Note: Descriptions are shown in the official language in which they were submitted.
CA 02296901 2000-O1-13
1
PIPERIDINE DERIVATES HAVING ANXIOLYTIC EFFECT
This application is a division of application serial N°
2,111,204 filed on June 12, 1992.
BACKGROUND OF THE INVENTION
Field of the Invention
The invention disclosed in patent application N° 2,111,204
relates to a class of piperidine compounds having
anxiolytic effect and potentially binding to the sigma
receptors and therefore being useful in the treatment of
psychic and neurologic disorders.
The present invention as claimed hereinafter relates to
others piperidine compounds from the same class of
compounds.
Description of the Related Art
Various related compounds are known from the prior art.
So, US patents Nos. 3,686,186 and 3,745,165 disclose spiro[phthalan-1,4'-
piperidine] and spiro[isochroman-3,4'-piperidine] compounds optionally having
a
benzyl substituent at the piperidine N-atom. The phthalan compounds are said
to be
useful as antidepressants as indicated by their ability to reverse reserpine
hypother-
mia, whereas the isochromane compounds are stated to be useful as hypotri-
glyceridemics.
German Offenlegungsschrift No. 2,458,176 and the corresponding US patent No.
3,985,889 generically describe inter alia 1,3-dihydrospiro(isobenzofuran-1,4'-
CA 02296901 2000-O1-13
la
piperidineJ or 1,3-dihydrospiro[isobenzofuran-1,3'-pyrrolidine] compounds sub-
stituted at the ring N-atom with lower alkyl, cycloalkyl or phenyl(C2_4)alkyl
and
optionally having an oxo group attached to the furan ring. The compounds are
alleged to be useful as tranquilizers as demonstrated by their ability to
display
effects on behaviour and reflex depression and on muscle relaxation, and they
are
claimed also to be useful in the treatment of pain as demonstrated in the 2-
phenyl-
1,4-quinone induced writhing assay in mice. However, only pharmacological data
for one such compound without an oxo substituent in the furan ring, i.e. the
com-
pound 1,3-dihydro-1'-methyl-spiro[isobenzofuran-1,4'-piperidineJ, are
presented,
to and only four such compounds having a substituent different from methyl on
the
piperidine N-atom are specifically disclosed, i. e. 1 '-cyclopropylmethyl-, 1
'-[3-(4-
fluoro-benzoyl)propyl]-, 1 '-[4,4-bis(4-fluorophenyl)butyl)- and 1 '-acetyl-
1,3-dihydro-
spiro[iso-benzofuran-1,4'-piperidine) . No indication of effect on sigma-
receptors is
given.
Japanese patent publication JP Kokai 55 143,980 generically describes inter
alia a
CA 02296901 2000-O1-13
2
very broad class of spiro[chromane-piperidine) compounds which are optionally
substituted at the piperidine N-atom with an alkyl, cycloalkyl, allyl, aryl,
aralkyl, or
arylcycloalkyl group and optionally substituted in the chromane ring with an
oxo
group. However, with respect to such spirochromane compounds having no oxo
substituent in the chromane ring only compounds having a methyl, phenyl or
benzyl
group at the piperidine N-atom are specifically disclosed. These compounds are
claimed to possess antiallergic activity and no suggestion of activity in the
central
nervous system is given.
~o European patent publication No'EP 0 414 289 A1 generically describes a
class of
1,2,3,4-tetrahydro-spiro[naphthalene-1,4'-piperidine) and 1,4-dihydro-
spiro[naph-
thalene-1,4'-piperidine) derivatives substituted at the piperidine N-atom with
a
"hydrocarbon" and aleged to have selective sigma receptor antagonistic
activity.
The term "hydrocarbon" as defined in said patent covers all possible straight
~s chained, cyclic, heterocyclic etc. groups; however, only compounds having
benzyl,
phenethyl, cycloalkylmethyl, furyl- or thienyl~nethyl or lower alkyl or
alkenyl as the
"hydrocarbon" substituent at the piperidine nitrogen atom being specifically
disclosed. The compounds are stated to displace tritiated di-tolyl guanidine
(DTG)
from sigma sites with potencies better than 200 nM. As a particularly
preferred
2o compound is mentioned 1 '-benzyl-1,2,3,4-tetrahydro-spiro[naphthalene-1,4'-
piperidi-
ne). European patent publication No EP 0 445 974 A2 generically describes the
corresponding spiro(indane-1,4'-piperidin'e) and spiro[benzocycloheptene-5,4'-
piperidine) derivatives. Again the compounds are only stated to displace
tritiated di-
tolyl guanidine (DTG) from sigma sites with potencies better than 200 nM
EP Application No. EP-A2-0 431 943 relates to a further extremely broad class
of
spiropiperidine compounds substituted at the piperidine N-atom. Said compounds
are alleged to be useful as antiarrhythmics and for impaired cardiac pump
function.
so Said application exemplifies several compounds, the majority of which
contain an
oxo andlor a sulfonylamino substituent in the spiro cyclic ring system. Of the
remainder compounds, the main part has another polar substituent, such as
vitro,
amino, . a fused imidazo group etc. attached to the spiro nucleus and/or they
have
CA 02296901 2000-O1-13
3
some polar substituents, such as sulfonylamino, vitro, amino, etc. in the
substituent
on the piperidine N-atom. Furthermore some of the compounds have heteroaryl
alkyl substituents on the piperidine N-atom, whereas only a very few of those
compounds exemplified do not have such substituents, i.e. a couple of 6-
methoxjr-
s spiro[2H-1-benzopyran-2,4'-piperidinesJ having a phenylsubstituent on the
piperi-
dine N-atom and a few spiro[3H-1-benzopyran-3,4'-piperidineJ's having a
benzyl,
phenethyl, hexyl or heptyl substituent on the piperidine N-atom. No suggestion
or
indication of effect of the compounds on the sigma receptors is given.
io US patent No. 4,420,485 discloses 1'-[3-(6-fluoro-1,2-benzoisoxazol-3-
yl)propylJ-
spiro[benzofuran-2(H),4'-piperidinesJ optionally having one or two
substituents in
the benzofuran ring. The compounds are claimed to be useful as
antihypertensives.
No mention or suggestion of effects in the treatment of psychic or
neurological
disorders is given.
is
German Offenlegungsschrift No 28 27 874 corresponding to U.S. Patent No.
4,251,538 generically discloses a class of 3-[4-(4-phenyl-piperidin-1-yl)-
butyl]- or 3-
[4-(4-phenyl-tetrahydropyridyl-1-yl)-butyljindole derivatives optionally
substituted in
the indole, piperidinyl or tetrahydropyridyl and/or phenyl groups. The
compounds
2o are said to show dopamine agonist effects and serotonin reuptake inhibiting
effects
in the central nervous system, and accordingly to be useful in the ,treatment
of
Parkinson's disease and depression. However, no documentation for such-effects
is
given in the specification, no pharmacological data at all being given, and
certainly
no indication or suggestion of effect on the sigma receptor is given.
Furthermore, the majority of the compounds listed in the specification, of
which
obviously only a few have actually been prepared, are tetrahydropyridyl
compounds
and/or they have an oxo substituent in the butyl chain. Only a few piperidyl
com-
pounds without an oxo substituent in the butyl chain are mentioned, i.e. 3-[4-
(4-
so phenyl-1-piperidyl)-butyl]-indole and the 1-methyl-, 1-phenyl- and 2-methyl-
derivatives thereof as well as derivatives thereof substituted with halogen,
methyl or
trifluoromethyl in the 4-phenyl substituent on the piperidyi group thereof.
With
respect to physical data only melting point for one such compounds is given.
. CA 02296901 2000-O1-13
4
International Patent Application No WO 91 /09594 published on July 11, 1991
relates i.a. to sigma receptor ligands being 4-phenyl-piperidine compounds and
having an optionally substituted "heteroaryl"-alkyl, -alkenyl, -alkynyl, -
alkoxy or -
alkoxy-alkyl substituent on the piperidine N-atom. The term "heteroaryl"-
alkyl~is
s defined by mention of a very broad class of such substituents. However only
four N-
substituted 4-phenyl-piperidine compounds are specifically disclosed which are
all 1-
(phenyl-lower alkyl)-4-phenyl-piperidines and only four compounds having a
"heteroaryl"-alkyl substituent are specifically mentioned, which are all
piperazine
(and not piperidine) compounds.The compounds are stated to be antipsychotics.
~o
From studies of the biology and function of sigma receptors, evidence has been
presented that sigma receptor ligands may be useful in the treatment of
psychosis
and movement disorders, such as dystonia and tardive dyskinesia, and motor
disturbances associated with Huntington's chorea or Tourette~s syndrome and in
~s Parkinson~s disease (Walker, J. M. et al, Pharmacological Reviews, 1990,
42, 355).
The known sigma receptor ligand rimcazole clinically shows effects in the
treatment
- of psychosis (Snyder, S.H., Largent, B.L. J. Neuropsychiatry 1989, 1, 7) and
a
group of sigma receptor ligands have been descibed to show antihallucinogenic
activity in animal models (International Patent Publication No WO 9103243).
Furthermore, sigma receptor ligands have been reported to be involved in
modula-
tion of NMDA receptor mediated events in the brain and to act as anti-ischemic
agents in in vivo tests (Rao, T. S. et al, Molecular Pharmacology, 1990, 37,
978). In
addition to ischemia they may also be useful in the treatment of other such
NMDA
2s receptor mediated events, e.g. epilepsy and convulsion.
Also, some sigma receptor ligands have been found to show anti-amnesic effects
in
an animal model (Early et al., Brain Research 1991, 546, 281-286).
Sigma ligands have been shown to influence central acetylcholine levels in
animal
ao models (Matsuno et al, Brain Research 1992, 575, 3i 5-319; Junien et al,
Eur. J.
Pharm. 1991, 200, 343-345) and may, therefore, have potential in the treatment
of
senile dementia of the Alzheimer type.
CA 02296901 2003-07-25
Finally, some guanid;.ne derivatives having sigma receptor
activity ha«e been c_;.iscl.c~sed ~:c> be use:Eul as anx.iolytics
(International Fatent fublicat.ie.on N° fnJC9019067; .
Accordingly, agents xaorertly a<-_-ting on the sigma receptors
in t:he central nerv~~~:} z;> svst zm ar_E: believed to be of
potential use in the t~he..rapy of: such conditions.
DETAILED DESCRIPTION OF THE INVENTION
As described and ~:~.aimed in parent application N°
2, 111, 204, i.t has n<~w been f.o.and that.- a certain class of
piperidine compounds bind at ;~:~gma receptors w.i.th potencies
which generally are ~_-3 o_Nders of_ magnit:ude hi<~her than the
potency limit indi.cai~e~u in Fu.r_~~pean patent pub_Licat:ion EP 0
414 289 Al. Furthermore the compounds of said class have
been found to show ar:~ioLytic e7ffect:s _n an_~mal. models..
Said class of compoa:ncts as bzoadly disclosed hereinafter
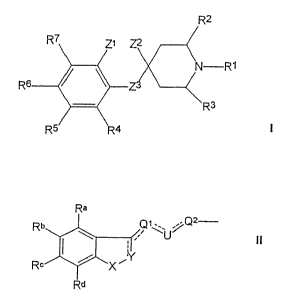
consists of compound. h.ava_na tine general Formula I
Rz
Z2
2 ~ N-R~
Rs Zs ~ I
R
R' R;
wherein R~ is
a) a group -D-13-A-R
wherein B is an up to 19 membered spacer group selected from alkylene,
alkenylene and alkynylene which may be branched or straight chain and
optionally substituted with hydroxy, which again may b~e esterified with an
aliphatic carboxylic acid having from two to twentyfour carbon atoms,
inclusive,
CA 02296901 2000-O1-13
5a
W
A is a bond or a divalent group selected from O, S, SO, S02, and _N~N- '
W being O or S and the dotted line designating an optional bond ;
R is alkyl, alkenyl, cycloalkyl, cycloalkenyl, phenyl, cyc9oalkylalkyl,
cycloalkenyl-
alkvl, phenylalkyl, diphenylalkyl, any alkylgroup optionally being substituted
with
CA 02296901 2000-O1-13
6
one or two hydroxy groups, which again may be optionally esterified with an
aliphatic carboxylic acid having from two to twentyfour carbon atoms
inclusive,
and any phenyl group being optionally substituted with one or more
substituents
in the phenyl ring; and
s D is CReR9 where R8 and R9 are independently selected from the substituents
- defined below for R4 - R~, or a cycloalkylene group; or
b) a group having the general Formula II
Ra
Rb
~\~U~%
II
Rc
Rd
wherein X is CHR~o, O, S, SO, S02 or NR~o, Rio being hydrogen, lower alkyl or
alkenyl, cycloalkyl or cycloalkylalkyl, cycloalkenyl~ or cycloalkenylalkyl,
acyl,
aminoalkyl, mono- or dialkylaminoalkyl, sulfonyl or. arylalkyl or phenyl
optionally
~s substituted with one or more substituents independently selected from the
following: halogen, lower alkyl, lower alkoxy, hydroxy, trifluoromethyl, and
cyano, or Rio is a hetero aromatic group, preferably 2-thienyl, 3-thienyl, 2-
furanyl, 3-furanyl, 2-thiazolyl, 2-oxazolyl, 2-imidazolyl, 2-pyridyl, 3-
pyridyl, or 4-
pyridyt;
one or two of the dotted lines may be a bond;
when the dotted line emanating from Y indicates a bond, Y is N or CH; or
when said dotted line indicates no bond, Y is CH2, NH, C=O or C=S;
Ra - Rd are independently selected from hydrogen, halogen, lower alkyl, lower
alkoxy, hydroxy; lower alkylthio, lower alkylsulphonyl, lower alkyl- or
dialkyl-
2s ~ amino, cyano, trifluoromethyl; or trifluoromethylthio;
U is CH2, O or S; or
when, one of the dotted lines emanating from U indicates a bond, U is CH;
the bond between U and Q1 or Q2, respectively, may also be a triple bond and
in
such case U is °C";
ao Q~ is selected from a bond, alkylene or alkenylene and Q2 is alkylene
having at
CA 02296901 2000-O1-13
7
least two C-atoms, alkenylene or a group QZ'D wherein Cl~' is as defined for
C~
and D is as defined above, Q~ and Q2 having together from 2 to 20 carbon
atoms and being optionally substituted with one or more hydroxy groups, any
such hydroxy group being optionally esterified with an aliphatic carboxylic
acid
having from two to twentyfour carbon atoms inclusive; and
RZ and R3 are independently hydrogen, lower alkyl or they may be linked
together
thereby forming an ethylene or propylene bridge;
R4 to R~ are independently selected from hydrogen, halogen, lower alkyl ,
lower
alkoxy, hydroxy, lower alkylthio, lower alkyl- or dialkylamino, cyano,
trifluoromethyl,
or trifluoromethylthio; and .
i) Z~ and Z2 are linked together in which case: Z~ is CH2, O or S;
Z2 and Z3 are independently (CH2)~, n being 0 or 1, O or S, with the proviso
that
Z? may not be S or O when Z2 is S or O, and that Z2 and Z3 may not both be
(CH2)~ wherein n is 0:
or Zl and Z2 may together represent a group -CH=CH-;
or when Z3 is (CH2)" wherein n is 0, Z~ and Z2 may together represent a 3-
membered divalent group, optionally containing one unsaturated bond, and
optionally containing one O- or S-heteroatom; or
ii) when R~ is a group as defined in b) Z~,and Z2 may also be unlinked, in
which
case: Z~ is a group as defined for R4 - R~, ZZ is hydrogen and Z3 is (CH2)"
wherein n is 0;
with the proviso that when Z~ - Z3 are as defined in i) wherein Z3 is (CH2)~
where n
is 0, and Z~ and Z2 together represent a 2- or 3-mernbered divalent
hydrocarbon
group, optionally containing one unsaturated bond, and R~ is a group defined
in a)
3o then D-B-A-R may not be phenyl-C.~.3-alkyl, lower alkyl or lower alkenyl;
and acid addition salts or prodrugs thereof.
