Note: Descriptions are shown in the official language in which they were submitted.
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
TITLE OF THE INVENTION
PYRROLIDINE AND PIPERIDINE MODULATORS OF CHEMOKINE
RECEPTOR ACTIVITY
BACKGROUND OF THE INVENTION
Chemokines are chemotactic cytokines that are released by
a wide variety of cells to attract macrophages, T cells, eosinophils,
basophils and neutrophils to sites of inflammation (reviewed in Schall,
C okin , 3 165-183 (1991) and Murphy, Rev. Immun., ~, 593-633 (1994)).
There are two classes of chemokines, C-X-C (a) and C-C ((3), depending
on whether the first two cysteines are separated by a single amino acid
(C-X-C) or are adjacent (C-C). The a-chemokines, such as interleukin-8
(IL-8), neutrophil-activating protein-2 (NAP-2) and melanoma growth
stimulatory activity protein (MGSA) are chemotactic primarily for
neutrophils, whereas (3-chemokines, such as R,ANTES, MIP-1a, MIP-lei
monocyte chemotactic protein-1 (MCP-1), MCP-2, MCP-3 and eotaxin
are chemotactic for macrophages, T-cells, eosinophils and basophils
(Deng, et al., Na re, 3~",1, 661-666 (1996)).
The chemokines bind specific cell-surface receptors
belonging to the family of G-protein-coupled seven-transmembrane-
domain proteins (reviewed in Horuk, Trends Pharm. Sci., x,159-165
(1994)) which are termed "chemokine receptors." On binding their
cognate ligands, chemokine receptors transduce an intracellular signal
though the associated trimeric G protein, resulting in a rapid increase
in intracellular calcium concentration. There are at least seven human
chemokine receptors that bind or respond to ~i-chemokines with the
following characteristic pattern: CCR-1 (or "CKR-1" or "CC-CKR-1")
[MIP-la, MIP-1~3, MCP-3, RANTES] (Ben-Barruch, et al., J. Biol
Chem., 2~, 22123-22128 {1995); Beote, et al, ~Cg~l, ~, 415-425 (1993)); CCR-
2A and CCR-2B (or "CKR-2A"/"CKR-2A" or "CC-CKR-2A"/"CC-CKR-
2A") [MCP-1, MCP-3, MCP-4]; CCR-3 (or "CKR-3" or "CC-CKR-3")
[eotaxin, RANTES, MCP-3] (Combadiere, et al., J. Bio~. Chem., '~70,
16491-16494 (1995); CCR-4 (or "CKR-4" or "CC-CKR-4") [MIP-la,
R,ANTES, MCP-1] (Power, et al., J. Biol. Chem., ~Ql ,19495-19500 (1995));
CCR-5 (or "CKR-5" or "CC-CKR-5") [MIP-la, RANTES, MIP-lei]
-1-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(Sanson, et al., ~~gchemistrv, ~5, 3362-3367 (1996)); and the Duffy blood-
group antigen [RANTES, MCP-1] (Chaudhun, et al., J. Biol. Chem., 269,
7835-7838 (1994)). The (3-chemokines include eotaxin, MIP ("macrophage
inflammatory protein"), MCP ("monocyte chemoattractant protein") and
RANTES ("regulation-upon-activation, normal T expressed and
secreted").
Chemokine receptors, such as CCR-1, CCR-2, CCR-2A,
CCR-2B, CCR-3, CCR-4, CCR-5, CXCR-3, CXCR-4, have been implicated
as being important mediators of inflammatory and immunoregulatory
disorders and diseases, including asthma and allergic diseases, as well
as autoimmune pathologies such as rheumatoid arthritis and
atherosclerosis. Accordingly, agents which modulate chemokine
receptors would be useful in such disorders and diseases.
A retrovirus designated human immunodeficiency virus
(HIV-1) is the etiological agent of the complex disease that includes
progressive destruction of the immune system (acquired immune
deficiency syndrome; AIDS) and degeneration of the central and
peripheral nervous system. This virus was previously known as LAV,
HTLV-III, or ARV.
Certain compounds have been demonstrated to inhibit the
replication of HIV, including soluble CD4 protein and synthetic
derivatives (Smith, et al., Science, 238, 1704-1707 (1987)), dextran sulfate,
the dyes Direct Yellow 50, Evans Blue, and certain azo dyes (U.S. Patent
No. 5,468,469). Some of these antiviral agents have been shown to act by
blocking the binding of gp120, the coat protein of HIV, to its target, the
CD4 gyycoprotein of the cell.
Entry of HIV-1 into a target cell requires cell-surface CD4
and additional host cell cofactors. Fusin has been identified as a cofactor
required for infection with virus adapted for growth in transformed T-
cells, however, Eosin does not promote entry of macrophagetropic
viruses which are believed to be the key pathogenic strains of HIV in
vivo. It has recently been recognized that for efficient entry into target
cells, human immunodeficiency viruses require the chemokine
receptors CCR-5 and CXCR-4, as well as the primary receptor CD4
(Levy, ~V. Engl. J. Med., 33(20), 1528-1530 (Nov. 141996). The principal
-2-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
cofactor for entry mediated by the envelope glycoproteins of primary
macrophage-trophic strains of HIV-1 is CCRS, a receptor for the ~3-
chemokines RANTES, MIP-la and MIP-lei (Deng, et al., Nature, ~1,
661-666 (1996)). HIV attaches to the CD4 molecule on cells through a
region of its envelope protein, gp120. It is believed that the CD-4 binding
site on the gp120 of HIV interacts with the CD4 molecule on the cell
surface, and undergoes conformational changes which allow it to bind to
another cell-surface receptor, such as CCR5 and/or CXCR-4. This
brings the viral envelope closer to the cell surface and allows interaction
between gp41 on the viral envelope and a fusion domain on the cell
surface, fusion with the cell membrane, and entry of the viral core into
the cell. It has been shown that ~i-chemokine ligands prevent HIV-1
from fusing with the cell (Dragic, et al., ure, 3~1, 667-673 (1996)). It
has further been demonstrated that a complex of gp120 and soluble CD4
interacts specifically with CCR-5 and inhibits the binding of the natural
CCR-5 ligands MIP-la and MIP-1(3 (Wu, et al., Nature, X84,179-183
(1996); Trkola, et al., Nature, x,184-187 (1996)).
Humans who are homozygous for mutant CCR-5 receptors
which do not serve as co-receptors for HIV-1 in vitro appear to be
unusually resistant to HIV-1 infection and are not
immunocompromised by the presence of this genetic variant t e,
~, 722-725 (1996)). Absence of CCR-5 appears to confer protection from
HIV-1 infection (Naturg, ~8 , 668-669 (1996)). Other chemokine receptors
may be used by some strains of HIV-1 or may be favored by non-sexual
routes of transmission. Although most HIV-1 isolates studied to date
utilize CCR-5 or fusin, some can use both as well as the related CCR-2B
and CCR-3 as co-receptors (Nature Medicine, 2_(11),1240-1243 (1996)).
Nevertheless, drugs targeting chemokine receptors may not be unduly
compromised by the genetic diversity of HIV-1 (Zhang, et al., N re,
,~, 768 (1996)). Accordingly, an agent which could block chemokine
receptors in humans who possess normal chemokine receptors should
prevent infection in healthy individuals and slow or halt viral
progression in infected patients. By focusing on the host's cellular
immune response to HIV infection, better therapies towards all subtypes
of HIV may be provided. These results indicate that inhibition of
-3-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
chemokine receptors presents a viable method fox the prevention or
treatment of infection by HIV and the prevention or treatment of AIDS.
The peptides eotaxin, RANTES, MIP-la, MIP-1(3, MCP-l,
and MCP-3 are known to bind to chemokine receptors. As noted above,
the inhibitors of HIV-1 replication present in supernatants of CD8+ T
cells have been characterized as the (3-chemokines RANTES, MIP-la
and MIP-1(3. PCT Patent Publication WO 97/10211 and EPO Patent
Publication EP 0,673,928 disclose certain piperidines as tachykinin
antagonists.
SUMMARY OF THE INVENTION
The present invention is directed to compounds which are
modulators of chemokine receptor activity and are useful in the
prevention or treatment of certain inflammatory and immunoregulatory
disorders and diseases, including asthma and allergic diseases, as well
as autoimmune pathologies such as rheumatoid arthritis and
atherosclerosis. The invention is also directed to pharmaceutical
compositions comprising these compounds and the use of these
compounds and compositions in the prevention or treatment of such
diseases in which chemokine receptors are involved.
The present invention is further concerned with compounds
which inhibit the entry of human immunodeficiency virus (HIV) into
target cells and are of value in the prevention of infection by HIV, the
treatment of infection by HIV and the prevention and/or treatment of the
resulting acquired immune deficiency syndrome {AIDS). The present
invention also relates to pharmaceutical compositions containing the
compounds and to a method of use of the present compounds and other
agents for the prevention and treatment of AIDS and viral infection by
HIV.
-4-
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
DETAILED DESCRIPTION OF THE INVENTION
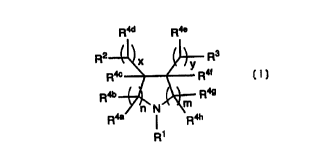
The present invention is directed to compounds of
formula I:
Raa Rae
R2 X Rs
R4c y Rat
R4b R49
N ~~
R4a ~ R4h
R1
I
wherein:
R1 is -X-R8 , wherein X is selected from the group consisting
of
(1) -CH2-,
(2) -CH2CH2-,
(3) -CH2CH2CH2-,
(4) -CH(C1_g alkyl)-,
(5) -CO-,
(6) -SO2-,
(7) -CONH2-, and
(8) -CONH(C1_6 alkyl)-,
and wherein
R8 is a selected
from:
phenyl, naphthyl,
biphenyl,
fluorenyl,
dihydronaphthyl,
tetrahydronaphthyl, octahydronaphthyl, C1_g
alkyl,
C5_g cycloalkyl, cyclohexenyl, adamantyl, and
heteroaryl,
which may be unsubstituted or substituted,
where the
substituents are independently selected from:
(a) hydroxy,
(b) C1_galkyl,
(c) -O-C1_galkyl,
(d) halogen,
(e) trifluoromethyl,
(f) phenyl
-5-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(g) -C02(C1_g alkyl), and
(h) -CONH2;
R2 is selected from the group consisting of
--N~ R5 ~--N / R5 ~--N N- R5
-N S ~-N S,,O N S\O
a O
.
O
i~
N O N N~S~ R~
O
O
N N~ R7
_ R
N N
wherein R5 is a selected from:
(1) -NR6C0-O-R7, wherein R6 is hydrogen, C1_6 alkyl or
C1_6 alkyl-C~_6 cycloalkyl, and R7 is C1_g alkyl,
C~_g cycloalkyl, benzyl or phenyl,
which is unsubsituted or substituted with halo,
C1_galkyl, C1_galkoxy or trifluoromethyl,
(2) -phenyl, which is unsubsituted or substituted with halo,
C1_galkyl, C1_3alkoxy or trifluoromethyl,
-6-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(3) -C1_galkyl-phenyl, which is unsubsituted or substituted
with halo, C1_galkyl, C1_galkoxy or trifluoromethyl,
(4) -O-C1_galkyl-phenyl, which is unsubsituted or substituted
with halo, C1_3alkyl, C1_3alkoxy or trifluoromethyl,
(5) -C1_4alkyl-O-C1_4alkyl-phenyl, which is unsubsituted or
substituted with halo, C1_3alkyl, C1_3alkoxy or
trifluoromethyl,
(6) -hydrogen,
(7) -C1_galkyl,
(8) -OH,
(9) -C02{C1_g alkyl), and
(10) -CO-NR6-(CO_3 alkyl)-R7;
R3 is selected from the group consisting of:
phenyl, naphthyl, biphenyl, fluorenyl, dihydronaphthyl,
tetrahydronaphthyl, octahydronaphthyl, C1_g alkyl,
C5_g cycloalkyl, cyclohexenyl, adamantyl, and heteroaryl,
which may be unsubstituted or substituted, where the
substituents are independently selected from:
(a) hydroxy,
(b) C1_galkyl,
(c) -O-C1_galkyl,
(d) halogen,
(e) trifluoromethyl,
(f) phenyl
(g) -C02(C1_g alkyl), and
(h) -CONH2;
R,4a~ R,4b~ R,4c~ R,4d~ R4e~ R,4f R,4g~ and R4h are independently selected
from the group consisting of:
(I) hydrogen, and
(2) C1_g alkyl;
_ '7 _
*rB
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
m is an integer selected from 0, 1 and 2, and n is an integer selected
from 0, 1 and 2, wherein the sum of m + n is 1, 2, 3 or 4;
x is an integer selected from 0, 1, 2 and 3;
y is an integer selected from 0, 1 and 2;
and pharmaceutically acceptable salts thereof and individual
diastereomers thereof.
Preferred compounds of the present invention include those
of formula Ia:
R2 R3
N
R'
Ia
wherein:
Rl, RZ and R3 are defined above;
and pharmaceutically acceptable salts and individual diastereomers
thereof.
Preferred compounds of the present invention include those
of formula Ib:
R2 R3
N
R~
Ib
wherein:
_g_
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
R1 is -X-R$, wherein X is selected from the group consisting of:
(1) -CH2-,
(2) -CH2CH2-,
(3) -CH(C1_g alkyl)-,
(4) -CO-,
(5) -S02-,
(6) -CONH2-, and
(7) -CONH(C1_g alkyl)-,
and wherein R$ is a selected from:
phenyl, naphthyl, biphenyl, fluorenyl, dihydronaphthyl,
tetrahydronaphthyl, octahydronaphthyl, C1_g alkyl,
C5_$ cycloalkyl, cyclohexenyl, adamantyl, and heteroaryl,
which may be unsubstituted or substituted, where the
substituents are independently selected from:
(a) hydroxy,
(b) C1_galkyl,
(c) -O-C 1-galkyl,
(d) halogen,
(e) trifluoromethyl,
(f) phenyl
(g) -C02(C1_g alkyl), and
(h) -CONH2;
R2 is selected from the group consisting of:
N~ Rs
O
--N S.O ~-N S ~ ~-N 0
~O
> >
wherein R5 is a selected from:
-9-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(1) -NRSCO-O-R7, wherein Rs is hydrogen, C1_g alkyl or
C1_g alkyl-C5_g cycloalkyl, and R7 is C1_g alkyl,
C5_6 cycloalkyl, benzyl or phenyl, which is unsubsituted or
substituted with halo, C1_3alkyl, C1_galkoxy or
trifluoromethyl,
(2) -phenyl, which is unsubsituted or substituted with halo,
C1_3alkyl, C1_3alkoxy or trifluoromethyl,
(3) -C1_galkyl-phenyl, which is unsubsituted or substituted
with halo, C1_galkyl, C1_3alkoxy or trifluoromethyl,
(4) -hydrogen,
(5) -C1_galkyl,
(6) -OH,
(7) -C02(C1_g alkyl), and
(8) -CO-NR6-(Cp_3 alkyl)-R7;
R3 is selected from the group consisting of:
phenyl, naphthyl, biphenyl, fluorenyl, dihydronaphthyl,
tetrahydronaphthyl, octahydronaphthyl, C1_g alkyl,
C5_g cycloalkyl, cyclohexenyl, adamantyl, and heteroaryl,
which may be unsubstituted or substituted, where the
substituents are independently selected from:
(a) hydroxy,
(b) C1_3alkyl,
(c) -O-C1_3alkyl,
(d) halogen,
(e) trifluoromethyl,
(f) phenyl
(g) -C02(C1_6 alkyl), and
(h) -CONH2;
and pharmaceutically acceptable salts thereof and individual
diastereomers thereof.
-10-
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
More preferred compounds of the present invention include
those of formula Ic:
R
N>
R
Ic
wherein
R1 is -X-R8, wherein X is selected from the group consisting of
(1) -CH2-, and
(2) -CO-,
and wherein R8 is a selected from cyclohexyl, cyclopentyl,
naphthyl, unsubstituted phenyl or substituted phenyl ,
where the substituents on phenyl are independently selected
from halogen and methyl;
R5 is a selected from:
(1) -phenyl, which is unsubsituted or substituted with halo,
C1_3alkyi, C1_galkoxy or trifluoromethyl, and
(3) -C1_6alkyl-phenyl, which is unsubsituted or substituted
with halo, C1_3alkyl, C1-galkoxy or trifluoromethyl
Highly preferred compounds of the present invention
include those of formula Id:
R
N>
-11-
*rB
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Id
wherein R5 is a selected from:
(1) -phenyl, which is unsubsituted or substituted with halo,
C1_3alkyl, C1_3alkoxy or trifluoromethyl, and
(3) -Cg_4alkyl-phenyl, which is unsubsituted or substituted
with halo, C1_3alkyl, C1_3alkoxy or trifluoromethyl.
In the present invention it is preferred that
R1 is -X-R8, wherein X is selected from the group consisting of
(1) -CH2-,
(2) -CH2CH2-,
(3) -CH(C1_g alkyl)-,
(4) -CO-,
(5) -S02-,
(6) -CONH2-, and
(7) -CONH(C1_g alkyl)-,
and wherein R8 is a selected from:
phenyl, naphthyl, biphenyl, fluorenyl, dihydronaphthyl,
tetrahydronaphthyl, octahydronaphthyl, C1_g alkyl,
C5_g cycloalkyl, cyclohexenyl, adamantyl, and heteroaryl,
which may be unsubstituted or substituted, where the
substituents are independently selected from:
(a) hydroxy,
(b) C1_galkyl,
(c) -O-C1_galkyl,
(d) halogen,
(e) trifluoromethyl,
(f) phenyl
(g) -C02(C1_g alkyl), and
(h) -CONH2.
In the present invention it is more preferred that
Rl is -X-R8, wherein X is selected from the group consisting of
(1) -CH2-, and
(2) -CO-,
-12-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
and wherein R8 is a selected from:
phenyl, naphthyl, C1_6 alkyl, cyclohexyl, cyclopentyl, pyridyl,
quinolyl, thiophenyl, indolyl, benzoxazolyl and
benzthiazolyl,
which may be unsubstituted or substituted, where the
substituents are independently selected from:
(a) chloro,
(b) fluoro,
(c) -O-CH3,
(d) -CH3,
(e) trifluoromethyl,
(f) -C02CH3, and
(g) -CONH2.
In the present invention it is highly
preferred that
R1 is selected
from the
group consisting
of:
(1) -CH2-phenyl,
(2) -CO-phenyl,
(3) -CH2-(2-chlorophenyl),
(4) -CO-(2-chlorophenyl),
(5) -CH2-(2-naphthyl),
(6) -CO-(2-naphthyl),
(7) -CH2-cyclopentyl,
(8) -CO-cyclopentyl,
(9) -CH2-cyclohexyl, and
(10) -CO-cyclohexyl.
In the present invention it is most preferred that
Rl is selected from the group consisting of:
(1) -CH2-cyclopentyl,
(2) -CO-cyclopentyl,
(3) -CH2-cyclohexyl, and
(4) -CO-cyclohexyl.
-13-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
In the present invention it is preferred that
R2 is selected from the group consisting of:
O
N S O N S N O
O
> > >
wherein R5 is a selected from:
(1) -NR6C0-O-R7, wherein R6 is hydrogen, C1_g alkyl or
C1_g alkyl-C5_g cycloalkyl, and R7 is C1_6 alkyl,
C5_g cycloalkyl, benzyl or phenyl,
which is unsubsituted or substituted with halo, CFg,
C1_galkyl, C1_galkoxy or trifluoromethyl,
(2) -phenyl, which is unsubsituted or substituted with halo,
CFg, C1_galkyl, C1_3alkoxy or trifluoromethyl,
(3) -C1_galkyl,
(4) -C 1_6alkyl-phenyl, which is unsubsituted or substituted
with halo, CF3, C1_3alkyl, C1_3alkoxy or trifluoromethyl,
(5) -C02(C1_g alkyl), and
(6) -CO-NR6-(Cp_g alkyl)-R7.
In the present invention it is more preferred that
R2 is:
N~ Rs
wherein R5 is selected from:
(1) -NR6C0-O-R7, wherein R6 is hydrogen, C1_g alkyl or
C1_g alkyl-C5_g cycloalkyl, and R7 is C1_g alkyl,
-14-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
C5_g cycloalkyl, benzyl or phenyl,
which is unsubsituted or substituted with halo, CFg,
C1_3alkyl, C1_galkoxy or trifluoromethyl,
(2) -phenyl, which is unsubsituted or substituted with halo,
CF3, C1_galkyl, C1_3alkoxy or trifluoromethyl,
(3) -C1_6alkyl-phenyl, which is unsubsituted or substituted
with halo, CF3, C1_3alkyl, C1_3alkoxy or trifluoromethyl,
and
(4) -CO-NR,s-(CO_g alkyl)-R7.
In the present invention it is still more preferred that
R2 is:
N~ Rs
wherein R5 is selected from:
(1) -phenyl, which is unsubsituted or substituted with halo,
CFg, C1_galkyl, C1_galkoxy or trifluoromethyl, and
(2) -C1_galkyl-phenyl, which is unsubsituted or substituted
with halo, CFg, C1_galkyl, C1_galkoxy or trifluoromethyl.
In the present invention it is even more preferred that
R2 is:
wherein R5 is a selected from:
(1) -phenyl, which is unsubsituted or substituted with halo,
C1_galkyl, C1_galkoxy or trifluoromethyl, and
{3) -C3_4alkyl-phenyl, which is unsubsituted or substituted
with halo, C1_galkyl, C1_3alkoxy or trifluoromethyl.
In the present invention it is highly preferred that
-15-
*rB
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98117755
R2 is:
--N
O
--N N~O
CH2CH3
O
-N N~O
i
or
O
--N N~OCH3
In the present invention it is preferred that
R3 is selected from the group consisting of:
phenyl, naphthyl, biphenyl, fluorenyl, dihydronaphthyl,
tetrahydronaphthyl, octahydronaphthyl, C1_g alkyl,
Cb_g cycloalkyl, cyclohexenyl, adamantyl, and thienyl,
which may be unsubstituted or substituted, where the
substituents are independently selected from:
(a) hydroxy,
(b) C1_galkyl,
(c) -O-C1_galkyl,
(d) halogen,
-16-
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
(e) trifluoromethyl,
(f) phenyl
(g) -C02(C1_g alkyl), and
(h) -CONH2.
In the present invention it is more preferred that
R3 is phenyl or thienyl, which may be unsubstituted or substituted,
where the substituents are independently selected from:
(a) chloro,
(b) fluoro,
(c) bromo,
(d) trifluoromethyl, and
(e) -O-CH3.
In the present invention it is most preferred that
R3 is phenyl or thienyl.
In the present invention it is preferred that
R4a~ R,4b~ R,4c~ R,4d~ R,4e~ R4f R,4g~ and R4h are independently selected
from the group consisting of
(1) hydrogen, and
(2) C1_g alkyl.
In the present invention it is more preferred that
R4a, R4b, R4c~ R,4e~ R4f R,4g~ and R4h are each hydrogen and
wherein R4d is selected from the group consisting of
(1) hydrogen, and
(2) CH3.
In the present invention it is most preferred that
R,4a~ g,4b~ R,4c~ R,4e~ R4f R4g~ and R4h are each hydrogen.
In the present invention it is most preferred that
R4d is selected from the group consisting of
(1) hydrogen, and
-17-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(2) CHg.
In the present invention it is preferred that m is 1 and
n is 1.
In the present invention it is preferred that x is 1.
In the present invention it is preferred that y is 0.
The compounds of the instant invention have at least two
asymmetric centers at the ring junction of the substitutents bearing R2
and R3. Additional asymmetric centers may be present depending upon
the nature of the various substituents on the molecule. Each such
asymmetric center will independently produce two optical isomers and it
is intended that all of the possible optical isomers and diastereomers in
mixtures and as pure or partially purified compounds are included
within the ambit of this invention. The relative configurations of the
most preferred compounds of this invention are of the trans orientation,
i.e. as depicted:
R4d Rae R4d R4e
R2-~,~ x Rs R~ x ,~ Rs
R4c ~ y R4t R4~ y R4t
R4b R49 R4b R49
n N m n N
R4a ( R4h R4a ~ 4h
R
R1 or R1
The independent syntheses of these dlastereomers or their
chromatographic separations may be achieved as known in the art by
appropriate modification of the methodology disclosed herein. Their
absolute stereochemistry may be determined by the x-ray
crystallography of crystalline products or crystalline intermediates
which are derivatized, if necessary, with a reagent containing an
asymmetric center of known absolute configuration.
_18_
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
As appreciated by those of skill in the art, halo or halogen as
used herein are intended to include chloro, fluoro, bromo and iodo.
Similarly, C1-g, as in C1_galkyl is defined to identify the group as having
1, 2, 3, 4, 5, or 6 carbons, such that C1_6alkyl specifically includes
methyl, ethyl, propyl, butyl, pentyl, hexyl, and cyclohexyl. The term
"heteraaryl" as used herein is intended to include the following groups:
benzimidazolyl, benzofuranyl, benzoxazolyl, furanyl, imidazolyl, indolyl,
isooxazolyl, isothiazolyl, oxadiazolyl, oxazolyl, pyrazinyl, pyrazolyl,
pyridyl, pyrimidyl, pyrrolyl, quinolyl, tetrazolyl, thiadiazolyl, thiazolyl,
thienyl, and triazolyl.
Exemplifying the invention is the use of the compounds
disclosed in the Examples and herein.
Specific compounds within the present invention include a
compound which selected from the group consisting of:
\ I -N \ I , -N Ph
~S
(+/-) ~ ~ ~ (+/-)
N N
S02Ph S02Ph
/ /
S ~.O
N Ph
(+~-) ~ \
N' (+/-) Nl
ph S02Ph
-19-
CA 02298813 2000-O1-28
WO 99/09984 PCT1US98/17755
N \
S N S
(+~-) N> ~ ~ (+i-) N,
S02Ph S02Ph
O
N S'O N S, O
S02Ph S02Ph
/ /
N g ~ \ I N Ph
O
(+~-) ~ ~ (+~-)
N N
S02Ph Ph
N~ Ph /
\ I N Ph
(+/_> l
N
OS i \ O ,S S
/
-20-
CA 02298813 2000-O1-28
WO 99!09984 PCT/US98/17755
Ph N )-- Ph
v'S
O~ \ \
/ /
\ I N Ph
Ph ~+/->
N
O'S
O~ \
/
,O
\ N O \ N ~S
~O
(+/-) N> ~ ~ (+/-> N
1
Ph ~ Ph
/
/ \ I N Ph
\ ( N S
(+/_)
N
(+/_)
Ph /
-21-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
I N Ph / I
\ N~ Ph
(+/-)
N / I (+/-) N /
O~ N \ \ I
H
\ I N Ph \ I, N Ph
(+/-) N~ (+/-) N~
N~S N~O
O
N~/
N \ I N Ph
t+/-)
\
(+/-)
/ H
( N Ph
(+/-) N Ph
N
HsC _ I \
/ O~ Ph
-22-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
\ / \ /
Ph y J._ ~ N ~- Ph
\ I N Ph \ I N Ph
(+l-) Nl (+l-) Nl
CI Ph
CI CI
\ I N Ph \
N S
(+/-) N> (+/-) N>
Ph ~ Ph
-23-
CA 02298813 2000-O1-28
WO 99109984 PCT/US98/17755
CI CI
N S'~ O \ I N
S,,O
(+~-) N, ~ ~ (+/-) N
'Ph 'Ph
\ I N Ph / ~ Ph
\ N
(+/ ) Nl OCH (+i-) N~
3
O I \ O I \ OCH3
/ r
CI
/
\ I N o
c+/-) ~ / \ I /
N
Ph
CH3
'~,
~N
N CI \
(+/_) \ I /
I/
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
CI
\ / \ /
\ \
I/ I/
- /
CH3 \ ~ N Ph
N
\ (+,.) N
N
(+/_) \ ~ / O \
/ OCH3
\ ~ ,~
\ ~ N
(+~_)
CI (+,-) NJ
\ CI
O
CI / CI
-25-
CA 02298813 2000-O1-28
WO 99/09984 PC'f/US98/17755
\ ~, N \ I N
/ ~ /
(+~-) N~ CI (+/-) N~ CI
O' \ O' \
CI I ~ I / CI
\ N
/ ~+/-~
N~ CI
O'
CI I
CI
\ /
I/ \I
NJ
NJ
o=s=o
/I
\ \
-26-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
CH3 /
\ N O
N
N \ (+~-) N~ CI
(+/ ) \ ~ / \
O'
/ I /
N N .
O-
O (+/-)
N CI
_ \
N
U
(+~_) N~ CFs
O' \
_ \ _ \
N ,, I / ~ ~ N-- I /
~J ~ ~J ~''
(+~') N~ C02CH3 (+~') N~ CH3
O~ ~ \ O~ ~ \
/ /
-27-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
\ _ \
N l / ~ ~ N -
'=.
(+/.~ Ni F (+/_) N~
O' \ O'
N' I / i., /
\ N \_
(+/_)
NI
N
~~O (+/-) O \
I
/ CONH2 I ~ N
N,-~,, \ ~ N",~-~'~.. \
"~ N ~ N
(+/-) (+/-)
O' ~ N O' S
~/
Br
v
N~- ~ ~ N
(+,
(+/-)
/
-28-
*rB
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
_ \ /
N- I /
v
\ / N- ~ /
(+,_) Ni
c+~.> N
O \ C02CH3
I ~\
/ i
\ CH3
N - I _/
(+/.) N
O'
C02CH3
/ N _
\ / N- ~ i
(+/-) ~+~.) N~
0
Br /
N.,,, \ I N...-,,, \
N "~ N
(+~-) o I \ \ (+~-)
/ /
-29-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
_-
N ~ / N OH
N N
t+~-) I \ (+~->
N /
N \I
(+/-) N~ CH3 (+i->
N
I\ ~\
/ /
C02CH2Ph
..N Ni \ N~O
(+~-> ~ CH2CH3
N N
O I \ \ (+~->
CI / I /
-30-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
N N O
O
N
(+/-)
/
'-"_ /
N N O
O
N
(+/-)
/
i
Me HO .
N N
rv
N
:+/-)
(+/-)
-31-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
\ N''. \
N~
(+/_)
\
/
N'''' \
\ ,.
N
(+/_) O ~ /
N''~
N'
(+/-) ~ \
C
(+/-)
-32-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
N
I N- ?-N~O
~/
(+/_) O
(+/->
N ~ ~ ~ ~ , N N
U
N' N'
(+/-) I ~ (+/-)
/ /
N N ~/
N/ N
(+/-) I ~ (+/-)
/ /
-33-
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
- \/ S
N
(+/-)
c+i->
N~,,, / I N..~,, . \ I
\ ~
N~ CH3 ~ N~ ~ I
(+~-) O (+~-) O \
I~
F
O N N
N~ (+/-> Nl N,CH2CH3
(+~-) C02CH2Ph
O'
I
-34-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
N
N~CH2CH3 N
O \ C02CH2Ph (+/-) N, N.CH2CH3
C02CH2Ph
O,
N C02Et
,CH2CH3
N (+~-)
C02CH2Ph I \
O
N~ N
~J / /
O
(+/-)
-35-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
N''''~~, \
,O N
N
(+/_) ,--.
~I
'N~'~ \
~O N
N
(+/-)
O'
O ,-,,, / ~ O ,,,
~O~N \ ~O~ \
N~ Nl
CI
(+/ ) O (+/ ) O \
O
,, \
~O
N
N
(+/_) t. \
O \
-36-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
\ \
N I \ N \
+/-) N N Y (+/-)
O~ N ~ O
j / CH2CH3 ' /
\ ~ O
N
N-OMe
N, Me
(+/_>
O
N
~N
Me
(+/->
,, N
N
Me
N
(+/-)
-37-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
\ f/
N N
(+/-) N~ N~C02CH2Ph (+/-) Nl
CH2CH3
O, O,
CI
O
N
Me N \
(+i-)
/
N y
N
(+/_) N \ Me-/
/
-N,., l \ N.,.,,,
' N~
(+/-~ (+/-) O
-38-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
N~,,, \
v
' N
(+/') O ~ ~ \
-N ,-,, \
\
' N O H
N
(+/-)
N,-,, \ ~ N..-,-.
/ \ wr / \ v
N Ph N C02Me
(+/-) ,_
0~,,. O
(+/-)
-N'~,, \
\ ,.
' N
-.."1
(+/-) O ~ / \ N H
-39-
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
I\ ~ v
N- I /
/ (+/-) NJ
\
N- I /
I ~ U
/
(+~.) N
\ /
y _
F
(+~-)
-40-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
/ \ N / \
(+~-)
/ \ ~N-.
(+/-) N
0
i
o ~ \
N%,
U
N>
t+~_) i
i
(+~-)
/ \
-m-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(+~-)
/ \
V-.,
u-
N
(+l-) O i /
- 42 -
*rB
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/177SS
O
-' N~_
N
N
(+~-)
i
O
-" N~~_~
N
N)
(+~-)
\ / ,.,N
N
N
O
(+/_)
-43-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
:'~ N
(+/-)
.-- ~,'" N
N
(+/-)
O
~~~~, ~ - ~~~~,
~s NJ ~s NJ
\ I ~O N \ I ~O N
(+/-) (+/-)
w
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
F
O'S-N w," ~ O'S,N w~,
I ~ ~O
\ N \ N
F F (+~-)
(+~-) F
w
I~ I~
.N,,,
N
(+~-)
'O
- 45 -
C
CI
O
o-~(
~ ~N =. ~ F
Nl
(+~-)
o-
CA 02298813 2000-O1-28
WO 99/09984 PCTlUS98/17755
N
O
(+/-)
---., N \ / F
N
0 I ~ (+/-)
i
~ i
.,-N \ / F
N
(+/-)
i
F
F
N \
N
~O
(+/-)
~CI
CI
-46-
CA 02298813 2000-O1-28
WO 99/099$4 PCT/US98/17755
F
F
Nl
0 (+/-)
F
r
N F
N~
o (+/-)
i
I
-47-
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98117755
N ~ ~ F
N
o (+/-)
C
CI
F
F \
'- N
N~
~ i (+~-)
N~ N
(+~-)
N : ~ /
N~
(+~-) ~ \
-48-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
/ \
/ \ N ,-
N
(+~-)
W
CI
cl
\ /
\ / N
N
(+~-)
H~,.. ~
~''' H
H
N
N/
(+~-)
O
-49-
*rB
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
/ \ ~N ,-
N
(+~-) O / Br
\ I
/ \ ~N .--
N
(+~-)
O'
F
/ \
N
N
(+~-)
O'
I
-50-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
_.
N O
~N~
(+~-)
1
N-.,
N
(+~-)
O' /
I
N-
(+~-)
Br
-51-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
N :,,
(+/-)
F
~O
N :,
O
N~ /
l
o-
~I
N
-_ N
O O N ,;.
+/-
( )
N+
(+/-)
-52-
CA 02298813 2000-O1-28
WO 99/09984 PCT/fJS98/17755
-' N
/ I O~N ~1
\\ N .,,._
O
(+/_) \
N
/ \, ~N ..~. _
._ \ +/_ \ /
()
/\
s
~N
N
~N .,;.
/ \ -
-_. (+/-) ~ /
-53-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
N
1
~N ....
(+/_)
N
~N .::
w
-_ (+/-)
/
N~
I
3= =O
(+/_) /
~S
N-,. ( /
N'
(+/-)
- 54 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
N
O- -O
(+~-)
~ ~S
N
N
(+/_) O
-55-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
/ \
(+~-)
(+~-)
- v
N-'-
(+~ ) N
O~
-56-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
N-
(+/-)
rv
O N~~,,, ~ /
O~N Nl
(+/-)
-5?-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
/
\ / ~ --
--.
(+/-)
\ / N-. .--
(+/_) _N_
0
N- ~
\
N =; ...._.
(+/-) N
O
~N~
(+/-)
_~8_
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1
N -'
v/
c+/_) N
0
N-
~+/_)
F
\ ~N-
N>
~+/-) ! \
-59-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(+~-)
(+~-)
N-
(+~-)
-60-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
C CI
N .'
i
v
(+~-)
0
\ ~ N
N
,,,..
O
(+~-)
o, i o
-si-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(+/-) N
O'
(+/-)
o~Co / \
N--~N
i
(+/-) N
O' '~J
/
O
N N-, -.._
N
(+/_) O
-62-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
/\
O N~N .._....
-;
(+/-) N
O' '~
/ \
O ---~
N~N-: _
(+/_) N
O'
//O
/ \
O N__~N-;
(+/-) N
O
and pharmaceutically acceptable salts thereof and individual
diastereomers thereof.
The subject compounds are useful in a method of
modulating chemokine receptor activity in a patient in need of such
modulation comprising the administration of an effective amount of the
compound.
