Note: Descriptions are shown in the official language in which they were submitted.
CA 02298792 2000-02-11
04645.0601
PHOSPHONATE ADDITIVES FOR NONAQUEOUS
ELECTROLYTE IN RECHARGEABLE CELLS
BACKGROUND OF INVENTION
The present invention generally relates to an alkali metal
electrochemical cell, and more particularly, to a rechargeable
alkali metal cell. Still more particularly, the present
invention relates to a lithium ion electrochemical cell activated
with an electrolyte having an additive provided to achieve high
charge/discharge capacity, long cycle life and to minimize the
first cycle irreversible~capacity. According to the present
invention, the preferred 'additive to the activating electrolyte
is a phosphonate compound. A phosphite compound is another name
for a phosphonate compound and is also preferred for the present
invention.
Alkali metal rechargeable cells typically comprise a
carbonaceous anode electrode and a lithiated cathode electrode.
Due to the high potential of the cathode material (up to 4.3V vs.
Li/Li+ for Lil_xCo02) and the low potential of the carbonaceous
anode material (0.01V vs. Li/Li+ for graphite) in a fully charged
lithium ion cell, the choice of the electrolyte solvent system is
limited. Since carbonate solvents have high oxidative stability
toward typically used lithiated cathode materials and good
kinetic stability toward carbonaceous anode materials, they are
generally used in lithium ion cell electrolytes. To achieve
CA 02298792 2003-11-03
..
C,". ~"y ~: '~:,; I i w: s;~ ~. ~;. ~:'c"-~ .'F: : : ~ i <.. ... .:.' f? : ~:3
".C3, ~ :'..':~, : E~cA''' 3, %S;.' -.:, .~ ~. s.. .
r ~ :: , y ~N'.s ~' ::"~ .~. t;~ 33'~ h f ' ~y ;: ~:. c:
:"4~.,i..Y 'C ~c..: :1 y"'.:''e~.?~~f::.~'.'S %:Lif:~~: , ~.:...3.~':i c:~.
~~~i.~.;~:.:.. :.< r" Lr... ,... ~'..";ZYC.'..~..".'.~
L::%zA~.~.',~t3F:3.<'~'':.~" t. i3-~i:~3~. ;~:~.~:~.~.f;:wv.#. ...
.:;:>~'3;~'xx:' t~c:3~.~"''r.:T'tv, %z~~~~~ i'~ ~i..%.,wCwif.
:'~.~ Yi':)3?.ck~'.i? ' ~' ;..;~Y ~ L ,
C: .-i ' .'s...:;;:r3,,t'...,'~:,~.:- .. :..'tn~:'~:'S'v , ~;t'.- ~.. .. ~
.,.y~ ..~i
.: ..NY'~,~'.:....<%C .. y ~.:,.t;~. . .~.vi
~'..;f~:~"~.~i.~''..''~.3.r'. ..",. ..,fii..«"f~>:a..'~.~y"' i.~:. ~..,
~~.'su:~%.>., fx.i...~:: u:~?~.xyl,~~_.'~,~.,.... hyC,'E,~'.'s!v':'-
~~'~.~..f'.~.;.:~..~. Ci.<..;i w.<v';:Sj~.l..::. :':.'~~.w.2~1 t:.f~
",',:.~''.'~. ~. .~.!f~:.'._'. ?."~-~ f i:.;, "y
iY~Ci... . . . . . "" ~ i_ < ;
~f~~:3..~:":~~';~~'.~5.::.~sy'~'3%a~.':~~.'", .:;~y!'~,:~;.~: ~i~C~.
:~:',.",%Ci~.":a ~.!':~~f~ie:x:';1'~::.3.i.'~:,
s~'>A.~r ~.%.. ~. t ~ S. ' ~~, i:. ' .., ~ , %.. , ~i ~ ~ ' :: 3. i , :.y, ~i
i~ s ..i S > :.. ~,.. ;i ~. %. S. '~: ,~'..: r~ ~: ;.~. ~'.: ;.3 C'~
f~t:.;.%°';~'.'..?,"#:3,t,'x~'''
~;~a.~'::'f.:F.~.:~.'~ij~!'s':"E:3s3.'~3:i.f. '5l;.. 'i ":a.'-% ..
r;~5:::;AA~.r~ _~.Y~ :~5,~'.-'
", .....~ !"%i~ .'.3;...%~ .:>,. , ...
. ~.: i/
w3 " ~ ' "::3. ~:: .'~.. ~-E ~~ ~;; ,. '.:.' ~".: ~' f ,~ .. M~ ~; w. . L3 ~'
, ~. ,. ~.' e'. ~ ;,i ~; L':3 ~~3. ! . C" .~. ,;. s ,; 'M, .~ ~~ ;. 't.'~.
~i "~vii~"'~~~Y Y ~ .,...Y
.:1. ,.: .,. ..., . a'3e~. c3 . ~: R. ~ N3, '. . '~.'c.??"~ . :.~ ~' . r; :~ -
~ swj :: ~. ,n;;:.:."~5. ~s~t Sv'x~: ~: ~'.i i~ ;:', ~ ;' " ".
.~. ?'>i ::
' ~ ,.:; ~.~k ~' :. ..
.. :i: f3 ~~ ; . S ~. i '::' ' ~. s" ~':.. ~,3,:~~ '..< as3r.~5. :.'.w( f~; ~
~3 ~':: ..: ...:.. ,., r: ~. t~? ~. ".. t"..." .... .: c., .. .~,'t','~.'.;'
''..~,'~..~:.~?,'~'~;~w...,J. ~i.~tr'~':.~'.~~ ."Y..';.S.L:~~.'~:~.....,...
:::'i,~:;<~ s:>.y:-:Y,~: rr..::> . ..~; ,."
.: .. =%x' ~' ~t.:,..c._,~, .....,, y ..,...c,~
,..:..'s.~:l.3fZ.~~.~,:$.~..,.. ~w r ,
f'.'...i~r,:F:';~Y . f~'.y Y~C>:$i:z'f:r,=; s.~.%'r~~",s , E~'~,' ~ ~Y1.:33F
'',:';l'.. ~.:,:.."..F.>t:'i:~~~ ~'E: i,~%';;~.',:°.~''.~
%,p..i.~. ,.'.:.z" .'.
..... : . ~"e.; : ~~ ~' ::Y~..':~.~ 'i.. L~,r .t'..i.~~~.i 'f:~~~... ~.,.:.~ ~
:...i.~ v~'v.~.~.. .. r:.:~ r, ~.. $_~f~.i.~~.':
.. ~J w
:~.;::. <'".~.' e::. r'~i '~f~:F:' .., ~3,~'s~F.-:~i~:-,a..t~' :~.~:~
"'°wCt ~::-'sy - i,;:: ;~
,; ~.,. ..,~ .:°. f': :i. :: ,~ #'~~: ~.;,;.. x' ; ..~..
~i:.:. ~...~.~~ a~ s.~"r~~.~.5-'.C~3 !:YG,~':~,~ ~... C'~M<'~ ~ C:t.''a~..
;.f.?.iF ,. ~a ~ !,~.~..f..~ <.?~ ~Y~.~(a:.':~i~ t3.~.~ ...
A.'i.~ ~~."tF."i?.~~?v>3? :?~h~. '.:~.~'>s:'c~.~':: :,~' ;.':%zn'
~~~'."~y:a.:1.:3~.,:.~~:~ r'.i'':~~..~:c.~%~i...:..'.'~~~ ~?;o
C~.i:if'~ t,i!:. :. ~':: ~..~fi~'rs't.'~. s, :,i.~ ''~'".~.:...f~:a:Y.~:.'~'~Y
iYf::.%.vi::>7~::.... .....',.~''~w'~"' ~':' 'n:> ~ Y: ".!.
,. .. .. .,~ .~": S ~4.. ~." . ...".. H. .~.>~,?~ i3:
.., f.~y~J.~.,~:"..i..~.Y?~.:;:aL:;. ,s~.f"~,~ "?.-~'.''~y~ ;:if3.~ u~::.~'
r~f'~::""': ~.~Y%" ~' ~ ' .~5,, ~'.'";:
v. ~..:t:5~~ ::3.
....'~... 3'.i.2w' _, Z .ia"y ; ~~~5iys °..~ i s~,: :. . .. , i%!""':
eti..:>. f'..2.i ~~ i..'s,'-::,~..' l 'Cv: >;;.. .. '~ f' ~'' . '; f..~.. ~
'~,;:;:,~
'= ~.~. , , ~A..>:i E.:.>~:c~~.cs.:~..'F.
c:~ '~:. : s~'t f.'"'. '<~-~-,';'~
~'I~~~ . ~i~%: a%'~: ~ hwuw.:~....~.'~~'t",:. fc:3.?":ate: r";y't.':.~.~:
',~:~:r:t'':'~:>> ,..Y.3.~.f:: <y~:~~%;~i~ ~.'t:'y'
L~:c~~: F '~.:~ ., i::.v.~; 7. .L t.: L.s:3..~. ;"' ,'' C~ ,~v;:~ ;
::.~yA:'~r',: s~>~t"j, i;~%z ~:. ~:~ ~: a" '~ 'i ik !" f.", ~: ~~~ .3..y 3.
t~. ~':
...
'..:" ... , ;~'°. ..., _ ~~ " ~.,i" s.~'~ .3 %°s' : ~.~~~:.'~ :
~a~i; . ' ';, : ~ :e : ' ~:~ . .">.? : ~;; , ~ ';' ~3 .