CA 02296901 2003-07-25
~la
The present invfentior~ a:-is u.la:im~~d herei.r:aftt~r is restricted
to the compounds i: general f_ormu~_a (I) as defined
here:inabove, wherein RI ifof the genr~r_al f.orr:ula (II) and
wherein in this farmula (~=I)
X is NR10, R1.0 be.inr~ 4-f:l.uorophe:y~' ~ ;
one or two of ttw:~ dashed ~_roes maw; be a bound;
when t::~e dashed l..ine err<<.~natincl from Y indicates a
bound, Y iS CH;
or when said dashed _line indicates no bound, Y is CH2;
Ra to Rd are independently selected fro>m the group
consisting of_ hydrage;,, haloga-~~, lawer alkyl , Lower alkoxy,
hydroxy, lower a:Lkyi.t:ruio, .i.:~wer aLkylsu7_phonyl, lower
alkylamino, lower dialkyl.aminc, cyano, triflucromethyl and
trifluoromethylthio;
U is CH2, C~, or ~; or
when, one of the dash~.d lines emanating from U
indicates a bound, ~. is CH; the bond between Ql or Q2,
respectively, and LJ may also i:>e a triple k~ond. and in such
case U is C;
Q1 is selected Yrom a bourud, C1-Clg alkylene or C2-Clg
alkenylene a.nd Q2 is .2>-C~0 alkyLene, C2_20 alkenylene or a
grou.F:~ Q2D whez:e:i_n ?°'= is a C2-C;20 alky_Lene or C2_20
alkenylene, and h ias defir:ed below, wherein the total
number of carbon atc:~~s :in Q1 ~n<~ Q~' Ls from ? to 20, and
being optionally SLibStit_uted with c:ne a~- rnore hydroxy
groups, any such hydr~~>::~y r~roLr~r~ bein~~ o~>t=ional:ly esterified
with an alit>hatic carboxylic arid having from two to twenty
four carbon atoms in~::l.usi_ve; arid
CA 02296901 2004-04-O1
7b
R2 and R3 are independently hydrogen, lower alkyl or
they may be linked together to form an ethylene or
propylene bridge;
R4 to R~ are independently selected from the group
consisting of hydrogen, halogen, lower alkyl, lower alkoxy,
hydroxy, lower alkylthio, lower alkylamino, lower
dialkylamino, cyano, trifluoromethyl, and trifluoro-
methylthio;
D is CR8R9 where R8 and R9 are independently selected
l0 from the substituents defined above for R4-R~, or C3-Cg
cycloalkylene group; and
Z1 is selected from the group consisting of hydrogen,
halogen, lower alkyl, hydroxy, lower alkylthio, lower
alkylamino, lower dialkylamino, cyano, trifluoromethyl, and
trifluoromethylthio;
Z2 is hydrogen; and
Z3 is a bound.
The present invention as claimed is also directed to the
use of the above piperidine compounds or acid addition
20 salts or prodrugs thereof for the manufacture of a
pharmaceutical preparation for the treatment of anxiety,
CA 02296901 2000-O1-13
psychosis, epilepsy, convulsion, movement disorders, motor disturbances, amne-
sia, cerebrovascular diseases, senile dementia of the Alzheimer type or
Parkinson's
disease.
s Some of the compounds of the general Formula I may exist as optical isomers
thereof; and such optical isomers are also embraced by the invention.
The term alkyl is intended to mean a C~-C2o straight chain or branched alkyl
group
and similarly alkenyl means a C2-C2o straight chain or branched alkenyl, group
~o having one or more unsaturated bonds in the chain. The term cycloalkyl
designates
a carbocyclic ring having 3-8 carbon atoms inclusive or a bicyclic or
tricyclic
carbocycle, such as adamantyl.
The terms lower alkyl, lower alkoxy, lower alkylthio, etc. designate such
branched or
~s unbranched groups having from one to six carbon atoms inclusive. Exemplary
of
. such groups are methyl, ethyl, 1-propyl, 2-propyl, 1-butyl, 2-butyl, 2-
methyl-2-propyl,
2-methyl-1-propyl, methoxy, ethoxy,l-propoxy, 2-propoxy, methylthio,
ethylthio, 1-
propylthio, 2-propylthio, methylsulphonyl, ethylsulphonyl, or the like.
2o Halogen means fluoro, chloro, bromo or iodo.
The term "sulfonyl" is used in the meaning alkyl or aryl substituted sulfonyl,
and
similarly "acyl" is used in the meaning alkyl- or arylcarbonyl
2s The term "one or two of the dotted lines may be a bond" is intended to mean
that
each of the dotted lines may or may not represent a bond, i.e. that the ring
and the
side chain respectively may or may not have a double bond in the positions of
the
dotted lines in Formula Il, provided that only two at a time indicate a bond
and that
adjacent dotted lines do not both indicate a bond.
The: optional substituents in the phenyl groups in the definition of R may
indepen-
dently be selected from halogen, lower alkyl, lower alkoxy, hydroxy, lower
alkylthio,
lower alkylsulphonyl, lower alkyl- or dialkylamino, cyano, trifluoromethyl, or
trifluoro-
CA 02296901 2000-O1-13
9
methylthio. Each phenyl group may carry one or more substituents.
The acid addition salts of the invention are pharmaceutically acceptable salts
of the
compounds of Formula I formed with non-toxic acids. Exemplary of such organic
s salts are those with malefic, fumaric, benzoic, ascorbic, embonic, succinic,
oxalic,
bis-methylenesalicylic, methanesulfonic, ethanedisulfonic, acetic, propionic,
tartaric,
salicylic, citric, gluconic, lactic, malic, mandelic, cinnamic, citraconic,
aspartic,
stearic, palmitic, itaconic, glycolic, p-amino-benzoic, glutamic, benzene
sulfonic and
theophylline acetic acids, as well as the 8-halotheophyllines, for example 8-
bromo-
~o theophylline. Exemplary of such inorganic salts are those with
hydrochloric, ,hydro-
bromic, sulfuric, sulfamic, phosphoric and nitric acids.
The movement disorders and motor disturbances which may be treated by the
preparation according to the invention are e.g. dystonia and tardive
dyskinesia and
is motor disturbances associated with Huntington's chorea or Tourette's
syndrome.
Dystonia may be acute or tardive and may be caused by neuroleptics or have
another reason.
Cerebrovascular diseases are such disorders caused by cerebral infarction,
2o cerebral hemorrhage, cerebral arteriosclerosis, subarachnoid hemorrhage,
cerebral
thrombosis, cerebral embolism, or the like, e.g. ischemia, hypoxia, anoxia.
The compounds of the invention have been found to displace tritiated di-tolyl
guanidine (DTG) from sigma sites in vitro with potencies better than about 40
nM,
2s and the great majority better than 1 nM, i.e. they are binding at the sigma
receptors
much more potently than sigma receptor ligands such as e.g. BMY 14802 and
rimcazole. Furthermore, most of the present compounds have proven to be very
selective ligands on the sigma receptors. For example as compared to the as
adrenoceptors and dopamine D2 receptors, the main part of the present
compounds
so have been found to show ratios of binding affinities (ICso alpha/sigma and
dopami-
ne/sigma, respectively) of 30-10000. Furthermore, the compounds have proven to
show very potent anxiolytic effects in an animal behaviour test in extremely
low
doses i.e. with ECso values in the ng - ug/kg range.
CA 02296901 2000-O1-13
When Z~ and Z2 are linked together, preferably at least one of Z~, Z2 and Z3
designates O or S, and more preferably Z3 is (CH2)~ wherein n is 0, and Z2 is
"O" or
"S" and Z~ is CH2 or Z~ and Z2 together represent CHZ-0-CH2. Other preferred
groups are those wherein Z~ is CH2, Z2 is O and Z3 is CH2; or Z3 is O and Z~-
Z2-is
s CH=CH; or Z~ is O, Z3 is O and Z2 is (CH2)~ where n =0,
Particularly preffered compounds are:
1'-(3-Adamantyloxy-1-propyl)spiro[3H2-benzopyran-3,4'-piperidine];
~0 1'-[4-(1-Benzyl-3-indolyl)-1-butyl]-spiro[iso,benzofuran-1 (3H),4'-
piperidine];
1'-(3-(3-Phenylimidazolidin-2-on-1-yl)-1-propyl)spiro[isobenzofuran-1 (3I~,4'-
piperidine];
1'-[4-[1-(4-Fluorophenyl)-3-indolyl]-1-butyl)-spiro[isobenzofuran-1 (3H),4'-
piperidine];
1,4-Dihydro-1'-[4-[1-(4-Fluorophenyl)-3-indolyl]-1-butyl]spiroj3H 2-benzopyran-
3,4'-
~s piperidine];
1'-(4-(1-p-Toluenesulfonyl-3-indolyl)-1-butyl)spiro[isobenzofuran-1 (3I->7,4'-
piperidine];
1'-[4-[5-Fluoro-1-(4-fluorophenyl)-3-indolyl]-1-butyl]spiro[isobenzofuran-1
(3N),4'-
piperidine];
6-Fluoro-1'-[4-[1-(4-fluoropheriyl)-3-indolyl]-1-butyl]spiro[isobenzofuran-1
(3H),4'-
2o piperidine];
1'-[4-[1-(4-Methylphenyl)-3-indolyl]-1-butyl]spiro[1 H-2-benzopyran-4(3H),4'-
piperidineJ;
1-(4-Fluorophenyl)-3-j4-(4-(4-fluorophenyl)-1-piperidyl)-1-butyl]indole;
1'-[4-(1-(2-Thiazolyl)-3-indolyl)-1-butyl]spiro[isobenzofuran-1 (3H),4'-
piperidine];
Zs 1'-[3-(5-Fluorobenzofuran-3-yl)-1-propyl]spiro[isobenzofuran-1 (3H),4'-
piperidine];
1'-[4-(5-Fluorobenzofuran-3-yl)-1-butyl)-spiro[isobenzofuran-1 (3H),4'-
piperidine];
1'-[4-[1-(4-Fluorophenyl)-5-trifluoromethylindazol-3-yl]-1-
butyl]spiro[isobenzofuran-
1 (3H),4'-piperidine];
4-Fluorophenyl-3-[4-(4-(4-fluorophenyl)-1-piperidinyl)-1-butyl]-5-
trifluoromethyl-
ao indazol;
1'-(4-(1,2-Benzisoxazol-3-yl)-1-butyl)spiro[isobenzofuran-1 (31,4'-
piperidine]; and
1'-[3-(Benzo[b]thiophen-3-ylthio)-1-propyl]spiro[isobenzofuran-1 (3H),4'-
piperidine].
CA 02296901 2000-O1-13
11
Some of the compounds used in accordance with the invention are novel, and
accor-
dingly in another aspect the present invention relates to novel piperidine
derivatives
having the general Formula I as defined above with the proviso that
if R1 is a group of the Formula II as defined above under b), Z1, Z2, and Z3
are .as
defined above under ii), X is NH, Y is CH and the dotted line emanating from Y
indicates a bond, then -Q1-U-GTR- may not be alkyl having less than 5 carbon
atoms;
if R1 is a group of the Formula II as defined above wherein Z3 is O, Z1 is
CH2, Z2 is
(CH2j~, n being 0, X is O, Y is N, the dotted line emanating from Y indicates
a
1o bond, and -Q1-U-Cx- indicates -(CH2)3, then R~ may not be fluoro;
if Z3 is O, 22 and Z1 are both CH2 and D-B-A-R is optionally substituted
phenethyl,
then R6 may not be methoxy; and
if Z1 is O, and Z2 and Z3 are both CH2, then D-B-A-R may not be phenethyl, or
optionally hydroxy substituted hexyl or heptyl.
The present invention also provides a pharmaceutical composition comprising at
least one novel piperidine compound according to the invention.as defined
above or
a pharmaceutically acceptable acid addition salt thereof or prodrug therefore
in
combination with one or more pharmaceutically acceptable carriers or diluents.
The pharmaceutical compositions of this invention or those which are
manufactured
in accordance with this invention may be administered by any suitable route,
for
example orally in the form of tablets, capsules, powders, syrups, etc., or
parenteral-
ly in the form of solutions for injection. For preparing such compositions
methods
2s well known in the art may be used, and any pharmaceutically acceptable
carriers,
diluents, exipients, or other additive usually used in in the art may be used.
Conveniently, the compounds of the invention are administered in unit dosage
form
containing said compound in an amount of about 0.01 to 50 mg.
The total daily dose usually ranges of about 0.05 - 100 mg, and most
preferably
about 0.1 to 20 mg of the active compound of the invention.
- CA 02296901 2000-O1-13
12
The compounds of Formula I may be prepared by:
a) reducing the amide carbonyl of a compound of Formula III
R2
R 2' ZZ O
v
N-~
Rs ~ ~ Za 'R»
R3
R5 R4 III
wherein R2 - R~ and Z1 - Z3 are as previously defined and R11 is such a group
that
CH2-R11 is a group comprised by the definition of R1;
b) alkylating a compound of Formula IV
R2
R Z~ ZZ
Rs ~ ~ Z3 NH
R3 .
Rs R4 (V
wherein R2 - R~ and Z1 - Z3 are as previously defined, with an alkylating
reagent of
the formula R1-V wherein R1 is as previously defined and V is a suitable
leaving
1s group such as halogen, mesylate or tosylate;
c) reductive alkylation of amines of Formula IV with aldehydes of the formula
R11-
CHO or carboxylic acids of the formula R11-COOH or ketones of the formula R12-
CO-R13 wherein R2-R~, R11, and Z1-Z3 are as previously defined and R12 and R1s
2o are such groups that R1z-CH-R13 is a group comprised by the definition of
R1;
d) reducing the C=Y1 double bond in a compound of Formula V
R2
R Z~ Z2 Ra
~N-GT2~ ,D' Rn
Rs ~ ~ Zs~ U I
R3 Y: \
Rs Ra X Rc V
Rd
- CA 02296901 2000-O1-13
13
wherein Ra-Rd, X, U, Q~, C~, R2-R~, and Z~-Z3 are as previously defined and Y~
is
CH or N;
e) oxidizing a compound of Formula VI to an oxo compound of Formula VII:
R2
R Z' ZZ Ra
Rs ~ ~ Za N ~~U~O~ ~ Rb
R3 ~ \ ( ---
Rs Ra X ~ Rc
Rd VI
Rz
R Z~ Zz Ra
~N-('12~U,0' ~ Re
Rs ~ ~ Z3
3
Rs Ra R O X ~Rc
Rd VII
wherein Ra-Rd, X, U, Q~, Q2, R2-R~, and Z~-Z3 are as previously defined;
io f) alkylating a compound of Formula VIII for obtaining a compound of
general
Formula I wherein X = NR~o; Rio' being lower alkyl, alkenyl, cycloalkyl,
cycloalkyl-
alkyl, cycloalkenyl or cycloalkenylalkyl,
R2
R Z~ Z2 Ra
Rs ~ ~ Z3 N ~?~U~~~ , ~ ' Rb
R3 Y~ N \ ~ c _.
Rs R4 H d R VIII
R
1s
wherein Ra-Rd, Y, U, Q~, Q2, R2-R~, and Z~-Z3 are as previously defined, with
an
alkylating reagent of the formula Rio=V~, wherein R1o'is as previously defined
and
V1 is a suitable leaving group, e.g. halogen, mesylate, or tosylate; or
acylating a
compound of the Formula VIII with an acylating reagent having the formula
R~o'=CO-
2o Hal, wherein Hal is halogen and R~o"is a group which together with CH2 form
the
group R~o', and subsequently reducing the acylated compound of Formula VIII;
CA 02296901 2000-O1-13
14
g) arylating a compound of Formula VIII for obtaining a compound of general
Formula I wherein X = NRIO'", R1o"' being optionally substituted phenyl or
heteroaryl,
with an arylating agent of the formula Ar-V1 wherein Ar is optionally
substituted
. phenyl or heteroaryl and V1 is as previously defined;
h) for obtaining a compound of general Formula I wherein X = N-CO-R1o' or N-CO-
Ar, wherein R1o' and Ar are as previously defined, acylating a compound of
Formula
VIII with an acylating agent of the formula R1o'-CO-Hal or Ar-CO-Hal. wherein
Hal is
as previously defined;
i) for obtaining a compound of general Formula I wherein X = N-S02-R1o' or N-
SOZ-
Ar, wherein R1o' and Ar are as previously defined, sulfonylating a compound of
Formula VIII with a sulfonylating agent of the formula R1o'-S02-Hal or Ar-S02-
Hal,
wherein Hal is as previously defined;
j) reductive alkylation of compounds of Formula VI11 with carbonyl compounds
of
formula R14-CO-R1$ wherein R14 and R~5 are such groups that R~4-CH-R~5 is a
group R1o as previously defined;
2o k) making a ring closure reaction of the hydrazone IX to the indazole
derivative X
RZ R'°-NH
Ra
R Z~ ZZ ~N
_ ~ R
s ~ ~ 3 \N C~~ U ~O ~ b base
R Z ~ ~ I
R3 Hal Rc
R5 R4 Rd IX
R2
R Z~ Z2 Ra
Rs ~ ~ Za N ~2\ U'Q' ~ Rb
I I
R3 N\
c
Rs Ra Rio d R
R
wherein Ra-Rd, U, Q1, Q2, R2-R~. Rlo, and Z1-Z3 are as previously defined and
Hal
Zs is halogen;
CA 02296901 2000-O1-13
I) reducing the quarternized pyridine derivative of Formula XI
R Zt R2
t_
Rs ~ ~ ~ 'N R~
Rs Ra ~R3 Xi
s in which Rt-R~. Zt and Vt are as previously defined;
whereupon the compound of Formula I is isolated as the free base or a
pharmaceuti-
cally acceptable acid addition salt thereof.
to The reduction according to method a) may preferably be carried out in an
inert
organic solvent such as diethyl ether or tetrahydrofuran in the presence of
lithium
aluminium hydride at reflux temperature.
