Note: Descriptions are shown in the official language in which they were submitted.
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
-1
~y-ARYL OR HETEROARYL-PIPERA~IN-1-YLMETHYL)-
Qackgtound of the Invention
The present invention relates to 2-(4-aryl or heteroaryl-piperazin-1-ylmethyl)-
1 H-
indole derivatives possessing central dopaminergic activity. Such compounds
are
useful in the treatment of Central Nervous Systems (CNS) disorders. This
invention
also relates to a method of using such compounds in the treatment of the above
disorders in mammals, especially humans, and the pharmaceutical compositions
useful
therefor.
It is generally known that dopamine receptors seem to be important for many
functions in the animal body. For example, altered functions of these
receptors
participate in the genesis of psychosis, drug addiction, compulsive disorders,
bipolar
disorders, vision, emesis, sleep, feeding, teaming, memory, sexual behavior,
regulation
of immunological responses and blood pressure. Since these n~ceptors control a
great
number of pharmacological events, not all of them are presently known, there
is a
possibility that compounds acting preferentially on D4 dopamine n:ceptor may
exert a
wide range of therapeutic effects in humans.
The 2-(4-aryl or heteroaryl-piperazin-1-ylmethyl~l H-indole derivatives of the
present invention, including forms of tautomers, enantiomers and acceptable
acid
addition salts, are centrally acting D4-dopamine receptor agonists and thus
are useful
as cognition enhancers and treatment of CNS diseases, such as Parkinsons
disease,
Alzheimer's disease, teaming and memory abnormalities. Another feature of this
invention provides for the use of combinations of compounds of the present
invention in
conjunction with 01, D2, D3 or D5 dopamine receptor agonists, such as L- dopa
and
D2 agonists, in treatment of CNS diseases, such as Parkinson's disease,
Alzheimer's
disease, attention deficit disorder and learning and memory abnormalities.
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
2-
Summary of ~ Inve
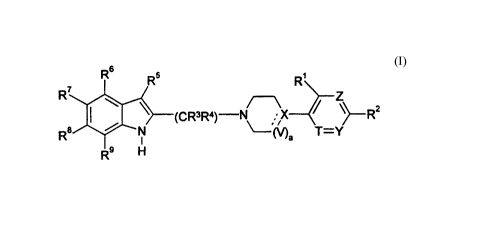
The present invention relates to a compound of the formula
Rs R5 R'
R Z
(CR3R4) N ~ \~R2
Rs / N ~ Ma T-Y
or the pharmaceutically acceptable salt thereof, wherein the broken line
represents an optional double bond;
a is 0 or 1, wherein when a is 0, X may form an optional double bond with the
carbon adjacent to V;
V is CHR'° wherein R'° is hydrogen or (C~-Cs)alkyl;
T is nitrogen or CH;
X is nitrogen or CR" wherein R" is hydrogen, (C~-Cs)alkyl, (C~-Cs)alkoxy,
hydroxy or cyano;
Y and Z are each independently nitrogen or CR'2 wherein R'Z is hydrogen,
chloro, bromo, trifluoromethyl, trifluoromethoxy, cyano, (C~-Cs)alkoxy or (C~-
Cs)alkyl;
R' is hydrogen, fluoro, chloro, bromo, trifluoromethyl, trifluoromethoxy,
cyano or
(C~-~s)alkyl;
R2, Rs, R', R8 and R9 are each independently selected from hydrogen, fluoro,
chloro, bromo, trifluoromethyl, trifluoromethoxy, cyano, (C~-Cs)aikoxy and (C~-
Cs)alkyl;
R3 and R° are each independently hydrogen or (C~-C$)alkyl; and
R5 is hydrogen, (CI-Cs)alkoxy, trifluoromethyl, cyano, (C~-Cs)alkyl or R'3C0-
wherein R'3 is amino, (C~-Cs)alkyiamino, ((C~-Cs)alkyl)2amino, (C~-Cs)alkyl,
(Cs-
C~°)aryl;
or when a is 1, R' and R'° may be taken together with the carbons to
which they
are attached to form a compound of the formula
CA 02297486 2000-O1-13
' WO 99/09025 PCT/IB98/01198
_3-
A--(B)b
-Z 2
II
T-Y
R7 h
wherein the broken lines represent optional bonds;
T, X, Y, Z, R2, R3, R', R5, R6, R', R8 and R9 are defined as above;
b is 0 or 1; and
A and B are each independently CH, CH2, oxygen, sulfur, NH or nitrogen;
with the proviso that when X is nitrogen, the optional double bond between X
and V does not exist;
with the proviso that when b is 0, the optional double bond between A and B
does not exist; and
with the proviso that when b is 1, A and B cannot both be oxygen or sulfur.
The term "alkyl", as used herein, unless otherwise indicated, includes
saturated
monovalent hydrocarbon radicals having straight, branched or cyclic moieties
or
combinations thereof.
The term "alkoxy", as used herein, inGudes O-alkyl groups wherein "alkyl" is
defined above.
The term "trealtng", as used herein, refers to reversing, alleviating,
inhibiting the
progress of, or preventing the disorder or condition to which such term
applies, or one
or more symptoms of such disorders or condition. The term "treatment', as used
herein, refers to the act of treating, as "treating" is defined immediately
above.
The term "disorders of the dopamine system", as referred to herein, refers to
disorders the treatment of which can be effected or faalitated by altering
(i.e., increasing
or decreasing) dopamine mediated neurotransmission.
The compounds in acxordance with the present invention, being ligands for
dopamine receptor subtypes, especially the dopamine D4 receptor, within the
body, are
accordingly of use in the treatment of disorders of the dopamine system.
The compound of formula 1 may have chiral centers and therefore exist in
different enantiomeric forms. This invention relates to al) optical isomers
and
stereoisomers of the compounds of formula t and mixtures thereof.
CA 02297486 2000-O1-13
- WO 99/09025 PCT/IB98/01198
~-
Preferred compounds of formula I include those wherein X is nitrogen.
Other preferred compounds of formula I include those wherein Y and Z are each
CR'2 wherein R'2 is hydrogen or fluoro.
Other preferred compounds of formula I include those wherein R2 is hydrogen,
fluoro or chloro.
Other preferred compounds of formula I include those wherein R3, R4 and R5 are
hydrogen.
Other preferred compounds of formula I include those wherein R' is fluoro or
chloro.
Other preferred compounds of formula I include those wherein R9 is fluoro,
chloro, bromo or alkoxy.
More preferred compounds of formula I include those wherein X is nitrogen; Y
and Z are each CR'3 wherein R'3 is hydrogen or fluoro; R2 is hydrogen fluoro
or chloro;
R3, R' and R5 are hydrogen; R' is fluoro or chloro; and R9 is fluoro, chloro,
.bromo or
alkoxy.
Spedfic prefen~ed compounds of formula 1 include the following:
2-[4-(3-Trifluoromethyl-phenyl)-piperazin-1-ylmethyl]-1 H-indole;
5-Fluoro-2-[4-(3-trifluoromethyl-phenyl)-piperazin-1-ylmethyl]-1 H-indole;
5-Fluoro-2-[4-(4-fluoro-phenyl)-piperazin-1-ylmethyl]-1 H-indole;
5-Fluo~o-2-[4-(4-fluoro-phenyl)-piperazin-1-ytmethyl]-1 H-indole;
5-Fluoro-2-(4-pyridin-2-yl-piperazin-1-ylmethyi)-1 H-indole;
2-[4-(6-Chloro-pyridazin-3-yl)-piperazin-1-ylmethyl]-5-fluoro-l H-indole;
5-Fluoro-2-(4-[5'-fluoro]pyridin-2-yl-piperazin-1-ylmethyl)-1 H-indole;
2-(4-pyridin-2-yl-piperazin-1-ylmethyl)-1 H-azaindole;
5-Fluoro-2-(4-pyridin-2-yl-piperazin-1-ylmethyly-1 H-azaindole; and
2-[4-(4-fluoro-phenyl)-piperazin-1-ylmethylJ-1H-azaindole.