~':tla..,,l:
2.:'~:::rNZ<~.~:.. ~::if: ._.,(;5r:. ,tw.t~';;%~;~..';;.3''°;_w:w5~::
r43: . .%~_#%~ ~:%. ,...,, ..':.:: ~< ?
,;:,. W 1..:... ~ .' C,~.
~l.'~. r'. ~.~ .:.. J .x.. .,:. ~. ~. ~... ,. ~w( '. .'.~. ,~ ~( Yy'!:>....
Y'S.~, i: S.> :'... ".~.A.. :'-. S~L~ f ,. , ,... V,'~?x ~ .,.
.% C:~ -. ~ ~y' , ~. ::. _ . .~ ., ~.~ :. s v ,.
<'.:3.~:'f _::~;~~'~"v~w ~. ' i:.'M ~:.:~~s~;:,~s~' ~ 13;~:3.f:.:~':;..
~:~.i.~:.'~. .a,. w ;~~ ~ ~3c: ~..; ..:..~.:.~<-~
i. C: '~> ; ,. 'i;%.~'F . :.. .. G~'~' i.s. ~', ":. .~~. t ~ ~:, ;,. , t ..,
4a :- 'i ; r~ ;- : ~: r-:: ~ :-? r :": c.~. . - D ,,.. ; a ,.
,....~.~....F'.~ ....... ,.,"...~_~;:~ ..,..,~' v"".w. :::c3~_<1,.3~:'~.~
;~,.3~:~::~.:'a:'~
'r-~ i:~ ~: ~. <~ ~. ~~':3 t~ r.~s.:. :a.~,. ~ .. ""%.'~: ~x'.'. .;..,~:3.r' ?
j.~' C : , :,y .' Y :f: w. o-~ j-; ~:
.. .: . -~ ~ -.. ~...~::~:~..~~. ~.~. .:: ij:~,.... Y:.~.,3.'.~; ~: ~ ie~: w
~f
.. ~ .. ,.:.... C !'.. C;
,. : v. ~.. ~~ t~; t~,i ::; t ~; '?~ W .. .~" :'.. i..'~ ~.
.:.~.~ .f.:;... ~F:~~ 1~:;.s.~:
CA 02298792 2000-02-11
- 3 - 04645.0601
cycle irreversible capacity, the lower the cell capacity in
subsequent cycles and the lower the cell efficiency. Thus, it is
desirable to minimize or even eliminate the first cycle
irreversible capacity in lithium ion cells while at the same time
maintaining the low temperature cycling capability of such cells.
According to the present invention, these objectives are
achieved by providing an organic phosphonate or phosphite in the
quaternary solvent electrolyte. Lithium ion cells activated with
these electrolytes exhibit lower first cycle irreversible
capacities relative to cells activated with the same quaternary
solvent electrolyte devoid of the phosphonate additive. As a
result, cells including the phosphonate additive present higher
- subsequent cycling capacity than control cells. The cycleability
of the present invention cells at room temperature, as well as at
low temperatures, i.e., down to about -40~C, is as good as cells
activated with the quaternary electrolyte devoid of a phosphonate
additive.
SUMMARY OF THE INVENTION
It is commonly known that when an electrical potential is
initially applied to lithium ion cells constructed with a carbon
anode in a discharged condition to charge the cell, some
permanent capacity loss occurs due to the anode surface
passivation film formation. This permanent capacity loss is
called first cycle irreversible capacity. The film formation
process, however, is highly dependent on the reactivity of the
electrolyte components at the cell charging potentials. The
electrochemical properties of the passivation film are also
dependent on the chemical composition of the surface film.
The formation of a surface film is unavoidable for alkali
metal systems, and in particular, lithium metal anodes, and
CA 02298792 2000-02-11
- 4 - 04645.0601
lithium intercalated carbon anodes due to the relatively low
potential and high reactivity of lithium toward organic
electrolytes. The ideal surface film, known as the solid-
electrolyte interphase (SEI), should be electrically insulating
and sonically conducting. While most alkali metal, and in
particular, lithium electrochemical systems meet the first
requirement, the second requirement is difficult to achieve. The
resistance of these films is not negligible, and as a result;
impedance builds up inside the cell due to this surface layer
formation which induces unacceptable polarization during the
charge and discharge of the lithium ion cell. On the other hand,
if the SEI film is electrically conductive, the electrolyte
decomposition reaction on the anode surface does not stop due to
the low potential of the lithiated carbon electrode.
Hence, the composition of the electrolyte has a significant
influence on the discharge efficiency of alkali metal systems,
and particularly the permanent capacity loss in secondary cells.
For example, when 1.0M LiPF6/EC:DMC=30:70 is used to activate a
secondary cell, the first cycle irreversible capacity is
approximately 35 mAh/g of graphite. However, under the same
cycling conditions, the first cycle irreversible capacity is
found to be approximately 65 mAh/g of graphite when 1. OM
LiPF6/EC:DMC:EMC:DEC=45:22:24.8:8.2 is used as the electrolyte.
Further, lithium ion cells activated with the binary solvent
electrolyte of ethylene carbonate and dimethyl carbonate cannot
be cycled at temperatures less than about -11°C. The quaternary
solvent electrolyte of the previously referenced patent
application, which enables lithium ion cells to cycle at much
lower temperatures, is a compromise in terms of providing a wider
temperature application with acceptable cycling efficiencies. It
would be highly desirable to retain the benefits of a lithium ion
CA 02298792 2000-02-11
- 5 - 04645.0601
cell capable of operating at temperatures down to as low as about
-40aC while minimizing the first cycle irreversible capacity.
According to the present invention, this objective is
achieved by adding a phosphonate additive in the above described
quaternary solvent electrolytes. In addition, this invention may
be generalized'to other nonaqueous organic electrolyte systems,
such as binary solvent and ternary solvent systems,.as well as
the electrolyte systems containing solvents other than mixtures
of linear or cyclic carbonates. For example, linear or cyclic
ethers or esters may also be included as electrolyte components.
Although the exact reason for the observed improvement is not
clear, it is hypothesized that the phosphonate additive competes
with the existing electrolyte components to react on the carbon
anode surface during initial lithiation to form a beneficial SEI
film. The thusly formed SEI film is electrically more insulating
than the film formed without the phosphonate additive and, as a
consequence, the lithiated carbon electrode is better protected
from reactions with other electrolyte components. Therefore,
lower first cycle irreversible capacity is obtained.
The phosphonate or phosphate additives are defined herein as
organic mono-alkyl alkyl or dialkyl alkyl phosphonate compounds
or phosphorous acid (phosphonic acid) provided as a co-solvent
with the other nonaqueous eletrolyte solvents. The organic
phosphonate additives are in a condensed phase which makes them
easy to handle in electrolyte preparation.
These and other objects of the present invention will become
increasingly more apparent to those skilled in the art by
reference to the following description and to the appended
drawings.
CA 02298792 2000-02-11
6 - 04645.0601
BRIEF DESCRIPTION OF THE DRAWINGS
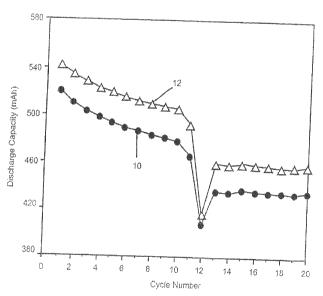
Fig. 1 is a graph showing trhe averaged discharge capacity
through twenty cycles for twp groups of lithium-ion cells, one
group activated with a quaternary carbonate solvent mixture
devoid of a phosphonate additive in comparison to a similarly
constructed cell group having the phosphonate electrolyte
additive.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
A secondary electrochemical cell constructed according to
the present invention includes an anode active material selected
from Groups IA, IIA, or IIIB of the Periodic Table of Elements,
including the alkali metals lithium, sodium, potassium, etc. The
preferred anode active material comprises lithium.
In secondary electrochemical systems, the anode. electrode
comprises a material capable of intercalating and de-
intercalating the alkali metal, and preferably lithium. A
carbonaceous anode comprising any of the various forms of carbon
(e. g., coke, graphite, acetylene black, carbon black, glassy
carbon, etc.) which are capable of reversibly retaining the
lithium species, is preferred. Graphite is particularly
preferred due to its relatively high lithium-retention capacity.
Regardless of the form of the carbon, fibers of the carbonaceous
material are particularly advantageous because the fibers have
excellent mechanical properties which permit them to be
fabricated into rigid electrodes that are capable of withstanding
degradation during repeated charge/discharge cycling. Moreover,
the high surface area of carbon fibers allows for rapid
charge/discharge rates. A preferred carbonaceous material for
the anode of a secondary electrochemical cell is described in
U.S. Patent No. 5,443,928 to Takeuchi et al., which is assigned
CA 02298792 2003-11-03
.i..k~1'C': C.fsi ':#.''~~~'i':f.': f~-::. :~~.i:'r.'.
~,~',~.~'..~i;:~.f...~.':~~::. :~..~~:'i:'..:1.~.~..i3~~~3.,
~. ...;J.~u.>.a:.>~~ ~.i.7f.'.':..ti~#..;.i~:,.7.,a~s yJ'S;~f~.,:.
C~.~.ftt.i~.~ .,.....n )j "'x'.~ '.sr...~:.~~~ .~ ~ ..
~..s::J.E..~. ~,H~ 't'..~ NY ,.~
%~i;..~ 5itv: ~'ti~::; s :'~'l v'i.::;~.~#:4:. ~~'rl':~'Ay.:: ':y:
H';.'.~..~~. Wit:: tw' ~ ;:.#"3. ..~':a:'s.~:. ~ ~'.: C: ~...i
irie~..~,~~~1~.. ~i.ir~.Y.rC."#~,i",:. y7~ i'Z ~:::.#~3.;'i~'~"
f,~.,#.~..:.~.~..#.f2~fi. s.yi#..:....... ~.;s
~.~,',,'..~.~.~..~:::.i.!2.r;;?:;.SJ' ,..