The amides of Formula III are conveniently prepared by treating piperidine
deriva-
ts tives of Formula IV with suitable carboxylic. acid chlorides of formula Rt
t-COCI in
the presence of base (potassium carbonate or triethylamine). When Rt ~-CHZ
designates a group of formula -D-B-A-R the corresponding carboxylic acid
chlorides
of formula Rt t-COCI are either commercially available or prepared according
to
standard procedures.
When R»-CH2 designates a group of Formula Il the corresponding carboxylic acid
chlorides of Formula XII,
~~Q?.~ Dt Ra Rb
CI
Y~ X \ Rc
Rd XI~
2s
wherein Ra-Rd, X, Y, U, and Qt are as previously defined and Q2' is such a
group
that Q2-CHz is a group Q2 as previously defined, are prepared from the
correspond-
ing carboxylic acids by known methods.
CA 02296901 2000-O1-13
16
The piperidine derivatives of Formula IV where Z~ and Z2 are linked together,
are
prepared as follows:
Spiro[isobenzofuran-1 (31-1),4'-piperidine] according to the method described.
by
s Marxer et al, J. Org. Chem. 1975, 40, 1427.
2,3-Dihydro-spiro[1 N-indene-1,4'-piperidinej and 3,4-dihydro-spiro[naphtalene-
1 (2M
,4'-piperidine] according to the method described in French Patent. No.
1,335,831.
~0 1'-Methyl-spiro[benzo[cjthiophene-1 (3f-~,4'-piperidinej according to the
method
described by Parham et al, J. Org. Chem. 1976, 41, 2628. The corresponding
demethylated derivative was obtained by treatment with ethyl chloroformate
followed by alkaline hydrolysis of the intermediary ethyl carbamate.
1'-Phenylmethyl-spiro[1 H-2-benzopyran-4(3H),4'-piperidinej according to the
method described by Yamamoto et al, J. Med. Chem., 1981, 24, 194. The corres-
ponding debenzylated derivative is obtained by hydrogenation in the presence
of a
palladium catalyst.
20 3,4-Dihydro-1'-phenylmethyl-spiro[2H-1-benzopyran-2,4'-piperidinej
according to the
method described by Yamamoto et al, Chem. Pharm. Bull. 1981, 29, 3494. The cor-
responding debenzylated derivative is obtained by treatment with ethyl chloro-
formate followed by alkaline hydrolysis of the intermediary ethyl carbamate.
zs 1'-Phenylmethyl-spiro[2H-1-benzopyran-2,4'-piperidinej is obtained
according to the
method described by Yamamoto et al, Chem. Pharm. Bull. 1981, 29, 3494. The cor-
responding debenzylated derivative is obtained by hydrogenation in the
presence of
a palladium catalyst.
so 1'-Phenylmethyl-spiro[3H-2-benzopyran-3,4'-piperidine]-1 (41-1)-one
according to the
method described by Yamamoto et al, J. Med. Chem. 1981, 24, 194. Reduction
with
lithium aluminium hydride followed by treatment with phosphoric acid according
to
the procedure described by Marxer et al, J. Org. Chem. 1975, 40, 1427 yields
1,4-
~
CA 02296901 2000-O1-13
17
dihydro-1'-phenylmethyl-spiro[3H-2-benzopyran-3,4'-piperidine] which is
debenzyl-
ated by hydrogenation in the presence of a palladium catalyst.
1'-Benzylspiro[4H-1-benzopyran-4,4'-piperidine] is obtained by a method
analogous-
s ly to the method described in EP 0 4i 4 289 A1 for the synthesis of 1'-
benzyl-1,4-
dihydrospiro[naphtalene-1,4'-piperidine]. Hydrogenation in the presence of a
palladium catalyst gave 2,3-dihydrospiro[4N-1-benzopyran-4,4'-piperidine].
Spiro[1,3-benzodioxole-2,4'-piperidine] is obtained by refluxing 1-
ethoxycarbonyl-4-
o piperidinone and catechol in toluene solution in the presence of p-
toluenesulfonic
acid with continous removal of water followed by removal of the benzyl group
by
hydrogenation in the presence of a palladium catalyst.
The substituents Rz-R~ are introduced by applying suitably substituted
starting com-
as pounds to methods analogously to the above mentioned.
The piperidine derivatives of Formula IV where Z~ and Z2 are not linked are
prepared by known methods, see e.g. US Pat. No. 2,891,066; McElvain et al J.
Amer. Chem. Soc. 1950, 72, 3134; Bally et al Chem.Ber.1887, 20, 2590.
zo
Alkylation of a compound of Formula IV according to method b) is conveniently
performed in an inert organic solvent such as a suitably boiling alcohol or
ketone,
preferably in the presence of a base (potassium carbonate or triethyl amine)
at
reflux temperature:
zs
The alkylating reagents of formula R~-V where R~ designates -D-B-A-R wherein A
is
0, S, or a bond and D, B, and R are as previously defined, are prepared by
standard literature methods. The corresponding sulfoxides and sulfones are
obtained by oxidation of the sulfides according to methods well known in the
art.
' CA 02296901 2000-O1-13
t8
~o
W
Such alkylating agents in which A represents a group ~N~N~ wherein W is O
or S are prepared by the method disclosed in DE-OS No 2035370.
The preparations of alkylating reagents of formula R~-V where Rt designates a
s group having the general Formula II are illustrated by examples in the
following
reaction schemes. In the formulas of the reaction schemes Ra-Rd, V and Ar are
as
previously defined, and E designates a 1-piperidyl group of general Formula
IV.
Indenes of Formula II are conveniently prepared according to Scheme 1, in~
which
Ra- F~ and V are as previously defined.
Ra
Rb O
S~~~OCH3 Method 1
(\
/ '/ ~ ----
Rc O
Rd
Ra Ra
Rb OH p Rb OH
\ OCH3 Method ~ I ~ OH
Rc ~ ~ Rc v
Rd Rd
Ra
Rb ~ OH
Method 3 I / I , Method 4
Rc
Rd
Ra
Rb \ V
R° ~'
Rd
Scheme ~
CA 02296901 2000-O1-13
19
Method 1 is a conventional Reformatsky condensation performed with activated
zinc. Method 2 consists of a lithium aluminium hydride reduction pertormed in
an
inert organic solvent such as diethyl ether or tetrahydrofuran at reflux
temperature.
The elimination in Method 3 is preferably performed in a suitable alcohol,
e.g.
methanol, in the presence of a strong mineral acid, e.g. concentrated
hydrochloric
acid. Preferably, Method 4 is treatment with methanesulfonyl chloride in the
presence of methyl amine in dichloromethane, thus giving the corresponding
yo methanesulfonate, but may alternatively be conversion of the hydroxy group
to a
halogen by means of a suitable reagent, e.g. thionyl choride.
Indanes of Formula II are prepared according to Scheme 2, in which Ra - Rd and
V
are as previously defined.
CA 02296901 2000-O1-13
Ra
Rb \ O
OCH3
+ Br / Method 1a
R' O
Rd
Ra Ra
b
R ( \ I / OCH3 Rb \ / / OCH3
/ O +
Rc Rc ~ / O
Rd Rd
Method 2
Ra Ra
Rb \ OH Rb OH
\ /
Rc / Rc ~ /
Rd Rd
Ra
Method 4 Rb OH
\ y/ ~/
R'
Ra
Rd
Rb \ V Method 5
Ra
R' / Rb OH
. Rd ( \ v \/
Rc
Rd
Method 4
Ra 1
Rb V
\ ~ \i
/
Rc
Rd
Scheme 2
Method 7 a is analogous to Method 1 with the modification that elimination of
water
is performed directly on the crude product by means of concentrated
hydrochloric
acid. The mixture of isomers obtained is reduced according to Method 2. One of
the
CA 02296901 2000-O1-13
21
isomers obtained is isolated while the remaining mixture is hydrogenated in a
conventional Parr apparatus (Method 5) in the presence of a suitable noble
metal
catalyst, e.g. palladium or platinum.
s Indoles of Formula 11 are most conveniently prepared either from indole
alkanoic
acid, exemplified by indole butanoic acid as described in Eur. Pat. Appl. No.
376607, or according to Scheme 3, in which Ra - Rd, Ar and V are as previously
defined.
Ra Ra O
Rb \ O Rc O~ ~
Method 5 ~ \ " OEt
/
R° N~COOCH3 R~ / N~COOCH
3
Rd Ar Rd Ar
R8
O
Rb \
Method 6 I / I OEt
R~ N J
Rd Ar
Ra . Ra
Rb I \ I OOH Rb \ O~ V
/ Method 4 ~ /
R~ N J -.~ R~ N
Rd Ar Rd Ar
Scheme 3
The starting indoxyl ester is prepared by the method described in US Pat. No.
4,710,500 and references therein. The alkylation in Method 5 is performed in
an
inert, suitably boiling, organic solvent, e.g. an alcohol or a ketone,
preferably in the
is presence of a base (potassium carbonate or triethyl amine). The
decarboxylation in
Method 6 is preferably performed thermally, after hydrolysis of the diester to
the
diacid, in a suitable inert solvent, e.g. quinoline, dimethyl formamide, or N-
methyl-2-
pyrrolidinone (NMP) in the presence of copper.
- CA 02296901 2000-O1-13
22
Indol-3-yloxypropyl derivatives can also be obtained by heating a mixture of 3-
acetoxyindoles and 1,3-propylene glycol in the presence of sulfuric acid,
thereby
obtaining 3-(indol-3-yloxy)-1-propanol derivatives which are converted to th.e
s corresponding methanesulfonates or halides by Method 4.
Benzofuranes and 2,3-dihydrobenzofuranes of Formula I! are prepared according
to
Schemes 4 and 5, in which Ra - Rd and E are as previously defined.
Ra Ra
Rb COOH Rb
COOCH3
-Method;
R ~ -p- R~ y0i
Rd Rd
Ra
Rb COOCH3
Metho~ ~ ~ i Method 2
Rc / O ~ --.
Rd
Ra Ra
b
R ~ ~ i OH Rb ~ COOCH3
Method 9 I I
R O ~ ~ / ~ COOCH3
R O
Rd Rd
Ra
Rb COOH
Method 11
-s. i
R ~ of
Rd
Scheme 4
Method 7 is a conventional conversion to the methyl ester by treating the
carboxylic
. acid with thionyl chloride followed by addition of methanol to the
intermediate
carboxylic acid chloride. The 2,3-dihydrobenzofuranes are obtained by
reduction
1s with magnesium (Method 8) at this stage, while the benzofuranes are
obtained by
CA 02296901 2000-O1-13
23
skipping this step. Method 9 consists of conversion of the alcohol group to
the
corresponding chloride by means of thionyl chloride followed by treatment with
dimethyl malonate in a suitable solvent, e.g. NMP, in the presence of base,
preferably potassium tert-butoxide. Method 11 is similar to Method 6 except
the
s decarboxylation is performed without addition of copper.
Ra Ra
0
Rb ~ COON Rb
~ 'E
Method 12 ~ / ;
Rc ~~O ~ Rc O
Rd Rd
Method 2
Ra Ra
b R
R ~ ~ , ON b ~ CI
/ ~ Method 13 ~ '
Rc O Rc / OJ
Rd Rd ,
Method 4 ~ Method 14
Ra Ra
Rb Rb
~ ~ V ~ , v RCN
/ ;
Rc ~ ,0 Rc ~ ,O
Rd Rd
Method 15
Ra Ra
Rb ~ E Rb ~ __
'COON
Method 12
Rc / O J O r---- Rc / O J
Rd Rd
Scheme 5
Method 12 consists of a conventional conversion of the carboxylic acid to the
amide
via the carboxylic acid chloride. Prolongation of the side chain is
accomplished by
1o conversion of the hydroxy group to the chloride by treatment with
preferably thionyl
CA 02296901 2000-O1-13
24
chloride in dichloromethane in the presence of a few drops of dimethyl
formamide
(Method 7 3) followed by treatment with a cyanide salt, e.g. potassium
cyanide, in a
suitable aprotic dipolar solvent, preferably dimethyl sulfoxide, at 100 - 200
°C
(Method 14). Hydrolysis of the cyano group is accomplished with mineral acid.
at
elevated temperature (Method 15).
Benzo[bjthiophenes and 2,3-dihydrobenzo[bjthiophenes of Formula II are
prepared
by methods analogous to the methods described in Schemes 4 and 5. Benzo[bjthio-
phen-3-ylmethyloxyethyl derivatives are prepared from methyl benzo[b]thiophene-
3-
1o carboxylate by reduction to the methanol derivative with lithium aluminium
hydride
followed by alkylation with ethyl bromoacetate and subsequent reduction to
benzo[bjthiophen-3-ylmethyloxyethanol derivatives which are converted to the
target compounds via Method 4. Benzofuran-3-ylmethyloxyethyl derivatives are
prepared analogously.
Benzo[bjthiophene-S,S-dioxide derivatives are obtained .by oxidation of the
corres-
ponding benzo[bjthiophene derivatives according to standard literature
methods.
2,3-Dihydroindoles of Formula II are prepared by methods analogous to method
d).
Indolones of Formula II are prepared by methods analogous to method e).
2o Indazoles of Formula II are prepared by methods analogous to method j).
Reductive alkylation of amines of Formula IV according to method c) is
performed
by standard literature procedures. The aldehydes , carboxylic acids, and
ketones of
formulas R11-CHO, R11-COOH, and R12-CO-R13, respectively, are either commer-
cially available or are prepared according according to standard procedures or
according to methods analogous to the methods described in Schemes 1-5.
Reduction of the C=Y1 double bond according to method d) is conveniently per-
formed by catalytic hydrogenation in an alcohol with a platinum catalyst or by
so hydrogenation with diborane or a diborane precursor such as trirnethyl
amine or
dimethyl sulfide complex in tetrahydrofuran or dioxane from 0 °C to
reflux tempera-
ture, followed by acid catalyzed hydrolysis of the intermediate borane
derivative.
°
CA 02296901 2000-O1-13
Alternatively, the double bond can be reduced by sodium borohydride in
methanol in
the presence of trifluoroacetic acid (see e.g. Berger et a! J. Med. Chem. 7
977, 20,
600).
s Oxidation of a compound of Formula VI according to method d) is performed
according to Szabo-Pustay et al., Synthesis , 1979, 276.
Alkylation, acylation or sulfonylation of compounds of Formula VIII according
to
methods f), h) and i), respectively, are performed in an inert solvent such as
a
1o suitably boiling alcohol or ketone, preferably in the presence of a base
(potassium
carbonate or methyl amine). The alkylating reagents R1o'-V1, the acylating
reagents
R1o"-CO-V1, R1o'-CO-V1, and Ar-CO-V1, and the sulfonylation reagents R1o'-S02-
V1,
and Ar-S02-V1 respectively, are commercially available or are prepared by
standard
procedures.
Arylation of a compound of Formula VIII according to method g) is most
convenient-
ly performed by applying the well known Ullmann reaction. Tfie arylating
reagents
Ar-V1 are commercially available.
2o Reductive alkylation of compounds of Formula VIII according to method j)
are
performed by standard literature methods.
The ring closure of compounds of Formula IX according to method k) is most
conveniently performed by heating a compound of Formula IX in a suitable inert
2s organic solvent such as dimethyl formamide in the presence of a base,
preferably
potassium tent-butoxide.
Hydrazones of Formula IX are prepared according to Scheme 6, in which Ra--R~,
R1o and Hal are as previously defined.
CA 02296901 2000-O1-13
26
Ra Ra O .
Rb \ CN Rb
Method 16 ~ \ v v OOH
/
Rc Hal Rc / Hal
Rd Rd
Method 17
Method 18
Re R2 NHR~o
Rb O Z2 z' R~ b Ra N
\ ~(CHz)a"N/~ R
~Zs ~ ~ Rs I ~ v ~ OOH
Rc Hal Ra Rc / Hal
Rd Ra Rs Rd
Method 17
Ra ~NHR~° R2
Re N Z2 Zo R~
~(CHZ)4-N~Z3 ~ ~ Rs
Rc / Hal R3
Rd Ra Rs
Scheme 6
Method 16 is an addition of the Grignard reagent of 4-chloro-1-butanol to o-
halo-
benzonitriles under standard conditions for Grignard reactions. Conversion to
the
s hydrazone (Method 17) is accomplished by reflux in ethanol in the presence
of a
hydrazine. Alternatively, the hydroxy derivative resulting from Method 16 .may
be
converted to the amine via the corresponding mesylate analogous to Method 4
and
a) before conversion of the keto group.