The present invention also relates to a method for treating disorders of the
dopamine system including psychotic disorders (affective psychosis,
schizophrenia, and
schizoaffecfive disorders), movement disorders (extrapyramidal side effects
from
neuroleptic agents, neuroleptic malignant syndrome, tardive dyskinesia, Gilles
De La
Tourette's syndrome, Parkinson's disease or Huntington's disease),
gastrointestinal
disorders (gastric acid secretion or emesis), d~emical abuse, chemical
dependencies,
substance abuse, vascular and cardiovascular disorders (congestive heart
failure and
CA 02297486 2003-08-25
65920-62
_5.
hypertension), ocular disorders and sleep disorders in a mammal, comprising
administering to said mamri~al an amount of a D4 dopamine receptor selective
compound
according to formula I, or a pharmaceutically acceptable salt thereof, that is
effective in
treating such disorder.
The present invention also relates to a method for treating disorders of the
dopamine system including psychotic disorders (affective psychosis,
schizophrenia, and
schizoaffective disorders), movement disorders (extrapyramidal side effects
from
neuroleptic agents, neuroleptic malignant syndrome, tardive dyskinesia, Gilies
De La
Toc~ette's syndrome, Parkinson's disease or Huntington's disease),
gastrointestinal
disorders (gastric acid secretion or emesis), chemical abuse, chemical
dependenaes,
substance abuse, vascular and cardiovascular dison~ers (congestive heart
failure and
hypertension), ocular disorders and sleep disorders in a mammal, comprising
administering to said mammal an amount of a D4 dopamine receptor selective
compound
according to formula I, or a pharmaceutically acceptable salt thereof, in
conjunction with
one or more D7, D2, D3 or D5 dopamine receptor agonists, that is effective in
treating
such disorder.
The present invention also relates to a pharmaceutical composition for
treating
disorders of the dopamine system including movement disorders (extrapyramidal
side
effects from r~euroleptic agents, neuroleptic malignant syndrome, tardive
dyskinesia,
G~les De La Tourette's syndrome, Parkinson's disease or Huntington's disease),
gastrointestinal disorders (gastric add secretion or emesis), chemical abuse,
d~emical
dependencies, substance abuse, vascular and cardiovasartar disorders
(congestive heart
failure and hypertension), ocular disorders and steep disorders in a mammal,
wmprising
a D4 dopamine receptor selective compound
axording to formula I, or a pham~aceutically acceptable salt thereof,
and a pharmaceutically acceptable carrier or diluent.
The present invention also relates to a pharmaceutical composition for
treating
disorders of the dopamine system including psychotic disorders (affective
psychosis,
schQOphrenia, and schizoaffedive disorders), movement disorders
(extrapyramidal side
effects from neuroleptic agents, neuroleptic malignant syndrome, tardive
dyskinesia,
Gilles De La Tourette's syndrome, Parkinson's disease or Huntington's
disease),
gastrointestinal disorders (gastric add secretion or emesis), diemical abuse,
chemical
CA 02297486 2003-08-25
65920-62
-6-
dependencies, substance abuse, vascular and cardiovascular
disorders (congestive heart failure and hypertension),
ocular disorders and sleep disorders in a mammal, comprising
administering to said mammal an amount of a D4 dopamine
receptor selective compound according to formula I, or a
pharmaceutically acceptable salt thereof, in conjunction
with one or more D1, D2, D3 or D5 dopamine receptor
agonists, that is effective in treating such disorder.
The present invention also relates to a use, for
treating a disorder o:E the dopamine system in a mammal, of
an amount of a D4 dopamine receptor selective compound
according to formula :I, or a pharmaceutically acceptable
salt thereof, that is effective in treating the disorder.
The present invention also relates to a use of a
D4 dopamine receptor selective compound according to
formula I, or a pharmaceutically acceptable salt thereof, in
the manufacture of a medicament for treating a disorder of
the dopamine system in a mammal.
The present invention also relates to a commercial
package comprising a pharmaceutical composition of the
invention and a written matter describing instructions for
the use thereof.
i
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
-7.
Detailed Description of the Inven~t'lon
The following reaction Schemes illustrate the preparation of the
compounds of the present invention. Unless otherwise indicated a, T, V, X, Y,
Z, R',
R2, R3, R4, R5, Rs, R', R8 and Re in the reaction Schemes and the discussion
that follow
are defined as above.
Scheme 1
Rs Rs R~
R \ /~ Z
I / \~ (CR3R4)-COOH + N ,: X ~ ~~--R2
Rs N ~(~a T=Y
R9 H
III IV
7
Rs R5 R~
R'
\ Z
~ (CR3R4) X ~ ~~---R2
Ra ~ N ~-(~ T=Y
a
09 H
i
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
.g_
Schp~
Rs
Rv
IV
O
s H
VI
1'
rc Rs R1
R ~ \ ~ X - ~ Z R2
Re ~ N ~ ~(V)e T=Y
Rs H O
V
2
I
CA 02297486 2000-O1-13
WO 99/09025 PCT/1B98/01198
In reaction 1 of Scheme 1, the compounds of formula III and IV are coupled to
form the corresponding compound of formula I by first treating 111 with O-, N-
dimethyl
hydroxylamine hydrochloride, dicydohexylcarbodiimide and a base, such as
fiethylamine, in a polar aprotic solvent, such as methylene chloride. The
hydroxamide
intermediate so formed is reduced, using a reducing agent such as lithium
aluminum
hydride, in a polar aprotic solvent, such as tetrahydrofuran. The reductive
amination of
the aldehyde intermediate so formed is accomplished by reacting the aldehyde
with the
compound of the formula IV in the pn~ence of sodium triacetoxyborohydride and
a
polar aprotic solvent, such as dichloroethane. The reaction mixture is
stirred, under
inert atmosphere, at room temperature for a time period between about 40 hours
to
about 56 hours, preferably about 48 hours.
In reaction 1 of Scheme ~, the compounds of formula VI, wherein L is a leaving
group such as chloro, bromo, methoxy or any activated ester derivative such as
para-
nitro phenyl ester, hydroxy benzotriazole ester, N-hydroxysuccinimide ester or
hydroxy,
and IV are coupled to form the corresponding methanone compound of formula III
by
reacting VI. and IV in the presence of diisopropylethylamine, carbodiimide or
a
dehydrating agent and a polar aprotic solvent, such as methylene chloride, or
in form of
mixtures containing, if desired, combinations of organic solvents or water
such as
combinations of cyclic and acyclic mono and dialkylamides, (C~-C4) alcohols,
halogenated solvents, or acyclic and cyclic alkylethers at temperatures
ranging from
about 0°C to about 150°C, preferabiey about 0°C or the
boiling point of the same
solvent mixture. Addition of an acid acceptor such as an alkalicarbonate, a
tertiary
amine or a similar reagent may be useful.
In reaction 2 of Scheme 2, the methanone compound of formula V is converted
to the corresponding compound of formula I, wherein R3 and R~ are hydrogen, by
reducing V with a reducing agent, such as lithium aluminum hydride or a borane
derivative, in the presence of a polar aprotic solvent, such as
tetrahydrofuran, for a time
period between about 10 hours to about 14 hours, preferably about 12 hours.
In each of the above reactions, pressure is not critical. Pressures in th
range of
about 0.5 atmospheres to 3 atmospheres are suitable, and ambient pressure
(generally,
about one atmosphere) is preferred as a matter of convenience. Also, for those
reactions where the preferred temperature varies with the particular compounds
reacted, no prefer-ed temperature is stated. For such reactions, preferred
CA 02297486 2000-O1-13
- WO 99/09025 PCT/IB98/01198
-10-
temperatures for particular reactants may be determined by monitoring the
reaction
using thin layer chromatography.
The novel compounds of the formula I and the pharmaceutically acceptable salts
thereof (herein "the therapeutic compounds of this invention's are useful as
dopaminergic
agents, i.e., they possess the ability to alter dopamine mediated
neurotransmission in
mammals, including humans. They are therefore able to function as therapeutic
agents in
the treatment of a variety of conditions in mammals, the treatment or
prevention of which
can be effected or fiaalitated by an increase or decxease in dopamine mediated
neurotransmission.
The compounds of the formula I that are basic in nature are capable of forming
a
wide variety of different salts with various inorganic and organic aads.