The present invention is directed to the use of the foregoing
spiro-substituted azacycles as modulators of chemokine receptor activity.
In particular, these compounds are useful as modulators of the
chemokine receptors, including CCR-1, CCR-2, CCR-2A, CCR-2B, CCR-
3, CCR-4, CCR-5, CXCR-3, and/or CXCR-4.
-63-
CA 02298813 2000-O1-28
WO 99/09984 PCT/CTS98/17755
The utility of the compounds in accordance with the present
invention as modulators of chemokine receptor activity may be
demonstrated by methodology known in the art, such as the assay for
CCR-1 and/or CCR-5 binding as disclosed by Van Riper, et al., J.J. Exn.
~., 177, 851-856 (1993), and the assay for CCR-2 and/or CCR-3 binding
as disclosed by Daugherty, et al., J. Exn. Med., 1$~, 2349-2354 (1996). Cell
lines for expressing the receptor of interest include those naturally
expressing the receptor, such as EOL-3 or THP-1, or a cell engineered to
express a recombinant receptor, such as CHO, RBL-2H3, HEK-293. For
example, a CCR3 transfected AML14.3D10 cell line has been placed on
restricted deposit with American Type Culture Collection in Rockville,
Maryland as ATCC No. CRL-12079, on April 5, 1996. The utility of the
compounds in accordance with the present invention as inhibitors of the
spread of HIV infection in cells may be demonstrated by methodology
known in the art, such as the HIV quantitation assay disclosed by
Nunberg, et al., J. Vyrologv, 6~ (9), 4887-4892 (1991).
In particular, the compounds of the following examples had
activity in binding to the CCR-5 receptor or to the CCR-3 receptor in the
aforementioned assays, generally with an IC50 of less than about 10 ~,M.
Such a result is indicative of the intrinsic activity of the compounds in
use as modulators of chemokine receptor activity.
Mammalian chemokine receptors provide a target fOT
interfering with or promoting eosinophil and/or lymphocyte function in
a mammal, such as a human. Compounds which inhibit or promote
chemokine receptor function, are particularly useful for modulating
eosinophil and/or lymphocyte function for therapeutic purposes.
Accordingly, the present invention is directed to compounds which are
useful in the prevention and/or treatment of a wide variety of
inflammatory and immunoregulatory disorders and diseases, including
asthma and allergic diseases, as well as autoimmune pathologies such
as rheumatoid arthritis and atherosclerosis.
For example, an instant compound which inhibits one or
more functions of a mammalian chemokine receptor (e.g., a human
chemokine receptor) may be administered to inhibit (i.e., reduce or
prevent) inflammation. As a result, one or more inflammatory
_ gø -
*rB
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
processes, such as leukocyte emigration, chemotaxis, exocytosis (e.g., of
enzymes, histamine) or inflammatory mediator release, is inhibited.
For example, eosinophilic infiltration to inflammatory sites (e.g., in
asthma) can be inhibited according to the present method.
Similarly, an instant compound which promotes one or
more functions of a mammalian chemokine receptor (e.g., a human
chemokine) is administered to stimulate (induce or enhance) an
inflammatory response, such as leukocyte emigration, chemotaxis,
exocytosis (e.g., of enzymes, histamine) or inflammatory mediator
release, resulting in the beneficial stimulation of inflammatory
processes. For example, eosinophils can be recruited to combat parasitic
infections.
In addition to primates, such as humans, a variety of other
mammals can be treated according to the method of the present
invention. For instance, mammals including, but not limited to, cows,
sheep, goats, horses, dogs, cats, guinea pigs, rats or other bovine, ovine,
equine, canine, feline, rodent or murine species can be treated.
However, the method can also be practiced in other species, such as
avian species (e.g., chickens).
Diseases and conditions associated with inflammation and
infection can be treated using the method of the present invention. In a
preferred embodiment, the disease or condition is one in which the
actions of eosinophils and/or lymphocytes are to be inhibited or
promoted, in order to modulate the inflammatory response.
Diseases or conditions of humans or other species which
can be treated with inhibitors of chemokine receptor function, include,
but are not limited to: inflammatory or allergic diseases and conditions,
including respiratory allergic diseases such as asthma, allergic
rhinitis, hypersensitivity lung diseases, hypersensitivity pneumonitis,
eosinophilic pneumonias (e.g., Loeffler's syndrome, chronic
eosinophilic pneumonia), delayed-type hypersentitivity, interstitial lung
diseases (ILD) (e.g., idiopathic pulmonary fibrosis, or ILD associated
with rheumatoid arthritis, systemic lupus erythematosus, ankylosing
spondylitis, systemic sclerosis, Sjogren's syndrome, polymyositis or
dermatomyositis); systemic anaphylaxis or hypersensitivity responses,
-65-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
drug allergies (e.g., to penicillin, cephalosporins), insect sting allergies;
autoimmune diseases, such as rheumatoid arthritis, psoriatic arthritis,
multiple sclerosis, systemic lupus erythematosus, myasthenia gravis,
juvenile onset diabetes; glomerulonephritis, autoimmune thyroiditis,
Behcet's disease; graft rejection (e.g., in transplantation), including
allograft rejection or graft-versus-host disease; inflammatory bowel
diseases, such as Crohn's disease and ulcerative colitis;
spondyloarthropathies; scleroderma; psoriasis (including T-cell
mediated psoriasis) and inflammatory dermatoses such an dermatitis,
eczema, atopic dermatitis, allergic contact dermatitis, urticaria;
vasculitis (e.g., necrotizing, cutaneous, and hypersensitivity vasculitis);
eosinphilic myositis, eosinophilic fasciitis; cancers with leukocyte
infiltration of the skin or organs. Other diseases or conditions in which
undesirable inflammatory responses are to be inhibited can be treated,
including, but not limited to, reperfusion injury, atherosclerosis, certain
hematologic malignancies, cytokine-induced toxicity (e.g., septic shock,
endotoxic shock), polymyositis, dermatomyositis.
Diseases or conditions of humans or other species which
can be treated with promoters of chemokine receptor function, include,
but are not limited to: immunosuppression, such as that in individuals
with immunodeficiency syndromes such as AIDS, individuals
undergoing radiation therapy, chemotherapy, therapy for autoimmune
disease or other drug therapy (e.g., corticosteroid therapy), which
causes immunosuppression; immunosuppression due congenital
deficiency in receptor function or other causes; and infectious diseases,
such as parasitic diseases, including, but not limited to helminth
infections, such as nematodes (round worms); (Trichuriasis,
Enterobiasis, Ascariasis, Hookworm, Strongyloidiasis, Trichinosis,
filariasis); trematodes (flukes) (Schistosomiasis, Clonorchiasis),
cestodes (tape worms) (Echinococcosis, Taeniasis saginata,
Cysticercosis); visceral worms, visceral larva migrans (e.g., Toxocara),
eosinophilic gastroenteritis (e.g., Anisaki spp., Phocanema ssp.~,
cutaneous larva migrans (Ancylostona braziliense, Ancylostoma
caninum).
-66-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
The compounds of the present invention are accordingly
useful in the prevention and treatment of a wide variety of inflammatory
and immunoregulatory disorders and diseases.
In another aspect, the instant invention may be used to
evaluate putative specific agonists or antagonists of chemokine
receptors, including CCR-1, CCR-2, CCR-2A, CCR-2B, CCR-3, CCR-4,
CCR-5, CXCR-3, and CXCR-4. Accordingly, the present invention is
directed to the use of these compounds in the preparation and execution
of screening assays for compounds which modulate the activity of
chemokine receptors. For example, the compounds of this invention are
useful for isolating receptor mutants, which are excellent screening
tools for more potent compounds. Furthermore, the compounds of this
invention are useful in establishing or determining the binding site of
other compounds to chemokine receptors, e.g., by competitive inhibition.
The compounds of the instant invention are also useful for the
evaluation of putative specific modulators of the chemokine receptors,
including CCR-1, CCR-2, CCR-2A, CCR-2B, CCR-3, CCR-4, CCR-5,
CXCR-3, and CXCR-4. As appreciated in the art, thorough evaluation of
specific agonists and antagonists of the above chemokine receptors has
been hampered by the lack of availability of non-peptidyl (metabolically
resistant) compounds with high binding affinity for these receptors.
Thus the compounds of this invention are commercial products to be
sold for these purposes.
The present invention is further directed to a method for the
manufacture of a medicament for modulating chemokine receptor
activity in humans and animals comprising combining a compound of
the present invention with a pharmaceutical carrier or diluent.
The present invention is further directed to the use of these
compounds in the prevention or treatment of infection by a retrovirus, in
particular, the human immunodeficiency virus (HIV) and the
treatment of, and delaying of the onset of consequent pathological
conditions such as AIDS. Treating AIDS or preventing or treating
infection by HIV is defined as including, but not limited to, treating a
wide range of states of HIV infection: AIDS, ARC (AIDS related
complex), both symptomatic and asymptomatic, and actual or potential
-67-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
exposure to HIV. For example, the compounds of this invention are
useful in treating infection by HIV after suspected past exposure to HIV
by, e.g., blood transfusion, organ transplant, exchange of body fluids,
bites, accidental needle stick, or exposure to patient blood during
surgery.
In a preferred aspect of the present invention, a subject
compound may be used in a method of inhibiting the binding of a human
immunodeficiency virus to a chemokine receptor, such as CCR-5 and/or
CXCR-4, of a target cell, which comprises contacting the target cell with
an amount of the compound which is effective at inhibiting the binding
of the virus to the chemokine receptor.
The subject treated in the methods above is a mammal,
preferably a human being, male or female, in whom modulation of
chemokine receptor activity is desired. "Modulation" as used herein is
intended to encompass antagonism, agonism, partial antagonism
and/or partial agonism. The term "therapeutically effective amount"
means the amount of the subject compound that will elicit the biological
or medical response of a tissue, system, animal or human that is being
sought by the researcher, veterinarian, medical doctor or other
clinician.
The term "composition" as used herein is intended to
encompass a product comprising the specified ingredients in the
specified amounts, as well as any product which results, directly or
indirectly, from combination of the specified ingredients in the specified
amounts. By "pharmaceutically acceptable" it is meant the carrier,
diluent or excipient must be compatible with the other ingredients of the
formulation and not deleterious to the recipient thereof.
The terms "administration of and or "administering a"
compound should be understood to mean providing a compound of the
invention or a prodrug of a compound of the invention to the individual
in need of treatment.
Combined therapy to modulate chemokine receptor activity
and thereby prevent and treat inflammatory and immunoregulatory
disorders and diseases, including asthma and allergic diseases, as well
as autoimmune pathologies such as rheumatoid arthritis and
-68-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
atherosclerosis, and those pathologies noted above is illustrated by the
combination of the compounds of this invention and other compounds
which are known for such utilities.
For example, in the treatment or prevention of
inflammation, the present compounds may be used in conjunction with
an antiinflammatory or analgesic agent such as an opiate agonist, a
lipoxygenase inhibitor, such as an inhibitor of 5-lipoxygenase, a
cyclooxygenase inhibitor, such as a cyclooxygenase-2 inhibitor, an
interleukin inhibitor, such as an interleukin-1 inhibitor, an NMDA
antagonist, an inhibitor of nitric oxide or an inhibitor of the synthesis of
nitric oxide, a non-steroidal antiinflammatory agent, or a cytokine-
suppressing antiinflammatory agent, for example with a compound
such as acetaminophen, asprin, codiene, fentanyl, ibuprofen,
indomethacin, ketorolac, morphine, naproxen, phenacetin, piroxicam,
a steroidal analgesic, sufentanyl, sunlindac, tenidap, and the like.
Similarly, the instant compounds may be administered with a pain
reliever; a potentiator such as caffeine, an H2-antagonist, simethicone,
aluminum or magnesium hydroxide; a decongestant such as
phenylephrine, phenylpropanolamine, pseudophedrine, oxymetazoline,
ephinephrine, naphazoline, xylometazoline, propylhexedrine, or levo-
desoxy-ephedrine; an antiitussive such as codeine, hydrocodone,
caramiphen, carbetapentane, or dextramethorphan; a diuretic; and a
sedating or non-sedating antihistamine.
The present invention is further directed to combinations of
the present compounds with one or more agents useful in the prevention
or treatment of AIDS. For example, the compounds of this invention
may be effectively administered, whether at periods of pre-exposure
and/or post-exposure, in combination with effective amounts of the AIDS
antivirals, immunomodulators, anti-infectives, or vaccines known to
those of ordinary skill in the art.
ANTIVIRALS
Drug Name Manufacturer Indication
-69-
*rB
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
HoechstJBayer HIV infection,
AIDS, ARC
(non-nucleoside
reverse
transcriptase
inhibitor)
141 W94 Glaxo Wellcome HIV infection,
AIDS, ARC
(protease inhibitor)
1592U89 Glaxo Wellcome HIV infection,
AIDS, ARC
Acemannan Carrington Labs ARC
(Irving, TX)
Acyclovir Burroughs Wellcome HIV infection,
AIDS
,
ARC, in
combination with
AZT
AD-439 Tanox Biosystems HIV infection,
AIDS
,
ARC
AD-519 Tanox Biosystems HIV infection,
AIDS
,
ARC
Adefovir dipivoxilGilead Sciences HIV infection
AL-721 Ethigen ARC, PGL
(Los Angeles, CA) HIV positive,
AIDS
Alpha InterferonGlaxo Wellcome Kaposi's sarcoma,
HIV in combination
w/R,etrovir
Ansamycin Adria Laboratories ARC
LM 427 (Dublin, OH)
Erbamont
(Stamford, CT)
-70-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Antibody which Advanced BiotherapyAIDS, ARC
neutralizes pH Concepts
labile alpha aberrant(Rockville, MD)
Interferon
AR177 Aronex Pharm HIV infection,
AIDS,
ARC
beta-fluoro-ddA Nat'1 Cancer InstituteAIDS-associated
diseases
(-) 6-Chloro-4(S)-Merck HIV infection,
cyclopropylethynyl- AIDS, ARC
4(S)-trifluoro- (non-nucleoside
methyl-1,4-dihydro- reverse
2H-3,1-benzoxazin- transcriptase
2-one inhibitor)
CI-1012 Warner-Lambert HIV-1 infection
Cidofovir Gilead Science CMV retinitis,
herpes,
papillomavirus
Curdlan sulfate AJI Pharma USA HIV infection
Cytomegalovirus MedImmune CMV retinitis
immune globin
Cytovene Syntex sight threatening
Ganciclovir C MV
peripheral CMV
retinitis
Delaviridine Pharmacia-Upjohn HIV infection,
AIDS, ARC
(protease inhibitor)
Dextran Sulfate Ueno Fine Chem. AIDS, ARC, HIV
Ind. Ltd. (Osaka, positive asymptomatic
Japan)
ddC Hoffinan-La Roche HIV infection,
AIDS,
Dideoxycytidine ARC
-71-
*rB
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
ddI Bristol-Myers SquibbHIV infection, AIDS,
Dideoxyinosine ARC; combination
with AZT/d4T
DMP-450 AVID HIV infection,
(Camden, NJ) AIDS, ARC
(protease inhibitor)
EL10 Elan Corp, PLC HIV infection
(Gainesville, GA)
Efavirenz, DMP- DuPont-Merck HIV infection,
266 Pharmaceuticals AIDS, ARC
(non-nucleoside
reverse
transcriptase
inhibitor)
Famciclovir Smith Kline herpes zoster,
herpes simplex
FTC Emory University HIV infection,
AIDS, ARC
(reverse transcriptase
inhibitor)
GS 840 Gilead HIV infection,
AIDS, ARC
(reverse transcriptase
inhibitor)
GW 141 Glaxo Welcome HIV infection,
AIDS, ARC
(protease inhibitor)
GW 1592 Glaxo Welcome HIV infection,
AIDS, ARC
(reverse transcriptase
inhibitor)
-72-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
HBY097 Hoechst Marion HIV infection,
Roussel AIDS, ARC
(non-nucleoside
reverse transcriptase
inhibitor)
Hypericin VIMRx Pharm. HIV infection,
AIDS,
ARC
Recombinant HumanTriton Biosciences AIDS, Kaposi's
Interferon Beta (Almeda, CA) sarcoma, ARC
Interferon alfa-n3Interferon SciencesARC, AIDS
Indinavir Merck HIV infection,
AIDS,
ARC, asymptomatic
HIV positive, also
in
combination with
AZT/ddI/ddC
ISIS 2922 ISIS PharmaceuticalsCMV retinitis
KNI-272 Nat'1 Cancer InstituteHIV-assoc.
diseases
Lamivudine, 3TC Glaxo Wellcome HIV infection,
AIDS, ARC
(reverse
transcriptase
inhibitor); also
with AZT
Lobucavir Bristol-Myers SquibbCMV infection
Nelfinavir Agouron HIV infection,
Pharmaceuticals AIDS, ARC
(protease inhibitor)
Nevirapine Boeheringer HIV infection,
Ingleheim AIDS, ARC
(protease inhibitor)
Novapren Novaferon Labs, HIV inhibitor
Inc.
(Akron, OH)
-73-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Peptide T Peninsula Labs AIDS
Octapeptide (Belmont, CA)
Sequence
Trisodium Astra Pharm. CMV retinitis,
HIV
Phosphonoformate Products, Inc infection, other
CMV
infections
PNU-140690 Pharmacia Upjohn HIV infection,
AIDS, ARC
(protease inhibitor)
Probucol Vyrex HIV infection,
AIDS
RBC-CD4 Sheffield Med. HIV infection,
Tech (Houston TX) AIDS, ARC
Ritonavir Abbott HIV infection,
AIDS, ARC
(protease inhibitor)
Saquinavir Hoffmann- HIV infection,
LaRoche AIDS, ARC
(protease inhibitor)
Stavudine; d4T Bristol-Myers SquibbHIV infection,
AIDS,
Didehydrodeoxy- ARC
thymidine
Valaciclovir Glaxo Wellcome genital HSV & CMV
infections
Virazole Viratek/ICN asymptomatic HIV
Ribavirin (Costa Mesa, CA) positive, LAS,
ARC
VX-478 Vertex HIV infection,
AIDS,
ARC
Zalcitabine Hoffmann-La Roche HIV infection,
AIDS,
ARC, With AZT
-74-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Zidovudine; AZT Glaxo Wellcome HIV infection,
AIDS,
ARC, Kaposi's
sarcoma, in
combination with
other therapies
IM~' RS
UNO
MODULATO
Dru~~Tame . Indication
-
Manufacturer
AS-101 Wyeth-Ayerst AIDS
Bropirimine Pharmacia Upjohn advanced AIDS
Acemannan Carrington Labs, AIDS, ARC
Inc.
(Irving, TX)
CL246,738 American Cyanamid AIDS, Kaposi's
Lederle Labs sarcoma
EL10 Elan Corp, PLC HIV infection
(Gainesville, GA)
Gamma interferon Genentech ARC, in combination
w/TNF {tumor
necrosis factor)
Granulocyte Genetics Institute AIDS
Macrophage Colony Sandoz
Stimulating
Factor
Granulocyte Hoeschst-Roussel AIDS
Macrophage Colony Immunex
Stimulating
Factor
Granulocyte Schering-Plough AIDS, combination
Macrophage Colony w/AZT
Stimulating Factor
HIV Core Particle Rorer seropositive HIV
Immunostimulant
-75-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
IL-2 Cetus AIDS, in combination
Interleukin-2 w/AZT
IL-2 Hoffman-La Roche AIDS, ARC, HIV,
in
Interleukin-2 Immunex combination w/AZT
IL-2 Chiron AIDS, increase in
CD4
Interleukin-2 cell counts
(aldeslukin)
Immune Globulin Cutter Biological pediatric AIDS,
in
Intravenous (Berkeley, CA) combination w/AZT
(human)
IMREG-1 Imreg AIDS, Kaposi's
(New Orleans, LA) sarcoma, ARC, PGL
IMREG-2 Imreg AIDS, Kaposi's
(New Orleans, LA) sarcoma, ARC, PGL
Imuthiol Diethyl Merieux Institute AIDS, ARC
Dithio Carbamate
Alpha-2 Schering Plough Kaposi's sarcoma
Interferon w/AZT, AIDS
Methionine- TNI Pharmaceutical AIDS, ARC
Enkephalin (Chicago, IL)
MTP-PE Ciba-Geigy Corp. Kaposi's sarcoma
Muramyl-Tripeptide
Granulocyte Amgen AIDS, in combination
Colony Stimulating w/AZT
Factor
Remune Immune Response immunotherapeutic
Corp.
rCD4 Genentech AIDS, ARC
Recombinant
Soluble Human
CD4
rCD4-IgG AIDS, ARC
hybrids
Recombinant Biogen AIDS, ARC
Soluble Human
CD4
-76-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Interferon Hoffman-La Roche Kaposi's sarcoma
Alfa 2a AIDS, ARC, in
combination w/AZT
SK&F106528 Smith Kline HIV infection
Soluble T4
Thymopentin Immunobiology HIV infection
Research Institute
Tumor Necrosis Genentech ARC, in combination
Factor; TNF w/gamma Interferon
ANTI-INFECTIVES
Drug Name Manufacturer Indication
Clindamycin with Pharmacia Upjohn PCP
Primaquine
Fluconazole Pfizer cryptococcal
meningitis,
candidiasis
Pastille Squibb Corp. prevention of
Nystatin Pastille oral candidiasis
Ornidyl Merrell Dow PCP
Eflornithine
Pentamidine LyphoMed PCP treatment
Isethionate (IM (Rosemont, IL)
& IV)
Trimethoprim antibacterial
Trimethoprim/sulfa antibacterial
Piritrexim Burroughs Wellcome PCP treatment
Pentamidine Fisons Corporation PCP prophylaxis
isethionate for
inhalation
Spiramycin Rhone-Poulenc cryptosporidial
diarrhea
-77-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Intraconazole- Janssen Pharm. histoplasmosis;
851211 cryptococcal
meningitis
Trimetrexate Warner-Lambert PCP
OTHER
Drug Name Manufacturer Indication
Daunorubicin NeXstar, Sequus Karposi's sarcoma
Recombinant Human Ortho Pharm. Corp.severe anemia
Erythropoietin assoc. with AZT
therapy
Recombinant Human Serono AIDS-related wasting,
Growth Hormone cachexia
Leukotriene B4 - HIV infection
Receptor Antagonist
Megestrol Acetate Bristol-Myers Squibbtreatment of
anorexia assoc.
w/AIDS
Soluble CD4 Protein- HIV infection
and Derivatives
Testosterone Alza, Smith Kline AIDS-related wasting
Total Enteral Norwich Eaton diarrhea and
Nutrition Pharmaceuticals malabsorption
related to AIDS
It will be understood that the scope of combinations of the
compounds of this invention with AIDS antivirals, immunomodulators,
anti-infectives or vaccines is not limited to the list in the above Table, but
includes in principle any combination with any pharmaceutical
composition useful for the treatment of AIDS.
Preferred combinations are simultaneous or alternating
treatments of with a compound of the present invention and an inhibitor
of HIV protease and/or a non-nucleoside inhibitor of HIV reverse
_ 78 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
transcriptase. An optional fourth component in the combination is a
nucleoside inhibitor of HIV reverse transcriptase, such as AZT, 3TC,
ddC or ddI. Preferred agents for combination therapy include:
Zidovudine, Lamivudine, Stavudine, Efavirenz, Ritonavir, Nelfinavir,
Abacavir, Indinavir, 141-W94 (4-amino-N-((2 syn,3S)-2-hydroxy-4-phenyl-
3-((S)-tetrahydrofuran-3-yloxycarbonylamino)-butyl)-N-isobutyl-
benzenesulfonamide), N-(2(R)-hydroxy-1(S)-indanyi)-2(R)-phenylmethyl-
4-(S )-hydroxy-5-( 1-(4-(2-benzo [b] furanylmethyl)-2(S)-N'(t-butylcarbox-
amido)-piperazinyl))-pentaneamide, and Delavirdine. A preferred
inhibitor of HIV protease is indinavir, which is the sulfate salt of N-
(2(R)-hydroxy-1(S)-indanyl)-2{R)-phenylmethyl-4-(S)-hydroxy-5-( 1-(4-(3-
pyridyl-methyl)-2(S)-N'-(t-butylcarbo-xamido)-piperazinyl))-pentane-
amide ethanolate, and is synthesized according to U.S. 5,413,999.
Indinavir is generally administered at a dosage of 800 mg three times a
day. Other preferred inhibitors of HIV protease include nelfinavir and
ritonavir. Preferred non-nucleoside inhibitors of HIV reverse
transcriptase include (-) 6-chloro-4(S)-cyclopropylethynyl-4{S)-
trifluoromethyl-1,4-dihydro-2H-3,1-benzoxazin-2-one, which may be
prepared by methods disclosed in EP 0,582,455. The preparation of ddC,
ddI and AZT are also described in EPO 0,484,071. These combinations
may have unexpected effects on limiting the spread and degree of
infection of HIV. Preferred combinations with the compounds of the
present invention include the following: (1) Zidovudine and
Lamivudine; (2) Stavudine and Lamivudine; (3) Efavirenz; (4) Ritoavir;
(5) Nelfinavir; (6) Abacavir; (7) Indinavir; (8) 141-W94; and (9)
Delavirdine. Preferred combinations with the compounds of the present
invention further include the following (1) indinavir, with efavirenz or (-)
6-chloro-4(S)-cyclopropylethynyl-4(S)-trifluoromethyl-1,4-dihydro-2H-3,1-
benzoxazin-2-one, and, optionally, AZT and/or 3TC and/or ddI and/or
ddC; (2) indinavir, and any of AZT and/or ddI and/or ddC.
In such combinations the compound of the present
invention and other active agents may be administered separately or in
conjunction. In addition, the administration of one element may be
prior to, concurrent to, or subsequent to the administration of other
agent(s).
-79-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
The compounds of the present invention may be
administered by oral, parenteral (e.g., intramuscular, intraperitoneal,
intravenous, ICV, intracisternal injection or infusion, subcutaneous
injection, or implant), by inhalation spray, nasal, vaginal, rectal,
sublingual, or topical routes of administration and may be formulated,
alone or together, in suitable dosage unit formulations containing
conventional non-toxic pharmaceutically acceptable carriers, adjuvants
and vehicles appropriate for each route of administration. In addition to
the treatment of warm-blooded animals such as mice, rats, horses,
cattle, sheep, dogs, cats, monkeys, etc., the compounds of the invention
are effective for use in humans.
The pharmaceutical compositions for the administration of
the compounds of this invention may conveniently be presented in
dosage unit form and may be prepared by any of the methods well known
in the art of pharmacy. All methods include the step of bringing the
active ingredient into association with the carrier which constitutes one
or more accessory ingredients. In general, the pharmaceutical
compositions are prepared by uniformly and intimately bringing the
active ingredient into association with a liquid carrier or a finely divided
solid carrier or both, and then, if necessary, shaping the product into the
desired formulation. In the pharmaceutical composition the active
object compound is included in an amount sufficient to produce the
desired effect upon the process or condition of diseases. As used herein,
the term "composition" is intended to encompass a product comprising
the specified ingredients in the specified amounts, as well as any
product which results, directly or indirectly, from combination of the
specified ingredients in the specified amounts.
The pharmaceutical compositions containing the active
ingredient may be in a form suitable for oral use, for example, as tablets,
troches, lozenges, aqueous or oily suspensions, dispersible powders or
granules, emulsions, hard or soft capsules, or syrups or elixirs.
Compositions intended for oral use may be prepared according to any
method known to the art for the manufacture of pharmaceutical
compositions and such compositions may contain one or more agents
selected from the group consisting of sweetening agents, flavoring
-80-
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
agents, coloring agents and preserving agents in order to provide
pharmaceutically elegant and palatable preparations. Tablets contain
the active ingredient in admixture with non-toxic pharmaceutically
acceptable excipients which are suitable for the manufacture of tablets.
These excipients may be for example, inert diluents, such as calcium
carbonate, sodium carbonate, lactose, calcium phosphate or sodium
phosphate; granulating and disintegrating agents, for example, corn
starch, or alginic acid; binding agents, for example starch, gelatin or
acacia, and lubricating agents, for example magnesium stearate,
stearic acid or talc. The tablets may be uncoated or they may be coated by
known techniques to delay disintegration and absorption in the
gastrointestinal tract and thereby provide a sustained action over a
longer period. For example, a time delay material such as glyceryl
monostearate or glyceryl distearate may be employed. They may also be
coated by the techniques described in the U.S. Patents 4,256,108;
4,166,452; and 4,265,874 to form osmotic therapeutic tablets for control
release.
Formulations for oral use may also be presented as hard
gelatin capsules wherein the active ingredient is mixed with an inert
solid diluent, for example, calcium carbonate, calcium phosphate or
kaolin, or as soft gelatin capsules wherein the active ingredient is mixed
with water or an oil medium, for example peanut oil, liquid paraffin, or
olive oil.
Aqueous suspensions contain the active materials in
admixture with excipients suitable for the manufacture of aqueous
suspensions. Such excipients are suspending agents, for example
sodium carboxymethylcellulose, methylcellulose, hydroxy-
propylmethylcellulose, sodium alginate, polyvinyl-pyrrolidone, gum
tragacanth and gum acacia; dispersing or wetting agents may be a
naturally-occurring phosphatide, for example lecithin, or condensation
products of an alkylene oxide with fatty acids, for example
polyoxyethylene stearate, or condensation products of ethylene oxide
with long chain aliphatic alcohols, for example
heptadecaethyleneoxycetanoi, or condensation products of ethylene oxide
with partial esters derived from fatty acids and a hexitol such as
-81-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
polyoxyethylene sorbitol monooleate, or condensation products of
ethylene oxide with partial esters derived from fatty acids and hexitol
anhydrides, for example polyethylene sorbitan monooleate. The aqueous
suspensions may also contain one or more preservatives, for example
ethyl, or n-propyl, p-hydroxybenzoate, one or more coloring agents, one
or more flavoring agents, and one or more sweetening agents, such as
sucrose or saccharin.
Oily suspensions may be formulated by suspending the
active ingredient in a vegetable oil, for example arachis oil, olive oil,
sesame oil or coconut oil, or in a mineral oil such as liquid paraffin. The
oily suspensions may contain a thickening agent, for example beeswax,
hard paraffin or cetyl alcohol. Sweetening agents such as those set forth
above, and flavoring agents may be added to provide a palatable oral
preparation. These compositions may be preserved by the addition of an
anti-oxidant such as ascorbic acid.
Dispersible powders and granules suitable for preparation
of an aqueous suspension by the addition of water provide the active
ingredient in admixture with a dispersing or wetting agent, suspending
agent and one or more preservatives. Suitable dispersing or wetting
agents and suspending agents are exemplified by those already
mentioned above. Additional excipients, for example sweetening,
flavoring and coloring agents, may also be present.
The pharmaceutical compositions of the invention may also
be in the form of oil-in-water emulsions. The oily phase may be a
vegetable oil, for example olive oil or arachis oil, or a mineral oil, for
example liquid paraffin or mixtures of these. Suitable emulsifying
agents may be naturally- occurring gums, for example gum acacia or
gum tragacanth, naturally-occurring phosphatides, for example soy
bean, lecithin, and esters or partial esters derived from fatty acids and
hexitol anhydrides, for example sorbitan monooleate, and condensation
products of the said partial esters with ethylene oxide, for example
polyoxyethylene sorbitan monooleate. The emulsions may also contain
sweetening and flavoring agents.
Syrups and elixirs may be formulated with sweetening
agents, for example glycerol, propylene glycol, sorbitol or sucrose. Such
-82-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
formulations may also contain a demulcent, a preservative and
flavoring and coloring agents.
The pharmaceutical compositions may be in the form of a
sterile injectable aqueous or oleagenous suspension. This suspension
may be formulated according to the known art using those suitable
dispersing or wetting agents and suspending agents which have been
mentioned above. The sterile injectable preparation may also be a sterile
injectable solution or suspension in a non-toxic parenterally-acceptable
diluent or solvent, for example as a solution in 1,3-butane diol. Among
the acceptable vehicles and solvents that may be employed are water,
Ringer's solution and isotonic sodium chloride solution. In addition,
sterile, fixed oils are conventionally employed as a solvent or suspending
medium. For this purpose any bland fixed oil may be employed
including synthetic mono- or diglycerides. In addition, fatty acids such
as oleic acid find use in the preparation of injectables.
The compounds of the present invention may also be
administered in the form of suppositories for rectal administration of the
drug. These compositions can be prepared by mixing the drug with a
suitable non-irritating excipient which is solid at ordinary temperatures
but liquid at the rectal temperature and will therefore melt in the rectum
to release the drug. Such materials are cocoa butter and polyethylene
glycols.
For topical use, creams, ointments, jellies, solutions or
suspensions, etc., containing the compounds of The present invention
are employed. (Fox purposes of this application, topical application shall
include mouth washes and gargles.)
The pharmaceutical composition and method of the present
invention may further comprise other therapeutically active compounds
as noted herein which are usually applied in the treatment of the above
mentioned pathological conditions.
In the treatment or prevention of conditions which require
chemokine receptor modulation an appropriate dosage level will
generally be about 0.01 to 500 mg per kg patient body weight per day
which can be administered in single or multiple doses. Preferably, the
dosage level will be about 0.1 to about 250 mg/kg per day; more preferably
-83-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
about 0.5 to about 100 mg/kg per day. A suitable dosage level may be
about 0.01 to 250 mg/kg per day, about 0.05 to 100 mg/kg per day, or about
0.1 to 50 mg/kg per day. Within this range the dosage may be 0.05 to 0.5,
0.5 to 5 or 5 to 50 mg/kg per day. For oral administration, the
compositions are preferably provided in the form of tablets containing 1.0
to 1000 milligrams of the active ingredient, particularly 1.0, 5.0, 10.0,
15Ø 20.0, 25.0, 50.0, 75.0, 100.0, 150.0, 200.0, 250.0, 300.0, 400.0, 500.0,
600.0, 750.0, 800.0, 900.0, and 1000.0 milligrams of the active ingredient
for the symptomatic adjustment of the dosage to the patient to be treated.
The compounds may be administered on a regimen of 1 to 4 times per
day, preferably once or twice per day.
It will be understood, however, that the specific dose level
and frequency of dosage for any particular patient may be varied and
will depend upon a variety of factors including the activity of the specific
compound employed, the metabolic stability and length of action of that
compound, the age, body weight, general health, sex, diet, mode and
time of administration, rate of excretion, drug combination, the severity
of the particular condition, and the host undergoing therapy.
Several methods for preparing the compounds of this
invention are illustrated in the following Schemes and Examples.
Starting materials are made from known procedures or as illustrated.
Several methods for preparing the compounds of this
invention are illustrated in the following Schemes and Examples.
_g4_
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
SCHEME 1
R~ Rad
X R3 {~N R3
Rac Raf CN-H II Rac Raf
n N~~m ~ n N~~m
R1 {iPr)2NEt III R'
I CH3CN or iBuCN
R'~ NaBH3CN,
O R3 NaB(OAc)~H Rad
R~ Raf ~N-H II or ~~N
N~~m NaBH4 Rac Raf
n
MeOH, CH2CI2, or EtCl2, ~ n N~~m
mol. sieves III R~
IV
X' R3 ~~N R3
Rac Raf C~N'_H II Rac Raf
N~~m ~ n N~~m
n Ri (iPr)2NEt CH2CI2 R1
V VI
'~--' R4c Raf
4f LiAIHa or BH3~THF
' n N ~m
THF or Et20 VII o1
In one protocol, the compounds of the present invention are
prepared by alkylating heterocycle I (wherein X is a leaving group such
as, for example, bromide, iodide, methanesulfonate, p-toluenesulfonate,
trifluoromethanesulfonate) with cyclic amine II under appropriate
conditions to provide compound III (Scheme 1). Cyclic amine II is
-85-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
available commercially or can be prepared using the methods given
below.
Alternatively, heterocycle IV, bearing a carbonyl group, can
be combined with the cyclic amine II and the intermediate imine or
iminium species is reduced to tertiary amine III under homogenous
conditions (e.g. using sodium cyanoborohydride, sodium borohydride, or
sodium triacetoxyborohydride) or in the presence of hydrogen and a
heterogeneous catalyst (e.g. palladium on carbon or Raney nickel).
In an alternative embodiment of the present invention,
heterocycle V, bearing an activated acyl side chain (wherein X', for
example, is a chloride or bromide atom, or is a hydroxybenzotriazole
residue from activation of the corresponding carboxylic acid with HOBt
in the presence of a suitable carbodiimide) is allowed to react with cyclic
amine II to provide the corresponding tertiary amide VI (Scheme 1).
Compound VI can then be treated with a suitable reducing agent (e.g.
diborane; borane in THF; borane dimethylsulfide, or lithium aluminum
hydride) to provide the desired product VII.