The reduction according to method 1) is most conveniently performed by
catalytic
hydrogenation in an alcohol with a platinum catalyst. Compounds of Formulas V,
VI,
and VIII are prepared by methods a), b), j), k), or I). Compounds of Formula
XI are
prepared by treating a compound of formula R~-V~ with a 4-aryl-pyridine in a
suitably boiling alcohol.
CA 02296901 2000-O1-13
27
In the following the invention is further illustrated by examples which,
however, may
not be construed as limiting:
s
1'-Butylspiro[isobenzofuran-1 (3t-~,4'-piperidine], oxalate, 1 a.
A mixture of spiro[isobenzofuran-1 (3H),4'-piperidine] (2 g), n-butyl bromide
(4 g),
potassium carbonate (5 g), and potassium iodide (0.2 g) in 100 ml of 4-methyl-
2-
io pentanone was refluxed for 16 h. After cooling the reaction mixture was
washed
with 100 ml of water and concentrated in vacuo. The title compound
crystallized as
the oxalate salt from acetone by addition of oxalic acid. Recrystallized from
an
ethanol/ether mixture. Yield: 1.2 g, mp: 171-73 °C. -
~s In a similar manner was also prepared:
1'-Pentylspiro[isobenzofuran-1 (31-x,4'-piperidineJ, oxalate, 1 b, mp: 170-72
°C.
20 1'-(4-Phenyl-1-butyl)spiro[isobenzofuran-1 (3f~,4'-piperidineJ, fumarate,
2a
To an ice cooled solution of 4-phenyl-1-butanol (20 g) and methyl amine (15 g)
in
200 ml of dichloromethane, a solution of methanesulfonyl chloride (12 ml) in
50 ml
of dichloromethane was added dropwise. After stirring for 1 h at 10 °C
the reaction
2s mixture was washed with water. Extraction of the water phase with dichloro-
methane, drying of the combined organic phases over MgSO 4 followed by removal
of the volatile components in vacuo, gave 29 g of a slightly yellow oil, 4-
phenyl-1-
butyl methanesulfonate, which was sufficiently pure for use in the next step.
A mixture of spiro[isobenzofuran-1 (3H),4'-piperidine] (2 g), 4-phenyl-1-butyl
so methanesulfonate (6 g) and potassium carbonate (14 g) in 75 m) of 4-methyl-
2
pentanone was refluxed for 20 h. After cooling the reaction mixture was
filtered and
concentrated in vacuo. The remaining viscous oil was applied to a silica gel
column
(eluent: etherlmethanol/triethyl amine = 93:5:2) giving a colorless oil which
crystal-
CA 02296901 2000-O1-13
28
lined as the fumarate salt, 2a, from acetone by addition of fumaric acid.
Yield: 0.7
g, mp: 197-99 °C.
In a similar manner were also prepared:
s 1'-(4-Cyclohexyl-1-butyl)spiro[isobenzofuran-1(3H),4'-piperidineJ, oxalate,
2b, mp:
139-42 °C.
2,3-Dihydro-1'-(4-phenyl-1-butyl)spiro[1H-indene-1,4'-piperidine], fumarate,
2c, mp:
207-11 °C.
1'-(4-Phenyl-1-butyl)spiro[benzo[c]thiophene-1 (3H),4'-piperidine], maleate,
2d, mp:
to 176-77 °C.
3,4-Dihydro-1'-(4-phenyl-1-butyl)spiro[naphtalene-1 (2!-~,4'-piperidineJ,
fumarate, 2e,
mp: 191-93 °C.
1'-(4-Phenyl-1-butyl)spiro[1 H 2-benzopyran-4(3H),4'-piperidineJ, maleate, 2f,
mp:
169-70 °C.
~s 1,4-Dihydro-1'-(4-phenyl-1-butyl)spiro[3H-2-benzopyran-3,4'-piperidineJ,
maleate,
2g, mp: 152-53 °C.
3,4-Dihydro-1'-(4-phenyl-1-butyl)spiro[2H-1-benzopyran-2,4'-piperidineJ,
oxalate,
2h, mp: 155-57 °C.
1'-(4-Phenyl-1-butyl)spiro[2H 1-benzopyran-2,4'-piperidineJ, fumarate, 2i, mp:
184-
20 85 °C.
1'-(3-Cyclohexyloxy-1-propyl)spiro[isobenzofuran-1 (3H),4'-piperidine],
fumarate, 2j,
mp: 154-55 °C.
1'-(3-Phenoxy-1-propyl)spiro[isobenzofuran-1 (3H),4'-piperidine], 2k, mp: 143-
44 °C.
1'-(3-Phenoxy-1-propyl)spiro[3N-2-benzopyran-3,4'-piperidineJ, maleate, 21,
mp:
2s 171-73 °C.
1'-(3-Adamantyloxy-1-propyl)spiro[3H-2-benzopyran-3,4'-piperidineJ, maleate,-
2m,
mp: 221-24 °C.
1'-(3-Methylthio-1-propyl)spiro[isobenzofuran-1 (3H),4'-piperidineJ, oxalate,
2n, mp:
126-27 °.
so 1'-(3-Cyclohexylthio-1-propyl)spiro[isobenzofuran-1(3H),4'-piperidineJ,
oxalate, 20,
mp: 170-74 °C.
1'-(3-Phenylthio-1-propyl)spiro[isobenzofuran-1 (3H),4'-piperidine],
oxalate,2p, 152-
55 °C.
CA 02296901 2000-O1-13
29
1'-(3-Methylsulfonyl-1-propyl)spiro(isobenzofuran-1 (3/-x,4'-piperidinej, 2q,
mp: 163-
64 °C.
1'-(3-Cyclohexylsulfonyl-1-propyl)spiro[isobenzofuran-1 (31-x,4'-piperidine],
2r, mp:
118-20 °C.
s 1'-(3-Phenylsulfonyl-1-propyl)spiro[isobenzofuran-1(31,4'-piperidine]., 2s,
mp: 197-
202 °C.
8'-(4-Phenyl-1-butyl)spiro(isobenzofuran-1 (3I-~,3'-8-
azabicyclo[3,2,1]octane],
maleate, 2t, mp: 180-81 °C.
1'-[4-(3-Indolyl)-1-butyl]-spiro[isobenzofuran-1(3t~,4'-piperidine], 2u, mp:
150=55 °C.
io 1'-[4-(3-Indolyl)-1-butyl]-spiro[1 H-2-benzopyran-4(3H),4'-piperidinej,
oxalate, 2v,
mp: 222-25 °C.
1'-[5-(3-Indolyl)-1-pentyl]-spiro[1 H-2-benzopyran-4(3l->7,4'-piperidine],
oxalate, 2x,
mp: 145-46 °C. ,
1'-[6-(3-Indolyl)-1-hexylj-spiro[1 H-2-benzopyran-4(3f->7,4'-piperidinej,
oxalate, 2y,
~s mp: 117-18 °C.
1'-[4-(5,6-Dichloro-3-indolyl)-1-butyl]-spiro[isobenzofuran; 1 (3f-~,4'-
piperidine], 2z,
mp: 122-23 °C.
1'-[4-(5-Fluoro-3-indolyl)-1-butyl]-spiro[isobenzofuran-1 (31-x,4'-
piperidine], 2aa, mp:
185-87 °C.
20 1'-[4-(1-Methyl-3-indolyl)-1-butylj-spiro[isobenzofuran-1(3fn,4'-
piperidine], 2bb, mp:
101-2 °C.
3-[4-(4-Phenyl-1-piperidyl)-1-butyljindole, 2cc, mp: 131-32 °C.
3-[4-(4-(3,4-Dichlorophenyl)-1-piperidyl)-1-butyl]indole, 2dd, mp: 118-19
°C.
5,6-Dichloro-3-[4-(4-(4-fluorophenyl)-1-piperidyl)-1-butyl]-indole, Zee, mp:
120-21 °C.
2s 3-[6-(4-(4-Fluorophenyl)-1-piperidinyl)-1-hexyljindole, 2ff, mp: 90-91
°C.
3-[4-(4-(2-Methoxyphenyl)-1-piperidinyl)-1-butyl)indol, oxalate, 2gg, mp: 183-
88 °C.
1'-[4-(1-Benzyl-3-indolyl)-1-butyl]-spiro[isobenzofuran-1 (3f-~,4'-
piperidine], 2hh, mp:
166-68 °C.
3-[4-(4-(4-Fluorophenyl)-1-piperidyl)-1-butyljindol-2-one, 2ii, mp: 108-10
°C.
30 6-Fluoro-1'-(4-(3-indolyl)-1-butyl)spiro[isobenzofuran-1 (31-x,4'-
piperidinej, 2jj, mp:
189-91 °C.
CA 02296901 2000-O1-13
EXAMPLE 3
1'-(4-(3-Cyclohexylimidazolidin-2-on-1-yl)-1-butyl)spiro[isobenzofuran-1
(3M,4'-
piperidine], hydrochloride, 3a.
5
A mixture of 1-cyclohexyl-3-(4-chloro-1-butyl)-2-imidazolidinon (2.0 g,
prepared
according to the method described in Ger. Offen. 2035370), spiro[isobenzofuran-
1 (31-~,4'-piperidineJ (1.5 g), potassium carbonate (4.4 g), and potassium
iodide (0.1
g) in 60 ml of methyl isobutyl ketone was refluxed for 17 h. After cooling to
room
~o temperature the mixture was filtered and the solvent removed in vacuo. The
remaining oil was purified by column chromatography (silica gel, eluent: ethyl
acetate/heptane/triethyl amine = 9:1:1 ). The title compound crystallized as
the
hydrochloride from an acetone/ether mixture by addition of en etheral solution
of
HCI. Yield: 1.9 g, mp: 203-7 °C.
is
In a similar manner were also prepared:
1'-(2-(3-Phenylimidazolidin-2-on-1-yl)-1-ethyl)spiro[isobenzofuran-1 (3f~,4'-
piperidineJ, hydrochloride, 3b, mp: 151-54 °C.
1'-(3-(3-Phenylimidazolidin-2-on-1-yl)-1-propyl)spiro[isobenzofuran-1 (3l-~,4'-
20 piperidineJ, hydrochloride, 3c, mp: 232-50 °C.
1'-(2-(3-Cyclohexylimidazolidin-2-on-1-yl)-1-ethyl)spiro[isobenzofuran-1 (31-
~,4'-
piperidine], hydrochloride, 3d, mp: 160-61 °C:
1'-Propyl-spiro[isobenzofuran-1 (3f~,4'-piperidine], maleate, 4a.
To a mixture of spiro[isobenzofuran-1 (3I-~,4'-piperidine] (3 g), potassium
carbonate
( 5 g), and 100 ml of water in 100 ml of toluene, propionyl chloride (3 g) was
added
so dropwise. After stirring for 3 h at room temperature the toluene phase was
sepa-
rated, washed with water and concentrated in vacuo.
The remaining oil was dissolved in 100 ml of tetrahydrofuran and lithium
aluminium
hydride (1 g) was added. After 3 h of reflux the reaction mixture was cooled
and,
CA 02296901 2000-O1-13
31
successively, water (2 ml), 9 N NaOH (1 ml), and water (5 ml) was added. The
mixture was filtered and concentrated in vacuo and the titel compound
crystallized
as the maleate salt, 4a, from ethyl acetat by addition of malefic acid.
Recrystallized
from an acetone/ether mixture. Yield: 0.7 g, mp: 107-9 °C.
s
In a similar manner were also prepared:
1'-(5-Methyl-1-hexyl)-spiro(isobenzofuran-1 (3l-f),4'-piperidine], oxalate,
4b, mp: 162-
64 °C.
1'-(2-Phenyl-1-ethyl)-spiro[isobenzofuran-1 (3f-fj,4'-piperidine], maleate,
4c, mp: 161-
~0 63 °C.
1'-(3-Phenyl-1-propyi)-spiro[isobenzofuran-1(3f-~,4'-piperidine], maleate, 4d,
mp:
142-44 °C.
1'-(5-Phenyl-1-pentyl)-spiro[isobenzofuran-1 (31-x,4'-piperidine], oxalate,
4e, mp: 115-
17 °C.
15 1'-(6-Phenyl-1-hexyl)-spiro[isobenzofuran-1(31-r),4'-piperidine], oxalate,
4f, mp: 156-
57 °C.
1'-Octadecanyl-spiro[isobenzofuran-1 (3l-~,4'-piperidine], oxalate, 4g, mp:
208-10
°C.
20 SAMPLE 5
1'-[4-[1-(4-Fluorophenyl)-3-indolyl]-1-butyl]-spiro[isobenzofuran-1 (3t~,4'-
piperidineJ,
5a.
2s A mixture of 2u (3 g), 1-fluoro-4-iodobenzene (5 g), copper powder (0.5 g),
and
potassium carbonate (2 g) in 50 ml of NMP was kept at 160-170 °C for 5
h. After
filtration, water was added followed by extraction with ether. Removal of
solvent in
vacuo gave a red oil which was applied to a silica gel column (eluent: ethyl
acetate). The title compound, 5a, crystallized as the oxalate salt from
acetone by
so addition of oxalic acid. Yield: 1.3 g, mp: 169-70 °C.
In a similar manner were also prepared:
1,4-Dihydro-1'-[4-[1-(4-Fluorophenyl)-3-indolyl]-1-butyl]spiro[3N-2-benzopyran-
3,4'-
piperidineJ, maleate, 5b, mp: 142-43 °C.
CA 02296901 2000-O1-13
32
1'-[4-[1-(3-Thienyl)-3-indolyl]-1-butyl]spiro[isobenzofuran-1 (3f~,4'-
piperidine], 5c,
mp: 182-83 °C.
1'-[4-[1-(2-Thienyl)-3-indolyl]-1-butylJspiro[isobenzofuran-1 (31-x,4'-
piperidine], 5d,
mp: 198-202 °C.
s 1'-[4-[1-(3-Furanyl)-3-indolylJ-1-butyl]spiro[isobenzofuran-1(31-n,4'-
piperidine], 5e,
mp: 141-42 °C.
1-(4-Fluorophenyl)-3-[4-(4-phenyl-1-piperidyl)-1-butylJindole, oxalate, 5f,
mp: 171-
73 °C.
1'-[4-[1-(4-Pyridyl)-3-indolylJ-1-butylJspiro[isobenzofuran-1 (3f-~,4'-
piperidi ne],
~o oxalate, 5g, mp: 127-29 °C.
1'-(4-(1-Methanesulfonyl-3-indolyl)-1-butyl)spiro[isobenzofuran-1 (3f-~,4'-
piperidine],
oxalate, 6a.
A solution of NaOH (20 g) in water (20 ml) was cooled to 10 °C and a
solution of 4-
(3-indolyl)-1-butanol (4 g) in methylene chloride (60 ml) was added together
with
tetrabutylammonium hydrogensulfate (0.8 g). Methanesulfonyl chloride (2.5 ml)
in
2o methylene chloride (25 ml) was added dropwise at 15 °C followed by
stirring for 20
min. at room temperature. The phases were separated and the organic phase
washed with water. Drying over magnesium sulfate and removal of solvent in
vacuo gave an oil which was purified by column chromatography (silica gel,
eluent:
ether/methylene chloride/heptane = 1:1:1) giving 1.8 g of a heavy oil, 4-(1-
2s methanesulfonyl-3-indolyl)-1-butyl methanesulfonate which was converted to
the
title compound by the method described in EXAMPLE 2. The oxalate salt crystal-
lized from acetone by addition of oxalic acid. Yield: 1.0 g, mp: 83-85
°C.
In a similar manner were also prepared:
so 1'-(4-(1-p-Toluenesulfonyl-3-indolyl)-1-butyl)spiro[isobenzofuran-1(3!-x,4'-
piperidine],
oxalate, 6b, mp: 201-4 °C.
6-Fluoro-1'-(4-(1-(2-thienyl)sulfonyl-3-indolyl)-1-butyl)spiro[isobenzofuran-1
(3f~,4'-
piperidine], oxalate, 6c, mp: 184-86 °C.
CA 02296901 2000-O1-13
33
EXAMPLE 7
1'-(4-(1-Acetyl-3-indolyl)-1-butyl)spiro[isobenzofuran-1(3H),4'-piperidine],
oxalate, 7.
s A solution of acetyl chloride (0.8 ml) in methylene chloride (10 ml) was
added
dropwise at 15 °C to a mixture of 2u (1.8 g), sodium hydroxide (1 g),
and tetrabutyl-
ammonium hydrogensulfate (0.2 g) in methylene chloride (40 ml). After stirring
for
1 h at room temperature water was added, the organic phase separated and dried
over magnesium sulfate. Filtration and removal of solvent in vacuo gave a
viscous
oil which was purified by column chromatography (silica gel, eluent:
heptane/ethyl
acetate/triethyl amine = 60:40:4). The title compound crystallized as the
oxalate
salt from acetone by addition of oxalic acid. Yield: 0.45 g, mp: 139-40
°C.