Although such salts
must be pham~aceuticalty acceptable for administration to animals, it is often
desirable in
practice to initially isolate a compound of the formula I from the reaction
mixture as a
pharmaceutically unacceptable salt and then simply convert the latter back to
the free
base compound by treatment with an alkaline reagent and subsequently convert
the latter
free base to a pharmaceutically acceptable acid addition salt. The aad
addition salts of
the base compounds of this invention are readily prepared by treating the base
compound
with a substantially equivalent amount of the chosen mineral or organic acid
in an
aqueous solvent medium or in a suitable organic solvent, such as methanol or
ethanol.
Upon careful evaporation of the solvent, the desired solid salt is readily
obtained. The
desired acid salt can also be precipitated from a solution of the free base in
an organic
solvent by adding to the solution an appropriate mineral or organic aad.
The therapeutic compounds of this invention can be administered orally,
transdermally (e.g. through the use of a patch), parenterally or topically.
Oral
administration is preferred. In general, these compounds are most desirably
administered
in dosages ranging from about 0.1 mg up to about 1000 mg per day, or 1 mg to
1000 mg
per day in some cases, although variations may occur depending on the weight
and
condition of the person being treated and the particular route of
administration chosen. In
some instances, dosage levels below the lower limit of the aforesaid range may
be more
than adequate, while in other cases still larger doses may be employed without
causing
any harmful side effect, provided that such larger doses are first divided
into several small
doses for administration throughout the day.
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98101198
-11-
The therapeutic compounds of the invention may be administered alone or in
combination with pharmaceutically acceptable carriers or diluents by either of
the two
routes previously indicated, and such administration may be carried out in
single or
mukiple doses. More particularly, the novel therapeutic compounds of this
invention can
be administered in a wide variety of different dosage forms, i.e., they may be
combined
with various pharmaceutically acceptable inert carriers in the form of
tablets, capsules,
lozenges, troches, hard candies, powders, sprays, creams, salves,
suppositories, jellies,
gels, pastes, lotions, ointments, elixirs, syrups, and the like. Such carriers
include solid
diluents or fillers, sterile aqueous media and various non-toxic organic
solvents, for
example. Moreover, oral pharmaceutical compositions can be suitably sweetened
and/or
flavored.
For oral administration, tablets containing various excipients such as
microcrystalline cellulose, sodium dtrate, cak~um carbonate, dicalcium
phosphate and
glydr>e may be employed along with various disintegrants such as starch (and
preferably
com, potato or tapioca starch), alginic aad and certain complex silicates,
together with
granulation binders like polyvinylpyrrolidone, sucrose, gelatin and acaaa.
Additionally,
lubricating agents such as magnesium stearate, sodium lauryl sulfate and talc
are often
very useful for tabletting purposes. Solid compositions of a similar type may
also be
employed as fillers in gelatin capsules; preferred materials in this
connection also inducts
lactose or milk sugar as well as high molecular weight polyethylene glycols.
Wtren
aqueous suspensions andlor elixirs are desired for oral administration, the
active
ingredient may be combined with various sweetening or flavoring agents,
coloring matter
or dyes, and, if so desired, emulsifying and/or suspending agents as well,
together with
such diluents as water, ethanol, propylene glycol, glycerin and various like
combinations
thereof.
For parenteral administration, solutions of a compound of the present
invention in
either sesame or peanut oil or in aqueous propylene glycol may be employed.
The
aqueous solutions should be suitably buffered if necessary and the liquid
diluent first
rendered isotonic. These aqueous solutions are suitable for intravenous
injection
purposes. The oily solutions are suitable for infra-articular, intramuscular
and
subcutaneous injection purposes. The preparation of all these solutions under
sterile
conditions is readily accomplished by standard pharmaceutical techniques well
known to
those skilled in the art.
CA 02297486 2003-08-25
65920-62
-12-
Additionally, it is also possible to administer the compounds of the present
invention topically when treating inflammatory conditions of the skin and this
may
preferably be done by way of creams, jelf~es, gels, pastes, ointments and the
like, in
accordance with standard pharmaceutical practice.
The ability of compounds to bind to mammalian dopamine receptors, and the
relative ability of compounds of this invention to inhibit [3H]-spiperone
binding to human
dopamine D4 receptor subtypes expressed in donal cell tines was measured using
the
following procedure.
p~ Rece r finding Abii~~r
The determination of D, receptor binding ability has been described by Van
Tol, et
al. (Nature, 1991, 350, 610). Clonal cell lines expressing the human dopamine
D4
receptor are harvested and homogenized ~polytron) in a 50 mM Tris:HCl (pH 7.4
at 4 °C)
buffer containing 5 mM EDTA, 1.5 mM calcium chloride. (CaCI~, 5 mM magnesium
chloride (MgCl2), 5 mM potassium chloride (KCI) and 120 mM sodium chloride
(NaCI).
The homogenates are centrifugated for 10-15 min. at 48,000 g, and the
resulting pellets
resuspended in a buffer at a concentration of 150-250 mglml. For saturation
experiments,
0.75 ml aliquots of tissue homogenate are incubated in triplicate with
increasing
concentrations of (3H]-spiperone (70.3 Cilmmol; 10-3000 pM final
concentration) for 30-
120 minutes at 22 °C in a total volume of i ml. For competition binding
experiments,
assays are initiated by the addition of 0.75 ml of membrane and incubated in
duplicate
with the indicated concentrations of competing ligands (10'"-10'~ M) andlor
[3Hr
spiperone (100-300 pM) for 60-120 min at 22°C. Assays are terminated by
rapid filtration
through a Brandell cell harvester and the fitters subsequently monitored for
tritium as
described by Sunahara, R.K et al. (Nafure, 1990, 346, 76). For all
experiments, specific
j3H]spiperone binding is defined as that inhibited by 1-10 mM (+rbutadamol.
Binding data
are analyzed by non-linear least square curve-fitting. The compounds of the
Examples
were tested in this assay, and all were found to have binding affinities (K;)
for the
displacement of [31i}-spiperone of less than 2 micromolar.
*Trade-mark
CA 02297486 2003-08-25
65920-62
-13-
Human D4 ~oeDtoLmodulation of CAMP formation
Chinese hamster ovary (CHO) cells expressing the fiuman D4.4 dopamine
receptor were obtained from Dr. H. Van Tol (Ctarke Institute of Psychiatry,
Toronto),
and were grown to confluence in Minimal Essential Alpha Media (Gibe)
supplemented
with 2.5% Fetal Bovine Serum (not heat inactivated), 2.5°~ Equine Senrm
(heat
inactivated), and 500 irglml Genetidn. Monotayers were disrupted and cells
disloged
with 5 mM ethylenediaminetetcaacetic acid (EDTA) and resuspended in phosphate
buffered saline buffer containing 5 mM magnesium chloride, 30 mM
hydroxyethylpiperazine-N-ethanesuff~ic acid (HEPES), 300 NM 3-isobutyl-1-
methyl-
xanthine (IBMX, a phosphodiesterase inhibitor), and 5.6 mM dextrose. Cells
75 (approximately 200,OOOItube) were exposed to 5 NM forskolin (an adenylate
cydase
activator), forskolin plus test compounds or quinpirole (a D4 receptor
agonist), or
forskoCn plus quinpirole plus antagonist for 11 minutes. In experiments with
antagonists, cells were exposed to antagonists 11 minutes prior to agonist
challenge.
The effect of test compounds in the abser~oe of the agonist quinpirole was
used to judge
agonist activity. D4 agonists produce an inhibition of cAMP accumulation which
can be
reversed by D4 receptor antagonists. The reaction was terminated with the
addition of
6N perchioric add, and samples neutralized with 5N potassium hydroxide and 2M
Tris
buffer. Cydic AMP levels were measured using a commercially available
competifrve
binding kit (Amecsham). !C~ values were calculated by linear regression
analysis of
the concentration-response curves. K; values were calculated using the
equation: K;
IC~I(1 + [agonist]I[agonist ECM]} (Minneman and Johnson, 1984).
The present invention is illustrated by the following examples, but it is not
limited
to the details thereof.
2-14-(G-Ghloro~?vD~dazin-3-yrl)-oinerazin-1-vlmethvll-5-fluoro-l H-indote
A mixture of 5 gm of 5-8uoro 2 indole carboxylic add, 2.74 gm of O-, N-
dimethyl
hydroxylamine hydrochloride, 3.89 ml triethylamine and 5.76 gm of
dicydohexylcarbodiimide in 35 ml methylene chloride is stin-ed at ambient
temperature
until a tan precipitate is fomned. The solid is removed by filtration, the
residue
concentrated and purified on Si02 (25%) EtOAc in Hexane) obtained are 3.6 gm
(64°~)
of the N-0-dimethyl 2 indole hydroxamide.