SCHEME 2
R4d
HO R (Ph0)2P(O)N3, HN3, or
R4c R4r Zn(N3)~PYridine)2 N3 Rs
1 R4~ R4f
n N - Jm Ph P, DEAD, imidazole
toluene ~ n N~~m
V III IX R 1
R4d R4d
N3 R3 H2 R3
R4c R4t H2, 10% Pd/C R~ Rat
n N~~m MeOH ~ n N~~m
IX R~ X R~
-86-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Ar Ar
Ar
LiAIHa TsCI, pyridine
O O"O THF HO ~OH er
Ph3P, CBra X
CH3CN
XI XII XIII
Rad
Rad
H2Rac RRaf XIII Ar N Rs
Rac Raf
n R1 m (iPr)2NEt, iPrCN ~ N~~m
X ny
XIV R
An alternative preparation of the target compounds is
carried out as shown in Scheme 2. Treatment of alcohol VIII with zinc
azide bis(pyridine) complex in the presence of triphenylphosphine and
diethyl azodicarboxylate, or with diphenylphosphoryl azide, or with
hydrazoic acid, provides azide IX. Reduction of IX, for example, with
hydrogen and palladium on carbon, affords primary amine X. This
amine can be doubly alkylated with a bis-electrophile such as XIII under
basic conditions, to provide the desired chemokine receptor modulator
XIV. Bis-electrophiles can be prepared from substituted glutaric
anhydride derivatives such as XI by reduction to diol XII followed by
double activation, using, for example, p-toluenesulfonyl chloride in
pyridine, or triphenylphosphine carbon tetrabromide in acetonitrile, to
provide XIII (where X = Br or OTs).
-87-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
SCHEME 3
r ~Me~i(Me)3 catalytic TFA
O N CH2C12
OMe P
XV XVI
X
(iBu)zAIH HO- Ar Dess-Martin
periodinane
THF/toluene N~ CH2CI2
X'
XVIII p
H
r C~N-H NaB(OAc)3H CN- r
X~ N mol. sieves, CH2C12 ~ N
P~ Ph''
H2, 20% Pd/C
CN-;, Ar
NH4 HC02
N,
MeOH XXI
H
The preparation of compounds within the scope of the
instant invention which bear a 1,3,4-trisubstituted pyrrolidine
framework is detailed in Scheme 3. Treatment of a trans-cinnamic ester
such as XV with N-benzyl-N-methoxymethyl-N-(trimethylsilyl)-
methylamine (XVI) in the presence of a substoichiometric amount of an
acid such as TFA, titanium tetrafluoride lithium fluoride or cesium
fluoride according to the procedure of Padwa et al (J. Org. Chem. 1887,
52, 235) preferentially affords the 3,4-trans pyrrolidine XVII. Executing
this sequence starting from the cis-cinnamic ester results in
preferential formation of the 3,4-cis pyrrolidine. Reduction of ester
_8g_
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
XVII, for example, with diisobutylaluminum hydride, lithium
aluminium hydride, or sodium bis(2-methoxyethoxy)aluminum
hydride, provides the primary alcohol XVIII. Oxidation to the aldehyde
XIX can be carried out under numeroua conditions, such as with the
Dess-Martin periodinane, with DMSO and oxalyl chloride at low
temperature, followed by triethylamine (Swern oxidation), or with
various chromium trioxide-based reagents (see March J. "Advanced
Organic Chemistry", 4th ed., John Wiley & Sons, New York, pp. 1167-
1171 (1992)). Reductive amination with cyclic amine II then provides
diamine XX, which can itself be a chemokine receptor modulator.
Alternatively, The N-benzyl group is cleaved in a hydrogen atmosphere
in the presence of 20% palladium on carbon to provide the secondary
amine XXI.
SCHEME 4
CN Ar R~CHO, NaBH CN CN , Ar
3
~I N mol. sieves, HOAc XXII N
H MeOH
N- C~ - Ar
Ar R1X, (iPr)2NEt N
Nl
~I H DMF, XXIII
-89-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
RaCOCI, (iPr)2NEt
N- Ar CH2C12 CN- Ar
or
~I N R8C02H, BOP-CI XXIV N
(iPr)2NEt n~Ra
CH2C12
CAN- Ar
C~N- Ar R8g02C1, EtOAc
N>
XXI N Aq. NaHC03 XXV 01 i
H OS.Re
The 1-unsubstituted pyrrolidine XXI may be further
functionalized as shown in Scheme 4. Reductive amination with
suitable aldehydes under standard conditions provides the tertiary
amine XXII. For subunits with a primary or secondary aliphatic carbon
linked to nitrogen, alkylation with a suitable halide, methanesulfonate,
p-toluene-sulfonate, etc. is carried out under standard conditions to
provide N-alkylated pyrrolidine XXIII. Alternatively, secondary amine
XXI are acylated with, for example, acid chlorides or bromides, or
activated esters such as HOBt esters (prepared by treating the precursor
carboxylic acid with a suitable carbodiimide in the presence of HOBt) or
a symmetrical or mixed anhydride, to give amide XXIV. The
sulfonamide XXV is prepared under standard conditions by exposing
XXI to an alkyl or aryl sulfonyl chloride in the presence of a suitable base
to neutralize the formed hydrogen chloride.
_g0_
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
SCHEME 5
OH R4t Me i(Me)3 OHC. Ar
catalytic TFA R4c ' Rat
R Ar ~ CH2C12 N>
XXVI Ph XVI PhJ XXVII
NaBH3CN,
OHC Ar NaB OAc H
Rac R4r N-H ( or )3 CN ac ' Ar 4r
R R
II NaBH4
J N
MeOH, CH2C12, or EtCl2, XXVIII
Ph XXVII mol. sieves Ph
CN Ar H2, 20% Pd/C CN Ar
R~ '~ R4f NH4+HC02 R4~ ~ R4t
XXVIII N/ MeOH XXIX N/
PhJ H
Compounds possessing geminal substituents on positions 3
or 4 (or on both C3 and C4) of the pyrrolidine ring are prepared by the
method shown in Scheme 5. Cycloaddition of unsaturated aldehyde
XXVI with reagent XVI in the presence of a substoichiometric amount
of an acid such as TFA or titanium tetrafluoride according to the
procedure of Padwa et al (J. Org. Chem. 1987, 52, 235) provides
pyrrolidine aldehyde XXVII. Reductive amination with cyclic amine II
under standard conditions provides diamine XXVIII, which is
debenzylated in a hydrogen atmosphere with palladium on charcoal
catalyst, to afford the secondary pyrrolidine XXI~~. Compound XXIX is
further functionalized according to the chemistry detailed in Scheme 4
for compound XXI.
-91-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
SCHEME 6
Tf
(Me3Si)2NLi, THF ~ Pd{Ph3P)4, Et3N
NJ C ~ NJ CO, DMF, MEOH
Bn ~ Bn
XXX N N{S02CF3)2 XXXI
02Me 1 ) ArMgCI, CuBr2, CN
Me3SiCl, Et20, THF LiAIH4
2) iPrMgCI, THF Ar
N
Bn N-H II N
i
XXXII XXXIII Bn
N N
Ar H2, Pd/C
Ar
XXXIV MeOH
NJ XXXV
Bn
The synthesis of one framework for piperidine-based
chemokine receptor modulators is given in Scheme 6. Enolate formation
of the 4-piperidone derivative XXX followed by formation of the vinyl
triflate with either 2-[N,N-bis(trifluoromethylsulfonyl)amino]-5-
chloropyridine or with N-phenyltriflimide provides compound ~I.
Palladium-catalysed carbonylation in the presence of methanol then
affords unsaturated ester XXXII. Conjugate addition of an aryl
magnesium halide reagent in the presence of a copper catalyst and
chlorotrimethylsilane to this species, followed by treatment with the
magnesium salt of a suitable cyclic amine, then yields amide XXXIII.
Reduction with lithium aluminum hydride or borane~THF affords the
tertiary amine XXXIV, which is hydrogenated under standard
conditions to the secondary piperidine XXXV. This compound is
-92-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
alkylated, acylated or sulfonated by analogy to the conditions described
for compound XXI in Scheme 4.
SCHEME 7
O O OTf O
OMe (Me3Si)2NLi, THF
~OMe
NJ I
Boc Boc
N' _ N SO CF
( 2 3)2 XXXVII
XXXVI Ar O
iPrM CI, THF
Pd(Ph~P)4, Et3N ~ OMe
ArSnMe3 or ArB(OH)2 NJ N-H II
DMF Boc
XXXVIII
Ar O Ar
N~ 1 ) Mg, MeOH N
N~ 2) AIH3, THF N'
Boc Boc
XXXIX Ar XL
TFA, CH2C12 N
N'
XLI
Synthesis of a piperidine derivative with an alternate
presentation of the 3- and 4-substituents is given in Scheme 7.
Formation of the enolate of ketoester XXXXVI (prepared from
commercially available 3-carbomethoxy-4-oxopiperidine and Boc
anhydride) followed by addition of either 2-[N,N-bis(trifluoro-
methylsulfonyl)amino]-5-chloropyridine or N-phenyltriflimide provides
vinyl triflate XX~~VII. Palladium-mediated coupling with a suitable
-93-
*rB
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
aryl stannane or aryl boronic acid provides unsaturated ester XX~~VIII.
Treatment of this compound with the magnesium salt of a suitable cyclic
amine then affords amide XXXIX, which can be reduced successively
with magnesium metal in methanol followed by alane in THF, to provide
the tertiary amine XL. Removal of the Boc group under standard acidic
conditions yields secondary amine XLI, which can be alkylated, acylated
or sulfonated by analogy to the conditions described for compound XXI in
Scheme 4.
SCHEME 8
O O 1 ) HOBT, EDAC
'N O
HO ~ t 2) ROH R, N
XLII Bu O ~ OtBu
XLIII
O O 1 } HOBT, EDAC O
'N
HO ~ tB 2) RR'NH R~ N
a N OtBu
XLII
R' XLIV
O O 1 ) HOBT, EDAC p
'N O
HO ~ t 2) Me0(Me)NH N
O Bu Me0-N U OtBu
XLII Me XLV
O O
RMgX or RLi O
N--~
Me0-N ' t N
O Bu R
OtBu
Me XLV
XLVI
-94-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
O
p O N H~TFA
'N TFA, CH2C12 R,O
R''O ~ t
O Bu XLVII
XLIII
O
O N O TFA, CH2C12 R'N NH~TFA
R'N ~ tBu
R XLVIII
R'
XLIV
O
O O TFA, CH2C12 NH~TFA
~N--~ R
R OtBu
XLIX
XLVI
The cyclic amine II employed in the preceding Schemes can
be obtained commercially in many cases or is prepared by a number of
procedures. For example, as shown in Scheme 8, compound XLII, the
N-t-butoxycarbonyl protected form of isonipecotic acid (4-piperidine-
carboxylic acid) is activated under standard conditions, for example with
a carbodiimide, and converted into ester XLIII or amide XLIV.
Alternatively, acid XLII is converted into the N-methyl-N-methoxy
amide, XLV, which upon reaction with organomagnesium and
organolithium reagents forms the ketone XLVI. The Boc group of
XLIII, XLIV and XLVI is removed under acidic conditions to provide
secondary amines XLVII, XLVIII and XLIX, respectively.
-95-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
SCHEME 9
O O 1 ) Oxalyl Chloride O
N CAN N
HO 2) NaN3; toluene, ~ OBn
OBn acetone O'
L 3) heat LI
O O
v
N N ROH R,
Cite v --
OBn O H N OBn
LI LII
O R. O
N ~N-~ RR'NH N N \N-
--~.
OBn R' H OBn
LI LIII
R\ p H2; Pd/C
O N N R,
H ~ gn MeOH O N NH
H
LII LIV
R O H2; Pd/C
N N N R,
H ~ MeOH N N NH
R' OBn R, H
Llll
LV
Alternatively, CBZ-protected piperidine L is allowed to react
with oxalyl chloride and then sodium azide, to provide the corresponding
acyl azide, which can then be thermally rearranged to isocyanate LI
(Scheme 9). Compound LI is treated with an alcohol ROH or an amine
RR'NH to form carbamate LII or urea LIII, respectively, each of which
is deprotected with hydrogen in the presence of palladium on carbon to
secondary amines LIV or LV.
_9g_
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
SCHEME 10
O NaH
O N N--~ -'' Q N N--~
CHI H OBn DMF CH ~ OBn
LI I'
x = 1-3 ( 2)" LVI
NaH, DMF
O N N-~ R~X O N N--
H OBn R' OBn
LII LVII
O H ; Pd/C
N ~N--~ ~ ~ N ~N H
(CH2)X~ OBn MeOH (CH2)xJ
LVI LVIII
O H ~ Pd/C
O N ~N--~ 2' O N N H
R' OBn MeOH R~
LVII LIX
If the carbamate LII has R = -(CH2)xCH2Cl, where x = 1-3,
then treatment with a suitable base, such as sodium hydride, lithium
hexamethyldisilazide or potassium t-butoxide, can induce cyclization to
compound LVI (Scheme 10). For other R groups, carbamate LII is
treated with an alkylating agent R'X,where R' = primary or secondary
alkyl, allyl, propargyl or benzyl, while X = bromide, iodide, tosylate,
mesylate or trifluoromethanesulfonate, in the presence of a suitable
base, such as sodium hydride, lithium hexamethyldisilazide or
potassium t-butoxide, to give derivative LVII; a similar process can be
employed for urea LIII. In each case, removal of the CBZ protecting
group under standard conditions provides the secondary amines LVIII
and LIX.
-97-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
SCHEME 11
Me Me
O t-BuOH; CuCI ~ O
,N N~ Me O H--~N
C' __
LI OBn DMF LX OBn
Me~ ~ TFA O
Me~O H N --- TFA~NH2~N-
CH CI
OBn z 2 OBn
LX LXI
O
TFA NH ~N--~ RCOCI, CH2CI2 O
~ 2 R NI-~N--
LXI OBn Pyridine H
LXII OBn
O
O ROCOCI, CH2CI2 O
TFA~NH2~N-~ R~O~N N
OBn Pyridine H
OBn
LII
RR'NCOCI, CH2CI2
TFA~NH2 N--~ Pyridine R~ ~ ~ /~1 O
-' N
LXI OBn or , i ~ H~N
RNCO (R = H) R ~ ~---~ LIII OBn
TFA~NH2 N~O RS02C1 , CH~12 ~S~ O
R N N
LXI OBn pyridine H--C
O Bn
LXIII
Additional derivatization of a piperidine with nitrogen
functionality at C4 is carried out as shown in Scheme 11. For example,
if the ring nitrogen is protected with a CBZ group, as with isocyanate LI,
treatment with tert-butyl alcohol in the presence of copper(I) chloride,
provides Boc derivative LX. This compound is selectively deprotected to
the free amine LXI. This amine is acylated with an acid chloride, a
chloroformate, an isocyanate, or a carbamyl chloride, to provide
compounds LXII, LII or LIII. Alternatively, amine LXI is sulfonated
with an alkyl or arylsulfonyl chloride, to give sulfonamide LXIII.
Compounds LXII and LXIII optionally is alkylated under the conditions
given above for the preparation of LVII from LII.
-98-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
SCHEME 12
O H ; Pd/C
2
R H N R H NH
OBn MeOH
LXII LXIV
O H2; Pd/C
R~ R
O H \N H
O H N~ B MeOH
O n
LII LIV
R~ O H2; Pd/C O
N H N
R~ pgn MeOH R~ N H \N H
R'
LIII
LV
H2; Pd/C
,O
R N N-~ ,S,
H OBn MeOH R H NH
LXIII
LXV
As shown in Scheme 12, removal of the CBZ group under
reductive conditions gives the desired secondary amines LIV, LXIV,
LV, and LXV.
_99_
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/1775~
SCHEME 13
Me~ ~ H2, Pd/C Me~
Me~.O N N-~ Me O N NH
LX H OBn MeOH H
Raa
O Rs LXVI
Me~ ~ Rac Raf
Me~O N N H + 1
H ~ n N Jm IV
LXVI R~
Me
NaBH3CN, MeOH Me~ ~ R'~
Me O N N R3
mol. sieves LXVII H Rac Raf
Rad C N.(~)m
MeCOCI HCI~NH2 N R3
Rac Raf
MeOH LXVIII ~ n N~~m
R~
Functionalization of the piperidine also is carried out after
it has been coupled with an Nl substituent. For example, as shown in
Scheme 13, reductive deprotection of CBZ derivative LX yields secondary
amine LXVI. Reductive amination with an appropriate aldehyde or
ketone fragment (such as IV) provides piperidine LXVII. Removal of
the Boc group under acidic conditions then gives primary amine
LXVIII. This primary amine is then functionalized by analogy to the
chemistry given in Scheme 11. Cyclic amines (compound II) with
spirocyclic functionality are prepared in some cases using methods
described in the literature; more specifically, as described in Ong, H. H.
et al, Journal of Medicinal Chemistry, 1983,26, 981-986, and Chen et al,
US Patent No. 5,536,716. None of the compounds in these references are
disclosed to be chemokine receptor modulators.
-100 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98117755
SCHEME 14
OBn ~OBn
1) pyridine, toluen /e
~\ N CH3CN N
2) CF3C02H, 60°C
3) NaBH4, MeOH \
i
HN~NH3CI CHO R ~ / N
LXXI H
LXIX LXX
R2-X
O
~OBn
H
N ~HCI Me3Sil; HCI
or
H2, Pd/C; HCI
R ~ \ ~ R_
/ N
LXXIII R~ LXXII R
Substituted spiro(indoline-3,4'-piperidine) derivatives can be
prepared as shown in Scheme 14 starting from the substituted
phenylhydrazine LXIX and the aldyhyde LXX. Following the Fischer
indole reaction and reduction of the intermediate imine with a mild
reducing agent such as sodium borohydride, the indoline LXXI can be
combined with an electrophile such as an acyl chloride or a sulfonyl
chloride. The protecting group on compound LXXII, for example a
benzyloxycarbonyl group, can be removed by treatment with hydrogen in
the presence of palladium on carbon or by exposure to trimethylsilyl
iodide, to give the deprotected substituted spiro(indoline-3,4'-piperidine)
LXXIII.
-101-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
BOC
~oc methyl phenyl
N sulfoxide KOtBu, tBuOH
L D AC, THF H O 50°C
S~O
LXXIV
/ LXXV ~ LXXVIII
R '
Boc Boc ~ ~ gH
N N
SOCl2, PhMe Br
----- ~ K2C03, 40°C
'OH 2,6-lutidine
LXXVI C
LXXVI I
~oc ~oc H HCI
N Bu3SnH, AIBN N 1) TFA N
Br phH 0 2) HCI, MeOH
- ~ \ ~ \
\- S \J
LXXIX R LXXX R LXXXI R
Preparation of spiro(2,3-dihydrobenzothiophene-3,4'-
piperidine) derivatives is shown in Scheme 15. Reaction piperidone
LXXIV with the lithium salt of methyl phenyl sulfoxide affords adduct
LXXV. Base-mediated elimination-rearrangement and basic cleavage
provides the allylic alcohol LXXVI. The alcohol is converted to
rearranged allylic chloride LXXVII with thionyl chloride in toluene in
the presence of 2,6-lutidine as a proton scavenger. Displacement of the
chloride with the 2-bromothiophenol LXXVIII provides allylic sulfide
LXXIX, which can be cyclized under radical conditions to give spiro(2,3-
dihydrobenzothiophene-3,4'-piperidine) LX~~X. Cleavage of the t-
butoxycarbonyl group under standard conditions, such as trifluoroacetic
acid, then provides the desired spirocycle LX~I.
-102-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
SCHEME 16
R
~e
NaH Me02C
F + _ - \F
C C! N J
O LXXXII LXXXIII LXXXIV Me
LiAIH4
NaH ~ ~ ~NMe
.... H
THF ~ DMF
LXXXVI
LXXX'
I N
Me
Me O CI
1,2-dichloroethane, H~HCI
reflux
""", JI I
Spiro(2,3-dihydrobenzofuran-3,4'-piperidine) derivatives are
prepared as illustrated in Scheme 16. Treatment of an appropiately
substituted ester of 2-fluorophenylacetate (I~XII) with
mechlorethamine hydrochloride (LXXXIII) under basic conditions
provides piperidine LXXXIV, which on treatment with a strong
reducing agent such as lithium aluminum hydride produces the
corresponding 4-(hydroxymethyl) compound LXX~~V. Cyclization with
base provides benzofuran LXXXVI, and cleavage of the N-methyl group
is then carried out using 1-chloroethyl chloroformate or other suitable N-
demethylating agents, to provide the desired intermediate LXX~~VII.
-103-
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
Ot-Bu CF3C02H H~TFA
LXXXVI I I .XXXIX
HN3, H~,S04 H H
rt to 45 deg C
w. X~.i
Spiro(2-oxo-1,2,3,4-tetrahydroquinoline-4,4'-piperidine) and
spiro(1-oxo-1,2,3,4-tetrahydroisoquinoline-4,4'-piperidine) are prepared
as shown in Scheme 17. Starting from the spiro(2-oxoindane-3,4'-
piperidine) I~XVIII (described in Claremon, D.A. et al, fro can
Patent 0 431943 943 A2, Evans, B.E. et , U.S. Patent 5,091,38 .?, Davis, L.
et al, U.S. Patent 4,420,485, all of which are incorporated by reference,
and Parham et al, Journal of Organic Chemistry, 41, 2628 (1976)),
deprotection of the piperidine nitrogen is carried out by treatment with
acid, for example trifluoroacetic acid, to provide ketone LXXXIX. After
protection as the trifluoroacetamide, the product is exposed to hydrazoic
acid in the presence of sulfuric acid. Heating of this mixture effects a
Schmidt rearrangement, to provide both tetrahydroquinoline XC and the
tetrahydroisoquinoline XCI. These spiro compounds are then separated
and coupled to functionalized aldehydes by the methodology given above.
Cyclic amines (compound II) which are 4-arylpiperazines
functionality are prepared using methods described in the following
Schemes. Starting materials are made from known procedures or as
illustrated. Substituted purines are prepared as disclosed in US
5,057,517; imidazo(1.2-a}pyrazinyl, as disclosed in US 4,242,344; (1,2,4)-
triazolo(1.5-a)pyrazinyl as disclosed in J. Org. Chem, 1974, 39, 2143 and
-104-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
J.C.S. Perkin I, 1980, 506; 1,7-naphthyridinyl as disclosed in J. Org.
Chem. 1963, 28, 1753; furo(3.2-c)pyridinyl as disclosed in J. Heterocyclic
Chem., 1982 ,19, 1207; and substituted 6-H-7,8-dihydro-thiopyrano(3.2-
d)pyrimidyl as disclosed in Arch. Int. Pharmacodyn. 1988, 280, pp302-
313.
Optionally, Compound III formed in the alkylation step is
further modified in subsequent reactions. In one illustration of such an
approach, the piperazine fragment may contain a vitro group, which is
reduced to the amine after the coupling step. The resulting amine is
further modified by acylation to provide the desired compounds. The
piperazine fragment may also contain a protecting group such as a
benzyl ester or a t-butyl ester. After reductive amination the protecting
group is removed and the resulting acid is further reacted to provide
additional analogs. Alternatively, the aldehyde portion may also contain
a protecting group such as a t-butoxycarbonyl for an amino function.
After reductive amination, the t-butoxycarbonyl group is removed by
treatment with a strong acid such as trifluoroacetic acid, formic acid or
hydrochloric acid and the resulting amine may be acylated to provide
other analogs.
The piperazine starting materials used in the coupling
reaction are prepared using methods described in the literature; more
specifically as described in US Patent No. 5,057,517; US Patent No.
4,242,344; J. Org. Chem, 1974, 39, 2143 and J.C.S. Perkin I, 1980, 506; J.
Org. Chem. 1963, 28, 1753; J. Heterocyclic Chem., 1982 ,19, 1207; Arch.
Int. Pharmacodyn. 1986, 280, pp302-313 ; Meurer, L.. C. et al., J. Med.
Chem., 1992, 35, 3845-3857. None of these published compounds are
disclosed to be chemokine receptor modulators. Alternatively, the
piperazine substrates is prepared as illustrated in Schemes 18-21.
-105-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
SCHEME 18
CN HN~ K2Cp3, DMF
/ F N~Boc 150°C
N H2
CN H2, Raney Ni
1000 psi, 80°C
/ N I /
N
XCII ~N~Boc XCIII ~N~
Boc
NHR NHR
acylation I ~ CF3C02H
or / N or ~ /
sulfonylation ~ HCI, EtOAc N
N~ Boc N.
XCIV XCV H
Substituted 4-arylpiperazines are prepared from
appropriate fluorobenzene derivative as shown in Scheme 18. Thus,
reaction of 2-fluorobenzonitrile with 1-t-butoxycarbonylpiperazine in the
presence of a base such as K2COg gives 1-t-butoxycarbonyl-4-(2-
cyanophenyl)-piperazine (compound XCII). Reduction of the cyano
group by hydrogenation in the presence of Raney nickel or by other
known methods gives benzyl amine XCIII, which is acylated or
sulfonylated, to provide piperazine XCIV. The t-butoxycarbonyl
protecting group is removed under acidic conditions, for example by
treatment with trifluoroacetic acid or anhydrous HCl to give 1-
unsubstituted piperazine XCV which can be used in the reductive
amination or alkylation steps described in Scheme 1. Similar reactions
using 2-chloro-nitrobenzene in the place of 2-fluorobenzonitrile provides
compounds containing a substituted aniline. Analogs containing a
benzoic acid or its derivatives are prepared by substituting 2-
fluorobenzoic acid in this sequence.
-106 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
CHO HN~ K2C03, DMF
F N~Boc 150 °C
CHO H
NaBH4, MeOH
N
~ ' N
XCVI ~N~Boc
XCVII ~N~Boc
X
MeS02Cl, Et3N I ~ X = Br or
or Ph3P; CBr4 ~ N~ OS02Me
XCVIII ~N~Boc
H-N~~ N N
CF3C02H
NaH, DMF ~ N or
HCI, EtOAc N
XCIX ~N~Boc N.
C H
Arylpiperazine derivatives containing heterocyclic
substituents are synthesized as shown in Scheme 19. Reaction between
2-fluorobenzaldehyde and 1-t-butoxycarbonylpiperazine gives 1-t-
butoxycarbonyl-4-(2-formylphenyl)-piperazine (compound XCVI).
Reduction of this aldehyde and treatment of the alcohol XCVII with
methanesulfonyl chloride gives XCVIII (X = mesylate), while treatment
of XCVII with triphenylphosphine and carbon tetrabromide gives
XCVIII (X = bromide). Displacement of the leaving group by a
heterocycle such as imidazole in the presence of a base provides
piperazine XCIX. Removal of the t-butoxycarbonyl protecting group
-107-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
under standard anhydrous acidic conditions furnishes compound C
which is used in the coupling reactions described in Scheme I.
SCHEME 20
N HN~ 80-150 °C
CI N~Boc
N CF3C02H ~ N
or
N~ HCI, EtOAc ~ N
~N~Boc ~NI ~H
Ci CII
Preparation of piperazines containing a heteroaryl
substituent is outlined in Scheme 20. Reaction of 1-t-butoxycarbonyl-
piperazine with a chloro substituted heteroaromatic compound such as
8-chloro-1,7-naphthyridine or 8-chloro-(1,2,4)-triazolo(1,5-a)pyrazine
gives N-protected piperazine CI. Removal of the t-butoxycarbonyl
protecting group under standard conditions by treatment with acid
provides piperazine CII for use in the coupling steps outlined in Scheme
1.
-108-
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
SCHEME 21
CHO H
I ~ CH3MgBr, THF ~ CHs
N
XCVI ~N~Boc N
CIII ~N'goc
1 ) Diethyl N H
azodicarboxylate, 2 RS02CI, RCOCI,
Ph3P, phthalimide I ~ CH3 R(R')NCOCI, RNCO,
2) NH2NH2~H20 ~ N~ or ROCOCI
CIV " N'Boc
H,N~X-R
I ~ ~CH~ 1 ) CF3C02H
N
or
~N.Boc HCI, EtOAc
CV
H,N~X-R
~CH3 where X = -S02-,-CO-, -OC(O)-,
I / -CONH-, or -CONR'-
N
CVI ~N'H
Preparation of piperazines containing a heteroaryl
substituent on a branched side chain is outlined in Scheme 21. Reaction
of aldehyde XCVI whose synthesis is described in Scheme 19 with a
carbon nucleophile such as a Grignard reagent, for example methyl
magnesium bromide, provides the benzylic alcohol CIII. Conversion to
the benzylic amine can be carried out by treatment of the alcohol with
potassium phthalimide in the presence of diethyl azodicarboxylate and
triphenyl phosphine, followed by heating with hydrazine hydrate, to give
the free primary amine CIV. Conversion to CIV can also be carried out
by activation of the hydroxyl group with a alkyl- or arylsulfonyl chloride,
-109 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
such as p-toluenesulfonyl chloride, to give a benzylic sulfonate ester.
The sulfonate ester is then displaced with ammonia or a primary or
secondary amine. Alternatively, the sulfonate ester can be displaced
with a suitable salt of the azide anion, such as sodium azide, zinc azide,
or tetrabutylammonium azide, and the resulting alkyl azide can be
reduced to primary amine CIV with hydrogen gas in the presence of a
suitable catalyst, such as 5% palladium on carbon. Alternatively, the
alkyl azide can be reduced by treatment with triphenyl phosphine
followed by hydrolysis to afford CIV. Benzylic amine CIV can then be
derivatized with a number of electrophilic reagents, such as alkyl or aryl
sulfonyl chlorides, carboxylic acid chlorides, carboxylic acid anhydrides,
alkyl chloroformates, carbamyl chlorides or alkyl or aryl isocyanates to
provide sulfonamides, carboxamides, ureas, or carbamates CV. These
intermediates can then be deprotected under acidic conditions to remove
the Boc group to provide free piperazine CVI for use in the coupling
reactions described in Scheme I.
-110 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
SCHEME 22
I 1 ) Di-isopropylcarbodiimide,
( / NH2 \ -Boc DIEA, DMAP
,S~
O O ~,.~'' 2) CF3C02H
A OH B
\ / R8S02C1, DIEA
or
H
/ .N ,,,,, NH
R COCI, DIEA
C O
\
1 ) (SiMe3)CHN2
_ Rs
I / .N ,,,.. N X 2) NH Ra Rb
()
O~' ~O 50°C, overnight
O X = S02, CO
D
\ ~ 1) BH3~MezS, 50°C Ra Re
N-X~Rs 2) 1% HCI, MeOH Rb~N~.,~'' N~S~O
Rbi ~~,,,,, 0 9
50 C, overni ht
O E [For X = SO 2] F
1 ) BH 3~MezS, 50°C Ra Re
Ra R8
~N-.-~
-X' 2) 1% HCI, MeOH Rb~Nw..~'' G
N ,.
50°C, overnight (For X = CO]
E
-111-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Preparation of target pyrrolidines using solid support
technology is outlined in Scheme 22. Coupling of intermediate B to a
commercially available 4-sulfamylbenzoyl polystyrene resin A (or a alkyl
sulfamyl resin} is carried out with di-isopropylcarbodiimide or with
other activating agents, for example dicyclohexylcarbodiimide, EDAC,
oxalyl chloride, etc. Agents that result in the formation of the
symmetrical anhydride from B (which then serves as the acylating
agent) are also suitable for this purpose. Removal of the Boc group is
carried out with trifluoroacetic acid or other acidic reagents, to give
resin-bound pyrrolidine C. This intermediate is then coupled with
sulfonyl chlorides or carbonyl chlorides in the presence of a suitable
amine, preferably a hindered tertiary amine such as
diisopropylethylamine (DIEA), lutidine, DBU, etc., to provide the N-
functionalized pyrrolidine D. Alkylation of the acyl sulfonamide
nitrogen can be carried out with trimethylsilyldiazomethane,
diazomethane, with bromoacetonitrile in the presence of DBU and DMF,
or under Mitsunobu conditions with a phenol such as
pentafluorophenol. Reaction of the resulting N-alkylated intermediate
with an amine NH(Ra)Rb at a temperature between 0 and 140°C,
preferably around 50°C, for 4-24 hr, preferably about 14 hr, then
cleaves
the pyrrolidine from the resin as amide E. Reduction of the newly
formed amide (and other amide functionality, if present) with borane
methyl sulfide complex (or other hydride reducing agents, such as
borane-pyridine, borane-THF, lithium aluminum hydride, lithium di-
(sec)butyl borohydride, etc) followed by hydrolysis with dilute hydrogen
chloride in methanol at a temperature between 0 and 140°C, preferably
around 50°C, for 4-24 hr, preferably about 14 hr, provides either
sulfonamide F or amine G.
Cyclic amines (compound II) from Scheme 1 which are
spirocyclic piperidines are prepared using azacyclic starting materials
prepared using methods described in the literature; more specifically, as
described in Claremon, D.A. ~t ~, European Patent Publication 0 431
943, Evans, B.E. e~~, U.S. atent 5,091,387, Davis, L. g~,~l, U.S. Patent
4,420,485, and Parham ~ al, Journal of Organic Chemistry, ~1, 2628
-112 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(1976). None of the compounds in the foregoing references are alleged to
be chemokine receptor modulators.
In some cases the order of carrying out the foregoing
reaction schemes may be varied to facilitate the reaction or to avoid
unwanted reaction products. The following examples are provided for
the purpose of further illustration only and are not intended to be
limitations on the disclosed invention.
EXAMPLE 1
1-Benzenesulfonyl-3-(RS)-(spiro[2,3-dihydrobenzothiophene-3,4'-
p~neridin-1'-vll )methyl-4-(SR)-~henylpyrrolidine
Step A: 1-Phenylmethyl-3-(SR)-carbomethoxy-4-(SR)-
phenvlpvrrolidine
A solution of 1.98 g (12.2 mmol) of methyl (Z)-cinnamate
and 5.80 g (24.4 mmol) N-(methoxymethyl)-N-(trimethylsilylmethyl)-
benzylamine in 30 mL of CH2C12 at 0 °C was treated with 20 drops of
trifluoroacetic acid and stirred cold for 1 h. The reaction mixture was
partitioned between 200 mL of ether and 100 mL of sat'd NaHC03 and the
layers were separated. The organic layer was washed with 100 mL of
sat'd NaCl, dried over MgS04 and concentrated in uacuo. Flash
chromatography on 150 g of silica gel using 20:1 v/v CH2Cl2/ether as the
eluant afforded 2.61 g (72%) of the title compound as an oil. 1H NMR (500
MHz, CDC13): $ 2.83 (t, J= 8.7, 1H), 3.09-3.19 (m, 6H), 3.16 (s, 3H), 3.46-
3.51 (m, 1H), 3.73-3.79 (m, 3H), 7.19-7.42 ( lOH).
Mass Spectrum (NH3-CI): m/z 296 (M+1).
Step B: 1-Phenylmethyl-3-(SR)-hydroxymethyl-4-(SR)-
p enYl_pyrrolidine
A solution of 2.61 g (8.8 mmol) of 1-phenylmethyl-3-(SR)-
carbomethoxy-4-(SR)-phenylpyrrolidine (from Example 1, Step A) in 100
mL of THF at -78 °C was treated with 9.2 mL of 1.5 M diisobutyl-
aluminum hydride solution in toluene. The reaction was warmed to 0
°C and stirred for 1 h. The reaction was quenched with 50 mL of sat'd
-113 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
sodium potassium tartrate solution, diluted with 100 mL of ether and
stirred at rt for 20 h. The layers were separated and the organic layer
was washed with 75 mL of H20, dried over MgS04 and concentrated in
vacuo. Flash chromatography on 100 g of silica gel using 1:1 v/v, then
3:2 v/v EtOAc/hexanes as the eluant afforded 1.99 g (84%) of the title
compound as a solid. 1H NMR (500 MHz, CDC13): 8 2.56-2.63 (m, 1H),
2.80 (dd, J= 4.3, 9.4, 1H), 2.91-2.98 (m, 2H), 3.05 (dd, J= 6.9, 9.6, 1H),
3.27
(dd, J= 5.0, 10.7, 1H), 3.44 (dd, J= 5.5, 10.8, 1H), 3.62 (q, J= 9.0, 1H),
3.76
(ABq, J= 24.2, 2H), 7.22-7.40 (lOH). Mass Spectrum (NH3-CI): m/z 268
(M+1).