1'-[3-[1-(4-Fluorophenyl)-3-indolyloxy)-1-propylJspiro[isobenzofuran-1 (31-
~,4'-
piperidine), oxalate, 8a.
A mixture of 3-acetyloxy-1-phenylindole (24 g), 1,3-dihydroxypropane (240 ml),
and
2o conc. sulfuric acid (10 ml) was heated to 80 °C for 2 h. Water was
added followed
by extraction with ether. The ether phase was dried over magnesium sulfate
followed by removal of solvent in vacuo giving 3-(1-phenyl-3-indolyloxy)-1-
propanol,
sufficiently pure for use in the next step.
The title compound was obtained by the method described in EXAMPLE 2 using
~s spiro[isobenzofuran-1 (3l-!),4'-piperidineJ and. crystallized as the
oxalate salt from
acetone by addition of oxalic acid. Yield: 2 g, mp: 151-54 °C.
In a similar manner were also prepared:
1'-(3-(6-Chloro-1-(4-fluorophenyl)-3-indolyloxyJ-1-propyl;spiro[isobenzofuran-
30 1 (31-r),4'-piperidine], oxalate, 8b, mp: 180-81 °C.
1'-[3-[5-Chloro-1-(4-fluorophenyl)-3-indolyloxy]-1-propyl]spiro[isobenzofuran-
1 (3f~,4'-piperidineJ, oxalate, 8c, mp: 115-18 °C.
CA 02296901 2000-O1-13
34
5-Chloro-1-(4-fluorophenyl)-3-[3-(4-(4-methylphenyl)-1-piperidinyl)-1-
propyloxyjindol, 8d, mp: 111-12 °C.
1'-[4-[1-(4-Fluorophenyl)-3-indolylj-1-butyljspiro[1 H 2-benzopyran-4(3f-~,4'-
piperidinej, maleate, 9a.
A tetrahydrofuran solution (500 ml) of methyl 4-(3-indolyl)-butyrate (103 g)
was
to added dropwise to a suspension of lithium aluminium hydride (25 g) in
tetrahydro-
furan (1000 ml) at 40 °C followed by stirring for 1 h at room
temperature. Usual
work-up gave 4-(3-indolyl)-1-butanol (96 g) as an oil. Arylation with 1-fluoro-
4-
iodobenzene according to the method described in EXAMPLE 5 yielded 4-[1-[4-
fluorophenyl)-3-indolyl]-1-butanol which was converted to the title compound
9a by
~s the method described in EXAMPLE 2 using spiro[1 H-2-benzopyran-4(31~,4'-
piperidine]. The maleate salt crystallized from acetone by addition of malefic
acid.
Yield: 1.5 g, mp: 189-90 °C.
In a similar manner were also prepared:
20 1'-[4-(5-Fluoro-1-(4-fluorophenyl)-3-indolylj-1-butyljspiro(isobenzofuran-1
(3l-~,4'-
piperidinej, oxalate, 9b, mp: 164-65 °C.
1'-(4-[1-(4-Fluorophenyl)-3-indolylJ-1-butyljspiro[benzo[cjthiophene-1 (3l-
~,4'-
piperidine], maleate, 9c, mp: 179=80 °C.
8'-[4-[1-(4-Fluorophenyl)-3-indolylJ-1-butylspiro[isobenzofuran-1 (31-x,3'-8-
2s azabicyclo[3,2,1joctanej, maleate, 9d, mp: 161-62 °C.
6-Fluoro-1'-[4-[1-(4-fluorophenyl)-3-indolylj-1-butyl]spiro[isobenzofuran-1
(3f-~,4'-
piperidinej, hydrochloride, 9e, mp: 227-31 °C.
1'-[4-[ 1-(4-Fluorophenyl)-3-indolylj-1-butylj-6-isopropylspiro(isobenzofuran-
1 (31~,4'-
pip~eridine], oxalate, 9f, mp: 129-44 °C.
so 7-Fluoro-1'-[4-[1-(4-fluorophenyl)-3-indolylj-1-butyljspiro[isobenzofuran-
1(3f-~,4'-
piperidinej, oxalate, 9g, mp: 186-89 °C.
1'-[4-[1-(4-Fluorophenyl)-3-indolylj-1-butyl]-5-methylspiro[isobenzofuran-1
(3f-fj,4'-
piperidine), oxalate, 9h, mp: 154-56 °C.
CA 02296901 2000-O1-13
1'-[4-[1-(4-Methylphenyl)-3-indolyl]-1-butyl]spiro[1 H-2-benzopyran-4(3!-~,4'-
piperidine], fumarate, 9i, mp: 186-88 °C.
- 1'-[4-[5-Fluoro-1-(3-thienyl)-3-indolyl]-1-butyl]-spiro[isobenzofuran-1 (3f-
~,4'-
piperidine], fumarate, 9j, mp: 184-86 °C. -
s 1'-[4-[1-(3-Pyridinyl)-3-indolyl]-1-butyl]spiro[1 H-2-benzopyran-4(3H),4'-
piperidine],
fumarate, 9k, mp: 185-87 °C.
1-(4-Fluorophenyl)-3-[4-(4-(4-fluorophenyl)-1-piperidyl)-1-butyl]indole,
oxalate, 91,
mp: 190-91 °C.
1-(4-Fluorophenyl)-3-[4-(4-(4-methylphenyl)-1-piperidyl)-1-butyl]indole,
maleate,
l0 9m, mp: 130-32 °C.
1-(4-Fluorophenyl)-3-[4-(4-(4-isopropylphenyl)-1-piperidyl)-1-butyl]indole,
maleate,
9n, mp: 160-62 °C.
1-(4-Fluorophenyl)-3-[4-(4-(4-dimethylaminophenyl)-1-piperidyl)-1-
butyl]indole,
fumarate, 90, mp: 180-82 °C.
~s 1-Phenyl-3-[4-(4-(4-fluorophenyl)-1-piperidyl)-1-butyl]indole, oxalate, 9p,
mp: 174-
76 °C.
1'-[4-(1-(2-Thiazolyl)-3-indolyl)-1-butyl]spiro[isobenzofuran-1 (3H),4'-
piperidine],
fumarate, 9q, mp: 165-67 °C
6-Trifluoromethyl-1'-[4-[ 1-(4-fluorophenyl)-3-indolyl]-1-butyl]spi
ro[isobenzofuran-
20 1 (3H),4'-piperidine], fumarate, 9r, mp: 100-105 °C.
4-Fluoro-1'-[4-[1-(4-fluorophenyl)-3-indolyl]-1-butyl]spiro[isobenzofuran-1
(31-~,4'-
piperidine], oxalate, 9s, mp: 160-63 °C.
1-(4-Fluorophenyl)-3-[4-(4-(3-trifluoromethylphenyl)-1-pi peridyl)-1-
butyl]indole,
maleate, 9t, mp: 112-13 °C.
2s 2,3-Dihydro-1'-[4-[1-(4-fluorophenyl)-3-indolyl]-1-butyl]spiro[4H 1-
benzopyran-4,4'-
piperidine], oxalate, 9u, mp: 187-92 °C.
6-Fluoro-1'-[4-[5-fluoro-1-(4-fluorophenyl)-3-indolyl]-1-
butyl]spiro[isobenzofuran-
1 (H),4'-piperidine], oxalate, 9v, mp: 144-46 °C.
ao EXAMPLE 10
1'-(4-(Benzo[b]thiophen-3-yl)-1-butyl)spiro[isobenzofuran-1 (3H),4'-
piperidine],
maleate, 10a.
CA 02296901 2000-O1-13
36
A~solution of methyl benzo[b]thiophen-3-ylacetate (69 g) in dry
tetrahydrofuran (100
ml) was added dropwise to a suspension of lithium aluminium hydride (10 g) in
dry
tetrahydrofuran (500 ml) at room temperature followed by reflux for 1 h.
Hydrolysis
with water, filtration and removal of solvent gave an oil which was applied to
a
s silica gel column (eluent: methylene chloride) giving 34.5 g of
benzo[b]thiophen-3-
ylethanol as an oil.
The product was dissolved in 200 ml of methylene chloride, thionyl chloride
(20 ml)
was added followed by reflux for 5 h. Removal of solvent and excess thionyl
chloride in vacuo gave 3-(2-chloro-1-ethyl)-benzo[b]thiophene as an oil (45
g).
~o The chloride was converted to 4-benzo[b]thiophen-3-yl-1-butanol via
treatment with
dimethyl malonate followed by hydrolysis, decarboxylation and reduction
according
to the method described in EXAMPLE 11.
The title compound 10a was prepared from 4-benzo[b]thiophen-3-yl-1-butanol and
spiro[isobenzofuran-1 (3I-~,4'-piperidine) by the method described in EXAMPLE
2.
15 Yield: 2.2 g, mp: 144-45 °C.
In a'similar manner were also prepared:
1,4-Dihydro-1'-(4-(benzo[b]thiophen-3-yl)-1-butyl)spiro[3H-2-benzopyran-3,4'-
piperidine], maleate, 10b, mp: 172-73 °C.
20 1'-(4-{5-Methylbenzo[b]thiophen-3-yl)-1-butyl)spiro[isobenzofuran-1 (3I-
~,4'-
piperidine], maleate, 10c, mp: 164-65 °C.
EXAMPLE 11
zs 2,3-Dihydro-5-fluoro-3-[3-(4-(4-fluorophenyl)-1-piperidinyl)-1-
propyl]benzofuran,
11 a.
To a suspension of 5-fluorobenzofuran-3-carboxylic acid (118 g) in 800 ml of
dichloromethane, thionyl chloride (200 ml) and dimethyl formamide (1 ml) were
so added. After reflux for 3 h the reaction mixture was concentrated in vacuo,
and the
remaining oil dissolved in 800 mi of dichloromethane. Methanol (1.5 I) was
slowly
added and the mixture stirred for 1 h. Removal of solvents in vacuo left
methyl 5-
fluorobenzofuran-3-carboxylate (125 g) as an oil.
CA 02296901 2000-O1-13
37
The oil was dissolved in methanol (1.8 I) and magnesium turnings (7 g) was
added.
After the reaction had started additional magnesium (80 g) was added
portionswise
during 1.5 h keeping the reaction temperature at 30-40 °C. The reaction
mixture
was stirred for 1 h followed by addition of aq. ammonium chloride. Extraction
with
s ether, drying the ether phase over sodium sulfate followed by removal of the
solvent in vacuo, left a viscous oil, methyl 2,3-dihydro-5-fluorobenzofuran-3-
carboxylate (120 g).
The oil was dissolved in 500 ml of dry ether and added dropwise to a
suspension
of lithium aluminium hydride (32 g) in 600 ml of dry ether. The mixture was
refluxed
~o for 3 h followed by hydrolysis with water. Filtration and removal of
solvent in vacuo
gave 2,3-dihydro-5-fluoro-3-hydroxymethyl-benzofuran (95 g) which was
converted
to 155 g of 2,3-dihydro-5-fluorobenzofuran-3-ylmethyl methanesulfonate by the
method described in EXAMPLE 2.
Dimethyl malonate (262 g) was dissolved in NMP (2 I) and potassium tert-
butoxide
~s (202 g) was added portionwise keeping the temperature at 15-20 °C.
The mixture
was heated to 60 °C and a solution of 2,3-dihydro-5-fl4orobenzofuran-3-
ylmethyl
methanesulfonate (155 g) in NMP (150 ml) was added dropwise. The mixture was
stirred for 4 h at 70-75 °C followed by addition of cold water.
Extraction with ether,
drying the ether phase over magnesium sulfate, followed by removal of solvent
in
2o vacuo gave dimethyl 2-(2,3-dihydro-5-fluorobenzofuran-3-ylmethyl)malonate
(160
g) as an oil, which was sufficiently pure for the further synthesis.
The oil was dissolved in 2 I of ethanol and a mixture of 120 g of solid
potassium
hydroxide and 200 ml of water was added followed by reflux for 2 h. The
reaction
mixture was concentrated in vacuo , water was added followed by extraction
with
2s ether. The water phase was acidified with concentrated hydrochloric acid
and
extracted with ethyl acetate. Drying of the organic phase over magnesium
sulfate
and removal of solvent in vacuo gave a viscous oil which was dissolved in NMP
(1
I) and kept at 150 °C for 2 h. Addition of water and extraction with
ether gave, after
drying over magnesium sulfate and removal of solvent in vacuo , 77 g of 3-(2,3-
30 dihydro-5-fluorobenzofuran-3-yl)-propionic acid. Reduction with lithium
aluminium
hydride by the method described above yielded 51 g of 3-(2,3-dihydro-5-fluoro-
benzofuran-3-yl)-1-propanol which was converted to 3-(2,3-dihydro-5-fluoro-ben-
zofuran-3-yl)-1-propyl methanesulfonate by the method described in EXAMPLE 2.
CA 02296901 2000-O1-13
38
The title compound, 11a, was prepared from 3-(2,3-dihydro-5-fluoro-benzofuran-
3-
yl)-1-propyl methanesulfonate (4.2 g) and 4-(4-fluorophenyl)piperidine (5.5 g)
by
the method described in EXAMPLE 2. Yield: 1.8 g, mp: 83-85 °C.
In a similar manner was also prepared:
s 1'-[3-(2,3-Dihydro-5-fluoro-benzofuran-3-yl)-1-propyl]spiro[isobenzofuran-1
(3l-~,4'-
piperidine],1'I b, mp: 62-63 °C.
l0 1'-[4-(2,3-Dihydro-5-fluoro-benzofuran-3-yl)-1-butyl]-spiro[isobenzofuran-1
(3f~,4'-
piperidine], oxalate,12.
3-(2,3-Dihydro-5-fluoro-benzofuran-3-yl)-1-propanol, prepared as described in
EX-
AMPLE 11, (51 g) was dissolved in 300 ml of dichloromethane and 0.5 ml of
~s dimethyl formamide was added. During 20 min 50 ml of thionyl chloride was
added dropwise followed by stirring for 3 h. Ice cold water was added, the
organic
phase separated, dried over magnesium sulfate and concentrated in vacuo giving
46 g of 3-(3-chloro-1-propyl)-2,3-dihydro-5-fluoro-benzofuran as an oil.
Sodium cyanide (12 g) was suspended in dirnethyl sulfoxide (180 ml) and heated
to
20 80 °C. A solution of 3-(3-chloro-1-propyl)-2,3-dihydro-5-fluoro-
benzofuran (42 g) in
40 ml of dimethyl sulfoxide was added dropwise followed by heating to 140
°C for
15 min. After cooling ether and water were added, the ether phase separated,
washed with water and dried over magnesium sulfate. Removal of solvent in
vacuo
left a viscous oil which was applied to a silica gel column giving 4-(2,3-
dihydro-5
2s fluoro-benzofuran-3-yl)butyronitrile as an oil (20 g).
The cyanide was dissolved in 100 ml of glacial acetic acid followed by
addition of
concentrated hydrochloric acid (200 ml). After reflux for 5 h, water was added
followed by extraction with ethyl acetate. Drying of the organic phase over
magne
sium sulfate and removal of solvent in vacuo gave 4-(2,3-dihydro-5-fluoro
so benzofuran-3-yl)butyric acid (20 g).
The acid (8 g) was dissolved in 50 ml of dichloromethane and 0.5 ml of
dimethyl
formamide was added. Thionyl chloride (20 ml) was added followed by reflux for
1.5 h. The reaction mixture was concentrated twice with heptane in vacuo
giving 4-
CA 02296901 2000-O1-13
39
(2,3-dihydro-5-fluoro-benzofuran-3-yl)-butyric acid chloride (7 g) as an oil.
A solution of spiro[isobenzofuran-1 (3f~,4'-piperidine) (2 g) and methyl amine
(3 ml)
in dichloromethane (50 ml) was cooled to 5 °C and a solution of 4-(2,3-
dihydro-5-
fluoro-benzofuran-3-yl)-butyric acid chloride (3 g) in dichloromethane (25 ml)
was
s added dropwise. After stirring for 1 h at room temperature the reaction
mixture was
washed with salt water and dried over magnesium sulfate. Removal of the
solvent
in vacuo gave 4.4 g of a viscous oil which was dissolved in 25 ml of dry
tetrahydro-
furan and added dropwise to a suspension of lithium aluminium hydride (2.6 g)
in
60 ml of dry tetrahydrofuran. The reaction mixture was heated to reflux for
2'h and
to hydrolyzed with water. Filtration and removal of solvent in vacuo gave a
viscous oil
which was applied to a silica gel column (eluent: heptane/ethyl acetate/
methyl
amine = 70:25:5) giving 2.9 g of the title compound, 12, which crystallized as
the
oxalate salt from acetone by addition of oxalic acid. Yield: 2.2 g, mp: 102-3
°C.
ss EXAMPLE 13
1'-[4-(2,3-Dihydro-3-indolyl)-1-butyl]spiro[1;3-benzodioxole-2,4'-piperidine],
oxalate,
13a.