*Trade-mark
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98L01198
-14-
3.9 gm of N-O-dimethyl 2 indole hydroxylamide is added over a period of 5
minutes to a cold suspension (~0 C) of 0.67 gm LiAIH4 in 30 ml tebahydrofuran.
The
mixture is stirre for an hour (-40 C-> -30 C) treated with a saturated aqueous
solution of
sodium sulfate and wamzed to ambient temperature. The solvent is separated
after
addition of solid sodiumsulfate and concentrated until a solid precipitate is
fomned (2.94
gm of 5-fluoro 2-indolecarboxaldehyde.
A mixture of 0.96 gm of 4-(5-chloro-phenyl)-piperazine, 1.0 gm of 5-Fluoro, 2-
indolecarboxaldehyde and 1.2 gm of sodium triacetoxyborohydride in 50 ml
dichloroethane is stirred under nitrogen at ambient temperature for 48 hours.
The
solvent is removed and the residue portioned between 100 ml EtOAc and 20 ml
NaOH
(1 N). The organic layer is washed with water (2x20m1) and brine (1x10 ml) and
concentrated. The residue is purified on Si02 (eluent: 5°r6 methanol in
methy(ene
chloride) to yield 1.02 gm of a cream colored solid which has a mp.: 204-205
C°).
A mixture of 1.0 mmol of 5-fluoro, 2-indole carboxylicacidchforide and 230 mg
of
meta-triftuoromethylphenylpiperazine and 129 mg of diisopropylethylamine in 10
ml
methylenchloride is kept at ambient temperature for 12 hours. Water is added,
the
organic layers separated, washed with wate, dried over sodium sulfate and
concentrated to yield 296 mg of the title compound. MP: 198°C.
EXAMPLE 3
A solution of 275 mg of 5-Fluoro-1 H-indol-2-yl)-[4-(3-trifluoromethyl-phenyl)-
piperazin-1-yl]methanone in 5 ml anhydrous tetrahydrofurane is kept under an
inert gas
atmosphere and is treated at ambient temperature with 2.11 ml of a 1 M
solution of
Lithiumaluminumhydride in tetrahydrofurane. After 12 hours the mixture is
treated with
78 NI 15°~ Sodium Hydroxide solution and again 234 NI water. After
addition of
magnesiumsulfate the organic layer is separated and concentrated to a yellow
oil (240
mg). This oil is disolved in ether and treated with an ether solution of
hydrochloric acid
until a precipitate is formed. The precipitate is collected, dried under
vacuum.
The title compounds of Examples 4- were prepared by a methods analogous to
that described in Example 1-3.
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
-15-
ALE 4
2-f4-l3-Trifluoromethyl-ohenyj)-Qjyrlmethy~-1 H-indol-5- of
MP: 188-190°C; HRSMS 375.15.
E~SAmPLE.~
2-t4-l3-Trifluoromet~yl-,ohe~,l1)~-Qj~Lyr~Yjl'-1 H-indole
MP: 192-194°C; HRSMS 359.15.
EXBMP~E 6
l1 H-Indol-2-yl,~-(4-l2-vitro-r~henyrj)~-pj~l~razin-1-yr~-methanon~
MP: 186-189°C.
EXAMPLE 7
f 5-Fluoro-1 H-indol-2-vll-(4-,(2-vitro-p~py j)-I~,hera"n-1 Y~- methaneng
M P: 184-188°C.
EXAMPLE 8
r5-Fluoro-1 H-indol-2-vl)-t4-r3-trifluoromethy~~py j)-pneraz~n-~- y~]~~-
methanene
MP: 198°C.
~MPLE,~
3-t4-l1 H-Indol-2-yrlmethyl)~-pj~nerazin-1-yjj-benzo(d)isothia7ole
MP: 150-152°C; MRSMS 348.12.
EXAMPLE 10
5-Fluoro-2-f4-r3-trifluoromethvl-phenyl)~-oioerazin-1-yrlmethy~l-1 H- indole
MP: 196-197°C; HRSMS 377.148.
EXAMPLE 11
2-r4-Naohthalen-1-yrl-pQgrazin-1-vlmetlbyj)-1 H-indole
MP: 238-239°C; HRSMS 341.19.
EXAMPLE 12
2-(4-r2-Nitro-~yj~-~,yrlmeth~-1 H-indole
MP: 210-211 °C; HRSMS 336.16.
EXAMPLE 13
5-Fluoro-2-f4-l2-vitro-p,~enyrl~-oioerazin-1 ;yrlmi; t~7C'[j'-1 H-indole
MP: 236°C; HRSMS 354.14.
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
-16-
EXAMPLE 14
5-Fiuoro-2-(4-na~hthalen-1-yrl-ni~razin-1-grime hy,~-1 H-indole
MP: 249-250°C; HRSMS 359.18.
EXAMPLE 15
5-Fluoro-2-~(4-oyrridin-2 yrt-~iy ethyrl~-1 H-indole
MP: 242°C; HRSMS 310.15.
MP:
EXAMPLE 17
5-F(uoro-2-(4-oyrimidin-2-yl-~!oerazin-1-yr methyr~-1 H-indole
MP: 199°C; HRSMS 311.16.
EXAMPLE 18
l5-Fluoro-1H-indol-2-~~~~(4-oyrridin- -yr1-~,pera~in-1-Yj~l_methanone
M P: 214-218°C.
EXAMPLE 19
2-{~y 'dm in-2=yr~p~,perazin-1-ylmethyriy-1 H-indole
MP:
EXAMPLE 20
(1 H-indoi-2_yrl)-(4-,Qyridin- yl-oiperazin-1-yrt)-methanon~
MP:198-200°C.
' EXAMPLE 21
2-{~y 'din-2-yi-pjQerazin-1-yr#metbyril-1 H-indole
'3C NMR {CDCI3, 75 MHz) d 45.29, 53.03, 55.96, 77.44, 101.94, 107.29,
110.91, 113.52, 119.70, 120.28, 121.69, 128.40, 135.53, 136.37, 137.61,
148.00,
159.55.
'H NMR (CDCI3, 250 MHz) d 2.6 (m, 4H), 3.6 (m, 4H), 3.7 {s, 2H), 6.4 (s, 1H),
6.7 (m, 2H), 7.1-7.6 (m, 4H), 8.2 (m, 1 H), 8.7 (br. s, 1 H).
GC-MS, tR = 4.468 min., M' = 292, (M-162) = 130.
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
-17-
F.~A~P-1E,22
~(2'a;3'a ,B. 6'a(~-1-~(,4-Fluoro-~y~)~(~"-p~~rl-1 ~2'.3'.3'a.4'.6'a-hexahydro-
~ntalen-2'-y~j~-oi~ine dihydrochloride
MP: 250-253°C. Analysis calculated for CZ,H2~FNZ~2 HCI~0.75 H20: C,
66.28;
H, 7_07; N, 6.44. Found: C, 66.18; H, 6.76; N, 6.56.
1 o EXA~~'1.E~
5'a. 6'a6)-5'-[4-(4-Fluoro~~ enyrl)~~erazin-1-yr~-2'-ohenyrl-octahydro-
Rentalen-2'-of maleate
MP: 206-207.5°C. Analysis calculated for C24H~FN20Ø75
C4H40,,~0.75 H20:
C, 67.41; H, 7.02; N, 5.82. Found: C, 67.24; H, 6.77; N, 5.68.
EXAMPLE 24
(2'a;3'a . 5(i 'oc. 6'aJ~~-1-(4-Fiuoro-~gpyr[)~(5'-~y~ahyrdro~~entalen-2'-vll-
~~e~zine dihydrochloride
MP: 255-256.5°C. Analysis calculated for C2,H~FN2~2HCL0.25 H20: C,
65.23;
H, 7.18; N, 6.34. Found: C, 65.40; H, 7.02; N, 6.38.