Step C: 3-(SR)-Hydroxvmethvl-4-(SR)-phenylRyrrolidine
A mixture of 302 mg ( 1.1 mmol) of 1-benzyl-3-(SR)-
hydroxymethyl-4-{SR)-phenylpyrrolidine (from Example 1, Step B) and
154 mg of 20% Pd(OH)~C was hydrogenated at 40 psi on a Paar
apparatus for 1 h. The catalyst was filtered and the filtrate was
concentrated in vacuo to afford 196 mg (98%) of the title compound as an
oil. 1H NMR (500 MHz, CDC13): 8 2.53-2.57 (m, 2H), 3.06-3.60 (6H), 7.24-
7.35 (5H). Mass Spectrum (NH3-CI): m/z 178 (M+1).
Step D: 1-(Benzenesulfonyl)-3-(SR)-benzenesulfonyloxymethyl-4-
(SR)-phenyl_Ryrrolidine
A solution of 196 mg ( 1.1 mmol) of 3-(SR)-hydroxymethyl-4-
(SR)-phenylpyrrolidine (from Example 1, Step C) and 27 mg (0.2 mmol) of
4-{N,N-dimethylamino)pyridine in 5 mL of pyridine at 0 °C was treated
with 488 mg (2.8 mmol) of benzenesulfonyl chloride. The cooling bath
was removed and the resulting mixture was stirred at rt for 1 h. The
reaction mixture was treated with an additional 290 mg of
benzenesulfonyl chloride and the resulting mixture was stirred at rt for
1 h. The reaction mixture was partitioned between 50 mL of ether and 20
mL of sat'd CuS04 and the layers were separated. The organic layer
was washed with 30 mL of H20, 30 mL of sat'd NaCI, dried over MgS04
and concentrated in vacuo. Flash chromatography on 30 g of silica gel
using3:2 v/v hexanes/ether afforded 340 mg (67%) of the title compound
as a solid. 1H NMR (500 MHz, CDC13): 8 2.65-2.72 (m, 1H), 3.21 (dd, J=
-114 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
7.8, 10.3, 1H), 3.44-3,67 (6H), 6.94-?.90 (15H). Mass Spectrum (NH3-CI):
m/z 458 (M+1).
Step E: 1-(Benzenesulfonyl)-3-(SR)-iodomethyl-4-(SR)-
enyl_pyrrolidine
A solution of 234 mg (0.51 mmol) of 1-(benzenesulfonyl)-3-
(SR)-benzenesulfonyloxymethyl-4-(SR)-phenylpyrrolidine (from Example
1, Step D) and 1.1 g (7.7 mmol) of sodium iodide in 8 mL of acetone was
heated at reflux for 20 h. The reaction mixture was partitioned between
50 mL of ether and 30 mL of H20 and the layers were separated. The
organic layer was washed with 30 mL of sat'd NaCl and dried over
MgS04. The aqueous layers were combined and extracted with 30 mL of
ether. The extract was dried; the organic layers were combined and
concentrated in va~cuo. Flash chromatography on 25 g of silica gel using
3:2 v/v hexanes/ether as the eluant afforded 211 mg (96%) of the title
compound as an oil. 1H NMR (500 MHz, CDC13): 8 2.43 (t, J= 9.8, 1H),
2.64-2.74 (m, 2H), 3.30 (dd, J= 2.5, 7.8, 1H), 3.47 (q, J= 6.7, 1H), 3.67-3.74
(3H), 7.05-7.95 (lOH). Mass Spectrum (NH3-CI): m/z 428 (M+1).
Step F: 1-Benzenesulfonyl-3-(RS)-(spiro[2,3-dihydrobenzo-
thiophene-3,4'-piperidin-1'-yl] )methyl-4-(SR)-
phenvlRvrrolidine
A solution of 86 mg (0.2 mmol) of 1-(benzenesulfonyl)-3-(SR)-
iodomethyl-4-(SR)-phenylpyrrolidine (from Example 1, Step E) and 124
mg (0.6 mmol) of spiro[2,3-dihydrobenzothiophene]-3,4'-piperidine in 2
mL of isobutyrnitrile was heated at 100 °C for 48 h. The reaction
mixture
was cooled and partitioned between 50 mL of ether and 25 mL of sat'd
NaHC03 and the layers were separated. The organic layer was dried
over MgS04 and concentrated in vacuo. Flash chromatography on 15 g
of silica gel using 2:1 v/v hexanes/ether afforded 57 mg (56%) of the title
compound as an oil. 1H NMR (500 MHz, CDCl3): 8 1.45-3.74 (19H), 7.02-
7.96 (14H). Mass Spectrum (NH3-CI): m/z 505 (M+1).
EMPLE 2
-115 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1-Benzenesulfonyl-3-(RS)-(spiro [2,3-dihydrobenzothiophene-3,4'-
pi eridin-1'- l~methyl-4-(SR)-~hg~y~,pvrrolidine, S oxide
A solution of 34 mg (0.07 mmol) of 1-benzenesulfonyl-3-(RS)-
(spiro[2,3-dihydrobenzothiophene-3,4'-piperidin-1'-yl])methyl-4-(SR)-
phenylpyrrolidine (from Example 1) in 2 mL of THF at 0 °C was treated
with a solution of 45 mg (0.07 mmol) of oxone in 1 mL of H20 and the
resulting mixture was stirred cold for 3 min. The reaction was
quenched with 2 mL of 1:1 v/v sat'd NaHS03/sat'd NaHC03 and
extracted with 4 x 20 mL of CH2C12. The extracts were dried over MgS04
and concentrated in uczcuo. Flash chromatography on 15 g of silica gel
using 49:1 v/v CH2C12JMeOH as the eluant afforded 26 mg (74%) of the
title compound as an oil. 1H NMR (500 MHz, CDCl3): 8 1.27-3.78 (19H),
7.01-7.96 (14H). Mass Spectrum (NH3-CI): 521 (M+1).
EXAMPLE 3
1-Benzenesulfonyl-3-(RS)-(spiro[2,3-dihydrobenzothiophene-3,4'-
pineridin-1'X11)methyl-4-(SR)-phenylpvrrolidine~ ~,S dioxide
A solution of 12 mg (0.02 mmol) of 1-benzenesulfonyl-3-(RS)-
(spiro[2,3-dihydrobenzothiophene-3,4"-piperidin-1'-yl] )methyl-4-(SR)-
phenylpyrrolidine (from Example 1) in 2 mL of THF at 0 °C was treated
with a solution of 32 mg (0.05 mmol) of oxone in 1 mL of H20. The
cooling bath was removed and the resulting solution was stirred at rt for
30 min. The reaction was quenched with 2 mL of 1:1 v/v sat'd
NaHS03/sat'd NaHC03 and extracted with 4 x 20 mL of CH2C12. The
extracts were dried over MgS04 and concentrated in uacuo. Flash
chromatography on 10 g of silica gel using 3:2 v/v hexanes/EtOAc as the
eluant afforded 8 mg (fi0%) of the title compound as an oil. Mass
Spectrum (NH3-CI): 537 (M+1).
EXAMPLE 4
1-Benzenesulfonyl-3-(SR)-(spiro[2,3-dihydrobenzothiophene-3,4'-
~i~eridin-1'-yll )methyl-4-(SR);nhenvlnvrrolidine
-116 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
The title compound was prepared in 6 steps from methyl (Z)-
cinnamate using procedures analogous to those described in Example 1.
1H NMR (500 MHz, CDC13): 8 1.45-3.76 (m, 19H), 7.10-7.92 (m, 14H).
Mass Spectrum (NH3-CI): 505 (M+1).
1-Benzenesulfonyl-3-(SR)-(spiro[2,3-dihydrobenzothiophene-3,4'-
~ineridin-1'-vll )methyl-4-(SR)-phenYl_Rvrrolidine, S-oxide
The title compound was prepared from 1-benzenesulfonyl-3-
(SR)-(spiro [2,3-dihydrobenzothiophene-3,4'-piperidin-1'-yl] )methyl-4-
(SR)-phenylpyrrolidine (from Example 4) using a procedure analogous
to that described in Example 2. 1H NMR (500 MHz, CDC13): s 1.25-3.76
(19H), 7.09-7.91 (14H). Mass Spectrum (NH3-CI): 521 (M+1).
1-Benzenesulfonyl-3-(SR)-(spiro[2,3-dihydrobenzothiophene-3,4'-
pineridin-1' yll)met yl-4-(SR)-phen~p3~rrolidine, S S-dio~~ide
The title compound was prepared from 1-benzenesulfonyl-3-
(SR)-(spiro[2,3-dihydrobenzothiophene-3,4'-piperidin-1'-yl] )methyl-4-
(SR)-phenylpyrrolidine (from Example 4) using a procedure analogous to
that described in Example 3. Mass Spectrum (NH3-CI): 537 (M+1).
EXAMPLE 7
1-Benzenesulfonyl-3-(RS)-((4-phenyl) piperidin-1-yl)methyl-4-(SR)-
Dhenvlnvrrolidine
The title compound was prepared in 6 steps from methyl (Z)-
cinnamate using procedures analogous to those described in Example 1.
4-(Phenyl)piperidine was substituted for spiro[2,3-dihydrobenzo-
thiophene]-3,4'-piperidine in Step 6. 1H NMR (500 MHz, CDC13): 81.69-
3.73 (17H), 7.03-7.96 (15H). Mass Spectrum (NH3-CI): 461 (M+1).
EXAMPLE 8
-117 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1-Benzenesulfonyl-3-(SR)-((4-phenyl) piperidin-1-yl)methyl-4-(SR)-
phenylpvrrolidine
The title compound was prepared in 6 steps from methyl
(E)-cinnamate using procedures analogous to those described in
Example 1. 4-(Phenyl)piperidine was substituted for spiro(2,3-
dihydrobenzothiophene]-3,4'-piperidine in Step 6. 1H NMR {500 MHz,
CDC13): 81.29-3.75 {17H), 7.12-7.92 (15H). Mass Spectrum (NH3-CI): 461
(M+1).
EXAMPLE 9
1-Phenylmethyl -3-(SR)-((4-phenyl) piperidin-1-yl)methyl-4-(SR)-
pe y~pyrrolidine
Step A: 1-Phenylmethxl -3-(RS)-formal -4-(SR)-phenyl"pvrrolidine
A solution of 0.87 mL (10.0 mmol) of oxalyl chloride in 50 mL
of CH2C12 at -75 °C was treated with 1.10 mL ( 15.0 mmol) of DMSO
maintaining the internal temperature below -65 °C. The resulting
mixture was stirred cold for 10 min and then 1.34 g (5.0 mmol) of 1-
phenylmethyl -3-(RS)-hydroxymethyl-4-(SR)-phenylpyrrolidine (from
Example 4) was added in one portion as a solid. The resulting mixture
was stirred cold for 30 min and then 8.70 mL (50 mmol) of DIEA was
added maintaining the internal temperature below -60 °C. The cooling
bath was removed and the reaction was allowed to warm to 0 °C . The
reaction was quenched with 50 mL of H20; the resulting mixture was
diluted with 100 mL of CH2Cl2 and 50 mL and the layers were separated.
The organic layer was washed with 50 mL of H20, dired over MgS04 and
concentrated in vacuo. Flash chromatography on 70 g of silica gel using
4:1 v/v hexanes/ether afforded 1.00 g (75%) of the title compound as an
oil.
1H NMR (300 MHz, CDC13): b 2.61 (dd, J= 9.0, 7.6, 1H), 2.86 (app t, J=
8.6), 2.99-3.06 (m, 1H), 3.10-3.20 (m, 2H), 3.62-3.69 (m, 1H), 3.66 and 3.69
(ABq, J= 15.6, 2H), 7.23-7.40 (lOH), 9.73 (d, J= 1.8, 1H).
-118 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Step B: 1-Phenylmethyl -3-(SR)-((4-phenyl) piperidin-1-yl)methyl-
4-(SR)-phenylpvrrolidine
A solution of 680 mg (2.6 mmol) of 1-phenylmethyl -3-(RS)-
formyl -4-(SR)-phenylpyrrolidine (from Example 9, Step B) and 420 mg
(2.6 mmol) of (4-phenyl)piperidine in 25 mL of dichloroethane at 0 °C
was
treated with 635 mg (3.0 mmol) of sodium triacetoxy-borohydride. The
cooling bath was removed and the resulting mixture was stirred at rt for
2h. The reaction mixture was partitioned between 100 mL of EtOAc and
50 mL of sat'd NaHC03 and the layers were separated. The organic layer
was washed with 50 mL of sat'd NaCl, dried over MgS04 and
concentrated in vacuo. Flash chromatography on 50 g of silica gel using
10:1 v/v hexanes/EtOAc + 1% TEA as the eluant afforded 1.01 g of the title
compound as an oil. 1H NMR (500 MHz, CDC13): 81.65-2.02 (6H), 2.42-
3.03 (11H), 3.72 (AB q, J= 35.4, 2H), 7.20-7.43 (m, 15H). Mass Spectrum
(NH3-CI): 411 (M+1).
The compounds in Examples 10-29 were prepared using
procedures analogous to those described in Example 9 substituting the
appropriate secondary amine for (4-phenyl)piperidine in Example 9, Step
B.
EXAMPLE 10
1-Phenylmethyl -3-(SR)-(spiro[(indan-1-one -3,4'-piperidin-1'-yl])methyl-
4-(SR)-phenvlRyrrolidine
1H NMR (500 MHz, CDCl3): 81.35 (dd, J= 2.2, 12.8, 1H), 1.45 (d, J= 10.8,
1H), 1.89-2.09 (4H), 2.50-2.74 (9H), 2.92-3.02 (3H), 3.72 (AB q, J= 34.8, 2H),
7.19-7.41 (11H), 7.53 (d, J= 7.7, 1H), 7.64 (t, J= 7.3, 1H), 7.71 (d, J= 7.6,
1H).
Mass Spectrum (NH3-CI): 451 (M+1).
EXAMPLE 11
1-Phenylmethyl -3-(RS)-(spiro(2,3-dihydrobenzothiophene-3,4'-piperidin-
1'-yll )methyl-4-(SR)-phen~pyrrolidine
-119 -
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
1H NMR (500 MHz, CDC13): S 1.66-2.08 (8H), 2.49-3.01 (8H), 3.22 (s, 2H),
3.72 (AB q, J= 35.9, 2H), 7.08-7.41 (14H). Mass Spectrum (NH3-CI): 455
(M+1).
EXAMPLE 12
1-Phenylmethyl -3-(RS)-((4-(N-methoxycarbonyl-N-cyclohexylmethyl)-
amino),~~eridin-1-yl)methyl-4-(SR)-pheny~pyrroliding
1H NMR (500 MHz, CDC13): 8 7.23-7.49 (lOH), 3.98 (s, 2H), 3.67 (s, 3H),
3.28-3.30 (2H). 3.09-3.11 (m , 2H), 2.91-2.96 (5H). 2.80 (d, J= 9.9, 1H). 2.62-
2.70 (1H). 2.52-2.60 (1H). 2.45-2.47 (1H). 1.53-2.06 (10H). 1.17-1.28 (3H).
0.85-0.91 (3H). Mass Spectrum (NH3-CI): 504 (M+1).
EXAMPLE 13
1- - 4-b 1 - ol' ine
The title compound was prepared from 22 mg of 1-Benzyl-3-
(SR)-formyl-4-(SR)-phenylpyrrolidine, 0.013 mL of 4-benzyl-piperidine
and 24 mg of sodium triacetoxyborohydride using a procedure analogous
to that described in Example 9 to provide 14 mg of the title compound.
RF: 0.39 (50% EtOAc in hexanes). 1H NMR (300 MHz, CDC13): 8 1.2-1.9
(m, 8H), 2.3-3.0 (m, 11H), 3.7 (ABq, 2H), 7.1-7.4 (m, 15H). Mass Spectrum
(NH3-CI): 425.3 (M+H).
EXAMPLE 14
1-Benzyl-3-(SR)-(4-hydroxypiperidin-1-ylmethyl)-4-(SR)-phenyl-
Ryrrolidine
The title compound was prepared from 500 mg of 1-Benzyl-3
(SR)-formyl-4-(SR)-phenylpyrrolidine, 196 mg of 4-hydroxypiperidine and
597 mg of sodium triacetoxyborohydride using a procedure analogous to
that described in Example 9, Step B to provide 348 mg of the title
compound. RF: 0.16 (5% MeOH in CH2C12). 1H NMR (300 MHz, CDC13):
81.35-1.54 (m, 2H), 1.69-1.83 (m, 2H), 1.93-2.05 (m, 2H), 2.36-2.71 (m, 7H),
-120 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
3.89-3.01 (m, 3H), 3.55-3.72 (m, 3H), 7.14-7.38 (m, lOH). Mass Spectrum
(NH3-CI): 351.3 (M+H).
1-Benzyl-3-(SR)-( 1,2-Biphenyl-3-(SR)-methyl-4-methylamino-2-(SR)-
butanol-N-ylmethvl)-4-(SR)-~~pvrrolidine
The title compound was prepared from 103 mg of 1-Benzyl-3-
(SR)-formyl-4-(SR)-phenylpyrrolidine, 105 mg of 1,2-Biphenyl-3-(SR)-
methyl-4-methylamino-2-(SR)-butanol-hydrochloride and 120 mg of
sodium triacetoxyborohydride using a procedure analogous to that
described in Example 9, Step B to provide 76 mg of the title compound:
RF: 0.53 (50% Acetone in hexanes). 1H NMR (300 MHz, CDC13): 8 0.98-
1.12 (m, 3H), 1.98-2.62 (m, 9H), 2.79-3.24 (m, 5H), 3.39 (ABq, J = 13 Hz,
2H), 3.60-3.72 (m, 3H), 7.06-7.45 (m, 20H). Mass Spectrum (NH3-CI):
519.3 (M+H).
EXAMPLE 16
1-Benzvl-3-(SR)-(4-oxQpin~ridin-1 ylmethvl)-4-(SR)- hen~pvrrolidine
To a solution of 0.14 mL of oxalylchloride in 3 mL of CH2Cl2
at -70 oC was added 0.16 mL of DMSO. After 5 min 270 mg of 1-Benzyl-3-
(SR)-(4-hydroxypiperidin-1-ylmethyl)-4-(SR)-phenylpyrrolidine (from
Example 14) in 3 mL of CH2C12 was added. After stirring for 10 min, the
reaction was warmed to 0 oC for 45 min. The reaction was quenched
with H20 and partitioned between 100 mL H20 and 100 mL CH2C12.
After separating phases, the aqueous layer was extracted with 100 mL
CH2C12. The combined organic layers were washed with 100 mL brine,
dried over Na2S04 and concentrated under vacuum. The residue was
purified by flash chromatography eluting with 2% MeOH in CH2C12 to
provide 256 mg of the title compound as a light yellow oil.
RF: 0.40 (5% MeOH in CH2C12). 1H NMR (300 MHz, CDC13): 8 2.2-2.4 (m,
4H), 2.4-2.7 (m, 9H), 2.9-3.1 (m, 3H), 3.7 (ABq, 2H), 7.1-7.4 (m, lOH). Mass
Spectrum (ESI): 349.3 (M+H).
-121-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
EXAMPLE 17
1-Benzyl-3(SR)-(4-(N-(benzyloxycarbonyl)-N-sec-butylamino)-piperidin-1-
vlmethyl)-4-(SR)-~y~,vrrolidine
The title compound was prepared from 20 mg of 1-Benzyl-3-
(SR)-formyl-4-(SR)-phenylpyrrolidine, 24 mg of 4-(N-(benzyloxycarbonyl)-
N-secbutylamino)piperidine-hydrochloride, 0.013 mL of DIEA and 24 mg
of sodium triacetoxyborohydride using a procedure analogous to that
described in Example 9, Step B to provide 32 mg of the title compound.
HPLC (YMC "Octyl" 4.6 x 250 mm column, 60:40 v/v H20/CH3CN + 0.5%
TFA, 1.5 mL/min, 200 nm): Retention Time: 12.99 min. 1H NMR (300
MHz, CDC13): 8 0.70-0.82 (m, 3H), 1.16 (t, J = 6.4 Hz, 3H), 1.37-1.95 (m,
lOH), 2.34-2.66 (m, 6H), 2.83-2.99 (m, 4H), 3.69 (ABq, J = 13 Hz, 2H), 5.11
(s, 2H), 7.14-7.39 (m, 15H). Mass Spectrum (ESI): 540.6 (M+H).
EXAMPLE 18
1-Benzyl-3(SR)-(4-(N-(benzyloxycarbonyl)-N-isopropylamino)-piperidin-1-
vlmethvl)-4-(SR)-nhenylpvrrolidine
The title compound was prepared from 20 mg of 1-Benzyl-3-
(SR)-formyl-4-(SR)-phenylpyrrolidine, 23 mg of 4-(N-(benzyloxy-
carbonyl)-N-isopropylamino)piperidine-hydrochloride, 0.013 mL of DIEA
and 26 mg of sodium triacetoxyborohydride using a procedure analogous
to that described in Example 9, Step B to provide 31 mg of the title
compound. HPLC (YMC "Octyl" 4.6 x 250 mm column, 60:40 v/v
H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200 nm): Retention Time: 9.99
min. 1H NMR (300 MHz, CDC13): b 1.17-2.00 (m, 14H), 2.34-2.72 (m, 6H),
2.82-3.01 (m, 4H), 3.68 {ABq, J = 13 Hz, 2H), 5.12 (s, 2H), 7.13-7.42 (m,
15H). Mass Spectrum (ESI): 526.2 (M+H).
EXAMPLE 19
1-Benzyl-3-(SR)-( 1,1-diphenyl-3-methylamino-1-propanol-N-ylmethyl)-4-
( SR)-nhen~,pvrrolidine
-122-
CA 02298813 2000-O1-28
WO 99/09984 PCTlUS98/17755
The title compound was prepared from 21 mg of 1-Benzyl-3-
(SR)-formyl-4-(SR)-phenylpyrrolidine, 24 mg of 1,1-diphenyl-3-methyl-
amino-1-propanol-hydrochloride, 0.013 mL of DIEA and 24 mg of sodium
triacetoxyborohydride using a procedure analogous to that described in
Example 9, Step B to provide 32 mg of the title compound. HPLC (YMC
"Octyl" 4.6 x 250 mm column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5
mL/min, 200 nm): Retention Time: 7.74 min. 1H NMR (300 MHz,
CDC13): 8 2.07-2.96 (m, 16H), 3.67 (s, 2H), 7.13-7.50 (m, 20H). Mass
Spectrum (ESI): 491.3 (M+H).
EXAMP~F 20
1-Benzyl-3-(SR)-(4-{2-tolyl)-piperidin-1-ylmethyl)-4-(SR)-
g~enylpvrrolidine
The title compound was prepared from 21 mg of 1-Benzyl-3-
(SR)-formyl-4-(SR)-phenylpyrrolidine, 16 mg of 4-(2-tolyl)-piperidine-
hydrochloride, 0.013 mL of DIEA and 23 mg of sodium triacetoxy-
borohydride using a procedure analogous to that described in Example 9,
Step B to provide 27 mg of the title compound. HPLC {YMC "Octyl" 4.6 x
250 mm column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200
nm): Retention Time: 6.69 min. 1H NMR (300 MHz, CDC13): 8 1.58-1.74
(m, 6H), 1.91-1.99 (m, 2H), 2.28 (s, 3H), 2.47-2.82 (m, 6H), 2.98-3.00 (m,
3H), 3.70 (ABq, J = 13 Hz, 2H), 7.04-7.40 (m, 14H). Mass Spectrum (ESI):
425.2 (M+H).
EXAMPLE 21
1-Benzyl-3(SR)-(4-(N-(isopropyloxycarbonyl)-N-methylenecyclohexyl-
amino)uineridin-1-vlmethvl~-4-(S~,)-nhenvlpvrrolidine
The title compound was prepared from 22 mg of 1-Benzyl-3-
(SR)-formyl-4-(SR)-phenylpyrrolidine, 23.5 mg of 4-(N-{isopropyloxy-
carbonyl)-N-methylenecyclohexylamino)piperidine-hydrochloride, 0.013
mL of DIEA and 23 mg of sodium triacetoxy-borohydride using a
procedure analogous to that described in Example 9, Step B to provide 28
mg of the title compound. RF: 0.34 (50% EtOAc in hexanes). 1H NMR
-123-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(300 MHz, CDC13): 8 0.82-0.92 (m, 2H), 1.10-1.25 (m, 9H), 1.44-1.89 (m,
13H), 2.35-2.52 (m, 4H), 2.62-2.68 (m, 2H), 2.83-2.98 (m, 6H), 3.65 (ABq, J =
13 Hz, 2H), 4.84-4.91 (m, 1H), 7.16-7.37 (m, lOH). Mass Spectrum (ESI):
532.3 (M+H).
EXAMPLE 22
1-Benzyl-3-(SR)-(4-phenyl-1, 2, 3, 6-tetrahydropyridin-1-ylmethyl)-4-(SR)-
p~enylpyrrolidine
The title compound was prepared from 20 mg of 1-Benzyl-3-
(SR)-formyl-4-(SR)-phenylpyrrolidine, 16 mg of 4-phenyl-1, 2, 3, 6-
tetrahydropyridine-hydrochloride, 0.013 mL of DIEA and 23 mg of
sodium triacetoxyborohydride using a procedure analogous to that
described in Example 9, Step B to provide 24 mg of the title compound.
RF: 0.37 (50% EtOAc in hexanes). 1H NMR (300 MHz, CDC13): 8 2.40-2.71
(m, 9H), 2.91-3.16 (m, 5H), 3.67 (ABq, J = 13 Hz, 2H), 5.98-6.00 (m, 1H),
7.07-7.41 (m, 15H). Mass Spectrum (ESI): 409.1 (M+H).
EXAMPLE 23
1-Benzyl-3-(SR)-~4-phenyl-pinerazin-lylmethyl)-4-(SR)-phenyl~~rrrolidine
The title compound was prepared from 20 mg of 1-Benzyl-3-
(SR)-formyl-4-(SR)-phenylpyrrolidine, 0.013 mL of 4-phenyl-piperazine
and 23 mg of sodium triacetoxyborohydride using a procedure analogous
to that described in Example 9, Step B to provide 28 mg of the title
compound. RF: 0.42 (50% EtOAc in hexanes) 1H NMR (300 MHz,
CDCi3): 8 2.36-2.54 (m, 8H), 2.62-2.67 (m, 1H), 2.90-3.08 (m, 7H), 3.66
(ABq, J =13 Hz, 2H), 6.79-6.88 (m, 3H), 7.14-7.38 (m, 12H). Mass
Spectrum (ESI): 412.2 (M+H).
1-Benzyl-3-(SR)-(1, 2, 3, 4-tetrahydroisoquinolin-2-ylmethyl)-4-(SR)-
~Yl_pvrrolidine
-124 -
CA 02298813 2000-O1-28
WO 99/099$4 PCT/US98/17755
The title compound was prepared from 20 mg of 1-Benzyl-3-
(SR)-formyl-4-(SR}-phenylpyrrolidine, 0.010 mL of 1, 2, 3, 4-tetrahydro-
isoquinoline and 25 mg of sodium triacetoxyborohydride using a
procedure analogous to that described in Example 9, Step B to provide 25
mg of the title compound. RF: 0.51 (50% EtOAc in hexanes). 1H NMR
(300 MHz, CDC13): 8 2.51-2.78 (m, 9H), 2.94-3.00 (m, 3H), 3.40-3.69 (m,
4H}, 6.91-6.94 (m, 1H), 7.02-7.38 (m, 13H). Mass spec. (ESI): 383.2 (M+H).
EXAMPLE 25
1-Benzyl-3-(SR)-(2, 3, 4, 5-tetrahydro-1H-benzo[d]-azepin-3-ylmethyl)-4-
( SR)-~ylRyrrolidine
The title compound was prepared from 20 mg of 1-Benzyl-3-
(SR)-formyl-4-(SR)-phenylpyrrolidine, 28 mg of 2, 3, 4, 5-tetrahydro-1H-
benzo[d]-azepine-picrate salt, 0.013 mL of DIEA and 24 mg of sodium
triacetoxyborohydride using a procedure analogous to that described in
Example 9, Step B to provide 14.5 mg of the title compound. RF: 0.53 (50%
EtOAc in hexanes). 1H NMR (300 MHz, CDC13): 8 2.42-3.02 (m, 16H),
3.67 (ABq, J = 13 Hz, 2H), 7.00-7.38 (m, 14H). Mass Spectrum (ESI): 397.1
(M+H).
EXAMPLE ,~6_
1-Benzyl-3-{SR)-(4-phenyl-perhydro-azepin-1-ylmethyl)-4-(SR)-
phen~pyrrolidine
The title compound was prepared from 20 mg of 1-Benzyl-3-
(SR)-formyl-4-(SR)-phenylpyrrolidine, 13 mg of 4-phenyl-perhydro-
azepine and 25 mg of sodium triacetoxyborohydride using a procedure
analogous to that described in Example 9, Step B to provide 18.5 mg of the
title compound. RF: 0.37 (50% EtOAc in hexanes). 1H NMR (300 MHz,
CDC13): 81.48-1.83 (m, 6H), 2.37-2.69 (m, lOH), 2.88-3.01 (m, 3H), 3.67
(ABq, J = 13 Hz, 2H}, 7.09-7.39 (m, 15H). Mass Spectrum (CI): 425.3
(M+H).
EXAMPLE 27
-125-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1-Benzyl-3-(SR)-(4-carboethoxy-piperidin-1-ylmethyl)-4-(SR)-
phen~Rvrrolidine
The title compound was prepared from 400 mg of 1-Benzyl-3-
(SR)-formyl-4-(SR)-phenylpyrrolidine, 0.24 mL of ethyl isonipecotate and
480 mg of sodium triacetoxyborohydride using a procedure analogous to
that described in Example 9, Step B to provide 363 mg of the title
compound. RF: 0.31 (50% EtOAc in hexanes). 1H NMR (300 MHz,
CDCl3): 81.22 (t, J = 7.1 Hz, 3H), 1.54-1.89 (m, 6H), 2.13-2.21 (m, 1H), 2.33-
2.64 (m, 6H), 2.77-2.97 (m, 4H), 3.65 (ABq, J = 13 Hz, 2H), 4.09 (q, J = 7.1
Hz, 2H), 7.14-7.37 (m, lOH). Mass Spectrum (CI): 407.3 (M+H).
EXAMPLE 28
1-Benzyl-3-(SR)-{4-(2, 3-naphthalenecarboxamid)-piperidin-1-ylmethyl)-4-
(SR)-phenylpyrrolidine
To a solution of 35 mg of 1-Benzyl-3-(SR)-(4-hydroxy-
piperidin-1-ylmethyl)-4-(SR)-phenylpyrrolidine, 23 mg of 2, 3-
naphthalenecarboximide and 31 mg of triphenylphosphine in 0.2 mL of
THF at room temperature was added 0.019 mL of diethylazodicarb-
oxylate. After 7 h the reaction was diluted with 10 mL of brine and
extracted with 2X10 mL Et20. The combined organic layers were dried
over MgS04 and concentrated under vacuum. The residue was purified
by flash chromatography eluting with a gradient of 0 to 4% MeOH in
CH2C12 to give 12 mg of the title compound. RF: 0.36 (5% MeOH in
CH2C12). 1H NMR (300 MHz, CDCl3): 8 1.49-1.70 (m, 4H), 1.91-2.01 (m,
2H), 2.36-2.78 (m, 7H), 2.92-3.03 (m, 3H), 3.68 (ABq, J = 13 Hz, 2H), 4.07-
4.15 (m, 1H), 7.14-7.40 (m, lOH), 7.65-7.70 (m, 2H), 8.01-8.06 (m, 2H), 8.30
(s, 2H). Mass Spec (CI): 530.3 (M+H).
EXAMPLE 29
1-Phenylmethyl -3-(SR)-(4-{2-keto-benzimidazol-1-yl)piperidin-1-
1)~thvl-4-, (SR)-nhenylpyrrolidine
-126-
*rB
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98117755
1H NMR (500 MHz, CDC13): 81.59-3.03 (16H), 3.67-3.?2 (m, 2H), 4.24-4.30
(m, 1H), 7.04-7.39 (14H), 9.27 (s, 1H). Mass Spectrum (NH3-CI): 467
(M+1). HPLC (YMC "Octyl" 4.6 x 250 mm column, 60:40 v/v
H20/CH3CN + 0.5% TFA, 1.5 mlJmin, 200 nm): 3.4 min.
EXAMPLE 30
1-(4-Chloro)benzenesulfonyl-3-(SR)-((4-phenyl) piperidin-1-yl)methyl-4-
(SR)-nhenylpvrrolidine
Step A: 3-(SR)-((4-Phenyl)piperidin-1-yl)methyl-4-(SR)-
phenylpvrrolidine
A mixture of 232 mg (0.6 mmol) of 1-phenylmethyl-3-(SR)-
((4-phenyl) piperidin-1-yl)methyl-4-(SR)-phenylpyrrolidine (from
Example 9, Step B), 894 mg (14.1 mmol) of ammonium formate and 116
mg of 20% Pd(OH)2 on carbon in 10 mL of MeOH was heated at reflux for
lh. The reaction mixture was cooled and filtered througha pad of Celite.
The reaction flask and filtered solids were rinsed with 50 mL of EtOAc
and the filtrate was concentrated in vacuo. The residue was partitioned
between 50 mL of EtOAc and 30 mL of sat'd NaHC03 and the layers were
separated. The organic layer was dried over MgS04. The aqueous layer
was extracted with 50 mL of EtOAc; the extract was dried and the
organic layers were combined and concentrated in vacuo to afford 190
mg (95%) of the title compound as an oil. 1H NMR (500 MHz, CDC13): 8
1.63-1.80 (m, 4H), 1.93-2.05 (m, 2H), 2.39-2.53 (m, 5H), 2.83 (d, J~ 10.7,
1H), 2.93-3.02 (m, 2H), 3.0$ (t, J= 9.6, 1H), 3.47-3.53 (m, 1H), 5.19 (br s,
1H), 7.19-7.38 (lOH). Mass Spectrum (NH3-CI): 321 (M+1).
Step B: 1-(4-Chloro)benzenesulfonyl-3-(SR)-((4-phenyl) piperidin-1-
yl methyl-4-(SR)-pheny~DVrrolidine
A mixture of 16 mg (0.05 mmol) of 3-(SR)-((4-phenyl)-
piperidin-1-yl)methyl-4-(SR)-phenylpyrrolidine (from Example 30, Step
A) in 2 mL EtOAc and 1 mL of sat'd NaHC03 was treated with 21 mg
(0.95 mmol) of (4-chloro)benzenesulfonyl chloride. After 30 min, the
-127-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
mixture was partitioned between 20 mL of EtOAc and 10 mL of sat'd
NaHC03 and the layers were separated. The organic layer was washed
with 10 mL of sat'd NaCI, dried over MgS04 and concentrated in vacuo.
Flash chromatography on 15 g of silica gel using 4:1 v/v, then 2:1 v/v
hexanes/ether as the eluant afforded 14 mg (56%) of the title compound
as an oil. 1H NMR (500 MHz, CDC13): b 1.45-3.73 (17H), 7.13-7.33 (lOH),
7.56 (d, J= 8.5, 2H), 7.84 (d, J= 8.4, 2H). Mass Spectrum (NH3-CI): 495
(M+1).
The compounds in Examples 31-35 were prepared using
procedures analogous to those described in Example 30 substituting the
appropriate aryl sulfonyl chloride for (4-chloro)benzenesulfonyl chloride
in Example 30, Step B.
EXAMPLE 31
1-(2-Thiophene)sulfonyl-3-(SR)-((4-phenyl) piperidin-1-yl)methyl-4-(SR)-
phenvlnvrrolidine
1H NMR (500 MHz, CDC13): 81.74-3.81 ( 17H), 7.14-7.33 ( 12H), 7.67 (m,
1H). Mass Spectrum (NH3-CI): 467 (M+1).
F~~AAMPLE 32
1-(1-Napthalene)sulfonyl-3-(SR)-((4-phenyl) piperidin-1-yl)methyl-4-(SR)-
nhenvhvrrolidine
1H NMR (500 MHz, CDC13): 81.64-2.85 (12H), 3.00 (q, J= 8.5, 1H), 3.32 (t,
J= 9.4, 1H), 3.40 (t, J= 8.7, 1H), 3.82 (t, J= 10.1, 1H), 3.87 (br t, J= 9.0,
1H),
7.10-7.33 (lOH), 7.57-7.72 (m, 3H), 7.97 (d, J= 8.2, 1H), 8.11 (d, J= 8.2,
1H),
8.31 (d, J= 7.4, 1H), 8.90 (d, J= 8.5, 1H). Mass Spectrum (NH3-CI): 511
(M+1).