2o To a solution of 25b ( 4 g) and BH3-NMe3 (10 g) in 100 ml of dioxane, conc.
hydrochloric acid (12 ml) was added. After stirring for 0.5 h the mixture was
refluxed for 2 h. The mixture was cooled to room temperature and 6 N
hydrochloric
acid (40 ml) was added followed by reflux for 1 h. The reaction mixture was
made
alkaline with aq. sodium hydroxide and extracted with dichloromethane. Drying
of
2s the organic phase over magnesium sulfate and removal of the solvent in
vacuo
gave an orange oil which was applied to a silica gel column (eluent: ethyl
acetate/-
heptane/triethyl amine = 70:28:2). The title compound, 13a, crystallized as
the
oxalate salt from acetone by addition of oxalic acid. Yield: 2.3 g, mp: 182-84
°C.
so In a similar manner were also prepared:
1'-[4-(2,3-Dihydro-3-indolyl)-1-butyljspiro[isobenzofuran-1 (3f~,4'-
piperidine],
oxalate, 13b, mp: 161-64 °C.
CA 02296901 2000-O1-13
1'-[4-(2,3-Dihydro-3-indolyl)-1-butyl]spiro[1 H-2-benzopyran-4(31-i),4'-
piperidineJ,oxalate, 13c, mp: 105-7 °C.
2,3-Dihydro-3-[4-(4-(2-methoxyphenyl)-1-piperidinyl)-1-butyl]indol, oxalate,
13d, mp:
160-62 °C.
s
1'-[2-[5-Chloro-1-(4-fluorophenyl)-3-indolyloxy]-1-ethyl]spiro[isobenzofuran-1
(3I-~,4'-
piperidine], oxalate,14a.
~o
To a solution of methyl 5-chloro-1-(4-fluorophenyl)-3-indolone-2-carboxylate
(50 g)
and potassium carbonate (40 g) in 500 ml of acetone, ethyl bromoacetate (35 g)
in
100 ml of acetone was added dropwise under reflux. After reflux for 6 h the
mixture
was filtered and the solvent removed in vacuo. Addition of water and
extraction
~s with ether gave, after drying over magnesium sulfate and removal of solvent
in
vacuo, a viscous oil, methyl 5-chloro-3-ethoxycarbonylmethoxy-1-(4-
fluorophenyl)-
indo(e-2-carboxylate, 62 g.
The oil was dissolved in 800 ml of ethanol and 30 g of potassium hydroxide
added.
Reflux for 4 h, addition of 4 I of crushed ice followed by acidification with
hydrochlo-
2o ric acid gave a colorless solid which was dissolved in 250 ml of NMP.
Copper
bronze (5 g) was added and the mixture kept at 200 °C for 4 h. Addition
of water
and extraction with ethyl acetate gave, after drying over magnesium sulfate
and
removal of solvent in vacuo, 27 g of 5-chloro-1-(4-fluorophenyl)-3-indolyloxy
acetic
acid.
2s The acid was dissolved in 500 ml of tetrahydrofuran and lithium aluminium
hydride
(4 g) was added. After reflux for 3 h, the reaction mixture was hydrolyzed
with
water, filtered, and concentrated in vacuo giving 18 g of 5-chloro-1-(4-
fluorophenyl)-
3-(2-hydroxyethyloxy)-indole as an oil.
The oil was dissolved in 250 ml of dichloromethane and triethyl amine (10 ml)
was
so added. Methanesulfonyl chloride (10 ml) was added dropwise at 0-5 °C
followed by
stirring for 4 h. The reaction mixture was washed with water, dried over
magnesium
sulfate and concentrated in vacuo, leaving 5-chloro-1-(4-fluorophenyl)-3-
indolyloxyethyl methanesulfonate (21 g) as a viscous oil.
- CA 02296901 2000-O1-13
41
1'-[2-[5-Chloro-1-(4-fluorophenyl)-3-indolyloxy]-1-ethyl]spiro[isobenzofuran-1
(3f->7,4'-
piperidineJ, 14a, was prepared from 5-chloro-1-(4-fluorophenyl)-3-
indolyloxyethyl
methanesulfonate (3 g) and spiro[isobenzofuran-1 (3H),4'-piperidine] by the
method
described in EXAMPLE 2 followed by purification by column chromatography
(silica
s gel, eluent: ethyl acetate). The title compound crystallized as the oxalate
salt from
acetone. Yield: 0.8 g, mp: 216-17 °C.
In a similar manner was also prepared:
5-Chloro-1-(4-fluorophenyl)-3-[2-(4-(4-fluorophenyl)-1-piperidinyl)-1-
ethyloxy]indol,
l0 14b, mp: 102-5 °C.
1'-[3-(5-Fluorobenzofuran-3-yl)-1-propyl]spiro[isobenzofuran-1 (31-~,4'-
piperidine],
~s oxalate, 15.
The title compound was prepared from 3-(5-fluorobenzofuran-3-yl) propionic
acid (3
g) and spiro[isobenzofuran-1 (3 h~,4'-piperidine] (2 g) by the method
described in EX-
AMPLE 12. 3-(5-Fluoro-benzofuran-3-yl)-propionic acid was prepared by a proce-
zo dure similar to the preparation of 3-(2,3-dihydro-5-fluorobenzofuran-3-yl)-
propionic
acid as described in EXAMPLE 11, but omitting the reduction with magnesium
turnings. The title compound, 8, crystallized as the oxalate salt from acetone
by
addition of oxalic acid. Yield: 2.6 g, mp: 157-59 °C.
2s EXAMPLE 16
1'-[4-(5-Fluorobenzofuran-3-yl)-1-butyl]-spiro[isobenzofuran-1 (3l-~,4'-
piperidine],
oxalate, 16.
3o The title compound was prepared from 4-(5-fluorobenzofuran-3-yl) butyric
acid (3.5
g) and spiro[isobenzofuran-1 (3l-~,4'-piperidine] (3 g) by the method
described in
EXAMPLE 12. 4-(5-Fluoro-benzofuran-3-yl) butyric acid was prepared by a
procedure analogous to the preparation of 4-(2,3-dihydro-5-fluoro-benzofuran-3-
yl)
CA 02296901 2000-O1-13
42
butyric acid as described in EXAMPLE 12, applying 3-(5-fluorobenzofuran-3-yl)-
1-
propanol instead of the corresponding dihydro analogue. 3-(5-Fluoro-benzofuran-
3-
yl)-1-propanol is prepared by the procedure described in EXAMPLE 11, but
omitting the reduction with magnesium turnings. The title compound, 16 ,
crystal-
s lized as the oxalate salt from acetone by addition of oxalic acid. Yield:
4.8 g, mp:
154-56 °C.
~0 1'-(4-(1-(4-Fluorophenyl)-5-trifluoromethylindazol-3-ylJ-1-
butylJspiro(isobenzofuran-
1 (31-x,4'-piperidine), 17a.
To a suspension of magnesium turnings (135 g) in 300 ml of dry
tetrahydrofuran,
ethyl bromide (140 g) dissolved in 500 ml of dry tetrahydrofuran was slowly
added
~s followed by reflux for 20 min. A solution of 4-chloro-1-butanol (274 g) in
500 ml of
tetrahydrofuran was added dropwise at reflux temperature. After stirring for
20 min,
the Grignard solution was filtered and added portionwise to a solution of 2-
chloro-4-
trifluoromethyl-benzonitril (200 g) in 600 ml of dry tetrahydrofuran. The
reaction
mixture, was stirred for 16 h at ,room temperature followed by addition of 2 N
2o hydrochloric acid and ice. Extraction with ether, drying of the ether phase
over
magnesium sulfate and removal of solvent in vacuo left a viscous oi! which was
applied to a silica gel column (eluent: dichloromethane/ether = 3:1) giving 4-
(2-
chloro-5-trifluoromethylbenzoyl)-1-butanol (101 g) as an oil.
The oil (80 g) was dissolved in 800 ml of ethanol and hydrazine hydrate (160
ml)
zs was added followed by refluxing for 20 h. The reaction mixture was cooled
and
concentrated in vacuo. Water was added followed by extraction with ether.
Drying
of the ether phase over magnesium sulfate and removal of solvent in vacuo gave
79 g of the hydrazone of 4-(2-chloro-5-trifluoromethyl-benzoyl)-1-butanol as
an oil.
The hydrazone (20 g) was dissolved in dimethyl formamide and potassium tert-
so butoxide (10 g) was added. The mixture was kept at 100-120 °C for 30
min
followed by addition of aq. ammonium chloride. Extraction with ethyl acetate,
drying
of the ethyl acetate phase over magesium sulfate, and removal of solvent in
vacuo
gave a viscous oil which was applied to a silica gel column (eluent: ethyl
acetate)
CA 02296901 2000-O1-13
43
giving crystalline 4-(5-trifluoromethyl-3-indazolyl)-1-butanol (2.7 g, mp: 177-
79 °C).
The indazole (2.7 g) was arylated with 1-fluoro-4-iodobenzene (5 g) by the
method
described in EXAMPLE 5 giving 4-[1-(4-fluorophenyl)-5-trifluoromethyl-3-
indazolylJ-
1-butanol (2.7 g, mp: 77-79 °C) which was converted to the
corresponding metha-
s nesulfonate by the method described in EXAMPLE 2. The title compound, 17a,
was prepared from the methanesulfonate (2 g) and spiro[iso-benzofuran-1 (3H
),4'-
piperidine] (2 g) by the method described in EXAMPLE 2. Yield: 1.7 g, mp: 74-
76°C.
1o In a similar manner was also prepared:
4-Fluorophenyl-3-[4-(4-(4-fluorophenyl)-1-piperidinyl)-1-butyl]-5-
trifluoromethyl-
indazol, 17b, mp: 124-25 °C.
1s
1'-[4-(5-Trifluoromethylindazol-3-yl)-1-butyl]-spiro[isobenzofuran-1 (3H),4'-
piperidine], 18.
4-(2-Chloro-5-trifluoromethylbenzoyl)-1-butanol, prepared as described in EXAM-
2o PLE 17, was converted to the corresponding methanesulfonate by the method
described in EXAMPLE 2. Treatment with spiro[isobenzofuran-1 (3H),4'-
piperidine]
according to the method described in EXAMPLE 2 gave 1'-[4-(2-chloro-5-
trifluoromethylbenzoyl)-1-butyl]-spiro[isobenzofuran-1(3H),4'-piperidine].
Conver-
sion to the corresponding hydrazone and ring closure according to the method
25 described in EXAMPLE 17 gave the title compound, 18, mp: 146-47 °C.
EXAMPLE 19
1'-(4-(1,2-Benzisoxazol-3-yl)-1-butyl)spiro[isobenzofuran-1 (3H),4'-
piperidine],
so oxalate, 19a, mp: 164-65 °C.
A solution of 1,2-benzisoxazole-3-acetic acid (prepared according to G. Casini
et al,
J. Het. Chem. 6, )969, 279) (18 g), ether saturated with dry HCI (150 ml) and
CA 02296901 2000-O1-13
44
methanol (200 ml) was stirred for 2 h at room temperature. Removal of the
volatiles in vacuo gave methyl 1,2-benzisoxazole-3-acetate (17 g) as an oil.
The oil was dissolved in tetrahydrofuran (100 ml) and added dropwise to a
suspen
sion of lithium aluminium hydride (6 g) in tetrahydrofuran (200 ml) at 0-10
°C
s followed by stirring for 30 min at 15 °C. Usual work-up gave 2-(1,2-
benzisoxazol-3
yl)ethanol (13 g) as an oil.
The ethanol derivative (13 g) was converted to the corresponding
methanesulfonate by the method described in EXAMPLE 2 (yield: 20 g}.
A solution of the methanesulfonate (20 g) in dimethyl sulfoxide (20 ml) was
added
to to a suspension of sodium cyanide (15 g) in dimethyl sulfoxide (40 ml) at
70 °C
followed by stirring for 30 min at 70-80 °C. Water and ether was added,
the phases
separated and the ether phase dried over magnesium sulfate. Removal of solvent
in vacuo gave 3-(1,2-benzisoxazol-3-yl)propionitrile a solid (13 g, mp: 67
°C).
The nitrite was dissolved in methanol (200 ml) and HCI-saturated ether (200
ml)
~s was added followed by stirring for 16 h at room temperature. The reaction
mixture
was concentrated in vacuo, water and ether added, arid the phases separated.
Drying of the ether phase over magnesium sulfate and removal of solvent in
vacuo
gave~methyl 3-(1,2-benzisoxazo!-3-yl)propionate (13 g) as an oil.
By repeating the above mentioned steps the 3-(1,2-benzisoxazol-3-yl)propionate
2o was converted to methyl 4-(1,2-benzisoxazol-3-yl)butyrate which was reduced
with
lithium aluminium hydride according to the procedure described above to 4-(1,2
benzisoxazol-3-yl)-1-butanol. By the method described in EXAMPLE 2, 4-(1,2
benzisoxazol-3-yl)-1-butyl methanesulfonate was obtained.
The title compound 19a was obtained from 4-(1,2-benzisoxazol-3-yl)-1-butyl
2s methanesulfonate (3.4 g) and spiro[isobenzofuran-1 (3N),4'-piperidine) (2
g) by the
procedure described in EXAMPLE 2. The oxalate salt crystallized from acetone
by
addition of oxalic acid. Yield: 2.4 g, mp: 164-65 °C.
In a similar manner were also prepared: '
3-[4-(4-(4-Fluorophenyl)-1-piperidyl)-1-butyl]-1,2-benzisoxazole, oxalate,
19b, mp:
so 174-75 °C.
1-(4-(1,2-Benzisoxazol-3-yl)-1-butyl)spiro[3H-2-benzopyran-3,4'-piperidine],
oxalate,
19c, mp: 162-63 °C.
CA 02296901 2000-O1-13
3-[4-(4-(2,6-Dichlorophenyl)-1-piperidyl)-1-butyl] -1,2-benzisoxazol,
fumarate, y9d,
mp: 196-97 °C.
s
1'-(3-(1,2-Benzisoxazol-3-yl)-1-propyl)spiro[isobenzofuran-1 (3!-x,4'-
piperidine],
oxalate, 20, mp: 131-32 °C.
The title compound 20 was prepared from 3-(1,2-benzisoxazol-3-yl)-1-propanbl
(3.2
1o g, prepared as described in EXAMPLE 19 and spiro[isobenzofuran-1 (3 I-~,4'-
pipe-
ridine] (2 g) by the method described in EXAMPLE 2. The product crystallized
as
the oxalate salt from acetone by addition of oxalic acid. Yield: 2.3 g, mp:
131-32°C.
3-[3-(4-(4-Fluorophenyl)-piperidin-1-yl)-1-propyloxy]-1,2-benzisothiazole, 21.
A mixture of 4-(4-fluorophenyl)-piperidine (15 g), ethyl 3-bromopropionate (20
g),
and potassium carbonate (14 g) in methyl isobutyl ketone was refluxed for 16
h.
2o Filtration and removal of solvent in vacuo gave 26 g of crude ethyl 3-(4-(4-
fluorophenyl)-1-piperidyl)propionate as an oil which was used directly in the
next
step.
The oil was dissolved in dry tetrahydrofuran (70 ml) and added dropwise to a
suspension of lithium aluminium hydride (6.5 g) in dry tetrahydrofuran (250
ml) at
2s 15 °C under nitrogen gas. After stirring for 30 min at room
temperature, water (6.5
ml), 10 N sodium hydroxide (7 ml), and water (30 ml) were added, subsequently.
Filtration and removal of solvent gave 20 g of crude 3-(4-(4-fluorophenyl)-1-
piperidyl)-1-propanol as an oil which was sufficiently pure for use in the
next step.
A solution of 3-(4-(4-fluorophenyl)-1-piperidyl)-1-propanol (10 g) in dry
toluene (150
so ml) was treated portionwise with a 50% xylene suspension of sodium hydride
(3 g).
A solution of 3-chloro-1,2-benzisothiazole (3.6 g) in dry toluene (30 ml) was
added
dropwise at room temperature followed by stirring for 1.5 h at room
temperature.
Ice was added, the phases separated and the aqeous phase extracted with ether.
CA 02296901 2000-O1-13
46
Drying of the combined organic phases over magnesium sulfate and removal of
solvents in vacuo gave an oil which was applied to column chromatography
(silica
gel, eluent: ethyl acetate/heptane/triethyl amine = 50:50:4). The title
compound 21
crystallized from a mixture of isopropyl ether/heptane. Yield: 1.5 g, mp: 91-
92 °C.~
1'-(4-(1,2-Benzisothiazol-3-yl)-1-butyl)spiro[isobenzofuran-1 (3M,4'-
piperidine],
oxalate, 22.