5....t.. 3~?..) !:~.:::.~:.. t~7lv~ar.'.'~N.~ if.,#f.'~~ :~ ~,,srki.i.A.~ ~ t
.,.._, ~ ~ fk,.. :.~....f:~~ ~. i:.C:A.:.r.~;.~:.xfw''<:~: ;.~C~S. #. ~.:, ,
t7:.i..,.." '~.A:.S'.~..~.t;:?~?:.y:. ~.;.~'i.f?~'5~:::.fd.~: ~%v's~'.,~~, ;
~i'~~ .:~. ~ r~",' tw.i ~4--,~.::fi.-y ,.. .
~'4%~. ~..~~~y.~.....~~~t.. yF.4.;.,n,»..ki.:~ti.~.iZ..
i:7~:.:.E!~~::.#.~' ;:'.v:ii'~k r ?~Cf.:..~Y'C'~:.~~3..:'~S,~:a,.:~
s..,A:.~'..~5. t~7,~"~""3 ,r 'F..r~ r..t.,7, ..,,~;..:.
:. .. 'f~. ... .:.~.~.i..'#....:: r f~'.'t:.~.r. Fi:.;.,.',...,;..~.F::,.
~:::':s'r.~a""'fw's~: , ~'~ :~. G:~.t~';~ ~''?t~~E.: ;~~ ~ ~.:: <.:."
".:.~'.'~:'F:: ~.';:4v.:~'f~' ;w=, ~r?."",; of'f ,j :::.~ ''~..... 'N~~
e;'~~~~:.~:;.F.~:. :~:'.3.~ :.~~~~~~;.y' ~t3~",'f:~ :..: tv7~ ... .~.~:.~:~:w.
:'.:'::. . .
... t ;. ~#?..'.. C'. w ::i ~.~.. ~.. :: ., , a : ~;'
~,.'~~~~~.~~ii::~'.~ ~'~:3.'F...:. t,)~. f-,~ 1. t:''.."'.~:: ''"7i' v~::3 .7
~#..v~t.3, ~ ~~"s~ ,~...~'.~'.,.~ ~ 'Y'i;j~".3.s'ef:,3 f?~''
Si~..S<'s',~'.s.~~E.~.h.!;~ ~~lk~~~.::.~,.fvf~~.l.~~w~ ~.W. C.Ci ~.:'SY~ r
~..#. :.~''i~'.i;~..:\...~'f.G~:~"' ~.i~w......~~k,
'~''.~':~cr.' f:'t'~tv7:~~,i.''f~:i~;~'~;!'?.~"4s~1'~w. ~.~:~ .,... z,:'<;'>
..._ F>:%:.~r::~~~E:C~Z ~,:~~', ti:~." .~.5.';:'r:
irki. ~.f''' %::~':~..~.': ;i%.'s~t., 's".S.C'~.~. .::> ~~3L': C'~~'~ti"~.sw
<"~.~...~.~.;~.:;. i.~47:#:~ w'~'~~.E_i~~ y
~w~7,~~ t tys:,~~'~. ~",::.:'Y'~', :~.f.~.t:.~:C3."~,~A ~."' .":f7...~5~:.~f
~' ~..-.,<
,. :~zy - . ; v~t:.~ ... ".". ,. ~:#. Z:~ :. > .~':y~
;'~c..<..~5..'....~:k:.~ c~~'#:~ L:f'kI~f~::a'.e"p-.~::,~s. 'f;:~y Gs.
'T%~~:;~.."ss. ~'.SyY 4~ t'~'....,. t,:.:3.:a''c:-'' ~.'~~' t~~:,vt'.'~; r~<'
:..., ..~ ,.. ... 3. . .,
I~a::~r;~~. :. r% ,,,. .,%iH,i':.' ~#~.~iV~,3 .. v E::~ '' ~ i. ~~:.:~:"d~.M
~~_
'Yt'~ f ~:'.,..~. ~.s'>~f. ~'"..ii...i...~ t .
~~.~ f # .;... 't,"b%?:fu.'c"r: :t~'s~:? t ~t ~:?,~.y ;<:'~~s.sF#.~:
~3#,'#,i~f~~' ~;~:a~.'' ~J~' ~,.i~~;i~~fw''a :7.":a ~.~~'..;?~:,
i.3f:.'c~'#: r'.~~tykri:~:'~~.~''~1~_ :~~'i~~'~. ..!.w i:4. .3:ih3~:'."~..~",E
::i::'~~~ ;'~i. s''s:>q- ;y-: ~~y;
:~...E.<%;~; c~~'E w".~::s,.'~::..i~"~.~' ~:?~i :~~~.1.~':: ;;.r~~ <''-~'"~~.
~i:i>.s~d'~"
r'~..:~',~t~. .,:i:3.t,...<..~Z3~'.:~.~': tjfw"~ ,,.
;:~~::::f.?.~~~t't.f;.~:,'~y :'."L:.3. . ~.'.~::~:'r.'.~'>~7',.i.,su:,:3~'r'~
f'-''.i~~::W;~.2;5~::f,..~ "..,
.j. ~. ~~..:.:#. ~N'~'~. t~ ,'; '~,'Fif,~ ~. ~':.~'.~ .'~.. ...... '~'.'~a:.vi
~ :#. t ~ i ~:. <''x.a~k,~.'-.~'~' ' ;',~ ~ .'~.. #,"
e::w~ ~;E~:%~.'::~:A..~."" r: '~
:~it:i ~, c: ~.~. .
~'ai:":.. ~>~.~.~.'.a w?~ :i~t.#<..:~~ :~.>.'i.'''2~~%,::::7.~i.::
~i:.~~,"",v?v':::L.; ~.."'.,.s""~.;'~t~~-' ;~~;,~:.t~..,.....~.~.
w.Xfi:.~t.~<':~~ f:3~~."~~.v~L:fs, ::°:#.i.:.~~.w~~t~,
.:~;'~..',.i.C,;,.~.r~.~r~~, i'~.~'.~t:. ~.~~.~.~~:w.t::.F". t,y~'
t.',:,~,:~:;h
;<~~:;..!~ f.' f2.i:.' , i.#.!~~~:~E.~#~::, ....Gfc~~...E.,#.~:i.,
.~~'.~..f~:i>.~A.F,#~i#~ ~~~sz;w5~ S:: ;; , yn .:~°.
~.~::.';3:~..;'~'r M-w'i3~'#. ~#..'~.~~t~~. , f'~":W'i~.~.~. :°t,~
r a;E_: :~~::3.'#'3:~,.:3~'3f'-.:.;sM , '.'M'f~:kt::ftur~:
T.'i.'~~~,~.~~.~~~~t~ ;".i'~r. # ~<~'.":~ '..i~.'.~,;:~E.~.ti':'
.:..i.:..'~c'~i 5~ . :.J.:.A~S:::~;~:r ~~.r,..Li's_.S,..... .-~s,.. , c'~:.:.:
f':.
S:#.~#.#.fi .L.t.#.;.SSr.:;_" fL'u ~,k....w~~ ..
s.~~:~"v7~.F:: ..:.~.i:'.C:?s~~"a.t,.kz1 .f..t;7 f:'cT~
~":fA~::f"i~..'.f:;C:~Etw ~.'_.~~' F#.s.".;:'.?."~,"',ws~'~.~.;.fZS.~
.3.tS'. 'J C~.~.~ T..''~'~ .:~..:C. , ~",'v'.aS:~.~~:'.~i .v~.'~.u~ :~ f~
~w.... ~. yiw f s #y'. ~~' ~:>/i " ray.. ~ f~ ::>
r -.. ~. .. F.3. ..... r. ' , ."~ :: c :.. ;. .#. s r: ~%'.', a .. ~. ,#. f~
.~.
A..p'r ~ :#~2:; ~~:~.':~.~C.~~i.~ ~t ' f.~~.' '~r~;t,~'n ht :. ~ r ., r
'4;E.1. ..f c ,
..y ...~.~ : .iy7~,~j, f .~J~: :,.'~,:..::'.~...~..:..i ,
:..~.~t...~..5:3'S.u.:.C':'
V.:C~:~;;L~;.,.~..>...~.. ~..":.,' !f~C~.:..~...'~..'~l~t~'::,;i
.#.~:.~.,.'~5.'~C". ~ '~.<'"~~'r%~~' ~'.'t~~
(.~i 4. . r .5. .,. .., .. i.i ~ C.~ v. 7:., y ; ~ 5:~..~.~ ~ ~St~i ~,~ ~;~.
Z'.1 i.. u:.
.»..a:'"..~~ ~',..>i: "w ~'~.:C..3~.S.G.~.'C': .
CA 02298792 2000-02-11
- 8 - 04645.0601
Metals such as nickel, aluminum, titanium and stainless steel in
powder form are also useful as conductive diluents when mixed
with the above listed active materials. The electrode further
comprises a fluoro-resin binder, preferably in a powder form,
such as PTFE, PVDF, ETFE, polyamides and polyimides, and mixtures
thereof.
To discharge such secondary cells, the lithium ion
comprising the cathode is intercalated into the carbonaceous
anode by applying an externally generated electrical potential to
recharge the cell. The applied recharging electrical potential
serves to draw the alkali metal ions from the cathode material,
through the electrolyte and into the carbonaceous anode to
saturate the carbon comprising the anode. The resulting LiXC6
electrode can have an x ranging between 0.1 and 1Ø The cell is
then provided with an electrical potential and is discharged in a
normal manner.