EXAMPLE 33
1-(2-Napthalene)sulfonyl-3-(SR)-((4-phenyl) piperidin-1-yl)methyl-4-(SR)-
uhenvlnvrrolidine
-128-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1H NMR (500 MHz, CDC13): 81.64-2.86 (m, 12H), 2.92 (q, J= 8.5, 1H), 3.26
(t, J= 8.0, 1H), 3.38 (t, J= 8.7, 1H), 3.?8-3.84 (m, 2H), 7.10-7.33 (11H),
7.64-
7.71 (m, 2H), 7.91-8.05 (m, 3H), 8.49 (s, 1H). Mass Spectrum (NH3-CI):
511 (M+1).
EXAMPLE ~4
1-(8-Quinoline)sulfonyl-3-(SR)-((4-phenyl) piperidin-1-yl)methyl-4-(SR)-
nhenvlnvrrolidine
1H NMR (500 MHz, CDC13): b 1.64-2.87 (12H), 2.96 (q, J= 9.2, 1H), 3.41 (t,
J= 9.2, 1H), 3.58 (t, J= 9.4, 1H), 4.26-4.32 (m, 2H), 7.10-7.32 ( lOH), 7.58
(dd,
J= 4.3, 8.2, 1H), 7.66 (t, J= 8.0, 1H), 8.07 (d, J= 8.2, 1H), 8.29 (d, J= 8.2,
1H),
8.56 (d, J= 7.4, 1H), 9.12 (d, J= 3.8, 1H). Mass Spectrum (NH3-CI): 512
(M+1).
PLE 35
1-(4-Biphenyl)sulfonyl-3-(SR)-((4-phenyl) piperidin-1-yl)methyl-4-(SR)-
nhenvlnvrrolidine
1H NMR (500 MHz, CDC13): 81.64-2.89 (12H), 2.97 (q, J= 8.5, 1H), 3.24 (t,
J= 9.6, 1H), 3.36 (t, J= 8.7, 1H), 3.75-3.79 (m, 2H), 7.14-7.33 (14H), 7.46
(m,
1H), 7.52 (m, 1H), 7.66 (m, 1H), 7.80 (m, 1H), 7.97 (m, 1H). Mass
Spectrum (NH3-CI): 537 (M+1).
The compounds in Examples 36-62 were prepared using
procedures analogous to those described in Example 30, 59, or 63
substituting the appropriate aromatic or aliphatic acid chloride for (4-
chloro)benzenesulfonyl chloride in Example 30, Step B, the appropriate
aromatic or aliphatic carboxylic acid for 1-fluorene carboxylic acid in
Example 59 or the appropriate aromatic or aliphatic acid chloride for
nicotinoyl chloride ~ HCl in Example 63.
-129 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1-Benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-phenyl-
Rvrrolidine
1H NMR (500 MHz, CDCl3): b 1.64-4.21 (17H), 7.17-7.62 (15H). Mass
Spectrum (NH3-CI): 425 (M+1).
EXAMPLE 37
1-(2-Chloro)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
pheny~~yrrolidine
1H NMR (500 MHz, CDC13): b 1.27-4.27 (17H), 7.17-7.47 (14H). Mass
Spectrum (NH3-CI): 459 (M+1).
EXAMPLE 38
1-(3-Chloro)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
pheny,~,Rvrrolidine
1H NMR (500 MHz, CDCl3): 81.27-4.20 (17H), 7.17-7.59 (14H). Mass
Spectrum (NH3-CI): 459 (M+1).
EXAMPLE 39
1-(4-Chloro)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
phenvhvrrolidine
1H NMR (500 MHz, CDCl3): 81.27-4.20 (17H), 7.17-7.58 (14H). Mass
Spectrum (NH3-CI): 459 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200 nm): 7.2
min.
EXAMPLE 40
1-(2-Methoxy)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
l~ne ylRyrrolidine
1H NMR (500 MHz, CDC13): 81.66-4.24 (20H), 3.87 (s, 3H), 6.90-7.41 (14H).
Mass Spectrum (NH3-CI): 455 (M+1).
-130-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1-(3-Methoxy)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
nhenvlDVrrolidine
1H NMR (500 MHz, CDCl3): 81.50-4.20 (20H), 3.83 (s, 3H), 6.94-7.38 (14H).
Mass Spectrum (NH3-CI): 455 (M+1).
EXAMPLE 42
1-(4-Methoxy)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
~envlnvrrolidine
Mass Spectrum (NH3-CI): 455 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200 nm): 9.8
min.
PLE 43
1-(3,5-Dichloro)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
phenvhvrrolidine
1H NMR (500 MHz, CDC13): 81.60-3.17 (12H), 3.85 (t, J= 8.7, 1H), 3.50-
3.58 (m; 1H), 3.74 (dd, J= 9.3, 12.3, 1H), 3.81 (dd, J= 7.6, 10.6, 1H), 4.11-
4.19
(m, 1H), 7.18-7.47 (13H). Mass Spectrum (NH3-CI): 492 (M+1).
EXAMPLE ~,4
1-(3,4-Dichloro)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
nhenvlnv~rQlidine
1H NMR (500 MHz, CDC13): 81.60-3.16 (12H), 3.42 (br t, 1H), 3.55 (q, J=
12.9, 1H), 3.83 (dd, J= 9.6, 12.3, 1H), 4.11-4.19 (m, 1H), 7.18-7.72 (13H).
Mass Spectrum (NH3-CI): 492 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200 nm): 19.5
min.
EXAMPLE 45
-131-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1-(2,6-Dichloro)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
~h~vl~v~rolidine
1H NMR (500 MHz, CDCl3): $1.60-3.25 (11H), 3.34 (t, J= 9.4, 1H), 3.57-
3.61 (m, 2H), 3.81 (dd, J= 8.0, 12.6, 1H), 4.20-4.26 (m, 2H), 7.18-7.39 {13H)
Mass Spectrum (NH3-CI): 492 (M+1).
EXAMPLE 46
1-(2,4-Dichloro)benzoyl-3-(SR)-({4-phenyl)piperidin-1-yl)methyl-4-(SR)-
nnenvmvrrolinine
1H NMR (500 MHz, CDC13): b 1.60-3.02 (9H), 3.09-3.20 (m, 2H), 3.34 {t, J=
9.8, 1H), 3.51-3.61 (m, 2H), 3.73 (dd, J= 8.7, 12.6, 1H), 4.17-4.25 (m, 2H),
7.18-7.49 (13H). Mass Spectrum (NH3-CI): 492 (M+1). HPLC (YMC
"Octyl" 4.6 x 250 mm column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5
mL/min, 200 nm): 16.9 min.
EXAMPLE 47
1-(2,3-Dichloro)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-{SR)-
p envlRyrrolidine
1H NMR (500 MHz, CDC13): S 1.60-4.24 (17H), 7.17-7.55 {13H). Mass
Spectrum (NH3-CI): 492 (M+1).
EXAMPLE 48
1-(2,5-Dichloro)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-{SR)-
phenvlDVrrolidine
1H NMR (500 MHz, CDC13): 81.60-3.20 {12H), 3.37 (br t, J= 9.8, 1H), 3.53
(dd, J= 8.7, 12.4, 1H), 3.62 (t, J= 10.1, 1H), 3.?3 (dd, J= 8.9, 12.3, 1H),
4.17-
4.25 (m, 2H), 7.17-7.40 (I3H). Mass Spectrum (NH3-CI): 492 (M+1).
HPLC (YMC "Octyl" 4.6 x 250 mm column, 60:40 v/v H20/CH3CN + 0.5%
TFA, 1.5 mL/min, 200 nm): 15.4 min
-132-
*rB
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
EXAMPLE 49
1-(2-Trifluoromethyl)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-
(SR)-nhenvlnvrrolidine
1H NMR (500 MHz, CDC13): b 1.46-2.14 (5H), 2.32-3.19 (6H), 3.26 (t, J=
10.3, 1H), 3.52-3.58 (m, 2H), 3.76 (dd, J= 8.7, 12.6, 1H), 4.19-4.27 (m, 2H),
7.17-?.77 (14H). Mass Spectrum (NH3-CI): 493 (M+1). HPLC (YMC
"Octyl" 4.6 x 250 mm column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5
mL/min, 200 nm): 12.7 min
~~PLE 50
1-(2-Methoxycarbonyl)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-
(SR)-phenvlnvrrolidine
1H NMR (500 MHz, CDCl3): 81.63-2.12 (5H), 2.34-3.27 (10H), 3.40-3.52
(3H), 3.69-3.79 {3H), 4.09-4.17 (m, 2H), 7.14-7.48 (14H). Mass Spectrum
(NH3-CI): 483 (M+1).
EXAMPLE 51
1-(2-Methyl)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
phenvlnvrrolidine
1H NMR (500 MHz, CDC13): b 1.67-3.77 (18H), 2.35-2.40 (3H), 4.18-4.29
(2H), 7.16-7.40 (14H). Mass Spectrum (NH3-CI): 439 (M+1). HPLC (YMC
"Octyl" 4.6 x 250 mm column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5
mL/min, 200 nm): 9.3 min
VIPL_E 52
1-(2-Fluoro)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
nhenvlnvrrolidine
1H NMR (500 MHz, CDCl3): 81.71-2.20 (6H), 2.40-2.80 (4H), 2.98-3.17
(3H), 3.35-3.84 (3H), 4.17-4.28 (m, 1H), 7.00-7.48 (14H). Mass Spectrum
-133 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(NH3-CI): 443 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm column, 60:40
v/v H20/CH3CN + 0.5% TFA, 1.5 mlJmin, 200 nm): 8.6 min
1-(2-Bromo)benzoyl-3-{SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
uhenvlnvrrolidine
1H NMR (500 MHz, CDC13): 8 0.91-3.78 ( 15H), 4.21-4.26 (2H), 7.17-7.64
(14H). Mass Spectrum (NH3-CI): 503 (M+1).
EXAMPLE 54
1-(Cyciohexanoyl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
Izhenvlpvrrolidine
1H NMR (500 MHz, CDC13): 81.22-2.11 ( 16H), 2.29-2.65 (5H), 2.88-3.54
(5H), 3.94-4.09 (2H), 7.19-7.39 (10H). Mass Spectrum (NH3-CI): 431
(M+1). HPLC (YMC "Octyl" 4.6 x 250 mm column, 60:40 v/v H20/CH3CN
+ 0.5% TFA, 1.5 mL/min, 200 nm): 11.8 min.
EXAMPLE 55
1-(Cyclopentanoyl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
phenvlnvrrolidine
1H NMR (500 MHz, CDCl3): b 1.50-3.56 (24H), 3.80-4.09 (2H), 7.19-7.38
(lOH). Mass Spectrum (NH3-CI): 417 (M+1).
EXAMPLE 56
1-(3-Methoxycarbonyl)benzoyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-
(SR)-nhenvlnyrrolidine
1H NMR (500 MHz, CDCl3): 81.50-3.16 (12H), 3.39-4.22 (11H), 7.16-8.27
(14H). Mass Spectrum (NH3-CI): 483 (M+1). HPLC (YMC "Octyl" 4.6 x
250 mm column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200
nm): 8.6 min.
-134-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
EXAMPLE 57
1-(Phenylacetyl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
nhenvlRvrrolidine _
1H NMR (500 MHz, CDC13): b 1.71-4.15 (19H), 7.21-7.3? (15H). Mass
Spectrum (NH3-CI): 439 (M+1) .
~~A,MPLE 58
1-(Isonicotinoyl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
~henyl~yrrolidine
1H NMR (500 MHz, CDC13): S 7.16-8.78 (9H), 1.27-4.19 (22H). Mass
Spectrum (NH3-CI): 426 (M+1).
EXAMPLE 59
1-(Nicotinoyl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
nhen~Ryrrolidine _
A solution of 20 mg (0.06 mmol) of 3-(RS)-((4-phenyl)-
piperidin-1-yl)methyl-4-(SR)-phenylpyrrolidine (from Example 30, Step
A), 19 mg (0.19 mmol) of TEA and 6 mg (0.05 mmol) of 4-DMAP in 2 mL
of CH2C12 (2 mL) was treated with 28 mg (0.16 mmol) of nicotinoyl
chloride ~ HCl and was stirred at rt for 2 h. The reaction was
concentrated in vacuo . Preparative thin layer chromatography (silica
gel GF, 20x20 cm, 1000 microns) using 92:$ v/v MeOH/CH2C12 + 0.5%
NH40H as the eluant afforded 20 mg (71%) of the title compound as a
yellow foam. 1H NMR (500 MHz, CDCl3): 8 7.17-8.88 (9H), 1.27-4.24
(22H). Mass Spectrum (NH3-CI): 426 (M+1).
PLE 60
1-(2-Thiophenecarbonyl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
nhenvlRyrrolidine
-135-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1H NMR (500 MHz, CDC13): 8 7.05-7.75 (13H), 1.80-4.52 (1?H). Mass
Spectrum (NH3-CI): 426 (M+1).
EXAMPLE 61
1-( 1-Naphthoyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
~henx~g«rrolidine
1H NMR (500 MHz, CDC13): b 7.12-7.98 (17H), 1.28-4.37 (17H). Mass
Spectrum (NH3-CI): 475 (M+1).
EPLE 62
1-(2-Naphthoyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
phenyl~rolidine
1H NMR (500 MHz, CDC13): 8 7.12-7.98 (17H), 1.28-4.37 (17H). Mass
Spectrum (NH3-CI): 475 (M+1).
EXAMPLE 63
1-(1-Fluorenecarbonyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
~henvl~~olidine
A solution of 10 mg (0.03 mmol) of 3-(RS)-((4-
phenyl)piperidin-1-yl)methyl-4-(SR)-phenylpyrrolidine (from Example
30, Step A), 10 mg (0.07 mmol) of DIEA and 10 mg (0.04 mmol) of bis(2-
oxo-3-oxazolidinyl)phosphinic chloride (BOP-Cl) in 2 mL of CH2C12 (2
mL) at 0 °C was treated with 7 mg (0.16 mmol) of 1-fluorene carboxylic
acid. The cooling bath was removed; the reaction was stirred at rt for 2 h
and then was concentrated in uacuo . Preparative thin layer
chromatography (silica gel GF, 20x20 cm, 1000 microns) using 95:5 v/v
MeOH/CH2C12 as the eluant afforded 14 mg (88%) of the title compound
as a foam. 1H NMR (500 MHz, CDC13): S 7.12-7.87 (17H), 1.28-4.13 (19H).
Mass Spectrum (NH3-CI): 513 (M+1).
EXAMPLE ~,4
-136-
CA 02298813 2000-O1-28
WO 99!09984 PCT/US98/17755
1-(9-Fluorenecarbonyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
uhenvlnvrrolidine
1H NMR (500 MHz, CDCl3): 8 6.92-7.82 (17H), 1.28-5.13 (19H). Mass
Spectrum (NH3-CI): 513 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200 nxn): 21.5
mm.
EXAMPLE 65
1-(4-Fluorenecarbonyl)-3-(RS)-{(4-phenyl)piperidin-1-yl)methyl-4-(SR)-
phenylpyrrolidine
1H NMR (500 MHz, CDC13): 8 7.11-7.79 (17H), 1.28-4.43 {19H). Mass
Spectrum (NH3-CI): 513 (M+1).
EXAMPLE 66
1-(1-Adamantanecarbonyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl-4-
(SR)-phenvlnvrrolidine
1H NMR (500 MHz, CDC13): S 7.21-?.38 (lOH), 1.74-4.08 (32H). Mass
Spectrum (NH3-CI): 483 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mlJmin, 200 nm): 26.2
mm.
The compounds described in Examples 6?-71 were prepared
using procedures analogous to those described in Examples 1 and 9
substituting the appropriately substituted (E)-cinnamate for methyl (Z)-
cinnamate in Example 1, Step A.
EXAMPLE 67
1-Phenylmethyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-{2-
chloro)phen3~lpvrrolidine
1H NMR (500 MHz, CDC13): 8 1.52-1.76 (m, 4H), 1.95-2.04 (m, 2H), 2.39-
2.60 (m, 5H), 2.67 (t, J= 8.4, 1H), 2.81 (d, J= 11.2, 1H), 3.00 (t, J= 8.7,
2H),
-137-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
3.11 (t, J= 7.3, 1H), 3.61 (q, J= 6.6, 1H), 3.72 (ABq, J= 48.3, 2H), 7.11-7.41
(13H), 7.5? (d, J= 7.8, 1H). Mass Spectrum (NH3-CI): 445 (M+1).
EXAMPLE 68
1-Phenylmethyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-(3-
chloro) envlpvrrolidine
IH NMR (500 MHz, CDC13): b 1.62-1.82 (m, 4H), 1.93-1,97 (m, 1H), 2.01
2.05 (m, 1H), 2.42-2.49 (m, 5H), 2.70-2.73 (m, 1H), 2.75-2.80 (m, 1H), 2.93
IO 3.02 (m, 4H), 3.70 (ABq, J= 12.8, 2H), 7.17-7.43 (14H). Mass Spectrum
(NH3-CI): 445 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm column, 60:40
v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200 nm): 11.0 min.
EXAMPLE 69
1-Phenylmethyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-(3-
~hloro) hen~p~~rrolidine
IH NMR (500 MHz, CDC13): b 1.58-1.82 (m, 4H), 1.97 (q, J= I1.5, 2H), 2.42-
2.53 (m, 5H), 2.68 (t, J= 6.5, 1H), 2.79 (d, J= 11.0, 1H), 2.93-3.04 (rn, 4H),
3.70 (ABq, J= 36.6, 2H), 7.22-7.41 (14H). Mass Spectrum (NH3-CI): 445
(M+1). HPLC (YMC "Octyl" 4.6 x 250 mm column, 60:40 v/v H20/CH3CN
+ 0.5% TFA, 1.5 mL/min, 200 nm): 9.0 min.
EXAMPLE 70
1-Phenylmethyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-(3-
bromo)phen~pyrrolidine
1H NMR {500 MHz, CDCl3): 81.60-2.03 (7H), 2.38-3.04 (lOH), 3.68 (ABq,
J= 36.6, 2H), 7.14-7.57 (14H). Mass Spectrum (NH3-CI): 489 (M+1).
EXAMPLE 71
1-Phenylmethyl-3-(SR)-(spiro[indan-1-one-3,4'-piperidin-1'-yl]methyl)-4-
(SR)-(3,4-di-chloro)~henyl_R3~rrolidine
-138-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
The title compound was prepared using procedures
analogous to those described in Example 1, Steps A and B (employing
methyl (Z)-(3,4-dichlorophenyl)propenoic acid in place of methyl (Z)-
cinnamate) and Example 9, Steps A and B (employing spiro[indan-1-one-
3,4'-piperidine] in place of 4-phenylpiperidine). Mass Spectrum (ESI)
m/e 519 (M+1).
The compounds in Examples 72-76 were prepared from 1-
phenylmethyl -3-(RS)-((4-(N-methoxycarbonyl-N-cyclohexyl-
methyl)amino)piperidin-1-yl)methyl-4-(SR)-phenylpyrrolidine (from
Example 12) using procedures analogous to those described in Example
30 substituting the appropriate acid chloride for (4-chloro)benzene-
sulfonyl chloride in Example 30, Step B.
EXAMPLE 72
1-( 1-Naphthoyl)-3-(RS)-{(4-(N-methoxycarbonyl-N-cyclohexyl-
methyl)amino)pi~eridin-1-vl)meth 1-4-(S )- henylpyrrqli i a
1H NMR (500 MHz, CDC13): 8 7.13-7.97 (12H), 0.89-4.34 (33H). Mass
Spectrum (NH3-CI): 568 (M+1).
EXAMPLE 73
1-Cyclohexanoyl-3-(RS)-((4-(N-methoxycarbonyl-N-cyclohexyl-
~yl)amino)pi~e~din-~vl)methyl-4-(SR)-phenyl~vrrolidine
1H NMR (500 MHz, CDCl3): S 7.20-7.37 (5H), 0.88-4.05 (44H). Mass
Spectrum (NH3-CI): 524 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mlJmin, 200 nm): 21.1
min.
1-Cyclopentanoyl-3-(RS )-({4-(N-methoxycarbonyl-N-cyclohexyl-
~aethyl)amine)~iueridin-1-vl)methyl-4-(SR)-~y~vrrolidine
-139 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1H NMR (500 MHz, CDCl3): 8 7.21-7.37 (5H), 0.89-4.11 (42H). Mass
Spectrum (NH3-CI): 510 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200 nm): 15.9
min.
EXAMPLE 7~
1-(2-Chlorobenzoyl)-3-(RS)-((4-{N-methoxycarbonyl-N-cyclohexyl-
met vl)amino)nineri~iin-1- lv )methyl-4-(SR);phenylpy~~olidine
1H NMR (500 MHz, CDCl3): 8 7.19-7.46 (9H), 0.89-4.21 (33H). Mass
Spectrum (NH3-CI): 552 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v HZO/CH3CN + 0.5% TFA, 1.5 mL/min, 200 nm): 16.9
min.
EXAMPLE ?6
1-Benzoyl-3-(RS)-((4-(N-methoxycarbonyl-N-cyclohexylmethyl)-
amino)niDeridin-1-vl)methyl-4-(SR)-uhen~pvrrolidine
1H NMR (500 MHz, CDCl3): 8 7.20-7.59 ( 10H), 0.89-4.15 (33H). Mass
Spectrum (NH3-CI): 518 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200 nm): 13.5
min.
EXAMPLE ? 7
1-Phenylmethyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-(3-
biphenyl)Rvrrolidine
A mixture of 34 mg (0.07 mmol) of 1-phenylmethyl -3-(SR)-
((4-phenyl)piperidin-1-yl)methyl-4-(SR)-(3-bromo)phenylpyrrolidine
(from Example 70), 19 mg (0.09 mmol) of benzene boronic acid, 32 mg
(0.30 mmol) of Na2C03 and 5.0 mg (0.004 mmol) of tetrakis(triphenyl-
phosphine)palladium{0) in 3 mL of 2:1 v/v toluene/EtOH was stirred in
an oil bath set at 95 °C for 20 h. The reaction mixture was partitioned
between 20 mL of ether and 10 mL of H20 and the layers were separated.
-140 -
CA 02298813 2000-O1-28
WO 99/09984 PCTIUS98/17755
The organic layer was washed with 10 mL of 0.5 N KHS04, 10 mL of sat'd
NaHC03, 10 mL of sat'd NaCI, dried over MgS04 and concentrated in
vacuo. Flash chromatography on I5 g of silica gel using 2:1 v/v, then 1:1
v/v hexanes/ether as the eluant afforded 15 mg (44%) of the title
compound as a solid. 1H NMR (500 MHz, CDCl3): b 1.22-4.20 (19H), 7.01-
8.20 (19H). Mass Spectrum (NH3-CI): 487 (M+1).
EXAMPLE 78
1-Phenylmethyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-(3-
methvl)uhenvlnvrrolidine _
A mixture of 42 mg (0.08 mmol) of 1-phenylmethyl -3-(SR)-
((4-phenyl)piperidin-1-yl)methyl-4-(SR)-(3-bromo)phenylpyrrolidine
{from Example 70), 4fi mg (0.26 mmol) of tetramethyltin and 6 mg (0.09
mmol) of bis(triphenylphosphine)palladium dichloride in 2 mL of DMF
was heated at 100 °C for 24 h. The reaction mixture was cooled and
partitioned between 20 mL of ether and 10 mL of H20 and the layers were
separated. The organic layer was washed with 10 mL of sat'd NaHC03,
10 mL of sat'd NaCI, dried over MgS04 and concentrated in vacuo. Flash
chromatography on 16 g of silica gel using 3:2 v/v hexanes/ether as the
eluant afforded 22 mg (61%) of the title compound as an oil. 1H NMR
(500 MHz, CDC13): 81.65-1.78 {m, 3H), 1.94-2.02 (m, 2H), 2.38 (s, 3H),
2.42-3.03 (m, 12H), 3.72 (ABq, J= 13.0, 2H), 7.02-7.42 (m, 14H). Mass
Spectrum (NH3-CI): 425 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200 nm): 6.9
min.
EXAMPLE 79
1-Phenylmethyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-(3-
methoxvcarbonyl hen~pwrrolidine
Carbon monoxide gas was bubbled through a mixture of 42
mg (0.08 mmol) of 1-phenylmethyl-3-(SR)-((4-phenyl)piperidin-1-
yl)methyl-4-(SR)-(3-bromo)phenylpyrrolidine (from Example 70), 24 p,L
-141-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
{0.17 mmol) of TEA and 40 mg (0.03 mmol) of tetrakis(triphenyl-
phosphine)palladium(0) in 2.5 mL of 4:1 v/v DMF/MeOH and the
resulting mixture was stirred under an atmosphere of CO at 70 °C for 12
h. The reaction mixture was cooled, filtered and the filtrate was
concentrated in vacuo. The residue was partitioned between 20 mL of
EtOAc and 10 mL of sat'd NaHC03 and the layers were separated. The
organic layer was washed with 10 mL of sat'd NaCI, dried over MgS04
and concentrated in vacuo. Flash chromatography on 10 g of silica gel
using 4:1 v/v hexanes/EtOAc as the eluant afforded 15 mg (38%) of the
title compound as an oil. 1H NMR (500 MHz, CDCl3): 8 1.54-3.11 (17H),
3.71 (ABq, J= 39.6, 2H), 3.94 (s, 3H), 7.19-7.42 (11H), 7.59 (d, J= 7.8, 1H),
7.88 (d, J= 7.8, 1H), 8.08 (s, 1H). Mass Spectrum (NH3-CI): 469 (M+1).
F~AMPLE 80
1-(2-Chlorobenzoyl)-3-(RS)-((4-(N-phenylmethoxycarbonyl-N-
ethvl)amino)~iperidin-1-~)methvl-4-(SR) phen~pyrrolidine
Step A: 1-(2-Chloro)benzoyl-3-(RS)-hydroxymethyl-4-(SR)-
phen~pvrrolidine
A mixture of 290 mg (1.6 mmol) of 3-(RS)-hydroxy-methyl-4-
(SR)-phenylpyrrolidine (from Example 4) in 10 mL of EtOAc and 5 mL of
sat'd NaHC03 was treated with 0.25mL (2.0 mmol) of (2-chloro)-benzoyl
chloride and stirred at rt for 20 min. The mixture was diluted with 25
mL of EtOAc and 10 mL of H20 and the layers were separated. The
organic layer was washed with 15 mL of 2 N HCI, 15 mL of sat'd
NaHC03, 15 mL of sat'd NaCI, dried over MgS04 and concentrated in
vacuo. Flash chromatography on 12 g of silica gel using 2:1 v/v
CH2C12/EtOAc as the eluant afforded 146 mg of the title compound as an
oil. 1H NMR (500 MHz, CDC13): 8 1.69 (br s, 1H), 2.50-2.63 (1H), 3.20-3.74
(6H), 4.09-4.22 (1H), 7.20-7.42 {9H).
Step B: 1-(2-Chloro)benzoyl-3-(RS)-formyl -4-(SR)-
phen~nvrrolidine
-142 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
The title compound was prepared from 1-(2-chloro)benzoyl-
3-(RS)-hydroxymethyl-4-(SR)-phenylpyrrolidine (from Example 80, Step
A) using a procedure analogous to that descibed in Example 9, Step A.
Mass Spectrum (NH3-CI): 314 (M+1).
Step C: 1-(2-Chlorobenzoyl) -3-(RS)-((4-(N-phenylmethoxy-
carbonyl-N-ethyl)amino)piperidin-1-yl)methyl-4-(SR)-
lzhenvluvrrolidine
A mixture of 80 mg (0.25 mmol) of 1-(2-chloro)benzoyl-3-
(RS)-formyl -4-(SR)-phenylpyrrolidine (from Example 90, Step B) and 90
mg (0.30 mmol) 4-(N-phenylmethoxycarbonyl-N-ethylamino)-piperidine
HCl and 45 ~.L (0.32 mmol) of TEA in 2 mL of dichloroethane was treated
with 105 mg (0.5 mmol} of sodium triacetoxyborohydride and the
resulting mixture was stirred at rt for 3 h. The reaction mixture was
partitioned between 30 mL of ether and 10 mL of 1 N NaOH and the
layers were separated. The organic layer was washed with 10 mL of
sat'd NaCl, dried over MgS04 and concentrated in vacuo. Flash
chromatography on 8 g of silica geI using 1:1 v/v hexanes/EtOAc + 1%
TEA as the eluant afforded 110 mg (77%) of the title compound as an oil.
Mass Spectrum (NH3-CI): 561 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200 nm): 11.3
min.
The compounds described in Examples 81-84 were prepared
using procedures analogous to those described in Example 80
substituting the appropriate acid chloride for (2-chloro)benzoyl chloride
in Example 80, Step A.
EXAMPLE 81
1-(Benzoyl)-3-(RS)-((4-(N-phenylmethoxycarbonyl-N-ethyl)amino)-
~,i p ' in-1-yl)methyl-4-(SR)-pheny~~yrrolidir.~e
1H NMR (500 MHz, CDC13): b 1.02-4.40 (22H), 5.10-5.16 (2H), 7.00-8.15
(15H).
-143-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
~~"E1MPLE 82
1-( 1-Napthoyl)-3-(RS)-((4-(N-phenylmethoxycarbonyl-N-ethyl)amino)-
p~ ' in-1-vl)methyl-4-(SR)- hoe y,~g~~dine
1H NMR (500 MHz, CDCl3): 81.05-4.50 (22H), 5.10-5.16 (2H), 7.12-7.97
(17H).
1-(Cyclohexanoyl)-3-(RS)-((4-(N-phenylmethoxycarbonyl-N-ethyl)amino)-
~i, ' in-1-yl)methvl-4-(SR)- hen~pvrrolidine _
1H NMR (500 MHz, CDC13): b 1.11-4.02 (33H), 5.14 (app s, 2H), 7.20-7.36
(lOH).
EXAMPLE 84
1-(Cyclopentanoyl)-3-(RS)-((4-(N-phenylmethoxycarbonyl-N-ethyl)amino)-
in-1-yl)methvl-4-(SR)-uhenxlnvrrolidine
1H NMR (500 MHz, CDC13): S 1.11-4.48 (31H), 5.13 (app s, 2H), 7.20-?.36
(lOH).
EXAMPLE 85
1-(2-Phenylethyl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
ul~envlpyrrolidine
A mixture of 50 mg (0.16 mmol) 3-(SR)-((4-phenyl)piperidin-
1-yl)methyl-4-(SR)-phenylpyrrolidine (from Example 30, Step A) and 37
mg (0.31 mmol) of phenylacetaldehyde and 100 mg 4 A powdered
molecular sieves in 3 mL of MeOH was treated with 18 mg (0.28 mmol) of
sodium cyanoborohydride and 2 drops of HOAc and stirred at rt for 18 h.
The mixture was filtered onto a pad of Celite; the pad and flask were
rinsed well with MeOH (25 mL). The filtrate was concentrated in vacuo
and the residue was partitioned between 25 mL of EtOAc and 15 mL of
sat'd NaHC03 and the layers were separated. The organic layer was
dried over MgS04 and concentrated in vacuo. Flash chromatography on
-144-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
15 g of silica gel using 2:1 v/v hexanea/EtOAc afforded 32 mg (48%) of the
title compound as an oil. 1H NMR (500 MHz, CDC13): S 1.66-2.04 (6H),
2.42-3.13 (15H), 7.20-7.37 (15H). Mass Spectrum (NH3-CI): 427 (M+1).
The compounds described in Examples 86-88 were prepared
using a procedure analogous to that described in Example 85
substituting the appropriate aldehyde for phenylacetaldehyde.
EXAMPLE 86
1-(3-Phenylpropyl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-{SR)-
phenvlnvrrolidine
1H NMR (500 MHz, CDC13): S 1.65-2.03 (7H), 2.41-3.05 (16H), 7.21-7.3?
(15H). Mass Spectrum (NH3-CI): 439 {M+1). HPLC (YMC "Octyl" 4.6 x
250 mm column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200
nm): 11.9 min.
EXAMPLE 8?
1-(Cyclohexylmethyl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
nhenvlpvrrolidine
1H NMR (500 MHz, CDC13): 8 7.19-7.36 (17H), 1.03-3.04 (30H). Mass
Spectrum (NH3-CI): 417 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mlJmin, 200 nm): 7.2
min.
EXAMPLE 88
1-((1-Napthyl)methyl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
~h~nylt~v~rolidine
1H NMR (500 MHz, CDC13): 8 7.18-8.40 (17H), 1.28-4.14 (19H). Mass
Spectrum (NH3-CI): 461 (M+1). HPLC {YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200 nm): 8.4
min.
-145-
CA 02298813 2000-O1-28
WO 99109984 PCT/US98/17755
PLE 89
1-((N-Phenylcarbamoyl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
nhenvlDVrrolidine
A solution of 50 mg (0.16 mmol) of 3-(SR)-((4-phenyl)-
piperidin-1-yl)methyl-4-(SR)-phenylpyrrolidine (from Example 30, Step
A) and 43 ~t.L (0.25 mmol) of DIEA in 2 mL of CHZCl2 was treated with 34
p,L (0.31 mmol) of phenyl isocyanate and stirred at rt for 2 h. The
reaction mixture was partitioned between 20 mL of EtOAc and 10 mL of
H20 and the layers were separated. The organic layer was dried over
MgS04 and concentrated in vacuo. Flash chromatography on 15 g of
silica gel using 2:1 v/v hexanes/EtOAc as the eluant afforded 39 mg (57%)
of the title compound as an oil. 1H NMR (500 MHz, CDC13): 81.70-1.83
(4H), 1.93-1.97 (m, 1H), 2.09-2.13 (m, 1H), 2.40-2.49 (3H), 2.68-2.73 (m, 1H),
2.91 (d, J= 11.0, 1H), 3.04 (d, J= 11.0, 1H), 3.12 (q, J= 9.2, 1H), 3.37 (t,
J=
9.0, 1H), 3.54 (t, J= 9.6, 1H), 3.95-4.01 (m, 2H), 6.31 (s, 1H), 7.03-7.48
(15H).
Mass Spectrum (NH3-CI): 440 (M+1).
EXAMPLE 90
1-((1-(RS)-Phenylethyl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
nhenvlDVrrolidine
A solution of 54 mg (0.17 mmol) of 3-(SR)-((4-phenyl)-
piperidin-1-yl)methyl-4-(SR)-phenylpyrrolidine (from Example 30, Step
A), 62 mg (0.34 mmol) of (~)-(1-phenyl)ethyl bromide and 53 N.L (0.30
mmol) of DIEA in 3 mL of CH3CN was heated at reflux for 1 h. The
reaction mixture was cooled and partitioned between 25 mL of ether and
10 mL of sat'd NaHC03 and the layers were separated. The organic
layer was dried over MgS04 and concentrated in vacuo. Flash
chromatography on 15 g of silica gel using 3:2 v/v hexanes/EtOAc as the
eluant afforded 46 mg (64%) of the title compounds as a mixture of
inseparable racemic diastereomers. Mass Spec (NH3-CI): 425 (M+1).
EXAMPLE 91
-146 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1-(Benzothiazol-2-yl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
phenvlnvrrolidine
A mixture of 54 mg (0.16 mmol) of 3-(SR)-((4-
phenyl)piperidin-1-yl)methyl-4-(SR)-phenylpyrrolidine (from Example
30, Step A), 53 mg (0.31 mmol) of (2-chloro)benzothiazole and 65 mg (0.47
mmol) of K2C03 in 3 mL of DMF was heated at 80 °C for 1 h. The
reaction mixture was cooled, partitioned between 25 mL of ether and 15
mL of H20 and the layers were separated. The organic layer was dried
over MgS04 and concentrated in vacuo. Flash chromatography on 15 g
of silica gel using 1:1 v/v hexnaes/EtOAc as the eluant afforded 16 mg
(23%) of the title compound as an oil. 1H NMR (500 MHz, CDC13): 81.79-
3.04 (12H), 3.29 (q, J= 8.9, 1H), 3.55 (t, J= 9.0, 1H), 3.69 (t, J= 9.6, 1H),
4.03-
4.11 (m, 2H), 7.07-7.64 (14H). Mass Spectrum (NH3-CI): 454 (M+1).
EXAMPLE 92
1-(Benzoxazol-2-yl)-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
h~nvl~vrrolidine
The title compound was prepared using a procedure
analogous to that described in Example 91 substituting (2-chloro)benz-
oxazole for (2-chloro)benzothiazole. 1H NMR (500 MHz, CDC13): 81.61-
3.28 ( 12H), 3.25 (q, J= 8.7, 1H), 3.59 (t, J= 9.6, 1H), 3.75 (t, J= 9.0, 1H),
4.16
(dd, J= 7.8, 10.5, 2H), 7.01-7,41 (14H). Mass Spectrum (NH3-CI): 438
(M+1).