~o
4-(1,2-Benzisothiazol-3-yl)butyric acid (C. Branca et al, Phytochemistry 14,
1975,
2545) (17 g) was dissolved in dry toluene (500 ml) and cooled to -10
°C. Di-tert -
butyl aluminium hydride (120 ml of an 1 M solution in toluene) was added
dropwise
at -10 °C followed by stirring for 2 h at room temperature. Dilute
sulfuric acid (2 M,
is 300 ml) was added, the phases separated, the aqeous phase extracted with
ether,
and the combined organic phases dried over magnesium sulfate. Removal of
solvents in vacuo left a viscous oil which was purified by column
chromatography
(silica gel, eluent: ether). 4-(1,2-Benzisothiazol-3-yl)-1-butanol (5.2 g) was
obtained
as an oil.
zo The title compound 22 was obtained from 4-(1,2-benzisothiazol-3-yl)-1-
butanol and
spiro[isobenzofuran-1 (3f-~,4'-piperidine] by the method described in EXAMPLE
2.
The oxalate salt crystallized from acetone by addition of oxalic acid. Yield:
2 g, mp:
151-52 °C.
2s EXAMPLE 23
1-(4-Fluorophenyl)-3-[4-[3-(4-fluorophenyl)-8-azabicyclo[3,2,1 ]-oct-2-en-8-
yl]-1-
butyl]-indol, 23.
ao A mixture of dry ether (600 ml) and 15% BuLi in hexane (500 ml) was cooled
to -45
°C. A solution of 4-bromo-1-fluorobenzene (145 g) in dry ether (350 ml)
was added
dropwise at -45 °C followed by stirring for 1 h. A solution of 8-methyl-
8-
azabicyclo[3,2,1]octan-3-one (85 g) in dry ether (400 ml) was added dropwise
at -
CA 02296901 2000-O1-13
47
50 °C followed by stirring for 30 min, the temperature raising to -20
°C. The
reaction mixture was poured into 2 M hydrochloric acid and the phases
separated.
The ether phase was extracted with 2 M hydrochloric acid and the combined
aqeous phases made alkaline with aqeous NaOH. Extraction with ethyl acetate,
s drying of the organic phase over magnesium sulfate and removal of solvent in
vacuo, gave 3-(4-fluorophenyl)-3-hydroxy-8-methyl-8-azabicyclo[3,2,1 ]octane
(96 g)
as a solid, mp: 169 °C.
The product was dissolved in trifluoroacetic acid (500 ml) followed by reflux
for 1 h.
The reaction mixture was concentrated in vacuo, water added and the mixture
~o made alkaline with aqeous NaOH (pH > 9). Extraction with ethyl acetate,
drying of
the organic phase over magnesium sulfate, and removal of solvent in vacuo gave
3-
(4-fluorophenyl)-8-methyl-8-azabicyclo(3,2,1]oct-2-ene as a solid (91 g, mp:
62-63
°C).
The product was dissolved in 1,1,1-trichloroethane (550 ml) and heated to 70
°C. A
is solution of 2,2,2-trichloroethyl chloroformate (14 ml) in 1,1,1-
trichloroethane (25 ml)
was added dropwise at 70 °C followed by reflux .for 1 h. Additional
2,2,2-
trichloroethyl chloroformate (24 ml) was added followed by reflux for 5 h.
Removal
of volatiles in vacuo and purification by column chromatography (silica gel,
eluent:
methylene chloride) gave 3-(4-fluorophenyl)-8-(2,2,2-
trichloroethyloxycarbonyl)-8-
2o azabicyclo[3,2,1]oct-2-ene (59 g) as an oil.
(4-Fluorophenyl)-8-(2,2,2-trichloroethyloxycarbonyl)-8-azabicyclo[3,2,1]oct-2-
ene
(17 g) was dissolved in glacial acetic acid (170 ml) followed by addition of
water
(20 ml). The mixture was heated to 50 °C and zink dust (40 g) was added
in
portions. After stirring for 2 h at 50 °C the mixture was filtered and
concentrated in
2s vacuo. Water was added and the mixture made alkaline with aqeous NaOH.
Extraction with ethyl acetate, drying of the organic phase over magnesium
sulfate
and removal of solvent in vacuo left 3-(4-fluorophenyl)-8-azabicyclo(3,2,1]oct-
2-ene
(7 g) as an oil.
The title compound 23 was prepared from 3-(4-fluorophenyl)-8-azabicyclo[3,2,i
]oct-
so 2-ene according to the method described in EXAMPLE 9. Yield: 1.9 g, mp: 74-
75°C.
CA 02296901 2000-O1-13
48
EXAMPLE 24
3-[4-(3-(4-Fluorophenyl)-8-azabicyclo[3.2.1 ]octan-8-yl]-1-butyl]-1,2-
benzisoxazole,
oxalate, 24, mp 169-170.
A solution of 3-(4-fluorophenyl)-8-azabicyclo[3,2,1]oct-2-ene (10 g) was
dissolved in
glacial acetic acid (150 ml). Platinum oxide (0.5 g) was added followed by
treat-
ment with hydrogen gas at 3 atm of pressure in a conventional Parr apparatus.
Filtration and removal of solvent in vacuo left a viscous oil. Water was
adde'ti and
~o the mixture made alkaline (pH > 9) with aqeous NaOH. Extraction with ethyl
acetate, drying of the organic phase over magnesium sulfate and removal of
solvent in vacuo gave 3-(4-fluorophenyl)-8-azabicyclo[3,2,1]-octane (9 g) as
an oil.
The title compound 24 was obtained from 3-(4-fluorophenyl)-8-
azabicyclo[3,2,1 ]octane and 4-(1,2-benzisoxazol-3-yl)-1-butyl
methanesulfonate
~s (prepared as described in EXAMPLE 19) by the method described in EXAMPLE 2.
The oxalate salt crystallized from acetone by addition of oxalic acid. Yield.
1.1 g,
mp: 169-70 °C.
EXAMPLE 25
1'-(4-Phenyl-1-butyl)-spiro[1,3-benzodioxole-2,4'-piperidine], maleate, 25a.
A mixture of 1-ethoxycarbonyl-4-piperidinone (17 g), pyrocatechol (13 g) and p-
toluenesulfonic acid (2.5 g) in 250 ml of dry toluene was refluxed with
continous
2s removal of water. After 3 h the reaction mixture was concentrated in vacuo
, 2%
NaOH (200 ml) was added followed by extraction with dichloromethane. After
drying over magnesium sulfate and removal of solvent in vacuo , the remaining
red
oil was applied to a silica gel column (eluent: ethyl acetate/ heptane = 1:1 )
giving
22 g of a slightly yellow oil, 1'-ethoxycarbonyl-spiro[1,3-benzodioxole-2,4'
ao piperidine].
The oil was dissolved in ethanol (250 ml), NaOH (10 g) and water (20 ml) were
added and the mixture refluxed for 20 h. Removal of the solvents in vacuo ,
addition of salt water followed by extraction with dichloromethane gave, after
drying
CA 02296901 2000-O1-13
49
the organic phase over magnesium sulfate and removal of the solvent in vacuo,
a
red oil which was applied to a silica gel column (eluent: ethyl
acetate/methanol/tri-
ethylamine = 4:5:1) yielding 10 g of colorless crystals, spiro[1,3-
benzodioxole-2,4'-
piperidine], mp: 108-10 °C.
s The title compound, 25a, prepared from 4-phenyl-1-butyl methanesulfonate
(2.3 g)
and spiro[1,3-benzodioxole-2,4'-piperidinej (1.9 g) according to the method
describ-
ed in EXAMPLE 2, crystallized as the maleate salt from acetone by addition of
malefic acid. Yield: 1.6 g, mp: 156-57 °C.
~o In a similar manner was also prepared:
1'-[4-(3-Indolyl)-1-butyl]-spiro[1,3-benzodioxole-2,4'-piperidine], 25b, mp:
144-49 °C.
~s 1'-[4-(4-Fluorophenyl)-4-hydroxy-1-butyl)-spiro[isobenzofuran-1 (31-x,4'-
piperidine],
oxalate, 26a.
1'-(3-(4-Fluorobenzoyl)-1-propyl)-spiro[isobenzofuran-1 (3i-~,4'-piperidine],
oxalate
(2 g), obtained from 1-chloro-3-(4-fluorobenzoyl)-propane and
spiro[isobenzofuran-
20 1 (3l-i),4'-piperidinej by the method described in EXAMPLE 1, was dissolved
in 100
ml of EtOH. Platinum oxide (0.1 g) was added and the mixture was hydrogenated
in a Parr apparatus at 3 atm of hydrogen pressure for 16 h. Filtration and
concen-
tration in vacuo gave, after addition of a mixture of ethyl acetate/acetone,
crystal-
fine 26a. Yield: 1.5 g, mp: 75-80 °C.
In a similar manner were also prepared:
1'-(4-Phenyl-4-hydroxy-1-butyl)-spiro[isobenzofuran-1 (3f-~,4'-piperidinej,
26b, mp:
139-40 °C.
1'-(4-Cyclohexyl-4-hydroxy-1-butyl)spiro[isobenzofuran-1 (31,4'-piperidine],
oxalate,
so 26c, mp: 87-89 °C.
CA 02296901 2000-O1-13
EXAMPLE 27
1'-(4-(1 H-Inden-3-yl)-1-butyl)-spiro[isobenzofuran-1 (3f-/~,4'-piperidine],
27.
s A suspension of activated zinc (80 g) in dry tetrahydrofuran (200 ml) was
heated to
reflux and a few crystals of iodine was added. A solution of 1-indanone (100
g) and
methyl 4-bromo-crotonate (200 g) in 500 ml of dry tetrahydrofuran was added
dropwise followed by reflux for 1 h. After cooling, ice and aq. ammonium
chloride
was added followed by extraction with dichloromethane. Removal of the solvent
in
io vacuo gave a viscous oil which was purified on a silica gel column (eluent:
dichloro-
methane/ether = 1:1 ) giving methyl 4-(1-hydroxyindan-1-yl)-crotonate (78 g).
A solution of the crotonate (20 g) was dissolved in 200 ml dry ether and added
dropwise to a suspension of lithium aluminium hydride (10 g) in 150 ml of-dry
ether. After reflux for 1 h, the reaction mixture was hydrolyzed with water.
Filtration,
is drying of the ether phase over magnesium sulfate, and removal of solvent in
vacuo
gave 4-(1-hydroxyindan-1-yl)-1-butanol (18 g) as an oil.
The alcohol (16 g) was dissolved in methanol (250 ml) and concentrated
hydrochlo-
ric acid (40 ml) was added. After stirring for 30 min at room temperature, the
reaction mixture was concentrated in vacuo, water was added and the mixture
Zo extracted with ether. Drying of the ether phase over magnesium sulfate and
removal of solvent in vacuo gave a viscous oil which was applied to a silica
gel
column (eluent: dichloromethane/ether = 9:1) giving 4-((1!-~inden-3-yl)-1-
butanol
(1.8 g).
By the method described in EXAMPLE 2, the alcohol was converted to the
2s corresponding methanesulfonate and the title compound, 27, was obtained
using
spiro[isobenzofuran-1 (31-i),4'-pi-peridineJ, mp: 79-80 °C.
so 1'-[4-(Indan-1-yl)-1-butyl]-spiro[isobenzofuran-1 (3f-~,4'-piperidineJ,
oxalate 28.
A suspension of activated zinc (40 g) in dry tetrahydrofuran (150 ml) was
heated to
reflux and a few crystals of iodine was added. A solution of 1-indanone (50 g)
and
CA 02296901 2000-O1-13
51
methyl 4-bromo-crotonate (100 g) in 400 ml of dry tetrahydrofuran was added
dropwise followed by reflux for 1 h. After cooling, ice was added and the pH
adjusted to 1 with concentrated hydrochloric acid followed by extraction with
dichloromethane. Concentration of the organic phase left a viscous oil which
was
s dissolved in methanol (400 ml). Concentrated hydrochloric acid (100 ml) was
added and the mixture stirred for 30 min. Removal of solvents in vacuo,
addition of
water, and extraction with ether gave, after drying over magnesium sulfate and
removal of solvent in vacuo, an oil (34 g). The oil was dissolved in 300 ml of
dry
ether and added dropwise to a suspension of lithium aluminium hydride (21 g).
~o After reflux for 2 h, the reaction mixture was hydrolyzed with water.
Filtration and
removal of solvent in vacuo gave a mixture of isomers from which 4-(1-indanyl)-
but-
3-en-1-of (6 g) could be isolated by column chromatography (silica gel,
eluent:
dichloromethane/ether = 9:1 ). The remaining mixture of isomers (9 g) was dis-
solved in 150 ml of methanol and hydrogenated at 3 atm of hydrogen pressure in
~s a conventional Parr apparatus in the presence of 5% palladium on charcoal
(6 g)
for 12 h. Filtration and removal of solvent in vacuo gave 6 g of.4-(1-indanyl)-
1-
butanol.
The alcohol was converted to the title compound; 28, viavthe corresponding
methanesulfonate following the procedures described ~in EXAMPLE 2 using
2o spiro[isobenzofuran-1 (31,4'-piperidine], mp: 114-15 °C.
EXAMPLE ~,,9
1'-[4-( 1-Indanyl)-but-3-en-1-yl]-spiro[isobenzofuran-1 (3f-!),4'-
2s piperidine], oxalate, 29.
4-(1-Indanyl)-but-3-en-1-ol, prepared as described in EXAMPLE 28, was
converted
to the corresponding methanesulfonate by the method described in EXAMPLE 2.
The title compound was prepared by the method described in EXAMPLE 2 using
ao spiro[isobenzofuran-1 (3I-i),4'-piperidine), mp: 108-9 °C.
CA 02296901 2000-O1-13
52
1'-(4-(2,3-Dihydro-1-(4-fluorophenyl)-3-indolyl)-1-butyl)spiro[isobenzofuran-1
(31~,4'-
s piperidine], oxalate, 30.
To a solution of 5a (2 g) in trifluoroacetic acid (30 ml), sodium
cyanborohydride (0.5
g) in methanol {25 ml) was added dropwise. After stirring for 2 h at room
tempera-
ture the mixture was concentrated in vacuo , ethyl acetate (50 ml) was added
~o followed by washing with 2 N sodium hydroxide (2 x 50 ml). Drying of the
organic
phase over sodium sulfate and removal of solvent in vacuo gave the title base
which crystallized as the oxalate salt from acetone by addition of oxalic
acid. Yield:
0.7 g, mp: 172-73 °C.
is EXAMPLE 31
1'-[3-(Benzo[b]thiophen-3-ylthio)-1-propyl]spiro[isobenzofuran-1 (31-x,4'-
piperidine],
maleate,3la.
zo A solution of benzo[b]thiophen-3-one (20 g), 3-mercaptopropionic acid (25
ml), 2 N
hydrochloric acid (3 ml) in xylene (80 ml) was refluxed for 18 h. Water (100
ml) and
ether (300 ml) were added and the phases separated. The ether phase was
extracted with 150 ml 2 N NaOH followed by acidification of the alkaline phase
with
conc. hydrochloric acid. Extraction with ether, drying of the ether phase over
zs magnesium sulfate and removal of solvent gave 15.6 g of a viscous oil, 3-
(benzo[b]thiophen-3-ylthio)-propionic acid.
The acid was dissolved in 100 ml of tetrahydrofuran and added dropwise to a
suspension of lithium aluminium hydride (4 g) in tetrahydrofuran (150 ml).
After
reflux for 3 h, the reaction was quenched with water followed by usual work-up
ao giving 12.7 g of 3-(benzo[b]thiophen-3-ylthio)-1-propanol as an oil.
The title compound 31 a was obtained by the method described in EXAMPLE 2
using spiro[isobenzofuran-1 (3t-i),4'-piperidine] and crystallized as the
maleate from
acetone by addition of malefic acid. Yield: 0.9 g, mp: 154-55 °C.
CA 02296901 2000-O1-13
53
In a similar manner was also prepared:
1'-(3-(Benzo[b]thiophen-3-ylthio)-1-propyl]spiro(3H-2-benzopyran-3,4'-
piperidine],
maleate, 31 b, mp: 169-70 °C.
1'-[4-(2,3-Dihydro-benzo[b]thiophen-3-yliden)-1-butyl]spiro[isobenzofuran-1
(3H),4'-
piperidine]-S,S-dioxide, maleate, 32.
to
A solution of 4-(3-benzo[b]thiophen-3-yl)-1-butyl methanesulfonate (4.4 g),
pre-
pared as described in EXAMPLES 10 and 2, in glacial acetic acid (12 ml) was
treated dropwise with aqeous 30 % hydrogen peroxide at room temperature
followed by heating to 80 °C for 20 min. Upon cooling the product, 4-(3-
~s benzo[b]thiophen-S,S-dioxide-3-yl)-1-butyl methanesulfonate, crystallized.