.E?SBMPLE 2525
(2'a. 3'a~3. 5'a. 6'a~~?-2-Fluoro-4-(4-(5'-hyd~r-5'-phenyl-~rdro-nentalen-2'-
yu-oioerazin-1-yrll-benzonitrile maleate
MP: 207-207.5°C. Analysis calculated for C25H28FN30~ C4H,O4: C,
66.78; H,
6.18; N, 8.06. Found: C, 66.64; H, 6.06; N, 8.14.
ALE 26
(2'a. 3'a j~, 5'a. 6'a(~)-2-Fluoro-4-[4-~(3'. 3'a, 4'. 5'. 6'. 6'a-
hexahvdro(isobenzofuran-1 ~(~H)~. 2'(1'H)~- nice ta_len]-5'-yrl)~-1-
hnerazinvll-benznnirr;lp
MP: 221-221.5°C. Analysis calculated for C~H28FN30~ C4H,O4~O.5
H2O: C,
66.41; H, 6.13; N, 7.74. Found: C, 66.33; H, 6.26; N, 7.61.
(2'a. 3'a (~. 5'a. 6'a~)-5'-[4-~(2-Methoxv-ohenyrl)-izoerazin-1-yrl)-2'-n
MP: 188-189°C. Analysis calculated for C25H~NZO2~ C4H4O4: C, 68.48;
H, 7.13;
N, 5.51. Found: C, 68.64; H, 7.10; N, 5.81.
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
-18-
E)(AMPLE 28
'a(~, 5'a-6'a(~3~4-Fluoro-i~henyrl)-5'-j4-(5-fluoro-hy~rimidin-2-yr1)~-
oioera'in-
1-y)1-octa dro-p~ntalen-2'-of maleate
MP: 219.5-220°C. Analysis calculated for C22H2gF2N4O~ C,H,0,~0.5
H20: C,
59.41; H, 5.94; N, 10.66. Found: C, 59.76; H, 5.89; N, 10.65.
EXAMPLE 29
(2'a. 3'ap. 5'a. 6'ap)-2-Fluoro-4~(øj5'-(4-fluoro-ohenyrl)-5'-hydro~yr-octa
rdro-
oentalen-2'-y~-pj,yJ~benzonitrile maleate
MP: 204-204.5°C. Analysis calculated for C25HZ~F2N3O~ C4H4O4~H2O: C,
62.47;
H, 5.97; N, 7.54. Found: C, 62.77; H, 5.74; N, 7.58.
~ 5 ~S~E 30
(2'a-3'a3'a~J3. 5'a. 6'a(~)~-2'-(4-Fluoro-ohe i)-5'-[4-(4-fluoro-ohenyl)-
pjoerazin-1-vll-
octah~y~-oentalen-2'-of maleate
MP: 209-209.5°C. Analysis calculated for C2,H28F2N20~ C4H~O4: C,
65.36; H,
6.27; N, 5.54. Found: C, 65.65; H, 6.25; N, 5.34. '
EXAMPLE 31
(2'a. 3'a(i_. 6'a(~)~-5-Fluoro-2-j4-(5'-~x)-1'.2'.3'.3'a.4'.6'a-
hexahyr~oentalen-
2'-yrl)-~iperazin-1-yr~]-~yPmidine maleate
MP: 202-203°C. Analysis calculated for C~H25FN4~ C,H~O,,: C, 64.99;
H, 6.08;
N, 11.66. Found: C, 64.67; H, 6.00; N, 11.79.
EXAMPLE 32
(2'a. 3'af3. 6'a(~r2-Fluoro-4-(4-(5'-hhen-yl-1'.2'.3'.3'a.4'.6'a-hexahyr~-
alpn-
2 =y~,)-,p~Qerazin-1-yrJ)-benzonitrile maleate
MP: 172-173°C. Analysis calculated for C25H2BFN3~ C4H,,O4: C, 69.17;
H, 6.00;
N, 8.34. Found: C, 69.06; H, 5.88; N, 8.57.
EXAMPLE 33
(2'a. 3'at~. 5'a. 6'a(~)-5-Fluoro-2-(4-~~5'-I~henyl-octahyr,~-,~gntaten-2'-vl)-
MP: 211.5-212°C. Analysis calculated for C~H2~FN4~ C4H,O4: C,
64.72; H,
6.48; N, 11.61. Found: C, 64.67; H, 6.43; N, 11.82.
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
-19-
F.~SBM PEE 34
(2'~c. 3'a~3. 5'a. 6'a ~)-2-Fluoro-4-(4-(5'-pb~pyrl-octahyr~j~gl~ntalen-
2'_,vl)-
ploerazin-1-yrlj-benzonitrile maleate
MP: 195-196°C. Analysis calculated for C~H28FN3~ C,H4O4: C, 68.89;
H, 6.38;
N, 8.31. Found: C, 68.99; H, 6.47; N, 8.30.
EXAMPLE 35
( 'ac. 3'aB. 5'a. 6'ati)-2-Fluoro-4-(ø[5'-(2-trifluoromethyl-l~gpyr[ -octahyr~
oentalen-2'-y!()-QIQerazin-1-yj~benzonitrile maleate
MP: 192-193°C. Analysis calculated for C~H2~F,N3~ C,,H4O4: C, 62.82;
H, 5.45;
N7.33. Found: C, 62.87; H, 5.22; N, 7.27.
EXAMPLE 36
(2'oc. 3'ali. 6'a(~)-2-Fluoro-4-.(4-j5-(2-mw ~~yr,!)-1' ~' '~' ~'a d' ~'a-
hexahydro-~~entalen-2'-ylj~ razin-1-y~)~benzonitrile maleate
MP: 155-156°C. Analysis calculated for C,~H2sFN30~ C,H,O~~0.25H20:
C,
66.96; H, 6.09; N, 7.81. Found: C, 67.00; H,6.05; N, 7.82.
~.XAMPLE 37
(2'a. 3'aj~ 5'a.. 6'app,)-2-Fluoro-4-(4-(5~2-methoxv-oh, envy)-
octa~y~,hentalen_
2'-ylj-Qjy~)-be~onitrile maleate
MP: 176-177°C. Analysis calculated for C28H~FN30~ C,H404~0.50H20:
C,
66.16; H, 6.48; N, 7.71. Found: C, 66.20; H, 6.31; N, 7.69.
EXAMPLE 38
(2'oci3'afi. 5'a. 6'a~3y-2-Fluoro-4-f4-j5'-(1 H-indol-3-yl)-octahyr~-
oentalewll-
~iherazin-1~,~J~-benzonitrile maleate
MP: 226-227°C. Analysis calculated for C~H~FN,,~ C4H4O,: C, 68.37;
H, 6.11;
N, 10.29. Found: C, 68.17; H, 6.24; N, 10.20.
EXAMPLE 39
( 'a. 3'a(~ 5'~c. 6'a(~)-2-Fluoro-4.-t4-[5'-(2-methanesulfonyla~y~)-octahyr~
oentalen-2'-y~J-~yr~)-benzonitrile maleate
MP: 179-180°C. Analysis calculated for C~H~FN302S~ C,,H4O4~0.25
H2O: C,
61.25; H, 5.91; N, 7.14. Found: C, 61.26; H, 6.32; N, 6.76.
CA 02297486 2000-O1-13
WO 99/09025 PCTIIB98/01198
-20-
~,FLE 40
j2'a 3'a~~ 5'(~ 6',ati)-' 2-Fluoro-4-(4-~(~~'. 3'a. 4'. 5'. 6'. 6'a-
hexa~yr~,~Gsobenz~furan-1(~~,~ 2'~(1'H)-oentalenl-5'-vl)-1-oioerazinvll-
benzonitrile
MP >260°C. Analysis calculated for C~H~FN30~ CH,,03S: C, 63.14; H,
6.27;
N, 8.18. Found: C, 63.12; H, 6.66; N, 8.00.
j ' 'a ji. 5'a. 6'aI3)~-2-Fluoro-4-j4-~(3. 3'. 3'a. 4. 4'. 5'. 6'. 6'a-
hexahyrdrosoiro{Z~
1-benzoQyrran- '~'~-nentalenl~yrl)~-1-Qjy!!]-~~onitrile maleate
MP: 176-177°C. Analysis calculated for CZ7H2gFN3O2~ C4H404~0.50
H20: C,
65.25; H, 5.82; N, 7.36. Found: C, 65.52; H, 6.06; N, 7.19.
j~ a. 6'a ~~)-2-Fluoro-4-j~,(3. 3'. 3'a. 4. 4'. 5'. 6'. 6'a-hexahyrdroso~(~,~,
1-~oovran-2.2'(.tb.~entalenk,~ld)~-1-QjYl1-~~onitrile maleate
MP: 179-180°C. Analysis calculated for C27H2gFN3O2~ C4H4O4: C,
66.30; H,
5.74; N, 7.48. Found: C, 66.17; H, 6.07; N, 7.34.