1-Benzyl-3-(SR)-(4-(N-methoxy, N-methyl-carboxamide)-piperidin-1-
methyl)-4-(SR)- eny~yr~oliding
To a solution of 225 mg of 1-benzyl-3-(SR)-(4-carboethoxy-
piperidin-1-ylmethyl)-4-(SR)-phenylpyrrolidine (from Example 27) and 83
mg of N-methoxy-methylamine hydrochloride in 1.5 mL of THF at -20 oC
was added 0.83 mL of isopropylmagnesium chloride (2M in THF). The
reaction was allowed to warm to 0 oC over 30 min. The reaction was
quenched with 1N NaHC03. The mixture was partitioned between 50
-147-
CA 02298813 2000-O1-28
WO 99/09984 PCT/pS98/17755
mL of EtOAc and 50 mL of 1N NaHC03. After separating layers, the
organic phase was washed with 50 mL of brine, dried over Na2S04 and
concentrated under vacuum. The residue was purified by flash
chromatography eluting with a 2.5% MeOH in CH2C12 to give 159 mg of
the title compound. RF: 0.26 (5% MeOH in CH2Cl2). 1H NMR (300 MHz,
CDC13): S 1.52-1.90 (m, 6H), 2.34-2.71 (m, 7H), 2.88-3.02 (m, 4H), 3.15 (s,
3H), 3.59-3.71 (m, 5H), 7.13-7.38(m, lOH). Mass Spectrum (CI): 422.3
(M+H).
EXAMPLE 94
1-Benzyl-3-(SR)-(4-(N-methyl-N-phenyl-carboxamide)-piperidin-1-
ylmethy )-4-(SR)-phenylpvrrolidine
The title compound was prepared from 35 mg of 1-benzyl-3-
(SR)-(4-carboethoxy-piperidin-1-ylmethyl)-4-(SR)-phenylpyrrolidine (from
Example 29), 0.015 mL of N-methylaniline and 0.070 mL of
isopropylmagnesium chloride (2M in THF) using a procedure analogous
to that described in Example 93 to provide 12 mg of the title compound.
RF: 0.30 (5% MeOH in CH2C12). 1H NMR (300 MHz, CDC13): S 1.36-1.86
(m, 6H), 2.02-2.94 (m, 11H), 3.22 (s, 3H), 3.63 (ABq, J = 13 Hz, 2H), 7.10-
7.41 (m, 15H). Mass Spectrum (CI): 468.3 {M+H).
X~PLE 95
1-Benzyl-3-(SR)-(4-(N-benzyl-N-methyl-carboxamide)-piperidin-1-
ylmethvl)-4-(SR)-phenylRvrrolidine _
The title compound was prepared from 35 mg of 1-benzyl-3-
(SR)-(4-carboethoxy-piperidin-1-ylmethyl)-4-(SR)-phenylpyrrolidine (from
Example 29), 0.017 mL of N-benzyl-N-methylamine and 0.070 mL of
isopropylmagnesium chloride (2M in THF) using a procedure analogous
to that described in Example 93 to provide 7.5 mg of the title compound.
RF: 0.25 (5% MeOH in CH2C12). 1H NMR (300 MHz, CDCl3): S 1.49-1.90
(m, 6H), 2.30-2.51 (m, 5H), 2.60-2.73(m, 2H), 2.84-3.02 (m, 7H), 3.59-3.72
-148-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(m, 2H), 4.51, 4.57 (2S, 2H), 7.10-7.37 (m, 15H). Mass Spectrum (CI):
482.4 (M+H).
EXAMPLE 96
1-Benzyl-3-(SR)-(4-(N-benzyl-N-ethyl-carboxamide)-piperidin-1-ylmethyl)-
4-(SR)-nhenvlpvrrolidine
The title compound was prepared from 30 mg of 1-benzyl-3-
(SR)-(4-carboethoxy-piperidin-1-ylmethyl)-4-(SR)-phenylpyrrolidine, 0.022
mL of N-benzyl-N-methyiamine and 0.075 mL of isopropyl-magnesium
chloride (2M in THF) using a procedure analogous to that described in
Example 93 to provide 16 mg of the title compound. RF: 0.30 (5% MeOH
in CH2Cl2). 1H NMR (300 MHz, CDC13): 81.04-1.I3 (m, 3H), 1.44-1.94 (m,
6H), 2.27-3.01 (m, 11H), 3.23, 3.37 (2q, J = 7.1 Hz, 2H), 3.58-3.72 (m, 2H),
4.49, 4.57 (2s, 2H), 7.11-7.37 (m, 15H). Mass Spectrum (CI): 496.3 (M+H).
EXAMPLE 97
1-Benzyl-3-(SR)-(4-(N-methyl-N-phenethyl-carboxamide)-piperidin-1-
y methyl)-4-(S~phenylpyrrolidine
The title compound was prepared from 37.5 mg of 1-benzyl-
3-(SR)-(4-carboethoxy-piperidin-1-ylmethyl)-4-(SR)-phenylpyrrolidine,
0.020 mL of N-methyl-N-phenethylamine and 0.070 mL of isopropyl-
magnesium chloride (2M in THF) using a procedure analogous to that
described in Example 93 to provide 25.5 mg of the title compound. RF:
0.20 (5% MeOH in CH2C12). 1H NMR (300 MHz, CDC13): b 1.20-1.97 (m,
7H), 2.27-2.49 (m, 4H), 2.61-2.70 (m, 2H), 2.78-3.00 (m, 9H), 3.46-3.71 (m,
4H), 7.09-7.37 (m, 15H). Mass Spectrum (CI): 496.3 (M+H).
EXAMPLE 98
1-Benzyl-(SR)-[3-methyl-3-((4-phenyl)piperidin-1-yl)methyl]-4-(SR)-
nhenvhwrrolidine
-149-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Step A: 1-Benzyl-(RS)-[3-methyl-3-formyl]-4-(SR)-phenyl
uvrrolidine
A solution of 150 mg (1.0 mmol) of a-methyl
cinnamaldehyde, 400 mg (1.67 mmol) of N-(methoxymethyl)-N-
(trimethylsilylmethyl) benzylamine and 25 mg (0.2 mmol) of titanium
tetrafluoride in 5 mL of CH3CN was stirred at 50 °C for 18 h. The
reaction mixture was cooled and concentrated in uacuo. The residue
was partitioned between 50 mL of ether and 25 mL of 50% sat'd NaCI and
the layers were separated. The orgainc layer was dried over MgS04.
The aqueous layer was extracted with 50 mL of ether; the extract was
dried, combined with the original organic layer and concentrated in
uacuo. Flash chromatography on 12 g of silica gel using 10:1 v/v
hexanes/ether as the eluant afforded 203 mg (75%) of the title compound
as an oil. 1H NMR (500 MHz, CDCl3): 8 0.72 (s, 3H), 2.40 (d, J= 12.0, 1H),
2.88 (t, J= 9.5, 1H), 3.19-3.22 (m, 2H), 3.68 and 3.76 (ABq, J= 40.5, 2H),
7.20-7.39 (lOH), 9.65 (s, 1H).
Step B: 1-Benzyl-(SR)-[3--methyl-3-((4-phenyl)piperidin-1
1 methyll-4-(SR)-~hen,~lpyrrolidine
The title compound was prepared from 1-benzyl-(RS)-[3-
methyl-3-formyl]-4-(SR)-phenylpyrrolidine (from Example 98, Step A)
and(4-phenyl)piperidine using a procedure analogous to that described
in Example 9. 1H NMR (500 MHz, CDC13): $ 0.69 (s, 3H), 1.72-1.79 (4H),
2.25-2.45 (4H), 2.35 and 2.79 (ABq, J= 220.0, 2H), 2.89 (t, J= 8.5, 1H), 2.90-
2.95 (m, 1H), 3.05 (app t, J= 9.0, 1H), 3.17 (app t, J= 8.0, 1H), 3.62 and
3.74
(ABq, J= 60.5, 2H), 7.17-7.39 (15H).
1-Benzyl-3-(SR)-( 1-(RS)-((4-phenyl)piperidin-1-yl)ethyl)-4-(SR)-
phenylpyrrolidine and 1-Benzyl-3-(SR)-(1-(SR)-((4-phenyl)piperidin-1-
vl)ethvl )-4-( SR)-,Rhen~py~rolidin a
Step A: ~-Benzyl-3-(RS)-acetvl-4-(SR)-~henylgyrrolidine
-150 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
The title compound was prepared from (E)-4-phenyl-3-
butan-2-one using a procedure analogous to that described in Example l,
Step A. 1H NMR (400 MHz, CDC13): 8 2.07 (s, 3H), 2.72 (m, 1H), 2.83-2.87
(m, 1H), 2.97-3.02 (2H), 3.21 (app q, J= 8.4, 1H), 3.58 (app q, J= 6.8, 1H),
3.64-3.68 (2H), 7.20-7.38 (15H); Mass Spectrum (NH3-CI): 280 (M+1).
Step B: 1-Benzyl-3-(SR)-(1-(RS)-((hydroxy)ethyl)-4-(SR)-
phenylpyrrolidine and 1-Benzyl-3-(SR)-(1-(RS)-
(h,~xy)ethyl)-4~(SR~~nvlpvrrolidine
A solution of 750 mg (2.7 mmol) of 1-benzyl-3-(RS)-acetyl-4-
(SR)-phenylpyrrolidine (from Example 99, Step A) in 10 mL of MeOH at 0
°C was treated with 150 mg of NaBH4 and was stirred cold for 30 min.
The reaction was quenched with 5 mL of sat'd NaHC03 and
concentrated in vacuo. The residue was partitioned between 50 mL of
ether and 25 mL of H20 and the layers were separated. The organic
layer was washed with 25 mL of sat'd NaCI, dried over MgS04 and
concentrated in uacuo. Flash chromtography on 35 g of silica gel using
4:1 v/v, then 2:1 v/v hexanes/EtOAc afforded 375 mg of (~)-Diastereomer
B1 and 159 mg of (~)-Diastereomer B2 (70% total yield). For (t)-
Diastereomer B1: 1H NMR (300 MHz, CDC13): d 1.11 (d, J= 6.4, 3H), 2.19-
2.25 (m, 1H), 2.34 (app t, J= 8.8, 1H), 2.79 (dd, J= 7.0, 9.2, 1H), 2.90 (dd,
J=
2.4, 9.4), 3.03 (app t, J= 8.8, 1H), 3.44-3.51 (m, 1H), 3.65 (app s, 2H), 3.93-
4.00 (m, 1H), ?.1?-7.33 (lOH). For (t)-Diastereomer B2: 1H NMR (300
MHz, CDCl3): d 1.12 (d, J= 6.4, 3H), 2.19-2.25 (m, 1H), 2.39-2.43 (m, 1H),
2.71 (app t, J= 10.6, 1H), 3.07 (dd, J= 3.2, 9.6, 1H), 3.22-3.33 (2H), and
3.65
and 3.72 (ABq, J= 20.0, 2H), 3.85-3.91 (m, 1H), 7.17-7.33 (lOH).
Step C: 1-Benzyl-3-(SR)-(1-(RS)-((azido)ethyl)-4-(SR)-phenyl
pyrrolidine and 1-Benzyl-3-(SR)-(1-(RS)
(azido)ethyl)-4-(SR)-pheny~pvrrolidine
A mixture of 147 mg (0.52 mmol) of (~)-Diastereomer B1
(from Example 99, Step B), 262 mg (1.0 mmol) of triphenylphosphine, 68
mg (1.0 mmol) of imidazole and 231 mg (0.75 mmol) of zinc azide,
bis(pyridine) complex in 3 mL of CH2Cl2 at 0 °C was treated with a
solution of 174 mg ( 1.0 mmol) of diethylazodicarboxylate in 1 mL of
-151-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
CH2C12. The cooling bath was removed and the reaction was stirred at
rt for 1 h. The reaction was filtered through a pad of Celite and the
filtrate was concentrated in uacuo. Flash chromatography on 12 g of
silica gel using 17:3 v/v hexanes/ether as the eluant afforded 129 mg
(81%) of one of the title compounds (Diatereomer C1) as an oil: 1H NMR
(300 MHz, CDC13): S L18 (d, J= 6.4, 3H), 2.26-2.35 (m, 1H), 2.63-2.69 (m,
2H), 2.86-3.09 (3H), 3.49-3.58 (m, 1H), 3.63 and 3.70 (ABq, J= 21.4, 2H),
7.18-7.38 (lOH); IR (cm-1, neat) 2104.
(t)-Diastereomer B2 was reacted similarly to afford Diastereomer C2 as
an oil: 1H NMR (500 MHz, CDCl3): d 1.22 (d, J= 6.5, 3H), 2.33-2.42 (2H),
2.66-2.?0 (m, IH), 2.88-2.93 (m, 2H), 3.17 (app q, J= 6.5, 1H), 3.47-3.52 (m,
1H), 3.61 and 3.66 (ABq, J= 26.0, 2H), 7.I5-7.35 (10H).
Step D: 1-Benzyl-3-(SR)-(1-(RS)-((4-phenyl)piperidin-lyl)ethyl)-4-
(SR)-phenylpyrrolidine and 1-Benzyl-3-(SR)-(1-(SR)-((4-
phen~~peridin-1-vl)ethvl)-4-(SR~vlRyrrolidine
A mixture of 20 mg of 10% palladium on carbon and 128 mg
(0.42 mmol) of (t)-Diastereomer C1 (from Example 99, Step B) in 4 mL of
methanol was stirred under an atmosphere of H2 for 20 h. The reaction
mixture was filtered through a pad of Celite and the filtrate was
concentratedin vacuo. The residue, 181 mg (0.37 mmol) of (~)-1,5-bis(4-
toluenesulfonyloxy)-3-phenylpentane and 175 mL (1.0 mmol) of DIEA
were dissolved in 5 mL of isobutyrylnitrile and the mixture was stirred
at 100 °C for 20 h. The reaction mixture was cooled and partitioned
between 50 mL of CH2C12 and 20 mL of 1.0 N NaOH and the layers were
separated. The organic layer was dried over MgS04 and concentrated in
vacuo. Flash chromatography on 8 g of silica gel using 40:1 v/v
hexanes/EtOAc + 1.5% TEA as the eluant afforded 70 mg of impure
product. Flash chromatography of this material on 8 g of silica gel
using 100:1:0.1 v/v/v CH2Cl2/MeOH/NH40H as the eluant afforded 54 mg
(30%) of one of the title compounds (Diastereomer D1) as an oil: 1H NMR
(500 MHz, CDC13): 8 0.82 (d, J= 6.5, 3H), 1.53-1.61 (m, 1H), 1.71-1.77 (3H),
2.08 (app t, J=11.0,1H), 2.36-3.00 (11H), 3.58 and 3.74 (ABq, J= ?8.5, 2H),
7.15-7.45 (15H); Mass Spectrum (NH3-CI): 425 (M+1).
-152-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(~)-Diastereomer C2 was reacted similarly to afford (t)-Diastereomer D2
as an oil: IH NMR (500 MHz, CDC13): 8 0.87 (d, J= 6.5, 3H), 1.03-1.11 (m,
1H), 1.31-L34 (m, 1H), 1.67-1.?? (2H), 1.93 (dt, J= 2.0, 11.5, 1H), 2.18-2.22
(m, 1H), 2.26-2.38 (3H), 2.50 (dt, J= 3.0, 11.0, 1H), 2.54-2.60 (m, 1H), 2.65-
2.68 (m, 1H), 2.73-2.76 (m, 1H), 2.84 (app t, J= 8.5, 1H), 2.92 (app t, J=
8.0,
1H), 3.27-3.30 (m, IH), 3.59 and 3.67 (ABq, J= 38.0, 2H), 7.11-7.35 ( 15H); );
Mass Spectrum (NH3-CI): 425 (M+1). HPLC (YMC "Octyl" 4.6 x 250 mm
column, 60:40 v/v H20/CH3CN + 0.5% TFA, 1.5 mL/min, 200 nm): 6.0
min.
HXAMPLE 100
1-(2-Chloro)benzoyl-3-(SR)-( 1-(RS)-((4-phenyl)piperidin-1-yl)ethyl)-4-(SR)-
phenylpyrrolidine or 1-(2-chloro)benzoyl-3-(SR)-(1-(SR)-((4-
Rhen~pi e~din-1-vl)ethyl)-4-(SR)- henylRyrrolidine
The title compound was prepared from (~)-Diastereomer D2
(from Example 99, Step D) using procedures analogous to those
described in Example 9, Step B and Example 37: 1H NMR (500 MHz,
CDCl3): 8 0.80-1.00 (4H),1.22-1.64 (5H), 1.98-2.67 (6H), 3.28-3.54 (3H), 4.03-
4.11 (m, 1H), 7.02-7.43 (m, 14H); Mass Spectrum (NH3-CI): 473 (M+1).
HPLC (YMC "Octyl" 4.6 x 250 mm column, 60:40 v/v H20/CH3CN + 0.5%
TFA, 1.5 mL/min, 200 nm): 10.7 min.
EXAMPLE ~O1
N- z h R - h 1 i ri i -1- th 1 - i a ' 'n
Step A: N-Benzyl-4-trifluoromethanesulfonyl-1, 2, 5, 6-
tetrah~R3~ridine
To a solution of N-benzyl-4-piperidone in 27 mL of THF at -78
oC was added 6.0 mL of lithium bis(trimethylsilyl)amide (1.OM in THF).
After stirring at -78 oC for 25 min, the reaction was warmed to 0 oC for 5
min, then retooled to -78 oC. After adding 2.54 g of 2-[N, N-
bis(trifluoromethyl-sulfonyl)amino]-5-chloropyridine, the reaction was
stirred for 1 h at -78 oC, quenched with 1N NaHC03 and warmed to room
temperature. The reaction was partitioned between 100 mL of 1N
-153 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
NaHC03 and 100 mL of Et20. After separating phases, the aqueous
layer was extracted with 100 mL of Et20. The combined organic layers
were dried over Na2S04 and concentrated under vacuum. The residue
was purified by flash chromatography eluting with 10% EtOAc in
hexanes to provide 737 mg of the title compound: 1H NMR (300 MHz,
CDC13): b 2.4 (m, 2H), 2.7 (t, 2H), 3.1 (m, 2H), 3.6 (s, 2H), 5.7 (m, 1H), 7.2-
7.4 (m, 5H). RF: 0.52 (20% EtOAc in hexanes).
Step B: N-Benzyl-4-carbomethoxy-1, 2, 5, 6-tetrahydro-
pyridine
To a solution of 253 mg of triflate (from Example 101, Step A)
and 0.21 mL of TEA in 1.2 mL of MeOH and 7.8 mL of DMF was added
367 mg of tetrakis(triphenylphosphine)palladium(0). After bubbling
carbon monoxide through the solution for 20 min, the reaction was
warmed to 65 oC under 1 atm of CO for 7.5 h. After cooling to room
temperature, the reaction was concentrated under vacuum. The
residue was dissolved in 100 mL of EtAOc and washed with 100 mL 1N
NaHC03. After separating layers the aqueous phase was extracted with
100 EtAOc. The combined organic layers were dried over Na2S04 and
concentrated under vacuum. The residue was purified by flash
chromatography eluting with 20% EtOAc in hexanes to provide 125 mg of
the title compound: 1H NMR (300 MHz, CDCl3): b 2.4 (m, 2H), 2.6 (t, 2H),
3.1 (m, 2H), 3.6 (s, 2H), 3.7 (s, 3H), 6.9 (m, 1H), 7.2-7.4 (m, 5H). RF: 0.56
(50% EtOAc in hexanes).
Step C: N-Benzyl-4-carbomethoxy-3-phenyl-~neridine
To a suspension of 59 mg of ester (from Example 101, Step
B), 4.4 mg of CuBr2 and 0.096 mL of TMSCI in 2.5 mL of Et20 at -45 oC
was added 0.125 mL of phenylmagnesium bromide (3.0M in Et20). After
stirring at -45 oC for 1 h, the reaction was quenched with 10% NH40H.
The reaction was partitioned between 10 mL of H20 and 10 mL of Et20.
After separating layers, the aqueous phase was extracted with 10 mL of
Et20. The combined organic layers were dried over MgS04 and
concentrated under vacuum. The residue was purified by flash
-154 -
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
chromatography eluting with 20% Et20 in hexanes to provide 12 mg of
the title compound: 1H NMR (300 MHz, CDCl3): S 1.8-1.9 (m, 1H), 2.0-2.1
(m, 1H), 2.3-2.4 (m, 1H), 2.6-2.7 (m, 1H), 2.8-2.9 (m, 2H), 3.1-3.2 (m, 1H),
3.2-3.3 (m, 1H), 3.4 (s, 3H), 3.6 (s, 2H), 7.2-7.4 (m, 8H), 7.4-7.5 (m, 2H).
RF: 0.40 (50% Et20 in hexanes).
Step D: N-Benzyl-4-(4-phenylpiperidinecarboxamide)-3-phenyl-
piperidine
To a solution 12 mg of methyl ester (from Example 101, Step
C) and 9 mg of 4-phenylpiperidine in 0.5 mL of THF at -20 aC was added
0.030 mL isopropylmagnesium chloride (2M in THF). The reaction was
stirred at -20 oC for 1 h, then warmed to room temperature. The
reaction was quenched with saturated NaCI and partitioned between 10
mL of brine and 10 mL of EtOAc. After separating layers the aqueous
phase was extracted with 10 mL EtOAc. The combined organic layers
were dried over Na2S04 and concentrated under vacuum. The residue
was purified by flash chromatography eluting with 100% EtOAc to
provide 8 mg of the title compound: 1H NMR (300 MHz, CDCl3): 8 1.4-2.1
(m, 6H), 2.3-2.8 (m, 4H), 2.9-3.4 (m, 5H), 3.6-3.9 (m, 3H), 4.6-4.8 (m, 1H),
6.9-7.4 (m, 15H); Mass Spectrum (CI): 439.2 (M+H). RF: 0.28 (100%
EtOAc).
Step E: N-Benzyl-3-(SR)-phenyl-4-(SR)-(4-phenylpiperidin-1-
ylmethvl)-~peridine
To a solution of 8 mg of amide (from Example 101, Step D) in
1 mL of THF at 0 oC was added 3 mg of lithium aluminumhydride.
After 2 h at 0 oC the reaction was quenched with saturated Rochelle salts
and partitioned between 10 mL of Rochelle salts and 10 mL of EtOAc.
After separating layers the aqueous phase was extracted with 10 mL
EtOAc. The combined organic layers were dried over Na2S04 and
concentrated under vacuum. The residue was purified by flash
chromatography eluting with 25% EtOAc in hexanes to provide 3 mg of
the title compound: 1H NMR (300 MHz, CDC13): 8 1.5-2.1 (m, 6H), 2.1-2.6
-155-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
(m, 5H), 2.7-3.2 (m, 7H), 3.4-3.6 (m, 3H), 7.1-7.4 {m, 13H), 7.7-7.8 (m, 2H);
Mass Spectrum (CI): 425.2 (M+H). RF: 0.47 (50% EtOAc in hexanes).
EXAMPLE 102
N-Tertbutoxycarbonyl-4-(SR)-phenyl-3-(SR)-(4-phenylpiperidin-1-
vlmethvl)-nineridine
Step A: N-Tertbutoxycarbonyl-4-phenyl-3-(4-phenylpiperidine-
carboxamide)-1 2, 5; 6-tetrahYdro~~ridine
To a solution of 14.4 mg 4-phenylpiperidine in 1.6 mL
CH2C12 at 0 oC was added 0.035 mL of DIEA, 26.5 mg of 1-(t-
butoxycarbonyl)-4-phenyl-1,2,5,6-tetrahydronicotinic acid and 26.5 mg of
BOP-Cl (Bis(2-oxo-3-oxazolidinyl)-phosphinic chloride). After 4 h at 0 oC
and 1 h at room temperature, the reaction was diluted with 50 mL of
EtOAc and washed with 50 mL H20, 50 mL 1N NaHC03, 50 mL H20 and
50 mL brine. The organic phase was dried over Na2S04 and
concentrated under vacuum. The residue was purified by flash
chromatography eluting with 35% EtOAc in hexanes to provide 22 mg of
the title compound: 1H NMR (300 MHz, CDC13): 8 1.5-1.8 (m, 13H), 2.1-
2.6 (m, 3H), 2.8-3.0 (m, 2H), 3.6-4.5 (m, 5H), 4.6-4.8 (m, 1H), 6.8 (br d,
1H),
7.1-7.5 (m, 9H). RF: 0.4? (50% EtOAc in hexanes).
Step B: N-Tertbutoxycarbonyl-4-phenyl-3-(4-phenylpiperidine-
~a~boxamide)pineridine
To a solution of 11 mg of amide (from Example 102, Step A)
in 0.5 mL MeOH at room temperature was added 26 mg of magnesium
turnings. After 3 h at room temperature the reaction was concentrated
under vacuum. The residue was suspended in 10 mL of EtOAc and
washed with 10 mL of brine. After separating the layers, the aqueous
phase was extracted with 10 mL EtOAc. The combined organic layers
were dried over Na2S04 and concentrated under vacuum. The residue
was taken on to Step C without further purification.
-156 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Step C: N-Tertbutoxycarbonyl-4-phenyl-3-(4-phenylpiperidin-1-
vlmeth~piperidine _
To a solution of the amide (from Example 102, Step B) at 0 oC
in 0.5 mL of THF was added 0.3 mL A1H3 (0.2M in THF). After 1.5 h at 0
oC the reaction was quenched with brine. The reaction was partitioned
between 10 mL of EtOAc and 10 mL of brine. After separating the layers,
the aqueous phase was extracted with 10 mL EtOAc. The combined
organic layers were dried over Na2S04 and concentrated under vacuum.
The residue was purified by flash chromatography eluting with 30%
EtOAc in hexanes to provide 4 mg of the title compound: 1H NMR (300
MHz, CDCi3): S 1.4-2.1 (m, 19H), 2.2-3.1 (m, 7H), 4.2-4.6 (m, 2H), 7.1-7.4
(m, 10H); Mass Spectrum (ESI): 435.5 (M+H). RF: 0.60 (50% EtOAc in
hexanes).
EX~PLE 103
N-Sulfonyl-4-(SR)-phenyl-3-(SR)-(4-phenylpiperidin-1-ylmethyl)-
~in~ridine
Step A: 4-(SR)-Phenyl-3-(SR)-(4-phenylpiperidin-1-ylmethyl)-
pmeriaine
To a solution of 5.5 mg of carbamate (from Example 102,
Step C) in 0.6 mL of CH2C12 at room temperature was added 0.4 mL
trifluoroacetic acid. After I h at room temperature, volatiles were
removed under vacuum. The crude product taken onto Step B without
further purification.
Step B: N-Sulfonyl-4-(SR)-phenyl-3-(SR)-(4-phenylpiperidin-1-
ylmeth~~~eridine
To a solution of the above deprotected amine (from Example
103, Step A) in 1 mL of CH2C12 was added 0.014 mL of DIEA, 0.002 mL of
benzenesulfonyl chloride and a catalytic amount of
dimethylaminopyridine. After stirring at room temperature for 20 h,
the reaction was diluted with 25 mL of CH2C12 and washed with 25 mL
of 1N NaHC03. After separating layers, the organic phase was dried
-15?-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
over Na2S04 and concentrated under vacuum. The residue was purified
by flash chromatography eluting with 20% EtOAc in hexanes to provide 3
mg of the title compound: 1H NMR (300 MHz, CDC13): 8 1.5-2.9 (m, 17H),
4.0-4.2 (m, 2H), 7.1-7.4 (m, 12H), 7.5-?.7 (m, 2H), 7.8-7.9 (m, 1H); Mass
spec. (ESI): 475.3 (M+H). RF: 0.44 (50% EtOAc in hexanes).
EXAMPLE 104
N-Sulfonyl-4-(SR)-phenyl-3-(SR)-(4_phenylpiperidin) pi~eridine
Step A: N-Tertbutoxycarbonyl-4-phenyl-3-(carbomethoxy)-1, 2, 5,
6-tetrahvdropyridine
To a solution of 153 mg of 1-(t-butoxycarbonyl)-4-phenyl-
1,2,5,6-tetrahydronicotinic acid in 1 mL of THF and 1 mL of MeOH was
added 0.6 mL of TMSCHN2 (2M in hexanes). The reaction was stirred at
room temperature for 2 h and concentrated under vacuum. The residue
was purified by flash chromatography eluting with 20% EtOAc in
hexanes to provide 116 mg of the title compound: 1H NMR (300 MHz,
CDC13): 8 1.5 (s, 9H), 2.5-2.6 (m, 2H), 3.5 (s, 3H), 3.6 (t, 2H), 4.2-4.3 (m,
2H), 7.1-7.2 (m, 2H), 7.2-7.4 (m, 3H). RF: 0.68 (50% EtOAc in hexanes).
Step B: N-Tertbutoxycarbonyl-4-phenyl-3-(carbomethoxy)-
pineridine
To a solution of 110 mg of ester (from Example 104, Step A)
in 5 mL of MeOH at room temperature was added 345 mg of magnesium
turnings. After 1.5 h at room temperature the reaction became
exothermic and was cooled with an ice bath. The reaction was warmed
to room temperature overnight. The reaction was filtered through a pad
of celite. The filtrate was concentrated uner vacuum. The residue was
suspended in 25 mL of EtOAc and washed with 25 mL of brine. After
separating the layers, the aqueous phase was extracted with 25 mL
EtOAc. The combined organic layers were dried over Na2S04 and
concentrated under vacuum to give 89 mg of a residue which was taken
onto Step C without further purification: 1H NMR (300 MHz, CDC13): S
-158 -
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
1.4-1.9 (m, 11H), 2.6-3.3 (m, 4H), 3.5 (s, 3H), 4.2-4.6 (m, 2H), 7.1-7.4 (m,
5H).
Step C: N-Tertbutoxvcarbonvl-4-4-phenyl-3-(carboxy~pi_pers~~rp
To a solution of 89 mg of methyl ester (from Example 104,
Step B) in 1.5 mL EtOH was added 1.0 mL of 0.5N NaOH. After 36 h the
reaction was concentrated under vacuum. The residue was dissolved in
25 mL H20 and washed with 25 mL Et20. After separating layers, the
aqueous phase was acidified to pH 2 using 0.5N KHS04. The aqueous
phase was extracted with 3X25 mL of CH2C12. The combined extracts
were dried over Na2S04 and concentrated under vacuum to give 58 mg of
a white foam which was taken on without further purification.
Step D: N-Tertbutoxycarbonyl-4-phenyl-3-(aminobenzyloxy-
carbonvl)-piperidine
To a solution of 58 mg of carboxylic acid (from Example 104,
Step C) in THF at -10 oC was added 0.029 mL of NMM and 0.034 mL of
isobutylchloroformate. After stirring at room temperature for 50 min,
the reaction was cooled to -10 oC and 68 mg of NaN3 in 0.35 mL of H20
was added. After stirring an additional 20 min at -10 oC, the reaction
was partitioned between 10 mL of Et20 and 10 mL of H20. After
separating layers, the organic phase was washed with 10 mL of brine
and dried over Na2S04 and concentrated under vacuum to give the
crude acyl azide (IR 2135 cm-1). A solution of the acyl azide in 1 mL of
toluene was warmed to 80 ~C for 40 min to produce an isocyanate (IR
2256 cm-1). To the solution of the isocyanate was added 0.044 mL of
benzyl alcohol. After warming to 80 oC for 2 h, the reaction was
partitioned between 25 mL of Et20 and 10 mL of 1N NaOH. After
separating layers, the organic phase was washed with 10 mL of brine,
dried over Na2S04 and concentrated under vacuum. The residue was
purified by flash chromatography eluting with 50% Et20 in hexanes to
provide 35 mg of the title compound: 1H NMR (340 MHz, CDC13): 8 1.4-1.9
(m, 11H), 2.5-2.8 (m, 2H), 3.7-3.8 (m, 1H), 4.1-4.6 (m, 3H), 4.8-5.0 (m, 2H),
7.1-7.4 (rn, lOH). RF: 0.17 (50% Et20 in hexanes).
-159 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Step E: ~I-Tertbutoxycarbo~wl-4-phenyl-3-amino-pine~~~ne
A solution of 35 mg of benzyl carbamate (from Example 104,
Step D) in 1 mL of EtOH containing 6 mg of 10% Pd-C was stirred under 1
Atm of H2 for 24 h. The reaction mixture was filtered through a pad of
celite and concentrated under vacuum to give 24 mg of a residue which
was taken onto Step F without further purification.
Step F: N-Tertbutoxycarbonyl-4-(SR)-phenyl-3-(SR)-(4
phenvlniperidin-1-vl)-piueridine
To a solution of amine (from Example 104, Step E) and (~)-1,
5-dibromo, 3-phenyl-pentane in 0.5 mL of isobutylnitrile was added 0.080
mL of DIEA and a catalytic amount of tetrabutyl-ammonium iodide.
After warming the reaction to 95 oC for 22 h, the mixture was
partitioned between 10 mL of CH2C12 and 10 mL 1N NaOH. After
separating layers, the aqueous phase was extracted with 10 mL of
CH2C12. The combined organic layers were dried over Na2S04 and
concentrated under vacuum. The residue was purified by flash
chromatography eluting with 10% EtOAc in hexanes to provide 10 mg of
the title compound: 1H NMR (300 MHz, CDC13): 81.2-1.9 (m, 16H), 2.1-
2.4 (m, 2H), 2.6-3.0 (m, 6H), 4.0-4.4 (m, 2H), 7.0-7.4 (m, lOH). RF: 0.45
(20% EtOAc in hexanes).
Step G: 4-(SR)-Phenyl-3-(SR)-(4-nherylp~ueridin-1-y~~,~P
To a solution of 10 mg of tert-butylcarbamate (from Example
104, Step F) in 0.6 mL of CH2Cl2 at room temperature was added 0.4 mL
trifluoroacetic acid. After 2 h at room temperature, volatiles were
removed under vacuum. The crude product taken onto Step H without
further purification.
Step H: N-Sulfonyl-4-(SR)-phenyl-3-(SR)-(4-phenylpiperidin)-
Ri_neridine
To a solution of the amine (from Example 104, Step G) in 1
mL of CH2C12 was added 0.028 mL of DIEA, 0.004 mL of benzenesulfonyl
chloride and a catalytic amount of dimethylamino-pyridine. After
stirring at room temperature overnight, the reaction was diluted with 25
-160-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
mL of CH2Cl2 and washed with 25 mL of 1N NaHC03. After separating
layers, the organic phase was dried over Na2S04 and concentrated
under vacuum. The residue was purified by flash chromatography
eluting with 20% EtOAc in hexanes to provide 8 mg of the title
compound: 1H NMR (300 MHz, CDC13): 8 1.2-1.7 {m, 4H), 1.8-2.1 (m, 3H),
2.2-2.3 (m, 3H), 2.5-2.8 (m, 4H), 3.0-3.1 (m, 1H), 3.8-3.9 (m, 1H), 4.0-4.1
(m,
1H), ?.0-?.3 (m, lOH), 7.5-7.7 (m, 3H), 7.8-7.9 (m, 2H); Mass Spectrum
(EI): 461.2 (M+H). RF: 0.36 (50% EtOAc in hexanes).
The 1-benzyl pyrrolidine derivatives in Examples 105 - 124
were prepared using procedures analogous to those described in
Example 1 Steps A and B 9 and in Example 9. What is varied is the
cinnamate selected to prepare the pyrrolidine precursor (Example 1 Step
A and B) and the amine selected for the transformation in Example 9.
Other Examples are prepared by 1) removal of the benzyl group by
procedures described in Example 1, Step C, or Example 30, Step A and 2)
by acylation with an acid chloride or carboxylic acid by procedures
analogous to Example 30, Step B or Example 63, respectively. In some
Examples, the stereoisomers of the racemic title compounds are
separated by HPLC chromotography utilizing chiral columns as
indicated.
EXAMPLE 105
1-Benzyl-3-(spiro [indan-1-one-3,4'-piperidin-1'-yl] methyl)-4-(3,4-di-
chloro)pheny~y;~rolidine (S~,ereoisomers 1 and 2)
The stereoisomers of Example ?1 were separated by HPLC
{CHIR,ALPAK AD, 2X25 cm, 2.5% iPrOH: hexanes) to give faster and
slower moving isomers.
Stereoisomer 1 Mass Spectrum (ESI) m/e 519 (M+1).
Stereoisomer 2 Mass Spectrum (ESI) m/e 519 (M+1).