Yield:
3.7 g, mp: 100-1 °C.
The methanesulfonate (1.4 g) was treated with spi,ro[isobenzofuran-1 ( 3H),4'-
piperidine]-HCI (1.2 g) according to the method described in EXAMPLE 2 giving
the
title compound 32 which crystallized as the maleate from acetone by addition
of
ao malefic acid. Yield: 1 g, mp: 186-87 °C.
EXAMPLE 33
1'-[4-(2,3-Dihydro-benzo[b]thiophen-3-yl)-1-butyl]spiro[isobenzofuran-1
(3H),4'-
Zs piperidine]-S,S-dioxide, maleate, 33.
Oxidation of 4-(benzo[b]thiophen-3-yl)butyric acid (8.5 g) according to the
method
described in EXAMPLE 32 gave the corresponding S,S-dioxide (9.2 g).
A solution of the acid (4 g) in tetrahydrofuran (25 ml) was added dropwise to
a
so suspension of lithium aluminium hydride (1.3 g) in tetrahydrofuran (50 m1)
at 0 °C
followed by stirring at room temperature for 2 h. Usual work-up yielded 3 g of
4
(2,3-dihydro-benzo[b]thiophen-S,S-dioxide-3-yl)-1-butanol which was converted
to
the title compound by treatment with spiro[isobenzofuran-1 (3H),4'-piperidineJ
a CA 02296901 2000-O1-13
54
according to the method described in EXAMPLE 2. The product crystallized as
the
fumarate from an acetone/ethanol mixture upon addition of fumaric acid. Yield:
1.5
g, mp: 197-98 °C.
s ~,XAMPLE 34
1'-[3-(Benzo[b]thiophen-3-yloxy)-1-propyl)spiro[isobenzofuran-1 (31,4'-
piperidine],
maleate, 34.
~o A mixture of benzo[b]thiophen-3-one (30 g), ethyl 3-bromopropionate (73 g),
potassium carbonate (55 g), potassium iodide (1.3 g) and acetone (600 ml) was
refluxed for 18 h. Filtration and removal of solvent in vacuo a red oil which
was
dissolved in ether (200 ml) and dried over magnesium sulfate. The ether
solution
was added dropwise to a suspension of lithium aluminium hydride (5 g) in ether
~s (100m1) followed by reflux for 1 h. Usual work-up gave a viscous oil which
was
applied to a silica gel column (eluent: isopropyl ether) giving 3-
(benzo[b]thiophen-3-
yloxy)-1-propanol (0.54 g) as an oil. .
The title compound was obtained according to the method described in EXAMPLE
2' using spiro[isobenzofuran-1 (3H),4'-piperidine] and crystallized as the
maleate salt
2o from an acetone/ether mixture by addition of malefic acid. Yield: 0.34 g,
mp: 116-17
°C.
EXAMPLE 35
2s 1'-[2-(Benzo[b]thiophen-3-ylmethyloxy)-1-ethyl]spiro[isobenzofuran-1(3f~,4'-
piperidineJ, fumarate, 35a.
An ether solution (50 ml) of methyl benzojb]thiophene-3-carboxylate (15 g) was
added dropwise to a suspension of lithium aluminium hydride (3.5 g) in ether
(100
so ml) followed by reflux for 2 h. Usual work-up gave benzo[b]thiophen-3-yl
methanol
(13.5 g) as an oil.
The oil was dissolved in tetrahydrofuran (50 ml) and added dropwise to a
suspen-
sion of NaH (5 g of an 80% paraffinsuspension) in tetrahydrofuran (100 ml)
CA 02296901 2000-O1-13
followed by reflux for 1 h. Ethyl bromoacetate (35 g) in tetrahydrofuran (50
ml) was
added at 60 °C followed by reflux for 1 h. Additional ethyl
bromoacetate (20 g) was
added followed by reflux for 7 h. The reaction was quenched with water and the
volatiles removed in vacuo. The remaining oil was applied to a silica gel
column
s (eluent: ethyl acetate/heptane 2:8) giving ethyl benzo[bJthiophen-3-
ylmethyloxyacetate (5 g) as an oil. Reduction with lithium aluminium hydride
as
described above yielded 3.8 g of 2-(benzo[b)thiophen-3-ylmethyloxy)ethanol as
an
oil.
The title compound 35a was obtained by the method described in EXAMPLE 2
~o using spiro[isobenzofuran-1(3f-I),4'-piperidineJ and crystallized as the
fumarate salt
from an acetonelethanol mixture by addition of fumaric acid. Yield: 0.55 g,
mp: 149-
50~°C.
In a similar manner was also prepared:
~s 1'-[2-(5-Fluorobenzofuran-3-ylmethyloxy)-1-ethyljspiro[isobenzofuran-1(3f-
!),4'-
piperidine), oxalate, 35b, mp: 148-49 °C.
1'-[2-(Benzofuran-3-ylmethyloxy)-1-ethyl)-4-fluorospiro[isobenzofuran-1 (31-
~,4'-
piperidine], oxalate, 35c, mp: 120-22 °C.
2o EXAMPLE 36
1'-[4-(1-(2-Dimethylamino-1-ethyl)-3-indolyl)-1-butylJspiro[isobenzofuran-1
(3f~f),4'-
piperidine), dihydrochloride, 36.
zs A solution of 4-(3-indolyl)-1-butanol (5 g) in dry DMF {50 ml) was cooled
to 10 °C
followed by treatment with potassium r-butoxide {3 g). After stirring for 5
min. 2-
chloro-N,N-dimethylacetamide (3.5 g) dissolved in dry DMF (10 ml) was added
dropwise at 10-15 °C. After stirring for 1 h at room temperature water
was added
followed by extraction with ethyl acetate. Drying of the organic phase over
magne-
ao sium sulfate and removal of solvent in vacuo left a viscous oil, 4-(1-
dimethylamino-
carbony!methyl-3-indolyl)-1-butanol (7 g) which was converted to 1'-[4-(1-
dimethyl-
aminocarbonylmethyl-3-indolyl)-1-butyl]spiro[isobenzofuran-1 (3f-~,4'-
piperidine] by
the method described in EXAMPLE 2 using spiro[isobenzofuran-1 (3 H),4'-
CA 02296901 2000-O1-13
56
piperidine]. Yield: 5.7 g of a viscous oil. Conventional reduction with
lithium
aluminium hydride gave the title compound 36 which crystallized as the dihydro-
chloride from acetone by addition of hydrochloric acid. Yield: 2.4 g, mp: 244-
45 °C.
s PHARMACOLOGY
Some of the compounds of Formula I have been tested according to an
established
and reliable pharmacological test as follows:
to INHIBITION OF 3H-DTG BINDING TO SIGMA RECEPTORS IN RAT BRAIN IN
VITRO.
By this method the inhibition by drugs of the binding of 2 nM 3H-DTG (1,3,di-o-
tolyl
guanidine) to sigma receptors in homogenates or membranes from rat brain
without cerebellum is determined in vitro as modified from Weber et al. Proc.
Natl.
Acad. Sci. 1986, 83, 8784.
Tissue preparations:
Homogenate: Rats (150-250 g) are decapitated and the brains (without
cerebellum)
2o quickly removed and placed on ice, weighed and homogenized in 100 vol ice-
cold
(0 °C) 50 mM Tris-buffer (pH 7.7) in an ethanol rinsed glass/teflon
homogenizer at
0 °C and kept on ice until use.
P2-membranes: Brains are homogenized in 10 vol 0.32 M sucrose in an ethanol
2s rinsed glass/teflon homogenizer with 10 strokes up and down. The homogenate
is
centrifuged for 10 min at 900 x g m at 4 °C. The supernatant is
decanted -and
centrifuged for 20 min at 50,000 g m at 4 °C. The resulting pellet is
resuspended in
vol ice-cold 50 nM Tris-buffer (pH 7.7) and incubated for 30 min. at 37
°C. The
membrane suspension is then centrifuged for further 20 min. at 50,000 g m at 4
°C.
ao The pellet is resuspended in 50 vol. of ice-cold Tris-buffer and used
immediately.
CA 02296901 2003-07-25
5I
Binding analysis:
0.5 ml 50 mM Tris-buffer (pH 7.7), 0.25 ml displacer (6 x 100 ~M DTG, 6 x
[test
compound), or Tris-buffer), and 0.25 ml 6 x 2 nM sH-DTG are mixed into 5 ml
s plastic test tubes and kept at 4 °C until use. The binding reaction
is initiated by
mixing 0.5 ml tissue suspension into this solution and incubate at 25
°C for 20 min.
Glass fiber filters (Whatman*GFIB) are placed on the filter machine which is
then
closed tightly. Just before filtration vacuum is applied, and the filters
washed with
0.1% PEI solution from a spray bottle followed by one wash with Tris-buffer.
io The binding reaction is stopped by filtration of the assay mixture at
reduced
pressure (750 mbar) followed by further 3 washes with 5 ml ice-cold Tris-
buffer.
The filters are then placed in counting vials and 4 ml scintillation solution
added.
The vials are counted in a Beckmann scintillation counter.
~5 Buffers and solutions:
50 mM Tris-buffer pH 7.7: 7.38 g Trizma - 7.7 plus distilled H 20 ad i liter.
100 ml 10% polyethylenimine (PEI): i00 ml dest. H 20 is added to approx. 20 g
50% PEI which is solubilized by stirring and heating. Diluted (1+99) before
use.
6 x 2 nM 3H-DTG: The exact volume depends on the actual concentration of the
2o batch, but is made as close as possible to 12 nM. The containers for the
radioac
tive solution is rinsed in 96% ethanol before use.
6 x 100 pM DTG: 14.36 mg/100 ml is kept frozen in 10 ml aliqouts.
3H-DTG was obtained from NEN Research Products, Du Pont Denmark. Specific
activity 62.3 Ci/mmol.
2S
The known sigma receptor ligands BMY 14802 and rimcazole were included in the
test for comparison purposes.
* (morgue de commerce)
CA 02296901 2000-O1-13
58
Table y
3H DTG Binding Data
Com oun IC50 nM Com oun~ IC50 nM Com oun dtC50 nM
is 7.3 4c . 2.9 11a 0.31
1b 3.7 4d 1.1 11b 0.63
2a 0.25 4e 0.20 1 2 0.30
2b 0.07 4 f 0.20 13a 3.7
2c 0.51 4 46 13b 1.0
2d 0.77 5a 0.33 13c 4.8
2e 1.2 5b 1.1 __ 2.1
13d
2f 0.95 5c 0.25 14a 24
2 0.36 _ 0.24 14b 3.8
5d
2h 1.4 5e ___ 1 5 0.42
4.7
2 i 4.3 5 f 0.04 1 6 0.1 5
2 ~ 0.07 5 _ 17a 0.1 1
4.0
2k 0.19 6a 0.05 17b 0.46
21 3.8 6b 0.17 18a 0.56
2m 0.47 6c <0.1 19a 0.08
2n 6.0 7 0.21 19b 0.49
20 0.26 8a 0.21 19c 0.84
2 0.32 8b 1.7 19d 4.4
2 430 8c 0.66 20 0.63
2 r ' 3.0 8d 1 1 21 0.2
2s 3.1 9a 0.34 2 2 0.05
2t 5.4 9b 0.06 23 2.5
2u 0.41 9c _ 24 i,g
0.14
2v 1 .8 9d _ _ 2 6 25a 1 1
2x 1 .6 9e 0.31 25b 3 6
2 0.84 9f 27 26a O,Bg
2z 1.5 9 4.2 26b 1.9
2aa 0.3 9h 2.3 26c 0.14
2bb 0.30 __ 0.62 2 7 0.1 1
9 i T
2cc 1 .1 9 ' 0.1 1 2 8 0.15
2dd 1 . 7 9 k 0 . 9 2 2 9 0 .1 2
Zee 1 .0 9 I p.14 3 0 0.07
2ff 0.43 9m 1.6 31a 0.23
3.3 9n 6.3 31 b 2.6
2hh 0.02 90 6.7 3 2 0.89
2 i i 5 . 8 9 __ 0 . 2 8 3 3 0 . 9 5
2~~ 1.4 g 0.12 34 0.27
3a 2.2 9 r 0.42 35a 0.73
3b 4.8 9s 5 6 35b ( 2.8
3c 1.0 9t 3.2 36 4.5
3d 7.3 1 Oa 0.09
4a - 23 10b 1.3 BMY 14802 230
4b 0.53 10c 0.35 rimcazole 1 80
I
CA 02296901 2000-O1-13
59
I t is seen from Table I that the compounds used in the present invention are
very
potent sigma receptor ligands as compared to the reference compounds which are
known in the art to be sigma receptor ligands, the potencies for the compounds
tested being better than about 40 nM, and for most of the compounds tested
better
s than about 1 nm.
Furthermore, the ability of the present compounds in inhibiting the binding of
3H-
Prazosin to al adrenoceptors in membranes from rat brain were determined in
vitro
according to Hyttel, J et al, J. Neurochem, 1985, 44, 1615; Skarsfeldt, T. et
al, Eur.
~o J. Pharmacol. 1986, 125, 323.
Additionally the compounds of the invention were tested with respect to
dopamine
D2 receptor binding activity according to van der Welde et al, Eur: J.
Pharmacol.
1987, 134, 211.
For most compounds, the affinities for a~ adrenoceptors and D2 receptors were
weak as compared to the potent binding to sigma receptors. Thus many of the
com-
pounds are very selective sigma receptor ligands, having ratios of binding
affinities
(ICso alpha/ICso sigma and ICso dopamine/ICso sigma, respectively) of 30-
10000.
LIGHT/DARK DISCRIMINATION TEST IN RATS.
The test was carried out in accodance with F.C.Colpaert et al.,
Psychopharmacolo-
gy (1985) 86: 45-54. The test used Wistar WU rats.
2s The test was conducted using a two compartment activity box in which the
actions
of anxiolytic compounds to reduce aversion against a brightly-lit environment
may
be readily detected. The box is designed as an open-top experimental box one
third of which was partitioned from the rest, painted black and illuminated
with red
light. The remainder of the box was painted white and brightly illuminated.
The
so floor of each area was lined into squares. Behavioural changes were
recorded.
Data obtained from dose groups were analysed using single factor analysis of
variance, and Dunnett's t-test. Test compounds were given intraperitoneally 45
min before testing.
_ CA 02296901 2000-O1-13
Several compounds have been tested in this test model and showed significant
anxiolytic activities with EDso values in the ng-~g/kg dose range.
FORMULATION EXAMPLES
5
The pharmaceutical formulations of the invention may be prepared by
conventional
methods in the art.
For example: Tablets may be prepared by mixing the active ingredient with
ordinary
adjuvants and/or diluents and subsequently compressing the mixture in a conven
io tional tabletting machine. Examples of adjuvants or diluents comprise: corn
starch,
potato starch, talcum, magnesium stearate, gelatine, lactose, gums, and the
like.
Any other adjuvants or additives usually used for such purposes such as
colourings,
flavourings, preservatives etc. may be used provided that they are compatible-
with
the active ingredients.
~s Solutions for injections may be prepared by dissolving the active
ingredient and
possible additives in a part of the solvent for injection, preferably sterile
water,
adjusting the solution to desired volume, sterilization of the solution and
filling in
suitable ampules or vials. Any suitable additive conventionally used in the
art may
be added, such as tonicity agents, preservatives, antioxidants, etc.
Typical examples of recipes for the formulation of the invention are as
follows:
1) Tablets containing 0.5 milligrams of Compound 3c calculated as the frbese:
2s Comp. 3c 0.5 mg
Lactose 18 mg
Potato starch 27 mg
Sucrose 58 mg
Sorbitol 3 mg
so Talcum 5 mg
Gelatine 2 mg
Povidone 1 mg
Magnesium stearate 0.5 mg
CA 02296901 2000-O1-13
61
2) Tablets containing 5 milligrams of Compound 2m calculated as the free base:
Comp. 2m 5 mg
Lactose 16 mg
Potato starch 45 mg
s Sucrose 106 mg
Sorbitol 6 mg
Talcum 9 mg
Gelatine 4 mg
Povidone 3 mg
~o Magnesium stearate0.6 mg
3) Syrup containing
per milliliter:
Comp. 6b 2.5 mg
Sorbitol 500 mg
is Tragacanth . 7 mg
Glycerol 50 mg
Methyl-paraben 1 mg
Propyl-paraben _0.1 mg
Ethanol 0.005 ml
2o Water ad 1 ml
4) Solution for injection containing per milliliter:
Comp. 17 a 1 mg
Acetic acid 17.9 mg
2s Sterile water ad 1 ml
5) Solution for injection containing per milliliter:
Comp. 17b 0.1 mg
Sorbitol 42.9 mg
so Acetic acid 0.63 mg
Sodium hydroxide 22 mg
Sterile water ad 1 ml