An alternate secondary cell construction comprises
intercalating the carbonaceous material with the active alkali
material before the anode is incorporated into the cell. In this
case, the cathode body can be solid and comprise, but not be
limited to, such materials as manganese dioxide, silver vanadium
oxide, copper silver vanadium oxide, titanium disulfide, copper
oxide, copper sulfide, iron sulfide, iron disulfide and
fluorinated carbon. However, this approach is compromised by the
problems associated with handling lithiated carbon outside o~f the
cell. Lithiated carbon tends to react when contacted by air.
The secondary cell of the present invention includes a
separator to provide physical segregation between the anode and
cathode active electrodes. The separator is of an electrically
insulative material to prevent an internal electrical short
circuit between the electrodes, and the separator material also
CA 02298792 2003-11-03
...,.. s~~"3,~'r;z,f~..~.:...~.yy wnza'.,F~.:,s,.~w,..,.~v'~.':
'ash°.'.'. v :5:. v.,;_:vs%.~t: c'~"3%.~~. t.'n...~~fi.:.~~
:~;.'.f~,;~v'~;
?':3~:~~?~ ...,.x ~iiw: f~'.:<::i".:~3 %y~ z: ~'':.f;~.~.w,~'_ w.~-
3.A:;:?r.3...~.:.'s.i ~' ,s~~.~::.~ 'C'~~;,".':. .~~'~.~z~y~,~ ~:3.~~._f"
:;..:
,S 7 f ~: ~ F ~ ~ Y :i :.. ~i .. f~ ,, w, f'# ~~ :.'~ 'wf.:L ~. ~?.t~:k ~ ~v
~'~ <w w '-c.'', tY''.' '' ~ ~"3 ~ ~.:. .~. %~ ~. 'f f ~:: .': i
:...:.i't~' :.C3:..~ ~~ .i ::' J;
f 4-.' f~'S~;V,>:'> .,r~': ~'. Li;.).~.'f'2 a ~:..~ L sl ~..~ $.'t ~~.. a'~.
.~. ~'..~. . ~": ~'t 4. ',. v..' '. ~i ..y ~ f-' ~~ ,~.~5 > ;. f~ a.., ;.. yh
rW ; ,-'.:.~ r., "
V cue' .....- .;t..L.~ 1. ~.~3..~ ._ .. "........,'c~S.S,<. ,.:~ .~~:.:'C::
'~'~'..~'f.~. ..;~.a~'"~.a"~ t~~F.s.~'"'~..'tiL:~ ~'.'~'ti;~
~',.c.~','s~,:~,:.:.i~.:~'3.y."a.,:~:::.~:G~t~, ,'~..'s~..''c~~'~~~.:l.f~,:
..':. .~'ea !:'''...
'' , > a w :'.: ~:1. . . '. . . ,.
'r x::.~': 2,7.s. u.~'~':.' .. ~~%,::, ~t, w~ ~~ ~ 'Y~".~.~::c~' ~..;. ~ :~.~
s,. C~~i~::~''~.:. slGii .~'z:, ....,.. ~f:~:.a....:!;
>:7~'~:N:s~..>:SA..A .."'., .':~rw;t~: . :':yZ%k"A .~:cxs~~:- t~."...y..,
..n,..
. . ... .. .. .3 ._~ c 3. ;~. . , <.. wt C.. .~ ", .w. .~ ,~ ~. .:: ~~ ~ ~i ,
~> ;w. . . ~..i ~.. ~ t : ' "'~ w x ,
vC'w:t"" K,i.~'~.~:
..~l.w : .~ ,~ _'' ,t ~~
,.~'3~li~f.r:'. ':.,'5 .., ~.v~C:.~.~. °t.:..; ~ ~w~..'''~?~' -.~.~lt"-
..'~.w.f.'3!~:: ~~~..''F.s~.'.:~~ :.~'~'"%a 3~~,'~F.'' :3.
t
~.r~ :~~: .:.:.,~..'~.~. ~);.'-C-~"~...f,'~3f.~'rw~C~': y~...:::.~~..'t"i:i
s.<. ' ~W~..:-M,.i~y .'~_.~i~~.~'",'.~ ~'~'t ~ :'~'::: :' > øA ~rtx
~ ' t' ~' ". ..~... ... .. ~~ ,. , 3 ... .,...,. '"_'.~ ~ ~'~J . ~:~5.
r.'3.fi~': ' '' :'t>.1. ~Y!~~z~ ,'~.,. .3 ~.:..:..~. s.::~t~i.''~.~"fC~
?.')::, ixt~~~r:s'; <'.~.~'?~~' s~'~...i.t..:.':i.3~;.%:? ~
;I'..~.s~:,F3t.'..'~_:.i.t3I3
~...'i ~'f~~...~f:~. sr.:.~ Z3i.a~,':~~:~'.~5'ii '.,. ,. ,....~.;.v~~t~.'s3.
.A.s".'3.~.a% ~ ~':: > = az-= .z... ; is
''..~ k 5 ~.~ "s. ~.~ .~. C~F i ~. 5;'t. i,. .. %.1:::: ..
'-.~.,:.,.F.ss. ~:.~~.:i:" . ..vy;: ',.;F::~j.>"~~.....~..;'.tr.
.~1.~.~~:;.r.~,_:~,,..~.:;...7 .'t53.f.:.~~,i::.,e:::
~.C.~~u....~.~:fsij"av~;5f3 ;...Sv.~~.';.
:.'~..'..w'S~f~'J..ii.3.~.~ti:.'.!C.',.."..,, .~.. , ~. !:"Y'~:~ r~,~~ ii::>..
. ~y ..:~,~ ,f~:'a'>'Y': » y~
.. " 3.v3~..~.,~ ':>.Y: ~:?,_,...~.~~,'~;.~.~.t.;,yC.-
:.,~..:.!,~..(.LfZ::'Z3's"~:i:y. ~z~.#'~>t?~lt'~
%':.r' ~if:'' .,:.:.Y.3.~::~'~''S<~.~.'...._t~'':if.~_.~.i.5..'
F~E'~'~''r~r~'5 ~, Fa ' r.t.y~.~ ~,' j..~,.»y. r.!-...
~ .. .~ . ~ ....i.:. s..~~<;..~~.~~~5~~.~. ..,~FCr.'. '.,.,. ,..,..'.
....~.:.a.'..'..~ ".. '...v..!~F.k:.: ...._
!,S.if.. ~..<~.:':.~~..'.~ri i i°<,~.. ~.~..~ C."~. ~~...~.
.:r:7~;~:~Li.:.. ~Ct~ir''. f. :.... Si:.,e..:.e"r~>'eJ'LJA'f.."'v'.~' r~ø.
;. .. , '.~ ~. .. ai , ..t s., ~ 1 ~~:.F~:
:vF,:..~t.:.~~~~,~.'.~ 5,_yG,'Yy,w...'..!~::i''~.
s':lc'~.~..':':~':.....~.3.~.L: yi.s"': a>s:-; rs_~.,_t t-~s~
. . c .. ~ v ~.~, .:. a : ai,'.3.'-.. ~,ai 4% ~ .H ~ ~ ';'j~ , f,. s:F...7 ~::
~
~i , ~~r:"'~w ~ ~s'~~.s:~'t~.~,,, ~'?C:~ i~:~,~~'~y.r:r"s~?s s~~~tM~" ~ a.
f.?~.F° ~s3 Mz:.X'~.:z....:5 . ;.';: ._:T~:3~.~':;a ...
s x
3s.~''rT y~..,s:.'..j...w ~:3't'...~.,4yF.~,3.'wT'3~;~~."~~1~.~.f.''.'t''~E
.s=' 3'-~: s :' f~i F~:#,~.,?.~..~"'..~.%~~..~~~
t~:.<~>~..~....44e,''~.>~"sMs~ ,.i.~5f~:'.'.'." 4~~;."..
~:. . ~..''y ~'. s#. ?~y ~,aF:: c:~~' s
. ,!'
:~,~'~ry_~.s~~E :' i...~?t''F 'l'~.. ~'~'~:~ ;.:.i~~.~;~#~~ s"..sE'-'v: ~'
?..;.:., ~S ~ 4't ~if3~.~ft : "''~.~.~"'~ 3s...:E.~
~':~f~s.t~'~,x'3.~:'i~i'~F~:
e~i't~ia""3"c~'$"zu,3.~~~.~.yy r3>~~:L:.~'f~3s~.~" i~'':~.w?h.' ~'.~~
s"~''sr.,I_f~t~:zs:3~~,Tt~ v~'Ia:~:'x~;~s~.~ ~~;~.~~~t~3sMtic:
~'.~. Cn s ;~. ".. .. '.~r" A~: Y S ; .._ , .:: '' ~ $t'~F7:1~'A. Yw . . ".~,
't ".. : ::.2
C.' ~..' .3.<T > . ~3. w!' i. ~' :. L: ~ ,~ ,...1...'.~:~. ; ; '..5' ~ . : s s
.,: ~ . as
.. e;S~:~,:.,~~. t:..~. f; .~. r'.~ 4't ~.:~.i.. c:..s.. _.,..
:f$
t':.i.3~~.w,~Y'.. °.~';. '. :'c~:a,i..t~:3.%k%'.:~..,'.;"~.: ~?;~y~-
,s,~.''~,~-~~~y.;y,> i;.:.. sa t ~kt~~.i':1.,....'.. < ~y.'sT <. r
".3G~.w,;;t''':.'.,. ~,C:~,:'.~.#~~'J. ,~ ..