EXAMPLE 106
1-Benzyl-3-((4-phenylpiperidin-1-yl)methyl)-4-(3-chloro)phenyl
pvrrolidine (Stereoisomers 1 and 2)
-161-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
The stereoisomers of Example 69 were separated by HPLC
(CHIRALCEL OD, 2X25 cm, 5% iPrOH: hexanes) to give faster and
slower moving isomers. Stereoisomer 1 Mass Spectrum (ESI) m/e 455
(M+1), [a]D = -24.2 (c. = 1.48, CHC13) Stereoisomer 2 Mass Spectrum
(ESI) m/e 455 (M+1), [a]D = +24.6 (c. = 1.46, CHClg)
EXAMPLE 107
1-Benzyl-3-((4-phenylpiperidin-1-yl)methyl)-4-(4-chloro)phenyl
Rvrrolidine (Stereoisomers 1 and 2)
The stereoisomers were separated by HPLC (CHIRALCEL
OD, 2X25 cm, 5% iPrOH: hexanes) to give faster and slower moving
isomers. Stereoisomer 1 Mass Spectrum (ESI) m/e 455 (M+1), [a]D =
27.3 (c. = 1.034, CHC13) Stereoisomer 2 Mass Spectrum (ESI) m/e 455
(M+1), [a]D = +26.8 (c. = 0.99, CHC13)
EXAMPLE 108
1-Benzoyl-3-((4-phenylpiperidin-1-yl)methyl)-4-(3,4-dichloro)phenyl-
Rvrrolidine (Stereoisomers 1 and 2)
The stereoisomers were separated by HPLC (CHIR,ALPAK
AD, 2X25 cm, 10% iPrOH: hexanes) to give faster and slower moving
isomers. Stereoisomer 1 Mass Spectrum (ESI) m/e 493 (M+1).
Stereoisomer 2 Mass Spectrum (ESI) m/e 493 (M+1).
EXAMPLE 109
1-3,5-Dichlorobenzoyl-3-((4-phenylpiperidin-1-yl)methyl)-4-(3,4-
dichloro)uhenvlnvrrolidine (Stereoisomers 1 and 2)
The stereoisomers were separated by HPLC (CHIRALPAK
AD, 2X25 cm, 8% iPrOH: hexanes) to give faster and slower moving
isomers. Stereoisomer 1 Mass Spectrum (ESI) m/e 561 (M+1).
Stereoisomer 2 Mass Spectrum (ESI) m/e 561 (M+1).
EXAMPLE 110
1-Benzoyl-3-(SR)-((4-phenylpiperazin-1'-yl)methyl)-4-(SR)-phenyl
pyrrolidine
-162-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
The title compound was prepared from 1-benzyl-3-(SR)-((4-
phenylpiperazin-1'-yl]methyl)-4-(SR)-(3,4-di-chloro) phenylpyrrolidine
(Example 23). Mass Spectrum (ESI) mfe 426 (M+1).
E.'~MPLE 111
1-Benzyl-3-(SR)-((4-benzylpiperazin-1-yl)methyl)-4-(SR)-phenyl
Rvrrolidine
Mass Spectrum (ESI) m/e 426 (M+1).
EXAMPLE 112
1-(1-Naphthoyl)-3-(SR)-((4-benzylpiperazin-1-yl)methyl)-4-{SR)-phenyl
,pyrrolidine
Mass Spectrum (ESI) m/e 489 (M+1).
EXAMPLE 113
1-Benzoyl-3-{(4-benzylpiperidin-1-yl)methyl)-4-phenylpyrrolidine
f Stereoisomers 1 and 2)
The stereoisomers were separated by HPLC (CHIRALPAK
AD, 2X25 cm, 15% iPrOH: hexanes) to give faster and slower moving
isomers. Stereoisomer 1 Mass Spectrum (NH3-CI) m/e 439 (M+1)
Stereoisomer 2 Mass Spectrum (NH3-CI) m/e 439 (M+1)
EXAMPLE 114
1-Benzoyl-3-((4-(2-phenyleth-1-yl)piperidin-1-yl)methyl)-4-
nhenvlnvrrolidine (Stereoisomers 1 and 2)
The stereoisomers were separated by HPLC (CHIRALPAK
AD, 2X25 cm, 10% iPrOH: hexanes) to give faster and slower moving
isomers. Stereoisomer 1 Mass Spectrum {NH3-CI) m/e 453 (M+1)
Stereoisomer 2 Mass Spectrum (NH3-CI) m/e 453 (M+1)
~XA~PLE 115
1-Benzoyl-3-(SR)-((4-(3-phenylprop-1-yl)piperidin-1-yl)methyl)-4-(SR)-
y,~gyrrolidine
Mass Spectrum (ESI) m/e 467 (M+1).
-163-
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
EXAMPLE 116
1-Benzyl-3-(SR)-((4-phenylpiperidin-1-yi)methyl)-4-(SR)-(3,4-
dimethyl)nhenvlpvrrolidine
Mass Spectrum (PB-NH3/CI): m/e 439 (M+1)
EXAMPLE 117
1-Benzoyl-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-(3,4-
dimethyl)nhenyi_pvrrolidine
Mass Spectrum (PB-NH3/CI): m/e 453 (M+1).
EXAMPLE 118
1-( 1-Naphthoyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-(3,4-
dimethyl) hen~pvrrolidine
Mass Spectrum (PB-NH3/CI): m/e 503 (M+1).
EXAMPLE 119
1-Benzyl-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-(3,4-
difluoro)phen_ylpvrrolidine
Mass Spectrum Mass Spectrum (NH3/CI): m/e 447 (M+1).
EXAMPLE 120
1-(3,4-dichlorobenzoyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-
(3.4-difluorophenvl)Ryrrolidine
Mass Spectrum (NH3/CI): m/e 529 (M+1).
EXAMPLE 121
1-(2,3-dichlorobenzoyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-
(,~,4-difluoro,Rhenyl)Ry~olidine
Mass Spectrum (NH3/CI): m/e 529 (M+1).
1-(1-Naphthoyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-(3,4-
difluoro hoe yl)Rvrrolidine
Mass Spectrum (NH3/CI): m/e 511 (M+1).
-164 -
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
EXAMPLE 123
1-(Benzoyl)-3-(SR)-((4-(2-methoxyphenyl)piperidin-1-yl)methyl)-4-(SR)-
phenvlpyrrolidine
Mass Spectrum (ESI) m/e 455 (M+1).
EXAMPLE 124
1-(Benzoyl)-3-{(4-(2-methoxyphenyl)piperidin-1-yI)methyl)-4-
p,~g~,g~pvrrolidine (Stereoisomers 1 and 2)
The stereoisomers of Example 123 were separated by HPLC
(ChiralPAK AD, 2X25 cm, 10% iPrOH: hexanes) to give faster and
slower moving isomers. Stereoisomer 1 Mass Spectrum (ESI) m/e 455
(M+1), [a]D = -51 (c. = 0.65, CHC13) Stereoisomer 2 Mass Spectrum
(ESI) m/e 455 (M+1), [a]D = +45 (c. = 0.64, CHCl3)
EXAMPLE 125
1-( 1-Naphthoyl)-3-(SR)-((4-(4-fluoro)phenylpiperidin-1-yl)methyl)-4-(SR)-
phenylpvrrolidine
Step 1. 1-(1-Naphthoyl)-3-(SR)-hydroxymethyl-4-(SR)-
phenylpyrrolidine
To a solution of 610 mg (3.47 mmol) of 3-(SR)-hydroxymethyl-
4-{SR)-phenylpyrrolidine (Example 1, Step C) in 30 mL of methylene
chloride (anhydrous) was added 661 mg (3.47 mmol) of 1-naphthoyl
chloride and 795 mg (7.58 mmol) of triethylamine at 0 oC and the
reaction mixture was stirred for 2 h. The reaction mixture was
partitioned between 100 mL of methylene chloride and 15 mL of aq
NaHC03. The aqueous layer was extracted with methylene chloride (20
mL x 3). The combined organic layers were dried over Na2S04, filtered,
and concentrated. The residue was purified by silica gel
chromatography with hexane / acetone = 3:1 to afford the title compound
as a white solid. 1H NMR (CDC13) b 3.15-3.32 (m, 2H), 3.42-3.56 (m, 2H),
3.82 (m, 1H), 4.17-4.31 (m, 1H), 7.10-7.36 (m, 6H), 7.42-7.92 (m, 6H); Mass
Spectrum (PB-NH3/CI): m/e 332 (M+1).
-165-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/i7755
Step 2. 4-(1-Naphthovl)-3-(SR)-formyl-4 (SR) RhPn~p3rrrolidine
To a solution of 0.45 mL (5.2 mmol) of oxalyl chloride in 20
mL of methylene chloride (anhydrous) was added slowly 0.74 mL (10.4
mmol) of methyl sulfoxide at -78 oC and the solution was stirred for 10
min. To this was added 0.7 g (2.6 mmol) of 1-(1-naphthoyl)-3-(SR)
hydroxymethyl-4-(SR)-phenylpyrrolidine in 10 mL of methylene chloride
(anhydrous) and the reaction mixture was stirred at -78 oC for 15 min.
To the reaction mixture was then added 1.8 mL ( 13 mmol) of
triethylamine and the reaction mixture was stirred at -78 oC for 10 min.
The cooling bath was removed and the reaction was warmed to room
temperature for 40 min. The reaction mixture was poured into 100 mL
of ether, washed with 30 mL of aq NaHC03, dried oven Na2S04~ and
concentrated to afford the title compound as a colorless oil which was
used without further purification. 1H NMR (CDC13) 8 7.16-7.59 (m, 9H),
7.86-7.92 (m, 3H), 9.59, 9.7? (s, 1H, two rotamers); Mass Spectrum (PB-
NH3/CI): m/e 330 (M+1).
Step 3. 4-(4-Fluoro ,~hen~~iperidine
A mixture of 2.00 g (9.36 mmol) of 4-(4-fluorophenyl)-1,2,3,6-
tetrahydropyridine hydrochloride (Aldrich) and 400 mg of palladium (5%
on activated carbon) in 40 mL of methanol (anhydrous) was pressurized
to 50 psi with hydrogen gas at room temperature for 4 h. The reaction
mixture was then filtered through a plug of celite, and the filtrate was
washed with a mixture of 5 : 1 methylene chloride / ammonia (2M in
MeOH) and concentrated. The residue was partitioned between 100 mL
of methylene chloride and 15 mL of aq. NaHC03. The aqueous layer was
extracted with methylene chloride (20 mL x 3). The combined organic
layers were dried over Na2S04 and concentrated to give the title
compound as a white solid. 1H NMR (CDC13) 8 2.03 (m, 2H), 2.23 (m,
2H), 2.76 (m, 1H), 3,04 (m, 2H), 3.64 (m, 2H), 7.00 (t, 2H, J = SHz), 7.21 (m,
2H); Mass Spectrum (PB-NH3/CI): m/e 180 (M+1).
Step 4. 1-(1-Naphthoyl)-3-(SR)-((4-(4-fluoro)phenylpiperidin-1-
y )methyl)-4-(SR)-~nYlpyrrolidine
-166 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98117755
To a solution of 20 mg (0.063 mmol) of 1-(1-naphthoyl)-3-
formyl-4-(SR)-phenylpyrrolidine and 26.7 mg of sodium
triacetoxyborohydride in 5 mL of 1,2-dichloroethane (anhydrous) was
added 22.7 mg (0.126 mmol) of 4-(4-fluorophenyl)piperidine and the
reaction mixture was stirred at room temperature for 3 h. The reaction
mixture was partitioned between 30 mL of methylene chloride and 5 mL
of aq NaHC03. The aqueous layer was extracted with methylene
chloride (10 mL x 3). The combined organic layer was dried over Na2S04
and concentrated. The residue was purified by silica gel
chromatography with hexane / acetone = 3:1 to afford the title
compound. 1H NMR (CDC13) 8 1.60-1.79 (m, 3H), 1.90-2.18 (m, 2H), 2.26-
2.57 (m, 2H), 2.68-2.93 (m, 2H), 6.94-7.38 (m, 6H), 7.40-7.60 (m, 3H); Mass
Spectrum (PB-NH3/CI): m/e 493 (M+1).
EXAMPLE 126
1-(1-Naphthoyl)-3-(SR)-((4-benzylpiperidin-1-yl)methyl)-4-(SR)-phenyl
R3~o]idine
The title compound was prepared as described in Example
125. Mass Spectrum (PB-NH3/CI): m/e 489 (M+1).
EXAMPLE 127
1-(1-Naphthoyl)-3-(SR)-((4-(3-phenylprop-1-yl)piperidin-1-yl)methyl)-4-
(SR)-n envlRyrrolidine _
The title compound was prepared as described in Example
125. Mass Spectrum (PB-NH3/CI): m/e 517 (M+1).
EXAMPLE 128
1-(3,4-dichlorobenzoyl)-3-(SR)-((4-(4-fluoro)phenylpiperidin-1-yl)methyl)-
4-(SR)-~ylRvrrolidine
The title compound was prepared from 3-(SR)-
hydroxymethyl-4-(SR)-phenylpyrrolidine according to procedures
described in Example 125 using 3,4-dichlorobenzoyl chloride in place of
1-naphthoyl chloride. Mass Spectrum (PB-NH3/CI): m/e 511 (M+1).
-167-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
EXAMPLE 129
1-(3,4-Dichlorobenzoyl)-3-(SR)-((4-(3-phenyiprop-1-yl)piperidin-1-
yl)methvl)-4-(SR)-phenxlpvrrolidine -
The title compound was prepared as described in Examples
125 and 126. Mass Spectrum (PB-NH3/CI): m/e 535 (M+1).
Examples 130 -134 were prepared from 3-(SR)-(spiro[indan-
1-one-3,4'-piperidin-1'-yl]methyl)-4-(SR)-phenylpyrrolidine (from
deprotection of Example 10) by acylation with an acid chloride or
carboxylic acid by procedures analogous to Example 30, Step B or
Example 63, respectively.
EXAMPLE 130
1-(1-Naphthoyl)-3-(SR)-(spiro[indan-1-one-3,4'-piperidin-1'-yi]methyl)-4-
(SR)-~henvlp~~rrolidine
Mass Spectrum (PB-NH3/CI): m/e 515 (M+1).
EXAMPLE 131
1-( 2-Naphthoyl)-3-( SR)-( spiro [indan-1-one-3, 4'-piperidin-1'-yl] methyl )-
4-
(SR)-~ylpyrrolidine
Mass Spectrum (PB-NH3/CI): m/e 515 (M+1).
EXAMPLE 132
1-Benzoyl-3-(SR)-(spiro[indan-1-one-3,4'-piperidin-1'-yl]methyl)-4-(SR)-
p~enYl_pvrrolidine
Mass Spectrum (PB-NH3/CI): m/e 465 (M+1).
EXAMPLE 133
1-(3,4-Dichlorobenzoyl)-3-(SR)-(spiro[indan-1-one-3,4'-piperidin-1'-
llmet vl)-4-(SR)-phenylpyrrolidine
Mass Spectrum (PB-NH3/CI): m/e 533 (M+1) (35C1), 535 (37C1).
~~.~I~s~4
1-(2,3-Dichlorobenzoyl)-3-(SR)-(spiro[indan-1-one-3,4'-piperidin-1'-
311methy~4-(SR)-nheriy~,Rvrrolidine
-168 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Mass Spectrum (PB-NH3/CI): m/e 533 (M+1) (35C1), 535 (37C1).
Examples 135 - 137 were prepared by procedures analogous
to those described in Example 85 substituting the appropriate aldehyde
for phenylacetaldehyde.
1-(1-Naphthylmethyl)-3-(SR)-(spiro[indan-1-one-3,4'-piperidin-1'-
y ethyl)-4-(SR)-Rhen~pyrrolidine
Mass Spectrum (PB-NH3/CI): m/e 501 (M+1).
EXAMPLE 136
1-(2-Naphthylmethyl)-3-(SR)-(spiro[indan-1-one-3,4'-piperidin-1'-
yl]methyl)-4-(SR)-phenylpyrrolidine
Mass Spectrum (PB-NH3/CI): m/e 501 (M+1).
EXAMPLE 137
1-(3,4-Dichlorobenzyl)-3-(SR)-(4-phenylpiperidin-1-yl)methyl)-4-(SR)-
~henyl-Ryrrolidine
Mass Spectrum (PB-NH3/CI): m/e 479 (M+1).
Examples 138-148 were prepared from 3-(SR)-((4-
phenylpiperidin-1'-yl)methyl)-4-(SR)-phenyl pyrrolidine (Example 30,
Step A) by acylation with an acid chloride or carboxylic acid by
procedures analogous to Example 30, Step B or Example 63, respectively.
In some Examples, the stereoisomers of the racemic title compounds are
separated by HLPC chromotography ustilizing chiral columns as
indicated.
EXAMPLE 138
1-(2-Methylnaphth-1-oyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-
g~enylgyrrolidine
Mass Spectrum (PB-NH3/CI): m/e 489 (M+1).
-169 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
EXAMPLE 139
1-(2-Ethoxynaphth-1-oyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-
p.~enYl~yrrolidine
Mass Spectrum (PB-NH3/CI): m/e 519 (M+1).
PLE 140
1-(4-Trifluoromethylbenzoyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-
~SR)-phenylpyrrolidine
Mass Spectrum (PB-NH3/CI): m/e 493 (M+1).
EXAMPLE 141
1-(4-Bromobenzoyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-
phenyl~vrrolidine
Mass Spectrum (PB-NH3/CI): m/e 503 (M+1), (79 Br), 505 (81 Br).
EXAMPLE 142
1-(4-Phenylbenzoyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-
~hen~lRyrrolidine
Mass Spectrum (PB-NH3/Ci): m/e 501 (M+1).
EXAMPLE 143
1-(4-Methylbenzoyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-
phenylpyrrolidin~,~
Mass Spectrum (PB-NH3/CI): m/e 439 (M+1).
1-(3-Phenyl-n-propion-1-oyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-
( SR)-phenylRyrrolidine
Mass Spectrum (PB-NH3/CI): m/e 453 (M+1).
EXAMPLE 145
1-(4-Iodobenzoyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-
nhenv~~rrolidine
Mass Spectrum (PB-NH3/CI): m/e 551 (M+1).
-1~0 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1-(4-Fluorobenzoyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-
~henylRvrrolidine
Mass Spectrum (PB-NH3/CI): m/e 443 (M+1).
F~MPLE 147
1-(3-Bromobenzoyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-
pheny~R~~rolidine
Mass Spectrum (PB-NH3/CI): m/e 503 (M+1), (79 Br), 505 (81 Br).
EXAMPLE 148
1-(2-hydroxybenzoyl)-3-(SR)-((4-phenylpiperidin-1-yl)methyl)-4-(SR)-
ylRy~rolidine
Mass Spectrum (PB-NH3/CI): m/e 441 (M+1).
1-Phenylmethyl-3-(S)-((4-phenylpiperidin-1-yl)methyl)-4-(S)-
phenylRyrrolidine f(SS)-stereoisomer of Example 91
Step 1. (S)-N-Cinnamoyl-4-benzvl-2-oxazolidinone
To a solution of 10 g of cinnamic acid (67.5 mmol) in 200 mL
of THF was added 11.3 mL of triethylamine (81 mmol). The solution was
cooled to -78 °C under nitrogen and to this was added a solution of 9.1
mL
of pivaloyl chloride (74.2 mmol) in 100 mL THF. The reaction mixture
was allowed to warm to 0 °C over 1 h, then was cooled to -78 °C.
Meanwhile, a solution of 12.0 g (67.2 mmol) of (S)-benzyl-2-
oxazolidinone in 100 mL of THF was cooled to -78 °C under nitrogen. To
this was added 46.5 mL of nbutyllithium (1.62M, mmol) and the solution
was stirred for 30 min at -78 °C. This was added via cannula to the
first
solution. After addition was complete, the solution was allowed to warm
to room temperature for 2 h.
The reaction was quenched by addition of saturated aqueous
NH4C1 and the mixture was extracted with ethyl acetate. The organic
layer was washed with brine, dried over MgS04, and filtered. The
solution was concentrated and the residue was purified by
-171-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
chromatography on silica gel using ethyl acetate-hexane (4:1) to afford
the title compound; 1H NMR (CDC13) 8 2.86 (d, AB, J = 9.5 Hz, 1H), 2.88
(d, AB, J = 9.5 Hz, 1H), 3.38 (d, AB, J = 3 Hz, 1H), 3.41 (d, AB, J = 3 Hz,
1H}, 4.2-4.3 (m, 2H), 4.82 (m, 1H), 7.25-7.46 (m, 8H), 7.65(m, 1H), 7.95 (dd,
AB, J = 15,5 Hz, 18 Hz, 2H); Mass spectrum (ESI) m/e = 308 (M+1).
Step 2. (S)-N-(1-Benzyl-4-(S)-phenyl-3-(R)-pyrrolidinylcarbonyl)-4-
benzyl-2-oxazolidinone and (S)-N-(1-Benzyl-4-(R)-phenyl-3-
(S)-pvrrolidin~lcarbonyl)-4-ben~yl-2-oxazolidinone
To a solution of 15.0 g of (S)-N-cinnamoyl-4-benzyl-2-
oxazolidinone (Step 1, 48.8 mmol) in 150 mL of CH2C12 at -10 °C was
added 23 g (97.6 mmol) of N-(methoxymethyl-N-trimethylsilylmethyl-
benzylamine. Then 1 mL of trifluoroacetic acid was added and the
solution was stirred at room temperature for 1 h. The solution was
poured into saturated NaHC03 solution and the layers were separated.
The aqueous layer was washed with CH2C12 and the combined organic
extracts were washed with brine, dried over MgS04, filtered, and
concentrated. the residue was purified by chromatography on silica gel
using ethyl acetate-hexane (4:1) to afford the title compound (S)-N-[(1-
Benzyl)-4-(S)-phenyl-3-(R)-pyrrolidinylcarbonyl]-4-benzyl-2-
oxazolidinone. 1H NMR (CDC13) 8 2.76 (m, 1H), 2.85 (m, 1H), 2.96 (m,
IH), 3.25-3.4 (m, 3H), 3.72 (d, AB, J = 13 Hz, 1H), 3.85 (d, AB, J = 13 Hz,
1H), 4.05-4.24 (m, 4H), 4.66 (m, 1H), 7.20 - 7.45 (m, 15H); Mass spectrum
(ESI) m/e = 441 (M+1); [a]D = +106 (c. 1.03, CHC13)
Futher elution of the above column using ethyl acetate-
hexane (4:1) afforded the title compound (S)-N-[(1-benzyl)-4-(R)-phenyl-3-
(S)-pyrrolidinylcarbonyl]-4-benzyl-2-oxazolidinone; 1H NMR (CDC13) S
2.78 (m, 2H), 2.85 (m, 1H), 3.19 (dd, J = 2.5 Hz, 13.5 Hz, 1H), 3.25-3.35 (m,
3H), 3.71 (d, AB, J = 13 Hz, 1H), 3.81 (d, AB, J = 13 Hz, 1H), 4.15-4.24 (m,
4H), 4.36 (m, 1H), 4.72 (m, 1H) 7.1 (m, 1H), 7.20 - 7.45 (m, 14H); Mass
spectrum (ESI) m/e = 441 (M+1); [a]D = +1.4 (c. = 4.366, CHC13)
Step 3. 1-Benzvl-3-(R)-hvdrQ~ymethyl-4-(S)- henvlpyrrolidine
To a solution of 5.34 gm of (S)-N-[(1-benzyl)-4-(S)-phenyl-3-
(R)-pyrrolidinylcarbonyl]-4-benzyl-2-oxazolidinone (Step 2, 12.12 mmol)
-172-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
in 50 mL of tetrahydrofuran was added 25 mL of a solution of LiAlH4 in
tetrahydrofuran (1.02 M, 25 mmol). The solution was stirred at room
temperature for 18 h, then was quenched by addition of aqueous KOH
solution. The mixture was filtered and the residue was washed with
ether. The combined organic filtrate was dried over MgS04, filtered,
and concentrated. The residue was purified by chromatography on
silica gel using ethyl acetate - hexane (1:1) to afford the title compound.
1H NMR (CDCl3) 8 2.4-2.6 (m, 2H), 2.8-3.0 (m, 2H), 3.10-3.5 (m, 3H), 3.6-
3.85 (m, 4H), 7.20 - 7.45 (m, lOH); Mass spectrum (ESI} m/e = 267 (M+1) ;
[a]D = +8.74 (c.= 1.42, CHCIg)
Step 4. 1-Benzyl-3-(R)-formyl-4-(S)-phenylpvrrolidine
A solution of 1.31 mL (15 mmol) of oxalyl chloride in 20 mL
of dry CH2C12 was cooled to -78 °C under nitrogen. To this was added
1.78 mL (25 mmol) of dimethyl sulfoxide and the mixture was stirred at -
78 °C for 15 min. A solution of 2.67 g ( 10 mmol) of 1-benzyl-3-(R}-
hydroxymethyl-4-(S)-phenylpyrrolidine in 10 mL of dry CH2C12 was
added and the mixture was stirred at -78 °C for 1 h. Then 4.88 mL (35
mmol) of triethylamine were added and the solution was allowed to
warm to room temperature over 1 h. The reaction mixture was diluted
with 100 mL of ether, poured onto 200 mL of water and the layers were
separated. The aqueous layer was washed with ether and the combined
organic extracts were washed with 0.1 M HCl, saturated NaHC03
solution and brine. The ethereal solution was dried over MgS04,
filtered, and concentrated to afford the title compound, which was used
directly in the next step. Mass Spectrum (ESI) m/e 265 (M+1).
Step 5. 1-Benzyl-3-(S)-((4-phenylpiperidin-1-yl)methyl)-4-(S)-
uhenvlDVrrolidine
To a solution of 2.68 g ( 10 mmol) of 1-benzyl-3-(R)-formyl-4-
(S)-phenylpyrrolidine in 10 mL of 1,2-dichlorethane was added 2.0 g (12
mmol) of 4-phenylpiperidine. To this was added 3.2 g (15 mmol) of
sodium triacetoxyborohydride and the solution was stirred at room
temperature for 1 h. The reaction was poured into 50 mL of saturated
-173-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
NaHC03 solution and the mixture was extracted with three portions of
ethyl acetate. The combined organic extracts were washed with brine,
dried over MgS04, filtered and concentrated. The residue was purified
by chromatography on silica gel using ethyl acetate-hexane (1:1) to afford
the title compound. 1H NMR (CDC13) 8 1.6-1.85 (m, 4H), 1.98 (dq, J = 7
Hz, J = 2 Hz, 2H), 2.4-2.56 (m, 4H), 2.62 (m, 1H), 2.72 (m, 1H), 2.83 (m,
1H), 3.04 (m, 4H), 3.69 (d, AB, J = 7 Hz, 1H), 3.77 (d, AB, J = 7 Hz, 1H),
7.2-?.6 (m, 15H); Mass Spectrum (ESI) m/e 411 (M+1); [a]D = +26.0 (c.=
1.56, CHC13)
EXAMPLE 150
1-( 1-Benzyl)-3-(R)-((4-phenylpiperidin-1-yl)methyl)-4-(R)-
p~y~Ryrrolidine f(RR)-stereoisomer of Examt~le 91
Step 1. 1-Benz ~~1-3-(S)-by rox~methyl-4-(R)-phen~pvrrolidine
To a solution of 6.55 gm of (S)-N-[(1-Benzyl)-4-(R)-phenyl-3-
(S)-pyrrolidinylcarbonyl]-4-benzyl-2-oxazolidinone (Example lE, Step 2,
14.87 mmol) ) in 50 mL of tetrahydrofuran was added 30 mL of a solution
of LiAlH4 in tetrahydrofuran (1.02M, 30 mmol). The solution was
stirred at room temperature for 18h, then was quenched by addition of
aqueous KOH solution. The mixture was filtered and the residue was
washed with ether. The combined organic filtrate was dried over
MgS04, filtered, and concentrated. The residue was purified by
chromatography on silica gel using ethyl acetate - hexane (1:1) to afford
the title compound. 1H NMR (CDC13) 8 2.4-2.6 (m, 2H), 2.8-3.0 (m, 2H),
3.10-3.5 (m, 3H), 3.6-3.85 (m, 4H), 7.20 - 7.45 (m, lOH); Mass spectrum
(ESI) m/e = 267 (M+1); [a]D = -8.90 (c. = 1.39, CHCIg)
Step 2. ~.-Benzvl-3-(S)-formvl-4-(R)-phenvlDVrrolid~ne
A solution of 1.31 mL (15 mmol) of oxalyl chloride in 20 mL
of dry CH2C12 was cooled to -78 °C under nitrogen. To this was added
1.78 mL (25 mmol) of dimethyl sulfoxide and the mixture was stirred at -
78 °C for 15 min. A solution of 2.67 g (10 mmol) of 1-benzyl-3-(S)-
hydroxymethyl-4-(R)-phenylpyrrolidine in 10 mL of dry CH2C12 was
-174-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
added and the mixture was stirred at -78 °C for 1 h. Then 4.88 mL (35
mmol) of triethylamine was added and the solution was allowed to warm
to room temperature over 1 h. The reaction mixture was diluted with
100 mL ether, poured into 200 mL of water, and the layers were
separated. The aqueous layer was washed with ether and the combined
organic extracts were washed with 0.1 M HCI, saturated NaHC03
solution and brine. The ethereal solution was dried over MgS04,
filtered, and concentrated to afford the title compound, which was used
directly in the next step. Mass Spectrum: (ESI) m/e 265 (M+H)
Step 3. 1-Benzyl-3-(R)-((4-phenylpiperidin-1-yl)methyl)-4-(R)-
phenylpvrrolidine
To a solution of 2.68 g (10 mmols) of 1-Benzyl-3-(S)-formyl-4-
(S)-phenylpyrrolidine in 10 mL of 1,2-dichlorethane was added 2.0 g (12
mmol) of 4-phenylpiperidine. To this was added 3.2 g (15 mmol) of
sodium triacetoxyborohydride and the solution was stirred at room
temperature for 1 h. The reaction was poured into 50 mL of saturated
NaHC03 solution and the mixture was extracted with three portions of
ethyl acetate. The combined organic extracts were washed with brine,
dried over MgS04, filtered and concentrated. The residue was purified
by chromatography on silica gel using ethyl acetate-hexane (1:1) to afford
the title compound. 1H NMR (CDC13) d 1.6-1.85 (m, 4H), 1.98 (dq, J = 7
Hz, J = 2 Hz, 2H), 2.4-2.56 (m, 4H), 2.62 (m, 1H), 2.72 (m, 1H), 2.83 (m,
1H), 3.04 (m, 4H), 3.69 (d, AB, J = 7 Hz, 1H), 3.77 (d, AB, J = 7 Hz, 1H),
7.2-7.6 (m, 15H); Mass Spectrum: (ESI) m/e 411 (M+1); [a]D = -26.0 (c. _
1.56, CHC13)
EXAMPLE 151
1-( 1-Naphthoyl)-3-(R)-((4-phenyl)piperidin-1-yl)methyl)-4-(R)-
enyl_~vrrolidine I(RR)-stereoisomer of Example 611
The title compound was prepared from 3-(S)-
hydroxymethyl-4-(R)-phenylpyrrolidine (Example 150) according to
procedures described in Example 150).
Mass Spectrum (ESI) m/e 475 (M+1), [a]D = -39.6 (c. = 1.10, CHC13)
-175-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
EXAMPLE 152
1-(1-Naphthoyl)-3-(S)-((4-phenyl)piperidin-1-yl)methyl)-4-(S)-
a ~gprrolidine f(SS)-stereoisomer of Examule 611
The title compound was prepared from 3-(R)-
hydroxymethyl-4-(S)-phenylpyrrolidine (Example 150) according to
procedures described in Example 149).
Mass Spectrum (ESI) m/e 475 (M+1), [a]D = +39.4 (c. = 1.10, CHCig)
E-XAMPLE 153
1-( 1-Naphthoyl)-3-(S)-((4-(4-fluoro)phenyl)piperidin-1-yl)methyl)-4-(S)-
y.~Rvrrolidine f(SS)-stereoi omer o Examt~le lAl
The title compound was prepared from 3-(R)-
hydroxymethyl-4-(S)-phenylpyrrolidine (Example 149) according to
procedures described in Example 125).
Mass Spectrum (PB-NH3/CI): m/e 491 (M+1).
[a]D = +19.5° (c. = 0.60, CHC13)
EXAMPLE 154
1-( 1-Naphthoyl)-3-(R)-((4-(4-fluoro)-phenyl}piperidin-1-yl)methyl)-4-(R)-
p~enylpvrrolidine f(RR)-stereoisomer of Exam~lAl
The title compound was prepared from 3-(S)-
hydroxymethyl-4-(R)-phenylpyrrolidine (Example 150) according to
procedures described in Example 125).
Mass Spectrum (PB-NH3/CI): m/e 491 (M+1).
[a]D = -21.25° (c. = 0.60, CHCIg)
~,~~A1VIPLE 155
1-Benzoyl-3-(S)-((4-phenyl)piperidin-1-yl)methyl-4-(R)-phenylpyrrolidine
and_1-Benzoyl-3-(R)-((4-phenyl)piperidin-1-yl)methyl-4-(S)-
phenvlnvrrolidine
Resolution of 20 mg of 1-benzoyl-3-(SR)-((4-phenyl)piperidin-
1-yl)methyl-4-(RS)-phenylpyrrolidine (from Example 36) was carried out
via semi-preparative HPLC using a Chiralpak AD 2 x 25 cm column
with 70/30 v/v hexanes/iPrOH as the eluant at a flow rate of 9.0 ml/min
-176-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
and detection at 220 nM to afford 8.4 mg of 1-benzoyi-3-(S)-((4-
phenyl)piperidin-1-yl)methyl-4-(R)-phenylpyrrolidine and 8.2 mg of 1-
Benzoyl-3-(S)-((4-phenyl)piperidin-1-yl)methyl-4-(R)-phenylpyrrolidine.
Anah,~tical HPLC: Chiralpak AD 4.6 x 250 mm column, 70/30 v/v
hexanes/iPrOH, 0.5 mL/min, 220 nm. Retention times: 3-(S), 4-(R), 14:8
min; 3-(R), 4-(S), 24.0 min.
1-(2-Chlorobenzoyl)-3-(R)-((4-(N-phenylmethoxycarbonyl-N-
ethyl)amino)piperidin-1-yl)methyl-4-(S)-phenylpyrrolidine and 1-(2-
Chlorobenzoyl)-3-(S)-((4-(N-phenylmethoxycarbonyl-N-
ethyl)aminopineridin-1-vl)methyl-4-(R)-phenyl~yrrolidine
Resolution of 15 mg of 1-(2-chlorobenzoyl)-3-(RS)-((4-(N-
phenylmethoxycarbonyl-N-ethyl)amino)piperidin-1-yl)methyl-4-(SR)-
phenylpyrrolidine (from Example 80) was carried out via semi-
preparative HPLC using a Chiralcel OD 2 x 25 cm column with 60/40 v/v
hexanes/EtOH as the eluant at a flow rate of 9.0 ml/min and detection at
240 nM to afford 4.1 mg of 1-(2-chlorobenzoyl)-3-(R)-((4-(N-
phenylmethoxycarbonyl-N-ethyl)amino)piperidin-1-yl)methyl-4-(S)-
phenylpyrrolidine and 6.2 mg of 1-(2-chlorobenzoyl)-3-(S)-((4-(N-
phenylmethoxycarbonyl-N-ethyl)amino)piperidin-1-yl)methyl-4-(R)-
phenylpyrrolidine. Anal3rtical HPLC: Chiralcel OD 4.6 x 250 mm
column, 60/40 v/v hexanes/EtOH, 0.5 mL/min, 220 nm. Retention times:
3-(R), 4-(S), 14.7 min; 3-(S), 4-(R), 24.1 min.
Examples 157 and 158 were prepared in a manner
analogous to Examples 36-62.
EXAMPLE 157
1-(2-Methyl)propionyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
p envlg3~rrolidine
1H NMR (500 MHz, CDC13): 81.64-4.21 (17H), 7.17-7.62 (15H). Mass
Spectrum (NH3-CI): 391 (M+1).
-177-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
IPLE 158
1-(2-Ethyl)butanonyl-3-(SR)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
phenvlDVrrolidine
Mass Spectrum (NH3-CI): 419 (M+1).
EXAMPLE 159
1-Benzyl-3-(SR)-(4-(N-methyl-N-(3-phenylpropyl)-carboxamide)-
~ineridin-1-ylmethvl)-4-(SR)-nhenyluvrrolidine _
The title compound was prepared from 35 mg of N-benzyl-3-
(SR)-(4-carboethoxy-piperidin-1-ylmethyl)-4-(SR)-phenylpyrrolidine, 30
mg of N-methyl-N-(3-phenylpropyl)amine and 0.090 mL of
isopropylmagnesium chloride (2M in THF) using a procedure analogous
to that described in Example 93 to provide 29.5 mg of the title compound.
RF: 0.15 (5% MeOH in CH2C12). 1H NMR (300 MHz, CDC13): b 1.35-2.15
(m, lOH), 2.3-2.75 (m, 8H), 2.8-3.1 (m, 6H), 3.2 (m,lH), 3.4 (m, 1H), 3.6-3.8
(m, 2H), 7.1-7.4 (m, 15H). Mass Spectrum (CI): 510.7 (M+H).