(2'a-3'ail3. 5'a.. 6'a(~ 2-Fluoro-4~-f4-{5'-~(2-trifluoromethoxyr-ohenyrl)~-
octahydro-
ant~I n- '-y~l-oioerazin-1-y~~-benzonitrile maleate
MP: 126-128°C. NMR DMSO dg 8 7.70 (t, J=8.5 Hz, 1 H), 7.52 (d,
J=7.1 Hz,
1 H), 7.40-7.25 (m, 3H), 7.09 (d, J=13.6 Hz, 1 H), 6.96 (d, J=9.0 Hz, 1 H),
6.06 (s, 2H),
3.73-2.90 (br m, 10H), 2.65-2.54 (m, partially under DMSO, 1 H), 2.46-2.18 (m,
4H),
1.63-1.42 (m, 4H).
EXAMPLE 44
~(2_a.. 3'a~, 5'a 6'a~)-2-Ftuoro-4-{~[~(2-fluoro-phenyl)-octahyrdro-nentalen-
2'-
y!1-~iperazin-1-y!}-benzonitrile maleate
MP: 179-180.5°C. Analysis catculated for C25H27F2N3~ C,H4O4: C,
66.53; H,
5.97; N, 8.03. Found: C, 66.62; H, 6.24; N, 7.98.
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
-21-
EXAMPLE 45
(2'ac,3'a13. 5'a. 6'a(~)-2-Cyran (4-[5'-(2-fluoro-ohenyrl ahyrdro-oenta_ len-
?'_
yj]-oioerazin-1 girl[}-benzonitrile maleate
MP: 193-194°C. Analysis calculated for C~H~FN4~ C4H,04~0.50 H20: C,
66.78; H, 5.98; N, 10.38. Found: C, 66.99; H, 6.05; N, 10.34.
EXAMPLE 46
(2'a,3'a(i. 5'a.. 6'a j~J-2-Fluoro-4-[4-(5'-nyrridin-2-yrl-octah~,~aentalen-2'-
vl)-
pj~erazin-1-yjJ-benzonitrile dihydrochloride
MP: 203-206°C. Analysis calculated for C24H27FN4~ 2HCLH20: C, 59.88; H,
6.49; N, 11.63. Found: C, 59.55; H, 6.42; N, 11.47.
EXAMPLE 47
(2'a. 3'aB. 5'a,. 6'a(~-5-F(uoro-2-(4-[5'-(2-methoxv-ohenyrl)i-
octah~~npr,t~m.,-
~,'-vll-oioerazin-1-y_I};wrimidine mall~,ate
MP: 183.5-184.5°C. Analysis calculated for C23H~FN,O~ C,,H4O4: C,
63.26; H,
6.49; N, 10.93. Found: C, 63.21; H, 6.71; N, 10.82.
EXAMPLE 48
(2'a. 3'aB. 5'~c. 6'aB)-2-Fluoro-4-t4-[5'-(6-fluoro-2-axo- _ -dihv~
benzoimidazol-1-vl1-octahvdro-oentalen-2'-yrlj-pjp"razin-1-yj~-benzonitrile
dim __ yj~
MP: 219-222°C. Analysis calculated for C~HZ~FN50~ 2CH403S: C, 51.29; H,
5.38; N, 10.68. Found: C, 51.84; H, 5.57; N, 10.64.
EXAMPLE 49
( 'cz. 3'aB. 5'a.. 6'a(i)-2-Fluoro-4-~(4-j5'-(6-fluoro-2-methyrlbenzoimida2el-
1-vo-
octa iyrdro-~~alen-2'-yrl]-~ji razin-1-x[~-benzonitrile dimes to
MP: >260°C. Analysis calculated for Cz,H~FZNS~ 2CH403S~0.50 H20: C,
52.56;
H, 5.48; N, 10.57. Found: C, 52.64; H, 5.71; N, 10.57.
EXAMPLE 50
(2'oc. 3'a(~ 5'a.. 6'a~~)-5-Fluoro-2-(~(3'. 3'a. 4'. 5'. 6' 6'a-
hexahyrdrosoiro(isobenzofuran-1 ~(3H)~. 2'(1'H)-oentalenJ-5'-yrl)~perazin-1-
yrl]-oyrrimidine
MP = 186°C. NMR CDCI3 8 8.20 (s, 2H), 7.25-7.17 (m, 4H), 7.12-7.09 (m,
1 H),
5.00 (s, 2H), 3.79-3.71 (m, 4H), 2.72-2.44 (m, 7H), 2.20-2.13 (m, 2H), 2.17-
1.93 (m,
2H), 1.69-1.67 (s, 2H).
*rB
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
EXAMPLE 51
(~~, 5'a. 6'a(~)-5-Ruoro-2~~3'. 3'a. 4'. 5'. 6'. 6'a-
hexahy~i,(Q~oirofisobenzofuran-~(~),~(ld,)-oentalen]-5'-yl)-IZoerazin-1-vll-
ovrimidine
MP: 186-187°C. NMR CDCl3 b 8.18 (s, 2H),7.26-7.10 (m, 3H), 7.08-
7.06
(m,lH), 5.00 (s, 2H), 3.78-3.76 (br s, 4H), 2.78-2.73 (m, 2H), 2.66-2.54 (m,
5H), 2.32-
2.22 (m, 4H), 1.74-1.69 (m, 2H), 1.38-1.29 (m, 2H).
SAMPLE 52
~'~-3'a 5~ 'oc 6'ati)-1-Phenyrl-4-(3. 3'. 3'a.4.4'. 5'.6'. 6'a-
hexahvdrosoirof2H-1-
t~enzo~yrran-2 2'(1'H1-oentalen)-~-~,!]-~yrl~~al~I7~razine maleate
MP: 200-201 °C. Analysis calculated for C~H~N202~ C~H,O,: C, 69.48;
H, 6.61;
N, 5.40. Found: C, 69.48; H, 6.80; N, 5.44.
( '2 6. 3'a(~ 5'a6'a()~,~,-Phe~~rl-4-(3. 3'. 3'a. 4. 4'. 5'. 6'. 6'a-hexah~ dr
ros iro j2H-1-
~o~yrran-2.2'(1'H)-nentalent-5'-y~]-5'-yr~)~-p~erazine maleate
MP: 220-221 °C. Analysis calculated for C~H3pN2O2~ C4H4O4: C, 69.48;
H, 6.61;
N, 5.40. Found: G, 69.28; H, 6.84; N, 5.33.
( 'n 3'ap. 5'oc. 6'a~,~3-j5'-(4-Phen~La~oerazin-1-yl)- octahyrd~oentalen-2'-
vll-
1 H-indole maleate
MP: 232-232.5°C. Analysis calculated for C~H3~N3~ C4H4O4: C, 71.83;
H, 7.03;
N, 8.38. Found: C, 71.57; H, 7.38; N, 8.31.
(2'~ 3' 6'a(~-1-Phenyrl-4-(5'-~yl-1'.2'.3'.3'a.4'.6'a-hexahyrdro-oentalen-2'-
MP: 156-157°C. Analysis calculated for C~H~N202~2C4H40,: C, 66.65;
H,
6.29; N, 4.86. Found: C, 66.27; H, 6.57; N, 5.00.
~(2'oc 3'ad 5'a.. 6'a(~~-1-Phenyrl-4-~(5'-phenyrl-octahyrdro-oentalen-2'-yrl)-
oil~razine
MP: 217-218°C. Analysis caICUlated for CZ4H~N2~ C4H4O,: C, 72.70;
H, 7.41;
N, 6.06. Found: C, 72.28; H, 7.46; N, 6.01.