'~~~;;5~,''-~ f~'~'#sH>~..''.:F:~: f3::. ..... sv.~,.:.'i::'F~.~'.t3.~.~r's~'-
.''~'..'' .r,''.i.:.~;f:?~..~ i'°'s~Jv~~~'C'':1~~.'i. :~~.~~'.:~'.
~;":'~.,,"'.vx:J~.~..'~~lE~;
....~~ i'~ s ~':>~.~.> ~F,s,.':.~3.~. ~:.: ~'".~:~::Y'~~~~~~i~'i':_N.~.
i::<'.~.;:, .~.~3~ a'.-iF:''f':.~3.f.,;,.'....''.~.s' .,~ s:i:.:~..~'E.~i
:.',t':%y:rS, 4~i. t,, ~ I ~.~'t .. C.c''x'S ~ C~'t-I :':f? ~ .. 7' ~i :i r ~.
,K' .i. ~ 5'f.3. ss"y a?:,~ t:~'..~ .. : %'3 ~ ~"~>":: a''3.f;~~~~E
~"35;:'~ ~;~3~...~ s~;..5. ~'?.'e.,~..,.k~~: ~.... 3C. f~..<4'~e~. ~.s.. <..
?:'. ~ ~..~. f s~;..~ ~ ,. %~ , ~~ti ryyM' (
,_ ai ' ~.~:I. ~ ~_; ~.' F.,:,~.'i:
~.~..5...,;.f...f~i5.7~ .:i.~,i~ ...':t:x ,~..~.3.'~ J~;~.F.::~1~~...~.,......
~;~~ F.~~.3.~': ;7.~::/':;~3>'.> ~r.-~..~::r~s i"~ ~":st ,try.
. , ...~ ,/ ~.. at't.~ ~~ '. .t. .:. C~.~. i ~4. , .~ .><. ! v,~ o
.~ i .:., ~,Ct.'~, L~.~:'%~~ ~"F~..wfi:" w'.::r:r ..r,'s..,3.w~ °:t"3
~~~"~>a '~:.:%,-~.Y~~ ~ A-, ~ rj~.3
.. i . . M . . .. .~3. :~: s~ C. F .. ..,;., C
".:J<.5..:.~~:'~'Ai.,..''S.'~. ~~r>«:::,i::F.~..yr-.'.''''y.:.)
ri.''...;.'.:'...".~.~.<'~:.s ~y''~'-5C r ~Y ~~,y.~;.., ~ r.;.~...y. :Y,
.. ..Ny ;,.... ..W....:': ',.,~t~3i~.is.. ...,..,.'. t"~ ........
.~S..ct~.i.~E.~!,'.ta,~..:'.r
.... ...~z.' wI t,~~' ..... '.~"t ::: ~'3s~~'~t:L:.t.~~';'?i.~~:
uS,~.:..i%'~~3~ ,~;';<.~. :.W iv:~'=' s~~,>~~~".r'>~3.~,..~' :'~~'~i
CA 02298792 2000-02-11
- 10 - 04645.0601
alkali metal salt dissolved in a quaternary mixture of organic
carbonate solvents comprising dialkyl (non-cyclic) carbonates
selected from dimethyl carbonate (DMC), diethyl carbonate (DEC),
dipropyl carbonate (DPC), ethylmethyl carbonate (EMC),
methylpropyl carbonate (MPC) and ethylpropyl carbonate (EPC), and
mixtures thereof, and at least one cyclic carbonate selected from
propylene carbonate (PC), ethylene carbonate (EC), butylene
carbonate (BC) and vinylene carbonate (VC), and mixtures thereof.
Organic carbonates are generally used in the electrolyte solvent
system for such battery chemistries because they exhibit high
oxidative stability toward cathode materials and good kinetic
stability toward anode materials.
Preferred electrolytes according to the present invention
comprise solvent mixtures of EC:DMC:EMC:DEC. Most preferred
volume percent ranges for the various carbonate solvents include
EC in the range of about 10$ to about 50~; DMC in the range of
about 5$ to about 75~; EMC in the range of about 5$ to about 50$;
and DEC in the range of about 3$ to about 450. Electrolytes
containing this quaternary carbonate mixture exhibit freezing
points below -50oC, and lithium ion cells activated with such
mixtures have very good cycling behavior at room temperature as
well as very good discharge and charge/discharge cycling behavior
at temperatures below -20oC.
Known lithium salts that are useful as a vehicle for
transport of alkali metal ions from the anode to the cathode, and
back again include LiPFs, LiBF4, LiAsF6, LiSbF6, LiC104, LiA1C14,
LiGaCl4, LiC (SOZCF3) 3, LiN03, LiN (SOZCF3) 2, LiSCN, Li03SCF2CF3,
LiC6F5S03, LiOZCCF3, LiS03F, LiB (C6H5) 4 and LiCF3S03, and mixtures
thereof. Suitable salt concentrations typically range between
about 0.8 to 1.5 molar.
CA 02298792 2000-02-11
- 11 - 04645.0601
In accordance with the present invention, at least one
organic phosphonate additive, preferably a mono-alkyl alkyl or a
dialkyl alkyl phosphonate compound is provided as a co-solvent in
the electrolyte solution of the previously described alkali metal
ion or rechargeable electrochemical cell. The phosphonate
additive is preferably an alkyl phosphonate compound having the
general formula (R10) P (=0) (0R2) (R3) wherein R1, RZ and R3 are the
same or different, and they can be a hydrogen atom or a saturated
or unsaturated organic group containing 1 to 13 carbon atoms.
The greatest effect is found when dimethyl phosphonate, diethyl
phosphonate, dipropyl phosphonate, dibutyl phosphonate, diphenyl
phosphonate, dibenzyl phosphonate, dimethyl methylphosphonate,
diethyl methylphosphonate, dipropyl methylphosphonate, dibutyl
methylphosphonate, Biphenyl methylphosphonate, dibenzyl
methylphosphonate, dimethyl benzylphosphonate, ethyl
methylphosphonate, dimethyl diphenylmethylphosphonate and
phosphourous acid or phosphonic acid, and mixtures thereof are
used as additives in the electrolyte.
The above described compounds are only intended to be
exemplary of those that are useful with the present invention,
and are not to be construed as limiting. Those skilled in the
art will readily recognize phosphonate compounds which come under
the purview of the general formula set forth above and which will
be useful as additives for the electrolyte to achieve high
charge/discharge capacity, long cycle life and to minimize the
first cycle irreversible capacity according to the present
invention.
While not intending to be bound by any particular mechanism,
i.t is believed that due to the presence of the P=0 bond in the
-OP(=O)(R)O- functional group, the bond between oxygen and at
least one of the group R1 and Rz is severed and the phosphonate
CA 02298792 2003-11-03
... ~.! ,.~
.3..~i1.%? Y.'::~?~.~' < .w~: ."'. .~''' t3s~~.~.:.': :~ ~:?~F'~.~;3~':~'.;:~
cy~'x '~~'~:'.'.i~:. . ': 'r_'-: ; -tJ sr~ ~..'>:.:".'. r;Y' h.,.
.; ,. ~: t'~''.' ,. '.. ~, ._. r..
.~;.%a:"'"s r~:;,i ~'w:.'.'-~ ;a:~,'.F.".,rs:.sr#~..> E~'::: ~'",u.~~'~'.z'-~G
Mf3 h: ~~ ' .:3.j::.#.s~:~'s :'::~ ~'..? '
.'.:~ ~ .~. ~: ; : .,? C ~: ~..
~~3 ~. :.,i ~' ; : i : ~3 t~ '~.:w ' i. ~' ,''J" . ... .. .
:~~.:;.v~.3. .. f . r ~ . :'.c,:,%:F? ~'3~3.f.','~.~~',7?,:Ls#'twi'~:.r~r
"y,.: ;:>3rr.'. ' ~. i.F'# ".i.~F'i:
,'.i c:;. ~. ~:
'% 7~'.'v'''~%~~.i55..%:~~:.;' y~::-.?# r.;~~ s»y~~~ ~:r'::iL~:..~ .. . ,
.>:~,.,y,.,~ ~
.., tlww.." :. t.: _#.' ..
.. ~.: i~. t'y ~ ~, . . ~. .,'~J~' ;..;. ~. i.. .. '. ,. ~: ~~ j:... 3.'3.
r.~.~. .
~.s:3..~'.'i.~'.ii.":::''u,':._.,i.:3 Cts''~3.?..yt.~~.'... ~~~~".
..'.'~.~5::~~.:;,.t~,....~..:~:.i.~ ;:k ,~~',.~'.y ; l"'Ys
y ~=.t. .~f., 'J ... ... , , ~.. C .:':~..:. .~. ~n i.'t~Z,f.<. ?',3
..~'..i:':;.t:.i..j~.:#.~v'~''-.'. ~..~.... f.. y~-' ,'~.,.~J_%.
~.i.:.~.SJr.?~ 2f'~ C r) ?~ 't'nt y!~ :,~ i-,r~ . t:
, nn.#. ..CC ~'a~. J i.Cl.:.:~ ~,,, . ' . f:,.Y1
. . . . . .. : i., >.; . t , ''.. ?' . ».
...;.C: f~~'?.~"G2~~...:_~ j..'~~.':r'r.7~~L3~'#.f~~.C~
:..~,r~v..l'._'~_.~'..~i..'f.~~'~~., ''~.t':: t.-.,,. ;,~,;.. ~Fy.,..;1... a _
X... G...,r....:,....~:.1..'....v...C...f ~~~.'ir.~'.5~~~~::~~f.l_l.l.~'a.,.'~
t..~'.::~~.';~.:3:,.k:..i'..~.~.'..... C~z~sv~. :.'ice.<.''~.C~c,~"-!>~'.'.
