two different methods. In Method I,
the photochromic naphthopyrans of the Examples were
incorporated into polymeric samples. The quantity of
naphthopyran calculated to yield a 1.5 times 10-3 molal
solution was added to a flask containing 50 grams of a monomer
blend of 4 parts ethoxylated bisphenol A dimethacrylate (BPA
2E0 DMA), 1 part poly(ethyleneglycol) 600 dimethacrylate, and
0.033 weight percent 2,2'-azobis(2-methyl propionitrile)
(AIBN). The naphthopyrans were dissolved into the monomer
blend by stirring and gentle heating, if necessary. After a
clear solution was obtained, it was poured into a flat sheet
mold having the interior dimensions of 2.2 mm x 6 inches
(15.24 cm) x 6 inches (15.24 cm). The mold was sealed and
placed in a horizontal air flow, programmable oven set to
increase the temperature from 40°C. to 95°C. over a 5 hour
interval, hold the temperature at 95°C. for 3 hours and then
lower it to 60°C. for at least 2 hours before the end of the
curing cycle. After the molds were opened, the polymer sheets
were cut using a diamond blade saw into 2 inch (5.1
centimeters) test squares.
In Method II, the photochromic naphthopyran was
imbibed into test square polymerizates prepared from a
diethylene glycol bis(allyl carbonate) composition.
The test squares measured 1/8 inch (0.3 centimeters) x 2
inches (5.1 centimeters) x 2 inches (5.1 centimeters). In the
__....~..~__~._. _r._____.____ _._.. _.._. _~
CA 02293834 2004-04-07
-35-
imbibition process, the photochromic naphthopyran was
dissolved to form a 10 weight percent solution in a 1:9
mixture of ethyl cellulose: toluene. The solution was then
spin coated onto the test squares and allowed to dry. Samples
were then heated in a hot-air oven at 135-155°C for a period
of time sufficient to thermally transfer the photochromic into
the test squares., The residence time in the oven for the test
squares was adjusted to imbibe comparable amounts of the
naphthopyran compounds. This was done in order to yield a
comparable W absorbance at the lambda max of the compound in
the near W. After cooling,. the ethyl cellulose/toluene resin
film was removed from the test squares by washing with
acetone.
Part B
The photochromic test squares were tested for
photochromic response rates on an optical bench. Prior to
testing on the optical bench, the photochromic test squares
were exposed to 365 nanometer ultraviolet light for about 15
minutes to activate the photochromic compounds and then placed
into a 76°C oven for about 15 minutes to bleach or inactivate
the photochromic compounds. The test squares were then cooled
to room temperature, exposed to fluorescent room lighting for
at least 2 hours and then kept covered for at least 2 hours
prior to testing on an optical bench maintained at 75°F
(23.9°C). The bench was fitted with a 150 watt Xenon arc
lamp, a remote controlled shutter, a copper sulfate bath
acting as a heat sink for the arc lamp, a Schott WG-320 nm
cut-off filter which removes short wavelength radiation;
neutral density filters) and a sample.holder in which the
square to be tested was inserted. A collimated beam of light
from a tungsten lamp was passed through the square at a small
* Trade-mark
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
-36-
angle normal to the square. After passing through the square,
the light from the tungsten lamp was directed through a
photopic filter attached to a detector. The photopic filter
passes wavelengths such that the detector mimics the response
of the human eye. The output signals from the detectors)
were processed by a radiometer.
Change in optical density (DOD) was determined by
inserting a test square in the bleached state into the sample
holder, adjusting the transmittance scale to 100%, opening the
shutter from the Xenon lamp to provide ultraviolet radiation
to change the test square from the bleached state to an
activated (i.e., darkened) state, measuring the transmittance
in the activated state, and calculating the change in optical
density according to the formula D OD=log(100/%Ta) where %Ta
is the percent transmittance in the activated state and the
logarithm is to the base 10.
The D OD/Min, which represents the sensitivity of
the photochromic compound's response to UV light, was measured
over the first five (5) seconds of UV exposure, then expressed
on a per minute basis. The saturation optical density (OD)
was taken under identical conditions as the O OD/Min, except
UV exposure was continued for 20 minutes for the examples in
Table 1. The lambda max reported in Table l is the wavelength
in the visible spectrum at.which the maximum absorption of the
activated (colored) form of the photochromic compound in a
diethylene glycol bis(allyl carbonate) composition occurs.
The Bleach Rate (T 1/2) is the time interval in seconds for
the absorbance of the activated form of the naphthopyran in
the test squares to reach one half the highest absorbance at
room temperature (75°F, 23.9°C) after removal of the source of
activating light. Results for the Compounds of the Examples
are tabulated in Table 1.
...____~~...~_ _._ _ ..
CA 02293834 1999-12-03
WO 98/55457 PCT/US97/09687
-37-
TABLE 1
EXAMPLE LAMBDA MAX DOD/MIN ~ODQ Bleach
) S FNSTTTVrTV ~g (T1/2)
g~
~
SATI
COMPOL1NDS iVTSTBLE 0.64 , 193
1 511 "
,
1.36
2 541 0.29 0.95 292
3 545 0.24 0.31 88
4* 528 0.67 0.46 270
5 520 0.31 0.88 70
6 591 0.27 0.30 70
7 584 0.20 0.51 200
8 618 0.16 0.07 19
* Test square prepared by Method II. All others were
prepared by Method I.
The results of Table 1 show that a range of
values for bleach rate, SOD at saturation, and
sensitivity are obtained for the Example Compounds 1
through 8 of the present invention depending on the
nature of substituents R1, R2, R3, and B and B'.
Although the present invention has been described
with reference to the specific details of particular
embodiments thereof, it is not intended that such details be
regarded as limitations upon the scope of the invention
except as and to the extent that they are included in the
accompanying claims.