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
-23-
EXAMPLE 57
'a.3'a 5'~I. oc.6'aj~)~r~Fluoro-2-methyl-1-j5'-(4-ohenyrj~~nerazirwl-yr[)~-
octahyr~,Q-
r~entalen-2'-y~]-1 H-benzoimidazole dimaleate
MP: 203-205°C. Analysis calculated for C~H3fFN,,~ 2C,H,0~~0.50
H20: C,
61.90; H, 6.11; N, 8.49. Found: C, 61.96; H, 6.01; N, 8.58.
EXAMPLE 58
p.pentalran-~
(2'a. 3'aB. 5'B. 6'a~(5'-(4-Fluoro- ho enoxyr)~ahy~ ~-y~~_
MP: 177-178°C. Analysis calculated for C24H~FN20~ C4H404: C,
67.72; H,
6.70; N, 5.64. Found: C, 67.33; H, 6.82; N, 5.62.
EXAMPLE 59
(2'a. 3'aB. 5'B. 6'aBl-2-f5'-(4-Phenyl-pyrlyr~laentamn_~~_~l~_
MP: 235.5-236°C. Analysis calculated for C~H29N302~C,H,O,: C,
67.78; H,
6.26; N, 7.90. Found: C, 67.71; H, 6.37; N, 7.94.
EXAMPLE 80
(2'a_ 3'aB. 5'a. 6'aB)-N-(2-f5'-(4-(5-Ffuoro-~yrimidin-2-y~) a~iQera~in_1-VIl-
octahyrdro-oentalen-2'-~~-~,gB,yrj)-acetamide maleate
MP: 211.5-212°C. Analysis calculated for Cz4H~FN50~C4H40,: C,
62.33; H,
6.35; N, 12.98. Found: C, 62.07; H, 6.32; N, 12.87.
~ EXAMPLE 6_1
(2'a. 3'a~~. 'Sa. 6'aBl-N-(2-{5'-f4-(4-Cyrano-3-fluoro-~y~)-~~ra~
octahyrdro-pentalen-2'-y~}-phenyl)-acetamide maleate
MP: 197-199°C. Analysis calculated for C2~H3tFN40~C,H404: C, 66.18;
H, 6.27;
N, 9.96. Found: C, 66.06; H,6.20; N, 9.89.
EXAMPLE 62
(2'a3'aB. 5'a. 6'aj~)3-Fiuoro-4-~(4-(;~(2-oxo-2.3-dihyrdro-berl'oimuda~o,- I-1-
~I)-
octa ~ydro-oentalen-2'-yj]-~jy~~-benzonitrile mesyrlate
MP >260°C. Analysis calculated for CZgH28FN50~CH403S~0.50 H20: C,
58.89;
H, 6.04; N, 12.72. Found: C, 59.01; H, 6.06; N, 12.71.
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
-24-
~XAAAPLE 63
j~a 3'a(i 5'a 6'a(~)~-{;~-j~(5-Fluoro-nyrim' in-2-vl)-oinerazin-1-vll-
octahvdro-
~~y~1-1.~dihvrdlro-ber~oimidazol-2~ mesyrlate
MP >260°C. Analysis calculated for C~H2TFNsO~CH403S: C, 55.58; H,
6.04; N,
16.20. Found: C, 55.48; H, 5.87; N, 16.41.
~,~ pM LE 64
(~'a 3'a(i. 5'a. 6'a[~)~(~-[~yano-3-fluoro-ohenyl)~-oioerazin-1-vll-
o~tiyrdro-ce~ n-2'-y1)-benzamide ,
MP 198.5-200°C. Analysis calculated for C~H~FN40~ C4H404~0.50 H20:
C,
64.62; H, 6.15; N, 10.05. Found: C, 64.84; H, 6.01; N, 10.03.
EXAMPLE 65
j?'n 3'a(i 5'a. 6'a(~-N~,,S'-j4-Phenyl-oj~azin-1-yrl)~-octahyrdro-oentalen-2'-
vll-
MP: 211-212.5°C. Analysis calculated for C25H3~N30~ C4H40,,~0.25
H20: C,
68.28; H, 7.01; N, 8.23. Found: C, 68.17; H, 6.94; N, 8.18.
~PLE 66
j ' 'a~,~~i. 6'aj~)-2-Fluoro-4-f4-(5'-f4-fluoro-nhenoxv)-octahyrdro-oentalen-
2'-
xIJ-oi,y~}-benzonitrile maleate
MP: 192-193°C. Analysis calculated for C25H27F2N3O~ C4H4O4: C,
64.55; H,
5.79; N, 7.79. Found: C, 64.50; H, 5.80; N, 7.71.
EXAMPLE 67
'S a 6'aJ~)~-5-Fluoro-2-(4-(5'-~(4-fluoro-nhenoxv)-octa yrdro-p~ntalen-2'-
y~~inerazin-1y~-Qyrrimidine maleate
MP: 192-194°C. Analysis calculated for Cz2H~F2N40~ C,H4O,: C,
60.46; H,
5.85; N, 10.85. Found: C, 60.30; H, 5.82; N, 10.78.
EXAMPLE 68
j 'n 3'aj3 5'ti 6'a~-2-Fluoro-4-~(4-f5'-(2-oxo-2.3-dihyrdro-benzoimidazo!-1-
vl)-
octahyrdro-oentalen-2'-y~,oioerazin-1-y!}-benzonitrile maleate
MP: 170-177°C. NMR DMSO ds 8 10.89 (s, 1 H), 7.70 (t, J=8.4 Hz, 1
H), 7.30-
7.23 (m, 1 H), 7.11 (d, J=13.9 Hz, 1 H), 7.04-6.94 (m, 4H), 6.06 (s, 2H), 4.97-
4.82 (m,
1 H), 3.62-2.80 (br m, 1 OH), 2.75-2.63 (m, 2H), 2.60-2.50 (m partially under
DMSO
peak, 1 H), 2.48-2.36 (m, 2H), 1.60 (dd, J~=12.4 Hz, J2=6.6 Hz, 2H), 1.58-1.34
(m, 2H).
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
-25-
E)CAMPLE 6~
(2'a,. 3'aB. 5'a.. 6'aB)~-2-Fluon~-4-!4-j5'-~(3-methox~Lohenyrl)-octa yrdro-~
n al n-
2'-vll-~iy~~benzonitr!!e maleate
MP: 169-170°C. Analysis calccrlated for C~H~FN30~ C4H,,O4: C,
67.27; H,
6.40; N, 7.85. Found: C, 67.18; H, 6.52; N, 7.87.
EXAMPLE 70
( 'a. 3'a~3. 5'oc. 6'alit-2-Fluoro-4-(4-j5'-(4-methoxv-p~nvj)r,~-~entalen-
MP: 186-186.5°C. Analysis calculated for C~H~FN30~ C4H~04~0.25
H20: C,
66.71; H, 6.44; N, 7.78. Found: C, 66.70; H, 6.60; N, 7.60.
EXAMPLE 71
l2'oc. 3'aB. 5'a_ 6'aB~2-Fluoro-4-f~( '-m-tOlyrl-~~ydro-ne!~~alon-~'-vil-
~~erazin-1 ylj-benzonitrile maleate
MP: 198-198.5°C. Analysis calculated for C~H~FN3~ C4H4O4: C,
69.35; H,
6.60; N, 8.09. Found: C, 69.48; H, 6.74; N, 8.14.
EXAMPLE 72
(2'o~.3'aB.5'oc.6'aBl-2-Fluoro-4-j4-(5'-~yl-o~ahyr~pntalan-~'-y1)-I~l r"'~-
MP: 194-195° C. NMR DMSO ds S 7.70 (t, J=8.5Hz, 1H), 7.16-7.09 (m,
5H),
6.96 )d, J=8.7Hz, 1 H), 6.06 (s, 2H), 3.75-2.85 (m, 11 H), 2.55-2.43 (m
partially under
DMSO peak, 1 H), 2.40-2.23 (m with singlet Qa 2.26, 7H total), 1.63-1.32
(m,4H).
~CAMPLE 73
(2'B. 3'aB. 5'B. 6'aB)-1-j5'-(4-Fluoro-p h~ epoxy)i-octahy~,Q-,~~entalen-~'-
vll-4-
~~y!~d~~erazine maleate
MP: 174-175°C. Analysis calculated for C24H29FN20~ C4H,,O,: C,
67.72; H,
6.70; N, 5.64. Found: C, 67.82; H, 6.83; N, 5.59.