~:..~5 #'~.?.J~..''''~'.J~~r~~..i?. ~'.i fyY ~'~~~.~ ; i:~Ytw ::a:~ .~
r. .. ~.. ...... '...v. ...(:#.~'e..~~_J..Af:3.'..~C~:?,.'..,esJ
!:.~.~i~5.cr.iS.~?v
:':ii.i.'~::<:t.~.'.F: ~:~~?.''J~~:~::~~'.~.Z%'~y ..".;:,>.5j~a'#~.~5.,~:
~y'::.:~..:#_.:;?yY~e,'"',, ~.f,~ ~'i?~;se.::..~'.hr..w.'.",'~ sld~..'~i~3
., ~..: ~~3.~::.'~3"~.#.'~ a. < A.. ~.'.;r:'. t'"Er~.,M ~,j~ ~.:.i ~~..:~
..::..~:'. ~:. R.~. .~. r ,r %~ 4'~':.'s. .i...: ~~' r~''~ '~; ~" ~ s~' .> ~.-
= L~ a. .;
_, ." ..... ... ,r. ,., 4 .» M ,#. ~~ .., ... '..~ >
~. ~: tr'.' :... .: > ~~:e~~t ,3.. 't3 w ri,; "~ f Mt f - ~ ~; ,~
:' c rn.. r :'t .~~ y. u.. ~. :.. f'~ ~; . ,.. S. ~'L'.2 ~~L: ~ ~~ :..'".'~.
~. w '.r'.~°.' i .~~. .r~_' ",.. . ~ 5J' .'t.
ri
......i.._ '
.T'....3x:~~~.'f. C7.. ... S'i''.i..#:~S.f~ .,~.. ? .5 '~','..l.~o ~~":,,'s4..
.x.'7 ~.~.~rf: ' '' r~r J~
~~~'t'~.'f:s':~:. ~,. w ,~;H, .
.. 3 ~ ,;:i.t".3.t: ..: ~. v,
:.::yJt~;,..v;'~i::3.~:'r ~i~t,f.~.f:'.s?.I:7,r~ »r..;G~
):.'..~..'~.':Cy.'..." ::3.X'.~..''. e;t 3y....f' s..,.,r,~ .. ,,,g_ '~
'~'J_':.~..: .:.. ~'~';..~~~?::~'.' ...ne L. .~~.'..i._~a~~'t,.y~..~..'v
J_ > 1:: "',Yi
_~;~,.:. . .t''~ t;re~:~ 3.~'.J . f~,~'~, zsK'r3L's.;C~ "a ~;r;?al'~~:: f w. ~
' rs ?..:" '~.s~~~.''.
.. . ~_.:.. _:;: ;a~v: :~3 .......c'~
_~ '?,'.%t:~'?:? ~. '~ t3#3 ~'"~:'' " F :' ~ ~ :,~:yy
.~ t'3:#'... C::~.1~ ~~:s3~: ~'fJ~..: ~::;:> .,:%:3'w: ' ~'?~'"'~ .~,
..~;~i~3.'~' .
.. w ,. .. .. _...'.::~ _.;z.'.I:
i .<. ''....~'."'', '~...' .': ii: ?,~n ~ ~ ~.;, '...: ' . .~:~.' s. G ,. 4..
'~.. C~' ..
,'S.:7 r~~;y...~-~~.s~ii:f~.,:F.4C :..Li#3.~:.1.s''?_t,.'_r~?~.i_.ii#3.v
t~.~wj.#'»»!3
i 'c ~ w. L t:~,'s...% .:.. '., '" v''~..ip',',e c.....':. ~'..:. ~ ~~ ~.'.;,'
, . ~#:~ ..t~ 4 i ~~ .#, r r~ ;.
:i . :J ~..' .~ ~ fJ ~f. : i y :.. ~~~ :'i ik ' v. i~ i,.:. f ~.''.'"~ '; .~
,..
:....
i. a t ;j ?:" ~,'. ~.. "s ?, n: ~- . :'3. ...~'t'',.~..A. ~ 7:A' A. tw w :~'
..~ ., w5
..,..r3 ~vi .:#.'ws~'ru'", r! .. ..W '_:i:~.:: ~.ci~..i.sM
;';?:~.~.ia:~~l.%.f%#o '~~~~3.f~'..
'E?::j.::3~..~.i»s.,. t.C~i<r.', I"f':ciy' ',-~.i~~':~~:'3'"'~.~:'.:.'
t~;C'~'~_.r.'.V;.3.s~s.~_.s.-.. .~ii.>~3 s.... ~~ar.=;» ,~.~>::>"
:<~.w .~~.:...,,J.~ >. ,_.s,~...~.A.~
°,
1 . . f."~ ~ ~ i~ ~' . r :,:,,?. L:.~~ .t. "'~3.~ c;' ~ ~;'~.< t '. .~.?~ ;a
#:. ~: ::? t. s ~ :#.'~:.,''::..7 :i~; Z3 ~' i'~ .~.:.::I':.i. t'~~ :~F''~, ,.
s , ..: ..
~s.7~ .~.~..i'~.:.~.;:f~.f~ ;~...'~:.t'','r'A~:w.sjf i~',t .c.tJ''~itr.,s c3~3
'~'~ ~; .'.~'#~~; c:'t.~...i.,.. ~'~:~:.i:~.~.''. '
's, ~, ~.. _#. j
'h~~~=%?~'..~"J,.E: :s:3,.° ;.'s~:?'~~' i.,y... ..
C:~.~,~ti::f~~~c~~'3.~;.. f~~:. v~~?w :? ..~.<
"'s:~: ':f::' .~ ,..c?~::~... f:i'~'~;~,~'~':#. L;'~cA..'' ,.,. "~~r?.-3's :~
~ ~.:#.!". ?:?. w: ~"'-';;:s~3.i'~~:?~t~ ~:3C"~
'v''' Kd ~. ~ ~'~
~.. ~"..'::. " ~''~E'3.~.~: c,..:'.i %:3.6.:~.;;~:~'..~;.'~,'::iv. ,_' ~ ~ ,
.. ,'.__.~~-r,, ~s_
~~'' ~~- '~ ~ ~ , ""~ ~.~ (. ~ .. .... .J 4: Cue' n.. ,! :» ~. ~'~i~,~..:: i.
~:5 ~~. l .~ #
s 'a s~ u~ I ., '.. w~ ~ ;:v. s .f:'~ .,r. ,'.o c: t.: ,' "~. : ;:< ~~~ L3
.fit. s'. ~s~: ?". i ~; ~v: w. ;,". .. . r syy ~. ,'; 'f :::a ".: .;. ~ ~. ~.
~ F r~ 'M:~ ~':: '.:~ f. '-'i :.i
#.~.~>f:.:~...#. ~.'t.:1 r~,... ~:y3~'S~ilti~i.~.... ~?:YS~N;,C;3; Q_. t,
;55'1 ....~. a .":C.', ~
. #:. :, ., ~: ~. .J. , A... " :;:: f.i ',, f ~ ,: i. t.'3 ~~. Y~ :~; n
F,.'..'~y?'
39i:
~.'~::#. ~~ ~i '>.~: ;:::#. ~. .i s~.~. ;.. '' .~'''.'...,. ea ~: w.~i5, i.~.y
. ~ ~ r '~.'.c; ~ ~w t? s: _ . ~ ~.';.':_. s .' ;> f'3 ~ > '~rs ..,
.. .. >., .,. .t. ~:: "
,t 'fit f' , ;
.._.... ' w~~..3~' ~3?,"',';......'.it~~" ~c.r''','~''s'~.'i.~..';.:"::...
J,~3... .~..rc.»'.C.s,'a,,:'~" rw;::,;s.3_: ~v c.: ~,a~~';.., r~~r'r-.
»s.3..:.... ".,s.- " .:r:.L.._.~..'~Jw..y :".''~ :,3..:~?~:~;
3,,~.:<: ci.~"; .:''J~..j::;;~~~CS,.'"'s ~".,Z3.~.'J~''<~?:''::.~i''i.'
;~..~.~:.,'(ia.~~;.'~'f:.>, <1: C ~.'', ctf...:>'?' r> ~
j J ,.7.
~~: ~:.:. ~. :? w'w~ :~ .J :~ ~ w"?w' L. ~. : ~ ,
?~:c::z": .:3~..it".'J ~:' ,~;.%;?f_>, '~'s'":3~:? sy?~_~. '~if:~;C's?w:",
~:C;v~'i.y~.'::.'#..M.~.c'
f:~ .'~.t::':'3.~:I'~~. W'3<:c :'~:.~'lE~::~:3a~;;~::.i"'~.~:..~.:.'~...~..
"'~..'.i 'rsf:E_ w.'~ #:~~?f'?-.;. . Ya.r ;~.r;-r; ., ;:
. . ,...~3...... ...:_.~:'~;7:':J;::~:f3~'.:~, , .» ~.~'~t=:
CA 02298792 2000-02-11
- 13 - 04645.0601
electrochemical cell and is resistant to corrosion. The cathode
lead is welded to the positive terminal pin in the glass-to-metal
seal and the header is welded to the case containing the
electrode stack. The cell is thereafter filled with the
electrolyte solution comprising at least one of the phosphonate
additives described hereinabove and hermetically sealed such as
by close-welding a.stainless steel ball over the fill hole, but
not limited thereto. ..