EXAMPLE 160
1-Benzyl-3-(SR)-(4-(N-methyl-N-(4-phenylbutyl)-carboxamide)-piperidin-
1-ylmethvl)-4-(SR)-phenvlg~rolidine
The title compound was prepared from 52 mg of N-benzyl-3-
(SR)-(4-carboethoxy-piperidin-1-ylmethyl)-4-(SR)-phenylpyrrolidine, 3?
mg of N-methyl-N-(4-phenylbutyl)amine and 0.13 mL of
isopropylmagnesium chloride (2M in THF) using a procedure analogous
to that described in Example 93 to provide 23 mg of the title compound.
RF: 0.25 (5% MeOH in CH2C12). 1H NMR (300 MHz, CDC13): 8 1.4-1.9 (m,
lOH), 2.25-2.75 (m, 9H), 2.85-3.1 (m, 7H), 3.2 (m,lH), 3.4 (m, 1H), 3.65
(ABq, 2H), 7.1-7.4 (m, 15H). Mass Spectrum (CI): 524.3 (M+H).
EXAMPLE 161
-178-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1-Benzyl-3-(SR)-(4-(N-(3-phenylpropyl)-carboxamide)-piperidin-1-
methyl)-4-(SR)-phenvlnvrrolidine
The title compound was prepared from I51 mg of N-benzyl-
3-(SR)-(4-carboethoxy-piperidin-1-ylmethyl)-4-(SR)-phenylpyrrolidine,
0.105 mL of 3-phenylpropylamine and 0.55 mL of isopropylmagnesium
chloride {2M in THF) using a procedure analogous to that described in
Example 93 to provide 94.5 mg of the title compound. RF: 0.20 (5% MeOH
in CH2C12). 1H NMR (300 MHz, CDCl3}: b 1.4-2.0 (m, 9H), 2.35-2.7 (m,
8H), 2.85-3.0 (m, 4H), 3.2-3.3 (m, 2H), 3.4 (m, 1H), 3.65 (ABq, 2H), 7.1-7.4
(m, 15H). Mass Spectrum (CI): 496.8 (M+H).
1-Benzoyl-3-(SR)-(4-(2-tolyl)-piperidin-1-ylmethyl)-4-(SR)-
i5 pheny~Rvrrolidine
Step A: 3-(SR)-(4-(2-tolyl)-piperidin-1-ylmethyl)-4-(SR)-
u- hen~,pvrrolidine
The title compound was prepared from 15 mg of 1-Benzyl-3-
(SR)-(4-(2-tolyl)-piperidin-1-ylmethyl)-4-(SR)-phenylpyrrolidine (from
Example 20), 35 mg of ammonium formate and 8 mg of 10% Pd on
carbon in 1.5 mL of MeOH using a procedure analogous to that described
in Example 30, Step A (except Pd on carbon was substituted for 20%
Pd(OH)2) to provide the title compound, which was taken onto Step B
without further purification.
Step B: 1-Benzoyl-3-{SR)-(4-(2-tolyl)-piperidin-1-ylmethyl)-4-(SR)-
t~l~envluvrrolidine
The title compound was prepared from 3-(SR)-(4-(2-tolyl)-
piperidin-1-ylmethyl)-4-(SR)-phenylpyrrolidine (Step A), 0.014 mL of
DIEA, 12.5 mg of BOP-Cl and 5 mg of benzoic acid in 0.5 mL of CH2C12
using a procedure analogous to that described in Example 63 to provide
the title compound. RF: 0.45 (5% MeOH in CH2Cl2). IH NMR (300 MHz,
CDCl3): 8 1.4-4.5 (m, 20H), 7.05-7.6 (m, 14H). Mass Spectrum (CI): 439.4
(M+H).
-179 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Examples 163 to 169 were prepared by the general
procedures given Example 1, substituting the appropriate acyl chloride
or carboxylic acid (in the presence of EDAC and hydroxybenzotriazole)
for benzenesulfonyl chloride in Step D, and substituting the appropriate
piperidine derivative for spiro[2,3-dihydrobenzothiophene)-3,4'-
piperidine in step F. Alternatively, these compounds can be prepared as
described in Example 9, using 3-(SR)-hydroxymethyl-4-(SR)-
phenylpyrrolidine, the intermediate from Example 1, Step C.
EXAMPLE 163
1-(Cyclobutanoyl)-3-(SR)-(4-phenylpiperidin-1-ylmethyl)-4-(SR)-
ylRvrrolidine
Mass Spectrum (NH3-CI): 403 (M+1).
EXAMPLE 164
1-(2-Furanoyl)-3-(SR)-(4-phenylpiperidin-1-ylmethyl)-4-(SR)-
p~en~R olidine
Mass Spectrum (NH3-CI): 415 (M+1).
EXAMPLE 165
1-(3-Furanoyl)-3-(SR)-(4-phenylpiperidin-1-ylmethyl)-4-(SR)-
enylpvrrolidine
Mass Spectrum (NH3-CI): 415 (M+1).
EXAMPLE 166
1-(2-(SR)-(2, 3, 4, 5-Tetrahydrofuranoyl))-3-(SR)-(4-phenylpiperidin-1-
lme yl)-4-(SR)-nhenylRyrrolidine
-180-
CA 02298813 2000-O1-28
WO 99/09984 PCTNS98/17755
Mass Spectrum (NH3-CI): 419 (M+1).
AMPLE 167
1-Benzyl-3-(SR)-(4-phenylpiperidin-I-ylmethyl)-4-(SR)-(3-
fluoro hen3~1)pvrrolidine
The compound was prepared using procedures analogous
to those described in Examples 1 and 9 substituting the appropriately
substituted (E)-cinnamate for methyl (Z)-cinnamate in Example I, Step
A. Mass Spectrum (NH3-CI): 429 (M+1).
EXAMPLE 168
1-(2-Phenyl)benzoyl-3-(SR)-(4-phenylpiperidin-1-ylmethyl)-4-(SR)-
phenvlDVrrolidine
Mass Spectrum (NH3-CI): 501 (M+1).
EXAMPLE 169
1-Benzyl-3-(SR)-(4-(N-(benzyloxycarbonyl)-N-ethylamino)-piperidin-1-
y methyl)-4-(SR)-phenylR3~rrolidine
Mass Spectrum (NH3-CI): 512 (M+1).
Examples 170 to 174 were prepared as described in Ex. 63.
PLE 170
1-((2-methyl)cyclohexancarbonyl)-3-(RS)-((4-phenyl)piperidin-1-
vl)methyl-4-(SR) phenvl~vrrolidine
Mass Spectrum (NH3-CI): 445 (M+i).
EXAMPLE 17 ~
-181-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1-((2-carbomethoxy)benzoyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl-4-
(SR)-nhenvlnvrrolidine
Mass Spectrum (NH3-CI): 489 (M+I).
EXAMPLE 172
1-(indol-4-ylcarbonyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
phenvhvrrolidine
Mass Spectrum (NH3-CI): 464 (M+1).
EXAMPLE 173
1-(indol-3-ylcarbonyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
phenvlnvrrolidine
Mass Spectrum (NH3-CI): 464 (M+1)..
EXAMPLE 174
1-(indol-7-ylcarbonyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
nhenvlRyrrolidine
Mass Spectrum (NH3-CI): 464 (M+1).
PLE 175
1-((4-fluoro)napthalene-1-carbonyl)-3-(RS)-((4-phenyl)piperidin-1-
y )met yl-4-(SR)- h~ envlp~~rrolidine
The title compound was prepared as described in Example
59 using the acid chloride as an intermediate. Mass Spectrum (NH3-
CI): 493 (M+1).
AMPLE 176
1-(3,4,4a,5,6,7,8,8a octahydro-napthalene-1-carbonyl)3-(RS)-((4-
~ en~~ix~eridin-1-vl)methy 4-(SR)~henylpyrrolidine
-182-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Step A: 1-Trifluoromethanesulfonyl-3,4,4a,5,6,7,8,8a octahydro-
nanthal-1-ene _
To a solution of 155 mg (1.02 mmol) of decalone in 3 mL of
THF at -78o C was added 1.43 mL (1.43 mmol) of lithium
bis(trimethylsilyl)amide ( 1.0 M in THF). After stirring at -78o C for 1
hour, 560 mg (1.43 mmol) of 2-[N,N-bis(trifluoromethyl-sulfonyl)amino]-
5-chloropyridine was added. After stirring for an additional 2 hours the
reaction was warmed to -20o C and quenched with a saturated solution
of ammonium chloride (10 mL). The reaction mixture was diluted with
H20 (50 mL), and extracted with CH2Cl2 (3 x 30 mL). The combined
organic extracts were washed with brine, dried (Na2S04), and
concentrated in vacuo. The residue was purified by flash
chromatography eluting with 0-15% EtOAc in hexanes to afford the
triflate (90 mg, 30%) as a colorless oil. 1H NMR (CDC13, 500 MHz) 8 5.69-
5.71 (m, 1H), 2.21-2.29 (m, 2H), 2.07-2.29 (m, 2H), 1.57-1.88 (m, 4H), 1.27-
1.45 {m, 4H), 1.10-1.18{m, 2H) ppm.
Step B: ~.-Carboxylic acid-3 4 4a 5 6,7,8 8a octahydro-napthal 1 ene
To a solution of 90 mg ( 0.32 mmol) of triflate (from Step A)
in 2 mL of DMF was added 126 mg (1.28 mmol) of potassium acetate
followed by 12 mg (0.016 mmol) of Pd(PPh3)2(OAc)2. After bubbling
carbon monoxide through the solution for 5 min, the reaction was
stirred at room temperature. After 3 hours the reaction was diluted
with H20 (50 mL), 0.5N HCl solution to a pH of 3 and extracted with
CH2Ci2 (3 x 30 mL). The combined organic extracts were washed with
brine, dried (Na2S04), and concentrated in vacuo to yield a black oil. The
oil was purified using flash chromatography (? g of silica gel 60, 40% v/v
ethyl acetate/hexanes) to afforded 33 mg (53%) of the title compound as a
yellow crystalline solid.lH NMR (CDC13, 500 MHz) 8 6.92-6.93 (m, 1H),
2.37-2.40 (m, 1H), 2.23-2.27 (m, 2H), 2.02-2.03 (m, 1H)1.60-1.81 (m, 4H),
1.17-1.45 (m, 5H), 0.90-0.93(m, 1H) ppm.
Step C: 1-(3,4,4a,5,6,7,8,8a octahydro-napthalene-1-carbonyl)3-(RS)-
((4-phenyl) ~~eridin-1-vl)methyl 4-(SR)-phen~l~yrroli one
-183-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
The title compound was prepared using 3,4,4a,5,6,7,8,8a
octahydro-napthal-1-ene-1-carboxylic acid (from Step B) and procedures
analogous to those described in Example 63.
Mass Spectrum (NH3-CI): 483 (M+1).
XLE 177
1-(3,4 dihydro-napthalene-1-carbonyl)-3-(RS)-((4-phenyl)piperidin-1-
yl)methvl 4-(SR -~hen_ylpvrrolidine
Step A: 1-Trifluo~omethanesulfonvl-3 4 dihydro-nanthalen~P
The title compound was prepared using alpha-tetralone and
procedures analogous to those described in Example 176, Step A.1H
NMR (CDC13, 500 MHz) b 7.19-7.38 (m, 4H), 6.04 (t, 1H, J = 4.8 Hz), 2.89 (t,
2H, J = 8.2 Hz), 2.51-2.56 (m, 2H) ppm.
Step B: 3,4 Dih rLdro-nanthalene-1-carbo~vlic acid
The title compound was prepared using the triflate from
Step A and procedures analgous to those described in Example 176, Step
B.1H NMR (CDC13, 500 MHz) 8 7.92 (d, 1H, J = 7.6 Hz), 7.41 (t, 1H, J = 4.8
Hz), 7.18-7.28 (m, 3H), 2.81 (t, 2H, J = 7.8 Hz), 2.45-2.49 (m, 2H) ppm.
Step C: 1-(3,4 dihydro-napthalen-1-carbonyl)3-(RS)-((4-phenyl)
pineridin-1-yl)methyl 4-(SR)-nhenylRyrrolidine
The title compound was prepared using 1-carboxylic acid-
3,4 dihydro-napthal-1-ene (from Step B) and procedures analgous to
those described in Example 63. Mass Spectrum (NH3-CI): 477 (M+1).
EXAMPLE 178
1-(5,6,7,8 tetrahydronapthalen-1-carbonyl)-3-(RS)-((4-phenyl)piperidin-1-
1 methvl 4-(SR~uhenylRvrrolidine -
Step A: ~-Trifluoromethanesuifonvl-~,f~7 ptra ydronapthalene
-184 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
To a solution of 5,6,7,8 tetrahydro-1-napthol (155 mg, 1.02
mmol) in 3 mL of CH2C12 at Oo C was added triethylamine (257 mg, 2.54
mmol) followed by 900 mg (2.54 mmol) of 2-[N,N-bis(trifluoromethyl-
sulfonyl)amino]-5-chloropyridine. The reaction was allowed to warm to
room temperature. After stirring overnight the reaction was diluted
with H20 (50 mL), and extracted with CH2C12 (3 x 30 mL). The combined
organic extracts were washed with brine, dried (Na2S04), and
concentrated in vacuo. The residue was purif ed by flash
chromatography eluting with 0-15% EtOAc in hexanes to afford the
triflate (281 mg, 60%) as a colorless oil.lH NMR (CDC13, 500 MHz) 8 7.06-
7.18 (m, 3H), 2.80-2.84 (m, 4H), 1.80-1.96 (m, 4H) ppm.
Step B: 5 6 ?,8 Tetrahydronapthalene-1-carboxylic acid
The title compound was prepared using the triflate from
Step A and procedures analogous to those described in Example 176, Step
B.1H NMR (CDCl3, 500 MHz) b 7.85 (d, 1H, J = 7.8 Hz), 7.28-7.30 (m,
1H),7.19 (t, 1H, J = 7.6 Hz), 3.15-3.18 (m, 2H), 2.84-2.87 (m, 2H), 1.82-1.83
(m, 4H) ppm.
Step C: 1-(5,6,7,8 tetrahydro-napthalene-1-carbonyl)-3-(RS)-((4-
~envl)~iperidin-1-yl)met yl4-(SR)-,phenylR3rrrolidine
The title compound was prepared using 5,6,7,8 tetrahydro
napthalene-1-carboxylic acid (from Step B) and procedures analogous to
those described in Example 63. Mass Spectrum (NH3-CI): 479 (M+1).
EXAMPLE 179
1-((Adamant-1-yl)methyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
nnenvmvrrolialne
To a solution of 1-(1-adamantanecarbonyl)-3-(RS)-((4-
phenyl)piperidin-1-yl)methyl-4-(SR)-phenylpyrrolidine (10 mg, 0.021
mmol) in 2 mL of THF was added lithium aluminum hydride (2 mg,
0.042 mmol). The reaction was heated to reflux. After 2 hours the
reaction was quenched with H20 (.5 mL), 15% NaOH solution (0.5 mL),
H20 (1.0 mL) and diluted with a saturated solution of Rochelle salts (50
-185-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
mL) and CH2C12 (50 mL). After stirring for 1 hour the mixture was
extracted with CH2C12 (3x50 mL). The combined organic extracts were
washed with brine, dried (Na2S04), and concentrated in vacuo to yield 9
mg of the title compound. Mass Spectrum (NH3-CI): 469 (M+1).
EXAMPLE 180
1-((7-indole)methyl)-3-{RS)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-
phenylpvrrolidine
The title compound was prepared using 1-(indol-7-
carbonyl)-3-{RS)-((4-phenyl)piperidin-1-yl)methyl-4-(SR)-phenyl-
pyrrolidine (from Example 174) and procedures analogous to those
described in Example 179. Mass Spectrum (NH3-CI): 450 (M+1).
EXAMPLE 181
1-(2(R)-phenyl-cyclohexyl-1(S)-methyl)-3-(RS)-((4-phenyl)piperidin-1-
vl)methyl-4-(SR)-phen~Rvrrolidine
The title compound was prepared using 1-((2R)-phenyl-
cyclohexane-1-(S)-carbonyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl-4-
(SR)-phenylpyrrolidine (from Example 183) and procedures analogous to
those described in Example 179. Mass Spectrum (NH3-CI): 493 {M+1).
EXAMPLE 182
1-( 2(R)-phenyl-cyclohex-3-ene-1(S)-carbonyl)-3-(RS)-((4-phenyl)piperidin-
1- )methyl4-(SR)-,phenvlp~rrrolidine
Step A: 1(R)-Dhen~~vclohex-5-ene-2(S)-carbonyl chloride
To a solution of 155 mg (1.02 mmol) of 1(R)-phenyl-2{S)-
carboxy cyclohex-5-ene (See U.S. Patent #5,750,549, Example 158, Step B)
in 3 mL of CH2C12 at Oo C was added oxalyl chloride (100 uL) followed by
DMF (1 drop). The reaction was allowed to warm to room temperature.
After stirring 1 hour the reaction was concentrated in vacuo, redissolved
-186 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
in CH2C12 and concentrated to a yellow solid which was used directly in
the next step.
Step B: 1-( 2(R)-phenyl-cyclohex-3-ene-1(S)-carbonyl)-3-(RS)-((4-
lg,~enyl)pineridin-1-vl)methyl 4-(SR)-phenvlnvrrolidine
The title compound was prepared using the acid chloride
from Step A and procedures analogous to those described in Example 59.
Mass Spectrum (NH3-CI): 493 (M+1).
EXAMPLE 183
1-(2(R)-phenyl-cyclohexane-1(S)-carbonyl)-3-(RS)-((4-phenyl)piperidin-1-
vl)methyl-4-(SR)- h~envlpyrrolidine
To a solution of 14 mg (0.03 mmol) of 1-(2(R) phenyl-
cyclohex-3-ene-1(S)-carbonyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl4-
(SR)-phenylpyrrolidine (from Example 182, Step B) in 2 mL of MeOH was
added 10% Pd/C (7.0 mg). The reaction mixture was hydrogenated at 50
psi on a Parr apparatus for 4 hrs. The reaction was filtered thru Celite,
washed with MeOH and the filtrate was concentrated in vacuo to afford
10 mg of the title compound as an oil. Mass Spectrum (NH3-CI): 507
(M+1).
EXAMPLE 184
1-(2(S)-phenyl-cyclohex-3-ene-1(R)-carbonyl)-3-(RS)-((4-phenyl)piperidin-
1 meth3rl 4-(SR)- henvlpyrrolidine
The title compound was prepared using 1(S)-phenyl-2(R)-
carboxy-cyclohex-5-ene and procedures analogous to those described in
Example 182, Steps A and B. Mass Spectrum (NH3-CI): 505 (M+1).
EXAMPLE 185
1-(2(S)-phenyl-cyclohexane-1(R)-carbonyl)-3-(RS)-((4-phenyl)piperidin-1-
)met vl-4-(SR)-~hen~pvrrolidine
-187-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
The title compound was prepared using 1-(2(S)-phenyl-
cyclohex-3-ene-1(R)-carbonyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl 4-
~SR)-phenylpyrrolidine (from Example 184) and procedures analogous to
those described in Example 183. Mass Spectrum (NH3-CI): 507 (M+1),
EXAMPLE 186
1-(2(R)-phenyl) cyclohex-3-ene-1(R)-carbonyl)-3-(RS)-((4-phenyl)piperidin-
1-vl)methvl 4-(SR)-phen3~l,pvrrolidinP
Step A: 1(R)-Rhen~)-carbomethox 5 ene
~.~yclohex
To a solution of 900 mg (4.45 mmol) of 1(R)-phenyl-2(S)-
carboxy cyclohex-5-ene (See U.S. Patent #5,750,549, Example 158, Step B)
in a 4:1 solution of MeOH/THF was added trimethylsilyldiazomethane
until a yellow color persisted After stirring at room temperature for 3
hour the reaction was concentrated in vacuo and the residue was
purified by flash chromatography eluting with 0-15% EtOAc in hexanes
to afford the ester (300 mg) as a colorless oil.lH NMR (CDC13, 500 MHz) 8
7.19-7.30 (m, 5H), 5.98-6.01 (m, 1H), 5.76-5.79 (m, 1H),3.89 (s, 1H),3.50 (s,
3H), 2.95-2.98 (m, 1H), 2.16-2.32 (m, 2H),1.80-1.87 (m, 2H) ppm.
Step B: 1(R)-Dhenvl-2(S)-h drox~et~l-cvclohex-5-ene
A solution of 300 mg (1.38 mmol) of 1(R)-phenyl-2(S)-
carbomethoxy-cyclohex-5-ene (from Step A) in 30 mL of CH2C12 at 0 °C
was treated with 2.3 mL of 1.5 M diisobutylaluminum hydride solution
in toluene. After stirring for 30 minutes the reaction was quenched with
50 mL of sat'd sodium potassium tartrate solution, diluted with 100 mL
of ether and stirred at rt for 20 h. The layers were separated and the
organic layer was washed with 75 mL of H20, dried over MgS04 and
concentrated in vaccuo. Flash chromatography on 100 g of silica gel
using 5-25% v/v EtOAc/hexanes as the eluant afforded 185 mg (72%) of
the title compound as an oil.lH NMR (CDCl3, 500 MHz) 8 7.23-7.34 (m,
5H), 5.95-5.97 (m, 1H), 5.77-5.79 (m, IH),3.66 (s, 1H), 3.23-3.32 (m, 2H),
2.14-2.25 (m, 3H),1.51-1.62 (m, 3H) ppm.
-188-
* rB
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Step C: ~.(R)-Dhenvl-2(S)-carboxald h de-cyciohex ~ ene
A solution of 0.09 mL (1.0 mmoi) of oxalyl chloride in 4 mL
of CH2C12 at -75 °C was treated with 0.142 mL (2.0 mmol) of DMSO
maintaining the internal temperature below -65 °C. The resulting
mixture was stirred cold for 10 min and then 75 mg (0.40 mmol) of 1(R)-
phenyl-2(S)-hydroxymethyl-cyclohex-5-ene (from Step B) was added in
one portion . The resulting mixture was stirred cold for 30 min and then
.547 mL (4.0 mmoi) of DIEA was added maintaining the internal
temperature below -60 °C. The cooling bath was removed and the
reaction was allowed to warm to room temperature and stirred for 45
minutes. The reaction was quenched with 50 mL of H2O; the resulting
mixture was extracted with CH2C12 (3x50 mL). The combined organic
extracts were washed with brine, dried (Na2S04), and concentrated in
vacuo. The residue was purified with flash chromatography (70 g of
silica gel; 0-25% v/v ethyl acetate/hexanes) afforded 67 mg (90%) of the
title compound as an oil. 1H NMR (CDCl3, 500 MHz) 8 9.52 (d, 1H, J = 1.8
Hz), 7.23-7.34 (m, 5H), 5.99-6.03 (m, 1H), 5.84-5.87 (m, 1H),3.99 ( s, 1H),
2.77-2.81 (m, 1H), 2.28-2.34 (m, 1H), 2.16-2.22 (m, 1H), 1.59-1.91 (m, 2H)
ppm.
Step D: L(R)-uhenvl-2(R) carboxaldeh .e cvclohex 5 eyne
The aldehyde 67 mg (from Step E) was taken up in MeOH (2
mL) and treated with NaOMe (0.5 mL of 0.32 M in MeOH) at room temp
for 3 hours. The reaction mixture was quenched with H20 (50 mL) and
extracted with EtOAc (3 x 30 mL). The combined organic extracts were
washed with brine, dried (Na2S04), and concentrated in vacuo to afford
the epimerized aldehyde (54 mg, 81%) as a colorless oil. 1H NMR (CDC13,
500 MHz) 8 9.71 (d, 1H, J = 1.3 Hz), 7.23-7.35 (m, 5H), 5.92-5.95 (m, 1H),
5.70-5.73 (m, 1H),3.79-3.81 ( m, 1H), 2.61-2.64 (m, 1H), 2.20-2.22 (m, 2H),
1.76-1.99 (m, 2H) ppm.
Step E: 1(R)-phenyl-2(R)-carboxY-_cyclohex 5 ene
To a solution of the aidehyde (from Step D) 54 mg (2.36
mmol) in THF (3 mL) at 0°C was added sulfamic acid (430 uL, 1M aq.),
NaH2P04 (160 uL, 2.7M aq.), and NaC102 (430 uL, 1M aq.). The reaction
-189-
CA 02298813 2000-O1-28
WO 99/09984 PCTlUS98/17755
mixture was allowed to warm to room temp and stirred for 18 h. The
reaction mixture was then quenched by addition of H20 (50 mL), and
extracted with CH2C12 (3 x 30 mL). The combined organic extracts were
washed with brine, dried (Na2S04), and concentrated in vc~cuo. The
residue was purified with flash chromatography (70 g of silica ge1;25%
v/v ethyl acetate/hexanes with .5% acetic acid ) to afford 31 mg (54%) of
the carboxylic acid.lH NMR (CDCl3, 500 MHz) b 7.23-?.33 (m, 5H), 5.88-
5.92 (m, 1H), 5.fi5-5.68 (m, 1H), 3.75-3.78 (m, 1H), 2.62-2.67 (m, 1H), 1.88-
2.24 (m, 4H) ppm.
Step F 1-(2(R)-phenyl-cyclohex-3-ene-1(R)-carbonyl)-3-(RS)-((4-
~henvl)niperidin-1- l~h~vl-4-(SR)- heny~p~nrrolidine
The title compound was prepared using 1(R)-phenyl-2(R)-
carboxy-cyclohex-5-ene (from Step E) and procedures analogous to those
described in Example 63. Mass Spectrum (NH3-CI): 505 (M+1).
EXAMPLE 187
1-(2(R)-phenyl-cyclohexane-1(R)-carbonyl)-3-(RS)-((4-phenyl)piperidin-1-
)met yl-4-(SR)-phenyl-nvrrolidine
The title compound was prepared using 1-(2(R)-phenyl-
cyclohex-3-ene-1(R)-carbonyl)-3-(RS)-((4-phenyl)piperidin-1-yl)methyl 4-
,(SR)-phenylpyrrolidine (from Example 186) and procedures analogous to
those described in Example 14. Mass Spectrum (NH3-CI): 507 (M+1).
EXAMPLE 188
1-(2(S)-phenyl-cyclohex-3-ene-1(S)-carbonyl)-3-(RS)-((4-phenyl)piperidin
1-y~)methvl 4-(SR)-nhenvhvrrolidine
The title compound was prepared starting with 1(S)-phenyl-
2(R)-carboxy-cyclohex-5-ene and procedures analogous to those described
in Example 186, Steps A-F. Mass Spectrum (NH3-CI): 505 (M+1).
EXAMPLE 189
-190-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
1-(2(S)-phenyl-cyclohexane-1(S)-carbonyl)-3-(RS)-((4-phenyl)piperidin-1-
yl)methvl-4-(SR)- 1 Pn~pyrrolidine
The title compound was prepared using 1-(( 2(S) phenyl)
cyclohex-3-ene-1(S)-carbonyl)3-(RS)-((4-phenyl)piperidin-1-yl)methyl 4-
ASR)-phenylpyrrolidine (from Example 188) and procedures analogous to
those described in Example 183. Mass Spectrum (NH3-CI): 507 (M+1).
The compounds in Examples 190 to 218 were prepared
according to the procedures given in Example 220 (carried out in single
compounds format, wherein the mixing and archiving steps are
omitted). Example 219 was prepared by coupling of 4-(3-phenylpropyl)-
piperidine with 3-(RS)-carboxy-4-ASR)-phenylpyrrolidine. followed by
reduction with BMS as in examples 190-218. Acylation of the pyrrolidine
nitrogen with cyclohexane carbonyl chloride provided the final product.
x
R
Ry
EXAMPLE Rx Ry Rz Spectral Data
1V1~
51s (M+1)
N-
1V1~
° 546 (M+1)
N-
V1~J:
560 (M+1)
-191-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
C-
'~ -~ 558 (M+1)
D
w~ a as vVV-11GA jn~.
/ \ ~ 558 (M+1)
c-
/ \ °~~~ 550 (M+1)
C
196 Ph ,~ o o MS:
o :S'' / 548 (M+1)
N-
S
19? Ph CH2-c-Hex w MS:
459 {M+1)
198 Ph ~ ~ MS:
0 'W ~ , 509 (M+1)
~ S
Rx N~ Rz
N>
Ry
EXAMPLE Rx Ry Rz HPLC MS
(Method)a (M+1)
199 Ph O~ ,O Ph 8.19 min 461.3
Ph'S "~ (A)
-192-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Ph ~' ~ Ph 8.43 min 475.4
s
I ~ ~ (A)
201 Ph F3c I °'sy Ph 8.75 min 529.4
(A)
~2 Ph ~~ .~ Ph 8.48 min 475.4
S
I ~ ~. (A)
Ph ~' %~ Ph 8.27 min 479.3
s
~ ~ (A)
F
204 Ph F ~~S ~ Ph 8.33 min 479.3
(A)
Ph ~~ S ~ Ph 8.45 min 495.1
~ ~ (A)
c
206 Ph ci I ~~'s~ Ph 8.61 min 495.1
(A)
20'7 Ph ° S ° Ph 8.46 min 475.4
w
(A>
208 Ph I ~ 4.60 min 431.3
(B)
209 Ph 4.18 min 518.4
Phi O~ N~
J ()
210 w phi 4.88 min 483.5
Me0 ~
(B)
-193-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
211 Ph ~ I ph~~ 6.21 min 453.3
(C)
2~ Ph ~ ~ Ph~'~ 5.61 min 467.3
(C)
213 Ph ~ phi. 5.82 min 391.3
(C)
214 Ph ~ , Ph~~ 6.21 min 459.3
s' ~ (c)
215 Ph ~ I ~ phi 6.52 min 503.3
i (C)
6
216 Ph ~ II 5.53 min 496.4
Ph ~O~N''
(C)
217 Ph ~ 'I~I 6.29 min 542.4
Ph ~O~N''
(C)
218 Ph 'I~I 4.88 min 565.4
Ph ~O~N''
i J (C)
w I N
219 Ph pt,~ 5.25 min 473.1
T ~ (C)
a HPLC Column: Zorbax SB-C8 4.6x75mm, 3.5 micron. Conditions: A;
-> 100 % CH3CN/H20 w/0.1% TFA over 10 min. B; 10 -> 100 %
CH3CN/H20 w/0.1% TFA over 7.5 min. C; 10 -> 100 % CH3CN/H20
w/0.1% TFA over 7.5 min.
5
EXAMPLE 220
Preparation of 21x19 combinatorial library
Commercially available 4-sulfamylbenzoyl polystyrene resin
10 (4.2 g /4.8 mmol), 1-(t-butoxycarbonyl)-3-(RS)-carboxy-4-(SR)-
phenylpyrrolidine (29 mmol, 6 equiv), diisopropylethylamine (DIEA; 15
-194 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
mmol, 3 equiv} and DMAP (1.2 mmol, 0.25 equiv) were suspended in 40
mL of 1/1 methylene chloride / THF. Diisopropylcarbodiimide (15 mmol,
3 equiv) was added and the mixture was agitated by rotary stirring
overnight. The resin was then washed sequentially with 1/1 methylene
chloride / THF, THF and methylene chloride (4x40 mL each). The resin
was split into 22 equal portions and each was washed with methylene
chloride (3x5mL). Each of the 21 pools was treated with 40 % TFA /
methylene chloride for 30 min then washed with methylene chloride (5x5
mL). To each of the 21 pools of resin, 5 mL of a 0.4 M methylene chloride
solution in the Y subunit (2 mmol, 5.3 equiv; see structures below) was
added followed by DIEA (3 mmol, 8 equiv). The mixtures were shaken
and let stand for 30 min. The resin was washed with methylene
chloride, THF and 1/1 methylene chloride / THF (4x5mL each). 80 mg of
resin from each pool was archived for deconvolution and the remaining
resin in the 22 flasks was then combined in 7/3 dichloroethane / DMF
and mixed for 2h. The combined resin was split into 19 equal portions
and each portion was washed with THF (3xlmL). A solution of
trimethylsilyldiazomethane (1.0 M in 1/1 THF / Hexane, 1.5 mL) was
added, the mixtures were shaken and let stand for 2 h. The resin in
each pool was washed with 1 mL of THF and treated with
trimethylsilyldiazomethane as above. The resin was washed with THF,
methylene chloride and ether, then dried under nitrogen and
transferred to a flask containing the Z subunit (2 equiv; see structures
below) in 0.35 mL THF. The suspension was heated to 50°C overnight..
To each of the 19 library pools was added 250 mg of scavenging resin (see
below) and the mixtures were let stand overnight. The resin was filtered
off and the samples were concentrated. A solution of borane-
methylsulfide (0.5 M in dioxane, 0.5 mL) was added and the pools were
heated to 50 °C for 3 h. The solvent was removed and the residue was
dissolved in 2 mL of 1% HCl / methanol and heated to 50 °C overnight.
The solvent was removed and the pools were redissolved in 2 mL of 1%
HCi / methanol then concentrated again. This was repeated a second
time. The samples were then lyophilized from acetonitrile / water to give
the 19 pools indexed by their Z subunits as light brown solids.
-195-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Scavenging resin was prepared by reacting commercially
available aminomethyl polystyrene ( 10 grams) with a solution of
pentafluorophenyl chlorothionoformate and DIEA (50 mL of 0.5 M in
each reagent, in methylene chloride). After 30 min the resin was
washed with methylene chloride and treated with a 0.5 M solution of
DIEA in DMF. After washing with DMF, THF, methylene chloride and
ether (3x50 mL each) the scavenging resin was dried under nitrogen.
Y Subunits:
O cl o
cl o '- / cl\s,, ' '',
F F I ~ ..~ O
CI
F
yS~CI CI g o $ o _
~~o ~ ~ ~ ~ o=
o ~I cl
o s CI o..s~ ~ ~ C~ F SCI O
I \ / _
c> ~S ~
CI F FOi y CI C1H~N
o ~ O _ cl
CI C/ ~ ~ F Q=S \ / D=S O F
CI
C CI F F Fi3C
F CI
I i
/ \ ~ a=s=o
s-cl ~ cl ors ~ ~ o s ~
O ~ ~ CI CI CI C
o ~ II
,N
O
CI
-196 -
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
_ N3C
/-\ / \ ,-.~ _ /-\
NVN ~ ~ NUN \ / N\.-/N
N N
V
G-
~N ~ ~ ~ G- N , C~CHa
N ~ C1 p '~~ N 1'
/ ...- / N C~-
~N ~ ~ F ~N ~ ~ I
_ ~ N
O' CI S%O
CH3 N,J p~ cH3
N
N
N /
N o=S V
0
~CH~ / CHI
N"O ~ I CIH
GH
N~ O N O~
CH3 N~o
~I oH,
N~ ~ N~ o
The compounds in Examples 221 and 222 were prepared
from 4-carboxy-3-phenylpiperidine (which can be obtained from N-
benzyl-4-carbomethoxy-3-phenyl-piperidine (from Example 101, Step C)
by hydrolysis with aqueous sodium hydroxide followed by hydrogenolysis
in ethanol with 10% palladium on carbon and hydrogen gas) followed by
reaction with cyclohexanecarbonyl chloride to provide 1-cyclohexyl-
carbonyl-4-carboxy-3-phenylpiperidine. Treatment of the latter
compound with the appropriate secondary amine in the presence of EDC
followed by reduction of both amides with borane-methyl sulfide then
provided the indicated final compounds.
-197-
CA 02298813 2000-O1-28
WO 99/09984 PCT/US98/17755
Rz
~N ~
Rx
N
Ry
EXAMPLE Rx Ry RZ HPLC MS
(Method)a (M+I)
221 Ph Pn ~ 4.27 min 473.4
222 Ph Ph ~.QJ~N~4.11 min 532.4
i
While the invention has been described and illustrated with
reference to certain particular embodiments thereof, those skilled in the
art will appreciate that various adaptations, changes, modifications,
substitutions, deletions, or additions of procedures and protocols may be
made without departing from the spirit and scope of the invention. For
example, effective dosages other than the particular dosages as set forth
herein above may be applicable as a consequence of variations in the
responsiveness of the mammal being treated for any of the indications
with the compounds of the invention indicated above. Likewise, the
specific pharmacological responses observed may vary according to and
depending upon the particular active compounds selected or whether
there are present pharmaceutical carriers, as well as the type of
formulation and mode of administration employed, and such expected
variations or differences in the results are contemplated in accordance
with the objects and practices of the present invention. It is intended,
therefore, that the invention be defined by the scope of the claims which
follow and that such claims be interpreted as broadly as is reasonable.
-198-