. 5'ac. 6'aj~~2-Fluoro-4-j4-~(5'-o-tolyt-oct yrdro-oentalen-?~_yl)-p r ~in-
MP: 198-199°C. Analysis calculated for C~H3oFN3~ C,H,O,:. C, 69.35;
H, 6.60;
N, 8.09. Found: C, 69.13; H, 6.69; N, 8.12.
CA 02297486 2000-O1-13
- WO 99/09025 PCT/IB98/01198
26-
EXAMPLE 75
;t'~~'n 3'af3 5'a. 6'a(i)-1-Phenyr-J~(~'-(3-ovrrolidin-1-vlmethv!-ohenvl)-
octahvdro-
~~entalen-2'-yJ1-oioerazine dimaieate
MP: 163.5-164°C. Analysis calculated for C29H39N3~ 2C4H,04: C,
67.15; H,
7.16; N, 6.35. Found: C, 66.81; H, 7.22; N, 6.27.
FxAMPLE 76
(,~'n 3'a(i ~'n 6'a~y-5-Fluoro-2 ~~(3'. 3'a. 4'. 5'. 6'. 6'a-hexahvdro-3'a.6'a-
dimeth~l~eirofisobenzofuran-1 (3H). 2'(1'H)-oentalenl-5'-vl)-1-aioerazinvlt-
ovrimidine
MP: 224.5-225°C. Analysis calculated for C25H3~FN,0~ C,H,Od~0.25
H2O: C,
64.13; H, 6.59; N, 10.32. Found: C, 64.25; H, 6.68; N, 10.14.
' ' ~,g,~ j~)-5-Fluoro-2-(~(3'. 3'a. 4'. 5'. 6'. 6'a-hexahydro-3'a.6'a-
~oirofisobenzofuran-1 ~(~jj)~. 2'(1'H)-nentalent-5'-vl)-1-oioerazinvll-
ovrimidine
MP: 222-223°C. NMR DMSO dg S 8.58 (s, 2H), 7.34-7.30 (m, 1 H), 7.28-
7.25 (m,
3H), 6.04 (s, 2H), 4.94 (s, 2H), 3.65-2.75 (br m, 9H), 2.20-2.12 (m, 2H), 1.94
(AB
quartet, a ~ 37.8Hz, J=13.2Hz, 4H), 1.54 (br t, J=11.7Hz, 2H), 1.21 (s, 6H).
j2'a.-3',8j~, 5'~, 6'a(~,~-{4-(5'-(1.3-Dioxo-l.3-dihyrdro-isoindol-2-yrl)~-
octahyrdro-
~nt~l n- 'yll-nioerazin-1-yrl}-2-fluoro-benzonitrile maleate
MP: 224-224.5°C. Analysis calculated for C2~HnFN402~ C4H4O4: C,
64.80; H,
5.44; N, 9.75. Found: C, 64.85; H, 5.56; N, 9.74.
,(2'a-3'a3'a~ 5'a 6'a~)-2-(5'l~(5-FI~~o~Ry~ . . in-2-vl)-oic~erazin-1-yll-
octahyrdro-
~g,~Jen-2'-y~~soindole-1.3-dione maleate
MP: 241.5-242°C. Analysis calculated for C24H~FN502~ C4H4O4: C,
60.97; H,
5.48; N, 12.70. Found: C, 60.66; H, 5.55; N, 12.44.
CA 02297486 2000-O1-13
WO 99109025
-27-
rcrns9slo~ i 9s
E)CA MPLE
80
m' (i 5' 6'a -2-Fluon~-4-(4-(33'3'a S' 6'a-hexahvdrosniro(2H-
'~' ~ 4 6'.
4'
r~ a (
a
~fluora nzoovran2 1'H]:~-y 11-~vl)-1-oiuerazinvll-benzoniirile
1 2'(, maleate
MP: 219-220°C. Analysis calculated for C2,H~F2N402~ C,H404~0.50
H20: C,
59.46; H, 5.55; N, 9.90. Found: C, 59.86; H, 5.70; N, 9.40.
EXAMPLE 81
(~'ti 3'a(i 5'a 6'atil-2-Fluoro-4-(4-~(y ~' 3'a 4 4'. 5'. 6'. 6'a-
hexahvdro~ir~2H-
a inre-'.-s.~nzooyran- '(1'H~-]~-.X~-~Y!?:~Rl~Yl1-~~onitrile maleate
MP: 216.5-217°C. Analysis calculated for C24H~F2N,02~ C,H40~: C,
60.43; H,
5.43; N, 10.07. Found: C, 60.39; H, 5.47; N, 9.90.
EXAMPLE 82
' , 5'ae. 6'2~j~,_5-Flu~rr~~~~(5'-o-tolYl-octahvdro-oentalen-2'-vl)-ninerazin-
MP: 204-205°C. Analysis calculated for C~H~FN4~ C~H4O4: C, 65.31;
H, 6.70;
N, 11.28. Found: C, 65.38; H, 6_77; N, 11.32.
EXAMPLE 83
'Q 'a(~, 5'a 6'a(~}-,~-{~-(~(4-Fluoro-R enyl)~oerazin-1-yl]-octahvdro-
MP: 217-218°C. Analysis calculated for C25H~FN,O~ C,,H,04: C,
64.91; H,
6.20; N, 10.44. Found: C, 64.57; H, 6.28; N, 10.18.
EXAMPLE $~
~2'Q. 3'a~, 5'a-6'a(~,-2-(5'.~(g-pheny~plpr~~t octa_hvdro-oentalen-2'-vloxvl-
MP: 161-162°C. Analysis calculated for C25H~N40~ C4H4O4: C, 67.16;
H, 6.61;
N, 10.80. Found: C, 67.05; H, 6.66; N, 10.59.
EXAMPLE 85
~'a 3'a(i 5'a 6'a~},-5-Chloro-2-{~j~2-methom-phenyJi]~-octahvdro-nentalen-
~,'-vll-oioerazin-1-vl1-oyrimidine maleate
MP: 199.5-200°C. Analysis calculated for C23H29CfN,0~ C4H4O,: C,
61.30; H,
6.29; N, 10.59. Found: C, 61.05; H, 6.31; N, 10.83.
CA 02297486 2000-O1-13
WO 99/09025 PCT/IB98/01198
2&
EXAI PM LE 86
j~'h 3'a~,, ~ 5'a. 6'a(~r5-Chloro-2-(4-(5'-o-to It -octah~ dt ro-oentalen-2'-
vl>-
,_y~Qymmidine maleate
MP: 200-200.5°C. Analysis calculated for C~H~CIN4~ C4H4O4: C,
63.21; H,
6.48; N, 10.92. Found: C, 62.97; H, 6.33; N, 11.29.
EXAMPLE 87
j2'a. 3'a(i. 5'a. 6'a(~,~-,(5'-(4-(3.4-Difluoro-ohenyrl)-oihen3zin-1-y~j-
octahydro-
~nt~! n- '-yl}-isoindole-1.3-dione maleate
MP: 221.5-222°C. Analysis calculated for C~H2~F2N3O2~ C,H4O4: C,
63.48; H,
5.51; N, 7.46. Found: C, 63.28; H, 5.51; N, 7.64.
~ 5 F.~SAm.eLE~
j~13. 3'a~~ 5'a. 6'a~)-2~(5'-(4-(4-Fluoro-ohen~~)~-ninerazin-1-yJ~-octahmdro-
~entafen-2'-yJJ~-isoindole-1.3-dione maleate
MP: 209-210°C. Analysis calculated for C26H2aFN302~
C4H404~0.50Hz0: C,
64.51; H, 5.95; N, 7.52. Found: C, 64.47; H, 5.91; N, 7.66.
ALE 89
j2'(i_. 3'a~3. 5'a. 6'a ~}~-{5'-j4-13.4-Difluoro-nhen»)-~Qerazin-1-y~~-octahv~
oenta, len-2'-y(j)_1I.3~iihydro-benzoimidazol-2-one maleate
MP: 201-202°C. Analysis calculated for C25HZ8F2N40~
CdH,04~0.50H20: C,
61.80; H, 5.90; N, 9.94. Found: C, 62.10; H, 5.80; N, 9.56.