The above assembly describes a case-negative cell, which is
the preferred construction of the exemplary cell of the present
invention. As is well known to those skilled in the art, the
exemplary electrochemical system of the present invention can
also be constructed in a case-positive configuration.
The following examples describe the manner and process of an
electrochemical cell according to the present invention, and set
forth the best mode contemplated by the inventors of carrying out
the invention, but are not construed as limiting.
EXAMPLE I
Six lithium ion cells were constructed as test vehicles.
The cells were divided into two groups of three cells. One group
of cells was activated with a quaternary carbonate solvent system
electrolyte devoid of a phosphonate additive while the remaining
cells had the same electrolyte but including the phosphonate
additive. Except for the electrolyte, the cells were the same.
In particular, the cathode was prepared by casting a LiCo02
cathode mix on aluminum foil. The cathode mix contained 91$ _.
LiCo02, 6~ graphite additive and 3$ PVDF binder, by weight. The
anode was prepared by casting an anode mix containing 91.7°s
CA 02298792 2000-02-11
- 14~ - 04645.0601
graphite and 8.3$ PVDF binder, by weight, on a copper foil. An
electrode assembly was constructed by placing one layer of
polyethylene separator between the cathode and the anode and
spirally winding the electrodes to fit into an AA sized
cylindrical stainless steel can. The cells were activated with
an electrolyte of EC:DMC:EMC:DEC=45:22:24.8:8.2 having 1. OM LiPF6
dissolved therein (group 1). The group 2 cells fabricated
according to the present invention further had 0.05M dibenzyl
phosphonate (DBPI) dissolved therein. Finally, the cells were
hermetically sealed.
All six cells were then cycled between 4.1V and 2.75V. The
charge cycle was performed under a 100 mA constant current until
the cells reach 4.1V. Then, the charge cycle was continued at
4.1V until the current dropped to 20 mA. After resting for 5
minutes, the cells were discharged under a 100 mA constant
current to 2.75 V. The cells were rested for another 5 minutes
before the next cycle.
The initial average charge and discharge capacities of both
groups of cells are summarized in Table 1. The first cycle
irreversible capacity was calculated as the difference between
the first charge capacity and the first discharge capacity.
Table 1
First Cycle Capacities and Irreversible Capacities
1st Charge 1st Discharge Irreversible
Group (mAh) (mAh)
1 641.4 ~ 1.5 520.2 ~ 9.5 121.2 ~ 10.7
2 635.1 ~ 13.9 541.7 ~ 4.3 93.4 ~ 18.2
CA 02298792 2000-02-11
- 15 - 04645.0601
The data in Table 1 clearly demonstrate that both
groups of cells had similar first cycle charge capacities.
However, the first cycle discharge capacities are quite
different. The group 2 cells activated with the electrolyte
containing the dibenzyl phosphonate additive had significantly
higher first cycle discharge capacities than that of the group 1
cells (approximately 4.1g higher). As a result, the group 2
cells also had about 23~ lower first cycle irreversible capacity
than that of the group I cells.
EXAMPLE II
After the initial cycle, the cycling of the six cells
continued for a total of 10 times under. the same cycling
conditions as described in Example I. The discharge'capacities
and the capacity retention of each cycle are summarized in Table
2. The capacity retention is defined as the capacity percentage
of each discharge cycle relative to that of the first cycle
discharge capacity.
Table 2
Cycling Discharge Capacity and Capacity Retention
Group 1 Group 2
Capacity Retention Capacity Retention
Cycle # (mAh)
(~h)
1 520.2 100.0 541.7 100.0
2 510.2 98.1 534.1 98.6
_. 3 503.4 96.8 528.0 97.5
4 497.6 95.7 522.7 96.5
493.2 94.8 519.0 95.8
6 489.4 94.1 515.3 95.1
CA 02298792 2000-02-11
- 16 - 04645.0601
Group 1 Group 2
Capacity Retention Capacity Retention
Cycle (mAh) ($) (mAh)
#
7 486.1 93.4 512.4 94.6
8 483.2 92.9 '509.8 94.1
9 480.2 92.3 507. 4 93.7
478.2 91.9 505.5 X3.3
The data in Table 2 demonstrate that the group 2 cells with
the dibenzyl phosphonate additive consistently presented higher
discharge capacities in all cycles. In addition, this higher
capacity was not realized at the expense of lower cycle life. The
group 1 and 2 cells had essentially the same cycling capacity
retention throughout the various cycles.
EXAMPLE III
After the above cycle testing described in Example II, the
cells were charged according to the procedures described in
Example I. Then, the cells were discharged under a 1000 mA
constant current to 2.75 V then a five minute open circuit rest,
followed by a 500 mA constant current discharge to 2.75 V then a
five minute open circuit rest, followed by a 250 mA con.stant
current discharge to 2.75 V then a five minute open circuit rest
and, finally, followed by a 100 mA constant current discharge to
2.75 V then a five minute open circuit rest. The averaged total
capacities under each discharge rate are summarized in Table 3
and the comparison of averaged discharge efficiency (defined as $
capacity of a 100 mA constant current discharge) under the
various constant currents are summarized in Table 4. In Table 3,
CA 02298792 2000-02-11
- 17 - 04645.0601
the discharge capacities are cumulative from one discharge
current to the next.
Table 3
Discharge Capacities (mAh) under Various Currents
Group 1000 mA 500 mA 250 mA 100 mA
1 277.8 439.8 459.8 465.9
2 231.6 460.9 484.2 492.3
Table 4
Discharge Efficiency (~) under Various Currents
Group 1000 mA 500 mA 250 mA 100 mA
1 59.7 94.4 98.7 100.0
2 47.0 93.6 98.3 100.0
The data in Table 3 indicate that the group 2 cells with
phosphonate additive delivered increased discharge capacity in
comparison to the group 1 control cells under a discharge rate
equal to or less than 500 mA (approximately a 1C rate). Under a
higher discharge rate (1000 mA, approximately a 2C rate),
however, the group 1 control cells delivered higher capacity than
that of the group 2 cells. The same trends are also shown in
Table 4. Under a 500 mA or lower discharge current, the group 2
cells presented similar discharge efficiencies than that of the
group 1 cells. Under a higher discharge current (i.e. 1000 mA),
CA 02298792 2000-02-11
- 18 - 04645.0601
the group 1 control cells afforded a higher discharge efficiency
than that of the group 2 cells.
EXAMPLE IV
After the above discharge rate capability test, all the
cells were fully charged according to the procedure described in
Example I. The six test cells were then stored on open circuit
voltage (OCV) at 37°C for two weeks. Finally, the cells were
discharged and cycled for eight more times. The ~ of self-
discharge and the capacity retention were calculated and are
shown in Table 5.
Table 5
Rates of Self-Discharge and After Storage Capacity Retention
Group Self-Discharge ($) Capacity Retention
1 12.6 93.4
2 15.0 93.6
The data in Table 5 demonstrate that both groups of cells
exhibited similar self-discharge rates and similar after storage
capacity retention rates. However, since the group 2 cells had
higher discharge capacities than that of the group 1 cells, the
capacities of the group 2 cells were still higher than that of
the group 1 cells, even though they presented similar self-
discharge and capacity retention rates. A total of 20 cycles
were obtained and the results are summarized in Fig. 1. In
CA 02298792 2003-11-03
.. -: w> w
,~c~~~::1.E:F>. .... , C'3_3:: :f' :.~'s 's~;:3.i~
E::r~~t";..',.°~.~t.: ~z::v'~ :'3."::~3; ~:~,3~~: i~~%F?:~.'cxC:~.-'~~
.. "~~ _.. . ~. ,.;,. ~. i.... :'~.~,~5;~' ~~,.,:;;'slni~~.~C; i(:~v..t1
'N'~:~~ '~ #. ":'. ~. ;t":: L1:~I ';..~. ftv ~.3::: ;'%' A.% :5:':~t w
v;;"z~~: ~:. ~ ~.y.:;" i ~',!:~. .. ,. _;~.'; .''". ~7~': : ~ s .:
;':.'~:~c..:='~:
v:%.''~. s_: '~ '~'tYw,'~;. 3 .:.~:.f 't..'~.i ! y~~:y fJi3 »'~;:<% , ,,
i~'~C'3~;:J.3..._~.t'~'. ~E~t::
,J .. . ecLJ:.:.!2Y..C"
........'..','~::C'~'.;~.riCr~'a,j~ '.,;.~.a':~..,......,~:'..'4-.~ta
f,~,~.;~y.'~,~t.a..~. ~.. it '~ i ;7::!'~v r.::> " :;,t~;;-: 'Yr
.,.. .. ~ ,. :.: ~.'... , ~.., ~.~$. ~. ~:. .. ,.: ., ,.....:. E., y~ ~
~'Y~~~',... .:. ... w. ... ,.
Z_.~_~.f'C:..~..~.~yY t~ Si .Ci..':.'.,y.';.:,. .
.;~::.','~'.~5~.~~:' i::i:3 .",~,~.:>ryc~,-r't:i'.~,'~. .%.~':, 'c.''','y.~'f~
i:::.%.'ri~.i..' ~'li'3~3. .. _,:~~.,i.:..t.:~.':7t: :jw~:
:~.c~Z.Y~":F: r%z~~~:%~ ~r .~. ~ yy:E~. .i.~~'~ %.'.~' ~::~iF ,~:.
:,!E~..'tc:.'v :,:.~. ,... >:f~t:3;j°.''~:a3.:~..~t:.~'
w s.
;~uE~'_'.~_~:_t ~~ .,'~%';r.:;7~:~~3.,',~"::J3 ~:.'3 w~3~: z~'t ts~.~'';;:
w'~~5':::..f:..?.,.."f.s ~.~3,>~~ w~. y.~.,~f::~wr~;:k
r~''~'..~'~3?vi~~:f.t:~~.~ f::.~~:~. ~'J~~::i:.:t,.~~~.:'.JS.~a~~.?V'
...fi_~~:...t....::rt' ~.,.:t,.~.~'.~'..;, .i'~'~'.~~rC;w ..~..'if~'~~.'
i'
'Ci%:'~~~~:~. s. ny.;~ . .~:~>~,s"~ z''"..~.%~~~~. rZ~ ~'~"~..~,~S'~:~:t-
C"'t"y ~'::» twv ,. ~ .. ,.',t:7~'f~%tj~,~,~:~.,~....'.J %'~:.i
..'.'.~.;';;':w o ~~.....;.:'"a.., .y..~i'~ ~;:%:~.~:. ~~'.~'%.'%~?~~i:~
c~j':, .~.~:'<,M'"~. %' ::~" :r.-. ~'' '"'' )!~ ~(~1
S.i._.. ...5. ~'.~~.w j;'i°.~... 4..'t.7)).\
~.i.::~. w..22tv' %..5~'s.~.':r'~ .,...:..., ~'.~,r.''., r r..har~~, r:"::',
's~.3 ~':" S- .:._iy..: '# ';,.y..r ; "~ _:
y,:: ~:~;.~.:.:.,: :~~~; f ....,. .~~:~~.._~_~:~..,. ~:.,,:,.r:~~t.3.
_, - . A ~ ;.. , ..t a ~, 3 q~ ~~
~, ~.'" . .:' ~ :~,'l, ,. > .i'~.'~ F ~ ~ ; ~3~.i'~i ~:. ,'.% h7~ t" ~ ~~. ..
.. .'r ;~3 ;~ ~ , ~"'t~~ .. .2.." v..°'s.:':.' _, .... . ~~:~.~:
~r7., ? ? 7,: a f " ~ 5 : =~ .~ .. ;4 ~' . : . .
:.. ". ~, ~..~.:8. ..aj:'~A~Ssi'..;~~..i.~~,s..: 'i'f.<..:...5. ~~:S-y
4y.:~.C'k':.~'.., '.~i"~~', .~,i.. :~~:.~..f..:. ~.'w: '' .,
~ ~' "hiS.'; ;<3t:~.C'~ ::. ~i,',., ,~,.,~. .~.:..;....,~ ";.'w~
~,.4~J..;i.3'',i:::. ~"'.u?~ ,~.:.° ~'..t_3 i.:v'~~'t~', u.
..;...~,~:..~~'... ~..'.'t~:~.
:ict~.~. , '~c'3,Wrt~ ~: , .:. ~' ~ f'zf~.;.''. ;;: w ........ ~s: _=z,3<~E ~
t.~ ~',',~?".e':> ~": ..' i~:: '.: ,.,....:,:.
H,~~'tyil#..3;.'~, '~'.~3.f::."~':#C'~' 3,~i.~.y;,'% f;?: ~~~:: ~...t;~
.~tj~'~.f;: ti.':'y'ic'~.:~ :::s: ~s'~~'~f" A3~',:':':
:'.-"; ~C~.. '."~'~,a: ;. ~,~x !,~.~~t,:. ~...~.it~. ,~ w w'"',. ~.~L..'
C.'~..e'~.'~:. a < ~ ~..~ :.;.~' .~s: fl ~:4i ~,e.:a .
~' i'ls. _s..4.~~'., ~~llJ.v.,..'', is a1 ' ~:~''~''~3f~ii~,.a:,..Y ;~.~~"'~'
S. I ..
,, 'C' .. . ... (. i2.. ''.', ;;,. - ~y3.~J'w.. ~.'i.....,.$.~.. r~ :..3 , 4~
,
'~.i:.3~,.~:~#4 : , i3~~~, s<:~~i . ~"'w ~~._.~'~":3:,. ~';>~f~~.J3i. :i.~.~.
:~1.: 4~{.:.'Ws..'.3. s'.?sn~~.."zs ~~,(.~v i4ly~n~'.'3;
'u ~,'3>. :z'~ .. ~:~~~~'ltJ~'3c>~~.ia y~f.~%x'~'.,:1.'~;.r~ ~'?c~~; .... ;"~
.:: sz~~,.N '-; . "~ ~'~ ~: ' z s~iL3s:~f~ ; ..~~ 4:.'.:'',:
?. t E. i >.. ~ . . . ... ;_. y .'x C.'. L.. ~~
w
'~..';i . -- s, . . ~ y ~y
~.~,w.',~..x3:y~~.',yi.i:. ''i$,'~,~.i~si-.~c~t.i'~.1~~':.C". c:E.i~t:i
i:.:~.~.','5:'.r:.~AY.,t, si:::.~':.':y:'~.':::<.,'~ vcr-.,r i~is3~:.,~'.2
r w~ n r'c.~, i' ~ .~~;7~~3 t , ~.,,.~
s' 3.~:-~" ~. ;. c:~ 'r' ~,.~ r~ . ~w. .7, w ~' ~ ''.' :~3 i~iC~. .-'z t:; ci
': ~ ,,;rE: '" '.~ .~%i n. '' ' , :: f'
~: I . s-~ . ' ,.. .. f:: i'~'. .':: ~: w ~ ; ' r~ , s ? '. ,.
1 ,.:.~',:_:. .~ JZ" .r.iyri:.. :'' L. 3.:i.:. ';.3~".:;~:5:3~'y'
.:..~.i...~'E.i :~~f~;.'j.;_ ~.':y::'...
~.. y. f '. t,:,~ ~ ~ .y ~. A~ t'$~ h :.. :"~ i..: ~;,. fit.. ~.. i 3 ~.) ~m.
~:r,..; : "~. ~ .,. ..ii3 :.,.; .~. . , t ~ ~..:. ~ . ~..:;. ~': ~
:'.":~,~;w.; ~.': ~~~ ~ :. ._, :; ~'s 6 Z.h~. _:. n' S%'~'' ~ : r
~.~:'.","~:.~3.'S.~'.CSi. C<~f: '''.-.~~'.a:;~:~J:.~',
~:i.3 a~a;:. v3~.3. :.::.':'~:..':.~:~3~ ~'f' ';?:'c ~.:j, ~.'f.. ...,~
~'z.::~ ~..'v'i~::f~ ;:#'i%~~: ~. i'3 f' ..
~':.y'.:f: tyy~: -;%'x'".i~~.~t.ir.3,c~.w.sy; ia.~'..~.%;.'<.3C'. :~
vr~~:try'.. ;~;~:,v, -:v ~ -y~ ~~.r.~ ~- ~ c., '
s~. .. 5, ,_._ "~.3..'~..:.,:.,. .~ w,._...__7.:.. ,s~..'~.
:~...:._~f.~'1%3..i:::3,~'
~i%::-A....ci'~.~i::;;:5~''e.%~f:.: '~~~ .., ~'x %; ~
.~..'~,.~..,:3...f.~~.~:,'; i.':.~.lc.'".:~ ~'"E''r;3~'::>ri % f,~.;,: n
t~p:~:'. ~. w ,,w ~_... f;:_s. ...,...,. ,..tV' ~ w
:~.i.e..~ti ~~.'.rr.:'.~':Et;?~..'t.~ '~.5.~.':~'~~, f~~'~'!'~~:
_.~~.~'.~.~'.4rs.''',..'.,..:..i.::.c.... .;~:'t°';,: ':::~i ;'~~~:rr~
'~' ~' ~ t f. . ~, :'... ~,:. ~'f' .... _ . , n ,. y '~.,.. .i, ,t'.....: J,
:: ~.'r.~ i':::'.'. 'i':.'i,;' s.).~'. ~v . ~,
. . ;,,:~ ~~ ;i'~, a. %%.i't :.:; ~: v: ,~ .., r~ ~~~- ~; ~: ::~ 3y~:; :.
i:'s.F:'~'3: .. ...,. .. ' ° ,.,
Lir.
'w'r%t..z!,e ; ~ ~::.~. .. ~,::.~.~'. i3~.3.
CA 02298792 2000-02-11
- 20 - 04645.0601
the carbon anode surface. Therefore, for lithium ion cells, at
least one of the R groups in the phosphonate additive having the
general formula (R10) P (=O) (0R2) (R3) should be either hydrogen
(acidic proton) or a saturated or unsaturated organic group
containing 1 to 13 carbon atoms.
While not intended to be bound by any particular theory, it
is believed that due to the presence of the -OP(=0)(R)0-
functional group, the reductive cleavage of at least owe of the
ORland ORZ bonds in the phosphonate additives of the present
invention may produce lithium phosphonate or the lithium salt of
a phosphonate reduction product of the general formula 0=P(O-
Li)"(OR)m (n = 1 or 2; m = 0 or 1) deposited on the anode surface.
This anode surface film is ionically more conductive than the
film formed in the absence of the additives and is responsible
for the improved performance of the lithium-ion cells.
The concentration limit for the phosphonate additive is
preferably about O.OO1M to about 0.40M. The beneficial effect of
the phosphonate additive will not be apparent if the additive
concentration is less than about O.OO1M. On the other hand, if
the additive concentration is greater than about 0.40M, the
beneficial effect of the additive will be canceled by the
detrimental effect of higher internal cell resistance due to the
thicker anode surface film formation and lower electrolyte
conductivity.
It is appreciated that various modifications to the
inventive concepts described herein may be apparent to those of
ordinary skill in the art without departing from the spirit and
scope of the present invention as defined by the appended claims.